Abstract
Amyotrophic lateral sclerosis (ALS) is characterized by progressive degeneration of motor neurons. Astrocytes from diverse ALS models induce motor neuron death in co-culture. Enhancing NAD+ availability, or increasing the expression of the NAD+-dependent deacylases SIRT3 and SIRT6, abrogates their neurotoxicity in cell culture models. To determine the effect of increasing NAD+ availability in ALS mouse models we used two strategies, ablation of a NAD+-consuming enzyme (CD38) and supplementation with a bioavailable NAD+ precursor (nicotinamide riboside, NR). Deletion of CD38 had no effect in the survival of two hSOD1-linked ALS mouse models. On the other hand, NR-supplementation delayed motor neuron degeneration, decreased markers of neuroinflammation in the spinal cord, appeared to modify muscle metabolism and modestly increased the survival of hSOD1G93A mice. In addition, we found altered expression of enzymes involved in NAD+ synthesis (NAMPT and NMNAT2) and decreased SIRT6 expression in the spinal cord of ALS patients, suggesting deficits of this neuroprotective pathway in the human pathology. Our data denotes the therapeutic potential of increasing NAD+ levels in ALS. Moreover, the results indicate that the approach used to enhance NAD+ levels critically defines the biological outcome in ALS models, suggesting that boosting NAD+ levels with the use of bioavailable precursors would be the preferred therapeutic strategy for ALS.
Keywords: astrocytes, motor neurons, nicotinamide riboside, NMNAT2, SIRT3, SIRT6
Introduction
Nicotinamide adenine dinucleotide (NAD+) is an essential redox molecule and a key player in several signaling pathways that govern fundamental biological processes (Berger et al., 2004; Canto et al., 2015). Redox reactions involve the transfer of reducing equivalents between the oxidized (NAD+) and reduced (NADH) forms of the nucleotide. Although, this electron carrier function is critical for catabolic reactions and energy production, it does not cause any net loss of NAD+. On the other hand, the signaling processes in which NAD+ is used as a co-substrate in multiple enzymatic reactions lead to its degradation. Three distinct families of enzymes use NAD+ as co-substrate: poly(ADP-ribose) polymerases (PARPs), ADP-ribosyl cyclases (e.g. CD38) and sirtuins (Pehar et al., 2018).
Sirtuins (Sir2-like enzymes) are NAD+-dependent deacylases that play a key role in transcription, DNA repair, metabolism, and oxidative stress resistance (Imai and Guarente, 2014). Modulating NAD+ availability appears to regulate endogenous sirtuin activity and has been shown to be a potential therapeutic approach for age-related diseases (Katsyuba and Auwerx, 2017; Lautrup et al., 2019; Pehar et al., 2018).
In order to maintain viability, cells have to continuously synthesize NAD+. NAD+ neosynthesis can occur from L-tryptophan (kynurenine pathway), nicotinic acid (Priess-Handler pathway) or nicotinamide riboside (NR) (Belenky et al., 2007a; Bieganowski and Brenner, 2004; Ruddick et al., 2006). However, since all the major NAD+-consuming enzymes generate nicotinamide (NAM) as a byproduct, eukaryotic cells have evolved a rescue pathway capable of re-synthesizing NAD+ from NAM. The enzyme nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the conversion of NAM and 5’-phosphoribosyl-1-pyrophosphate to nicotinamide mononucleotide (NMN). NR is also converted into NMN by nicotinamide riboside kinases (NRKs) (Bieganowski and Brenner, 2004). Subsequently nicotinamide mononucleotide adenylyl transferases (NMNATs) transfer adenine from ATP to NMN to generate NAD+ (Di Stefano and Conforti, 2013; Garten et al., 2009). All the biosynthetic pathways converge at the level of dinucleotide formation catalyzed by NMNATs.
Amyotrophic lateral sclerosis (ALS) is characterized by the progressive degeneration of motor neurons in the spinal cord, brain stem, and motor cortex. Motor neuron death leads to muscle weakness and paralysis. Typically, death occurs due to respiratory paralysis in three to five years from the time of symptoms onset (Brown and Al-Chalabi, 2017). Approximately 5–10% of the ALS cases have a familial history of the disease (familial ALS, FALS) and are most frequently linked to a dominant mutation. The rest of the cases do not have a familial history (sporadic ALS, SALS) and may result from a yet unidentified environmental exposure or genetic mutations (Renton et al., 2014). The first ALS-linked gene identified was superoxide dismutase 1 (SOD1) (Rosen et al., 1993). Mutations in SOD1 account for up to 20% of FALS and 1–2% of apparently SALS cases. Mutations in several other genes, like TAR DNA binding protein (TARDBP) and fused in sarcoma (FUS) can also be found in FALS and SALS cases, while a repeat expansion in C9orf72 (chromosome 9, open reading frame 72) is the most common genetic cause of ALS (Brown and Al-Chalabi, 2017; Ravits et al., 2013; Renton et al., 2014). In the sera of patients with ALS, significant alterations in the level of key molecules of the tryptophan–nicotinamide metabolic pathway (e.g. circulating nicotinamide) have been reported. This observation may originate from impaired microbiome-derived nicotinamide metabolism in patients with ALS. Accordingly, nicotinamide supplementation appears to be protective in an ALS (hSOD1G93A) mouse model (Blacher et al., 2019).
Astrocytes play a key role determining motor neuron fate in ALS models, and primary astrocytes over-expressing mutant hSOD1 or mutant hFUS induce motor neuron death in co-culture (Kia et al., 2018; Nagai et al., 2007; Vargas et al., 2006). In line with these observations, astrocytes differentiated from human postmortem ALS spinal cord-derived progenitor cells and astrocytes obtained from the trans-differentiation of fibroblasts from FALS and SALS patients are also toxic for motor neurons in co-culture (Haidet-Phillips et al., 2011; Meyer et al., 2014).
Notably, therapeutic strategies aimed at reverting astrocyte-mediated toxicity increase motor neuron survival and improve motor performance in ALS mouse models (de Boer et al., 2014; Miquel et al., 2014; Song et al., 2016; Vargas et al., 2008). Treatment with NAD+ precursors enhance NAD+ availability in mutant hSOD1-expressing astrocytes, leading to increased resistance to oxidative stress and the reversal of their toxicity toward co-cultured motor neurons (Harlan et al., 2019; Harlan et al., 2016). Here we sought to determine the effect of enhancing NAD+ availability in the ALS-like pathology developed by hSOD1G93A mice. Our results show that NR supplementation delays motor neuron degeneration, decreases markers of neuroinflammation in the spinal cord, appears to modify muscle metabolism and extends the survival of hSOD1G93A mice. In addition, we present evidence of altered expression of SIRT6 and NAD+ synthesizing enzymes in ALS patients, which can be directly relevant to the pathophysiology of the disease. Since NR appears to be orally bioavailable and safe in humans (Trammell et al., 2016), our data suggests this would be the preferred therapeutic strategy to enhance NAD+ levels in ALS.
Materials and methods
Reagents-
All chemicals and reagents were from Sigma-Aldrich unless otherwise specified. Primers were obtained from Integrated DNA Technologies (see supplemental table 1).
Animals-
B6.Cg-Tg(SOD1*G93A)1Gur/J mice (Gurney et al., 1994) were obtained from The Jackson Laboratory and maintained in hemizygosis in a C57BL/6J background. Littermates were randomly assigned to a control diet (Teklad 2016S) or a test diet containing NR 2.4g/kg (2016S Teklad + NR). Ad libitum feeding of this modified diet translates in approximately 400 mg/kg/day of NR in adult mice (Zhang et al., 2016). hSOD1H46R/H48Q mice were provided by Dr. David Borchelt [35] and have been backcrossed into C57BL/6J pure background for more than 10 generations. C57BL/6J.129 CD38−/− mice have been previously described (Partida-Sanchez et al., 2001). In order to generate the animals for this study, hemizygous hSOD1G93A males were mated with CD38(−/−) females to obtain breeders with the following genotype CD38(+/−)/hSOD1G93A(+/−) and CD38(+/−)/hSOD1G93A(−/−). Then, CD38(+/−)/hSOD1G93A(+/−) males were mated with CD38(+/−)/hSOD1G93A(−/−) females to obtain the genotypes analyzed in the study. The same breeding strategy was used to generate hSOD1H46R/H48Q mice in a CD38 knockout background. For life-span studies, end-point was determined by the inability of the animal to right itself within 20 seconds when placed on its side. Mice that were unable to right themselves within 20 seconds were euthanized and recorded as dead. Mice were weighed two times per week and disease onset was retrospectively determined as the time when mice reached peak body weight. Hind-limb grip strength was determined using a grip strength meter (San Diego Instruments). Tests were performed by allowing the animal to grasp the grid with both hind-limbs and pulling the animal straight away from the grid until it released the platform. Grip strength was measured once a week, in each session the average peak force of three attempts was recorded. For the animals in the life-span study, relative quantitative PCR was used to estimate hSOD1 gene copy number. One of the male breeders that fathered the litters used in these studies was used as a reference for copy number estimation. Animals with less relative gene copy number that the reference breeder were not used in the study. All animal procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH. The Animal Care and Use Committee of MUSC (Animal Welfare Assurance number A3428–01) approved the animal protocol pertinent to the experiments reported in this publication.
