Abstract
The Sec7 domain ADP-ribosylation factor (Arf) guanine nucleotide exchange factors (GEFs) are found in all eukaryotes, and are involved in membrane remodeling processes throughout the cell. This review is focused on members of the GBF/Gea and BIG/Sec7 subfamilies of Arf GEFs, all of which use the class I Arf proteins (Arf1-3) as substrates, and play a fundamental role in trafficking in the endoplasmic reticulum (ER)—Golgi and endosomal membrane systems. Members of the GBF/Gea and BIG/Sec7 subfamilies are large proteins on the order of 200 kDa, and they possess multiple homology domains. Phylogenetic analyses indicate that both of these subfamilies of Arf GEFs have members in at least five out of the six eukaryotic supergroups, and hence were likely present very early in eukaryotic evolution. The homology domains of the large Arf1 GEFs play important functional roles, and are involved in interactions with numerous protein partners. The large Arf1 GEFs have been implicated in several human diseases. They are crucial host factors for the replication of several viral pathogens, including poliovirus, coxsackievirus, mouse hepatitis coronavirus, and hepatitis C virus. Mutations in the BIG2 Arf1 GEF have been linked to autosomal recessive periventricular heterotopia, a disorder of neuronal migration that leads to severe malformation of the cerebral cortex. Understanding the roles of the Arf1 GEFs in membrane dynamics is crucial to a full understanding of trafficking in the secretory and endosomal pathways, which in turn will provide essential insights into human diseases that arise from misregulation of these pathways.
Keywords: Guanine nucleotide exchange factor, GBF1, BIG1, BIG2, ADP-ribosylation factor, Small G protein
Introduction
The distinguishing feature of eukaryotic cells is their internal membrane organization. Membrane-bound organelles such as the endoplasmic reticulum (ER), the Golgi apparatus, and endosomes, ensure that specialized functions are carried out in the appropriate delimited environment. Each organelle possesses a characteristic lipid and protein composition, but is highly dynamic and linked to other organelles via trafficking pathways. There are two major membrane trafficking systems in eukaryotes, the secretory and the endocytic systems. The secretory pathway transports secreted and membrane proteins synthesized in the ER to their final destination (plasma membrane (PM), cell exterior or intracellular organelle). Endocytosis pathways transport material from the cell exterior to intracellular organelles such as the lysosome (or vacuole in yeasts).
The Golgi apparatus is a structurally complex and highly dynamic organelle that is found at the crossroads of the secretory and endosomal membrane trafficking pathways. The mechanisms that generate and maintain Golgi structure are not well understood. However, activation of the ADP-ribosylation factor 1 (Arf1) small G protein is absolutely required to maintain Golgi structure and function in eukaryotic cells, since inhibiting Arf1 activation by drugs or mutations causes complete disassembly of the Golgi apparatus and a complete block in trafficking pathways through the Golgi (Klausner et al. 1992; Jackson 2009). Membrane trafficking in eukaryotic cells is mediated by transport vesicles that bud from a donor compartment, then are targeted to and fuse with an acceptor compartment (Bonifacino and Glick 2004; Behnia and Munro 2005). Arf1 is required for vesicle budding, where its activation results in recruitment of effectors such as coat complexes to membranes. Coat complexes deform membranes and concentrate cargo into a membrane domain to form a vesicle that carries the cargo from the donor to acceptor compartment. Targeting of a vesicle to its correct acceptor compartment membrane requires tethering molecules that link the vesicle and target membranes at a distance. A series of steps ensues that result in engagement of SNARE proteins, which mediate membrane fusion (Bonifacino and Glick 2004). The proteins involved in vesicle budding, cargo sorting, tethering, and fusion are highly conserved in evolution (Behnia and Munro 2005).
Recent advances in genome sequencing efforts have allowed definition of six major supergroups of eukaryotes (Adl et al. 2005). Interestingly, phylogenetic studies have indicated that the Last Common Eukaryotic Ancestor (the LCEA) possessed a complex endomembrane system and that many of the major families of proteins involved in trafficking arose prior to the divergence of the eukaryotic supergroups (Jekely 2003; Dacks and Field 2007; Gurkan et al. 2007; Kloepper et al. 2007; Pereira-Leal 2008). The LCEA probably already had small G proteins including primordial Arf and Rab proteins, coat complexes, membrane tethering complexes, and SNAREs (Gurkan et al. 2007; Koumandou et al. 2007; Kloepper et al. 2008; Pereira-Leal 2008; Dacks et al. 2009).
There are nine subfamilies of Sec7 domain Arf guanine nucleotide exchange factors (GEFs) in eukaryotes (Cox et al. 2004). In this review, we will focus on two of these subfamilies, the GBF/Gea and BIG/Sec7 GEFs. Other well-studied subfamilies of Arf GEFs include the ARNO/cytohesin, SYT1, SYT2, EFA6, and BRAG subfamilies, which are in general smaller proteins, and which function primarily in endosomal–PM trafficking pathways (Shin and Nakayama 2004; Casanova 2007; Gillingham and Munro 2007). Of the nine subfamilies of Arf GEFs, only those of the GBF/Gea, BIG/Sec7, ARNO/cytohesin, and RalF subfamilies have significant activity on the class I Arfs, Arf1–Arf3. RalF is the founding member of a family of bacterial Arf1 GEFs, first found in Legionella pneumophila and Rickettsia prowazekii (Nagai et al. 2002; Amor et al. 2005). These proteins do not arise from the bacteria themselves, but were incorporated into these bacterial genomes through horizontal transfer from their eukaryotic host (Nagai et al. 2002). We will not discuss this special subfamily of Arf1 GEFs in this review. Among the eukaryotic Arf1 GEFs, there has been some controversy as to whether ARNO/cytohesin GEFs actually use class I Arfs as substrates in vivo, as they localize primarily to the endosomal–PM system where Arf6 functions. However, a key paper from the Donaldson laboratory has provided new insights into this issue through identification of a GTPase cascade involving both Arf1 and Arf6 in cells (Cohen et al. 2007). Cohen et al. demonstrated that interaction of Arf6-GTP with the PH domain of ARNO is essential for its recruitment to the PM, where it then activates Arf1. Structural and biochemical analyses showing release of an autoinhibitory interaction within ARNO by Arf6-GTP provide further molecular insight into this regulatory cascade (DiNitto et al. 2007). Hence, although the ARNO/cytohesin proteins are bona fide Arf1 GEFs, we will focus this review on the GBF/Gea and BIG/Sec7 Arf1 GEFs, which function in Golgi and endosomal membrane trafficking pathways.
The GBF/Gea and BIG/Sec7 subfamilies are related, with members sharing sequence similarity principally in five homology domains (Mouratou et al. 2005). The majority of these Arf1 GEFs are high molecular weight proteins, on the order of 200 kDa, and for this reason they have been referred to as the large Arf GEFs. The GBF/Gea and BIG/Sec7 Arf1 GEFs function in internal membrane systems such as the Golgi apparatus, the trans-Golgi network (TGN) and endosomal pathways (Zhao et al. 2002; Shin and Nakayama 2004; Park et al. 2005; Casanova 2007; Gillingham and Munro 2007).
Although the GBF/Gea and BIG/Sec7 Arf1 GEFs share a common domain structure, they clearly form two distinct subfamilies (Cox et al. 2004; Mouratou et al. 2005). Members of each subfamily have different localizations and functions, with GBF/Gea GEFs in general functioning in the ER-early Golgi membrane system and BIG/Sec7 GEFs functioning in the late-Golgi and endosomal membrane systems. Mammalian GBF1 localizes primarily to the intermediate compartment between the ER and the Golgi (ERGIC) and to cis-Golgi membranes (Kawamoto et al. 2002; Zhao et al. 2002; Garcia-Mata et al. 2003), and yeast Gea1p and Gea2p localize to the cis-Golgi (Chantalat et al. 2003). In plants, all the Arf GEFs fall into the GBF/Gea and BIG/Sec7 subfamilies, so the situation is more complicated. Like its yeast and mammalian counterparts, the A. thaliana Arf1 GEF GNL1 localizes to and functions at the cis-Golgi, but another member of the GBF/Gea subfamily, GNOM, localizes to and functions in the endosomal membrane system, although it still retains a capacity to function in the early Golgi (Richter et al. 2007). To compensate for the lack of endosomal–PM Arf GEFs, plants have thus diversified the functions of the GBF/Gea subfamily members, and possibly those of the BIG/Sec7 subfamily as well. In mammals and yeast, the BIG/Sec7 proteins localize to and function at the trans-Golgi, the TGN and endosomes (Franzusoff et al. 1991; Shinotsuka et al. 2002a; Shinotsuka et al. 2002b; Zhao et al. 2002; Charych et al. 2004; Shin et al. 2004).
In this review, we will discuss the evolution of these Arf1 GEFs, and present an analysis of conserved domains that are common to both subfamilies as well as those that are specific to either the GBF/Gea or BIG/Sec7 proteins. We will describe the interacting partners of these Arf1 GEFs, indicating which of the homology regions of these proteins are involved in each interaction. Finally, we will describe studies that have implicated the Arf1 GEFs in human disease, including recent studies describing specific inhibitors of members of the GBF/Gea subfamily of Arf1 GEFs.
Phylogenetic analysis of the Arf1 GEFs
The number of complete genome sequences has increased dramatically in the past few years, and now are available in public databanks for a wide variety of both prokaryotes and eukaryotes. Analyses of these genome sequences have indicated that there are six major supergroups of eukaryotes that diverged from the LCEA (Adl et al. 2005). These supergroups are the Excavata, the Chromalveolata, the Archaeplastidia, the Opisthokonta, the Amoebozoa, and the Rhizaria. Fungi, including the well-studied model system Saccharomyces cerevisiae and mammals, are part of the same supergroup, the Opisthokonta. Complete genome sequences from members of five of the six eukaryotic supergroups are available, with the Rhizaria being the one exception. We searched the complete genome sequences of representative members of these five supergroups for members of the two subfamilies of large Arf1 GEFs, and found members of both the GBF/Gea and the BIG/Sec7 proteins (Tables 1, 2). Humans have two BIG/Sec7 proteins, called BIG1 and BIG2, and only one GBF/Gea subfamily member, GBF1. Arabidopsis, which like other plant species of the Archaeplastida, have only GBF/Gea and BIG/Sec7 Arf1 GEF subfamilies and no others, have five BIGs and three GBFs. Paramecium tetraurelia, a protozoan that belongs to the Chromalveolata supergroup, has nine BIG/Sec7 members, but no GBF/Gea sequences. The model organism Saccharomyces cerevisiae has one member of the BIG/Sec7 subfamily, Sec7p, and two members of the GBF/Gea subfamily, Gea1p and Gea2p (likely due to a recent genome duplication event). The human pathogen Cryptosporidium parvum, a member of the Apicomplexa, has no BIG/Sec7 proteins, and only one GBF/Gea sequence. The C. parvum GBF protein shares a high level of homology with plant GBF proteins such as the Arabidopsis thaliana GNOM protein. The Apicomplexa are characterized by the apicoplast, an organelle which is thought to be the remnant of an endosymbiotic algal cell (Abrahamsen et al. 2004; Huang et al. 2004). It is likely that the C. parvum GBF gene, like other plant-like genes in this organism, was transferred from the primordial endosymbiosed algal cell to the nucleus of the Apicomplexan ancestor that engulfed it (Huang et al. 2004). The closely related Cryptosporidium hominis species has a very divergent Arf GEF that appears never-the-less to be a member of the GBF/Gea subfamily (Xu et al. 2004).
