Abstract
Background—
Ebstein anomaly and tricuspid valve dysplasia are rare congenital tricuspid valve malformations associated with high perinatal mortality. The literature consists of small, single-center case series spanning several decades. We performed a multicenter study to assess the outcomes and factors associated with mortality after fetal diagnosis in the current era.
Methods and Results—
Fetuses diagnosed with Ebstein anomaly and tricuspid valve dysplasia from 2005 to 2011 were included from 23 centers. The primary outcome was perinatal mortality, defined as fetal demise or death before neonatal discharge. Of 243 fetuses diagnosed at a mean gestational age of 27±6 weeks, there were 11 lost to follow-up (5%), 15 terminations (6%), and 41 demises (17%). In the live-born cohort of 176 live-born patients, 56 (32%) died before discharge, yielding an overall perinatal mortality of 45%. Independent predictors of mortality at the time of diagnosis were gestational age <32 weeks (odds ratio, 8.6; 95% confidence interval, 3.5–21.0; P<0.001), tricuspid valve annulus diameter z-score (odds ratio, 1.3; 95% confidence interval, 1.1–1.5; P<0.001), pulmonary regurgitation (odds ratio, 2.9; 95% confidence interval, 1.4–6.2; P<0.001), and a pericardial effusion (odds ratio, 2.5; 95% confidence interval, 1.1–6.0; P=0.04). Nonsurvivors were more likely to have pulmonary regurgitation at any gestational age (61% versus 34%; P<0.001), and lower gestational age and weight at birth (35 versus 37 weeks; 2.5 versus 3.0 kg; both P<0.001).
Conclusion—
In this large, contemporary series of fetuses with Ebstein anomaly and tricuspid valve dysplasia, perinatal mortality remained high. Fetuses with pulmonary regurgitation, indicating circular shunt physiology, are a high-risk cohort and may benefit from more innovative therapeutic approaches to improve survival.
Keywords: Ebstein anomaly; echocardiography; heart defects, congenital; mortality; tricuspid valve insufficiency
Ebstein anomaly and tricuspid valve dysplasia (EA/TVD) are rare congenital tricuspid valve malformations1,2 characterized by apical displacement of the valve or leaflet deformation, respectively.3 In severe cases of dysplasia, the tricuspid valve orifice may become unguarded.4,5 Although there is a broad morphologic spectrum, these malformations lead to the same hemodynamic burden, namely, tricuspid regurgitation (TR) and its pathophysiological sequelae. Although older children and adults with EA/TVD may be asymptomatic for years, the diagnosis of EA/TVD in the perinatal period carries a poor prognosis. In the fetus, severe TR may lead to cardiomegaly, hydrops, and arrhythmia, with demise rates as high as 48%.6 Among prenatally diagnosed patients who survive to live birth, hemodynamic instability, cyanosis, and respiratory compromise are common. Although neonatal mortality approached 80 to 85% in early series,7,8 various single-center series have reported reduced mortality in the past 2 decades, ranging from 17% to 56%.9–12
Fetal risk factors for perinatal mortality have been identified, including lack of antegrade flow across the pulmonary valve and retrograde duct flow9–12 and fetal distress.12 However, studies were limited by small sample sizes, with Yu et al12 reporting the largest series to date with 46 prenatally diagnosed patients. The prognostic value of indices of cardiomegaly, such as the cardiothoracic area (CTA) ratio and the right atrial area index, has been mixed.9–13 Importantly, hemodynamic factors with potentially important influences on perinatal mortality, such as right ventricular pressure and the presence of pulmonary regurgitation (PR), have not been investigated.
Since our understanding of fetuses diagnosed with EA/TVD is derived from single-center experiences, often spanning long periods of time to capture sufficient patients for analysis, we embarked on a multicenter study across North America to investigate a large cohort of fetuses in the contemporary era of medical and surgical management. In the present study, we aim to report the current perinatal outcomes of EA/TVD and to investigate clinical and fetal echocardiographic predictors of perinatal mortality.
Methods
Study Design and Patient Selection
We performed a multicenter, retrospective cohort study among fetuses diagnosed in the recent era, January 2005 to September 2011. Thirty fetal echocardiography laboratories in the United States and Canada were contacted; 23 agreed to participate. Each center obtained institutional review board approval with a waiver of informed consent. De-identified clinical and echocardiographic data, from the time of diagnosis to neonatal hospital discharge (if applicable), were sent to the lead site and core laboratory at Boston Children’s Hospital for review and analysis. If multiple fetal echocardiograms were performed, the first and last complete studies in gestation were reviewed.
We included singleton fetuses with EA/TVD. We excluded fetuses with complex associated cardiac anomalies, such as congenitally corrected transposition of the great arteries or left heart obstruction, those with hypertrophied and hypoplastic right ventricles consistent with pulmonary atresia with intact ventricular septum, and those with TR with an otherwise normal-appearing tricuspid valve (TV), as may occur with cardiomyopathy or premature closure of the ductus arteriosus.
Primary Outcome
The primary outcome was perinatal mortality, defined as fetal demise or neonatal death before hospital discharge. A secondary outcome was fetal demise only. Additional outcomes limited to descriptive analyses included lost to follow-up and elective termination of pregnancy. Patients were categorized as lost to follow-up when the mother did not return to the diagnosing center for additional prenatal care or delivery, and there was no documentation of fetal demise or termination.
Clinical Variables
Prenatal clinical variables included maternal age at diagnosis; maternal medical history, including medications and substance use; family history of congenital heart disease; gestational age (GA) at diagnosis (determined from the time of the first complete fetal echocardiogram at the referring center); results of amniocentesis, obstetric ultrasound, and magnetic resonance imaging (MRI), if performed; diagnosis of arrhythmia; and administration of medications for fetal indication, such as digoxin for arrhythmia or steroids for prematurity. Peripartum data consisted of whether delivery was performed for fetal distress and the mode of delivery. Postnatal variables included GA and weight at birth, Apgar scores, and the diagnosis of a genetic abnormality or syndrome.
Fetal Echocardiograms
The fetal echocardiograms were reviewed independently at the lead site by readers blinded to clinical outcome. Both the first and last complete studies in gestation were reviewed, if applicable. To assess for cardiomegaly, the CTA ratio was calculated from measurement in the transverse plane. Two-dimensional measurements were obtained at the hinge points of the atrioventricular valves in diastole and of the semilunar valves in systole. These measurements were converted to gestational age–adjusted z-scores based on internally collected Boston Children’s Hospital data. TR was assessed qualitatively as none, mild (narrow jet of regurgitation), or ≥moderate (broad jet) and quantitatively by calculating the vena contracta width in the lateral dimension. The maximum TR jet velocity in systole was measured if an adequate Doppler pattern was present. The TV inflow pattern was documented as normal if biphasic and abnormal if partially fused or monophasic. The presence or absence of PR was noted. Right ventricular (RV) and left ventricular (LV) systolic function were assessed qualitatively and categorized as normal or depressed. The presence of a pericardial or pleural effusion, ascites, and subcutaneous/scalp edema were noted; fetal hydrops was defined as fluid accumulation in ≥2 of these compartments.
