Summary
Background
Stroke is a leading cause of death and disability worldwide. Despite considerable improvements in diagnosis and treatment, little is known about the short-term and long-term prognosis after a first stroke in low-income and middle-income countries, including China. We aimed to assess the short-term and long-term risk of recurrent stroke and mortality after a first stroke for each of the major pathological stroke types.
Methods
This population-based cohort study included adults aged 35–74 years without disability who were recruited to the China Kadoorie Biobank (CKB). A baseline survey was conducted in ten geographical areas (five urban, five rural) in China, and participants had clinical measurements recorded. Participants were followed up by monitoring death registries and by electronic linkage to health registries and health insurance claims databases, with follow-up until Jan 1, 2017. Participants were excluded from analyses if they had a previous history of stroke, transient ischaemic attack, or ischaemic heart disease at baseline. All incidences of fatal and non-fatal stroke during the study period were recorded by type (ischaemic stroke, intracerebral haemorrhage, subarachnoid haemorrhage, and unspecified type). Primary outcome measures were 28-day mortality, recurrent stroke, major vascular events (recurrent stroke, myocardial infarction, or vascular death), vascular mortality, and all-cause mortality.
Findings
Of 512 715 individuals in the CKB, 489 586 participants without previous ischaemic heart disease and stroke at recruitment were included, of whom 45 732 (42 073 [92%] confirmed by brain imaging) had a stroke during the study period. The mean age was 59·3 years (SD 9·8) for participants who had a stroke (54% women) and 50·8 years (10·3) for participants with no stroke (60% women). 36 588 (80%) of the incident cases of stroke were ischaemic stroke, 7440 (16%) were intracerebral haemorrhage, 702 (2%) were subarachnoid haemorrhage, and 1002 (2%) were an unspecified stroke type. 28-day mortality was 3% (95% CI 3–4) for ischaemic stroke, 47% (46–48)for intracerebral haemorrhage, 19% (17–22; 52% for rural areas and 32% for urban areas) subarachnoid haemorrhage, and 24% (22–27) for unspecified stroke. Among participants who survived stroke at 28 days, 41% (41–42) had recurrent stroke at 5 years (ischaemic stroke 41% [41–42], intracerebral haemorrhage 44% [42–46], subarachnoid haemorrhage 22% [18–27], unspecified stroke type 40% [35–44]) and mortality at 5 years was 17% ([17–18] ischaemic stroke 16% [15–16], intracerebral haemorrhage 28% [26–29], subarachnoid haemorrhage 16% [12–20], unspecified stroke type 15% [12–19]). After a first ischaemic stroke, 91% of recurrent strokes were also ischaemic stroke; after an intracerebral haemorrhage, 56% of recurrent strokes were intracerebral haemorrhage, and 41% of recurrent strokes were ischaemic stroke.
Interpretation
After a first stroke, the risk of recurrence or death within 5 years was high among this population of Chinese adults. Urgent improvements to secondary prevention of stroke in China are needed to reduce these risks.
Funding
Wellcome Trust, Medical Research Council, British Heart Foundation, Cancer Research UK, Kadoorie Charitable Foundation, Chinese Ministry of Science and Technology, National Natural Science Foundation of China.
