Abstract
It appears that electronic cigarettes (EC) are a less harmful alternative to conventional cigarette (CC) smoking, as they generate substantially lower levels of harmful carcinogens and other toxic compounds. Thus, switching from CC to EC may be beneficial for smokers. However, recent accounts of EC- or vaping-associated lung injury (EVALI) has raised concerns regarding their adverse health effects. Additionally, the increasing popularity of EC among vulnerable populations, such as adolescents and pregnant women, calls for further EC safety evaluation. In this state-of-the-art review, we provide an update on recent findings regarding the neurological effects induced by EC exposure. Moreover, we discuss possible neurotoxic effects of nicotine and numerous other chemicals which are inherent both to e-liquids and EC aerosols. We conclude that in recognizing pertinent issues associated with EC usage, both government and scientific researchers must address this public health issue with utmost urgency.
Keywords: e-cigarette, electronic cigarette, vaping, neurotoxicity, brain, neurodevelopment
1. E-cigarettes as an alternative to conventional tobacco products
Tobacco smoking is considered the most preventable disease cause worldwide (Samet, 2013). The association between tobacco smoking and pathological conditions, such as cancer, cardiovascular diseases or neurodevelopmental diseases is well established (Burns, 2003; Lagiou and Lagiou, 2017; Nguyen et al., 2018; Sales et al., 2019). Although the consumption of conventional cigarettes (CC) has decreased, alternatives such as electronic cigarettes (EC, widely known as e-cigarettes, e-cigs or vaping) emerged, and their usage in the last decade has increased (Dai and Leventhal, 2019).
EC are battery-operated devices designed to release vapors of nicotine and/or other substances to be inhaled in aerosolized form, intended to provide an analog sensation to tobacco smoking, absent actual smoke (Haddad et al., 2019; Tegin et al., 2018). The EC basic structure consists of a battery, a heating element, and a liquid containing cartridge. The increase in the airflow activates the heating element which is in contact with the liquid (e-liquid or e-juice). This results in liquid vaporization and formation of aerosol, which is released and inhaled by the user. When heated, some constituents in the e-liquid (e.g. particular solvents) can generate a vapor that closely resembles tobacco smoke. However, no burn or flame is generated, which means that carbon monoxide and many other toxic combustion products are not present in the aerosol (Hatsukami et al., 2019; Kaisar et al., 2016; Patil et al., 2019; Stone and Marshall, 2019; Zhang et al., 2018a).
Thus, EC are considered less harmful than smoking regular tobacco cigarettes (Amrock et al., 2015; Farsalinos and Polosa, 2014), and are marketed as an alternative, safe and effective form of smoking-cessation therapy. A recent randomized clinical trial has shown a higher rate (18%) of sustained (1 year) abstinence from CC when EC were used, whereas standard nicotine replacement therapies were less effective (9.9%) (Hajek et al., 2019). Other studies have shown weak, or negative effect of EC in encouraging smoking cessation (Kalkhoran and Glantz, 2016; Patil et al., 2019; Worku and Worku, 2019).
Even though EC are free of many hazardous substances generated by CC, they contain other chemicals that may be toxic (Farsalinos and Polosa, 2014; Gupta et al., 2019; Zulkifli et al., 2018). Moreover, an increasing number of cases of severe EC-linked lung injury (CDC, 2020) challenges the idea that EC are a safe alternative to CC.
2. Major health concerns associated with e-cigarettes
Since the primary patent filing in 2003 and further launch on the Chinese (2004), European (2006) and the US (2007) markets, the use of EC is continuously growing, especially among young individuals (MacDonald and Middlekauff, 2019; McCubbin et al., 2017; Tegin et al., 2018). Recent prevalence of EC consumption has been estimated at 3.5–5.5% in the general population, where most of the EC users were also recognized as CC smokers (Dai and Leventhal, 2019; Yoong et al., 2018). Of importance, during recent years (2014–2018) the prevalence of EC smoking in the general population has slightly declined or remained stable, but increased significantly (46.2%) among young adults (age 18–24), making EC the most common nicotine product used by young adults (data from the US) (Dai and Leventhal, 2019). An analogous trend has been observed for adolescents – an increased use from 0.6% in 2011 to 4.9% in 2018 among middle school students, and from 1.5% to 20.8% among high school students in the same period (Gentzke et al., 2019). Similar trends have also been described in Great Britain, Hong Kong, and Taiwan (Bauld et al., 2017; Chang et al., 2017; Chen et al., 2019; SAHM, 2020).
There are advantages to EC usage. For instance, it has been shown that abstaining from CC smoking by using EC may improve respiratory outcomes among ex-CC smokers (Campagna et al., 2016; Cibella et al., 2016; Polosa et al., 2014; Polosa et al., 2016a; Polosa et al., 2016b). Nonetheless, several cross-sectional surveys of adolescents have shown that EC use is associated with increased risks of respiratory and asthma symptoms and asthma-related school absenteeism (Cho and Paik, 2016; Choi and Bernat, 2016; McConnell et al., 2017; Wang et al., 2016). The association of EC use and self-reported chronic respiratory disorders has been also reported in adults (Perez et al., 2019; Wang et al., 2018; Wills et al., 2019). Clinical studies focusing on pulmonary changes following EC exposure have shown mixed results, with some studies reporting impaired respiratory outcomes (Chaumont et al., 2019; Marini et al., 2014; Meo et al., 2019; Vardavas et al., 2012) and others showing no significant changes (Boulay et al., 2017; Ferrari et al., 2015; Flouris et al., 2013; Polosa et al., 2017). However, increased incidence (recognized recently in the US as a nationwide outbreak) of EC or vaping product use-associated lung injury (EVALI) has raised significant concern. As of January 14, 2020, there were 2,668 hospitalized EVALI cases (including 60 deaths) and nearly half the patients required intensive care to treat respiratory failure (CDC, 2020). Most patients diagnosed with EVALI were younger than 35 years-of-age and have reported the use of EC products containing tetrahydrocannabinol (THC) (82%) acquired primarily from informal sources (78%). Therefore, the outbreak has been linked to EC products containing THC, although the precise cause of the disease remains unknown (see section 4.2.) (Blount et al., 2019; CDC, 2020; Ellington et al., 2020; King et al., 2020).
Another significant health hazard associated with EC usage is cardiovascular diseases (Benowitz and Fraiman, 2017). A cross-sectional analysis by the National Health Interview Survey revealed that daily EC use was linked to increased odds of myocardial infarction (Alzahrani et al., 2018). Several studies have examined the acute effects of EC on vascular function using biomarkers such as flow-mediated dilation (Carnevale et al., 2016), circulating endothelial progenitor cells (Antoniewicz et al., 2016), pulse wave velocity (Vlachopoulos et al., 2016), and others (Chaumont et al., 2018; Franzen et al., 2018), providing evidence for EC-induced vascular damage. Notably, the transient abnormalities of these biomarkers may represent pharmacological effect of nicotine (Benowitz and Burbank, 2016; Benowitz and Fraiman, 2017). Similarly, the main hemodynamic changes following acute EC exposure, such as increase in heart rate and blood pressure (Middlekauff et al., 2014; Vansickel and Eissenberg, 2013; Yan and D’Ruiz, 2015), or the acute cardiac sympathetic activation (Moheimani et al., 2017) were also associated with the presence of nicotine in EC aerosols.
The nicotine delivered EC systems may also have a detrimental effect on juveniles. EC are particularly popular among youngsters (Dai and Leventhal, 2019; Gentzke et al., 2019). The central nervous system (CNS) remains under continuous development and is associated with intense experience-dependent plasticity in the prefrontal cortex, which regulates cognition, emotion, and decision-making (Bernheim et al., 2013; Dwyer et al., 2009; Yuan et al., 2015). The neuronal nicotinic acetylcholine receptors (nAChRs) are critical regulators of brain development, which exhibit higher expression pattern during CNS formation and maturation (Dwyer et al., 2008; Dwyer et al., 2009; Ehlinger et al., 2016). Therefore, nicotine is able to increase neuronal activity with a greater effect in the adolescent brain when compared to the adult (Ehlinger et al., 2016; Shram et al., 2007). Furthermore, chronic nicotine exposure during adolescence has long-term consequences on cognitive behavior associated with diminished cognitive function, which could lead to reduced attention span and enhanced impulsivity in adults (Counotte et al., 2011; Counotte et al., 2009; Trauth et al., 2000). EC associated nicotine exposure during adolescence leads to aberrant activation of nAChRs, and further lifelong changes in neuronal signaling, influencing behaviors such as addiction, cognition and emotional regulation (Tobore, 2019; Yuan et al., 2015). In fact, a strong negative association of white matter integrity with tobacco use by adolescents has been attributed to the increasing popularity of EC (Thayer et al., 2020). EC use has been linked to illicit drug use, mental health problems, and impulsivity in university students (Grant et al., 2019). EC have been shown to have greater addictive potential when compared to CC in educated young adults (Jankowski et al., 2019) (the mechanism behind nicotine addiction and neurotoxicity has been further elaborated in section 6.1). Vaping also potentiates some neurological conditions, such as increased frequency of epileptic seizures (Wharton et al., 2019).
A risk upon use of EC during pregnancy has also emerged (Scherman et al., 2018). One of the pioneering (US population-based) studies reported that the prevalence of daily EC use among pregnant women was relatively low (0,6 %; 2 of 326); however, it revealed numerous misconceptions about EC among pregnant women, for example, approx. 30% of pregnant women did not realize that EC contained nicotine or could be addictive (Mark et al., 2015). Moreover, several studies reported higher rate of EC usage among pregnant woman: 4.9% (Kurti et al., 2017), 6.8% (Cardenas et al., 2019a), 6,5–8.5% (Wagner et al., 2017), 7.0% (Kapaya et al., 2019), probably due to the common perception of EC as a safer alternative to CC. The detrimental effects of nicotine and other CC-derived chemicals on fetal development are well established (Bruin et al., 2010; Faber et al., 2017). However, the perception among pregnant women of EC as a minor or moderate health hazard (Ashford et al., 2016; McCubbin et al., 2017) might impair this positive trend. Although human studies investigating the impact of maternal vaping on offspring’s health are missing (Cardenas et al., 2019b; NAS, 2018), it is likely that nicotine and other toxicants released by EC present a health risk to the developing fetus. Indeed, EC use during pregnancy increased the risk of smallness-for-gestational-age (Cardenas et al., 2019a). Additionally, animal studies have shown that exposure to EC during early development impairs major organ system development and function. For instance, pregnant rats exposed to EC containing nicotine showed a marked decrease in blood flow in both maternal uterine and fetal umbilical circulation, which led to a reduction in offspring weight (Orzabal et al., 2019). Maternal EC exposure in mice has been shown to impair embryo implantation and cause weight reduction in female offspring (Wetendorf et al., 2019). Maternal exposure to EC has also been shown to negatively affect the offspring’s cardiac (Palpant et al., 2015), lung (Chen et al., 2018a) and kidney (Li et al., 2019) development. The general impact of EC exposure on animal pregnancy and early development has been recently reviewed elsewhere (Greene and Pisano, 2019; Orzabal and Ramadoss, 2019), and known neurodevelopmental effects will be discussed in section 3.
Numerous studies suggest health hazards subsequent to EC usage especially in teenagers and pregnant women. This is due to the increasing popularity of EC with these groups, and simultaneously the higher vulnerability to nicotine and other chemicals present in EC vapors, affecting in particular the developing brain. Therefore, in this paper, we provide an overview of current findings regarding EC impact to the brain and we also discuss neurotoxic effects of chemicals found in both e-liquids and EC vapors (Figure 1).
Figure 1.
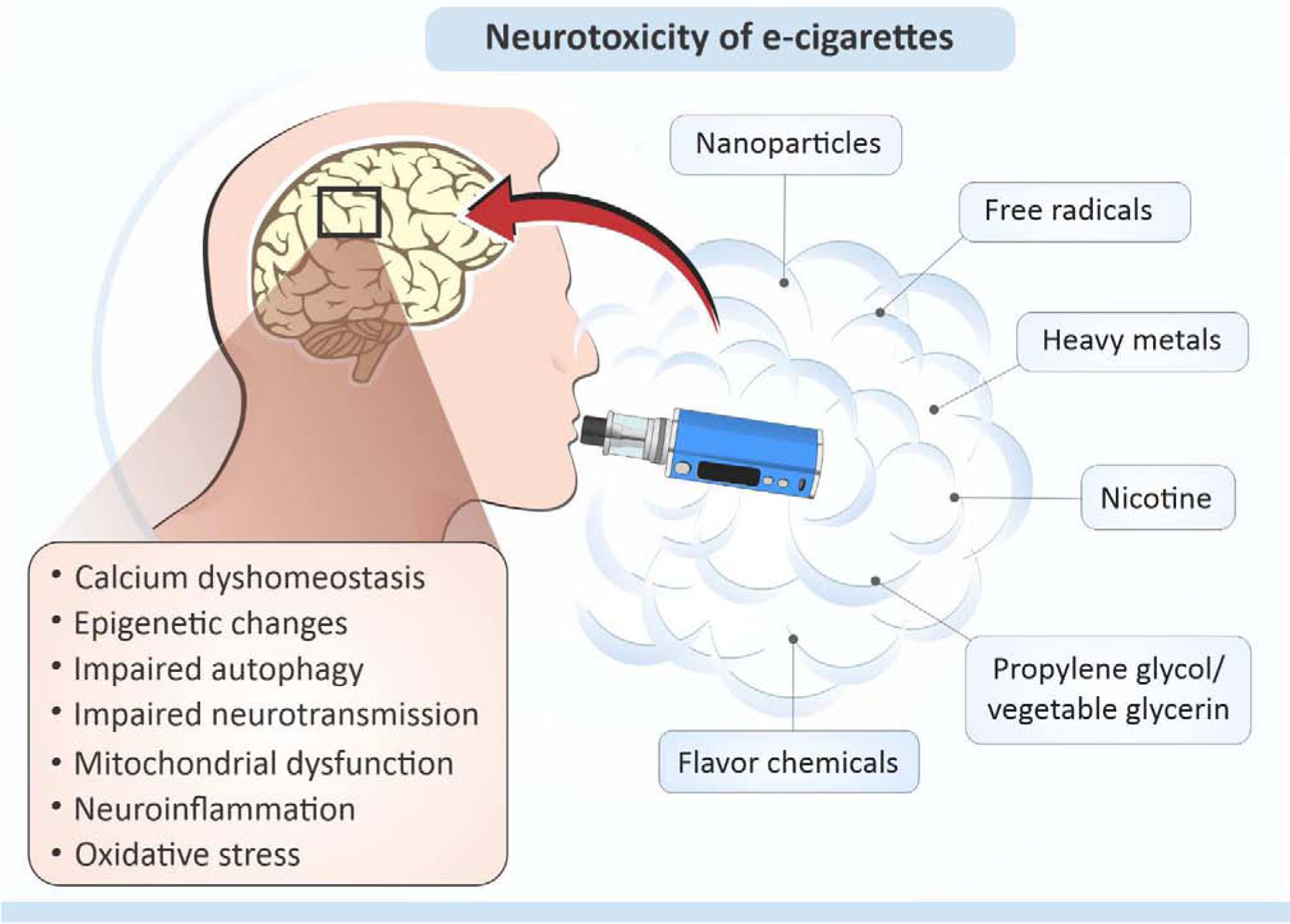
Neurotoxic effects of compounds found in e-cigarettes (see text for details).
