Abstract
Dromedary camels have been implicated consistently as the source of Middle East respiratory syndrome coronavirus (MERS-CoV) human infections and attention to prevent and control it has focused on camels. To understanding the epidemiological role of camels in the transmission of MERS-CoV, we utilized an iterative empirical process in Geographic Information System (GIS) to identify and qualify potential hotspots for maintenance and circulation of MERS-CoV, and produced risk-based surveillance sites in Kenya. Data on camel population and distribution were used to develop camel density map, while camel farming system was defined using multi-factorial criteria including the agro-ecological zones (AEZs), production and marketing practices. Primary and secondary MERS-CoV seroprevalence data from specific sites were analyzed, and location-based prevalence matching with camel densities was conducted. High-risk convergence points (migration zones, trade routes, camel markets, slaughter slabs) were profiled and frequent cross-border camel movement mapped. Results showed that high camel-dense areas and interaction (markets and migration zones) were potential hotspot for transmission and spread. Cross-border contacts occurred with in-migrated herds at hotspot locations. AEZ differential did not influence risk distribution and plausible risk factors for spatial MERS-CoV hotspots were camel densities, previous cases of MERS-CoV, high seroprevalence and points of camel convergences. Although Kenyan camels are predisposed to MERS-CoV, no shedding is documented to date. These potential hotspots, determined using anthropogenic, system and trade characterizations should guide selection of sampling/surveillance sites, high-risk locations, critical areas for interventions and policy development in Kenya, as well as instigate further virological examination of camels.
Electronic supplementary material
The online version of this article (10.1007/s10393-018-1317-6) contains supplementary material, which is available to authorized users.
Keywords: MERS-CoV, camel, Kenya, hotspot, transmission, risk
Introduction
Emerging and re-emerging infectious diseases continue to threaten global health security. Zoonotic diseases or pathogens account for 70% of emerging infectious diseases (EIDs) and 60% of human pathogens/diseases (Woolhouse and Gowtage-Sequeria 2005; Jones et al. 2008). Major zoonotic EIDs have occurred over the last 20 years including but not limited to Middle East respiratory syndrome coronavirus (MERS-CoV) (Zumla et al. 2016). MERS-CoV was first diagnosed in human in 2012 in Saudi Arabia (Zaki et al. 2012) as a novel zoonotic virus responsible for more than 1918 laboratory confirmed cases including 729 fatalities by February 2017 (WHO 2016; FAO 2017).
The epidemiology of MERS-CoV is not well established, but epidemics in humans have occurred sporadically with geographic range restricted to the Middle East/Arabian Peninsula (Khalafalla et al. 2014; Sabir et al. 2016). In addition, most human cases have been associated with healthcare settings, with a fifth of virus detections reported among healthcare workers (Mackay and Arden 2015a, b). Travel-related MERS-CoV links have also been established, and these remain a threat to other regions in view of rapidity and intensity of travels (Fanoy et al. 2014). Since the discovery of MERS-CoV in 2012 (Zaki et al. 2012), serologic and molecular evidences have demonstrated that the virus in dromedary camels is genetically very similar to MERS-CoV in humans; hence, the conclusion that dromedary camels may serve as reservoirs for human infections (Nowotny and Kolodziejek 2014; Dudas and Rambaut 2016). Bats are also considered as the likely primary source of zoonotic beta-coronaviruses (Memish et al. 2013).
In recent past, the role of camels as major contributors to food security and livelihood in the arid and semi-arid parts of Sub-Saharan and North Africa has been threatened by the emergence of MERS-CoV (Jores 2015), not because the virus cause severe disease in camels but because of potential loss of export markets of camel products due to avoidance by humans. In addition, Somalia, Sudan and Ethiopia with high seroprevalence of MERS-CoV antibodies (Müller et al. 2014) sometimes move their camels through Kenya. Hence, attention to prevent MERS-CoV has focused on camels. Although no human case of MERS-CoV has been reported in Kenya, serologic evidence for circulation of the virus in camels dates back to 1992 (Corman et al. 2014).
To date, information on the epidemiology of MERS-CoV in humans, animals and the environment is scanty and details on the virus persistence and transmission (animal to animal, animal to human, human to human, and in the environment) is lacking (Mackay and Arden 2015a). The exact mode of transmission to humans from camels and other possible animal sources remains undefined (Reusken et al. 2013; Mackay and Arden 2015a, b; Adney et al. 2016). However, sequencing data has suggested that MERS-CoV originated from bat ancestors and may have undergone a recombination event in the spike protein, possibly in dromedary camels in Kenya including other Eastern African regions before its exportation to the Arabian Peninsula along the camel trading routes (Omrani et al. 2015).
The Greater Horn of Africa (HOA) covering Djibouti, Eritrea, Ethiopia, Kenya, Somalia, South Sudan, Sudan, Uganda is home to over 70% of the world camel population (Farah et al. 2004; FAOSTAT 2015). The Region is a major exporter of live camels and meat to Egypt and the Gulf Cooperation Council countries of Bahrain, Kuwait, Oman, Qatar, Saudi Arabia and United Arab Emirates (Mahmoud 2010). Egypt is a major consumer of a significant amount of camel meat from Ethiopia, Eritrea and the Sudan, and most of the “Ethiopian camels” are thought to originate from or have spent part of their lifetime grazing in Kenya and Somalia.
The detection of MERS antibodies in Camels in HOA, a region with significant trade links to infected countries, has triggered the need for greater eco-epidemiological understanding of MERS-CoV in the HOA (Corman et al. 2014; Müller et al. 2014; Deem et al. 2015). Already regional and country efforts have been initiated. The USAID’s Emerging Pandemic Threats (EPT-2) program of GHSA has extended financial support to Kenya, Ethiopia, Egypt and Jordan to undertake MERS-CoV surveillance in livestock and wildlife in order to generate useful information for prevention and control.
To understand the epidemiology of the virus in Kenya, the delineation of hotspots and risk mapping of the camel production zones are important for surveillance, rapid response and policy formulation and to reduce the risk of long-distance transmission of the virus. In addition, in view of the limited resources available for livestock disease surveillance, the mapping will enable the country to prioritize and focus surveillance, understand trade patterns and evaluate risk behaviors. This study was carried to identify and map MERS-CoV potential hotspots along the camel value chain in Kenya using literature reviews, empirical and anecdotal evidences, expert opinion, field surveys and interviews.
Materials and Methods
Extensive literature review on camel production and marketing in Kenya was carried out to analyze camel population and densities, document existing farming and marketing practices and generate data to map stakeholders involved in the camel value chain and understand the global perspective. The review also revealed knowledge gaps and the combination of approaches mentioned above was used to arrive at criteria for risk mapping.
