Abstract
The Brevinin peptides are antimicrobial agents obtained from frog skin secretions. Brevinin-2R has attracted many attentions due to its very low hemolytic activity, cationic property, and high affinity to cancer cells. Moreover, it has shown little toxicity against normal mammalian cells, while having killed several tumor cell lines by activation of lysosome-mitochondrial death pathway. In this review, we introduced the Brevinin superfamily with a focus on its therapeutic applications. Next, some unique properties of Brevinins were briefly discussed, including their ability to stimulate insulin secretion, dendritic cell maturation, and wound healing. In this context, we also provide information about the decoration of nanoparticles, such as cerium nano-oxide, by Brevinins. Finally, we addressed their potential for anti-tumor and drug design applications.
Keywords: Antimicrobial peptide, Brevinin, Bioactive peptide, Cancer, Frog skin secretions
Introduction
Bioactive peptides are biomolecules with different effects on cellular behavior in a way that could promote the biological functions of the body, thereby might ultimately improve human health (Perez Espitia et al. 2012). Numerous properties have been introduced for bioactive peptides, such as, antimicrobial (Li et al. 2012), anti-oxidant (Power et al. 2013), anti-thrombotic (Khiari et al. 2014), anti-hypertensive (Pokora et al. 2014), and immunomodulatory (Haney and Hancock 2013) activities.
Attention to bioactive peptides has dramatically increased in recent years to design and develop effective drugs with minimal side-effects for the treatment of different diseases, like cancer, diabetes, inflammatory and infectious disorders (Seo et al. 2012). Commercial development of peptide drugs has raised from 14.1 million dollars in 2011 to 25.4 million dollars in 2018, and led researchers to carry out extensive studies in this field. More than 500 peptide drugs are under study in different clinical trial phases. About 60 drugs, based on peptides, have confirmed up to now, and according to ongoing studies, this number will have strong potential to in the near future (Fosgerau and Hoffmann 2015).
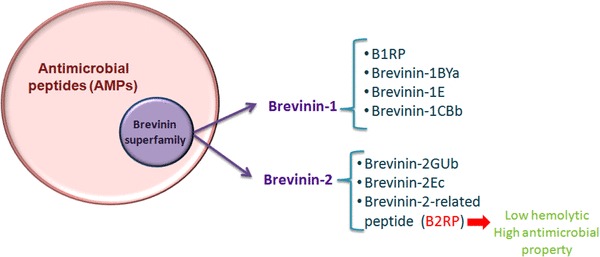
One of the main sources of bioactive peptides, including antimicrobial peptides (AMPs), is the skin of amphibian that acts as a physical and chemical barrier against pathogens. In fact, most of AMPs are cationic and some are anionic (Lai et al. 2007). Generaly, the AMP peptides contain less than 50 amino acids in length and many studies have demonstrated the contribution of these peptides to immune defense (Daum et al. 2012; Sadredinamin et al. 2016). Brevinines are natural AMPs and have been found in various species of frogs. According to database of anuran and defense peptides (DADP), over 350 types of Brevinines are identified with two main families: Brevinine 1 and Brevinine 2 (Novkovic et al. 2012).
This review was intended to introduce the Brevinin superfamily and its therapeutic properties. Next, we briefly dealt with some unique properties of Brevinins, such as their activity to provoke insulin secretion, dendritic cell maturation, and wound healing. Ultimately, some recommendations were suggested with regard to their therapeutic potentials.
Structure of Brevinin Superfamily
Morikawa et al. isolated these unique AMPs from the skin of the frog, Rana brevipoda porsa for the first time in 1992, and introduced them as Brevinin-1 and -2. Brevinine-1 has 24 amino acid with FLPVLA GIAAKVVPALFCKITKKC sequences, and Brevinine-2 is composed of 33 amino acid with GLLDSLKGFAATAGKGVLQSLLSTASCKLAKTC sequences (Morikawa et al. 1992). The Brevinin superfamily presents common characteristics, however several studies have revealed that the amino acid sequences of Brevinin-1 and -2 are poorly conserved across species with at least four invariant residues (Conlon et al. 2005, 2004). Members of this superfamily contain “Rana box”, which consists of disulfide-bridged cyclic heptapeptide (Cys18-(Xaa)4-Lys-Cys24), and shows cationic and amphipathic properties.
Different kinds of frogs from the genus “Rana” were investigated to isolate Brevinin peptide, such as, Rana okinavana, Rana septentrionalis, and Rana boylii, which have led to the identification of B1R, B1RP, Brevinin-1BYa, and B2RP-ERa, respectively (Hossain et al. 2011). These cationic peptides lack a well-defined secondary structure in water, even though having the potential to form amphipathic α-helices in biological solution (Kwon et al. 1998; Powers and Hancock 2003). The presence of Rana box was thought to be responsible for the antibacterial activity of the Brevinin family in preliminary experiments; nevertheless, this idea has been refuted in later studies (Conlon et al. 2005). Nowadays, it has been postulated that the interaction of α-helical structures with anionic lipid bilayers of bacterial cell membranes is mainly related to antimicrobial properties (Conlon et al. 2014). To confirm this idea, a study by Won et al. showed that the N-terminal domain of Brevinin-1 (residues 1–13) is the critical region for antimicrobial activity (Won et al. 2004). Since Brevinin-2 was separated from the skin of the rain frog R. brevipoda parse (reclassified as Pelophylax porosus), divergent members of this family are widely discovered. To date, Brevinin-2 peptides have not been identified in any North American frogs of the Ranidae family (Conlon et al. 2009b). The Brevinin-2 related peptides (B2RPs) are 21-amino-acid peptide purified by ultrafiltration and ion exchange chromatography from the skin of Rana frog species (R. septentrionalis) which can be found in the north shores of the Caspian Sea and Russia (Li et al. 2013). Although B2RPs exhibit sequence similarity to Brevinin-2 peptides, the C-terminal heptapeptide domain (Cys-Lys-Xaa4-Cys) is not present as compared with Eurasian species. Besides, some studies have demonstrated that this cyclic domain in Brevinin-2 is not necessary for its antimicrobial activity (Bevier et al. 2004).