3. E-cigarettes exposure affects the brain (animal studies)
3.1. Neurotoxicity of EC aerosols
Despite growing awareness of the potential hazard of EC usage, little is known about their neurotoxic effect. To date, only a few studies have addressed the neurotoxicity of EC, particularly during neurodevelopment. The impact of maternal exposure to EC aerosols on offspring cognitive performance has been evaluated in Balb/c mice. Dams were exposed during and after pregnancy to ambient air, EC aerosols with, or without nicotine. The study showed deficits in short-term memory, reduced anxiety and hyperactivity in offspring following maternal EC exposure (Nguyen et al., 2018). Interestingly, in human maternal CC exposure, particularly heavy smoking (>20 cigarettes/day) has been associated with increased anxiety and other internalizing behaviors in young children (Moylan et al., 2015). Memory deficits were likely caused by nicotine (stronger effect), while reduced anxiety (elevated plus maze tests) was observed in both groups exposed to EC aerosols with and without nicotine, which suggests the neurotoxic effect of other EC constituents. Moreover, exposure to nicotine-free EC aerosols significantly increased global DNA methylation soon after birth, affected histone acetyltransferases, and induced changes within genes linked to neurological activity (Nguyen et al., 2018). The effect of switching from a CC to EC during pregnancy has been also evaluated. One group of Balb/c female mice was exposed to tobacco CC smoke before gestation, during gestation and lactation, while another group was exposed to cigarette smoke before gestation, followed by EC aerosols during gestation and lactation. Continuous tobacco cigarette smoke exposure resulted in marked neurological deficits in the offspring, whereas switching to EC during pregnancy reduced neurological deficits, as compared to CC; however, some neurological damage was still observed. The offspring from mothers switching to EC exhibited normal exploration activity, but showed epigenetic changes – a decrease in global DNA methylation, aurora kinase (Aurk) A and B gene expression (important for cellular division), and a reduction in neuronal cell numbers in the hippocampus, as compared to the control (Nguyen et al., 2019). In a similar study, investigating the replacement of CCs to EC during gestation and lactation, the effect of nicotine, as well as L-carnitine (as a neuroprotectant) were evaluated. CC-induced upregulation of mRNA expression of neuropeptide Y (NPY), NPY Y1 receptor, proopiomelanocortin downstream components, and inducible nitric oxide synthase (iNOS), were normalized in Balb/c offspring due to replacement by EC (with nicotine), but only partially normalized due to L-carnitine supplementation. Interestingly, nicotine-free EC exposure significantly increased mRNA expression of brain NPY and iNOS in pups (Chen et al., 2018b). Epigenetic changes were also observed in offspring of C57BL/6 mice dams exposed to aerosols from EC (with and without nicotine) during gestation and lactation. A comparison of global gene expression changes in the frontal cortex of EC-exposed offspring (one-month-old) revealed that the nicotine-free group (both males and females) had the highest number of unique gene expression changes. Transcriptome alterations in both sexes and treatment groups (with and without nicotine) were all significantly associated with downstream adverse neurobiological outcomes (based on ingenuity pathway analysis, IPA) (Lauterstein et al., 2016). In EC-exposed C57BL/6 mice pups, hippocampal gene expression of neurotrophins, nerve growth factor receptor and brain-derived neurotrophic factor (Bdnf), and serum levels of inflammatory markers, interleukin (IL): IL-1β, IL-2, and IL-6 were significantly reduced irrespective of nicotine presence (Zelikoff et al., 2018). Moreover, exposure to EC aerosols without nicotine enhanced expression of calcium-binding adaptor molecule 1, a specific marker of activated microglia, in the cornus ammonis 1 region of the hippocampus, but not in other brain regions. Analysis of genes associated with the CNS inflammation using frontal cortex transcriptome data revealed, that male offspring exposed early in life to EC aerosols without nicotine had 27 altered genes that overlapped with the IPA “affects inflammation of the CNS” molecule list, while females from the same group had 24 altered genes that fall into that criteria. For pups exposed to EC containing nicotine, in males 6 genes and in females 13 genes (from the list) were altered (Zelikoff et al., 2018). This effect might be due to the dual function of nicotine as both a developmental neurotoxicant and neuroprotectant (see section 4.1). Acute EC vapor exposure (without nicotine) for three days increased reactive oxygen species (ROS) formation in the frontal cortex and down-regulation (mRNA and protein) of a neuronal nitric oxide synthase NOS (nNOS) in whole brain of the adult C57BL/6 mice. Many of these changes were prevented by genetic deletion of phagocytic NADPH oxidase (NOX2), suggesting that EC vapors induce NOX2-dependent prooxidative and inflammatory milieu in the brain (Kuntic et al., 2019). Again, these studies demonstrate that EC components other than nicotine can cause damage to the brain. When the neurological effects of EC vapor vs. CC smoke exposure (three 30-min sessions/day for 7 weeks, producing similar nicotine exposure of 16.8 mg/day) was evaluated in young (one-month-old) male Balb/c mice, both EC and CC led to similarly elevated brain cotinine and nicotine levels, as well as urine cotinine levels. Moreover, both exposures caused the up-regulation of α4β2-containing nAChRs in different brain areas. Mice exposed to CC smoke showed a wide range of behavioral alterations, related to the withdrawal symptoms (locomotor activity, episodic memory, and emotional responses) 24 h after cessation, while mice exposed to EC showed less severe outcomes (Ponzoni et al., 2015). Adult male C57BL/6 mice exposed for 6 months to EC vapors containing nicotine (24 mg/ml) showed altered homeostasis of several neurotransmitters in the mesocorticolimbic areas: levels of dopamine decreased in the striatum, γ-aminobutyric acid (GABA) decreased in the frontal cortex, glutamate increased in the striatum, glutamine increased in both the striatum and frontal cortex, whereas no changes in serotonin levels were observed, suggesting that chronic EC use may result in the development of nicotine dependence (Alasmari et al., 2019). Exposure of adult male C57BL/6J mice to EC vapor (24 mg/ml nicotine) for 1 week decreased brain glucose uptake and glucose transporters (GLUT) GLUT1 and GLUT3 expression under normoxic and ischemic conditions (Sifat et al., 2018). Similarly, 2 weeks exposure to EC (24 mg/ml nicotine) lead to the oxidative stress, loss of blood-brain barrier (BBB) integrity, neurovascular inflammation, and worsening of post-ischemic brain injury at the rate analogous to CC (Kaisar et al., 2017), which suggest that EC usage could enhance ischemic brain injury and/or stroke risk. EC exposure has also been shown to alter brain lipid profiles. Adult Sprague Dawley rats exposed to EC vapors (18mg/ml nicotine) for 4 or 8 weeks showed a significant increase in saturated fatty acids and decrease in cholesterol and oxysterols levels in the brain, which as authors suggest, could contribute to the development of neurodegenerative diseases (Cardenia et al., 2018).
3.2. Neurotoxicity of EC liquids
Besides vapor exposure, there is also a risk related to the ingestion of e-liquids – a few fatal, or near-fatal cases from purposeful or incidental e-liquid exposures have been reported (Belkoniene et al., 2019; Chen et al., 2015; Hua and Talbot, 2016; Ordonez et al., 2015; Payne et al., 2017; Thornton et al., 2014). Thus, the potential neurotoxicity of e-liquids should be also considered. When e-liquid (28 μl/kg of body weight) with, or without nicotine (0.5 mg/kg), has been intraperitoneally injected to adult rats for 4 weeks, it altered cognitive functions of animals, with the more pronounced effect in nicotine-free group, which corresponded larger decrease in hippocampal cell viability, when compared to nicotine-containing group. Yet, e-liquid treatment did not affect motor function and cortical cell viability in both groups (Golli et al., 2016). Toxicity of 35 commercially available EC refills at concentrations 0.001–1% has been compared in vitro in mouse neural stem cells (mNSC) and other cell types – human embryonic stem cells (hESC) and human pulmonary fibroblasts (hPF). Overall, hESCs and mNSCs were more sensitive to refill solutions than hPF. Refill products varied from non-cytotoxic to highly cytotoxic, demonstrating variability and the importance of evaluating multiple marketed samples. Interestingly, cytotoxicity was not correlated with nicotine levels, but with the number and concentration of flavor chemicals. Of note, doses applied in the study were 100 times lower (maximum concentration of 1%) than a potential dose of human exposure (Bahl et al., 2012). The possible molecular mechanism behind EC neurotoxicity was revealed in the recent in vitro study. In mNSCs, EC liquid and aerosol exposures (up to 24h, 0.3–1%) altered mitochondrial dynamics and mitochondrial membrane potential, caused mitochondrial hyperfusion and swelling (Zahedi et al., 2019). Moreover, EC induced increased production of mitochondrial superoxide levels, protein oxidation, mitochondrial DNA aggregation, and impaired autophagy clearance. Observed mitochondrial damage was likely due to impaired calcium homeostasis and attributed mostly to the nicotine presence. Despite excessive mitochondrial damage, mNSCs exposure to e-liquids and aerosols did not lead to mitophagy in most scenarios, except for the treatment with 1% menthol-containing EC group (Zahedi et al., 2019).
Taken together, exposure to EC aerosols and liquids may cause developmental delays, neurobehavioral changes and cognitive deficits, which imply their neurotoxic potential. On a molecular level, EC-induced changes in the brain were associated with massive epigenetic alterations, mitochondrial damage, oxidative stress, inflammation, calcium and neurotransmitter dyshomeostasis (Figure 1). Interestingly, many detrimental effects of EC exposure were observed regardless of nicotine presence, suggesting either the neuroprotective role of nicotine or neurotoxicity of other EC constituents.
4. Neurotoxicity of e-liquid and EC aerosol constituents
ECs are generally considered safer than CC – the mainstream cigarette smoke delivered about 1500 times more harmful and potentially harmful constituents than EC aerosols (Tayyarah and Long, 2014). Albeit, the EC emission is not free from dangerous chemicals. Despite the absence of combustion products and toxicants such as carbon monoxide, and very low levels of tobacco-specific nitrosamines, EC may increase exposure to other toxic or potentially toxic compounds, some of them are not present in conventional tobacco smoke. Typically, EC liquid contains solvent carriers (>75%) – usually propylene glycol (PG) and/or vegetable glycerin (VG), water (20%), flavoring chemicals (10%), and nicotine (2%), and similar proportions of these constituents are found in EC-derived aerosols (Tayyarah and Long, 2014). Moreover, EC aerosols contain additional substances, such as formaldehyde and acrolein (Kaisar et al., 2016; Sleiman et al., 2016), and numerous trace substances, such as heavy metals, phenolic compounds, polycyclic aromatic hydrocarbons, and other, potentially harmful chemicals. The neurotoxic properties of common EC constituents have been reviewed.
4.1. Nicotine
Nicotine is the most studied constituent of EC exhibiting neurotoxic properties. The nicotine levels in e-liquids vary widely among products, with a range from 0 to 87.2 mg/ml (NAS, 2018). Actual nicotine intake is only partially dependent on the nicotine concentration in e-liquid. Other factors, such as device characteristics (e.g., generations/models, heat/power settings) and user behavior (e.g., experience, puff topography) are also important determinants of systemic nicotine exposure (DeVito and Krishnan-Sarin, 2018). There is substantial evidence that nicotine intake from EC among experienced users is equivalent to that from CC (NAS, 2018). A study measuring urinary and salivary nicotine equivalents in chronic EC users found that long-term use of EC is associated with roughly similar daily nicotine intake compared with CC users (Shahab et al., 2017). EC can deliver nicotine rapidly to the human brain, although brain nicotine accumulation from EC was smaller than that from CC in both males (24%) and females (32%) (Solingapuram Sai et al., 2019). Yet the effects of EC use on resting state functional brain connectivity and withdrawal symptoms were similar to those observed for CC, and other forms of nicotine administration, suggesting analogous addictive properties (Hobkirk et al., 2018). As described earlier (section 3), many neurological and neurodevelopmental effects observed in animals exposed to EC may be attributed to the nicotine neurotoxicity and its addictive properties (Alasmari et al., 2019; Javadi-Paydar et al., 2019; Li et al., 2018).
4.1.1. Neurotoxic effect of nicotine and mechanisms of nicotine addiction
Developmental neurotoxicity of nicotine is well established – prenatal exposure of the fetus to nicotine is likely to disturb the balance of cholinergic transmission and hence result in various behavioral and developmental impairments in the offspring, as well as perinatal morbimortality (Bruin et al., 2010; Cauley et al., 2018; Slikker et al., 2005; Slotkin and Seidler, 2015). General nicotine toxicity related to cholinergic overstimulation (Lott and Jones, 2019). Many psychopharmacological effects of nicotine are related to their addictive properties. Nicotine delivered by non-tobacco mode (EC) can be as popular or addictive as a CC. This is evidenced by the recent use of EC that are devoid of tobacco, but allegedly, provide pure nicotine (Alasmari et al., 2019). Nicotine as an addictive substance stimulates the central reward pathway, believed to be primarily driven by a select dopaminergic trajectory emanating from the ventral tegmental area (VTA) and terminating in nucleus accumbens (NACC), is commonly referred to as “mesolimbic reward pathway”. The significance of this pathway in conferring “pleasure” or “reward” by natural stimuli such as food or sex, or various addictive drugs was established decades ago (NIDA, 2016; Wise, 1996). More recently it has been demonstrated that deficits in this circuit is likely the cause of social interaction impairment in children with autism (Supekar et al., 2018).
The actions of nicotine are primarily mediated through nicotinic receptors. These receptors are ligand-gated ion channels that were first described at the neuromuscular junction and were extensively investigated due to their abundance in electric ray also called torpedo fish (Changeux, 2012; Keesey, 2005). Nicotinic receptors are also abundant in the autonomic ganglia and the CNS. However, the subunit structure of nicotinic receptors at these three sites differ from each other. Thus, at the neuromuscular junction, nicotinic receptors are either in the embryonic form, composed of α1, β1, γ, and δ subunits or in the adult form composed of α1, β1, δ, and ε subunits. In the autonomic ganglia, these receptors are primarily composed of α3–β4 subunit combination whereas in the CNS, the homomeric (all one type of subunit, e.g. α7) or heteromeric (at least one α and one β subunit combination) are the most abundant (Dani, 2015; Gotti et al., 2019). Currently, 12 different neuronal nicotinic receptor subunits (α2–α10 and β2–β4) have been identified. Neuronal nicotinic receptors, primarily composed of α4β2 subunit and strategically located in the VTA and NACC, are critically involved in mediating the rewarding effects of nicotine as well as dependence and addiction to it (Dani, 2015; Gotti et al., 2019). It has been demonstrated that α4 β2 and also α6-containing subunits in VTA and NACC are not only involved in addictive behavior of nicotine, but also other drugs, such as alcohol (Gotti et al., 2019; Mineur and Picciotto, 2008; Rahman et al., 2016; Tolu et al., 2017). Moreover, a recent study implicates α6 in nicotine-induced structural plasticity in mouse and human induced pluripotent stem cells -derived dopaminergic neurons (Collo et al., 2018).