Copyright
© 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Introduction
Stroke is a leading cause of death and permanent disability worldwide. Around three-quarters of the global burden of stroke deaths (approximately 6·5 million per year) and associated disability-adjusted life years (113 million) now occur in low-income and middle-income countries, including China.1 In recent decades, the incidence and mortality rates of first stroke cases have declined progressively in high-income countries, but have not changed or have increased in many low-income and middle-income countries.1 Stroke is the leading cause of death and disability in China, accounting for more than 3 million new cases and around 2 million deaths in 2013,2, 3 and a higher proportion of strokes are intracerebral haemorrhages in the Chinese population than in western populations.2, 3
Previous large, nationwide studies in China have provided reliable estimates for stroke prevalence,4 incidence,3 and mortality,1 and have assessed the role of major risk factors for stroke.5, 6 However, there is little reliable contemporary evidence in China (and many other low-income and middle-income countries) about the case-fatality rate or recurrent risk after first stroke of different types. Previous such studies have been constrained by small sample sizes,7, 8 a focus on particular stroke types or minor cases,7, 8, 9 short duration of follow-up,3 or restriction to hospital-based rather than community-based settings.10 As in most high-income countries, considerable improvements have been made in the diagnosis of stroke (ie, widespread use of brain imaging), the acute treatment of stroke (eg, thrombolysis and antiplatelet therapy), and long-term secondary prevention (eg, blood-pressure lowering, antiplatelet and lipid-lowering therapy) in the past few decades in China.11 However, considerable uncertainty persists about whether such advances have resulted in any substantial improvement in both the short-term and long-term prognosis after first stroke in the general population. Reliable contemporary evidence on the natural history of stroke types in low-income and middle-income countries is needed to guide the delivery of appropriate services for the treatment and prevention of stroke.
Research in context.
Evidence before this study
We searched PubMed from inception to Sept 1, 2019, for studies that had investigated the recurrence or mortality after stroke and major stroke types, using the search terms (“stroke” or “Ischemic stroke” or “haemorrhagic stroke” or “Chinese” and “recurrence” or “mortality or fatality”) in articles published in English. In a national representative survey on stroke incidence and mortality, recurrence was not reported and patients were only followed up for 1 year. Although considerable improvements have been made in the diagnosis and treatment of stroke, substantial uncertainty persists about whether such advances have resulted in any appreciable improvements in prognosis after a first stroke in China.
Added value of this study
In this prospective study of adults recruited into the China Kadoorie Biobank during 2004–08, 489 586 participants without previous cardiovascular disease events were followed up for 9 years to record cases of first incident and recurrent stroke and cause-specific mortality. Risks of death and recurrent stroke were estimated separately by stroke type. 45 732 participants had a first stroke, including 36 588 (80%) individuals who had an ischaemic stroke, 7440 (16%) who had an intracerebral haemorrhage, 702 (2%) who had a subarachnoid haemorrhage, and 1002 (2%) who had an unspecified stroke type. The prognosis after a first stroke was poor, with 28-day mortality of 3% after ischaemic stroke, 47% after cerebral haemorrhage, 19% after subarachnoid haemorrhage, and 24% after unspecified stroke, with higher 28-day mortality in rural than urban areas, particularly for intracerebral haemorrhage (52% vs 32%). Among stroke survivors at 28 days, 41% had recurrent stroke and 17% died by 5 years after first stroke.
Implications of all the available evidence
Despite progress in the treatments for stroke worldwide, the short-term and long-term prognosis following stroke is still poor in middle-income countries such as China, highlighting the urgent need for further improvements in secondary prevention of stroke in these countries.
We present relevant findings from a 9-year follow-up of a population-based study of 0·5 million adults from ten diverse geographical areas in China. The aims of this study were to assess the 28-day case-fatality rates after first stroke for the major pathological stroke types; to estimate the short-term and long-term risks of recurrent stroke and mortality after a first stroke event by stroke type; and to investigate the pathological types of recurrent stroke after a first stroke of different types.
Methods
Study design and participants
Details of the design and methods used in the China Kadoorie Biobank (CKB) have been previously reported.12, 13 In brief, the 2004–08 baseline survey was conducted in ten geographical areas (five urban, five rural) of China, chosen from China's nationally representative Disease Surveillance Points. These ten areas were chosen to maximise regional and social diversity, differences in risk exposures, and differences in burden of major diseases, including stroke. In each area, all permanent residents aged 35–74 years without disability were invited to participate. 512 715 individuals (including a small number of individuals outside the target age range) agreed to participate in the study.
Trained health workers administered laptop-based questionnaires at local study clinics, which collected detailed information on sociodemographic status, smoking, alcohol consumption, diet, physical activity, and medical history. All participants also had several clinical measurements recorded, including height, weight, waist and hip circumference, lung function, blood pressure, and heart rate, and had a blood sample collected for long-term storage. Previous international, national and local ethics approval was obtained and all participants provided written informed consent to participate in the study and for their health status to be monitored by linkage with their electronic health records.
