Abstract
The bed nucleus of the stria terminalis (BNST) is emerging as a critical region in multiple psychiatric disorders including anxiety, PTSD, and alcohol and substance use disorders. In conjunction with growing knowledge of the BNST, an increasing number of studies examine connections of the BNST and how those connections impact BNST function. The importance of this BNST network is highlighted by rodent studies demonstrating that projections from other brain regions regulate BNST activity and influence BNST-related behavior. While many animal and human studies replicate the components of the BNST network, to date, structural connections between the BNST and insula have only been described in rodents and have yet to be shown in humans. In this study, we used probabilistic tractography to examine BNST-insula structural connectivity in humans. We used two methods of dividing the insula: 1) anterior and posterior insula, to be consistent with much of the existing insula literature; and 2) eight subregions that represent informative cytoarchitectural divisions. We found evidence of a BNST-insula structural connection in humans, with the strongest BNST connectivity localized to the anteroventral insula, a region of agranular cortex. BNST-insula connectivity differed by hemisphere and was moderated by sex. These results translate rodent findings to humans and lay an important foundation for future studies examining the role of BNST-insula pathways in psychiatric disorders.
Keywords: Anterior Insula, BNST, DTI, structural connectivity, agranular insula
Introduction
The bed nucleus of the stria terminalis (BNST) has increasingly become a region of interest due to its proposed role in numerous psychiatric disorders including anxiety, post-traumatic stress disorder (PTSD), and alcohol and substance use disorders (Avery et al., 2016; Lebow and Chen, 2016; Shackman and Fox, 2016). The BNST is a small region in the basomedial forebrain connected to the amygdala through the stria terminalis. Over the last few decades, rodent studies established the BNST’s role in sustained negative affect, arousal, autonomic nervous system activation, social behaviors, hormone production, learning, and the generation and perpetuation of the stress response (for reviews see Ch’ng et al., 2018; Crestani et al., 2013; Davis et al., 2010). Early studies of human BNST function have linked altered BNST function or connectivity to social anxiety (Clauss et al., 2019), generalized anxiety disorder (Buff et al., 2017), anxiety disorders (Torrisi et al., 2019), panic disorder (Brinkmann et al., 2017a), and PTSD (Brinkmann et al., 2017b; Rabellino et al., 2018). Therefore, evidence from rodent and human studies converge to highlight the BNST as a critical region in psychiatric disorders (see Figure 1).
Figure 1:
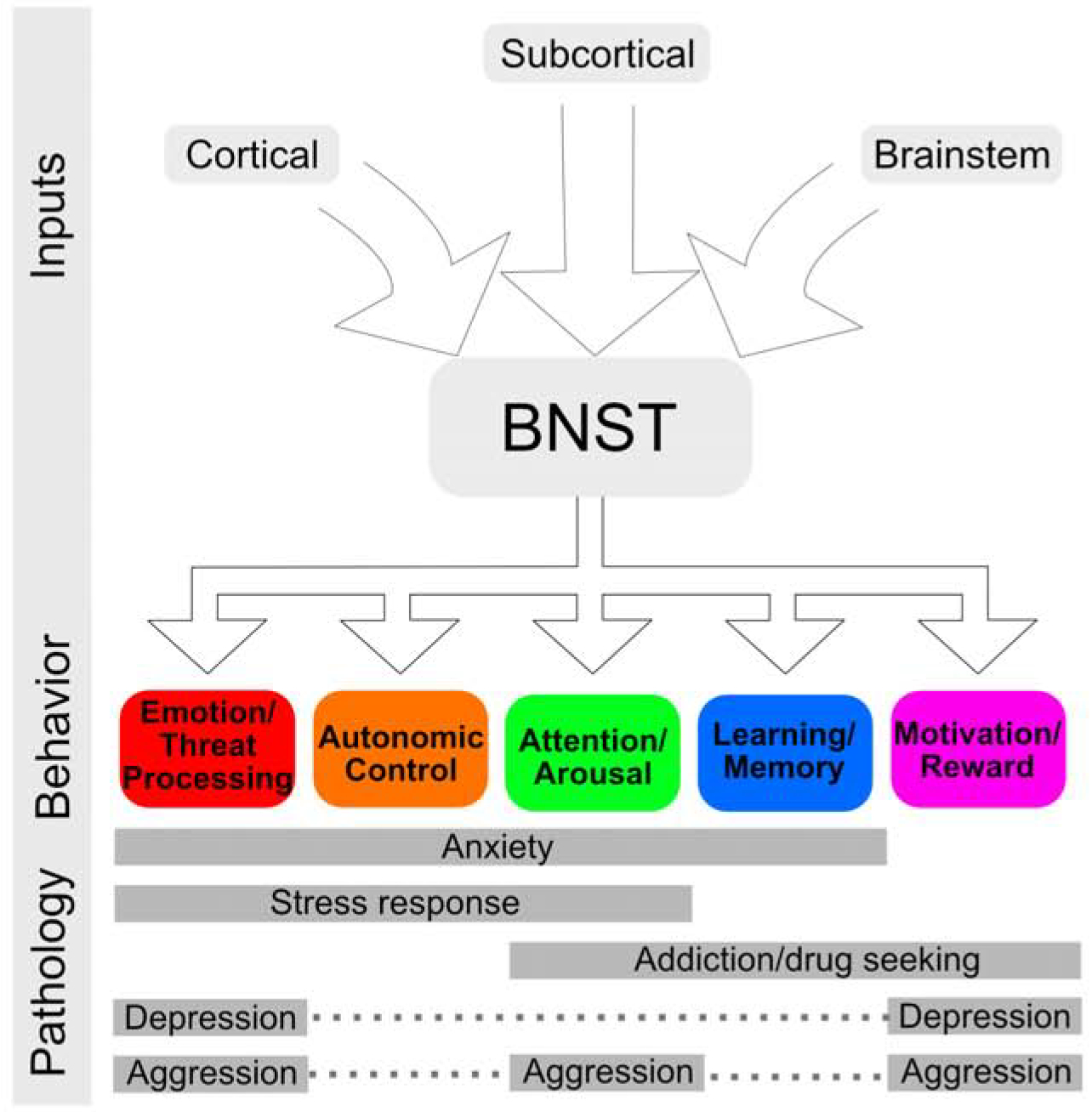
Overview of bed nucleus of the stria terminalis (BNST) inputs and role in behavior and pathology.
