Abstract
Background
Methotrexate is considered the preferred disease‐modifying anti‐rheumatic drug (DMARD) for the treatment of rheumatoid arthritis, but controversy exists on the additional benefits and harms of combining methotrexate with other DMARDs.
Objectives
To compare methotrexate and methotrexate‐based DMARD combinations for rheumatoid arthritis in patients naïve to or with an inadequate response (IR) to methotrexate.
Methods
We systematically identified all randomised controlled trials with methotrexate monotherapy or in combination with any currently used conventional synthetic DMARD , biologic DMARDs, or tofacitinib. Three major outcomes (ACR50 response, radiographic progression and withdrawals due to adverse events) and multiple minor outcomes were evaluated. Treatment effects were summarized using Bayesian random‐effects network meta‐analyses, separately for methotrexate‐naïve and methotrexate‐IR trials. Heterogeneity was explored through meta‐regression and subgroup analyses. The risk of bias of each trial was assessed using the Cochrane risk of bias tool, and trials at high risk of bias were excluded from the main analysis. The quality of evidence was evaluated using the GRADE approach. A comparison between two treatments was considered statistically significant if its credible interval excluded the null effect, indicating >97.5% probability that one treatment was superior.
Main results
158 trials with over 37,000 patients were included. Methotrexate‐naïve: Several treatment combinations with methotrexate were statistically superior to oral methotrexate for ACR50 response: methotrexate + sulfasalazine + hydroxychloroquine (“triple therapy”), methotrexate + several biologics (abatacept, adalimumab, etanercept, infliximab, rituximab, tocilizumab), and tofacitinib. The estimated probability of ACR50 response was similar between these treatments (range 56‐67%, moderate to high quality evidence), compared with 41% for methotrexate. Methotrexate combined with adalimumab, etanercept, certolizumab, or infliximab was statistically superior to oral methotrexate for inhibiting radiographic progression (moderate to high quality evidence) but the estimated mean change over one year with all treatments was less than the minimal clinically important difference of five units on the Sharp‐van der Heijde scale. Methotrexate + azathioprine had statistically more withdrawals due to adverse events than oral methotrexate, and triple therapy had statistically fewer withdrawals due to adverse events than methotrexate + infliximab (rate ratio 0.26, 95% credible interval: 0.06 to 0.91). Methotrexate‐inadequate response: In patients with an inadequate response to methotrexate, several treatments were statistically significantly superior to oral methotrexate for ACR50 response: triple therapy (moderate quality evidence), methotrexate + hydroxychloroquine (low quality evidence), methotrexate + leflunomide (moderate quality evidence), methotrexate + intramuscular gold (very low quality evidence), methotrexate + most biologics (moderate to high quality evidence), and methotrexate + tofacitinib (high quality evidence). There was a 61% probability of an ACR50 response with triple therapy, compared to a range of 27% to 64% for the combinations of methotrexate + biologic DMARDs that were statistically significantly superior to oral methotrexate. No treatment was statistically significantly superior to oral methotrexate for inhibiting radiographic progression. Methotrexate + cyclosporine and methotrexate + tocilizumab (8 mg/kg) had a statistically higher rate of withdrawals due to adverse events than oral methotrexate and methotrexate + abatacept had a statistically lower rate of withdrawals due to adverse events than several treatments.
Authors' conclusions
We found moderate to high quality evidence that combination therapy with methotrexate + sulfasalazine+ hydroxychloroquine (triple therapy) or methotrexate + most biologic DMARDs or tofacitinib were similarly effective in controlling disease activity and generally well tolerated in methotrexate‐naïve patients or after an inadequate response to methotrexate. Methotrexate + some biologic DMARDs were superior to methotrexate in preventing joint damage in methotrexate‐naïve patients, but the magnitude of these effects was small over one year.
Plain language summary
Methotrexate alone or in combination with other medications for rheumatoid arthritis
Researchers in the Cochrane Collaboration conducted a review of the effects of methotrexate either taken alone or with other disease‐modifying anti‐rheumatic drugs (DMARDs) for people with rheumatoid arthritis. After searching for all relevant studies up to January 19, 2016, they found 158 studies with over 37,000 people. These studies were published between 1985 and 2016 and were between 12 weeks and 2 years in duration. Their findings are summarised below:
In people with rheumatoid arthritis, compared to taking methotrexate alone:
‐The combination of methotrexate + sulfasalazine + hydroxychloroquine and methotrexate + most biologic DMARDs improves disease activity. Other treatment combinations (methotrexate + hydroxychloroquine, methotrexate + leflunomide, methotrexate + gold injections) may improve disease activity in people who do not respond to methotrexate alone.
‐The combinations of methotrexate + several biologic DMARDs (adalimumab, etanercept, certolizumab, or infliximab) reduces joint damage (as seen on x‐rays) slightly over one year in patients who have not taken methotrexate before.
‐The combinations of methotrexate + azathioprine, methotrexate + cyclosporine and methotrexate + tocilizumab (8 mg/kg) probably increases the chance of stopping the medication due to a side effect.
What is rheumatoid arthritis and what is methotrexate and other disease‐modifying anti‐rheumatic drugs?
When you have rheumatoid arthritis (RA) your immune system, which normally fights infection, attacks the lining of your joints. This makes your joints swollen, stiff and painful. There is no cure for RA at present, so the treatments aim to relieve pain and stiffness and improve your ability to move. Fortunately, there are many medications that can control the disease effectively. These medications are known as disease‐modifying anti‐rheumatic drugs, or DMARDs. Methotrexate is widely regarded as the preferred DMARD for most patients with RA as it works well for most patients and is generally well tolerated. Methotrexate can be used by itself or can be combined with other DMARDs. These other DMARDs include medications that have been available and used for many years (such as sulfasalazine and hydroxychloroquine), as well as newer more expensive treatments (biologic DMARDs and tofacitinib). It is important to understand how all of these treatments compare in terms of the benefits and side effects.
What happens to people with rheumatoid arthritis who take methotrexate combined with other disease‐modifying anti‐rheumatic drugs?
A) People who have not taken methotrexate before:
ACR 50 (number of tender or swollen joints and other outcomes such as pain and disability)
‐61 out of 100 people who took methotrexate + sulfasalazine + hydroxychloroquine and 56 to 67 people out of 100 who took methotrexate + biologic DMARDs or tofacitinib experienced improvement in the symptoms of their rheumatoid arthritis, compared to 41 out of 100 people who took methotrexate alone.
X‐rays of the joints:
‐People who took methotrexate combined with adalimumab, etanercept, certolizumab, or infliximab had a small reduction in the progression of joint damage (Sharp‐van der Heijde score) over one year compared to oral methotrexate, but the estimated amount of damage even with oral methotrexate was very small (2.6 point increase).
Stopping the medication due to a side effect
‐36 out of 100 people who took methotrexate + azathioprine had to stop the medication due to a side effect, compared to 8 people out of 100 who took methotrexate alone.
B) People who have taken methotrexate before:
ACR 50 (number of tender or swollen joints and other outcomes such as pain and disability)
‐61 out of 100 people who took methotrexate + sulfasalazine + hydroxychloroquine and 27 to 64 people out of 100 who took methotrexate + biologic DMARDs or tofacitinib experienced improvement in the symptoms of their rheumatoid arthritis, compared to 13 out of 100 people who took methotrexate alone.
X‐rays of the joints:
‐No treatment resulted in a significant reduction in the amount of joint damage seen on x‐rays over one year.
Stopping the medication due to a side effect
‐21 out of 100 people who took methotrexate + cyclosporine and 12 out of 100 people who took methotrexate + tocilizumab (8 mg/kg) had to stop the medication due to a side effect, compared to 7 people out of 100 who took methotrexate alone.
Background
Description of the condition
Rheumatoid arthritis (RA) is a systemic autoimmune disease manifesting primarily as a symmetric and erosive polyarthritis, affecting between 0.5 and 1% of the adult population (Kvien 2004). Patients with RA experience pain, functional limitation and a significant decline in their health‐related quality of life (Kvien 2004). New treatment approaches with early and intensive treatment targeted to a goal of remission or low disease‐activity can significantly improve outcomes (Knevel 2010).
Description of the interventions
Disease‐modifying anti‐rheumatic drugs (DMARDs) target pathways of inflammation responsible for joint swelling and damage and are the cornerstone of treatment for RA. DMARDs can be classified based on their structure and mechanism of action (Smolen 2014). Conventional synthetic DMARDs are derived synthetically without a specific molecular target in mind and found to have activity in the treatment of RA. Methotrexate (MTX) is considered the preferred conventional synthetic DMARD, based on its excellent benefit to toxicity profile (Singh 2016; Smolen 2014a). The conventional synthetic DMARDs most commonly used in combination with methotrexate are hydroxychloroquine, sulfasalazine and leflunomide. Less commonly used conventional synthetic DMARDs include intra‐muscular gold, cyclosporine and azathioprine.
Biologic DMARDs, in contrast to conventional synthetic DMARDs, are derived through biologic processes and are designed to target specific cells or proteins involved in the inflammatory response. Biologic DMARDs are newer treatments for RA, with the first biologic DMARD (etanercept) approved for RA in 1998. Biologic DMARDs currently in use for RA include: abatacept, rituximab and tocilizumab and the anti‐tumour necrosis factor (TNF) inhibitors (adalimumab, certolizumab, etanercept, golimumab, infliximab). Targeted synthetic DMARDs are the most recent class of medications approved for use in RA. Like conventional synthetic DMARDs, they are developed through synthetic methods, and like biologic DMARDS, they are designed to target specific cellular processes. Tofacitinib is the first targeted synthetic DMARD in use for the treatment of RA.
How the intervention might work
The pathophysiology of RA is complex, involving an interplay between genetic risk factors and environmental triggers, and both innate and adaptive immune responses (McInnes 2011). Methotrexate likely works through multiple mechanisms, including the promotion of adenosine‐mediated anti‐inflammatory effects, increased apoptosis of T cells, and reduction of cell proliferation (Braun 2009; Tian 2007). Absorption of oral methotrexate varies between individuals and is improved with parenteral administration, particularly at doses > 15mg/week (Hoekstra 2004; Schiff 2014). The addition of other conventional synthetic DMARDs to methotrexate may improve control of disease activity through the targeting of complementary immunopathologic mechanisms, although little is known about the mechanism of action of many conventional synthetic DMARDs (Bingham 2001). Biologic DMARDs work through inhibition of cytokines involved in RA pathogenesis including TNF‐alpha (anti‐TNF therapy) and interleukin‐6 (tocilizumab), T‐cell co‐stimulation blockade (abatacept) and B‐cell depletion (rituximab). Tofacitinib inhibits Janus kinase 1 (JAK1) and Janus kinase 3 (JAK3), intracellular tyrosine kinases involved in signal transduction.
Why it is important to do this overview
Methotrexate‐based treatments form the core of rheumatoid arthritis (RA) treatment. Methotrexate is recommended as the first DMARD for most patients with RA, and methotrexate co‐prescription is generally recommended when using biologic DMARDs or the recently approved tofacitinib (Singh 2016; Smolen 2014a). Combining methotrexate with other conventional synthetic DMARDs, however, is more controversial. A trial of conventional synthetic DMARD combination therapy prior to biologic therapy is not currently recommended by either major rheumatology guideline, although each provides the option (Singh 2016; Smolen 2014a). Understanding the comparative benefits and harms of these treatments is essential to inform decision‐making, as biologic DMARD therapy and tofacitinib costs over 10‐20 times that of methotrexate and most conventional synthetic DMARDs. It is important to maximize the use of conventional synthetic DMARDs that are safe and effective, while at the same time avoiding unnecessary delays in administering biologic therapy by using treatments of no proven benefit over methotrexate monotherapy.
Network (mixed treatment) meta‐analyses are a natural avenue of comparative effectiveness research, as they combine all direct and indirect evidence to estimate treatment effects between all treatments of interest (Jansen 2011). If treatments A and B are in the same study, there is direct evidence linking A and B. If they are compared in separate studies to a common comparator C, then the A‐C and B‐C studies allow an indirect comparison of A and B. Longer chains of indirect comparisons (A‐B, B‐C, C‐D) are also possible. Considering indirect evidence is critical if a treatment decision must be made and the treatments have not been directly compared in a head‐to‐head trial. Indirect evidence is also important to consider when treatments have been directly compared, as it adds to the entire body of evidence and may help refine the precision in estimation of the treatment effect (Jansen 2011).
A previous Cochrane network meta‐analysis examined the relative effects of different biologic therapies through indirect comparisons, and found some differences between agents (Singh 2009). Our review expands on this, by including combination therapy with methotrexate + conventional synthetic DMARDs. A previous Cochrane traditional (non‐network) meta‐analysis did not find an additional overall benefit with combination therapy over methotrexate alone (Katchamart 2010). By including indirect evidence we expand the evidence base to draw from. For example, three trials have been published that have compared combination therapy with methotrexate + sulfasalazine + hydroxychloroquine versus methotrexate + anti‐TNF therapy (RACAT 2013; SWEFOT 2012; TEAR 2012). The inclusion of these trials in a network meta‐analysis adds indirect evidence on the relative effects of methotrexate + sulfasalazine + hydroxychloroquine compared to all other treatments in the network.
Objectives
To compare methotrexate‐based DMARD treatments for rheumatoid arthritis in patients naïve to or after an inadequate response (IR) to methotrexate.
Methods
This is an overview of reviews, as some of the interventions of interest have been previously evaluated through Cochrane reviews (Katchamart 2010; Singh 2009). It differs from a traditional overview of reviews though, as we are considering all comparisons between any intervention of interest and will therefore include trials not previously reviewed.
Criteria for considering reviews for inclusion
We included RCTs or controlled clinical trials (CCTs) of at least 12 weeks duration that contained any intervention of interest (defined in detail below). After identifying all studies, trials that could not be linked within the network to another intervention of interest through a shared comparator were excluded. For example, if we identified a trial comparing methotrexate to hydroxychloroquine, the trial would be included if another trial was available that compared hydroxychloroquine to methotrexate + hydroxychloroquine (or any other treatment of interest). In this example, the two trials (methotrexate vs. hydroxychloroquine and hydroxychloroquine vs. methotrexate + hydroxychloroquine) allow an indirect comparison to be made between the treatments of interest (methotrexate, methotrexate + hydroxychloroquine). Similarly, if a trial contained more than 2 arms, each arm was included only if it provided direct or indirect evidence on the treatments of interest.
Trials were divided into 3 groups for all analyses, characterised by prior medication exposure: 1) Methotrexate‐naïve; 2) Methotrexate‐inadequate response (IR); 3) Anti‐TNF‐ inadequate response. Methotrexate‐IR and TNF‐IR trials are trials where the protocol required all patients to have tried and failed methotrexate or anti‐TNF therapy respectively. Trials that included a mix of methotrexate‐naïve patients and patients who had tried methotrexate previously were classified as ‘partial‐exposure’ trials and included in the methotrexate‐naïve analyses, unless subgroup data was available separately for methotrexate‐naïve and methotrexate‐IR patients. We subsequently excluded the TNF‐IR trials, as the identified studies formed a network that included only trials of biologic therapy (i.e. – no studies evaluated conventional synthetic DMARD combination therapy). The comparative benefits and harms of biologic therapy (with and without concomitant methotrexate) have been evaluated in 2 previous Cochrane Overview of Reviews (Singh 2009; Singh 2011). We had pre‐specified in the protocol to not report analyses that overlapped completely with this review.
As the networks of methotrexate‐naïve and methotrexate‐IR trials were analysed separately, the decision to include trials that provided only indirect comparisons between interventions of interest was specific to each network. For example, if studies of methotrexate vs. hydroxychloroquine and hydroxychloroquine vs. methotrexate + hydroxychloroquine were identified, they had to be in the same network (both methotrexate naïve or methotrexate‐IR) to provide an indirect comparison and be eligible for inclusion.
Types of studies
Randomised controlled trials (RCTs) or controlled clinical trials (CCTs). CCTs were defined as per the Cochrane Handbook, as trials where randomisation was not truly random (i.e. quasi‐randomised), or trials where double blinding was used but randomisation was not mentioned (Higgins 2011).
Types of participants
Adults (age > 18 years) with RA, according to 1958, 1987 or 2010 classification criteria (Aletaha 2010; Arnett 1988; Ropes 1958).
Types of interventions
The interventions considered in this review were:
Oral methotrexate monotherapy
Parenteral methotrexate monotherapy (subcutaneous or intra‐muscular)
Methotrexate combined with conventional synthetic DMARDs. Conventional synthetic DMARDs were limited to: anti‐malarials (hydroxychloroquine/chloroquine), sulfasalazine, leflunomide, cyclosporine, intra‐muscular gold and azathioprine.
Methotrexate combined with biologic DMARDs, including anti‐TNF inhibitors (adalimumab, certolizumab, etanercept, golimumab, infliximab), abatacept, rituximab, and tocilizumab.
Methotrexate combined with tofacitinib
No dose restriction was applied to conventional synthetic DMARDs, given the variability of dosing in clinical practice. Biologic DMARDs and tofacitinib were limited to currently recommended doses or dose equivalents, specifically:
adalimumab 40 mg subcutaneously every two weeks;
certolizumab 200 mg subcutaneously every two weeks after initial dosing of 400 mg subcutaneously at baseline, two, and four weeks;
etanercept 50 mg subcutaneously every week or 25 mg subcutaneously twice weekly;
golimumab 50 mg subcutaneously every four weeks;
golimumab 2 mg/kg IV every 8 weeks after initial dosing at baseline and 4 weeks;
infliximab 3 mg/kg intravenously every eight weeks after initial dosing at baseline, two and six weeks;
abatacept every four weeks intravenously at ˜10 mg/kg (500 mg in patients < 60 kg, 750 mg in patients 60 kg to 100 kg and 1000 mg in patients > 100 kg), after the initial dosing regimen at baseline, two and four weeks;
abatacept 125 mg subcutaneously with or without an intravenous loading dose of ˜ 10 mg/kg
rituximab, two 1000 mg IV doses two weeks apart;
tocilizumab every four weeks intravenously at 4 mg/kg or 8 mg/kg. The two doses of intravenous tocilizumab were analysed separately, as the approved dosing varies by country (Furst 2013).
tocilizumab 162 mg subcutaneously every week
tofacitinib 5 mg orally twice daily
We excluded trials that evaluated the effect of corticosteroids as an intervention, as this was not the objective of the review. We included trials that required or allowed corticosteroids as part of the treatment arm if the corticosteroids were applied equally across arms.
Types of outcome measures
Major outcomes
Benefits (efficacy)
American College of Rheumatology (ACR)‐50 response (Felson 1995)
Radiographic progression as a continuous variable, measured by Larsen, Sharp or modified Larsen/Sharp scores including Scott‐modified Larsen, Genant‐modified Sharp and van der Heijde‐modified Sharp (Ory 2003)
Harms (toxicity)
Withdrawals due to adverse events, including death.
Minor outcomes
Benefits (efficacy)
ACR‐20 and ACR‐70 responses (Felson 1995).
Disease activity score (DAS) (van der Heijde 1992) or DAS28 (Prevoo 1995). DAS28 in its original form includes 4 variables: ESR, patient global assessment of disease activity, tender and swollen joint counts (DAS28‐4‐ESR). It has been modified to include just 3 variables (excluding global assessment) and with the CRP instead of ESR. We extracted the original DAS28‐4‐ESR if it was reported in multiple formats.
DAS28 remission, defined as a DAS28 <2.6.
European League Against Rheumatism (EULAR) response (moderate or good) (van Gestel 1996)
Radiographic non‐progression, as defined by the study's definition of radiographic progression. If multiple definitions were used in a study, we extracted the result that used the highest threshold for defining progression. We therefore used values in the following order of preference: minimal clinically important difference > smallest detectable change > any progression.
Swollen joint count.
Withdrawals due to inefficacy.
Pain (Visual Analogue Scale).
Functional limitation, as measured by the Health Assessment Questionnaire‐Disability Index (HAQ‐DI) (Fries 1982) or modified HAQ (mHAQ) (Pincus 2005).
Fatigue, as defined by the study.
Harms (toxicity)
Serious adverse events (SAE), as defined by the study.
Serious infections, as defined by the study.
Gastrointestinal (GI) side effects, excluding liver and oral toxicity (e.g. – aphthous ulcers).
Elevated transaminases (ALT or AST). If multiple definitions were provided we used the lowest threshold for an abnormal value.
Hematological toxicity (low haemoglobin, leucopenia/neutropenia or thrombocytopenia). If multiple definitions were provided we used the lowest threshold for an abnormal value.
Combined (efficacy and toxicity)
Combined withdrawals due to inefficacy or adverse events
The three major outcomes were selected to encompass key treatment benefits (improvement in disease activity (ACR‐50), and inhibition of radiographic progression) and harms (withdrawals due to adverse events). Minor efficacy outcomes included other composite measures of disease activity and selected patient‐reported outcomes (pain, functional limitation and fatigue). Toxicity outcomes were limited to selected outcomes that can be evaluated within the context of a randomised trial (i.e. those that occur with sufficient frequency over short‐term follow‐up) and which were hypothesised to have clinically important differences between the interventions of interest. A low threshold for abnormal lab values was chosen to increase the ability to detect a safety signal. All outcomes were prespecified in the protocol.
Search methods for identification of reviews
An electronic database search was performed in MEDLINE (including in process and non‐indexed citations), EMBASE (including EMBASE classic) and Cochrane Central Register of Controlled Trials (CENTRAL) from inception to January 19, 2016. As each intervention contained methotrexate, the search strategies contained subject headings and keywords for "rheumatoid arthritis", "methotrexate" and "randomised controlled trial" (Appendix 1; Appendix 2; Appendix 3). The database search strategies were adapted from a previously published Cochrane review (Katchamart 2010). We also searched the trial registries ClinicalTrials.gov (http://clinicaltrials.gov/) and the International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) using the search term "rheumatoid arthritis AND methotrexate". Finally, we performed hand‐searches for abstracts from American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) conferences (2009‐2015) and reviewed all existing Cochrane reviews to ensure no relevant trials were missed. All languages were included.
Data collection and analysis
Selection of reviews
Two review authors (GH, ChB) independently screened articles for inclusion by title or abstract and full‐text if necessary. Disagreements were resolved by consensus and if not possible, by discussion with a third review author (ClB).
Data extraction and management
Three review authors working in pairs (GH, ChB, DD) abstracted relevant data from included studies on an Excel spreadsheet. A detailed data extraction template was developed and piloted on 5 articles. Changes were made to address inconsistencies and the 5 articles were then re‐extracted. Trial characteristics and baseline patient characteristics were extracted by one author (GH) and confirmed by a second (ChB or DD); outcome data were extracted independently, with disagreements resolved through discussion.
Dichotomous efficacy outcomes were abstracted as the number of patients with an event and the total number of patients in each arm, on an intention‐to‐treat basis as the number of patients randomised to the arm who received at least one dose of medication or, if this was not reported, the total number of patients randomised to the arm. Continuous efficacy outcomes were abstracted as the mean, standard deviation, and the total number of patients for the final values and change in values from baseline. Toxicity outcomes were extracted as the number of events and treatment exposure (person‐years) in each arm. If the total number of events was not reported, we used the total number of patients with at least 1 event, which should be a close approximation for uncommon events.
Assessment of methodological quality of included reviews
The methodological quality of included trials was assessed using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). Studies were graded as having a "low risk", "high risk" or "unclear risk" of bias across the seven specified domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias (Higgins 2011). Other sources of bias included baseline imbalances in co‐interventions (particularly corticosteroids) and other biases identified during the review (not pre‐specified). Outcomes were divided into three categories (radiographic outcomes, withdrawals, other clinical outcomes) as the risk of bias could differ across several domains, as discussed below. For each of the three outcome categories we also judged an overall risk of bias. The overall ROB rating included a judgment across all domains for that outcome, although not all domains were equally weighted. The main domains that affected the overall ROB were blinding and incomplete outcome data.
The domains “blinding of participants and personnel” and “blinding of outcome assessment” were assessed separately for radiographic and non‐radiographic outcomes (which included withdrawals and all other clinical outcomes). Blinding of the outcome assessor occurs separately for radiographic versus other outcomes, and the impact of not blinding participants on the risk of bias for radiographic outcomes was felt to be “unclear”, whereas it was judged to be “high” for all other outcomes. The domain “incomplete outcome data” was assessed separately for each of the 3 outcome categories. The proportion of missing data, the balance of missing data between treatment arms and the methods of handling missing data may vary between clinical and radiographic outcomes. The outcome ‘withdrawals’ was judged at low risk of bias even if the withdrawal rate from the trial was high and/or imbalanced between arms. An exception was early‐escape trials, where high or imbalanced rates of early escape resulted in a higher risk of bias.
To improve the consistency between raters we developed a template for the risk of bias (ROB) assessment, based on Cochrane guidance, but with specific context‐specific clarifications and examples. For trials that were previously included in Cochrane reviews and graded using the Cochrane risk of bias, one review author (GH) reassessed the risk of bias to ensure the results agree with those published. If there was a discrepancy in the ratings or if the prior Cochrane review did not distinguish outcome categories as we did, the ROB was assessed independently by a second reviewer (ChB or DD). For studies not included in existing Cochrane reviews, two review authors (GH, and ChB or DD) independently assessed the risk of bias. Any disagreements were resolved by consensus and if not possible, by discussion with a third review author (ChB or DD).
Data synthesis
Data analysis
Random‐effects Bayesian network meta‐analyses were fitted for each outcome measure. The models used account for the correlation in multi‐arm trials, and have been previously published (Ades 2006; Dias 2013). Random‐effects models allow the treatment heterogeneity we expect, given the clinical heterogeneity amongst the trials. Uninformative prior probability distributions were used for all parameters. Markov chain Monte Carlo sampling was used to obtain samples from posterior distributions, with 10,000 burn‐in iterations followed by 10,000 monitoring iterations. Convergence was assessed by running three chains, inspecting the sampling history plots and calculating Gelman–Rubin–Brooks (GBR) statistics (Brooks 1998). Model fit was assessed using residual deviance and Deviance Information Criterion (DIC). All data analyses were performed using R statistical software version 3.1.2 (www.r‐project.org) with rjags package version 3‐14 running Just Another Gibbs Sampler (JAGS) version 3.4.0.
Measures of treatment effect
For the primary analyses, trials with a high risk of bias for that outcome category were excluded. Treatment effects on dichotomous outcomes were evaluated using odds ratios (ORs). For continuous outcomes that were measured on the same or very similar scale (DAS, DAS28, HAQ‐DI/mHAQ, pain VAS), treatment effects were evaluated as mean differences, with final values or change in values from baseline; we used final values if both final and change values were reported. For continuous outcomes measured on different scales (radiographic progression, swollen joint count, fatigue) treatment effects were evaluated using standardised mean differences (SMD). For SMD, we performed separate analyses for change and final values, as it is not recommended to combine the two in the same analysis (Higgins 2011). SMD were estimated by dividing the modeled change or final value in each arm by the within‐trial pooled standard deviation of the change or final value. We summarized withdrawals due to adverse events and other minor toxicity outcomes as rate ratios to allow for differences in exposure between arms in early escape and crossover trials.
For all outcomes we reported the posterior median (point estimate) and 95% credible interval for all treatments effects relative to oral methotrexate. For the major outcomes we reported the effects and probability of superiority for all pair‐wise comparisons. We considered an effect to be ‘significant’ if the 95% credible interval (CrI) excluded the null effect. This equates to a 97.5% probability of superiority (one‐tailed) of one treatment over another.