Humans postmortem samples-
Deidentified lumbar spinal cord samples from non-ALS controls and ALS patients were obtained from the NIH NeuroBioBank. Demographic data and cause of death are provided in supplemental table 2.
Immunofluorescence and histochemistry-
Antigen retrieval and staining in paraffin embedded tissues was performed as previously described (Vargas et al., 2008). Mice lumbar spinal cord sections were stained with anti-GFAP (Novus, NBP2–29415, Lot 2670–1P180807) and anti-IBA1 (Fujifilm Wako, 013–27691, Lot PTJ0731) antibodies. Nuclei were counterstained with DAPI (4(,6-Diamidino-2-phenylindole dihydrochloride). Several sections (the number is indicated in the corresponding figure legend) from 4 animals in each group were stained and imaged concurrently. Immunofluorescent images were captured in a Zeiss LSM 880 NLO microscope (Carl Zeiss) with identical settings for all experimental groups. Image quantification was performed with Imaris image analysis software 9.1.2 (Oxford Instruments). A same size area of interest in the ventral horn was selected for each image. Individual channel intensities were quantified following background subtraction using the threshold cutoff feature to subtract background fluorescence. Motor neuron numbers were determined in 10 μm serial sections across the lumbar spinal cord stained with cresyl violet. Two independent observers blinded to the genotype and treatment of the samples counted every fifth section and a total of 9–10 sections per animal were analyzed. Human spinal cord sections were stained with anti-NMNAT2 (Santa Cruz, SC-134935, Lot C2410) or anti-SIRT6 (LSbio, LS-B5589, Lot 36791) antibodies, developed with the Dako liquid DAB+ substrate chromogen system (Agilent) and counter stained with hematoxylin. All sections were stained and developed concurrently. Images were captured in a Zeiss Axiovert 200 microscope (Carl Zeiss).
Real-time PCR and western blot analysis-
RNA extraction, RNA retrotranscription, real-time PCR and western blot analysis were performed as previously described (Pehar et al., 2014; Vargas et al., 2008).
Statistical analysis-
Survival and onset data was analyzed with Kaplan-Meier curves and log rank test. Groups of at least 4 animals were used for biochemical analysis and all data are reported as mean ± SD. Comparisons between two groups were performed with an unpaired t-test. Multiple group comparisons were performed with two-way ANOVA with Tukey’s post-test and differences were declared statistically significant if p ≤ 0.05. All statistical computations were performed using GraphPad Prism 6.0 (GraphPad Software).
Results
To determine the effect of increasing NAD+ availability in ALS mouse models we used two strategies. A transgenic approach, ablation of a NAD+ consuming enzyme (CD38), and a dietary supplementation approach with nicotinamide riboside (NR, a bioavailable NAD+ intermediate). Originally described as an ectoenzyme, CD38 is also present in the endoplasmic reticulum, and in the nuclear and mitochondrial membrane (Aksoy et al., 2006). Knockout of CD38 results in a significant increase in the steady state levels of NAD+ in the brain; with reported changes ranging from 2- to 10-fold increases (Aksoy et al., 2006; Young et al., 2006). We used two different ALS mouse models overexpressing a mutant hSOD1, the ALS-linked mutation G93A (hSOD1G93A) and the experimental mutation H46R/H48Q (hSOD1H46R/H48Q), in a CD38 knockout background. We observed that the ablation of CD38 does not modify the survival of the two hSOD1-linked ALS mouse models used (Figure 1).
Figure 1. CD38 ablation does not extend survival in mutant hSOD1-linked ALS mouse models.
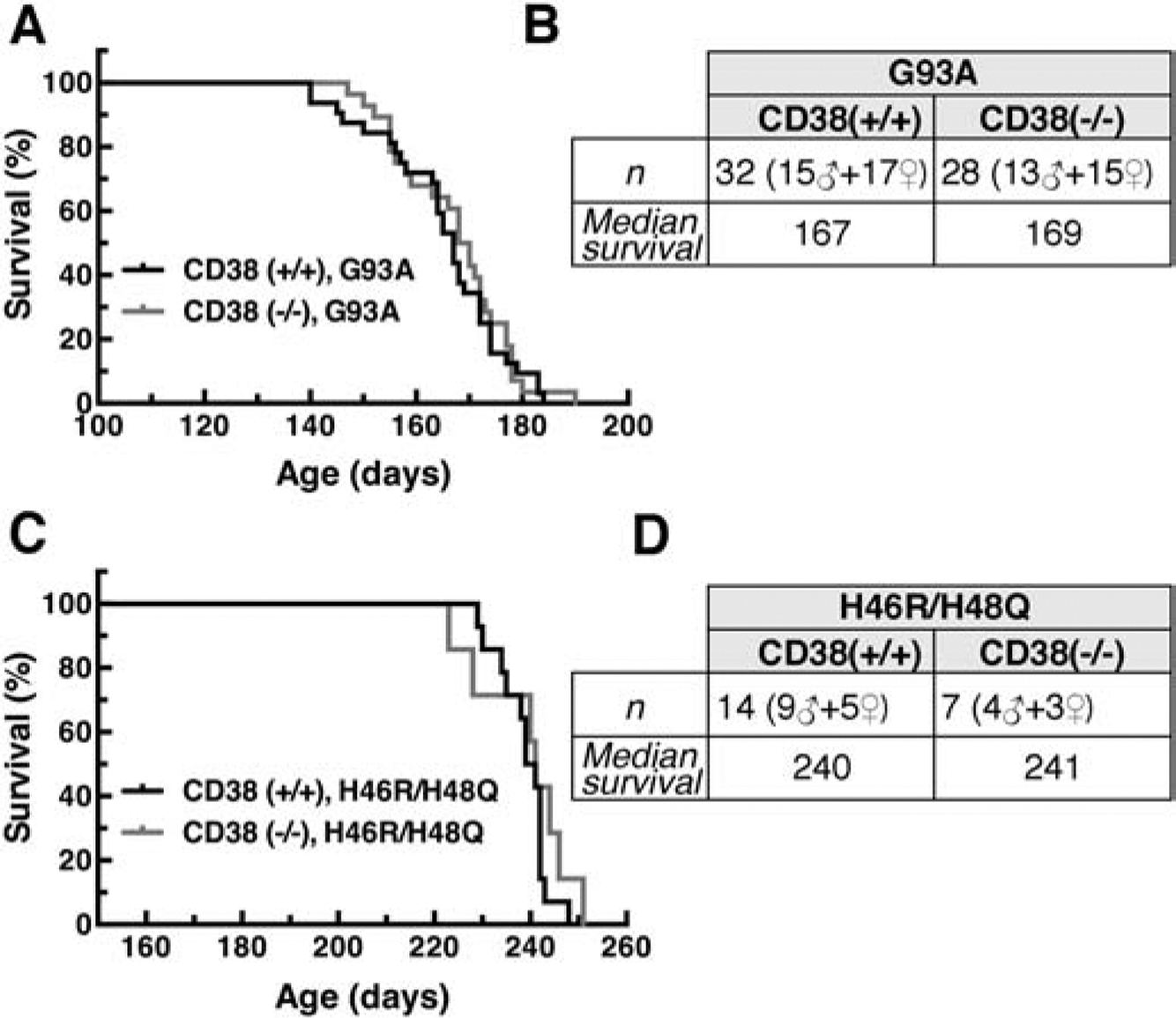
A) Median survival in CD38(+/+)/hSOD1G93A and CD38(−/−)/hSOD1G93A mice. Curves are not significantly different. B) Summary of the data presented in (A). C) Median survival in CD38(+/+)/hSOD1H46R/H48Q and CD38(−/−)/hSOD1H46R/H48Q mice. Curves are not significantly different. D) Summary of the data presented in (C).
