Abstract
Fibrosis is involved in the majority of cardiovascular diseases and is a key contributor to end-organ dysfunction. In the current study, the antifibrotic effects of recombinant human relaxin-2 (serelaxin; RLX) and/or the AT2R agonist CGP42112 (CGP) were compared with those of the established AT1R antagonist, candesartan cilexetil (CAND), in a high salt-induced cardiac fibrosis model. High salt (HS; 5%) for 8 weeks did not increase systolic blood pressure in male FVB/N mice, but CAND treatment alone significantly reduced systolic blood pressure from HS-induced levels. HS significantly increased cardiac interstitial fibrosis, which was reduced by either RLX and/or CGP, which were not additive under the current experimental conditions, while CAND failed to reduce HS-induced cardiac fibrosis. The antifibrotic effects induced by RLX and/or CGP were associated with reduced myofibroblast differentiation. Additionally, all treatments inhibited the HS-induced elevation in tissue inhibitor of matrix metalloproteinases-1, together with trends for increased MMP-13 expression, that collectively would favor collagen degradation. Furthermore, these antifibrotic effects were associated with reduced cardiac inflammation. Collectively, these results highlight that either RXFP1 or AT2R stimulation represents novel therapeutic strategies to target fibrotic conditions, particularly in HS states that may be refractory to AT1R blockade.
Keywords: AT2 receptor, CGP42112, serelaxin, candesartan cilexetil, high salt, cardiac fibrosis, inflammation
Fibrosis is a hallmark of several cardiovascular diseases (CVDs) and directly contributes to organ dysfunction.1,2 Fibrosis is initiated by tissue injury that activates the innate immune system, resulting in local inflammation in the affected tissue.3,4 Inflammatory cells such as macrophages release pro-fibrotic mediators that contribute to accumulation of extracellular matrix (ECM) components.5,6 These cells release transforming growth factor (TGF)-β1, which is considered the major pro-fibrotic mediator that drives the development of fibrosis.7−10 TGF-β1 triggers the differentiation of fibroblasts into myofibroblasts, which are the key cellular drivers for collagen production within an extracellular matrix (ECM).11,12 At the same time, matrix metalloproteinases (MMPs)13,14 are the main enzymes for collagen degradation, the activities of which are tightly regulated by tissue inhibitors of metalloproteinases (TIMPs).15 Therefore, the balance between collagen production and degradation is essential to maintain homeostasis of ECM.
However, in situations of persistent tissue injury and inflammation, there is ongoing activation of TGF-β1-myofibroblast-induced collagen production, leading to unbalanced collagen production and degradation that contributes to organ fibrosis. Chronic high salt (HS) intake has been long recognized as a major contributor to the development of CVD globally.16−18 Indeed, HS-induced heart disease has been largely applied in preclinical studies, including the studies of cardiac fibrosis.19−23
Despite the alarming statistics of the current incidence of fibrotic diseases,24,25 there are very few effective treatments that can halt disease progression. Inhibitors that target the renin angiotensin system (RAS) such as angiotensin II (Ang II) type 1 receptor blockers (ARBs) or angiotensin converting enzyme inhibitors (ACEi) are recognized as frontline therapies for many CVDs that may eventuate in cardiac fibrosis. Indeed, ARBs (e.g., losartan) and ACEi (e.g., lisinopril) have been shown to reduce cardiac fibrosis in hypertensive patients.26−28 However, in other clinical trials with larger patient size, their ability to improve end-organ damage (fibrosis) beyond reducing blood pressure is limited.29−31 Therefore, novel therapies are urgently needed for the treatment of organ fibrosis.
Growing evidence has suggested that activation of the Ang II type 2 receptor (AT2R) is protective in various preclinical models.32−34 Direct AT2R stimulation has been shown to reduce fibrosis in lung,35 kidney,36−39 and heart.40−42 These antifibrotic effects caused by AT2R stimulation were normally associated with anti-inflammatory effects.34 Importantly, the expression of AT2R was upregulated during pathological states,43−46 which suggests AT2R is a potential novel therapeutic target for CVD.
The ovarian and cardiovascular hormone relaxin, via stimulation of relaxin family peptide receptor 1 (RXFP1), has been consistently shown to reduce organ fibrosis in various experimental models.47,48 Interestingly, we have previously reported that the antifibrotic actions mediated by relaxin were blocked by an AT2R antagonist.49 More recently, we have now found that the antifibrotic effects induced by relaxin could also be inhibited by an AT1R antagonist, which collectively suggests a crosstalk between AT1R, AT2R, and RXFP1.50 In the current study, the antifibrotic effects of recombinant human relaxin-2 (serelaxin; RLX), AT2R agonist, CGP42112 (CGP), and AT1R antagonist, candesartan cilexetil (CAND) were compared in HS-induced cardiac fibrosis. CGP is a peptidomimetic compound that was first described in 198951 and was instrumental in defining Ang II receptor subtypes as AT1R and AT2R. Importantly, CGP is still one of the most selective experimental tools with which to probe AT2R function since, based on radioligand binding data, it has higher affinity than Ang II or Ang III for AT2R. Moreover, it has 10-fold greater binding affinity for AT2R than the nonpeptide AT2R agonist C21 and exhibited >40,000-fold AT2R:AT1R selectivity.52 Given that the antifibrotic effects due to AT2R stimulation are based almost exclusively on the use of C21,35−42 it was of interest to determine if CGP evoked antifibrotic effects. To this end, we used a HS-induced model of cardiac fibrosis that represents elevated salt ingestion noted in most western diets. It was hypothesized that AT2R stimulation using CGP would be equi-effective with RLX and CAND as an antifibrotic agent in the heart. In addition, additive effects were investigated by using combinations of RLX with CGP or CAND.
Materials and Methods
Materials
CGP42112 was obtained from GL Biochem (Shanghai) Ltd. (Shanghai, China). Candesartan cilexetil was generously obtained from Astra Zeneca (Sweden), and recombinant H2 relaxin was generously provided by Corthera Inc. (San Carlos, CA; a subsidiary of Novartis AG, Basel, Switzerland).
Animals and Treatments
All experiments were approved by the Institutional Animal Ethics Committees at Monash University (MARP/2013/118), which adhere to the Australian Code of Practice for the Care and Use of Laboratory Animals.
Male FVB/N mice, 10–12 weeks old, were obtained from the Monash Animal Research Precinct and were housed in the Department of Phamacology animal holding facility, while under experimentation in standard mouse cages at 21 ± 3 °C, with a 12 h light/dark cycle with free access to food and water. Mice were fed a HS diet (5% NaCl; SF05-038 diet containing 5% NaCl; Specialty Feeds Western Australia) for 4 weeks before drug administration. After a 4-week HS diet, mice were randomized to continue with the HS diet, or the HS + CGP, HS + RLX, HS + CAND, HS + RLX + CGP, or HS + RLX + CAND diet for another 4 weeks (from weeks 5–8). A control group of mice were fed a normal salt (NS; 0.5% NaCl) diet for 8 weeks. Animals were randomly allocated to treatment groups by an independent investigator who ensured different drug treatments occurred contemporaneously across animal groups. Investigators were blinded to treatments and subsequent histological analyses.
CGP (1.44 mg/kg/day53,54) and RLX (0.5 mg/kg/day49,55) were used at concentrations that were effective in other experimental models of inflammation (for CGP) and fibrosis (for RLX), and were diluted with saline and delivered by subcutaneously implanted osmotic mini-pumps (model 2004, Alzet). This dose of RLX can produce 20–50 ng/mL of circulating RLX56 which is within the physiological range found in pregnant rodents.57 Candesartan cilexetil (2 mg/kg/day58,59) was delivered in drinking water over the 4-week treatment period. CAND was replaced in drinking water every 2–3 days. Dose calculations for CAND were based on drinking rates of mice that had already been on a HS diet for 4 weeks and were therefore well established (∼3 mL per day per mouse).
Blood Pressure Measurements
Systolic blood pressure (SBP) was measured at week 0 (prior to HS diet), week 4 (prior to treatments), and week 8 (after treatments), using tail cuff plethysmography60 (MC4000 BP Analysis Systems; Hatteras Instruments Inc.). At least 15 measurements were performed to obtain the average for each animal at each time point.