Dealing with missing data
For all trials, we sought data from clinical trial registries (www.clinicaltrials.gov, http://www.who.int/ictrp/en/), US Food and Drug Administration (FDA), European Medicines Agency (EMEA) and drug manufacturer web sites. For dichotomous outcomes, if only percentages were reported, the actual number of events was calculated from the percentage and total number of patients and rounded to the nearest whole number. For continuous measures, if standard deviations (SD) were not available, they were calculated from standard errors (se), 95% confidence intervals, or exact p‐values from t‐tests, using formulas published in the Cochrane Handbook (Higgins 2011). If only medians and inter‐quartile ranges (IQR) were presented, these were extracted, with the SD calculated as IQR divided by 1.34, which assumes a normal distribution of outcomes. If no variance data was available, we used the baseline standard deviation as the final standard deviation, if available. For toxicity outcomes, if the drug exposure was not available, it was calculated. Withdrawals were assumed to occur at a constant rate, unless specific information was available to permit a more accurate calculation.
All data conversions above were performed using R statistical software version 3.1.2 (www.r‐project.org). Automated functions for each calculation (e.g.‐ converting an exact p‐value to a standard deviation) were developed, validated against examples provided in the Cochrane Handbook (Higgins 2011) and then used by two independent reviewers working in pairs (GH, ChB, DD).
If data were presented only in graphical format it was extracted digitally. Images in the highest resolution available were digitised and extracted using the software program GraphClick (version 3.0.2, Arizona Software). If another outcome or another time point on the same graph was also reported as a numerical value, it was used as an internal validation of the data extraction procedure. All graphical data were extracted by two independent reviewers (GH, ChB) and averaged or corrected if an obvious discrepancy was apparent.
Assessment of heterogeneity
Analyses were performed separately for trials of methotrexate‐naïve and methotrexate‐IR patients, as we judged patients in these two types of trials too clinically heterogeneous to pool. We assessed statistical heterogeneity by calculating the between‐study variance in the random‐effects model. For the major outcomes, we evaluated the consistency of the direct and indirect evidence through ‘node‐splitting’, which separates the direct and indirect evidence for a comparison where both direct and indirect evidence exist (Dias 2010). Direct effects were determined through a Bayesian fixed‐effects model. A fixed‐effect model was used as there were typically too few trials to estimate a between study variance. Node‐splitting for most comparisons was carried out using the R package gemtc (version 0.6); comparisons using standardised mean differences were not supported by the package, so were programmed directly. The results of the node‐splitting analyses were used to inform the GRADE quality assessments, as described below.
Meta‐regression and sensitivity analyses
For ACR50 response we explored clinical heterogeneity through meta‐regression for the following variables separately for methotrexate‐naïve and methotrexate‐IR populations:
Response rate to oral methotrexate (post‐hoc)
Disease duration
MTX dose ≥ 15 mg/week
Duration of trial
Baseline swollen joint count
Baseline HAQ‐DI
Year of publication of trial
Time‐point of assessment
The meta‐regression models included a covariate for the treatment effect relative to oral methotrexate. We assumed that this was the same for all treatments as there were too few trials to specify separate covariate for each comparison. For example, the effect of trial duration on the OR comparing methotrexate + etanercept to methotrexate was assumed to be the same as its effect on the OR comparing methotrexate + adalimumab to methotrexate. If a trial did not contain an arm with oral methotrexate, the trial was included in the analysis, but the treatment effect was not adjusted. For the meta‐regression analysis of the response rate to oral methotrexate, we used the modelled response rate as opposed to the actual response rate in each trial to limit the effect of regression to the mean (Sharp 1996). To determine if there was an association between prior methotrexate use and ACR50 response, we performed an additional meta‐regression analysis where all trials were included and the prior methotrexate status (methotrexate‐naïve versus methotrexate‐IR) was defined through a covariate.
Additional post‐hoc sensitivity analyses were performed. We fitted fixed‐effect models for the major outcome ‘radiographic progression’ as there were few trials available to estimate a random‐effect. To further evaluate the effect of differing time‐points of assessment, we performed analyses using 6‐month and 12‐month data separately, using interim data from a trial if available. We also performed an analysis where we used ‘pre‐rescue’ data instead of end‐trial data for all ‘rescue’ trials; if pre‐rescue data was not reported, the trial was excluded. For the methotrexate‐naïve analysis we performed an additional analysis where we excluded trials that included some patients with prior methotrexate exposure. For the methotrexate‐IR network we included a sensitivity analysis where we included SWEFOT (SWEFOT 2012). SWEFOT was a major trial comparing triple therapy to methotrexate + infliximab that was excluded from our main analysis for ACR50 response because it had a high risk of bias for clinical outcomes, resulting from its open label design and high withdrawal rate.
We also did sensitivity analyses around several modeling assumptions. In the protocol, we had planned to use odds ratios to pool withdrawals due to adverse events. However, we changed the analyses to rate ratios given the differences in exposure between arms in early escape and crossover trials. As a sensitivity analysis, we compared the rate ratios with odds ratios, in which we used the total exposure (in patient months) in each arm as the denominator, instead of the number of patients. The model estimates the effect on the monthly odds of an outcome, assuming independence between months, and should approximate the rate ratio from a Poisson model.
The choice of prior distribution for the between study variance may affect the estimated treatment effects, although this effect has been found to be small in analyses of ten or more studies (Lambert 2005). For the primary analysis, we followed published guidance and chose a prior that was vague but realistic (Lambert 2005). We then did sensitivity analyses using an additional uninformative prior and potentially informative priors of Turner et al (odds ratio for ACR50 response and rate ratio for withdrawals due to adverse events) (Turner 2012) and Rhodes et al (radiographic progression) (Rhodes 2015).
Presentation of key results ("Summary of findings" table)
The three major outcomes were presented in the Summary of Findings (SoF) tables, separately for the methotrexate‐naïve and methotrexate‐IR analyses. We converted the average treatment effect for each outcome into an absolute response by using an assumed (baseline) value for oral methotrexate. For all analyses, the assumed baseline value was the median from a bayesian random effects model of the oral methotrexate arms. For ACR50 response, we used all trials in the analysis to estimate the assumed probability of response. For radiographic progression, we calculated the assumed mean over one year on the Sharp‐van der Heijde scale (van der Heijde 2000) from the trials that reported this outcome for oral methotrexate. We then calculated the absolute effect for each treatment by using this assumed value and the mean differences for each treatment relative to oral methotrexate on the Sharp‐van der Heijde scale, which we calculated by multiplying the standardized mean differences by the pooled within arm standard deviation for studies that used the Sharp‐van der Heijde scale. For withdrawals due to adverse events, we estimated the assumed rate at one year from the available trials and converted it to an absolute probability by using the rate ratio, assuming that the time to withdrawal over one year was exponentially distributed for each person.
We used recently published GRADE (Grading of Recommendations Assessment, Development and Evaluation) guidance for assessing the quality of evidence from a network meta‐analysis (Puhan 2014). First, the quality of evidence for each direct comparison was evaluated using the GRADE domains of study limitations, inconsistency, imprecision, indirectness and publication bias (Guyatt 2008). Second, the quality of the indirect evidence (determined through ‘node‐splitting’ (Dias 2010)) was evaluated by considering the precision of the indirect estimate, the quality of the direct comparisons that formed the indirect evidence and the likelihood of ‘intransitivity’. Intransitivity exists if there is heterogeneity in the trials that form the different comparisons within the indirect evidence. The indirect evidence can be complex. A ‘first‐order’ indirect comparison is formed by 2 trials that share a common treatment arm. A ‘second‐order’ comparison will have 2 intermediary treatments (i.e.‐ the indirect comparison of treatments A and D in trials of A vs B, B vs C and C vs D). For the quality assessment we focused on first‐order comparisons, as recommended by GRADE (Puhan 2014). We then rated the quality of evidence for the network meta‐analysis, based on the quality ratings for the direct and indirect evidence.
Results
This review is also published as an abridged version that presents the results for the major outcomes (Hazlewood 2016). As each article underwent a separate peer‐review process, there are slight differences in the text of the two manuscripts, but the results in both are identical. The abridged version also has several open‐access appendices, which we refer to in this review when appropriate.
Search results
Our search identified 9817 unique records. After title/abstract and full‐text review, 412 potentially eligible records remained (Figure 1). 204 were further excluded because they could not be linked within any network; the comparators for these trials were most commonly experimental (not approved) DMARDs (Figure 1). After excluding the 11 trials in a TNF‐IR population that overlapped with a prior Cochrane review (Table 1), 197 records remained, representing 158 unique trials. Two records were pending classification and not included, as the full‐text article was not available.
1.
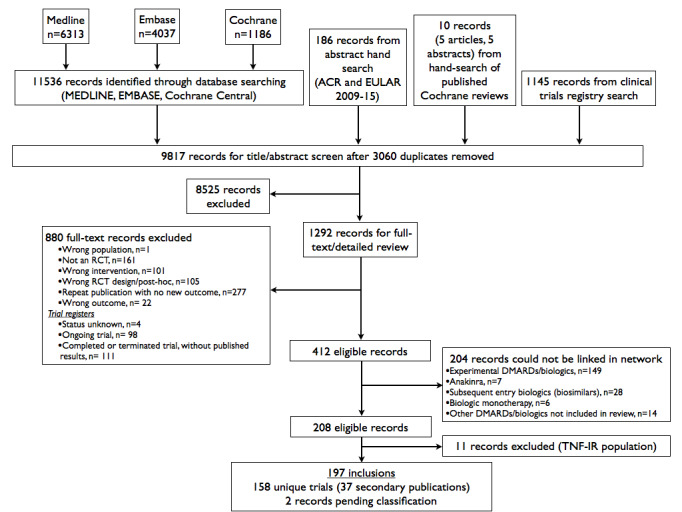
Search flow chart
1. Characteristics of excluded studies.
| Study ID | Reason for exclusion |
| Kim 2012 | RCT that compared ETN+methotrexate vs. ETN+DMARD; DMARD comparator not standardized |
| Kremer 2010 | Wrong dose interval of IV golimumab (given Q 12 weeks) |
| De Stefano 2010 | Unclear study design; compared methotrexate+TNF to LEF+TNF (n=120), but also appears to have randomised pts to 3 different TNF within each subgroup ; no response from e‐mail to corresponding author to confirm |
| Saunders 2008 | Strategy trial that compared triple therapy to step‐up therapy (starting with SSZ). No pre‐switch data available |
| Taylor 2006 | Wrong dose of IFX (5 mg/kg) |
| Keystone 2004 | PBO only given for 8 weeks |
| Cohen 2001 | Optional extension to 2 years of ULTRA |
| Drosos 2000 | Extension study of Drosos 1998 |
| Calguneri 1999 | Compared triple therapy to methotrexate‐based 2‐drug therapy or monotherapy; 2‐drug combination therapy and monotherapy not standardized |
| Willkens 1996 | Open‐label extension of Wilkens 1992 (Optional switch at 24 weeks to open‐label trial) |
| Usova 1993 | Interim report of Sigidin Ya 1994 |
| Peterfy 2011 | Abstract with unclear methods; uncertain is PBO patients crossed over to RTX at 6 months. Only outcome available is SAE, which is only reported at study end. |
| Gao 2004 | Abstract with no outcome of interest |
| Beals 2013 | Abstract only with IFX dosing intervals not reported |
| Dougados 2014 | 52 week results of ACT‐RAY; ACT‐RAY required open‐label addition of DMARDs beyond 24 weeks in a treat‐to target approach; therefore considered a strategy trial beyond 24 weeks. |
| Burmester 2013 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Cohen 2006 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Combe 2013 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Emery 2008 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Furst 2007 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Keystone 2008 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Kume 2012 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Strand 2012 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| NCT01283971 2014 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Strand 2015 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
| Manders 2015 | TNF‐inadequate response trial (all patients required to have failed anti‐TNF therapy) |
Description of included reviews
Overall trial characteristics
The 158 trials included over 37,000 patients across the arms included in this review (Table 2, with full study details available in Web appendix 2 of abridged review, Hazlewood 2016). Seventeen articles were available only as an abstract, although additional data were available through www.clinicaltrials.gov for 9 of these. An additional trial was available only as a trial register with results available through www.clinicaltrials.gov. Ten articles were in languages other than English.
2. Summary of trial characteristics.
| Medication | Studies (N) | Patients (n) | Year published, median (range) | Early escape design, % of studies (n pts) | Trial duration*, wks, median (range) | methotrexatedose >15mg/wk**, % of studies (n pts) | Disease duration, yrs, median (range) | Swollen joint count, median (range) | Low, % (n pts) | Unclear, % (n pts) | High, % (n pts) |
| MTX + biologic DMARDs/tofacitinib | |||||||||||
| MTX + etanercept | 10 | 2448 | 2007 (1999‐2014) | 0% | 38 (12‐52) | 50% (n=1833) | 2 (0.5‐13) | 13.9 (11‐24) | 10% (n=424) | 70% (n=1965) | 20% (n=59) |
| MTX + infliximab | 13 | 2806 | 2006 (2000‐14) | 8% (n=264) | 26 (13‐54) | 46% (n=1990) | 7.6 (0.4‐10.5) | 15 (5‐21.5) | 31% (n=898) | 54% (n=1824) | 15% (n=84) |
| MTX + adalimumab | 16 | 4465 | 2010 (2003‐15) | 38% (n=1936) | 24 (12‐104) | 50% (n=1809) | 2.5 (0.1‐11.7) | 16.3 (8.7‐22.5) | 25% (n=2142) | 69% (n=2258) | 6% (n=65) |
| MTX + rituximab | 4 | 1262 | 2008 (2004‐12) | 25% (n=342) | 24 (24‐104) | 50% (n=683) | 8.6 (0.9‐11.5) | 20.9 (20.2‐21.6) | 25% (n=80) | 75% (n=1182) | 0% |
| MTX + abatacept | 10 | 3612 | 2012 (2005‐15) | 0% | 25 (17‐52) | 60% (n=3014) | 6.4 (0.5‐9.3) | 17.1 (10‐22.4) | 60% (n=2496) | 40% (n=1116) | 0% |
| MTX + tocilizumab | 10 | 4859 | 2012 (2006‐16) | 50% (n=3671) | 24 (16‐52) | 60% (n=2729) | 6.6 (0.4‐9.2) | 13.7 (6.4‐20.1) | 10% (n=553) | 60% (n=2765) | 30% (n=1541) |
| MTX + certolizumab | 7 | 2680 | 2012 (2008‐15) | 71% (n=1561) | 24 (24‐52) | 29% (n=1119) | 6 (0.3‐9.6) | 21 (16.4‐22.5) | 0% | 29% (n=1192) | 71% (n=1488) |
| MTX + golimumab | 6 | 1640 | 2012 (2008‐13) | 83% (n=1570) | 24 (16‐52) | 50% (n=1132) | 6.9 (3.2‐8.7) | 13.5 (11.6‐15.4) | 0% | 100% (n=1640) | 0% |
| MTX + tofacitinib | 4 | 749 | 2012 (2011‐15) | 50% (n=621) | 24 (12‐52) | 50% (n=621) | 8.7 (0.7‐9.1) | 14.7 (14.1‐14.9) | 0% | 75% (n=268) | 25% (n=481) |
| SUBTOTAL | 80 | 24 521 | 2011 (1999‐2016) | 31% (n=9965) | 24 (12‐104) | 50 (n=14930) | 6.3 (0.1‐13) | 16.4 (5‐24) | 21% (n=6593) | 61% (n=14210) | 18% (n=3718) |
| Comparative effectiveness trials | |||||||||||
| Head to head biologic DMARDs/ tofacitinib | 4 | 1658 | 2010 (2006‐2014) | 25% (n=501) | 27 (26‐104) | 50% (n=1077) | 7.8 (1.8‐11.3) | 16.6 (15.9‐20.6) | 25% (n=431) | 50% (n=1147) | 25% (n=80) |
| MTX + biologic DMARDs vs. MTX + conventional synthetic DMARDs | 4 | 1382 | 2012 (2012‐2013) | 0% | 63 (16‐104) | 25% (n=353) | 0.5 (0.3‐5.2) | 12 (11.2‐12.8) | 50% (n=1108) | 0% | 50% (n=274) |
| SUBTOTAL | 8 | 3040 | 2012 (2006‐2014) | 12% (n=501) | 27 (16‐104) | 38% (n=1430) | 5.2 (0.3‐11.3) | 15.9 (11.2‐20.6) | 38% (n=1539) | 25% (n=1147) | 38% (n=354) |
| MTX + conventional synthetic DMARDs | |||||||||||
| MTX + azathioprine | 1 | 209 | 1992 | 0% | 24 | 0% | 8.6 | 17.3 | 0% | 100% (n=209) | 0% |
| MTX + hydroxychloroquine/ chloroquine | 7 | 452 | 2005 (1993‐2012) | 0% | 26 (12‐52) | 0% | 4 (0.3‐7.7) | 10.7 (8.2‐27.3) | 14% (n=82) | 14% (n=40) | 71% (n=330) |
| MTX + sulfasalazine | 6 | 639 | 2000 (1994‐2007) | 0% | 52 (24‐76) | 0% | 0.4 (0.2‐5) | 16.7 (9.8‐22.6) | 0% | 67% (n=515) | 33% (n=124) |
| MTX + cyclosporine | 9 | 1100 | 2003 (1995‐2008) | 11% (n=120) | 48 (16‐104) | 22% (n=64) | 1.1 (0.2‐10.3) | 13.6 (11‐19) | 0% | 89% (n=1076) | 11% (n=24) |
| MTX + sulfasalazine + hydroxychloroquine | 4 | 503 | 2005 (1996‐2013) | 0% | 91 (13‐104) | 0% | 4.4 (0.5‐8.6) | 17.1 (9.5‐29.8) | 0% | 75% (n=463) | 25% (n=40) |
| MTX + leflunomide | 3 | 921 | 2006 (2002‐15) | 0% | 24 (16‐36) | 67% (n=455) | 6.2 (0.7‐11.6) | 14.3 (10.7‐18) | 0% | 33% (n=263) | 67% (n=658) |
| MTX + IM gold | 1 | 65 | 2005 | 0% | 48 | 100% (n=65) | 3.2 | 11 | 0% | 100% (n=65) | 0% |
| SUBTOTAL | 31 | 3889 | 2003 (1992‐2015) | 3% (n=120) | 48 (12‐104) | 16% (n=584) | 1.1 (0.2‐11.6) | 13.6 (8.2‐29.8) | 3% (n=82) | 61% (n=2631) | 35% (n=1176) |
| MTX vs conventional synthetic DMARD monotherapy | |||||||||||
| Placebo | 5 | 324 | 1985 (1985‐93) | 20% (n=52) | 14 (12‐18) | 0% | 9.5 (4.8‐14) | 27.5 (24‐30.9) | 0% | 100% (n=324) | 0% |
| Azathioprine | 5 | 257 | 1991 (1987‐94) | 0% | 24 (24‐52) | 0% | 12 (8.7‐13.9) | 14.6 (9.5‐21.9) | 0% | 40% (n=106) | 60% (n=151) |
| IM gold | 5 | 489 | 1991 (1988‐2001) | 0% | 48 (26‐52) | 20% (n=99) | 4 (1.2‐6) | 14 (13.9‐15.2) | 0% | 60% (n=249) | 40% (n=240) |
| sulfasalazine | 2 | 211 | 1998 (1995‐2002) | 0% | 24 | 0% | 4 (1.4‐6.6) | 9.3 | 0% | 50% (n=126) | 50% (n=85) |
| Cyclosporine | 2 | 203 | 1999 (1998‐2000) | 0% | 65 (26‐104) | 50% (n=100) | 3.8 (2.2‐5.5) | 13.1 (12.2‐14) | 0% | 0% | 100% (n=203) |
| Leflunomide | 16 | 3258 | 2002 (1999‐2014) | 12% (n=567) | 24 (12‐52) | 25% (n=927) | 3.9 (0.5‐6.8) | 11.8 (8.2‐16.5) | 0% | 50% (n=1598) | 50% (n=1660) |
| Hydroxychloroquine | 2 | 409 | 2006 (2000‐12) | 0% | 24 | 0% | 1.5 (1‐2.1) | NA | 0% | 0% | 100% (n=409) |
| sc vs. oral MTX | 2 | 467 | 2009 (2008‐10) | 50% (n=383) | 24 | 100% (n=467) | 0.2 | 15 | 0% | 50% (n=383) | 50% (n=84) |
| SUBTOTAL | 39 | 5618 | 2000 (1985‐2014) | 10% (n=1002) | 24 (12‐104) | 21% (n=1593) | 4.5 (0.2‐14) | 14 (8.2‐30.9) | 0% | 51% (n=2786) | 49% (n=2832) |
| TOTAL | 158 | 37 068 | 2008 (1985‐2016) | 20% (n=11 588) | 24 (12‐104) | 35% (n=18537) | 4.8 (0.1‐14) | 15.1 (5‐30.9) | 13% (n=8214) | 57% (n=20774) | 30% (n=8080) |
Abbreviations: DMARD, disease‐modifying anti‐rheumatic drug; IM, intra‐muscular; methotrexate, methotrexate; pts, patients; sc, subcutaneous; SJC, swollen joint count
Trials are grouped by comparator and sorted by the year of the first trial for within each class, for illustrative purposes. Patient characteristics, including the number of patients, only include the arms considered in the review. *Trial duration for efficacy outcomes; some studies had longer follow‐up for safety outcomes **Studies where the dose of methotrexate could be confirmed as >= 15 mg/wk. In some studies, methotrexate was dosed across a range of values that included 15 mg/wk but the actual dose was not provided. See Web appendix 2 of Hazlewood 2016 for further details.
Of the 158 trials, 80 (51%) compared methotrexate + biologic DMARDs or tofacitinib to methotrexate monotherapy or made comparisons of different dosing formulations (subcutaneous or intravenous) of the same biologic DMARD (Table 2). There were only eight ‘comparative effectiveness’ trials, with four providing head‐to‐head comparisons of different biologic DMARDs/tofacitinib and four comparing methotrexate + biologic DMARDs to methotrexate + conventional synthetic DMARD therapy (methotrexate + sulfasalazine + hydroxychloroquine in three of the four trials). Eleven trials were a strategy or crossover design (Web appendix 2 of Hazlewood 2016). An early escape design was more common in trials of methotrexate + biologic DMARDs/tofacitinib with no active comparator (31%) than comparative effectiveness trials of methotrexate + biologic DMARDs/ tofacitinib (12%) or trials of conventional synthetic DMARD combination or monotherapy (3% and 10%) (Table 2). Trials of the four biologic DMARDs/ tofacitinib with the most recent year of first publication (certolizumab, golimumab, tocilizumab, tofacitinib) had high rates of early‐escape design, at ≥ 50% of trials (Table 2).
The trials ranged in duration from 12 to 104 weeks and had similar median disease duration across the medication classes (Table 2). The median baseline swollen joint count across the trials was high at 15, with a similar distribution across medication classes. Methotrexate dosing varied across studies and was variably reported. The dose of methotrexate could be confirmed as >= 15 mg/week in 50% of biologic DMARDs/ tofacitinib trials and only 16% and 21% in trials evaluating methotrexate + conventional synthetic DMARD combination therapy or conventional synthetic DMARD monotherapy. The risk of bias for the main outcome category (non‐radiographic outcomes excluding withdrawals) varied across trials and medication classes (Table 2) and is discussed further in the section “methodological quality of included studies.”
Two trials were performed on an early RA population and included some patients who did not meet established criteria for RA (EMPIRE 2014; tREACH 2013). Both trials had high percentages of patients meeting 2010 criteria (88%) (tREACH 2013) and 94% (EMPIRE 2014) and presented subgroup data for patients meeting 2010 criteria for their main outcomes. We included these trials, using data for the subgroup meeting 2010 criteria, if available.
Characteristics of trial networks
The connections between the trials for the methotrexate‐naïve and methotrexate‐IR populations are presented in Figure 2. The network diagrams include all trials; the actual number of trials for each analysis varied. Two trials had data available for both networks. One trial had subgroup available for methotrexate‐naïve and methotrexate‐IR patients for ACR responses (O'Dell 2002). The other trial was a four‐arm trial (TEAR 2012). Two arms of this trial compared methotrexate + sulfasalazine + hydroxychloroquine versus methotrexate + etanercept in patients’ naïve to methotrexate and were included in the methotrexate‐naïve analysis. The other two arms compared a strategy of adding sulfasalazine + hydroxychloroquine vs. etanercept to methotrexate in patients with an inadequate response to methotrexate and were included in the methotrexate‐IR network.
2.
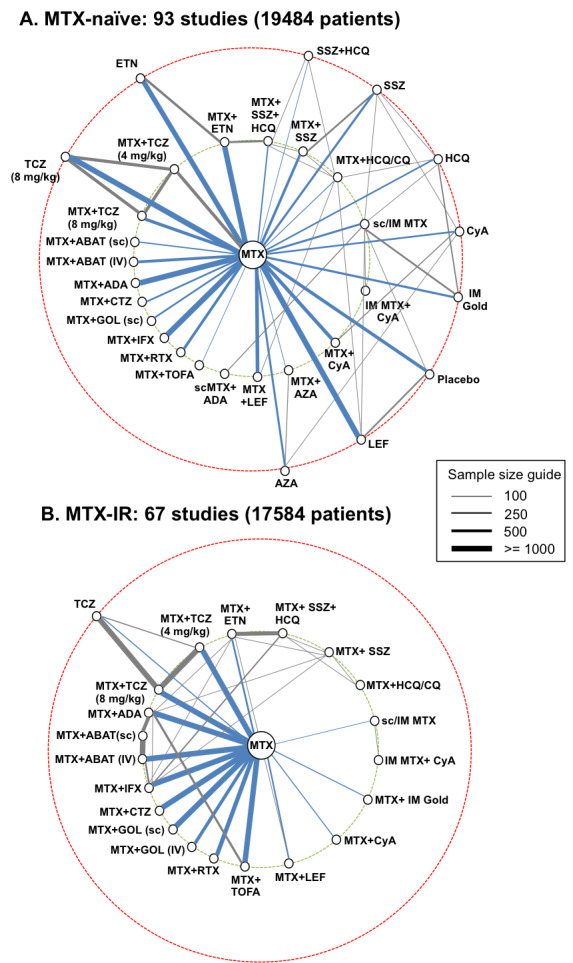
Networks of included studies for methotrexate‐naïve (A) and methotrexate‐inadequate response populations (B). Each line represents a direct comparison between two treatments from one or more trials. The line thickness is directly proportional to the total sample size of all trials for that comparison (line length has no meaning). Biologic/targeted synthetic DMARDs are shown on the left of each network and conventional synthetic DMARDs on the right. Treatments on the innermost circle (green hashed line) are treatments of interest, whereas treatments on the outermost circle (red hashed line) are other treatments that form links between treatments of interest. Comparisons to methotrexate are shown in blue. Two trials were included in both analyses.
Abbreviations: ABAT, abatacept; ADA, adalimumab; AZA, azathioprine; CTZ, certolizumab; CQ, chloroquine; CyA, cyclosporine; ETN, etanercept; GOL, golimumab; HCQ, hydroxychloroquine; IFX, infliximab; IM, intra‐muscular; IV, intravenous; LEF, leflunomide; , methotrexate; RTX, rituximab; sc, subcutaneous; SSZ, sulphasalazine; TCZ, tocilizumab; TOFA, tofacitinib
Methotrexate‐naïve network
The methotrexate‐naïve analysis included 93 trials with over 19000 patients (Figure 2). Most comparisons of methotrexate+ biologic DMARDs were to methotrexate, with no head‐to‐head comparisons between different biologic DMARDs. Trials evaluating conventional synthetic DMARD therapy were generally smaller than biologic trials, but were more inter‐connected. Only one trial connected biologic DMARDs to combination therapy with methotrexate + conventional synthetic DMARDs (TEAR 2012). Ten treatments contributed only indirect evidence to comparisons between the treatments of interest (shown in the outer circle in Figure 2). Eight of the 93 trials (9%) included patients with some prior exposure to methotrexate, and the remainder were methotrexate‐naïve (Web appendix 2 of Hazlewood 2016). Of these eight trials, the percentage of patients at baseline with prior methotrexate use at baseline ranged from 1% (Jaimes‐Hernandez 2012) to 64% (Takeuchi 2013b).