Table 1.
Sequences used for the phylogenetic analysis and multiple alignments
| Super-groups | Groups | Species | Protein name | Accession number | Size in amino acids |
|---|---|---|---|---|---|
| Opisthokonta | Mammals | Canis familiaris | BIG1 Canf | XP_535095.2 | 1,849 |
| GBF1 Canf | XP_850976.1 | 1,872 | |||
| Equus caballus | BIG1 Equc | XP_001494609.1 | 1,840 | ||
| GBF1 Equc | XP_001499167.1 | 1,858 | |||
| Homo sapiens | BIG1 Homsa | Q9Y6D6.2 | 1,849 | ||
| BIG2 Homsa | Q9Y6D5.3 | 1,785 | |||
| GBF1 Homsa | Q92538.2 | 1,859 | |||
| Monodelphis domestica | BIG1 Mond | XP_001368081.1 | 1,849 | ||
| GBF1 Mond | XP_001369356.1 | 1,861 | |||
| Mus musculus | BIG1 Musm | XP_001473216 | 1,846 | ||
| GBF1 Musm | NP_849261.2 | 1,861 | |||
| Pan troglodytes | BIG1 Pant | XP_519797.2 | 1,849 | ||
| GBF1 Pant | XP_521592.2 | 1,859 | |||
| Rattus norvegicus | BIG1 Ratna | EDM11544.1 | 1,846 | ||
| GBF1 Ratna | XP_001066196.1 | 1,862 | |||
| Invertebratesss | Anopheles gambiae | BIG Anoga | XP_319652.3 | 1,662 | |
| GBF Anoga | XP_321598.4 | 2,000 | |||
| Drosophila melanogaster | BIG Droma | NP_609675.2 | 1,653 | ||
| GBF Droma | NP_610761.2 | 1,983 | |||
| Nasonia vitripennis | BIG Nasv | XP_001605970.1 | 1,818 | ||
| GBF Nasv | XP_001601088.1 | 1,767 | |||
| Tribolium castaneum | BIG Tric | XP_972785.1 | 1,663 | ||
| GBF Tric | XP_967092.1 | 1,742 | |||
| Fungi | Candida glabrata | SEC7 Cang | XP_445724.1 | 1,825 | |
| GEA Cang | XP_447385.1 | 1,454 | |||
| Kluyveromyces lactis | GEA Klula | XP_454979.1 | 1,387 | ||
| SEC7 Klula | XP_453828.1 | 1,848 | |||
| Saccharomyces cerevisiae | GEA1 Sacca | P47102.1 | 1,408 | ||
| GEA2 Sacca | P39993 | 1,459 | |||
| SEC7 Sacca | P11075.2 | 2,009 | |||
| Schizosaccharomyces pombe | SEC71 Schp | Q9UT02.1 | 1,811 | ||
| SEC72 Schp | Q9P7V5.1 | 1,822 | |||
| GEA Schp | CAB75411.1 | 1,462 | |||
| Amoebozoa | Mycetozoa | Dictyostelium discoideum | BIG Dicda | XP_635808.1 | 1,886 |
| GBF Dicda | XP_635561.1 | 1,748 | |||
| Archaeplastida | Flowering | Arabidopsis thaliana | BIG1 Arat | AAF76474.1 | 1,750 |
| BIG2 Arat | NP_191645.1 | 1,793 | |||
| BIG3 Arat | NP_195533.1 | 1,698 | |||
| BIG4 Arata | O65490 | 1,711 | |||
| GNL1 Arat | Q9FLY5 | 1,443 | |||
| GNL2 Arat | NP_197462.1 | 1,375 | |||
| GNOM Arata | Q42510.1 | 1,451 | |||
| Oryza sativa | BIG Orysa | AAM00191.1 | 1,789 | ||
| GBF3 Orysa | EAY91294.1 | 1,456 | |||
| Vitis vinifera | BIG Vitv | CAO24490.1 | 1,653 | ||
| GBF3 Vitv | CAN61434.1 | 1,433 | |||
| Algae | Chlamydomonas reinhardtii | BIG Chlra | XP_001694943.1 | 2,150 | |
| GBF Chlra | XP_001700166.1 | 1,490 | |||
| Ostreococcus tauri | BIG Ostta | CAL51840.1 | 1,743 | ||
| Chromalveolata | Alveolata | Tetrahymena thermophila | BIG Tetta | XP_001031228.2 | 1,842 |
| Cryptosporidium parvum | GBF Crypa | XP_625855.1 | 1,800 | ||
| Excavata | Protist | Trichomonas vaginalis | BIG Triva | XP_001327074.1 | 1,305 |
| GBF Triva | XP_001316830.1 | 1,204 |
Table 2.
Additional sequences used only for phylogenetic analysis
| GEF subfamily | Species | Protein name | Accession number | Size in amino acids |
|---|---|---|---|---|
| GBF/Gea | Canis lupus familiaris | GBF1 Canf | XP_850976.1 | 1,872 |
| Danio rerio | GBF1 Danr | XP_694714.3 | 2,059 | |
| Gallus gallus | GBF1 Galg | XP_421632.2 | 1,852 | |
| Caenorhabditis elegans | GBF Caee | NP_499522.2 | 2,005 | |
| Strongylocentrotus purpuratus | GBF Strp | XP_001193947.1 | 1,447 | |
| Hydra magnipapillata | GBF Hydm | XP_002167603.1 | 1,705 | |
| Ciona intestinalis | GBF Cioi | XP_002128867.1 | 1,842 | |
| Neurospora crassa | GBF Neuc | XP_962693.2 | 1,588 | |
| Ustilago maydis | GBF Ustm | XP_757309.11738 | 1,738 | |
| Monosiga brevicollis | GBF Monb | XP_001742906.1 | 1,541 | |
| Coprinopsis cinerea okayama | GBF Copo | XP_001833466.1 | 1,499 | |
| BIG/Sec7 | Rattus norvegicus | BIG2 Ratn | NP_851597 | 1,791 |
| Canis lupus familiaris | BIG1 Canf | XP_859391.1 | 1,859 | |
| Danio rerio | BIG1 Danr | XP_001923315.1 | 1,833 | |
| Gallus gallus | BIG1 Galg | XP_418283.2 | 1,537 | |
| Caenorhabditis elegans | BIG Caee | NP_001129800.1 | 1,628 | |
| Strongylocentrotus purpuratus | BIG Strp | XP_791125.2 | 1,396 | |
| Ciona intestinalis | BIG Cioi | XP_002123264.1 | 1,689 | |
| Neurospora crassa | BIG Neuc | XP_962785.2 | 1,948 | |
| Ustilago maydis | BIG Ustm | XP_760498.1 | 2,038 | |
| Monosiga brevicollis | BIG Monb | XP_001748607.1 | 1,786 | |
| Coprinopsis cinerea okayama | BIG Copo | XP_001837141.1 | 1,910 | |
| Paramecium tetraurelia | GGG1 Part | XP_001428865 | 1,615 | |
| GGG2 Part | XP_001437414 | 1,615 | ||
| GGG3 Part | XP_001429046 | 1,598 | ||
| GGG4 Part | XP_001431359 | 1,599 | ||
| GGG5 Part | XP_001450751 | 1,473 |
Phylogenetic analysis of the BIG/Sec7 and GBF/Gea subfamilies of Arf GEFs using members from five eukaryotic supergroups indicates a clear separation of these two subfamilies (Fig. 1). The entire Arf GEF sequences were used for the phylogenetic analysis, not just the Sec7 domains as in previous studies (Cox et al. 2004; Mouratou et al. 2005). Using three different programs, the same tree topology was obtained, with high confidence levels for a separation of the BIG/Sec7 and GBF/Gea sequences into two different clades (Fig. 1). Hence the presence of two subfamilies of large Arf GEFs is ancient, and it is likely that both GBF/Gea and BIG/Sec7 proteins existed within the LCEA before the separation of the different eukaryotic lineages. The nine protozoan Paramecium tetraurelia Arf GEFs, including the five denoted GGG1-5, clearly fall into the BIG/Sec7 subfamily (Fig. 1).
Fig. 1.
