Abstract
Diseases transmitted between animals and people have made up more than 50% of emerging infectious diseases in humans over the last 60 years and have continued to arise in recent months. Yet, public health and animal disease surveillance programs continue to operate independently. Here, we assessed whether recent emerging zoonotic pathogens (n = 143) are known to cause morbidity or mortality in their animal host and if so, whether they were first detected with an animal morbidity/mortality event. We show that although sick or dead animals are often associated with these pathogens (52%), only 9% were first detected from an animal morbidity or mortality event prior to or concurrent with signs of illness in humans. We propose that an animal morbidity and mortality reporting program will improve detection and should be an essential component of early warning systems for zoonotic diseases. With the use of widespread low-cost technology, such a program could engage both the public and professionals and be easily tested and further incorporated as part of surveillance efforts by public health officials.
Electronic supplementary material
The online version of this article (doi:10.1007/s10393-014-0988-x) contains supplementary material, which is available to authorized users.
Keywords: zoonotic diseases, disease detection, disease surveillance, morbidity, mortality
Over the last 60 years, more than half of the emerging infectious diseases appearing in humans have been transmitted from animals (Jones et al. 2008), and of these, 72% are of wildlife origin. The numbers are likely to increase as zoonoses continue to appear, such as the avian Influenza H7N9 virus which emerged in China in the spring of 2013 from direct contact with poultry (Gao et al. 2013; Li et al. 2014) and the Middle East respiratory syndrome coronavirus (MERS-CoV) that emerged in countries near the Arabian Peninsula in 2012 of putative animal origins (Assiri et al. 2013, Reusken et al. 2013), specifically from dromedary camels (Alagaili et al. 2014). These zoonotic disease outbreaks have posed and continue to pose substantial burdens on public health systems, global politics, wildlife populations, as well as local and global economies (Leroy et al. 2004; Rouquet et al. 2005; LaDeau et al. 2007; Karesh et al. 2012). For example, the West Nile virus (WNV), which was primarily transmitted to humans from infected mosquitoes and bird reservoir hosts, resulted in over 30,000 reported cases of encephalitis and caused 1,350 human deaths in the United States from 1999 through January 2011 (Centers for Disease Control and Prevention 2011). In 1 year (2002), the cost of WNV was over $20 million for the state of Louisiana alone (Zohrabian et al. 2004) and prompted several local and state agencies to initiate expensive mosquito-control programs. On a global scale, zoonotic disease outbreaks are estimated to have cost over US$20 billion in direct and US$ 200 billion in indirect costs between 2000 and 2010; the Severe Acute Respiratory Syndrome (SARS) virus was one of the most devastating, costing over US$ 40–50 billion in losses in East Asia and Canada alone (World Bank 2010).
Moreover, the effects of such zoonotic pathogens can be severe on both wildlife and human populations. The Ebola virus, which causes hemorrhagic fever in humans and non-human primates, had caused, as of 2009, >1,500 human deaths since its emergence in Democratic Republic of Congo (formerly Zaire) in 1976 (Centers for Disease Control and Prevention 2010). Non-human primate numbers drastically declined (Walsh et al. 2003) in the Republic of Congo alone during the two-year Ebola outbreak from 2001 to 2003 (e.g., 56% in gorillas and 89% for chimpanzees, Leroy et al. 2004). Equally devastating was the introduction of WNV into the Americas, which lead to a precipitous population decline for North American birds, most notably in the American Crow (Corvus brachyrhynchos) whose numbers declined by 45% since the emergence of the virus (LaDeau et al. 2007).
When an animal first contracts a pathogen such as highly pathogenic avian influenza (HPAI H5N1) or West Nile virus, an immunological response ensues and animals exhibit clinical symptoms of the disease such as severe weakness, tremors, incoordination, and general lethargy (Komar et al. 2003, Brown et al. 2006). In some cases, the animals die shortly after being infected without the onset of clinical signs (Brown et al. 2008) or the animal survives infection, seroconverts, and is no longer infectious. When the infection is fatal, the animal dies within a week of pathogen infection and disease emergence (Komar et al. 2003, Brown et al. 2006, 2008). However, certain infections in animals are asymptomatic such as H7N9 infections in poultry (WHO 2013).
