Abstract
Sialylated N-glycans play essential roles in the immune system, pathogen recognition and cancer. This review approaches the sialylation of N-glycans from three perspectives. The first section focuses on the sialyltransferases that add sialic acid to N-glycans. Included in the discussion is a description of these enzymes’ glycan acceptors, conserved domain organization and sequences, molecular structure and catalytic mechanism. In addition, we discuss the protein interactions underlying the polysialylation of a select group of adhesion and signaling molecules. In the second section, the biosynthesis of sialic acid, CMP-sialic acid and sialylated N-glycans is discussed, with a special emphasis on the compartmentalization of these processes in the mammalian cell. The sequences and mechanisms maintaining the sialyltransferases and other glycosylation enzymes in the Golgi are also reviewed. In the final section, we have chosen to discuss processes in which sialylated glycans, both N- and O-linked, play a role. The first part of this section focuses on sialic acid-binding proteins including viral hemagglutinins, Siglecs and selectins. In the second half of this section, we comment on the role of sialylated N-glycans in cancer, including the roles of β1-integrin and Fas receptor N-glycan sialylation in cancer cell survival and drug resistance, and the role of these sialylated proteins and polysialic acid in cancer metastasis.
Keywords: Sialic acid, Polysialic acid, Sialyltransferase, Golgi, Selectins, Siglecs
The synthesis of sialylated glycans on proteins and lipids in the secretory pathway is catalyzed by twenty Golgi localized sialyltransferases (STs) with distinct substrate and linkage specificity [reviewed in Harduin-Lepers et al. (2005)]. As highlighted in the introduction to this thematic issue (Gabius and Roth 2017), glycan structures, including those that contain sialic acid (Sia), encode an amazing array of information that is essential for health and can be altered in disease. In this review, we will provide a general overview of N-glycan sialylation, the structure and function of the STs that catalyze these reactions, the mechanisms of their Golgi localization, substrate recognition and the function of sialic acid on N-glycans in health and disease. Please also see the article by A. P. Corfield in this issue for general information on protein N-glycosylation (Corfield 2017), as well as the article by J. Kopitz concerning the basics of lipid glycosylation (Kopitz 2017). We would also like to direct your attention to a recent special issue of Trends in Biochemical Sciences entitled The Magic of the Sugar Code with forward by Gabius (2015) for a broad overview of all forms glycosylation and their function. Finally, there are numerous excellent reviews referenced throughout the text on all aspects of N-glycan sialylation that we encourage the reader to consider. We also want to sincerely apologize to the authors of many fine papers that we did not have the room to include.
Sialyltransferases: structure and function
This discussion will focus on the STs involved in the modification of N-glycans in mammalian cells including the ST6Gal-I and ST6Gal-II, ST3Gal-IV and ST3Gal-VI, and the polysialyltransferases (polySTs), ST8Sia-II and ST8Sia-IV (see Fig. 1 for the structures formed by these STs on N-glycans). We will discuss these enzymes’ substrates and general function, their common domain structure and conserved sequences, the molecular structure of the ST6Gal-I, the ST catalytic mechanism and mechanism underlying the protein specificity of polysialylation.
Fig. 1.
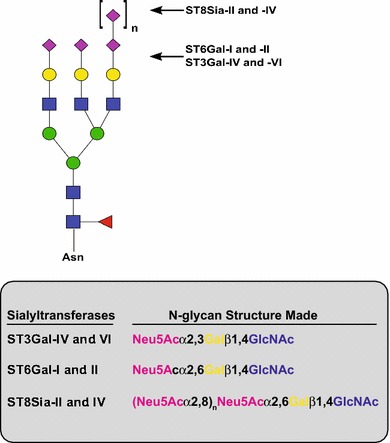
N-glycan structures synthesized by α2,3-, α2,6- and α2,8-sialyltransferases. In this review, we have focused on the sialyltransferases that add Sia to N-glycans. The ST3Gal enzymes may also modify O-glycans and glycolipids, while the role of ST6Gal-II in modifying protein-bound N-glycans has not been unequivocally demonstrated. The polysialyltransferases, ST8Sia-II and ST8Sia-IV, synthesize polySia chains of 8 to greater than 400 units long on a preexisting Sia that is many times α2,6-linked. Shown is a triantennary N-glycan as a model and does not imply that the activity of these enzymes in any way is restricted to this type of N-glycan. Pink diamond, Neu5Ac; yellow circle, galactose (Gal); blue square, N-acetylglucosamine (GlcNAc); green circle, mannose (Man); red triangle, fucose (Fuc)
ST6Gal-I and ST6Gal-II
In the early 1970s, a number of laboratories identified ST activities in several mammalian tissues (Bartholomew et al. 1973; Carlson et al. 1973a, b; Hudgin and Schachter 1972). In 1977, an α2,6-ST from bovine colostrum that used Galβ1,4GlcNAc as an acceptor was isolated and characterized (Paulson et al. 1977a, b). This enzyme was later called ST6Gal-I. Cloning of this enzyme’s coding sequence from a rat liver cDNA library revealed its amino acid sequence and confirmed that the soluble forms found in body fluids, such as colostrum, breast milk and blood, are proteolytically truncated versions of those found in the cell (Weinstein et al. 1987). Subsequent work suggested that this ST is cleaved in a late Golgi or post-Golgi compartment (Kitazume-Kawaguchi et al. 1999; Ma et al. 1997) and that several proteases could be involved, including cathepsin D and BACE (Kitazume et al. 2001; Lammers and Jamieson 1988). The ability of soluble glycosyltransferases to catalytically function outside the Golgi was questioned until recently (see discussion below). A second α2,6-ST, ST6Gal-II, was identified, cloned and characterized (Takashima et al. 2002). While this enzyme can use the Galβ1,4GlcNAc structure of oligosaccharides as an acceptor, its shows poor activity with this structure on glycoproteins and glycolipids, and consequently, its in vivo function is unclear.
The expression of ST6Gal-I is tissue specific, and its expression is regulated by multiple transcriptional promoters (Svensson et al. 1992; Wang et al. 1993; Wen et al. 1992). A constitutive, ubiquitous promoter maintains ST6Gal-I expression at low but steady levels, particularly in liver. However, an inducible, liver-specific P1 promoter was shown to drive high ST6Gal-I expression during inflammation and the observed increase in cleaved and secreted ST6Gal-I in the blood (Appenheimer et al. 2003). This inducible pool was shown to be important as a regulator of myelopoiesis/hematopoiesis (Jones et al. 2010; Nasirikenari et al. 2014); however, the source of CMP-Sia that would serve as a donor in these serum ST reactions was unclear. Recent work has demonstrated that activated platelets release the CMP-Sia that serves as the donor for circulating ST6Gal-I, allowing for the remodeling of the glycans of hematopoietic progenitor cells (Lee et al. 2014). Additional in vivo support for the biosynthetic role of circulating ST6Gal-I comes from a recent study showing that ST6Gal-I-deficient B cells express IgGs that are modified with α2,6-sialylated glycans and these are synthesized by circulatory ST6Gal-I released from the liver and CMP-Sia from activated platelets (Jones et al. 2016).
ST3Gal-IV and ST3Gal-VI
These STs add a single Sia in an α2,3-linkage to terminal Gal residues of glycoproteins and glycolipids. ST3Gal-IV has been reported to sialylate Galβ1,4(3)GlcNAc found on N-glycans, as well as core-2, core-3 or core-4 O-glycans that carry GlcNAc (Yang et al. 2012), and Galβ1,3GalNAc terminated structures in glycoproteins or glycolipids (Kitagawa and Paulson 1994; Sasaki et al. 1993). The ST3Gal-VI enzyme uses Galβ1,4GlcNAc acceptors on glycoproteins and glycolipids, but has additional specificity for the glycolipid moieties of its acceptors. As both enzymes use the type II lactosamine structure (Galβ1,4GlcNAc), they were proposed to be involved in the synthesis of the sialyl Lewis X (sLeX) determinant on leukocyte E-, L- and P-selectin ligands (Kitagawa and Paulson 1994). These ligands are required for leukocyte binding to selectins on endothelial cells, and their slow rolling and tethering prior to extravasation. Please see the discussion of selectins and their ligands below, and the article in this issue by Mayer et al. for a review of C-type lectins (Mayer et al. 2017). For additional insights into lectin structure, function and analysis, please see the article by Manning et al. in this issue (Manning et al. 2017), and other excellent reviews published elsewhere (Gabius et al. 2015, 2016; Solis et al. 2015).
ST8Sia-II and ST8Sia-IV
The demonstrated role of polysialic acid (polySia) in cell migration and plasticity in the brain prompted the search for the polySTs [reviewed in Colley et al. (2014), Rutishauser (2008), Schnaar et al. (2014)]. Originally called STX and PST-1, ST8Sia-II and ST8Sia-IV polymerize long chains of α2,8-linked Sia that can extend from 8 to over 400 residues at the termini of both N-linked and O-linked glycans (Sato and Kitajima 2013). Structural analysis of the polysialylated N-glycans of bovine neural cell adhesion molecule (NCAM) shows that polySia is added to hybrid, bi-, tri- and small numbers of tetra-antennary complex glycans, with terminal α2,6-linked Sia as the preferred acceptor (von Der Ohe et al. 2002; Wuhrer et al. 2003). Expression of neuropilin-2 (NRP-2) and the polySTs in COS-7 cells revealed that ST8Sia-IV exclusively polysialylates NRP-2 and that polySia is found on sialylated core-1 and core-2 O-glycans (Rollenhagen et al. 2013). What sets polysialylation apart from other glycosylation reactions is that it is protein specific, is found on very few glycoprotein substrates in mammalian cells and, as such, requires the polySTs to recognize and bind these substrates prior to modification of their glycans (Colley 2010; Colley et al. 2014). Interestingly, the two polySTs do show substrate preferences. For example, while the neural cell adhesion molecule, NCAM, can be polysialylated by both polySTs, NRP-2 is exclusively polysialylated by ST8Sia-IV, and SynCAM-1 is exclusively polysialylated by ST8Sia-II, in the cells evaluated so far (Galuska et al. 2010; Rollenhagen et al. 2012, 2013).
The expression and roles of polySia as part of the capsule of neuroinvasive bacteria and during the development of the mammalian nervous system have been appreciated for some time and are comprehensively documented in the book Polysialic Acid-From Microbes to Man edited by J. Roth, U. Rutishauser and F. A. Troy II (Roth et al. 1993). In mammals, polySia is found on N- and O-linked glycans and is essential for cell migration and plasticity during nervous system development and to maintain these processes in select areas of the adult brain such as the hippocampus, olfactory bulb and hypothalamus [reviewed in Colley et al. (2014), Rutishauser (2008), Schnaar et al. (2014)]. More recent work has demonstrated a role for polySia in regeneration of damaged neurons (El Maarouf et al. 2006; El Maarouf and Rutishauser 2010; Zhang et al. 2007a, b), and in liver development and regeneration (Tsuchiya et al. 2014). In addition, many cancer cells upregulate polySia, and its expression correlates with increased invasion and metastasis (Colley et al. 2014; Falconer et al. 2012). For sometime, polySia was believed to exert its effects exclusively through an anti-adhesive mechanism; however, more recently, its ability to impact signaling, either directly by controlling protein–protein interactions or indirectly by serving as a reservoir for signaling molecules, has been appreciated (Colley et al. 2014; Schnaar et al. 2014). Interestingly, polySia is also being used as a less immunogenic and more biodegradable substitute for polyethylene glycol (PEG) to enhance the stability and circulating half-life of therapeutic proteins [reviewed in Bader and Wardwell (2014), Colley et al. (2014)], and as part of nanoparticles for drug delivery (Zhang et al. 2014, 2016). Please see below for a discussion of the protein specificity of polysialylation, its role cancer, and the article in this issue by Higuero and colleagues that discusses the role of glycans, including polySia, in CNS and PNS development and function (Higuero et al. 2017).
Sialyltransferase domain organization and conserved sequence motifs
The elucidation of the primary structure of the ST6Gal-I (Weinstein et al. 1987), and the cloning of other ST coding sequences [reviewed in Harduin-Lepers et al. (2005)], allowed the definition of the domain structure of STs and the identification of conserved sequences. The STs, and indeed all Golgi glycosyltransferases involved in N-linked glycosylation, are type II membrane proteins that possess short N-terminal cytoplasmic tails followed by transmembrane (TM) regions (signal anchors), and proteolytically sensitive stem or stalk regions that tether a large lumenal catalytic domain to the membrane (Paulson and Colley 1989) (Fig. 2b). Conserved sequences called sialylmotifs have been identified in the ST family that play roles in CMP-Sia and glycan acceptor recognition, and in the catalytic mechanism (Fig. 2a) [reviewed in Audry et al. (2011), Datta and Paulson (1997)]. Mutational analyses by Datta and Paulson and others demonstrated that the large sialylmotif (SML) participates in the binding of CMP-Sia donor (Datta and Paulson 1995), while the small sialylmotif (SMS) participates in interactions with both CMP-Sia and acceptor substrates (Datta et al. 1998), and the very small sialylmotif (SMVS) is involved in the catalytic reaction (Datta 2009; Jeanneau et al. 2004). Jeanneau and colleagues identified another sialylmotif called motif 3 and provided support for its involvement in acceptor recognition and catalytic efficiency in the ST3Gal-I enzyme (Jeanneau et al. 2004). The polySTs have two additional conserved sequences (Fig. 2). The polybasic region (PBR) is found at the stem-catalytic domain border and includes selected basic residues involved in substrate recognition and binding (Foley et al. 2009; Zapater and Colley 2012). The polyST domain (PSTD) is a 32-amino acid region adjacent to the small sialylmotif (Nakata et al. 2006). Basic residues in this region are required for catalytic activity and are proposed to play a role in forming an extended binding groove for the growing polySia chain (Foley et al. 2009; Nakata et al. 2006). Structural evidence supporting the roles of these conserved ST and polyST sequences is described below.
Fig. 2.

Sialyltransferase conserved sequences, domain structure and sequences involved in Golgi localization. a The positions of the conserved sequences found in all STs, the sialylmotifs, are shown using a generic polyST structure. SML, large sialylmotif; SMS, small sialylmotif; 3, motif 3; VS (or SMVS in text), very small sialylmotif. These sequences are involved in glycan substrate and CMP-Sia donor binding as well as catalysis. A key catalytic His residue mentioned in the text is the first residue in the SMVS. Also shown are two sequences conserved in the polySTs, the polybasic region (PBR) and polysialyltransferase domain (PSTD), which are involved in substrate recognition (via protein–protein interactions—PBR) and that are predicted to form a basic surface or groove for the growing polySia chain (PBR and PSTD). The disulfide formed between the SML and the SMS is conserved across all STs, while the disulfide bond linking the C-terminus is essential for the polySTs. b Sialyltransferase domain structure (left) and Golgi localization sequences (right). STs are type II membrane proteins with short, N-terminal cytoplasmic tails followed by relatively short transmembrane (TM) regions, proteolytically sensitive stem regions followed by large catalytic domains that contain the sialylmotifs and face the Golgi lumen. Golgi glycosylation enzymes are localized by multiple mechanisms. Shown is a summary of sequences and mechanisms involved in Golgi localization
Sialyltransferase structure and catalytic mechanism
To date, the X-ray crystal structures of three STs with well-defined substrate specificity have been solved including that of CstII, a bifunctional α2,3/α2,8-ST from Campylobacter jejuni (Chiu et al. 2004), porcine ST3Gal-I, which transfers Sia in an α2,3-linkage to Gal residues on O-glycans (Rao et al. 2009), and human (Kuhn et al. 2013) and rat (Meng et al. 2013) ST6Gal-I. The ST6Gal-I adopts a glycosyltransferase-A (GT-A) variant twofold that consists of a seven stranded twisted β sheet flanked by 14 α-helices (Kuhn et al. 2013; Meng et al. 2013). Both the CstII and ST3Gal-I also adopt a GT-A fold (Chiu et al. 2004; Rao et al. 2009), and all three enzymes share high levels of similarity in their β sheets, which contain the conserved sialylmotifs. These three STs exhibit less similarity in their helical and loop segments and in the arrangement of the final strand of the β sheet; all seven β strands are parallel in CstII and ST3Gal-I, while the last strand (β7) of ST6Gal-I is inserted into the sheet in an antiparallel orientation due to an α-helical insertion between it and the preceding strand (β6) (Kuhn et al. 2013; Meng et al. 2013). The catalytic domain of ST6Gal-I also has a large N-terminal extension not found in ST3Gal-I that may be involved in substrate binding (Kuhn et al. 2013). All six cysteines in the ST6Gal-I are engaged in disulfide bonds (Kuhn et al. 2013; Meng et al. 2013). Two disulfide bonds are critical for activity (Datta et al. 2001; Hirano et al. 2012): One between Cys181 and Cys332 links sialylmotifs SML and SMS and is conserved across all STs, and the other between Cys350 and Cys361 holds a loop above the active site that forms a “lid” over the nucleotide sugar-binding pocket (Meng et al. 2013). The former disulfide bond is also conserved in ST3Gal-I (Rao et al. 2009). The latter disulfide bond is unique to ST6Gal-I, although ST3Gal-I maintains a “lid” above the active site in the absence of a disulfide bond (Meng et al. 2013; Rao et al. 2009). This “lid” in both STs is unstructured in the absence of CMP-Sia but forms a more ordered structure in the presence of CMP-Sia (Meng et al. 2013). Evidence for two critical disulfide bonds has also been reported for the polyST, ST8Sia-IV, and in this case, a single cysteine remains unpaired (Angata et al. 2001) (see Fig. 2a).