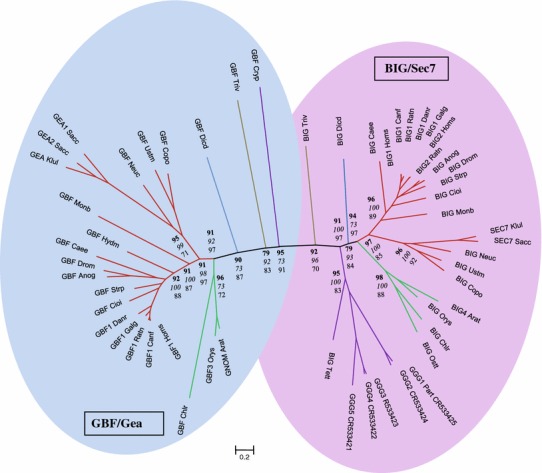
Unrooted phylogenetic tree of the BIG/Sec7 and GBF/Gea Arf GEFs using the full-length sequence of each protein. Three separate analyses were performed using the programs MrBayes (Bayesian inference), PAUP (maximum parsimony), and PhyML (maximum likelihood). Bootstrap percentages are shown for each, with results using MrBayes in bold, PAUP in italics, and a PhyML in normal text. The sequences used for the analysis are shown in Tables 1 and 2. Branches of the tree are color coded to indicate the eukaryotic supergroup that each sequence belongs to: Opisthokonta (red), Archaeplastidia (green), Amoebozoa (blue), Chromalveolata (violet), and Excavata (beige)
Homology domains of the GBF/Gea and BIG/Sec7 Arf1 GEFs
As described previously, the large Arf1 GEFs have five major sequence homology domains that are common to members of both the GBF/Gea and the BIG/Sec7 subfamilies (Mouratou et al. 2005). These domains are the DCB and the HUS domains upstream of the catalytic Sec7 domain, and HDS1, HDS2, and HDS3 that lie downstream of the Sec7 domain (Fig. 2). This analysis was based on sequence data from organisms found primarily within two eukaryotic supergroups. When representative members of five of the six eukaryotic supergroups are analyzed, the five common homology domains are present in the Arf1 GEFs of most of the organisms examined, in both GBF/Gea and BIG/Sec7 subfamily members (Figs. 3, 4, 5, 6, 7, 8). However, HDS2 and HDS3 are the least conserved domains, particularly for the GBF/Gea proteins, and we were unable to find evidence for these domains in the GBF protein of the Chromalveolata supergroup member C. parvum.
Fig. 2.
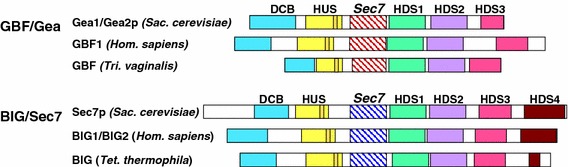
Domain structure of the BIG/Sec7 and GBF/Gea Arf GEFs. Positions of the homology domains are shown for representative sequences of the Opisthokonta supergroup (Saccharomyces cerevisiae, Homo sapiens), and of the Excavata and Chromalveolata supergroups (Trichomonas vaginalis, Tetrahymena thermophila); positions of domains and sizes of proteins are drawn to scale. The only homology domain unique to one subfamily is the BIG/Sec7 HDS4 sequence; only a portion of the region shown in Fig. 10 is conserved in the Chromalveolata members that we analyzed (amino acid residues 1,600–1,665 of the Tetrahymena thermophila BIG protein, corresponding to amino acids 1,675–1,740 of human BIG1). The orange box within the HUS domain represents the highly conserved HUS box, sequence Y/F Φ N Y/F D C D/E/N (Φ: hydrophobic)
Fig. 3.
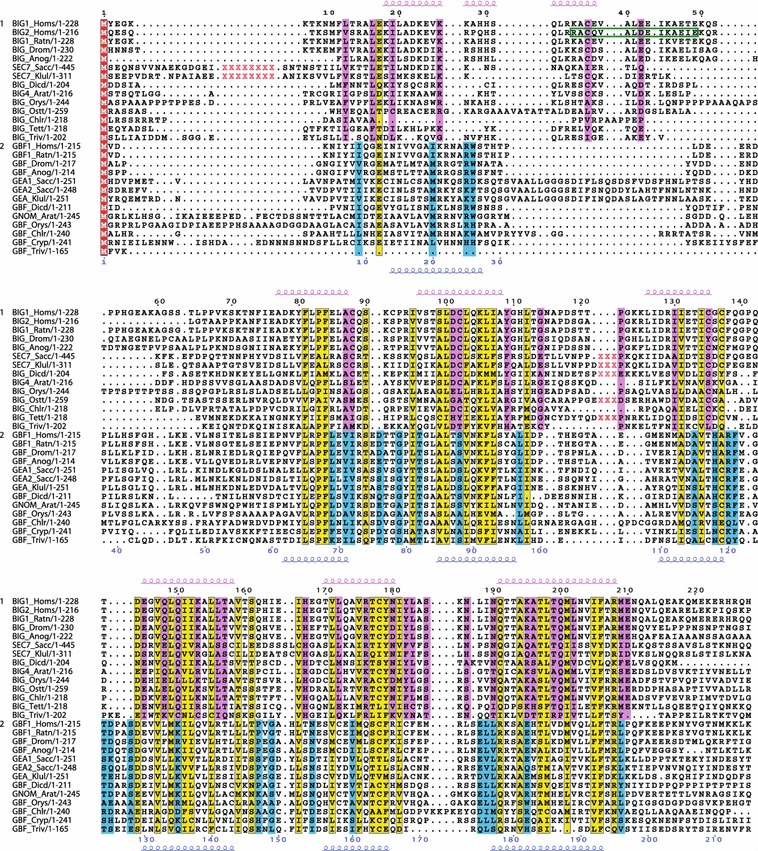
Conserved residues within the N-terminal region of the BIG/Sec7 and GBF/Gea subfamilies of Arf GEFs containing the DCB domain. Multiple sequence alignment showing conserved residues specific to the BIG/Sec7 subfamily (pink), specific to the GBF/Gea subfamily (blue), or both subfamilies (yellow). The most highly conserved portion of this region contains the DCB domain. Secondary structure prediction of alpha-helical regions is shown above alignment in pink for BIG/Sec7 sequences, and below alignment in blue for GBF/Gea sequences. Deletions in sequences are indicated by red Xs, and correspond to: S18-G217, S319-P336 in SEC7_Sacc, R17-M82, S186-P202 in SEC7_Klul, P106-G109 in BIG_Dicd, V120-H154 in BIG_Ostt, V89-N116 in BIG_Chlr, and K101-D113 in BIG_Tett. Protein sequence alignments were created using Clustal W 1.83 (Thompson et al. 1994) and T-coffee (Notredame et al. 2000) with default parameters. The multiple alignments were manually adjusted and edited using BioEdit version 7.0.8 (http://www.mbio.ncsu.edu/bioedit/bioedit.html). The GBF/Gea and BIG/Sec7 alignments (done separately) were imported into BioEdit, then these were manually corrected to correspond to the combined alignment. Aligned sequences were displayed with ESPript (Gouet et al. 1999) using the BLOSUM62 matrix with a similarity global score of 0.15 and a difference score between conserved groups of 0.5. Secondary structure predictions on multiple alignments were performed at the Pôle Bioinformatique Lyonnais (http://pbil.univ-lyon1.fr/) and the consensus of three different programs (the PHD, Predator, and GOR IV) is indicated. Green box indicates AKAP domain of BIG2
Fig. 4.
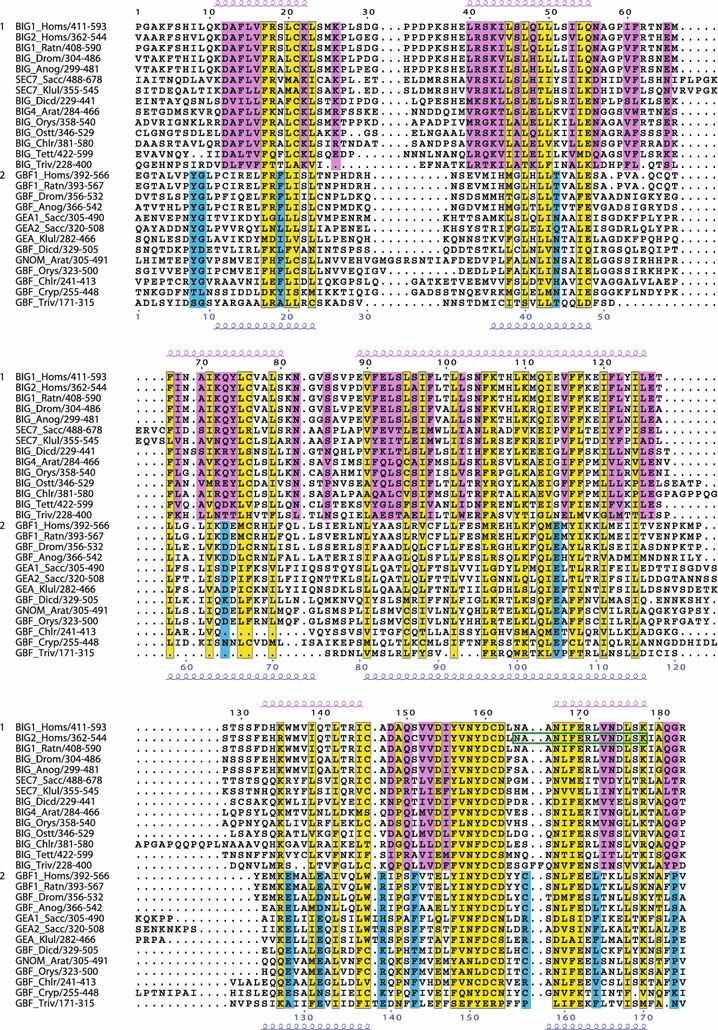
Conserved residues within the N-terminal region containing the HUS domain. Multiple sequence alignment showing conserved residues specific to the BIG/Sec7 subfamily (pink), specific to the GBF/Gea subfamily (blue), or both subfamilies (yellow). Secondary structure prediction as in Fig. 2. Green box indicates AKAP domain of BIG2
Fig. 5.
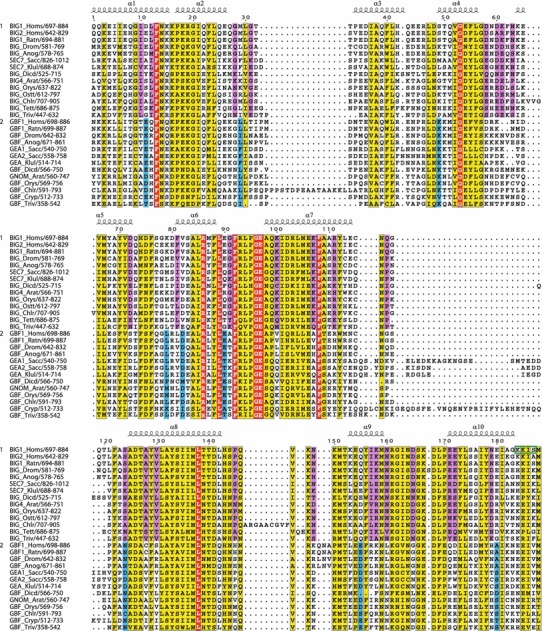
Conserved residues within the Sec7 domain. Multiple sequence alignment showing conserved residues specific to the BIG/Sec7 subfamily (pink), specific to the GBF/Gea subfamily (blue), or both subfamilies (yellow). Invariant residues in all the sequences are shown in red. Alpha-helical regions from crystal structures of Sec7 domains is shown. Green box indicates PKA phosphorylation site identified in BIG1
Fig. 6.