Data Mining
Global-level data on the status of infection, transmission and affected/implicated species of animals for MERS-CoV as well as previous seroprevalence studies among camels in Kenya were mined for useful quantitative and qualitative datasets. Furthermore, field data on the camel production systems in Kenya, marketing practices, population dynamics and densities as well as on trade networks, linkages and movement routes for camels in the HOA were collected and supplemented by available peer-reviewed and gray literature (FAO 2016). Documents were obtained from government repositories, commissioned reports, conference papers, institutional analyses and peer-reviewed literature using search engines (Google Scholar and PubMed). Duplicate reports were removed from the search and data search and filtration was carried out by two independent researchers leaving a total of 54 documents for evaluation. Relevant stakeholders were interviewed to clarify sectoral data. Country and county-level camel populations (2015 estimates) were obtained from Directorate of Livestock Production (DLP), State Department of Livestock, Ministry of Agriculture, Livestock and Fisheries (MoALF).
Field Data Collection
Between July and December 2016, field evaluations, rapid appraisals and validation studies were carried out in Laikipia, Isiolo and Marsabit Counties on camel production systems, marketing, trade volumes and migration routes. Using analytical hierarchical method in decision making to generate consensus opinions (Saaty 2008), semi-structured questions and check list were utilized to gather data from key informants: County veterinary staff (n = 5); County Animal production staff (n = 3); Meat inspectors (n = 1); Livestock Market Association members (n = 1); Camel traders (n = 13); Herders (n = 4); Livestock marketing cooperative (n = 1); Camel meat trader (Butcher) (n = 1); Primary marketers (n ≈ 150 to 250 persons); Secondary marketers(n ≈ 300–400 persons); other key informants (n = 2); total (n = 481–681 persons). The data obtained were triangulated by independent field visits to six (6) selected secondary markets (two in each of the three counties) with additional data gathered from two camel slaughter slabs. A Market Profiling Tool (MPT) (Supplementary material 1a) was developed and utilized to capture market-level quantitative and qualitative data. Description of the infrastructure and biosecurity status of the six markets and two slaughter slabs was achieved through observations of the investigators.
Trade and migration routes were updated through participatory geographic information systems (pGIS) involving focused group meetings (FGM) with randomly selected local community members (n = 30) to cross-validate the data above. Specifically, ten members of each community were chosen based on gender/age disaggregation including four men, three women and three youths. This selection also cut across functional roles (men: rearers/owner, take camels on long-distant travels and responsible for monies arising from camel sales; women: do daily chores around camels in the households like milking restraints and processing/marketing of bye-products; youth (both sexes inclusive) usually drive the camels daily to the fields/markets). Each group listed major markets and other features relevant to the camel sector such as watering points and pasture fields. Community base maps were provided with key topographic features in mini-workshops, and missing features were compiled based on visual routes used for trade and within-country migration. Each group made presentations which was critiqued by group members before group consensus was reached. Communities’ views and perception on the geospatial–temporal attributes per community was obtained through non-structured interviews and the outputs of FGM. All spatial locations and features provided were geocoded using Epicollect 5® (Imperial College, London) through follow-up field walks and mapping with the key informants. All geospatial data were again cross-matched with details obtained from the interviews. Based on the mined data, interviews, FGM and field walks and county-level data on camels, density map was created (Fig. 1).
Figure 1.
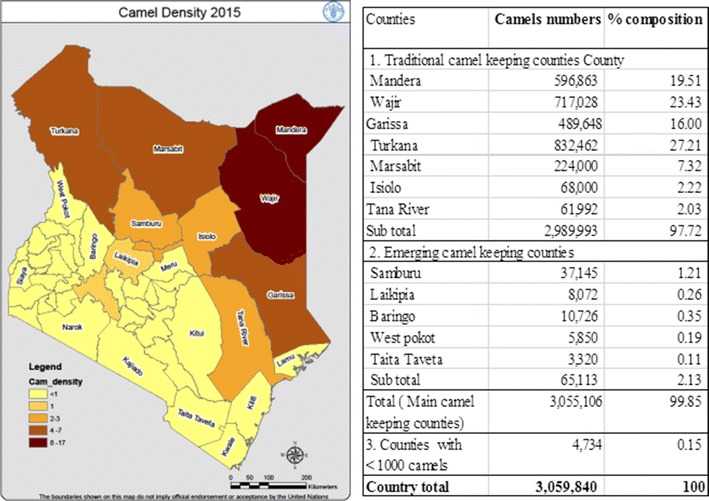
Map of camel population densities in Kenya and Table 1 Camel population in major camel keeping counties. Source: Director of Livestock production (DLP) 2015 livestock population estimates.
Data Analysis
The tabular data (Table 1) and DLP-MoALF camel population data were linked with spatial data and using map calculator; the camel density per county was developed (Fig. 1). Camel production systems were analyzed and reclassified based on the different agro-ecological zones of the country and also disaggregated by Counties. Agro-ecologically, the country has been zoned into the following: I (humid with mean annual rainfall of 1100–2700 mm), II (sub-humid with mean annual rainfall of 1000–1600 mm), III (semi-humid with mean annual rainfall of 800–1400 mm), IV (semi-humid to semi-arid with mean annual rainfall of 600–1100 mm), V (semi-arid with mean annual rainfall of 450–900 mm), VI (arid with mean annual rainfall of 300–550 mm) and VII (very arid with mean annual rainfall of 150–350 mm) (Sombroek et al. 1982).
Table 1.
Camel production systems per agro-ecological zones and population growth and projection, 2009–2015. Source: FAOSTAT (2015). http://faostat3.fao.org.
| Production system | Agro-ecological zones (climate type) | Locations |
|---|---|---|
| Traditional Pastoralism | VII, VI, V (Semi-arid to very Arid) | Mandera, Wajir, Garissa, Tana River, Marsabit, Turkana. Baringo, Samburu Isiolo, Laikipia, Kajiado |
| Semi-Sedentary system including ranching and Peri-urban camel production | V (Semi-arid) | Isiolo, Laikipia, Kajiado |
| Commercial ranching | V (Semi-arid) | Laikipia, Taita Taveta |
| Camel population growth trend in Kenya between 2009 and 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015* | Mean annual growth (%)# |
| Estimated | 2,971,111 | 3,030,600 | 3,091,200 | 2,864,732 | 2,899,244 | 2,937,262 | 3,059,840 | 0.56% |
| Percentage difference (%) | NA | 2.00 | 2.00 | − 7.33 | 1.20 | 1.31 | 4.17 | 3.36 (absolute growth) |
Details of persons who participated in VCA and pGIS interviews and their functional roles: County veterinary staff (n = 5): provision of animal health services and regulation of diseases control; County Animal production staff (n = 3): provision animal production advisory services; Meat inspectors (n = 1): meat hygiene services; Livestock Market Association members (n = 1): management of livestock markets; Camel traders (n = 13): trade in camels; Herders (n = 4): grazing, security, milking, watering of camels; Livestock marketing cooperative (n = 1): promotion of efficient livestock marketing and development; Camel meat trader (Butcher) (n = 1): slaughter and sale of camel meat; Primary marketers (n ≈ 150 to 250 persons); Secondary marketers(n ≈ 300-400 persons); other key informants (n = 2); total (n = 481 – 681 persons).