Histology and Immunofluorescence Staining
One section from the midzone of left ventricle (LV) was embedded with OCT and immersed in methylbutane/isopentane (Merck) for slow-freezing using liquid nitrogen. Cardiac sections were cut at 5 μm using a Cryostat (Leica; CM1860). Collagen deposition was identified from frozen cardiac tissue sections that were stained with 0.05% picrosirius red (Polysciences Inc., Warrington, PA).
Frozen heart sections (5 μm) were used for immunofluorescence staining of cardiac α-SMA expression (as a marker of differentiated myofibroblasts) (ab5694, 1:1000 dilution; Abcam), phosphorylated-IκB (p-IκB; as a marker of NF-κB activity) (no. 28595, 1:200 dilution; Cell Signaling Technology) or monocyte chemoattractant protein (MCP)-1 (sc-28879, 1:1000; Santa Cruz). Alexa Fluor 594 Goat Anti-Rabbit IgG secondary antibody (1:1000 dilution) was used to detect α-SMA staining while Alexa Fluor 488 Goat Anti-Rabbit IgG secondary antibody (1:500 dilution) was used to detect p-IκB and MCP-1 staining within the LV.
All images were taken under 200× magnification and quantified from an average of 6–8 fields of view for each heart section using ImageJ software.
Hydroxyproline Assay
The apical region of the heart was lyophilized to dry weight, hydrolyzed in 6 M hydrochloric acid and evaluated for hydroxyproline content as described previously.49,55 Collagen concentration was then calculated according to hydroxyproline values.61
Western Blot
The total protein content from homogenized LV tissue was extracted using 1.5× Laemmli buffer containing 25% glycerol, 7.5% SDS, 250 mM Tris-HCl at pH 6.8 and 0.001 g bromophenol blue. Homogenized samples were sonicated 3 × 3 s with ice chilling in-between and heated at 37 °C for 10 min. Samples were then centrifuged at 13 000 rpm for 30 min at 4°C. Total protein amount was determined by reducing agent and detergent compatible (RCDC) protein assay (Bio-Rad). The protein content was quantified using ProteinQuant-Lowry software (SoftMax Pro) at 750 nm. Equal amounts of total protein (25 μg) from each sample were electrophoresed on 10% acrylamide gels at 200 V for 40 min to 1 h in the presence of 1× running buffer (diluted from 10× buffer, no. 161–0732, Bio-Rad) and transferred to Turbo Mini-size LF PVDF membranes (no. 170–4272, Bio-Rad) in the Transfer-Blot Turbo transfer system (Bio-Rad). Membranes were probed with primary antibodies to either TGF-β1 (no. sc-146, 1:1000 dilution; Santa Cruz Biotechnology), MMP-13 (ab75606, 1:300 dilution; Abcam) TIMP-1 (ab38978, 1:1000 dilution; Abcam) or GAPDH ((ab8245, 1:20,000 dilution; Abcam); and the appropriate secondary antibodies (at 1:10,000 dilution) using Dako antirabbit or Jackson immune research antimouse antibodies, respectively. Protein bands of interest were quantified by measuring optical densities per unit area of each band and corrected for any differences in protein loading by normalizing to GAPDH. All protein expressions were assessed as relative ratio to the NS group.
Quantitative Real-Time PCR
Gene expression levels of RXFP1, AT1aR, and AT2R were determined. Because of the lack of tissue samples, only NS and HS tissue could be assessed for receptor expression. RNA was extracted from LV of NS (n = 7) and HS (n = 6) mice using the RNeasy fibrous tissue mini kit (Qiagen) and treated with deoxyribonuclease 1. RNA was assessed for purity (260/280 and 260/230 ratios) and yield using a spectrophotometer. One microgram of RNA was reverse transcribed into cDNA (iScript Supermix, Bio-Rad Life Sciences, New South Wales, Australia). Gene expression was determined with TaqMan gene expression assays (RXFP1: Mm01220214_m1; Agtr1a: Mm01957722_s1; Agtr2: Mm01341373_m1; GAPDH: Mm99999915_g1) in 10 μL reaction volumes containing 25 ng of cDNA, using a Qiagen Rotor Gene Q system. The comparative cycle threshold (CT) method was used to determine expression of RXFP1, AT1aR, and AT2R using GAPDH as the reference gene, as performed previously.50
Statistical Analysis
All results were expressed as mean ± standard error of mean (s.e.m.). All statistical plots and analyses were performed using the Prism program (GraphPad Prism 7 software). All statistical comparisons except SBP were conducted using one-way analysis of variance (ANOVA) followed by Tukey’s posthoc correction test for multiple comparisons between groups. SBP analysis was also performed by two-way repeated measures (RM) ANOVA which allowed for within-group analysis over time and between groups. For all results, P < 0.05 was deemed statistically significant.
Results
Systolic Blood Pressure, Ventricular Weight (VW) to Body Weight (BW) Ratio, and Receptor Gene Expressions Were Not Affected by HS (5%) Diet
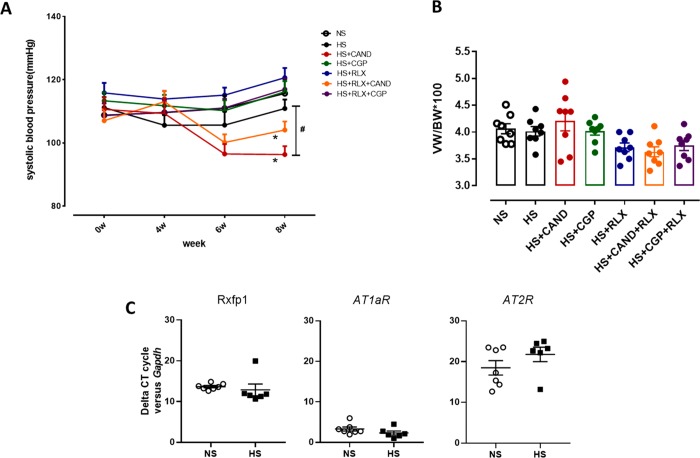
SBP was measured before the HS diet and after 4- and 8-weeks of HS diet. The HS diet did not significantly increase SBP over the 8-week period (Figure 1A), compared to corresponding measurements obtained from mice fed a NS diet or versus its own baseline SBP. Mice were fed a HS diet and treated with either CAND, CGP, RLX, or RLX in combination with CAND or CGP from weeks 5–8 of the HS diet. Both candesartan-treated groups reduced SBP versus their own predrug levels after 2 weeks of treatment. Only CAND significantly reduced SBP (by 20–25 mHg; p < 0.05 vs HS alone) compared to corresponding measurements obtained from mice fed the HS diet alone, after 4 weeks of treatment (Figure 1A). However, CAND alone, or combined with RLX, reduced SBP compared with the HS over the entire treatment period (p < 0.05, 2-way RM ANOVA). Body weight was measured prior to study commencement and after 4 weeks of high salt diet and again 4 weeks later after drug treatments (see Table S-1). These results indicate that there were generally similar increases in body weight (∼2–3 g) across all groups over the first 4 weeks of HS alone. Moreover, none of the drug treatments stopped weight gain. Cardiac hypertrophy was indirectly measured by using ventricular weight (VW) to body weight (BW) ratio. The eight-week HS diet did not increase the VW to BW ratio, and this was not further affected by any treatment (Figure 1B). In addition, the left ventricle mRNA expression of RXFP1, AT1aR, and AT2R was measured by rtPCR. All three receptors were detected in the left ventricular with the rank order of expression being AT1aR > RXFP1 > AT2R. However, receptor expression was not affected by the HS diet for any subtype (Figure 1C).
Figure 1.