One emerging area of research investigates BNST connections with other brain regions, which is essential to understanding how the BNST functions as part of a network. Early human studies have replicated connectivity findings from rodent research, identifying a small number of brain regions with strong structural connections to the BNST, including the hippocampus, striatum, thalamus, and amygdala (Avery et al., 2014; Dong et al., 2001; Dong and Swanson, 2006; Krüger et al., 2015; Weller and Smith, 1982). Of these brain regions, the amygdala has been the most thoroughly investigated across species. For example, amygdala-BNST structural connectivity has been demonstrated in rodents (de Guglielmo et al., 2019; Reynolds and Zahm, 2005), non-human primates (Nauta, 1961; Novotny, 1977; Oler et al., 2017) and humans (Avery et al., 2014; Krüger et al., 2015). The successful translation of BNST-amygdala connectivity across species has opened the door to using animal findings to conduct hypothesis-directed DTI studies to uncover novel BNST connections in humans.
Established by rodent studies, an important brain region connected with the BNST is the insula—a large, heterogeneous structure located deep in the Sylvian fissure. Projections from the insula to the BNST have been shown in rodents, particularly from the anterior portion of the insula (e.g. Centanni et al., 2018; Reynolds and Zahm, 2005; Shin et al., 2008). These projections also have critical functional significance: a recent study reported that inhibiting anterior insula projections to the BNST decreases negative affect in rodents that were abstinent following chronic alcohol use (Centanni et al., 2019). A BNST-insula connection could also provide insight into other psychiatric disorders, as both the insula and BNST have been associated with substance use disorder, anxiety, and PTSD (e.g. Avery et al., 2016; Clauss et al., 2019; Faulkner et al., 2018; Figel et al., 2019; Rabellino et al., 2018; Terasawa et al., 2013). However, a critical first step is determining if a structural connection between the BNST and insula exists in humans.
Despite evidence from rodent models, it remains unknown whether the BNST and insula are structurally connected in humans. Several DTI studies have investigated insula connectivity (e.g. Cerliani et al., 2012; Cloutman and Lambon Ralph, 2012; Ghaziri et al., 2018, 2017; Nomi et al., 2018) or BNST connectivity (Avery et al., 2014; Krüger et al., 2015), yet a BNST-insula structural connection has not been reported. There are several potential explanations. First, most structural connectivity studies evaluate the insula as a single region. However, the insulais a large region with much heterogeneity that would be obscured by examining the insula as a whole. Due to this variation in insula anatomy, histology, and function (e.g. Nieuwenhuys, 2012), some studies divide the insula along an anatomical boundary, known as the central insular sulcus, to create an anterior and posterior insula (e.g. Ham et al., 2012). The anterior insula is primarily associated with cognition and emotion, and the posterior insula with sensory interoception (for reviews see Craig, 2010a; Gogolla, 2017). The anterior and posterior division, however, does not reflect the underlying cytoarchitecture of the insula. Human studies are therefore difficult to directly translate from the rodent literature, which primarily relies on cytoarchitecture (for reviews see Gogolla, 2017; Nieuwenhuys, 2012). More specifically, the anterior insula of the rodent is agranular cortex, a type of cortex seen in a small anteroventral region within the human anterior insula. Thus, known rodent connections between the BNST and insula could be harder to detect in humans when the insula is typically examined as a single region or divided into two or three subregions. Second, in insula connectivity studies, a BNST connection might have been overlooked due to neuroimaging advancements only recently permitting the evaluation of the BNST in humans (Avery et al., 2014; Krüger et al., 2015; Theiss et al., 2017; Torrisi et al., 2015). As a result, most in vivo imaging atlases don’t include the BNST, thus connectivity with the BNST would likely be missed or attributed to a neighboring brain region.
For the two BNST structural connectivity studies, the lack of a BNST-insula connection might result from the scope of the studies. Both studies characterized only the strongest structural connections of the BNST and would not have reported more modest connections. For example, the first BNST structural connectivity study identified the most highly connected brain regions using a standard method for segmenting the entire brain into regions (Avery et al., 2014). The second study defined the three major white matter pathways of the BNST and replicated BNST connections to many of the brain regions described in the initial study (Krüger et al., 2015). Together, these first human studies revealed the most robust structural connections of the BNST, uncovering a BNST network that provides a foundation for future studies. Moving forward, the BNST network in humans can be compared to what is already known from rodents, and discrepancies, such as the BNST-insula connection, can be investigated using specific, hypothesis-driven studies.
This study aimed to determine whether a BNST-insula structural connection exists in humans. To overcome the presented limitations of prior structural connectivity studies, we use a previously validated BNST mask and two methods for dividing the insula—an anterior/posterior insula division, for comparison with previous human studies, and subregion insula masks, to account for important cytoarchitectural divisions of the human insula. Based on previous studies in humans that show anterior insula connectivity with other limbic regions, we hypothesized that the anterior insula would have greater structural connectivity with the BNST relative to the posterior insula. We further hypothesized that within the anterior insula, the most anteroventral insula subregion (agranular cortex) would show the strongest connectivity, consistent with rodent studies (Reynolds and Zahm, 2005).
Methods
Participants
The current study used the diffusion tensor imaging (DTI) scans of 81 healthy controls. Participants were aged 18 – 57 years (mean ± SD = 30 ± 11 years), 46% female, and 83% right handed. The ethnicities of the participants were: 70% White/Caucasian, 22% Black/African-American, and 7% Asian. The original scans were collected as part of two ongoing studies and data from this sample has previously been published (Avery et al., 2014). The Vanderbilt University Institutional Review Board approved the studies, and written informed consent was obtained for each participant. Participants were eligible for the studies by meeting the following criteria: 1) no current or prior mental health disorders based on evaluation with the Structured Clinical Interview for the DSM IV; 2) no psychotropic medication use in the previous 6 months; and 3) high quality DTI data. Of the 89 participants with high quality DTI scans, 8 participants were excluded for excessive motion (> 5mm or 3 degrees of motion in any direction across the diffusion series), resulting in a final sample of 81 participants. Preprocessed scans were visually inspected for processing failures (e.g., skull stripping failure), and all failures at this stage were corrected.