Statistical Analysis
Descriptive data are reported as frequency (%), mean±standard deviation, or median (minimum, maximum) where appropriate. Data on patients LFTU and pregnancy terminations are presented but were not compared with the other groups owing to the small numbers. As mentioned previously, such cases were also not included in analyses of associations with mortality.
Univariable analysis of factors associated with perinatal mortality was performed using the independent samples t test or the Wilcoxon rank sum test for continuous variables and χ2 analysis or Fisher exact test for categorical variables where appropriate. Variables with a 2-sided P value of <0.05 were considered for inclusion in forward-stepwise multivariable logistic regression models. For continuous variables, such as CTA ratio, TV annulus diameter z-score, and TR jet velocity, receiver operator characteristic analyses were performed to identify thresholds that optimized the sensitivity and specificity of their association with survival. However, for a variety of reasons, including normal distribution of the values, lack of clear threshold effects with relatively modest explanatory power, and clinically inconvenient cut-off values, only the continuous forms of these variables were included in the multivariable models. Based on preliminary analyses treating the center as a fixed effect in the models, we decided not to perform mixed modeling methods to adjust for potential center-level effects. Odds ratios (OR) are presented with 95% confidence intervals (CIs).
We first investigated variables associated with perinatal mortality at the time of diagnosis. Because the GA at diagnosis ranged considerably and the disease is often progressive, we sought to determine whether there was a natural GA threshold for differences in perinatal outcome. Receiver operator characteristic analysis was performed, with GA at diagnosis grouped into 2-week bins and plotted against outcome. This analysis demonstrated significantly worse outcomes among fetuses diagnosed at <32 weeks. Therefore, subanalyses were performed for perinatal mortality, and fetal demise, as well, in this higher-risk GA cohort. Finally, we analyzed the subset of patients who had late-gestation echocardiograms performed, defined as >30 weeks, to determine whether there were unique late-gestation variables related to third trimester fetal demise or neonatal death. If both the first and last fetal echocardiograms in gestation occurred after 30 weeks, the later study was used.
Results
Of 307 patients sent to the lead site, 261 were eligible for inclusion and 243 had at least 1 complete fetal echocardiogram available for review. The median number of patients from each center was 9 (1–23). Table 1 demonstrates the maternal and fetal characteristics of the population. Only 1 mother was known to be taking lithium at the time of diagnosis.
Table 1.
Maternal and Fetal Characteristics (n=243)
| Maternal characteristics | |
| Maternal age at diagnosis, y | 29.9±6.4 |
| Family history of congenital heart disease (n=227)* | 23 (10) |
| Fetal characteristics | |
| Gestational age at diagnosis, wk | 27.0±5.9 |
| Amniocentesis performed (n=176)* | 55 (31) |
| Abnormal† | 11 (20) |
| Trisomy 21 | 8 (15) |
| Other | 3 (5) |
| Obstetric ultrasound performed (n=186)* | 176 (95) |
| Abnormal/extracardiac anomaly | 50 (28) |
| Fetal MRI performed (n=196)* | 18 (9) |
| Abnormal fetal lung volumes | 10 (56) |
| Arrhythmia | 15 (6) |
| Treatment for arrhythmia or ventricular dysfunction | 18 (7) |
| Administration of steroids for lung maturity | 44 (18) |
Values are presented as mean±standard deviation or frequency (%).
Numbers in parentheses indicate the number of fetuses for which the variable in question (affirmative or negative) was reported.
Abnormal among studies performed.
The overall perinatal outcome for the 243 fetuses is depicted in Figure 1. There were 15 known pregnancy terminations, all but 1 of which occurred at less than 24 weeks GA (median, 21 weeks GA [14–31]). There were 41 fetal demises, and the median GA at demise was 30 weeks (22–37). Among live-born patients, information regarding 2 patients was insufficient to determine survival to neonatal hospital discharge; thus, further analysis refers to the cohort of 241 fetuses. Excluding pregnancies categorized as LFTU or termination, perinatal mortality was 45% (97/215). When all eligible fetuses were analyzed, regardless of the availability of a complete echocardiogram (n=261), the proportion of patients in each perinatal outcome group was unchanged.
Figure 1.
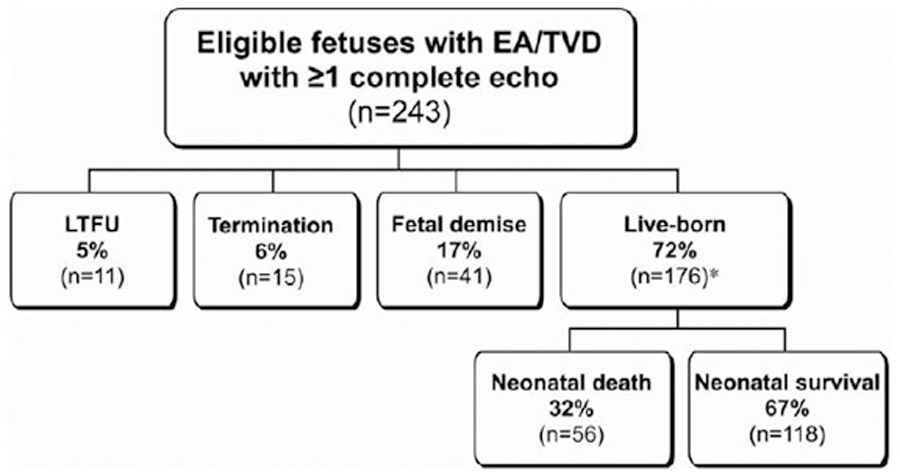
Flow diagram depicting perinatal outcomes for the population of eligible fetuses with at least 1 complete fetal echocardiogram for review. *Data were insufficient to determine survival to neonatal hospital discharge for 2 patients. EA/TVD indicates Ebstein anomaly/tricuspid valve dysplasia; and LTFU, lost to follow-up.
Table 2 demonstrates the characteristics of the various perinatal outcome groups at the time of diagnosis. The lost to follow-up and termination groups were diagnosed at an earlier GA. Aside from earlier diagnosis, however, cardiovascular and other parameters in these fetuses did not appear to differ markedly from fetuses that survived or experienced a perinatal death. Perinatal outcome according to GA at diagnosis is depicted in Figure 2. Perinatal mortality was higher among fetuses diagnosed at <32 weeks GA in comparison with those diagnosed later. Among fetuses diagnosed at <32 weeks, mortality was 54% and without a significant survival difference according to GA at diagnosis.
Table 2.