Methotrexate‐inadequate response network
The methotrexate‐IR analysis included 67 trials with over 17patients (Figure 2). As compared to the methotrexate‐naïve network, the connections between conventional synthetic DMARDs were fewer and smaller in size. Connections between methotrexate + biologic DMARDs and methotrexate, in contrast, were large in size. The four head‐to‐head trials of biologic therapy formed links between several biologic therapies and all four trials comparing methotrexate+ biologic therapy to methotrexate + conventional synthetic DMARDs were included in this network.
Methodological quality of included reviews
The risk of bias of the trials varied considerably across trials and across each domain (Figure 3, with details for each study available as an appendix on Cochrane Musculoskeletal web site). Random sequence generation and allocation concealment were rarely rated as high risk of bias, although many studies were rated as ‘unclear’ for failing to provide details beyond “randomised”. The risk of bias for ‘blinding of participants’ and ‘blinding of outcome assessment’ was higher for non‐radiographic outcomes than for radiographic outcomes. For non‐radiographic outcomes, 28% and 25% of trials were rated as high risk of bias for blinding of participants and blinding of outcome assessment, respectively.
3.
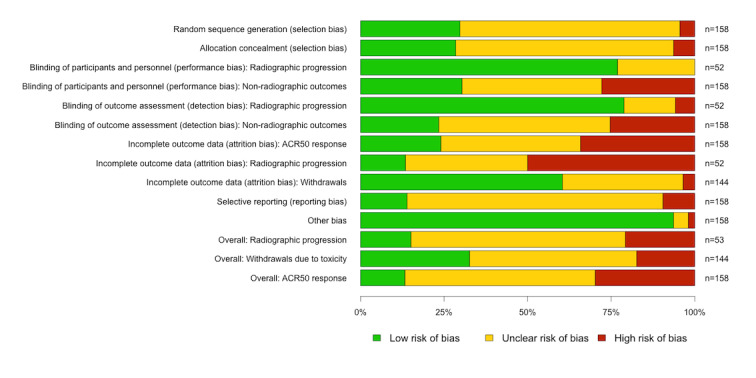
The risk of bias for the domain ‘incomplete outcome data’ varied across the outcome categories. The outcome ‘withdrawals’ had a low or unclear risk of bias for most studies; studies rated as ‘unclear’ were typically early escape studies. In contrast, the risk of bias for incomplete outcome data was high in 50% and 34% of trials for radiographic and non‐radiographic outcomes respectively. The risk of bias for the domain ‘selective outcome reporting’ was generally unclear, as most studies did not have a protocol available. The risk of bias in the domain ‘other bias’ was generally low.
The overall risk of bias was high in 21%, 17% and 30% of trials for radiographic outcomes, withdrawals and non‐radiographic outcomes respectively. Some important differences in the risk of bias across medication classes were observed. Trials assessing biologic DMARDs or tofacitinib had the lowest percentage of trials with a high risk of bias (19%), although exceptions existed. Five of the 7 certolizumab trials were rated as high risk of bias for the main outcome category (non‐radiographic outcomes, excluding withdrawals), due to high rates of withdrawal and/or early escape, often with imbalance between treatment arms.
Two of the four trials that compared methotrexate + biologic DMARDs to methotrexate + conventional synthetic DMARDs were rated as high risk of bias for non‐radiographic clinical outcomes (excluding withdrawals). One was a small open‐label study published only as an abstract (Joo 2012). The other was a larger open‐label trial with relatively high rates of incomplete outcome data (SWEFOT 2012).
Effect of interventions
Major outcomes
Methotrexate‐naïve network
ACR50
Twenty‐nine trials with 10697 patients were included in this analysis. The combination of methotrexate plus several biologic DMARDs (intravenous abatacept, adalimumab, etanercept, infliximab, rituximab and tocilizumab 8 mg/kg) and methotrexate + tofacitinib were statistically significantly superior to oral methotrexate (Table 3). In pair wise comparisons, methotrexate + etanercept was statistically significantly superior to methotrexate + certolizumab, methotrexate + sc golimumab, methotrexate + hydroxychloroquine/chloroquine, methotrexate + sulfasalazine and sc/IM methotrexate (Web appendix 3 of Hazlewood 2016).
3. Summary of findings.
| Intervention |
Absolute risk (95% CrI) |
Treatment effect relative to oral methotrexate (95% CrI) |
Quality of the evidence | Comments* |
| MTX‐naïve | ||||
| ACR50 (29 studies; 10697 patients) | No of events/1000 patients at 1 year | Odds ratio | ||
| MTX | 405 | Reference treatment | ||
| MTX + abatacept (IV) | 555 (407 to 699) | 1.84 (1.01 to 3.42) | High | NNTB 7 (3 to500) 37% (0.5 to 73) improvement |
| MTX + abatacept (SC) | 574 (390 to 730) | 1.98 (0.94 to 3.97) | High | Not statistically significant |
| MTX + adalimumab | 588 (508 to 661) | 2.10 (1.52 to 2.87) | High | NNTB 5 (4 to 10) 45% (25 to 63) improvement |
| IM/sc MTX + adalimumab | 601 (353 to 805) | 2.22 (0.80 to 6.06) | Moderate (imprecision) | Not statistically significant |
| MTX + certolizumab | 504 (361 to 646) | 1.49 (0.83 to 2.68) | Moderate (study limitations) | Not statistically significant |
| MTX + etanercept | 671 (578 to 757) | 3.00 (2.02 to 4.59) | High | NNTB 4 (3 to 6) 66% (43 to 87) improvement |
| MTX + golimumab (sc) | 476 (315 to 638) | 1.33 (0.68 to 2.59) | Moderate (study limitations) | Not statistically significant |
| MTX + infliximab | 580 (470 to 719) | 2.03 (1.30 to 3.77) | High | NNTB 6 (3 to 15) 43% (16 to 78) improvement |
| MTX + rituximab | 622 (469 to 750) | 2.42 (1.30 to 4.42) | High | NNTB 5 (3 to 16) 54% (16 to 85) improvement |
| MTX + tocilizumab (4 mg/kg) | 529 (392 to 665) | 1.66 (0.95 to 2.92) | Moderate (study limitations) | Not statistically significant |
| MTX + tocilizumab (8 mg/kg) | 565 (426 to 696) | 1.91 (1.09 to 3.36) | High | NNTB 6 (3 to 48) 40% (5 to 72) improvement |
| MTX + tofacitinib | 674 (416 to 864) | 3.04 (1.05 to 9.37) | Moderate | NNTB 4 (2 to 91) 66% (3 to 113) improvement |
| MTX + cyclosporine | 539 (370 to 695) | 1.72 (0.86 to 3.36) | Low (indirectness, imprecision, study limitations) | Not statistically significant |
| IM/sc MTX + cyclosporine | 516 (234 to 803) | 1.57 (0.44 to 6.01) | Low (imprecision, study limitations) | Not statistically significant |
| MTX + hydroxychloroquine/ chloroquine | 346 (136 to 663) | 0.78 (0.23 to 2.90) | Moderate (indirectness) | Not statistically significant |
| MTX + sulfasalazine | 427 (219 to 654) | 1.10 (0.41 to 2.78) | Low (indirectness, imprecision, study limitations) | Not statistically significant |
| MTX + sulfasalazine + hydroxychloroquine | 612 (442 to 765) | 2.32 (1.17 to 4.79) | Moderate (indirectness) | NNTB 5 (3 to 27) 51% (9 to 89) improvement |
| IM/sc MTX | 434 (288 to 595) | 1.13 (0.59 to 2.16) | Moderate (study limitations) | Not statistically significant |
| Radiographic progression (18 studies; 7594 patients) | Mean change on Sharp‐VdH scale over 1 year (points) | Standardized mean difference | ||
| MTX | 2.34 | Reference | ||
| MTX + abatacept (IV) | 1.11 (−1.29 to 3.47) | −0.20 (−0.60 to 0.19) | Moderate (imprecision) | Not statistically significant |
| MTX + adalimumab | 0.09 (−1.52 to 1.88) | −0.37 (−0.64 to −0.08) | High | 96% (20 to 165) improvement |
| MTX + certolizumab | −0.01 (−1.74 to 1.74) | −0.39 (−0.68 to −0.10) | Moderate (study limitations) | 100% (26 to 174) |
| MTX + etanercept | 0.12 (−1.19 to 1.67) | −0.37 (−0.59 to −0.11) | High | 95% (29 to 151) improvement |
| MTX + golimumab (sc) | 1.57 (−0.87 to 4.08) | −0.13 (−0.53 to 0.29) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + infliximab | −0.26 (−2.59 to 2.10) | −0.43 (−0.82 to −0.04) | High | 111% (10 to 211) improvement |
| MTX + rituximab | 0.03 (−2.40 to 2.42) | −0.38 (−0.79 to 0.01) | Moderate (imprecision) | Not statistically significant |
| MTX + tocilizumab (4 mg/kg) | 0.84 (−1.64 to 3.30) | −0.25 (−0.66 to 0.16) | Moderate (study limitations) | Not statistically significant |
| MTX + tocilizumab (8 mg/kg) | 0.14 (−2.28 to 2.54) | −0.37 (−0.77 to 0.03) | Moderate (study limitations) | Not statistically significant |
| MTX + tofacitinib | 1.09 (−2.78 to 5.17) | −0.21 (−0.85 to 0.47) | Moderate (imprecision) | Not statistically significant |
| MTX + cyclosporine | 1.07 (−0.68 to 2.94) | −0.21 (−0.50 to 0.10) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + sulfasalazine + hydroxychloroquine | 2.14 (−2.18 to 6.69) | −0.03 (−0.75 to 0.72) | Moderate (imprecision) | Not statistically significant |
| Withdrawals due to adverse events (37 studies; 10528 pt‐years) | No of events/1000 patients in 1 year | Rate ratio | ||
| MTX | 76 | Reference | ||
| MTX + abatacept (IV) | 52 (15 to 163) | 0.70 (0.21 to 2.35) | High | Not statistically significant |
| MTX + abatacept (sc) | 71 (15 to 310) | 0.97 (0.20 to 4.89) | Moderate (imprecision) | |
| MTX + adalimumab | 88 (46 to 153) | 1.21 (0.63 to 2.18) | High | Not statistically significant |
| IM/sc MTX + adalimumab | 60 (5.1 to 458) | 0.81 (0.07 to 8.06) | Moderate (imprecision) | Not statistically significant |
| MTX + etanercept | 59 (33 to 117) | 0.80 (0.45 to 1.64) | High | Not statistically significant |
| MTX + golimumab (sc) | 164 (49 to 520) | 2.36 (0.67 to 9.67) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + infliximab | 175 (69 to 448) | 2.53 (0.94 to 7.81) | Moderate (imprecision) | Not statistically significant |
| MTX + rituximab | 61 (17 to 204) | 0.83 (0.22 to 3.01) | High | Not statistically significant |
| MTX + tocilizumab (4 mg/kg) | 96 (35 to 249) | 1.33 (0.46 to 3.77) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + tocilizumab (8 mg/kg) | 158 (61 to 384) | 2.26 (0.82 to 6.38) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + tofacitinib | 66 (13 to 293) | 0.90 (0.17 to 4.56) | Moderate (imprecision) | Not statistically significant |
| MTX + azathioprine | 356 (113 to 842) | 5.79 (1.58 to 24.31) | Moderate (indirectness) | NNTH 4 (1.3 to 27) 368% (49 to 1008) worsening |
| MTX + cyclosporine | 77 (28 to 166) | 1.06 (0.37 to 2.38) | Moderate (indirectness) | Not statistically significant |
| IM/sc MTX + cyclosporine | 491 (71 to 999) | 8.89 (0.98 to 139.30) | Very low (extreme imprecision, indirectness) | Not statistically significant |
| MTX + hydroxychloroquine/ chloroquine | 98 (30 to 392) | 1.35 (0.40 to 5.26) | Low (imprecision) | Not statistically significant |
| MTX + sulfasalazine | 95 (49 to 190) | 1.31 (0.67 to 2.78) | Moderate (indirectness) | Not statistically significant |
| MTX + sulfasalazine + hydroxychloroquine | 49 (21 to 109) | 0.67 (0.28 to 1.51) | Moderate (imprecision) | Not statistically significant |
| IM/sc MTX | 131 (42 to 399) | 1.85 (0.56 to 6.69) | Moderate (imprecision) | Not statistically significant |
| MTX‐inadequate response | ||||
| ACR50 (45 studies; 12549 patients) | No of events/1000 patients at 1 year | Odds ratio | ||
| MTX | 127 | Reference | ||
| MTX + abatacept (IV) | 357 (290 to 437) | 3.81 (2.80 to 5.33) | High | NNTB 4 (3 to 6) 181% (128 to 244) improvement |
| MTX + abatacept (sc) | 377 (284 to 488) | 4.16 (2.72 to 6.53) | High | NNTB 4 (3 to 6) 197% (123 to 284) improvement |
| MTX + adalimumab | 389 (330 to 462) | 4.37 (3.38 to 5.89) | High | NNTB 4 (3 to 5) 206% (160 to 264) improvement |
| MTX + etanercept | 642 (456 to 818) | 12.31 (5.76 to 30.78) | Moderate (study limitations) | NNTB 2 (1.4 to 3) 406% (259 to 544) improvement |
| MTX + golimumab (sc) | 395 (273 to 539) | 4.49 (2.57 to 8.01) | Moderate (study limitations, indirectness) | NNTB 4 (2 to 7) 211% (115 to 324) improvement |
| MTX + golimumab (IV) | 343 (207 to 514) | 3.58 (1.79 to 7.25) | Moderate (study limitations) | NNTB 5 (3 to 13) 170% (63 to 305) improvement |
| MTX + infliximab | 335 (264 to 422) | 3.46 (2.46 to 5.00) | High | NNTB 5 (3 to 7) 164% (108 to 232) improvement |
| MTX + rituximab | 343 (241 to 477) | 3.59 (2.18 to 6.27) | High | NNTB 5 (3 to 9) 170% (90 to 276) improvement |
| MTX + tocilizumab (4 mg/kg) | 273 (171 to 399) | 2.57 (1.42 to 4.56) | High | NNTB 7 (4 to 23) 114% (35 to 214) improvement |
| MTX + tocilizumab (8 mg/kg) | 377 (264 to 499) | 4.16 (2.46 to 6.85) | High | NNTB 4 (3 to 7) 197% (108 to 293) improvement |
| MTX + tofacitinib | 441 (325 to 568) | 5.42 (3.31 to 9.01) | High | NNTB 3 (2 to 5) 247% (156 to 347) improvement |
| MTX + hydroxychloroquine/ chloroquine | 566 (241 to 871) | 8.94 (2.18 to 46.14) | Low (high imprecision) | NNTB 2 (1.3 to 9) 346% (90 to 586) improvement |
| MTX + IM gold | 704 (228 to 988) | 16.34 (2.03 to 553.42) | Very low (extreme imprecision) | NNTB 2 (1.2 to 10) 454% (80 to 678) improvement |
| MTX + leflunomide | 453 (245 to 703) | 5.69 (2.23 to 16.27) | Moderate (imprecision) | NNTB 3 (2 to 8) 257% (93 to 454) improvement |
| MTX + sulfasalazine | 267 (67 to 667) | 2.50 (0.49 to 13.76) | Low (high imprecision) | Not statistically significant |
| MTX + sulfasalazine + hydroxychloroquine | 605 (394 to 818) | 10.51 (4.46 to 30.81) | Moderate (study limitations) | NNTB 2 (1.4 to 4) 376% (210 to 544) improvement |
| Radiographic progression (10 studies; 3238 patients) | Mean change on Sharp‐VdH scale over 1 year (points) | Standardized mean difference | ||
| MTX | 3.35 | Reference | ||
| MTX + abatacept (IV) | 1.45 (−5.85 to 8.80) | −0.30 (−1.44 to 0.85) | Moderate (imprecision) | Not statistically significant |
| MTX + abatacept (sc) | 0.26 (−9.65 to 11.10) | −0.48 (−2.03 to 1.21) | Moderate (imprecision) | Not statistically significant |
| MTX + adalimumab | 0.51 (−6.42 to 7.96) | −0.44 (−1.53 to 0.72) | Moderate (study limitations, imprecision) | Not statistically significant |
| MTX + etanercept | −0.49 (−12.09 to 11.06) | −0.60 (−2.41 to 1.21) | Moderate (imprecision) | Not statistically significant |
| MTX + golimumab (sc) | 2.44 (−2.77 to 7.66) | −0.14 (−0.96 to 0.67) | Low (study limitations, inconsistency, indirectness, imprecision) | Not statistically significant |
| MTX + golimumab (IV) | 0.52 (−6.56 to 7.98) | −0.44 (−1.55 to 0.73) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + infliximab | −1.08 (−8.34 to 6.35) | −0.69 (−1.83 to 0.47) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + sulfasalazine + hydroxychloroquine | 0.70 (−9.58 to 11.05) | −0.41 (−2.02 to 1.20) | Low (indirectness, imprecision) | Not statistically significant |
| Withdrawals due to adverse events (53 studies; 9950 pt‐years) | No of events/1000 patients in 1 year | Rate ratio | ||
| MTX | 73 | Reference | ||
| MTX + abatacept (IV) | 54 (31 to 90) | 0.76 (0.44 to 1.30) | High | Not statistically significant |
| MTX + abatacept (sc) | 39 (21 to 72) | 0.55 (0.28 to 1.03) | Moderate (indirectness) | Not statistically significant |
| MTX + adalimumab | 100 (67 to 155) | 1.44 (0.95 to 2.30) | High | Not statistically significant |
| MTX + certolizumab | 99 (56 to 196) | 1.42 (0.79 to 2.99) | Low (study limitations, indirectness) | Not statistically significant |
| MTX + etanercept | 89 (40 to 195) | 1.28 (0.56 to 2.92) | Moderate (study limitations) | Not statistically significant |
| MTX + golimumab (sc) | 72 (28 to 184) | 1.02 (0.39 to 2.78) | Low (study limitations, indirectness) | Not statistically significant |
| MTX + golimumab (IV) | 92 (26 to 370) | 1.32 (0.36 to 6.31) | Low (study limitations, imprecision) | Not statistically significant |
| MTX + infliximab | 112 (70 to 179) | 1.62 (0.99 to 2.70) | High | Not statistically significant |
| MTX+ rituximab | 141 (53 to 376) | 2.07 (0.74 to 6.45) | Moderate (imprecision) | Not statistically significant |
| MTX + tocilizumab (4 mg/kg) | 112 (67 to 191) | 1.63 (0.95 to 2.90) | High | Not statistically significant |
| MTX + tocilizumab (8 mg/kg) | 118 (74 to 188) | 1.71 (1.01 to 2.84) | High | NNTH 22 (9 to 1000) 62% (1.4 to 158) |
| MTX + tofacitinib | 87 (52 to 152) | 1.24 (0.74 to 2.26) | High | Not statistically significant |
| MTX + cyclosporine | 212 (84 to 503) | 3.27 (1.20 to 9.57) | Low (indirectness, imprecision) | NNTH 7 (2 to 91) 190% (15 to 589) worsening |
| MTX + IM gold | 260 (35 to 999) | 4.12 (0.49 to 102.75) | Very low (extreme imprecision) | Not statistically significant |
| MTX + leflunomide | 127 (53 to 290) | 1.86 (0.74 to 4.68) | Moderate (imprecision) | Not statistically significant |
| MTX + sulfasalazine + hydroxychloroquine | 125 (62 to 249) | 1.82 (0.87 to 3.92) | Moderate (imprecision) | Not statistically significant |
*The number needed to treat and number needed to harm were calculated as 1/absolute risk difference, where absolute risk difference = corresponding ‐ assumed risk. The percent improvement or worsening was calculated as the absolute risk difference divided by the assumed risk.
Abbreviations: IM, intra‐muscular; IV, intravenous; methotrexate, methotrexate; NNTB; number needed to benefit; NNTH, number needed to harm; OR, odds ratio; sc, subcutaneous; SMD, standardized mean difference
Methotrexate + sulfasalazine + hydroxychloroquine was the only conventional synthetic DMARD combination that was statistically significantly superior to oral methotrexate. This comparison was based on indirect evidence, and was judged to be ‘moderate’ quality, as one the 2 trials comparing methotrexate + etanercept to methotrexate included patients with partial methotrexate exposure (TEMPO 2004). The other trial, however, which was larger and included only methotrexate‐naïve patients, found a nearly identical treatment effect (COMET 2008). The magnitude of the estimated probability of ACR50 response was similar between triple therapy (61.2%, 95% credible interval 44.2 to 76.5) and the other DMARDs that had a statistically significant benefit relative to oral methotrexate (point estimate range 56‐67%) (Table 3). In comparison, the estimated probability of ACR50 response with oral methotrexate was 40.5%. In pair wise comparisons, we found no statistically significant difference between triple therapy and methotrexate plus any biologic DMARD, although we could not rule out an important difference, as the credible intervals were wide for some comparisons (Web appendix 3 of Hazlewood 2016).
Radiographic progression
Eighteen trials with 7594 patients were included in this analysis. The combinations of methotrexate plus several biologic DMARDs (adalimumab, certolizumab, etanercept, infliximab) were associated with a statistically significant reduction in radiographic progression relative to oral methotrexate (Table 3). There were no statistically significant differences between treatments in pair‐wise comparisons (Web appendix 3 of Hazlewood 2016). The sizes of the effects for all interventions relative to oral methotrexate were small. The expected radiographic progression was 2.34 points over one year with oral methotrexate (the reference treatment) and lower for all other treatments, which is below the minimal clinically important difference of 5 units on the Sharp‐van der Heijde scale (Table 3). There was no statistically significant difference between any treatments when final (instead of change from baseline) values were used (data not shown).
In post‐hoc sensitivity analyses using fixed‐effects models, the point estimates were nearly identical to the random‐effects model, but the credible intervals were not as wide, resulting in several biologic DMARDs (+methotrexate) that were statistically significantly superior to oral methotrexate (Web appendix 3 of Hazlewood 2016). Methotrexate + sulfasalazine + hydroxychloroquine was the only conventional synthetic DMARD combination with outcome data available, and was not statistically significantly superior to oral methotrexate in either the random‐effects or fixed‐effect models (Web appendix 3 of Hazlewood 2016).
Withdrawals due to adverse events
Thirty‐seven trials with a total follow‐up of 10528 patient‐years were included in this analysis. The combination of methotrexate + azathioprine had a statistically significant higher rate of withdrawals due to adverse events compared to oral methotrexate and several other treatments (Table 3 and Web appendix 3 of Hazlewood 2016). There were no statistically significant differences in pair wise comparisons between different biologic DMARDs (+ methotrexate). Methotrexate + sulfasalazine + hydroxychloroquine was associated with a statistically significant reduction in withdrawals due to adverse events than methotrexate + infliximab (rate ratio 0.26, 95% credible interval 0.06 to 0.91, Web appendix 3 of Hazlewood 2016).
Methotrexate‐inadequate response network
ACR50
Forty‐five trials with 12549 patients were included in this analysis. Several treatments were statistically significantly superior to oral methotrexate for ACR50 response (Table 3). The results reached statistical significance for the combination of methotrexate and several conventional synthetic DMARDs (sulfasalazine + hydroxychloroquine, hydroxychloroquine, leflunomide, or intra‐muscular gold), methotrexate + all biologic DMARDs with available evidence, and methotrexate + tofacitinib. The estimated probability of an ACR50 response with triple therapy was 60.5% (39.4% to 81.8%) and varied widely for other treatments (point estimate range 27‐70%). We found no evidence for certolizumab, as the available trials were judged to be at high risk of bias. In general, the credible intervals in the pair wise comparisons between different treatments combinations were wide, although some estimates reached statistical significance (Web appendix 3 of Hazlewood 2016): methotrexate + etanercept was superior to the combination of methotrexate + most biologic DMARDs, and methotrexate + sulfasalazine + hydroxychloroquine was superior to methotrexate + the biologic DMARDs intravenous abatacept, infliximab, and tocilizumab 4 mg/kg.
The quality of the evidence for methotrexate + sulfasalazine + hydroxychloroquine versus methotrexate was judged to ‘moderate’ as some minor inconsistencies existed in the findings of the two trials that compared triple therapy with MTX + etanercept (RACAT 2013; TEAR 2012), and because the study design of one of the trials was judged to indirectly address the comparison of interest (TEAR 2012) (Web appendix 4 of Hazlewood 2016). This trial randomised patients at baseline a step‐up to triple therapy versus a step‐up to methotrexate + etanercept, only if an inadequate response to methotrexate was found after 6 months (TEAR 2012).
Radiographic progression
Ten trials with 3238 patients were included in this analysis. We found no statistically significant differences between any treatment and oral methotrexate in the random‐effects model (Table 3 and Web appendix 3 of Hazlewood 2016). The predicted change in Sharp‐vDH score over 1 year was small for each treatment, similar to the methotrexate‐naïve analysis (Table 3). The results using final (versus change) values were similar, with no statistically significant differences between any treatments (data not shown).
Similar to the analysis in methotrexate naïve patients, the credible intervals were more precise in the post‐hoc fixed effect model, resulting in several treatments that reached statistical significance relative to oral methotrexate (methotrexate + abatacept (intravenous and subcutaneous), adalimumab, etanercept, intravenous golimumab, and infliximab) (Web appendix 3 of Hazlewood 2016). The point estimate favoured methotrexate + sulfasalazine + hydroxychloroquine in the comparison of methotrexate + sulfasalazine + hydroxychloroquine versus oral methotrexate, but the result was not statistically significant [SMD: ‐0.40 (95%CrI: ‐0.84 to 0.04)].
Withdrawals due to adverse events
Fifty‐three trials with a total follow‐up of 9950 patient‐years were included in this analysis. Methotrexate plus ciclosporin and methotrexate plus tocilizumab 8 mg/kg were the only treatments with statistically significant higher rates of withdrawals due to adverse events relative to oral methotrexate (Table 3). In pair wise comparisons, MTX plus subcutaneous abatacept and methotrexate plus intravenous abatacept were associated with a statistically significant lower rate of withdrawals due to adverse events than several treatments, including methotrexate plus biologic DMARDs and triple therapy (Web appendix 3 of Hazlewood 2016).
Minor efficacy outcomes
Methotrexate‐naïve network
Overall, the point estimates were similar across minor outcomes for each intervention; interventions that were favoured for one outcome were generally favoured for the others (Table 4). Credible intervals varied by outcome, however, and often included the null effect. Credible intervals were particularly wide for dichotomous outcomes that are difficult to attain (ACR70, DAS28 remission), and for the continuous outcomes swollen joint count and fatigue. The mean difference in HAQ‐DI for treatments that were statistically significantly superior than MTX were generally close to the threshold of 0.22 for a minimum clinically important difference (Table 4).