Effects of RLX (0.5 mg/kg/day), CGP (1.44 mg/kg/day), CAND (2 mg/kg/day) or RLX with CAND or CGP on (A) systolic blood pressure measured by tail cuff and (B) ventricular weight (VW) to body weight (BW) ratio in FVB/N mice fed a high salt (HS, 5% NaCl) diet for 8 weeks. (C) Left ventricular expression of RXFP1, AT1aR, and AT2R mRNA levels from NS- and HS-treated mice; shown in the comparative cycle of threshold fluorescence at which RXFP1, AT1aR, and AT2R mRNA expression was detected, relative to the internal housekeeping gene GAPDH, by real-time PCR analysis. All treatments were given for 4 weeks, between weeks 5–8 of the HS diet. At the same time, a group of mice were fed a normal salt (NS, 0.5% NaCl) diet for comparison. All data are expressed as mean ± s.e.m. (n = 6–8 per group): (#) P < 0.05 vs HS for systolic blood pressure at 8 weeks (one-way ANOVA with Tukey correction for multiple comparisons); (∗) P < 0.05 for systolic blood pressure-time interaction compared with HS group (2-way repeated measures ANOVA).
CGP and/or RLX, but Not CAND, Reduced HS-Induced Cardiac Fibrosis
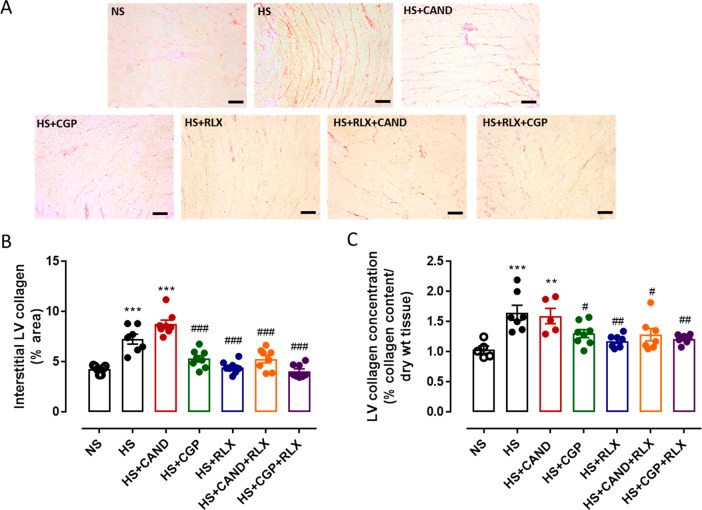
We compared the effects of RLX (0.5 mg/kg/day, at a dose that is antifibrotic49,55) and the AT2R agonist, CGP (1.44 mg/kg/day, at a dose that is organ protective53,54), with CAND (2 mg/kg/day, at a dose that is antifibrotic58), in male FVB/N mice that had been on a HS diet for 4 weeks, at which time there was already elevated cardiac fibrosis.19,62 Cardiac fibrosis was measured using picrosirius red which stains for interstitial collagens I and III63 as well as by hydroxyproline analysis that measures total LV collagen concentration.64 Compared to mice fed a NS diet, mice fed a HS diet for 8 weeks had significantly increased cardiac interstitial fibrosis by 60–100% using both methods (both p < 0.001 vs NS alone; Figure 2). Importantly, this HS-induced cardiac fibrosis was reversed by either RLX (p < 0.01 vs HS alone) or CGP (p < 0.05 vs HS alone) alone toward levels observed in the NS diet group. Furthermore, there was no additive antifibrotic effect when these compounds were combined, such that the combined effects of both drugs reduced LV fibrosis to a similar extent as either drug alone. However, CAND alone failed to reduce HS-induced cardiac fibrosis, while RLX and CAND in combination reduced HS-induced cardiac fibrosis (p < 0.05 vs HS alone) to a similar level as RLX alone (Figure 2).
Figure 2.
(A) Representative images for interstitial fibrosis stained with picrosirius red (indicated by red) in left ventricle (LV) sections obtained from FVB/N mice fed on a normal salt (NS, 0.5% NaCl) diet or mice fed on a high salt (HS, 5% NaCl) diet in the presence or absence of RLX (0.5 mg/kg/day), CGP (1.44 mg/kg/day), CAND (2 mg/kg/day), or RLX with CAND or CGP. Scale bar for all images = 50 μm. (B) Group data of % area of interstitial fibrosis in LV sections, or (C) total tissue collagen measured by hydroxyproline assay in LV tissue from same animal groups in which picrosirius red staining was performed (n = 7–8 per group). All data are expressed as mean ± s.e.m. (∗∗) P < 0.01, (∗∗∗) P < 0.001 vs NS; (#) P < 0.05, (##) P < 0.01, (###) P < 0.001 vs HS, determined by One-Way ANOVA with Tukey correction for multiple comparisons.
GCP and/or RLX, but Not CAND, Reduced HS-Induced Fibrogenesis in the Heart
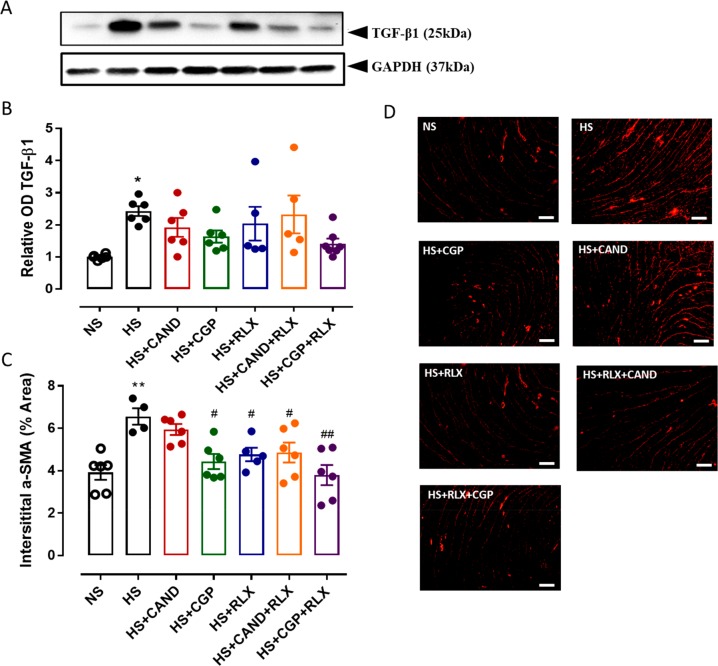
TGF-β1 is considered the major pro-fibrotic mediator of collagen synthesis contributing to fibrosis development.7−10 Here, cardiac TGF-β1 protein (expressed as a dimer sized at 25 kDa65) was measured by Western blot analysis. Cardiac TGF-β1 expression increased by ∼1.5-fold after an 8-week HS diet in comparison to corresponding levels from the NS fed mice (Figure 3A,B). However, mean TGF-β1 expression levels in all treated groups were not significantly different from either the HS or NS groups (Figure 3B and Figures S-1 and S-2).
Figure 3.
(A) Representative Western blots of TGF-β1 (25 kDa) and GAPDH (37 kDa) from LV obtained from FVB/N mice fed on a normal salt (NS, 0.5% NaCl) diet or mice fed on a high salt (HS, 5% NaCl) diet in the presence or absence of RLX (0.5 mg/kg/day), CGP (1.44 mg/kg/day), CAND (2 mg/kg/day), or RLX with CAND or CGP (n = 5–7 per group). (B) Densitometric quantification of Western blots of TGF-β1 protein expression from indicated groups. Loading was adjusted by normalizing to GAPDH protein expression for each animal. All treatments were presented as a ratio to NS. (C) α-SMA expression quantified as % area from indicated groups. (n = 4–6 per group). All data are expressed as mean ± s.e.m. (∗) P < 0.05, (∗∗) P < 0.01 vs NS; (#) P < 0.05, (##) P < 0.01 vs HS, determined by One-Way ANOVA with Tukey correction for multiple comparisons. (D) Representative immunofluorescence staining images of α-SMA from same treatment groups as panel C. Scale bar for all images = 50 μm.