The CMP-Sia-binding pocket of ST6Gal-I is located in a well-defined cavity formed by SML residues (Kuhn et al. 2013). Meng and colleagues performed modeling and molecular dynamics simulation to evaluate CMP-Sia donor and acceptor substrate-binding sites (Meng et al. 2013). They found that the majority of the interactions between CMP-Sia and the enzyme arise from interactions with the nucleotide portion rather than with the monosaccharide portion, consistent with the previously observed binding affinities for these groups (Blume et al. 2006; Meng et al. 2013). These interactions include hydrogen bonds with the hydroxyl groups of the ribose and the phosphate group of CMP, and hydrophobic stacking between the ribose ring and Phe208. The observed extension of the glycerol chain and C5 N-acetyl group of the Sia toward the solvent explains why CMP-Sia modified at O9 and the N-acetyl group can be tolerated by STs (Meng et al. 2013).
The structure reported by Kuhn and coworkers includes a glycan from the partner molecule inserted into the ST6Gal-I active site (Kuhn et al. 2013). This structure, and the modeling and molecular dynamics simulations of donor and acceptor binding by Meng et al. (2013), provides insights into the specificity of Sia addition to substrates. Consistent with the inverting SN2-like direct displacement reaction mechanism of STs, a base is required for deprotonation of a galactose (Gal) residue for the addition of Sia (Audry et al. 2011). SMVS residues, His370 in the ST6Gal-I and His319 in the ST3Gal-I, are shown to be in proximity of the acceptor substrates in the respective crystal structures and are likely to serve in this capacity (Kuhn et al. 2013; Meng et al. 2013; Rao et al. 2009). In fact, the regiospecificity of Sia addition is explained by the orientation of the acceptor oxygen on Gal (O3 for the ST3Gal-I and O6 for the ST6Gal-I) toward the respective catalytic His, while the other hydroxyl groups of the Gal are “locked” into their positions by specific hydrogen bonds in and around the enzyme active site. Moreover, mutation of these catalytic His residues in these proteins, as well as the analogous residues in polySTs, ST8Sia-II and ST8Sia-IV, results in complete inactivation of these enzymes (Jeanneau et al. 2004; Kitazume-Kawaguchi et al. 2001; Zapater and Colley 2012).
The polySTs and protein-specific polysialylation
The polySTs are unusual in that, while they can modify typical sialylated N- and O-linked glycans, polySia is found on a limited number of mammalian glycoproteins. These include, the neural cell adhesion molecule, NCAM (Finne et al. 1983; Hoffman et al. 1982; Rothbard et al. 1982), the synaptic cell adhesion molecule, SynCAM-1 (Galuska et al. 2010), neuropilin-2 (NRP-2) (Curreli et al. 2007), the C–C chemokine receptor type 7 (CCR7) (Kiermaier et al. 2016), E-selectin ligand-1 (Werneburg et al. 2016), the α subunit of the voltage-dependent sodium channel (James and Agnew 1987; Zuber et al. 1992), CD36 scavenger receptor in human milk (Yabe et al. 2003), and the polySTs themselves, which are capable of autopolysialylation (Close et al. 2000; Mühlenhoff et al. 1996). The protein specificity of polysialylation was suggested by the limited number of polyST substrates and the finding that free oligosaccharides, or proteins like fetuin that are not physiologically polysialylated, are poor polyST substrates in vitro (Angata et al. 2000).
Using NCAM as a model substrate, and subsequent studies with NRP-2, our laboratory has determined that polysialylation is indeed protein specific in that the polySTs must recognize the substrate through an initial protein–protein interaction that allows it to dock and then modify glycans in an adjacent domain [reviewed in Colley (2010), Colley et al. (2014) and Bhide et al. (2016)] (Fig. 3). For NCAM, the first fibronectin type III repeat (FN1) serves as the polyST recognition and docking site for the polysialylation of two N-glycans in the adjacent Ig5 domain (Close et al. 2003). Removing the FN1 domain from the full length NCAM molecule eliminates its polysialylation and its ability to bind to ST8Sia-IV (Mendiratta et al. 2005; Thompson et al. 2011). Likewise, the meprin-A5 protein-μ tyrosine phosphatase (MAM) domain of NRP-2 is required for the recognition and polysialylation of the O-glycans in the adjacent linker region (Bhide et al. 2016). Acidic residues in FN1 are key for NCAM polysialylation, and our data indicate that they bind to specific basic residues in the PBR of the polySTs (Mendiratta et al. 2005, 2006; Thompson et al. 2011, 2013). Similarly, mutation of specific acidic residues in the MAM domain of NRP-2 results in reduction in NRP-2 polysialylation by ST8Sia-IV. For NCAM polysialylation, after binding to the FN1 domain, ST8Sia-IV makes secondary contacts with the Ig5 domain that appear to stabilize the interaction to further promote polysialylation (Thompson et al. 2013). Similar secondary contacts have not been identified for NRP-2. In summary, studies of NCAM and NRP-2 polysialylation have established a two-step paradigm for protein-specific polysialylation that includes substrate recognition and docking of the enzyme on one domain and polysialylation of glycans on an adjacent domain (Fig. 3).
Fig. 3.

The protein-specific polysialylation of NCAM and NRP-2. The polysialylation of NCAM and NRP-2 requires that the polySTs recognize and bind an acidic patch or surface on the domain adjacent to the domain or region that carries the glycans to be polysialylated. NCAM consists of five immunoglobulin domains (Ig1–Ig5) and two fibronectin type III repeats (FN1 and 2). NRP-2 consists of two complement homology (CUB) domains, two coagulation factor V/VIII homology (F5/8) domains and one Meprin-A5 protein-μ tyrosine phosphatase (MAM) domain. N-glycans are shown as Vs, O-glycans are shown as horizontal lines, and polySia chains are shown in red. For NCAM polysialylation, either ST8Sia-II or ST8Sia-IV recognize and dock on an acidic patch on the first fibronectin type III repeat (FN1) to allow the polysialylation of two N-glycans in the adjacent Ig5 domain (left). Likewise, for NRP-2, acidic residues of the MAM domain are required for ST8Sia-IV to polysialylate O-glycans in the adjacent linker region between the MAM domain and the second F5/8 domain (F5/8 #2)
Recently, structural models of ST8Sia-IV were published by two groups that address the protein specificity of polysialylation and mechanism of polySia chain elongation (Volkers et al. 2015; Zhou et al. 2015). Three-dimensional modeling suggested that the previously made mutations in basic residues of the conserved PBR and PSTD sequences that resulted in loss of NCAM polysialylation may have altered the enzyme secondary structure and this hindered substrate recognition (Zhou et al. 2015). On the other hand, biophysical studies from our laboratory suggest a direct electrostatic interaction between residues in the NCAM FN1 domain and ST8Sia-IV PBR (Bhide et al. 2015). In another study, the structure of ST8Sia-IV was modeled based on the X-ray crystal structure of another α2,8-sialyltransferase, ST8Sia-III. This modeling, and docking of the previously solved NCAM Ig5-FN1 structure from our laboratory (Foley et al. 2010), suggested that the PBR and PSTD regions together form an extended basic groove providing both substrate recognition and an electropositive surface to guide the growing polySia chain (Volkers et al. 2015).
The cell biology of sialylation
Glycan sialylation in the secretory pathway requires that STs, their CMP-Sia nucleotide sugar donor and the appropriate substrates are colocalized in the Golgi (Fig. 4d). In this section, we will discuss the synthesis of Sia, CMP-Sia and sialylated glycans, including the unique localization of the CMP-Sia synthetase enzyme in the vertebrate nucleus, its transport into the Golgi via the CMP-Sia transporter (CST) and the mechanisms localizing the STs and other glycosyltransferases in the Golgi.
Fig. 4.
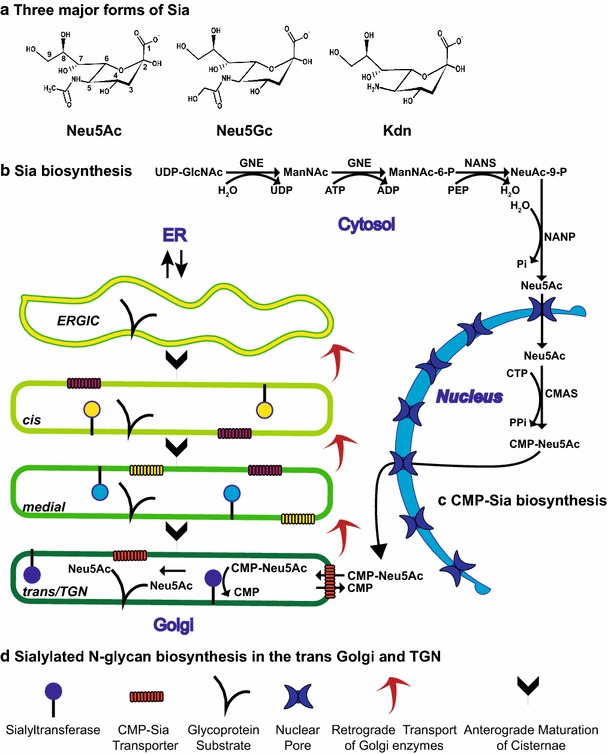
Sialic acid structure and the compartmentation of Sia, CMP-Sia and sialylated N-glycan biosynthesis. a Three major forms of Sia are shown. b The pathway for Neu5Ac biosynthesis in the cytosol. GNE, UDP-GlcNAc 2-epimerase/ManNAc Kinase; NANS, N-acetylneuraminic acid synthase; NANP, N-acetylneuraminic acid phosphatase. c The pathway for CMP-Sia biosynthesis in the nucleus is shown. Both Neu5Ac and CMP-Neu5Ac are small enough to flow into and out of the nucleus through nuclear pores. CMAS, CMP-sialic acid synthetase. d The pathway for sialylated N-glycan biosynthesis in the trans Golgi and TGN. According to the cisternal maturation model, Golgi enzymes are localized/retained in the Golgi by continuous retrograde transport in COPI-coated vesicles or tubules and cisternae-containing cargo proteins are “matured” by the sequential introduction of glycosylation enzymes. The sialyltransferase reaction in the Golgi trans cisternae and TGN is highlighted. CMP-Sia, like other nucleotide sugar donors, is transported into the Golgi by a specific CMP-Sia transporter (CST). It is then used in the transferase reaction with the release of CMP which is transported to the cytosol by the CST in exchange for CMP-Sia
The biosynthesis of sialic acids
The most abundant Sias in mammals are N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) (Fig. 4a). Two to three million years ago, humans lost the ability to synthesize Neu5Gc owing to a 92 base pair deletion in the cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) gene causing a frameshift and elimination of activity (Chou et al. 1998). However, Neu5Gc from dietary sources such as red meat can be incorporated in human sialoglycoproteins and gangliosides and this incorporation has recently been shown to promote inflammation and tumor progression (Samraj et al. 2015). Kdn (2-keto-3-deoxy-d-glycerol-d-galacto-nononic acid) is another form of Sia that has a hydroxyl group instead of acetyl amino group at C5 (Fig. 4a). It is overexpressed in numerous cancers, possibly due to hypoxia-mediated changes in expression level of Kdn processing enzymes [reviewed in Pearce and Läubli (2016)]. Acetylated Sias play important roles in embryogenesis, development and immunological processes (Mandal et al. 2015). Additionally, sulfated Sias, found on gangliosides in mammals, have been reported but not well studied (Kitajima et al. 2015).
The biosynthesis of Neu5Ac is a highly regulated process and begins with the glycolysis product, fructose-6-phosphate, which is diverted to the synthesis of hexosamines by the enzyme glutamine fructose 6-phosphate amidotransferase (Hinderlich et al. 2015; Varki and Schauer 2009). Following the acetylation of the free amine group of glucosamine-6-phosphate (GlcNH2-6-P) to form N-acetylglucosamine-6-phosphate (GlcNAc-6-P), an epimerization reaction converts GlcNAc-6-P to GlcNAc-1-phosphate (GlcNAc-1-P) which is then condensed with UTP to form UDP-GlcNAc. Subsequently, the action of the bifunctional enzyme, UDP-GlcNAc 2-epimerase/ManNAc Kinase (GNE), commits UDP-GlcNAc to the Sia biosynthesis pathway (Fig. 4b).
In the first step, GNE catalyzes the hydrolysis of UDP-GlcNAc to UDP-ManNAc, which is then epimerized to form free ManNAc (Fig. 4b). CMP-Sia is a negative feedback inhibitor of the epimerization step, and its levels serve to regulate the amount of Sia synthesized by the cell (Weiss et al. 1989). In the second GNE-catalyzed step, ManNAc is phosphorylated to form ManNAc-6-phosphate. Consistent with its role as the “master regulator” of Sia biosynthesis, eliminating GNE expression in mice leads to a complete loss of sialylation and embryonic lethality, highlighting the importance of Sia in development (Schwarzkopf et al. 2002). Mutations in Gne have also been shown to be responsible for sialuria, a rare disease in which Sia accumulates due to a loss of GNE feedback inhibition by CMP-Sia; this disease is characterized by delayed skeletal muscle development and microcytic anemia (Seppala et al. 1999; Weiss et al. 1989). In addition, other mutations in Gne have been shown to cause a rare, autosomal recessive form of progressive muscular dystrophy called GNE myopathy or hereditary inclusion body myopathy (HIBM) (Eisenberg et al. 2001).
Free cytosolic Sia (Neu5Ac) is generated from ManNAc-6-P in two steps (Fig. 4b). First N-acetylneuraminic acid synthase (NANS) catalyzes the condensation of ManNAc-6-P with phosphoenol pyruvate to produce Neu5Ac-9-phosphate (Neu5Ac-9-P). Second, N-acetylneuraminic acid phosphatase (NANP) removes the phosphate group from Neu5Ac-9-P to generate Neu5Ac (Varki and Schauer 2009). Notably, biallelic mutations in NANS gene were recently shown to be responsible for severe developmental delay and skeletal dysplasia in infants (van Karnebeek et al. 2016). The levels of free Sia in both prokaryotes and eukaryotes are likely controlled by cytosolic sialate pyruvate-lyases that hydrolyze Neu5Ac to ManNAc and pyruvate (Schauer et al. 1999).
The biosynthesis and transport of CMP-Sia
Cytidine monophosphate-N-acetylneuraminic acid (CMP-Sia) is the nucleotide sugar donor used in all ST reactions. Free Sia is activated by the transfer of cytidine monophosphate (CMP) from CTP to the hydroxyl group at C2 by CMP-Sia synthetase (CMAS) (Fig. 4c) (Sellmeier et al. 2015). Importance of CMP-Sia was demonstrated in CMAS-knockout mice where it was shown to cause kidney failure and death three days after birth (Weinhold et al. 2012). Unlike other vertebrate sugar activating enzymes that are localized in the cytosol, the CMAS is localized in the nucleus (Sellmeier et al. 2015). Mouse CMAS possesses a key monopartite nuclear localization signal (K198RPRR) that also contains residues important for catalytic activity. Mutagenesis studies demonstrated that activity and nuclear localization are separable and that nuclear localization is not required for activity [reviewed in Sellmeier et al. (2015)]. Why CMAS enzymes from all vertebrate species studied are localized in the nucleus in the steady state is unclear, but not surprisingly, they also have nuclear export signals that allow them to enter the cytoplasm for import into the Golgi. Interestingly, the CMAS from Drosophila melanogaster is a membrane protein localized in the Golgi (Viswanathan et al. 2006). The reasons for this differential localization remain unknown.