Procedures and outcomes
After the baseline survey, the vital status of participants was monitored regularly through Disease Surveillance Point death registries, supplemented by annual checks with local residential records and active confirmation by contacting local street committees or village administrators.13 The causes of death, usually available from official death certificates, were supplemented by available medical records. In the small proportion of deaths (<5%) occurring without recent medical attention, standard procedures (verbal autopsy) were used to determine the probable causes of death based on symptoms and signs provided by informants (usually family members).14
All hospitalised cases of stroke (and other diseases) were identified by electronic linkage, via a unique personal identification number, to established registries of major diseases (ie, stroke, ischaemic heart disease, cancer, and diabetes) and to local health insurance claims databases for any hospital admissions, which covered more than 97% of study participants (these procedures also yielded a few additional deaths that had not been identified through death registries). To minimise bias due to loss to follow-up, any uninsured participants were also actively followed up by annual home visits in addition to annual checking of death and disease registries for any hospital admissions with stroke and other diseases and deaths in such participants.
All fatal and non-fatal stroke cases reported by different sources were coded by trained medical staff, who were blinded to other personal information, using the International Classification of Diseases 10th revision (ICD-10), with further checking and review done centrally by a clinical research fellow. Any hospital-reported cases of first stroke also underwent separate clinical adjudication, involving retrieval and review of original medical records and brain imaging reports by clinical specialists in China using a bespoke web-based system.
First incident and recurrent strokes
For first incident stroke, the major pathological types examined were ischaemic stroke (ICD-10 code I63), including lacunar infarction and non-lacunar infarction; intracerebral haemorrhage (I61); subarachnoid haemorrhage (I60); and unspecified stroke (I64). Ischaemic stroke was defined as a focal neurological dysfunction lasting for more than 24 h with or without neuroimaging evidence of a cerebral infarct. Lacunar infarction was defined as stroke with neuroimaging evidence of an infarct in the brain of less than 1·5 cm in diameter on CT or MRI reports, with or without focal neurological deficits, including lacunar syndrome. Non-lacunar infarction was any other type of ischaemic stroke, excluding lacunar infarction. Intracerebral haemorrhage was defined as neurological dysfunction caused by haemorrhage into the brain parenchyma or the ventricular system, excluding those induced by injury, with or without neuroimaging evidence of brain haemorrhage. Subarachnoid haemorrhage was defined as neurological dysfunction considered to be caused by haemorrhage into the subarachnoid space, excluding those induced by injury, with or without relevant neuroimaging evidence. 92% of the reported first stroke cases had their diagnosis confirmed by brain imaging (CT or MRI). Radiological reports (but not the brain imaging) of individuals with non-fatal stroke were reviewed by Chinese neurologists online.
The primary outcome measures were 28-day mortality, recurrent stroke, major vascular events, vascular mortality, and all-cause mortality. Recurrent stroke was defined as any hospitalised stroke events (ICD-10 I60, I61, I63, I64) occurring more than 24 h after the onset of a known first stroke. Major vascular events were defined as recurrent stroke, myocardial infarction (I21), or vascular death (I00–99), whichever was reported first.
Statistical analysis
The present analysis included all participants who had a stroke between enrolment and Jan 1, 2017, without a previous history of stroke or transient ischaemic attack, or ischaemic heart disease at baseline. The incidences of first stroke were standardised by sex, area, and age group to the overall CKB population for each decade of age at the time of diagnosis, excluding those with previous ischaemic heart disease or a previous stroke or transient ischaemic attack and are presented as incidence per 100 000 person-years. 28-day mortality was calculated as the proportion of participants who died within 28 days after a first incident stroke.