4.1.2. Nicotine as a neuroprotectant and cognitive enhancer
In addition to their role in nicotine addiction, nAChRs have also been implicated in various neurological diseases such as Parkinson’s (PD), Alzheimer’s (AD), epilepsy, as well as neuropsychiatric disorders such as schizophrenia and depression (Brown et al., 2013; De Biasi, 2015; Dineley et al., 2015; Hurley and Tizabi, 2013; Tizabi, 2016; Tizabi and Getachew, 2017; Tizabi et al., 2010). Although in many studies, nicotine is presented as a neuroprotectant (Tizabi, 2016). In regard to depression, an antidepressant effect of nicotine has been well established by preclinical (Djuric et al., 1999; Kalejaiye et al., 2013; Semba et al., 1998; Tizabi et al., 2009; Tizabi et al., 2010; Tizabi et al., 1999; Tizabi et al., 2000), as well as few clinical studies (Cook et al., 2007; McClernon et al., 2006; Pomerleau and Pomerleau, 1992; Salin-Pascual et al., 1995). Moreover, high incidence of smoking among depressed patients may be considered as an attempt at self-medication with nicotine, which also contributes to the difficulty in quitting smoking by such population (Cook et al., 2007; Edwards and Kendler, 2011; Moreno-Coutino et al., 2007; Spring et al., 2008). The antidepressant effects of nicotine are believed to be primarily mediated by α4-β2 and to some extent by α7 subtypes (Alzarea and Rahman, 2019; Barreto et al., 2014; Picciotto et al., 2015; Rahman, 2015). Neuroprotective effects of nicotine and its applicability to neurological disease, particularly PD has been documented (Hoskin et al., 2019; Tizabi and Getachew, 2017). In cellular models of substantia nigra neurons, nicotine has been shown to protect against salsolinol and aminochrome that selectively damage the dopaminergic cells (Copeland et al., 2007; Das and Tizabi, 2009; Munoz et al., 2012; Ramlochansingh et al., 2011). Nicotine protection against toxicity induced by iron (Fe) and manganese (Mn), excess of which has been implicated in PD, were also recently reported (Getachew et al., 2019). Earlier studies verified the protection of nicotine in PD-like symptoms induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin in various animal studies, including non-human primates (Quik et al., 2015; Quik et al., 2006). Postulated mechanisms of nicotine action include inhibition of astrocyte activation and hence inflammatory suppression (Bagdas et al., 2018; Liu et al., 2012; Patel et al., 2017), modulation of synaptic plasticity via interaction with the neurotrophic mediators, especially Bdnf (Bagdas et al., 2018; Edwards and Kendler, 2011; Hoskin et al., 2019; Liu et al., 2012; Patel et al., 2017; Quik et al., 2015). The latter mechanism may also be at least partially responsible for the antidepressant effects of nicotine (Bagdas et al., 2018; Liu et al., 2012; Patel et al., 2017; Quik et al., 2015). It is noteworthy that nicotine activation of presynaptic receptors on various neuronal networks can enhance the release of a number of neurotransmitters such as dopamine, norepinephrine, serotonin, and glutamate, all of which have been implicated in mood regulation (Gotti et al., 2019; Liu et al., 2018; Miller, 2019). Curiously, there exists a substantial co-morbidity between PD and depression, which poses a further challenge in treatment options as depression itself may be an indication of latent neurodegeneration, particularly in late life (Baquero and Martin, 2015; Burgut et al., 2006; Hurley and Tizabi, 2013). In addition, nicotine role in inflammatory processes, apoptosis, oxidative stress, protein aggregation, and neuroplasticity, may also extend its beneficial effects to other CNS diseases, such as autism schizophrenia, epilepsy or Tourette syndrome (De Biasi, 2015; Dineley et al., 2015; Tizabi, 2016; Tizabi et al., 2019).
Cognitive enhancing effects of nicotine have also been amply verified, as reviewed by (Besson and Forget, 2016). This contention is supported by a number of animal studies showing that nicotine can block or reverse the cognitive impairment induced by chronic stress (Andreasen et al., 2011; Shang et al., 2017); glutamate N-methyl-D-aspartate (NMDA) receptor antagonist (Andre et al., 2011; Jacklin et al., 2012); lipopolysaccharide (Wei et al., 2015) and methamphetamine (Vieira-Brock et al., 2015), as well a clinical study showing beneficial effects of nicotine patch in mild cognitive impairment (Newhouse et al., 2012).
In summary, nicotine is a neurotoxicant and highly addictive drug, but with great therapeutic potential in various neurodegenerative and neuropsychiatric disorders. However, beneficial, neuroprotective effects of nicotine exposure may be easily nullified due to the presence in EC liquids and vapors, other harmful and neurotoxic compounds.
4.2. Solvents
The most common solvents and simultaneously the primary ingredients (>75%) of e-liquids are PG and/or VG (Tayyarah and Long, 2014). Additionally, other solvents in lower concentrations have been detected in commercial e-liquids (Hutzler et al., 2014), e.g. ethylene glycol (WHO, 2002). Both PG and VG are generally recognized as safe (GRAS) and approved by the Food and Drug Administration (FDA) by ingestion. Results of ninety-day rat study (funded by the tobacco industry) revealed low toxicity of inhaled PG/VG mixtures in a number of endpoints (measured in blood, lung, and liver), and only the addition of nicotine produced adverse effects (Phillips et al., 2017). Nevertheless, other studies provide evidence that at certain conditions PG/VG may produce adverse health effects. For glycerin and its pure form glycerol, animal studies have shown relatively low toxicity both in acute (Becker et al., 2019) and chronic exposures (Gad et al., 2006), also via inhalation (Renne et al., 1992), and toxic effects have been observed only due to dramatic overdose (Andresen et al., 2009; Traudt et al., 2014). For PG, a solvent widely applied in the pharmaceutical, cosmetic and food industry, an airway and ocular irritability from occupational exposure have been reported (Varughese et al., 2005; Wieslander et al., 2001). PG exposure has been associated with toxic effects on the peripheral system (LaKind et al., 1999; Levy et al., 1995) and the CNS disturbances, such as drowsiness and confusion (Arulanantham and Genel, 1978; Lim et al., 2014). There is a limited, but consistent evidence, that very high doses of PG administered orally or intravenously produce toxic effects in humans, related to osmotic changes in the blood, and lactic acid formation, secondary to the PG metabolism (Zosel et al., 2010). For example, PG is a common solvent for intravenous phenobarbitals used for the treatment of neonatal convulsions, thus such treatment may lead to PG levels exceeding safety thresholds (millimolar levels) (Pouwels et al., 2019), resulting in the developing brain damage. Single intraperitoneal injection of PG (2–10 ml/kg) induced widespread apoptosis in the brain of developing C57BL/6 mice at the rate similar to the damage induced by ethanol (Lau et al., 2012). High dose (70% and 35%) PG injections caused axonal degeneration and intraneural inflammation in the sciatic nerve of young female CD1 mice (Belavy et al., 2013), and low dose (0.5–5%) exposure stimulated release of calcium from mitochondrial stores in cerebrocortical synaptosomes isolated from Wistar rats (Satoh et al., 2004). Cytotoxicity of solvents depends on theirs concentrations – liquids containing PG and VG solutions up to 1% were not cytotoxic to mNSCs and other stem cells (Bahl et al., 2012), and aerosols produced from such liquids did not affect mitochondria integrity (Zahedi et al., 2019). However, when 80% PG/ 20% water mixture was aerosolized at 5V, it produced significant cytotoxicity in peripheral cell lines (Behar et al., 2018). When female Balb/c mice were exposed for 2 weeks to EC vapors from three different e‐liquids containing PG 70%/glycerol 30%, PG 100%, or glycerol 100%, they showed altered expression of circadian molecular clock genes in the brain and other organs (e.g. liver or kidney). Considering the role of the circadian molecular clock in biological homeostasis and disease, these alterations could potentially facilitate the onset of adverse neurological conditions (Lechasseur et al., 2017). On the other hand, developmental exposure to PG 100% vapors from EC in C57BL/6J mice reduced pups’ body weight, but had no effect on neurobehavioral outcomes (Smith et al., 2015).
The toxicity of EC solvents may also reflect their pyrolytic properties. When heated in EC device (150–350°C), both PG and VG produce mist which contains numerous toxic carbonyls, such as acrolein, acetaldehyde, and formaldehyde in levels which may reach similar to those found in tobacco smoke (Conklin et al., 2018; Goniewicz et al., 2014; Hutzler et al., 2014; Jensen et al., 2015; Rubinstein et al., 2018; Sleiman et al., 2016). For example, formaldehyde is a well-established environmental neurotoxicant linked to neurodegeneration (Songur et al., 2010; Tulpule and Dringen, 2013). Additionally, during vaping at high voltage (5V) formaldehyde reacts with EC solvents and forms potentially toxic formaldehyde hemiacetal (Jensen et al., 2015; Salamanca et al., 2017). Acrolein is also a ubiquitous environmental pollutant linked to neurodegeneration, due to its pro-inflammatory and pro-oxidative properties (Wang et al., 2017). Analogously, acetaldehyde has been shown to be neurotoxic via oxidative stress (Yan et al., 2016), calcium dyshomeostasis (Cui et al., 2019) and NMDA receptor activation (Wan et al., 2000). Many other products of solvents pyrolysis found in EC vapor exhibit hazardous properties. These include: glycidol (Sleiman et al., 2016), which is a developmental neurotoxicant (Akane et al., 2014; Kawashima et al., 2017), and glyoxal and methylglyoxal (Sleiman et al., 2016), which are potent glycating agents mediating the formation of advanced glycation end-products , linked to neurodegeneration (Wetzels et al., 2017).
Another potentially toxic chemical used as solvent in EC is vitamin E acetate (α-tocopherol acetate; ester of α-tocopherol and acetic acid), which has recently been detected in the majority of THC-containing products and samples of lung fluid from EVALI patients (Blount et al., 2019; Lewis et al., 2019; Taylor et al., 2019), suggesting a possible link to this vaping-related illnesses. The FDA reports that most outbreak-associated THC products contain vitamin E acetate, at an average concentration of 50% (23– 88%) (it is used as THC solvent because of similar viscosity) (FDA, 2020). Vitamin E acetate is commonly used in the dietary supplements and in cosmetics as a precursor of vitamin E (Desmarchelier et al., 2013), and to date has not been associated with adverse health outcomes, and is recognized as GRAS (FDA, 2019) for ingestion only. Although the safety profile (including neurotoxicity) of vitamin E acetate inhalation is not known. In rats, unlike vitamin E, vitamin E acetate inhalation does not attenuate the inflammatory response to bacterial lipopolysaccharide toxicity in lungs (Hybertson et al., 2005). Additional studies to examine the effects of inhaling aerosolized vitamin E acetate are required.
4.3. Flavor chemicals
Numerous studies demonstrated that flavoring is an important factor for EC users (Audrain-McGovern et al., 2016; Nonnemaker et al., 2016). Flavoring is particularly appealing to youth and inexperienced smokers, which are more likely to initiate vaping through flavored products (Harrell et al., 2017; Krusemann et al., 2019; Schneller et al., 2019; Soneji et al., 2019; Zare et al., 2018). Moreover, recent data suggest that popular flavor chemicals may trigger a reward mechanism in mice (Avelar et al., 2019). Therefore, flavorings in EC are currently one of the major targets for regulatory authorities, alarmed by an outbreak of EVALI and fatal cases particularly among young EC users (Layden et al., 2019).
Hundreds of different flavor chemicals have been detected in e-liquids, with several chemicals present in the majority of commercial products (Hua et al., 2019; Omaiye et al., 2019b). Although flavorants detected in EC are usually considered as GRAS in food products, they have not been safety tested for exposure routes other than ingestion and for chronic exposures, thus their perniciousness when aerosolized and inhaled is often unrecognized (Barrington-Trimis et al., 2016). In fact, numerous flavorings, e.g. menthol, menthone, maltol, ethyl maltol, vanillin, ethyl vanillin, cinnamaldehyde, ethyl cinnamate, benzyl alcohol, benzaldehyde, eugenol, p-anisaldehyde, triacetin, and 2,5-dimethypyrazine, have been effectively transferred to EC aerosols that may cause cytotoxicity, mostly to the respiratory system (Behar et al., 2018; Hickman et al., 2019; Hua et al., 2019; Kosmider et al., 2016; Omaiye et al., 2019a; Sassano et al., 2018; Sherwood and Boitano, 2016). Multiple studies have implied that the high number and concentration of flavor chemicals are critical for cellular and CNS toxicity of e-liquids (Behar et al., 2018; Cervellati et al., 2014; Hua et al., 2019; Otero et al., 2019). Nonetheless, no difference between various flavors of e-liquids was observed in eliciting neurobehavioral outcomes in mice (Nguyen et al., 2018), and to date, no other study has addressed neurotoxicity of EC-derived flavoring chemicals. Therefore, there is a huge gap in understanding the effect of flavorants delivered through the EC vapors on the brain, which urgently needs to be evaluated.
Yet, the possible neurotoxic effect of these substances can be predicted based on toxicity evoked in other conditions. For instance, Kaur et al. reviewed data on the toxicity of flavoring chemicals in tobacco and non-tobacco products in the context of pulmonary diseases, and discussed possible pathomechanisms associated with oxidative stress, inflammation and DNA damage (Kaur et al., 2018). Diacetyl and acetyl propionyl, found in the majority of commercial e-liquids and aerosols in levels exceeding the safety limits (Farsalinos et al., 2015a), have been linked to the occupational “popcorn” lung disease (Holden and Hines, 2016). Menthol and ethyl maltol have been found in some commercial EC refill fluids in levels respectively 30 and 100 times their cytotoxic concentration, while one refill product contained 34% of cinnamaldehyde, which was more than 100,000 times its cytotoxic level (Omaiye et al., 2019b). Menthol is a flavorant commonly found in CC. It is of low toxicity and is often used as a natural medicine for cold or pain. However, a case of fatal occupational exposure (Kumar et al., 2016), and a nearly fatal case of chronic exposure through menthol-rich cough droplets (Baibars et al., 2012) have been reported. Moreover, menthol may produce some powerful neurological effects (Henderson et al., 2017; Thompson et al., 2018) – it has been shown to affect cigarette smoking-related behaviors, and at the molecular level, to modulate nAChRs and alter nicotine metabolism, as reviewed in (Wickham, 2015). The other popular chemical flavorant in EC – farnesol has also been shown to produce reward-related behavior in male mice, associated with the upregulation of α6* nAChRs; it enhanced locomotor activity and affected dopaminergic and GABAergic transmissions (Avelar et al., 2019). Maltol toxicity has been shown in neuroblastoma cell lines derived from mouse (Neuro 2a) and human (IMR 32), and in primary murine fetal hippocampal neurons (Hironishi et al., 1996), although this flavorant can also act as neuroprotectant, attenuating oxidative stress and nuclear factor-κB (NF-κB)-dependent signaling pathway (Mi et al., 2018; Song et al., 2015). Cinnamaldehyde is one of the most frequent flavorant in EC (Omaiye et al., 2019b), and cinnamaldehyde-containing e-liquids and aerosols have been shown to produce cytotoxicity, genotoxicity and adversely affect cellular functions at low concentrations in peripheral tissues (Clapp et al., 2017; Hickman et al., 2019; Sassano et al., 2018). For instance, at sub-cytotoxic concentrations, cinnamaldehyde decreased cell growth, attachment and spreading, altered cell morphology, motility, and increased DNA strand breaks in hPF (Behar et al., 2016). Cinnamaldehyde in e-liquids has been shown to alter mitochondrial function (respiration, glycolysis), reduce adenosine triphosphate (ATP) levels, and suppress ciliary beat frequency in bronchial epithelial cells (Clapp et al., 2019).