Following the synthesis of CMP-Sia in the nucleus, it makes its way into the cytoplasm and then is transported into the Golgi cisternae to be used by STs (Fig. 4d). Nucleotide sugars that serve as donors in glycosyltransferase reactions are imported into the Golgi lumen by specific transporters (reviewed in Caffaro and Hirschberg (2006); Hirschberg et al. (1998); Zhao and Colley (2008)). The CMP-Sia transporter (CST) is a type III (multi-spanning) transmembrane protein (10 TM regions) localized in medial–trans Golgi, which functions as an antiporter to import CMP-Sia into the Golgi in exchange for CMP (Zhao et al. 2006). A CHO cell mutant, Lec2, has a defect in CST function and is widely used to study transporter function and the role of sialylation in glycoprotein and glycolipid function (Eckhardt et al. 1998). The creation of CST mutants and chimeric proteins using regions from the UDP-Gal transporter has identified specific TM regions and residues critical for the specificity and mechanism of CMP-Sia transport [reviewed in Hadley et al. (2014), Zhao and Colley (2008)]. While a four-amino acid sequence (IIGV) at the carboxy-terminus of the CST is required for its ER export (Zhao et al. 2006), little is known about the Golgi localization mechanism of mammalian nucleotide sugar transporters. Interestingly, a GDP-Man transporter in yeast, Vrg4p, possesses membrane proximal lysine residues in its carboxy-terminal tail (KQKK) that bind to COPI coats on transport vesicles to maintain its Golgi localization (Abe et al. 2004). A similar carboxy-terminal motif is not found in the mammalian CST.
The biosynthesis of sialylated N-glycans in the Golgi
The N-linked glycosylation of proteins is initiated in the endoplasmic reticulum (ER) with the en bloc transfer of a preformed oligosaccharide structure to the consensus sequence Asn-X-Ser/Thr. This structure is subsequently processed by glycosidases and glycosyltransferases in the ER and Golgi [reviewed in Moremen et al. (2012)] (please see the articles by Corfield (2017) and Roth and Zuber (2017) in this theme issue for a review of this process). While the glycosylation enzymes in the Golgi are localized in the cisternae essentially in the order in which they act to modify the new glycans, there is distinct overlap (Rabouille et al. 1995). Sias are typically added to N-glycans on proteins in the trans most cisternae and trans Golgi network (TGN) of the Golgi. In fact, the TGN (then called the trans tubular network) was first visualized in rat hepatocytes by Roth and colleagues (Roth et al. 1985) using an antibody against the ST6Gal-I. In these cells, the ST6Gal-I was found in both the trans cisternae and TGN; however, later work demonstrated that the distribution of this ST diverged in different cell types (Roth et al. 1986). Specifically, these investigators demonstrated that the ST6Gal-I is localized in the trans cisternae of the Golgi in intestinal goblet cells, but across all Golgi cisternae, with the exception of the “fenestrated first cis cisterna” in adjacent intestinal absorptive cells.
Glycosylation enzyme localization in the Golgi
Studies by many groups over the last 20 years have concluded that multiple mechanisms serve to localize glycosylation enzymes in the Golgi [reviewed in Banfield (2011), Colley (1997), Derby and Gleeson (2007)]. Following the cloning of the coding sequences of several glycosyltransferases, including the ST6Gal-I, investigators began to evaluate what sequences were required for the localization of these enzymes in the Golgi. At that time (mid-1980s), the prevailing view was that the Golgi was comprised of a series of “stable” cisternae that contained specific glycosylation enzymes (Kornfeld and Kornfeld 1985). In the “stable compartments/vesicular transport model,” cargo from the ER entered the Golgi at its cis face, traversed the cisternae in vesicular carriers and exited to post-Golgi compartments from the trans face. Each cisterna or group of cisternae was defined by the presence of specific glycosylation enzymes. Cargo proteins were envisioned to move in an anterograde fashion between compartments in COPI-coated vesicles, and glycoproteins modified sequentially by the glycosylation enzymes resident in each cisterna (Glick and Luini 2011; Glick and Nakano 2009). At this time, investigators searched for sequences in the Golgi glycosyltransferases that allowed their retention in the Golgi cisternae.
This stable compartments/vesicular transport mechanism of movement through the Golgi came into question when researchers noticed that cells could transport cargo that is too large to fit into small transport vesicles (Bonfanti et al. 1998; Mironov et al. 2001), and evidence suggested that COPI vesicles functioned in retrograde rather than anterograde transport (Rabouille and Klumperman 2005). This brought the field back to the cisternal progression or “cisternal maturation model” that was supported by the early studies of morphologists [reviewed in Mollenhauer and Morré (1991)] and has been supported by recent studies by several groups [for example, Losev et al. (2006), Matsuura-Tokita et al. (2006)]. In this mechanism, a new cisterna forms at the cis face of the Golgi by the combined anterograde transport of protein cargo from the ER in COPII-coated vesicles and the retrograde transport of cis Golgi enzymes in COPI-coated vesicles. This cisterna and its cargo “mature” as medial and then trans Golgi enzymes are transported in a retrograde fashion from earlier cisterna. At the level of the TGN, cargo is sorted into different carriers for constitutive or regulated secretion, transport to the endosome/lysosome system, or for return to earlier portions of the Golgi. It is possible that both mechanisms function in the same cell and that tubules as well as vesicles participate in movement of membrane and protein between compartments (Glick and Luini 2011; Mollenhauer and Morré 1991). With the reemergence of the cisternal maturation model, researchers began to consider the possibility that the localization of Golgi glycosyltransferases depended upon their incorporation into retrograde transport vesicles.
On this backdrop, investigators have generally concluded that Golgi glycosylation enzymes are localized in the Golgi via multiple mechanisms that may include sequences in different parts of the protein, namely the TM region (bilayer thickness mechanism), lumenal sequences (oligomerization/kin recognition mechanism) and cytoplasmic tail (COPI vesicle incorporation) (Fig. 2c) [reviewed in Banfield (2011)]. The ST6Gal-I has been used extensively as a model for these studies. Munro first noted that Golgi proteins on average had shorter TM regions than plasma membrane proteins (Munro 1991, 1995a) and that the width of the membrane increases along the secretory pathway, from ER to Golgi to plasma membrane, due to an increase in the concentration of cholesterol in the membrane (Bretscher and Munro 1993). These observations led him to propose the “bilayer thickness model” that suggested that Golgi proteins were not able to partition into carriers moving toward the plasma membrane because of their shorter TM regions (Munro 1998). This model was tested by altering the TM region of the full length ST6Gal-I and creating chimeric proteins. It was concluded that the 17-amino acid TM region and flanking sequences are critical for Golgi localization and that the length rather than amino acid composition of this region was essential (Dahdal and Colley 1993; Munro 1991, 1995b). On the other hand, it was also observed that fusing the ST6Gal-I cytoplasmic tail and TM region onto two different reporter proteins was not able to completely retain these chimeric proteins in the Golgi, suggesting that the enzyme’s lumenal sequences might mediate another process that increased retention efficiency (Dahdal and Colley 1993).
Machamer and her colleagues provided support for an oligomerization-based mechanism for the cis Golgi localization of the coronavirus E1 protein (Swift and Machamer 1991; Weisz et al. 1993). Their oligomerization model was extended and elaborated upon for Golgi glycosylation enzymes by Nilsson and colleagues (Nilsson et al. 1994, 1996) who provided evidence that enzymes residing in the same cisterna, such as the medial Golgi enzymes, GlcNAcT1 and mannosidase II, form “kin” complexes. These observations gave rise to the “kin recognition hypothesis” where hetero-oligomers form between enzymes in the same Golgi cisterna via their TM and lumenal sequences to prevent their incorporation into transport vesicles and movement to later compartments (Nilsson et al. 1993). Work by a number of other laboratories implicated oligomerization as a potential mechanism for glycosylation enzyme Golgi localization (Chen et al. 2000; Fenteany and Colley 2005; Opat et al. 2000). Differences in trafficking behavior between two isoforms of the ST6Gal-I, STtyr and STcys, which have a single-amino acid difference in their catalytic domain at position 123, suggested that oligomerization is a major factor in the stable Golgi localization of this ST. Both isoforms are equally active (Chen et al. 2003); however, the STcys is stably Golgi localized, while the STtyr is transiently Golgi localized and moves to a post-Golgi compartment where it is cleaved and secreted (Ma et al. 1997). Stable Golgi localization was correlated with pH-dependent oligomer formation; 100% of the STcys, and only 13% of the STtyr formed Triton-insoluble oligomers at pH 6.3 (pH of late Golgi), and neither isoform formed oligomers when membranes were solubilized at pH 8.0 (Chen et al. 2000). More recent studies provided support for the kin recognition model by demonstrating that Golgi N-linked and O-linked glycosylation enzymes form pH-dependent oligomers (Hassinen et al. 2011). Using FRET and FRAP experiments, this group found that the medial Golgi enzymes GnTI and GnTII, and the trans Golgi enzymes GalT-1 and ST6Gal-I, individually form homodimers in the ER and then form cisterna-specific, pH-dependent, hetero-oligomers in the Golgi (GnT-I/GnT-II and GalT-I/ST6Gal-I) (Rivinoja et al. 2012).
A more complete picture of the mechanisms involved in ST6Gal-I Golgi localization was revealed when we simultaneously evaluated the role of the cytoplasmic tail, TM region and oligomerization in STcys Golgi localization (Fenteany and Colley 2005). Individually altering the sequence or length of the TM region or deleting the core amino acids of the cytoplasmic tail did not alter the stability of STcys Golgi localization or its oligomerization. However, making both of these changes simultaneously led to impaired oligomerization and movement of the STcys to compartments where it was cleaved and secreted. We concluded that the ST6Gal-I STcys isoform is localized by mechanisms independently mediated by its cytoplasmic tail and TM region and that oligomerization was likely a secondary event that resulted after a concentration of this protein in the Golgi.
With the reemergence of the cisternal maturation mechanism for protein transport through the Golgi, the cytoplasmic tails of glycosylation enzymes would be expected to play important roles in their incorporation into retrograde COPI transport vesicles. Golgi glycosylation enzymes have been found in COPI vesicles, but how they are incorporated into these vesicles was not clear because their cytoplasmic tails lacked known COPI-binding motifs (Nilsson et al. 2009). Work done by Banfield and colleagues identified a peripheral membrane protein, Vps74p, that binds as a sorting receptor to COPI coats and to a pentameric sequence in the cytoplasmic tails of yeast Golgi glycosyltransferases (Tu et al. 2008). The mammalian homolog of Vps74p, GOLPH3, was later shown to bind to coatomer (Tu et al. 2012) and the cytoplasmic tails of both the core-2 GlcNAcT-I (C2GnT-I) (Ali et al. 2012) and the protein O-linked mannose-1,2-GlcNAcT-1 (POMGnTI) (Pereira et al. 2014) to control their Golgi localization. A LLRRR motif in the cytoplasmic tail of C2GnT-1 was shown to be required for GOLPH3 binding (Ali et al. 2012). Further studies demonstrated that GOLPH3 allows the incorporation of both C2GnT-1 and ST6Gal-I, but not GalT-1, into COPI-coated vesicles (Eckert et al. 2014). GOLPH3 is a phosphatidylinositol 4-phosphate-binding protein and is enriched in the TGN due to the prevalence of this phospholipid in this compartment. Consequently, it has been hypothesized that GOLPH3 interactions and COPI vesicle incorporation serve to prevent glycosyltransferases from escaping the Golgi and moving to the cell surface (Eckert et al. 2014). As GOLPH3-binding sequences, which consist of conjoined hydrophobic and basic sequences (LKKK in ST6Gal-I (predicted), LLRRR in C2GnT-1), are not found in all Golgi enzymes, it has been suggested that the alterations in the expression of GOLPH3 may also variably regulate Golgi enzyme localization and the types of glycans expressed by cells (Eckert et al. 2014).
The integrity of the secretory pathway and glycosylation are also tightly linked via the COG complex. The role of the COG complex in maintaining Golgi structure and efficient glycosylation was appreciated when several congenital disorders of glycosylation (CDGs) were identified that were the result of mutations in various COG subunits [reviewed in Reynders et al. (2011), Rosnoblet et al. (2013)]. The COG complex is an eight-subunit heteromeric complex organized in two lobes (A and B) that serves as a tethering complex for the interaction of incoming vesicles with target membranes in COPI-mediated retrograde transport in the Golgi and between the Golgi and ER. Mutations in COG subunits lead to changes in Golgi structure, deficits in glycan synthesis, and alterations in retrograde trafficking and the localization and/or stability of Golgi proteins, including glycosylation enzymes [reviewed in Rosnoblet et al. (2013)]. Mislocalization of several Golgi glycosylation enzymes has been observed when lobe A and B subunits are depleted. Notably, Peanne et al. (2011) observed that the stability of GalT1 and ST6Gal-I was compromised in cells depleted of lobe B subunits (Peanne et al. 2011). However, more recent work suggests that each COG subunit is essential for the complex’s function (Bailey Blackburn et al. 2016).
Removal of sialic acid from glycoconjugates
Four neuraminidases in mammalian cells, NEU1, 2, 3 and 4, have been shown to cleave α-linked Sia residues from glycoconjugates. These enzymes, also called sialidases, exhibit differences in cellular localization and substrate specificity [reviewed in Miyagi and Yamaguchi (2012), Monti et al. (2010), Pearce and Läubli (2016)]. They are characterized by two types of conserved sequences: the aspartate box (-Ser-X-Asp-X-Gly-X-Thr-Trp-), which can appear multiple times in each enzyme’s sequence, and the RIP sequence (-Phe/Tyr-Arg-Ile-Pro-) (Miyagi and Yamaguchi 2012). NEU1 is found in the lysosome and on the cell surface and is the most highly expressed of this sialidase family, but shows the least conservation with the other members. It exclusively acts on glycoproteins and preferentially cleaves α2,3 linkages over α2,6 or α2,8 linkages (Miyagi and Tsuiki 1984). Interestingly, in lysosomes it is found in a complex with carboxypeptidase protective protein/cathepsin A (PPCA) and β-galactosidase that is key for its activity and localization (Bonten et al. 2009; d’Azzo et al. 1982; Galjart et al. 1988). Mutations in NEU1 DNA lead to the lysosomal storage disease, sialidosis, while a defect in PPCA leads to a combined deficiency in both NEU1 and β-galactosidase activity, called galactosialidosis (d’Azzo and Bonten 2010). NEU1 traffics to the cell surface in exovesicles following cell stimulation (lysosomal exocytosis), and interestingly, NEU1 itself controls lysosomal exocytosis by controlling LAMP1 sialylation (Machado et al. 2015). In addition, NEU1, released from cells in exosomes, was shown to trigger loss of polySia from the surface of Ra2 microglial cells or Neuro2A neuroblastoma cells co-cultured with Ra2 cells in response to an inflammatory stimulus (lipopolysaccharide). Chemical inhibition of NEU1 using Sia analog GSC-649 or its knockdown rescued this shedding of polySia (Sumida et al. 2015). In addition, NEU1 is believed to control several aspects of the immune response by the desialylation of key molecules, such as Toll-like receptor 4 (TLR4) and adhesion molecules involved in the recruitment of leukocytes to inflammatory sites (Miyagi and Yamaguchi 2012). NEU2 is localized in the cytosol, has a broad cleavage specificity, has a neutral pH optimum and has been suggested to act on cytosolic free glycans arising from non-lysosomal breakdown (Miyagi and Yamaguchi 2012). NEU3 is localized to plasma membrane and exclusively hydrolyzes Sias on gangliosides, preferring either the α2,3- or α2,8-linkages found in GM3 and GD3 gangliosides. Interestingly, the ability of NEU3 to convert GT1b and GD1a to monosialic GM1 plays a role in axon growth and regeneration (reviewed in (Higuero et al. 2017) in this issue). See also the article by Kopitz (2017) in this issue for more information on glycolipids and the article by Kaltner et al. (2017) in this issue on the functional consequences of galectin binding to glycolipids once Sia is removed. Two isoforms of NEU4 have been reported, and they are believed to direct the localization of NEU4 to lysosomes, mitochondria or the ER in different tissues. NEU4 is believed to act on gangliosides as well as sialyl Lewisx (sLex) and sialyl Lewisa (sLea) antigens (Läubli and Borsig 2010b; Miyagi and Yamaguchi 2012; Pearce and Läubli 2016).
While all four neuraminidases have been implicated in cancer progression, NEU1 and NEU3 have been most well studied (Miyagi et al. 2012; Miyagi and Yamaguchi 2012; Pearce and Läubli 2016). One group provided evidence that NEU1 is a positive regulator of the epithelial-to-mesenchymal transition (EMT) and cancer progression in pancreatic cancer via its desialylation of the EGF receptor (Gilmour et al. 2013). In contrast, another group provided evidence that NEU1 is a negative modulator of metastasis in colorectal cancer (Uemura et al. 2009). The latter observation appears to be more in line with the upregulation of STs in cancer and the resulting anti-apoptotic and pro-metastatic effects of increased sialylation (see below). Most recently, d’Azzo and colleagues demonstrated that the low NEU1 levels in metastatic sarcomas promote lysosomal exocytosis of lysosomal hydrolases which in turn enhances ECM degradation and invasion (Machado et al. 2015). The ganglioside-specific sialidase activity of NEU3 has been demonstrated to stimulate a number of signaling pathways to increase proliferation and block apoptosis in cancer (Pearce and Läubli 2016). The roles of NEU2 and NEU4 in cancer are summarized in (Miyagi and Yamaguchi 2012; Pearce and Läubli 2016).