Once activated, TGF-β1 triggers the differentiation of fibroblasts into myofibroblasts, which are the major cellular driver of fibrosis progression,11,12 and are distinguished from fibroblasts by their expression of α-smooth muscle actin (α-SMA).66 Consistent with the findings detailed above, HS significantly increased myofibroblast differentiation in the LV of HS fed mice (p < 0.01 vs NS alone), which was reversed by either CGP, RLX, or both combined (all p < 0.05 vs HS alone; Figure 3C,D). However, CAND itself did not significantly reduce HS-induced myofibroblast differentiation, whereas the combination of CAND and RLX exhibited a similar reduction of myofibroblast differentiation (p < 0.05 vs HS alone) to that of RLX alone (Figure 3C,D).
CGP, RLX, and CAND All Facilitated Greater Collagen Degradative Capacity Which Was Impaired by HS
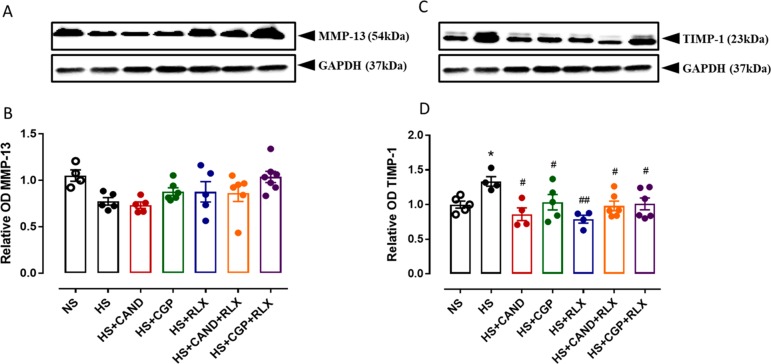
Extracellular matrix (ECM) synthesis is counterbalanced by degradation resulting in healthy ECM homeostasis. The degradation of ECM components is achieved by MMPs.13,14 MMP-13 is the main cardiac collagenase within the myocardium of rodents, which is responsible for cleaving collagen types I and III.67 The activity of MMPs is tightly regulated by TIMPs.15 The protein expression of MMP-13 and TIMP-1 in the heart was measured by Western blot analysis in the current study. Compared with NS-fed mice, HS-fed mice tended to have reduced LV MMP-13 protein expression levels (by ∼25–30%; Figure 4A,B), while at the same time having increased TIMP-1 levels (by ∼30–40%; p < 0.05; Figure 4C,D), which would favor collagen accumulation being increased in the heart of HS-fed mice. Although none of the treatments significantly affected LV MMP-13 levels, GCP combined with RLX increased MMP-13 levels toward those observed in hearts from NS-fed mice (Figure 4A,B), while all treatments reversed the HS-induced increase in TIMP-1 levels (all p < 0.05 vs HS alone; Figure 4C,D).
Figure 4.
Representative Western blots of (A) MMP-13 (54 kDa) and (C) TIMP-1 (23 kDa) from LV obtained from FVB/N mice fed on a normal salt (NS, 0.5% NaCl) diet or mice fed on a high salt (HS, 5% NaCl) diet in the presence or absence of RLX (0.5 mg/kg/day), CGP (1.44 mg/kg/day), CAND (2 mg/kg/day), or RLX with CAND or CGP (n = 4–6 per group). Densitometric quantification of (B) MMP-13 and (D) TIMP-1 protein expressions from indicated groups. Protein loading was adjusted by normalizing to GAPDH protein expression for each animal. All treatments were presented as a ratio to NS. All data are expressed as mean ± s.e.m. (∗) P < 0.05 vs NS; (#) P < 0.05, (##) P < 0.01 vs HS, determined by One-Way ANOVA with Tukey correction for multiple comparisons.
HS-Induced Cardiac Inflammation Was Inhibited by CGP, RLX, and CAND
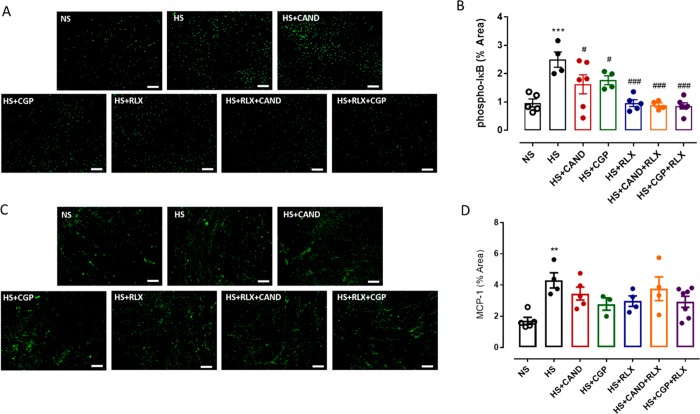
Chronic inflammation is considered the initial trigger for the development of organ fibrosis.4,5 Nuclear factor-kappa B (NF-κB) regulates inflammatory responses via directly controlling pro-inflammatory cytokine gene expression (such as the monocyte chemoattractant protein (MCP)-1) and facilitating macrophage infiltration.68 The activity of NF-κB is controlled by the inhibitor of κB (I-κB).68 In the current study, phosphorylated I-κB (p-I-κB; Figure 5A,B) and MCP-1 (Figure 5C,D) expressions were both significantly increased (by ∼1.5–2 fold; both p < 0.01 vs HS alone) in the LV. While CAND or CGP alone partially reduced the HS-induced p-I-kB expression levels (both p < 0.05 vs HS alone), RLX alone and the combination treatments fully reversed the p-I-kB expression levels back to those measured in NS-fed mice (all p < 0.001 vs HS alone; Figure 5A,B). A similar trend was observed for MCP-1 expression, although this did not reach statistical significance (Figure 5C,D).
Figure 5.
Representative images for (A) p-IκB and (C) MCP-1 in LV sections obtained from FVB/N mice fed on a normal salt (NS, 0.5% NaCl) diet or mice fed on a high salt (HS, 5% NaCl) diet in the presence or absence of RLX (0.5 mg/kg/day), CGP (1.44 mg/kg/day), CAND (2 mg/kg/day) or RLX with CAND or CGP. Scale bar for all images = 50 μm. Group data of % area of (B) p-IκB and (D) MCP-1 in LV sections from indicated groups (n = 4–6 per group). All data are expressed as mean ± s.e.m. (∗∗) P < 0.01, (∗∗∗) P < 0.001 vs NS; (#) P < 0.05, (###) P < 0.001 vs HS, determined by One-Way ANOVA with Tukey correction for multiple comparisons.
Discussion
This study investigated, for the first time, the antifibrotic effects of the RXFP1 agonist, RLX, and AT2R agonist, CGP, in comparison to a current standard of care ARB therapy, candesartan cilexetil, in a HS-induced murine model of cardiac disease. Mice fed a HS (5% NaCl) diet for 8 weeks underwent significantly increased LV inflammation, TGF-β1 expression, myofibroblast differentiation, interstitial and total collagen deposition (fibrosis), TIMP-1 expression, and a trend toward reduced MMP-13 levels, in the absence of any marked changes in SBP. AT1aR was well expressed in LV while RXFP1R and AT2R were less abundantly expressed in LV but were unaltered by the HS diet, enabling all three drugs to target their respective receptors in the heart. This model thus, allowed for the direct antifibrotic evaluation of the treatments investigated, in the absence of increased BP as a confounding variable.
To this extent, both RLX or CGP demonstrated similar, marked reductions in HS-induced LV fibrosis, which was associated with the ability of these drugs to reduce pro-fibrotic mediators (TGF-β1 and myofibroblast differentiation) that contribute to aberrant ECM/collagen deposition, together with a situation favoring a net increase in ECM/collagen degradation (increased MMP-13: TIMP-1 ratio) and reduced cardiac inflammation (NF-κB and MCP-1 expression). In contrast, CAND itself failed to demonstrate any antifibrotic efficacy in this HS model, despite being active and able to lower SBP in HS fed mice after 4 weeks of treatment. As there were no marked additional effects of RLX when combined with CGP over either therapy alone, collectively, these results suggested that either direct AT2R stimulation or RXFP1 activation leading to RXFP1-AT2R crosstalk49 most likely induced similar antifibrotic efficacy and may represent novel therapeutic strategies to target fibrotic conditions, particularly in HS states that may be refractory to AT1R blockade.
