Abstract
Objective:
To study the long-term risk of de novo mental health conditions in women who underwent hysterectomy with bilateral ovarian conservation compared with age-matched referent women.
Method:
Using the Rochester Epidemiology Project records-linkage system, we identified a historical cohort of 2,094 women who underwent hysterectomy with ovarian conservation for benign indications at age ≥18 years and with an index date between 1980 and 2002 in Olmsted County, Minnesota. Each woman was age-matched (±1 year) to a referent woman residing in the same county who had not undergone hysterectomy or any oophorectomy prior to the index date. These two cohorts were followed historically to identify de novo mental health conditions. We estimated hazard ratios (HRs) and 95% confidence intervals (95% CIs) using Cox proportional hazards models adjusted for 20 pre-existing chronic conditions and other potential confounders. We also calculated absolute risk increases (ARIs) and reductions (ARRs) at 30 years of follow-up.
Results:
Over a median follow-up of 21.9 years, women who underwent hysterectomy at any age experienced increased risks of de novo depression (adjusted HR 1.26; 95% CI 1.12–1.41; ARI 6.6%) and anxiety (adjusted HR 1.22; 95% CI 1.08–1.38; ARI 4.7%). The association for depression increased significantly with younger age at hysterectomy but did not vary significantly by indication. Interactions were not significant for anxiety.
Conclusions:
Hysterectomy, even with ovarian conservation, is associated with an increased long-term risk of de novo depression and anxiety, especially when performed in women who are younger.
Keywords: hysterectomy, depression, anxiety, epidemiology, cohort study
Over 400,000 hysterectomies with or without bilateral oophorectomy are performed in the United States annually; thus, hysterectomy is the most common gynecological surgery.1, 2 The risk of increased morbidity and mortality after bilateral oophorectomy with or without concurrent hysterectomy has been established. In particular, bilateral oophorectomy has been associated with increased risk of anxiety, depression, and dementia.3, 4 However, emerging research has demonstrated that also hysterectomy with both ovaries conserved may be associated with long-term, harmful effects.5–8 The association may be explained by a cause-effect inversion, by a confounding by indication, or by biological effects of hysterectomy. For example, it has been hypothesized that hysterectomy may have detrimental effects on the ovaries or directly on other organs and systems via ovarian-independent mechanisms.9–11 A possible endocrine activity of the endometrium, both during pregnancy and in nonpregnant women, has been discussed in the more recent literature, including our recent letter in Menopause.12–14
The association of hysterectomy with mental health outcomes has been debated. Several studies that assessed depressive symptoms immediately prior to hysterectomy have shown a general improvement in symptoms and quality of life after surgery.15–17 However, women with gynecologic problems and women undergoing hysterectomy have been shown to have lower quality of life than the general population.18, 19 Therefore, short-term improvement after surgery may indicate normalization of symptoms that were acutely worsened by the gynecologic condition. Indeed, most of the studies that showed an improvement were based on patient recall or on a preoperative evaluation performed within weeks before the surgery.
A study based on a national insurance database in Taiwan demonstrated an increase in depression in women who underwent hysterectomy with ovarian conservation, after excluding women with a preoperative diagnosis of depression.20 In addition, two longitudinal studies from Australia have shown consistently higher risks of depressive symptoms in women undergoing hysterectomy compared with perimenopausal and postmenopausal women.21, 22 The risk of depression was higher in women who underwent a concurrent bilateral oophorectomy compared with hysterectomy with ovarian conservation.21, 22 In addition, menopause in itself may be a time of vulnerability to depression;23 however, depression improved over time regardless of surgical status in a longitudinal study of the menopausal transition.16
Hysterectomy with ovarian conservation has been previously associated with the risk of dementia;8, 24 however, in some studies it was not possible to clearly separate hysterectomy with and without a concurrent bilateral oophorectomy.25, 26 A 2018 study on a rat model suggested that hysterectomy with ovarian conservation may have a specific detrimental effect on the working memory cognitive domain.14 The authors suggested that hysterectomy, with or without ovarian conservation, may have important effects on brain aging and endocrine aging.14 Other studies have suggested an association of hysterectomy with substance abuse disorders, particularly in relation to the postoperative use of narcotic pain medications.27–29 We are not aware of previous studies of the association between hysterectomy and schizophrenia or psychosis. As shown in detail by the results of this study, the cumulative incidence during the follow-up was higher for depression and anxiety but lower for dementia, substance abuse disorders, and schizophrenia or psychosis.
The aim of this study was to investigate the long-term associations of hysterectomy with bilateral ovarian conservation with a broad range of aging-related mental health conditions, after accounting for preoperative mental health diagnoses and several potential confounders.30
METHODS
Study population and overall study design
A subset of women was identified from the Mayo Clinic Study of Uterine Disease and Health (MCSUD), as described elsewhere.31–34 This subset comprised 2,094 Olmsted County, Minnesota resident women who underwent hysterectomy with ovarian conservation for a benign indication at age ≥18 years and with an index date between January 1, 1980 and December 31, 2002 (23 years). All data for this study were obtained using the Rochester Epidemiology Project (REP) medical records-linkage system that includes the complete inpatient and outpatient records of all the major medical care providers in Olmsted County, Minnesota. Details about the REP and about the Olmsted County population have been reported elsewhere.35
As described previously, the procedural codes for hysterectomy and the diagnostic codes for surgical indication were extracted from the REP electronic indices to identify the women who underwent hysterectomy.33 Our ascertainment of hysterectomy was formally validated.33 The manual review of a random sample of 100 women with a procedural code for hysterectomy confirmed that all women had undergone hysterectomy with full details about the surgery and the pathology (100% positive predictive value). In addition, the type of hysterectomy (abdominal vs. vaginal) also had a 99% predictive value.33 Finally, our exclusion of bilateral oophorectomy has also been validated in a previous study.36
The date of hysterectomy was considered the index date. For each woman who underwent hysterectomy, we used simple random sampling to identify one referent woman matched by age (±1 year) who resided in Olmsted County on the index date, and who had not undergone a hysterectomy or oophorectomy (unilateral or bilateral) prior to the index date. Women who did not authorize the use of their medical records for research were excluded. All research activities were approved by the institutional review boards at Olmsted Medical Center and Mayo Clinic.