*2015-Estimates from Directorate of Livestock Production. #Note that the total absolute growth for the 7-year period was 3.36%.
The visual routes, trade patterns and migration routes were drawn on ArcGIS and ArcMap using the available dataset and the details from pGIS, and the base map routes for trade and migration. Digitized group presentations standardized by consensus were overlaid with the topographic maps to ascertain the viability of trekking in the terrain with special focus on existence of steep ridges, cliff or geological fault lines that may impede movement. Finally, county-level camel seroprevalence to MERS-CoV were obtained (Table 2) to finalize risk maps.
Table 2.
Seroprevalence of MERS-CoV in dromedary camels in selected counties of Kenya.
| County | Production system | Camel density (per km square) | Seroprevalence | Source |
|---|---|---|---|---|
| Laikipia | Ranching (commercial) | 1 | 6.9% | Corman et al. (2014) |
| Nomadic pastoralism | 46.95% | Deem et al. (2015) | ||
| Isiolo | Nomadic pastoralism | 2–3 | 16.7% | Corman et al. (2014) |
| Peri-urban camel production system (PUCPS) | Not done | – | ||
| Turkana | Nomadic pastoralism | 2–3 | 9.0% | Corman et al. (2014) |
| Wajir | Nomadic pastoralism | 4–7 | 59.0% | Corman et al. (2014) |
| Marsabit | Nomadic pastoralism | 2–3 | 72.1% | Corman et al. (2014) |
| Mandera | Nomadic pastoralism | 8–18 | 56.2% | Corman et al. (2014) |
| Nakuru (Naivasha) | Commercial ranch | <1 | 12.0% | This study |
Results
Camel Production in Kenya
Based on the available dataset, camel production is an integral part of the Kenya pastoral farming system and is mostly resident in the northern rangelands and arid and semi-arid (ASAL) counties (Fig. 1). However, this trend appears to be changing and currently the central (Laikipia, Baringo and West Pokot) and southern (Kajiado and Taita Taveta) rangelands counties are slowly gaining prominence as key camel-producing areas (Fig. 1 and Table 1). Based on field interviews, camels are reared mainly for subsistence and as dairy animals for commercial milk production but less for meat production in Kenya.
Two management systems used for camel production in Kenya are the traditional pastoralism (Nomadic and transhumance Pastoralism) and the semi-sedentary system (Peri-urban market oriented systems, ranching and off-farm camel management for ecotourism, camel safaris and camel racing) with very limited commercial system (Table 1). County-level characteristics and production systems are available in Supplementary material 1b.
The traditional pastoralism is highly extensive and is the most prevalent system constituting over 98% of the total camel population while the semi-intensive system constitutes approximately 1%, and mainly from Isiolo, Laikipia and Kajiado. Using the 2014 livestock census, the camel population in Kenya was ≈ 2.9 million heads (FAOSTAT 2015). An estimated absolute population growth for the year 2009 until 2014 was 3.36% with a mean annual growth of 0.56%. The year on year annual increase was ≈ 2.0, 2.0, − 7.3, 1.2, 1.3, and 4.2% for 2010, 2011, 2012, 2013, 2014 and 2015, respectively. In 2012, there was a severe depression (> 7%) in the growth rate for camel production in Kenya and the highest increase was projected for 2015.
In terms of camel heads (absolute numbers) and population densities, Kenya has approximately 3.1 million camels with Turkana (27.3%), Wajir (23.4%), Mandera (19.5%) and Garissa (16.0%) having the highest population in that order, while Mandera remains the camel densest community with approximately 8–17 camels per square kilometer (Fig. 1). In addition, camel densities continue to reduce as one transit from the extreme northeast to the southwest part of Kenya (Fig. 1).
Livestock Movement, Trade and Market Linkages
Marketing of livestock (cattle, camels, sheep and goats) in Isiolo, Laikipia and Marsabit is representative of what transpires in other Kenyan pastoralist communities. The in and out-flows of people and livestock to the markets, and the volumes vary significantly across the different categories of markets and also the months of the year (Tables 3, 4, 5). The closer a market is to a terminal (final destination of the camel), the higher the level and volumes of traders, diversity of actors and animal numbers (Tables 3, 4, 5). Additionally, livestock from secondary markets are transported to many destinations and have linkages with long-distant markets. Using the MPT, it was inferred that the risk of intra- and interspecies transmission of disease is considered higher in secondary (terminal) markets compared with the primary (bush) markets (Table 3). More cattle, sheep and goats were traded through the markets compared with camels. Sale of donkeys was sparingly done and was reported only in Isiolo livestock auction yard. Seasonal variations in sales were influenced by annual migration of livestock to locations away from the markets in search of forage and water (Fig. 2a, b); during such movements, there fewer livestock are available for sale in the markets. Similarly, fewer animals were presented to the markets during the wet months of late March up until early June, and in the months of October to December. During these months, camels and cows are at their peaks in terms of production and productivity, and lush pastures are abundant, with resultant increase volume of milk for the pastoral household and the need to sell off animals to purchase food stuff is not warranted. Interviews with the traders suggested that the price of livestock skyrocketed during the low offtake period, but this increased prices of livestock do not seem to trigger increased supply of animals by the pastoralists.
Table 3.
Comparison of trade volume, livestock sources and origin–destination between primary and secondary markets in Marsabit, Kenya. Source: Marsabit Livestock office and Merille market LMA.
| Merille secondary market | Illaut primary market | ||
| Numbers traded in single market day | Camels | 100 | 10 |
| Cattle | 200 | 10 | |
| Sheep and goats | 1000 | 300 | |
| Donkeys | 0 | 7 | |
| Source | Many—Illaut market, Korr market, bush markets in Laisamis sub-county, Bargoi in Samburu and Producers in Laisamis sub-county | Few—bush markets in Kargi/South Horr and Korr/Ngurunit wards | |
| Destination | Far and wide—Moyale(camels), Isiolo, Meru and Nairobi(sheep and goats) | Limited destination—Merille market, Kargi | |
| Actors involved | Many—highly accessible (Isiolo–Marsabit road) | Few- poor accessibility by road | |
Table 4.