Several flavor chemicals may pyrolyze or react with other components of EC liquids and produce new, potentially harmful chemicals. For instance, flavor aldehydes, including benzaldehyde, cinnamaldehyde, citral, ethyl vanillin and vanillin rapidly react with PG after mixing, producing aldehyde PG acetals, detected in many commercial e-liquids (Erythropel et al., 2019). Up to 40% of flavor aldehyde content was converted to acetals, with 50–80% preservation in EC vapors, and these acetals were shown to activate aldehyde-sensitive irritant receptors and aldehyde-insensitive irritant receptors in human embryonic kidney cells HEK-293T (Erythropel et al., 2019). Interestingly, several flavor-derived chemicals may have protective properties. For example, sweeteners, such as sucrose, glucose, and sorbitol in EC yielded a significant amount of 5-hydroxymethylfurfural and furfural (Abraham et al., 2011; Adams et al., 1997; Soussy et al., 2016), which exhibit neuroprotective and antioxidant effect via activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)/ antioxidant response element (ARE) signaling pathway (Ya et al., 2017), and apurinic/ apyrimidinic endonuclease/ redox factor-1 (Zhang et al., 2015). In addition, vanillin exhibits antioxidant activity beneficial in cancer therapy (Bezerra et al., 2016) and neuroprotection at a high dose (Ho et al., 2011; Yan et al., 2017).
4.4. Other organic compounds
Additional organic compounds exhibiting neurotoxic properties have been detected in e-liquids, e.g. toluene, p,m-xylene, ethyl acetate, benzene, ethanol, and methanol, with some exceeding safety limits (Lim and Shin, 2017). Benzene was detected in EC aerosols generated from different liquids and devices, reaching maximal levels of 5000 μg/m3, which are significantly lower than from CC (200,000 μg/m3), but still may produce adverse health effects, especially due to repeated exposures (Pankow et al., 2017). The increase in many toxic volatile organic chemicals has also been detected in the urine of adolescent EC users; however at levels lower than in CC/EC dual users (Rubinstein et al., 2018). Xylene, N,N-dimethylformamide (dimethylfuran), and acrylonitrile levels were higher in the urine of EC users than non-smokers (Lorkiewicz et al., 2019). Moreover, some cancerogenic tobacco-specific nitrosamines, e.g. N-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone) have been detected in EC aerosols (Goniewicz et al., 2014) and in EC users (Bustamante et al., 2018; Kotandeniya et al., 2015), although at levels orders of magnitude lower than produced by CC. Potential carcinogenic and neurotoxic phthalates, diethyl phthalate and diethylhexyl phthalate , have been also found in e-liquids (Oh and Shin, 2015).
4.5. Heavy metals and other trace elements
Several neurotoxic heavy metals, such as arsenic (As), cadmium (Cd), lead (Pb), manganese (Mn), zinc (Zn), nickel (Ni), aluminum (Al), tin (Sn), chromium (Cr) and copper (Cu) have been found in EC vapors (Gaur and Agnihotri, 2019; Goniewicz et al., 2014; Halstead et al., 2019; Hess et al., 2017; Mikheev et al., 2016; Olmedo et al., 2018; Williams et al., 2015; Zhao et al., 2019). These metals mostly originate from components of EC atomizer (metallic coil), but may also be present in e-liquids (Beauval et al., 2016). Although the majority of studies have shown significantly lower concentrations of metals in EC vapors than in CC smoke (Goniewicz et al., 2014; Palazzolo et al., 2016), some of them were at similar, or even higher level, e.g. Ni (Halstead et al., 2019; Palazzolo et al., 2016; Williams et al., 2013), Cu (Lerner et al., 2015a), Cr (Halstead et al., 2019), Al and Pb (Williams et al., 2013), raising concerns about their health effect (Halstead et al., 2019; Olmedo et al., 2018). Although risk assessment analyses have shown that inhalation exposures to these metals in EC users are unlikely to exceed safety limits (Farsalinos and Rodu, 2018; Farsalinos et al., 2015b), EC-derived metals can contribute to the overall increase of the internal dose, as has been demonstrated for Cr, Ni (Aherrera et al., 2017), Cd and Pb (Jain, 2019). EC users showed also higher serum levels of selenium (Se), silver (Ag), vanadium (V), lanthanides and other rare earth elements (Badea et al., 2018). Thus, the use of EC may increase exposure to hazardous metals affecting human health (Gaur and Agnihotri, 2019). Moreover, mixtures of different metals and other components, even at levels that are considered below the individual the lowest-observed-adverse-effect level (LOAEL) can have additive or synergistic effects (Andrade et al., 2017; Karri et al., 2016). Albeit numerous metals detected in EC vapors exhibit neurotoxic properties when inhaled (Lebedova et al., 2018; Lucchini et al., 2012; Mate et al., 2017; Wallin et al., 2017), to date no study has specifically addressed the effects of EC-derived metals on the brain
4.6. Nanoparticles
The aerosol emitted by EC contains a high concentration of nanoparticles , which despite the small size (<100 nm), display a significant toxicological impact (Zoroddu et al., 2014). Nanoparticles have a bigger surface area per unit mass, increasing their catalytic potential when compared to larger particles. Moreover, small size allows easier penetration and transport through cellular barriers to various organs, including the brain. Nanoparticle exposure has been linked to developmental neurotoxicity (Umezawa et al., 2018; Wu et al., 2015; Zhang et al., 2018b) and other brain disorders (Engin and Engin, 2019; Mushtaq et al., 2015; Sharma and Sharma, 2012; Teleanu et al., 2019).
Typical EC generates high concentrations of nanoparticles and fewer bigger particles (<10 μm) when compared to CC smoke (Schripp et al., 2013). One puff of aerosol emitted from one type of EC device contained approximately 2×106 nanoparticles/cm3 (<100 nm), particularly of toxic metals, such as Sn, Cr and Ni (Williams et al., 2013). In another study, emission from different e-liquids contained 107–108 nanoparticles/cm3 (5–50 nm), with metals representing approximately 10% of the total nanoparticle mass, while the rest were unknown chemicals (Mikheev et al., 2016). Interestingly, the emission of nanoparticles was dependent on the flavor – it was significantly higher for tobacco-flavored EC than for menthol-flavored EC (Lee et al., 2017). The chemical content and subsequent health hazards of EC-derived nanoparticles require further investigation. Yet one conclusion can be drawn – the smaller the particle size, the higher its ability to produce free radicals (Abdal Dayem et al., 2017), which are additional harmful components of aerosols emitted by EC.
4.7. Free radicals
Many EC constituents, like metallic coil, metals, solvents, flavorings and other components of e-liquids, trigger the generation of harmful free radicals and ROS (Tobore, 2019). Thus, EC users may be exposed to ROS, which in excess cause oxidative stress, a key factor contributing to aging and many diseases, including neurodevelopmental (Dowell et al., 2019; Rock and Patisaul, 2018) and neurodegenerative disorders (Carvalho et al., 2017; Singh et al., 2019). Smoking-related cerebral oxidative stress is a potential mechanism promoting neurodegeneration and increased risk for neurodegenerative diseases (Durazzo et al., 2016).
Activating the EC heating element and aerosolizing the e-liquid produce ROS which may be inhaled and circulate in the body. The amount of ROS produced by EC is generally lower than in CC smoke (10–1000 times) (Goel et al., 2015; Ito et al., 2019; Shein and Jeschke, 2019; Son et al., 2019; Zhao et al., 2018), although considering different usage patterns, the daily exposure could be of comparable levels (Son et al., 2019). Moreover, several conditions (Lerner et al., 2015a) and the new generation of high power EC devices (Haddad et al., 2019) promote the generation of ROS levels analogous to CC. In addition to device settings, e-liquid composition affects the amount of ROS produced – higher ROS emission in EC vapors is dependent on concentration of VG (Haddad et al., 2019; Son et al., 2019) and flavors (Lerner et al., 2015b; Son et al., 2019), but not nicotine (Haddad et al., 2019). Moreover, the age and stage of the EC heating element can affect ROS emission (Lerner et al., 2015b).
EC vapors induce ROS production in vitro in the respiratory system (Anderson et al., 2016; Chatterjee et al., 2019; Ganapathy et al., 2017; Lee et al., 2019; Putzhammer et al., 2016; Scott et al., 2018; Zhao et al., 2018) and other cell types (Di Biase et al., 2018; Shaito et al., 2017). EC-induced oxidative stress is linked to the toxicity of EC vapors in mice lungs (Lerner et al., 2015b), liver (Espinoza-Derout et al., 2019), and rat testicles (Vivarelli et al., 2019). Moreover, increased ROS production and other oxidative stress markers are found in the serum of young healthy individuals using EC for a short time (acute exposure) (Chatterjee et al., 2019). The effect of EC and CC aerosol extracts on cerebrovascular function was evaluated in mouse brain microvascular endothelial cells (mBMECs). Both extracts showed similar properties – increased intracellular ROS levels as well as the expression of Nrf2 and its downstream effector (Sivandzade and Cucullo, 2019). Moreover, both extracts decreased BBB integrity and increased permeability, however, the effect was stronger for CC exposure (Sivandzade and Cucullo, 2019). Similarly, EC and CC treatment promoted ROS-associated mitochondrial depolarization in the vascular endothelial cell line bEnd.3, but only CC induced mitochondrial depolarization in mBMECs, and both exposures upregulated the transmembrane Fe exporter Slc40a1, which is crucial for maintaining cellular Fe and redox homeostasis (Kaisar et al., 2018).
5. Conclusions and perspectives
To date, no published study has evaluated the effects of EC exposure on brain function, and only a few studies have addressed this issue in animals. For instance, mice studies revealed that EC exposure during pregnancy affects neurobehavioral outcomes in offspring, characterized by deficits in short-term memory or anxiety. At the molecular level, EC exposure induced broad epigenetic alterations, mitochondrial dysfunction, inflammation, oxidative stress, calcium, and neurotransmitter dyshomeostasis, all of which may account for the EC associated neurotoxic effects (Figure 1). In addition to nicotine, numerous neurotoxic chemicals, particles, and radicals have been found in EC liquids and aerosols. Some of them may pose a potential hazard for EC users due to their presence at levels exceeding safety limits (e.g. flavorants diacetyl and acetyl propionyl). Others, which are present at low concentrations (e.g. heavy metals), may seem harmless, but due to the cumulative, synergistic effects of various factors that exhibit analogous properties, and target toxic pathways such as induction of oxidative stress, mitochondrial impairment, or inflammation, may also contribute to the neurological outcomes observed following EC exposure. However, studies are limited and oftentimes do not adequately reflect real-life scenarios; for instance, little is known about the long-term effects of EC use. One of the major difficulties in studying EC toxicity is a broad heterogenicity in the composition of e-liquids available on the market. The lack of regulation governing e-liquid formulations has led to a situation when dozens of different solvents, hundreds of flavorants, and great variability in nicotine concentrations may be found in such products. That makes it extremely difficult to determine the composition and levels of chemicals released in aerosols, and subsequently, identify the possible cause of health problems related to EC exposure (e.g. EVALI). This causes also barrier for standardization of experimental designs or comparison of the experimental outcomes.
Another important public health issue related to EC is its increasing popularity among adolescents and pregnant women. Modern design, attractive flavoring, and common belief that EC are a safe alternative to conventional tobacco products, make EC appealing particularly to younger generations. And that may have severe consequences on the health of these vulnerable populations. Additional research is needed in order to fully estimate both the overall safety of EC usage and its specific effects on the brain. Although from the data available we can already conclude that EC should not be ignored as a potential health hazard, and current guidelines regarding EC use should be carefully reconsidered. Future studies should be profitably directed at the aforementioned issues. Ultimately, a balance should be struck between science and technology development, while recognizing pertinent issues associated with EC usage, with government and scientific researchers addressing this public health in an urgent manner.
Highlights.
Electronic cigarettes (EC) are marketed as alternatives to conventional cigarette (CC) smoking
However, recent accounts of EC- or vaping-associated lung injury (EVALI) have raised concerns regarding their adverse health effects.
The increased popularity of EC among vulnerable populations, such as adolescents and pregnant women, calls for further safety evaluation.
EC may have neurotoxic effects due to nicotine and other chemicals inherent both to e-liquids and EC aerosols.
Acknowledgments
This work has been supported by the National Institute of Environmental Health Sciences (R01ES07331, R01ES10563 and R01ES020852).