Characteristics of the Perinatal Outcome Groups at the Time of Prenatal Diagnosis (n=241)
| LTFU (n=11) | Termination (n=15) | Fetal Demise or Neonatal Death (n=97) | Neonatal Survival (n=118) | |
|---|---|---|---|---|
| Maternal age at diagnosis, y | 29.9±6.0 | 32.6±5.5 | 29.6±6.3 | 29.9±6.6 |
| GA at diagnosis, wk | 24.5±5.3 | 20.4±4.1 | 25.9±5.0 | 29.1±5.8 |
| CTA ratio | 0.40±0.12 | 0.38±0.12 | 0.47±0.12 | 0.40±0.11 |
| TV annulus diameter z-score | 4.7±3.3 | 6.0±3.1 | 6.8±3.0 | 4.3±2.7 |
| ≥ Moderate TR | 9 (82) | 13 (87) | 86 (89) | 84 (71) |
| TR jet velocity,* m/s (n=174) | 2.4±0.6 | 2.0±0.7 | 2.3±0.6 | 2.8±0.7 |
| No antegrade PV flow | 5 (45) | 9 (60) | 65 (67) | 46 (39) |
| Pulmonary regurgitation | 4 (36) | 3 (20) | 44 (45) | 24 (20) |
| Depressed RV function | 3 (27) | 5 (33) | 37 (38) | 31 (26) |
| Depressed LV function | 3 (27) | 1 (7) | 22 (23) | 11 (9) |
| Pericardial effusion | 3 (27) | 2 (13) | 33 (34) | 17 (14) |
| Hydrops | 0 (0) | 1 (7) | 10 (10) | 4 (4) |
Values are presented as mean±standard deviation or frequency (%). CTA indicates cardiothoracic area; GA, gestational age; LTFU, lost to follow-up; LV, left ventricle; PV, pulmonary valve; RV, right ventricle; TR, tricuspid regurgitation; and TV, tricuspid valve.
Data not available for all patients.
Figure 2.
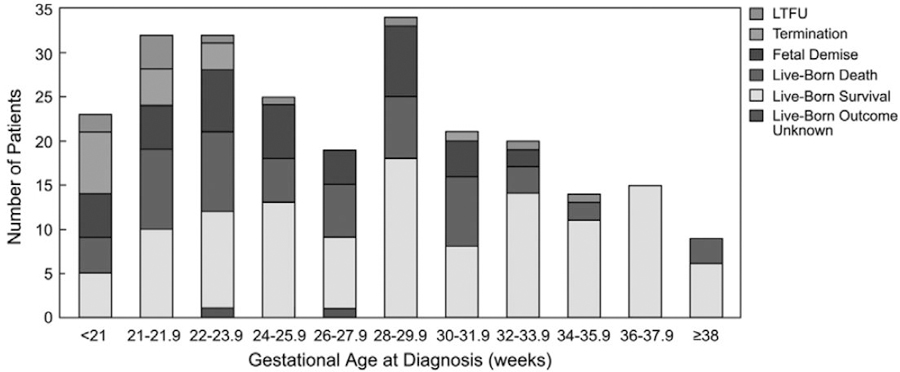
Perinatal outcome by gestational age at diagnosis. LTFU indicates lost to follow-up.
Clinical and fetal echocardiographic factors associated with perinatal mortality at the time of diagnosis are summarized in Table 3. In the entire cohort, significant associations by multivariable analysis included GA at diagnosis <32 weeks, larger TV annulus diameter z-score, the presence of PR, and a pericardial effusion. Multivariable analysis limited to the cohort of fetuses diagnosed at <32 weeks revealed nearly the same associations with perinatal mortality: TV annulus diameter z-score (nonsurvivors 6.9±3.1 versus survivors 4.0±2.5; P<0.001; OR, 1.4; 95% CI, 1.2–1.6) and the presence of PR (44% versus 14%; P=0.04; OR, 3.4; 95% CI, 1.0–11.2). The relationship among CTA ratio, TV annulus diameter z-score, and perinatal mortality in this cohort, with or without PR, is demonstrated in Figure 3. Fetuses with larger CTA ratios and TV annulus diameter z-scores and PR fared worse. With regard to fetal demise only, the same variables as the cohort diagnosed <32 weeks GA were found to be significant by multivariable analysis: TV annulus diameter z-score (7.7±3.0 versus 4.9±2.9; P<0.001; OR, 1.3; 95% CI, 1.2–1.5) and PR (54% versus 22%; P=0.008; OR, 3.0; 95% CI, 1.3–6.9).
Table 3.
Clinical and Echocardiographic Factors at the Time of Prenatal Diagnosis Associated With Perinatal Mortality (n=215)
| Nonsurvivors (n=97) | Survivors (n=118) | Unadjusted UVA OR (95% CI) | Unadjusted UVA P Value | MVA* OR (95% CI) | MVA P value | |
|---|---|---|---|---|---|---|
| Maternal age at diagnosis, y | 29.6±6.3 | 29.9±6.6 | 0.99 (0.95–1.04) | 0.79 | ||
| GA at diagnosis, wk | 25.9±5.0 | 29.1±5.8 | 0.90 (0.85–0.95) | <0.001 | ||
| GA at diagnosis <24 wk | 39 (40) | 26 (22) | 2.4 (1.3–4.3) | 0.004 | ||
| GA at diagnosis <32 wk | 87 (90) | 73 (62) | 5.6 (2.6–11.8) | <0.001 | 8.6 (3.5–21.0) | <0.001 |
| CTA ratio† | 0.47±0.12 | 0.40±0.11 | 1.6‡ (1.2–2.0) | <0.001 | ||
| ≥ Moderate TR | 86 (89) | 84 (71) | 3.1 (1.5–6.5) | 0.003 | ||
| TV annulus diameter z-score | 6.8±3.0 | 4.3±2.7 | 1.4 (1.2–1.5) | <0.001 | 1.3 (1.2–1.5) | <0.001 |
| TR vena contracta diameter, cm | 0.5±0.2 | 0.4±0.2 | 4.5 (1.2–17.8) | 0.03 | ||
| TR jet velocity,† m/s (n=152) | 2.3±0.6 | 2.9±0.7 | 0.25 (0.14–0.45) | <0.001 | ||
| Abnormal TV inflow pattern† (n=171) | 28 (36%) | 31 (33%) | 1.2 (0.6–2.3) | 0.60 | ||
| PV annulus z-score | −1.9±1.7 | −1.6±1.4 | 0.9 (0.8–1.1) | 0.25 | ||
| No antegrade PV flow | 65 (67) | 46 (39) | 3.6 (2.0–6.4) | <0.001 | ||
| Retrograde duct flow | 78 (80) | 62 (53) | 4.7 (2.4–9.1) | <0.001 | ||
| Pulmonary regurgitation | 44 (45) | 24 (20) | 3.4 (1.9–6.3) | <0.001 | 2.9 (1.4–6.2) | <0.001 |
| Depressed RV function | 37 (38) | 31 (27) | 1.8 (1.0–3.2) | 0.05 | ||
| Depressed LV function | 22 (23) | 11 (9) | 2.9 (1.3–6.2) | 0.009 | ||
| Pericardial effusion | 32 (33) | 16 (14) | 3.1 (1.6–6.2) | 0.001 | 2.5 (1.1–6.0) | 0.04 |
| Hydrops | 10 (10) | 4 (4) | 3.3 (1.0–10.8) | 0.05 | ||
| Arrhythmia | 4 (4) | 9 (8) | 0.5 (0.2–1.7) | 0.52 |
Values are presented as mean±standard deviation or frequency (%). CI indicates confidence interval; CTA, cardiothoracic area; GA, gestational age; LV, left ventricle; MVA, multivariable analysis; OR, odds ratio; PV, pulmonary valve; RV, right ventricle; TR, tricuspid regurgitation; TV, tricuspid valve; and UVA, univariable analysis.