Synthetic Analoges of Brevinin Peptides
All living cell sorounded by one or two membranes composed of lipids and proteins. Cell and bacteria membranes provide protection against extracellular environtment and ply a key role in the exchange phenomena berween the cytoplasm and the outer space. The AMPs serve as the nonspecific innate immunity system by interacting with lipids of the bacterial and fungal cell membranes. Despite the great potential of AMPs as natural antibiotics, their application as pharmaceuticals are limited due to their lack of selectivity on exposured cells membrane (Ruiz et al. 2014). There have been some studies to reduce the hemolytic activity of B2RP, for example, some analogues of B2RP were developed by the substitution of Leu 18 to Lys cause decreases in both antimicrobial and hemolytic activity. In contrast, substitution of Asp 4 to Lys cause sensible increases in antimicrobial activity against Escherichia coli and Staphylococcus aureus without significant changes in hemolytic activity against human erythrocytes (Conlon et al. 2009a; Deslouches and Di 2017). More recently, Vineethkumar et al. (2017) reported the C-terminal amidation of Brevinin1 HYba1 and HYba 2 improved their antimicrobial activity against Aeromonas sobria (a fish pathogen). Exploiting this post-translational modification, the positive charge of the peptides increased and intensified the electrostatic interaction of peptides with the anionic membrane of bacteria. Besides, they revealed that the 3D-structure of these peptides was stabilized by internal disulfide bridge (Tv et al. 2017). Also, another study reoprted the synthesis of 17 analogs of B1CTcu5 peptide by C- and N-terminal amino acid substitution or deletion and concluded that both regions are crucial for peptide function. It was demonstrated that the N-terminal region attributed to antibacterial property, while the C-terminal contributes to conformational stability (Parvin et al. 2015). Hossain et al. prepared an analogue of Brevinin-1BYa (isolated from R. boylii) through replacing disulphide bridge with a stable dicarba bond. Dicarba-Brevinin-1BY increased antimicrobial activity against both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria in comparison with unmodified Brevinin-1BYa (Fig. 1). Growth inhibitory potential against methicillin-resistant S. aureus (MRSA) and multidrug resistant Acinetobacter baumannii (MDRAB) was observed, as well (Hossain et al. 2011).
Fig. 1.
Brevinin superfamily as a member of antimicrobial peptides
Antimicrobial Effect of Brevinin Peptide
Antimicrobial peptides protect the host against pathogens (Conlon et al. 2009a; Holroyd 2003), and modulate harmful effects of the inflammatory responses (Ali et al. 2002). These peptides have different action levels in vertebrates and mostly exist in the skin, mucous surfaces and granules of the immune cells (Rao 1995). The AMPs do not only belong to vertebrates, but also are produced in all kinds of animals (Hancock 1997; Jenssen et al. 2006). Some AMPs are stored in skin granular glands of amphibian. They frequently have the α-helical structure and are released on the skin surface when the sympatic nerve is damaged or in the presence of other environmental stimulations (Chan et al. 2006). Antimicrobial effects arise from the interplay between cationic peptides and the anionic membrane of pathogens (Pálffy et al. 2009). The number and distribution of positive charges can be a selective advantage for the microbial membrane.
There are three main mechanisms for the interaction between the plasma membrane and Brevinins family (Chan et al. 2006; Sitaram and Nagaraj 1999):
Barrel stave model: In this model, antimicrobial activity of peptide is based on the pore formation in the membrane. The polar residues constitute the inner part of the channel, while the hydrophobic ones are in contact with the membrane phospholipids in the outer part (Savelyeva et al. 2014).
Carpet like model: Integration and uniformity of the membrane are disrupted in this model (Chan et al. 2006; Shai 1999).
Toroidal model: A mechanical stress and replacement of monomers on both sides of the membrane result in the membrane instability and thereby loss of its integrity.
Almost all Brevinin superfamily possess high antimicrobial activity against Gram-positive and Gram-negative bacteria, as well as fungal pathogens. The application of Brevinins as antimicrobial agents is limited because of their strong hemolytic property, however some studies have attempted to moderate this negative effect via structure modification. A good example is the replacement of the C-terminal box to central position in Brevinin-1E, which, in turn, decreased hemolytic activity and maintained antibacterial property (Kumari and Nagaraj 2001). Among this family of peptides, Brevinin-2R is an exception; it shows low hemolytic activity and has a broad-spectrum antimicrobial property (Bevier et al. 2004). The multidrug-resistant bacteria (MDRB) infections have spread in recent years owing to extensive application of antibiotics in the food industry, medicine, and agriculture. It is of utmost importance to develop new antibacterial agents for the treatment of life threating health problems caused by MDRB, including MRSA, Gram-positive MDRB resistant to not only the beta-lactam antibiotics, but also erythromycin, ciprofloxacin, and gentamicin (Borde and Kern 2012), and extended-spectrum β-lactamase (ESBL) E. coli, Gram-negative MDRB with restricted antibiotic options for its treatment (Nuotio et al. 2013). Some Brevinin family members, such as dicarba derivative of bevinin-1BYa (Hossain et al. 2011), Brevinin-2TS (Conlon et al. 2006), and Brevinin-2CE (Zhang et al. 2014), have been investigated for the treatment of MRSA, as the most difficult infection to treat. In conjunction with that, Brevinin-2CE and B2RP have been found to have antimicrobial potential against multidrug-resistant A. baumannii (Al-Ghaferi et al. 2010; Zhang et al. 2014). In addition to anti-bacterial action of the Brevinins family, some scholars have addressed their antiviral properties. Yasin et al. confirmed the antiviral activity of Brevinin-1 (isolated from the skin of Asian frog P. porosus) against herpes simplex virus type 1 and 2. They exhibited that the Brevinin-1 anti-viral properties were maintained even after reduction and carboxamidomethylation, while cysteine modification diminished hemolytic and cytotoxic effects (Yasin et al. 2000). Brevinin-1BYa from R. boylii and B2RP-ERa from Hylarana erythraea were documented to inhibit HSV-1 infection of MDBK cells. Brevinin-1BYa prevented HSV-1 infection via inactivation of free virions or disturbance intracellular virus replication. However, it caused high cytotoxicity in the MDBK cells (Conlon et al. 2014). Some derivatives of Brevinin-1 revealed remarkable antiviral activities against HSV by direct impacts on the viruses lipid membrane, suggesting new therapeutic potentials of Brevinins for treatment of resistance in viral diseases, like Ebola, SARS or HIV/AIDS (Yasin et al. 2000).