Roles of sialic acid in health and disease
Sia is a unique monosaccharide. With its negative charge at physiological pH, it is comparable to a phosphate group, albeit larger, and also can be modified at several positions by acetyl, sulfate and other groups (Schauer 2009; Varki and Schauer 2009). As a result, Sia can impact protein conformation and oligomerization, and the interactions of proteins with other proteins and the extracellular matrix. Not surprisingly, Sia expression is dynamic, changes during development and is altered in numerous diseases including immune disorders and cancer (Dall’Olio et al. 2014; Pearce and Läubli 2016; Varki and Gagneux 2012). Sialylated glycans are binding partners for lectins that control key processes in health and disease (selectins, Siglecs, viral cell attachment proteins), impact the ligand binding and signaling of receptors (β1-integrin) and regulate cell migration during development and in cancer. These functions of Sia, with a particular focus on those involving sialylated N-linked glycans, will be discussed below.
Sia and its binding proteins in viral infection
Sia on both glycoproteins and glycolipids (gangliosides) is used as an entry receptor by many different viruses including influenza viruses, adenoviruses, coronaviruses, rotaviruses, toroviruses and reoviruses [reviewed in Matrosovich et al. (2015), Neu et al. (2011), Stencel-Baerenwald et al. (2014)]. Binding to host cell receptors, followed by membrane fusion (either plasma membrane or endosomal membrane), allows viruses to deposit their genetic material in host cells and replicate (Fig. 5). The influenza A and B viruses have two Sia recognition molecules: the hemagglutinin (HA) that recognizes the sialylated glycoconjugate on the host cell surface and possesses sequences essential for membrane fusion, and the neuraminidase (NA), an acetylesterase that cleaves Sia and is critical for virion release following viral replication (Fig. 5). In contrast, for influenza C the ability to recognize its receptor (9-O-acetylated Sia), to fuse with the membrane and to cleave Sia is all combined in a hemagglutinin-esterase-fusion (HEF) protein (Matrosovich et al. 2015). Other viruses, such as coronaviruses, toroviruses and Newcastle disease virus have hemagglutinin-esterase (HE) or hemagglutinin-neuraminidase (HN) proteins, respectively (Neu et al. 2011). Both the influenza viruses and type 3 reoviruses use sialylated N-glycans as receptors (Stencel-Baerenwald et al. 2014). For type 3 reoviruses, a cell attachment protein, σ1, recognizes Sia allowing tethering to the host cell membrane and secondary binding by the junctional adhesion molecule, JAM-A (JAM-1) (Matrosovich et al. 2015; Stencel-Baerenwald et al. 2014). The structure of the reovirus σ1 in complex with α2,3-, α2,6- and α2,8-sialyllactose has been recently solved (Reiter et al. 2011). Below, we discuss in more detail Sia binding by influenza HA and NA.
Fig. 5.
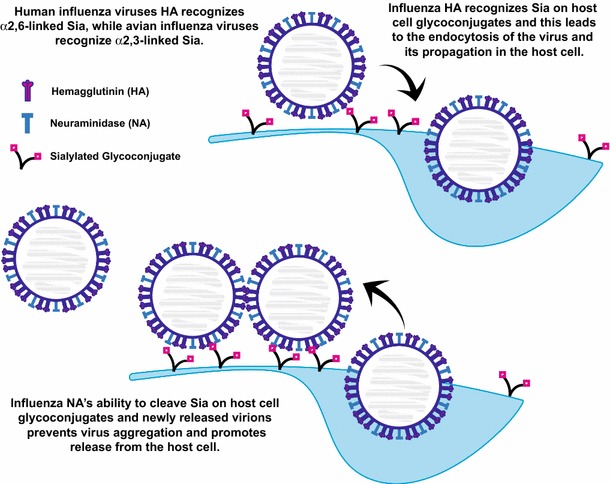
Influenza virus hemagglutinin and neuraminidase recognize Sia residues on host cell glycoconjugates. The hemagglutinin (HA) proteins on the influenza virus membrane recognize Sia residues on host cell surface glycoconjugates. This interaction allows the endocytosis of the virus and its propagation inside the host cell. Release of the newly made virions from the host cell can be hampered by HA–Sia interactions between the host cell surface glycoconjugates and the virus. Sia-mediated interactions between viruses can also cause aggregation at the host cell surface. The viral neuraminidase (NA) cleaves Sia allowing the release of the virus from the host cell and the dispersion of aggregates. Many influenza drugs are designed to block the activity of the NA. Influenza viruses that infect humans recognize α2,6-linked Sia, while influenza viruses that recognize avian species recognize α2,3-linked Sia
The influenza HA is a homotrimeric receptor, is responsible for both host recognition and membrane fusion and is essential for viral entry and replication (Fig. 5) (Skehel and Wiley 2002). The HA proteins from influenza viruses that infect humans and birds recognize distinct Sia linkages, with avian viruses binding preferentially to α2,3-linked Sia and human viruses binding preferentially to α2,6-linked Sia (Rogers and Paulson 1983; Rogers et al. 1983). Avian viruses are believed to be transmitted orally, while human viruses enter through the respiratory track (Wilks et al. 2012). Consistent with this notion, α2,3-linked Sia is found in bird’s intestinal epithelium and only in the lower respiratory tract of humans, while α2,6-linked Sia is enriched on bronchial epithelium of the human upper respiratory tract (Shinya et al. 2006). Others have demonstrated a further distinction between avian and human viruses and shown that avian viruses infect ciliated cells while human viruses infect non-ciliated cells in the airway epithelium (Matrosovich et al. 2004a, 2007). In 1988, Weis and colleagues reported the structure of the HA in complex with Sia and identified the Sia-binding pocket and key hydrogen bonds stabilizing this interaction (Weis et al. 1988).
Influenza pandemics that lead to significant loss of life typically result when a highly virulent avian-like virus infects humans (Taubenberger and Kash 2010; Wilks et al. 2012). This can result from the oral transmission of the avian virus to humans, and/or reassortment of genetic segments when an avian and human virus infects the same host (for example, swine) that leads to a “human-specific” HA being paired with other proteins from an avian strain, or the accumulation of mutations in avian HAs that make them “human adapted” (Taubenberger and Kash 2010; Wilks et al. 2012). To this point, an interesting study by Chandrasekaran et al. (2008) evaluated the sialylated structures recognized by human-adapted H1N1 and H3N2 viruses in contrast with the avian influenza virus H5N1. Evaluation of the glycan structures on a human upper respiratory epithelial cell line revealed a preponderance of α2,6-sialylated structures, many with multiple lactosamine repeats. Structural evaluation showed that all three monosaccharides in the Neu5Acα2,3Galβ1,4GlcNAc structure form contacts with the HA-binding site and that these sugars form a cone-like structure. In contrast, the binding contacts of a Neu5Acα2,6Galβ1,4GlcNAc structure allow more flexibility and suggest that this glycan can assume not only a cone-like structure but also an umbrella-like structure in the HA-binding pocket. They also predicted that the length and branching of the oligosaccharide will influence the HA contacts made by α2,6-sialylated structures permitting longer α2,6-Sia terminated glycans to form an umbrella-like structure, while shorter α2,6-Sia terminated glycans to form a cone-like structure. Reevaluation of HA–glycan co-crystal structures, as well as direct binding assays, demonstrated that the two human-adapted HAs (H1 and H3) had accumulated mutations that allowed them to make more contacts with α2,6-Sia in the umbrella-like form, and to bind to upper respiratory tract tissues, and in particular the apical membranes of tracheal tissue sections that are enriched in α2,6-sialylated glycans with longer branches (Chandrasekaran et al. 2008). However, a simple alteration of HA specificity in the avian virus is likely not the whole story since other viral proteins including the NA are key regulators of infection and virulence (Matrosovich et al. 2004b; Taubenberger and Kash 2010).
Influenza virions bud from the surface of the host cell, but many times aggregate or get trapped by binding to surface Sia. They are released from the cell by a tetrameric NA that is also found on the viral capsid (Fig. 5) (Matrosovich et al. 2015; Wilks et al. 2012). NA inhibitors such as oseltamivir (Tamiflu), zanamivir (Relenza) and peramivir (Rapivab) have been developed to treat influenza infections (Burnham et al. 2013). Studies of drug-resistant influenza strains have demonstrated that a balance between the contrasting activities of HA and NA dictates viral growth, transmission and host adaptation (Baigent and McCauley 2001; Gaymard et al. 2016b). For example, oseltamivir-resistant strains isolated after 2007 exhibited a particular mutation in NA, which coexisted with a new variant of HA, leading to a virus with better “viral fitness” likely due to a readjusted HA–NA balance (Gaymard et al. 2016a; Rameix-Welti et al. 2008).
Siglecs and their role in the immune response
Siglecs are another family of Sia-binding lectins that are implicated in a variety of immune cell diseases (Macauley et al. 2014; O’Reilly and Paulson 2009). Here we will briefly introduce the Siglec family and describe how the interaction of two family members (Siglec-2 or CD22 and Siglec-10 or Siglec-G in mouse) with their sialylated ligands modulates the B cell response and autoimmunity (Fig. 6).
Fig. 6.
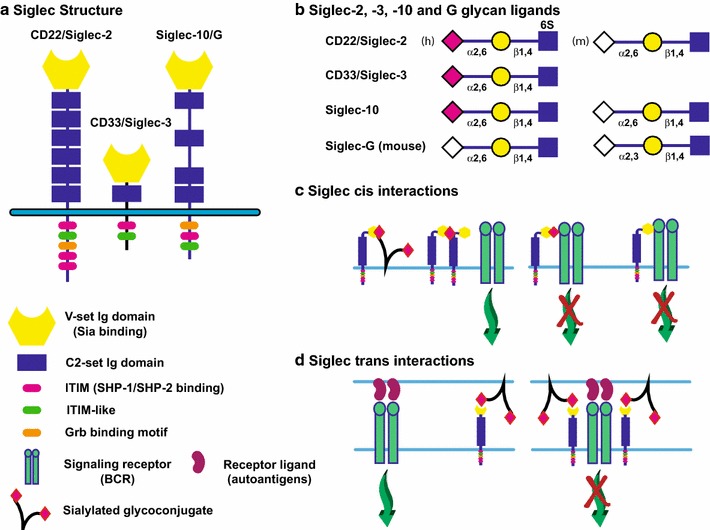
Siglecs and their role in the regulation of the immune response. a Siglecs share a common structure with an N-terminal V-set Ig domain that recognizes Sia, followed by a variable number of C2-set Ig domains, a transmembrane region and cytoplasmic tail. The cytoplasmic tail of Siglecs contains a number of binding sequences. Inhibitory Siglecs contain immunoreceptor tyrosine-based inhibitory motif (ITIM) sequences that allow SHP-1 and SHP-2 binding and inhibition of signaling, in addition to other signaling molecule-binding sequences. Activating Siglecs (not shown) possess a basic residue in their TM regions that allow the binding of DAP12, an immunoreceptor tyrosine-based activation motif (ITAM) adaptor protein that promotes the activation of signaling. Examples of Siglecs that bind sialylated N-glycan structures are shown. b The sialylated ligands recognized by selected Siglecs are shown. Siglec-10 (human) and Siglec-G (mouse) are considered orthologs; however, the human protein recognizes only α2,6-linked Neu5Ac (pink diamond) or Neu5Gc (white diamond) structures, while the mouse Siglec recognizes both α2,3- and α2,6-linked Sia in the Neu5Gc form. c Siglecs can bind ligands in cis. An inhibitory Siglec can be sequestered away from an activating receptor by binding to other sialylated ligands, including itself (left). Conversely, binding to an activating receptor directly or via Sia residues on its glycans can inhibit its signaling (right). d Siglecs can bind ligands in trans. These interactions can either sequester the Siglec away from an activating receptor (left), or if sialylated ligands are close to the ligands for the activating receptor, this interaction may enhance the ability of the Siglec to inhibit the activating receptor (right). Please see Varki and Crocker (2009), Macauley et al. (2014) for more details on Siglec structure and function
Siglecs are type I membrane proteins and immunoglobulin (Ig) superfamily (IgSF) proteins with multiple Ig domains including a single N-terminal V-set Ig domain for Sia ligand binding linked to 1–17 C2-set Ig domains (see Fig. 6a for examples) (Macauley et al. 2014; Varki and Crocker 2009). As lectins of the IgSF, Siglecs are also categorized as I-type lectins (Varki and Crocker 2009). A number of Siglecs have been identified in humans that fall into two groups. The first group is conserved across mammals and includes Siglec-1 (sialoadhesin), Siglec-2 (CD22), Siglec-4 (MAG) and Siglec-15. The second group is equivalent to the CD33 (Siglec-3) family (five identified in mice, nine identified in humans). This second group of Siglecs is not ubiquitously found in all mammals and shows rapid evolution by gene duplication, conversion, exon loss and exon-shuffling events (Angata et al. 2004; Padler-Karavani et al. 2014). Siglecs exhibit specificity for distinct sialylated glycan structures; however, when similar specificities exist, the comparable Siglecs may be expressed in different cell types and/or as part of an inhibitory/activating Siglec pair (see below). For a complete list of glycan structures bound by various human and mouse Siglecs, and the cell types in which they are expressed, please refer to Macauley et al. (Macauley et al. 2014).
Siglecs function in the innate and adaptive immune system to allow the discrimination between “self” and “nonself” (Macauley et al. 2014). As Sia-binding proteins, they effectively recognize “self” markers on the surfaces of cells in a cis (same cell) or trans (adjacent cell/organism) fashion, leading to the modulation of the immune response and preventing autoimmunity (Fig. 6c, d). Pathogens like group B Streptococcus exploit this mechanism for immune response downregulation by coating themselves in “self”-like sialylated glycans. The cytoplasmic tails of what are known as “inhibitory” Siglecs have immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which recruit phosphatases SHP-1 and SHP-2 to negatively regulate downstream signaling pathways (Fig. 6a) (Avril et al. 2004; Paul et al. 2000). In contrast, “activating” Siglecs have a positively charged residue in their transmembrane region that can be bound by the immunoreceptor tyrosine-based activation motif (ITAM) adaptor protein, DAP12, leading to recruitment of the SYK kinase to activate downstream signaling pathways (Blasius et al. 2006; Macauley et al. 2014). The evolution of “activating” Siglecs (Siglec-14, Siglec-15 and Siglec-16) and their murine counterpart (Siglec-H) was suggested to be a response to pathogens exploiting inhibitory Siglecs to suppress host immune response. In support of this idea, most humans express two pairs of Siglecs, with each pair exhibiting the same glycan-binding activity but with different functional activities. For example, Siglec-5 (inhibitory) is expressed with Siglec-14 (activating), while Siglec-11 (inhibitory) is expressed with Siglec-16 (activating) (Macauley et al. 2014).
Peripheral B cells express both CD22 (Siglec-2) and Siglec-10 (Siglec-G in mice) that work together to prevent the generation of self-reactive B cells and autoantibodies by inhibiting the signaling downstream of the B cell receptor (BCR), thereby contributing to a process called B cell tolerance [reviewed in Macauley et al. (2014), Paulson et al. (2012)]. Human CD22 recognizes Neu5Acα2,6Galβ1,4(6S)GlcNAc (6S indicates sulfation of C6 hydroxyl of GlcNAc residue), while mouse CD22 recognizes the unsulfated structure containing Neu5Gc (Fig. 6b). Human Siglec-10 recognizes Neu5Acα2,6Galβ1,4GlcNAc, while mouse Siglec-G recognizes both Neu5Gcα2,6Galβ1,4GlcNAc and Neu5Gcα2,3-Galβ1,4GlcNAc (Fig. 6b) [reviewed in Macauley et al. (2014)]. Both of these Siglecs are inhibitory and possess ITIMs and other signaling molecule-binding sites in their cytoplasmic tails.