Ascertainment of chronic conditions present at the index date
Chronic conditions present at the index date were obtained electronically from the diagnostic indices of the REP. We considered 18 chronic conditions used by the Department of Health and Human Services (DHHS) to define multi-morbidity, but added to the list also anxiety and obesity (total of 20 conditions listed in Supplemental Digital Content 1).37–39 All of the five mental health conditions considered in this study are part of the modified DHHS list: depression, anxiety, substance abuse disorders (drugs and alcohol), dementia (and other disorders of cognition), schizophrenia and psychosis, hyperlipidemia, hypertension, diabetes, obesity, cardiac arrhythmias, coronary artery disease, congestive heart failure, stroke, arthritis, cancer (all types), asthma, chronic obstructive pulmonary disease, osteoporosis, chronic kidney disease, and hepatitis. Women were required to have at least two diagnostic codes for a given condition separated by more than 30 days to reduce the risk of false positive diagnoses. Before 1994, a finer dating of the codes was not available; thus, a one-year separation of codes was required. We included all chronic conditions documented in the records-linkage system at any time before the index date.
Ascertainment of mental health conditions during follow-up
The primary outcomes of the study were the five mental health conditions included in the modified DHHS list. The mental health outcomes were obtained electronically from the REP indices, and required at least two diagnostic codes separated by more than 30 days (or more than one year) as described above. However, to include those conditions that were first identified at death (such as depression-related suicide or accidental death due to substance abuse), a single diagnostic code found anywhere on a death certificate was also sufficient.
Statistical analysis
Each mental health condition was evaluated separately, and women with that condition diagnosed before the hysterectomy (or index date for referent women) were excluded from the analysis to consider only de novo conditions. The duration of follow-up was calculated from the index date to the earliest of four endpoints: diagnosis of the specific mental health outcome, date of death, last contact within the REP, or the end of the study (December 31, 2015). Cumulative incidence curves were estimated using the Kaplan-Meier method, and absolute risks were derived from the adjusted Kaplan-Meier curves at 15 and 30 years. Differences between the two cohorts were measured using the absolute risk increase (ARI) or reduction (ARR), obtained by subtracting the two absolute risks. In addition, Cox proportional hazards models were used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) using age as the time scale and with women entering the risk set at their respective index ages.
The Kaplan-Meier curves and the Cox models were adjusted using inverse probability weights derived from a logistic regression model including 20 pre-existing chronic conditions, years of education (≤12, 13–16, >16, unknown), race (white vs. nonwhite), and age and calendar year at index date (continuous). Robust sandwich covariance estimates were used in the Cox models to account for women included in both cohorts (referent women with subsequent hysterectomy), and for the use of estimated weights.
Analyses were performed for all women combined, and stratified by age at hysterectomy (18–35, 36–50, and >50 years) and by surgical indication (leiomyomas, menstrual disorders, and uterine prolapse). Formal tests of interaction across strata were performed. The inverse probability weights were derived separately within each stratum to maximize the balance of the adjustment variables. We performed four sets of sensitivity analyses to 1) exclude women with any of the 20 chronic conditions prior to the index date; 2) to censor women at the time of subsequent unilateral or bilateral oophorectomy (both women with hysterectomy and referent women) or hysterectomy (referent women); 3) to additionally adjust for number of children (0, 1, 2, ≥3 children, unknown); and 4) to additionally adjust for number of medical contacts during follow-up treated as a time-dependent continuous variable (and excluding the medical contacts in the first six months after index date). Analyses were performed using the SAS version 9.4 software package (SAS Institute, Inc., Cary, NC), and tests of statistical significance were conducted at the 2-tailed alpha level of 0.05.
RESULTS
Description of the hysterectomy and referent cohorts
Between 1980 and 2002, 2,094 women underwent hysterectomy with ovarian conservation for a benign indication. A total of 529 women (25.3%) were age 18–35 years at the time of hysterectomy,1,294 (61.8%) were 36–50 years, and 271 (12.9%) were older than 50 years (Supplemental Digital Content 2). The median age at index date was 40.0 years (IQR 35.0–44.0). Indications for hysterectomy with ovarian conservation included uterine leiomyomas (n=827, 39.5%), prolapse (n=425, 20.3%), menstrual disorders (n=534, 25.5%; including heavy and irregular menstrual bleeding), and other non-cancer indications (n=308, 14.7%). Vaginal hysterectomy was performed in 1,709 women (81.6%).
The median length of capture in the records-linkage system before the index date was 22.5 years (interquartile range [IQR] 13.7–32.2) for women with hysterectomy and 19.3 (IQR 8.2–27.0) for referent women. The median length of follow-up after the index date was 21.9 years (IQR 14.2–28.7) for both cohorts combined, 22.5 years (IQR 15.2–28.8) for women with hysterectomy, and 21.3 years (IQR 13.7–28.6) for referent women. Therefore, the median age at the end of follow-up was 62.0 years (IQR 55.0–71.0) for both cohorts combined, 62.0 years (IQR 55.0–72.0) for women with hysterectomy, and 62.0 years (IQR 54.0–71.0) for referent women. Overall, the median density of medical contacts during follow-up was 7.3 per year (IQR 4.3–11.4) for the women with hysterectomy and 6.2 per year (IQR 3.6–9.9) for referent women (excluding contacts in the first 6 months after the index date). However, when we truncated the follow-up at the first diagnosis of de novo depression, the median length of follow-up and the median density of contacts were virtually identical for women with or without hysterectomy. The same pattern was observed for de novo anxiety (data not shown). A total of 293 women (14.0%) died in the hysterectomy cohort and 306 (14.6%) in the referent cohort.
Conditions present at baseline and adjustments
At baseline (index date), women in the hysterectomy group were more likely to have previous diagnoses of depression, anxiety, hyperlipidemia, obesity, asthma, and chronic obstructive pulmonary disease compared with referent women (Supplemental Digital Content 1). Women who underwent hysterectomy also had fewer years of education and higher gravidity and parity at baseline compared to the referent group (Supplemental Digital Content 2). These differences were most pronounced for the youngest women. The two overall cohorts were not highly imbalanced on baseline characteristics before the adjustments using inverse probability weights (each standardized difference of means was <25% of the SD), and the adjustments improved the balance successfully (each standardized difference of means was <5% of the SD after weighting). The range of the weights used in the overall analysis was 0.6–2.5 for the women who underwent hysterectomy and 0.6–2.8 for the referent women.