In a study, Wang et al. examined anti-HIV-1 activities of about 30 peptides. They developed a library by rearrangement of Aurein residues as a template peptide. The sequence pattern of their B5 peptide was similar to Brevinin-2DYd, but this peptide appeared ineffective for HIV-1 inhibition (Wang et al. 2010). It seems that more studies are needed to elaborate on the efficiency of the Brevinin family against viruses.
The Role of Brevinin Peptides in the Innate Immunity System
The innate immune system is activated by the release of pro-inflammatory cytokines, namely interferon-γ (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-8, and so forth from mononuclear cells (Semple and Freedman 2010). Popovic et al. explored the role of Brevinin-2GU (isolated from Hylarana guentheri) (Conlon et al. 2008) and B2RP-ERa in producing pro-inflammatory and anti-inflammatory cytokines from peripheral blood mononuclear cells (PBMCs). They stimulated these cells by concanavalin A (ConA), a plant mitogen that triggered cytokines production under specified in vitro conditions. Both Brevinins declined the release of TNF-α from ConA-stimulated PBMCs at 20 µg/mL, while none exerted a significant effect on the IFN-γ release in these cells (Table 1). Only B2RP-ERa provoked anti-inflammatory cytokines, including TGF-β, IL-4, and IL-4 (Popovic et al. 2012). It seems that there is no correlation between antimicrobial efficiency and anti-inflammatory properties; for instance, IDR-1, an antimicrobial peptide derived from human cathelicidin, unraveled antimicrobial activity against both Gram-positive and Gram-negative pathogens, while reducing pro-inflammatory cytokine responses (Scott et al. 2007). Recently, one study reported the stimulation of pro-inflammatory IL-1β and -8 cytokines at different concentrations of Brevinin-2R in human lung epithelial adenocarcinoma cell line (A549). As well documented in the pertained literature, Brevinin-2R is speculated to possess low hemolytic activity however the viability of A549 cells decreased non-considerably (20%) following the treatment with 5–20 µg/mL of B2R peptide (Asoodeh et al. 2013). Similarly, it was showed that Brevinin-2R increased the expression of IL-1β and -6 genes in human liver carcinoma cells (HepG2) in a dose-dependent manner (Homayouni-Tabrizi et al. 2015). Further research should be carried out to elucidate immunogenicity of Brevinins.
Table 1.
The effects of Brevinin peptides on pro-inflammatory and anti-inflammatory cytokines
| Peptide | Action | Cell source | References | |
|---|---|---|---|---|
| Pro-inflammatory | ||||
| TNF-α | Brevinin-2GUb/B2RP-ERa | Inhibit | ConA-stimulated PBMC | Popovic et al. (2012) |
| INF-γ | Brevinin-2GUb | Inhibit | Unstimulated PBMC | Popovic et al. (2012) |
| IL-1β | Brevinin-2R | Stimulate | A549/HepG2 | Homayouni-Tabrizi et al. (2015) |
| IL-6 | Brevinin-2R | Stimulate | HepG2 | Asoodeh et al. (2013) |
| IL-8 | Brevinin-2R | Stimulate | A549 | Homayouni-Tabrizi et al. (2016) |
| Anti-inflammatory | ||||
| TGF-β | B2RP-ERa | Stimulate | Both ConA-stimulated/unstimulated PBMC | Popovic et al. (2012) |
| IL-4 | B2RP-ERa | Stimulate | Both ConA-stimulated/unstimulated PBMC | Popovic et al. (2012) |
| IL-10 | B2RP-ERa | Stimulate | ConA-stimulated PBMC | Popovic et al. (2012) |
Anti-cancer Effects of Brevinin
Since Brevinin-2R does not form pores in the cell plasma membrane (like many toxins) and exhibits low hemolytic activity against human erythrocytes, it has begun to attract interest from scholars for further investigations as a novel anti-cancer peptide. A preliminary study revealed anti-cancer properties of B2R in a semi-selective manner. It was proposed that B2R could afford to kill cancer cells (Jurkat, BJAB, MCF-7, L929, A549) via lysosome-mitochondrial cross talk during cell death in the caspase independent pathway. Nevertheless, cells, which either over-expressed Bcl2 or suppressed BNIP3 expression, were observed to resist anti-cancer effects of B2R. Furthermore, in this study the cytotoxicity influence of individual B2R (10 µg/mL) were also compared to its combination with doxorubicin and cisplatin (50 µg/mL) in Jurkat and MCF-7 cells over short time periods (4 h). The results revealed that B2R was more toxic than doxorubicin and cisplatin in these cells. Brevinin-2R triggered cell death by reduction of the mitochondrial membrane potential as well as cellular ATP levels, and yet elevation of reactive oxygen species (ROS) generation (Ghavami et al. 2008). Interestingly, B2R showed low cytotoxicity against non-cancerous cells, such as PBMCs and human CD3 + T cells (Ghavami et al. 2008). This phenomenon might arise from variations of the outer membrane surface of cancer cells unlike normal cells. Some studies have demonstrated that the negatively charged phospholipid phosphatidylserines (PS) increase in the outer leaflet of the human tumor cell membranes (Ran et al. 2002; Utsugi et al. 1991). Others have substantiated these findings and suggested the PS presence to detect metastasis (Riedl et al. 2011). It is speculated that B2R low cytotoxicity against non-cancerous cells, however direct studies to investigate this mechanism are needed for confirmation. Similar studies have proposed some analogues of Brevinin-1, namely Brevinin-1CEa, to carry anti-cancer impacts against the growth of MCF-7 (breast cancer) and HeLa (cervical cancer) cells (Yu et al. 2009). Additionally, anti-cancer properties of Brevinin-1EMa derivatives were investigated against seven tumor cell lines, such as A498 (kidney), A549 (lung), HCT116 (colon), MKN45 (stomach), PC-3 (prostate), SK-MEL-2 (skin), and SK-OV-3 (ovary). (Kang et al. 2012). There was a report concerning the anti-cancer function of modified Brevinin, like dicarba-Brevinin-1BYa. Elevated cytotoxicity against human erythrocytes, MDA-MB-231 (breast carcinoma), and HepG2 (hepatoma) cancer cells occurred following treatment with dicarba-Brevinin (Hossain et al. 2011).