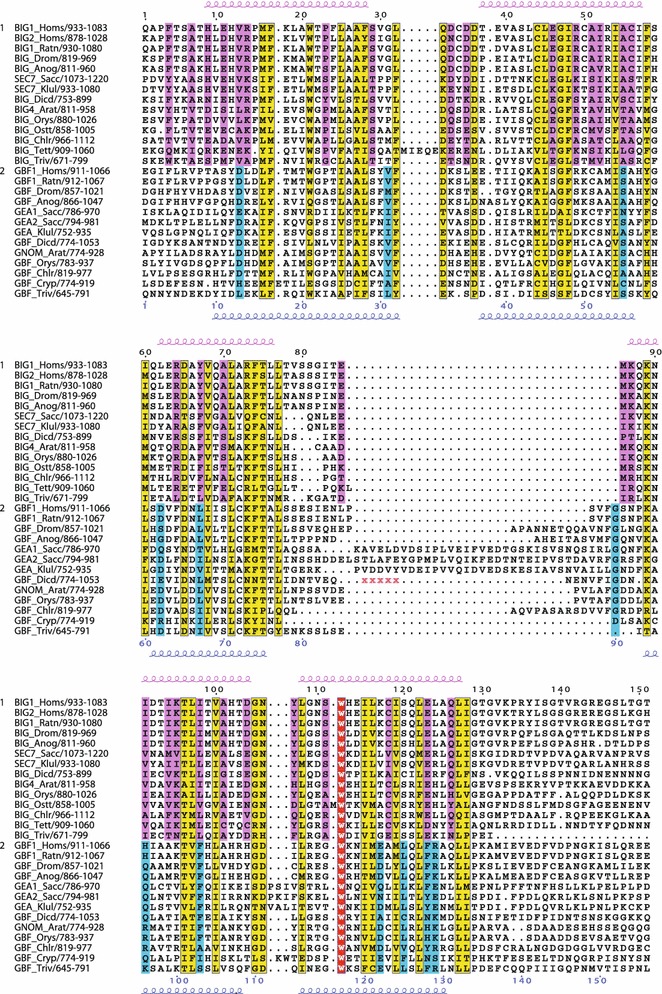
Conserved residues within the C-terminal region including the HDS1 domain. Multiple sequence alignment showing conserved residues specific to the BIG/Sec7 subfamily (pink), specific to the GBF/Gea subfamily (blue), or both subfamilies (yellow). Invariant residues in all the sequences are shown in red. Secondary structure prediction as in Fig. 2. Deletions in sequences are indicated by red Xs, and correspond to: Q858-Y982 in GBF_Dicd
Fig. 7.
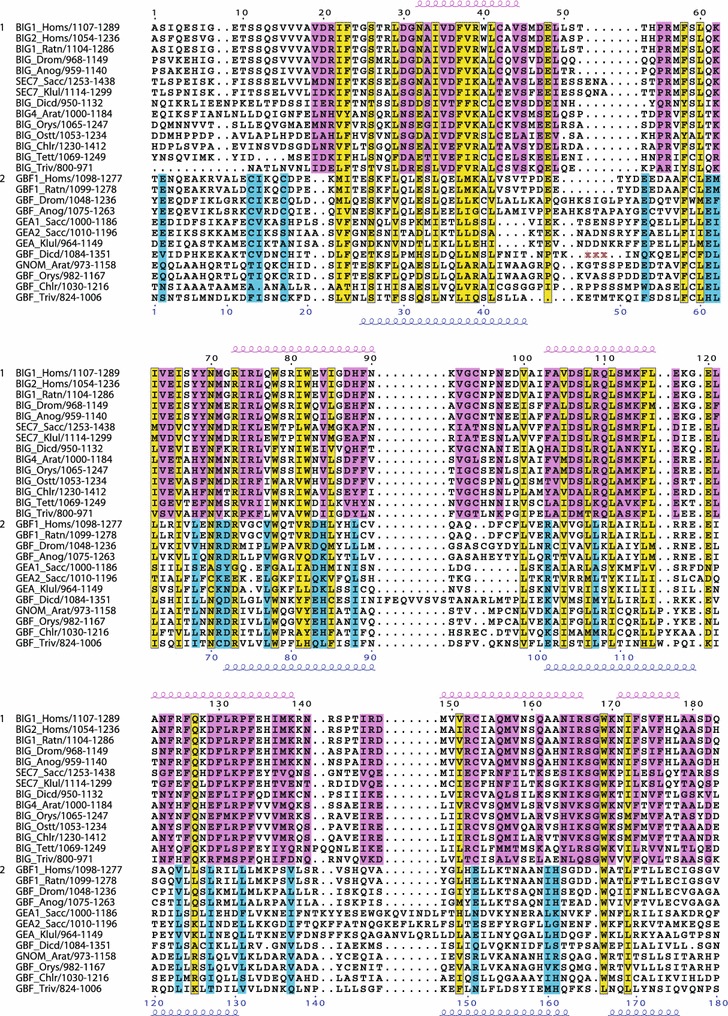
Conserved residues within the C-terminal region including the HDS2 domain. Multiple sequence alignment showing conserved residues specific to the BIG/Sec7 subfamily (pink), specific to the GBF/Gea subfamily (blue), or both subfamilies (yellow). Secondary structure prediction as in Fig. 2. Deletions in sequences are indicated by red Xs, and correspond to: Q1133-Y1211 in GBF_Dicd
Fig. 8.
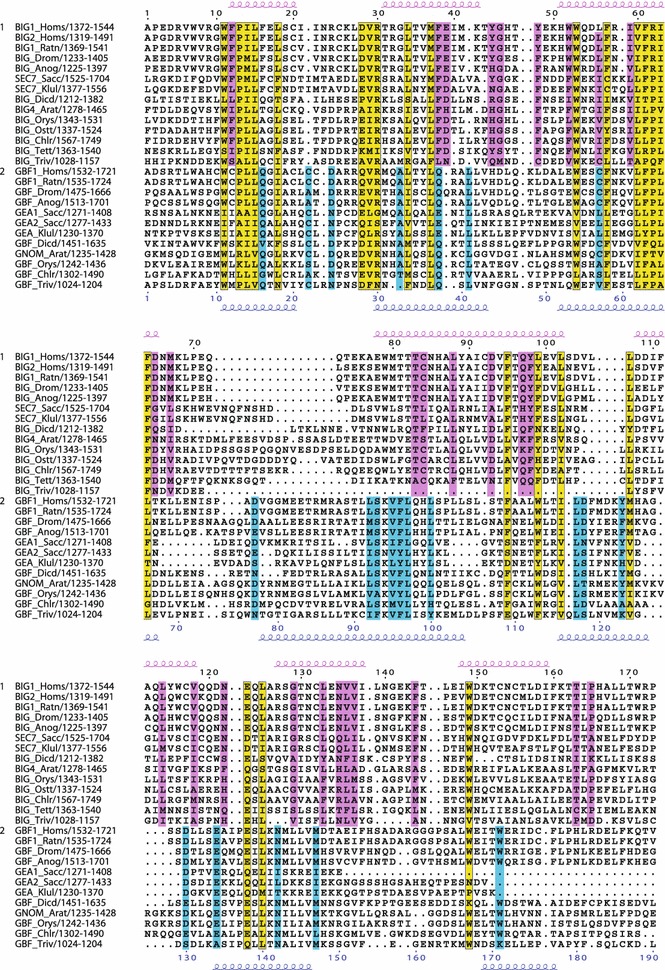
Conserved residues within the C-terminal region including the HDS3 domain. Multiple sequence alignment showing conserved residues specific to the BIG/Sec7 subfamily (pink), specific to the GBF/Gea subfamily (blue), or both subfamilies (yellow). Secondary structure prediction as in Fig. 2. This domain is longer than that proposed by Mouratou et al.; their coordinates for selected sequences are BIG1_Homs (1372-1489), BIG_Drom (1233-1350), SEC7_Sacc (1525-1704), BIG4_Arat (1278-1410), and hence all are 55 amino acids shorter than the domain proposed here. Coordinates in Mouratou et al. for GBF/Gea sequences are: GBF1_Homs (1532-1644), GBF1_Drom (1475-1666), GEA2_Sacc (1277-1433), GNOM_Arat (1235-1349), making the HDS3 domain defined here 50–80 amino acids longer than the one originally proposed
Because the GBF/Gea and the BIG/Sec7 proteins have distinct localizations and functions, it is pertinent to ask the question of whether there exist homology regions specific to each of the two subfamilies of large Arf1 GEFs. Examination of homology regions both upstream and downstream of the Sec7 domain indicated that among the representatives of all five eukaryotic supergroups, only one domain, specific to the BIG/Sec7 subfamily, lies outside of the common domains (Fig. 10). All other regions of high sequence conservation specific to either the GBF/Gea or the BIG/Sec7 subfamilies that were present among members of all the eukaryotic supergroups we examined were within the common homology regions DCB, HUS, HDS1, HDS2, and HDS3. Hence it appears that there is a highly conserved function and/or structure of the large Arf1 GEFs that is preserved in both the subfamilies, and specific functions are superposed on this common structural organization. Overall, there is a higher level of sequence homology between the BIG/Sec7 subfamily members than among GBF/Gea sequences.
Fig. 10.