Results of overall analyses
Supplemental Digital Content 3 shows the results of univariable analyses for the effect of the major potential confounding variables on the mental health conditions. Tables 1–2 show the results of our cohort analyses overall and in strata by age at hysterectomy and by indication. Among women without anxiety or depression at the index date, those who underwent hysterectomy with ovarian conservation experienced a higher risk of de novo anxiety or depression compared with referent women. The incidence curves started to diverge approximately 2.5 years after the index date for depression and 7.5 years for anxiety (Figure 1). The risk of depression was increased overall (adjusted HR 1.26; 95% CI 1.12–1.41, Table 1), with a 6.6% ARI at 30 years compared with referent women (Table 3). The risk of anxiety was increased overall (adjusted HR 1.22; 95% CI 1.08–1.38, Table 1), with a 4.7% ARI at 30 years compared with referent women (Table 3). By contrast, the risk of substance abuse disorders, dementia, and schizophrenia were not significantly increased in women who underwent hysterectomy with ovarian conservation (Table 1).
TABLE 1.
Cumulative incidence of mental health conditions overall and in strata by age at hysterectomy with ovarian conservation
| Hysterectomy |
Referent women |
Unadjusted modelsa |
Adjusted modelsb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | N at risk | Person-years | N of events | N at risk | Person-years | N of events | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P |
| Overall | ||||||||||
| Depression | 1,872 | 32,540 | 636 | 1,950 | 33,418 | 506 | 1.29 (1.15–1.44) | <0.001 | 1.26 (1.12–1.41) | <0.001 |
| Anxiety | 1,941 | 35,279 | 554 | 1,992 | 35,252 | 423 | 1.31 (1.16–1.48) | <0.001 | 1.22 (1.08–1.38) | 0.001 |
| Substance abuse disorders | 2,054 | 43,065 | 104 | 2,069 | 40,905 | 65 | 1.51 (1.11–2.04) | 0.008 | 1.35 (0.99–1.85) | 0.06 |
| Dementia | 2,089 | 44,221 | 143 | 2,091 | 41,396 | 132 | 0.98 (0.78–1.24) | 0.87 | 0.94 (0.74–1.19) | 0.59 |
| Schizophrenia and psychosis | 2,087 | 44,356 | 51 | 2,082 | 41,447 | 47 | 0.99 (0.67–1.46) | 0.94 | 0.93 (0.62–1.39) | 0.73 |
| Age 18–35 years | ||||||||||
| Depression | 483 | 7,969 | 189 | 511 | 8,512 | 130 | 1.54 (1.23–1.92) | <0.001 | 1.47 (1.17–1.86) | 0.001 |
| Anxiety | 494 | 8,651 | 168 | 518 | 9,084 | 106 | 1.67 (1.31–2.11) | <0.001 | 1.45 (1.12–1.88) | 0.005 |
| Substance abuse disorders | 520 | 10,820 | 41 | 523 | 10,201 | 22 | 1.74 (1.05–2.90) | 0.03 | 1.27 (0.70–2.29) | 0.44 |
| Dementia | 529 | 11,363 | 15 | 529 | 10,487 | 12 | 1.24 (0.59–2.58) | 0.57 | 0.66 (0.29–1.53) | 0.33 |
| Schizophrenia and psychosis | 528 | 11,397 | 3 | 529 | 10,526 | 4 | 0.68 (0.15–3.03) | 0.61 | 0.73 (0.15–3.50) | 0.69 |
| Age 36–50 years | ||||||||||
| Depression | 1,140 | 20,467 | 377 | 1,190 | 21,095 | 304 | 1.28 (1.10–1.48) | 0.001 | 1.25 (1.07–1.45) | 0.004 |
| Anxiety | 1,192 | 22,253 | 332 | 1,225 | 22,213 | 278 | 1.19 (1.02–1.39) | 0.03 | 1.11 (0.94–1.30) | 0.21 |
| Substance abuse disorders | 1,265 | 27,234 | 53 | 1,279 | 26,143 | 31 | 1.64 (1.06–2.54) | 0.03 | 1.43 (0.90–2.25) | 0.13 |
| Dementia | 1,294 | 28,068 | 51 | 1,293 | 26,583 | 34 | 1.44 (0.94–2.22) | 0.09 | 1.37 (0.89–2.12) | 0.15 |
| Schizophrenia and psychosis | 1,290 | 28,003 | 27 | 1,288 | 26,486 | 17 | 1.52 (0.84–2.74) | 0.17 | 1.34 (0.74–2.45) | 0.33 |
| Age >50 years | ||||||||||
| Depression | 249 | 4,104 | 70 | 249 | 3,812 | 72 | 0.90 (0.65–1.24) | 0.51 | 0.91 (0.65–1.28) | 0.59 |
| Anxiety | 255 | 4,375 | 54 | 249 | 3,954 | 39 | 1.22 (0.82–1.82) | 0.33 | 1.21 (0.80–1.85) | 0.37 |
| Substance abuse disorders | 269 | 5,012 | 10 | 267 | 4,561 | 12 | 0.74 (0.33–1.65) | 0.46 | 0.86 (0.37–1.98) | 0.72 |
| Dementia | 266 | 4,790 | 77 | 269 | 4,326 | 86 | 0.77 (0.57–1.04) | 0.08 | 0.81 (0.59–1.10) | 0.18 |
| Schizophrenia and psychosis | 269 | 4,956 | 21 | 265 | 4,435 | 26 | 0.69 (0.39–1.22) | 0.21 | 0.71 (0.39–1.28) | 0.25 |
Hazard ratios calculated using Cox proportional hazards models with age as the time scale.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline (index date), years of education (≤12, 13–16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). These adjustments were performed separately in each stratum to maximize the balance at baseline. Overall interactions by age (18–35, 36–50, >50 years) were assessed for each mental health condition: depression P=0.002, anxiety P=0.28, substance abuse disorders P=0.20, dementia P=0.81, and schizophrenia and psychosis P=0.68.
TABLE 2.