MVA models were developed with and without TR jet velocity owing to the relatively large number of patients with missing data. In the model with TR jet velocity, this potential covariate was not retained; therefore, only the model without TR jet velocity is presented.
Data not available for all patients.
ORs are for every 0.1 difference in the CTA ratio (ie, 0.4 vs 0.5).
Figure 3.
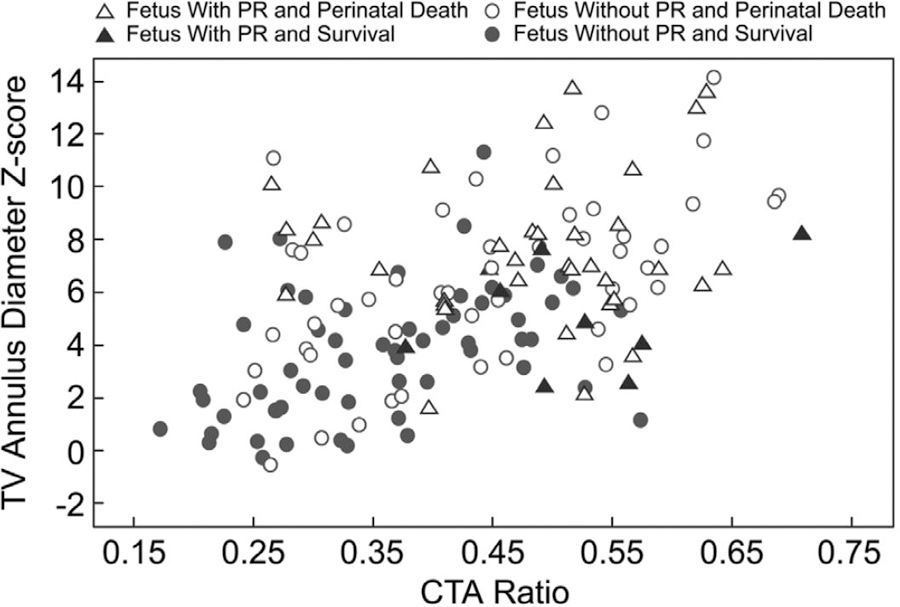
Scatterplot of tricuspid valve (TV) annulus diameter z-score and cardiothoracic area (CTA) ratio for patients diagnosed <32 weeks gestational age. PR indicates pulmonary regurgitation.
Three-quarters of the cohort (n=165) had a late-gestation fetal echocardiogram performed. Among these patients, there were 4 lost to follow-up and 1 termination; therefore, 160 patients were included in the analysis. The late-gestation factors associated with perinatal mortality are presented in Table 4. Fetuses who had PR at any point (at the time of diagnosis or at the time of the last fetal echocardiogram) were nearly twice as likely to experience fetal demise or neonatal death (61% versus 34%; P<0.001).
Table 4.
Late Gestation (>30 Weeks) Fetal Echocardiographic Findings Associated With Perinatal Mortality (n=160)
| Nonsurvivors (n=54) | Survivors (n=106) | Unadjusted UVA OR (95% CI) | Unadjusted UVA P Value | MVA Model 1 OR (95% CI) | MVA Model 1 P value | MVA Model 2* OR (95% CI) | MVA Model 2 P value | |
|---|---|---|---|---|---|---|---|---|
| GA at last fetal echocardiogram, wk | 33.4±2.6 | 34.6±2.4 | 0.80 (0.69–0.93) | 0.003 | ||||
| CTA ratio† (n=152) | 0.53±0.1 | 0.45±0.1 | 1.9‡ (1.4–2.6) | <0.001 | 1.7‡ (1.1–2.6) | 0.01 | 1.5‡ (1.1–2.1) | 0.05 |
| ≥ Moderate TR† (n=160) | 49 (91) | 79 (75) | 3.2 (1.2–9.0) | 0.02 | ||||
| TV annulus diameter z-score | 6.5±2.9 | 4.9±3.0 | 1.2 (1.1–1.3) | 0.002 | ||||
| TR jet velocity,† m/s (n=110) | 2.3±0.7 | 3.0±0.7 | 0.23 (0.12–0.47) | <0.001 | 0.28 (0.13–0.55) | <0.001 | ||
| No antegrade PV flow† (n=159) | 39 (74) | 49 (46) | 3.2 (1.5–6.5) | 0.002 | 3.6 (1.3–10.2) | 0.017 | ||
| Retrograde duct flow† (n=157) | 47 (89) | 62 (60) | 5.3 (2.1–13.5) | <0.001 | ||||
| Pulmonary regurgitation | 24 (44) | 27 (26) | 2.4 (1.2–4.7) | 0.016 | ||||
| Depressed RV function | 33 (61) | 36 (34) | 3.1 (1.6–6.0) | 0.001 | ||||
| Depressed LV function | 21 (39) | 15 (14) | 3.9 (1.8–8.4) | <0.001 | 3.6 (1.5–8.5) | 0.003 | ||
| Pericardial effusion | 24 (44) | 26 (25) | 2.5 (1.2–4.9) | 0.01 | ||||
| Hydrops | 12 (22) | 7 (7) | 4.0 (1.5–11.0) | 0.006 |
Values are presented as mean±standard deviation or frequency (%). CI indicates confidence interval; CTA, cardiothoracic area; GA, gestational age; LV, left ventricle; MVA, multivariable analysis; OR, odds ratio; PV, pulmonary valve; RV, right ventricle; TR, tricuspid regurgitation; TV, tricuspid valve; and UVA, univariable analysis.
MVA model 1 was developed with TR jet velocity and MVA model 2 was developed without TR jet velocity, owing to the relatively large number of patients with missing data.
Data not available for all patients.
ORs are for every 0.1 difference in the CTA ratio (ie, 0.4 vs 0.5).