Cumulative mortality after first incident stroke was calculated as one minus Kaplan-Meier survival probability. The cumulative event rates (95% CIs) for recurrent stroke and other cardiovascular disease outcomes were calculated by the cumulative incidence function, treating death from any cause as a competing risk. The cumulative incidence of competing risks was estimated using the cmprsk package in R and the corresponding 95% CIs were based on the log-minus-log transformation.15, 16 The event rates were estimated for all participants who had a first incident stroke, and separately by stroke type. Event rates were also calculated separately by sex and area (urban or rural). In analyses of multiple events (vascular or non-vascular deaths; recurrent strokes classified by type; myocardial infarction, angina, or other cardiac diseases), different event types were treated as competing risks in the analyses. Unless otherwise specified, recurrent cases of stroke, major vascular events, or deaths were restricted to those occurring more than 28 days after a first stroke event. All analyses were done using R 3.5.0.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. YC, RCl, and ZC had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
By Jan 1, 2017 (the censoring date for the present analyses), 44 066 (8·6%) participants had died and 4751 (<1%) were lost to follow-up, mostly because participants moved out of the study area.
Of 512 715 individuals in the CKB, 23 129 had a previous history of stroke, transient ischaemic attack, or ischaemic heart disease at baseline and were excluded from these analyses. 489 586 participants were included, of whom 45 732 had a stroke during the study period and 443 854 had no stroke (table 1).
Table 1.
Baseline characteristics of participants by type of stroke
|
Stroke type |
Any stroke (n=45 732) | No stroke (n=443 854) | |||||
|---|---|---|---|---|---|---|---|
| IS (n=36 588) | ICH (n=7440) | SAH (n=702) | Unspecified (n=1002) | ||||
| Age group, years | |||||||
| 30–39 | 974 (2·7%) | 285 (3·8%) | 52 (7·4%) | 36 (3·6%) | 1347 (2·9%) | 76 013 (17·1%) | |
| 40–49 | 5662 (15·5%) | 1122 (15·1%) | 148 (21·1%) | 166 (16·6%) | 7098 (15·5%) | 143 628 (32·4%) | |
| 50–59 | 12 096 (33·1%) | 2202 (29·6%) | 259 (36·9%) | 282 (28·1%) | 14 839 (32·4%) | 135 833 (30·6%) | |
| 60–69 | 12 122 (33·1%) | 2467 (33·2%) | 172 (24·5%) | 323 (32·2%) | 15 084 (33·0%) | 67 199 (15·1%) | |
| 70–79 | 5734 (15·7%) | 1364 (18·3%) | 71 (10·1%) | 195 (19·5%) | 7364 (16·1%) | 21 181 (4·8%) | |
| Age, years | 59·3 (9·6) | 59·6 (10·2) | 56 (10·1) | 59·6 (10·4) | 59·3 (9·8) | 50·8 (10·3) | |
| Sex | |||||||
| Men | 16 380 (44·8%) | 3913 (52·6%) | 265 (37·7%) | 455 (45·4%) | 21 013 (45·9%) | 179 122 (40·4%) | |
| Women | 20 208 (55·2%) | 3527 (47·4%) | 437 (62·3%) | 547 (54·6%) | 24 719 (54·1%) | 264 732 (59·6%) | |
| Area | |||||||
| Rural | 17 606 (48·1%) | 5618 (75·5%) | 447 (63·7%) | 530 (52·9%) | 24 201 (52·9%) | 253 985 (57·2%) | |
| Urban | 18 982 (51·9%) | 1822 (24·5%) | 255 (36·3%) | 472 (47·1%) | 21 531 (47·1%) | 189 869 (42·8%) | |
| Highest education | |||||||
| Primary school or no formal education | 20 079 (54·9%) | 5317 (71·5%) | 433 (61·7%) | 649 (64·8%) | 26 478 (57·9%) | 222 431 (50·1%) | |