Cumulative incidence of mental health conditions in strata by hysterectomy indicationa
| Hysterectomy |
Referent women |
Unadjusted modelsb |
Adjusted modelsc |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | N at risk | Person-years | N of events | N at risk | Person-years | N of events | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P |
| Leiomyomas | ||||||||||
| Depression | 726 | 12,369 | 223 | 759 | 12,449 | 198 | 1.13 (0.94–1.37) | 0.19 | 1.12 (0.92–1.35) | 0.25 |
| Anxiety | 762 | 13,315 | 199 | 772 | 12,989 | 161 | 1.20 (0.98–1.48) | 0.07 | 1.14 (0.93–1.40) | 0.22 |
| Substance abuse disorders | 807 | 16,179 | 33 | 817 | 15,468 | 21 | 1.50 (0.88–2.57) | 0.14 | 1.36 (0.78–2.36) | 0.27 |
| Dementia | 824 | 16,613 | 59 | 825 | 15,628 | 48 | 1.14 (0.79–1.65) | 0.48 | 1.11 (0.77–1.61) | 0.57 |
| Schizophrenia and psychosis | 824 | 16,663 | 23 | 823 | 15,639 | 17 | 1.29 (0.69–2.39) | 0.43 | 1.21 (0.64–2.28) | 0.56 |
| Menstrual disorders | ||||||||||
| Depression | 482 | 8,585 | 174 | 500 | 8,901 | 136 | 1.31 (1.05–1.64) | 0.02 | 1.30 (1.03–1.63) | 0.03 |
| Anxiety | 497 | 9,277 | 156 | 510 | 9,504 | 112 | 1.43 (1.12–1.81) | 0.003 | 1.24 (0.97–1.60) | 0.09 |
| Substance abuse disorders | 524 | 11,367 | 33 | 528 | 11,036 | 20 | 1.54 (0.89–2.68) | 0.12 | 1.19 (0.64–2.21) | 0.57 |
| Dementia | 534 | 11,849 | 21 | 533 | 11,247 | 23 | 0.90 (0.50–1.61) | 0.72 | 0.71 (0.37–1.35) | 0.30 |
| Schizophrenia and psychosis | 532 | 11,790 | 13 | 532 | 11,272 | 7 | 1.82 (0.73–4.55) | 0.20 | 1.28 (0.46–3.52) | 0.64 |
| Uterine prolapse | ||||||||||
| Depression | 387 | 6,903 | 128 | 403 | 6,966 | 105 | 1.23 (0.96–1.59) | 0.11 | 1.20 (0.92–1.56) | 0.17 |
| Anxiety | 398 | 7,380 | 119 | 411 | 7,301 | 87 | 1.35 (1.04–1.76) | 0.03 | 1.20 (0.92–1.57) | 0.18 |
| Substance abuse disorders | 421 | 9,111 | 22 | 417 | 8,258 | 12 | 1.64 (0.81–3.31) | 0.17 | 1.58 (0.77–3.24) | 0.22 |
| Dementia | 423 | 9,173 | 50 | 425 | 8,310 | 46 | 0.89 (0.60–1.33) | 0.58 | 0.91 (0.60–1.37) | 0.65 |
| Schizophrenia and psychosis | 423 | 9,272 | 13 | 421 | 8,323 | 18 | 0.62 (0.30–1.25) | 0.18 | 0.55 (0.27–1.13) | 0.10 |
A total of 308 women with other non-cancer indications for hysterectomy were not included in the stratified analyses.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline (index date), years of education (≤12, 13–16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). These adjustments were performed separately in each stratum to maximize the balance at baseline. Overall interactions by indication (leiomyomas, menstrual disorders, uterine prolapse) were assessed for each mental health condition: depression P=0.12, anxiety P=0.23, substance abuse disorders P=0.55, dementia P=0.21, and schizophrenia and psychosis P=0.44.
Fig. 1.
Cumulative incidence curves for mental health conditions in women who underwent hysterectomy with ovarian conservation compared with referent women (all ages). The curves were adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13–16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). The number of women at risk varied across conditions because we excluded women with the specific condition on the index date. Note the different scales used for the y-axis to better show differences.
TABLE 3.
Absolute risk of de novo mental health conditions, overall and in strata by age at hysterectomy and by indication
| Condition | Hysterectomy |
Referent women |
Absolute risk difference, %b |
|||
|---|---|---|---|---|---|---|
| Absolute risk at 15 years, %a (95% CI) | Absolute risk at 30 years, %a (95% CI) | Absolute risk at 15 years, %a (95% CI) | Absolute risk at 30 years, %a (95% CI) | 15 years | 30 years | |
| Overall | ||||||
| Depression | 26.2% (24.2–28.4) | 42.4% (39.7–45.3) | 21.8% (19.8–23.9) | 35.8% (33.1–38.8) | 4.4% | 6.6% |
| Anxiety | 20.6% (18.8–22.6) | 36.2% (33.5–39.1) | 16.4% (14.7–18.3) | 31.5% (28.8–34.4) | 4.2% | 4.7% |
| Substance abuse disorders | 3.5% (2.8–4.5) | 6.5% (5.3–8.0) | 2.9% (2.2–3.8) | 4.7% (3.6–6.2) | 0.6% | 1.8% |
| Dementia | 2.7% (2.0–3.5) | 10.5% (8.7–12.7) | 3.5% (2.7–4.4) | 11.2% (9.4–13.4) | −0.8% | −0.7% |
| Schizophrenia and psychosis | 1.0% (0.6–1.6) | 3.3% (2.4–4.5) | 1.4% (0.9–2.0) | 3.7% (2.7–5.0) | −0.4% | −0.4% |
| Age 18–35 years | ||||||
| Depression | 31.8% (27.5–36.6) | 47.5% (42.1–53.2) | 22.0% (18.1–26.6) | 35.5% (30.2–41.4) | 9.8% | 12.0% |
| Anxiety | 25.0% (21.0–29.5) | 43.1% (37.6–49.1) | 18.3% (14.8–22.6) | 33.0% (27.8–38.9) | 6.7% | 10.1% |