Annual mean volume of livestock traded in selected secondary markets in Laikipia, Marsabit and Isiolo Counties, Kenya. Source: County livestock movement report.
| Market volumes | |||||||
|---|---|---|---|---|---|---|---|
| County | Markets | Seasons | Camels | Cattle | Sheep and goats | Donkeys | |
| Laikipia | Secondary | Rumuruti Market | Low | 0 | 250 | 1000 | 0 |
| Normal | 0 | 400 | 1500 | 0 | |||
| High | 0 | 750 | 2500 | 0 | |||
| Doldol Market | Low | 5 | 40 | 150 | 0 | ||
| Normal | 10 | 60 | 300 | 0 | |||
| High | 15 | 100 | 600 | 0 | |||
| Marsabit | Merile | Low | 20 | 80 | 600 | 0 | |
| Normal | 50 | 120 | 800 | 0 | |||
| High | 80 | 200 | 1200 | 0 | |||
| Moyale | Low | 20 | 50 | 200 | 0 | ||
| Normal | 50 | 50 | 200 | 0 | |||
| High | 60 | 70 | 800 | 0 | |||
| Isiolo | Secondary | Isiolo auction yard | Low | 0 | 200 | 200 | 20 |
| Normal | 0 | 300 | 500 | 12 | |||
| High | 0 | 800 | 2000 | 12 | |||
| Duse | Low | 2 | 0 | 70 | 0 | ||
| Normal | 7 | 0 | 150 | 0 | |||
| High | 15 | 0 | 500 | 0 | |||
Table 5.
Seasonal variations in volume of livestock traded in the six profiled markets. Source: County livestock markets reports and key informant interviews.
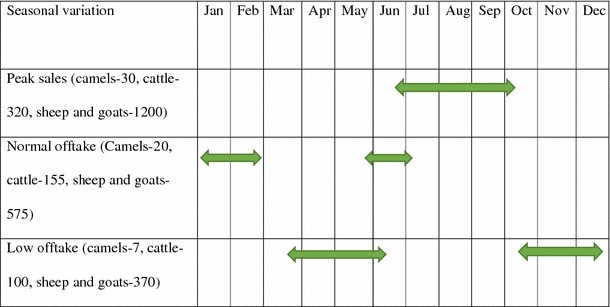
Figure 2.
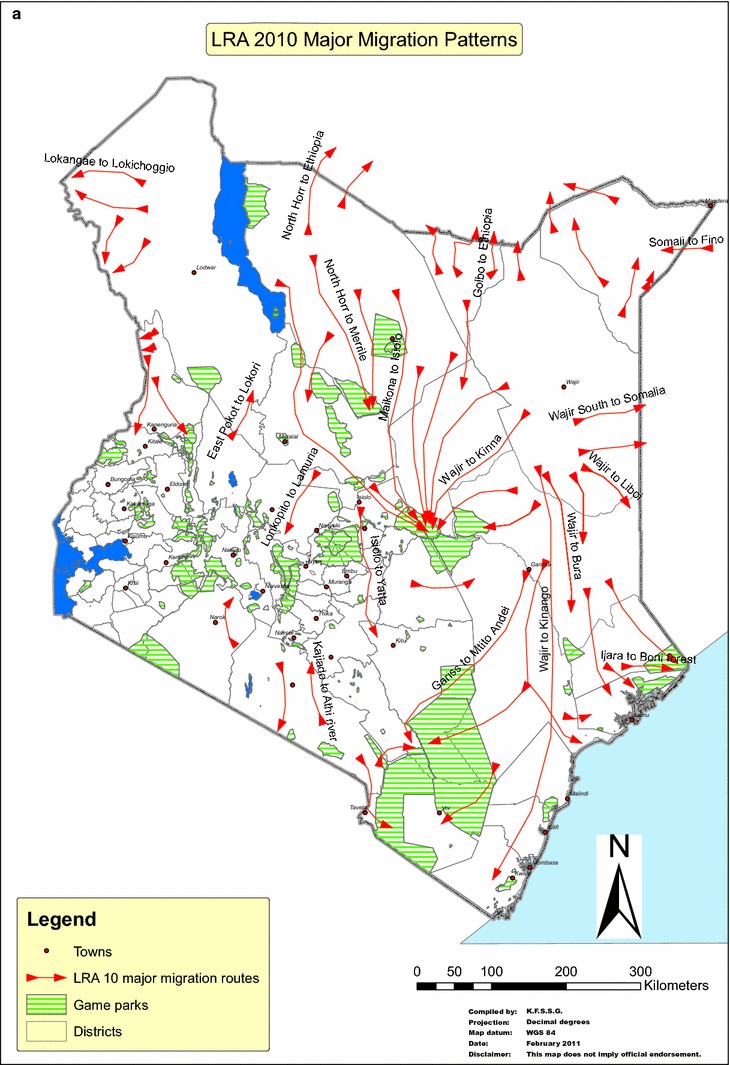
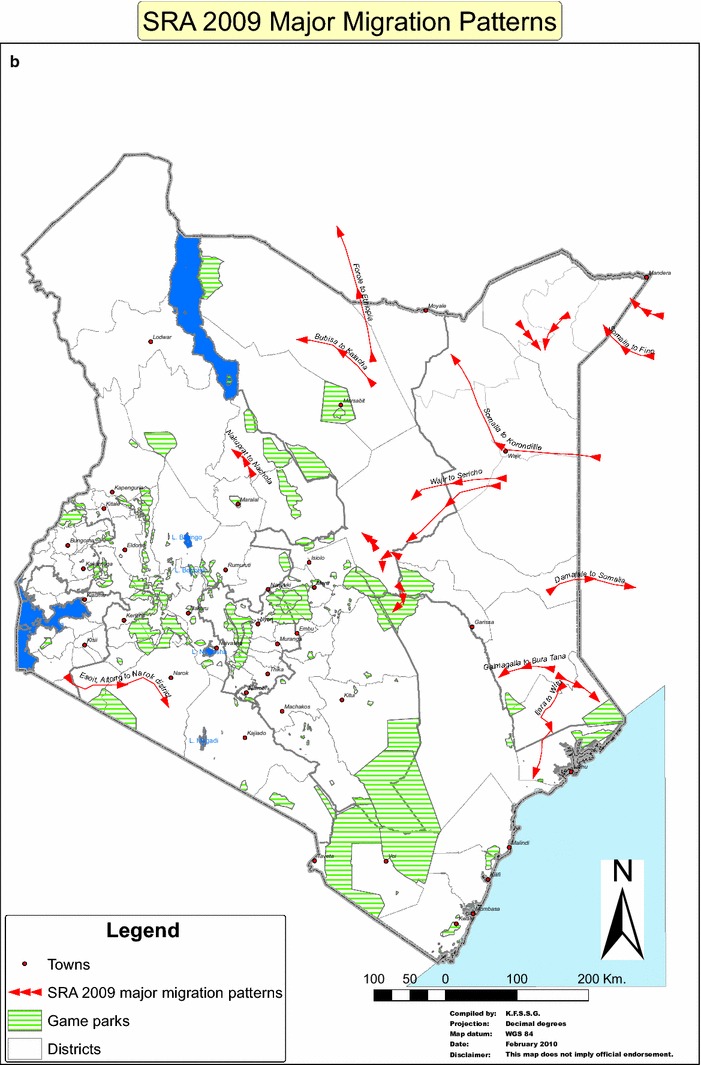
Impact assessment and major migration patterns of livestock associated with a long rain assessment (LRA–April to October), and b short rain assessment (SRA–November to March). The long and short rains influenced the patterns of movement of animal annually. Vegetations improve in-country during long rains, and these significantly influence in-migration. Conversely, dry seasons and period of short rains are characterized by sparse vegetations which cause out-migration of camels and other ruminant livestock.