NR is orally bioavailable in animals and humans (Airhart et al., 2017; Dellinger et al., 2017; Trammell et al., 2016; Zhang et al., 2016). NR treatment increases intracellular NAD+ concentration and enhances NAD+-dependent sirtuin activity in different culture and animal models (Belenky et al., 2007b; Harlan et al., 2016; Zhang et al., 2016). Ad libitum feeding of a NR supplemented diet, that translates in approximately 400 mg/kg/day of NR in adult mice, modestly extended the survival of hSOD1G93A mice (Figure 2). Although mice in control and NR diet had similar median onset (Figure 2A, C), NR appeared to slow down disease progression as reflected by a significant delay in the age at which mice displayed 10% and 15% body weight loss (as percentage of peak body weight) (Figure 2D, E). The NR diet also improved hindlimb grip strength around the age of symptoms onset (Figure 2F). In addition, NR supplementation decreased glial activation in the spinal cord of early symptomatic mice, as determined by immunofluorescence against astrocyte (Gfap) and microglia (Iba-1) markers (Figure 3A–C). The neuroprotective effect conferred by NR is evidenced by a delay in the loss of large motor neurons in the lumbar spinal cord of hSOD1G93A mice on the experimental diet (an average of about 11% more motor neurons was observed in the NR diet group, Figure 3D). Moreover, we observed a significant decrease in the expression of several inflammatory markers highly expressed by glial cells in the CNS (Figure 3E–H). Because we have shown that the NAD+-dependent enzymes, SIRT3 and SIRT6 are important regulators of motor neuron survival in in vitro models of ALS, we analyzed the expression levels of Sirt3 and Sirt6 in these experimental animals (Harlan et al., 2019; Harlan et al., 2016). Although NR supplementation has no effect on their expression level, we found that the mRNA levels of both enzymes are significantly downregulated in the spinal cord of hSOD1G93A mice (Figure 3I, J). These changes are most likely linked to the onset of the neurodegenerative process, and were not observed in the spinal cord of young hSOD1G93A mice (Supplemental Figure 1A).
Figure 2. Dietary NR supplementation modestly extends survival in hSOD1G93A mice.
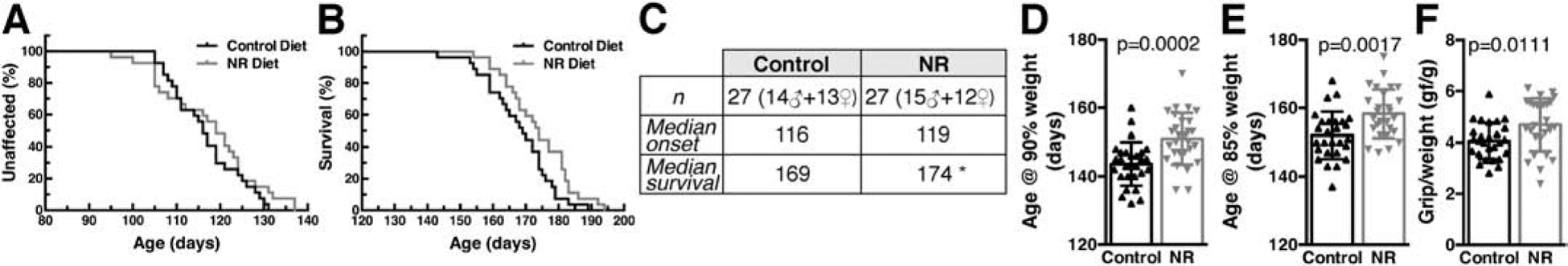
A) Disease onset in hSOD1G93A mice on control or nicotinamide riboside (NR)-supplemented diet. Onset was determined as the age at peak body weight. Curves are not significantly different. B) NR increased the median survival of hSOD1G93A mice from 169 days to 174 days. Survival curves are significantly different p<0.05 (Log-rank test, χ2=4.1). C) Summary of the data presented in (A) and (B) (*p<0.05). D) Age at which hSOD1G93A mice on control or NR-supplemented diet exhibited 10% weight loss (age at 90% of peak body weight, mean ± SD). E) Age at which hSOD1G93A mice on control or NR-supplemented diet exhibited 15% weight loss (age at 85% of peak body weight, mean ± SD). F) Ratio of hind-limb grip strength to body weight at 110 days of age in hSOD1G93A mice on control or NR-supplemented diet (mean ± SD). The number and sex of the animals for panels (D), (E) and (F) are the same as in (C).
Figure 3. Dietary NR supplementation decreases glial activation and delays motor neuron loss in the spinal cord of hSOD1G93A mice.
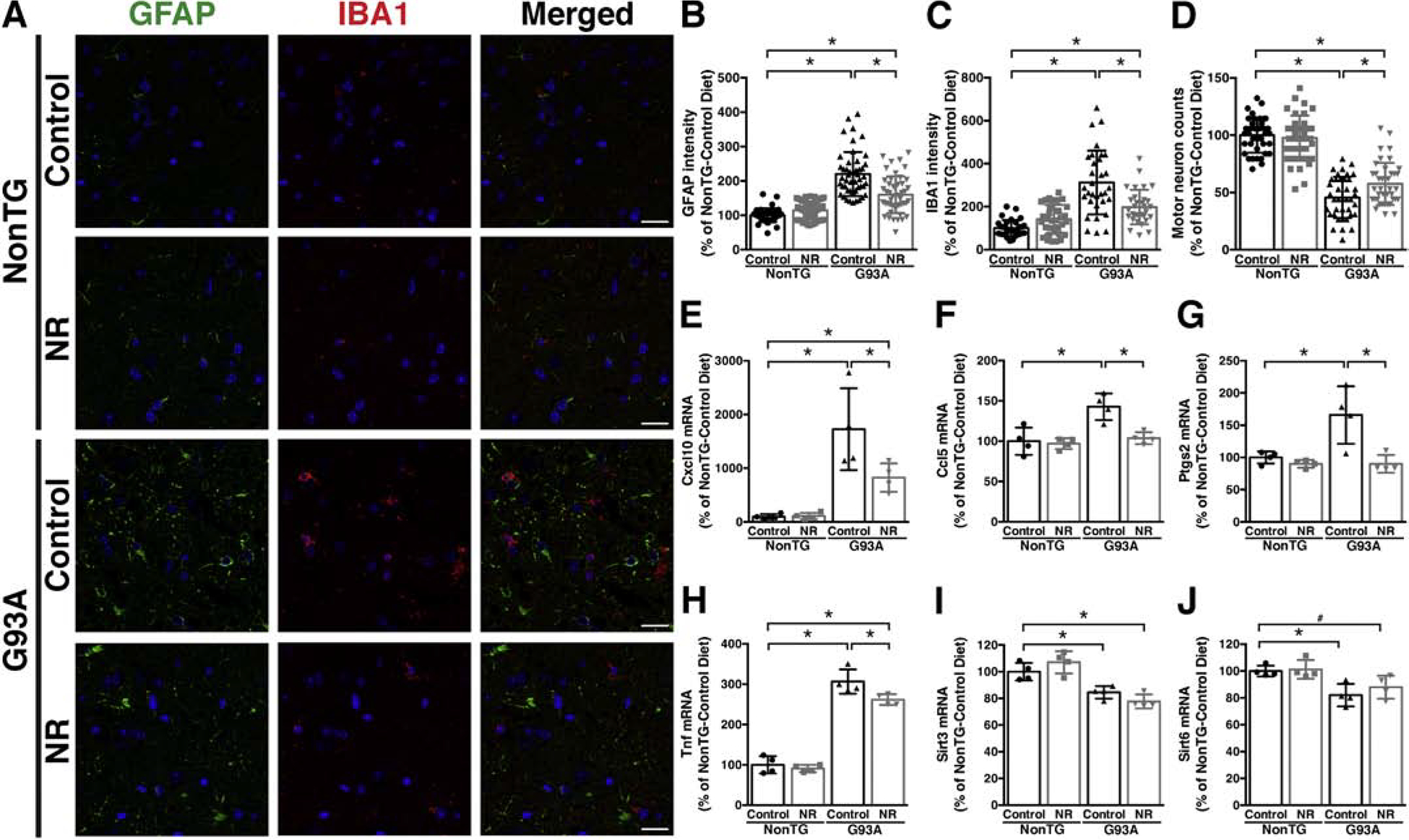
A) Representative microphotographs showing GFAP (green) and IBA1 (red) immunofluorescence in the anterior horn of the lumbar spinal cord from early symptomatic (120 days-old) hSOD1G93A (G93A) and aged-match non-transgenic (NonTG) mice on control or nicotinamide riboside (NR)-supplemented diet. Nuclei were counterstained with DAPI. Scale bar, 20 μm. B) Quantification of relative GFAP fluorescence intensity in images from the anterior horn of the lumbar spinal cord of mice treated as in (A) (10–13 images per animal, n=4 mice per treatment group). C) Quantification of relative IBA1 fluorescence intensity in images from the anterior horn of the lumbar spinal cord of mice treated as in (A) (7–8 images per animal, 4 animals per treatment group). D) Number of large motor neurons in the ventral horn of the lumbar spinal cord of mice treated as in (A) (9–10 sections per animal, n=4 mice per treatment group). E–J) Total RNA was extracted from the lumbar spinal cord of early symptomatic (120 days-old) G93A mice and NonTG littermates on control or NR-supplemented diet and Cxcl10, Ccl5, Ptgs2, Tnf, Sirt3, and Sirt6 mRNA levels were determined by real-time PCR and corrected by Rplp0 mRNA levels (n=4 mice per group). For all panels data are expressed as percentage of NonTG-control diet mice (mean ± SD). *p<0.05 (2-way ANOVA). #p<0.05 (t-test).