Experiments in mouse models have established the role of CD22 and Siglec-G in immune system regulation. CD22-knockout mice exhibit a hyper-responsive immune system due to an inability to inhibit BCR-induced Ca2+ signaling (Nitschke 2005; Nitschke et al. 1997; O’Keefe et al. 1996; Otipoby et al. 1996; Sato et al. 1996). Siglec-G-knockout mice exhibit an enhanced B1 cell lifespan and an altered BCR repertoire (Jellusova et al. 2010a). A double knockout of both of these Siglecs in mice leads to systemic autoimmunity (Jellusova et al. 2010b). On the other hand, the ST6Gal-I-knockout mouse that lacks CD22 ligands exhibits reduced signaling upon B cell stimulation (Hennet et al. 1998). A double knockout of CD22 and ST6Gal-I restores normal B cell signaling linking the diminished B cell signaling of the ST6Gal-I-knockout mouse to the ligand-binding function of CD22 (Collins et al. 2006). Interestingly, these investigators showed that there was an increase in CD22 colocalization with the BCR in clathrin-rich microdomains and a reduced CD22 homo-oligomerization in mice lacking ST6Gal-I, CD22 ligands, and exhibiting reduced BCR signaling, suggesting that CD22 interactions with the BCR are not dependent upon the presence of α2,6-Sia (Collins et al. 2006; Ghosh et al. 2006). More recently, Müller and colleagues showed that mice expressing CD22 with a mutated ligand-binding domain exhibited reduced BCR-induced Ca2+ signaling and this could be explained by the inability of the mutant CD22 to bind cell surface α2,6-Sia ligands in cis (including those on adjacent CD22 molecules) and its increased association with and inhibition of the BCR (Fig. 6c) (Müller et al. 2013). On the other hand, expressing Siglec-G with a mutated ligand-binding domain in mice led to an increase in B1 cell Ca2+ signaling and expansion of the B1 cell population, and this was linked to a decrease in the Siglec-G–BCR interaction (Fig. 6c) (Hutzler et al. 2014). Based on these and other experiments, the authors propose that sialylated glycans displayed by the IgM portion of the BCR are typically bound by Siglec-G to control BCR signaling (Fig. 6c). Several other studies have also suggested the possibility that simultaneous interaction of the BCR with autoantigens and CD22/Siglec-G with trans ligands on the same cell may bring these inhibitory Siglecs into proximity with the BCR in this “immunological synapse” to dampen self-reactive B cell signaling (Fig. 6d) reviewed in Macauley et al. (2014). In sum, a complex series of interactions between CD22 and Siglec-10/G and their ligands, including the BCR, coordinate to control B cell signaling and the autoimmune response.
Selectins and the roles they play in the immune system and cancer
Selectins are carbohydrate-binding proteins that minimally recognize the sialyl Lewis X structure (sLeX) (Neu5Acα2,3Galβ1,4[Fucα1,3]GlcNAcβ1-R) on N- and O-glycans of specific ligands (Fig. 7). They mediate the rolling adhesion of cells such as leukocytes, platelets and hematopoietic progenitors along vascular surfaces as they are recruited to locations of injury or infection [reviewed in Lowe (1997, 2003), McEver (2015), McEver and Zhu (2010), Sperandio et al. (2009)]. Selectins are Ca2+-dependent lectins and, as such, are considered C-type lectins. Please see the article by Mayer and colleagues in this thematic issue for a review of C-type lectins (Mayer et al. 2017). They are type I membrane proteins that consist of an N-terminal lectin domain, an EGF-like module, followed by 2–9 consensus repeats, a transmembrane region and a short cytoplasmic tail (McEver 2015; McEver and Zhu 2010). There are three selectins that differ in the number of consensus repeats and their expression patterns (Fig. 7a). P-selectin is stored in endothelial cell Weibel–Palade bodies and platelet α-granules and moves to the cell surface upon cell stimulation with thrombin, complement or histamine (McEver 2015). E-selectin is constitutively expressed on skin and bone endothelial cells, but in other tissue types, its expression on post-capillary venules is induced by pro-inflammatory cytokines like TNFα or IL-1β. L-selectin is constitutively expressed on the surface of leukocytes (Läubli and Borsig 2010b; McEver and Zhu 2010). Selectin–ligand interactions are involved in the recruitment of leukocytes to sites of inflammation, the homing of naïve lymphocytes to secondary lymphoid organs, the recruitment of hematopoietic stem cells to the bone marrow and platelets to sites of hemorrhage. In addition, expression of selectin ligands by cancer cells facilitates their metastasis and survival (Läubli and Borsig 2010b). We will describe some of these processes in more detail below.
Fig. 7.
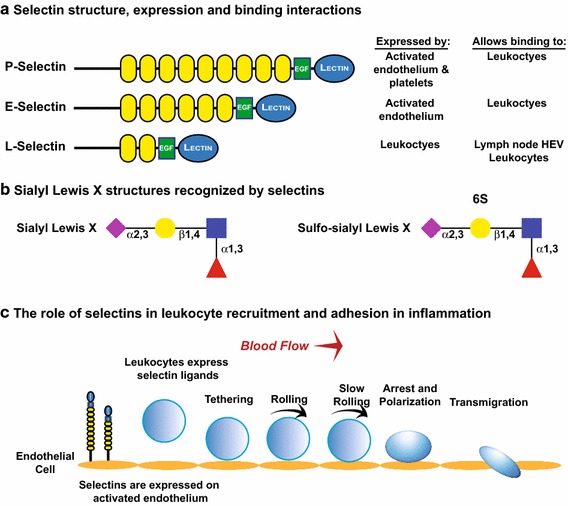
Selectins mediate binding to glycans capped with sialyl Lewis X structures to mediate cells interactions in inflammation, lymphocyte homing and metastasis. a Structures of P-, E- and L-selectins, cells on which they are expressed, and the binding they mediate. b Sialyl Lewis X and sulfo-sialyl Lewis X structures. Note that while all selectins recognize sialyl Lewis X, sulfo-sialyl Lewis X is primarily expressed by peripheral lymph node addressins (PNAds) recognized by L-selectin. c Selectin function in leukocyte recruitment and adhesion in inflammation (McEver and Zhu 2010). P- and E-selectins are expressed by activated endothelium and mediate recruitment, tethering and initial stages of leukocyte rolling on the endothelium. Signaling through selectin ligands on the leukocytes stimulates a conformational change in integrins expressed by leukocytes, leading to weak binding of their receptors on the endothelium and slowing their rolling. Release of chemokines from endothelium stimulates signaling from chemokine receptors on the leukocyte membrane, and these signals stimulate the conversion of integrins to forms with high affinity for ligands, and these high-affinity interactions lead to leukocyte arrest. Ultimately, the leukocyte migrates through the endothelium to the site of inflammation
The sialylated and fucosylated sLex structure capping N- and O-glycans is a key component of selectin ligands (Fig. 7b) (Lowe et al. 1990; Phillips et al. 1990). Several pieces of evidence show that ST3Gal-IV and ST3Gal-VI are the α2,3-sialyltransferases involved in sLeX synthesis. Combinatorial knockout of both STs led to a decrease in neutrophil binding to E- and P-selectins, selectin-dependent rolling, and lymphocyte homing (Yang et al. 2012). Other work suggests that ST3Gal-IV plays the major role in the synthesis of sLeX structures that mediate selectin-dependent adhesion and rolling. For example, it was recently demonstrated that ST3Gal-IV knockout results in significant reduction in the synthesis of sLea and sLex structures in HL-60 cells and in neutrophils derived from human hematopoietic stem cells. These cells show significant impairment in rolling and adhesion to the endothelial cells (Mondal et al. 2015). Fucosylation is also crucial for the function of all the selectins, as mice deficient in α1,3-fucosyltransferase, FucT-VII, exhibit impaired recruitment and extravasation of leukocytes to sites of inflammation (Lowe 1997; Maly et al. 1996).
Below, we will briefly review the role of selectins in inflammation and in lymphocyte homing [reviewed in McEver (2015), McEver and Zhu (2010), Rosen (2004)]. Leukocytes are recruited to the site of inflammation in a process that begins an interaction between endothelial cell selectins (predominantly P- and E-selectins) and their ligands on a leukocyte (Fig. 7c). These interactions allow the selectin ligands to transduce signals that convert leukocyte integrins to conformations with low affinity for their endothelial cell receptors such as ICAM-1 (ligand for αLβ2 integrin) and VCAM-1 (ligand for α4β1 integrin). These weak interactions allow the leukocytes to slowly roll on the surface of vascular wall and enable the release of immobilized chemokines from the endothelial cell membrane to stimulate signaling through chemokine G protein coupled receptors on the leukocyte membrane. These signals convert integrins to a high-affinity ligand-binding conformation that allows the arrest of cell rolling. Details of the signaling pathways involved in this process are found in (McEver 2015). The final step of leukocyte homing is transmigration through the vascular endothelium to the site of inflammation. Some of the well-characterized proteins carrying selectin ligands include P-selectin glycoprotein ligand-1 (PSGL-1), CD44 and E-selectin ligand-1 (ESL-1) [reviewed in Läubli and Borsig (2010b), McEver and Zhu (2010)]. Notably, PSGL-1 is the primary ligand for P- and L-selectins on leukocytes and optimal selectin binding requires not only the sLeX structure but also contacts with amino acids and sulfated tyrosines in the N-terminal region of PSGL-1 (McEver and Zhu 2010). On the other hand, E-selectin binds preferentially to PSGL-1, CD44 and E-selectin ligand-1 (ESL-1), but its binding to PSGL-1 does not require the presence of sulfate (McEver and Zhu 2010).
L-selectin is involved in the process of lymphocyte homing to secondary lymphoid organs, such as the lymph nodes, via interactions with selectin ligands on high endothelial venules (HEV) (Butcher and Picker 1996; Rosen 2004). In the case of naïve T cells, entry into the secondary lymphoid organs allows them to encounter antigens, while in the case of memory T cells that have already been primed with antigen, L-selectin allows them to localize in these lymphoid organs where they will proliferate once antigen is encountered again. L-selectin ligands on the high endothelial venules are collectively called peripheral lymph node addressins (PNAds) and include CD34, GlyCAM-1 and podocalyxin (Rosen 2004). Sulfated sLeX structures on both O- and N-glycans on these PNAds are key for L-selectin binding (Fig. 7b) [reviewed in McEver and Zhu (2010)].
Selectin interactions are deregulated in various diseases such as atherosclerosis, sepsis and cancer (Läubli and Borsig 2010b). The formation of atherosclerotic lesions is facilitated by the involvement of selectins, mainly P-selectin, on platelets (Collins et al. 2000; Dong et al. 2000). In addition, selectin knockout or inhibition prevents acute inflammation and tissue damage in mice (Mangell et al. 2007; Singbartl et al. 2000; Tedder et al. 1995) and reduces atherosclerotic lesions (Collins et al. 2000; Dong et al. 2000). Glycans capped with sLeX structures are enriched on the surface of cancer cells, and their presence is correlated with increased cancer progression and poor prognosis (Kannagi et al. 2004; Läubli and Borsig 2010b; Pinho and Reis 2015). Work done by several groups suggests that cancer cells employ selectin–ligand interactions to adhere to and extravasate through the endothelium and that ablation or inhibition of selectins reduces metastasis and tumor growth [for example, Biancone et al. (1996), Borsig et al. (2002), Büll et al. (2014a), Kim et al. (1998), Läubli and Borsig (2010a, b), Läubli et al. (2006)]. Notably, changes in sialylation have been observed following the induction of EMT, a process that allows cancer cells to break away from tumors, migrate and invade. For example, induction of EMT in colon cancer cells by EGF or basic FGF led to the upregulation of ST3Sia-I, ST3Sia-III and ST3Sia-IV that are involved in the synthesis of sLeX and sLea structures that serve as ligands for E-selectin, a selectin implicated in mediating the extravasation of circulating tumor cells into tissues for metastatic colonization (Sakuma et al. 2012). Low molecular weight heparins are potent inhibitors of metastasis, and a single dose of heparins attenuates lung metastasis, mainly via P-selectin inhibition (Borsig et al. 2002; Koenig et al. 1998). Likewise, fucosylated polysaccharide nanoparticles are potent inhibitors of P-selectin, as well as tumor growth and metastasis (Shamay et al. 2016).
Hypersialylation of β1-integrin and Fas receptor in cancer cells and their role in the increased motility and resistance to cell death and chemotherapy
It has been long appreciated that sialylated glycans on the surface of cancer cells are upregulated and contribute to increased aggressiveness, metastasis and invasion of a tumor by increasing cancer cell motility, extravasation from the circulation into tissues and resistance to cell death [reviewed in Büll et al. (2014a, b), Dall’Olio et al. (2014), Lu and Gu (2015), Pearce and Läubli (2016)]. In addition, hypersialylation of cancer cell surfaces enhances a cancer’s resistance to chemotherapy. In particular, ST6Gal-I has been reported to be upregulated in various cancers as a consequence of oncogenic Ras activation [for example, Le Marer et al. (1992), Seales et al. (2003, 2005b)]. ST6Gal-I expression has been correlated with the expression of stem cell markers in colon, pancreatic and ovarian cancers and directly shown to promote metastasis and survival/resistance to drug treatment (Schultz et al. 2013, 2016; Swindall et al. 2013). Below, we discuss how upregulation of ST6Gal-I by cancer cells and the increased sialylation of β1-integrin and the death receptor, FasR, controls cancer cell ECM adhesion and cell motility and resistance to apoptosis, respectively.
Bellis and colleagues have demonstrated that hypersialylation of β1-integrin in cancer cells impacts adhesion to extracellular matrix (ECM) and cell motility as well as resistance to cell death [reviewed in Schultz et al. (2012)]. Integrins are heterodimeric transmembrane receptors capable of bidirectional signaling. They can modulate intracellular signaling as a result of changes in the ECM, termed “outside-in signaling,” and can change their affinity for various ECM proteins-based various intracellular signaling events, termed “inside-out signaling” (Kim et al. 2011). The conformation and dimerization of integrins is important for their activity and influences cell adhesion, membrane detachment, migration, invasion and anchorage-dependent growth. The expression of oncogenic Ras leads to the upregulation of ST6Gal-I and the hypersialylation of the N-glycans of β1-integrin that is part of the integrin receptor for collagen (α1β1 and α2β1), fibronectin (α5β1) and laminin (α3β1) (Pochec et al. 2003; Pretzlaff et al. 2000; Seales et al. 2003; Semel et al. 2002; Shaikh et al. 2008). The sialylation of β1-integrin N-glycans alters the integrin receptor affinity for these ECM molecules. For example, hypersialylation of β1-integrins in ovarian adenocarcinoma cells results in increased cell motility by enhancing collagen binding and migration toward collagen (Christie et al. 2008; Seales et al. 2005a). These effects are believed to be caused by sialylation-mediated stabilization of β1-integrin in a conformation that has higher affinity for the particular ECM ligands (Shaikh et al. 2008).
Additional work by this group and others highlights the ability of cell surface α2,6-sialylation to block apoptosis and anoikis (cell death related to detachment from other cells or the ECM) (Amano et al. Amano et al. 2003, 2012; Liu et al. 2011; Sanchez-Ruderisch et al. 2011; Swindall and Bellis 2011). Early studies showed that the sialylation of T cell CD45 by ST6Gal-I blocks galectin-1 clustering of CD45 and resulting cell death (Amano et al. 2003). Please see the article in this thematic issue on galectins by Kaltner and colleagues for additional information on this family of proteins (Kaltner et al. 2017). More recently, Swindall and Bellis showed that α2,6-sialylation of FasR blocks binding of Fas-associated adaptor molecule (FADD) to the FasR death domain, thus inhibiting the formation of the death-inducing signaling complex (DISC) (Swindall and Bellis 2011). Internationalization and additional DISC formation was also hindered further blocking apoptotic signaling. Other studies demonstrated that the sialylation of α5β1 fibronectin receptor blocks galectin-1 binding and the induction of anoikis (Amano et al. 2012; Sanchez-Ruderisch et al. 2011). Interestingly, Amano et al. (2012) showed that p16INK4a, an anoikis-related tumor suppressor, acts to upregulate the α5β1 integrin and galectin-1 and simultaneously reduces sialylation by downregulating GNE and NANS, key enzymes in the Sia biosynthesis pathway (see Fig. 4b). Along these lines, overexpression of ST6Gal-I confers resistance to chemotherapy agents such as cisplatin, irinotecan and gemcitabine (Schultz et al. 2013, 2016; Swindall et al. 2013).
PolySia and metastasis
The expression of polySia has been strongly correlated with late stage, highly metastatic cancers including small cell and non-small cell lung carcinoma (SCLC and NSCLC), neuroblastoma, pancreatic cancer, pituitary tumors, Wilms’ tumor, rhabdomyosarcoma (Falconer et al. 2012) and, most recently, breast cancer (Wang et al. 2016). It is likely that the ability of polySia to abrogate cell adhesion and regulate signaling combine to exert its effects in cancer. Surprisingly, few studies have directly evaluated the role of polySia in cancer metastasis and those that did presumed that the polySia was modifying NCAM and influencing NCAM-mediated adhesion or signaling. Pioneering work by Scheidegger and colleagues compared two subclones of the NCI-H69 SCLC line and found that while both formed tumors when injected subcutaneously into nude mice, only the polySia expressing subclone formed significant numbers of intracutaneous metastases (Scheidegger et al. 1994). NCAM was expressed in these cells and was believed to be the only polysialylated protein. Later work by the Figarella-Branger group supported the role of polySia in cancer metastasis. They demonstrated that TE671 rhabdomyosarcoma cells generated lung metastases when injected into nude mice and that these could be decreased by repeated injections of EndoN, an enzyme that specifically cleaves polySia (Daniel et al. 2001). This group also showed that rat pituitary transplantable tumors that expressed high levels of polySia-NCAM were invasive and metastasized, while those that did not were benign (Daniel et al. 2000). In 2000, Tanaka et al. evaluated 57 NSCLC clinical cases and found that those tumors expressing polySia, but not NCAM, were more aggressive and metastatic than those expressing polySia-NCAM. Notably, ST8SiaII expression was highly correlated with tumor progression and later-stage tumors (Tanaka et al. 2000). The expression of NCAM was not correlated with tumor progression in this study, suggesting that the impact of polySia on carrier function rather than the sole presence of polySia might be more critical for tumor progression to later stages. As other polyST substrates like NRP-2, SynCAM-1 and ESL-1 have links to cancer, their polysialylation in NSCLC is of significant interest as a tumor marker and target for therapies.