Camel-Specific Sales, Marketing Network and Migration Routes
Camels were not traded in two (2) of the six (6) profiled markets–Rumuruti in Laikipia and Auction yard in Isiolo (Table 5). Furthermore, traded volumes for camels (n = 5–80) were significantly low compared with those of sheep, goats and cattle in all the surveyed markets.
Using the details of the pGIS conducted in Isiolo and Marsabit, the following trade routes have been identified: (1) Wajir–Isiolo–Meru–Mwingi–Athi River (Major route), (2) Merille–Moyale–Ethiopia–Djibouti–Egypt–Middle East (Major route), (3) Merille–Isiolo–Meru–Mwingi–Athi River-(Major route), (4) Wajir–North Horr (Maikoma ward)—Barter trade—heifers and return with bulls to Wajir (Minor route) (Fig. 3).
Figure 3.
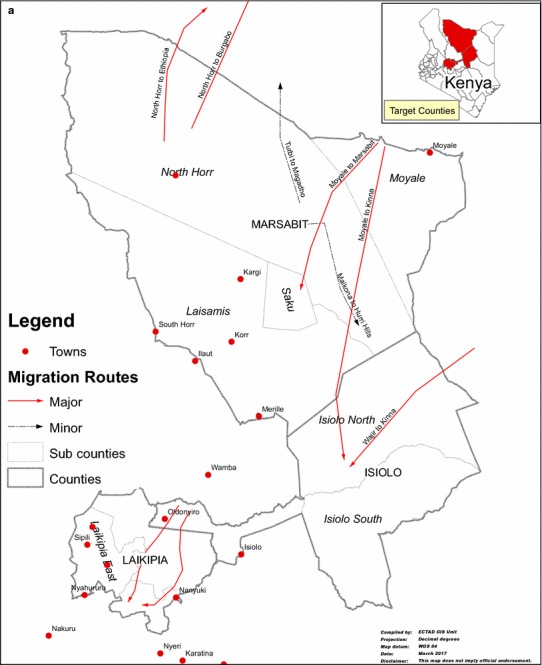
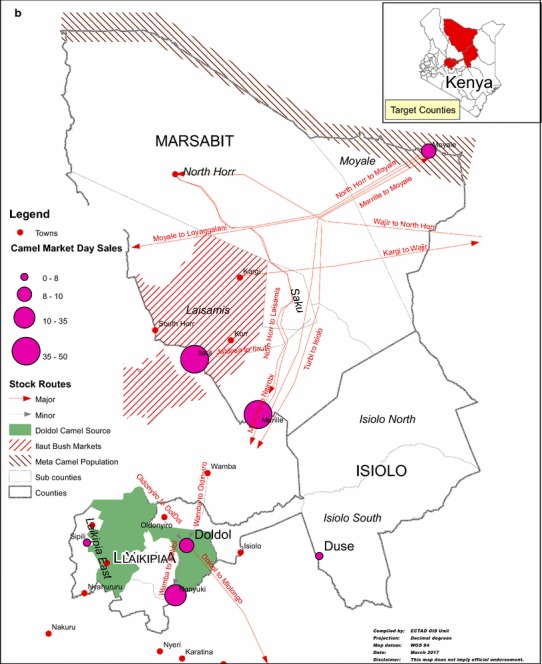
a Camel migration routes and b Market volumes and stock routes in some major camel-producing counties (Laikipia, Marsabit and Isiolo), Kenya.
Camels migrate from most counties to Kinna area of Isiolo, a dry season grazing area for cattle, sheep and goats where pastoralists congregate with their livestock herds for dry season grazing. Furthermore, camel stock route from Moyale to Marsabit involves barter trade in which mature bull camels—destined for the Djibouti holding ground for onward sale to the Middle East markets—are exchanged for female calves that are later sold in the Marsabit region (Fig. 3). Similar scenario was noted on the Wajir–North Horr stock route in which traders brought female calves from Wajir to exchange with mature bulls in North Horr which are then trekked to Wajir County and sold to neighboring Somalia.
Camel Slaughter in Selected Counties, Kenya
Although camel meat is not very popular meat among Kenyans, the trade in camel meat in Nanyuki and Isiolo towns is primarily a preserve of Somali traders. Seven such butcheries have been identified: three (3) in Nanyuki and four (4) in Isiolo. Few other such facilities may exist elsewhere within the country. Camels for slaughter are sourced directly from ranches/pastoral herds and also from the livestock markets of Doldol and Merille. Slaughtering of camels was carried out in Nanyuki and Isiolo town municipality slaughter slabs (Supplementary material 1c). Distinct slaughters slabs are used for camels, cattle, sheep and goats in each town but all within the same precincts. Stunning guns were used by the operators to immobilize the animals before slaughtering as required under the animals welfare regulations. However, the level of hygiene in the investigated slaughter slabs and biosecurity were poor.
Seroprevalence of MERS-CoV in Kenya Camel Herds
Dromedary camels from Kenya have presented with antibodies against MERS-CoV, and the virus has been in circulation for over 20 years based on serologic evidence (Jores 2015; Deem et al. 2015; Müller et al. 2014). Specifically, the following levels of seroprevalence MERS-CoV antibodies have been reported: Marsabit (72.1%), Wajir (59.0%), Mandera (56.2%), Laikipia (6.9–47.0%), Isiolo (16.7%), Nakuru (12.0%) and Turkana (9.0%). Seroprevalence was positively correlated with camel densities per counties with a correlation value of 0.55 (P = 0.20), frequency of animal and herd contacts but negatively correlated with absolute camel population per county (correlation value = − 0.06, P = 0.92).
Discussion
Risk Mapping and Prioritization of MERS-CoV Hotspots
Risk analysis and decision support to prioritize potential hotspots for MERS-CoV amplification and transmission should be based on empirical evidence. In this work, we have conducted camel production system characterization, market–related variables and practices as basis for determining MERS-CoV hotspots and risk nodes. These maps can serve as bases for future decision on risk-based disease surveillance in camels for Kenya and in the sub-region. Camel value chain should be investigated further to identify, qualify and quantify risks, critical control and intervention points (risky nodes), socio-anthropogenic risky behaviors and practices that facilitate the propagation of pathogens.