4. Treatment effects relative to oral methotrexate for all minor efficacy outcomes.
|
Outcome Treatment effect |
|||||||||||
| Intervention | ACR20 | ACR70 | DAS‐28 remission | EULAR response | Radiographic non‐progression (Y/N) | Withdrawals due to inefficacy | DAS‐28 | HAQ‐DI | Pain VAS | SJC, change from baseline | Fatigue, change from baseline |
| OR | OR | OR | OR | OR | OR | MD | MD | MD | SMD |
SMD (methotrexate‐naïve) MD (methotrexate‐IR) |
|
| MTX‐naïve | |||||||||||
| MTX + abatacept (IV) | ‐‐ | 1.99 (0.71 to 5.30) | 2.33 (1.23 to 4.37) | ‐‐ | 1.42 (0.74 to 2.72) | NE | ‐0.74 (‐1.50 to 0.04) | ‐0.20 (‐0.37 to ‐0.03) | ‐‐ | ‐‐ | ‐‐ |
| MTX + abatacept (sc) | 1.56 (0.71 to 3.48) | 2.10 (0.70 to 6.25) | 1.76 (0.87 to 3.61) | ‐‐ | ‐‐ | 0.40 (0.07 to 2.20) | ‐0.51 (‐1.29 to 0.30) | ‐0.15 (‐0.36 to 0.05) | ‐‐ | ‐‐ | ‐‐ |
| MTX + adalimumab | 1.92 (1.38 to 2.76) | 2.44 (1.44 to 4.06) | 2.29 (1.64 to 3.03) | 3.60 (0.27 to 46.35) | 2.96 (2.05 to 4.48) | 0.21 (0.08 to 0.55) | ‐0.82 (‐1.41 to ‐0.28) | ‐0.22 (‐0.31 to ‐0.14) | ‐5.79 (‐8.92 to ‐2.86) | ‐‐ | ‐0.19 (‐2.69 to 2.33) |
| IM/sc MTX + adalimumab | 2.78 (0.92 to 8.55) | 3.52 (0.74 to 15.93) | ‐‐ | ‐‐ | ‐‐ | NE | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + certolizumab | 1.43 (0.75 to 2.73) | 1.62 (0.59 to 4.41) | 2.16 (1.37 to 3.40) | 2.03 (0.16 to 27.02) | 4.24 (1.85 to 9.36) | 0.40 (0.09 to 1.77) | ‐0.60 (‐1.35 to 0.18) | ‐0.18 (‐0.34 to ‐0.02) | ‐4.47 (‐9.40 to 0.40) | ‐‐ | ‐‐ |
| MTX + etanercept | 2.66 (1.69 to 4.19) | 3.07 (1.58 to 6.06) | 2.74 (1.79 to 4.03) | ‐‐ | 2.92 (1.73 to 4.87) | 0.19 (0.07 to 0.46) | ‐‐ | ‐0.24 (‐0.34 to ‐0.14) | ‐11.04 (‐15.67 to ‐6.43) | ‐‐ | ‐0.28 (‐2.84 to 2.26) |
| MTX + golimumab (sc) | 1.41 (0.69 to 2.86) | 1.43 (0.48 to 4.25) | 1.50 (0.74 to 2.91) | 1.70 (0.13 to 22.03) | 2.07 (0.79 to 6.00) | 1.02 (0.15 to 6.71) | ‐‐ | ‐0.08 (‐0.27 to 0.11) | ‐‐ | ‐‐ | ‐‐ |
| MTX + infliximab | 2.16 (1.46 to 3.62) | 3.27 (1.74 to 7.23) | 1.52 (0.77 to 3.04) | ‐‐ | 3.09 (1.34 to 7.51) | 0.18 (0.04 to 0.86) | ‐0.57 (‐1.16 to 0.05) | ‐0.39 (‐0.56 to ‐0.22) | ‐‐ | ‐‐ | ‐‐ |
| MTX + rituximab | 2.30 (1.18 to 4.52) | 2.36 (0.83 to 6.79) | 3.27 (1.66 to 6.44) | ‐‐ | 2.19 (1.14 to 4.18) | ‐‐ | ‐1.19 (‐2.00 to ‐0.40) | ‐0.25 (‐0.43 to ‐0.07) | ‐‐ | ‐‐ | ‐‐ |
| MTX + tocilizumab (4 mg/kg) | 1.43 (0.78 to 2.64) | 1.63 (0.63 to 4.29) | 2.35 (1.31 to 4.20) | ‐‐ | ‐‐ | 0.30 (0.06 to 1.47) | ‐‐ | ‐0.27 (‐0.44 to ‐0.09) | ‐1.80 (‐7.40 to 3.79) | ‐‐ | ‐0.20 (‐2.73 to 2.36) |
| MTX+ tocilizumab (8 mg/kg) | 1.75 (0.94 to 3.21) | 2.10 (0.81 to 5.32) | 4.37 (2.46 to 7.82) | ‐‐ | ‐‐ | 0.07 (0.007 to 0.49) | ‐‐ | ‐0.24 (‐0.41 to ‐0.07) | ‐8.59 (‐13.95 to ‐3.12) | ‐‐ | ‐0.23 (‐2.75 to 2.33) |
| MTX + tofacitinib | 3.02 (1.05 to 9.65) | 1.06 (0.24 to 4.54) | 2.91 (0.82 to 12.59) | 4.63 (0.31 to 69.67) | ‐‐ | NE | ‐1.10 (‐2.25 to 0.05) | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + azathioprine | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | NE | ‐‐ | ‐‐ | ‐‐ | 0.006 (‐1.91 to 1.91) | ‐‐ |
| MTX + cyclosporine | 2.76 (1.09 to 7.39) | 1.86 (0.60 to 5.78) | 1.44 (0.86 to 2.45) | ‐‐ | 0.79 (0.27 to 2.12) | 0.85 (0.28 to 2.75) | ‐0.21 (‐0.78 to 0.35) | ‐0.19 (‐0.33 to ‐0.05) | ‐3.95 (‐10.81 to 2.77) | ‐‐ | ‐‐ |
| IM/sc MTX + cyclosporine | 1.01 (0.27 to 4.03) | 5.28 (0.85 to 33.45) | ‐‐ | ‐‐ | ‐‐ | NE | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + hydroxychloroquine/ chloroquine | 0.67 (0.19 to 2.28) | 0.74 (0.12 to 4.90) | ‐‐ | ‐‐ | ‐‐ | NE | ‐‐ | ‐0.17 (‐0.50 to 0.14) | 0.18 (‐9.06 to 9.64) | ‐0.77 (‐3.15 to 1.59) | ‐‐ |
| MTX + sulfasalazine | 1.51 (0.85 to 2.64) | 1.61 (0.36 to 8.21) | ‐‐ | 1.59 (0.12 to 20.34) | 2.24 (0.60 to 10.49) | 0.89 (0.22 to 3.82) | ‐‐ | ‐0.06 (‐0.41 to 0.31) | ‐5.94 (‐11.77 to 0.10) | ‐0.68 (‐1.88 to 0.48) | ‐‐ |
| MTX + sulfasalazine + hydroxychloroquine | 2.30 (1.12 to 4.92) | 1.49 (0.47 to 5.27) | ‐‐ | 2.63 (0.20 to 33.51) | 1.41 (0.52 to 3.63) | 0.29 (0.07 to 1.02) | ‐‐ | ‐0.23 (‐0.36 to ‐0.08) | ‐9.39 (‐16.65 to ‐2.33) | ‐1.09 (‐3.42 to 1.27) | ‐‐ |
| IM/sc MTX | 1.46 (0.71 to 2.91) | 1.38 (0.47 to 3.82) | ‐‐ | ‐‐ | ‐‐ | 0.46 (0.05 to 4.11) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX‐inadequate response | |||||||||||
| MTX + abatacept (IV) | 3.75 (2.93 to 4.84) | 4.17 (2.65 to 6.86) | 5.73 (3.41 to 10.73) | ‐‐ | ‐‐ | 0.20 (0.08 to 0.49) | ‐1.20 (‐1.55 to ‐0.86) | ‐0.32 (‐0.40 to ‐0.24) | ‐18.37 (‐23.15 to ‐13.49) | ‐0.60 (‐1.16 to ‐0.01) | ‐‐ |
| MTX + abatacept (sc) | 4.09 (2.99 to 5.96) | 5.00 (2.78 to 9.50) | 6.01 (3.07 to 13.97) | ‐‐ | ‐‐ | 0.47 (0.11 to 2.50) | ‐1.21 (‐1.74 to ‐0.70) | ‐0.31 (‐0.42 to ‐0.22) | ‐21.77 (‐27.48 to ‐15.81) | ‐0.64 (‐1.55 to 0.28) | ‐‐ |
| MTX + adalimumab | 3.60 (2.95 to 4.51) | 4.44 (3.06 to 6.89) | 4.82 (2.54 to 9.30) | 2.95 (1.13 to 7.99) | ‐‐ | 0.27 (0.10 to 0.71) | ‐0.86 (‐1.46 to ‐0.27) | ‐0.29 (‐0.37 to ‐0.21) | ‐12.41 (‐15.63 to ‐9.37) | ‐0.65 (‐1.07 to ‐0.24) | ‐‐ |
| MTX + certolizumab | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 0.35 (0.14 to 0.89) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + etanercept | 7.30 (4.15 to 13.19) | 11.33 (3.17 to 53.51) | ‐‐ | ‐‐ | ‐‐ | 0.59 (0.08 to 2.84) | ‐1.50 (‐2.33 to ‐0.67) | ‐0.20 (‐0.39 to ‐0.02) | ‐24.48 (‐41.54 to ‐9.75) | ‐‐ | ‐‐ |
| MTX + golimumab (sc) | 3.94 (2.84 to 5.40) | 5.97 (2.59 to 14.72) | 5.90 (2.41 to 15.59) | 3.85 (2.17 to 6.74) | 1.43 (0.30 to 7.07) | 0.33 (0.05 to 1.52) | ‐1.30 (‐1.90 to ‐0.68) | ‐0.37 (‐0.46 to ‐0.27) | ‐‐ | ‐‐ | ‐5.35 (‐7.68 to ‐3.05) |
| MTX + golimumab (IV) | 3.92 (2.43 to 6.56) | 5.31 (1.83 to 16.87) | 4.19 (1.27 to 14.41) | ‐‐ | 2.55 (0.31 to 20.55) | 0.53 (0.01 to 35.46) | ‐1.20 (‐1.98 to ‐0.44) | ‐0.32 (‐0.46 to ‐0.17) | ‐‐ | ‐‐ | ‐5.40 (‐8.18 to ‐2.59) |
| MTX + infliximab | 3.21 (2.49 to 4.12) | 3.67 (2.20 to 6.31) | 3.91 (1.86 to 9.37) | 3.33 (1.61 to 7.10) | 7.86 (0.82 to 66.77) | 0.26 (0.07 to 0.73) | ‐0.89 (‐1.33 to ‐0.45) | ‐0.29 (‐0.41 to ‐0.18) | ‐‐ | ‐0.47 (‐1.06 to 0.11) | ‐‐ |
| MTX + rituximab | 3.42 (2.34 to 5.08) | 3.55 (1.64 to 7.78) | 4.53 (1.07 to 23.14) | 3.56 (2.20 to 6.21) | ‐‐ | 0.13 (0.02 to 0.39) | ‐1.07 (‐1.68 to ‐0.48) | ‐0.16 (‐0.36 to 0.04) | ‐‐ | ‐0.46 (‐1.29 to 0.35) | ‐3.81 (‐6.75 to ‐0.70) |
| MTX + tocilizumab (4 mg/kg) | 2.49 (1.63 to 3.90) | 2.16 (0.89 to 5.07) | 7.44 (1.99 to 34.88) | 3.05 (1.40 to 6.63) | ‐‐ | 0.16 (0.03 to 0.57) | ‐1.11 (‐1.85 to ‐0.38) | ‐0.18 (‐0.37 to 0.01) | ‐11.10 (‐17.53 to ‐4.51) | ‐0.42 (‐1.23 to 0.39) | ‐3.26 (‐6.47 to ‐0.22) |
| MTX+ tocilizumab (8 mg/kg) | 3.77 (2.52 to 5.53) | 4.41 (2.06 to 9.32) | 16.16 (5.25 to 59.96) | 7.37 (3.24 to 16.20) | ‐‐ | 0.07 (0.02 to 0.24) | ‐1.71 (‐2.27 to ‐1.14) | ‐0.21 (‐0.39 to ‐0.02) | ‐16.10 (‐22.36 to ‐9.43) | ‐0.62 (‐1.44 to 0.19) | ‐4.60 (‐7.87 to ‐1.50) |
| MTX + tofacitinib | 4.14 (2.86 to 6.32) | 8.86 (4.19 to 19.60) | 4.40 (1.67 to 11.78) | 3.57 (1.38 to 9.35) | ‐‐ | 0.43 (0.09 to 1.58) | ‐0.82 (‐1.33 to ‐0.32) | ‐0.28 (‐0.42 to ‐0.14) | ‐11.79 (‐16.28 to ‐7.36) | ‐0.54 (‐1.23 to 0.07) | ‐3.76 (‐8.95 to 1.32) |
| MTX + cyclosporine | 5.63 (2.76 to 12.10) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 0.10 (0.003 to 0.90) | ‐‐ | ‐0.28 (‐0.46 to ‐0.10) | ‐9.55 (‐18.64 to ‐0.41) | ‐0.49 (‐1.34 to 0.35) | ‐‐ |
| MTX + hydroxychloroquine/ chloroquine | 3.24 (0.79 to 11.36) | 4.28 (0.41 to 53.49) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + IM gold | 3.91 (1.31 to 11.54) | NE | ‐‐ | ‐‐ | ‐‐ | 0.13 (0.01 to 0.90) | ‐‐ | ‐0.11 (‐0.42 to 0.17) | ‐6.00 (‐25.27 to 13.54) | ‐0.40 (‐1.33 to 0.52) | ‐‐ |
| MTX + leflunomide | 3.59 (1.86 to 6.82) | 5.20 (1.22 to 29.24) | ‐‐ | ‐‐ | ‐‐ | 0.58 (0.11 to 3.12) | ‐‐ | ‐0.33 (‐0.48 to ‐0.18) | ‐16.98 (‐24.20 to ‐9.60) | ‐0.45 (‐1.28 to 0.36) | ‐‐ |
| MTX + sulfasalazine | 1.47 (0.34 to 5.19) | 2.03 (0.13 to 29.43) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + sulfasalazine + hydroxychloroquine | 6.92 (3.48 to 14.02) | 5.13 (1.18 to 29.09) | ‐‐ | ‐‐ | ‐‐ | 0.84 (0.13 to 3.92) | ‐1.50 (‐2.52 to ‐0.51) | ‐0.18 (‐0.40 to 0.03) | ‐24.05 (‐41.74 to ‐8.45) | ‐‐ | ‐‐ |
Abbreviations: IM, intra‐muscular; IV, intravenous; MD, mean difference; MTX, methotrexate; NE, not estimable; OR, odds ratio; sc, subcutaneous; SMD, standardized mean difference
The results for the radiographic progression as a dichotomised variable (Yes/No) were similar to the assessment of radiographic progression as a continuous variable for the major outcome. Methotrexate + several biologic DMARDs (adalimumab, certolizumab, etanercept, or infliximab) were statistically significantly superior to oral MTX in both analyses. Methotrexate + rituximab was statistically superior to oral MTX only for the dichotomised outcome, but the credible interval for the continuous (major) outcome only narrowly excluded the null value (Table 3).
Methotrexate‐inadequate response network
The treatment effects for all minor efficacy outcomes for the methotrexate‐IR analysis demonstrated similar trends across outcomes for each intervention, similar to the methotrexate‐naïve analysis (Table 4). Again, credible intervals varied, with some not reaching statistical significance.
Minor toxicity outcomes
Methotrexate‐naïve network
There were few statistically significant differences between any treatment and oral methotrexate, although many events were rare, leading to very wide credible intervals (Table 5). There were no statistically significant differences in serious adverse events between any treatment and methotrexate. Methotrexate + sulfasalazine + hydroxychloroquine and methotrexate + sulfasalazine had a statistically significant increased rates of total gastrointestinal events (excluding oral and liver toxicity).
5. Treatment effects (rate ratios) relative to oral methotrexate for all minor toxicity outcomes and the combined outcome withdrawals due to toxicity/inefficacy.
| Intervention | Combined WDAE and WD inefficacy | Serious adverse events | Serious infections | Total GI events | Elevated ALT | Elevated AST | Anemia | Leukopenia | Thrombocytopenia |
| MTX‐naïve | |||||||||
| MTX + abatacept (IV) | 0.46 (0.13 to 1.64) | 0.96 (0.37 to 2.63) | 0.95 (0.21 to 4.71) | 0.66 (0.38 to 1.15) | 0.70 (0.11 to 4.45) | 0.49 (0.05 to 4.57) | 0.99 (0.05 to 19.95) | 0.42 (0.03 to 5.78) | NE |
| MTX + abatacept (sc) | 0.59 (0.15 to 2.18) | 0.86 (0.24 to 2.98) | NE | ‐‐ | 1.50 (0.12 to 22.19) | 2.37 (0.10 to 121.23) | NE | ‐‐ | ‐‐ |
| MTX + adalimumab | 0.70 (0.39 to 1.32) | 1.17 (0.73 to 1.98) | 1.74 (0.82 to 3.81) | 1.02 (0.71 to 1.51) | 1.19 (0.32 to 4.79) | 2.06 (0.21 to 21.43) | ‐‐ | 3.74 (0.15 to 284.29) | ‐‐ |
| IM/sc MTX + adalimumab | 0.32 (0.02 to 2.76) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + certolizumab | ‐‐ | 1.08 (0.43 to 2.81) | 0.90 (0.26 to 3.27) | 1.18 (0.63 to 2.24) | 1.50 (0.24 to 9.75) | ‐‐ | NE | ‐‐ | ‐‐ |
| MTX + etanercept | 0.47 (0.24 to 0.87) | 1.20 (0.66 to 2.67) | 0.87 (0.40 to 2.23) | 0.86 (0.61 to 1.21) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + golimumab (sc) | 1.44 (0.41 to 5.20) | 0.85 (0.34 to 2.10) | 1.27 (0.37 to 4.78) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + infliximab | 1.08 (0.42 to 3.78) | 1.35 (0.62 to 3.61) | 2.89 (0.82 to 10.28) | 1.08 (0.61 to 1.91) | NE | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + rituximab | ‐‐ | 1.26 (0.52 to 3.14) | 0.67 (0.20 to 2.19) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + tocilizumab (4 mg/kg) | 0.90 (0.29 to 2.87) | 1.29 (0.55 to 3.24) | 1.97 (0.60 to 7.84) | 1.00 (0.60 to 1.69) | 1.43 (0.25 to 8.01) | 0.85 (0.10 to 7.22) | ‐‐ | ‐‐ | ‐‐ |
| MTX + tocilizumab (8 mg/kg) | 1.28 (0.41 to 4.05) | 1.38 (0.59 to 3.48) | 1.82 (0.56 to 7.11) | 1.00 (0.60 to 1.68) | 2.02 (0.36 to 11.60) | 1.84 (0.23 to 14.87) | ‐‐ | ‐‐ | ‐‐ |
| MTX + tofacitinib | 0.39 (0.07 to 1.76) | 1.08 (0.14 to 12.62) | NE | 0.65 (0.21 to 1.96) | 1.36 (0.18 to 11.06) | 0.71 (0.04 to 10.63) | NE | NE | ‐‐ |
| MTX + azathioprine | 3.78 (1.04 to 14.80) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + cyclosporine | 0.95 (0.38 to 2.21) | 1.03 (0.42 to 2.51) | 0.55 (0.16 to 1.71) | 0.90 (0.59 to 1.38) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| IM/sc MTX + cyclosporine | 6.53 (0.67 to 87.88) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + hydroxychloroquine/ chloroquine | 1.63 (0.18 to 17.75) | ‐‐ | ‐‐ | 0.47 (0.008 to 6.51) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + sulfasalazine | 1.14 (0.53 to 2.70) | NE | NE | 1.90 (1.18 to 2.99) | 8.57 (0.64 to 250.91) | NE | ‐‐ | 10.42 (0.80 to 448.42) | ‐‐ |
| MTX + sulfasalazine + hydroxychloroquine | 0.41 (0.15 to 0.94) | 1.05 (0.44 to 2.52) | 0.75 (0.17 to 3.56) | 2.10 (1.19 to 3.96) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| IM/sc MTX | 1.39 (0.40 to 4.79) | 1.37 (0.38 to 4.83) | ‐‐ | 1.11 (0.65 to 1.92) | 0.34 (0.04 to 2.77) | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX‐inadequate response | |||||||||
| MTX + abatacept (IV) | 0.36 (0.20 to 0.62) | 0.96 (0.37 to 2.63) | 1.02 (0.25 to 2.87) | 1.06 (0.78 to 1.61) | 0.79 (0.22 to 2.69) | 1.49 (0.32 to 8.99) | 0.60 (0.06 to 3.57) | 1.56 (0.12 to 20.29) | 3.63 (0.28 to 112.95) |
| MTX + abatacept (sc) | 0.34 (0.15 to 0.72) | 0.86 (0.24 to 2.98) | 0.99 (0.19 to 4.16) | 0.82 (0.49 to 1.63) | 0.39 (0.05 to 1.78) | 0.85 (0.08 to 9.15) | 0.20 (0.005 to 4.98) | 7.38 (0.25 to 389.85) | NE |
| MTX + adalimumab | 0.59 (0.32 to 1.04) | 1.17 (0.73 to 1.98) | 2.63 (0.85 to 10.68) | 0.71 (0.48 to 1.10) | 0.96 (0.50 to 2.28) | 1.20 (0.60 to 2.87) | 1.08 (0.12 to 13.43) | 3.56 (0.58 to 28.07) | ‐‐ |
| MTX + certolizumab | 0.54 (0.30 to 1.01) | 1.08 (0.43 to 2.81) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + etanercept | 0.75 (0.24 to 2.19) | 1.20 (0.66 to 2.67) | NE | 0.77 (0.34 to 1.46) | 1.84 (0.43 to 7.27) | 1.93 (0.41 to 11.30) | NE | ‐‐ | ‐‐ |
| MTX + golimumab (sc) | 0.79 (0.30 to 2.02) | 0.85 (0.34 to 2.10) | 0.93 (0.09 to 11.41) | 2.05 (0.16 to 41.06) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + golimumab (IV) | 1.08 (0.26 to 5.45) | ‐‐ | NE | ‐‐ | 1.31 (0.35 to 4.63) | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + infliximab | 0.59 (0.29 to 1.21) | 1.35 (0.62 to 3.61) | 0.86 (0.25 to 2.41) | 1.03 (0.64 to 1.74) | 0.97 (0.28 to 2.90) | 1.76 (0.22 to 15.26) | 0.52 (0.009 to 13.98) | NE | 12.67 (0.30 to 1697.38) |
| MTX + rituximab | 0.31 (0.13 to 0.68) | 1.26 (0.52 to 3.14) | 0.49 (0.09 to 2.10) | 0.78 (0.45 to 1.32) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + tocilizumab (4 mg/kg) | 0.80 (0.41 to 1.53) | 1.29 (0.55 to 3.24) | 0.87 (0.09 to 6.77) | 1.02 (0.56 to 1.92) | 4.11 (0.76 to 25.37) | 0.82 (0.02 to 53.41) | ‐‐ | 9.03 (0.79 to 120.43) | ‐‐ |
| MTX + tocilizumab (8 mg/kg) | 0.66 (0.35 to 1.15) | 1.38 (0.59 to 3.48) | 2.52 (0.44 to 15.50) | 1.16 (0.72 to 2.00) | 3.56 (0.58 to 23.26) | 2.00 (0.15 to 83.94) | NE | 16.25 (1.48 to 205.84) | NE |
| MTX + tofacitinib | 0.56 (0.25 to 1.28) | 1.08 (0.14 to 12.62) | 4.55 (0.72 to 47.39) | 1.34 (0.73 to 2.50) | 1.19 (0.57 to 2.88) | 1.50 (0.67 to 3.59) | 1.51 (0.24 to 19.68) | 2.06 (0.33 to 29.33) | ‐‐ |
| MTX + cyclosporine | 1.37 (0.47 to 3.95) | 1.03 (0.42 to 2.51) | ‐‐ | 1.40 (0.69 to 2.86) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + IM gold | 0.37 (0.09 to 1.35) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 1.51 (0.05 to 101.78) | ‐‐ |
| MTX + leflunomide | 1.05 (0.35 to 3.09) | ‐‐ | ‐‐ | 1.67 (0.86 to 3.23) | 4.75 (1.16 to 20.70) | 3.91 (0.83 to 19.06) | ‐‐ | ‐‐ | ‐‐ |
| MTX + sulfasalazine | ‐‐ | NE | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| MTX + sulfasalazine + hydroxychloroquine | 0.88 (0.30 to 2.45) | 1.05 (0.44 to 2.52) | NE | 1.08 (0.40 to 2.56) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| IM/sc MTX | ‐‐ | 1.37 (0.38 to 4.83) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
Abbreviations: IM, intra‐muscular; IV, intravenous; MD, mean difference; MTX, methotrexate; NE, not estimable; sc, subcutaneous.
Methotrexate‐inadequate response network
Similar to the methotrexate‐naïve analysis, the credible intervals were very often very imprecise (Table 5). There were no statistically significant differences in serious adverse events between any treatment and methotrexate. Compared to oral methotrexate, methotrexate + leflunomide had a statistically significant higher rate of alanine aminotransferase elevations, and tocilizumab (8 mg/kg) had a statistically significant higher rate of leukopenia relative to oral methotrexate. Methotrexate + tocilizumab (4 mg/kg) also had a higher rate of leukopenia, but the result was not statistically significant.
Sensitivity analyses
Meta‐regression (ACR50 response)
The odds ratios comparing treatments to methotrexate were influenced by several study‐level characteristics (Table 6), although the adjusted treatment effects were similar to the main analysis for most comparisons (Figure 4; Figure 5). In the methotrexate‐naïve analysis, a more recent year of trial publication was associated with a statistically significant increased odds ratio for ACR50 response (Table 6). Higher doses of methotrexate were associated with lower odds ratios, although the result narrowly failed to reach statistical significance. For the methotrexate‐IR analysis, a higher placebo/oral methotrexate response rate was associated with a statistically significant, large decrease in the OR (0.59 times, 95% CrI: 0.43 to 0.75 ), although the model fit was not improved. A higher baseline disease duration was associated with a statistically significant increased OR in the methotrexate‐IR analysis, although with a small effect.