References
- Abe M, Noda Y, Adachi H, Yoda K. Localization of GDP-mannose transporter in the Golgi requires retrieval to the endoplasmic reticulum depending on its cytoplasmic tail and coatomer. J Cell Sci. 2004;117:5687–5696. doi: 10.1242/jcs.01491. [DOI] [PubMed] [Google Scholar]
- Ali MF, Chachadi VB, Petrosyan A, Cheng PW. Golgi phosphoprotein 3 determines cell binding properties under dynamic flow by controlling Golgi localization of core 2 N-acetylglucosaminyltransferase 1. J Biol Chem. 2012;287:39564–39577. doi: 10.1074/jbc.M112.346528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–7475. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- Amano M, et al. Tumour suppressor p16(INK4a)—anoikis-favouring decrease in N/O-glycan/cell surface sialylation by down-regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J. 2012;279:4062–4080. doi: 10.1111/febs.12001. [DOI] [PubMed] [Google Scholar]
- Angata K, Suzuki M, McAuliffe J, Ding Y, Hindsgaul O, Fukuda M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide acceptors by three distinct a2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J Biol Chem. 2000;275:18594–18601. doi: 10.1074/jbc.M910204199. [DOI] [PubMed] [Google Scholar]
- Angata K, Yen TY, El-Battari A, Macher BA, Fukuda M. Unique disulfide bond structures found in ST8Sia IV polysialyltransferase are required for its activity. J Biol Chem. 2001;276:15369–15377. doi: 10.1074/jbc.M100576200. [DOI] [PubMed] [Google Scholar]
- Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenheimer MM, et al. Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology. 2003;13:591–600. doi: 10.1093/glycob/cwg066. [DOI] [PubMed] [Google Scholar]
- Audry M, Jeanneau C, Imberty A, Harduin-Lepers A, Delannoy P, Breton C. Current trends in the structure-activity relationships of sialyltransferases. Glycobiology. 2011;21:716–726. doi: 10.1093/glycob/cwq189. [DOI] [PubMed] [Google Scholar]
- Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- Bader RA, Wardwell PR. Polysialic acid: overcoming the hurdles of drug delivery. Ther Deliv. 2014;5:235–237. doi: 10.4155/tde.13.153. [DOI] [PubMed] [Google Scholar]
- Baigent SJ, McCauley JW. Glycosylation of haemagglutinin and stalk-length of neuraminidase combine to regulate the growth of avian influenza viruses in tissue culture. Virus Res. 2001;79:177–185. doi: 10.1016/S0168-1702(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Bailey Blackburn J, Pokrovskaya I, Fisher P, Ungar D, Lupashin VV. COG complex complexities: detailed characterization of a complete set of HEK293T cells lacking individual COG subunits. Front Cell Dev Biol. 2016;4:23. doi: 10.3389/fcell.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield DK. Mechanisms of protein retention in the Golgi. Cold Spring Harb Perspect Biol. 2011;3:a005264. doi: 10.1101/cshperspect.a005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew BA, Jourdian GW, Roseman S. The sialic acids. XV. Transfer of sialic acid to glycoproteins by a sialyltransferase from colostrum. J Biol Chem. 1973;248:5751–5762. [PubMed] [Google Scholar]
- Bhide GP, Prehna G, Ramirez BE, Colley KJ. Biophysical investigation of NCAM-polysialyltransferase interaction. Glycobiology. 2015;25:1268. [Google Scholar]
- Bhide GP, Fernandes NR, Colley KJ. Sequence requirements for neuropilin-2 recognition by ST8SiaIV and polysialylation of Its O-glycans. J Biol Chem. 2016;291:9444–9457. doi: 10.1074/jbc.M116.714329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancone L, Araki M, Araki K, Vassalli P, Stamenkovic I. Redirection of tumor metastasis by expression of E-selectin in vivo. J Exp Med. 1996;183:581–587. doi: 10.1084/jem.183.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A, Angulo J, Biet T, Peters H, Benie AJ, Palcic M, Peters T. Fragment-based screening of the donor substrate specificity of human blood group B galactosyltransferase using saturation transfer difference NMR. J Biol Chem. 2006;281:32728–32740. doi: 10.1074/jbc.M600424200. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, et al. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/S0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Bonten EJ, et al. Heterodimerization of the sialidase NEU1 with the chaperone protective protein/cathepsin A prevents its premature oligomerization. J Biol Chem. 2009;284:28430–28441. doi: 10.1074/jbc.M109.031419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA. 2002;99:2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Büll C, den Brok MH, Adema GJ. Sweet escape: sialic acids in tumor immune evasion. Biochim Biophys Acta. 2014;1846:238–246. doi: 10.1016/j.bbcan.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Büll C, Stoel MA, den Brok MH, Adema GJ. Sialic acids sweeten a tumor’s life. Cancer Res. 2014;74:3199–3204. doi: 10.1158/0008-5472.CAN-14-0728. [DOI] [PubMed] [Google Scholar]
- Burnham AJ, Baranovich T, Govorkova EA. Neuraminidase inhibitors for influenza B virus infection: efficacy and resistance. Antiviral Res. 2013;100:520–534. doi: 10.1016/j.antiviral.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Caffaro CE, Hirschberg CB. Nucleotide sugar transporters of the Golgi apparatus: from basic science to diseases. Acc Chem Res. 2006;39:805–812. doi: 10.1021/ar0400239. [DOI] [PubMed] [Google Scholar]
- Carlson DM, Jourdian GW, Roseman S. The sialic acids. XIV. Synthesis of sialyl-lactose by a sialyltransferase from rat mammary gland. J Biol Chem. 1973;248:5742–5750. [PubMed] [Google Scholar]
- Carlson DM, McGuire EJ, Jourdian GW, Roseman S. The sialic acids. XVI. Isolation of a mucin sialyltransferase from sheep submaxillary gland. J Biol Chem. 1973;248:5763–5773. [PubMed] [Google Scholar]
- Chandrasekaran A, et al. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotechnol. 2008;26:107–113. doi: 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]
- Chen C, Ma J, Lazic A, Backovic M, Colley KJ. Formation of insoluble oligomers correlates with ST6Gal I stable localization in the Golgi. J Biol Chem. 2000;275:13819–13826. doi: 10.1074/jbc.275.18.13819. [DOI] [PubMed] [Google Scholar]
- Chen TL, Chen C, Bergeron NQ, Close BE, Bohrer TJ, Vertel BM, Colley KJ. The two rat alpha 2,6-sialyltransferase (ST6Gal I) isoforms: evaluation of catalytic activity and intra-Golgi localization. Glycobiology. 2003;13:109–117. doi: 10.1093/glycob/cwg015. [DOI] [PubMed] [Google Scholar]
- Chiu CP, et al. Structural analysis of the sialyltransferase CstII from Campylobacter jejuni in complex with a substrate analog. Nat Struct Mol Biol. 2004;11:163–170. doi: 10.1038/nsmb720. [DOI] [PubMed] [Google Scholar]
- Chou HH, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie DR, Shaikh FM, Lucas JA, III, Bellis SL. ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J Ovarian Res. 2008;1:3. doi: 10.1186/1757-2215-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close BE, Tao K, Colley KJ. Polysialyltransferase-1 autopolysialylation is not requisite for polysialylation of neural cell adhesion molecule. J Biol Chem. 2000;275:4484–4491. doi: 10.1074/jbc.275.6.4484. [DOI] [PubMed] [Google Scholar]
- Close BE, Mendiratta SS, Geiger KM, Broom LJ, Ho LL, Colley KJ. The minimal structural domains required for neural cell adhesion molecule polysialylation by PST/ST8Sia-IV and STX/ST8Sia-II. J Biol Chem. 2003;278:30796–30805. doi: 10.1074/jbc.M305390200. [DOI] [PubMed] [Google Scholar]
- Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley KJ. Structural basis for the polysialylation of the neural cell adhesion molecule. Adv Exp Med Biol. 2010;663:111–126. doi: 10.1007/978-1-4419-1170-4_7. [DOI] [PubMed] [Google Scholar]
- Colley KJ, Kitajima K, Sato C. Polysialic acid: biosynthesis, novel functions and applications. Crit Rev Biochem Mol Biol. 2014;49:498–532. doi: 10.3109/10409238.2014.976606. [DOI] [PubMed] [Google Scholar]
- Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BE, Smith BA, Bengtson P, Paulson JC. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat Immunol. 2006;7:199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- Corfield AP (2017) Protein glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1526-4 [DOI] [PMC free article] [PubMed]
- Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NM. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–30356. doi: 10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- Dahdal RY, Colley KJ. Specific sequences in the signal anchor of the beta-galactoside alpha-2,6-sialyltransferase are not essential for Golgi localization. Membrane flanking sequences may specify Golgi retention. J Biol Chem. 1993;268:26310–26319. [PubMed] [Google Scholar]
- Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M. Sialosignaling: sialyltransferases as engines of self-fueling loops in cancer progression. Biochim Biophys Acta. 2014;1840:2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Daniel L, Trouillas J, Renaud W, Chevallier P, Gouvernet J, Rougon G, Figarella-Branger D. Polysialylated-neural cell adhesion molecule expression in rat pituitary transplantable tumors (spontaneous mammotropic transplantable tumor in Wistar-Furth rats) is related to growth rate and malignancy. Cancer Res. 2000;60:80–85. [PubMed] [Google Scholar]
- Daniel L, Durbec P, Gautherot E, Rouvier E, Rougon G, Figarella-Branger D. A nude mice model of human rhabdomyosarcoma lung metastases for evaluating the role of polysialic acids in the metastatic process. Oncogene. 2001;20:997–1004. doi: 10.1038/sj.onc.1204176. [DOI] [PubMed] [Google Scholar]
- Datta AK. Comparative sequence analysis in the sialyltransferase protein family: analysis of motifs. Curr Drug Targets. 2009;10:483–498. doi: 10.2174/138945009788488422. [DOI] [PubMed] [Google Scholar]
- Datta AK, Paulson JC. The sialyltransferase “sialylmotif” participates in binding the donor substrate CMP-NeuAc. J Biol Chem. 1995;270:1497–1500. doi: 10.1074/jbc.270.15.8637. [DOI] [PubMed] [Google Scholar]
- Datta AK, Paulson JC. Sialylmotifs of sialyltransferases. Indian J Biochem Biophys. 1997;34:157–165. [PubMed] [Google Scholar]
- Datta AK, Sinha A, Paulson JC. Mutation of the sialyltransferase S-sialylmotif alters the kinetics of the donor and acceptor substrates. J Biol Chem. 1998;273:9608–9614. doi: 10.1074/jbc.273.16.9608. [DOI] [PubMed] [Google Scholar]
- Datta AK, Chammas R, Paulson JC. Conserved cysteines in the sialyltransferase sialylmotifs form an essential disulfide bond. J Biol Chem. 2001;276:15200–15207. doi: 10.1074/jbc.M010542200. [DOI] [PubMed] [Google Scholar]
- d’Azzo A, Bonten E. Molecular mechanisms of pathogenesis in a glycosphingolipid and a glycoprotein storage disease. Biochem Soc Trans. 2010;38:1453–1457. doi: 10.1042/BST0381453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Azzo A, Hoogeveen A, Reuser AJ, Robinson D, Galjaard H. Molecular defect in combined beta-galactosidase and neuraminidase deficiency in man. Proc Natl Acad Sci USA. 1982;79:4535–4539. doi: 10.1073/pnas.79.15.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby MC, Gleeson PA. New insights into membrane trafficking and protein sorting. Int Rev Cytol. 2007;261:47–116. doi: 10.1016/S0074-7696(07)61002-X. [DOI] [PubMed] [Google Scholar]
- Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.CIR.101.19.2290. [DOI] [PubMed] [Google Scholar]
- Eckert ES, Reckmann I, Hellwig A, Röhling S, El-Battari A, Wieland FT, Popoff V. Golgi phosphoprotein 3 triggers signal-mediated incorporation of glycosyltransferases into coatomer-coated (COPI) vesicles. J Biol Chem. 2014;289:31319–31329. doi: 10.1074/jbc.M114.608182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M, Gotza B, Gerardy-Schahn R. Mutants of the CMP-sialic acid transporter causing the Lec2 phenotype. J Biol Chem. 1998;273:20189–20195. doi: 10.1074/jbc.273.32.20189. [DOI] [PubMed] [Google Scholar]
- Eisenberg I, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- El Maarouf A, Rutishauser U. Use of PSA-NCAM in repair of the central nervous system. Adv Exp Med Biol. 2010;663:137–147. doi: 10.1007/978-1-4419-1170-4_9. [DOI] [PubMed] [Google Scholar]
- El Maarouf A, Petridis AK, Rutishauser U. Use of polysialic acid in repair of the central nervous system. Proc Natl Acad Sci USA. 2006;103:16989–16994. doi: 10.1073/pnas.0608036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer RA, Errington RJ, Shnyder SD, Smith PJ, Patterson LH. Polysialyltransferase: a new target in metastatic cancer. Curr Cancer Drug Targets. 2012;12:925–939. doi: 10.2174/156800912803251225. [DOI] [PubMed] [Google Scholar]
- Fenteany FH, Colley KJ. Multiple signals are required for alpha2,6-sialyltransferase (ST6Gal I) oligomerization and Golgi localization. J Biol Chem. 2005;280:5423–5429. doi: 10.1074/jbc.M412396200. [DOI] [PubMed] [Google Scholar]
- Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983;112:482–487. doi: 10.1016/0006-291X(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Foley DA, Swartzentruber KG, Colley KJ. Identification of sequences in the polysialyltransferases ST8Sia II and ST8Sia IV that are required for the protein-specific polysialylation of the neural cell adhesion molecule, NCAM. J Biol Chem. 2009;284:15505–15516. doi: 10.1074/jbc.M809696200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DA, Swartzentruber KG, Lavie A, Colley KJ. Structure and mutagenesis of neural cell adhesion molecule domains: evidence for flexibility in the placement of polysialic acid attachment sites. J Biol Chem. 2010;285:27360–27371. doi: 10.1074/jbc.M110.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabius H-J. The magic of the sugar code. Trends Biochem Sci. 2015;40:341. doi: 10.1016/j.tibs.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Gabius H-J, Roth J (2017) An introduction to the sugar code. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1521-9 [DOI] [PubMed]
- Gabius H-J, Kaltner H, Kopitz J, Andre S. The glycobiology of the CD system: a dictionary for translating marker designations into glycan/lectin structure and function. Trends Biochem Sci. 2015;40:360–376. doi: 10.1016/j.tibs.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Gabius H-J, Manning JC, Kopitz J, Andre S, Kaltner H. Sweet complementarity: the functional pairing of glycans with lectins. Cell Mol Life Sci. 2016;73:1989–2016. doi: 10.1007/s00018-016-2163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjart NJ, Gillemans N, Harris A, van der Horst GT, Verheijen FW, Galjaard H, d’Azzo A. Expression of cDNA encoding the human “protective protein” associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell. 1988;54:755–764. doi: 10.1016/S0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- Galuska SP, et al. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA. 2010;107:10250–10255. doi: 10.1073/pnas.0912103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard A, et al. Impact on antiviral resistance of E119V, I222L and R292K substitutions in influenza A viruses bearing a group 2 neuraminidase (N2, N3, N6, N7 and N9) J Antimicrob Chemother. 2016 doi: 10.1093/jac/dkw275. [DOI] [PubMed] [Google Scholar]
- Gaymard A, Le Briand N, Frobert E, Lina B, Escuret V. Functional balance between neuraminidase and haemagglutinin in influenza viruses. Clin Microbiol Infect. 2016 doi: 10.1016/j.cmi.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Bandulet C, Nitschke L. Regulation of B cell development and B cell signalling by CD22 and its ligands alpha2,6-linked sialic acids. Int Immunol. 2006;18:603–611. doi: 10.1093/intimm/dxh402. [DOI] [PubMed] [Google Scholar]
- Gilmour AM, et al. A novel epidermal growth factor receptor-signaling platform and its targeted translation in pancreatic cancer. Cell Signal. 2013;25:2587–2603. doi: 10.1016/j.cellsig.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Glick BS, Luini A. Models for Golgi traffic: a critical assessment. Cold Spring Harb Perspect Biol. 2011;3:a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Nakano A. Membrane traffic within the Golgi apparatus. Annu Rev Cell Dev Biol. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley B, Maggioni A, Ashikov A, Day CJ, Haselhorst T, Tiralongo J. Structure and function of nucleotide sugar transporters: current progress. Comput Struct Biotechnol J. 2014;10:23–32. doi: 10.1016/j.csbj.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology. 2005;15:805–817. doi: 10.1093/glycob/cwi063. [DOI] [PubMed] [Google Scholar]