Data Acquisition
Diffusion magnetic resonance image (MRI) data were acquired on two identical 3 Tesla Philips Achieva MRI scanners (Philips Healthcare, Inc.). Whole-brain diffusion weighted images were acquired using a pulsed-gradient spin echo, echo planar imaging (single-shot EPI) pulse sequence, and the following parameters: 96 × 96 matrix; voxel size = 2.5 mm isotropic; number of slices = 50; TE = 65 ms; TR = 8.5 s; SENSE acceleration factor = 2. 92 diffusion directions were acquired with a b value of 1600 s/mm2 and one T2-weighted volume with a b value of 0 s/mm2. High resolution T1-weighted anatomical images were collected with the following parameters: FOV = 256 mm; number of slices = 170; voxel size = 1 mm isotropic; gap = 0 mm.
Data Processing
The diffusion-weighted images were preprocessed and analyzed using FMRIB Software Library (FSL, version 5.0; Oxford Centre for Functional MRI of the Brain (FMRIB),UK; http://www.fmrib.ox.ac.uk/fsl/) and Matlab (Version R2018a, The MathWorks, Inc, Natick, MA). First, Eddy Current Correct from the FMRIB FSL toolbox was applied to correct for motion and eddy current distortions (Andersson et al., 2003). The brain extraction tool from the FMRIB FSL toolbox (Smith, 2002) was used to remove the non-brain tissue from the image. DTIFIT was then applied to align the diffusion tensors to the skull-extracted, eddy-corrected images. Finally, BEDPOSTX was used to estimate the diffusion of each voxel (samples = 5000), including the possibility of multiple crossing fibers.
Seed-based probabilistic tractography was used to determine the degree of structural connectivity between the insula and the BNST, with the BNST mask as a seed and the insula masks as targets. Only connections between ipsilateral BNST and insula subregions were evaluated, as previous rodent tracer studies have demonstrated consistent but more substantial ipsilateral, relative to contralateral, BNST connections with cortical regions (Coolen and Wood, 1998; McDonald et al., 1999; Sun et al., 1991; Wood and Swann, 2005). Therefore, for each participant, BNST connectivity values from both the left and right hemispheres were obtained for the 1) whole insula, 2) anterior and poster insula, and 3) insula subregions (see details below).
Region of Interest Masks
The BNST mask used for this study has previously been validated (for details see Avery et al., 2014). For the insula, we used previously published subregions masks (Figure 2) that were developed to account for insula anatomy and cytoarchitecture (Farb et al., 2013). Additionally, the subregion masks were combined to form anterior and posterior masks, using the central sulcus of the insula as the division between the anterior and posterior insula (Figure 2). The anterior mask consisted of the dorsal accessory gyrus (ACd), dorsal anterior short gyrus (ASd), middle short gyrus (MS), posterior short gyrus (PS), ventral anterior short gyrus (ASv), and ventral accessory gyrus (ACv) subregions. The posterior mask consisted of the anterior long gyrus (AL) and posterior long gyrus (PL) subregions. Finally, all subregions were combined to create a whole insula mask. For each participant, all masks were transformed into participant space and reviewed in native space to evaluate for anatomical accuracy.
Figure 2:

Divisions of the insula. Blue subregions (AL and PL) are posterior insula; all others are anterior insula. ACd = dorsal accessory gyrus; ACv = ventral accessory gyrus; ASd = dorsal anterior short gyrus; ASv = ventral anterior short gyrus; MS = middle short gyrus; PS = posterior short gyrus; AL = anterior long gyrus; PL = posterior long gyrus.
Voxel-based Connectivity
As this study is the first to investigate insula connectivity with the BNST, we also used a voxel-based approach to provide an illustration of the overall pattern of BNST connectivity within the insula. A voxel-based approach allows for the evaluation of connectivity patterns without the apriori anatomical boundaries set by regions of interest.
Validation Analysis
Three validation analyses were performed: 1) we evaluated whether the probabilistic tractography streamlines went through the expected white matter tracts; 2) we compared the streamlines for the expected white matter tracts relative to a neighboring white matter tract; and 3) we compared the BNST-insula results to connectivity between the BNST and both a positive and negative control region.
First, the pathway of BNST-insula connectivity has not been described in detail, but based on the available evidence (Nachtergaele et al., 2019; Schmahmann and Pandya, 2006) the most likely white matter tract is the extreme capsule, which runs medial to the insula (Nachtergaele et al., 2019; Schmahmann and Pandya, 2006). To assess whether the observed BNST-insula tracts ran through the extreme capsule, we performed a region of interest analysis. The extreme capsule regions of interest were created by manually tracing the white matter path in FSL according to theirs known anatomical location based on the Atlas of the Human Brain (Mai et al., 2015). Tracings were performed by EF and confirmed by JUB. Each participant’s probabilistic tractography map was thresholded for error (< 1%), warped to MNI space, and averaged into a group map. The group map was thresholded at > 50% overlap and masked with the extreme capsule masks.
Next, we compared probabilistic tractography streamlines between the extreme capsule and the external capsule, a white matter path that is close in proximity to the extreme capsule, to determine specificity. For the external capsule, we used the JHU atlas (Hua et al., 2008; Mori et al., 2005; Wakana et al., 2007) external capsule mask. Because the external and extreme capsules are in close proximity, we subtracted the extreme capsule mask from the external capsule mask to create independent masks. Each mask was transformed into native space, and the number of streamlines in the extreme versus external capsule masks were compared for each participant.
Finally, the BNST-insula connectivity values were compared with connectivity between the BNST and a positive control region and a negative control region. The positive and negative control regions were selected from previously published BNST structural connectivity data (Avery et al., 2014), with the central amygdala (CeA) as the positive control region and medial frontal gyrus (MFG) as the negative control region. The values from the positive and negative control regions also provide an estimate of the relative strength of the BNST-insula findings.
Statistical analyses
The effect of hemisphere was examined, as previous studies have shown connectivity differences between hemispheres (e.g. Baur et al., 2013; Gorka et al., 2017; Moran-Santa Maria et al., 2015; Onay et al., 2017; Ray et al., 2010). The effect of biological sex was examined because studies have reported sex differences in both the insula and BNST (e.g. Allen and Gorski, 1990; Avery et al., 2014; Chung et al., 2002; Lotze et al., 2019; Ruigrok et al., 2014).
To determine whether BNST connectivity differed between the anterior and posterior insula, a linear mixed model was performed with region (anterior/posterior), hemisphere (right/left), and sex (male/female) as fixed factors and participant as a random factor. Second, to determine whether BNST connectivity differed between insula subregions, a linear mixed model analysis was performed with subregion (ACd/ASd/MS/PS/AL/PL/ASv/ACv), hemisphere (left/right), and sex (male/female) as fixed factors and participant as a random factor. For both the linear mixed models, post-hoc analyses were used to explore significant interactions.