| Substance abuse disorders | 5.8% (3.9–8.5) | 9.9% (7.1–13.6) | 5.2% (3.3–7.9) | 7.9% (5.3–11.7) | 0.6% | 2.0% |
| Dementia | 0.3% (0.1–1.7) | 4.8% (2.6–8.7) | 1.1% (0.4–2.8) | 7.2% (4.3–11.9) | −0.8% | −2.4% |
| Schizophrenia and psychosis | 0.0% (0.0–0.0) | 1.1% (0.3–3.4) | 0.9% (0.3–2.6) | 0.9% (0.3–2.6) | −0.9% | 0.2% |
| Age 36–50 years | ||||||
| Depression | 25.2% (22.7–28.0) | 40.5% (37.0–44.1) | 21.6% (19.2–24.3) | 34.2% (30.8–37.9) | 3.6% | 6.3% |
| Anxiety | 20.2% (18.0–22.8) | 34.0% (30.7–37.5) | 17.3% (15.1–19.8) | 31.8% (28.5–35.3) | 2.9% | 2.2% |
| Substance abuse disorders | 2.9% (2.0–4.0) | 5.1% (3.8–6.8) | 2.4% (1.7–3.6) | 3.3% (2.4–4.7) | 0.5% | 1.8% |
| Dementia | 1.0% (0.6–1.8) | 6.4% (4.5–8.9) | 1.0% (0.5–1.8) | 5.4% (3.7–7.8) | 0.0% | 1.0% |
| Schizophrenia and psychosis | 0.5% (0.2–1.2) | 2.6% (1.7–4.2) | 0.4% (0.1–1.0) | 2.3% (1.3–3.9) | 0.1% | 0.3% |
| Age >50 years | ||||||
| Depression | 20.5% (15.5–26.7) | 40.4% (32.8–49.2) | 22.2% (16.9–28.9) | 48.5% (38.5–59.5) | −1.7% | −8.1% |
| Anxiety | 14.0% (9.8–19.6) | 34.0% (26.0–43.6) | 11.6% (7.8–17.0) | 32.6% (22.5–45.8) | 2.4% | 1.4% |
| Substance abuse disorders | 2.0% (0.8–5.0) | 7.9% (3.8–15.8) | 3.1% (1.4–6.6) | 8.3% (3.3–20.2) | −1.1% | −0.4% |
| Dementia | 14.5% (10.4–20.0) | 41.3% (33.4–50.2) | 19.3% (14.5–25.4) | 53.3% (43.7–63.5) | −4.8% | −12.0% |
| Schizophrenia and psychosis | 5.4% (3.0–9.5) | 12.1% (7.6–18.8) | 7.3% (4.5–12.0) | 18.7% (12.0–28.4) | −1.9% | −6.6% |
| Leiomyomasc | ||||||
| Depression | 25.0% (21.9–28.5) | 38.9% (34.5–43.6) | 23.1% (20.0–26.6) | 36.4% (31.9–41.3) | 1.9% | 2.5% |
| Anxiety | 20.7% (17.8–24.0) | 33.0% (28.9–37.6) | 17.1% (14.4–20.3) | 31.2% (26.9–36.0) | 3.6% | 1.8% |
| Substance abuse disorders | 2.8% (1.8–4.3) | 6.3% (4.3–9.3) | 2.6% (1.7–4.1) | 3.5% (2.2–5.4) | 0.2% | 2.8% |
| Dementia | 3.5% (2.4–5.1) | 11.3% (8.4–15.1) | 3.4% (2.2–5.1) | 11.1% (8.0–15.3) | 0.1% | 0.2% |
| Schizophrenia and psychosis | 1.2% (0.6–2.3) | 3.9% (2.4–6.4) | 1.6% (0.9–2.8) | 2.7% (1.6–4.5) | −0.4% | 1.2% |
| Menstrual disordersc | ||||||
| Depression | 27.6% (23.6–32.1) | 43.1% (38.1–48.6) | 24.1% (20.2–28.6) | 35.7% (30.5–41.4) | 3.5% | 7.4% |
| Anxiety | 22.4% (18.7–26.7) | 37.9% (32.7–43.7) | 18.3% (14.9–22.3) | 33.3% (28.2–39.2) | 4.1% | 4.6% |
| Substance abuse disorders | 4.9% (3.2–7.2) | 7.0% (4.9–10.0) | 3.7% (2.3–6.0) | 7.0% (4.5–10.9) | 1.2% | 0.0% |
| Dementia | 1.6% (0.8–3.4) | 6.2% (3.9–9.8) | 2.5% (1.4–4.6) | 9.3% (6.2–13.9) | −0.9% | −3.1% |
| Schizophrenia and psychosis | 1.2% (0.5–2.7) | 3.1% (1.7–5.6) | 0.4% (0.1–1.8) | 3.8% (1.9–7.4) | 0.8% | −0.7% |
| Uterine prolapsec | ||||||
| Depression | 25.2% (20.9–30.1) | 40.9% (35.1–47.4) | 20.9% (16.9–25.8) | 39.4% (32.7–47.0) | 4.3% | 1.5% |
| Anxiety | 19.6% (15.7–24.2) | 36.2% (30.3–42.9) | 14.9% (11.4–19.2) | 34.9% (28.5–42.4) | 4.7% | 1.3% |
| Substance abuse disorders | 2.9% (1.6–5.3) | 6.9% (4.2–11.2) | 2.6% (1.4–5.0) | 4.3% (2.1–8.6) | 0.3% | 2.6% |
| Dementia | 3.8% (2.3–6.3) | 18.6% (13.3–25.5) | 5.4% (3.4–8.4) | 18.0% (13.2–24.2) | −1.6% | 0.6% |
| Schizophrenia and psychosis | 0.7% (0.2–2.4) | 4.0% (2.0–7.7) | 2.3% (1.2–4.7) | 6.7% (4.0–11.3) | −1.6% | −2.7% |
Absolute cumulative risk at 15 and 30 years after index date calculated using the Kaplan-Meier method. The estimates were adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline (index date), years of education (≤12, 13–16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). These adjustments were performed separately in each stratum to maximize the balance at baseline.
Risk difference calculated as the absolute risk estimate for women who underwent hysterectomy minus the absolute risk estimate for the referent women.
A total of 308 women with other non-cancer indications for hysterectomy were not included in the analyses stratified by indication.
Results of stratified analyses
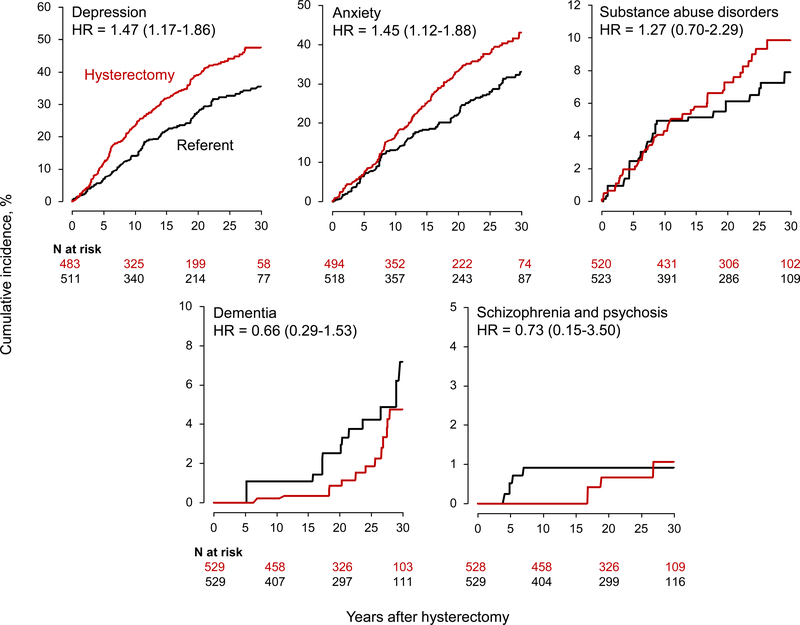
The increased risks of de novo depression and anxiety were more pronounced for women who underwent hysterectomy at age 18–35 years (Figure 2). In this younger age group, the risk of depression was increased nearly 50% (adjusted HR 1.47; 95% CI 1.17–1.86, Table 1), with a 12% ARI at 30 years compared with referent women (Table 3). The risk of anxiety was increased 45% (adjusted HR 1.45; 95% CI 1.12–1.88; Table 1), with a 10.1% ARI at 30 years compared with referent women (Table 3). The formal tests for interactions across the age strata were statistically significant for depression but not for the other mental health conditions (Table 1, footnote b).