Among live-born patients (n=174), the mean GA and weight at birth of nonsurvivors and survivors were 35.4±0.4 weeks versus 37.5±0.2 weeks and 2.5±0.1 kg versus 3.0±0.1 kg (both P<0.001), respectively. Figure 4 demonstrates the relationship among GA at diagnosis, GA at birth, and birth weight. Of 120 patients with further perinatal information for analysis, nonsurvivors were more likely to be induced for fetal distress (53% versus 28%; P=0.01) and to deliver by cesarean delivery (66% versus 48%; P=0.04). In the delivery room, Apgar scores also differed between nonsurvivors and survivors: 1-minute 3 versus 8 and 5-minute 6 versus 8, respectively (both P<0.001).
Figure 4.
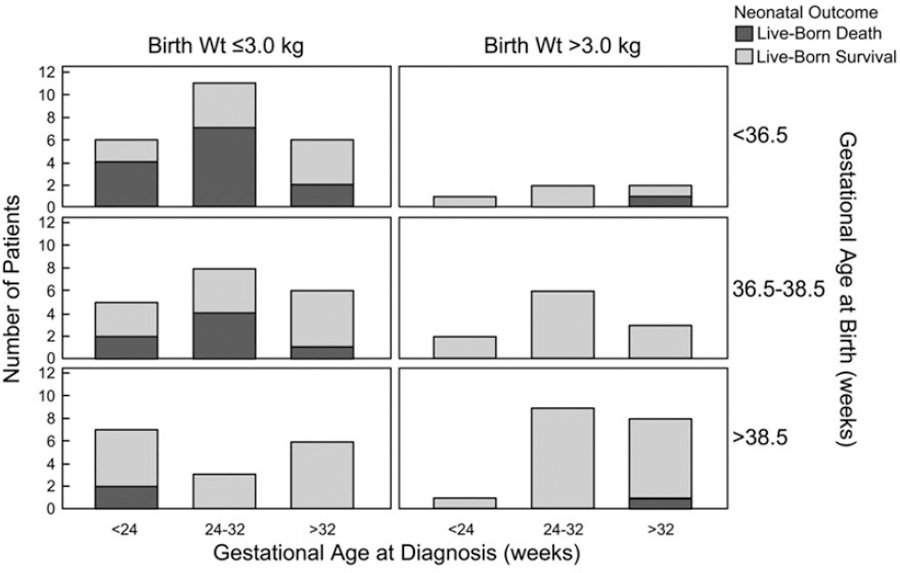
Neonatal outcome by gestational age at diagnosis, gestational age at birth, and birth weight (Wt).
After birth, the families of 7 patients elected for comfort care only. An additional 11 patients died within 24 hours of life; 4 deaths occurred in the delivery room. Of the remaining cohort (n=154; 2 patients with insufficient data), 71 patients (46%) had a neonatal procedure: cardiac surgery (n=53), interventional catheterization (n=12), or both (n=6). There was no significant mortality difference between patients who underwent a neonatal procedure versus those who did not (30% versus 20%; P=0.19), nor between patients who underwent cardiac surgery versus those who did not (32% versus 20%; P=0.09). Of the 38 patients who died beyond 24 hours of life, the median age at the time of death was 16 days (1–180). There were 9 patients who survived >30 days but died before hospital discharge. Nineteen patients (11%) were confirmed to have a genetic abnormality or syndrome before death or hospital discharge. The most common abnormalities detected were Trisomy 21 (n=11), CHARGE syndrome (n=2), and chromosome 1p36 deletion (n=2). Despite 2 patients receiving comfort care only, there was no significant relationship between the presence of a genetic abnormality or syndrome and mortality (P=0.69). Finally, there was no substantial difference in mortality by center, with both high-volume and low-volume centers performing similarly.
Discussion
In this contemporary, multicenter cohort of fetuses with EA/TVD, the perinatal mortality was 45%, substantially higher than other forms of congenital heart disease in the current era.14,15 However, in comparison with previous single-center series of EA/TVD over the past several decades, a greater proportion of fetuses survived to birth. This may be attributable to a combination of factors, including a lower rate of pregnancy termination and progress in prenatal diagnosis resulting in greater likelihood of identifying less severely affected fetuses. Importantly, neonatal mortality, at approximately one-third of live-born patients, was relatively unchanged in comparison with previous reports.6,9–11
Predictors of Mortality
While previous smaller studies focused on the lack of antegrade flow across the pulmonary valve or retrograde duct flow as the most important markers of mortality risk,9–12 we found that the presence of PR at the time of diagnosis was a more powerful hemodynamic indicator of risk by multivariable analysis. We believe that this finding is critical to understanding the pathophysiological aberrations that occur in the fetus with EA/TVD. In the context of severe TR, lack of antegrade flow across the pulmonary valve leads to retrograde duct flow, which increases afterload on the RV. Meanwhile, the chronic volume overload from TR leads to RV dilatation, which may accentuate underlying myocardial abnormalities. Because TR favors the high-capacitance systemic venous circuit, inadequate filling ensues, and, as a result of diminished preload, reduced contractile reserve, and increased afterload, the RV fails to generate adequate pressure. Lack of antegrade pulmonary valve flow, retrograde duct flow, and low RV pressure, all of which were found to be significantly associated with adverse outcome in our univariable analysis, appear to be necessary precursors to the development of PR. In this manner, PR serves as the final insult that completes the circular shunt, whereby an ineffective shunt bypasses both the systemic and pulmonary capillary beds, produces systemic steal, and contributes to further volume overloading of the RV. As a result of septal displacement and adverse ventriculo-ventricular interactions, there may be subsequent ineffective volume loading and dysfunction of the LV,16 leading to reduced systemic cardiac output, diminished end-organ perfusion, acidosis, and ultimately, demise in the fetus.
The other risk factors for perinatal mortality at the time of diagnosis by multivariable analysis were GA <32 weeks, larger TV annulus diameter z-score, and a pericardial effusion. More severe disease was likely detected by obstetric ultrasound earlier in pregnancy as a result of cardiomegaly or a fluid collection. In addition, perhaps the longer a fetus is exposed to the progressive pathophysiological aberrations of EA/TVD in utero, the more likely the patient is to experience its adverse sequelae. The relationship between mortality and TV annulus diameter z-score, insofar as a larger dimension was associated with a worse prognosis, underscores the importance of not only a stretched annulus from severe TR but also that of right-sided dilation and cardiomegaly that result from its hemodynamic burden. Right-sided dilation may cause ineffective LV loading and inadequate systemic cardiac output from direct compression, as mentioned previously, as well as by distortion of atrial septal anatomy diminishing right to left flow at the atrial level. Meanwhile, cardiomegaly may restrict pulmonary growth and lead to deleterious cardiorespiratory interactions, particularly in the postnatal setting. In this population of fetuses with structural heart disease, a pericardial effusion, regardless of size,17 was likely a harbinger of hydrops, which is well known to be associated with high perinatal mortality.18–20
The size of the TV annulus likely indicates a similar set of conditions as previously described by Celermajer et al.21 However, the right atrial area index was initially developed in neonates, and its ability to predict mortality among fetuses has yielded inconsistent results.9,10,12,13 We suspect that this variability may relate to the difficulty of performing the contour tracing in fetuses. An advantage of using the linear TV annulus diameter instead is that it is a commonly measured index on routine fetal echocardiography, which may augment its feasibility and reproducibility.