The most challenging issue, and to date, one of the most important for reducing the impacts of zoonotic diseases, is the early detection of viral emergence and movement over space and time. Viruses cannot be seen and are detected most commonly through the morbidity or mortality events they cause in their animal hosts. Despite the enormous economic and ecological impacts and despite the recognized utility of animals as sentinels for disease surveillance (Halliday et al. 2007; Rabinowitz et al. 2005), to date, few, if any, effective animal morbidity or mortality surveillance mechanisms exist globally. Moreover, the public health sector is slow to adopt strategies that include animals as sentinels. Here, we demonstrate, through our own review and examples of emerging infectious disease events, that most zoonotic diseases could have been detected first by animal morbidity/mortality events (in both domestic and wild animals), but because of a lack of organized and comprehensive surveillance, they are detected too late to limit the spread of the pathogen and the resulting impact it has on human populations.
Materials and Methods
We used the list of zoonotic diseases of wildlife origin compiled by Jones et al. (2008) to quantify the number of diseases emerging between 1940 and 2004 that (a) are known to cause disease or death in animal hosts and (b) were first detected through an animal morbidity/mortality event. Our general thesis is, an animal mortality monitoring system is an essential component to surveillance systems to minimize human morbidity and mortality impacts. We considered a zoonotic disease to have been first detected in animals if the detection of sick or dead animals preceded or were concurrent and associated with the emergence of the disease in humans as reported in peer-reviewed publications (Online Appendix 1). For example, dead voles infected with Francisella tularensis, which causes Tularemia, were found in the hay with which Swedish farmers worked at the time of the 1966 emergence and outbreak of the disease in these same farms (Dahlstrand et al. 1971); it was also previously known to cause die-offs in rodents (McCoy and Chapin 1912). We therefore considered the Tularemia outbreak first detected in animals. We concomitantly verified whether each pathogen was known to produce extended morbidity in their animal hosts (fever, diarrhea, or other symptoms) or was fatal. We performed our literature search using ISI web of Knowledge and Google Scholar using pathogens (common and scientific terms) as search terms or close variants of the combination of ‘first detected(ion)’ and ‘clinical representation in animals’, respectively. Each pathogen was tagged as “yes” if it had been shown to cause disease in non-human animals or “no/unknown” when it had been demonstrated not to cause disease or the results were inconclusive (Online Appendix I). Because the majority of zoonotic pathogens have a known wildlife origin (Jones et al. 2008), we restricted our analysis to the 143 pathogens listed as a combination of being (a) zoonotic; disease emerged via non-human to human transmission and (b) wildlife; zoonotic emerging infectious disease event caused by a pathogen with a wildlife origin in Jones et al. (2008).
Results and Discussion
We show that although 75 (52%) of the zoonotic pathogens listed in Jones et al. (2008) are known to produce extended morbidity or mortality in animal hosts (Online Appendix 1), only 13 of the 143 pathogens (9%), including two that do not produce signs of illness (Seoul and Whitewater Arroyo viruses; Table 1), were first detected in animals and subsequently reported prior to, or concurrent with, signs of morbidity and mortality in humans (Figure 1; Table 1). Therefore, for 64 (45%) more zoonotic pathogens listed here, outbreaks of these diseases could have been first detected in animals had a surveillance mechanism been in place (Figure 1). Furthermore, 10 of the 13 animal morbidity or mortality events reported were from domestic or peridomestic animals (Table 1). For example, the anthrax (Bacillus anthracis) outbreak of 1979 in Sverdslovsk (former USSR) was detected in sick and dying livestock (primarily sheep) shortly before the 64 documented human deaths (Meselson et al. 1994), most likely because sheep, and more generally, herbivores appear to be more susceptible to anthrax inhalation than humans (Young et al. 1946, Watson and Keir 1994). However, anthrax is well known to cause animal die-offs in the wild (Clegg et al. 2007; Mapesa et al. 2007), therefore, signs of the disease could have also been detected in wild animal populations. Comparatively, HPAI H5N1, which is fatal in some wild bird species (Brown et al. 2008), appeared simultaneously in a sick child in Hong Kong and in chickens at local poultry farms in the same year (1997; Subbarao et al. 1998). However, in both cases, no official and comprehensive surveillance program existed that would have systematically alerted local officials of the presence of a pathogen.
Table 1.