Effect of Brevinins on Insulin Release
The prevalence of diabetes (largely type 2) is on the rise due to the worldwide increase in obesity, insulin resistance, and dyslipidemia. The main strategy for treatment regards hyperglycemia control using a different kind of drugs, namely thiazolidinedione, metformin, and sulphonylureas. These medicines have limited efficacy, limited tolerability, and several side effects (Moller 2001). Consequently, it sounds essential to research and develop new natural types of therapeutic agents (Leidig-Bruckner et al. 2014). Surprisingly, beyond the antimicrobial activities of the Brevinin superfamily, a host of these peptides has shown to stimulate insulin secretion in vitro. In this way, therapeutic potentials of Brevinins for the treatment of diabetes mellitus (type 2) patients have been explored in few studies. The BRIN-BD11 is a rat insulin-secreting β-cell line and nominated as an approved model for insulin-release studies (McClenaghan et al. 1996). Most experiments have utilized BRIN-BD11 cells to indicate the insulin-release stimulation as a result of Brevinin peptides, such as Brevinin-1 Pa (Marenah et al. 2005), Brevinin-1E, -2Ec (Marenah et al. 2006), Brevinin-1CBb (Mechkarska et al. 2011), Brevinin-2GUb (Conlon et al. 2008), and Brevinin-2-related (B2RP) peptide (Abdel-Wahab et al. 2010). In another study, gaegurin-6, a peptide from the Brevinin-1 family, was presented to trigger insulin release from rat RINm5F insulinoma-derived cells (Kim et al. 2010). In a recent study, it was revealed that two members of the Brevinin-1 family, Brevinin-1ITa and -1ITb, could carry stimulative effects on insulin release from BRIN-BD11 clonal β-cells in a concentration-dependent manner. Although, Brevinin-1ITa failed to exhibit antimicrobial activity against Gram-negative bacterium E. coli (Conlon et al. 2017). The mechanism of insulin release by Brevinins could be due to the protective nature of these peptides along with their antimicrobial activities. Brevinins stimulate insulin secretion and reduce blood glucose in their predators as a defense mechanism since hypoglycemia might be fatal to some species, namely snakes and birds (Marenah et al. 2004). The exact molecular mechanism underlying this action is not fully elucidated however it appears that an increase in intracellular Ca2+ concentrations may induce insulin secretion (Kim et al. 2010). Since B2RPlack the C-terminal cyclic heptapeptide domain that enhances antimicrobial activity and reduces cytotoxicity, it has received much more attention than others. In this regard, some have intended to modify this peptide for insulin secretion. Abdel-wahab et al. modified B2RP by Asp4 → Lys substitution and accordingly insulin-releasing potency enhanced owing to elevation of cationicity. Nonetheless, other analogues, which were produced by amino acid substitution and contained more hydrophobic residues, developed reduced insulin-releasing abilities (Abdel-Wahab et al. 2010). Despite the evidence supporting the potential of Brevinins as novel insulinotropic peptides, it is worth mentioning that they are rapidly cleared from the circulation. This event is perceived as a negative factor for this kind of treatment. Therefore, additional studies are required to assess and validate Brevinins as potential therapeutic agents for the treatment of diabetes mellitus (Table 2).
Table 2.
Origins and primary structures of Brevinin peptides with stimulative impacts on insulin release
| Peptides | Species | Primary structures | Rate of action | References |
|---|---|---|---|---|
| Brevinin-1Pa | Lithobates pipiens | FLPIIAGVAAKVFPKIFCAISKKC | Marenah et al. (2005) | |
| Brevinin-1E, Brevinin-2Ec | Pelophylax saharicus |
FLPLLAGLAANFLPKIFCKITRKC GILLDKLKNFAKTAGKGVLQSLLNTASCKLSGQC |
Marenah et al. (2006) | |
| Brevinin-1CBb | Lithobates catesbeianus | FLPFIARLAAKVFPSIICSVTKKC | 119% of basal rate at a concentration of 30 nM | Mechkarska et al. (2011) |
| Brevinin-2GUb | Hylarana. guentheri | GVIIDTLKGAAKTVAAELLRKAHCKLTNSC | 139% of basal rate at a concentration of 0.1 µM | Conlon et al. (2008) |
| Brevinin-2-related peptide (B2RP) | Lithobates septentrionalis | GIWDTIKSMGKVFAGKILQNL | 148% of basal rate at a concentration of 1 µM | Abdel-Wahab et al. (2010) |
| Gaegurin-6 | Glandirana emeljanovi | FLPLLAGLAANFLPTIICKISYKC | Kim et al. (2010) | |
| Brevinin-1 ITa Brevinin-1 ITb | Rana italica |
IVPFLLGMVPKLVCLITKKC VFLGAIAQALTSLLGKLNH |
The basal rate in the presence of 5.6 mm glucose at a concentration of 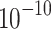 M of Brevinin-1ITa M of Brevinin-1ITa |
Conlon et al. (2017) |
Brevinin Effect on Wound Healing
Wound healing is a complex process containing cellular and molecular stimulation, which may cause inflammation, tissue formation, and ultimately tissue regeneration. People in Vietnam and south America apply amphibian skins for wound healing and inflammation treatment for years (Shores et al. 2007). Modern science has just recently investigated the wound healing properties of frog skin secretions and extractions (Liu et al. 2014; Piccolo et al. 2008). One of the these studies reported that the extract obtained from Rana ridibunda frog skin secretions (especially those products under 10 kDa) could accelerate the wound healing process (Mashreghi et al. 2013). Likewise, the efficacy of dermal (FS) and epidermal (RFS) sides of this frog skin was examined as wound dressing (Rezazade Bazaz et al. 2015). The main limitation of these studies regards the lack of sufficient characterizations of special compounds to determine the factor(s) involved in such properties. Furthermore, no study is available to compare the efficacy of Brevinin peptides and to focus on the approved standard of wound healing medicines. This process have been attributed to the collagen and lipid content of the frog skin (Kumar et al. 2002; Raghavan et al. 2010). What is more, the antimicrobial property of the skin secretion probably reduces wound infection and thus inducing the proliferation and migration of endothelial cells to the damaged site. Popovic et al. (2012) examined the therapeutic potential of the Brevinin-2GU, isolated from Hylarana guentheri, and B2RP-ERa from Hylarana erythraea (Al-Ghaferi et al. 2010) as a wound healing agent for common skin rashes. They found out that these peptides exhibited the bactericidal effect against Propionibacterium acnes as a major pathogenesis in acne vulgaris development (Popovic et al. 2012). Therefore, the influences of Brevinins on the wound healing process can be explained by their antimicrobial activities, to date.