Motor neuron degeneration and concomitant muscle denervation is accompanied by transcriptional and metabolic changes in the affected skeletal muscles. Changes include dysregulation of metabolic enzymes involved in fuel utilization and up-regulation of denervation markers [e.g., gamma and alpha1 subunits of the nicotinic acetylcholine receptor (Chrng and Chrna1), and ubiquitin C-terminal hydrolase L1 (Uchl1)] (Gonzalez de Aguilar et al., 2008; Palamiuc et al., 2015; Palma et al., 2016). NR appears to partially prevent the increase in Chrna1 and Uchl1 observed in the gastrocnemius muscle of hSOD1G93A mice, while no effect was observed in the expression of Chrng (Figure 4A–C). In addition, NR supplementation upregulates (or partially maintains) the expression of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (Pfkfb3) and carnitine palmitoyltransferase 1B (Cpt1b) in the gastrocnemius muscle of hSOD1G93A mice (Figure 4D, E). Interestingly, we observed a decrease in the expression of Pfkfb3 in non-transgenic mice after NR supplementation (Figure 4D). We observed no changes in the expression of Sirt6 in the gastrocnemius muscle of hSOD1G93A mice, while Sirt3 expression is significantly downregulated and the NR-supplemented diet restores (or maintains) normal Sirt3 expression level (Figure 4F, G).
Figure 4. Dietary NR supplementation partially decreases denervation markers and appears to increase metabolic flexibility in the gastrocnemius muscle of hSOD1G93A mice.
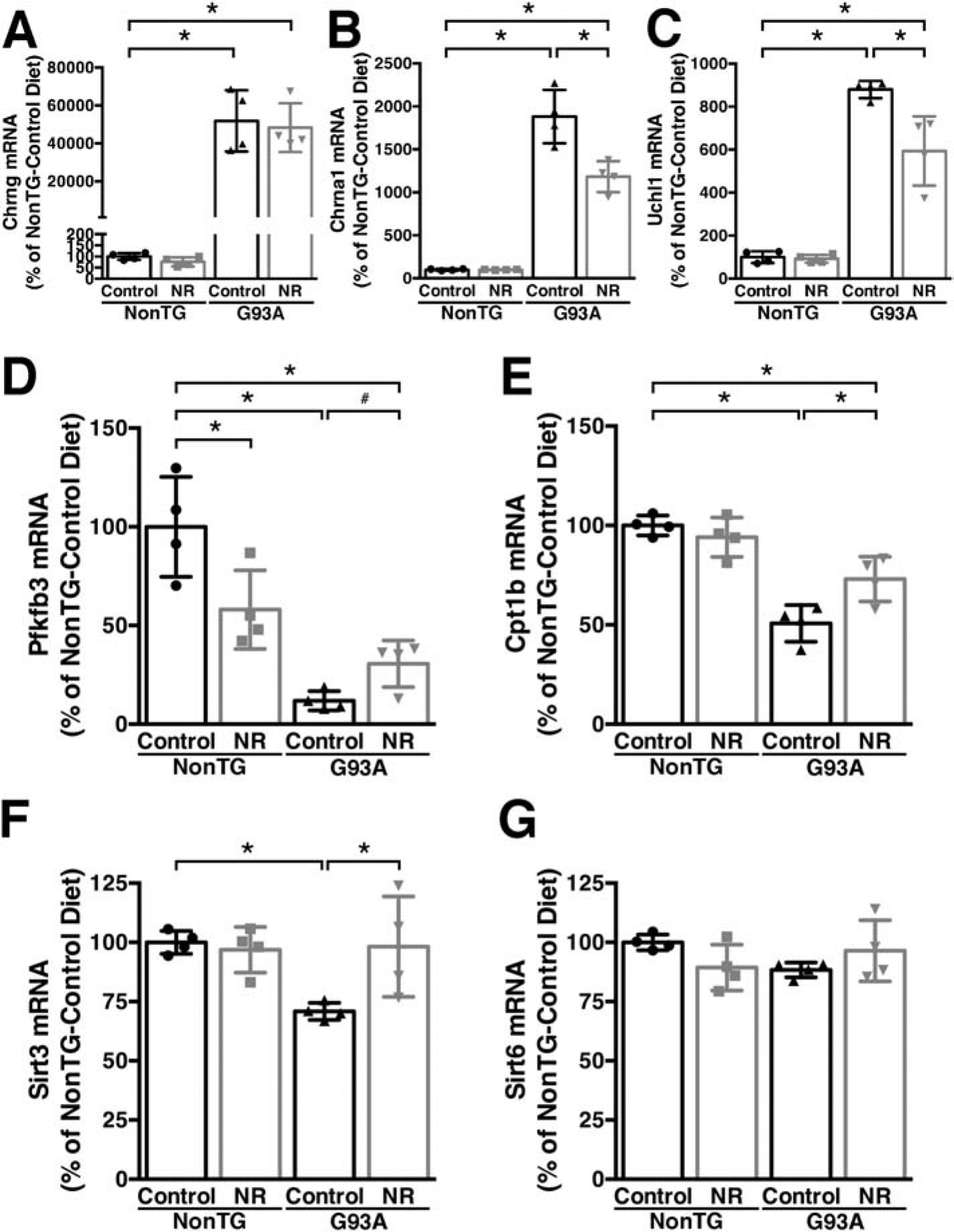
A–G) Total RNA was extracted from the gastrocnemius muscle of early symptomatic (120 days-old) hSOD1G93A (G93A) and non-transgenic (NonTG) mice on control or nicotinamide riboside (NR)-supplemented diet. mRNA levels for Chrng, Chrna1, Uchl1, Pfkfb3, Cpt1b, Sirt3 and Sirt6 were determined by real-time PCR and corrected by Rplp0 mRNA levels (n=4 mice per group). For all panels data are expressed as percentage of NonTG-control diet mice (mean ± SD). *p<0.05 (2-way ANOVA). #p<0.05 (t-test).
Since Sirt3 and Sirt6 expression is neuroprotective in in vitro models of ALS (Harlan et al., 2019; Harlan et al., 2016), and both enzymes are significantly downregulated in the spinal cord of hSOD1G93A mice (Figure 3I, J), we analyzed the expression of these sirtuins in the spinal cord of ALS patients. Distinct to the data found in hSOD1G93A mice, no significant changes were observed in the expression of SIRT3 in the spinal cord of ALS patients (Figure 5A, B, C and Supplemental Figure 2). However, SIRT6 expression was significantly decreased in the spinal cord of ALS patients when compared to non-ALS controls (Figure 5D, E, F and Supplemental Figure 2). Immunohistochemistry analysis confirmed a decrease in SIRT6 expression in the spinal cord of ALS patients, particularly evident in non-motor neuron cells (Figure 5G, H).
Figure 5. Decreased SIRT6 expression in the spinal cord of ALS patients.