Emerging Infectious Human Pathogens of Zoonotic Origin Detected in Non-human Hosts (Domestic or Wild) Prior to or Concurrent with Emergence in Human Populationsa
| Zoonotic pathogen | Non-human hostb |
|---|---|
| Bacillus anthracis | Sheep; domestic |
| Francisella tularensis | Voles; peridomestic |
| Hendra | Horses; domestic |
| Influenza A; H5N1 | Chickens; domestic |
| Kyasanur forest disease virus | Monkeys; wild |
| Menangle virus | Pigs; domestic |
| Salmonella typhimurium drug-res | Calves; domestic |
| Salmonella typhimurium multidrug-res | Calves; domestic |
| Seoul virusc | Rats; peridomestic |
| Venezuelan Equine Encephalitis virus | Horses, mules, donkeys; domestic |
| Whitewater Arroyo virusc | Rodents; wild |
| Yersinia pestis | Prairie dogs; wild |
| Yersinia pestis multiple drug-res | Rats; peridomestic |
aAn emerging pathogen is defined in Jones et al. (2008) as pathogens that make a first appearance in humans, those that have been known as human pathogens but reappear in higher incidence, as well as new strains of pathogens (see Jones et al. 2008 for detailed methodology of the list).
bAnimal in which the pathogen was detected concurrently or prior to emergence in humans and whether these animals were domestic or wild. See Online Appendix 1 for a full list of pathogens included in this analysis.
cPathogens that do not cause extended morbidity or mortality in non-human hosts.
Figure 1.
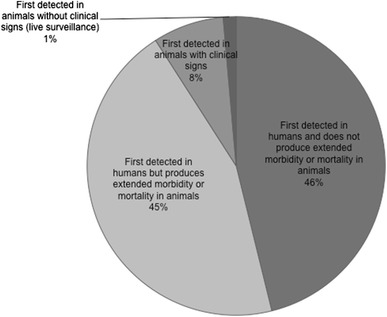
Proportion of emerging infectious human pathogens of zoonotic origin (Jones et al. 2008) that were first detected in humans versus animals (domestic or wild) prior to or concurrent with signs of illness in humans in relation to their pathogenicity in non-human hosts.
Recent reviews (Kuiken et al. 2005; Halliday et al. 2007; Gubernot et al. 2008; Olson et al. 2012) and empirical studies of zoonotic diseases (Eidson et al. 2001; Leroy et al. 2004; Rouquet et al. 2005) suggest that animal surveillance, including sampling of live animals (i.e., active sampling), is an essential component to predicting, responding to, and managing zoonotic disease emergence. We further propose that animal mortality monitoring of both domestic and wild animals (i.e., passive sampling) should be a well-integrated and complimentary component to any surveillance program to prevent the emergence, re-occurrence, and spread of such diseases. At least three empirical studies provide evidence that sampling both healthy (mammals; Levinson et al. 2013) and dead animals (Eidson et al. 2001; Rouquet et al. 2005; Komar and Olsen 2008) are effective strategies for zoonotic pathogen detection and disease prevention (Olson et al. 2012). Rouquet et al. (2005) demonstrated that a dead animal reporting mechanism would have likely prevented further spread of Ebola virus in West Africa. Human infections initially occurred by handling dead non-human primates, carcasses putatively heading to or present at markets for human consumption (gorillas, duiker, and chimpanzees; Leroy et al. 2004). During the outbreak, an Animal Mortality Monitoring Network (AMMN) was implemented briefly (2001–2003) in northeastern Gabon and northwestern Republic of Congo as a predictive and preventive measure for the spread of the Ebola virus (Rouquet et al. 2005). Over 60% of the 21 carcasses reported (primarily by hunters) and tested were infected with the Ebola virus. All animal deaths occurred before any apparent infection in humans. By reporting the dead primates, diagnostics were performed and local authorities were immediately alerted of infected carcasses putatively minimizing the continued risk of pathogen spread to humans. Corroborating this study is a recent review by Olson et al. (2012), which shows that sampling animal carcasses for the presence of the Ebola virus proved to be more effective in recovering the virus than live animal sampling. Because no known vaccine for Ebola exists, stopping the spread of the pathogen is essential. Similarly, dead crow (Corvus brachyrhynchos) reports in the northeastern United States helped to identify the geographical scope of West Nile virus outbreaks (Eidson et al. 2001; Mostashari et al. 2003) and preceded the onset of viral activity in humans (Eidson et al. 2001; 2005; Johnson et al. 2006). It was the reporting of dead crows that led many state officials to effectively pinpoint disease hotspots (Eidson et al. 2001) and ultimately helped to better understand disease dynamics (Marra et al. 2004; Kilpatrick 2011). However, these mechanisms were put in place long after the pandemic hit the United States (U.S. General Accounting Report 2000) and moreover, health officials in the affected states were not properly prepared to deal with dead bird reports or bird necropsies, which prevented officials from tracking the disease in a way that would allow quantification of virus prevalence. To this day, no systematic and extensive animal mortality surveillance program exists despite the ongoing threat of the next zoonotic disease outbreak and the demonstrated importance of such a network as an early warning system for disease emergence.