To determine the relative strength of BNST-insula connectivity, the BNST connectivity values to all insula masks were compared to BNST connectivity with a positive control (central amygdala) and a negative control (MFG) using t-tests. Effect sizes (Cohen’s d) were also calculated. For the relative strength analyses, a Bonferroni adjustment was used to control for multiple comparisons (adjusted values: anterior/posterior α = 0.025 (or 0.05/2), subregion α = 0.00625 (or 0.05/8)).
Statistical analyses were performed using R (R Core Team, 2017) with the lme4 (Bates et al., 2015) package for the linear mixed models and the emmeans (Lenth, 2019) package for post-hoc analysis.
Results
BNST Connectivity by Insula Region (Anterior vs Posterior)
BNST connectivity differed significantly by insula region (p < 0.001, Figure 3), with greater connectivity for the anterior relative to posterior insula. There was also a sex by region interaction (p < 0.05; Figure 3). The anterior and posterior difference reflected similarly strong anterior insula connectivity between males and females (p = 0.92) but lower posterior insula connectivity in males than female (p = 0.03). Greater anterior than posterior insula connectivity was driven by a significant region effect in males (p < 0.001) but not females (p = 0.78).
Figure 3:

Values of anterior and posterior insula structural connectivity with the BNST by sex. Greater BNST-anterior vs posterior insula connectivity in males (*** p < 0.001). Connectivity values are the log transformed number of streamlines between the BNST and each insula region.
BNST Connectivity by Insula Subregion
For the analysis of insula subregions, the main effects of subregion (p < .001) and hemisphere (p < 0.001) were qualified by subregion x hemisphere interaction (p = .008). Figure 4 shows the mean BNST connectivity results for each insula subregion by hemisphere. In both hemispheres, the ventral accessory gyrus (ACv), in the anteroventral insula, showed significantly greater BNST connectivity than all other subregions (all p < .001). Post-hoc comparisons of all subregion pairs are shown in Table 1. A post-hoc analysis of the main effect of hemisphere demonstrated hemispheric differences in BNST connectivity for insula dorsal accessory, dorsal anterior short, and ventral anterior short subregions (p = 0.02, 0.007, and 0.02 respectively, all left > right). There were also significant interactions of sex by subregion (p = .003) and sex by hemisphere (p = .05). For the post-hoc analysis of sex differences by subregion, none of the individual subregions showed significant sex differences, although the difference in mean values differed by subregion (see Supplement Figure 1). For the post-hoc analysis of sex differences by hemisphere, there was greater BNST connectivity in males in the left insula compared to the right (p < 0.001) but no hemisphere difference for females (p = 0.11).
Figure 4:

Values of BNST structural connectivity by insula subregion. Connectivity values are the log transformed number of streamlines between the BNST and each insula subregion. Note: ACd = dorsal accessory gyrus; ACv = ventral accessory gyrus; ASd = dorsal anterior short gyrus; ASv = ventral anterior short gyrus; MS = middle short gyrus; PS = posterior short gyrus; AL = anterior long gyrus; PL = posterior long gyrus.
Table 1:
BNST connectivity values comparing insula subregion by hemisphere
| Left Hemisphere | Right Hemisphere | |||
|---|---|---|---|---|
| Subregion comparison | Z score | p value | Z score | p value |
| ACd vs ASd | −3.00 | 0.01 | −3.21 | 0.01 |
| ACd vs MS | −4.75 | <0.001 | −1.10 | 0.54 |
| ACd vs PS | −7.93 | <0.001 | −5.64 | <0.001 |
| ACd vs AL | −8.17 | <0.001 | −3.17 | 0.01 |
| ACd vs PL | 1.12 | 0.53 | 4.42 | <0.001 |
| ACd vs ASv | 3.22 | 0.009 | 2.96 | 0.03 |
| ACd vs ACv | 8.08 | <0.001 | 8.46 | <0.001 |
| ASd vs MS | −1.75 | 0.24 | 2.11 | 0.18 |
| ASd vs PS | −4.94 | <0.001 | −2.43 | 0.095 |
| ASd vs AL | −5.17 | <0.001 | 0.039 | 0.97 |
| ASd vs PL | 4.11 | <0.001 | 7.63 | <0.001 |
| ASd vs ASv | 6.22 | <0.001 | 6.17 | <0.001 |
| ASd vs ASv | 11.07 | <0.001 | 11.67 | <0.001 |
| MS vs PS | −3.19 | 0.009 | −4.54 | <0.001 |
| MS vs AL | −3.42 | 0.005 | −2.07 | 0.18 |
| MS vs PL | 5.87 | <0.001 | 5.52 | <0.001 |
| MS vs ASv | 7.97 | <0.001 | 4.06 | <0.001 |
| MS vs ACv | 12.82 | <0.001 | 9.56 | <0.001 |
| PS vs AL | −0.23 | 0.81 | 2.47 | 0.10 |
| PS vs PL | 9.05 | <0.001 | 10.06 | <0.001 |
| PS vs ASv | 11.16 | <0.001 | 8.60 | <0.001 |
| PS vs ACv | 16.01 | <0.001 | 14.10 | <0.001 |
| AL vs PL | 9.28 | <0.001 | 7.59 | <0.001 |
| AL vs ASv | 11.39 | <0.001 | 6.13 | <0.001 |
| AL vs ACv | 16.24 | <0.001 | 11.63 | <0.001 |
| PL vs ASv | 2.11 | 0.14 | −1.46 | 0.43 |
| PS vs ACv | 6.96 | <0.001 | 4.04 | <0.001 |
| ASv vs ACv | 4.85 | <0.001 | 5.50 | <0.001 |
Note: ACd = dorsal accessory gyrus; ACv = ventral accessory gyrus; ASd = dorsal anterior short gyrus; ASv = ventral anterior short gyrus; MS = middle short gyrus; PS = posterior short gyrus; AL = anterior long gyrus; PL = posterior long gyrus.
To further explore BNST connectivity patterns, we performed a voxel-wise connectivity analysis across the insula (Figure 5). Voxel-wise analysis were consistent with subregion analysis; the strongest connectivity was observed in the ventral accessory gyrus, located in the anteroventral insula.
Figure 5:

Heatmap of insula-BNST structural connectivity. The heatmap was masked by the whole insula and thresholded at 80% to display the most connected voxels. The first two rows (x = 45 and x = 37) are right hemisphere and bottom two rows (x = −28 and x = −34) are left hemisphere.