Brevinin peptides have the advantages of low or no hemolytic activity even at high concentrations (no cytotoxicity against normal cells), and strong antimicrobial activity that provide a basis for their implication in wound healing. However, the short half-life of Brevinins in the circulation could significantly restrict their systemic administration and topical application.
Brevinin Functionalized Nanostructure
Ligand conjugation to nano-particles is a widely used technique that allows to extend application of functionalized nano-structures in diverse fields, such as gene or drug delivery systems (Askarian et al. 2017, 2015), biomedical imaging process, drug design, and vaccine development strategies (Sapsford et al. 2013). Casciaro et al. (2017) covalently conjugated an Esculentin-1a derivative, Esc (1–21) NH2, to gold nanoparticles (AuNPs) using poly(ethylene glycol) linkers. The Esculentin-1a is a frog skin antimicrobial peptide and isolated from Pelophylax lessonae/ridibundus. The antimicrobial activity of peptide-coated AuNPs increased about 15-fold against Pseudomonas aeruginosa bacterium more than free peptides (Casciaro et al. 2017). In accordance to extant studies, it seems that peptide-functionalized nano-particles can considerably enhance the antibacterial activity of biomolecules (Veerapandian and Yun 2011). Recently, cerium oxide nanoparticle (CNP) was conjugated to B2RP. The resultant complex (CNP-B2R) showed a higher cytotoxicity against cancer cell line (A549) than normal (HFLF-pI5) cell line (Homayouni-Tabrizi et al. 2016). Furthermore, this study benefited from the semi-selective anti-cancer activity of B2RP and the anti-oxidant property of cerium oxide nanoparticles, but further studies are necessary on CNP-B2R nano-structure stability and the validation of results by in vivo experiments. In general, the numbers of studies in which amphibian peptides undergo modification or functionalization with nano-particles are scarce. Given the potential of nano-structures for target modification of peptides, designing novel Brevinin-derived peptides through variant nanoparticle or different modifications holds promise for improving their current use or even extending their application in other fields (Fig. 2).
Fig. 2.
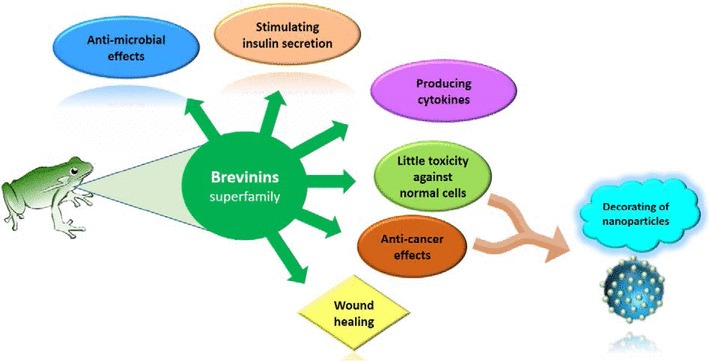
Different functions and application of Brevinin superfamily
Conclusions
The search for novel bioactive peptides of therapeutic promise is sever ending story as human health permanently face the complications caused by ongoing diseases as well as emerging disorders. Antimicrobial peptides (AMPs) are biomolecules found in skins of almost all organisms and act as a natural defense mechanism against pathogens. Some of these peptides have other unique characteristics besides antimicrobial properties, which make them intriguing effector molecules to employ in different applications. The Brevinin superfamily consists of AMPs derivate from frog skins and captivates many attentions owing to their protection against cancer cells, low hemolytic activity, insulin release stimulation, and wound healing properties. Brevinins also can counteract the infectious effects of bacteria, viruses, and pathogenic fungi. Furthermore, Brevinin 2R has presented a strong potential to become a cancer-specific peptide with low affinity toward non-malignant cells as well as non-hemolytic activity. Brevinin-1 and -2 derivatives (with or without peptide modifications) have been proven to stimulate insulin release, though the exact molecular mechanisms remain unknown. Wound healing properties of Brevinins are more likely to arise from their antimicrobial activity in spite of few studies. Another interesting aspect of AMPs generally and Brevinins exclusively is the possibility of chemical modifications by conjunction to nano-particle. Some studies have demonstrated that design of smart targeting delivery of these modified peptides affords both anti-cancer and antimicrobial activities. Despite the numerous investigations on different members of the Brevinin superfamily, the molecular mechanisms underlying their actions against pathogens and cancer cells are still in need for further research prior to the clinical applications. Of note, the precise and detailed mechanisms of their cellular uptake are unknown.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The article does not contain any studies with human participants or with animals performed by any of the authors.
Informed Consent
The article is a review manuscript and does not contain any photograph. However, the figures and tables are also prepared by the authors themselves.
Footnotes
Fatemeh Zohrab and Saeedeh Askarian have contributed equally to this manuscript.