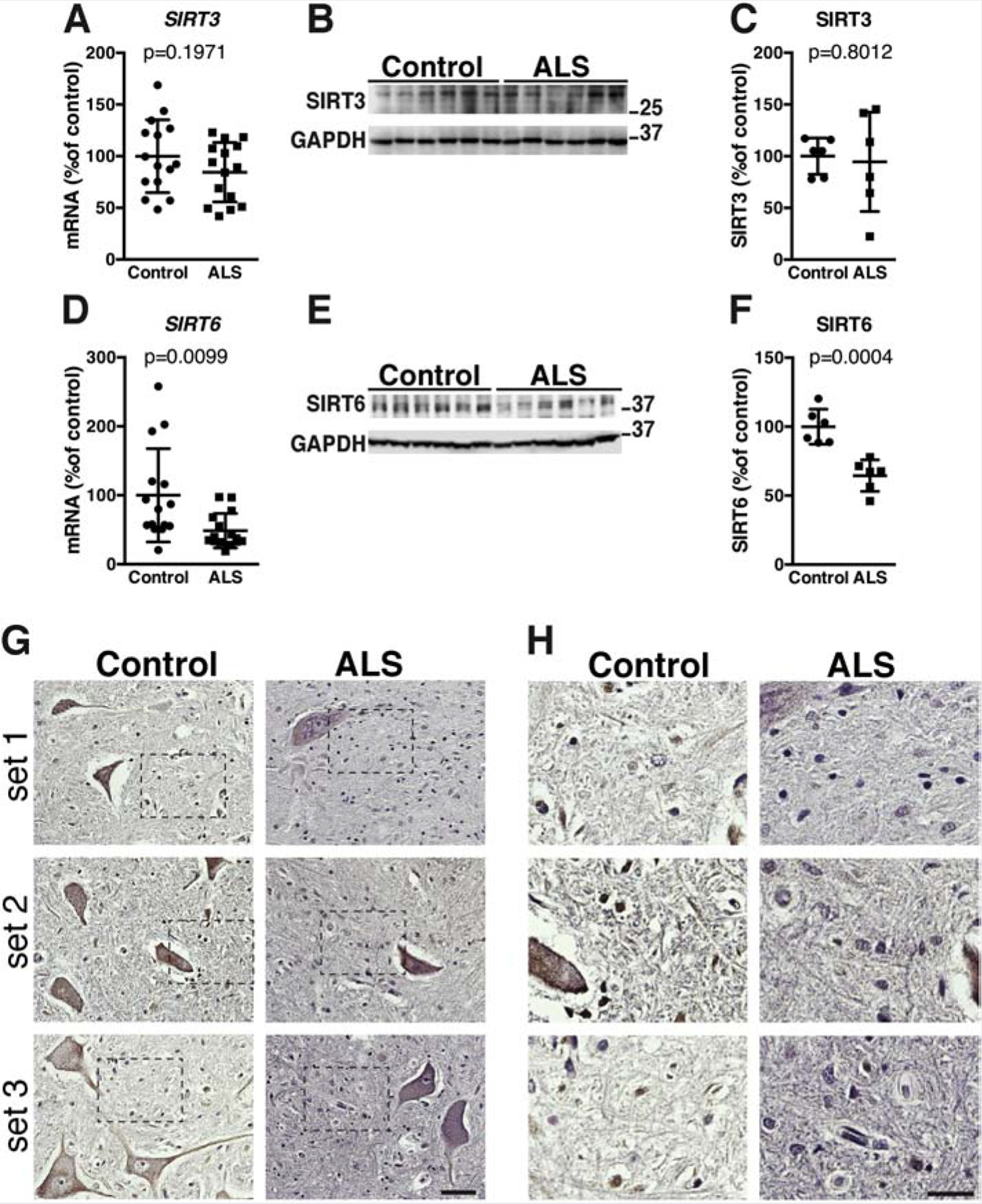
A) Total RNA was extracted from spinal cord tissue of non-ALS controls (control) and ALS patients. mRNA levels for SIRT3 were determined by real-time PCR and corrected by TBP mRNA levels. Data are expressed as percentage of controls (mean ± SD, n=15 in each group). B) SIRT3 protein levels in the spinal cord tissue of non-ALS controls (control) and ALS patients. GAPDH levels were used as loading control for normalization. C) Quantification of SIRT3 levels shown in (B) (mean ± SD, n=6 in each group). D) mRNA levels for SIRT6 were determined by real-time PCR in the spinal cord of non-ALS controls (controls) and ALS patients and corrected by TBP mRNA levels Data are expressed as percentage of controls (mean ± SD, n=15 in each group). E) SIRT6 protein levels in the spinal cord tissue of non-ALS controls (control) and ALS patients. GAPDH levels were used as loading control for normalization. F) Quantification of SIRT6 levels shown in (E) (mean ± SD, n=6 in each group). G) SIRT6 immunostaining in the ventral horn of the spinal cord from three different non-ALS controls (control) and three different ALS patients. Each set corresponds to control and ALS tissue sections mounted on the same slide. All sections were stained and developed concurrently. Scale bar: 50μm. H) Higher magnification of the areas highlighted in (G). The decrease in immunoreactivity was particularly evident in small nuclei surrounding motor neurons (likely glia). Scale bar: 25μm.
Enhancing NAD+ availability through NAD+ precursor supplementation is being considered to treat human diseases (Airhart et al., 2017; Dellinger et al., 2017; Martens et al., 2018; Trammell et al., 2016). Thus, it is essential to characterize changes in NAD+ biosynthetic pathways during pathological processes. We analyzed the expression level of several enzymes involved in NAD+ synthesis and found increased NAMPT and decreased NMNAT2 expression in the spinal cord of ALS patients, when compared to non-ALS controls (Figure 6). The increase in NAMPT mRNA data observed in ALS patients is in line with an overall increase in NAMPT protein expression previously described in these patients (Wang et al., 2017). Quantitative protein and immunohistochemistry analysis confirmed a decrease in NMNAT2 expression in the spinal cord of ALS patients, which appears particularly evident in motor neurons (Figure 6 G, H, I). Changes in Nmnat1 and Nmnat2 mRNA expression are also evident in the spinal cord early symptomatic hSOD1G93A mice but not in young asymptomatic mice (Supplemental Figure 1), further suggesting that these changes may be linked to the neurodegenerative process observed in ALS.
Figure 6. Altered expression of enzymes involved in NAD+ synthesis in the spinal cord of ALS patients.
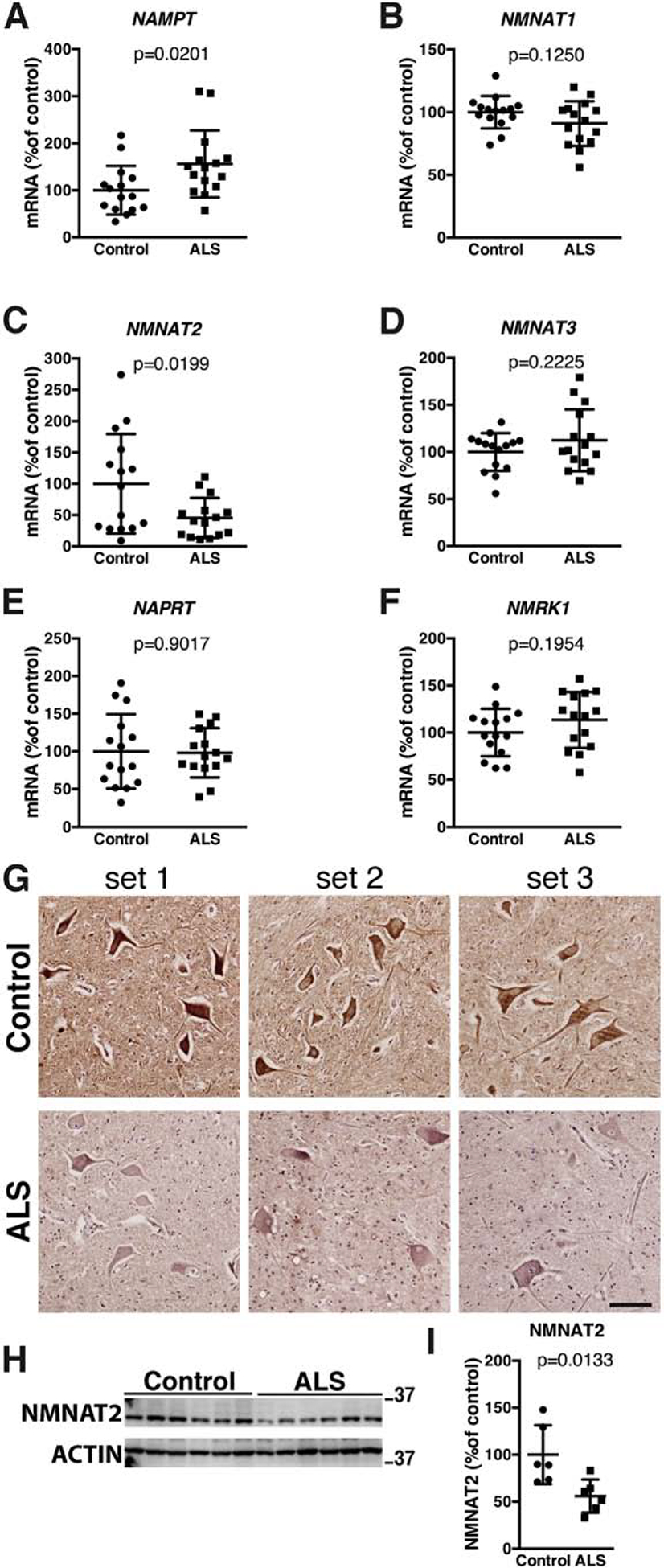
A–F) Total RNA was extracted from spinal cord tissue of non-ALS controls (controls) and ALS patients. mRNA levels for NAMPT, NMNAT1, NMNAT2, NMNAT3, NAPRT and NMRK1 were determined by real-time PCR and corrected by TBP mRNA levels. For all panels, data are expressed as percentage of controls (mean ± SD, n=15 in each group). G) NMNAT2 immunostaining in the ventral horn of the spinal cord from three different non-ALS controls (control) and three different ALS patients. Each set corresponds to a different control and ALS tissue sections mounted on the same slide. All sections were stained and developed concurrently. Scale bar: 100μm. H) NMNAT2 protein levels in the spinal cord tissue of non-ALS controls (control) and ALS patients. ACTIN levels were used as loading control for normalization. I) Quantification of NMNAT2 levels shown in (H) (mean ± SD, n=6 in each group).