Conclusion
Despite the significant increase and overall occurrence of zoonotic diseases, very few public health platforms incorporate animal sampling in their disease surveillance programs. Vital to reducing the spread and impact of zoonotic pathogens to humans and among animal populations is early detection and isolation, which first requires observations of the affected animal hosts either when they are sick or dead. Because the transmission route for zoonotic pathogens from animal host to humans is inherently complex, evidence can be presented to advocate for both active and passive animal surveillance (Olson et al. 2012). We suggest that, in part because of this complexity, both should co-occur in comprehensive surveillance programs for disease prevention in humans and wildlife. We have shown that 75 zoonotic pathogens are known to cause extended morbidity or mortality in their animal hosts, and we argue that the quick reporting of these sick or dead animals could have prevented the subsequent or concurrent emergence in humans. Active animal surveillance programs, although essential, are often costly and must be operated by extensively trained personnel thus limiting the scope of surveillance efforts. Passive animal reporting monitoring, on the other hand is a monitoring system that can employ low-cost technology available to civil servants (e.g., park rangers) and the public (e.g., hunters, farmers, citizens) of all income levels, greatly expanding the geographic range of surveillance efforts. If zoonotic disease surveillance and monitoring were limited to the sampling of live animals, it would greatly limit the scope of the search to a small proportion of the public trained for such sampling.
We therefore propose that integrating an animal morbidity and mortality surveillance program is fundamental for the success of public health surveillance systems. Pilot tests can easily be included in public health platforms initially in one to two zoonotic disease hotspots by merging public health surveillance systems with wildlife management efforts. Peri-urban regions near wildlife conservation areas would be ideal interfaces for such pilot tests and disease hotspots would provide a more opportunistic environment to evaluate the effectiveness of reporting sick or dead animals for pathogen detections. Recent use of mobile phone technology has proven highly successful in the public health sector (e.g., ‘Outbreaks Near Me’ application, 29–31, FrontlineSMS, 32; EpiSurveyor, 33). It also offers a similar utility for animal morbidity and mortality monitoring because it can easily be integrated in citizen science approaches that encourage people to report animal mortality events as well as prioritization of reporting in high-risk pathogen transmission zones, such as conservation areas and regions where agriculture and wildlife overlap. We propose that mobile-based data collection could facilitate and rapidly mobilize surveillance teams for pathogen sampling by linking reporting by the public and civil servants to alerting mechanisms with local authorities. Once local authorities are alerted, dead animals can be sampled for the presence of pathogens and surveillance efforts can be initiated if animals have tested positively for zoonotic pathogens, thus focusing surveillance on target animals, pathogens, or areas. The challenges inherent in any system requiring large-scale surveillance of potentially small targets are both in the survey and reporting. For animal morbidity or mortality surveillance, reports may be biased toward larger animals—not only because they are more visible but because carcasses may persist for a longer period of time (Santos et al. 2011)—, animals that are found near or in human habitations, or domestic animals (Table 1) because of their proximity to humans and financial value.
The success of these programs will largely depend on first implementation by international agencies and by continued efforts at a local level. Finally and equally importantly, animal morbidity and mortality reporting is also an indispensable tool for wildlife conservation as it will not only stop the spread of disease in wildlife populations but will also help to quickly mobilize rescue efforts for animals in distress.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank C. Chapman and A. M. Kilpatrick for valuable comments on the manuscript. This research was supported by the American people through the United States Agency for International Development (USAID) Emerging Pandemic Threats program, PREDICT project.