Abbreviations
- AD
Alzheimer’s disease
- Ag
silver
- Al
aluminum
- ARE
antioxidant response element
- As
arsenic
- ATP
adenosine triphosphate
- Aurk
aurora kinase
- BBB
blood-brain barrier
- Bdnf
brain-derived neurotrophic factor
- CC
conventional cigarettes
- Cd
cadmium
- CNS
central nervous system
- Cr
chromium
- Cu
copper
- EC
electronic cigarettes
- EVALI
electronic cigarette or vaping product use-associated lung injury
- FDA
Food and Drug Administration
- Fe
iron
- GABA
γ-aminobutyric acid
- GLUT
glucose transporters
- GRAS
generally recognized as safe
- hESC
human embryonic stem cells
- hPF
human pulmonary fibroblasts
- iNOS
inducible nitric oxide synthase
- IL
interleukin
- IPA
ingenuity pathway analysis
- LOAEL
lowest-observed-adverse-effect level
- mBMECs
mouse brain microvascular endothelial cells
- Mn
manganese
- mNSC
mouse neural stem cells
- NACC
nucleus accumbens
- nAChRs
nicotinic acetylcholine receptors
- Ni
nickel
- NMDA
N-methyl-D-aspartate
- NOX2
NADPH oxidase
- NF-κB
nuclear factor-κB
- nNOS
neuronal nitric oxide synthase
- NPY
neuropeptide Y
- Nrf2
nuclear factor erythroid 2-related factor 2
- Pb
lead
- PD
Parkinson’s disease
- PG
propylene glycol
- ROS
reactive oxygen species
- Se
selenium
- Sn
tin
- THC
tetrahydrocannabinol
- V
vanadium
- VG
vegetable glycerin
- VTA
ventral tegmental area
- Zn
zinc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdal Dayem A, Hossain MK, Lee SB, Kim K, Saha SK, Yang GM, Choi HY, Cho SG, 2017. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 18, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham K, Gurtler R, Berg K, Heinemeyer G, Lampen A, Appel KE, 2011. Toxicology and risk assessment of 5-hydroxymethylfurfural in food. Mol Nutr Food Res 55, 667–678. [DOI] [PubMed] [Google Scholar]
- Adams TB, Doull J, Goodman JI, Munro IC, Newberne P, Portoghese PS, Smith RL, Wagner BM, Weil CS, Woods LA, Ford RA, 1997. The FEMA GRAS assessment of furfural used as a flavour ingredient. Flavor and Extract Manufacturers’ Association. Food Chem Toxicol 35, 739–751. [DOI] [PubMed] [Google Scholar]
- Aherrera A, Olmedo P, Grau-Perez M, Tanda S, Goessler W, Jarmul S, Chen R, Cohen JE, Rule AM, Navas-Acien A, 2017. The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Environ Res 159, 313–320. [DOI] [PubMed] [Google Scholar]
- Akane H, Saito F, Shiraki A, Takeyoshi M, Imatanaka N, Itahashi M, Murakami T, Shibutani M, 2014. Downregulation of immediate-early genes linking to suppression of neuronal plasticity in rats after 28-day exposure to glycidol. Toxicol Appl Pharmacol 279, 150–162. [DOI] [PubMed] [Google Scholar]
- Alasmari F, Crotty Alexander LE, Hammad AM, Bojanowski CM, Moshensky A, Sari Y, 2019. Effects of chronic inhalation of electronic cigarette vapor containing nicotine on neurotransmitters in the frontal cortex and striatum of C57BL/6 mice. Front Pharmacol 10, 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani T, Pena I, Temesgen N, Glantz SA, 2018. Association between electronic cigarette use and myocardial infarction. Am J Prev Med 55, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzarea S, Rahman S, 2019. Alpha-7 nicotinic receptor allosteric modulator PNU120596 prevents lipopolysaccharide-induced anxiety, cognitive deficit and depression-like behaviors in mice. Behav Brain Res 366, 19–28. [DOI] [PubMed] [Google Scholar]
- Amrock SM, Zakhar J, Zhou S, Weitzman M, 2015. Perception of e-cigarette harm and its correlation with use among U.S. adolescents. Nicotine Tob Res 17, 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Majeste A, Hanus J, Wang S, 2016. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci 154, 332–340. [DOI] [PubMed] [Google Scholar]
- Andrade VM, Aschner M, Marreilha Dos Santos AP, 2017. Neurotoxicity of metal mixtures. Adv Neurobiol 18, 227–265. [DOI] [PubMed] [Google Scholar]
- Andre JM, Leach PT, Gould TJ, 2011. Nicotine ameliorates NMDA receptor antagonist-induced deficits in contextual fear conditioning through high-affinity nicotinic acetylcholine receptors in the hippocampus. Neuropharmacology 60, 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen JT, Henningsen K, Bate S, Christiansen S, Wiborg O, 2011. Nicotine reverses anhedonic-like response and cognitive impairment in the rat chronic mild stress model of depression: comparison with sertraline. J Psychopharmacol 25, 1134–1141. [DOI] [PubMed] [Google Scholar]
- Andresen H, Bingel U, Streichert T, Schmoldt A, Zoerner AA, Tsikas D, Just I, 2009. Severe glycerol intoxication after Meniere’s disease diagnostic-case report and overview of kinetic data. Clin Toxicol (Phila) 47, 312–316. [DOI] [PubMed] [Google Scholar]
- Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, Lundback M, 2016. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 255, 179–185. [DOI] [PubMed] [Google Scholar]
- Arulanantham K, Genel M, 1978. Central nervous system toxicity associated with ingestion of propylene glycol. J Pediatr 93, 515–516. [DOI] [PubMed] [Google Scholar]
- Ashford K, Wiggins A, Butler K, Ickes M, Rayens MK, Hahn E, 2016. E-cigarette use and perceived harm among women of childbearing age who reported tobacco use during the past year. Nurs Res 65, 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, Wileyto EP, 2016. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend 166, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelar AJ, Akers AT, Baumgard ZJ, Cooper SY, Casinelli GP, Henderson BJ, 2019. Why flavored vape products may be attractive: Green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function. Neuropharmacology 158, 107729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea M, Luzardo OP, Gonzalez-Antuna A, Zumbado M, Rogozea L, Floroian L, Alexandrescu D, Moga M, Gaman L, Radoi M, Boada LD, Henriquez-Hernandez LA, 2018. Body burden of toxic metals and rare earth elements in non-smokers, cigarette smokers and electronic cigarette users. Environ Res 166, 269–275. [DOI] [PubMed] [Google Scholar]
- Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI, 2018. New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: A focus on alpha7 nAChRs. Curr Neuropharmacol 16, 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P, 2012. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 34, 529–537. [DOI] [PubMed] [Google Scholar]
- Baibars M, Eng S, Shaheen K, Alraiyes AH, Alraies MC, 2012. Menthol toxicity: An unusual cause of coma. Case Rep Med 2012, 187039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero M, Martin N, 2015. Depressive symptoms in neurodegenerative diseases. World J Clin Cases 3, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto GE, Iarkov A, Moran VE, 2014. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Front Aging Neurosci 6, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Urman R, Leventhal AM, Gauderman WJ, Cruz TB, Gilreath TD, Howland S, Unger JB, Berhane K, Samet JM, McConnell R, 2016. E-cigarettes, cigarettes, and the prevalence of adolescent tobacco use. Pediatrics 138, e20153983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauld L, MacKintosh AM, Eastwood B, Ford A, Moore G, Dockrell M, Arnott D, Cheeseman H, McNeill A, 2017. Young people’s use of e-cigarettes across the United Kingdom: Findings from five surveys 2015–2017. Int J Environ Res Public Health 14, E973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauval N, Howsam M, Antherieu S, Allorge D, Soyez M, Garcon G, Goossens JF, Lo-Guidice JM, Garat A, 2016. Trace elements in e-liquids - development and validation of an ICP-MS method for the analysis of electronic cigarette refills. Regul Toxicol Pharmacol 79, 144–148. [DOI] [PubMed] [Google Scholar]
- Becker LC, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr., Shank RC, Slaga TJ, Snyder PW, Gill LJ, Heldreth B, 2019. Safety assessment of glycerin as used in cosmetics. Int J Toxicol 38, 6–22. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, Talbot P, 2016. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control 25, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar RZ, Luo W, McWhirter KJ, Pankow JF, Talbot P, 2018. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep 8, 8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belavy D, Sunn N, Lau Q, Robertson T, 2013. Absence of neurotoxicity with perineural injection of ultrasound gels: Assessment using an animal model. BMC Anesthesiol 13, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkoniene M, Socquet J, Njemba-Freiburghaus D, Pellaton C, 2019. Near fatal intoxication by nicotine and propylene glycol injection: A case report of an e-liquid poisoning. BMC Pharmacol Toxicol 20, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Burbank AD, 2016. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc Med 26, 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Fraiman JB, 2017. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 14, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A, Halfon O, Boutrel B, 2013. Controversies about the enhanced vulnerability of the adolescent brain to develop addiction. Front Pharmacol 4, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Forget B, 2016. Cognitive dysfunction, affective states, and vulnerability to nicotine addiction: A multifactorial perspective. Front Psychiatry 7, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra DP, Soares AK, de Sousa DP, 2016. Overview of the role of vanillin on redox status and cancer development. Oxid Med Cell Longev 2016, 9734816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Karwowski MP, Morel-Espinosa M, Rees J, Sosnoff C, Cowan E, Gardner M, Wang L, Valentin-Blasini L, Silva L, De Jesus VR, Kuklenyik Z, Watson C, Seyler T, Xia B, Chambers D, Briss P, King BA, Delaney L, Jones CM, Baldwin GT, Barr JR, Thomas J, Pirkle JL, 2019. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use-associated lung injury - 10 States, August-October 2019. MMWR Morb Mortal Wkly Rep 68, 1040–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay ME, Henry C, Bosse Y, Boulet LP, Morissette MC, 2017. Acute effects of nicotine-free and flavour-free electronic cigarette use on lung functions in healthy and asthmatic individuals. Respir Res 18, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, Ramlochansingh C, Manaye KF, Tizabi Y, 2013. Nicotine promotes survival of cells expressing amyloid precursor protein and presenilin: Implication for Alzheimer’s disease. Neurosci Lett 535, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC, 2010. Long-term consequences of fetal and neonatal nicotine exposure: A critical review. Toxicol Sci 116, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgut FT, Benaur M, Hencliffe C, 2006. Late-life depression: A neuropsychiatric approach. Expert Rev Neurother 6, 65–72. [DOI] [PubMed] [Google Scholar]
- Burns DM, 2003. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis 46, 11–29. [DOI] [PubMed] [Google Scholar]
- Bustamante G, Ma B, Yakovlev G, Yershova K, Le C, Jensen J, Hatsukami DK, Stepanov I, 2018. Presence of the carcinogen N’-nitrosonornicotine in saliva of e-cigarette users. Chem Res Toxicol 31, 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna D, Cibella F, Caponnetto P, Amaradio MD, Caruso M, Morjaria JB, Malerba M, Polosa R, 2016. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 46, 698–706. [DOI] [PubMed] [Google Scholar]
- Cardenas VM, Cen R, Clemens MM, Moody HL, Ekanem US, Policherla A, Fischbach LA, Eswaran H, Magann EF, Delongchamp RR, Boysen G, 2019a. Use of electronic nicotine delivery systems (ENDS) by pregnant women I: Risk of small-for-gestational-age birth. Tob Induc Dis 17, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VM, Fischbach LA, Chowdhury P, 2019b. The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: A systematic review of the literature. Tob Induc Dis 17, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenia V, Vivarelli F, Cirillo S, Paolini M, Canistro D, Rodriguez-Estrada MT, 2018. The effect of electronic-cigarettes aerosol on rat brain lipid profile. Biochimie 153, 99–108. [DOI] [PubMed] [Google Scholar]
- Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G, 2016. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 150, 606–612. [DOI] [PubMed] [Google Scholar]
- Carvalho AN, Firuzi O, Gama MJ, Horssen JV, Saso L, 2017. Oxidative stress and antioxidants in neurological diseases: Is there still hope? Curr Drug Targets 18, 705–718. [DOI] [PubMed] [Google Scholar]
- Cauley M, Hall BJ, Abreu-Villaca Y, Junaid S, White H, Kiany A, Slotkin TA, Levin ED, 2018. Critical developmental periods for effects of low-level tobacco smoke exposure on behavioral performance. Neurotoxicology 68, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2020. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
- Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G, 2014. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro 28, 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Tsai YW, Shiu MN, Wang YT, Chang PY, 2017. Elucidating challenges that electronic cigarettes pose to tobacco control in Asia: a population-based national survey in Taiwan. BMJ Open 7, e014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, 2012. The nicotinic acetylcholine receptor: The founding father of the pentameric ligand-gated ion channel superfamily. J Biol Chem 287, 40207–40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Tao JQ, Johncola A, Guo W, Caporale A, Langham MC, Wehrli FW, 2019. Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am J Physiol Lung Cell Mol Physiol 317, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont M, de Becker B, Zaher W, Culie A, Deprez G, Melot C, Reye F, Van Antwerpen P, Delporte C, Debbas N, Boudjeltia KZ, van de Borne P, 2018. Differential effects of e-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: A randomized crossover trial. Sci Rep 8, 10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, Starczewska E, Briki R, de Hemptinne Q, Zaher W, Debbas N, 2019. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol 316, L705–L719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BC, Bright SB, Trivedi AR, Valento M, 2015. Death following intentional ingestion of e-liquid. Clin Toxicol (Phila) 53, 914–916. [DOI] [PubMed] [Google Scholar]
- Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, Annissa T, McGrath KC, Sharma P, Oliver BG, 2018a. Maternal e-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol 58, 366–377. [DOI] [PubMed] [Google Scholar]
- Chen H, Li G, Chan YL, Nguyen T, van Reyk D, Saad S, Oliver BG, 2018b. Modulation of neural regulators of energy homeostasis, and of inflammation, in the pups of mice exposed to e-cigarettes. Neurosci Lett 684, 61–66. [DOI] [PubMed] [Google Scholar]
- Chen J, Ho SY, Leung LT, Wang MP, Lam TH, 2019. School-level electronic cigarette use prevalence and student-level tobacco use intention and behaviours. Sci Rep 9, 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Paik SY, 2016. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS One 11, e0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Bernat D, 2016. E-cigarette use among Florida youth with and without asthma. Am J Prev Med 51, 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibella F, Campagna D, Caponnetto P, Amaradio MD, Caruso M, Russo C, Cockcroft DW, Polosa R, 2016. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin Sci (Lond) 130, 1929–1937. [DOI] [PubMed] [Google Scholar]
- Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, Jaspers I, 2019. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol 316, 470–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, Jaspers I, 2017. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol 313, 278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, Cavalleri L, Zoli M, Maskos U, Ratti E, Merlo Pich E, 2018. Alpha6-containing nicotinic acetylcholine receptors mediate nicotine-induced structural plasticity in mouse and human iPSC-derived dopaminergic neurons. Front Pharmacol 9, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu XA, Chen LC, Riggs DW, Lorkiewicz P, Bhatnagar A, Srivastava S, 2018. Electronic cigarette-generated aldehydes: The contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci Technol 52, 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, 2007. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology (Berl) 192, 87–95. [DOI] [PubMed] [Google Scholar]
- Copeland RL Jr., Das JR, Kanaan YM, Taylor RE, Tizabi Y, 2007. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res 12, 61–69. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S, 2011. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci 14, 417–419. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T, 2009. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology 34, 299–306. [DOI] [PubMed] [Google Scholar]
- Cui J, Liu Y, Chang X, Gou W, Zhou X, Liu Z, Li Z, Wu Y, Zuo D, 2019. Acetaldehyde induces neurotoxicity in vitro via oxidative stress- and Ca(2+) imbalance-mediated endoplasmic reticulum stress. Oxid Med Cell Longev 2019, 2593742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Leventhal AM, 2019. Prevalence of e-cigarette use among adults in the United States, 2014–2018. Jama 322, 1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, 2015. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol 124, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das JR, Tizabi Y, 2009. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res 16, 194–204. [DOI] [PubMed] [Google Scholar]
- De Biasi M, 2015. Nicotine use in mental illness and neurological disorders, 1st ed Academic Press. [Google Scholar]