Fig. 2.
Cumulative incidence curves for mental health conditions in women who underwent hysterectomy with ovarian conservation at age 18–35 years compared with referent women. The curves were adjusted using inverse probability weights derived from a logistic regression model restricted to this age stratum, and including all 20 chronic conditions present at baseline, years of education (≤12, 13–16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). The number of women at risk varied across conditions because we excluded women with the specific condition on the index date. Note the different scales used for the y-axis to better show differences.
The risk of depression was increased significantly in women who underwent hysterectomy for menstrual disorders (adjusted HR 1.30; 95% CI 1.03–1.63; Table 2) with a 7.4% ARI at 30 years compared with referent women (Table 3), but not for leiomyomas or prolapse. The risks of anxiety, substance abuse disorders, dementia, or schizophrenia did not differ by indication for the hysterectomy (Table 2). The formal tests of interaction by surgical indication were not statistically significant for any of the mental health conditions (Table 2, footnote c).
Results of sensitivity analyses
In the first set of sensitivity analyses excluding women with any of the 20 pre-existing chronic conditions at the index date, the risks of depression and anxiety were still increased in the hysterectomy with ovarian conservation group (n=1,204) compared with referent women (n=1,433; Supplemental Digital Content 4). Results stratified by age were similar to the results in the full cohort, with some of the more extreme risks found in the younger age stratum (1.5-fold increased risk for depression and 1.7-fold for anxiety, Supplemental Digital Content 4). Hysterectomy for menstrual disorders was associated with a 1.4-fold increase of both depression and anxiety (Supplemental Digital Content 4), but there was no association with other mental disorders. The second set of sensitivity analyses censoring women at the time of subsequent oophorectomy (both women with hysterectomy and referent women) or hysterectomy (referent women) showed results similar to the primary analyses (data not shown). Results were also similar to the primary analyses in the third set of sensitivity analyses including number of children as an adjustment variable, and in the fourth set of sensitivity analyses including the number of medical contacts during follow-up (data not shown).
DISCUSSION
Women who underwent hysterectomy with ovarian conservation had a higher long-term risk of de novo diagnosis of depression and anxiety compared to referent women who did not have a hysterectomy at index date. The HRs were significantly higher for women who had hysterectomy at a young age with ARIs between 10% and 12% at 30 years. By contrast, the risks of substance abuse disorders, dementia, and schizophrenia were not significantly increased.
Comparison with other studies
Our findings are consistent with findings from other cohort studies and national databases that assessed the long-term risks of anxiety and depression both before and after the hysterectomy.20–22 Depressive symptoms and anxiety in the immediate preoperative period may be caused by the gynecologic condition or by the concerns about the surgery itself. Vandyk et al. attempted to distinguish longer-term anxiety (persistent or chronic anxiety) from shorter-term anxiety, and found that women who had longer-term anxiety were more likely to develop depressive symptoms within 6 months after the surgery.40 Our study focused on long-term anxiety and depression occurring both before and after the hysterectomy. To avoid confusion with the short term anxiety or depressed mood that may have been associated with the gynecological condition or surgery itself, we obtained depression and anxiety diagnoses that women received at any time before or after the index date from their medical records.
Contrary to some previous studies, we did not observe a significantly increased risk of dementia following hysterectomy.8, 24 However, our power to detect a difference in the risk of dementia in these relatively young cohorts was limited. The overall age of the end of follow-up was approximately 62 years. A longer follow-up of these cohorts is needed to further address this association. Hysterectomy with ovarian conservation was associated with the risk of substance abuse disorders in the unadjusted analyses; however, the statistical significance was lost in adjusted analyses. Because the hysterectomies included in the study were performed before 2002, they preceded the recent trend in overutilization of opioid treatments after surgeries.27–29
Possible interpretations of the findings
The association between hysterectomy with ovarian conservation and depression or anxiety may be caused by a cause-effect inversion. It is possible that some women did suffer from anxiety, depression, or other mental health conditions, which contributed to the decision to undergo a hysterectomy (cause-effect inversion). Indeed, we reported in this study that depression and anxiety diagnosed before the index date were more common in women who underwent hysterectomy (Supplemental Digital Content 1). However, our primary analyses excluded women affected by depression at index date when considering de novo depression as the outcome. The same was done when considering de novo anxiety as the outcome. Finally, in our first set of sensitivity analyses, we removed all women with any of the 20 chronic conditions recommended by the DHHS, thus eliminating the possible overlap or misclassification of anxiety, depression, or other mental disorders diagnosed before the index date (Supplemental Digital Content 4). Therefore, our findings in the majority of women do not appear to be explained by a cause-effect inversion.
Some inherited genetic variants or epigenetic modifications, or some early life events or behaviors may have predisposed women to manifest gynecological symptoms that prompted the hysterectomy, and independently predisposed the women to develop depression or anxiety after hysterectomy.41, 42 In this scenario, the association would be explained by confounding by indication. In support of a confounding by indication hypothesis is the higher risk of depression in the subset of women who underwent hysterectomy for menstrual disorders compared to leiomyomas or uterine prolapse (Table 2). However, none of the interactions by indication were statistically significant.