Whereas we have identified and ranked counties with high camel population density for prioritized surveillance, and as potential hotspots, socio-anthropogenic features, challenges of security, unique production systems, trade dynamics and unfavorable geographic features that discourage animal production and movements in certain areas may influence a re-prioritization. Based on these identified challenges, Mandera, Wajir, Garissa, Samburu, and Baringo counties, although were ranked among the high-density camel counties, were left out of the study. Such locations can only be investigated using specialized security-backed animal disease surveillance program. Marsabit and Turkana counties were ranked among the first five counties with high camel densities and had minimal security challenges, and were therefore selected for risk-based camel disease surveillance areas alongside Isiolo, Laikipia, and Nakuru counties with moderate camel densities.
Value Chain Nodes
Market-based surveillance should target secondary markets which are prone to higher risks compared with the primary markets. In this study Merille (operates every Tuesday with trade volumes of approximately 50 camels), Duse (Tuesday, 7 camels) and Moyale (Wednesday, 50 camels) were identified for such evaluation. Virological and serological surveillance should also focus on slaughter slabs in Isiolo and Nanyuki towns with average slaughter capacities of 48 and nine (9) camels per week because slaughter slab workers may be predisposed to camel-related zoonoses. Consideration for surveillance along the migration routes should focus on spatial–temporal convergence points including: (1) Losai thorny bushland in Laisamis Ward of Laisamis sub-county which is located southwest and adjacent to the Marsabit National Reserve as well as (2) Kinna Ward in Garbatulla sub-county in Isiolo county which receives highest rainfall, and is near to Nyambene hills in Meru. For trade routes, focused surveillance should target identified routes including: (1) Wajir–Isiolo–Meru–Mwingi–Athi River, (2) Merille–Moyale–Ethiopia–Djibouti–Egypt–Middle East, (3) Merille–Isiolo–Meru–Mwingi–Athi River, (4) Wajir–North Horr (Maikoma ward)—Barter trade—heifers and return with bulls to Wajir. Peri-urban camel production systems (PUCPS) are beginning to gain prominence especially around Isiolo town, and they comprise of traceable milking herds (usually 10–50 camels in size), which are grazed within the proximity (10–30 km) of the town. The camel–human interactions facilitated by this system as well as the potential zoonoses associated with milk and other products from these herds necessitated a special surveillance system. However, herd recruitment for programmed surveillance is complicated because the majority of such herds are owned by several (3–5) pastoralists who may have different programs.
The northern and eastern rangelands are traditional camel-producing areas in Kenya; however, camel production has emerged as an important activity in central and southern counties: West Pokot, Kajiado, Tana River, Elgeyo Marakwet and Laikipia due to influence of climate change, and conflicts associated with animal movement, cattle raiding and diseases; and as a strategy for livelihood diversification because camels thrive where cattle fail in unfavorable conditions (Guliye et al. 2007). Specifically, pastoralist preference and shift from cattle to camels and small ruminants has gained prominence in order to adapt to drastic changes in vegetation ecology. Whereas small ruminants are mainly browsers and utilize significantly less feed resource compared with cattle and will thrive better in sparse grassland, camels can live in hot arid environment and avoid hyperthermia, cope with drought and maintain body conditions, survive for a much longer period and travel for 5–7 days without food and water without impairment to their physiology (Ouajd and Kamel 2009).
The Counties under review (n = 14) account for approximately 90% of the national camel herd and are the most important camel-producing areas, consists of 2.4 million households and representing 26% (13 million individuals) of the Kenyan population (KNBS 2009). A significant proportion of this human population are at risk of losing livelihoods (trade bans that may be associated with ban on camels and camel products in the event of MERS-CoV), food security (milk and associated products) and infection with diseases like MERS-CoV and camel-associated human brucellosis in an uncontrolled camel farming sector. It becomes imperative that the county and national governments jointly develop industry support programs and implement animal disease control in these counties.
As camel production has gained more prominence in Kenya’s central and southern rangelands, better management methods are advocated. Production function (milk and milk-products) is relevant to the camel value chain particularly in sub-Saharan Africa (Noor 2013). The estimated tonnage and worth of total camel meat produced in 2013 in Kenya were put at between 700 and 10,000 tonnes and approximately Ksh 1 billion (≈ US$10 million), respectively, and between 200 and 350 million liters of camel milk was produced annually at a net worth of over Ksh 2 billion (≈ US$20 million) (Anonymous 2005; ZED 2015). The reproductive efficiency of camels under traditional pastoral conditions is low with short breeding season, late attainment of puberty and the long gestation period of approximately 13 months; over 90% of the Kenyan camel herds currently operate under this system and will need improvement (Kaufman and Binder 2002; Skidmore 2003). Potential export market can be explored in addition to local economy and a shift to more resident herding system (ranching) and commercialization can tremendously increase camel values and reduce the limitations associated with the traditional pastoralism (Kaufman and Binder 2002).
Traditional pastoralism requires large expanse of land, and it comes with inefficient resource utilization, prone to conflicts, have potential to disseminate disease over large areas and makes camel population census difficult. It, however, has the benefit of reduced disease intensification per unit space and limited animal–human contacts with implication for reduced zoonoses.
In the Arabian Peninsula, where high number of human cases of MERS-CoV has been recorded, camel production is highly intensive and human–animal (camel) contacts appear more intense (Nowotny and Kolodziejek 2014; Omrani et al. 2015; Sabir et al. 2016). To date, Kenya has recorded zero human cases of MERS-CoV. This observation could be due to limited animal–human contacts, lack of a MERS-CoV surveillance system, pathogen biology or the virus is absent. Currently, camels have been linked with the emergence of and zoonotic transmission of MERS-CoV (Dudas and Rambaut 2016) and countries in the HOA contribute significantly to the volumes of camels exported to North Africa and the Middle East, it becomes mandatory to implement risk-based surveillance in export markets such as Merille, Doldol, Moyale and Duse. Surveillance may include value chain nodal analysis, repeat sampling as well as socio-anthropological studies of actors and traders that may promote the emergence, circulation, amplification and intensification of MERS-CoV.
Although northeastern counties were important camel areas, whether transboundary movement through Mandera, Wajir, Marsabit and other counties in northeast Kenya, or camel residency influence the absolute population figures and the densities were not investigated in this study. Perhaps a national camel population study conducted during periods when vegetation index is favorable and animal movement is limited may present with a different outcome. National camel identification project that may assist with animal traceability (in-migration, out-migration and within-country movement) is necessary. Because there have been direct correlation between animal population densities and animal disease (Olive et al. 2016; Corman et al. 2014), targeted surveillance should be prioritized in these camel-dense counties. In this work, counties with high seroprevalence (Marsabit, Wajir and Mandera) were primary high transit areas for camel movements and were positively correlated with camel densities, frequent animal and herd contacts. Jores (2015) argued that antibody levels of nomadic camels are significantly higher than those from ranches and that herds in northeastern parts showed increased seropositivity than camels in the north western and our finding is in agreement with this assertion. Müller et al. (2014) have earlier reported that Kenya receives many camels from Somalia and Sudan where seroprevalence values were as high as over 90%. These herds enter Kenya through Mandera, Wajir and Garissa thereby increasing animal-to-animal contacts in cross-border camel meta-populations.