| Middle school or high school | 13 938 (38·1%) | 1931 (26·0%) | 244 (34·8%) | 317 (31·6%) | 16 430 (35·9%) | 196 457 (44·3%) | |
| College or university | 2571 (7·0%) | 192 (2·6%) | 25 (3·6%) | 36 (3·6%) | 2824 (6·2%) | 24 966 (5·6%) | |
| Annual household income, ¥ | |||||||
| <9999 | 11 301 (30·9%) | 3335 (44·8%) | 209 (29·8%) | 377 (37·6%) | 15 222 (33·3%) | 123 610 (27·8%) | |
| 10 000–19 999 | 11 822 (32·3%) | 2095 (28·2%) | 224 (31·9%) | 280 (27·9%) | 14 421 (31·5%) | 126 910 (28·6%) | |
| 20 000–34 999 | 8000 (21·9%) | 1291 (17·4%) | 159 (22·6%) | 199 (19·9%) | 9649 (21·1%) | 111 274 (25·1%) | |
| ≥35 000 | 5465 (14·9%) | 719 (9·7%) | 110 (15·7%) | 146 (14·6%) | 6440 (14·1%) | 82 060 (18·5%) | |
| Current smoker | |||||||
| Men | 9272 (56·6%) | 2414 (61·7%) | 160 (60·4%) | 254 (55·8%) | 12 100 (57·6%) | 111 930 (62·5%) | |
| Women | 802 (4·0%) | 133 (3·8%) | 23 (5·3%) | 20 (3·7%) | 978 (4·0%) | 5642 (2·1%) | |
| Current alcohol drinker | |||||||
| Men | 11 915 (72·7%) | 2525 (64·5%) | 182 (68·7%) | 305 (67·0%) | 14 927 (71·0%) | 138 459 (77·3%) | |
| Women | 7596 (37·6%) | 982 (27·8%) | 165 (37·8%) | 175 (32·0%) | 8918 (36·1%) | 95 510 (36·1%) | |
| Regular dietary intake* | |||||||
| Meat | 28 811 (78·7%) | 5427 (72·9%) | 582 (82·9%) | 793 (79·1%) | 35 613 (77·9%) | 369 906 (83·3%) | |
| Fish | 15 636 (42·7%) | 2427 (32·6%) | 311 (44·3%) | 438 (43·7%) | 18 812 (41·1%) | 209 947 (47·3%) | |
| Dairy | 9029 (24·7%) | 859 (11·5%) | 129 (18·4%) | 181 (18·1%) | 10 198 (22·3%) | 86 145 (19·4%) | |
| Fruit | 20 468 (55·9%) | 3295 (44·3%) | 415 (59·1%) | 522 (52·1%) | 24 700 (54·0%) | 266 159 (60·0%) | |
| Prevalent disease† | |||||||
| Diabetes | 2583 (7·1%) | 310 (4·2%) | 20 (2·8%) | 71 (7·1%) | 2984 (6·5%) | 10 329 (2·3%) | |
| Hypertension | 7960 (21·8%) | 1844 (24·8%) | 117 (16·7%) | 246 (24·6%) | 10 167 (22·2%) | 38 392 (8·6%) | |
| Systolic blood pressure, mm Hg | 141·5 (24·2) | 151·2 (26·6) | 138·3 (22·6) | 143 (25·7) | 143 (24·9) | 129·3 (20·2) | |
| Body-mass index (kg/m2) | 24·3 (3·5) | 23·3 (3·6) | 23·7 (3·5) | 23·8 (3·6) | 24·1 (3·5) | 23·5 (3·3) | |
| Random plasma glucose, mmol/L | 6·7 (3·2) | 6·5 (3·1) | 6·2 (2·7) | 6·6 (3·3) | 6·7 (3·2) | 6·0 (2·2) | |
Data are mean (SD) or n (%). 23 129 participants with self-reported previous ischaemic heart disease, stroke, or transient ischaemic attack at baseline were excluded. IS=ischaemic stroke. ICH=intracerebral haemorrhage. SAH=subarachnoid haemorrhage.
1–3 days per week or more often.
Self-reported at baseline survey.
The mean age at baseline was 59·3 years (SD 9·8) for participants who had a stroke during the study period and 50·8 years (10·3) for participants who had no stroke. 54·1% with stroke and 59·6% without stroke were women, and 47·1% with stroke and 42·8% without stroke resided in urban areas (table 1). During 4·72 million person-years of follow-up, 45 732 participants had a first incident stroke, including 36 588 (80%) ischaemic strokes, 7440 (16%) intracerebral haemorrhages, 702 (2%) subarachnoid haemorrhages, and 1002 (2%) unspecified stroke types (table 1). 42 073 (92%) of 45 732 stroke diagnoses were confirmed by brain imaging using CT or MRI.