Validation Analyses
We evaluated the group tractography map to determine if the tracts between the insula and BNST went through the extreme capsule. The tractography maps had a 99.69% overlap with the left extreme capsule and a 99.78% overlap with the right extreme capsule. At the individual participant level, 100% of participants had streamlines through the left and right extreme capsules.
Given the proximity of the external and extreme capsules, we evaluated the maximum number of streamlines though both the external and extreme capsule masks. 93% of participant maps (94% right hemisphere; 91% left hemisphere) had a greater maximum number of streamlines in the extreme capsule compared to the external capsule, providing some evidence for specificity to the extreme capsule.
To provide a relative estimate of the strength of the observed BNST-insula connections, we compared BNST connectivity values to a negative control region (MFG) and a positive control region (CeA). Data for all comparisons and effect sizes are provided in Table 2. For the insula regions (anterior, posterior), the left anterior insula showed significantly greater BNST connectivity than the MFG (p < .001). All BNST-insula regions were significantly less connected than the BNST-amygdala positive control (p < .001). For the insula subregions, left ventral anterior short, left ventral accessory, and right ventral accessory subregions showed significantly greater BNST connectivity compared to the MFG (p < .01). All insula subregions showed significantly less BNST connectivity than the CeA (p < 0.001).
Table 2:
Structural connectivity of insula regions to BNST compared to Central Amygdala-BNST and Medial Frontal Gyrus-BNST by hemisphere
| Central Amygdala Positive Control | Medial Frontal Gyrus Negative Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Subregion | Left Hemisphere | Right Hemisphere | Left Hemisphere | Right Hemisphere | ||||
| t | d | t | d | t | d | t | d | |
| ACd | 21.78* | 2.99 | 20.93* | 2.59 | −1.86 | −0.25 | 0.69 | 0.11 |
| ASd | 25.61* | 3.41 | 21.09* | 2.52 | 0.05 | 0.0074 | 2.24 | 0.34 |
| MS | 26.07* | 3.59 | 23.22* | 2.94 | 1.20 | 0.18 | 1.35 | 0.23 |
| PS | 26.20* | 3.76 | 25.33* | 3.31 | 3.09 | 0.44 | 3.78 | 0.62 |
| AL | 27.08* | 3.94 | 23.39* | 3.17 | 3.31 | 0.45 | 2.41 | 0.39 |
| PL | 21.36* | 3.34 | 20.43* | 2.79 | −2.60 | −0.34 | −1.78 | −0.26 |
| ASv | 22.79* | 3.04 | 20.60* | 2.81 | −4.43* | −0.59 | −0.92 | −0.15 |
| ACv | 17.63* | 2.27 | 16.57* | 2.05 | −7.52* | −0.92 | −3.90* | −0.58 |
| Anterior | 24.17* | 3.34 | 21.20* | 2.71 | −4.31* | −0.56 | −1.69 | −0.27 |
| Posterior | 23.58* | 3.74 | 22.63* | 3.18 | −0.87 | −0.12 | −0.87 | −0.14 |
Note: T values (t) and effect size (d) are reported, negative values indicate that insula-BNST connectivity > control-BNST connectivity;
= p < 0.00625 (subregions) or p < 0.025 (anterior/posterior). ACd = dorsal accessory gyrus; ACv = ventral accessory gyrus; ASd = dorsal anterior short gyrus; ASv = ventral anterior short gyrus; MS = middle short gyrus; PS = posterior short gyrus; AL = anterior long gyrus; PL = posterior long gyrus.
Discussion
The results of this study provide compelling evidence for a structural connection between the BNST and insula in humans. Using anterior-posterior divisions of the insula, the BNST had stronger connectivity with the anterior relative to the posterior insula. More specific subregion analysis identified the anteroventral portion of the insula as the most connected to the BNST bilaterally. Sex moderated several findings; BNST connectivity in males differed by both region (anterior > posterior) and hemisphere (left > right). Compared to control regions, the BNST has low levels of connectivity with most of the insula but was robustly connected with the left anterior insula, the left anteroventral insula (ventral accessory gyrus and ventral anterior short gyrus) and the right anteroventral insula (ventral accessory gyrus). To our knowledge, this is the first study to identify BNST-insula structural connectivity in humans— a critical first step to investigating a role for BNST-insula connectivity in psychiatric disorders.
The structural connection between the BNST and insula likely has functional relevance, as the BNST and insula are involved in many of the same neural processes. The BNST and anterior insula are both involved in emotion, feeding behaviors, attention, and autonomic and threat processing (for reviews see Craig, 2010; Crestani et al., 2013; Davis et al., 2010; Menon and Uddin, 2010). A number of rodent studies have shown anterior insula projections to the BNST (e.g. Centanni et al., 2019; Reynolds and Zahm, 2005), with less evidence for reciprocal connections (Dong and Swanson, 2006) suggesting largely unidirectional flow of information from the anterior insula to the BNST with possible, weak feedback from the BNST to the anterior insula. The weak feedback from the BNST to the insula was shown to project from the dorsomedial portion of the BNST, a region associated with integrating social information and influencing stress, mood, and reward circuitry (Lebow and Chen, 2016). Thus, feedback could serve to fine-tune the anterior insula’s integrated emotional response to incoming stimuli. Provided this directionality is conserved across species, the human anterior insula likely alters BNST activity. In humans the anterior insula has been associated with emotional regulation, suggesting that inputs from the insula to the BNST could initiate behavioral responses that translate emotional states into BNST-modulated behavioral changes including fight or flight or changes in motivated behaviors. Thus, our findings represent a first step towards translating rodent research of BNST-anterior insula connectivity in humans, which could have significant impact in our understanding of stress, anxiety, and addiction.