References
- Abdel-Wahab YHA, Patterson S, Flatt PR, Conlon JM. Brevinin-2-related peptide and its [D4K] analogue stimulate insulin release in vitro and improve glucose tolerance in mice fed a high fat diet. Horm Metab Res. 2010;42:652–656. doi: 10.1055/s-0030-1254126. [DOI] [PubMed] [Google Scholar]
- Al-Ghaferi N, et al. Antimicrobial peptides from the skin secretions of the South-East Asian frog Hylarana erythraea (Ranidae) Peptides. 2010;31:548–554. doi: 10.1016/j.peptides.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Ali MF, Lips KR, Knoop FC, Fritzsch B, Miller C, Conlon JM. Antimicrobial peptides and protease inhibitors in the skin secretions of the crawfish frog, Rana areolata. Biochim Biophys Acta. 2002;1601:55–63. doi: 10.1016/S1570-9639(02)00432-6. [DOI] [PubMed] [Google Scholar]
- Askarian S, Abnous K, Taghavi S, Oskuee RK, Ramezani M. Cellular delivery of shRNA using aptamer-conjugated PLL-alkyl-PEI nanoparticles. Colloids Surf B. 2015;136:355–364. doi: 10.1016/j.colsurfb.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Askarian S, Abnous K, Ayatollahi S, Farzad SA, Oskuee RK, Ramezani M. PAMAM-pullulan conjugates as targeted gene carriers for liver cell. Carbohyd Polym. 2017;157:929–937. doi: 10.1016/j.carbpol.2016.10.030. [DOI] [PubMed] [Google Scholar]
- Asoodeh A, Haghparast A, Kashef R, Chamani J. Pro-inflammatory cytokine responses of A549 epithelial cells to antimicrobial peptide brevinin-2R. Int J Pept Res Ther. 2013;19:157–162. doi: 10.1007/s10989-012-9328-6. [DOI] [Google Scholar]
- Bevier CR, Sonnevend A, Kolodziejek J, Nowotny N, Nielsen PF, Conlon JM. Purification and characterization of antimicrobial peptides from the skin secretions of the mink frog (Rana septentrionalis) Comp Biochem Physiol Part C. 2004;139:31–38. doi: 10.1016/j.cca.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Borde JP, Kern WV. Treatment of MRSA infections. Dtsch Med Wochenschr. 2012;137:2553–2557. doi: 10.1055/s-0032-1327283. [DOI] [PubMed] [Google Scholar]
- Casciaro B, Moros M, Rivera-Fernández S, Bellelli A, Jesús M, Mangoni ML. Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a (1–21) NH 2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 2017;47:170–181. doi: 10.1016/j.actbio.2016.09.041. [DOI] [PubMed] [Google Scholar]
- Chan DI, Prenner EJ, Vogel HJ. Tryptophan-and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta. 2006;1758:1184–1202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Conlon JM, Kolodziejek J, Nowotny N. Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochem Biophys Acta. 2004;1696:1–14. doi: 10.1016/j.bbapap.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Conlon JM, et al. Purification and characterization of antimicrobial peptides from the skin secretions of the carpenter frog Rana virgatipes (Ranidae, Aquarana) Regul Pept. 2005;131:38–45. doi: 10.1016/j.regpep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Conlon JM, et al. Antimicrobial peptides from the skin of the Tsushima brown frog Rana tsushimensis. Comp Biochem Physiol. 2006;143:42–49. doi: 10.1016/j.cbpa.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Conlon JM, et al. A potent, non-toxic insulin-releasing peptide isolated from an extract of the skin of the Asian frog, Hylarana guntheri (Anura:Ranidae) Regul Pept. 2008;151:153–159. doi: 10.1016/j.regpep.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Conlon JM, Ahmed E, Condamine E. Antimicrobial properties of brevinin-2-related peptide and its analogs: efficacy against multidrug-resistantAcinetobacter baumannii. Chem Biol Drug Design. 2009;74:488–493. doi: 10.1111/j.1747-0285.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- Conlon JM, Ahmed E, Coquet L, Jouenne T, Leprince J, Vaudry H, King JD. Peptides with potent cytolytic activity from the skin secretions of the North American leopard frogs, Lithobates blairi and Lithobates yavapaiensis. Toxicon. 2009;53:699–705. doi: 10.1016/j.toxicon.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Conlon JM, Mechkarska M, Lukic ML, Flatt PR. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides. 2014;57:67–77. doi: 10.1016/j.peptides.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Conlon JM, et al. Cytotoxic peptides with insulin-releasing activities from skin secretions of the Italian stream frog Rana italica (Ranidae. J Pept Sci. 2017;23(10):769–776. doi: 10.1002/psc.3025. [DOI] [PubMed] [Google Scholar]
- Daum JM, Davis LR, Bigler L, Woodhams DC. Hybrid advantage in skin peptide immune defenses of water frogs (Pelophylax esculentus) at risk from emerging pathogens. Infect Genet Evol. 2012;12:1854–1864. doi: 10.1016/j.meegid.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Deslouches B, Di YP. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017;8:46635. doi: 10.18632/oncotarget.16743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Ghavami S, et al. Brevinin-2R1 semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J Cell Mol Med. 2008;12:1005–1022. doi: 10.1111/j.1582-4934.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- Haney EF, Hancock RE. Peptide design for antimicrobial and immunomodulatory applications. Pept Sci. 2013;100:572–583. doi: 10.1002/bip.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd D. Atomic force microscopy: a novel tool for the analysis of the mechanism of action of antimicrobial peptides on target membranes. Stellenbosch: Stellenbosch University; 2003. [Google Scholar]
- Homayouni-Tabrizi M, Asoodeh A, Soltani M, Forghanifard MM. Antimicrobial peptide Brevinin-2R induces the secretion of a pro-inflammatory cytokine in HepG2 cells. J Basic Res Med Sci. 2015;2:2923. [Google Scholar]
- Homayouni-Tabrizi M, Asoodeh A, Mashreghi M, Rezazade Bazaz M, Kazemi Oskuee R, Darroudi M. Attachment of a frog skin-derived peptide to functionalized cerium oxide nanoparticles. Int J Pept Res Ther. 2016;22:505–510. doi: 10.1007/s10989-016-9531-y. [DOI] [Google Scholar]