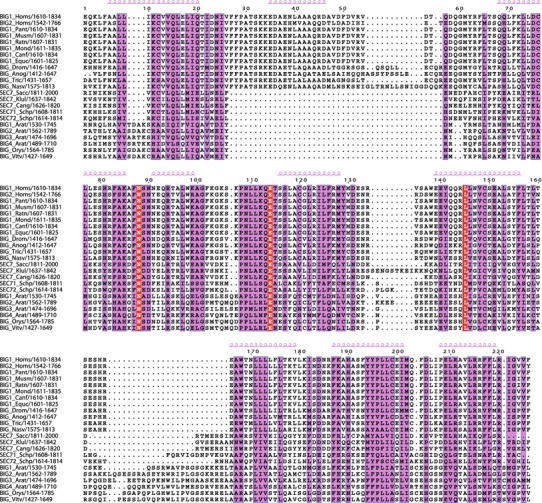
Conserved residues among members of the BIG/Sec7 subfamily within the C-terminal region including the HDS4 domain. Multiple sequence alignment showing conserved residues (pink) and invariant residues (red). Secondary structure prediction of alpha-helical regions is shown above alignment in pink
Outside of the catalytic Sec7 domain, which is the most highly conserved domain of the Arf1 GEFs, the N-terminal domains DCB and HUS have the next highest levels of homology, particularly among GBF/Gea family members. As described previously (Ramaen et al. 2007), the DCB domain was redefined to include residues upstream of the originally defined domain (Mouratou et al. 2005) (Fig. 3). The most highly conserved region outside of the catalytic Sec7 domain is the “HUS box,” a region of seven amino acids near the end of the HUS domain (Fig. 4) (Mouratou et al. 2005; Park et al. 2005). Including a wide range of the eukaryotic sequences confirms the high level of homology of this region, which has the consensus sequence Y/F Φ N Y/F D C D/E/N (where Φ stands for hydrophobic). The highly conserved aspartic acid residue within this motif is invariant in all but one of the sequences examined (Fig. 4). In vivo, this residue plays an important role in trafficking and in GBF/Gea function in both mammalian cells and in yeast (Park et al. 2005; Ramaen et al. 2007; Deng et al. 2009). On either side of this motif, there are residues conserved within each subfamily of the Arf1 GEFs, specific either for GBF/Gea or BIG/Sec7 proteins (Fig. 4).
In the catalytic Sec7 domain, the loop between helices α6 and α7 in yeast, plant, and mammalian large Arf1 GEFs has the invariant sequence FRLPGE, where the final residue is the glutamic acid finger that is essential for exchange activity (Goldberg 1998; Beraud-Dufour et al. 1999). When members of other eukaryotic families are included, only residues F and GE of this motif are invariant (Fig. 5). Significantly, the catalytic glutamic acid residue is invariant even in this wide range of eukaryotic organisms. Within the Sec7 domain itself, there are several residues that are highly conserved within each subfamily (Fig. 5). Again, there are more conserved residues among BIG/Sec7 subfamily members than among GBF/Gea sequences.
In the C-terminal homology regions HDS1, HDS2, and HDS3, the BIG/Sec7 sequences had a significantly higher level of homology than the GBF/Gea sequences (Figs. 6, 7, 8). Both HDS1 and HDS2 are more highly conserved among the BIGs than among the GBFs (Figs. 6, 7), with the second half of HDS2 showing a particularly high number of conserved residues among BIG/Sec7 proteins of diverse eukaryotic origin (Fig. 7). Indeed, the NCBI conserved domain database has identified a portion of the BIG/Sec7 HDS2 domain as highly conserved and named it DUF1981. In contrast, there are few if any residues in the second half of HDS2 that are conserved among all the GBF/Gea proteins (Fig. 7).
The HDS3 domain was described previously, but our analysis has indicated that this domain is 50–80 amino acids longer than that proposed by Mouratou et al. (2005), with the additional homology region located at the C-terminal end of the originally defined HDS3 domain (Fig. 8). Including representatives of the five eukaryotic super families indicates that there is only weak homology within this domain (Fig. 8), but when representative members of only fungi, plants, and mammals are included, a significant level of homology is found throughout the HDS3 domain among members of both BIG/Sec7 (not shown) and GBF/Gea subfamilies (Fig. 9).
Fig. 9.
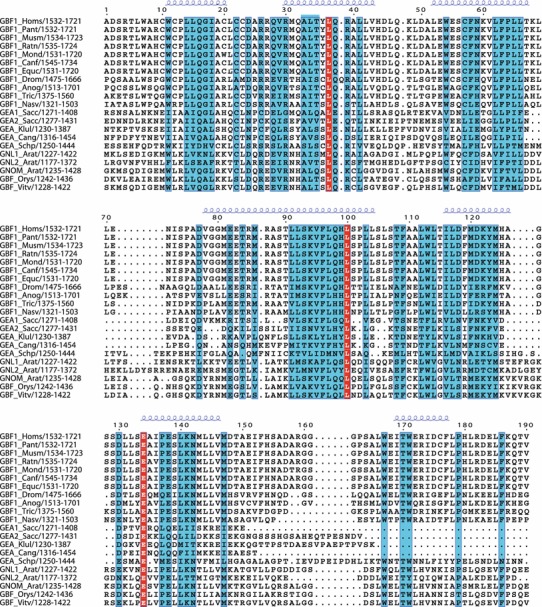
Conserved residues among members of the GBF/Gea subfamily within the region including the HDS3 domain. Multiple sequence alignment showing conserved residues (blue) and invariant residues (red). Only sequences from animals, fungi, and plants are included. Secondary structure prediction of alpha-helical regions is shown above alignment in blue
There is one homology region specific to a subfamily of the large Arf1 GEFs, which we call HDS4, and which is present only among BIG/Sec7 proteins (Fig. 10). No equivalent homology region can be found in the GBF/Gea proteins. We found HDS4 sequences in eukaryotic supergroups other than the Opisthokonta (animals, fungi) and Archaeplastida (plants, algae), but with less homology. Both Tetrahymena thermophila and Paramecium tetraurelia BIG sequences have a region homologous to amino acids 1,675–1,740 of the human BIG1 HDS4 domain (Fig. 2, data not shown). However, we did not find evidence for an HDS4 domain in the Amoebozoa or Excavata supergroup members Dictyostelium discoideum and Trichomonas vaginalis, respectively.
Interacting Partners of the large Arf1 GEFs
The highly conserved sequence homology domains of the GBF/Gea and the BIG/Sec7 Arf1 GEFs suggest that they have important, conserved functions in evolution, and are likely to be involved in protein–protein interactions. Indeed, studies from a number of laboratories have identified interacting proteins of the GBF/Gea and the BIG/Sec7 Arf1 GEFs (Table 3). It is important to point out that many of these interacting partners have been identified by yeast two-hybrid screens or by co-immunoprecipitation of proteins from cells, which demonstrate a physical association, but not a direct protein–protein interaction. Further studies are required to determine whether these interactions are direct or via a bridging protein. Many of the interactions described in Table 3 have been previously reviewed (Cox et al. 2004; Shin and Nakayama 2004; Mouratou et al. 2005; Casanova 2007; Anders and Jurgens 2008), so we will focus here on more recent results.
Table 3.
Interactions between the Arf1 GEFs and protein partners
| Arf GEF | Species | Interacting protein | Interaction domain | Technique showing direct interaction in vitro | References |
|---|---|---|---|---|---|
| Direct protein–protein interactions | |||||
| BIG/Sec7 subfamily | |||||
| BIG1 | Hs | Myosin IXb molecular motor | 1,305–1,849 | Epitope-tagged proteins purified from baculovirus, immunoaffinity column | Saeki et al. (2005) |
| Exo70 exocyst complex component | 1–643 | CoIP of in vitro-translated, epitope-tagged proteins | Xu et al. (2005) | ||
| BIG1 | DCB | Purification from E. coli, gel filtration | Ramaen et al. (2007) | ||
| BIG2 | Rn | β-subunit of GABA receptor gamma-aminobutyric acid type A receptor | 1,739–1849 | GST- and His-tagged proteins purified from E. coli, gel overlay assay | Charych et al. (2004) |
| Gea/GBF subfamily | |||||
| GBF1 | Hs | Rab1b small G protein | 1–380 | GST- and His-tagged proteins purified from E. coli, GST column | Monetta et al. (2007) |
| γ COP COPI coat subunit | nd | GST- and His-tagged proteins purified from E. coli, GST column | Deng et al. (2009), Golinelli-Cohen and Jackson, unpublished data | ||
| Gea1/2p | Sc | Drs2p aminophospholipid translocase | Sec7 | Purification from E. coli, transcription–translation in vitro, immunoaffinity column | Chantalat et al. (2004) |
| Sec21p COPI coat subunit | N-Sec7 | GST- and His-tagged proteins purified from E. coli, GST column | Deng et al. (2009) | ||
| Arf GEF | Species | Interacting protein | Interaction domain | Technique showing physical association | References |
|---|---|---|---|---|---|
| Physical associations (possibly direct but not demonstrated) | |||||
| BIG/Sec7 subfamily | |||||
| BIG1/2 | Hs | FKBP13 FK506-binding protein | 1–331 | 2-hybrid, coIP | Padilla et al. (2003) |
| PP1-γ protein phosphatase | nd | Kinase-anchoring proteins sequence, phosphorylation | Kuroda et al. (2007) | ||
| Hs | 3CD poliovirus protein | nd | Translation of 3CD in cell extracts, Western analysis of membrane fractions | Belov et al. (2007b) | |
| BIG1 | Hs | Fibrillarin part of snRNP particle U3 snoRNA | nd | CoIP | Padilla et al. (2008) |
| Integrin β1 | nd | Necessary for correct glycosylation of integrin β1 | Shen et al. (2007) | ||
| KIF21A kinesin motor protein | 885–1,849 | CoIP, colocalization | Shen et al. (2008) | ||
| La RNA binding protein | nd | CoIP | Padilla et al. (2008) | ||
| Nucleolin synthesis and maturation of ribosomes | nd | CoIP | Padilla et al. (2004, 2008) | ||
| Nucleoporin 62 import of protein to the nucleus | nd | CoIP | Padilla et al. (2004) | ||
| BIG2 | Hs | AMY-1 c-Myc interacting protein, AKAP-binding | DCB | CoIP, siRNA | Ishizaki et al. (2006) |
| R subunits of PKA regulatory subunits of type I protein kinase | 27–48 (RIα, RIβ), 284–301 (RIIα, RIIβ), 517–538 (RIα, RIIα, RIIβ) | 2-hybrid, coIP | Li et al. (2003) | ||
| TNFR1 tumor necrosis factor receptor | nd | Effect of siRNA/BFA on the release of TNFR1, colocalisation, co-IP | Islam et al. (2007, 2008) | ||
| Sec7p | Sc | COPI coat complex | 493–1,196 | GST-pulldown using purified GST-Sec7 fragments and yeast cytosol | Deitz et al. (2000) |
| COPII coat complex | 1,196–2,010 | GST-pulldown using purified GST-Sec7 fragments and yeast cytosol | Deitz et al. (2000) | ||
| Gea/GBF subfamily | |||||
| GBF1 | Hs | AMP-activated protein kinase (AMPK) | nd | Phospho-akt-substrate antibody | Miyamoto et al. (2008) |
| GBF1 | DCB and HUS | 2-hybrid, coIP | Ramaen et al. (2007) | ||
| GGA adaptor protein for clathrin | DCB–HUS | Colocalization, 2-hybrid, coIP, siRNA | Lefrancois and McCormick (2007) | ||
| p115 tether | 1,752–1,859 | 2-hybrid, coIP, GST-pulldown using cytosol, colocalization | Garcia-Mata and Sztul (2003) | ||
| 3A protein polio and coxsackieviruses protein | 1–566 | 2-hybrid, coIP, colocalization | Wessels et al. (2006), Belov et al. (2007a) | ||
| GNOM | At | CYP5 peptidyl-prolyl transisomerase | 1–146 | 2-hybrid, GST-pulldown using GST-CYP5 and Arabidopsis protein extracts | Grebe et al. (2000) |
| GNOM | DCB, GNOMΔDCB | 2-hybrid, in vivo complementation | Grebe et al. (2000), Anders et al. (2008) | ||
| Gea1/2p | Sc | Gmhlp Golgi integral membrane protein | HDS1–3 | 2-hybrid, co-IP, colocalization | Chantalat et al. (2003) |
| Scp160p KH-domain RNA binding protein | 2-hybrid, co-purification | Peyroche and Jackson (2001) | |||
Hs Homo sapiens, Rn Rattus norvegicus, Sc Saccharomyces cerevisiae, At Arabidopsis thaliana, nd not determined
The Arf1 GEF interactions for which a direct protein–protein interaction has been demonstrated are listed in the first part of Table 3. For BIG1, these include the exocyst subunit Exo70 (Xu et al. 2005), the Myosin IXb molecular motor (Saeki et al. 2005), and the BIG1 protein itself (Ramaen et al. 2007). The interactions with Exo70 and Myosin IXb suggest a role for BIG1 in targeting secretory vesicles to the PM. The BIG1 DCB domain has been demonstrated directly to form a dimer through purification of this domain from E. coli and gel filtration analysis (Ramaen et al. 2007). This data confirmed the initial observations for the Arabidopsis thaliana GNOM Arf1 GEF that the DCB domain is involved in dimerization (Grebe et al. 2000).