References
- Alagaili AN, Briese T, Mishra N, Kapoor V, Sameroff SC, de Wit E, et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5(2):e00884-14. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East Respiratory Syndrome Coronavirus Disease from Saudi Arabia: A descriptive study. Lancet Infectious Diseases. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Swayne DE. Experimental infection of swans and geese with Highly Pathogenic Avian Influenza Virus (H5N1) of Asian lineage. Emerging Infectious Diseases. 2008;14:136–142. doi: 10.3201/eid1401.070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Beck JR, Suarez DL, Swayne DE. Susceptibility of North American ducks and gulls to H5N1 Highly Pathogenic Avian Influenza Viruses. Emerging Infectious Diseases. 2006;12:1663–1670. doi: 10.3201/eid1211.060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011). http://www.cdc.gov/ncidod/dvbid/westnile/surv&control_archive.htm
- Centers for Disease Control and Prevention. Ebola Hemorrhagic Fever information packet (2010). http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/ebola.htm
- Clegg SB, Turnbull PCB, Foggin CM, Lindeque PM. Massive outbreak of anthrax in wildlife in the Malilangwe Wildlife Reserve, Zimbabwe. Veterinary Record . 2007;160:113–118. doi: 10.1136/vr.160.4.113. [DOI] [PubMed] [Google Scholar]
- Dahlstrand S, Ringertz O, Zetterberg B. Airborne tularemia in Sweden. Scandinavian Journal of Infectious Diseases. 1971;3:7–16. doi: 10.3109/inf.1971.3.issue-1.02. [DOI] [PubMed] [Google Scholar]
- Eidson M, Kramer L, Stone W, Hagiwara Y, Schmit K. Dead bird surveillance as an early warning system for West Nile virus. Emerging Infectious Diseases. 2001;7:631–635. doi: 10.3201/eid0704.017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson M, Schmidt K, Hagiwara Y, Anand M, Backenson PB, Gotham I, Kramer L. Dead crow density and West Nile virus monitoring, New York. Emerging Infectious Diseases. 2005;11:1370–1375. doi: 10.3201/eid1109.040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, et al. Human infection with a novel avian–origin Influenza A (H7N9) virus. New England Journal of Medicine. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- Gubernot DM, Boyer BL, Moses MS (2008) Animals as early detectors of bioevents: Veterinary tools and a framework for animal-human integrated zoonotic disease surveillance. Public Health Rep 123:300–315. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2289983/ [DOI] [PMC free article] [PubMed]
- Halliday JEB, Meredith AL, Knobel DL, Shaw DJ, de Bronsvoort BMC, Cleaveland S. A framework for evaluating animals as sentinels for infectious disease surveillance. Journal of the Royal Society Interface. 2007;4:973–984. doi: 10.1098/rsif.2007.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GD, Eidson M, Schmidt K, Ellis A, Kulldorff M. Geographic prediction of human onset of West Nile virus using dead crow clusters: An evaluation of year 2002 data in New York state. American Journal of Epidemiology. 2006;163:171–180. doi: 10.1093/aje/kwj023. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, et al. (2012) Ecology of zoonoses: Natural and unnatural histories. The Lancet 380:1936–1945. doi:10.1016/S0140-6736(12)61678-X [DOI] [PMC free article] [PubMed]
- Kilpatrick AM. Globalization, land use, and the invasion of West Nile virus. Science. 2011;334:323–327. doi: 10.1126/science.1201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, et al. (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging Infectious Diseases 9:311–322. [DOI] [PMC free article] [PubMed]
- Komar N, Olsen B. Avian influenza virus (H5N1) mortality surveillance. Emerging Infectious Diseases. 2008;14:1176–1178. doi: 10.3201/eid1407.080161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Leighton FA, Fouchier RAM, LeDuc JW, Peiris JSM, Schudel A, et al. Pathogen surveillance in animals. Science. 2005;309:1680–1681. doi: 10.1126/science.1113310. [DOI] [PubMed] [Google Scholar]