6. Meta‐regression for ACR50 response.
| MODEL FIT | ||||||||
| Beta coefficient, median (CrI) | Interpretation | Unadjusted analysis | Adjusted analysis | |||||
| DIC | Between‐study standard deviation | Total residual deviance (number of parameters) | DIC | Between‐study standard deviation | Total residual deviance (number of parameters) | |||
| MTX‐naïve | ||||||||
| MTX response rate | ‐1.7 (‐4.3 to 1.3) | Decrease in OR of 0.85 times (0.65 to 1.14) for every 10% increase in the response rate for MTX | 609.2 | 0.19 | 64.8 (64) | 610.9 | 0.21 | 64.6 (64) |
| Disease duration (years) | 0.008 (‐0.08 to 0.12) | Increase in OR of 1.01 times (0.92 to 1.12) for every year of disease duration | 585.1 | 0.21 | 61.5 (62) | 585.3 | 0.25 | 61.0 (62) |
| Duration of trial (weeks) | ‐0.002 (‐0.01 to 0.007) | Decrease in OR of 0.97 times (0.87 to 1.1) for every 12 weeks of trial duration | 609.2 | 0.19 | 64.8 (64) | 609.2 | 0.23 | 63.6 (64) |
| MTX dose >= 15 mg/week | ‐0.34 (‐0.80 to 0.03) | Decrease in OR of 0.71 times (0.45 to 1.0) for trials where the dose of MTX is >= 15 mg/wk | 609.2 | 0.19 | 64.8 (64) | 606.9 | 0.17 | 63.6 (64) |
| Year of publication of trial | 0.049 (0.008 to 0.10) | Increase in OR of 1.05 times (1.01 to 1.11) for each year later of publication (range of years 2000‐2015) | 609.2 | 0.19 | 64.8 (64) | 605.4 | 0.13 | 61.2 (64) |
| Swollen joint count | ‐0.04 (‐0.12 to 0.03) | Decrease in OR of 0.96 times (0.89 to 1.03) for every 1 additional swollen joint at baseline | 396.8 | 0.30 | 40.4 (40) | 396.4 | 0.23 | 40.3 (40) |
| DAS‐28 | 0.57 (‐0.29 to 1.6) | Increase in OR of 1.8 times (0.74 to 5.2) for every 1 additional point increase in DAS28 at baseline | 407.1 | 0.13 | 38.9 (40) | 407.6 | 0.12 | 38.2 (40) |
| MTX‐inadequate response | ||||||||
| MTX response rate | ‐5.3 (‐8.5 to ‐2.8) | Decrease in OR of 0.59 times (0.43 to 0.75) for every 10% increase in the response rate for MTX | 818.9 | 0.24 | 104.7 (97) | 825.3 | 0.24 | 105.1 (97) |
| Disease duration (years) | 0.10 (0.02 to 0.19) | Increase in OR of 1.11 times (1.02 to 1.21) for every year of disease duration | 740.4 | 0.27 | 91.9 (87) | 736.7 | 0.21 | 91.1 (87) |
| Duration of trial (weeks) | 0.001 (‐0.02 to 0.02) | Increase in OR of 1.02 times (0.83 to 1.22) for every 12 weeks of trial duration | 818.9 | 0.24 | 104.7 (97) | 819.3 | 0.26 | 104.0 (97) |
| MTX dose >= 15 mg/week | ‐0.17 (‐0.53 to 0.20) | Decrease in OR of 0.85 times (0.59 to 1.22) for trials where the dose of MTX is >= 15 mg/wk | 818.9 | 0.24 | 104.7 (97) | 818.3 | 0.27 | 102.4 (97) |
| Year of publication of trial | ‐0.03 (‐0.08 to 0.01) | Decrease in OR of 0.97 times (0.93 to 1.01) for each year later of publication (range of years 2000‐2015) | 818.9 | 0.24 | 104.7 (97) | 819.5 | 0.21 | 105.5 (97) |
| Swollen joint count | 0.02 (‐0.05 to 0.08) | Increase in OR of 1.02 times (0.95 to 1.09) for every 1 additional swollen joint at baseline | 728.0 | 0.26 | 92.0 (87) | 727.3 | 0.27 | 91.8 (87) |
| DAS‐28 | 0.23 (‐0.31 to 0.79) | Increase in OR of 1.26 times (0.73 to 2.20) for every 1 additional point increase in DAS28 at baseline | 546.7 | 0.25 | 71.1 (64) | 548.3 | 0.27 | 71.1 (64) |
Abbreviations: DAS: Disease Activity Score; DIC, deviance information criterion; IM, intra‐muscular; IV, intravenous; MD, mean difference; MTX, methotrexate; NE, not estimable; OR, odds ratio; sc, subcutaneous.
4.
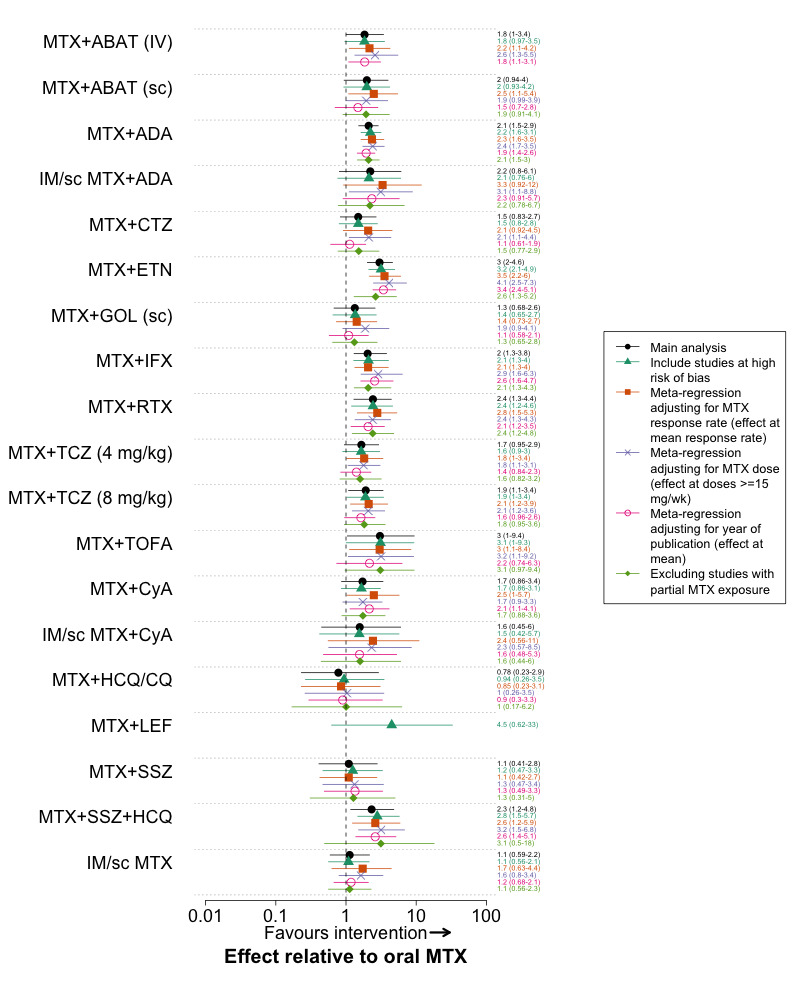
Selected meta‐regression and sensitivity analyses for ACR50 response in methotrexate‐naïve trials
Abbreviations: ABAT, abatacept; ADA, adalimumab; CQ, chloroquine; CyA, cyclosporine; ETN, etanercept; GOL, golimumab; HCQ, hydroxychloroquine; IFX, infliximab; IM, intra‐muscular; IV, intravenous; LEF, leflunomide; MTX, methotrexate; RTX, rituximab; sc, subcutaneous; SSZ, sulphasalazine; TCZ, tocilizumab
5.
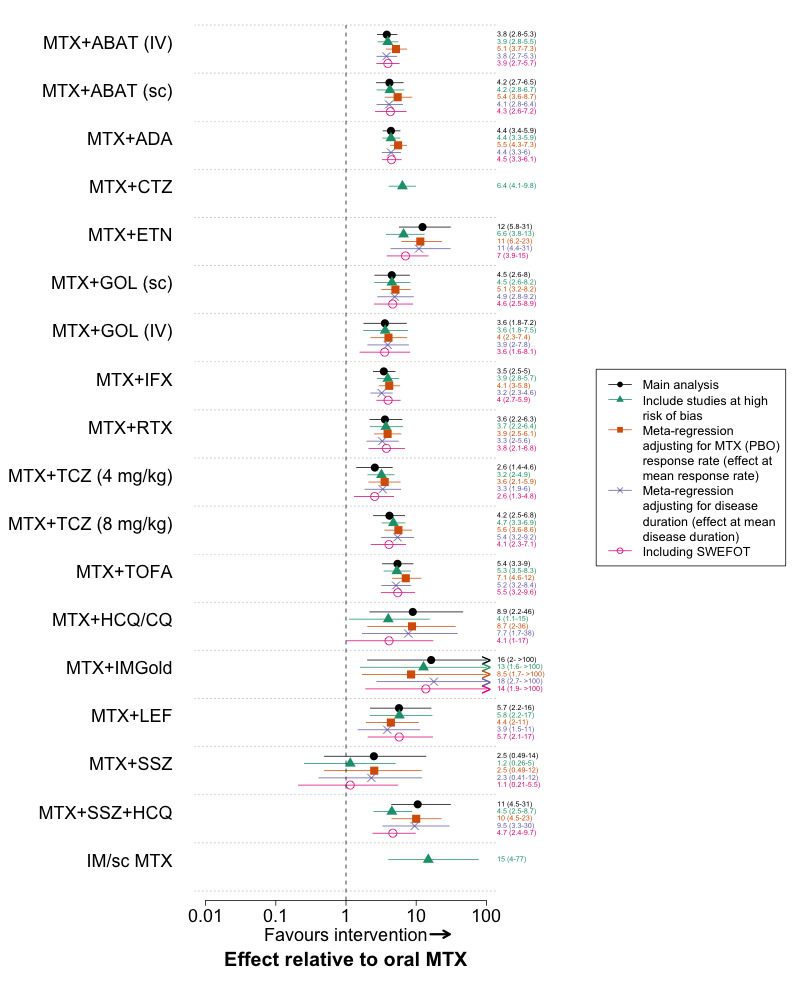
Selected meta‐regression and sensitivity analyses for ACR50 response in methotrexate‐inadequate response trials
Abbreviations: ABAT, abatacept; ADA, adalimumab; CTZ, certolizumab; CQ, chloroquine; ETN, etanercept; GOL, golimumab; HCQ, hydroxychloroquine; IFX, infliximab; IA, IM, intra‐muscular; IV, intravenous; LEF, leflunomide; MTX, methotrexate; RTX, rituximab; sc, subcutaneous; SSZ, sulphasalazine; TCZ, tocilizumab; TOFA, tofacitinib
When all studies (both methotrexate‐naïve and methotrexate‐IR) were included in the same network meta‐analysis and the network assignment was specified with a meta‐regression covariate, the OR of methotrexate‐IR trials were 2.05 times higher (95% CrI: 1.70‐2.48).
Additional sensitivity analyses
When trials at high risk of bias were included in the methotrexate‐IR analysis, methotrexate + certolizumab and sc/IM methotrexate were also statistically significantly superior to oral methotrexate. When studies with partial methotrexate exposure were excluded from the methotrexate‐naïve analysis, methotrexate + sulfasalazine + hydroxychloroquine was not statistically significantly superior to oral methotrexate for ACR50 response (Figure 4). The point estimate, however, was slightly higher than the main analysis and higher than any other treatment. Including SWEFOT in the methotrexate‐IR analysis for ACR50 response decreased the OR for methotrexate + etanercept and methotrexate + sulfasalazine + hydroxychloroquine relative to oral methotrexate, but each still had a large effect [methotrexate + etanercept: OR 7.0 (95%CrI: 3.9 to 15); methotrexate + sulfasalazine + hydroxychloroquine: OR 4.7 (95%CrI: 2.4 to 9.7)].
There was little change in the point estimates for ACR50 response at different time‐points of assessment, although the credible intervals were wider for several comparisons (Figure 6 and Figure 7). This supports the meta‐regression results where no association was found between trial duration and ACR50 response.
6.
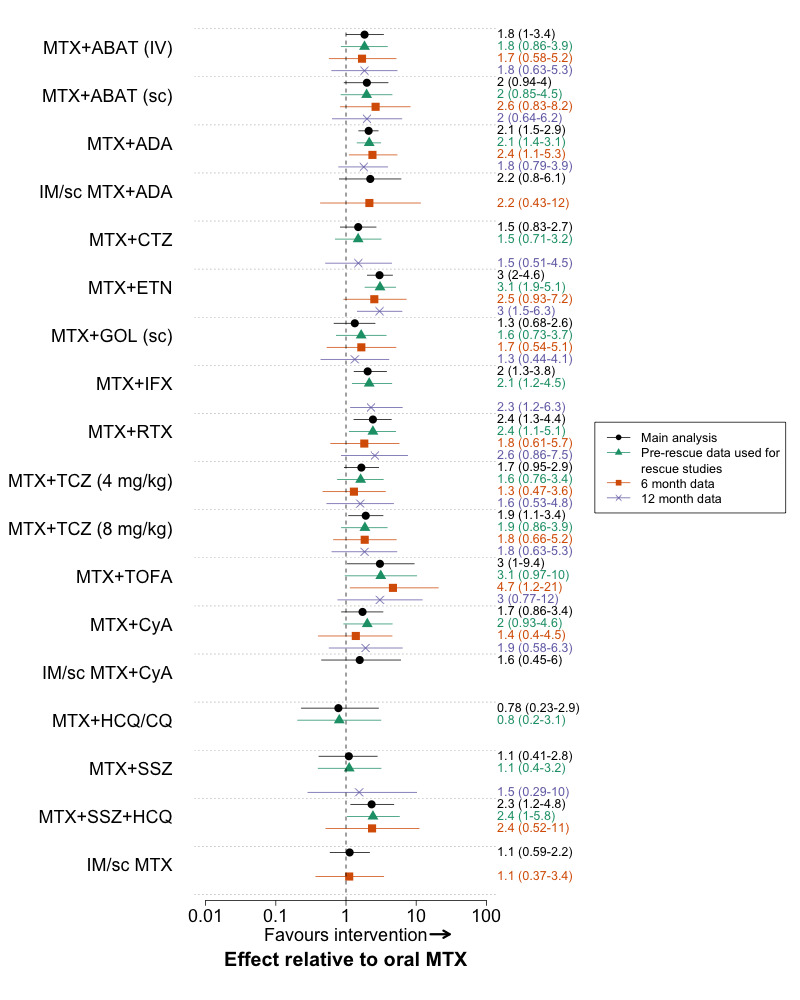
MTXSensitivity analyses for ACR50 response in methotrexate‐naïve trials for different time‐points of outcome assessment
Abbreviations: ABAT, abatacept; ADA, adalimumab; AZA, azathioprine; CQ, chloroquine; CyA, cyclosporine; ETN, etanercept; GOL, golimumab; HCQ, hydroxychloroquine; IFX, infliximab; IA, IM, intra‐muscular; IV, intravenous; MTX, methotrexate; RTX, rituximab; sc, subcutaneous; SSZ, sulphasalazine; TCZ, tocilizumab
7.
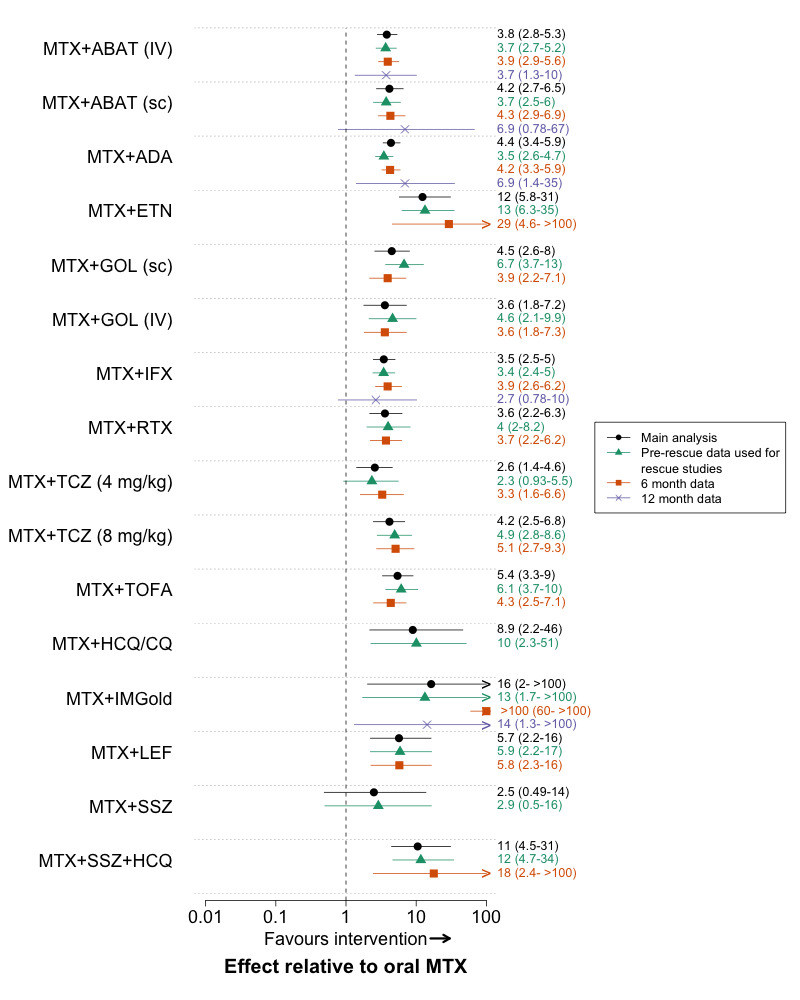
Sensitivity analyses for ACR50 response in methotrexate‐inadequate response trials for different time‐points of outcome assessment
Abbreviations: ABAT, abatacept; ADA, adalimumab; CTZ, certolizumab; CQ, chloroquine; ETN, etanercept; GOL, golimumab; HCQ, hydroxychloroquine; IFX, infliximab; IA, IM, intra‐muscular; IV, intravenous; LEF, leflunomide; MTX, methotrexate; RTX, rituximab; sc, subcutaneous; SSZ, sulfasalazine; TCZ, tocilizumab; TOFA, tofacitinib
Discussion
Summary of main results
Our systematic review and network meta‐analysis compared methotrexate and all currently used DMARD combinations with methotrexate. The main new finding from our review was that methotrexate + sulfasalazine + hydroxychloroquine (‘triple therapy’) was statistically significantly superior to oral methotrexate and similar to methotrexate + biologic therapy for ACR50 response, in both methotrexate‐naïve and methotrexate‐IR populations. We found a statistically significant benefit for other conventional synthetic DMARD combinations compared with oral methotrexate, but only after an inadequate response to methotrexate, and the magnitude of effect or quality of evidence was graded lower than for triple therapy. Most biologic DMARDs and tofacitinib, in combination with methotrexate, were superior to oral methotrexate for ACR50 response in both methotrexate‐naïve and methotrexate‐IR populations, although exceptions existed. In methotrexate‐naïve patients, the combinations of methotrexate + the biologic DMARDs subcutaneous abatacept, certolizumab, subcutaneous golimumab and tocilizumab (4 mg/kg IV) were not statistically significantly superior to oral methotrexate. Most treatments were well tolerated, with only methotrexate + azathioprine, methotrexate + cyclosporine and methotrexate + tocilizumab 8 mg/kg IV having statistically significantly more withdrawals due to adverse events than oral methotrexate in either the methotrexate‐naïve or methotrexate‐IR analyses.
The findings for radiographic progression merit careful consideration. Inhibition of radiographic damage is considered a key outcome when evaluating DMARDs in RA. The treatment effects for radiographic progression in the network meta‐analysis, however, were small, with wide credible intervals. For the main analysis, only four biologic DMARDs (adalimumab, certolizumab, etanercept, infliximab) were statistically significantly superior to oral methotrexate, and only in methotrexate‐naïve patients. For all network meta‐analyses we decided a priori to use a random‐effects model, as it is generally recommended in network meta‐analyses (Jansen 2014). In post‐hoc sensitivity analyses using fixed‐effects models, most biologic DMARDs (+ methotrexate) were statistically significantly superior to oral methotrexate. Importantly, even with a fixed‐effects model, methotrexate + sulfasalazine + hydroxychloroquine was not statistically significantly superior to oral methotrexate in either methotrexate‐naïve or methotrexate‐IR patients. Thus, although the effect is not robust to modeling assumptions, radiographic progression represents an important outcome that may distinguish triple therapy from methotrexate+biologic DMARDs. The magnitude of these effects was small over 1 year and below the minimal clinically important difference of 5 units on the Sharp‐vDh scale (Bruynesteyn 2002). Radiographic damage is cumulative though, so small effects over 1 year may have important long‐term consequences.
The meta‐regression analysis yielded some interesting results. Perhaps most importantly, we demonstrated that methotrexate‐IR trials were associated with a 2‐fold higher increase in odds ratios for ACR50 response. Thus, prior methotrexate response is a strong effect‐modifier of the clinical response and pooling studies in methotrexate‐naïve and methotrexate‐IR patients will yield biased estimates that are difficult to relate to clinical practice. While meta‐regression allowed us to demonstrate this effect, we preferred the approach of separating the analyses into methotrexate‐naïve and methotrexate‐IR trials to specifying the network assignment through meta‐regression. The meta‐regression coefficient was only specified for trials that included an oral methotrexate arm, as there were too few trials to estimate the effect separately for each comparison. Thus, the treatment effects from trials that compare 2 treatments other than oral methotrexate will not be adjusted, making it difficult to apply the results to clinical practice.
Overall completeness and applicability of evidence
This review included 158 trials with over 37000 patients, and multiple pre‐specified outcomes that were deemed to be important for therapeutic decision‐making in RA. We employed a rigorous approach to trial identification and outcome abstraction, and thus have confidence that the results encompass the best RCT evidence of the comparative benefits and harms for the treatments of interest.
Our results have direct relevance to therapeutic decision‐making in RA. The role of combination therapy with conventional synthetic DMARDs was considered controversial. A trial of conventional synthetic DMARD combination therapy prior to biologic therapy is not currently recommended by either major rheumatology guideline, although each provides the option (Singh 2016; Smolen 2014a). Given the similarity of methotrexate + sulfasalazine + hydroxychloroquine to methotrexate+ biologic DMARDs for the major efficacy outcome (ACR50 response) in both methotrexate‐naïve and methotrexate‐IR patients and the 10‐20 fold difference in cost, our findings suggest that it would be difficult to justify the use of methotrexate + biologic DMARDs without an adequate trial of methotrexate + sulfasalazine + hydroxychloroquine. There is also evidence for other DMARD combinations after an inadequate response to methotrexate, so these could also be considered, although the evidence was less precise and judged as lower quality. An exception, where methotrexate + biologic DMARDs may be preferred prior to a trial of conventional synthetic DMARD combination therapy is patients at high risk for rapid radiographic progression, given the lack of methotrexate + sulfasalazine + hydroxychloroquine to inhibit radiographic damage relative to oral methotrexate. The effect on radiographic progression with methotrexate + biologic DMARDs was small, however, and only demonstrated with fixed‐effects models. Given this, a trial of triple therapy in patients at high risk of radiographic progression, with close monitoring and progression to biologic therapy in patients with an inadequate response and/or radiographic progression may be more appropriate.
We did not evaluate the effect of glucocorticoids, which are known to have a disease‐modifying effect, particularly in early RA (Gaujoux‐Viala 2014). We did not exclude trials with corticosteroids, however; our findings therefore relate to the effects of DMARD therapy independent of a corticosteroid effect. We limited adverse events to only those that were felt a priori to be adequately captured within the context of RCTs; we did not evaluate potential rare adverse events that are best using long‐term data via registries. Adverse events of biologic therapy, including rare events, have been previously reviewed in a Cochrane network meta‐analysis (Singh 2011). Our results should not be generalised to patients who have had an inadequate response to biologic therapy, as we did not include these trials. While the trials included patients with both established and early RA, the findings will be most applicable to patients with early RA, as they relate to patients naïve to or after an inadequate response to methotrexate. Our review did not evaluate biologic or conventional synthetic DMARD monotherapy, which may also be appropriate therapeutic options, particularly in patients with an intolerance or contraindication to methotrexate (Singh 2016; Smolen 2014a).
Our study was not designed to directly compare treatment strategies. Specifically, we did not directly compare the approach of starting with methotrexate monotherapy in methotrexate‐naïve patients and progressing to triple therapy versus the strategy of starting with triple therapy directly. As initial therapy, methotrexate monotherapy was effective in a significant proportion of patients in the studies in our review, and prior Cochrane reviews have established it's effectiveness relative to placebo (Lopez‐Olivo 2014). Thus, patients may prefer to start with methotrexate monotherapy and progress to triple therapy (or another effective combination) only if they have an inadequate therapeutic response to methotrexate. The estimates of absolute risk with each treatment can help inform this decision. Based on the included trials, ˜40% of patients naïve to methotrexate are expected to have an ACR50 response to oral methotrexate, compared to 60% for triple therapy. Patients may accept this difference in risk and reserve combination therapy if they fail to respond adequately to initial therapy with methotrexate monotherapy. Methotrexate + sulfasalazine + hydroxychloroquine was also associated with a small increase in total gastrointestinal events in methotrexate‐naïve patients, which may influence patients’ decisions.
Quality of the evidence
The quality of evidence was higher for ACR50 responses than for radiographic progression and withdrawals due to adverse events. For both radiographic progression and withdrawals due to adverse events, study limitations and the imprecision in the estimates were the primary reasons for downgrading the quality. The quality of evidence for ACR50 responses with triple therapy was judged as moderate. In both methotrexate‐naïve and methotrexate‐IR analyses, the estimates were based on indirect evidence. While having only indirect evidence available should not by itself result downgrading the quality of evidence, there are several ways in which the quality of evidence from a NMA can be downgraded (Puhan 2014). First, the estimate from the NMA may be imprecise. Second, the direct evidence that informs the indirect comparison may have important quality limitations. This was a reason for downgrading the quality of evidence for methotrexate + sulfasalazine + hydroxychloroquine in both methotrexate‐naïve and methotrexate‐IR analyses. Third, there may be intransitivity between the direct comparisons that form the indirect evidence. We judged the likelihood of intransitivity to be low for methotrexate + sulfasalazine + hydroxychloroquine relative to oral methotrexate and did not further downgrade the evidence. The trials informing the indirect evidence consisted largely of trials of methotrexate + sulfasalazine + hydroxychloroquine versus methotrexate + etanercept and trials of methotrexate + etanercept versus methotrexate. These trials were generally recent trials with appropriate methotrexate dosing and similar patient characteristics. The relatively large magnitude of effect for methotrexate + sulfasalazine + hydroxychloroquine adds confidence to the findings. This was particularly true for the methotrexate‐IR analysis, even at the lower range of the 95% credible interval (OR: 11, 95% CrI: 4.3 to 28). The two trials at low/moderate risk of bias that directly compared methotrexate and methotrexate + sulfasalazine + hydroxychloroquine also support the efficacy of triple therapy in methotrexate‐naïve/partially exposed patients (O'Dell 1996; tREACH 2013). Both trials demonstrated superiority of triple therapy for their primary outcome, but ACR responses were not assessed.
Potential biases in the overview process
The extent to which indirect evidence is considered can affect the results of a network meta‐analysis (Hawkins 2009). With our search strategy we included all direct and first‐order indirect evidence between the treatments of interest; we did not attempt to capture all second‐order indirect evidence. This would have required an additional search for all of the interventions in the network that provided only indirect evidence (shown in the outer circle of the network diagrams, Figure 2). This could then lead to the identification of new treatments, which would require further searching, if a ‘complete’ network was desired (Hawkins 2009). Ultimately, this could lead to the inclusion of almost every DMARD trial in RA. The contribution of the indirect evidence to the overall estimate from the network meta‐analysis, however, decreases quite rapidly as the ‘order’ of the comparison increases. To obtain the same precision around the treatment effect, each of 3 trials in a second‐order indirect comparison would have to have 3 times the number of patients as one trial providing a direct comparison (Hawkins 2009). In our study, most of the treatments that would potentially form second‐order indirect evidence were conventional synthetic DMARD monotherapy in the methotrexate‐naïve network. The trials connecting these treatments to the network were small, such that if a new trial were identified (e.g.‐ sulfasalazine versus placebo) the indirect evidence it contributed to would have a minimal effect on the estimates between the treatments of interest. A systematic review of all conventional synthetic DMARDs found few trials of DMARD monotherapy (Gaujoux‐Viala 2014). These trials typically made comparisons to placebo with few patients and very few measured ACR responses. We therefore expect the impact of excluding these trials to have a minimal impact on any of the treatment effects we have reported.
An ‘early escape’ design was common in trials of methotrexate + biologic DMARDs and methotrexate + tofacitinib, particularly in more recent trials. While this allows trials to ethically include a placebo arm, it presents challenges in interpreting and synthesizing the results. The proportion of patients remaining on the control treatment at trial end can be very low. Trials may also differ in how they impute the data in patients who enter early escape. The imputation can be applied equally to all arms; if patients fail to meet a given response at the time of early escape, they are imputed as a non‐responder, regardless of the treatment arm. Alternatively, a trial may impute data only for patients in the control (placebo) arm. One trial compared these approaches, and found that ACR20 responses in the active treatment arms increased from ˜50% if patients meeting criteria for early escape were considered non‐responders, to ˜60% if observed data was used (ORALSTD 2012). Thus, using observed data for the active treatment arms and applying a non‐responder imputation to the placebo arm, as done in some trials (GOFORWARD 2009; Kremer 2012), may inflate the treatment effect. We included a sensitivity analysis for ACR50 response using pre‐rescue data and found few differences in treatment effects.