For mammalian GBF1, two proteins have been shown to interact directly, the small G protein Rab1 (Monetta et al. 2007) and the COPI coat subunit gamma-COP (Deng et al. 2009). Rab1 plays an essential role in trafficking between the ER and cis-Golgi (Behnia and Munro 2005). Like the Arf small G proteins, Rabs cycle between GDP- and GTP-bound forms, and target effector proteins to membranes when in their active GTP-bound conformation. Rab proteins are primarily involved in membrane fusion, with roles in regulation of tethering complexes and the pairing of SNAREs which directly mediate fusion (Behnia and Munro 2005). GBF1 was shown to be an effector for Rab1 in vitro, and active Rab1 in cells was shown to increase the association of both GBF1 and COPI to membranes (Monetta et al. 2007). Interestingly, another Rab1 effector, the tethering protein p115, has also been shown to interact with GBF1 (Garcia-Mata and Sztul 2003). p115 not only acts to tether membranes in ER–Golgi trafficking, but also interacts directly with and promotes pairing of the ER–Golgi SNARE proteins, which are required for membrane fusion (Short et al. 2005). A precise molecular explanation for the Rab1–GBF1–p115 series of interactions has not been demonstrated, but suggests a role for GBF1 in coordination of vesicle budding and fusion processes.
The interaction between GBF1 and the gamma-COP subunit of the COPI coat complex is conserved from yeast to humans. This interaction is a direct protein–protein interaction in both systems (Deng et al. 2009; MPG-C and CLJ, unpublished data). Studies from several laboratories have indicated that GBF1 is involved specifically in recruitment of COPI to early Golgi membranes. Knockdown of GBF1 by RNAi inhibits COPI association with membranes without affecting other coats such as AP-1 (Ishizaki et al. 2008; Manolea et al. 2008; Deng et al. 2009). In contrast, knockdown of the BIG1 and BIG2 proteins prevents AP-1 association with TGN membranes, without affecting COPI (Ishizaki et al. 2008; Manolea et al. 2008). A model explaining this specificity has been proposed, whereby COPI interacts with the Arf1 GEF GBF1 (Gea1p and Gea2p in yeast) prior to nucleotide exchange on Arf1, so that the effector COPI is in place at the time Arf1-GTP is formed (Deng et al. 2009). This model offers an explanation for the observation that a single Arf protein, Arf1, can recruit at least five different coat complexes, including COPI, AP1/clathrin, GGA/clathrin, AP3, and AP4, to different sites within cells (Bonifacino and Lippincott-Schwartz 2003). Interestingly, all these coat complexes share sequence homology and are structurally similar (Bonifacino and Lippincott-Schwartz 2003; McMahon and Mills 2004), and function in a specific trafficking pathway (Bonifacino and Lippincott-Schwartz 2003; McMahon and Mills 2004). This is also true of the large Arf1 GEFs, whose function is required to recruit these coat complexes to membranes. One of the regions of gamma-COP that interacts with the Arf1 GEFs is the appendage domain (Deng et al. 2009), a region that is structurally conserved in all five coats recruited to membranes by Arf1-GTP (McMahon and Mills 2004). These results suggest the possibility that the different Arf1 GEFs within these two subfamilies regulate coat assembly via a common mechanism involving GEF-coat interactions.
An interaction between GBF1 and GGA coat complexes has been proposed, although it has not been determined if this interaction is direct (Lefrancois and McCormick 2007) (Table 3). GBF1 functions and localizes to the cis-Golgi, whereas the GGA proteins function and localize primarily to the trans-Golgi and TGN (Hirst et al. 2001; Puertollano et al. 2001; Mattera et al. 2003; Nakayama and Wakatsuki 2003; Puertollano et al. 2003). Severe perturbations of the early Golgi caused by interfering with GBF1 function also affect late-Golgi functions, where the GGA proteins act. Hence, the results of this study are likely due to a more indirect mechanism than by direct interaction of GBF1 and the GGAs.
An important role for the Arf1 GEFs in cell signaling has emerged with the identification of BIG1 and BIG2 as A-kinase anchoring proteins or AKAPs. AKAPs are PKA regulatory subunit binding proteins that act as scaffolds to compartmentalize signaling cascades. This function is accomplished through formation of multi-protein complexes that spatially restrict the PKA-cAMP signaling cascade at specific membrane sites within cells. BIG1 and BIG2 both contain AKAP domains that mediate binding of the regulatory subunit RIIβ of protein kinase A (PKA) (Figs. 2, 3), and BIG1 accumulates in nuclei upon increase in cAMP levels in cells (Li et al. 2003; Padilla et al. 2004; Citterio et al. 2006). Both BIG1 and BIG2 immunoprecipitated from HepG2 cells can be phosphorylated by recombinant PKA, and this results in a decrease in GEF activity (Kuroda et al. 2007). One PKA phosphorylation site in BIG1 has been identified, at the C-terminus of the Sec7 domain (Li et al. 2003) (Fig. 4). Furthermore, endogenous protein phosphatase 1-γ (PP1-γ) was found in a complex with BIG1 or BIG2 in microsomal fractions, indicating that a phosphatase of this signaling pathway is physically associated with BIG1 and BIG2 GEFs (Kuroda et al. 2007). A recent study from this group has shown that another regulatory enzyme in this cascade, phosphodiesterase PDE3A, is functionally associated with BIG1 and BIG2 (Puxeddu et al. 2009). PDE enzymes break down cAMP to terminate cAMP signaling, reversing its effects on BIG1 and BIG2. Puxeddu et al. showed that PDE3A (but not PDE4) co-immunoprecipitates with both BIG1 and BIG2, and that knockdown of PDE3A led to a partial redistribution of BIG1 and BIG2 from Golgi membranes to cytosol. In addition to the cAMP-PKA regulatory proteins themselves, an AKAP-binding protein AMY-1 has also been found to associate with the RIIβ-binding (AKAP) domains of both BIG1 and BIG2 (Ishizaki et al. 2006). In cells, AMY-1 TGN localization is abrogated in cells depleted of BIG2, but not of BIG1, indicating that BIG2 is the physiological partner of AMY-1 at the TGN (Ishizaki et al. 2006).
What are the functional consequences of BIG1 and BIG2 involvement as AKAPs in cAMP-PKA signaling? For BIG1, phosphorylation by PKA results in its redistribution from the Golgi and cytoplasm to the nucleus (Citterio et al. 2006). The precise function of BIG1 in the nucleus is not known, but it involves interactions with nuclear proteins including nucleolin, fibrillarin and RNA-binding protein La, as well as the small nuclear RNA snoU3 (Padilla et al. 2008).
The type I tumor necrosis factor receptor (TNFR1) is one of two receptors for TNF that mediates its effects on inflammation, apoptosis, and the innate immune response. TNFR1 can be released from cells by incorporation into exosome-like vesicles which are shed from the surface of cells (Hawari et al. 2004). BIG2 has been shown to play a role in regulating this process by two mechanisms, one involving its GEF activity, the other through its AKAP domains. Activation of Arf1 and Arf3 by BIG2 is required for extracellular release of TNFR1 in exosome-like vesicles, and involves a physical association between BIG2 and TNRF1 (Islam et al. 2007). In addition, cAMP-dependent protein kinase A signaling induces TNFR1 exosome-like vesicle release through binding of PKA regulatory subunit RIIβ to AKAP domains of BIG2 (Islam et al. 2008).
Arf1 GEFs and human disease
Mutations in the BIG2 Arf1 GEF have been linked to autosomal recessive periventricular heterotopia (ARPH), a disorder of neuronal migration that leads to severe malformation of the cerebral cortex (microcephaly) and severe developmental delay (Sheen et al. 2004). Two BIG2 disease alleles have been identified, including a frameshift mutation that results in truncation of the majority of the protein (Sheen et al. 2004). The disease symptoms are a result of the failure of a specific class of neurons to migrate from their point of origin in the lateral ventricular proliferative zone to the cerebral cortex (Sheen et al. 2004; Ferland et al. 2009). This defect arises from a defect in vesicular trafficking that alters the adhesion properties of these neurons (Ferland et al. 2009). Interestingly, mutations in other trafficking proteins can give rise to periventricular heterotopia in humans or in model systems, indicating that trafficking defects are likely the root cause of this disease (Ferland et al. 2009). Hence, the Arf1 GEFs not only serve essential roles in fundamental cell biological processes, but also play an important role in human development and neuronal function (Sheen et al. 2004).