A HS diet can be associated with 17% higher risk for CVD, by exacerbating hypertension and ventricular hypertrophy.18 HS-induced organ remodelling in preclinical studies can be blood pressure-dependent or -independent. Generally, 8% HS causes elevated BP in both salt-sensitive or salt-resistant animals;20,21,69,70 however, 4–5% HS generally only increases BP in salt-sensitive animals.23,71−73 Consistent with previous studies, in the current study, 5% HS diet for 8 weeks did not change SBP in FVB/N mice but did promote cardiac fibrosis and inflammation compared to that measured in NS-fed mice. As expected, CAND was the only treatment that reduced BP, while neither RLX nor CGP affected BP. Indeed, the organ-protective effects of AT2R stimulation have been consistently shown to be blood pressure independent,36,41 and similarly, the majority of studies have reported that the antifibrotic effects of RLX were independent of BP regulation.47
Mice fed a HS diet underwent increased cardiac inflammation and fibrosis within 4–5 weeks.22,62 Hence, all treatments evaluated in the current study informed on their potential to reverse established cardiac pathology. Consistent with previous findings, we reported there was increased cardiac inflammation, in this case, measured by using NF-κB and MCP-1 expression, following 8 weeks’ HS diet. It is well-known that NF-κB regulates inflammatory response directly by promoting cytokines production, such as MCP-1.68 The increased MCP-1 facilitates monocytes recruitment to a local injury site and results in increased profibrotic cytokine (e.g., TGF-β1) production and directly contributes to fibrosis development.6,74
Indeed, there is consistent evidence showing that RLX or activation of the AT2R were cardiovascular- and/or reno-protective.32−34,48,53,54,75 Importantly, we observed that RLX or CGP alone reversed HS-induced cardiac fibrosis and inflammation (NF-κB and MCP-1). While CGP treatment, (same dose for 2 weeks) had been reported to reduce renal inflammation in obese Zucker rats,54 there were no reports on the effects of this peptide on organ fibrosis. Therefore, the current study supports an antifibrotic role of chronic CGP treatment, as reported for C21,35−42 suggesting a class effect of AT2R agonists. Moreover, we have shown for the first time that there was no additional antifibrotic or anti-inflammatory effect caused by RLX and CGP combination in the heart. This finding might be explained by the crosstalk between AT2 and RXFP1 receptors. For example, we have recently reported that the antifibrotic effects of RLX was blocked by AT2R antagonist,49,50 suggesting the interaction between AT2 and RXFP1 receptors. Therefore, activation of either AT2 or RXFP1 receptor may lead to the same downstream signaling pathways to induce antifibrotic effects. However, single treatments with either RLX or CGP had nearly normalized a number of fibrotic and inflammatory markers to levels measured in NS-fed mice, therefore a model of more severe fibrosis may be required to determine if there are additive antifibrotic and anti-inflammatory effects of combination therapy.
Surprisingly, the AT1R antagonist CAND did not affect the elevated fibrosis caused by a HS diet, despite a significant reduction in blood pressure, although it significantly reduced cardiac NF-κB activity to a similar level as CGP. Since inflammatory responses after tissue injury are considered as the primary trigger for fibrogenesis,4,76 and previous studies have suggested the slow action of ARBs,77 a longer period of treatment might be required to observe a reversal of HS-induced fibrosis by CAND, given the earlier inhibitory effect on inflammation. Le Corvoisier et al. previously demonstrated that both irbesartan and ramipril only partially reduced HS diet (4%)-induced cardiac fibrosis after 8 weeks, noting that these treatments were initiated at the beginning of a HS diet,73 unlike in the current study. In any case, short-term treatment with either RLX and CGP reversed HS-induced cardiac fibrosis in the current study, suggesting that RXFP1 or AT2R stimulation provided greater antifibrotic efficacy compared with AT1R blockade, at least in the HS-induced cardiac fibrosis model. Recently, Chow et al. reported that the antifibrotic effect of RLX was inhibited by coadministration of CAND in the LV of isoprenaline-injured mice as well as the kidneys of HS-fed mice.50 In contrast, in the current study, CAND did not alter the ability of RLX to reduce HS-induced cardiac fibrosis and inflammation, which may relate to different AT1R and RXRP1 receptor densities in the heart vs kidney, which may impact on functional receptor interactions or crosstalk.50 Collectively, these data suggest that the inhibitory effect of CAND on RLX-mediated protective effects was organ specific, at least in the HS model.
With respect to signaling mechanisms, TGF-β1 is considered the major pro-fibrotic mediator that drives the development of organ fibrosis.7−10 Indeed, HS-induced cardiac and renal remodelling are closely related to increased TGF-β1 expression,20,69 which was dependent on activation of the AngII-AT1R axis.69 Consistent with these results, we showed that HS-evoked fibrosis was associated with marked increase in TGF-β1 and myofibroblast differentiation, which are the key drivers for collagen production.11,12 A reduction in TGF-β1 and myofibroblast differentiation after RXFP1 or AT2R activation has been consistently demonstrated in various experimental models previously36,38,49,78 although, in the present study, only CGP alone or combined with RLX reduced TGF-β1 expression, albeit nonsignificantly. In addition, while HS tended to reduce MMP-13, there was a marked increase in TIMP-1 levels that together would suggest a much reduced MMP-13/TIMP-1 ratio, that would lead to reduced collagen degradation in the HS state. Given that elevated TIMP-1 levels were significantly inhibited by RLX or CGP alone or in combination, with a trend for increased MMP-13 levels, these results are consistent with an increased MMP-13/TIMP-1 ratio after CGP or RLX treatment that favored the promotion of MMP-13-induced collagen degradation. Therefore, both inhibiting collagen production and promoting collagen degradation most likely contributed to AT2R- and RXFP1-mediated cardiac antifibrotic effects in the setting of the HS diet-induced disease pathology. Consistent with the fibrosis data presented, CAND itself failed to reduce HS-induced TGF-β1 and myofibroblast differentiation, although it partially but significantly reduced TIMP-1 levels, suggesting that it may have promoted collagen degradation to a certain extent, rather than inhibit myofibroblast-induced collagen production. Moreover, the fact that cardiac fibrosis was unaltered by CAND at this time suggests that there was a temporal dissociation between pro-fibrotic and degradative mechanisms and their sensitivity to the AT1R blockade, such that cardiac fibrosis predominated under these circumstances. Indeed, a longer treatment period for CAND may have been required to observe a CAND-induced reduction in fibrosis, as discussed earlier.
The current study assessed potential antifibrotic agents in a normotensive model with persistent tissue injury. However, the same pathological mechanisms are likely involved with or without a background of elevated BP. RLX and AT2R agonists induce their antifibrotic actions without regulating blood pressure, when applied to normotensive40,79 and hypertensive41,80 models of disease. Our findings are significant because, for the first time, they directly demonstrate that RXFP1 receptor and AT2R stimulation evoked rapid, marked antifibrotic effects in a model that was refractory to AT1R blockade. In addition, while it is acknowledged that end-organ damage such as fibrosis is exacerbated in hypertensive states, combinations such as ARBs and ACE inhibitors that may provide additional protection are actually contra-indicated due to adverse renal effects. Indeed, RLX and AT2R agonists may serve as adjunct antifibrotic therapies under such circumstances.
There were also a number of limitations with the current study. First, we have only examined male mice since they generally undergo more severe pathologically- induced fibrosis compared with their female counterparts.81 While the main aim of this study was not to determine sex differences of these treatments, the effects of gender on the treatments investigated could be considered in future studies. Second, individual treatments with either RLX or CGP reduced most fibrotic and inflammatory markers to the extent that it was difficult to assess additive effects of combination therapy. Third, we did not examine the effects of various treatments on receptor expression due to insufficient availability of remaining tissue samples. Finally, since we used normotensive animals, it will be important in future studies to use hypertensive models in which fibrosis is exacerbated, particularly since dual RAS inhibition is contra-indicated due to adverse systemic and renal effects. Therefore, in future studies, the use of more severe fibrotic models, with or without hypertension, will inform on whether or not submaximal effects of individual treatments, as used in the present study, can evoke additive antifibrotic effects.