The synthesis of adverse events in early escape trials is also challenging. Patients who crossover from placebo to active therapy often represent a substantial pt‐years of exposure; excluding these patients may obscure important safety signals. We therefore choose to summary all toxicity data with exposure‐adjusted estimates, using the on‐treatment data from early escape trials. This could potentially bias the estimates, as patients who crossover may differ in certain ways than patients assigned to the original treatment. We felt this to be less of a potential bias than excluding the patients who crossover. Some trials also only reported exposure‐adjusted data and we otherwise would have excluded these trials.
Agreements and disagreements with other studies or reviews
Multiple network meta‐analyses of biologic therapy in RA, including a Cochrane overview of reviews have been performed (Singh 2009; Thorlund 2013). This is the first review to our knowledge that has systematically compared all methotrexate‐based conventional synthetic DMARD and biologic DMARD/tofacitinib treatment approaches. A prior network meta‐analysis compared combination DMARD therapy with conventional synthetic DMARDs and biologic DMARDs for radiographic outcomes (Graudal 2014). Overall, the authors found that one conventional synthetic DMARD + one biologic DMARD was not superior to combination therapy with 2 or 3 conventional synthetic DMARDs for radiographic outcomes. There are several important differences with our study. First, the authors grouped conventional synthetic DMARD combination therapy according to the number of medications (2 or 3), whereas each biologic agent was considered separately. Grouping DMARD combinations that are commonly used with those that are rarely used (e.g. – combinations with bucillamine or auranofin) adds heterogeneity to the estimates and makes it difficult to apply the results to clinical practice. Second, trials in DMARD‐naïve and DMARD‐IR patients were grouped which, as we demonstrated, may bias the treatment effects. Third, we evaluated a range of outcomes beyond radiographic outcomes, covering multiple domains relevant to decision making. The number of trials included in our review (158) was also much greater than the 39 trials the authors included. Finally, they included trials where corticosteroids were part of the intervention (i.e.‐ applied different between arms). The results, therefore address a different research question.
Other traditional (non‐network) systematic reviews have evaluated conventional synthetic DMARD combination therapy (Choy 2005; Graudal 2010; Katchamart 2010). The reviews differed in their outcomes considered and inclusion criteria, particularly around the inclusion and exclusion of interventions with corticosteroids. Combined withdrawal due to inefficacy or adverse events was used as the primary outcome for 2 of the reviews (Choy 2005; Katchamart 2010), as it is commonly reported. Trials are not powered for this outcome, however, and it does not allow a separation of benefits and harms necessary to inform clinical decisions.
In contrast to other systematic reviews, we evaluated the risk of bias separately for different outcomes, which is the approach recommended by GRADE and Cochrane (Guyatt 2011; Higgins 2011). We also used recently published GRADE guidance for grading the quality of the evidence (Puhan 2014). While this approach requires subjective decisions, it should increase the transparency of these choices.
Authors' conclusions
Implications for practice.
On the basis of all available direct and indirect evidence, our results suggest that triple therapy (methotrexate + sulfasalazine + hydroxychloroquine) is effective in both methotrexate naïve and methotrexate inadequate response patients and not statistically different from methotrexate plus biologic therapy for controlling disease activity. Other conventional synthetic DMARD combinations, including methotrexate + hydroxychloroquine, methotrexate + leflunomide and the less commonly used methotrexate + intra‐muscular gold were superior to oral methotrexate after an inadequate response to methotrexate, although the quality of evidence or magnitude of effect was lower than for methotrexate + sulfasalazine + hydroxychloroquine. Some biologics in combination with methotrexate were statistically significantly superior to methotrexate for preventing joint damage in methotrexate‐naïve patients, but the magnitude of effect was of questionable clinical significance. For most treatments, withdrawals due to adverse events were similar and not statistically different from oral methotrexate. Given these findings and cost considerations, it would be difficult to justify the use of methotrexate + biologic DMARDs prior to an adequate trial of combination therapy with methotrexate + conventional synthetic DMARDs (preferably methotrexate + sulfasalazine + hydroxychloroquine).
Implications for research.
More head‐to‐head trials of active treatments are needed to help inform decision‐making in RA. These trials would add to the precision around the treatment effects of interest and would help confirm the consistency of the treatment effects derived through indirect evidence. The results of any new trial, however, should be viewed in context of the existing indirect and direct evidence that forms the entire evidence base.
Acknowledgements
Dr. Hazlewood was supported by a UCB/Canadian Rheumatology Association (CRA)/The Arthritis Society (TAS) Post‐Graduate Rheumatology Fellowship and an Alberta Innovates Health Solutions Clinical Fellowship.
Dr. Bombardier holds a Canada Research Chair in Knowledge Transfer for Musculoskeletal Care and a Pfizer Chair in Rheumatology, University of Toronto, Faculty of Medicine's Rheumatology Division.
Appendices
Appendix 1. MEDLINE search strategy
exp arthritis, rheumatoid/
((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
(felty$ adj2 syndrome).tw.
(caplan$ adj2 syndrome).tw.
(sjogren$ adj2 syndrome).tw.
(sicca adj2 syndrome).tw.
still$ disease.tw.
or/1‐7
Methotrexate/
Methotrexate.tw.
amet?opterine.tw.
mexate.tw.
Abitrexate.tw.
A Met?opterine.tw.
Antifolan.tw.
Emt?exate.tw.
Enthexate.tw.
Farmitrexate.tw.
Folex.tw.
Ledertrexate.tw.
Methoblastin.tw.
Methohexate.tw.
Methotrate.tw.
Methylaminopterin.tw.
Metotrexate.tw.
Mtx.tw.
Novatrex.tw.
Rheumatrex.tw.
or/9‐28
8 and 29
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/31‐38
exp animals/ not humans.sh.
39 not 40
30 and 41
Appendix 2. EMBASE search strategy
exp arthritis, rheumatoid/
((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
(felty$ adj2 syndrome).tw.
(caplan$ adj2 syndrome).tw.
(sjogren$ adj2 syndrome).tw.
(sicca adj2 syndrome).tw.
still$ disease.tw.
or/1‐7
methotrexate/
Methotrexate.tw.
mexate.tw.
Abitrexate.tw.
Amet?opterine.tw.
mexate.tw.
Abitrexate.tw.
A Met?opterine.tw.
Antifolan.tw.
Emt?exate.tw.
Enthexate.tw.
Farmitrexate.tw.
Folex.tw.
Ledertrexate.tw.
Methoblastin.tw.
Methohexate.tw.
Methotrate.tw.
Methylaminopterin.tw.
Metotrexat$.tw.
Mtx.tw.
Novatrex.tw.
Rheumatrex.tw.
or/9‐30
8 and 31
(random$ or placebo$).ti,ab.
((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
controlled clinical trial$.ti,ab.
RETRACTED ARTICLE/
or/33‐36
(animal$ not human$).sh,hw.
37 not 38
32 and 39
Appendix 3. CENTRAL search strategy
MeSH descriptor Arthritis, Rheumatoid explode all trees
((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat* or reumat* or revmarthrit*) near/3 (arthrit* or artrit* or diseas* or condition* or nodule*)):ti,ab
felty* near/2 syndrome:ti,ab
caplan* near/2 syndrome:ti,ab
sjogren* near/2 syndrome:ti,ab
sicca near/2 syndrome:ti,ab
still* next disease:ti,ab
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
MeSH descriptor Methotrexate explode all trees
Methotrexate:ti,ab
ametopterine:ti,ab
mexate:ti,ab
Abitrexate:ti,ab
"A Met?opterine":ti,ab
Antifolan:ti,ab
Emtexate:ti,ab
Enthexate:ti,ab
Farmitrexate:ti,ab
Folex:ti,ab
Ledertrexate:ti,ab
Methoblastin:ti,ab
Methohexate:ti,ab
Methotrate:ti,ab
Methylaminopterin:ti,ab
Metotrexate:ti,ab
mtx:ti,ab
Novatrex:ti,ab
Rheumatrex:ti,ab
(#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28)
(#8 AND #29)
Contributions of authors
Concept: GH
Title Registration: GH
Protocol draft: GH
Protocol editing: GH, ChB, GT, DM, ClB
Title and abstract review: GH, ChB
Data abstraction: GH, ChB, DD
Data analysis: GH, GT
Drafting the review: GH
Editing and revising the review: GH, ChB, GT, DM, DD, ClB
Sources of support
Internal sources
No sources of support supplied
External sources
-
Alberta Innovates Health Solutions Clinical Fellowship, Canada.
Salary support, 80% protected research time
Arthur JE Child chair in rheumatology outcomes research, Canada.
Declarations of interest
GH ‐ GH received $1500 from Amgen as honorarium for an advisory board meeting, but the total amount was transferred without obligations to a non‐profit organization. This was discussed with the Funding Arbiters who agreed that the review could be published.
ChB ‐ Speaker fees: Abbott, BMS, Pfizer, UCB; Consultant: Abbott, Roche, UCB, Amgen; Educational Grant‐in‐Aid: Roche, Amgen/Pfizer; Unrestricted travel grant: Celgene
GT ‐ Chair of drug safety monitoring board for a clinical trial sponsored by Smith & Nephew.
DM ‐ Health economics and outcomes research consultant through OptumInsight.
ClB ‐ Consultant: Abbott, AstraZeneca, Bristol‐Myers Squibb Canada, Janssen, Pfizer, UCB Canada; Advisory board: Janssen, Pfizer, Takeda Canada Inc; Research funding: Abbott, Amgen, Bristol‐Myers Squibb Canada, Janssen, Hoffman La‐Roche, Pfizer, UCB Canada Inb
DD ‐ None
New
References
References to included reviews
Abe 2006
- Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, Nagaya I. A multicenter, double‐blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. Journal of Rheumatology 2006;33:37‐44. [PubMed] [Google Scholar]
ACQUIRE 2011
- Genovese MC, Covarrubias A, Leon G, Mysler E, Keiserman M, Valente R, et al. Subcutaneous abatacept versus intravenous abatacept: A phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis and Rheumatism 2011;63:2854‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
ACT‐RAY 2013
- Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan P G, Mola EM, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24‐week symptomatic and structural results of a 2‐year randomised controlled strategy trial in rheumatoid arthritis (ACT‐RAY). Annals of the rheumatic diseases 2013;72:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
AGREE 2009
- Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate‐naive patients with early rheumatoid arthritis and poor prognostic factors. Annals of the Rheumatic Diseases 2009;68:1870‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2010
- Ahmed S. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis. Internal Medicine Journal. 2010; Vol. 40:20.
AIM 2006
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial.. Annals of Internal Medicine 2006;144:865‐76. [DOI] [PubMed] [Google Scholar]
AIM 2006 (Additional article #1)
- Russell AS, Wallenstein GV, Li T, Martin MC, Maclean R, Blaisdell B, et al. Abatacept improves both the physical and mental health of patients with rheumatoid arthritis who have inadequate response to methotrexate treatment. Annals of the Rheumatic Diseases 2007;66:189‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alam 2012
- Alam MK, Sutradhar SR, Pandit H, Ahmed S, Bhattacharjee M, Miah AH, et al. Comparative study on methotrexate and hydroxychloroquine in the treatment of rheumatoid arthritis. Mymensingh medical journal: MMJ 2012;21:391‐8. [PubMed] [Google Scholar]
AMBITION 2010
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez‐Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Annals of the Rheumatic Diseases 2010;69:88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
AMPLE 2014
- Schiff M, Weinblatt M E, Valente R, Heijde D, Citera G, Elegbe A, et al. Head‐to‐head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: Two‐year efficacy and safety findings from AMPLE trial. Annals of the rheumatic diseases 2014;73:86‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 1985
- Andersen PA, West SG, O'Dell JR, Via CS, Claypool RG, Kotzin BL. Weekly pulse methotrexate in rheumatoid arthritis. Clinical and immunologic effects in a randomized, double‐blind study. Annals of Internal Medicine 1985;103:489‐96. [DOI] [PubMed] [Google Scholar]
ARMADA 2003
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis and Rheumatism 2003;48:35‐45. [DOI] [PubMed] [Google Scholar]
ARMADA 2003 (Additional article #1)
- Yount S, Sorensen MV, Cella D, Sengupta N, Grober J, Chartash EK. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clinical and Experimental Rheumatology 2007;25:838‐46. [PubMed] [Google Scholar]
Arnold 1990
- Arnold MH, O'Callaghan J, McCredie M, Beller EM, Kelly DE, Brooks PM. Comparative controlled trial of low‐dose weekly methotrexate versus azathioprine in rheumatoid arthritis: 3‐Year prospective study. British Journal of Rheumatology 1990;29:120‐5. [DOI] [PubMed] [Google Scholar]
ASPIRE 2004
- Clair EW, Heijde DMFM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis & Rheumatism 2004;50:3432‐43. [DOI] [PubMed] [Google Scholar]
ASSET 2013
- Conaghan P G, Durez P, Alten R E, Burmester G R, Tak P P, Klareskog L, et al. Impact of intravenous abatacept on synovitis, osteitis and structural damage in patients with rheumatoid arthritis and an inadequate response to methotrexate: the ASSET randomised controlled trial. Annals of the rheumatic diseases 2013;72:1287‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
ATTEST 2008
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: A phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the Rheumatic Diseases 2008;67:1096‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
ATTRACT 2000
- Lipsky PE, Heijde DMFM, Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. New England Journal of Medicine 2000;343:1594‐602. [DOI] [PubMed] [Google Scholar]
ATTRACT 2000 (Additional article #1)
- Maini R, Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932‐9. [DOI] [PubMed] [Google Scholar]
AUGUST‐II 2011
- Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: Results of a phase II, randomized, placebo‐controlled trial. Arthritis and Rheumatism 2011;63:1782‐92. [DOI] [PubMed] [Google Scholar]
AVERT 2015
- Emery P, Burmester GR, Bykerk VP, Combe BG, Furst DE, Barre E, Karyekar CS, Wong DA, Huizinga TWJ. Evaluating drug‐free remission with abatacept in early rheumatoid arthritis: Results from the phase 3b, multicentre, randomised, active‐controlled AVERT study of 24 months, with a 12‐month, double‐blind treatment period. Annals of the Rheumatic Diseases 2015;74:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bao 2000
- Bao CD, Huang WQ, SL Chen, Gu YY. Treatment of rheumatoid arthritis with leflunomide: a double blind, randomised controlled study. Clinical Journal of Rheumatology 2000;4:44‐6. [Google Scholar]
Bao 2003
- Bao C, Chen S, Gu Y, Lao Z, Ni L, Yu Q, et al. Leflunomide, a new disease‐modifying drug for treating active rheumatoid arthritis in methotrexate‐controlled phase II clinical trial. Chinese Medical Journal 2003;116:1228‐34. [PubMed] [Google Scholar]
Bejarano 2008
- Bejarano V, Quinn M, Conaghan PG, Reece R, Keenan AM, Walker D, et al. Effect of the early use of the anti‐tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis & Rheumatism 2008;59:1467‐74. [DOI] [PubMed] [Google Scholar]
BEST 2005
- Goekoop‐Ruiterman YPM, Vries‐Bouwstra JK, Allaart CF, Zeben D, Kerstens PJSM, Hazes JMW, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the best study): A randomized, controlled trial. Arthritis and Rheumatism 2005;52:3381‐90. [DOI] [PubMed] [Google Scholar]
BEST 2005 (Additional article #1)
- Kooij SM, Vries‐Bouwstra JK, Goekoop‐Ruiterman YPM, Ewals JAPM, Han KH, Hazes JMW, et al. Patient‐reported outcomes in a randomized trial comparing four different treatment strategies in recent‐onset rheumatoid arthritis. Arthritis Care and Research 2009;61:4‐12. [DOI] [PubMed] [Google Scholar]
Braun 2008
- Braun J, Kastner P, Flaxenberg P, Wahrisch J, Hanke P, Demary W, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six‐month, multicenter, randomized, double‐blind, controlled, phase IV trial. Arthritis & Rheumatism 2008;58:73‐81. [DOI] [PubMed] [Google Scholar]
C‐EARLY 2015
- Emery P, Bingham CO, Burmester GR, Bykerk VP, Furst DE, Mariette X, Purcaru O, Coteur G, Lunen B, Weinblatt M. Improvements in patient‐reported outcomes and workplace and household productivity following 52 weeks of treatment with certolizumab pegol in combination with methotrexate in DMARD‐naive early rheumatoid arthritis patients: Results from the C‐early randomized, double‐blind, controlled phase 3 study. Annals of the Rheumatic Diseases 2015;74:712‐3. [Google Scholar]
C‐EARLY 2015 (Additional article #1)
- Weinblatt M, Bingham C, Burmester G, Bykerk VP, Furst DE, Mariette X, Heijde D, Tatla D, Arendt C, Mountian I, Lunen B, Emery P. Certolizumab pegol in combination with methotrexate in DMARD‐naive patients with active, severe, progressive rheumatoid arthritis: Results from a randomized, double‐blind, controlled phase 3 study. Arthritis and Rheumatology 2015;67 (suppl 10):Abstract number 968. [Google Scholar]
C‐OPERA 2014
- Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. The first early rheumatoid arthritis, certolizumab pegol, multicenter, double‐blind, randomized, parallel‐group study: C‐Opera, in patients fulfilling the 2010 ACR/EULAR classification criteria, demonstrates inhibition of joint damage progression. Annals of the Rheumatic Diseases. 2014; Vol. 73.
CARDERA 2008
- Choy EHS, Smith CM, Farewell V, Walker D, Hassell A, Chau L, et al. Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Annals of the Rheumatic Diseases 2008;67:656‐63. [DOI] [PubMed] [Google Scholar]
CareRA 2015
- Verschueren P, Cock D, Corluy L, Joos R, Langenaken C, Taelman V, Raeman F, Ravelingien I, Vandevyvere K, Lenaerts J, Geens E, Geusens P, Vanhoof J, Durnez A, Remans J, Vander Cruyssen B, Essche E, Sileghem A, Brabanter G, Joly J, Meyfroidt S, Elst K, Westhovens R. Methotrexate in combination with other DMARDs is not superior to methotrexate alone for remission induction with moderate‐to‐high‐dose glucocorticoid bridging in early rheumatoid arthritis after 16 weeks of treatment: The CareRA trial. Annals of the Rheumatic Diseases 2015;74:27‐34. [DOI] [PubMed] [Google Scholar]
CHARISMA 2006
- Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double‐blind randomized controlled clinical trial of the interleukin‐6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate.[Erratum appears in Arthritis Rheum. 2008 Mar;58(3):887]. Arthritis & Rheumatism 2006;54:2817‐29. [DOI] [PubMed] [Google Scholar]
Chen 2009
- Chen DY, Chou SJ, Hsieh TY, Chen YH, Chen HH, Hsieh CW, et al. Randomized, double‐blind, placebo‐controlled, comparative study of human anti‐TNF antibody adalimumab in combination with methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis. Journal of the Formosan Medical Association 2009;108:310‐19. [DOI] [PubMed] [Google Scholar]
Chen 2011
- Chen RL, Tao Y, Lin ZY, Huang CH, Huang WH. Efficacy and safety of adalimumab in patients with rheumatoid arthritis. [Chinese]. Chinese Journal of New Drugs 2011;20:152‐155+166. [Google Scholar]
Choy 2012
- Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology (Oxford, England) 2012;51:1226‐34. [DOI] [PubMed] [Google Scholar]
CIMESTRA 2006
- Hetland ML, Stengaard‐Pedersen K, Junker P, Lottenburger T, Ellingsen T, Andersen LS, et al. Combination treatment with methotrexate, cyclosporine, and intraarticular betamethasone compared with methotrexate and intraarticular betamethasone in early active rheumatoid arthritis: an investigator‐initiated, multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study. Arthritis & Rheumatism 2006;54:1401‐9. [DOI] [PubMed] [Google Scholar]
CIMESTRA 2006 (Additional article #1)
- Hetland ML, Stengaard‐Pedersen K, Junker P, Lottenburger T, Hansen I, Andersen LS, et al. Aggressive combination therapy with intra‐articular glucocorticoid injections and conventional disease‐modifying anti‐rheumatic drugs in early rheumatoid arthritis: Second‐year clinical and radiographic results from the CIMESTRA study. Annals of the Rheumatic Diseases 2008;67:815‐22. [DOI] [PubMed] [Google Scholar]
COMET 2008
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double‐blind, parallel treatment trial. The Lancet 2008;372:375‐82. [DOI] [PubMed] [Google Scholar]
COMET 2008 (Additional article #1)
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A, et al. Patient‐reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial.[Erratum appears in Ann Rheum Dis. 2011 Aug;70(8):1519]. Annals of the Rheumatic Diseases 2010;69:222‐5. [DOI] [PubMed] [Google Scholar]
Conaghan 2014
- Conaghan PG, Ostergaard M, Wu C, Heijde D, Irazoque‐Palazuelos F, Hrycaj P, Xie Z, Zhang R, Wyman BT, Bradley JD, Soma K, Wilkinson B. Effects of tofacitinib on bone marrow edema, synovitis, and erosive damage in methotrexate‐naive patients with early active rheumatoid arthritis (duration <2 years): Results of an exploratory phase 2 MRI study. Arthritis and Rheumatology 2014;66:S519‐S20. [Google Scholar]
Cuomo 2006
- Cuomo G, Molinaro G, Montagna G, Migliaresi S, Valentini G. A comparison between the Simplified Disease Activity Index (SDAI) and the Disease Activity Score (DAS28) as measure of response to treatment in patients undergoing different therapeutic regimens. [Italian]. Reumatismo 2006;58:22‐5. [DOI] [PubMed] [Google Scholar]
DANCER 2006
- Emery P, Fleischmann R, Filipowicz‐Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis & Rheumatism 2006;54:1390‐400. [DOI] [PubMed] [Google Scholar]
DANCER 2006 (Additional article #1)
- Mease PJ, Revicki DA, Szechinski J, Greenwald M, Kivitz A, Barile‐Fabris L, et al. Improved health‐related quality of life for patients with active rheumatoid arthritis receiving rituximab: Results of the Dose‐Ranging Assessment: International Clinical Evaluation of Rituximab in Rheumatoid Arthritis (DANCER) Trial. Journal of Rheumatology 2008;35:20‐30. [PubMed] [Google Scholar]
DE019 2004
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, Clinical, and Functional Outcomes of Treatment with Adalimumab (a Human Anti‐Tumor Necrosis Factor Monoclonal Antibody) in Patients with Active Rheumatoid Arthritis Receiving Concomitant Methotrexate Therapy: A Randomized, Placebo‐Controlled, 52‐Week Trial. Arthritis and Rheumatism 2004;50:1400‐11. [DOI] [PubMed] [Google Scholar]
Dougados 1999
- Dougados M, Combe B, Cantagrel A, Goupille P, Olive P, Schattenkirchner M, et al. Combination therapy in early rheumatoid arthritis: A randomised, controlled, double blind 52 week clinical trial of sulphasalazine and methotrexate compared with the single components. Annals of the Rheumatic Diseases 1999;58:220‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drosos 1998
- Drosos AA, Voulgari PV, Papadopoulos IA, Politi EN, Georgiou PE, Zikou AK. Cyclosporine A in the treatment of early rheumatoid arthritis. A prospective, randomized 24‐month study. Clinical and Experimental Rheumatology 1998;16:695‐701. [PubMed] [Google Scholar]
Durez 2007
- Durez P, Malghem J, Nzeusseu TA, Depresseux G, Lauwerys BR, Westhovens R, et al. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis & Rheumatism 2007;56:3919‐27. [DOI] [PubMed] [Google Scholar]
Edwards 2004
- Edwards JCW, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close DR, et al. Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. New England Journal of Medicine 2004;350:2572‐81. [DOI] [PubMed] [Google Scholar]
Edwards 2004 (Additional article #1)
- Strand V, Balbir‐Gurman A, Pavelka K, Emery P, Li N, Yin M, et al. Sustained benefit in rheumatoid arthritis following one course of rituximab: Improvements in physical function over 2 years. Rheumatology 2006;45:1505‐13. [DOI] [PubMed] [Google Scholar]
Elmuntaser 2014
- Elmuntaser K. Efficacy of leflunomide 100mg weekly compared to 10mg methotrexate weekly in patients with active rheumatoid arthritis. Clinical and Experimental Rheumatology 2014;83:S43. [Google Scholar]
Emery 2000
- Emery P, Breedveld FC, Lemmel EM, Kaltwasser JP, Dawes PT, Gomor B, et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology 2000;39:655‐65. [DOI] [PubMed] [Google Scholar]
Emery 2000 (Additional article #1)
- Sharp JT, Strand V, Leung H, Hurley F, Loew‐Friedrich I. Treatment with leflunomide slows radiographic progression of rheumatoid arthritis: results from three randomized controlled trials of leflunomide in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group.[Erratum appears in Arthritis Rheum 2000 Jun;43(6):1345]. Arthritis & Rheumatism 2000;43:495‐505. [DOI] [PubMed] [Google Scholar]
EMPIRE 2014
- Nam JL, Villeneuve E, Hensor EMA, Wakefield RJ, Conaghan PG, Green MJ, et al. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: The EMPIRE trial. Annals of the Rheumatic Diseases 2014;73:1027‐36. [DOI] [PubMed] [Google Scholar]
ERA 2000
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis.[Erratum appears in N Engl J Med 2001 Jan 18;344(3):240], [Erratum appears in N Engl J Med 2001 Jan 4;344(1):76]. New England Journal of Medicine 2000;343:1586‐93. [DOI] [PubMed] [Google Scholar]
ERA 2000 (Additional article #1)
- Kosinski M, Kujawski SC, Martin R, Wanke LA, Buatti MC, Ware JE, et al. Health‐related quality of life in early rheumatoid arthritis: impact of disease and treatment response. American Journal of Managed Care 2002;8:231‐40. [PubMed] [Google Scholar]
ESCAPE 2010
- Gerlag DM, Hollis S, Layton M, Vencovsky J, Szekanecz Z, Braddock M, et al. Preclinical and clinical investigation of a CCR5 antagonist, AZD5672, in patients with rheumatoid arthritis receiving methotrexate. Arthritis & Rheumatism 2010;62:3154‐60. [DOI] [PubMed] [Google Scholar]
Fedorenko 2012
- Fedorenko E, Lukina G, Sigidin Y. The best strategy of treatment in early rheumatoid arthritis patients: Comparative efficacy of four regimens. Rheumatology. 2012; Vol. 51:i40.
Fedorenko 2012 (Additional article #1)
- Fedorenko E, Lukina G, Sigidin Y, Karateev D, Luchikhina E, Nasonov E. Comparative analysis of safety data of four treatment regimens in early rheumatoid arthritis patients. Ann Rheum Dis. 2012; Vol. 71, issue Suppl 3:672.
Fedorenko 2012 (Additional article #2)
- Fedorenko E, Lukina G, Sigidin Y. Remission as the main therapeutic target: Comparative efficacy of four treatment regimens in early rheumatoid arthritis (RA) patients (PTS). Annals of the Rheumatic Diseases 2013;72(Suppl 1):A70. [Google Scholar]
Ferraccioli 2002
- Ferraccioli GF, Gremese E, Tomietto P, Favret G, Damato R, Poi E. Analysis of improvements, full responses, remission and toxicity in rheumatoid patients treated with step‐up combination therapy (methotrexate, cyclosporin A, sulphasalazine) or monotherapy for three years. Rheumatology 2002;41:892‐8. [DOI] [PubMed] [Google Scholar]
Ferraz 1994
- Ferraz MB, Pinheiro GR, Helfenstein M, Albuquerque E, Rezende C, Roimicher L, et al. Combination therapy with methotrexate and chloroquine in rheumatoid arthritis. A multicenter randomized placebo‐controlled trial. Scandinavian Journal of Rheumatology 1994;23:231‐6. [DOI] [PubMed] [Google Scholar]
Fiehn 2007
- Fiehn C, Jacki S, Heilig B, Lampe M, Wiesmuller G, Richter C, et al. Eight versus 16‐week re‐evaluation period in rheumatoid arthritis patients treated with leflunomide or methotrexate accompanied by moderate dose prednisone. Rheumatology International 2007;27:975‐9. [DOI] [PubMed] [Google Scholar]
FUNCTION 2013
- Burmester G, Rigby W, Vollenhoven R, Kay J, Rubbert‐Roth A, Kelman A, et al. Tocilizumab (TCZ) in combination and monotherapy versus methotrexate (MTX) in MTX‐naive patients (pts) with early rheumatoid arthritis (RA): Clinical and radiographic outcomes from a randomised, placebo‐controlled trial. Annals of the Rheumatic Diseases. 2013; Vol. 72:A63.