- LaDeau SL, Kilpatrick AM, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710–713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- Leroy EM, Rouquet P, Formenty P, Souquiere S, Kilbourne A, Froment J-M, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303:387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- Levinson J, Bogich TL, Olivial KJ, Epstein JH, Johnson CK, Karesh W, Daszak P. Targeting surveillance for zoonotic virus discovery. Emerging Infectious Diseases. 2013;19:743–747. doi: 10.3201/eid1905.121042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, et al. Epidemiology of human infections with avian Influenza A (H7N9) Virus in China. New England Journal of Medicine. 2014;370:520–532. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapesa MW, Atimnedi P, Tumwesigye C. Managing the 2004/05 anthrax outbreak in Queen Elizabeth and Lake Mburo National Parks, Uganda. African Journal of Ecology. 2007;46:24–31. [Google Scholar]
- Marra PP, Griffing S, Caffrey C, Kilpatrick AM, McLean R, Brand C, et al. West Nile virus and wildlife. BioScience. 2004;54:393–402. doi: 10.1641/0006-3568(2004)054[0393:WNVAW]2.0.CO;2. [DOI] [Google Scholar]
- McCoy GW, Chapin CW. Bacterium tularense, the cause of a plague like disease of rodents. Public Health Bull. 1912;53:17–23. [Google Scholar]
- Meselson M, Guillemin J, Hugh-Jones M, Langmuir A, Popova I, Shelokov A, Yampolskaya O. The Sverdlovsk anthrax outbreak of 1979. Science. 1994;266:1202–1208. doi: 10.1126/science.7973702. [DOI] [PubMed] [Google Scholar]
- Mostashari F, Kulldorff M, Hartman JJ, Miller JR, Kulasekera V. Dead bird clusters as an early warning system for West Nile virus activity. Emerging Infectious Diseases. 2003;9:641–646. doi: 10.3201/eid0906.020794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SH, Reed P, Cameron KN, Ssebide BJ, Johnson CK, Morse SS, et al. Dead or alive: Animal sampling during Ebola hemorrhagic fever outbreaks in humans. Emerging Health Threats. 2012;5:1–9. doi: 10.3402/ehtj.v5i0.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz PM, Gordon Z, Holmes R, Taylor B, Wilcox M, Chudnov D, Nadkarni P, Dein FJ. Animals as sentinels of human environmental health hazards: An evidence-based analysis. EcoHealth. 2005;2:26–37. doi: 10.1007/s10393-004-0151-1. [DOI] [Google Scholar]
- Reusken CBEM, Haagmans BL, Müller MA, Gutierrez C, Godeke G-J, Meyer B, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infectious Diseases. 2013;13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouquet P, Froment J-M, Bermejo M, Kilbourn A, Karesh W, Reed P, Kumulungui B, et al. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerging Infectious Diseases. 2005;11:283–290. doi: 10.3201/eid1102.040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SM, Carvalho F, Mira A. How long do the dead survive on the road? Carcass persistence probability and implications for road-kill monitoring surveys”. PLoS One. 2011;6:e25383. doi: 10.1371/journal.pone.0025383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- US General Accounting Office Report (2000) West Nile virus: Preliminary information on lessons learned. GAO/HEHS-00-142R. http://www.gao.gov/products/HEHS-00-142R
- Walsh PD, Abernethy KA, Bermejo M, Beyers R, De Wachter P, Akou ME, et al. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422:611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- Watson A, Keir D. Information on which to base assessments of risk from environments contaminated with Anthrax spores. Epidemiology & Infection. 1994;113:479–490. doi: 10.1017/S0950268800068497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank (2010) People, Pathogens and Our Plant, Vol 1: Towards a Once Health Approach for Controlling. Zoonotic Diseases Report 50833-GLB.
- World Health Organization (2013) Influenza at the human-animal interface. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/en/
- Young GA, Zelle MR, Lincoln RE. Respiratory pathogenicity of Bacillus anthracis spores: I. Methods of study and observations on pathogenesis. Journal of Infectious Diseases. 1946;79:233–246. doi: 10.1093/infdis/79.3.233. [DOI] [PubMed] [Google Scholar]
- Zohrabian A, Meltzer MI, Ratard R, Billah K, Molinari NA, Roy K, et al. West Nile virus economic impact, Louisiana, 2002. Emerging Infectious Diseases. 2004;10:1736–1744. doi: 10.3201/eid1010.030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