The standardised event rates were 778 (95% CI 107–113) per 100 000 person-years for ischaemic stroke (110 [107–113] lacunar infarction, 668 [661–676] non-lacunar infarction), 156 (153–160) for intracerebral haemorrhage, 15 (14–16) for subarachnoid haemorrhage, and 21 (20–22) for unspecified stroke types (appendix p 4). The event rate of ischaemic stroke was higher in those living in urban areas rather than rural areas (appendix p 4). In contrast, the event rate of intracerebral haemorrhage were higher in those living in rural areas rather than urban areas (appendix p 4).
Compared with those who had no stroke, individuals who developed a first stroke during the study period tended to be older, male, to reside in urban areas, and to have lower levels of education and household income at baseline (table 1). Compared with ischaemic stroke, individuals who had intracerebral haemorrhage were more likely to be men, to live in rural areas, and to have lower levels of education and income. Individuals who had intracerebral haemorrhage also tended not to regularly consume meat, fish, fruit, and dairy products, and tended to be current smokers and have higher mean systolic blood pressure than individuals who had ischaemic stroke. However, the two groups had a similar mean body-mass index and random plasma glucose concentration. The baseline characteristics of individuals who had subarachnoid haemorrhage were generally similar to those of individuals who had intracerebral haemorrhage, and those of individuals who had unspecified stroke were similar to those of individuals who had ischaemic stroke (table 1).
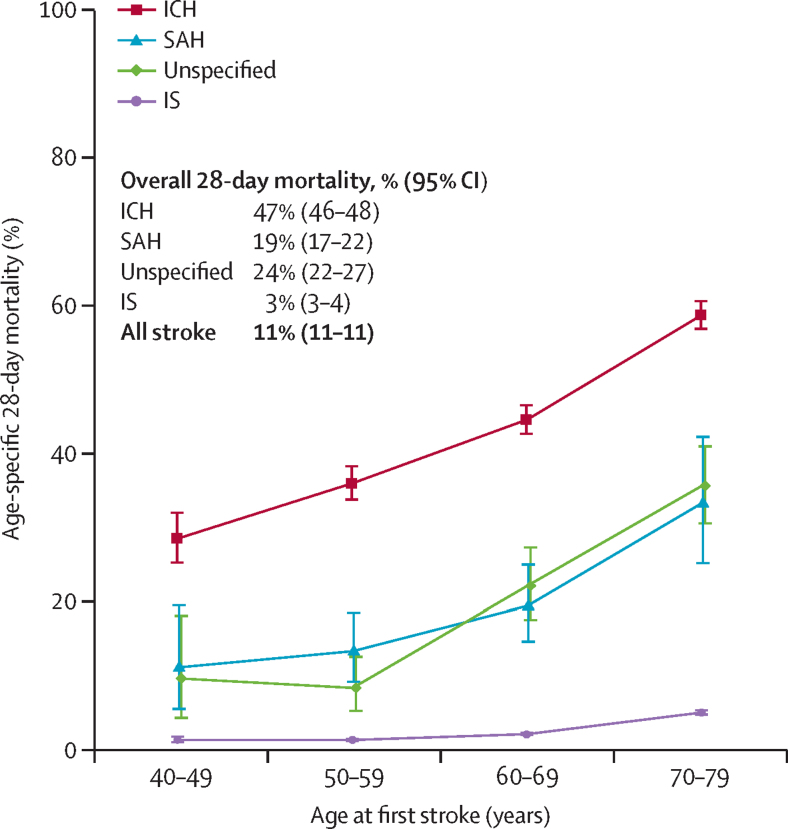
28-day mortality of individuals with first stroke was 11% (95% CI 11–11; figure 1), with higher age-specific mortality in men than women (13% [12–13] vs 10% [9–10]; appendix p 13), and in rural areas than urban areas (16% [15–16] vs 6% [6–6]; appendix p 13). Mortality increased with age, particularly for intracerebral haemorrhage (figure 1). 28-day mortality was highest for intracerebral haemorrhage (47% [46–48]) and lowest for ischaemic stroke (3% [3–4]). For ischaemic stroke, 28-day mortality was higher for non-lacunar infarction than for lacunar infarction (4% [3–4] vs 0·4% [0·2–0·6]; data not shown).