- Desmarchelier C, Tourniaire F, Preveraud DP, Samson-Kremser C, Crenon I, Rosilio V, Borel P, 2013. The distribution and relative hydrolysis of tocopheryl acetate in the different matrices coexisting in the lumen of the small intestine during digestion could explain its low bioavailability. Mol Nutr Food Res 57, 1237–1245. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Krishnan-Sarin S, 2018. E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol 16, 438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase A, Attorri L, Di Benedetto R, Sanchez M, 2018. Comparative effects between electronic cigarette and tobacco cigarette smoke on oxidative markers in cultured immune cells isolated from rats. Ann Ist Super Sanita 54, 300–307. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Pandya AA, Yakel JL, 2015. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci 36, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M, 1999. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav 67, 533–537. [DOI] [PubMed] [Google Scholar]
- Dowell J, Elser BA, Schroeder RE, Stevens HE, 2019. Cellular stress mechanisms of prenatal maternal stress: Heat shock factors and oxidative stress. Neurosci Lett 709, 134368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Korecka M, Trojanowski JQ, Weiner MW, R OH, Ashford JW, Shaw LM, 2016. Active cigarette smoking in cognitively-normal elders and probable Alzheimer’s disease is associated with elevated cerebrospinal fluid oxidative stress biomarkers. J Alzheimers Dis 54, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM, 2008. Nicotine and brain development. Birth Defects Res C Embryo Today 84, 30–44. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM, 2009. The dynamic effects of nicotine on the developing brain. Pharmacol Ther 122, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS, 2011. Nicotine withdrawal-induced negative affect is a function of nicotine dependence and not liability to depression or anxiety. Nicotine Tob Res 13, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlinger DG, Bergstrom HC, Burke JC, Fernandez GM, McDonald CG, Smith RF, 2016. Adolescent nicotine-induced dendrite remodeling in the nucleus accumbens is rapid, persistent, and D1-dopamine receptor dependent. Brain Struct Funct 221, 133–145. [DOI] [PubMed] [Google Scholar]
- Ellington S, Salvatore PP, Ko J, Danielson M, Kim L, Cyrus A, Wallace M, Board A, Krishnasamy V, King BA, Rose D, Jones CM, Pollack LA, 2020. Update: Product, substance-use, and demographic characteristics of hospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury - United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep 69, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin AB, Engin A, 2019. Nanoparticles and neurotoxicity: Dual response of glutamatergic receptors. Prog Brain Res 245, 281–303. [DOI] [PubMed] [Google Scholar]
- Erythropel HC, Jabba SV, DeWinter TM, Mendizabal M, Anastas PT, Jordt SE, Zimmerman JB, 2019. Formation of flavorant-propylene glycol adducts with novel toxicological properties in chemically unstable e-cigarette liquids. Nicotine Tob Res 21, 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza-Derout J, Shao XM, Bankole E, Hasan KM, Mtume N, Liu Y, Sinha-Hikim AP, Friedman TC, 2019. Hepatic DNA damage induced by electronic cigarette exposure is associated with the modulation of NAD+/PARP1/SIRT1 axis. Front Endocrinol (Lausanne) 10, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber T, Kumar A, Mackenbach JP, Millett C, Basu S, Sheikh A, Been JV, 2017. Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis. Lancet Public Health 2, e420–e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Kistler KA, Gillman G, Voudris V, 2015a. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res 17, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Polosa R, 2014. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf 5, 67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Rodu B, 2018. Metal emissions from e-cigarettes: a risk assessment analysis of a recently-published study. Inhal Toxicol 30, 321–326. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Voudris V, Poulas K, 2015b. Are metals emitted from electronic cigarettes a reason for health concern? A risk-assessment analysis of currently available literature. Int J Environ Res Public Health 12, 5215–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2019. Alpha-tocopherol acetate. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.8892
- FDA, 2020. Lung illnesses associated with use of vaping products. https://www.fda.gov/news-events/public-health-focus/lung-illnesses-associated-use-vaping-products#Analysis
- Ferrari M, Zanasi A, Nardi E, Morselli Labate AM, Ceriana P, Balestrino A, Pisani L, Corcione N, Nava S, 2015. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC Pulm Med 15, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsakis AM, Koutedakis Y, 2013. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol 25, 91–101. [DOI] [PubMed] [Google Scholar]
- Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, Dalhoff K, Mortensen K, Droemann D, 2018. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vasc Med 23, 419–425. [DOI] [PubMed] [Google Scholar]
- Gad SC, Cassidy CD, Aubert N, Spainhour B, Robbe H, 2006. Nonclinical vehicle use in studies by multiple routes in multiple species. Int J Toxicol 25, 499–521. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Manyanga J, Brame L, McGuire D, Sadhasivam B, Floyd E, Rubenstein DA, Ramachandran I, Wagener T, Queimado L, 2017. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One 12, 0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S, Agnihotri R, 2019. Health effects of trace metals in electronic cigarette aerosols-a systematic review. Biol Trace Elem Res 188, 295–315. [DOI] [PubMed] [Google Scholar]
- Gentzke AS, Creamer M, Cullen KA, Ambrose BK, Willis G, Jamal A, King BA, 2019. Vital signs: Tobacco product use among middle and high school students - United States, 2011–2018. MMWR Morb Mortal Wkly Rep 68, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Csoka AB, Aschner M, Tizabi Y, 2019. Nicotine protects against manganese and iron-induced toxicity in SH-SY5Y cells: Implication for Parkinson’s disease. Neurochem Int 124, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, Richie JP Jr., 2015. Highly reactive free radicals in electronic cigarette aerosols. Chem Res Toxicol 28, 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golli NE, Dallagi Y, Rahali D, Rejeb I, Fazaa SE, 2016. Neurobehavioral assessment following e-cigarette refill liquid exposure in adult rats. Toxicol Mech Methods 26, 435–442. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N, 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Marks M, Millar N, Wonnacott S, 2019. Nicotinic acetylcholine receptors 10.2218/gtopdb/F76/2019.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Lust K, Fridberg DJ, King AC, Chamberlain SR, 2019. E-cigarette use (vaping) is associated with illicit drug use, mental health problems, and impulsivity in university students. Ann Clin Psychiatry 31, 27–35. [PMC free article] [PubMed] [Google Scholar]
- Greene RM, Pisano MM, 2019. Developmental toxicity of e-cigarette aerosols. Birth Defects Res. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Tulsyan S, Bharadwaj M, Mehrotra R, 2019. Grass roots approach to control levels of carcinogenic nitrosamines, NNN and NNK in smokeless tobacco products. Food Chem Toxicol 124, 359–366. [DOI] [PubMed] [Google Scholar]
- Haddad C, Salman R, El-Hellani A, Talih S, Shihadeh A, Saliba NA, 2019. Reactive oxygen species emissions from supra- and sub-ohm electronic cigarettes. J Anal Toxicol 43, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ, 2019. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 380, 629–637. [DOI] [PubMed] [Google Scholar]
- Halstead M, Gray N, Gonzalez-Jimenez N, Fresquez M, Valentin-Blasini L, Watson C, Pappas RS, 2019. Analysis of toxic metals in electronic cigarette aerosols using a novel trap design. J Anal Toxicol, bkz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, Heath JW, Nayak P, Perry CL, Pechacek TF, Eriksen MP, 2017. Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev Med Rep 5, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Meier E, Lindgren BR, Anderson A, Reisinger S, Norton K, Strayer L, Jensen J, Dick L, Murphy S, Carmella S, Tang MK, Chen M, Hecht SS, O’Connor RJ, Shields PG, 2019. A randomized clinical trial examining the effects of instructions for electronic cigarette use on smoking-related behaviors, and biomarkers of exposure. Nicotine Tob Res, ntz233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA, 2017. Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology 42, 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, Rule AM, 2017. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ Res 152, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman E, Herrera CA, Jaspers I, 2019. Common e-cigarette flavoring chemicals impair neutrophil phagocytosis and oxidative burst. Chem Res Toxicol 32, 982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironishi M, Kordek R, Yanagihara R, Garruto RM, 1996. Maltol (3-hydroxy-2-methyl-4-pyrone) toxicity in neuroblastoma cell lines and primary murine fetal hippocampal neuronal cultures. Neurodegeneration 5, 325–329. [DOI] [PubMed] [Google Scholar]
- Ho K, Yazan LS, Ismail N, Ismail M, 2011. Toxicology study of vanillin on rats via oral and intra-peritoneal administration. Food Chem Toxicol 49, 25–30. [DOI] [PubMed] [Google Scholar]
- Hobkirk AL, Nichols TT, Foulds J, Yingst JM, Veldheer S, Hrabovsky S, Richie J, Eissenberg T, Wilson SJ, 2018. Changes in resting state functional brain connectivity and withdrawal symptoms are associated with acute electronic cigarette use. Brain Res Bull 138, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden VK, Hines SE, 2016. Update on flavoring-induced lung disease. Curr Opin Pulm Med 22, 158–164. [DOI] [PubMed] [Google Scholar]
- Hoskin JL, Al-Hasan Y, Sabbagh MN, 2019. Nicotinic acetylcholine receptor agonists for the treatment of Alzheimer’s dementia: An update. Nicotine Tob Res 21, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Omaiye EE, Luo W, McWhirter KJ, Pankow JF, Talbot P, 2019. Identification of cytotoxic flavor chemicals in top-selling electronic cigarette refill fluids. Sci Rep 9, 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Talbot P, 2016. Potential health effects of electronic cigarettes: A systematic review of case reports. Prev Med Rep 4, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y, 2013. Neuroinflammation, neurodegeneration, and depression. Neurotox Res 23, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A, 2014. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol 88, 1295–1308. [DOI] [PubMed] [Google Scholar]
- Hybertson BM, Chung JH, Fini MA, Lee YM, Allard JD, Hansen BN, Cho OJ, Shibao GN, Repine JE, 2005. Aerosol-administered alpha-tocopherol attenuates lung inflammation in rats given lipopolysaccharide intratracheally. Exp Lung Res 31, 283–294. [DOI] [PubMed] [Google Scholar]
- Ito S, Taylor M, Mori S, Thorne D, Nishino T, Breheny D, Gaca M, Yoshino K, Proctor C, 2019. An inter-laboratory in vitro assessment of cigarettes and next generation nicotine delivery products. Toxicol Lett 315, 14–22. [DOI] [PubMed] [Google Scholar]
- Jacklin DL, Goel A, Clementino KJ, Hall AW, Talpos JC, Winters BD, 2012. Severe cross-modal object recognition deficits in rats treated sub-chronically with NMDA receptor antagonists are reversed by systemic nicotine: implications for abnormal multisensory integration in schizophrenia. Neuropsychopharmacology 37, 2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB, 2019. Concentrations of cadmium, lead, and mercury in blood among US cigarettes, cigars, electronic cigarettes, and dual cigarette-e-cigarette users. Environ Pollut 251, 970–974. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Krzystanek M, Zejda JE, Majek P, Lubanski J, Lawson JA, Brozek G, 2019. E-cigarettes are more addictive than traditional cigarettes-a study in highly educated young people. Int J Environ Res Public Health 16, E2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, Taffe MA, 2019. Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug Alcohol Depend 198, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH, 2015. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 372, 392–394. [DOI] [PubMed] [Google Scholar]
- Kaisar MA, Prasad S, Liles T, Cucullo L, 2016. A decade of e-cigarettes: Limited research & unresolved safety concerns. Toxicology 365, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA, Sivandzade F, Bhalerao A, Cucullo L, 2018. Conventional and electronic cigarettes dysregulate the expression of iron transporters and detoxifying enzymes at the brain vascular endothelium: In vivo evidence of a gender-specific cellular response to chronic cigarette smoke exposure. Neurosci Lett 682, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA, Villalba H, Prasad S, Liles T, Sifat AE, Sajja RK, Abbruscato TJ, Cucullo L, 2017. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is metformin a viable countermeasure? Redox Biol 13, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalejaiye O, Bhatti BH, Taylor RE, Tizabi Y, 2013. Nicotine blocks the depressogenic effects of alcohol: Implications for drinking-smoking co-morbidity. J Drug Alcohol Res 2, 235709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoran S, Glantz SA, 2016. E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respir Med 4, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapaya M, D’Angelo DV, Tong VT, England L, Ruffo N, Cox S, Warner L, Bombard J, Guthrie T, Lampkins A, King BA, 2019. Use of electronic vapor products before, during, and after pregnancy among women with a recent live birth - Oklahoma and Texas, 2015. MMWR Morb Mortal Wkly Rep 68, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karri V, Schuhmacher M, Kumar V, 2016. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 48, 203–213. [DOI] [PubMed] [Google Scholar]
- Kaur G, Muthumalage T, Rahman I, 2018. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol Lett 288, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M, Watanabe Y, Nakajima K, Murayama H, Nagahara R, Jin M, Yoshida T, Shibutani M, 2017. Late effect of developmental exposure to glycidol on hippocampal neurogenesis in mice: Loss of parvalbumin-expressing interneurons. Exp Toxicol Pathol 69, 517–526. [DOI] [PubMed] [Google Scholar]
- Keesey J, 2005. How electric fish became sources of acetylcholine receptor. J Hist Neurosci 14, 149–164. [DOI] [PubMed] [Google Scholar]
- King BA, Jones CM, Baldwin GT, Briss PA, 2020. The EVALI and youth vaping epidemics - implications for public health. N Engl J Med 382, 689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Prokopowicz A, Kurek J, Zaciera M, Knysak J, Smith D, Goniewicz ML, 2016. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 71, 376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotandeniya D, Carmella SG, Pillsbury ME, Hecht SS, 2015. Combined analysis of N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in the urine of cigarette smokers and e-cigarette users. J Chromatogr B Analyt Technol Biomed Life Sci 1007, 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemann EJZ, Wenng FM, Pennings JLA, de Graaf K, Talhout R, Boesveldt S, 2019. Sensory evaluation of e-liquid flavors by smelling and vaping yields similar results. Nicotine Tob Res 2019, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Baitha U, Aggarwal P, Jamshed N, 2016. A fatal case of menthol poisoning. Int J Appl Basic Med Res 6, 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntic M, Oelze M, Steven S, Kroller-Schon S, Stamm P, Kalinovic S, Frenis K, Vujacic-Mirski K, Bayo Jimenez MT, Kvandova M, Filippou K, Al Zuabi A, Bruckl V, Hahad O, Daub S, Varveri F, Gori T, Huesmann R, Hoffmann T, Schmidt FP, Keaney JF, Daiber A, Munzel T, 2019. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur Heart J 2019, ehz772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti AN, Redner R, Lopez AA, Keith DR, Villanti AC, Stanton CA, Gaalema DE, Bunn JY, Doogan NJ, Cepeda-Benito A, Roberts ME, Phillips J, Higgins ST, 2017. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med 104, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagiou A, Lagiou P, 2017. Tobacco smoking and breast cancer: a life course approach. Eur J Epidemiol 32, 631–634. [DOI] [PubMed] [Google Scholar]
- LaKind JS, McKenna EA, Hubner RP, Tardiff RG, 1999. A review of the comparative mammalian toxicity of ethylene glycol and propylene glycol. Crit Rev Toxicol 29, 331–365. [DOI] [PubMed] [Google Scholar]
- Lau K, Swiney BS, Reeves N, Noguchi KK, Farber NB, 2012. Propylene glycol produces excessive apoptosis in the developing mouse brain, alone and in combination with phenobarbital. Pediatr Res 71, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterstein DE, Tijerina PB, Corbett K, Akgol Oksuz B, Shen SS, Gordon T, Klein CB, Zelikoff JT, 2016. Frontal cortex transcriptome analysis of mice exposed to electronic cigarettes during early life stages. Int J Environ Res Public Health 13, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde M, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, Meiman J, 2019. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - preliminary report. N Engl J Med 2019, 1–14. [DOI] [PubMed] [Google Scholar]