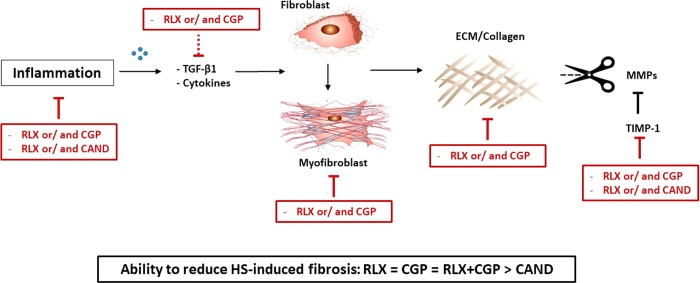
In conclusion, this is the first demonstration of an antifibrotic effect of the AT2R agonist CGP42112, which demonstrated similar antifibrotic efficacy to the RXFP1 agonist, RLX, either in isolation or in combination. Unlike CAND treatment, CGP or RLX, alone and in combination, inhibited collagen production as well as promoted factors that control the rate of collagen degradation as part of their antifibrotic effects against HS-induced cardiac fibrosis (see Figure 6).
Figure 6.
Potential mechanisms involved in the antifibrotic actions of RLX and CGP, with inhibitory mechanisms depicted by red line blocks. RXFP1 and AT2R stimulation reduced inflammatory and pro-fibrotic factors thereby inhibiting myofibroblast differentiation and ECM production while enabling ECM degradation by inhibiting TIMP-1. In contrast, CAND inhibited fewer mechanisms in this pathway. Line blocks represent inhibition; dotted line block represents inhibitory trend.
Collectively, these results provide additional evidence that either AT2R or RXFP1 stimulation leading to RXFP1-AT2R crosstalk represents novel therapeutic strategies to target fibrotic conditions in a BP-independent manner, particularly in HS states that may be less sensitive to AT1R blockade.
Acknowledgments
This work was supported in part by Grants from the National Health and Medical Research Council (NHMRC) of Australia (GNT1045848, GNT1101552 and GNT1127792), and NHMRC Senior Research Fellowships to C.S.S. (GNT1041766) and K.M.D. (GNT1041844).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.9b00095.
The authors declare no competing financial interest.
This article is made available for a limited time sponsored by ACS under the ACS Free to Read License, which permits copying and redistribution of the article for non-commercial scholarly purposes.
Supplementary Material
References
- Kong P.; Christia P.; Frangogiannis N. G. (2014) The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 71 (4), 549–74. 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D.; Liu Y. (2016) Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 12 (2), 68–70. 10.1038/nrneph.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W.; Booz G. W.; Wang Y.; Fan F.; Roman R. J. (2018) Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 820, 65–76. 10.1016/j.ejphar.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S. D.; Frangogiannis N. G. (2016) The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 119 (1), 91–112. 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. A.; Vannella K. M. (2016) Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44 (3), 450–62. 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P. M.; Nikolic-Paterson D. J.; Lan H. Y. (2019) Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144. 10.1038/s41581-019-0110-2. [DOI] [PubMed] [Google Scholar]
- Kuwahara F.; Kai H.; Tokuda K.; Kai M.; Takeshita A.; Egashira K.; Imaizumi T. (2002) Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106 (1), 130–5. 10.1161/01.CIR.0000020689.12472.E0. [DOI] [PubMed] [Google Scholar]
- Yu L.; Border W. A.; Huang Y.; Noble N. A. (2003) TGF-beta isoforms in renal fibrogenesis. Kidney Int. 64 (3), 844–56. 10.1046/j.1523-1755.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- Loboda A.; Sobczak M.; Jozkowicz A.; Dulak J. (2016) TGF-beta1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediators Inflammation 2016, 8319283. 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H. Y.; Chung A. C. (2012) TGF-beta/Smad signaling in kidney disease. Semin. Nephrol. 32 (3), 236–43. 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Mack M.; Yanagita M. (2015) Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 87 (2), 297–307. 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- Sun Y. B.; Qu X.; Caruana G.; Li J. (2016) The origin of renal fibroblasts/myofibroblasts and the signal that trigger fibrosis. Differentiation 92 (3), 102–7. 10.1016/j.diff.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Zitka O.; Kukacka J.; Krizkov S.; Huska D.; Adam V.; Masarik M.; Prusa R.; Kizek R. (2010) Matrix metalloproteinases. Curr. Med. Chem. 17 (31), 3751–68. 10.2174/092986710793213724. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Dong Y.; Tian X.; Tan T. K.; Liu Z.; Zhao Y.; Zhang Y.; Harris D.; Zheng G. (2013) Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World journal of nephrology 2 (3), 84–9. 10.5527/wjn.v2.i3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte D.; Heymans S. (2010) TIMPs and cardiac remodeling: ’Embracing the MMP-independent-side of the family’. J. Mol. Cell. Cardiol. 48 (3), 445–53. 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- O’Donnell M.; Mente A.; Yusuf S. (2015) Sodium Intake and Cardiovascular Health. Circ. Res. 116 (6), 1046–57. 10.1161/CIRCRESAHA.116.303771. [DOI] [PubMed] [Google Scholar]
- Morrison A. C.; Ness R. B. (2011) Sodium intake and cardiovascular disease. Annu. Rev. Public Health 32, 71–90. 10.1146/annurev-publhealth-031210-101209. [DOI] [PubMed] [Google Scholar]
- Cappuccio F. P. (2013) Cardiovascular and other effects of salt consumption. Kidney Int. Suppl. 3 (4), 312–5. 10.1038/kisup.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D. N.; Katayama I. A.; Oliveira I. B.; Rosa K. T.; Furukawa L. N.; Coelho M. S.; Casarini D. E.; Heimann J. C. (2010) Salt-induced cardiac hypertrophy and interstitial fibrosis are due to a blood pressure-independent mechanism in Wistar rats. J. Nutr. 140 (10), 1742–51. 10.3945/jn.109.117473. [DOI] [PubMed] [Google Scholar]
- Yu H. C.; Burrell L. M.; Black M. J.; Wu L. L.; Dilley R. J.; Cooper M. E.; Johnston C. I. (1998) Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 98 (23), 2621–8. 10.1161/01.CIR.98.23.2621. [DOI] [PubMed] [Google Scholar]
- Lal A.; Veinot J. P.; Leenen F. H. (2003) Prevention of high salt diet-induced cardiac hypertrophy and fibrosis by spironolactone. Am. J. Hypertens. 16 (4), 319–23. 10.1016/S0895-7061(02)03268-5. [DOI] [PubMed] [Google Scholar]
- Endemann D. H.; Touyz R. M.; Iglarz M.; Savoia C.; Schiffrin E. L. (2004) Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 43 (6), 1252–7. 10.1161/01.HYP.0000128031.31572.a3. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. E.; Rhaleb N. E.; D’Ambrosio M. A.; Nakagawa P.; Liu Y.; Leung P.; Dai X.; Yang X. P.; Peterson E. L.; Carretero O. A. (2015) Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin II-high salt-induced hypertension. J. Hypertens. 33 (1), 144–52. 10.1097/HJH.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L.; Sheppard D.; Duffield J. S.; Violette S. (2013) Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5 (167), 167sr1. 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- Olson A. L.; Gifford A. H.; Inase N.; Fernandez Perez E. R.; Suda T. (2018) The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur. Respir. Rev. 27 (150), 180077. 10.1183/16000617.0077-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez J.; Querejeta R.; Lopez B.; Gonzalez A.; Larman M.; Martinez Ubago J. L. (2002) Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105 (21), 2512–7. 10.1161/01.CIR.0000017264.66561.3D. [DOI] [PubMed] [Google Scholar]
- Lopez B.; Querejeta R.; Varo N.; Gonzalez A.; Larman M.; Martinez Ubago J. L.; Diez J. (2001) Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation 104 (3), 286–91. 10.1161/01.CIR.104.3.286. [DOI] [PubMed] [Google Scholar]