Discussion
Enhancing NAD+ availability has been shown to confer protection in animals models of Alzheimer’s disease (Gong et al., 2013; Hou et al., 2018; Sorrentino et al., 2017) and in in vitro models of ALS (Harlan et al., 2019; Harlan et al., 2016). Here we showed that a diet supplemented with NR modestly increases the survival of hSOD1G93A mice and it has a significant biological response in inflammatory and metabolic parameters measured in this ALS mouse model. While deletion of NAD+ consuming enzymes, like PARP1 and CD38 does increase NAD+ levels in several tissue types, our data show that ablation of CD38 does not confer protection in two hSOD1-linked ALS models. This result appears to be in line with previous published data showing that PARP1 inhibition does not extend the survival of hSOD1G93A mice (Andreassen et al., 2001).
Interestingly, while PARP1 and CD38 deletion or inhibition is protective in some models of neurodegeneration (Abeti et al., 2011; Blacher et al., 2015; Cardinale et al., 2015; Kauppinen et al., 2011); PARP1 or CD38 ablation confers no protection against sciatic nerve transection (Sasaki et al., 2009), and PARP1 deletion in multiple sclerosis models may even aggravate the pathology (Selvaraj et al., 2009). In neurodegenerative processes, dying cells are exposed to increased oxidative stress, and PARPs play a central role in DNA damage repair (Berger et al., 2018). In turn, CD38 plays a role in many complex biological processes such as stem cell differentiation and transfer of mitochondria between cells (Hayakawa et al., 2016; Kim et al., 2016; Wei et al., 2015). In addition, CD38 has a key role in neuropeptide release and Ca2+ homeostasis (Adebanjo et al., 1999; Jin et al., 2007). Consequently, while inhibition of PARPs and CD38 effectively enhances NAD+ availability, it can also adversely affect pathways that could be fundamental in mounting an endogenous protective response during a neurodegenerative process. Thus, at least in ALS, the available evidence suggests that inhibition of NAD+-consuming enzymes may not be beneficial, while boosting NAD+ with bioavailable precursors may be the most appropriate approach when the aim is to increase NAD+ availability for therapeutic purposes.
Importantly, despite the modest extension in survival observed with the experimental diet, NR has a clear and significant effect in the number of surviving spinal cord motor neurons and the level of gliosis and inflammatory markers expressed in the spinal cord (Figure 3). The latter observation could be due to either a direct effect of NR supplementation in astrocyte and microglia biology or a secondary outcome due to delayed muscle denervation and/or motor neuron degeneration. Previous published data on the beneficial role of Sirt3 and Sirt6 in ALS models (Buck et al., 2017; Harlan et al., 2019; Harlan et al., 2016; Song et al., 2013) prompted us to analyze the expression of these enzymes, and we found a significant decrease in their expression level in the spinal cord of symptomatic hSOD1G93A mice (Figure 3). Thus, during neuronal degeneration in this ALS model, Sirt3 and Sirt6 activity may not only be limited by co-substrate availability but also by a decrease in expression.
Our data confirms an earlier observation made in a smaller cohort of ALS patients that showed no change in SIRT3 mRNA expression (Korner et al., 2013). The discrepancy between the data on Sirt3 expression in the spinal cord of symptomatic hSOD1G93A mice and the data obtained in ALS patients highlights the need for additional studies to fully characterize the role of this sirtuin during motor neuron degeneration. On the other hand, we present for the first-time evidence of a significant downregulation of SIRT6 expression in the spinal cord of ALS patients (Figure 5). Since, SIRT6 can be neuroprotective, this observation lends further support to the rational of enhancing NAD+ availability to increase sirtuin activity in ALS. In this respect, it is worth noting that enhancing NAD+ availability will not necessarily increase the expression of the target enzyme but its activity.
Treatment with NAD+ precursors, like NR, reverses mitochondrial dysfunction and metabolic changes associated with aging and high-fat diet intake (Canto et al., 2012; Mills et al., 2016; Mouchiroud et al., 2013; Yoshino et al., 2011; Zhang et al., 2016). Moreover, NR supplementation has been shown to increase skeletal muscle NAD+ metabolome in aged human control subjects (Elhassan et al., 2019). Metabolic changes in affected muscles are one of the earlier changes observed in ALS animal models (Gonzalez de Aguilar et al., 2008; Palamiuc et al., 2015; Palma et al., 2016), and strategies aimed at overcoming metabolic constraints have been shown to be protective in these models (Dupuis et al., 2004; Manzo et al., 2019). Pfkfb3 controls both the synthesis and degradation of fructose-2,6-bisphosphate (F2,6BP), a regulatory molecule that controls glycolysis in eukaryotes (Bolanos et al., 2010). F2,6BP activates glycolysis through allosteric modulation of phosphofructokinase (Pfkm). Cpt1b is the rate-limiting enzyme in the utilization of long-chain fatty acids for beta-oxidation in muscle mitochondria (Lundsgaard et al., 2018). The expression of both enzymes is significantly downregulated in the gastrocnemius muscle of symptomatic hSOD1G93A mice (Figure 4) and NR partially restores Pfkfb3 and Cpt1b expression, suggesting that the experimental diet may improve metabolic flexibility in the affected skeletal muscle. Sirt3 regulates metabolic pathways in fuel-producing and fuel-utilizing tissues (Dittenhafer-Reed et al., 2015). Similar to the finding in the spinal cord, Sirt3 expression is significantly downregulated in the gastrocnemius muscle of symptomatic hSOD1G93A mice in the control diet, while Sirt3 levels remain unchanged in mice fed the NR-supplemented diet. The changes in gene expression observed in the hSOD1G93A mice on the NR supplemented diet can be due to a direct effect of NR reprograming the metabolism of the skeletal muscle or could be an indirect effect due to a delay in changes associated with muscle denervation and wasting (e.g. decrease in the expression of these enzymes as muscle wasting ensues).
A previous manuscript argued for a dysregulation of the amount of intracellular and extracellular levels of NAMPT in the spinal cord of ALS patients (Wang et al., 2017) but showed an overall increase in NAMPT expression. Our data confirms that there is a significant increase in NAMPT expression in the spinal cord of ALS patients (Figure 6). The variability of NMNAT2 mRNA expression in the spinal cord of non-ALS controls correlates with the significant variability of expression identified for this enzyme in human post-mortem brains (Ali et al., 2016). On the other hand, NMNAT2 mRNA expression in the lumbar spinal cord of ALS patients is tightly clustered and significantly downregulated (Figure 6C, Supplemental Figure 2). Similarly, Nmnat2 mRNA is significantly down-regulated in both the lumbar spinal cord and gastrocnemius muscle of early symptomatic hSOD1G93A mice (Supplemental Figure 1B, C). Of the three NMNAT isoforms, NMNAT2 has been shown to be an essential survival factor for maintenance of healthy axons (Conforti et al., 2014; Gerdts et al., 2016), and reduction of NMNAT2 expression below a threshold level triggers degeneration even in uninjured axons (Gilley et al., 2019). Moreover, NMNAT2 expression levels directly correlate with cognitive performance, and a decrease in NMNAT2 expression is observed in Alzheimer’s disease patients (Ali et al., 2016). NMNAT2 acts as a NAD+-synthesizing enzyme as well as a chaperone for protein refolding (Ali et al., 2016; Zhai et al., 2008). Hypomorphic human NMNAT2 variants cause skeletal muscle hypoplasia and fetal akinesia apparently due to defects in both NAD+ synthesis and chaperone functions (Lukacs et al., 2019). NMNAT2 overexpression provides neuroprotection in several models of neurodegeneration (Ali et al., 2013; Conforti et al., 2014), and consistent with its rate-limiting role in NAD+ synthesis, NAMPT overexpression is also protective in several models of neurodegeneration (Harlan et al., 2016; Jing et al., 2014; Pehar et al., 2018; Wang et al., 2016). Thus, the observed NAMPT upregulation in ALS patients could be a compensatory mechanism in response to a decrease in NMNAT2 expression.
A recent clinical trial compared the effect of a combination of NR and pterostilbene against placebo control in ALS patients (de la Rubia et al., 2019). ALS patients in the treatment group displayed significant improvements in the revised ALS functional rating scale, pulmonary function, muscular strength and in skeletal muscle/fat weight ratio compared to the placebo control group. Our results show that NAD+ precursor supplementation produces measurable changes in biological variables that are relevant in the context of motor neuron degeneration observed in hSOD1G93A mice and provides an initial mechanistic insight to the results observed in patients. While the extension in survival achieved by NR supplementation is modest, several transgenic approaches aimed at increasing sirtuins activity in this mouse model have provided a similar extension in survival (Herskovits et al., 2018; Watanabe et al., 2014). However, the apparent safety and simplicity of NAD+ boosting strategies (Airhart et al., 2017; de la Rubia et al., 2019; Dellinger et al., 2017; Trammell et al., 2016) justifies future efforts to improve NAD+ precursor delivery strategies to target certain tissues or cell types in order to achieve greater efficacy. In addition, we present evidence of altered expression of SIRT6 and NAD+ synthesizing enzymes in ALS patients, which can be directly relevant to the pathophysiology of disease. Taken together, our results indicate that enhancing NAD+ availability could be a potential therapeutic strategy for ALS.