FUNCTION 2013 (Additional article #1)
- Burmester G, Blanco R, Keiserman M, Rigby W, Vollenhoven R F, Dimonaco S M, et al. Tocilizumab (TCZ) as combination therapy and as monotherapy vs methotrexate (MTX) in MTX‐naive patients with early rheumatoid arthritis: Patient‐reported outcomes (PROS) from a randomized, placebo‐controlled trial. Annals of the Rheumatic Diseases. 2014; Vol. 73:672.
Furst 1989
- Furst DE, Koehnke R, Burmeister L F, Kohler J, Cargill I. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. Journal of Rheumatology 1989;16:313‐20. [PubMed] [Google Scholar]
Gerards 2003
- Gerards AH, Landewe RBM, Prins APA, Bruyn GAW, Goei The HS, Laan RFJM, et al. Cyclosporin A monotherapy versus cyclosporin A and methotrexate combination therapy in patients with early rheumatoid arthritis: a double blind randomised placebo controlled trial.[Erratum appears in Ann Rheum Dis. 2003 Nov;62(11):1126 Note: Bruijn, GA [corrected to Bruyn, GA]]. Annals of the Rheumatic Diseases 2003;62:291‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghosh 2008
- Ghosh B, Halder S, Ghosh A, Dhar S. Early rheumatoid arthritis: Clinical and therapeutic evaluation in a tertiary care centre in India. Indian Journal of Rheumatology 2008;3:48‐51. [Google Scholar]
Giacomelli 2002
- Giacomelli R, Cipriani P, Matucci Cerinic M, Fulminis A, Barattelli G, Pingiotti E, et al. Combination therapy with cyclosporine and methotrexate in patients with early rheumatoid arthritis soon inhibits TNFalpha production without decreasing TNFalpha mRNA levels. An in vivo and in vitro study. Clinical and Experimental Rheumatology 2002;20:365‐72. [PubMed] [Google Scholar]
GOBEFORE 2013
- Emery P, Fleischmann RM, Doyle MK, Strusberg I, Durez P, Nash P, et al. Golimumab, a human anti‐tumor necrosis factor monoclonal antibody, injected subcutaneously every 4 weeks in patients with active rheumatoid arthritis who had never taken methotrexate: 1‐year and 2‐year clinical, radiologic, and physical function findings of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study. Arthritis care & research 2013;65:1732‐42. [DOI] [PubMed] [Google Scholar]
GOBEFORE 2013 (Additional article #1)
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti‐tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate‐naive patients with active rheumatoid arthritis: Twenty‐four‐week results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study of golimumab before methotrexate as first‐line therapy for early‐onset rheumatoid arthritis. Arthritis and Rheumatism 2009;60:2272‐83. [DOI] [PubMed] [Google Scholar]
GOFORTH 2012
- Tanaka Y, Harigai M, Takeuchi T, Yamanaka H, Ishiguro N, Yamamoto K, et al. Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO‐FORTH study. Annals of the Rheumatic Diseases 2012;71:817‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOFORWARD 2009
- Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO‐FORWARD Study.[Erratum appears in Ann Rheum Dis. 2011 Jan;70(1):238]. Annals of the Rheumatic Diseases 2009;68:789‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOFORWARD 2009 (Additional article #1)
- Emery P, Fleischmann R, Heijde D, Keystone EC, Genovese MC, Conaghan PG, et al. The effects of golimumab on radiographic progression in rheumatoid arthritis: results of randomized controlled studies of golimumab before methotrexate therapy and golimumab after methotrexate therapy.[Erratum appears in Arthritis Rheum. 2012 Apr;64(4):1045]. Arthritis & Rheumatism 2011;63:1200‐10. [DOI] [PubMed] [Google Scholar]
GOFORWARD 2009 (Additional article #2)
- Genovese MC, Han C, Keystone EC, Hsia EC, Buchanan J, Gathany T, et al. Effect of golimumab on patient‐reported outcomes in rheumatoid arthritis: Results from the GO‐FORWARD study. Journal of Rheumatology 2012;39:1185‐91. [DOI] [PubMed] [Google Scholar]
GOFORWARD 2009 (Additional article #3)
- Keystone E, Genovese MC, Klareskog L, Hsia EC, Hall S, Miranda PC, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52‐week results of the GO‐FORWARD study.[Erratum appears in Ann Rheum Dis. 2011 Jan;70(1):238‐9]. Annals of the Rheumatic Diseases 2010;69:1129‐35. [DOI] [PubMed] [Google Scholar]
GOFURTHER 2013
- Weinblatt ME, Bingham III CO, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: Results of the phase 3, randomised, multicentre, double‐blind, placebo‐controlled GO‐FURTHER trial. Annals of the Rheumatic Diseases 2013;72:381‐9. [DOI] [PubMed] [Google Scholar]
GOFURTHER 2013 (Additional article #1)
- Bingham III CO, Weinblatt M, Han C, Gathany TA, Kim L, Lo KH, et al. The effect of intravenous golimumab on health‐related quality of life in rheumatoid arthritis: 24‐week results of the phase III GO‐FURTHER trial. Journal of Rheumatology 2014;41:1067‐76. [DOI] [PubMed] [Google Scholar]
GOFURTHER 2013 (Additional article #2)
- Westhovens R, Weinblatt ME, Han C, Kim L, Mack M, Lu J, et al. Health‐related quality of life of patients with rheumatoid arthritis achieving DAS28 remission, improvement in physical function and no radiographic progression after treatment with intravenous golimumab. Annals of the Rheumatic Diseases. 2014; Vol. 73:479‐480.
Gubar 2008
- Gubar EE, Bochkova AG, Bunchuk NV. Comparison of efficacy and tolerability of triple combination therapy (methotrexate+sulfasalazine+hydroxychloroquine) with methotrexate monotherapy in patients with rheumatoid arthritis. [Russian]. Terapevticheskii Arkhiv 2008;80:25‐30. [PubMed] [Google Scholar]
GUEPARD 2009
- Soubrier M, Puechal X, Sibilia J, Mariette X, Meyer O, Combe B, et al. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology (Oxford, England) 2009;48:1429‐34. [DOI] [PubMed] [Google Scholar]
Haagsma 1994
- Haagsma CJ, Riel PL, Rooij DJ, Vree TB, Russel FJ, van't Hof MA, et al. Combination of methotrexate and sulphasalazine vs methotrexate alone: a randomized open clinical trial in rheumatoid arthritis patients resistant to sulphasalazine therapy. British Journal of Rheumatology 1994;33:1049‐55. [DOI] [PubMed] [Google Scholar]
Haagsma 1997
- Haagsma CJ, Riel PL, Jong AJ, Putte LB. Combination of sulphasalazine and methotrexate versus the single components in early rheumatoid arthritis: a randomized, controlled, double‐blind, 52 week clinical trial. British Journal of Rheumatology 1997;36:1082‐8. [DOI] [PubMed] [Google Scholar]
Hamdy 1987
- Hamdy H, McKendry RJ, Mierins E, Liver JA. Low‐dose methotrexate compared with azathioprine in the treatment of rheumatoid arthritis. A twenty‐four‐week controlled clinical trial. Arthritis & Rheumatism 1987;30:361‐8. [DOI] [PubMed] [Google Scholar]
Hamilton 2001
- Hamilton J, McInnes IB, Thomson EA, Porter D, Hunter JA, Madhok R, et al. Comparative study of intramuscular gold and methotrexate in a rheumatoid arthritis population from a socially deprived area. Annals of the Rheumatic Diseases 2001;60:566‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
HITHARD 2013
- Detert J, Bastian H, Listing J, Weiss A, Wassenberg S, Liebhaber A, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD‐naive patients with early rheumatoid arthritis: HIT HARD, an investigator‐initiated study. Annals of the Rheumatic Diseases 2013;72:844‐50. [DOI] [PubMed] [Google Scholar]
HOPEFUL‐I 2014
- Takeuchi T, Yamanaka H, Ishiguro N, Miyasaka N, Mukai M, Matsubara T, et al. Adalimumab, a human anti‐TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: The HOPEFUL 1 study. Annals of the rheumatic diseases 2014;73:536‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2001
- Hu Y, Tu S, Liu P. A randomized, controlled, single‐blind trial of leflunomide in the treatment of rheumatoid arthritis. Journal of Tongji Medical University = Tong ji yi ke da xue xue bao 2001;21:72‐4. [DOI] [PubMed] [Google Scholar]
Huang 2009
- Huang F, Zhang FC, Bao CD, Tao Y, Gu JR, Xu JH, et al. [Adalimumab plus methotrexate for the treatment of rheumatoid arthritis: a multi‐center randomized, double‐blind, placebo‐controlled clinical study.]. Chung‐Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 2009;48:916‐21. [PubMed] [Google Scholar]
Huang 2012
- Huang Z, Yang B, Shi Y, Cai B, Li Y, Feng W, et al. Anti‐TNF‐alpha therapy improves Treg and suppresses Teff in patients with rheumatoid arthritis. Cellular Immunology 2012;279:25‐9. [DOI] [PubMed] [Google Scholar]
IMAGE 2012
- Tak PP, Rigby W, Rubbert‐Roth A, Peterfy C, Vollenhoven RF, Stohl W, et al. Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2‐Year results from the randomised controlled trial IMAGE. Annals of the Rheumatic Diseases 2012;71:351‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
IMAGE 2012 (Additional article #1)
Ishaq 2011
- Ishaq M, Muhammad JS, Hameed K, Mirza A I. Leflunomide or methotrexate? Comparison of clinical efficacy and safety in low socio‐economic rheumatoid arthritis patients. Modern Rheumatology 2011;21:375‐80. [DOI] [PubMed] [Google Scholar]
Islam 2000
- Islam MN, Alam MN, Haq SA, Moyenuzzaman M, Patwary MI, Rahman MH. Efficacy of sulphasalazine plus methotrexate in rheumatoid arthritis. Bangladesh Medical Research Council Bulletin 2000;26:1‐7. [PubMed] [Google Scholar]
Iwahashi 2014
- Iwahashi M, Inoue H, Matsubara T, Tanaka T, Amano K, Kanamono T, et al. Efficacy, safety, pharmacokinetics and immunogenicity of abatacept administered subcutaneously or intravenously in Japanese patients with rheumatoid arthritis and inadequate response to methotrexate: a Phase II/III, randomized study. Mod Rheumatol 2014;24(6):885‐91. [DOI] [PubMed] [Google Scholar]
Jaimes‐Hernandez 2012
- Jaimes‐Hernandez J, Melendez‐Mercado C I, Mendoza‐Fuentes A, Aranda‐Pereira P, Castaneda‐Hernandez G. Efficacy of leflunomide 100 mg weekly compared to low dose methotrexate in patients with active rheumatoid arthritis. Double blind, randomized clinical trial. [Spanish]. Reumatologia Clinica 2012;8:243‐9. [DOI] [PubMed] [Google Scholar]
Jeurissen 1991
- Jeurissen ME, Boerbooms AM, Putte LB, Doesburg WH, Mulder J, Rasker JJ, et al. Methotrexate versus azathioprine in the treatment of rheumatoid arthritis. A forty‐eight‐week randomized, double‐blind trial. Arthritis & Rheumatism 1991;34:961‐72. [DOI] [PubMed] [Google Scholar]
Jeurissen 1991 (additional article #1)
- Jeurissen ME, Boerbooms AM, Putte LB, Doesburg WH, Lemmens AM. Influence of methotrexate and azathioprine on radiologic progression in rheumatoid arthritis. A randomized, double‐blind study. Annals of Internal Medicine 1991;114:999‐1004. [DOI] [PubMed] [Google Scholar]
Joo 2012
- Joo K, Heejung K, Lim MJ, Kwon SR, Park W. Safety and efficacy of TNFa inhibitor versus leflunomide in patients with rheumatoid arthritis inadequately responding to methotrexate in Korea. International Journal of Rheumatic Diseases. 2012; Vol. 15:53‐4.
JRAPID 2014
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: the J‐RAPID randomized, placebo‐controlled trial. Mod Rheumatol 2014;24(5):715‐24. [DOI] [PubMed] [Google Scholar]
JRAPID 2014 (Additional article #1)
Kang 2012
- Kang YM, Park W, Park YE, Choe JY, Bae SC, Cho CS, Shim SC, Lee SK, Suh CH, Cha HS, Song YW, You B, Lee SS, Lee SH, Park MC. Efficacy and safety of certolizumab pegol (CZP) with concomitant methotrexate (MTX) in Korean rheumatoid arthritis (RA) patients (pts) with an inadequate response to MTX. Ann Rheum Dis. 2012; Vol. 71, issue Suppl3:666.
Kay 2008
- Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double‐blind, placebo‐controlled, dose‐ranging study.[Erratum appears in Arthritis Rheum. 2010 Nov;62(11):3518]. Arthritis & Rheumatism 2008;58:964‐75. [DOI] [PubMed] [Google Scholar]
Kim 2000
- Kim WU, Cho ML, Kim SI, Yoo WH, Lee SS, Joo YS, et al. Divergent effect of cyclosporine on Th1/Th2 type cytokines in patients with severe, refractory rheumatoid arthritis. Journal of Rheumatology 2000;27:324‐31. [PubMed] [Google Scholar]
Kim 2007
- Kim HY, Lee SK, Song YW, Yoo DH, Koh EM, Yoo B, et al. A randomized, double‐blind, placebo‐controlled, phase III study of the human anti‐tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR Journal of Rheumatology 2007;10:9‐16. [Google Scholar]
Kim 2013
- Kim J, Ryu H, Yoo DH, Park SH, Song GG, Park W, et al. A clinical trial and extension study of infliximab in Korean patients with active rheumatoid arthritis despite methotrexate treatment. Journal of Korean Medical Science 2013;28:1716‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraan 2000
- Kraan MC, Koster BM, Elferink JG, Post WJ, Breedveld FC, Tak PP. Inhibition of neutrophil migration soon after initiation of treatment with leflunomide or methotrexate in patients with rheumatoid arthritis: findings in a prospective, randomized, double‐blind clinical trial in fifteen patients. Arthritis & Rheumatism 2000;43:1488‐95. [DOI] [PubMed] [Google Scholar]
Kremer 2002
- Kremer JM, Genovese MC, Cannon GW, Caldwell JR, Cush JJ, Furst DE, et al. Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate. A randomized, double‐blind, placebo‐controlled trial.[Summary for patients in Ann Intern Med. 2002 Nov 5;137(9):I42; PMID: 12416973]. Annals of Internal Medicine 2002;137:726‐33. [DOI] [PubMed] [Google Scholar]
Kremer 2005
- Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve‐month results of a phase iib, double‐blind, randomized, placebo‐controlled trial.[Erratum appears in Arthritis Rheum. 2005 Oct;52(10):3321]. Arthritis & Rheumatism 2005;52:2263‐71. [DOI] [PubMed] [Google Scholar]
Kremer 2005 (Additional article #1)
- Kremer JM, Westhovens R, Leon M, Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. New England Journal of Medicine 2003;349:1907‐15. [DOI] [PubMed] [Google Scholar]
Kremer 2012
- Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez‐Reino J, et al. A phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis and rheumatism 2012;64:970‐81. [DOI] [PubMed] [Google Scholar]
Lan 2004
- Lan JL, Chou SJ, Chen DY, Chen YH, Hsieh TY, Young M Jr. A comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12‐week, double‐blind, randomized, placebo‐controlled study. Journal of the Formosan Medical Association 2004;103:618‐23. [PubMed] [Google Scholar]
Lao 2001
- Lao ZY, Ni LQ, Zhang ZF, Zhou JL. Leflunomide in treating rheumatoid arthritis: a double‐blind study. Chinese Journal of New Drugs and Clinical Remedies 2001;20:94‐7. [Google Scholar]
Lau 2002
- Lau CS. Leflunomide (LEF) versus low dose methotrexate (MTX) in adult Asian patients with active rheumatoid arthritis (RA). Abstract book, the 10th APLAR meeting. 2002; Vol. 141.
Li 2013
- Li Z, Zhang F, Kay J, Fei K, Han C, Zhuang Y, et al. Safety and efficacy of subcutaneous golimumab in chinese patients with active rheumatoid arthritis despite MTX therapy: Results from a randomized, placebo‐controlled, phase 3 trial. Annals of the Rheumatic Diseases 2013;72:A444. [DOI] [PubMed] [Google Scholar]
Lisbona 2012
- Lisbona MP, Maymo J, Solano A, Almirall M, Sanchez S, Pamies A, Navallas M, Carbonell J. Comparative assessment of methotrexate and leflunomide by magnetic resonance imaging in patients with early rheumatoid arthritis. Ann Rheum Dis. 2012; Vol. 71, issue Suppl3:603.
LITHE 2011
- Kremer JM, Blanco R, Brzosko M, Burgos‐Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: Results from the double‐blind treatment phase of a randomized placebo‐controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis and Rheumatism 2011;63:609‐21. [DOI] [PubMed] [Google Scholar]
Machein 2002
- Machein U, Buss B, Spiller I, Braun J, Rudwaleit M, Faerber L, et al. Effective treatment of early rheumatoid arthritis with a combination of methotrexate, prednisolone and cyclosporin. Rheumatology 2002;41:110‐1. [DOI] [PubMed] [Google Scholar]
MacIsaac 2014
- MacIsaac KD, Baumgartner R, Kang J, Loboda A, Peterfy C, DiCarlo J, Riek J, Beals C. Pre‐treatment whole blood gene expression is associated with 14‐week response assessed by dynamic contrast enhanced magnetic resonance imaging in infliximab‐treated rheumatoid arthritis patients. PLoS ONE 2014;9:no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchesoni 2003
- Marchesoni A, Battafarano N, Arreghini M, Panni B, Gallazzi M, Tosi S. Radiographic progression in early rheumatoid arthritis: a 12‐month randomized controlled study comparing the combination of cyclosporin and methotrexate with methotrexate alone. Rheumatology 2003;42:1545‐9. [DOI] [PubMed] [Google Scholar]
Marcora 2006
- Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti‐tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. American Journal of Clinical Nutrition 2006;84:1463‐72. [DOI] [PubMed] [Google Scholar]
MASCOT 2007
- Capell HA, Madhok R, Porter DR, Munro RAL, McInnes IB, Hunter JA, et al. Combination therapy with sulfasalazine and methotrexate is more effective than either drug alone in patients with rheumatoid arthritis with a suboptimal response to sulfasalazine: results from the double‐blind placebo‐controlled MASCOT study. Annals of the Rheumatic Diseases 2007;66:235‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsubara 2012
- Matsubara T, Inoue H, Iwahashi M, Yamazaki A, Takeuchi T, and the Japan Abatacept Study Group. A multi‐center, double‐dummy, double‐blind study of subcutaneous (sc) abatacept (aba) compared with intravenous (iv) aba in Japanese rheumatoid arthritis patients with inadequate response to methotrexate. Ann Rheum Dis. 2012; Vol. 71, issue Suppl3:197.
MEASURE 2015
- McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, et al. Effect of interleukin‐6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo‐controlled study. Ann Rheum Dis 2015;74(4):694‐702. [DOI: 10.1136/annrheumdis-2013-204345.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehrotra 2006
- Mehrotra A Mehrotra R. A comparative 3 year clinical study of combined leflunomide and methotrexate vs methotrexate alone as DMARD in rheumatoid arthritis. Internal Medicine Journal. 2006:A83.
METGO 2005
- Lehman AJ, Esdaile JM, Klinkhoff AV, Grant E, Fitzgerald A, Canvin J. A 48‐week, randomized, double‐blind, double‐observer, placebo‐controlled multicenter trial of combination methotrexate and intramuscular gold therapy in rheumatoid arthritis: Results of the METGO study. Arthritis and Rheumatism 2005;52:1360‐70. [DOI] [PubMed] [Google Scholar]
Morassut 1989
- Morassut P, Goldstein R, Cyr M, Karsh J, McKendry RJ. Gold sodium thiomalate compared to low dose methotrexate in the treatment of rheumatoid arthritis‐‐a randomized, double blind 26‐week trial. Journal of Rheumatology 1989;16:302‐6. [PubMed] [Google Scholar]
Mottaghi 2005
- Mottaghi P, Karimzade H. Does chloroquine decrease liver enzyme abnormalities induced by methoterexate in patients with rheumatoid arthritis?. Journal of Research in Medical Sciences 2005;10:135‐8. [Google Scholar]
NCT01248780 2013
- Centocor Inc. Study of Subcutaneous Golimumab in Chinese Patients With Active Rheumatoid Arthritis Despite Methotrexate Therapy. Registered clinical trial with results available through www.clinicaltrials.gov Last updated: August 12, 2013.
O'Dell 1996
- O'Dell JR, Haire CE, Erikson N, Drymalski W, Palmer W, Eckhoff PJ, et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. New England Journal of Medicine 1996;334:1287‐91. [DOI] [PubMed] [Google Scholar]
O'Dell 2002
- O'Dell JR, Leff R, Paulsen G, Haire C, Mallek J, Eckhoff PJ, et al. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: Results of a two‐year, randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2002;46:1164‐70. [DOI] [PubMed] [Google Scholar]
OPERA 2014
- Horslev‐Petersen K, Hetland M L, Junker P, Podenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab added to a treat‐to‐target strategy with methotrexate and intra‐articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: An investigator‐initiated, randomised, double‐blind, parallel‐group, placebo‐controlled Trial. Annals of the rheumatic diseases 2014;73:654‐61. [DOI] [PubMed] [Google Scholar]
OPTIMA 2013
- Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26‐week results from the randomised, controlled OPTIMA study. Annals of the rheumatic diseases 2013;72:64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
OPTIMA 2013 (Additional article #1)
- Emery P, Kavanaugh AF, Smolen J, Cifaldi MA, Chaves L, Guerette B, Vipin A. Combination therapy with adalimumab methotrexate significantly improved work ability, physical function, fatigue, and other patient‐reported outcomes in early rheumatoid arthritis: Results from a 26‐week analysis. Arthritis and Rheumatism. 2011; Vol. 63, issue Suppl 10:2189.
OPTION 2008
- Smolen JS, Beaulieu A, Rubbert‐Roth A, Ramos‐Remus C, Rovensky J, Alecock E, et al. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. Lancet 2008;371:987‐97. [DOI] [PubMed] [Google Scholar]
ORALSCAN 2013
- Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, Cardiel MH, Cohen S, Nash P, Song YW, Tegzova D, Wyman BT, Gruben D, Benda B, Wallenstein G, Krishnaswami S, Zwillich SH, Bradley JD, Connell CA, and the ORAL Scan Investigators. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four‐month phase III randomized radiographic study. Arthritis Rheum 2013; Vol. 65:559‐70. [DOI] [PubMed]
ORALSTD 2012
- Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Meijide JAG, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. New England Journal of Medicine 2012;367:508‐19. [DOI] [PubMed] [Google Scholar]
Pinheiro 1993
- Pinheiro GR, Helfenstein Junior M, Ferraz MB, Atra E. A short‐term randomized controlled study with methotrexate in rheumatoid arthritis. [Portuguese]. Revista da Associacao Medica Brasileira (1992) 1993;39:91‐4. [PubMed] [Google Scholar]
PREMIER 2006
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, PavelkaK, Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis and Rheumatism 2006;54:26‐37. [DOI] [PubMed] [Google Scholar]
PREMIER 2006 (Additional article #1)
- Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health‐related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: Results from a randomized multicenter study. Journal of Rheumatology 2012;39:63‐72. [DOI] [PubMed] [Google Scholar]
Quinn 2005
- Quinn MA, Conaghan PG, O'Connor PJ, Karim Z, Greenstein A, Brown A, et al. Very early treatment with infliximab in addition to methotrexate in early, poor‐prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: Results from a twelve‐month randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2005;52:27‐35. [DOI] [PubMed] [Google Scholar]
RA‐BEAM 2015
- Taylor PC, Keystone EC, Heijde D, Tanaka Y, Ishii T, Emoto K, Yang L, Arora V, Gaich CL, Rooney T, Schlichting DE, Macias W, Bono S, Weinblatt ME. Baricitinib versus placebo or adalimumab in patients with active rheumatoid arthritis (RA) and an inadequate response to background methotrexate therapy: Results of a phase 3 study. Arthritis and Rheumatology. 2015; Vol. 67.
RACAT 2013
- O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. New England Journal of Medicine 2013;369:307‐18. [DOI] [PubMed] [Google Scholar]
RAPID‐I 2008
- Keystone E, Heijde D, Mason D Jr, Landewe R, Vollenhoven R, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty‐two‐week, phase III, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study.[Erratum appears in Arthritis Rheum. 2009 May;60(5):1249]. Arthritis & Rheumatism 2008;58:3319‐29. [DOI] [PubMed] [Google Scholar]
RAPID‐I 2008 (Additional article #1)
- Strand V, Mease P, Burmester G R, Nikai E, Coteur G, Vollenhoven R, et al. Rapid and sustained improvements in health‐related quality of life, fatigue, and other patient‐reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Research & Therapy 2009;11:R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
RAPID‐II 2009
- Smolen J, Landewe R B, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: The RAPID 2 study. A randomised controlled trial. Annals of the Rheumatic Diseases 2009;68:797‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
RAPID‐II 2009 (Additional article #1)
- Strand V, Smolen JS, Vollenhoven RF, Mease P, Burmester GR, Hiepe F, et al. Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: Analysis of patient‐reported outcomes from the RAPID 2 trial. Annals of the Rheumatic Diseases 2011;70:996‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rau 1991
- Rau R, Herborn G, Karger T, Menninger H, Elhardt D, Schmitt J. A double‐blind comparison of parenteral methotrexate and parenteral gold in the treatment of early erosive rheumatoid arthritis: An interim report on 102 patients after 12 months. Seminars in Arthritis and Rheumatism 1991;21:13‐20. [DOI] [PubMed] [Google Scholar]
Rau 1997
- Rau R, Herborn G, Menninger H, Blechschmidt J. Comparison of intramuscular methotrexate and gold sodium thiomalate in the treatment of early erosive rheumatoid arthritis: 12 month data of a double‐blind parallel study of 174 patients. British Journal of Rheumatology 1997;36:345‐52. [DOI] [PubMed] [Google Scholar]
Rau 1997 (Additional article #1)
- Rau R, Herborn G, Menninger H, Sangha O. Progression in early erosive rheumatoid arthritis: 12 month results from a randomized controlled trial comparing methotrexate and gold sodium thiomalate. British Journal of Rheumatology 1998;37:1220‐6. [DOI] [PubMed] [Google Scholar]
Reece 2002
- Reece RJ, Kraan MC, Radjenovic A, Veale DJ, O'Connor PJ, Ridgway JP, et al. Comparative assessment of leflunomide and methotrexate for the treatment of rheumatoid arthritis, by dynamic enhanced magnetic resonance imaging. Arthritis and Rheumatism 2002;46:366‐72. [DOI] [PubMed] [Google Scholar]
Salaffi 1995
- Salaffi F, Carotti M, Cervini C. Serum soluble interleukin‐2 receptor levels in rheumatoid arthritis: Effect of methotrexate, sulphasalazine and hydroxychloroquine therapy. Clinical Rheumatology 1995;14:458‐63. [DOI] [PubMed] [Google Scholar]
Sarzi‐Puttini 2005
- Sarzi‐Puttini P, D'Ingianna E, Fumagalli M, Scarpellini M, Fiorini T, Cherie‐Ligniere EL, et al. An open, randomized comparison study of cyclosporine A, cyclosporine A + methotrexate and cyclosporine A + hydroxychloroquine in the treatment of early severe rheumatoid arthritis. Rheumatology International 2005;25:15‐22. [DOI] [PubMed] [Google Scholar]
SATORI 2009
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): Significant reduction in disease activity and serum vascular endothelial growth factor by IL‐6 receptor inhibition therapy. Modern Rheumatology 2009;19:12‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
SERENE 2010
- Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, et al. Efficacy and safety of different doses and retreatment of rituximab: A randomised, placebo‐controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Efficacy in MTX iNadequate rEsponders (SERENE)). Annals of the Rheumatic Diseases 2010;69:1629‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shashikumar 2010
- Shashikumar NS, Shivamurthy MC, Chandrashekara S. Evaluation of efficacy of combination of methotrexate and hydroxychloroquine with leflunomide in active rheumatoid arthritis. Indian Journal of Pharmacology 2010;42:358‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shim 2010
- Shim SC, Park SH, Bae SC, Song YW, MacAraeg GM, Delvaux A, et al. Efficacy and safety of abatacept in Korean patients with active rheumatoid arthritis and inadequate response to methotrexate: A phase III, multicenter, randomized, double‐blind, placebo‐controlled bridging study. International Journal of Rheumatic Diseases. 2010; Vol. 13:101.