- Hossain MA, et al. Synthesis, conformational analysis and biological properties of a dicarba derivative of the antimicrobial peptide, brevinin-1BYa. Eur Biophys J. 2011;40:555–564. doi: 10.1007/s00249-011-0679-2. [DOI] [PubMed] [Google Scholar]
- Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Ji HY, Lee BJ. Anticancer activity of undecapeptide analogues derived from antimicrobial peptide, Brevinin-1EMa. Arch Pharmacal Res. 2012;35:791–799. doi: 10.1007/s12272-012-0505-0. [DOI] [PubMed] [Google Scholar]
- Khiari Z, Rico D, Martin-Diana AB, Barry-Ryan C. Structure elucidation of ACE-inhibitory and antithrombotic peptides isolated from mackerel skin gelatine hydrolysates. J Sci Food Agric. 2014;94:1663–1671. doi: 10.1002/jsfa.6476. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JO, Jung JH, Lee SK, You GY, Park SH, Kim HS. Gaegurin-6 stimulates insulin secretion through calcium influx in pancreatic β Rin5mf cells. Regul Pept. 2010;159:123–128. doi: 10.1016/j.regpep.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Kumar K, Sai KP, Babu M. Application of frog (Rana tigerina Daudin) skin collagen as a novel substrate in cell culture. J Biomed Mater Res Part A. 2002;61:197–202. doi: 10.1002/jbm.10116. [DOI] [PubMed] [Google Scholar]
- Kumari VK, Nagaraj R. Structure-function studies on the amphibian peptide brevinin 1E: translocating the cationic segment from the C-terminal end to a central position favors selective antibacterial activity. J Pept Res. 2001;58:433–441. doi: 10.1034/j.1399-3011.2001.00924.x. [DOI] [PubMed] [Google Scholar]
- Kwon MY, Hong SY, Lee KH. Structure-activity analysis of brevinin 1E amide, an antimicrobial peptide from Rana esculenta. Biochim Biophys Acta. 1998;1387:239–248. doi: 10.1016/S0167-4838(98)00123-X. [DOI] [PubMed] [Google Scholar]
- Lai Y, Villaruz AE, Li M, Cha DJ, Sturdevant DE, Otto M. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol Microbiol. 2007;63:497–506. doi: 10.1111/j.1365-2958.2006.05540.x. [DOI] [PubMed] [Google Scholar]
- Leidig-Bruckner G, Grobholz S, Bruckner T, Scheidt-Nave C, Nawroth P, Schneider JG. Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr Disord. 2014;14:33. doi: 10.1186/1472-6823-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiang Q, Zhang Q, Huang Y, Su Z. Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides. 2012;37:207–215. doi: 10.1016/j.peptides.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Li A, Zhang Y, Wang C, Wu G, Wang Z. Purification, molecular cloning, and antimicrobial activity of peptides from the skin secretion of the black-spotted frog, Rana nigromaculata. World J Microbiol Biotechnol. 2013;29:1941–1949. doi: 10.1007/s11274-013-1360-y. [DOI] [PubMed] [Google Scholar]
- Liu H, et al. A potential wound healing-promoting peptide from frog skin. Int J Biochem Cell Biol. 2014;49:32–41. doi: 10.1016/j.biocel.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Marenah L, Flatt PR, Orr DF, McClean S, Shaw C, Abdel-Wahab YHA. Brevinin-1 and multiple insulin-releasing peptides in the skin of the frog Rana palustris. J Endocrinol. 2004;181:347354. doi: 10.1677/joe.0.1810347. [DOI] [PubMed] [Google Scholar]
- Marenah L, Flatt P, Orr D, Shaw C, Abdel-Wahab Y. Characterization of naturally occurring peptides in the skin secretion of Rana pipiens frog reveal pipinin-1 as the novel insulin-releasing agent. Chem Biol Drug Des. 2005;66:204–210. doi: 10.1111/j.1399-3011.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Marenah L, Flatt P, Orr D, Shaw C, Abdel-Wahab Y. Skin secretions of Rana saharica frogs reveal antimicrobial peptides esculentins-1 and -1B and brevinins-1E and -2EC with novel insulin releasing activity. J Endocrinol. 2006;188:1–9. doi: 10.1677/joe.1.06293. [DOI] [PubMed] [Google Scholar]
- Mashreghi M, Bazaz MR, Shahri NM, Asoodeh A, Mashreghi M, Rassouli MB, Golmohammadzadeh S. Topical effects of frog “Rana ridibunda” skin secretions on wound healing and reduction of wound microbial load. J Ethnopharmacol. 2013;145:793–797. doi: 10.1016/j.jep.2012.12.016. [DOI] [PubMed] [Google Scholar]
- McClenaghan NH, et al. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996;45:1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- Mechkarska M, et al. Peptidomic analysis of skin secretions from the bullfrog Lithobates catesbeianus (Ranidae) identifies multiple peptides with potent insulin-releasing activity. Peptides. 2011;32:203–208. doi: 10.1016/j.peptides.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- Morikawa N, Hagiwara K, Nakajima T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem Biophys Res Commun. 1992;189:184–190. doi: 10.1016/0006-291X(92)91542-X. [DOI] [PubMed] [Google Scholar]
- Novkovic M, Simunic J, Bojovic V, Tossi A, Juretic D. DADP: the database of anuran defense peptides. Bioinformatics. 2012;28:1406–1407. doi: 10.1093/bioinformatics/bts141. [DOI] [PubMed] [Google Scholar]
- Nuotio L, Schneitz C, Nilsson O. Effect of competitive exclusion in reducing the occurrence of Escherichia coli producing extended-spectrum beta-lactamases in the ceca of broiler chicks. Poultry Sci. 2013;92:250–254. doi: 10.3382/ps.2012-02575. [DOI] [PubMed] [Google Scholar]
- Pálffy R, Gardlík R, Behuliak M, Kadasi L, Turna J, Celec P. On the physiology and pathophysiology of antimicrobial peptides. Mol Med. 2009;15:51–59. doi: 10.2119/molmed.2008.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin A, Anand S, Asha R, Reshmy V, Sanil G, Kumar KS. Structure-activity relationship and mode of action of a frog secreted antibacterial peptide B1CTcu5 using synthetically and modularly modified or deleted (SMMD) peptides. PLoS ONE. 2015 doi: 10.1371/journal.pone.0124210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Espitia PJ, de Fátima Ferreira Soares N, dos Reis Coimbra JS, de Andrade NJ, Souza Cruz R, Medeiros A, Antonio E. Bioactive peptides: synthesis, properties, and applications in the packaging and preservation of food. Compr Rev Food Sci Food Saf. 2012;11:187–204. doi: 10.1111/j.1541-4337.2011.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo NS, Piccolo MS, Piccolo MS. The use of frogskin as a biological dressing for temporary cover of burn wounds. In: Eisenmann-Klein M, Neuhann-Lorenz C, editors. Innovations in plastic and aesthetic surgery. Berlin: Springer; 2008. pp. 129–137. [Google Scholar]