Conclusions
Enhancing NAD+ availability is being considered as a possible treatment for multiple diseases. The decrease in the expression of NMNAT2 and SIRT6 observed in the spinal cord of ALS patients suggest that normal NAD+ metabolism, as well as, the activity of this neuroprotective sirtuin may be compromised in these patients. This provides further support to the use of therapeutic approaches that increase NAD+ availability and/or potentiate sirtuin activity in ALS. Our data demonstrates that the strategy used to enhance NAD+ levels defines the biological outcome in ALS models. The results presented here support the use of NAD+ bioavailable precursors as the preferred strategy to enhance NAD+ levels in ALS.
Supplementary Material
The approach used to enhance NAD+ levels defines the biological outcome in ALS models.
Nicotinamide riboside delays motor neuron degeneration in hSOD1G93A-ALS mice.
CD38 ablation does not confer protection in hSOD1G93A-ALS mice.
The expression of NMNAT2 and SIRT6 decreases in the spinal cord of ALS patients.
Acknowledgements
This study was funded by NIH grant R01NS089640 and by the Swiss National Science Foundation grant 310030B-160318. This work used instrumentation and technical support provided by the Cell and Molecular Imaging Shared Resource Core, Hollings Cancer Center, Medical University of South Carolina (supported by P30 CA138313, P20 GM103542 and S10 OD018113). Human spinal cord tissue samples were obtained from the University of Maryland Brain and Tissue Bank through the NIH NeuroBioBank.
Abbreviations:
- ALS
amyotrophic lateral sclerosis;
- Chrna1
nicotinic acetylcholine receptor alpha1 subunit
- Chrng
nicotinic acetylcholine receptor gamma subunit
- Cpt1b
carnitine palmitoyltransferase 1B
- NAD+
oxidized nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- NAPRT
nicotinate phosphoribosyltransferase
- NMNAT
nicotinamide mononucleotide adenylyl transferase
- NMRK1
nicotinamide riboside kinase 1
- NR
nicotinamide riboside
- PARPs
poly(ADP-ribose) polymerases
- Pfkfb3
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- SIRT
sirtuin
- SOD1
superoxide dismutase 1
- Uchl1
ubiquitin C-terminal hydrolase L1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
References
- Abeti R, Abramov AY, Duchen MR, 2011. Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain : a journal of neurology 134, 1658–1672. [DOI] [PubMed] [Google Scholar]
- Adebanjo OA, Anandatheerthavarada HK, Koval AP, Moonga BS, Biswas G, Sun L, Sodam BR, Bevis PJ, Huang CL, Epstein S, Lai FA, Avadhani NG, Zaidi M, 1999. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat Cell Biol 1, 409–414. [DOI] [PubMed] [Google Scholar]
- Airhart SE, Shireman LM, Risler LJ, Anderson GD, Nagana Gowda GA, Raftery D, Tian R, Shen DD, O’Brien KD, 2017. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PloS one 12, e0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy P, White TA, Thompson M, Chini EN, 2006. Regulation of intracellular levels of NAD: a novel role for CD38. Biochemical and biophysical research communications 345, 1386–1392. [DOI] [PubMed] [Google Scholar]
- Ali YO, Allen HM, Yu L, Li-Kroeger D, Bakhshizadehmahmoudi D, Hatcher A, McCabe C, Xu J, Bjorklund N, Taglialatela G, Bennett DA, De Jager PL, Shulman JM, Bellen HJ, Lu HC, 2016. NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies. PLoS biology 14, e1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali YO, Li-Kroeger D, Bellen HJ, Zhai RG, Lu HC, 2013. NMNATs, evolutionarily conserved neuronal maintenance factors. Trends in neurosciences 36, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Friedlich A, Ferrante KL, Hughes D, Szabo C, Beal MF, 2001. Effects of an inhibitor of poly(ADP-ribose) polymerase, desmethylselegiline, trientine, and lipoic acid in transgenic ALS mice. Experimental neurology 168, 419–424. [DOI] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C, 2007a. NAD+ metabolism in health and disease. Trends in biochemical sciences 32, 12–19. [DOI] [PubMed] [Google Scholar]
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C, 2007b. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 129, 473–484. [DOI] [PubMed] [Google Scholar]
- Berger F, Ramirez-Hernandez MH, Ziegler M, 2004. The new life of a centenarian: signalling functions of NAD(P). Trends in biochemical sciences 29, 111–118. [DOI] [PubMed] [Google Scholar]
- Berger NA, Besson VC, Boulares AH, Burkle A, Chiarugi A, Clark RS, Curtin NJ, Cuzzocrea S, Dawson TM, Dawson VL, Hasko G, Liaudet L, Moroni F, Pacher P, Radermacher P, Salzman AL, Snyder SH, Soriano FG, Strosznajder RP, Sumegi B, Swanson RA, Szabo C, 2018. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br J Pharmacol 175, 192–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C, 2004. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502. [DOI] [PubMed] [Google Scholar]
- Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson MEV, Hansson GC, Gotkine M, Segal E, Elinav E, 2019. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. [DOI] [PubMed] [Google Scholar]
- Blacher E, Dadali T, Bespalko A, Haupenthal VJ, Grimm MO, Hartmann T, Lund FE, Stein R, Levy A, 2015. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Annals of neurology 78, 88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A, Moncada S, 2010. Glycolysis: a bioenergetic or a survival pathway? Trends in biochemical sciences 35, 145–149. [DOI] [PubMed] [Google Scholar]
- Brown RH, Al-Chalabi A, 2017. Amyotrophic Lateral Sclerosis. N Engl J Med 377, 162–172. [DOI] [PubMed] [Google Scholar]
- Buck E, Bayer H, Lindenberg KS, Hanselmann J, Pasquarelli N, Ludolph AC, Weydt P, Witting A, 2017. Comparison of Sirtuin 3 Levels in ALS and Huntington’s Disease-Differential Effects in Human Tissue Samples vs. Transgenic Mouse Models. Front Mol Neurosci 10, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J, 2012. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell metabolism 15, 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Menzies KJ, Auwerx J, 2015. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell metabolism 22, 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale A, Paldino E, Giampa C, Bernardi G, Fusco FR, 2015. PARP-1 Inhibition Is Neuroprotective in the R6/2 Mouse Model of Huntington’s Disease. PloS one 10, e0134482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Gilley J, Coleman MP, 2014. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nature reviews. Neuroscience 15, 394–409. [DOI] [PubMed] [Google Scholar]
- de Boer AS, Koszka K, Kiskinis E, Suzuki N, Davis-Dusenbery BN, Eggan K, 2014. Genetic validation of a therapeutic target in a mouse model of ALS. Sci Transl Med 6, 248ra104. [DOI] [PubMed] [Google Scholar]
- de la Rubia JE, Drehmer E, Platero JL, Benlloch M, Caplliure-Llopis J, Villaron-Casales C, de Bernardo N, AlarcOn J, Fuente C, Carrera S, Sancho D, GarcIa-Pardo P, Pascual R, JuArez M, Cuerda-Ballester M, Forner A, Sancho-Castillo S, Barrios C, Obrador E, Marchio P, Salvador R, Holmes HE, Dellinger RW, Guarente L, Estrela JM, 2019. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotrophic lateral sclerosis & frontotemporal degeneration 20, 115–122. [DOI] [PubMed] [Google Scholar]
- Dellinger RW, Santos SR, Morris M, Evans M, Alminana D, Guarente L, Marcotulli E, 2017. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD(+) levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano M, Conforti L, 2013. Diversification of NAD biological role: the importance of location. The FEBS journal 280, 4711–4728. [DOI] [PubMed] [Google Scholar]
- Dittenhafer-Reed KE, Richards AL, Fan J, Smallegan MJ, Fotuhi Siahpirani A, Kemmerer ZA, Prolla TA, Roy S, Coon JJ, Denu JM, 2015. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell metabolism 21, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP, 2004. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proceedings of the National Academy of Sciences of the United States of America 101, 11159–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, Cartwright DM, Oakey L, Burley CV, Jenkinson N, Wilson M, Lucas SJE, Akerman I, Seabright A, Lai YC, Tennant DA, Nightingale P, Wallis GA, Manolopoulos KN, Brenner C, Philp A, Lavery GG, 2019. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD(+) Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep 28, 1717–1728 e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Petzold S, Korner A, Imai S, Kiess W, 2009. Nampt: linking NAD biology, metabolism and cancer. Trends in endocrinology and metabolism: TEM 20, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Milbrandt J, DiAntonio A, 2016. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron 89, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Mayer PR, Yu G, Coleman MP, 2019. Low levels of NMNAT2 compromise axon development and survival. Human molecular genetics 28, 448–458. [DOI] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM, 2013. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiology of aging 34, 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez de Aguilar JL, Niederhauser-Wiederkehr C, Halter B, De Tapia M, Di Scala F, Demougin P, Dupuis L, Primig M, Meininger V, Loeffler JP, 2008. Gene profiling of skeletal muscle in an amyotrophic lateral sclerosis mouse model. Physiol Genomics 32, 207–218. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. , 1994. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK, 2011. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nature biotechnology 29, 824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan BA, Pehar M, Killoy KM, Vargas MR, 2019. Enhanced SIRT6 activity abrogates the neurotoxic phenotype of astrocytes expressing ALS-linked mutant SOD1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33, 7084–7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan BA, Pehar M, Sharma DR, Beeson G, Beeson CC, Vargas MR, 2016. Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing Amyotrophic Lateral Sclerosis-linked Mutant Superoxide Dismutase 1 (SOD1). The Journal of biological chemistry 291, 10836–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH, 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits AZ, Hunter TA, Maxwell N, Pereira K, Whittaker CA, Valdez G, Guarente LP, 2018. SIRT1 deacetylase in aging-induced neuromuscular degeneration and amyotrophic lateral sclerosis. Aging cell 17, e12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, Stevnsner TV, Mattson MP, Bohr VA, 2018. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proceedings of the National Academy of Sciences of the United States of America 115, E1876–E1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Guarente L, 2014. NAD+ and sirtuins in aging and disease. Trends in cell biology 24, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H, 2007. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45. [DOI] [PubMed] [Google Scholar]