The Arf1 GEFs have been shown to act as host factors for pathogens mediating human disease. Replication of several viruses has been shown to require GBF1, including poliovirus, coxsackievirus, and coronavirus. Poliovirus and coxsackievirus are enteroviruses belonging to the Picornaviridae family of positive strand non-enveloped RNA viruses. Despite the fact that enteroviruses are not enclosed by a membrane, their replication never-the-less depends completely on host cell membranes. Upon infection, enteroviruses cause a massive reorganization of the intracellular membranes of their host, including ER and Golgi membranes (Bienz et al. 1983; Salonen et al. 2005). Replication of poliovirus and coxsackievirus is completely inhibited by BFA, and it has been shown that GBF1 is the major target of this drug in poliovirus replication (Belov et al. 2007a; Belov et al. 2008). The enteroviral 3A protein has also been shown to block secretion in host cells, which inhibits the innate immune response of the host (Doedens et al. 1997; Wessels et al. 2006). This block in secretion also occurs via GBF1, through physical association of 3A with GBF1 which inhibits its function on Golgi membranes in the host cell (Wessels et al. 2006; Wessels et al. 2007). Hence, polio and coxsackieviruses subvert the host membrane by blocking GBF1 activity on host cell Golgi membranes, reorganizing these membranes, then activating GBF1 function on Arf1 for viral replication on these virally induced membrane structures.
Although a major role for GBF1 in enteroviral replication has been demonstrated, there is evidence that the BIG1 and the BIG2 Arf1 GEFs are also involved. Interestingly, different viral proteins have been shown to recruit different coats to virally reorganized membranes. In an in vitro system, it has been shown that the 3A protein recruits GBF1 to membranes (Belov et al. 2008), whereas the viral 3CD protein specifically recruits BIG1 and BIG2 (Belov et al. 2007b). Furthermore, 3A recruitment of GBF1 results in specific binding of COPI to membranes (Belov et al. 2008), whereas, 3CD recruits the GGA3 coat to membranes (Belov et al. 2007b). These observations support an intimate coupling between recruitment of specific GEFs with specific coats to membranes, and provide another line of evidence supporting a connection between GBF1 and COPI, and also a connection between BIG1/BIG2 and the late-Golgi coat GGA3.
In addition to polio and coxsackieviruses, the RNA replication of a number of positive-strand viruses is BFA-sensitive (Gazina et al. 2002; Molina et al. 2007; Tai et al. 2009). Among these is Hepatitis C virus (HCV), which has been shown recently to depend also on the Arf1 effectors COPI and phosphatidylinositol-4-kinase for its replication (Tai et al. 2009). These results strongly suggest that GBF1 is a required host factor for HCV replication. BFA also inhibits replication of other families of viruses, including the enveloped positive-strand RNA coronaviruses (Verheije et al. 2008). For one member of the coronaviruses, mouse hepatitis coronavirus, GBF1 and Arf1 have been demonstrated to be essential host factors for its RNA replication (Verheije et al. 2008).
Given the important roles of the Arf1 GEFs of the GBF/Gea and BIG/Sec7 subfamilies in human disease, development of drugs that specifically target these proteins is of medical interest. As mentioned above, BFA is a natural substance that is a potent and specific inhibitor of members of both of these subfamilies. Recently, a chemical (Golgicide A) which specifically inhibits GBF1, but not BIG1 or BIG2, has been described (Saenz et al. 2009). Golgicide A was identified in a screen for inhibitors of the cytotoxicity of Shiga toxin, a protein produced by the pathogenic bacterium Shigella dysenteriae (Saenz et al. 2009). This discovery, in addition to providing an important inhibitor of GBF1, demonstrates a crucial role for this Arf1 GEF as a host factor in S. dysenteriae infection.
Acknowledgments
This study was supported by a Chaire d’Excellence from the Agence Nationale de la Recherche, France. We thank Jacqueline Cherfils and Valerie Biou for assistance with bioinformatics programs and for critical reading of the manuscript.
References
- Abrahamsen MS, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- Adl SM, et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol. 2005;52:399–451. doi: 10.1111/j.1550-7408.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- Amor JC, et al. The structure of RalF, an ADP-ribosylation factor guanine nucleotide exchange factor from Legionella pneumophila, reveals the presence of a cap over the active site. J Biol Chem. 2005;280:1392–1400. doi: 10.1074/jbc.M410820200. [DOI] [PubMed] [Google Scholar]
- Anders N, Jurgens G. Large ARF guanine nucleotide exchange factors in membrane trafficking. Cell Mol Life Sci. 2008;65:3433–3445. doi: 10.1007/s00018-008-8227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders N, et al. Membrane association of the Arabidopsis ARF exchange factor GNOM involves interaction of conserved domains. Plant Cell. 2008;20:142–151. doi: 10.1105/tpc.107.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Belov GA, Altan-Bonnet N, Kovtunovych G, Jackson CL, Lippincott-Schwartz J, Ehrenfeld E. Hijacking components of the cellular secretory pathway for replication of poliovirus RNA. J Virol. 2007;81:558–567. doi: 10.1128/JVI.01820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov GA, Habbersett C, Franco D, Ehrenfeld E. Activation of cellular Arf GTPases by poliovirus protein 3CD correlates with virus replication. J Virol. 2007;81:9259–9267. doi: 10.1128/JVI.00840-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov GA, Feng Q, Nikovics K, Jackson CL, Ehrenfeld E. A critical role of a cellular membrane traffic protein in poliovirus RNA replication. PLoS Pathog. 2008;4:e1000216. doi: 10.1371/journal.ppat.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraud-Dufour S, Paris S, Chabre M, Antonny B. Dual interaction of ADP ribosylation factor 1 with Sec7 domain and with lipid membranes during catalysis of guanine nucleotide exchange. J Biol Chem. 1999;274:37629–37636. doi: 10.1074/jbc.274.53.37629. [DOI] [PubMed] [Google Scholar]
- Bienz K, Egger D, Rasser Y, Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983;131:39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/S0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- Casanova JE. Regulation of Arf activation: the sec7 family of Guanine nucleotide exchange factors. Traffic. 2007;8:1476–1485. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- Chantalat S, Courbeyrette R, Senic-Matuglia F, Jackson CL, Goud B, Peyroche A. A novel Golgi membrane protein is a partner of the ARF exchange factors Gea1p and Gea2p. Mol Biol Cell. 2003;14:2357–2371. doi: 10.1091/mbc.E02-10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, et al. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci. 2004;117:711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- Charych EI, et al. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Citterio C, Jones HD, Pacheco-Rodriguez G, Islam A, Moss J, Vaughan M. Effect of protein kinase A on accumulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) in HepG2 cell nuclei. Proc Natl Acad Sci USA. 2006;103:2683–2688. doi: 10.1073/pnas.0510571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell. 2007;18:2244–2253. doi: 10.1091/mbc.E06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Mason-Gamer RJ, Jackson CL, Segev N. Phylogenetic analysis of Sec7-domain-containing Arf nucleotide exchangers. Mol Biol Cell. 2004;15:1487–1505. doi: 10.1091/mbc.E03-06-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks JB, Field MC. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J Cell Sci. 2007;120:2977–2985. doi: 10.1242/jcs.013250. [DOI] [PubMed] [Google Scholar]
- Dacks JB, Peden AA, Field MC. Evolution of specificity in the eukaryotic endomembrane system. Int J Biochem Cell Biol. 2009;41:330–340. doi: 10.1016/j.biocel.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Deitz SB, Rambourg A, Kepes F, Franzusoff A. Sec7p directs the transitions required for yeast Golgi biogenesis. Traffic. 2000;1:172–183. doi: 10.1034/j.1600-0854.2000.010209.x. [DOI] [PubMed] [Google Scholar]
- Deng Y, Golinelli-Cohen MP, Smirnova E, Jackson CL. A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep. 2009;10:58–64. doi: 10.1038/embor.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNitto JP, et al. Structural basis and mechanism of autoregulation in 3-phosphoinositide-dependent Grp1 family Arf GTPase exchange factors. Mol Cell. 2007;28:569–583. doi: 10.1016/j.molcel.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens JR, Giddings TH, Jr, Kirkegaard K. Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis. J Virol. 1997;71:9054–9064. doi: 10.1128/jvi.71.12.9054-9064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, et al. Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia. Hum Mol Genet. 2009;18:497–516. doi: 10.1093/hmg/ddn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A, Redding K, Crosby J, Fuller RS, Schekman R. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J Cell Biol. 1991;112:27–37. doi: 10.1083/jcb.112.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Sztul E. The membrane-tethering protein p115 interacts with GBF1, an ARF guanine-nucleotide-exchange factor. EMBO Rep. 2003;4:320–325. doi: 10.1038/sj.embor.embor762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Szul T, Alvarez C, Sztul E. ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol Biol Cell. 2003;14:2250–2261. doi: 10.1091/mbc.E02-11-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazina EV, Mackenzie JM, Gorrell RJ, Anderson DA. Differential requirements for COPI coats in formation of replication complexes among three genera of Picornaviridae. J Virol. 2002;76:11113–11122. doi: 10.1128/JVI.76.21.11113-11122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. doi: 10.1016/S0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- Gouet P, Courcelle E, Stuart DI, Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- Grebe M, et al. A conserved domain of the Arabidopsis GNOM protein mediates subunit interaction and cyclophilin 5 binding. Plant Cell. 2000;12:343–356. doi: 10.1105/tpc.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan C, Koulov AV, Balch WE. An evolutionary perspective on eukaryotic membrane trafficking. Adv Exp Med Biol. 2007;607:73–83. doi: 10.1007/978-0-387-74021-8_6. [DOI] [PubMed] [Google Scholar]