Among fetuses with late-gestation echocardiograms, similar findings were identified with regard to the relationship between the underlying pathophysiology and perinatal mortality. In particular, low TR jet velocity (indicative of low RV pressure) suggests a failing RV, which augments the development of PR; and a larger CTA ratio, like TV annulus size, is a marker of cardiac chamber enlargement. In the second model without TR jet velocity (owing to the number of patients with missing data), both TV annulus size and CTA ratio, and depressed LV function, as well, were significantly associated with perinatal mortality. The relationship between depressed LV function in late gestation to mortality not only builds on previous investigations of LV function in smaller cohorts of fetuses with EA/TVD,22,23 but also underscores the significance of adverse ventriculo-ventricular interactions and decreased systemic cardiac output that may occur with disease progression.
Although it may be more clinically useful to propose multivariable models with dichotomization of continuous variables, the data, as depicted in Figure 3, did not support the robustness of this approach in this complex patient population with progressive disease. The continuous variables were kept intact to provide the best statistical resolution, but they also highlight the importance of understanding the pathophysiology that defines the highest-risk population of fetuses. There are several explanations as to why slightly different factors emerged in the late-gestation analysis. First, it was a smaller cohort of patients who, by virtue of surviving to the third trimester, exhibited a survival bias in comparison with the entire cohort at the time of diagnosis. Although the cohort was smaller, findings such as depressed LV function were more prevalent owing to the progressive nature of EA/TVD in utero. Finally, the development of specific features, that is, a pericardial effusion or hydrops, along with fetal distress and maternal illness in the third trimester, may have prompted earlier delivery, with a subset of those patients surviving to neonatal discharge.
Prenatal Evaluation and Monitoring
The identification of fetuses at high risk for perinatal mortality serves several important purposes. From a diagnostic standpoint, prenatal evaluation of such fetuses should be comprehensive. Surprisingly, less than one-third of patients in the current study had an amniocentesis performed. Among those, 20% of the results were abnormal. A similar proportion of patients were found to have at least 1 abnormality on obstetric ultrasound. Although the presence of a genetic abnormality or syndrome was not associated with perinatal mortality in our series, such a diagnosis, with or without extracardiac anomalies, has been shown to increase morbidity or mortality in other forms of congenital heart disease.24–26 The availability of this information may be critical to help families make informed decisions. In addition, the role of MRI to quantify fetal lung volumes in this population has not been studied, and, given the high proportion of patients with abnormal findings in the current study, it may be worthy of further investigation.
Serial monitoring should occur frequently in high-risk fetuses, especially in the third trimester. As opposed to other forms of congenital heart disease in which fetuses have relatively stable physiology in utero, fetuses with EA/TVD may rapidly decompensate. Therefore, a low threshold for performing nonstress testing or a biophysical profile is likely warranted.27 An abnormal test, however, must be interpreted cautiously in the context of GA and the relative risks associated with premature delivery, which both the current study and others22,23 suggest is associated with worse neonatal outcome. Unfortunately, the optimal timing of delivery cannot be definitively addressed by the current data, and a prospective study with a standardized approach among multiple centers may help guide clinical practice.
Future Directions
We have demonstrated that fetuses with PR, suggestive of a circular shunt, are at increased risk for mortality. An important question that arises is whether we can use our understanding of the natural history and pathophysiology of EA/TVD to improve outcomes. In particular, could better identification of high-risk patients prenatally help stratify management postnatally? The ductus arteriosus plays a critical role in the development and maintenance of the circular shunt, and early ductal closure has been associated with improved survival in neonates.28 However, if a fetus manifests low RV pressure with PR, then medical management or ductal closure alone may not be viable strategies. More innovative coordination of care, from the delivery room to the operating room, may need to be explored. While further analysis of neonatal risk factors and postnatal management strategies among this cohort is ongoing, the current study identifies a high-risk fetal subgroup that deserves a more thoughtful and comprehensive approach, beginning from the time of prenatal diagnosis.
Limitations
This study has several important limitations. Since it was performed retrospectively, there was no standardized protocol for fetal echocardiograms. The frequency of prenatal follow-up and the timing of the last complete echocardiogram in gestation varied considerably, which likely impacted the analysis of the late-gestation predictors of mortality in particular. The absence of a protocol also meant that some studies lacked important Doppler data, such as TR jet velocity and aortic valve indices, which can be used to quantify LV myocardial performance and output. Although an additional model was created to account for the missing TR jet velocity data, there were too few patients with aortic valve indices for analysis. Therefore, the relationship between decreased LV myocardial performance or output and mortality could not be adequately investigated in this series. Recognition of the potential importance of these factors may help guide clinicians to incorporate them into routine practice and ultimately to include them in prospective research protocols.
Categorization of TV morphology as EA or TVD was difficult in a large proportion of fetuses for a variety of reasons, including relatively modest apical displacement of the septal leaflet and poor image resolution. The correlation between echocardiographic and pathological findings for distinguishing these TV malformations has been demonstrated to be imperfect,7 and given the shared hemodynamic burden of TR, the distinction was not pursued in our analyses. The high proportion of patients with genetic abnormalities or syndromes and abnormal obstetric ultrasound findings may relate to ascertainment bias, because all patients were prenatally diagnosed. Perinatal management, including the indication for, timing, and mode of delivery, varied among institutions, which made it difficult to draw definitive conclusions regarding the influence of these potentially modifiable variables on mortality. Finally, based on preliminary analyses suggesting that center-level confounding was not important in the multivariable models, mixed modeling methods were not used to adjust for potential center-level effects.
Conclusions
Despite major advances in prenatal care and the diagnosis and management of congenital heart disease over the past several decades, perinatal mortality in fetuses with EA/TVD remains alarmingly high. Patients at highest risk are those with PR, suggestive of circular shunt physiology, as well as earlier GA at diagnosis, larger TV annulus size, and a pericardial effusion. This high-risk cohort may be targeted for novel prenatal therapies or a more unified perinatal approach, including timing of delivery and postnatal interventional strategy. Given the rarity and severity of EA/TVD diagnosed in fetal life, multicenter collaboration will remain essential for helping improve the care of these complex patients.
CLINICAL PERSPECTIVE.