Figure 1.
Age-specific 28-day mortality after a first stroke of different types
Mortality was calculated as the proportion of participants dying from any cause within 28 days after a first stroke. Whiskers indicate 95% CIs. IS=ischaemic stroke. ICH=intracerebral haemorrhagic stroke. SAH=subarachnoid haemorrhage.
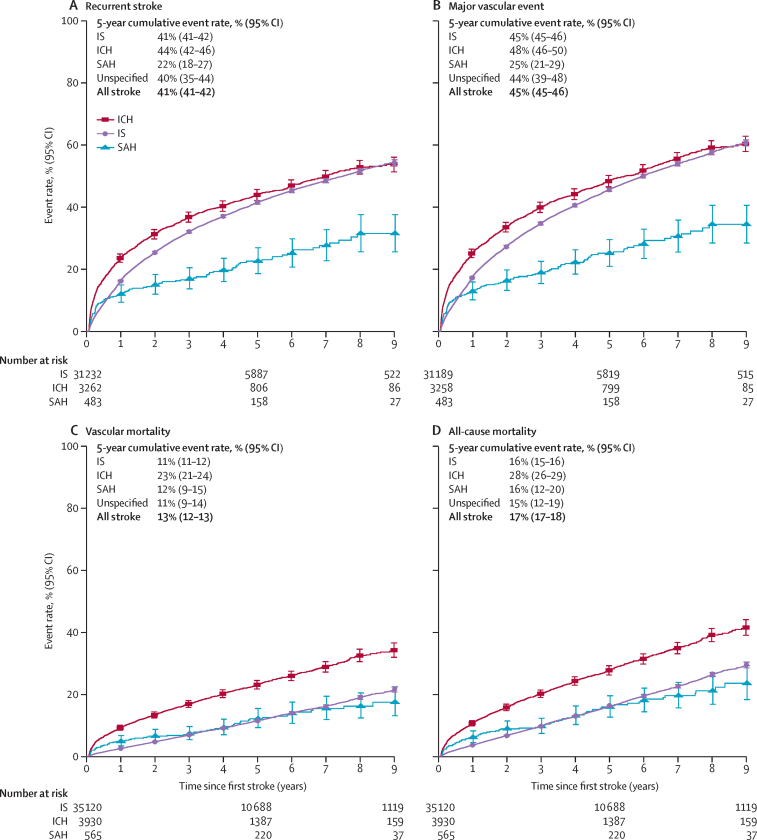
Among those who survived a first stroke, the cumulative recurrence rates from 28 days after the first stroke were 17% (95% CI 16–17) at 1 year, 41% (41–42) at 5 years, and 53% (52–54) at 9 years (table 2). All-cause mortality (predominantly from vascular causes) was 4% (4–4) at 1 year, 17% (17–18) at 5 years, and 30% (30–31) at 9 years. Individuals with intracerebral haemorrhage had higher post-28-day mortality than any other stroke type, with mortality rates of 11% (10–12) at 1 year, 28% (26–29) at 5 years, and 41% (39–44) at 9 years (appendix p 6), compared with 4% (3–4) at 1 year, 16% (15–16) at 5 years, and 29% (28–30) at 9 years for those with ischaemic stroke (appendix p 5). At 5 years, the all-cause mortality for those with intracerebral haemorrhage (28% [26–29]) was almost twice as high as that for other stroke types (figure 2). Stroke recurrence rates at 5 years were similar for those with intracerebral haemorrhage (44% [42–46]), ischaemic stroke (41% [41–42]), and unspecified stroke type (40% [35–44]), but were lower for those with subarachnoid haemorrhage (22% [18–27]). Mortality at 5 years was almost twice as high for individuals with non-lacunar infarction compared with lacunar infarction (17% [16–17] vs 9% [8–10]), and the stroke recurrence rates were similar (41% [40–42] vs 45% [43–47]; figure 3).