- Hassinen A, Pujol FM, Kokkonen N, Pieters C, Kihlström M, Korhonen K, Kellokumpu S. Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J Biol Chem. 2011;286:38329–38340. doi: 10.1074/jbc.M111.277681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuero AM, Díez-Revuelta N, Abad-Rodríguez J (2017) The sugar code in neuronal physiology. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1519-3 [DOI] [PubMed]
- Hinderlich S, Weidemann W, Yardeni T, Horstkorte R, Huizing M. UDP-GlcNAc 2-epimerase/ManNAc kinase (GNE): a master regulator of sialic acid synthesis. Top Curr Chem. 2015;366:97–137. doi: 10.1007/128_2013_464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, et al. Disulphide linkage in mouse ST6Gal-I: determination of linkage positions and mutant analysis. J Biochem. 2012;151:197–203. doi: 10.1093/jb/mvr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- Hoffman S, et al. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982;257:7720–7729. [PubMed] [Google Scholar]
- Hudgin RL, Schachter H. Evidence for two CMP-N-acetylneuraminic acid: lactose sialyltransferases in rat, porcine, bovine, and human liver. Can J Biochem. 1972;50:1024–1028. doi: 10.1139/o72-141. [DOI] [PubMed] [Google Scholar]
- Hutzler S, Ozgör L, Naito-Matsui Y, Klasener K, Winkler TH, Reth M, Nitschke L. The ligand-binding domain of Siglec-G is crucial for its selective inhibitory function on B1 cells. J Immunol. 2014;192:5406–5414. doi: 10.4049/jimmunol.1302875. [DOI] [PubMed] [Google Scholar]
- James WM, Agnew WS. Multiple oligosaccharide chains in the voltage-sensitive Na channel from electrophorus electricus: evidence for alpha-2,8-linked polysialic acid. Biochem Biophys Res Commun. 1987;148:817–826. doi: 10.1016/0006-291X(87)90949-1. [DOI] [PubMed] [Google Scholar]
- Jeanneau C, et al. Structure-function analysis of the human sialyltransferase ST3Gal I: role of N-glycosylation and a novel conserved sialylmotif. J Biol Chem. 2004;279:13461–13468. doi: 10.1074/jbc.M311764200. [DOI] [PubMed] [Google Scholar]
- Jellusova J, Düber S, Gückel E, Binder CJ, Weiss S, Voll R, Nitschke L. Siglec-G regulates B1 cell survival and selection. J Immunol. 2010;185:3277–3284. doi: 10.4049/jimmunol.1001792. [DOI] [PubMed] [Google Scholar]
- Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- Jones MB, Nasirikenari M, Feng L, Migliore MT, Choi KS, Kazim L, Lau JT. Role for hepatic and circulatory ST6Gal-1 sialyltransferase in regulating myelopoiesis. J Biol Chem. 2010;285:25009–25017. doi: 10.1074/jbc.M110.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MB, Oswald DM, Joshi S, Whiteheart SW, Orlando R, Cobb BA. B-cell-independent sialylation of IgG. Proc Natl Acad Sci USA. 2016;113:7207–7212. doi: 10.1073/pnas.1523968113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltner H, Toegel S, García Caballero G, Manning JC, Ledeen RW, Gabius H-J (2017) Galectins: their network and roles in immunity/tumor growth control. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1522-8 [DOI] [PubMed]
- Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiermaier E, et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science. 2016;351:186–190. doi: 10.1126/science.aad0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Paulson JC. Cloning of a novel alpha 2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J Biol Chem. 1994;269:1394–1401. [PubMed] [Google Scholar]
- Kitajima K, Varki N, Sato C. Advanced technologies in sialic acid and sialoglycoconjugate analysis. Top Curr Chem. 2015;367:75–103. doi: 10.1007/128_2013_458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazume S, Tachida Y, Oka R, Shirotani K, Saido TC, Hashimoto Y. Alzheimer’s beta-secretase, beta-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc Natl Acad Sci USA. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazume-Kawaguchi S, Dohmae N, Takio K, Tsuji S, Colley KJ. The relationship between ST6Gal I Golgi retention and its cleavage-secretion. Glycobiology. 1999;9:1397–1406. doi: 10.1093/oxfordjournals.glycob.a018856. [DOI] [PubMed] [Google Scholar]
- Kitazume-Kawaguchi S, Kabata S, Arita M. Differential biosynthesis of polysialic or disialic acid structure by ST8Sia II and ST8Sia IV. J Biol Chem. 2001;276:15696–15703. doi: 10.1074/jbc.M010371200. [DOI] [PubMed] [Google Scholar]
- Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitz J (2017) Lipid glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1518-4 [DOI] [PubMed]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kuhn B, Benz J, Greif M, Engel AM, Sobek H, Rudolph MG. The structure of human α-2,6-sialyltransferase reveals the binding mode of complex glycans. Acta Crystallogr D Biol Crystallogr. 2013;69:1826–1838. doi: 10.1107/S0907444913015412. [DOI] [PubMed] [Google Scholar]
- Lammers G, Jamieson JC. The role of a cathepsin D-like activity in the release of Gal beta 1-4GlcNAc alpha 2-6-sialyltransferase from rat liver Golgi membranes during the acute-phase response. Biochem J. 1988;256:623–631. doi: 10.1042/bj2560623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läubli H, Borsig L. Selectins as mediators of lung metastasis. Cancer Microenviron. 2010;3:97–105. doi: 10.1007/s12307-010-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Läubli H, Stevenson JL, Varki A, Varki NM, Borsig L. L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res. 2006;66:1536–1542. doi: 10.1158/0008-5472.CAN-05-3121. [DOI] [PubMed] [Google Scholar]
- Le Marer N, et al. The c-Ha-ras oncogene induces increased expression of beta-galactoside alpha-2,6-sialyltransferase in rat fibroblast (FR3T3) cells. Glycobiology. 1992;2:49–56. doi: 10.1093/glycob/2.1.49. [DOI] [PubMed] [Google Scholar]
- Lee MM, Nasirikenari M, Manhardt CT, Ashline DJ, Hanneman AJ, Reinhold VN, Lau JT. Platelets support extracellular sialylation by supplying the sugar donor substrate. J Biol Chem. 2014;289:8742–8748. doi: 10.1074/jbc.C113.546713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Swindall AF, Kesterson RA, Schoeb TR, Bullard DC, Bellis SL. ST6Gal-I regulates macrophage apoptosis via α2-6 sialylation of the TNFR1 death receptor. J Biol Chem. 2011;286:39654–39662. doi: 10.1074/jbc.M111.276063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Selectin ligands, leukocyte trafficking, and fucosyltransferase genes. Kidney Int. 1997;51:1418–1426. doi: 10.1038/ki.1997.194. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Lowe JB, Stoolman LM, Nair RP, Larsen RD, Berhend TL, Marks RM. ELAM-1—dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990;63:475–484. doi: 10.1016/0092-8674(90)90444-J. [DOI] [PubMed] [Google Scholar]
- Lu J, Gu J. Significance of β-galactoside α2,6 sialyltranferase 1 in cancers. Molecules. 2015;20:7509–7527. doi: 10.3390/molecules20057509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Qian R, Rausa FM, III, Colley KJ. Two naturally occurring alpha2,6-sialyltransferase forms with a single amino acid change in the catalytic domain differ in their catalytic activity and proteolytic processing. J Biol Chem. 1997;272:672–679. doi: 10.1074/jbc.272.1.672. [DOI] [PubMed] [Google Scholar]
- Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado E, et al. Regulated lysosomal exocytosis mediates cancer progression. Sci Adv. 2015;1:e1500603. doi: 10.1126/sciadv.1500603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly P, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/S0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Mandal C, Schwartz-Albiez R, Vlasak R. Functions and biosynthesis of O-acetylated sialic acids. Top Curr Chem. 2015;366:1–30. doi: 10.1007/128_2011_310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangell P, Mihaescu A, Wang Y, Schramm R, Jeppsson B, Thorlacius H. Critical role of P-selectin-dependent leukocyte recruitment in endotoxin-induced intestinal barrier dysfunction in mice. Inflamm Res. 2007;56:189–194. doi: 10.1007/s00011-007-6163-x. [DOI] [PubMed] [Google Scholar]
- Manning JC, Romero A, Habermann F, García Caballero G, Kaltner H, Gabius HJ (2017) Lectins: a primer for histochemists and cell biologists. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1524-6 [DOI] [PubMed]
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci USA. 2004;101:4620–4624. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004;78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M, Matrosovich T, Uhlendorff J, Garten W, Klenk HD. Avian-virus-like receptor specificity of the hemagglutinin impedes influenza virus replication in cultures of human airway epithelium. Virology. 2007;361:384–390. doi: 10.1016/j.virol.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Matrosovich M, Herrler G, Klenk HD. Sialic acid receptors of viruses. Top Curr Chem. 2015;367:1–28. doi: 10.1007/128_2013_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- Mayer S, Raulf M-K, Lepenies B (2017) C-type lectins: their network and roles in immunity/pathogen recognition. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1523-7 [DOI] [PubMed]
- McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiratta SS, Sekulic N, Lavie A, Colley KJ. Specific amino acids in the first fibronectin type III repeat of the neural cell adhesion molecule play a role in its recognition and polysialylation by the polysialyltransferase ST8Sia IV/PST. J Biol Chem. 2005;280:32340–32348. doi: 10.1074/jbc.M506217200. [DOI] [PubMed] [Google Scholar]
- Mendiratta SS, Sekulic N, Hernandez-Guzman FG, Close BE, Lavie A, Colley KJ. A novel alpha-helix in the first fibronectin type III repeat of the neural cell adhesion molecule is critical for N-glycan polysialylation. J Biol Chem. 2006;281:36052–36059. doi: 10.1074/jbc.M608073200. [DOI] [PubMed] [Google Scholar]
- Meng L, et al. Enzymatic basis for N-glycan sialylation: structure of rat α2,6-sialyltransferase (ST6GAL1) reveals conserved and unique features for glycan sialylation. J Biol Chem. 2013;288:34680–34698. doi: 10.1074/jbc.M113.519041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov AA, et al. Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae. J Cell Biol. 2001;155:1225–1238. doi: 10.1083/jcb.200108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T, Tsuiki S. Rat-liver lysosomal sialidase. Solubilization, substrate specificity and comparison with the cytosolic sialidase. Eur J Biochem. 1984;141:75–81. doi: 10.1111/j.1432-1033.1984.tb08159.x. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–896. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- Miyagi T, et al. Altered expression of sialidases in human cancer. Adv Exp Med Biol. 2012;749:257–267. doi: 10.1007/978-1-4614-3381-1_17. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morré DJ. Perspectives on Golgi apparatus form and function. J Electron Microsc Tech. 1991;17:2–14. doi: 10.1002/jemt.1060170103. [DOI] [PubMed] [Google Scholar]
- Mondal N, et al. ST3Gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood. 2015;125:687–696. doi: 10.1182/blood-2014-07-588590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti E, et al. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem. 2010;64:403–479. doi: 10.1016/S0065-2318(10)64007-3. [DOI] [PubMed] [Google Scholar]
- Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlenhoff M, Eckhardt M, Bethe A, Frosch M, Gerardy-Schahn R. Autocatalytic polysialylation of polysialyltransferase-1. EMBO J. 1996;15:6943–6950. [PMC free article] [PubMed] [Google Scholar]
- Müller J, et al. CD22 ligand-binding and signaling domains reciprocally regulate B-cell Ca2+ signaling. Proc Natl Acad Sci USA. 2013;110:12402–12407. doi: 10.1073/pnas.1304888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Sequences within and adjacent to the transmembrane segment of alpha-2,6-sialyltransferase specify Golgi retention. EMBO J. 1991;10:3577–3588. doi: 10.1002/j.1460-2075.1991.tb04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. A comparison of the transmembrane domains of Golgi and plasma membrane proteins. Biochem Soc Trans. 1995;23:527–530. doi: 10.1042/bst0230527. [DOI] [PubMed] [Google Scholar]
- Munro S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata D, Zhang L, Troy FA., II Molecular basis for polysialylation: a novel polybasic polysialyltransferase domain (PSTD) of 32 amino acids unique to the alpha 2,8-polysialyltransferases is essential for polysialylation. Glycoconj J. 2006;23:423–436. doi: 10.1007/s10719-006-6356-5. [DOI] [PubMed] [Google Scholar]
- Nasirikenari M, Veillon L, Collins CC, Azadi P, Lau JT. Remodeling of marrow hematopoietic stem and progenitor cells by non-self ST6Gal-1 sialyltransferase. J Biol Chem. 2014;289:7178–7189. doi: 10.1074/jbc.M113.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu U, Bauer J, Stehle T. Viruses and sialic acids: rules of engagement. Curr Opin Struct Biol. 2011;21:610–618. doi: 10.1016/j.sbi.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Slusarewicz P, Hoe MH, Warren G. Kin recognition. A model for the retention of Golgi enzymes. FEBS Lett. 1993;330:1–4. doi: 10.1016/0014-5793(93)80906-B. [DOI] [PubMed] [Google Scholar]
- Nilsson T, et al. Kin recognition between medial Golgi enzymes in HeLa cells. EMBO J. 1994;13:562–574. doi: 10.1002/j.1460-2075.1994.tb06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Rabouille C, Hui N, Watson R, Warren G. The role of the membrane-spanning domain and stalk region of N-acetylglucosaminyltransferase I in retention, kin recognition and structural maintenance of the Golgi apparatus in HeLa cells. J Cell Sci. 1996;109(Pt 7):1975–1989. doi: 10.1242/jcs.109.7.1975. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Au CE, Bergeron JJ. Sorting out glycosylation enzymes in the Golgi apparatus. FEBS Lett. 2009;583:3764–3769. doi: 10.1016/j.febslet.2009.10.064. [DOI] [PubMed] [Google Scholar]
- Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr Opin Immunol. 2005;17:290–297. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Nitschke L, Carsetti R, Ocker B, Köhler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7:133–143. doi: 10.1016/S0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- Opat AS, Houghton F, Gleeson PA. Medial Golgi but not late Golgi glycosyltransferases exist as high molecular weight complexes. Role of luminal domain in complex formation and localization. J Biol Chem. 2000;275:11836–11845. doi: 10.1074/jbc.275.16.11836. [DOI] [PubMed] [Google Scholar]
- O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otipoby KL, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- Padler-Karavani V, et al. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 2014;28:1280–1293. doi: 10.1096/fj.13-241497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SP, Taylor LS, Stansbury EK, McVicar DW. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood. 2000;96:483–490. [PubMed] [Google Scholar]
- Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- Paulson JC, Beranek WE, Hill RL. Purification of a sialyltransferase from bovine colostrum by affinity chromatography on CDP-agarose. J Biol Chem. 1977;252:2356–2362. [PubMed] [Google Scholar]
- Paulson JC, Rearick JI, Hill RL. Enzymatic properties of beta-d-galactoside alpha2 leads to 6 sialytransferase from bovine colostrum. J Biol Chem. 1977;252:2363–2371. [PubMed] [Google Scholar]
- Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Ann NY Acad Sci. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peanne R, Legrand D, Duvet S, Mir AM, Matthijs G, Rohrer J, Foulquier F. Differential effects of lobe A and lobe B of the conserved oligomeric Golgi complex on the stability of {beta}1,4-galactosyltransferase 1 and {alpha}2,6-sialyltransferase 1. Glycobiology. 2011;21:864–876. doi: 10.1093/glycob/cwq176. [DOI] [PubMed] [Google Scholar]
- Pearce OM, Läubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26:111–128. doi: 10.1093/glycob/cwv097. [DOI] [PubMed] [Google Scholar]
- Pereira NA, Pu HX, Goh H, Song Z. Golgi phosphoprotein 3 mediates the Golgi localization and function of protein O-linked mannose β-1,2-N-acetlyglucosaminyltransferase 1. J Biol Chem. 2014;289:14762–14770. doi: 10.1074/jbc.M114.548305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- Pochec E, Litynska A, Amoresano A, Casbarra A. Glycosylation profile of integrin alpha 3 beta 1 changes with melanoma progression. Biochim Biophys Acta. 2003;1643:113–123. doi: 10.1016/j.bbamcr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Pretzlaff RK, Xue VW, Rowin ME. Sialidase treatment exposes the beta1-integrin active ligand binding site on HL60 cells and increases binding to fibronectin. Cell Adhes Commun. 2000;7:491–500. doi: 10.3109/15419060009040306. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Klumperman J. Opinion: the maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–817. doi: 10.1038/nrm1735. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci. 1995;108(Pt 4):1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- Rameix-Welti MA, Enouf V, Cuvelier F, Jeannin P, van der Werf S. Enzymatic properties of the neuraminidase of seasonal H1N1 influenza viruses provide insights for the emergence of natural resistance to oseltamivir. PLoS Pathog. 2008;4:e1000103. doi: 10.1371/journal.ppat.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao FV, et al. Structural insight into mammalian sialyltransferases. Nat Struct Mol Biol. 2009;16:1186–1188. doi: 10.1038/nsmb.1685. [DOI] [PubMed] [Google Scholar]