Ebstein anomaly and tricuspid valve dysplasia are rare congenital tricuspid valve malformations associated with high perinatal mortality. Previous literature has consisted of single-center series, often spanning several decades. We report a series of 243 fetuses with Ebstein anomaly or tricuspid valve dysplasia from 23 centers across North America in the recent era. Unfortunately, perinatal mortality remained high at 45%, with one-third of patients not surviving to neonatal hospital discharge. Independent risk factors for mortality included gestational age at diagnosis of <32 weeks, larger tricuspid valve annulus z-score, the presence of pulmonary regurgitation, and a pericardial effusion. The presence of pulmonary regurgitation, in particular, signifies circular shunt physiology, which often culminated in mortality. An understanding of this unique physiology in utero may help clinicians better counsel expectant parents, develop and pursue novel treatment and perinatal management strategies, and ultimately improve mortality for fetuses with this rare and complex disease.
Acknowledgments
Sources of Funding
Dr Freud is supported by the National Institutes of Health (T32-HL007572) and the Kenrose Kitchen Foundation. This research was also supported by the Ossenbeck family of New York.
Footnotes
Guest Editor for this article was James S. Tweddell, MD.
Disclosures
None.
Contributor Information
Lindsay R. Freud, Boston Children’s Hospital, Department of Cardiology, Harvard Medical School, Department of Pediatrics, MA.
Maria C. Escobar-Diaz, Boston Children’s Hospital, Department of Cardiology, Harvard Medical School, Department of Pediatrics, MA.
Brian T. Kalish, Boston Children’s Hospital, Department of Medicine, Harvard Medical School, Department of Pediatrics, MA.
Rukmini Komarlu, Boston Children’s Hospital, Department of Cardiology, Harvard Medical School, Department of Pediatrics, MA.
Michael D. Puchalski, Primary Children’s Hospital, University of Utah School of Medicine, Department of Pediatrics, Division of Cardiology, Salt Lake City.
Edgar T. Jaeggi, Hospital for Sick Children, University of Toronto Faculty of Medicine, Department of Pediatrics, Division of Cardiology, ON, Canada.
Anita L. Szwast, Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Department of Pediatrics, Division of Cardiology, PA.
Grace Freire, All Children’s Hospital, Johns Hopkins Medicine, Department of Pediatrics, Division of Cardiology, St. Petersburg, FL.
Stéphanie M. Levasseur, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center, Department of Pediatrics, Division of Cardiology, New York.
Ann Kavanaugh-McHugh, Monroe Carell Jr. Children’s Hospital, Vanderbilt University School of Medicine, Department of Pediatrics, Division of Cardiology, Nashville, TN.
Erik C. Michelfelder, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Department of Pediatrics, The Heart Institute, OH.
Anita J. Moon-Grady, UCSF Benioff Children’s Hospital, University of California-San Francisco School of Medicine, Department of Pediatrics, Division of Cardiology.
Mary T. Donofrio, Children’s National Medical Center, Division of Cardiology, George Washington University School of Medicine and Health Sciences, Department of Pediatrics, Washington, DC.
Lisa W. Howley, Heart Institute Children’s Hospital Colorado, University of Colorado School of Medicine, Department of Pediatrics, Division of Cardiology, Aurora.
Elif Seda Selamet Tierney, Lucile Packard Children’s Hospital, Stanford School of Medicine, Department of Pediatrics, Division of Cardiology, Palo, Alto, CA.
Bettina F. Cuneo, Advocate Children’s Hospital, Oak Lawn, IL.
Shaine A. Morris, Texas Children’s Hospital, Baylor College of Medicine, Department of Pediatrics, Division of Cardiology, Houston.
Jay D. Pruetz, Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Department of Pediatrics, Division of Cardiology.
Mary E. van der Velde, University of Michigan Congenital Heart Center, C.S. Mott Children’s Hospital, University of Michigan Medical School, Department of Pediatrics, Division of Cardiology, Ann Arbor.
John P. Kovalchin, Nationwide Children’s Hospital, Ohio State University College of Medicine, Department of Pediatrics, Division of Cardiology, Columbus.
Catherine M. Ikemba, Children’s Medical Center, University of Texas Southwestern Medical School, Department of Pediatrics, Division of Cardiology, Dallas.
Margaret M. Vernon, Seattle Children’s Hospital, University of Washington School of Medicine, Department of Pediatrics, Division of Cardiology, Seattle.
Cyrus Samai, Children’s Healthcare of Atlanta, Emory University School of Medicine, Department of Pediatrics, Division of Cardiology, Atlanta, GA.
Gary M. Satou, Mattel Children’s Hospital, University of California-Los Angeles David Geffen School of Medicine, Department of Pediatrics, Division of Cardiology.
Nina L. Gotteiner, Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, Department of Pediatrics, Division of Cardiology, IL.
Colin K. Phoon, Hassenfeld Children’s Hospital, New York University School of Medicine, Department of Pediatrics, Division of Cardiology New York.
Norman H. Silverman, UCSF Benioff Children’s Hospital, University of California-San Francisco School of Medicine, Department of Pediatrics, Division of Cardiology; Lucile Packard Children’s Hospital, Stanford School of Medicine, Department of Pediatrics, Division of Cardiology, Palo, Alto, CA.
Doff B. McElhinney, Lucile Packard Children’s Hospital, Stanford School of Medicine, Department of Pediatrics, Division of Cardiology, Palo, Alto, CA.