In addition to animal population density, virus transmission might also be influenced by camel movement, socio-anthropological behavior of pastoralists, trade networks, and slaughter practices. We observed a poor level of hygiene in the investigated slaughter slabs, weak implementation of biosecurity and extensive trade networks facilitated limited but sometimes intense contacts between camels from the different counties and those from Sudan, Ethiopia and Somalia especially during droughts, when limited water resources are available for sharing within the affected ASALs. The animal movement is an integral component of livestock marketing, and these movements are potentially risky for long-distance infectious diseases transmission across substantial geographic areas.
Although the price of livestock skyrocketed during the low offtake period, it did not influence supply of animals by the pastoralists. Economics principle suggests that there is an inverse relationship between the supply and prices of goods and services if demand remains unchanged. It was expected that with the relatively high price and relative increase in demand, the supply chain should be triggered to meet the challenges. This position is true for most of the agricultural production enterprises; however, it will appear that pastoralists do not respond to market prices by increasing or decreasing production rather, and the role of livestock to them goes beyond the ordinary economic principles. Most farmers rather hold their herds/flocks more like an investment portfolio/bank and only draw from it as needs arise (Barrett et al. 2006).
Camel Trade and Migration Routes
Based on pGIS, four important trade routes have been identified above. It is pertinent to note that these trade routes traverse parts of the country’s ASAL terrains and cross one another in the course of animal movement mainly influenced by drought. At such periods, domestic and wild animals concentrate around watering points, river course and feed resources with increased level of interactions between domestic and wild animals. Such intense interaction may introduce and amplify disease pathogens and increase the likelihood of disease outbreaks. Such outbreaks are recorded periodically in Kinna, Cherab and Garbatulla areas of Isiolo, a dry season grazing area for cattle, sheep and goats (Wasonga et al. 2016). Furthermore, in the situation of acute food shortage for camels during drier periods, and as part of trade practices, some of the stock routes do trade by barter, e.g., (1) Moyale to Marsabit barters mature bull destined for the Djibouti and ultimately the Middle East markets for female calves to be sold in the Marsabit region; (2) Wajir–North Horr barters female calves from Wajir for mature bulls in North Horr which are then trekked to Wajir County and sold to neighboring Somalia (Fig. 3). The implications of these barters in the epidemiology of MERS-CoV and other camel diseases cannot be underestimated.
Although camel meat is not popular in Kenya, the trade in camel meat in Nanyuki and Isiolo towns remains a preserve of Somali traders. Seven such butcheries have been identified and (Supplementary material 1c). Hygiene and biosecurity levels in these niche markets should improve, and pre-slaughter inspections should be carried out on camels meant for slaughter.
The sero-evaluation for antibodies to MERS-CoV antibodies in dromedary camels from Kenya presented with location specific results. Previous serological works have suggested that Kenya may have had the MERS-Co virus in circulation (Jores 2015; Deem et al. 2015; Müller et al. 2014). Our conclusion is in agreement with these assertions, and we confirmed that camel population density per county, volume of camel movement through counties and intense animal–animal contacts are associated with high seropositivity. Jores (2015) had earlier argued that herds in northeastern parts showed increased seropositivity than camels in the north western parts of Kenya. East-African sub-regional herds enter Kenya through Mandera, Wajir and Garissa counties thereby increasing animal-to-animal contacts, and this may be responsible for the high level of seropositivity in camels from this axis.
We have demonstrated that Kenyan camels are predisposed to coronaviruses including MERS-CoV but no evidence suggests shedding of the virus based on the serologic result in this study. We have also determined potential hotspots based on empirical facts, system and trade characterization. Outputs from this study should guide careful selection of sampling and surveillance sites, high-risk locations, critical areas for interventions and policy development to support camel production and trade in Kenya. It is hoped that virological examination of Kenya’s camel will be conducted in due course to add to the understanding of epidemiology of MERS-CoV globally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was sponsored by the United States Agency for International Development through the MERS-CoV applied research activities in Middle East and North East Africa under the USAID’s Emerging Pandemic Threats Program (OSRO/GLO/505/USA). We thank the staff of the Directorate of Veterinary Services, State Department of Livestock, Ministry of Agriculture, Livestock and Fisheries. We acknowledge the contributions of the relevant Directors of Veterinary Services, County Department of Agriculture, Kenya.
References
- Adney DR, Brown VR, Porter SM, Bielefeldt-Ohmann H, Hartwig AE, Bowen RA. Inoculation of goats, sheep, and horses with MERS-CoV does not result in productive viral shedding. Viruses. 2016;8:230. doi: 10.3390/v8080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous (2005) Kenyan camels thrive where cattle cannot. New Agriculturist. Available at http://www.new-ag.info/en/focus/focusItem.php?a=1273. Accessed 1 April 2017
- Barrett CB, Bellemare MF, Osterloh SM (2006) Household-level livestock marketing behavior among Northern Kenyan and Southern Ethiopian Pastoralists. In: Pastoral Livestock Marketing in Eastern Africa Research and Policy Challenges, McPeak J, Little P (editors). 10.3362/9781780440323.002
- Corman VM, Jores J, Meyer B, Younan M, Liljander AM, Said MY, Gluecks I, Lattwein E, Bosch B-J, Drexler JF, Bornstein S, Drosten C, Müller MA. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerging Infectious Diseases. 2014;20(8):1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem SL, Fèvre EM, Kinnaird M, Browne AS, Muloi D, Godeke G-J, Koopmans M, Reusken CB. Serological evidence of MERS-CoV antibodies in dromedary camels (Camelus dromedaries) in Laikipia County. Kenya. PLoS ONE. 2015;10(10):e0140125. doi: 10.1371/journal.pone.0140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas G, Rambaut A. MERS-CoV recombination: implications about the reservoir and potential for adaptation. Virus Evolution. 2016;2(1):vev023. doi: 10.1093/ve/vev023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanoy EB, van der Sande MAB, Kraaij-Dirkzwager M, Dirksen K, Jonges M, van der Hoek W, Koopmans MPG, van der Werf D, Sonder G, van der Weijden C, van der Heuvel J, Gelinck L, Bouwhuis JW, van Gageldonk-Lafeber AB (2014) Travel-related MERS-CoV cases: an assessment of exposures and risk factors in a group of Dutch travellers returning from the Kingdom of Saudi Arabia, May 2014. Emerging Themes in Epidemiology 11:16. http://www.ete-online.com/content/11/1/16 [DOI] [PMC free article] [PubMed]