Strong evidence against confounding by indication is provided by our sensitivity analyses restricted to the majority of women who did not have any of the 20 chronic conditions at baseline. In these sensitivity analyses, the HR was higher for menstrual disorders, but was increased also for leiomyomas and uterine prolapse. The formal test for interaction by indication was not significant (Supplemental Digital Content 4). Also against a confounding hypothesis is the significant trend of greater HRs with younger age at hysterectomy. This trend is similar to the trend observed for risk of depression, anxiety, and dementia after bilateral oophorectomy, in which there is a direct endocrine effect of the surgery.3, 4 If our findings were due to confounding they would not be expected to change with age.
Although our study, like any other observational study, cannot determine causality, the higher incidence of depression and anxiety for women who had a hysterectomy at younger age could indicate an impact of the surgery on ovarian function. Some studies have shown that ovarian function may decline after hysterectomy.5, 9, 10 There is speculation that the decline in ovarian function is mediated either by a decrease in collateral blood flow between the uterus and the ovaries, or by a paracrine effect of the uterus on the ovaries.5, 11–13 It is also possible that hysterectomy affects brain aging and endocrine aging through some yet unknown mechanisms that are not mediated by an ovarian effect.12–14
Higher parity has been associated with hysterectomy, with women bearing more than 3 children reporting higher hysterectomy rates than women with less children and nulliparous women.43 In our cohort, women who underwent hysterectomy had higher parity, and this difference was more pronounced in women who had hysterectomy at age 18–35 years (Supplemental Digital Content 2). Only 8% of women who underwent hysterectomy at age 18–35 were nulliparous, and we do not know whether they desired future fertility. Facing the decision to have a hysterectomy prior to the end of childbearing could increase depression for some women. However, adjustment for parity did not modify our results.
We have controlled or removed the possible confounders and biases to the extent possible; however, the observed associations may be explained by some residual or yet unknown confounding variable. In addition, because we considered several outcome conditions, and conducted stratified analyses and sensitivity analyses, some of the significant findings may be due to chance. If we hypothesize that all the biases and confounding effects have been removed, that the women have a 30-year follow-up, and that the associations are attributable to hysterectomy, we can estimate the number needed to harm (NNH; defined as the inverse of ARI). In women who underwent hysterectomy, the NNH was 15 for depression and 21 for anxiety. NNH was even lower for women who underwent hysterectomy at age 18–35 years: 8 for depression and 10 for anxiety.
Strengths and limitations
Important strengths of this study include the large sample size of women with direct medical record documentation of hysterectomy and bilateral ovarian conservation (n=2,094). The comprehensive nature of the medical records-linkage system also allowed for a significant amount of information to be collected, with no reliance on self-report of mental health diagnoses, hysterectomy, or oophorectomy. In addition, the longitudinal nature of REP allowed us to assess the chronic conditions present at baseline and provided a long follow-up period. We used inverse probability weights to balance the hysterectomy and the referent cohorts at baseline on potential confounders. These methods are a powerful way to bring observational studies closer in interpretation to randomized clinical trials when the intervention (in our case, hysterectomy) cannot be ethically or feasibly randomized.44, 45
There were some limitations to the study. First, the REP indices, although comprehensive, may have missed some diagnoses. This under-diagnosis is particularly important for diagnoses such as mental health disorders that carry a social stigma. Second, electronic records are subject to incorrect coding in daily practice, and over-diagnosis may also have occurred. However, to address the chance of over-diagnosis, two diagnostic codes separated by more than 30 days (or more than one year) were required. Third, several confounders including marital status, desire for future children, alcohol consumption, occupation, physical activity, and income level were not available from electronic abstraction and could not be included in our analyses. However, our adjusted models included education, race, age and calendar year at the index date, and 20 pre-existing chronic conditions. If any of the unmeasured potential confounders were associated with the adjustment variables, their effect may have been indirectly controlled. Fourth, despite the long follow-up of the two cohorts (median of approximately 20 years), the women were relatively young at the end of follow-up (median of approximately 62 years); therefore, the study was underpowered to detect dementia and schizophrenia or psychosis. Finally, our findings could be in part explained by a surveillance bias because women who underwent hysterectomy may have had more intense contacts with health care providers after the hysterectomy.46 However, when we truncated the follow-up at the first diagnosis of depression or anxiety, the length of follow-up and the density of medical contacts were virtually identical in women with or without hysterectomy.
CONCLUSIONS
Hysterectomy, even with conservation of both ovaries, is associated with an increased risk of long-term mental health conditions, primarily anxiety and depression. In a subset of women, these associations may be due to a confounding by indication in which pre-existing depression and anxiety or pre-existing risk factors may have played a role. However, there is growing evidence that in another subset of women, hysterectomy may have deleterious effects on brain aging and endocrine aging. These effects may be mediated by an effect of hysterectomy on the ovaries, or the uterus may have direct effects on the brain and on other organs or systems that are not mediated by the ovaries.5, 11–14 Further research is needed on the possible direct effects of hysterectomy on mental health and on instruments to screen women before hysterectomy to assess long-term risk.
Supplementary Material
Figure showing case-control analyses for the chronic conditions present at index date. The conditions are presented in decreasing order of magnitude of the odds ratio. pdf
Table showing case-control analyses for characteristics at index date in women who underwent hysterectomy and age-matched referent women, overall and in women age 18–35 years at index date. pdf
Table showing univariable analyses for the effects of potential confounders (age, calendar year, race, and education) on each of the mental health outcome conditions. pdf
Table showing cumulative incidence of mental health conditions among women who did not have any of the 20 chronic conditions at index date, overall and in strata by age at hysterectomy and by indication. Pdf
Acknowledgements:
The authors thank Sondra Buehler and Kristi Klinger for formatting the manuscript and Cathy Schleck for assistance with the creation of the cohort.
Sources of funding: This study was supported by the Office of Research on Women’s Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Building Interdisciplinary Research Careers in Women’s Health (BIRCWH, K12 HD065987–2), the National Institute on Aging (R01 AG034676 and U54 AG044170), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD060503). WAR was partly supported by the National Institutes of Health (R01 AG034676, R01 AG052425, U54 AG044170, U01 AG006786, and P01 AG004875).
The abstract was presented at the Society for Gynecologic Investigation Annual Meeting, Florence, Italy, March 2014
Financial disclosure/conflicts of interest: Dr. Stewart receives funding from Bayer. Dr. Laughlin-Tommaso consults for Allergan, has a research grant from Bayer, is on the DSMB for the ULTRA trial (Halt Medical), and receives royalties from UpToDate for fibroid articles.
Footnotes
For the remaining authors, none were declared.