- Brilla C. G.; Funck R. C.; Rupp H. (2000) Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102 (12), 1388–93. 10.1161/01.CIR.102.12.1388. [DOI] [PubMed] [Google Scholar]
- Yusuf S.; Teo K. K.; Pogue J.; Dyal L.; Copland I.; Schumacher H.; Dagenais G.; Sleight P.; Anderson C. (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 358 (15), 1547–59. 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- Julius S.; Kjeldsen S. E.; Weber M.; Brunner H. R.; Ekman S.; Hansson L.; Hua T.; Laragh J.; McInnes G. T.; Mitchell L.; Plat F.; Schork A.; Smith B.; Zanchetti A. (2004) Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 363 (9426), 2022–31. 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- van Vark L. C.; Bertrand M.; Akkerhuis K. M.; Brugts J. J.; Fox K.; Mourad J. J.; Boersma E. (2012) Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur. Heart J. 33 (16), 2088–97. 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. S.; Vinh A.; McCarthy C. A.; Gaspari T. A.; Widdop R. E. (2008) AT2 receptors: Functional relevance in cardiovascular disease. Pharmacol. Ther. 120 (3), 292–316. 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdop R. E.; Jones E. S.; Hannan R. E.; Gaspari T. A. (2003) Angiotensin AT2 receptors: cardiovascular hope or hype?. Br. J. Pharmacol. 140 (5), 809–24. 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Del Borgo M.; Lee H. W.; Baraldi D.; Hirmiz B.; Gaspari T. A.; Denton K. M.; Aguilar M. I.; Samuel C. S.; Widdop R. E. (2017) Anti-fibrotic Potential of AT2 Receptor Agonists. Front. Pharmacol. 8, 564. 10.3389/fphar.2017.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce E.; Shenoy V.; Rathinasabapathy A.; Espejo A.; Horowitz A.; Oswalt A.; Francis J.; Nair A.; Unger T.; Raizada M. K.; Steckelings U. M.; Sumners C.; Katovich M. J. (2015) Selective activation of angiotensin AT2 receptors attenuates progression of pulmonary hypertension and inhibits cardiopulmonary fibrosis. Br. J. Pharmacol. 172 (9), 2219–31. 10.1111/bph.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelosa P.; Pignieri A.; Fandriks L.; de Gasparo M.; Hallberg A.; Banfi C.; Castiglioni L.; Turolo L.; Guerrini U.; Tremoli E.; Sironi L. (2009) Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J. Hypertens. 27 (12), 2444–51. 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- Matavelli L. C.; Huang J.; Siragy H. M. (2011) Angiotensin AT(2) receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension 57 (2), 308–13. 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulis C.; Chow B. S.; McKelvey M.; Steckelings U. M.; Unger T.; Thallas-Bonke V.; Thomas M. C.; Cooper M. E.; Jandeleit-Dahm K. A.; Allen T. J. (2015) AT2R agonist, compound 21, is reno-protective against type 1 diabetic nephropathy. Hypertension 65 (5), 1073–81. 10.1161/HYPERTENSIONAHA.115.05204. [DOI] [PubMed] [Google Scholar]
- Castoldi G.; di Gioia C. R.; Bombardi C.; Maestroni S.; Carletti R.; Steckelings U. M.; Dahlof B.; Unger T.; Zerbini G.; Stella A. (2014) Prevention of diabetic nephropathy by compound 21, selective agonist of angiotensin type 2 receptors, in Zucker diabetic fatty rats. American journal of physiology Renal physiology 307 (10), F1123–31. 10.1152/ajprenal.00247.2014. [DOI] [PubMed] [Google Scholar]
- Kaschina E.; Grzesiak A.; Li J.; Foryst-Ludwig A.; Timm M.; Rompe F.; Sommerfeld M.; Kemnitz U. R.; Curato C.; Namsolleck P.; Tschope C.; Hallberg A.; Alterman M.; Hucko T.; Paetsch I.; Dietrich T.; Schnackenburg B.; Graf K.; Dahlof B.; Kintscher U.; Unger T.; Steckelings U. M. (2008) Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction?. Circulation 118 (24), 2523–32. 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- Rehman A.; Leibowitz A.; Yamamoto N.; Rautureau Y.; Paradis P.; Schiffrin E. L. (2012) Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 59 (2), 291–9. 10.1161/HYPERTENSIONAHA.111.180158. [DOI] [PubMed] [Google Scholar]
- Lauer D.; Slavic S.; Sommerfeld M.; Thone-Reineke C.; Sharkovska Y.; Hallberg A.; Dahlof B.; Kintscher U.; Unger T.; Steckelings U. M.; Kaschina E. (2014) Angiotensin type 2 receptor stimulation ameliorates left ventricular fibrosis and dysfunction via regulation of tissue inhibitor of matrix metalloproteinase 1/matrix metalloproteinase 9 axis and transforming growth factor beta1 in the rat heart. Hypertension 63 (3), e60–7. 10.1161/HYPERTENSIONAHA.113.02522. [DOI] [PubMed] [Google Scholar]
- Jones E. S.; Vinh A.; McCarthy C. A.; Gaspari T. A.; Widdop R. E. (2008) AT2 receptors: functional relevance in cardiovascular disease. Pharmacol. Ther. 120 (3), 292–316. 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabito K. M.; Hilliard L. M.; Kett M. M.; Brown R. D.; Booth S. C.; Widdop R. E.; Moritz K. M.; Evans R. G.; Denton K. M. (2014) Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. American journal of physiology Renal physiology 307 (8), F901–7. 10.1152/ajprenal.00288.2014. [DOI] [PubMed] [Google Scholar]
- Carey R. M. (2005) Cardiovascular and renal regulation by the angiotensin type 2 receptor: the AT2 receptor comes of age. Hypertension 45 (5), 840–4. 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- Kaschina E.; Namsolleck P.; Unger T. (2017) AT2 receptors in cardiovascular and renal diseases. Pharmacol. Res. 125 (A), 39–47. 10.1016/j.phrs.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Samuel C. S.; Royce S. G.; Hewitson T. D.; Denton K. M.; Cooney T. E.; Bennett R. G. (2017) Anti-fibrotic actions of relaxin. British journal of pharmacology 174 (10), 962–76. 10.1111/bph.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H. H.; Shen M.; Samuel C. S.; Schlossmann J.; Bennett R. G. (2019) Relaxin and extracellular matrix remodeling: Mechanisms and signaling pathways. Mol. Cell. Endocrinol. 487, 59–65. 10.1016/j.mce.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B. S.; Kocan M.; Bosnyak S.; Sarwar M.; Wigg B.; Jones E. S.; Widdop R. E.; Summers R. J.; Bathgate R. A.; Hewitson T. D.; Samuel C. S. (2014) Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int. 86 (1), 75–85. 10.1038/ki.2013.518. [DOI] [PubMed] [Google Scholar]
- Chow B. S. M.; Kocan M.; Shen M.; Wang Y.; Han L.; Chew J. Y.; Wang C.; Bosnyak S.; Mirabito-Colafella K. M.; Barsha G.; Wigg B.; Johnstone E. K. M.; Hossain M. A.; Pfleger K. D. G.; Denton K. M.; Widdop R. E.; Summers R. J.; Bathgate R. A. D.; Hewitson T. D.; Samuel C. S. (2019) AT1R-AT2R-RXFP1 Functional Crosstalk in Myofibroblasts: Impact on the Therapeutic Targeting of Renal and Cardiac Fibrosis. J. Am. Soc. Nephrol. 30 (11), 2191–207. 10.1681/ASN.2019060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitebread S.; Mele M.; Kamber B.; de Gasparo M. (1989) Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem. Biophys. Res. Commun. 163 (1), 284–91. 10.1016/0006-291X(89)92133-5. [DOI] [PubMed] [Google Scholar]