- Pokora M, Zambrowicz A, Dąbrowska A, Eckert E, Setner B, Szołtysik M, Szewczuk Z, Zabłocka A, Polanowski A, Trziszka T, Chrzanowska J. An attractive way of egg white protein by-product use for producing of novel anti-hypertensive peptides. Food Chem. 2014;151:500–505. doi: 10.1016/j.foodchem.2013.11.111. [DOI] [PubMed] [Google Scholar]
- Popovic S, Urbán E, Lukic M, Conlon JM. Peptides with antimicrobial and anti-inflammatory activities that have therapeutic potential for treatment of acne vulgaris. Peptides. 2012;34:275–282. doi: 10.1016/j.peptides.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Power O, Jakeman P, FitzGerald R. Antioxidative peptides: enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids. 2013;44:797–820. doi: 10.1007/s00726-012-1393-9. [DOI] [PubMed] [Google Scholar]
- Powers JP, Hancock RE. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Raghavan KV, Babu M, Rajaram R, Sai KP. Efficacy of frog skin lipids in wound healing. Lipids Health Dis. 2010;9:74. doi: 10.1186/1476-511X-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Can Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- Rao AG. Antimicrobial peptides. Mol Plant-Microbe Interact. 1995;8:6–13. doi: 10.1094/MPMI-8-0006. [DOI] [PubMed] [Google Scholar]
- Rezazade Bazaz M, Mashreghi M, Mahdavi Shahri N, Mashreghi M, Asoodeh A, Behnam Rassouli M. Evaluation of antimicrobial and healing activities of frog skin on Guinea pigs wounds jundishapur. J Microbiol. 2015;8:e21218. doi: 10.5812/jjm.21218v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S, et al. In search of a novel target-phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochem Biophys Acta. 2011;1808:2638–2645. doi: 10.1016/j.bbamem.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J, Calderon J, Rondón-Villarreal P, Torres R. Analysis of structure and hemolytic activity relationships of antimicrobial peptides (AMPs) In: Castillo LF, Cristancho M, Isaza G, Pinzón A, Rodríguez JMC, editors. Advances in computational biology: proceedings of the 2nd colombian congress on computational biology and bioinformatics (CCBCOL) Cham: Springer International Publishing; 2014. p. 253258. [Google Scholar]
- Sadredinamin M, Mehrnejad F, Hosseini P, Doustdar F. Antimicrobial peptides (AMPs) Nov Biomed. 2016;4:70–76. [Google Scholar]
- Sapsford KE, et al. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113:1904–2074. doi: 10.1021/cr300143v. [DOI] [PubMed] [Google Scholar]
- Savelyeva A, Ghavami S, Davoodpour P, Asoodeh A, Łos MJ. An overview of Brevinin superfamily: structure, function and clinical perspectives. In: Grimm S, editor. Anticancer genes. Berlin: Springer; 2014. pp. 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MG, et al. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol. 2007;25:465. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MD, Won HS, Kim JH, Mishig-Ochir T, Lee BJ. Antimicrobial peptides for therapeutic applications: a review. Molecules. 2012;17:12276–12286. doi: 10.3390/molecules171012276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta. 1999;1462:55–70. doi: 10.1016/S0005-2736(99)00200-X. [DOI] [PubMed] [Google Scholar]
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20:493–508. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- Sitaram N, Nagaraj R. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim Biophys Acta. 1999;1462:29–54. doi: 10.1016/S0005-2736(99)00199-6. [DOI] [PubMed] [Google Scholar]
- Tv V, Asha R, Shyla G, George S. Post-translationally modified frog skin-derived antimicrobial peptides are effective against Aeromonas sobria. Microb Pathog. 2017;104:287–288. doi: 10.1016/j.micpath.2017.01.052. [DOI] [PubMed] [Google Scholar]
- Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Can Res. 1991;51:3062–3066. [PubMed] [Google Scholar]
- Veerapandian M, Yun K. Functionalization of biomolecules on nanoparticles: specialized for antibacterial applications. Appl Microbiol Biotechnol. 2011;90:1655–1667. doi: 10.1007/s00253-011-3291-6. [DOI] [PubMed] [Google Scholar]
- Vineethkumar T, Asha R, Shyla G, George S. Studies on the mode of membrane interaction of C-terminally amidated brevinin1 HYba1 and 2 peptides against bacteria. Int J Pept Res Ther. 2018;24:117–129. doi: 10.1007/s10989-017-9598-0. [DOI] [Google Scholar]
- Wang G, Watson KM, Peterkofsky A, Buckheit RW., Jr Identification of novel human immunodeficiency virus type 1-inhibitory peptides based on the antimicrobial peptide database. Antimicrob Agents Chemother. 2010;54:1343–1346. doi: 10.1128/AAC.01448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won HS, Kim SS, Jung SJ, Son WS, Lee B, Lee BJ. Structure-activity relationships of antimicrobial peptides from the skin of Rana esculenta inhabiting in Korea. Mol Cells. 2004;17:469–476. [PubMed] [Google Scholar]
- Yasin B, et al. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis. 2000;19:187–194. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- Yu F, Zhang L, Li J, Li X, Fu X, Shang D. Cloning of cDNAs encoding skin antimicrobial peptide precursors from Chinese brown frogs, Rana chensinensis and determination of antimicrobial, anticancer and haemolysis activity. Chin J Biotechnol. 2009;25:101–108. [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Sun Y, Liu Q, Wang X, Li Z, Hao J. In vitro synergistic activities of antimicrobial peptide brevinin-2CE with five kinds of antibiotics against multidrug-resistant clinical isolates. Curr Microbiol. 2014;68:685–692. doi: 10.1007/s00284-014-0529-4. [DOI] [PubMed] [Google Scholar]