The strongest BNST structural connectivity was in the anteroventral insula, consistent with what is known about the cytoarchitecture of the human insula and findings from rodent research. The human insula transitions in an anteroventral-posterodorsal gradient from agranular, to dysgranular, to granular cortex (Nieuwenhuys, 2012), with agranular referring to a lack of the granular layer of cortex (layer IV). Thus, the anteroventral portion of the insula is the location of the insula’s agranular cortex in humans. The human agranular insula is a small portion of the anterior insula, especially when compared to rodents in which the anterior insula is primarily agranular. Using a subregion that approximates the agranular cortex, in this case, the ventral accessory gyrus, allows for a more cytoarchitecturally accurate comparison to rodent studies of the anterior insula. The anteroventral insula results from our study recapitulate rodent studies demonstrating the greatest BNST structural connection with the agranular insula (Reynolds and Zahm, 2005). Functionally, the anteroventral insula has been implicated in emotional regulation and processing (for review see Klein et al., 2013) and the BNST is associated with anxiety and threat processing (for review see Davis et al., 2010). Thus, a structural connection between the BNST and anteroventral insula is also supported by a strong functional homology. Other studies specifically comparing ventral and dorsal anterior insula connectivity show selective connectivity between the ventral anterior insula and other limbic regions (e.g. Ghaziri et al., 2018; Nomi et al., 2018, 2016). This is also supported by studies in non-human primates where the agranular insula is structurally connected to other limbic regions (Augustine, 1996; Carmichael and Price, 1995). In summary, our results of a structural connection between the BNST and agranular insula in humans are a critical first step for translating rodent research with potential clinical impact.
Our findings of sex and hemispheric differences in connectivity are consistent with previous literature demonstrating sex differences and laterality in both the BNST and insula. Specifically, we found BNST structural connectivity with the posterior insula was greater in women compared to men. Although structural connectivity between the BNST and insula has not been examined previously, sex differences in both the BNST and insula have been demonstrated. One BNST study in humans found that over 70% of brain regions examined had greater BNST structural connectivity in females compared to males (Avery et al., 2014). Further, the BNST contributes to multiple sexually dimorphic functions including aggressive behavior, sexual behavior, and gonadotropin hormones secretion and in humans has greater volume in males than females (Allen and Gorski, 1990; Chung et al., 2002). For the insula, sex differences have been demonstrated in grey matter (e.g. Lotze et al., 2019; Ruigrok et al., 2014), resting state connectivity (e.g. Hong et al., 2014), and task-based functional connectivity (e.g. Macey et al., 2016; Moriguchi et al., 2014). Similarly for laterality, a diverse set of studies have reported hemispheric differences of the BNST (for examples see Avery et al., 2014; Gorka et al., 2017; Klumpers et al., 2017) and insula (for examples see Duerden et al., 2013; Menon and Uddin, 2010; Santangelo et al., 2019), though laterality is not always investigated, reported, nor found. Important next steps will be to replicate and expand on our sex and laterality differences in future studies. When evaluated in the context of these previous studies, the results of the current study emphasize the importance of continuing to consider sex and hemispheric differences in studies involving the BNST or insula.
Several limitations should be noted. First, the subregion masks used for this study were the result of combining anatomical and cytoarchitectural information, allowing us to isolate a subregion in the anteroventral region that approximated the agranular insula. While these subregions permitted a better comparison to rodent models, little consensus exists on the best way to divide the insula and using different subregions would likely influence the findings. Our results suggest that the anterior insula connectivity is likely driven by the ventral portion of the anterior insula, indicating that subdivisions beyond anterior and posterior insula are critical to accurately reflect insula heterogeneity. Second, the observed BNST-insula white matter connections were modest in strength. Likely the BNST-insula connection represents a smaller white matter tract compared to the major BNST white matter pathways previously found in humans: the stria terminalis, anterior pathway, and posterior pathway (Krüger et al., 2015). Therefore, replicating and extending our findings in other samples will be critical next steps. Third, DTI findings are not directional, meaning we are only able to hypothesize the directionality of the BNST-insula connection from rodent studies. Fourth, our findings suggest that the pathway uses the extreme capsule and, to a lesser extent, the external capsule; however, complete segregation of these pathways is challenging at this resolution and should be validated in future studies and animal models. Finally, while the sample size was larger than many previous structural connectivity studies, the sample sizes for males and females were smaller; thus, the sex interactions should be interpreted with caution and will need to be replicated in future studies.
The results of this study prompt interesting future directions. First, these findings suggest that future studies of the BNST networks should include the anterior insula, in addition to other key brain regions like the amygdala and hippocampus. Second, these results add to a growing literature illustrating the need to divide the insula into smaller subregions to better reflect the insula’s structural and functional heterogeneity. Research in other brain regions has benefitted from subdividing large areas of cortex, such as the prefrontal cortex and cingulate cortex. However, more work is needed to reach a consensus on the most appropriate way to divide the insula. Third, as the first study to demonstrate BNST-insula connectivity in humans, validation in future studies will be critical. A promising opportunity for validation and extension of these findings could be the use of a large, publicly available, diffusion data set with state-of-the-art scanners and protocols such as the Human Connectome Project (Van Essen et al., 2013). In addition, similarities between the cytoarchitecture of the human and non-human primate insula make non-human primates a compelling animal model to further investigate the BNST-insula connection. Finally, important next steps will be to examine BNST-insula connectivity alterations in clinical populations, including anxiety disorders and substance use disorders. Given recent rodent findings that insula projections to the BNST mediate negative affect and drug seeking behaviors, BNST-insula connectivity in humans has exciting potential clinical implications.
Supplementary Material
Supplemental Fig. 1. BNST connectivity values for left and right insula subregions by sex. Connectivity values are number of streamlines that has been log transformed to a normal distribution.
Acknowledgements
Research reported in this work was supported in part by funding from the National Institute on Alcohol Abuse and Alcoholism (R21AA025385, JUB and DGW), the National Institute of Mental Health (K01-MH083052, JUB; T32MH018921), Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1- TR000445 from the National Center for Research Resources/NIH), Vanderbilt Medical Scientist Training Program (through grant T32GM007347 from the National Institute of General Medical Sciences), the Vanderbilt Psychiatric Genotype/Phenotype Project, and the Charlotte and Donald Test Fund.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No authors have competing interests to declare. Declaration of interests: none.