REFERENCES
- 1.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007;110:1091–1095. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol 2013;122:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause 2018;25:1275–1285. [DOI] [PubMed] [Google Scholar]
- 4.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083. [DOI] [PubMed] [Google Scholar]
- 5.Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause 2018;25:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J 2011;32:745–750. [DOI] [PubMed] [Google Scholar]
- 7.Yeh JS, Cheng HM, Hsu PF, et al. Hysterectomy in young women associates with higher risk of stroke: a nationwide cohort study. Int J Cardiol 2013;168:2616–2621. [DOI] [PubMed] [Google Scholar]
- 8.Phung TK, Waltoft BL, Laursen TM, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 2010;30:43–50. [DOI] [PubMed] [Google Scholar]
- 9.Trabuco EC, Moorman PG, Algeciras-Schimnich A, Weaver AL, Cliby WA. Association of ovary-sparing hysterectomy with ovarian reserve. Obstet Gynecol 2016;127:819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG 2005;112:956–962. [DOI] [PubMed] [Google Scholar]
- 11.Stewart EA. Gonadotropins and the uterus: is there a gonad-independent pathway? J Soc Gynecol Investig 2001;8:319–326. [PubMed] [Google Scholar]
- 12.Lee SH, Lee SJ, Rho HJ. To the Editor. Menopause 2019;26:112. [DOI] [PubMed] [Google Scholar]
- 13.Laughlin-Tommaso SK, Smith CY, Weaver AL, Khan Z, Stewart EA, Rocca WA. In Reply. Menopause 2019;26:112–114. [DOI] [PubMed] [Google Scholar]
- 14.Koebele SV, Palmer JM, Hadder B, et al. Hysterectomy uniquely impacts spatial memory in a rat model: a role for the nonpregnant uterus in cognitive processes. Endocrinology 2019;160:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farquhar CM, Harvey SA, Yu Y, Sadler L, Stewart AW. A prospective study of 3 years of outcomes after hysterectomy with and without oophorectomy. Am J Obstet Gynecol 2006;194:711–717. [DOI] [PubMed] [Google Scholar]
- 16.Gibson CJ, Joffe H, Bromberger JT, et al. Mood symptoms after natural menopause and hysterectomy with and without bilateral oophorectomy among women in midlife. Obstet Gynecol 2012;119:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darwish M, Atlantis E, Mohamed-Taysir T. Psychological outcomes after hysterectomy for benign conditions: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2014;174:5–19. [DOI] [PubMed] [Google Scholar]
- 18.Rannestad T, Eikeland OJ, Helland H, Qvarnstrom U. The general health in women suffering from gynaecological disorders is improved by means of hysterectomy. Scand J Caring Sci 2001;15:264–270. [DOI] [PubMed] [Google Scholar]
- 19.Davies JE, Doyle PM. Quality of life studies in unselected gynaecological outpatients and inpatients before and after hysterectomy. J Obstet Gynaecol 2002;22:523–526. [DOI] [PubMed] [Google Scholar]
- 20.Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res 2015;68:186–191. [DOI] [PubMed] [Google Scholar]
- 21.Hickey M, Schoenaker DA, Joffe H, Mishra GD. Depressive symptoms across the menopause transition: findings from a large population-based cohort study. Menopause 2016;23:1287–1293. [DOI] [PubMed] [Google Scholar]
- 22.Wilson L, Pandeya N, Byles J, Mishra G. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women’s Health. Epidemiol Psychiatr Sci 2018;27:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares CN. Can depression be a menopause-associated risk? BMC Med 2010;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis 2012;10:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bove R, Secor E, Chibnik LB, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014;82:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocca WA, Henderson VW. Is there a link between gynecologic surgeries and Alzheimer disease? Neurology 2014;82:196–197. [DOI] [PubMed] [Google Scholar]
- 27.As-Sanie S, Till SR, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol 2017;130:1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA surgery 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol 2018;219:486.e481–486.e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-V. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 31.Blandon RE, Bharucha AE, Melton LJ 3rd, et al. Incidence of pelvic floor repair after hysterectomy: a population-based cohort study. Am J Obstet Gynecol 2007;197:664e661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melton LJ 3rd, Achenbach SJ, Gebhart JB, Babalola EO, Atkinson EJ, Bharucha AE. Influence of hysterectomy on long-term fracture risk. Fertil Steril 2007;88:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babalola EO, Bharucha AE, Schleck CD, Gebhart JB, Zinsmeister AR, Melton LJ 3rd. Decreasing utilization of hysterectomy: a population-based study in Olmsted County, Minnesota, 1965–2002. Am J Obstet Gynecol 2007;196:214e211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laughlin-Tommaso SK, Khan Z, Weaver AL, Schleck CD, Rocca WA, Stewart EA. Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation. Menopause 2016;23:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocca WA, Gazzuola Rocca L, Smith CY, et al. Cohort profile: the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) in Olmsted County, Minnesota (USA). BMJ Open 2017;7:e018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc 2016;91:1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen JW, Cohen SB, Banthin JS. The Medical Expenditure Panel Survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care 2009;47:S44–50. [DOI] [PubMed] [Google Scholar]
- 40.Vandyk AD, Brenner I, Tranmer J, Van Den Kerkhof E. Depressive symptoms before and after elective hysterectomy. J Obstet Gynecol Neonatal Nurs 2011;40:566–576. [DOI] [PubMed] [Google Scholar]
- 41.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab 1998;83:1875–1880. [DOI] [PubMed] [Google Scholar]
- 42.Weel AE, Uitterlinden AG, Westendorp IC, et al. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J Clin Endocrinol Metab 1999;84:3146–3150. [DOI] [PubMed] [Google Scholar]
- 43.Cooper R, Hardy R, Kuh D. Timing of menarche, childbearing and hysterectomy risk. Maturitas 2008;61:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010;15:234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med 2007;26:20–36. [DOI] [PubMed] [Google Scholar]
- 46.Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA 2011;305:2462–2463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure showing case-control analyses for the chronic conditions present at index date. The conditions are presented in decreasing order of magnitude of the odds ratio. pdf
Table showing case-control analyses for characteristics at index date in women who underwent hysterectomy and age-matched referent women, overall and in women age 18–35 years at index date. pdf
Table showing univariable analyses for the effects of potential confounders (age, calendar year, race, and education) on each of the mental health outcome conditions. pdf
Table showing cumulative incidence of mental health conditions among women who did not have any of the 20 chronic conditions at index date, overall and in strata by age at hysterectomy and by indication. Pdf