Shuai 2002
- Shuai ZW, Liu S, Shun GH, Xue JH, Xue SY. The phase II clinical trial of leflunomide in treatment of rheumatoid arthritis. Acta Universitatis Medicinalis Anhui 2002;37:41‐4. [Google Scholar]
Sigidin Ya 1994
- Sigidin Ya A, Usova SB. Comparative study of cyclosporin A in systemic rheumatoid arthritis. International Journal of Immunotherapy 1994;10:61‐5. [Google Scholar]
Singh 2000
- Singh CP, Kaur BG, Singh G, Singh N, Singh J. The open randomised trial of cyclosporine vs methotrexate in refractory rheumatoid arthritis. Journal of Internal Medicine of India 2000;3:19‐25. [Google Scholar]
Singh 2012
- Singh H, Mathur R, Arya S, Gupta V, Ghangas N, Singhania A, et al. Comparison of efficacy of combination of methotrexate and hydroxychloroquine with leflunomide alone in patients of rheumatoid arthritis. Indian Journal of Rheumatology 2012;7:S31. [Google Scholar]
START 2006
- Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo‐controlled trial.[Erratum appears in Arthritis Rheum. 2007 May;56(5):1675 Note: Dosage error in article text]. Arthritis & Rheumatism 2006;54:1075‐86. [DOI] [PubMed] [Google Scholar]
Suarez‐Almazor 1988
- Suarez‐Almazor ME, Fitzgerald A, Grace M, Russell AS. A randomized controlled trial of parenteral methotrexate compared with sodium aurothiomalate (myochrysine) in the treatment of rheumatoid arthritis. Journal of Rheumatology 1988;15:753‐6. [PubMed] [Google Scholar]
SURPRISE 2014
- Takeuchi T, Kaneko Y, Atsumi T, Tanaka Y, Inoh M, Kobayashi H, et al. Clinical and radiographic effects after 52‐week of adding tocilizumab or switching to tocilizumab in ra patients with inadequate response to methotrexate: Results from a prospective randomized controlled study (surprise study). Annals of the Rheumatic Diseases. 2014; Vol. 73:686.
SURPRISE 2014 (Additional article #1)
- Takeuchi T, Kaneko Y, Atsumi T, Tanaka Y, Inoh M, Kobayashi H, et al. Adding tocilizumab or switching to tocilizumab monotherapy in RA patients with inadequate response to methotrexate: 24‐week results from a randomized controlled study (surprise study). Annals of the Rheumatic Diseases 2013;72:A62‐A63. [Google Scholar]
SURPRISE 2016
- Kaneko Y, Atsumi T, Tanaka Y, Inoo M, Kobayashi‐Haraoka H, Amano K, Miyata M, Murakawa Y, Yasuoka H, Hirata S, Nagasawa H, Tanaka E, Miyasaka N, Yamanaka H, Yamamoto K, Takeuchi T. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52‐week results from a prospective, randomised, controlled study (SURPRISE study). Ann Rheum Dis 2016:208426. [DOI: 10.1136/annrheumdis-2015-208426] [DOI] [PMC free article] [PubMed] [Google Scholar]
SWEFOT 2012
- Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, Petersson IF, et al. Conventional combination treatment versus biological treatment in methotrexate‐refractory early rheumatoid arthritis: 2 year follow‐up of the randomised, non‐blinded, parallel‐group Swefot trial. Lancet 2012;379(9827):1712‐20. [DOI] [PubMed] [Google Scholar]
SWEFOT 2012 (Additional article #1)
- Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1‐year results of a randomised trial. The Lancet 2009;374:459‐66. [DOI] [PubMed] [Google Scholar]
Takeuchi 2013a
- Takeuchi T, Matsubara T, Nitobe T, Suematsu E, Ohta S, Honjo S, et al. Phase II dose‐response study of abatacept in Japanese patients with active rheumatoid arthritis with an inadequate response to methotrexate.[Erratum appears in Mod Rheumatol. 2013 Mar;23(2):236]. Modern Rheumatology 2013;23:226‐35. [DOI] [PubMed] [Google Scholar]
Takeuchi 2013b
- Takeuchi T, Miyasaka N, Zang C, Alvarez D, Fletcher T, Wajdula J, et al. A phase 3 randomized, double‐blind, multicenter comparative study evaluating the effect of etanercept versus methotrexate on radiographic outcomes, disease activity, and safety in Japanese subjects with active rheumatoid arthritis. Modern Rheumatology 2013;23:623‐33. [DOI] [PubMed] [Google Scholar]
Tam 2012
- Tam LS, Shang Q, Li EK, Wang S, Li RJ, Lee KL, et al. Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis ‐‐ a randomized trial. Journal of rheumatology 2012;39:2267‐75. [DOI] [PubMed] [Google Scholar]
Tanaka 2011
- Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators. Phase II study of tofacitinib (CP‐690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis care & research 2011;63:1150‐8. [DOI] [PubMed] [Google Scholar]
Tascioglu 2003
- Tascioglu F, Oner C, Armagan O. Comparison of low dose methotrexate and combination therapy with methotrexate and sulphasalazine in the treatment of early rheumatoid arthritis. Journal of Rheumatology and Medical Rehabilitation 2003;14:142‐9. [Google Scholar]
TEAR 2012
- Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, Stclair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: The treatment of early aggressive rheumatoid arthritis trial. Arthritis and Rheumatism 2012;64:2824‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
TEAR 2012 (Additional article #1)
- Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, Clair EW, et al. Two‐year radiographic results from the TEAR trial. Arthritis and Rheumatism. 2010; Vol. 62:1368.
TEMPO 2004
- Klareskog L, Heijde D, Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004;363:675‐81. [DOI] [PubMed] [Google Scholar]
TEMPO 2004 (Additional article #1)
- Heijde D, Klareskog L, Singh A, Tornero J, Melo‐Gomes J, Codreanu C, et al. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: The TEMPO trial. Annals of the Rheumatic Diseases 2006;65:328‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
TEMPO 2004 (Additional article #2)
- Heijde D, Klareskog L, Rodriguez‐Valverde V, Codreanu C, Bolosiu H, Melo‐Gomes J, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two‐year clinical and radiographic results from the TEMPO study, a double‐blind, randomized trial. Arthritis & Rheumatism 2006;54:1063‐74. [DOI] [PubMed] [Google Scholar]
TOMERA 2014
- Ducreux J, Durez P, Galant C, Toukap AN, Eynde B, Houssiau FA, et al. Global molecular effects of tocilizumab therapy in rheumatoid arthritis synovium. Arthritis and Rheumatology 2014;66:15‐23. [DOI] [PubMed] [Google Scholar]
TOMERA 2014 (Additional article #1)
- Durez P, Depresseux G, Avauk M, Toukap AN, Lauwerys B, Bellefon LM, Stoenoiu MS, Houssiau FA. Remission Induction in Early Active Rheumatoid Arthritis: Comparison of Tocilizumab Versus Methotrexate Monotherapy. Arthritis Rheum. 2012; Vol. 64, issue Suppl 10:S575.
tREACH 2013
- Jong PHP, Hazes JM, Barendregt PJ, Huisman M, Zeben D, Lubbe PA, et al. Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: First results of the tREACH trial. Annals of the Rheumatic Diseases 2013;72:72‐8. [DOI] [PubMed] [Google Scholar]
Trnavsky 1993
- Trnavsky K, Gatterova J, Linduskova M, Peliskova Z. Combination therapy with hydroxychloroquine and methotrexate in rheumatoid arthritis. Zeitschrift fur Rheumatologie 1993;52:292‐6. [PubMed] [Google Scholar]
Tugwell 1995
- Tugwell P, Pincus T, Yocum D, Stein M, Gluck O, Kraag G, et al. Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. The Methotrexate‐Cyclosporine Combination Study Group. New England Journal of Medicine 1995;333:137‐41. [DOI] [PubMed] [Google Scholar]
ULTRA 1999
- Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Archives of Internal Medicine 1999;159:2542‐50. [DOI] [PubMed] [Google Scholar]
van Jaarsveld 2000
- Jaarsveld CH, Jahangier ZN, Jacobs JW, Blaauw AA, Albada‐Kuipers GA, ter Borg EJ, et al. Toxicity of anti‐rheumatic drugs in a randomized clinical trial of early rheumatoid arthritis. Rheumatology 2000;39:1374‐82. [DOI] [PubMed] [Google Scholar]
Weinblatt 1985
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low‐dose methotrexate in rheumatoid arthritis. New England Journal of Medicine 1985;312:818‐22. [DOI] [PubMed] [Google Scholar]
Weinblatt 1999
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. New England Journal of Medicine 1999;340:253‐9. [DOI] [PubMed] [Google Scholar]
Weinblatt 2015
- Weinblatt ME, Mease P, Mysler E, Takeuchi T, Drescher E, Berman A, Xing J, Zilberstein M, Banerjee S, Emery P. The efficacy and safety of subcutaneous clazakizumab in patients with moderate‐to‐severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase IIb, randomized, double‐blind, placebo/active‐controlled, dose‐ranging study. Arthritis rheumatol 2015;67:2591‐600. [DOI] [PubMed] [Google Scholar]
Westedt 1994
- Westedt ML, Dijkmans BAC, Hermans J. Azathioprine compared with methotrexate for rheumatoid arthritis: An open randomized clinical trial. Revue du Rhumatisme (English Edition) 1994;61:523‐9. [PubMed] [Google Scholar]
Williams 1985
- Williams HJ, Willkens RF, Samuelson CO Jr, Alarcon GS, Guttadauria M, Yarboro C, et al. Comparison of low‐dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis & Rheumatism 1985;28:721‐30. [DOI] [PubMed] [Google Scholar]
Willkens 1992
- Willkens RF, Urowitz MB, Stablein DM, McKendry RJ Jr, Berger RG, Box JH, et al. Comparison of azathioprine, methotrexate, and the combination of both in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis & Rheumatism 1992;35:849‐56. [DOI] [PubMed] [Google Scholar]
Xia 2011
- Xia L, Lu J, Xiao W. Blockage of TNF‐alpha by infliximab reduces CCL2 and CCR2 levels in patients with rheumatoid arthritis. Journal of Investigative Medicine 2011;59:961‐3. [DOI] [PubMed] [Google Scholar]
Zhang 2004
- Zhang X, Cui Y, Luo RQ, Yao RY, Zhou H. [Methotrexate combined with leflunomide or hydroxychloroquine in the treatment of rheumatoid arteritis]. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2004;84:1038‐40. [PubMed] [Google Scholar]
Zhang 2006
- Zhang FC, Hou Y, Huang F, Wu DH, Bao CD, Ni LQ, et al. Infliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A preliminary study from China. APLAR Journal of Rheumatology 2006;9:127‐30. [Google Scholar]
References to excluded reviews
Beals 2013
- Beals C, Baumgartner R, Peterfy C, Balanescu A, Mirea G, Harabagiu A, et al. Treatment effects measured by dynamic contrast enhanced MRI and Ramris for rheumatoid arthritis. Annals of the Rheumatic Diseases. 2013; Vol. 72:A748.
Burmester 2013
- Burmester G R, Blanco R, Charles‐Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP‐690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: A randomised phase 3 trial. The Lancet 2013;381:451‐60. [DOI] [PubMed] [Google Scholar]
Calguneri 1999
- Calguneri M, Pay S, Caliskaner Z, Apras S, Kiraz S, Ertenli I, et al. Combination therapy versus monotherapy for the treatment of patients with rheumatoid arthritis. Clinical and Experimental Rheumatology 1999;17:699‐704. [PubMed] [Google Scholar]
Cohen 2001
- Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, et al. Two‐year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis and Rheumatism 2001;44:1984‐92. [DOI] [PubMed] [Google Scholar]
Cohen 2006
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: Results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis & Rheumatism 2006;54:2793‐806. [DOI] [PubMed] [Google Scholar]
Combe 2013
- Combe B, Dougados M, Logeart I, Lukas C, Mariette X, Puechal X, et al. An open‐label randomized controlled study to evaluate the efficacy of etanercept (ETN) versus rituximab (RTX), in patients with active rheumatoid arthritis previously treated with RTX and TNF blockers. Annals of the Rheumatic Disease 2013;71:667. [Google Scholar]
De Stefano 2010
- Stefano R, Frati E, Nargi F, Baldi C, Menza L, Hammoud M, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: Leflunomide‐anti‐TNF‐alpha versus methotrexate‐anti‐TNF‐alpha. Clinical Rheumatology 2010;29:517‐24. [DOI] [PubMed] [Google Scholar]
Dougados 2014
- Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, Gerli R, et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab‐based treatment strategies in rheumatoid arthritis: The ACT‐RAY study. Annals of the rheumatic diseases 2014;73:803‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drosos 2000
- Drosos AA, Voulgari PV, Katsaraki A, Zikou AK. Influence of cyclosporin A on radiological progression in early rheumatoid arthritis patients: A 42‐month prospective study. Rheumatology International 2000;19:113‐8. [DOI] [PubMed] [Google Scholar]
Emery 2008
- Emery P, Keystone E, Tony HP, Cantagrel A, Vollenhoven R, Sanchez A, et al. IL‐6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti‐tumour necrosis factor biologicals: results from a 24‐week multicentre randomised placebo‐controlled trial.[Erratum appears in Ann Rheum Dis. 2009 Feb;68(2):296]. Annals of the Rheumatic Diseases 2008;67:1516‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furst 2007
- Furst DE, Gaylis N, Bray V, Olech E, Yocum D, Ritter J, et al. Open‐label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: The opposite study. Annals of the Rheumatic Diseases 2007;66:893‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2004
- Gao JS, Wu H, Chen YJ, Li F, Tian J, X Xie. Observation on the short‐term curative effect and safety on combination use of LEF plus MTX in the treatment of active and severe RA. Abstract book, the 11th APLAR meeting. 2004:92.
Keystone 2004
- Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, Vries T, et al. Once‐Weekly Administration of 50 mg Etanercept in Patients with Active Rheumatoid Arthritis: Results of a Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Trial. Arthritis and Rheumatism 2004;50:353‐63. [DOI] [PubMed] [Google Scholar]
Keystone 2008
- Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, et al. Improvement in patient‐reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy. Arthritis and Rheumatism 2008;59(6):785‐93. [DOI] [PubMed] [Google Scholar]
Kim 2012
- Kim HY, Hsu PN, Barba M, Sulaiman W, Robertson D, Vlahos B, et al. Randomized comparison of etanercept with usual therapy in an Asian population with active rheumatoid arthritis: The APPEAL trial. International Journal of Rheumatic Diseases 2012;15:188‐96. [DOI] [PubMed] [Google Scholar]
Kremer 2010
- Kremer J, Ritchlin C, Mendelsohn A, Baker D, Kim L, Xu Z, et al. Golimumab, a new human anti‐tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: Forty‐eight‐week efficacy and safety results of a phase III randomized, double‐blind, placebo‐controlled study.[Erratum appears in Arthritis Rheum. 2010 Oct;62(10):3130]. Arthritis & Rheumatism 2010;62:917‐28. [DOI] [PubMed] [Google Scholar]
Kume 2012
- Kume K. Tocilizumab Improves Bone Mineral Density Compared with Abatacept in Patients with TNF Blockers‐Resistant Active Rheumatoid Arthritis: an Open Label Randomized Controlled Trial. Arthritis Rheum. 2012; Vol. 64, issue Suppl 10:S192‐S193.
Manders 2015
- Manders SHM, Kievit W, Adang E, Brus HL, Moens HJ, Hartkamp A, et al. Cost‐effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi‐centre randomised trial. Arthritis Research and Therapy 2015;17 (1)(134):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01283971 2014
- Hoffmann‐La Roche. A Study of RoActemra/Actemra (Tocilizumab) Versus Adalimumab in Combination With Methotrexate in Patients With Moderate to Severe Active Rheumatoid Arthritis And an Inadequate Response to Treatment With Only One TNF‐Inhibitor. Registered clinical trial with results available through www.clinicaltrials.gov Last updated: January 9, 2014.
Peterfy 2011
- Peterfy C, Emery P, Tak PP, Ostergaard M, DiCarlo J, Otsa K, et al. Rituximab (RTX) plus methotrexate (MTX) prevents bone erosion and joint‐space narrowing (JSN) and reduces synovitis, osteitis as shown on MRI: results from a randomised, placebo‐controlled trial in patients (pts) with rheumatoid arthritis (RA‐SCORE). Ann Rheum Dis 2011;70(Suppl 3):152. [Google Scholar]
Saunders 2008
- Saunders SA, Capell HA, Stirling A, Vallance R, Kincaid W, McMahon AD, et al. Triple therapy in early active rheumatoid arthritis: A randomized, single‐blind, controlled trial comparing step‐up and parallel treatment strategies. Arthritis and Rheumatism 2008;58:1310‐7. [DOI] [PubMed] [Google Scholar]
Strand 2012
- Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health‐related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: Results from the 24‐week randomized controlled radiate study. Rheumatology (United Kingdom) 2012;51:1860‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2015
- Strand V, Burmester GR, Zerbini CAF, Mebus CA, Zwillich SH, Gruben D, et al. Tofacitinib with methotrexate in third‐line treatment of patients with active rheumatoid arthritis: Patient‐reported outcomes from a phase III trial. Arthritis Care and Research 2015;67(4):475‐83. [PUBMED: 2015859898] [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor PC, Steuer A, Gruber J, McClinton C, Cosgrove DO, Blomley MJK, et al. Ultrasonographic and radiographic results from a two‐year controlled trial of immediate or one‐year‐delayed addition of infliximab to ongoing methotrexate therapy in patients with erosive early rheumatoid arthritis. Arthritis and Rheumatism 2006;54:47‐53. [DOI] [PubMed] [Google Scholar]
Usova 1993
- Usova SB, Sigidin IA. A comparative evaluation of the treatment results with cyclosporin A, methotrexate and azathioprine in rheumatoid arthritis patients (a preliminary report). [Russian]. Terapevticheskii Arkhiv 1993;65:65‐9. [PubMed] [Google Scholar]
Willkens 1996
- Willkens RF, Stablein D. Combination treatment of rheumatoid arthritis using azathioprine and methotrexate: a 48 week controlled clinical trial. The Journal of rheumatology 1996;Supplement. 44:64‐8. [PubMed] [Google Scholar]
Additional references
Ades 2006
- Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost‐effectiveness analysis. Pharmacoeconomics 2006;24:1‐19. [DOI] [PubMed] [Google Scholar]
Aletaha 2010
- Aletaha D, Neogi T, Silman A J, Funovits J, Felson DT, Bingham III CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580‐8. [DOI] [PubMed] [Google Scholar]
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315‐24. [DOI] [PubMed] [Google Scholar]
Bingham 2001
- Bingham S, Emery P. Combination therapy in rheumatoid arthritis. Springer Semin Immunopathol 2001;23:165‐83. [DOI] [PubMed] [Google Scholar]
Braun 2009
- Braun J, Rau R. An update on methotrexate. Curr Opin Rheumatol 2009;21:216‐23. [DOI] [PubMed] [Google Scholar]
Brooks 1998
- Brooks SP, Gelman A. General Methods for Monitoring Convergence of Iterative Simulations. J Comput Graph Stat 1998;7:434‐55. [Google Scholar]
Bruynesteyn 2002
- Bruynesteyn K, Heijde D, Boers M, Saudan A, Peloso P, Paulus H, et al. Determination of the minimal clinically important difference in rheumatoid arthritis joint damage of the Sharp/van der Heijde and Larsen/Scott scoring methods by clinical experts and comparison with the smallest detectable difference. Arthritis Rheum 2002;46:913‐20. [DOI] [PubMed] [Google Scholar]
Choy 2005
- Choy EHS, Smith C, Dore CJ, Scott DL. A meta‐analysis of the efficacy and toxicity of combining disease‐modifying anti‐rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology 2005;44:1414‐21. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med 2010;29:932‐44. [DOI] [PubMed] [Google Scholar]
Dias 2013
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Med Decis Making 2013;33:607‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727‐35. [DOI] [PubMed] [Google Scholar]
Fries 1982
- Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9:789‐93. [PubMed] [Google Scholar]
Furst 2013
- Furst DE, Keystone EC, So AK, Braun J, Breedveld FC, Burmester GR, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2012. Ann Rheum Dis 2013;72 Suppl 2:ii2‐34. [DOI] [PubMed] [Google Scholar]
Gaujoux‐Viala 2014
- Gaujoux‐Viala C, Nam J, Ramiro S, Landewe R, Buch MH, Smolen JS, et al. Efficacy of conventional synthetic disease‐modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014;73:510‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graudal 2010
- Graudal N, Jurgens G. Similar effects of disease‐modifying antirheumatic drugs, glucocorticoids, and biologic agents on radiographic progression in rheumatoid arthritis: Meta‐analysis of 70 randomized placebo‐controlled or drug‐controlled studies, including 112 comparisons. Arthritis and Rheumatism 2010;62:2852‐63. [DOI] [PubMed] [Google Scholar]
Graudal 2014
- Graudal N, Hubeck‐Graudal T, Tarp S, Christensen R, Jurgens G. Effect of combination therapy on joint destruction in rheumatoid arthritis: a network meta‐analysis of randomized controlled trials. PLoS One 2014;9:e106408. [DOI: 10.1371/journal.pone.0106408] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). J Clin Epidemiol 2011;64:407‐15. [DOI] [PubMed] [Google Scholar]
Hawkins 2009
- Hawkins N, Scott DA, Woods B. How far do you go? Efficient searching for indirect evidence. Med Decis Making 2009;29:273‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoekstra 2004
- Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, Laar M. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol 2004;31:645‐8. [PubMed] [Google Scholar]
Jansen 2011
- Jansen J P, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011;14:417‐28. [DOI] [PubMed] [Google Scholar]
Jansen 2014
- Jansen JP, Trikalinos T, Cappelleri JC, Daw J, Andes S, Eldessouki R, et al. Indirect treatment comparison/network meta‐analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR‐AMCP‐NPC Good Practice Task Force report. Value Health 2014;17:157‐73. [DOI: 10.1016/j.jval.2014.01.004] [DOI] [PubMed] [Google Scholar]
Katchamart 2010
- Katchamart W, Trudeau J, Phumethum V, Bombardier C. Methotrexate monotherapy versus methotrexate combination therapy with non‐biologic disease modifying anti‐rheumatic drugs for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD008495] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knevel 2010
- Knevel R, Schoels M, Huizinga TW, Aletaha D, Burmester GR, Combe B, et al. Current evidence for a strategic approach to the management of rheumatoid arthritis with disease‐modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2010;69:987‐94. [DOI] [PubMed] [Google Scholar]
Kvien 2004
- Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 2004;22:1‐12. [DOI] [PubMed] [Google Scholar]
Lambert 2005
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med 2005 Aug 15;24(15):2401‐28. [PUBMED: 16015676] [DOI] [PubMed] [Google Scholar]
Lopez‐Olivo 2014
- Lopez‐Olivo MA, Siddhanamatha HR, Shea B, Tugwell P, Wells GA, Suarez‐Almazor ME. Methotrexate for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD000957.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2011
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205‐19. [DOI] [PubMed] [Google Scholar]
Ory 2003
- Ory PA. Interpreting radiographic data in rheumatoid arthritis. Ann Rheum Dis 2003;62:597‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 2005
- Pincus T, Yazici Y, Bergman M. Development of a multi‐dimensional health assessment questionnaire (MDHAQ) for the infrastructure of standard clinical care. Clin Exp Rheumatol 2005;23:S19‐28. [PubMed] [Google Scholar]
Prevoo 1995
- Prevoo ML, 't Hof MA, Kuper HH, Leeuwen MA, Putte LB, Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism 1995;38:44‐8. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ 2014;349:g5630. [DOI: 10.1136/bmj.g5630] [DOI] [PubMed] [Google Scholar]
Rhodes 2015
- Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta‐analyses of continuous outcome data. J Clin Epidemiol 2015 Jan;68(1):52‐60. [PUBMED: 25304503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ropes 1958
- Ropes MW, Bennett GA, Cobb S, Jacox R, Jessar RA. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis 1958;9:175‐6. [PubMed] [Google Scholar]
Schiff 2014
- Schiff MH, Jaffe JS, Freundlich B. Head‐to‐head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug‐exposure limitations of oral methotrexate at doses >=15 mg may be overcome with subcutaneous administration. Ann Rheum Dis 2014;73(8):1549‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 1996
- Sharp SJ, Thompson SG, Altman DG. The relation between treatment benefit and underlying risk in meta‐analysis. BMJ 1996;313:735‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2009
- Singh JA, Christensen R, Wells GA, Suarez‐Almazor ME, Buchbinder R, Lopez‐Olivo MA, Tanjong Ghogomu E, Tugwell P. Biologics for rheumatoid arthritis: An overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2011
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008794.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2016
- Singh JA, Saag KG, Bridges SLJr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis & rheumatology 2016;68(1):1‐26. [DOI] [PubMed] [Google Scholar]
Smolen 2014
- Smolen JS, Heijde D, Machold KP, Aletaha D, Landewe R. Proposal for a new nomenclature of disease‐modifying antirheumatic drugs. Ann Rheum Dis 2014;73:3‐5. [DOI] [PubMed] [Google Scholar]
Smolen 2014a
- Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2013
- Thorlund K, Druyts E, Avina‐Zubieta JA, Wu P, Mills EJ. Why the findings of published multiple treatment comparison meta‐analyses of biologic treatments for rheumatoid arthritis are different: an overview of recurrent methodological shortcomings. Ann Rheum Dis 2013;72:1524‐35. [DOI: 10.1136/annrheumdis-2012-201574] [DOI] [PubMed] [Google Scholar]
Tian 2007
- Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis 2007;65:168‐73. [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International journal of epidemiology 2012 Jun;41(3):818‐27. [PUBMED: 22461129] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Heijde 1992
- Heijde DM, van't Hof MA, Riel PL, Leeuwen MA, Rijswijk MH, Putte LB. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis 1992;51:177‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Heijde 2000
- Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261‐3. [PubMed] [Google Scholar]
van Gestel 1996
- Gestel AM, Prevoo ML, 't Hof MA, Rijswijk MH, Putte LB, Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34‐40. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hazlewood 2016
- Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta‐analysis. BMJ 21 April 2016;353:i1777. [DOI] [PMC free article] [PubMed] [Google Scholar]