References
- Allen LS, Gorski RA, 1990. Sex difference in the bed nucleus of the stria terminalis of the human brain. J. Comp. Neurol 302, 697–706. 10.1002/cne.903020402 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J, 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888. [DOI] [PubMed] [Google Scholar]
- Augustine JR, 1996. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22 10.1016/S0165-0173(96)00011-2 [DOI] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU, 2016. The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology 41, 126–141. 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU, 2014. BNST neurocircuitry in humans. Neuroimage 91, 311–323. 10.1016/j.neuroimage.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Machler M, Bolker B, Walker S, 2015. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw 67, 1–48. [Google Scholar]
- Baur V, Hänggi J, Langer N, Jäncke L, 2013. Resting-State Functional and Structural Connectivity Within an Insula-Amygdala Route Specifically Index State and Trait Anxiety. Biol. Psychiatry 73, 85–92. https://doi.org/10.1016Zj.biopsych.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Tupak SV, Becker MPI, Herrmann MJ, Straube T, 2017a. Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychol. Med 1–14. https://doi.org/ DOI: 10.1017/S0033291717001192 [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Neumeister P, Tupak SV, Becker MPI, Herrmann MJ, Straube T, 2017b. Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Hum. Brain Mapp 38, 2190–2205. 10.1002/hbm.23513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buff C, Brinkmann L, Bruchmann M, Becker MPI, Tupak S, Herrmann MJ, Straube T, 2017. Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Soc. Cogn. Affect. Neurosci 12, 1766–1774. 10.1093/scan/nsx103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL, 1995. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol 363, 615–641. 10.1002/cne.903630408 [DOI] [PubMed] [Google Scholar]
- Centanni SW, Morris BD, Luchsinger JR, Bedse G, Fetterly TL, Patel S, Winder DG, 2019. Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology 44, 526–537. 10.1038/s41386-018-0257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JCW, Nanetti L, Crippa A, Gazzola V, D’Arceuil H, Keysers C, 2012. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum. Brain Mapp 33, 2005–2034. 10.1002/hbm.21338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng S, Fu J, Brown RM, McDougall SJ, Lawrence AJ, 2018. The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog. Neuro-Psychopharmacology Biol. Psychiatry 87, 108–125. [DOI] [PubMed] [Google Scholar]
- Chung WCJ, De Vries GJ, Swaab DF, 2002. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J. Neurosci 22, 1027–1033. 10.1523/JNEUROSCI.22-03-01027.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Benningfield MM, Blackford JU, 2019. Social anxiety is associated with BNST response to unpredictability. Depress. Anxiety 36, 666–675. 10.1002/da.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Lambon Ralph MA, 2012. Connectivity-based structural and functional parcellation of the human cortex using diffusion imaging and tractography. Front. Neuroanat 6, 34 10.3389/fnana.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Wood RI, 1998. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: Simultaneous anterograde and retrograde tract tracing. J. Comp. Neurol 399, 189–209. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2010. The sentient self. Brain Struct. Funct 1–15. 10.1007/s00429-010-0248-y [DOI] [PubMed] [Google Scholar]
- Crestani CC, Alves F, Gomes F, Resstel L, Corrêa FMA, Herman J, 2013Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr. Neuropharmacol 11, 141–159. 10.2174/1570159X11311020002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C, 2010. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Pomrenze MB, Crawford EF, Simpson S, Schweitzer P, Koob GF, Messing RO, George O, 2019. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat. Commun 10, 1238 10.1038/s41467-019-09183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW, 2001. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Rev 38, 192–246. 10.1016/S0165-0173(01)00079-0 [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW, 2006. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: Implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J. Comp. Neurol 494, 75–107. 10.1002/cne.20790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Arsalidou M, Lee M, Taylor MJ, 2013. Lateralization of affective processing in the insula. Neuroimage 78, 159–175. https://doi.org/10.1016Zj.neuroimage.2013.04.014 [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK, 2013. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb. Cortex 23, 114–126. 10.1093/cercor/bhr385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P, Ghahremani DG, Tyndale RF, Paterson NE, Cox C, Ginder N, Hellemann G, London ED, 2019. Neural basis of smoking-induced relief of craving and negative affect: Contribution of nicotine. Addict. Biol 24, 1087–1095. 10.1111/adb.12679 [DOI] [PubMed] [Google Scholar]
- Figel B, Brinkmann L, Buff C, Heitmann CY, Hofmann D, Bruchmann M, Becker MPI, Herrmann MJ, Straube T, 2019. Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. NeuroImage. Clin 22, 101735 10.1016/j.nicl.2019.101735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Boucher O, Houde J-C, Descoteaux M, Obaid S, Gilbert G, Rouleau I, Nguyen DK, 2018. Subcortical structural connectivity of insular subregions. Sci. Rep 8, 8596 10.1038/s41598-018-26995-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Houde J-C, Boucher O, Gilbert G, Descoteaux M, Lippe S, Rainville P, Nguyen DK, 2017. The Corticocortical Structural Connectivity of the Human Insula. Cereb. Cortex 27, 1216–1228. 10.1093/cercor/bhv308 [DOI] [PubMed] [Google Scholar]
- Gogolla N, 2017. The insular cortex. Curr. Biol 27, R580–R586. 10.1016/j.cub.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Gorka AX, Torrisi S, Shackman AJ, Grillon C, Ernst M, 2017. Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroimage 1–11. 10.1016/j.neuroimage.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham TE, de Boissezon X, Leff A, Beckmann C, Hughes E, Kinnunen KM, Leech R, Sharp DJ, 2012. Distinct frontal networks are involved in adapting to internally and externally signaled errors. Cereb. Cortex 23, 703–713. 10.1093/cercor/bhs056 [DOI] [PubMed] [Google Scholar]
- Hong J-Y, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, Smith SR, Tillisch K, Naliboff B, Mayer EA, 2014. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J. Neurosci 34, 14252–14259. 10.1523/JNEUR0SCI.1683-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S, 2008. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 39, 336–347. https://doi.org/10.1016Zj.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Ullsperger M, Danielmeier C, 2013. Error awareness and the insula: links to neurological and psychiatric diseases. Front. Hum. Neurosci 7, 14 10.3389/fnhum.2013.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Kroes MCW, Baas JMP, Fernández G, 2017. How human amygdala and bed nucleus of the stria terminalis may drive distinct defensive responses. J. Neurosci 37, 9645–9656. 10.1523/JNEUR0SCI.3830-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger O, Shiozawa T, Kreifelts B, Scheffler K, Ethofer T, 2015. Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex 66, 60–68. [DOI] [PubMed] [Google Scholar]