Wayne Tworetzky, Boston Children’s Hospital, Department of Cardiology, Harvard Medical School, Department of Pediatrics, MA.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Lang D, Oberhoffer R, Cook A, Sharland G, Allan L, Fagg N, Anderson RH. Pathologic spectrum of malformations of the tricuspid valve in prenatal and neonatal life. J Am Coll Cardiol 1991;17:1161–1167. [DOI] [PubMed] [Google Scholar]
- 4.Mohan JC, Passey R, Arora R. Echocardiographic spectrum of congenitally unguarded tricuspid valve orifice and patent right ventricular outflow tract. Int J Cardiol 2000;74:153–157. [DOI] [PubMed] [Google Scholar]
- 5.Wong KK, Farquharson DI, Duncan WJ. Unguarded tricuspid valvar orifice in the fetus. Cardiol Young 2004;14:557–559. doi: 10.1017/S1047951104005141. [DOI] [PubMed] [Google Scholar]
- 6.Hornberger LK, Sahn DJ, Kleinman CS, Copel JA, Reed KL. Tricuspid valve disease with significant tricuspid insufficiency in the fetus: diagnosis and outcome. J Am Coll Cardiol 1991;17:167–173. [DOI] [PubMed] [Google Scholar]
- 7.Sharland GK, Chita SK, Allan LD. Tricuspid valve dysplasia or displacement in intrauterine life. J Am Coll Cardiol 1991;17:944–949. [DOI] [PubMed] [Google Scholar]
- 8.Yetman AT, Freedom RM, McCrindle BW. Outcome in cyanotic neonates with Ebstein’s anomaly. Am J Cardiol 1998;81:749–754. [DOI] [PubMed] [Google Scholar]
- 9.McElhinney DB, Salvin JW, Colan SD, Thiagarajan R, Crawford EC, Marcus EN, del Nido PJ, Tworetzky W. Improving outcomes in fetuses and neonates with congenital displacement (Ebstein’s malformation) or dysplasia of the tricuspid valve. Am J Cardiol 2005;96:582–586. doi: 10.1016/j.amjcard.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Andrews RE, Tibby SM, Sharland GK, Simpson JM. Prediction of outcome of tricuspid valve malformations diagnosed during fetal life. Am J Cardiol 2008;101:1046–1050. doi: 10.1016/j.amjcard.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Barre E, Durand I, Hazelzet T, David N. Ebstein’s anomaly and tricuspid valve dysplasia: prognosis after diagnosis in utero. Pediatr Cardiol 2012;33:1391–1396. doi: 10.1007/s00246-012-0355-z. [DOI] [PubMed] [Google Scholar]
- 12.Yu JJ, Yun TJ, Won HS, Im YM, Lee BS, Kang SY, Ko HK, Park CS, Park JJ, Gwak M, Kim EA, Kim YH, Ko JK. Outcome of neonates with Ebstein’s anomaly in the current era. Pediatr Cardiol 2013;34:1590–1596. doi: 10.1007/s00246-013-0680-x. [DOI] [PubMed] [Google Scholar]
- 13.Roberson DA, Silverman NH. Ebstein’s anomaly: echocardiographic and clinical features in the fetus and neonate. J Am Coll Cardiol 1989;14:1300–1307. [DOI] [PubMed] [Google Scholar]
- 14.Khoshnood B, De Vigan C, Vodovar V, Goujard J, Lhomme A, Bonnet D, Goffinet F. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983–2000: a population-based evaluation. Pediatrics 2005;115:95–101. doi: 10.1542/peds.2004-0516. [DOI] [PubMed] [Google Scholar]
- 15.Fesslova V, Brankovic J, Boschetto C, Masini A, Prandstraller D, Perolo A, Ventriglia F, Macerola S, Crepaz R, Romeo C, De Luca F, Previtera A, Errico G. Changed outcomes of fetuses with congenital heart disease: new Italian multicentre study [published online ahead of print June 13, 2014]. J Cardiovasc Med (Hagerstown). doi: 10.2459/JCM.0b013e328365c325. Accessed February 8, 2015. [DOI] [PubMed]
- 16.Ishii T, Tworetzky W, Harrild DM, Marcus EN, McElhinney DB. Left ventricular function and geometry in fetuses with severe tricuspid regurgitation. Ultrasound Obstet Gynecol 2012;40:55–61. doi: 10.1002/uog.10115. [DOI] [PubMed] [Google Scholar]
- 17.Slesnick TC, Ayres NA, Altman CA, Bezold LI, Eidem BW, Fraley JK, Kung GC, McMahon CJ, Pignatelli RH, Kovalchin JP. Characteristics and outcomes of fetuses with pericardial effusions. Am J Cardiol 2005;96:599–601. doi: 10.1016/j.amjcard.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Wieczorek A, Hernandez-Robles J, Ewing L, Leshko J, Luther S, Huhta J. Prediction of outcome of fetal congenital heart disease using a cardiovascular profile score. Ultrasound Obstet Gynecol 2008;31:284–288. doi: 10.1002/uog.5177. [DOI] [PubMed] [Google Scholar]
- 19.Turgal M, Ozyuncu O, Boyraz G, Yazicioglu A, Sinan Beksac M. Non-immune hydrops fetalis as a diagnostic and survival problems: what do we tell the parents? J Perinat Med 2015;43:353–358. doi: 10.1515/jpm-2014-0094. [DOI] [PubMed] [Google Scholar]
- 20.Derderian SC, Jeanty C, Fleck SR, Cheng LS, Peyvandi S, Moon-Grady AJ, Farrell J, Hirose S, Gonzalez J, Keller RL, MacKenzie TC. The many faces of hydrops. J Pediatr Surg 2015;50:50–54; discussion 54. doi: 10.1016/j.jpedsurg.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celermajer DS, Cullen S, Sullivan ID, Spiegelhalter DJ, Wyse RK, Deanfield JE. Outcome in neonates with Ebstein’s anomaly. J Am Coll Cardiol 1992;19:104120100331046. [DOI] [PubMed] [Google Scholar]
- 22.Inamura N, Taketazu M, Smallhorn JF, Hornberger LK. Left ventricular myocardial performance in the fetus with severe tricuspid valve disease and tricuspid insufficiency. Am J Perinatol 2005;22:91–97. doi: 10.1055/s-2005-837739. [DOI] [PubMed] [Google Scholar]
- 23.Lasa JJ, Tian ZY, Guo R, Rychik J. Perinatal course of Ebstein’s anomaly and tricuspid valve dysplasia in the fetus. Prenat Diagn 2012;32:245–251. doi: 10.1002/pd.2939. [DOI] [PubMed] [Google Scholar]
- 24.Anaclerio S, Di Ciommo V, Michielon G, Digilio MC, Formigari R, Picchio FM, Gargiulo G, Di Donato R, De Ioris MA, Marino B. Conotruncal heart defects: impact of genetic syndromes on immediate operative mortality. Ital Heart J 2004;5:624–628. [PubMed] [Google Scholar]
- 25.Michielon G, Marino B, Oricchio G, Digilio MC, Iorio F, Filippelli S, Placidi S, Di Donato RM. Impact of DEL22q11, trisomy 21, and other genetic syndromes on surgical outcome of conotruncal heart defects. J Thorac Cardiovasc Surg 2009;138:565–570.e2. doi: 10.1016/j.jtcvs.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Patel A, Hickey E, Mavroudis C, Jacobs JP, Jacobs ML, Backer CL, Gevitz M, Mavroudis CD. Impact of noncardiac congenital and genetic abnormalities on outcomes in hypoplastic left heart syndrome. Ann Thorac Surg 2010;89:1805–1813; discussion 1813. doi: 10.1016/j.athoracsur.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, Lacey S, Lee W, Michelfelder EC Sr, Rempel GR, Silverman NH, Spray TL, Strasburger JF, Tworetzky W, Rychik J; American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Young and Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular and Stroke Nursing. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 28.Wald RM, Adatia I, Van Arsdell GS, Hornberger LK. Relation of limiting ductal patency to survival in neonatal Ebstein’s anomaly. Am J Cardiol 2005;96:851–856. doi: 10.1016/j.amjcard.2005.05.035. [DOI] [PubMed] [Google Scholar]