- Lebedova J, Novakova Z, Vecera Z, Buchtova M, Dumkova J, Docekal B, Blahova L, Mikuska P, Misek I, Hampl A, Hilscherova K, 2018. Impact of acute and subchronic inhalation exposure to PbO nanoparticles on mice. Nanotoxicology 12, 290–304. [DOI] [PubMed] [Google Scholar]
- Lechasseur A, Jubinville E, Routhier J, Berube JC, Hamel-Auger M, Talbot M, Lamothe J, Aubin S, Pare ME, Beaulieu MJ, Bosse Y, Duchaine C, Morissette MC, 2017. Exposure to electronic cigarette vapors affects pulmonary and systemic expression of circadian molecular clock genes. Physiol Rep 5, e13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC, 2017. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 16, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Ong SG, Zhou Y, Tian L, Bae HR, Baker N, Whitlatch A, Mohammadi L, Guo H, Nadeau KC, Springer ML, Schick SF, Bhatnagar A, Wu JC, 2019. Modeling cardiovascular risks of e-cigarettes with human-induced pluripotent stem cell-derived endothelial cells. J Am Coll Cardiol 73, 2722–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, Kurtzman R, Ossip DJ, Robinson R, McIntosh S, Rahman I, 2015a. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ Pollut 198, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I, 2015b. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy ML, Aranda M, Zelman V, Giannotta SL, 1995. Propylene glycol toxicity following continuous etomidate infusion for the control of refractory cerebral edema. Neurosurgery 37, 363–371. [DOI] [PubMed] [Google Scholar]
- Lewis N, McCaffrey K, Sage K, Cheng CJ, Green J, Goldstein L, Campbell H, Ferrell D, Malan N, LaCross N, Maldonado A, Board A, Hanchey A, Harris D, Callahan S, Aberegg S, Risk I, Willardson S, Carter A, Nakashima A, Duncan J, Burnett C, Atkinson-Dunn R, Dunn A, 2019. E-cigarette use, or vaping, practices and characteristics among persons with associated lung injury - Utah, April-October 2019. MMWR Morb Mortal Wkly Rep 68, 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chan YL, Nguyen LT, Mak C, Zaky A, Anwer AG, Shi Y, Nguyen T, Pollock CA, Oliver BG, Saad S, Chen H, 2019. Impact of maternal e-cigarette vapor exposure on renal health in the offspring. Ann N Y Acad Sci 1452, 65–77. [DOI] [PubMed] [Google Scholar]
- Li G, Saad S, Oliver BG, Chen H, 2018. Heat or burn? Impacts of intrauterine tobacco smoke and e-cigarette vapor exposure on the offspring’s health outcome. Toxics 6, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Shin HS, 2017. Determination of volatile organic compounds including alcohols in refill fluids and cartridges of electronic cigarettes by headspace solid-phase micro extraction and gas chromatography-mass spectrometry. Anal Bioanal Chem 409, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Lim TY, Poole RL, Pageler NM, 2014. Propylene glycol toxicity in children. J Pediatr Pharmacol Ther 19, 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hu J, Wu J, Zhu C, Hui Y, Han Y, Huang Z, Ellsworth K, Fan W, 2012. Alpha7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J Neuroinflammation 9, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhao J, Guo W, 2018. Emotional roles of mono-aminergic neurotransmitters in major depressive disorder and anxiety disorders. Front Psychol 9, 2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorkiewicz P, Riggs DW, Keith RJ, Conklin DJ, Xie Z, Sutaria S, Lynch B, Srivastava S, Bhatnagar A, 2019. Comparison of urinary biomarkers of exposure in humans using electronic cigarettes, combustible cigarettes, and smokeless tobacco. Nicotine Tob Res 21, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott EL, Jones EB, 2019. Cholinergic toxicity StatPearls Publishing LLC, Treasure Island (FL). [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B, 2012. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 33, 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A, Middlekauff HR, 2019. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag 15, 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini S, Buonanno G, Stabile L, Ficco G, 2014. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicol Appl Pharmacol 278, 9–15. [DOI] [PubMed] [Google Scholar]
- Mark KS, Farquhar B, Chisolm MS, Coleman-Cowger VH, Terplan M, 2015. Knowledge, attitudes, and practice of electronic cigarette use among pregnant women. J Addict Med 9, 266–272. [DOI] [PubMed] [Google Scholar]
- Mate Z, Horvath E, Papp A, Kovacs K, Tombacz E, Nesztor D, Szabo T, Szabo A, Paulik E, 2017. Neurotoxic effects of subchronic intratracheal Mn nanoparticle exposure alone and in combination with other welding fume metals in rats. Inhal Toxicol 29, 227–238. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED, 2006. Transdermal nicotine attenuates depression symptoms in nonsmokers: A double-blind, placebo-controlled trial. Psychopharmacology (Berl) 189, 125–133. [DOI] [PubMed] [Google Scholar]
- McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K, 2017. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med 195, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin A, Fallin-Bennett A, Barnett J, Ashford K, 2017. Perceptions and use of electronic cigarettes in pregnancy. Health Educ Res 32, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo SA, Ansary MA, Barayan FR, Almusallam AS, Almehaid AM, Alarifi NS, Alsohaibani TA, Zia I, 2019. Electronic cigarettes: Impact on lung function and fractional exhaled nitric oxide among healthy adults. Am J Mens Health 13, 1557988318806073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi XJ, Hou JG, Wang Z, Han Y, Ren S, Hu JN, Chen C, Li W, 2018. The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt and p53 signaling pathways. Sci Rep 8, 15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Park J, Moheimani RS, 2014. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol 64, 1740–1750. [DOI] [PubMed] [Google Scholar]
- Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI, 2016. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res 18, 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JJ, 2019. Depression’s journey from monoamines to glutamate. Psychiat Times 36, 1–5. [Google Scholar]
- Mineur YS, Picciotto MR, 2008. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol 75, 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR, 2017. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: Implications for cardiovascular risk. JAMA Cardiol 2, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Coutino A, Calderon-Ezquerro C, Drucker-Colin R, 2007. Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine Tob Res 9, 389–396. [DOI] [PubMed] [Google Scholar]
- Moylan S, Gustavson K, Overland S, Karevold EB, Jacka FN, Pasco JA, Berk M, 2015. The impact of maternal smoking during pregnancy on depressive and anxiety behaviors in children: The Norwegian mother and child cohort study. BMC Med 13, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz P, Huenchuguala S, Paris I, Cuevas C, Villa M, Caviedes P, Segura-Aguilar J, Tizabi Y, 2012. Protective effects of nicotine against aminochrome-induced toxicity in substantia nigra derived cells: Implications for Parkinson’s disease. Neurotox Res 22, 177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq G, Khan JA, Joseph E, Kamal MA, 2015. Nanoparticles, neurotoxicity and neurodegenerative diseases. Curr Drug Metab 16, 676–684. [DOI] [PubMed] [Google Scholar]
- NAS, 2018. Public health consequences of e-cigarettes. National Academies Press (US), Washington (DC). [PubMed] [Google Scholar]
- Newhouse P, Kellar K, Aisen P, White H, Wesnes K, Coderre E, Pfaff A, Wilkins H, Howard D, Levin ED, 2012. Nicotine treatment of mild cognitive impairment: A 6-month double-blind pilot clinical trial. Neurology 78, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, Gorrie CA, 2018. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem Res Toxicol 31, 601–611. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, Gorrie CA, 2019. Neurological effects in the offspring after switching from tobacco cigarettes to e-cigarettes during pregnancy in a mouse model. Toxicol Sci 172, 191–200. [DOI] [PubMed] [Google Scholar]
- NIDA, 2016. Understanding drug abuse and addiction: What science says. https://www.drugabuse.gov/understanding-drug-abuse-addiction-what-science-says.
- Nonnemaker J, Kim AE, Lee YO, MacMonegle A, 2016. Quantifying how smokers value attributes of electronic cigarettes. Tob Control 25, e37–43. [DOI] [PubMed] [Google Scholar]
- Oh JA, Shin HS, 2015. Identification and quantification of several contaminated compounds in replacement liquids of electronic cigarettes by gas chromatography-mass spectrometry. J Chromatogr Sci 53, 841–848. [DOI] [PubMed] [Google Scholar]
- Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, Aherrera A, Chen R, Hilpert M, Cohen JE, Navas-Acien A, Rule AM, 2018. Metal concentrations in e-cigarette liquid and aerosol samples: The contribution of metallic coils. Environ Health Perspect 126, 027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Pankow JF, Talbot P, 2019a. High-nicotine electronic cigarette products: Toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem Res Toxicol 32, 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, Talbot P, 2019b. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep 9, 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez JE, Kleinschmidt KC, Forrester MB, 2015. Electronic cigarette exposures reported to Texas poison centers. Nicotine Tob Res 17, 209–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzabal M, Ramadoss J, 2019. Impact of electronic cigarette aerosols on pregnancy and early development. Curr Opin Toxicol 14, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzabal MR, Lunde-Young ER, Ramirez JI, Howe SYF, Naik VD, Lee J, Heaps CL, Threadgill DW, Ramadoss J, 2019. Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl Res 207, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero CE, Noeker JA, Brown MM, Wavreil FDM, Harvey WA, Mitchell KA, Heggland SJ, 2019. Electronic cigarette liquid exposure induces flavor-dependent osteotoxicity and increases expression of a key bone marker, collagen type I. J Appl Toxicol 39, 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo DL, Crow AP, Nelson JM, Johnson RA, 2016. Trace metals derived from electronic cigarette (ECIG) generated aerosol: Potential problem of ECIG devices that contain nickel. Front Physiol 7, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpant NJ, Hofsteen P, Pabon L, Reinecke H, Murry CE, 2015. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PLoS One 10, e0126259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, Peyton DH, 2017. Benzene formation in electronic cigarettes. PLoS One 12, e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, McIntire J, Ryan S, Dunah A, Loring R, 2017. Anti-inflammatory effects of astroglial alpha7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-kappaB pathway and activation of the Nrf2 pathway. J Neuroinflammation 14, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S, Arakeri G, Patil S, Ali Baeshen H, Raj T, Sarode SC, Sarode GS, Awan KH, Gomez R, Brennan PA, 2019. Are electronic nicotine delivery systems (ENDs) helping cigarette smokers quit?-Current evidence. J Oral Pathol Med 2019. [DOI] [PubMed] [Google Scholar]
- Payne JD, Michaels D, Orellana-Barrios M, Nugent K, 2017. Electronic cigarette toxicity. J Prim Care Community Health 8, 100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MF, Atuegwu NC, Mead EL, Oncken C, Mortensen EM, 2019. Adult e-cigarettes use associated with a self-reported diagnosis of COPD. Int J Environ Res Public Health 16, 3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B, Titz B, Kogel U, Sharma D, Leroy P, Xiang Y, Vuillaume G, Lebrun S, Sciuscio D, Ho J, Nury C, Guedj E, Elamin A, Esposito M, Krishnan S, Schlage WK, Veljkovic E, Ivanov NV, Martin F, Peitsch MC, Hoeng J, Vanscheeuwijck P, 2017. Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints. Food Chem Toxicol 109, 315–332. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS, 2015. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology 96, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Cibella F, Caponnetto P, Maglia M, Prosperini U, Russo C, Tashkin D, 2017. Health impact of e-cigarettes: A prospective 3.5-year study of regular daily users who have never smoked. Scientific Reports 7, 13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Morjaria J, Caponnetto P, Caruso M, Strano S, Battaglia E, Russo C, 2014. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: evidence for harm reversal. Int J Environ Res Public Health 11, 4965–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Morjaria JB, Caponnetto P, Caruso M, Campagna D, Amaradio MD, Ciampi G, Russo C, Fisichella A, 2016a. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med 21, 99–108. [PubMed] [Google Scholar]
- Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A, Bruno CM, 2016b. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir Res 17, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, 1992. Euphoriant effects of nicotine in smokers. Psychopharmacology (Berl) 108, 460–465. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D, 2015. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacol 25, 1775–1786. [DOI] [PubMed] [Google Scholar]
- Pouwels PJW, van de Lagemaat M, van de Pol LA, Witjes BCM, Zonnenberg IA, 2019. Spectroscopic detection of brain propylene glycol in neonates: Effects of different pharmaceutical formulations of phenobarbital. J Magn Reson Imaging 49, 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzhammer R, Doppler C, Jakschitz T, Heinz K, Forste J, Danzl K, Messner B, Bernhard D, 2016. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One 11, e0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Bordia T, Zhang D, Perez XA, 2015. Nicotine and nicotinic receptor drugs: Potential for Parkinson’s disease and drug-induced movement disorders. Int Rev Neurobiol 124, 247–271. [DOI] [PubMed] [Google Scholar]
- Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, Kim A, Tyndale RF, Langston JW, Di Monte DA, 2006. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem 98, 1866–1875. [DOI] [PubMed] [Google Scholar]
- Rahman S, 2015. Targeting brain nicotinic acetylcholine receptors to treat major depression and comorbid alcohol or nicotine addiction. CNS Neurol Disord Drug Targets 14, 647–653. [DOI] [PubMed] [Google Scholar]
- Rahman S, Engleman EA, Bell RL, 2016. Recent advances in nicotinic receptor signaling in alcohol abuse and alcoholism. Prog Mol Biol Transl Sci 137, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlochansingh C, Taylor RE, Tizabi Y, 2011. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res 20, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne RA, Wehner AP, Greenspan BJ, Deford HS, Ragan HA, Westerberg RB, Buschbom RL, Burger GT, Hayes AW, Suber RL, Mosberg AT, 1992. 2-week and 13-week inhalation studies of aerosolized glycerol in rats. Inhal Toxicol 4, 95–111. [Google Scholar]
- Rock KD, Patisaul HB, 2018. Environmental mechanisms of neurodevelopmental toxicity. Curr Environ Health Rep 5, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Delucchi K, Benowitz NL, Ramo DE, 2018. Adolescent exposure to toxic volatile organic chemicals from e-cigarettes. Pediatrics 141, e20173557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHM, 2020. Protecting youth from the risks of electronic cigarettes. J Adolesc Health 66, 127–131. [DOI] [PubMed] [Google Scholar]
- Salamanca JC, Munhenzva I, Escobedo JO, Jensen RP, Shaw A, Campbell R, Luo W, Peyton DH, Strongin RM, 2017. Formaldehyde hemiacetal sampling, recovery, and quantification from electronic cigarette aerosols. Sci Rep 7, 11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales MPU, Araujo AJ, Chatkin JM, Godoy I, Pereira LFF, Castellano M, Tanni SE, Almeida AA, Chatkin G, Silva L, Goncalves CMC, Botelho C, Santos UP, Viegas CAA, Sestelo MR, Meireles RHS, Correa P, Oliveira MEM, Reichert J, Lima MS, Silva C, 2019. Update on the approach to smoking in patients with respiratory diseases. J Bras Pneumol 45, e20180314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R, 1995. Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) 121, 476–479. [DOI] [PubMed] [Google Scholar]
- Samet JM, 2013. Tobacco smoking: The leading cause of preventable disease worldwide. Thorac Surg Clin 23, 103–112. [DOI] [PubMed] [Google Scholar]
- Sassano MF, Davis ES, Keating JE, Zorn BT, Kochar TK, Wolfgang MC, Glish GL, Tarran R, 2018. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol 16, e2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh E, Murakami K, Nishimura M, 2004. Propylene glycol releases calcium from mitochondrial stores in rat cerebrocortical synaptosomes. Int J Neurosci 114, 1111–1118. [DOI] [PubMed] [Google Scholar]
- Scherman A, Tolosa JE, McEvoy C, 2018. Smoking cessation in pregnancy: a continuing challenge in the United States. Ther Adv Drug Saf 9, 457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller LM, Bansal-Travers M, Goniewicz ML, McIntosh S, Ossip D, O’Connor RJ, 2019. Use of flavored e-cigarettes and the type of e-cigarette devices used among adults and youth in the US-results from wave 3 of the population assessment of tobacco and health study (2015–2016). Int J Environ Res Public Health 16, 2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E, Salthammer T, 2013. Does e-cigarette consumption cause passive vaping? Indoor Air 23, 25–31. [DOI] [PubMed] [Google Scholar]
- Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, Grudzinska FS, Dosanjh D, Parekh D, Foronjy R, Sapey E, Naidu B, Thickett DR, 2018. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba J, Mataki C, Yamada S, Nankai M, Toru M, 1998. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry 43, 389–391. [DOI] [PubMed] [Google Scholar]
- Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, Feng J, Wang L, West R, 2017. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: A cross-sectional study. Ann Intern Med 166, 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaito A, Saliba J, Husari A, El-Harakeh M, Chhouri H, Hashem Y, Shihadeh A, El-Sabban M, 2017. Electronic cigarette smoke impairs normal mesenchymal stem cell differentiation. Sci Rep 7, 14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X, Shang Y, Fu J, Zhang T, 2017. Nicotine significantly improves chronic stress-induced impairments of cognition and synaptic plasticity in mice. Mol Neurobiol 54, 4644–4658. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Sharma A, 2012. Neurotoxicity of engineered nanoparticles from metals. CNS Neurol Disord Drug Targets 11, 65–80. [DOI] [PubMed] [Google Scholar]
- Shein M, Jeschke G, 2019. Comparison of free radical levels in the aerosol from conventional cigarettes, electronic cigarettes, and heat-not-burn tobacco products. Chem Res Toxicol 32, 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CL, Boitano S, 2016. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res 17, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD, 2007. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett 418, 286–291. [DOI] [PubMed] [Google Scholar]
- Sifat AE, Vaidya B, Kaisar MA, Cucullo L, Abbruscato TJ, 2018. Nicotine and electronic cigarette (e-cig) exposure decreases brain glucose utilization in ischemic stroke. J Neurochem 147, 204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kukreti R, Saso L, Kukreti S, 2019. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 24, 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F, Cucullo L, 2019. Assessing the protective effect of rosiglitazone against electronic cigarette/tobacco smoke-induced blood-brain barrier impairment. BMC Neurosci 20, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H, 2016. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ Sci Technol 50, 9644–9651. [DOI] [PubMed] [Google Scholar]
- Slikker WJ, Xu ZA, Levin ED, Slotkin TA, 2005. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction-developmental neurotoxicity of nicotine. Crit Rev Toxicol 35, 703–711. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, 2015. Prenatal nicotine alters the developmental neurotoxicity of postnatal chlorpyrifos directed toward cholinergic systems: Better, worse, or just “different?”. Brain Res Bull 110, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA, 2015. Adult behavior in male mice exposed to e-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS One 10, e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solingapuram Sai KK, Zuo Y, Rose J.E.y.z., Garg PK, Garg S, Nazih R, Mintz A, Mukhin AG, 2019. Rapid brain nicotine uptake from electronic cigarettes. J Nucl Med, 119.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Mishin V, Laskin JD, Mainelis G, Wackowski OA, Delnevo C, Schwander S, Khlystov A, Samburova V, Meng Q, 2019. Hydroxyl radicals in e-cigarette vapor and e-vapor oxidative potentials under different vaping patterns. Chem Res Toxicol 32, 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji SS, Knutzen KE, Villanti AC, 2019. Use of flavored e-cigarettes among adolescents, young adults, and older adults: Findings from the population assessment for tobacco and health study. Public Health Rep 134, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hong S, Iizuka Y, Kim CY, Seong GJ, 2015. The neuroprotective effect of maltol against oxidative stress on rat retinal neuronal cells. Korean J Ophthalmol 29, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songur A, Ozen OA, Sarsilmaz M, 2010. The toxic effects of formaldehyde on the nervous system. Rev Environ Contam Toxicol 203, 105–118. [DOI] [PubMed] [Google Scholar]
- Soussy S, El-Hellani A, Baalbaki R, Salman R, Shihadeh A, Saliba NA, 2016. Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tob Control 25, ii88–ii93. [DOI] [PubMed] [Google Scholar]
- Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, Vanderveen J, Hedeker D, 2008. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl) 196, 461–471. [DOI] [PubMed] [Google Scholar]
- Stone E, Marshall H, 2019. Tobacco and electronic nicotine delivery systems regulation. Transl Lung Cancer Res 8, S67–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Kochalka J, Schaer M, Wakeman H, Qin S, Padmanabhan A, Menon V, 2018. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain 141, 2795–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Wiens T, Peterson J, Saravia S, Lunda M, Hanson K, Wogen M, D’Heilly P, Margetta J, Bye M, Cole C, Mumm E, Schwerzler L, Makhtal R, Danila R, Lynfield R, Holzbauer S, 2019. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement - Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep 68, 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyarah R, Long GA, 2014. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol 70, 704–710. [DOI] [PubMed] [Google Scholar]
- Tegin G, Mekala HM, Sarai SK, Lippmann S, 2018. E-cigarette toxicity? South Med J 111, 35–38. [DOI] [PubMed] [Google Scholar]
- Teleanu DM, Chircov C, Grumezescu AM, Teleanu RI, 2019. Neurotoxicity of nanomaterials: An up-to-date overview. Nanomaterials (Basel) 9, e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer RE, Hansen NS, Prashad S, Karoly HC, Filbey FM, Bryan AD, Feldstein Ewing SW, 2020. Recent tobacco use has widespread associations with adolescent white matter microstructure. Addict Behav 101, 106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MF, Poirier GL, Davila-Garcia MI, Huang W, Tam K, Robidoux M, Dubuke ML, Shaffer SA, Colon-Perez L, Febo M, DiFranza JR, King JA, 2018. Menthol enhances nicotine-induced locomotor sensitization and in vivo functional connectivity in adolescence. J Psychopharmacol 32, 332–343. [DOI] [PubMed] [Google Scholar]
- Thornton SL, Oller L, Sawyer T, 2014. Fatal intravenous injection of electronic nicotine delivery system refilling solution. J Med Toxicol 10, 202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, 2016. Duality of antidepressants and neuroprotectants. Neurotox Res 30, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, 2017. Nicotinic receptor intervention in Parkinson’s disease: Future directions. Clin Pharmacol Transl Med 1, 14–19. [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Csoka AB, Manaye KF, Copeland RL, 2019. Novel targets for parkinsonism-depression comorbidity. Prog Mol Biol Transl Sci 167, 1–24. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Rezvani AH, Hauser SR, Overstreet DH, 2009. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-Hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry 33, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Hauser SR, Tyler KY, Getachew B, Madani R, Sharma Y, Manaye KF, 2010. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol Psychiatry 34, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E Jr., Janowsky DS, Kling MA, 1999. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 142, 193–199. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH, 2000. Depressive characteristics of FSL rats: Involvement of central nicotinic receptors. Pharmacol Biochem Behav 66, 73–77. [DOI] [PubMed] [Google Scholar]
- Tobore TO, 2019. On the potential harmful effects of e-cigarettes (EC) on the developing brain: The relationship between vaping-induced oxidative stress and adolescent/young adults social maladjustment. J Adolesc 76, 202–209. [DOI] [PubMed] [Google Scholar]
- Tolu S, Marti F, Morel C, Perrier C, Torquet N, Pons S, de Beaurepaire R, Faure P, 2017. Nicotine enhances alcohol intake and dopaminergic responses through beta2* and beta4* nicotinic acetylcholine receptors. Sci Rep 7, 45116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traudt CM, McPherson RJ, Studholme C, Millen KJ, Juul SE, 2014. Systemic glycerol decreases neonatal rabbit brain and cerebellar growth independent of intraventricular hemorrhage. Pediatr Res 75, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA, 2000. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res 880, 167–172. [DOI] [PubMed] [Google Scholar]
- Tulpule K, Dringen R, 2013. Formaldehyde in brain: An overlooked player in neurodegeneration? J Neurochem 127, 7–21. [DOI] [PubMed] [Google Scholar]
- Umezawa M, Onoda A, Korshunova I, Jensen ACO, Koponen IK, Jensen KA, Khodosevich K, Vogel U, Hougaard KS, 2018. Maternal inhalation of carbon black nanoparticles induces neurodevelopmental changes in mouse offspring. Part Fibre Toxicol 15, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg T, 2013. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res 15, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK, 2012. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141, 1400–1406. [DOI] [PubMed] [Google Scholar]
- Varughese S, Teschke K, Brauer M, Chow Y, van Netten C, Kennedy SM, 2005. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am J Ind Med 47, 411–418. [DOI] [PubMed] [Google Scholar]
- Vieira-Brock PL, McFadden LM, Nielsen SM, Smith MD, Hanson GR, Fleckenstein AE, 2015. Nicotine administration attenuates methamphetamine-induced novel object recognition deficits. Int J Neuropsychopharmacol 18, pyv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli F, Canistro D, Cirillo S, Cardenia V, Rodriguez-Estrada MT, Paolini M, 2019. Impairment of testicular function in electronic cigarette (e-cig, e-cigs) exposed rats under low-voltage and nicotine-free conditions. Life Sci 228, 53–65. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, Stefanadis C, Tousoulis D, 2016. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol 67, 2802–2803. [DOI] [PubMed] [Google Scholar]
- Wagner NJ, Camerota M, Propper C, 2017. Prevalence and perceptions of electronic cigarette use during pregnancy. Matern Child Health J 21, 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin C, Sholts SB, Osterlund N, Luo J, Jarvet J, Roos PM, Ilag L, Graslund A, Warmlander S, 2017. Alzheimer’s disease and cigarette smoke components: Effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-beta peptide aggregation. Sci Rep 7, 14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan JY, Wang JY, Wang Y, Wang JY, 2000. A comparison between acute exposures to ethanol and acetaldehyde on neurotoxicity, nitric oxide production and NMDA-induced excitotoxicity in primary cultures of cortical neurons. Chin J Physiol 43, 131–138. [PubMed] [Google Scholar]
- Wang JB, Olgin JE, Nah G, Vittinghoff E, Cataldo JK, Pletcher MJ, Marcus GM, 2018. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One 13, e0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MP, Ho SY, Leung LT, Lam TH, 2016. Electronic cigarette use and respiratory symptoms in Chinese adolescents in Hong Kong. JAMA Pediatr 170, 89–91. [DOI] [PubMed] [Google Scholar]
- Wang YT, Lin HC, Zhao WZ, Huang HJ, Lo YL, Wang HT, Lin AM, 2017. Acrolein acts as a neurotoxin in the nigrostriatal dopaminergic system of rat: Involvement of alpha-synuclein aggregation and programmed cell death. Sci Rep 7, 45741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Liu Q, Li D, Zheng Q, Zhou J, Li J, 2015. Acute nicotine treatment attenuates lipopolysaccharide-induced cognitive dysfunction by increasing BDNF expression and inhibiting neuroinflammation in the rat hippocampus. Neurosci Lett 604, 161–166. [DOI] [PubMed] [Google Scholar]
- Wetendorf M, Randall LT, Lemma MT, Hurr SH, Pawlak JB, Tarran R, Doerschuk CM, Caron KM, 2019. E-cigarette exposure delays implantation and causes reduced weight gain in female offspring exposed in utero. J Endocr Soc 3, 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels S, Wouters K, Schalkwijk CG, Vanmierlo T, Hendriks JJ, 2017. Methylglyoxal-derived advanced glycation endproducts in multiple sclerosis. Int J Mol Sci 18, E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton JD, Kozek LK, Carson RP, 2019. Increased seizure frequency temporally related to vaping: Where there’s vapor, there’s seizures? Pediatr Neurol 104, 66–67. [DOI] [PubMed] [Google Scholar]
- WHO, 2002. Ethylene glycol: Human health aspects https://apps.who.int/iris/handle/10665/42530 [Google Scholar]
- Wickham RJ, 2015. How menthol alters tobacco-smoking behavior: A biological perspective. Yale J Biol Med 88, 279–287. [PMC free article] [PubMed] [Google Scholar]
- Wieslander G, Norback D, Lindgren T, 2001. Experimental exposure to propylene glycol mist in aviation emergency training: Acute ocular and respiratory effects. Occup Environ Med 58, 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, To A, Bozhilov K, Talbot P, 2015. Strategies to reduce tin and other metals in electronic cigarette aerosol. PLoS One 10, e0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P, 2013. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 8, e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Pagano I, Williams RJ, Tam EK, 2019. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend 194, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 1996. Addictive drugs and brain stimulation reward. Annu Rev Neurosci 19, 319–340. [DOI] [PubMed] [Google Scholar]
- Worku D, Worku E, 2019. A narrative review evaluating the safety and efficacy of e-cigarettes as a newly marketed smoking cessation tool. SAGE Open Med 7, 2050312119871405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yu C, Tan Y, Hou Z, Li M, Shao F, Lu X, 2015. Effects of prenatal exposure to silver nanoparticles on spatial cognition and hippocampal neurodevelopment in rats. Environ Res 138, 67–73. [DOI] [PubMed] [Google Scholar]
- Ya BL, Li HF, Wang HY, Wu F, Xin Q, Cheng HJ, Li WJ, Lin N, Ba ZH, Zhang RJ, Liu Q, Li YN, Bai B, Ge F, 2017. 5-HMF attenuates striatum oxidative damage via Nrf2/ARE signaling pathway following transient global cerebral ischemia. Cell Stress Chaperones 22, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Zhao Y, Zhang X, 2016. Acetaldehyde induces cytotoxicity of SH-SY5Y cells via inhibition of Akt activation and induction of oxidative stress. Oxid Med Cell Longev 2016, 4512309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liu DF, Zhang XY, Liu D, Xu SY, Chen GX, Huang BX, Ren WZ, Wang W, Fu SP, Liu JX, 2017. Vanillin protects dopaminergic neurons against inflammation-mediated cell death by lnhibiting ERK1/2, P38 and the NF-kappaB signaling pathway. Int J Mol Sci 18, E389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XS, D’Ruiz C, 2015. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol 71, 24–34. [DOI] [PubMed] [Google Scholar]
- Yoong SL, Stockings E, Chai LK, Tzelepis F, Wiggers J, Oldmeadow C, Paul C, Peruga A, Kingsland M, Attia J, Wolfenden L, 2018. Prevalence of electronic nicotine delivery systems (ENDS) use among youth globally: a systematic review and meta-analysis of country level data. Aust N Z J Public Health 42, 303–308. [DOI] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, Leslie FM, 2015. Nicotine and the adolescent brain. J Physiol 593, 3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A, Phandthong R, Chaili A, Leung S, Omaiye E, Talbot P, 2019. Mitochondrial stress response in neural stem cells exposed to electronic cigarettes. iScience 16, 250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare S, Nemati M, Zheng Y, 2018. A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS One 13, e0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, Aschner M, 2018. Microglia activation and gene expression alteration of neurotrophins in the hippocampus following early-life exposure to e-cigarette aerosols in a murine model. Toxicol Sci 162, 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wang Z, Zhang K, Hou R, Xing C, Yu Q, Liu E, 2018a. Safety assessment of electronic cigarettes and their relationship with cardiovascular disease. Int J Environ Res Public Health 15, E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Di Y, Wu LY, He YL, Zhao T, Huang X, Ding XF, Wu KW, Fan M, Zhu LL, 2015. 5-HMF prevents against oxidative injury via APE/Ref-1. Free Radic Res 49, 86–94. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ding Y, He K, Li H, Gao F, Moehling TJ, Wu X, Duncan J, Niu Q, 2018b. Exposure to alumina nanoparticles in female mice during pregnancy induces neurodevelopmental toxicity in the offspring. Front Pharmacol 9, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Navas-Acien A, Ilievski V, Slavkovich V, Olmedo P, Adria-Mora B, Domingo-Relloso A, Aherrera A, Kleiman NJ, Rule AM, Hilpert M, 2019. Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ Res 174, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang Y, Sisler JD, Shaffer J, Leonard SS, Morris AM, Qian Y, Bello D, Demokritou P, 2018. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J Hazard Mater 344, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoroddu MA, Medici S, Ledda A, Nurchi VM, Lachowicz JI, Peana M, 2014. Toxicity of nanoparticles. Curr Med Chem 21, 3837–3853. [DOI] [PubMed] [Google Scholar]
- Zosel A, Egelhoff E, Heard K, 2010. Severe lactic acidosis after an iatrogenic propylene glycol overdose. Pharmacotherapy 30, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli A, Abidin EZ, Abidin NZ, Amer Nordin AS, Praveena SM, Syed Ismail SN, Rasdi I, Karuppiah K, Rahman AA, 2018. Electronic cigarettes: A systematic review of available studies on health risk assessment. Rev Environ Health 33, 43–52. [DOI] [PubMed] [Google Scholar]