- Bosnyak S.; Jones E. S.; Christopoulos A.; Aguilar M. I.; Thomas W. G.; Widdop R. E. (2011) Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin. Sci. 121 (7), 297–303. 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- Ali Q.; Wu Y.; Hussain T. (2013) Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int. 84 (5), 931–9. 10.1038/ki.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabuhi R.; Ali Q.; Asghar M.; Al-Zamily N. R.; Hussain T. (2011) Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: opposing effects in lean and obese Zucker rats. American journal of physiology Renal physiology 300 (3), F700–6. 10.1152/ajprenal.00616.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B. S.; Chew E. G.; Zhao C.; Bathgate R. A.; Hewitson T. D.; Samuel C. S. (2012) Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: the additional involvement of iNOS. PLoS One 7 (8), e42714. 10.1371/journal.pone.0042714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. S.; Zhao C.; Bathgate R. A.; Bond C. P.; Burton M. D.; Parry L. J.; Summers R. J.; Tang M. L.; Amento E. P.; Tregear G. W. (2003) Relaxin deficiency in mice is associated with an age-related progression of pulmonary fibrosis. FASEB J. 17 (1), 121–3. 10.1096/fj.02-0449fje. [DOI] [PubMed] [Google Scholar]
- Sherwood O. D. (2004) Relaxin’s physiological roles and other diverse actions. Endocr. Rev. 25 (2), 205–34. 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- Jones E. S.; Black M. J.; Widdop R. E. (2012) Influence of Angiotensin II Subtype 2 Receptor (AT(2)R) Antagonist, PD123319, on Cardiovascular Remodelling of Aged Spontaneously Hypertensive Rats during Chronic Angiotensin II Subtype 1 Receptor (AT(1)R) Blockade. Int. J. Hypertens. 2012, 543062. 10.1155/2012/543062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Zhao G.; Li H.; Liu X.; Wang S. (2012) Candesartan antagonizes pressure overload-evoked cardiac remodeling through Smad7 gene-dependent MMP-9 suppression. Gene 497 (2), 301–6. 10.1016/j.gene.2012.01.081. [DOI] [PubMed] [Google Scholar]
- Widdop R. E.; Li X. C. (1997) A simple versatile method for measuring tail cuff systolic blood pressure in conscious rats. Clin. Sci. 93 (3), 191–4. 10.1042/cs0930191. [DOI] [PubMed] [Google Scholar]
- Hossain M. A.; Samuel C. S.; Binder C.; Hewitson T. D.; Tregear G. W.; Wade J. D.; Bathgate R. A. (2010) The chemically synthesized human relaxin-2 analog, B-R13/17K H2, is an RXFP1 antagonist. Amino Acids 39 (2), 409–16. 10.1007/s00726-009-0454-1. [DOI] [PubMed] [Google Scholar]
- Hijmans R. S.; Shrestha P.; Sarpong K. A.; Yazdani S.; El Masri R.; de Jong W. H. A.; Navis G.; Vives R. R.; van den Born J. (2017) High sodium diet converts renal proteoglycans into pro-inflammatory mediators in rats. PLoS One 12 (6), e0178940. 10.1371/journal.pone.0178940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattouf R.; Younes R.; Lutomski D.; Naaman N.; Godeau G.; Senni K.; Changotade S. (2014) Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 62 (10), 751–8. 10.1369/0022155414545787. [DOI] [PubMed] [Google Scholar]
- Samuel C. S.; Unemori E. N.; Mookerjee I.; Bathgate R. A.; Layfield S. L.; Mak J.; Tregear G. W.; Du X. J. (2004) Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 145 (9), 4125–33. 10.1210/en.2004-0209. [DOI] [PubMed] [Google Scholar]
- Annes J. P.; Munger J. S.; Rifkin D. B. (2003) Making sense of latent TGFbeta activation. J. Cell Sci. 116 (Pt 2), 217–24. 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Löffek S.; Schilling O.; Franzke C.-W. (2011) Biological role of matrix metalloproteinases: a critical balance. Eur. Respir. J. 38 (1), 191–208. 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- Howes J. M.; Bihan D.; Slatter D. A.; Hamaia S. W.; Packman L. C.; Knauper V.; Visse R.; Farndale R. W. (2014) The recognition of collagen and triple-helical toolkit peptides by MMP-13: sequence specificity for binding and cleavage. J. Biol. Chem. 289 (35), 24091–101. 10.1074/jbc.M114.583443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. G.; Hayden M. S.; Ghosh S. (2011) NF-kappaB, inflammation, and metabolic disease. Cell Metab. 13 (1), 11–22. 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y.; Aoyama T.; Yokoyama C.; Okamoto C.; Komaki H.; Minatoguchi S.; Iwasa M.; Yamada Y.; Kawamura I.; Kawasaki M.; Nishigaki K.; Mikami A.; Suzuki F.; Minatoguchi S. (2015) High salt intake damages the heart through activation of cardiac (pro) renin receptors even at an early stage of hypertension. PLoS One 10 (3), e0120453. 10.1371/journal.pone.0120453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopona E. P. B.; Rocha V. F.; Furukawa L. N. S.; Oliveira I. B.; Heimann J. C. (2019) Myocardial hypertrophy induced by high salt consumption is prevented by angiotensin II AT2 receptor agonist. Nutr., Metab. Cardiovasc. Dis. 29 (3), 301–5. 10.1016/j.numecd.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Ali Q.; Patel S.; Hussain T. (2015) Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese Zucker rats. American journal of physiology Renal physiology 308 (12), F1379–85. 10.1152/ajprenal.00002.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakumar S. B.; Kundu S.; Metreveli N.; Tyagi S. C.; Sen U. (2013) Matrix Metalloproteinase Inhibition Mitigates Renovascular Remodeling in Salt-Sensitive Hypertension. Physiol. Rep. 1 (3), e00063. 10.1002/phy2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Corvoisier P.; Adamy C.; Sambin L.; Crozatier B.; Berdeaux A.; Michel J. B.; Hittinger L.; Su J. (2010) The cardiac renin-angiotensin system is responsible for high-salt diet-induced left ventricular hypertrophy in mice. Eur. J. Heart Failure 12 (11), 1171–8. 10.1093/eurjhf/hfq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald O.; Zymek P.; Winkelmann K.; Koerting A.; Ren G.; Abou-Khamis T.; Michael L. H.; Rollins B. J.; Entman M. L.; Frangogiannis N. G. (2005) CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96 (8), 881–9. 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- Kanai A. J.; Konieczko E. M.; Bennett R. G.; Samuel C. S.; Royce S. G. (2019) Relaxin and fibrosis: Emerging targets, challenges, and future directions. Mol. Cell. Endocrinol. 487, 66–74. 10.1016/j.mce.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzel A.; Muller D. N.; Hafler D. A.; Erdman S. E.; Linker R. A.; Kleinewietfeld M. (2014) Role of ″Western diet″ in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 14 (1), 404. 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M.; Matsuo T.; Fukuda R.; Ohta M.; Nagano H.; Shibouta Y.; Naka T.; Nishikawa K.; Imura Y. (1999) Effect of candesartan cilexetil (TCV-116) in rats with chronic renal failure. Kidney Int. 56 (3), 898–909. 10.1046/j.1523-1755.1999.00614.x. [DOI] [PubMed] [Google Scholar]
- Huuskes B. M.; Wise A. F.; Cox A. J.; Lim E. X.; Payne N. L.; Kelly D. J.; Samuel C. S.; Ricardo S. D. (2015) Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J. 29 (2), 540–53. 10.1096/fj.14-254789. [DOI] [PubMed] [Google Scholar]
- Samuel C. S.; Bodaragama H.; Chew J. Y.; Widdop R. E.; Royce S. G.; Hewitson T. D. (2014) Serelaxin is a more efficacious antifibrotic than enalapril in an experimental model of heart disease. Hypertension 64 (2), 315–22. 10.1161/HYPERTENSIONAHA.114.03594. [DOI] [PubMed] [Google Scholar]
- Lekgabe E. D.; Kiriazis H.; Zhao C.; Xu Q.; Moore X. L.; Su Y.; Bathgate R. A.; Du X. J.; Samuel C. S. (2005) Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension 46 (2), 412–8. 10.1161/01.HYP.0000171930.00697.2f. [DOI] [PubMed] [Google Scholar]
- Blenck C. L.; Harvey P. A.; Reckelhoff J. F.; Leinwand L. A. (2016) The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ. Res. 118 (8), 1294–312. 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.