- Hawari FI, et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lindsay MR, Robinson MS. GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell. 2001;12:3573–3588. doi: 10.1091/mbc.12.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Mullapudi N, Sicheritz-Ponten T, Kissinger JC. A first glimpse into the pattern and scale of gene transfer in Apicomplexa. Int J Parasitol. 2004;34:265–274. doi: 10.1016/j.ijpara.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Ishizaki R, Shin HW, Iguchi-Ariga SM, Ariga H, Nakayama K. AMY-1 (associate of Myc-1) localization to the trans-Golgi network through interacting with BIG2, a guanine-nucleotide exchange factor for ADP-ribosylation factors. Genes Cells. 2006;11:949–959. doi: 10.1111/j.1365-2443.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in membrane traffic between the trans-Golgi network and endosomes. Mol Biol Cell. 2008;19:2650–2660. doi: 10.1091/mbc.E07-10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A, Shen X, Hiroi T, Moss J, Vaughan M, Levine SJ. The brefeldin A-inhibited guanine nucleotide-exchange protein, BIG2, regulates the constitutive release of TNFR1 exosome-like vesicles. J Biol Chem. 2007;282:9591–9599. doi: 10.1074/jbc.M607122200. [DOI] [PubMed] [Google Scholar]
- Islam A, et al. cAMP-dependent protein kinase A (PKA) signaling induces TNFR1 exosome-like vesicle release via anchoring of PKA regulatory subunit RIIbeta to BIG2. J Biol Chem. 2008;283:25364–25371. doi: 10.1074/jbc.M804966200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL. Mechanisms of transport through the Golgi complex. J Cell Sci. 2009;122:443–452. doi: 10.1242/jcs.032581. [DOI] [PubMed] [Google Scholar]
- Jekely G. Small GTPases and the evolution of the eukaryotic cell. Bioessays. 2003;25:1129–1138. doi: 10.1002/bies.10353. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, et al. GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic. 2002;3:483–495. doi: 10.1034/j.1600-0854.2002.30705.x. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell. 2007;18:3463–3471. doi: 10.1091/mbc.E07-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepper TH, Kienle CN, Fasshauer D. SNAREing the basis of multicellularity: consequences of protein family expansion during evolution. Mol Biol Evol. 2008;25:2055–2068. doi: 10.1093/molbev/msn151. [DOI] [PubMed] [Google Scholar]
- Koumandou VL, Dacks JB, Coulson RM, Field MC. Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol Biol. 2007;7:29. doi: 10.1186/1471-2148-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda F, Moss J, Vaughan M. Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1 gamma. Proc Natl Acad Sci USA. 2007;104:3201–3206. doi: 10.1073/pnas.0611696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois S, McCormick PJ. The Arf GEF GBF1 is required for GGA recruitment to Golgi membranes. Traffic. 2007;8:1440–1451. doi: 10.1111/j.1600-0854.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- Li H, Adamik R, Pacheco-Rodriguez G, Moss J, Vaughan M. Protein kinase A-anchoring (AKAP) domains in brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) Proc Natl Acad Sci USA. 2003;100:1627–1632. doi: 10.1073/pnas.0337678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolea F, Claude A, Chun J, Rosas J, Melancon P. Distinct functions for Arf guanine nucleotide exchange factors at the Golgi complex: GBF1 and BIGs are required for assembly and maintenance of the Golgi stack and trans-Golgi network, respectively. Mol Biol Cell. 2008;19:523–535. doi: 10.1091/mbc.E07-04-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Arighi CN, Lodge R, Zerial M, Bonifacino JS. Divalent interaction of the GGAs with the Rabaptin-5-Rabex-5 complex. EMBO J. 2003;22:78–88. doi: 10.1093/emboj/cdg015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, et al. AMP-activated protein kinase phosphorylates Golgi-specific brefeldin A resistance factor 1 at Thr1337 to induce disassembly of Golgi apparatus. J Biol Chem. 2008;283:4430–4438. doi: 10.1074/jbc.M708296200. [DOI] [PubMed] [Google Scholar]
- Molina S, Sanz MA, Madan V, Ventoso I, Castello A, Carrasco L. Differential inhibition of cellular and Sindbis virus translation by brefeldin A. Virology. 2007;363:430–436. doi: 10.1016/j.virol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Monetta P, Slavin I, Romero N, Alvarez C. Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol Biol Cell. 2007;18:2400–2410. doi: 10.1091/mbc.E06-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouratou B, et al. The domain architecture of large guanine nucleotide exchange factors for the small GTP-binding protein Arf. BMC Genomics. 2005;6:20. doi: 10.1186/1471-2164-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Wakatsuki S. The structure and function of GGAs, the traffic controllers at the TGN sorting crossroads. Cell Struct Funct. 2003;28:431–442. doi: 10.1247/csf.28.431. [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Padilla PI, Chang MJ, Pacheco-Rodriguez G, Adamik R, Moss J, Vaughan M. Interaction of FK506-binding protein 13 with brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1): effects of FK506. Proc Natl Acad Sci USA. 2003;100:2322–2327. doi: 10.1073/pnas.2628047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla PI, Pacheco-Rodriguez G, Moss J, Vaughan M. Nuclear localization and molecular partners of BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP-ribosylation factors. Proc Natl Acad Sci USA. 2004;101:2752–2757. doi: 10.1073/pnas.0307345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla PI, Uhart M, Pacheco-Rodriguez G, Peculis BA, Moss J, Vaughan M. Association of guanine nucleotide-exchange protein BIG1 in HepG2 cell nuclei with nucleolin, U3 snoRNA, and fibrillarin. Proc Natl Acad Sci USA. 2008;105:3357–3361. doi: 10.1073/pnas.0712387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Hartnell LM, Jackson CL. Mutations in a highly conserved region of the Arf1p activator GEA2 block anterograde Golgi transport but not COPI recruitment to membranes. Mol Biol Cell. 2005;16:3786–3799. doi: 10.1091/mbc.E05-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Leal JB. The Ypt/Rab family and the evolution of trafficking in fungi. Traffic. 2008;9:27–38. doi: 10.1111/j.1600-0854.2007.00667.x. [DOI] [PubMed] [Google Scholar]
- Peyroche A, Jackson CL. Functional analysis of ADP-ribosylation factor (ARF) guanine nucleotide exchange factors Gea1p and Gea2p in yeast. Methods Enzymol. 2001;329:290–300. doi: 10.1016/S0076-6879(01)29090-9. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001;105:93–102. doi: 10.1016/S0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- Puertollano R, van der Wel NN, Greene LE, Eisenberg E, Peters PJ, Bonifacino JS. Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network. Mol Biol Cell. 2003;14:1545–1557. doi: 10.1091/mbc.02-07-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puxeddu E, et al. Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity. Proc Natl Acad Sci USA. 2009;106:6158–6163. doi: 10.1073/pnas.0901558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaen O, et al. Interactions between conserved domains within homodimers in the BIG1, BIG2, and GBF1 Arf guanine nucleotide exchange factors. J Biol Chem. 2007;282:28834–28842. doi: 10.1074/jbc.M705525200. [DOI] [PubMed] [Google Scholar]
- Richter S, et al. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature. 2007;448:488–492. doi: 10.1038/nature05967. [DOI] [PubMed] [Google Scholar]
- Saeki N, Tokuo H, Ikebe M. BIG1 is a binding partner of myosin IXb and regulates its Rho-GTPase activating protein activity. J Biol Chem. 2005;280:10128–10134. doi: 10.1074/jbc.M413415200. [DOI] [PubMed] [Google Scholar]
- Saenz JB, et al. Golgicide A reveals essential roles for GBF1 in Golgi assembly and function. Nat Chem Biol. 2009;5:157–165. doi: 10.1038/nchembio.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen A, Ahola T, Kaariainen L. Viral RNA replication in association with cellular membranes. Curr Top Microbiol Immunol. 2005;285:139–173. doi: 10.1007/3-540-26764-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen VL, et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36:69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- Shen X, Hong MS, Moss J, Vaughan M. BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein, is required for correct glycosylation and function of integrin beta1. Proc Natl Acad Sci USA. 2007;104:1230–1235. doi: 10.1073/pnas.0610535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Meza-Carmen V, Puxeddu E, Wang G, Moss J, Vaughan M. Interaction of brefeldin A-inhibited guanine nucleotide-exchange protein (BIG) 1 and kinesin motor protein KIF21A. Proc Natl Acad Sci USA. 2008;105:18788–18793. doi: 10.1073/pnas.0810104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Nakayama K. Guanine nucleotide-exchange factors for Arf GTPases: their diverse functions in membrane traffic. J Biochem. 2004;136:761–767. doi: 10.1093/jb/mvh185. [DOI] [PubMed] [Google Scholar]
- Shin HW, Morinaga N, Noda M, Nakayama K. BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol Biol Cell. 2004;15:5283–5294. doi: 10.1091/mbc.E04-05-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotsuka C, Waguri S, Wakasugi M, Uchiyama Y, Nakayama K. Dominant-negative mutant of BIG2, an ARF-guanine nucleotide exchange factor, specifically affects membrane trafficking from the trans-Golgi network through inhibiting membrane association of AP-1 and GGA coat proteins. Biochem Biophys Res Commun. 2002;294:254–260. doi: 10.1016/S0006-291X(02)00456-4. [DOI] [PubMed] [Google Scholar]
- Shinotsuka C, Yoshida Y, Kawamoto K, Takatsu H, Nakayama K. Overexpression of an ADP-ribosylation factor-guanine nucleotide exchange factor, BIG2, uncouples brefeldin A-induced adaptor protein-1 coat dissociation and membrane tubulation. J Biol Chem. 2002;277:9468–9473. doi: 10.1074/jbc.M112427200. [DOI] [PubMed] [Google Scholar]
- Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744:383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Tai AW, et al. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheije MH, et al. Mouse hepatitis coronavirus RNA replication depends on GBF1-mediated ARF1 activation. PLoS Pathog. 2008;4:e1000088. doi: 10.1371/journal.ppat.1000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels E, et al. A viral protein that blocks Arf1-mediated COP-I assembly by inhibiting the guanine nucleotide exchange factor GBF1. Dev Cell. 2006;11:191–201. doi: 10.1016/j.devcel.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Wessels E, Duijsings D, Lanke KH, Melchers WJ, Jackson CL, van Kuppeveld FJ. Molecular determinants of the interaction between coxsackievirus protein 3A and guanine nucleotide exchange factor GBF1. J Virol. 2007;81:5238–5245. doi: 10.1128/JVI.02680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, et al. The genome of Cryptosporidium hominis. Nature. 2004;431:1107–1112. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- Xu KF, Shen X, Li H, Pacheco-Rodriguez G, Moss J, Vaughan M. Interaction of BIG2, a brefeldin A-inhibited guanine nucleotide-exchange protein, with exocyst protein Exo70. Proc Natl Acad Sci USA. 2005;102:2784–2789. doi: 10.1073/pnas.0409871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lasell TK, Melancon P. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol Biol Cell. 2002;13:119–133. doi: 10.1091/mbc.01-08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]