- Reiter DM, Frierson JM, Halvorson EE, Kobayashi T, Dermody TS, Stehle T. Crystal structure of reovirus attachment protein σ1 in complex with sialylated oligosaccharides. PLoS Pathog. 2011;7:e1002166. doi: 10.1371/journal.ppat.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynders E, Foulquier F, Annaert W, Matthijs G. How Golgi glycosylation meets and needs trafficking: the case of the COG complex. Glycobiology. 2011;21:853–863. doi: 10.1093/glycob/cwq179. [DOI] [PubMed] [Google Scholar]
- Rivinoja A, Pujol FM, Hassinen A, Kellokumpu S. Golgi pH, its regulation and roles in human disease. Ann Med. 2012;44:542–554. doi: 10.3109/07853890.2011.579150. [DOI] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC, Daniels RS, Skehel JJ, Wilson IA, Wiley DC. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rollenhagen M, et al. Polysialylation of the synaptic cell adhesion molecule 1 (SynCAM 1) depends exclusively on the polysialyltransferase ST8SiaII in vivo. J Biol Chem. 2012;287:35170–35180. doi: 10.1074/jbc.M112.375642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen M, et al. Polysialic acid on neuropilin-2 is exclusively synthesized by the polysialyltransferase ST8SiaIV and attached to mucin-type O-glycans located between the b2 and c domain. J Biol Chem. 2013;288:22880–22892. doi: 10.1074/jbc.M113.463927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Rosnoblet C, Peanne R, Legrand D, Foulquier F. Glycosylation disorders of membrane trafficking. Glycoconj J. 2013;30:23–31. doi: 10.1007/s10719-012-9389-y. [DOI] [PubMed] [Google Scholar]
- Roth J, Zuber C (2017) Quality control of glycoprotein folding and ERAD: the role of N-glycan handling, EDEM1 and OS-9. Histochem Cell Biol 147(2). doi:10.1007/s00418-016-1513-9 [DOI] [PubMed]
- Roth J, Taatjes DJ, Lucocq JM, Weinstein J, Paulson JC. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985;43:287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Roth J, Taatjes DJ, Weinstein J, Paulson JC, Greenwell P, Watkins WM. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem. 1986;261:14307–14312. [PubMed] [Google Scholar]
- Roth J, Rutishauser U, Troy FA. Polysialic acid-from microbes to man. Advances in life sciences. Basel: Birkhäuser Verlag; 1993. [Google Scholar]
- Rothbard JB, Brackenbury R, Cunningham BA, Edelman GM. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982;257:11064–11069. [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Aoki M, Kannagi R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc Natl Acad Sci USA. 2012;109:7776–7781. doi: 10.1073/pnas.1111135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samraj AN, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ruderisch H, Detjen KM, Welzel M, André S, Fischer C, Gabius HJ, Rosewicz S. Galectin-1 sensitizes carcinoma cells to anoikis via the fibronectin receptor α5β1-integrin. Cell Death Differ. 2011;18:806–816. doi: 10.1038/cdd.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, et al. Expression cloning of a novel Gal beta (1-3/1-4) GlcNAc alpha 2,3-sialyltransferase using lectin resistance selection. J Biol Chem. 1993;268:22782–22787. [PubMed] [Google Scholar]
- Sato C, Kitajima K. Disialic, oligosialic and polysialic acids: distribution, functions and related disease. J Biochem. 2013;154:115–136. doi: 10.1093/jb/mvt057. [DOI] [PubMed] [Google Scholar]
- Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang ML, Tedder TF. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/S1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R, Sommer U, Kruger D, van Unen H, Traving C. The terminal enzymes of sialic acid metabolism: acylneuraminate pyruvate-lyases. Biosci Rep. 1999;19:373–383. doi: 10.1023/A:1020256004616. [DOI] [PubMed] [Google Scholar]
- Scheidegger EP, Lackie PM, Papay J, Roth J. In vitro and in vivo growth of clonal sublines of human small cell lung carcinoma is modulated by polysialic acid of the neural cell adhesion molecule. Lab Invest. 1994;70:95–106. [PubMed] [Google Scholar]
- Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–518. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MJ, Swindall AF, Wright JW, Sztul ES, Landen CN, Bellis SL. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J Ovarian Res. 2013;6:25. doi: 10.1186/1757-2215-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MJ, et al. The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res. 2016;76:3978–3988. doi: 10.1158/0008-5472.CAN-15-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf M, et al. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Singhal A, Bellis SL. Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin. Oncogene. 2003;22:7137–7145. doi: 10.1038/sj.onc.1206834. [DOI] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- Seales EC, Shaikh FM, Woodard-Grice AV, Aggarwal P, McBrayer AC, Hennessy KM, Bellis SL. A protein kinase C/Ras/ERK signaling pathway activates myeloid fibronectin receptors by altering beta1 integrin sialylation. J Biol Chem. 2005;280:37610–37615. doi: 10.1074/jbc.M508476200. [DOI] [PubMed] [Google Scholar]
- Sellmeier M, Weinhold B, Münster-Kühnel A. CMP-sialic acid synthetase: the point of constriction in the sialylation pathway. Top Curr Chem. 2015;366:139–167. doi: 10.1007/128_2013_477. [DOI] [PubMed] [Google Scholar]
- Semel AC, Seales EC, Singhal A, Eklund EA, Colley KJ, Bellis SL. Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J Biol Chem. 2002;277:32830–32836. doi: 10.1074/jbc.M202493200. [DOI] [PubMed] [Google Scholar]
- Seppala R, Lehto VP, Gahl WA. Mutations in the human UDP-N-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am J Hum Genet. 1999;64:1563–1569. doi: 10.1086/302411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh FM, Seales EC, Clem WC, Hennessy KM, Zhuo Y, Bellis SL. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp Cell Res. 2008;314:2941–2950. doi: 10.1016/j.yexcr.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay Y, et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci Transl Med. 2016;8:345–387. doi: 10.1126/scitranslmed.aaf7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. Influenza haemagglutinin. Vaccine. 2002;20(Suppl 2):S51–S54. doi: 10.1016/S0264-410X(02)00131-7. [DOI] [PubMed] [Google Scholar]
- Solis D, et al. A guide into glycosciences: How chemistry, biochemistry and biology cooperate to crack the sugar code. Biochim Biophys Acta. 2015;1850:186–235. doi: 10.1016/j.bbagen.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stencel-Baerenwald JE, Reiss K, Reiter DM, Stehle T, Dermody TS. The sweet spot: defining virus-sialic acid interactions. Nat Rev Microbiol. 2014;12:739–749. doi: 10.1038/nrmicro3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida M, et al. Rapid trimming of cell surface polysialic acid (polySia) by exovesicular sialidase triggers release of preexisting surface neurotrophin. J Biol Chem. 2015;290:13202–13214. doi: 10.1074/jbc.M115.638759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson EC, Conley PB, Paulson JC. Regulated expression of alpha 2,6-sialyltransferase by the liver-enriched transcription factors HNF-1, DBP, and LAP. J Biol Chem. 1992;267:3466–3472. [PubMed] [Google Scholar]
- Swift AM, Machamer CE. A Golgi retention signal in a membrane-spanning domain of coronavirus E1 protein. J Cell Biol. 1991;115:19–30. doi: 10.1083/jcb.115.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindall AF, Bellis SL. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem. 2011;286:22982–22990. doi: 10.1074/jbc.M110.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindall AF, Londono-Joshi AI, Schultz MJ, Fineberg N, Buchsbaum DJ, Bellis SL. ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 2013;73:2368–2378. doi: 10.1158/0008-5472.CAN-12-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Tsuji S, Tsujimoto M. Characterization of the second type of human beta-galactoside alpha 2,6-sialyltransferase (ST6Gal II), which sialylates Galbeta 1,4GlcNAc structures on oligosaccharides preferentially. Genomic analysis of human sialyltransferase genes. J Biol Chem. 2002;277:45719–45728. doi: 10.1074/jbc.M206808200. [DOI] [PubMed] [Google Scholar]
- Tanaka F, et al. Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Res. 2000;60:3072–3080. [PubMed] [Google Scholar]
- Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7:440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MG, Foley DA, Swartzentruber KG, Colley KJ. Sequences at the interface of the fifth immunoglobulin domain and first fibronectin type III repeat of the neural cell adhesion molecule are critical for its polysialylation. J Biol Chem. 2011;286:4525–4534. doi: 10.1074/jbc.M110.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MG, Foley DA, Colley KJ. The polysialyltransferases interact with sequences in two domains of the neural cell adhesion molecule to allow its polysialylation. J Biol Chem. 2013;288:7282–7293. doi: 10.1074/jbc.M112.438374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya A, et al. PolySia-NCAM modulates the formation of ductular reactions in liver injury. Hepatology. 2014 doi: 10.1002/hep.27099. [DOI] [PubMed] [Google Scholar]
- Tu L, Tai WC, Chen L, Banfield DK. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 2008;321:404–407. doi: 10.1126/science.1159411. [DOI] [PubMed] [Google Scholar]
- Tu L, Chen L, Banfield DK. A conserved N-terminal arginine-motif in GOLPH3-family proteins mediates binding to coatomer. Traffic. 2012;13:1496–1507. doi: 10.1111/j.1600-0854.2012.01403.x. [DOI] [PubMed] [Google Scholar]
- Uemura T, et al. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28:1218–1229. doi: 10.1038/onc.2008.471. [DOI] [PubMed] [Google Scholar]
- van Karnebeek CD, et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat Genet. 2016;48:777–784. doi: 10.1038/ng.3578. [DOI] [PubMed] [Google Scholar]
- Varki A, Crocker PR (2009) I-type lectins. In: Varki A et al (eds) Essentials of glycobiology, 2nd edn. Cold Spring Harbor, NY
- Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann NY Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Schauer R (2009) Sialic acids. In: Varki A et al (eds) Essentials of glycobiology, 2nd edn. Cold Spring Harbor, NY
- Viswanathan K, Tomiya N, Park J, Singh S, Lee YC, Palter K, Betenbaugh MJ. Expression of a functional Drosophila melanogaster CMP-sialic acid synthetase. Differential localization of the Drosophila and human enzymes. J Biol Chem. 2006;281:15929–15940. doi: 10.1074/jbc.M512186200. [DOI] [PubMed] [Google Scholar]
- Volkers G, et al. Structure of human ST8SiaIII sialyltransferase provides insight into cell-surface polysialylation. Nat Struct Mol Biol. 2015;22:627–635. doi: 10.1038/nsmb.3060. [DOI] [PubMed] [Google Scholar]
- von Der Ohe M, et al. Localization and characterization of polysialic acid-containing N-linked glycans from bovine NCAM. Glycobiology. 2002;12:47–63. doi: 10.1093/glycob/12.1.47. [DOI] [PubMed] [Google Scholar]
- Wang X, Vertino A, Eddy RL, Byers MG, Jani-Sait SN, Shows TB, Lau JT. Chromosome mapping and organization of the human beta-galactoside alpha 2,6-sialyltransferase gene. Differential and cell-type specific usage of upstream exon sequences in B-lymphoblastoid cells. J Biol Chem. 1993;268:4355–4361. [PubMed] [Google Scholar]
- Wang X, Li X, Zeng YN, He F, Yang XM, Guan F. Enhanced expression of polysialic acid correlates with malignant phenotype in breast cancer cell lines and clinical tissue samples. Int J Mol Med. 2016;37:197–206. doi: 10.3892/ijmm.2015.2395. [DOI] [PubMed] [Google Scholar]
- Weinhold B, et al. Deficits in sialylation impair podocyte maturation. J Am Soc Nephrol. 2012;23:1319–1328. doi: 10.1681/ASN.2011090947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J, Lee EU, McEntee K, Lai PH, Paulson JC. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987;262:17735–17743. [PubMed] [Google Scholar]
- Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Weiss P, Tietze F, Gahl WA, Seppala R, Ashwell G. Identification of the metabolic defect in sialuria. J Biol Chem. 1989;264:17635–17636. [PubMed] [Google Scholar]
- Weisz OA, Swift AM, Machamer CE. Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J Cell Biol. 1993;122:1185–1196. doi: 10.1083/jcb.122.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen DX, Svensson EC, Paulson JC. Tissue-specific alternative splicing of the beta-galactoside alpha 2,6-sialyltransferase gene. J Biol Chem. 1992;267:2512–2518. [PubMed] [Google Scholar]
- Werneburg S, Buettner FF, Erben L, Mathews M, Neumann H, Mühlenhoff M, Hildebrandt H. Polysialylation and lipopolysaccharide-induced shedding of E-selectin ligand-1 and neuropilin-2 by microglia and THP-1 macrophages. Glia. 2016;64:1314–1330. doi: 10.1002/glia.23004. [DOI] [PubMed] [Google Scholar]
- Wilks S, de Graaf M, Smith DJ, Burke DF. A review of influenza haemagglutinin receptor binding as it relates to pandemic properties. Vaccine. 2012;30:4369–4376. doi: 10.1016/j.vaccine.2012.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Geyer H, von der Ohe M, Gerardy-Schahn R, Schachner M, Geyer R. Localization of defined carbohydrate epitopes in bovine polysialylated NCAM. Biochimie. 2003;85:207–218. doi: 10.1016/S0300-9084(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Yabe U, Sato C, Matsuda T, Kitajima K. Polysialic acid in human milk. CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J Biol Chem. 2003;278:13875–13880. doi: 10.1074/jbc.M300458200. [DOI] [PubMed] [Google Scholar]
- Yang WH, Nussbaum C, Grewal PK, Marth JD, Sperandio M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood. 2012;120:1015–1026. doi: 10.1182/blood-2012-04-424366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapater JL, Colley KJ. Sequences prior to conserved catalytic motifs of polysialyltransferase ST8Sia IV are required for substrate recognition. J Biol Chem. 2012;287:6441–6453. doi: 10.1074/jbc.M111.322024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ghadiri-Sani M, Zhang X, Richardson PM, Yeh J, Bo X. Induced expression of polysialic acid in the spinal cord promotes regeneration of sensory axons. Mol Cell Neurosci. 2007;35:109–119. doi: 10.1016/j.mcn.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Wu D, Verhaagen J, Richardson PM, Yeh J, Bo X. Lentiviral-mediated expression of polysialic acid in spinal cord and conditioning lesion promote regeneration of sensory axons into spinal cord. Mol Ther. 2007;15:1796–1804. doi: 10.1038/sj.mt.6300220. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wardwell PR, Bader RA. In vitro efficacy of polysaccharide-based nanoparticles containing disease-modifying antirheumatic drugs. Pharm Res. 2014 doi: 10.1007/s11095-014-1329-z. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dong D, Li P, Wang D, Mu H, Niu H, Duan J. Novel pH-sensitive polysialic acid based polymeric micelles for triggered intracellular release of hydrophobic drug. Carbohydr Polym. 2016;139:75–81. doi: 10.1016/j.carbpol.2015.12.041. [DOI] [PubMed] [Google Scholar]
- Zhao W, Colley K (eds) (2008) Nucleotide sugar transporters of the Golgi apparatus. The Golgi Apparatus
- Zhao W, Chen TL, Vertel BM, Colley KJ. The CMP-sialic acid transporter is localized in the medial-trans Golgi and possesses two specific endoplasmic reticulum export motifs in its carboxyl-terminal cytoplasmic tail. J Biol Chem. 2006;281:31106–31118. doi: 10.1074/jbc.M605564200. [DOI] [PubMed] [Google Scholar]
- Zhou GP, Huang RB, Troy FA., II 3D structural conformation and functional domains of polysialyltransferase ST8Sia IV required for polysialylation of neural cell adhesion molecules. Protein Pept Lett. 2015;22:137–148. doi: 10.2174/0929866521666141019192221. [DOI] [PubMed] [Google Scholar]
- Zuber C, Lackie PM, Catterall WA, Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J Biol Chem. 1992;267:9965–9971. [PubMed] [Google Scholar]