- Farah KO, Nyariki DM, Ngugi RK, Noor IM, Guliye AY. The Somali and the camel: ecology, management and economics. Anthropologist. 2004;6(1):45–55. doi: 10.1080/09720073.2004.11890828. [DOI] [Google Scholar]
- FAOSTAT (2015) Food and agriculture data. Available at http://www.fao.org/faostat/en/#home. Accessed 23 June 2016
- Food and Agriculture Organization of the United Nations (2016) An overview of camel production system and value chain in Kenya. Unpublished report developed by ECTAD, FAO Kenya. Accessed 20 October 2017
- Food and Agriculture Organization of the United Nations (2017) MERS-CoV Situation Update, 22 March 2017. Available at http://www.fao.org/ag/againfo/programmes/en/empres/mers/situation_update.html. Accessed 28 March 2017
- Guliye AY, Noor IM, Bebe BO, Kosgey IS. Role of camels (Camelus dromedarius) in the traditional lifestyle of Somali pastoralists in northern Kenya. Outlook on Agriculture. 2007;36(1):29–34. doi: 10.5367/000000007780223669. [DOI] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jores J (2015) Middle east respiratory syndrome-coronavirus in camels: an overview for Sub-Saharan and North Africa. 10.12774/eod_cr.july2015.joresj [DOI]
- Kaufman BA, Binder C (2002) Production aims and functions of camels in Kenyan pastoral systems. In: Proceedings of collaborative research project on camel breed differentiation and pastoral camel breeding strategies within the KARI/EU agriculture/livestock research support programme for Kenya (ARSPII; Project No. 6ACP KE0161-KE 6003/001)
- Khalafalla AI, Lu X, Al-Mubarak AIA, Dalab AHS, Al-Busadah KAS, Erdman DD. MERS-CoV in upper respiratory tract and lungs of dromedary camels. Saudi Arabia. Emerging Infectious Disease. 2014;21(7):1153–1158. doi: 10.3201/eid2107.150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNBS (2009) National population and housing census 2009. Available at: https://international.ipums.org/international/resources/enum_materials_pdf/enum_instruct_ke2009a.pdf. Accessed 19 October 2017
- Mackay IM, Arden KE. MERS coronavirus: diagnostics, epidemiology and transmission. Virology Journal. 2015;12:222. doi: 10.1186/s12985-015-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay IM, Arden KE. Middle East respiratory syndrome: an emerging coronavirus infection tracked by the crowd. Virus Research. 2015;202:60–88. doi: 10.1016/j.virusres.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud HA (2010) Camel Marketing in the Northern Kenya/Southern Ethiopia Borderlands. FAC Pastoralist Theme, November 2010. FAC Pastoralist Theme.
- Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, Alhakeem R, Durosinloun A, Al Asmari M, Islam A, Kapoor A, Briese T, Daszak P, Al Rabeeah AA, Lipkin WI. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerging Infectious Deseases. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MA, Corman VM, Jores J, Meyer B, Younan M, Liljander AM, Bosch B-J, Lattwein E, Hilali M, Musa BE, Bornstein S, Park SS. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerging Infectious Diseases. 2014;20(12):2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor IM (2013) Characteristics, feeding and marketing practices of the emerging peri-urban camel production system in Isiolo county, Kenya. Unpublished Ph.D. Thesis, Egerton University, Kenya
- Nowotny N, Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Eurosurveillance. 2014;19(16):20781. doi: 10.2807/1560-7917.ES2014.19.16.20781. [DOI] [PubMed] [Google Scholar]
- Olive M-M, Chevalier V, Grosbois V, Tran A, Andriamandimby S, Durand B, Ravalohery J-P, Andriamamonjy S, Rakotomanana F, Rogier C, Heraud J-M. Integrated analysis of environment, cattle and human serological data: risks and mechanisms of transmission of rift valley fever in Madagascar. PLOS Neglected Tropical Diseases. 2016;10(7):e0004827. doi: 10.1371/journal.pntd.0004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A, Al-Tawfiq J, Memish Z. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathogens and Global Health. 2015;109(8):354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouajd S, Kamel B. Physiological particularities of dromedary (Camelus dromedarius) and experimental implications. Scandinavian Journal of Laboratory Animal Science. 2009;36(1):19–29. [Google Scholar]
- Reusken CB, Ababneh M, Raj VS, Meyer B, Eljarah A, Abutarbush S, Godeke GJ, Bestebroer TM, Zutt I, Müller MA, Bosch BJ, Rottier PJ, Osterhaus AD, Drosten C, Haagmans BL, Koopmans MP. Middle East respiratory syndrome coronavirus (MERS CoV) serology in major livestock species in an affected region in Jordan, June to September, 2013. Eurosurveillance. 2013;18(50):20662. doi: 10.2807/1560-7917.ES2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- Saaty TL. Decision making with the analytical hierarchy process. International Journal of Services Sciences. 2008;1:83–98. doi: 10.1504/IJSSCI.2008.017590. [DOI] [Google Scholar]
- Sabir JS, Lam TT, Ahmed MM, Li L, Shen Y, Abo-Aba SE, Qureshi MI, Abu-Zeid M, Zhang Y, Khiyami MA, Alharbi NS, Hajrah NH, Sabir MJ, Mutwakil MH, Kabli SA, Alsulaimany FA, Obaid AY, Zhou B, Smith DK, Holmes EC, Zhu H, Guan Y. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351(6268):81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- Skidmore JA. The main challenges facing camel reproduction research in the 21st century. Reproduction. 2003;61:37–47. [PubMed] [Google Scholar]
- Sombroek WG, Braun HMH, van der Pouw BJA (1982) Exploratory soil map and agro-climatic zone map of Kenya, 1980. Scale: 1:1,000,000. Exploratory soil survey report no. E1. Kenya Soil Survey Ministry of Agriculture—National Agricultural Laboratories, Nairobi, Kenya
- World Health Organization (WHO) (2016) WHO MERS-CoV Global Summary and risk assessment. Available at http://www.who.int/emergencies/mers-cov/mers-summary-2016.pdf?ua=1. Assessed 19 October 2017
- Woolhouse M, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerging Infectious Diseases. 2005;11(12):1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasonga O, Musembi J, Rotich K, Jarso I, King-Okumu C (2016) Vegetation resources and their economic importance in Isiolo County, Kenya. IIED, London. Available at http://pubs.iied.org/pdfs/10141IIED.pdf. Accessed 24 February 2017
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zoonotic and Emerging Disease Group (2015). Camel value chain in Kenya. Available at http://www.zoonotic-diseases.org/camel-value-chain-in-kenya/. Accessed 1 April 2017
- Zumla A, Dar O, Kock R, Muturi M, Ntoumi F, Kaleebu P, Eusebio M, Mfinanga S, Bates M, Mwaba P, Ansumana R, Khan M, Alagaili AN, Cotten M, Azhar EI, Maeurer M, Ippolito G, Petersen E. Taking forward a “One Health” approach for turning the tide against the Middle East respiratory syndrome coronavirus and other zoonotic pathogens with epidemic potential. International Journal of Infectious Diseases. 2016;47:5–9. doi: 10.1016/j.ijid.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