- Jing Z, Xing J, Chen X, Stetler RA, Weng Z, Gan Y, Zhang F, Gao Y, Chen J, Leak RK, Cao G, 2014. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34, 1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsyuba E, Auwerx J, 2017. Modulating NAD(+) metabolism, from bench to bedside. The EMBO journal 36, 2670–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen TM, Suh SW, Higashi Y, Berman AE, Escartin C, Won SJ, Wang C, Cho SH, Gan L, Swanson RA, 2011. Poly(ADP-ribose)polymerase-1 modulates microglial responses to amyloid beta. J Neuroinflammation 8, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia A, McAvoy K, Krishnamurthy K, Trotti D, Pasinelli P, 2018. Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia 66, 1016–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim T, Lee HR, Jang EH, Ryu HH, Kang M, Rah SY, Yoo J, Lee B, Kim JI, Lim CS, Kim SJ, Kim UH, Lee YS, Kaang BK, 2016. Impaired learning and memory in CD38 null mutant mice. Molecular brain 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner S, Boselt S, Thau N, Rath KJ, Dengler R, Petri S, 2013. Differential sirtuin expression patterns in amyotrophic lateral sclerosis (ALS) postmortem tissue: neuroprotective or neurotoxic properties of sirtuins in ALS? Neuro-degenerative diseases 11, 141–152. [DOI] [PubMed] [Google Scholar]
- Lautrup S, Sinclair DA, Mattson MP, Fang EF, 2019. NAD(+) in Brain Aging and Neurodegenerative Disorders . Cell metabolism 30, 630–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs M, Gilley J, Zhu Y, Orsomando G, Angeletti C, Liu J, Yang X, Park J, Hopkin RJ, Coleman MP, Zhai RG, Stottmann RW, 2019. Severe biallelic loss-of-function mutations in nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) in two fetuses with fetal akinesia deformation sequence. Experimental neurology 320, 112961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundsgaard AM, Fritzen AM, Kiens B, 2018. Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends in endocrinology and metabolism: TEM 29, 18–30. [DOI] [PubMed] [Google Scholar]
- Manzo E, Lorenzini I, Barrameda D, O’Conner AG, Barrows JM, Starr A, Kovalik T, Rabichow BE, Lehmkuhl EM, Shreiner DD, Joardar A, Lievens JC, Bowser R, Sattler R, Zarnescu DC, 2019. Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR, 2018. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nature communications 9, 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, Ditsworth D, Lagier-Tourenne C, Smith RA, Ravits J, Burghes AH, Shaw PJ, Cleveland DW, Kolb SJ, Kaspar BK, 2014. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proceedings of the National Academy of Sciences of the United States of America 111, 829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI, 2016. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell metabolism 24, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel E, Cassina A, Martinez-Palma L, Souza JM, Bolatto C, Rodriguez-Bottero S, Logan A, Smith RA, Murphy MP, Barbeito L, Radi R, Cassina P, 2014. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free radical biology & medicine 70, 204–213. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J, 2013. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 154, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S, 2007. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature neuroscience 10, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamiuc L, Schlagowski A, Ngo ST, Vernay A, Dirrig-Grosch S, Henriques A, Boutillier AL, Zoll J, Echaniz-Laguna A, Loeffler JP, Rene F, 2015. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol Med 7, 526–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Reyes-Ruiz JM, Lopergolo D, Roseti C, Bertollini C, Ruffolo G, Cifelli P, Onesti E, Limatola C, Miledi R, Inghilleri M, 2016. Acetylcholine receptors from human muscle as pharmacological targets for ALS therapy. Proceedings of the National Academy of Sciences of the United States of America 113, 3060–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, Randall TD, Lund FE, 2001. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nature medicine 7, 1209–1216. [DOI] [PubMed] [Google Scholar]
- Pehar M, Beeson G, Beeson CC, Johnson JA, Vargas MR, 2014. Mitochondria-Targeted Catalase Reverts the Neurotoxicity of hSOD1G93A Astrocytes without Extending the Survival of ALS-Linked Mutant hSOD1 Mice. PloS one 9, e103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Harlan BA, Killoy KM, Vargas MR, 2018. Nicotinamide Adenine Dinucleotide Metabolism and Neurodegeneration. Antioxidants & redox signaling 28, 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravits J, Appel S, Baloh RH, Barohn R, Brooks BR, Elman L, Floeter MK, Henderson C, Lomen-Hoerth C, Macklis JD, McCluskey L, Mitsumoto H, Przedborski S, Rothstein J, Trojanowski JQ, van den Berg LH, Ringel S, 2013. Deciphering amyotrophic lateral sclerosis: what phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotrophic lateral sclerosis & frontotemporal degeneration 14 Suppl 1, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Chio A, Traynor BJ, 2014. State of play in amyotrophic lateral sclerosis genetics. Nature neuroscience 17, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. , 1993. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62. [DOI] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA, 2006. Tryptophan metabolism in the central nervous system: medical implications. Expert reviews in molecular medicine 8, 1–27. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J, 2009. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 5525–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Soundarapandian MM, Chechneva O, Williams AJ, Sidorov MK, Soulika AM, Pleasure DE, Deng W, 2009. PARP-1 deficiency increases the severity of disease in a mouse model of multiple sclerosis. The Journal of biological chemistry 284, 26070–26084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Miranda CJ, Braun L, Meyer K, Frakes AE, Ferraiuolo L, Likhite S, Bevan AK, Foust KD, McConnell MJ, Walker CM, Kaspar BK, 2016. Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nature medicine 22, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Song Y, Kincaid B, Bossy B, Bossy-Wetzel E, 2013. Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: neuroprotection by SIRT3 and PGC-1alpha. Neurobiology of disease 51, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S, Counts SE, Auwerx J, 2017. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature 552, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C, 2016. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nature communications 7, 12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA, 2008. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 13574–13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L, 2006. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. Journal of neurochemistry 97, 687–696. [DOI] [PubMed] [Google Scholar]
- Wang X, Li H, Ding S, 2016. Pre-B-cell colony-enhancing factor protects against apoptotic neuronal death and mitochondrial damage in ischemia. Sci Rep 6, 32416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A, Han X, Wilkins HM, Swerdlow RH, Wang X, Ding S, 2017. Deletion of Nampt in Projection Neurons of Adult Mice Leads to Motor Dysfunction, Neurodegeneration, and Death. Cell Rep 20, 2184–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Ageta-Ishihara N, Nagatsu S, Takao K, Komine O, Endo F, Miyakawa T, Misawa H, Takahashi R, Kinoshita M, Yamanaka K, 2014. SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Molecular brain 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Lu Y, Hao B, Zhang K, Wang Q, Miller AL, Zhang LR, Zhang LH, Yue J, 2015. CD38 Is Required for Neural Differentiation of Mouse Embryonic Stem Cells by Modulating Reactive Oxygen Species. Stem cells 33, 2664–2673. [DOI] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S, 2011. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell metabolism 14, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Choleris E, Lund FE, Kirkland JB, 2006. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochemical and biophysical research communications 346, 188–192. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, Bellen HJ, 2008. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature 452, 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J, 2016. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