- Lebow MA, Chen AD, 2016. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R, 2019. emmeans: Estimated Marginal Means, aka Least-Squares Means
- Lotze M, Domin M, Gerlach FH, Gaser C, Lueders E, Schmidt CO, Neumann N, 2019. Novel findings from 2,838 adult brains on sex differences in gray matter brain volume. Sci. Rep 9, 1671 10.1038/s41598-018-38239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Rieken NS, Kumar R, Ogren JA, Middlekauff HR, Wu P, Woo MA, Harper RM, 2016. Sex differences in insular cortex gyri responses to the valsalva maneuver. Front. Neurol 7, 87 10.3389/fneur.2016.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Majtanik M, Paxinos G, 2015. Atlas of the Human Brain, Fourth. ed Elsevier Academic Press. [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M, 1999. Cortical afferents to the extended amygdala. Ann. N. Y. Acad. Sci 877, 309–338. 10.1111/j.1749-6632.1999.tb09275.x [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct 1–13. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Hartwell KJ, Hanlon CA, Canterberry M, Lematty T, Owens M, Brady KT, George MS, 2015. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict. Biol 20, 407–414. 10.1111/adb.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PC, Nagae-Poetscher L, 2005. MRI Atlas of Human White Matter, 1st ed Elsevier Science. [Google Scholar]
- Moriguchi Y, Touroutoglou A, Dickerson BC, Barrett LF, 2014. Sex differences in the neural correlates of affective experience. Soc. Cogn. Affect. Neurosci 9, 591–600. 10.1093/scan/nst030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtergaele P, Radwan A, Swinnen S, Decramer T, Uytterhoeven M, Sunaert S, van Loon J, Theys T, 2019. The temporoinsular projection system: an anatomical study. J. Neurosurg 1–9. 10.3171/2018.11.JNS18679 [DOI] [PubMed] [Google Scholar]
- Nauta WJ, 1961. Fibre degeneration following lesions of the amygdaloid complex in the monkey. J. Anat 95, 515–31. [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, 2012. The insular cortex, in: Progress in Brain Research pp. 123–163. 10.1016/B978-0-444-53860-4.00007-6 [DOI] [PubMed] [Google Scholar]
- Nomi JS, Farrant K, Damaraju E, Rachakonda S, Calhoun VD, Uddin LQ, 2016. Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Hum. Brain Mapp 37, 1770–1787. 10.1002/hbm.23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Schettini E, Broce I, Dick AS, Uddin LQ, 2018. Structural connections of functionally defined human insular subdivisions. Cereb. Cortex,28(10):3445–3456. doi: 10.1093/cercor/bhx211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny GE, 1977. A direct ventral connection between the bed nucleus of the stria terminalis and the amygdaloid complex in the monkey (Macaca fascicularis). J. Hirnforsch 18, 271–84. [PubMed] [Google Scholar]
- Oler JA, Tromp DPM, Fox AS, Kovner R, Davidson RJ, Alexander AL, McFarlin DR, Birn RM, Berg B, DeCampo DM, Kalin NH, Fudge JL, 2017. Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non-human primate: neuronal tract tracing and developmental neuroimaging studies. Brain Struct. Funct 222, 21–39. 10.1007/s00429-016-1198-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onay A, Yapici Eser H, Ulasoglu Yildiz C, Aslan S, Tali ET, 2017. A combined VBM and DTI study of schizophrenia: bilateral decreased insula volume and cerebral white matter disintegrity corresponding to subinsular white matter projections unlinked to clinical symptomatology. Diagnostic Interv. Radiol 23, 390–397. 10.5152/dir.2017.16519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A language and environment for statistical computing
- Rabellino D, Densmore M, Harricharan S, Jean T, McKinnon MC, Lanius RA, 2018. Resting-state functional connectivity of the bed nucleus of the stria terminalis in post-traumatic stress disorder and its dissociative subtype. Hum. Brain Mapp 39, 1367–1379. 10.1002/hbm.23925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Hanson C, Hanson SJ, Bates ME, 2010. fMRI BOLD response in high-risk college students (part 1): during exposure to alcohol, marijuana, polydrug and emotional picture cues. Alcohol Alcohol 45, 437–443. 10.1093/alcalc/agq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS, 2005. Specificity in the Projections of Prefrontal and Insular Cortex to Ventral Striatopallidum and the Extended Amygdala. J. Neurosci 25, 11757–11767. 10.1523/JNEUROSCI.3432-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J, 2014. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev 39, 34–50. https://doi.org/10.1016Zj.neubiorev.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo AM, Sawiak SJ, Fryer T, Hong Y, Shiba Y, Clarke HF, Riss PJ, Ferrari V, Tait R, Suckling J, Aigbirhio FI, Roberts AC, 2019. Insula serotonin 2A receptor binding and gene expression contribute to serotonin transporter polymorphism anxious phenotype in primates. Proc. Natl. Acad. Sci 116 (29), 14761–14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D, 2006. Fiber Pathways of the Brain Oxford University Press, New York. [Google Scholar]
- Shackman AJ, Fox AS, 2016. Contributions of the central extended amygdala to fear and anxiety. J. Neurosci 36, 8050–8063. 10.1523/JNEUR0SCI.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J-W, Geerling JC, Loewy AD, 2008. Inputs to the ventrolateral bed nucleus of the stria terminalis. J. Comp. Neurol 511, 628–657. 10.1002/cne.21870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum. Brain Mapp 17, 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD, 1991. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res. Bull 27, 651–662. 10.1016/0361-9230(91)90041-H [DOI] [PubMed] [Google Scholar]
- Terasawa Y, Shibata M, Moriguchi Y, Umeda S, 2013. Anterior insular cortex mediates bodily sensibility and social anxiety. Soc. Cogn. Affect. Neurosci 8, 259–266. 10.1093/scan/nss108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss JD, Ridgewell C, McHugo M, Heckers S, Blackford JU, 2017. Manual segmentation of the human bed nucleus of the stria terminalis using 3T MRI. Neuroimage 146, 288–292. 10.1016/j.neuroimage.2016.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Alvarez GM, Gorka AX, Fuchs B, Geraci M, Grillon C, Ernst M, 2019Resting-state connectivity of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in clinical anxiety. J. Psychiatry Neurosci 44, 1–11. 10.1503/jpn.180150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, Grillon C, Ernst M, 2015. Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Hum. Brain Mapp 36, 4076–4088. 10.1002/hbm.22899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K, Consortium W-MHCP, 2013. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79. 10.1016/j.neuroimage.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S, 2007. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36, 630–644. 10.1016/j.neuroimage.2007.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller KL, Smith DA, 1982. Afferent connections to the bed nucleus of the stria terminalis. Brain Res 232, 255–270. 10.1016/0006-8993(82)90272-4 [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM, 2005. The bed nucleus of the stria terminalis in the Syrian hamster: Subnuclei and connections of the posterior division. Neuroscience 135, 155–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. BNST connectivity values for left and right insula subregions by sex. Connectivity values are number of streamlines that has been log transformed to a normal distribution.