Table 2.
Cumulative event rate of recurrent stroke, major vascular events, vascular mortality, and all-cause mortality (n=45 732)
| 28 days | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | |
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent stroke | ||||||||||
| Events | 0 | 5616 | 8140 | 9685 | 10 623 | 11 298 | 11 722 | 11 995 | 12 161 | 12 230 |
| No events or death | 35 654 | 24 916 | 18 535 | 13 723 | 9953 | 7008 | 4799 | 2937 | 1565 | 668 |
| Deaths | 0 | 655 | 1067 | 1391 | 1634 | 1786 | 1910 | 2002 | 2064 | 2077 |
| Censored | 0 | 4467 | 7912 | 10 855 | 13 444 | 15 562 | 17 223 | 18 720 | 19 864 | 20 679 |
| Cumulative event rate, % (95% CI) | 0 | 17% (16–17) | 26% (25–26) | 32% (31–33) | 37% (36–37) | 41% (41–42) | 45% (44–45) | 48% (47–49) | 51% (50–52) | 53% (52–54) |
| Major vascular event* | ||||||||||
| Events | 0 | 5963 | 8731 | 10 461 | 11 552 | 12 324 | 12 816 | 13 148 | 13 346 | 13 426 |
| No events or death | 35 605 | 24 839 | 18 448 | 13 646 | 9872 | 6933 | 4738 | 2894 | 1547 | 660 |
| Deaths | 0 | 347 | 548 | 698 | 807 | 877 | 937 | 975 | 1001 | 1005 |
| Censored | 0 | 4456 | 7878 | 10 800 | 13 374 | 15 471 | 17 114 | 18 588 | 19 711 | 20 514 |
| Cumulative event rate, % (95% CI) | 0 | 18% (17–18) | 27% (27–28) | 35% (34–35) | 40% (40–41) | 45% (45–46) | 49% (49–50) | 53% (53–54) | 57% (56–58) | 60% (59–61) |
| Vascular mortality | ||||||||||
| Events | 0 | 1208 | 1972 | 2597 | 3113 | 3545 | 3885 | 4105 | 4284 | 4362 |
| No events or death | 40 370 | 33 334 | 27 525 | 22 097 | 17 001 | 12 562 | 9078 | 5984 | 3321 | 1368 |
| Deaths | 0 | 426 | 729 | 962 | 1168 | 1298 | 1416 | 1496 | 1550 | 1569 |
| Censored | 0 | 5402 | 10 144 | 14 714 | 19 088 | 22 965 | 25 991 | 28 785 | 31 215 | 33 071 |
| Cumulative event rate, % (95% CI) | 0 | 3% (3–3) | 6% (5–6) | 8% (8–8) | 10% (10–10) | 13% (12–13) | 15% (15–16) | 17% (17–18) | 20% (20–21) | 23% (22–24) |
| All-cause mortality | ||||||||||
| Events | 0 | 1634 | 2701 | 3559 | 4281 | 4843 | 5301 | 5601 | 5834 | 5931 |
| No events or death | 40 370 | 33 334 | 27 525 | 22 097 | 17 001 | 12 562 | 9078 | 5984 | 3321 | 1368 |
| Censored | 0 | 5402 | 10 144 | 14 714 | 19 088 | 22 965 | 25 991 | 28 785 | 31 215 | 33 071 |
| Cumulative event rate, % (95% CI) | 0 | 4% (4–4) | 8% (7–8) | 11% (10–11) | 14% (14–14) | 17% (17–18) | 21% (20–21) | 24% (23–24) | 27% (27–28) | 30% (30–31) |
Time-points are time since first stroke.
Stroke, myocardial infarction, and vascular mortality.
Figure 2.
Estimated cumulative event rates of recurrent stroke, major vascular events, vascular mortality, and all-cause mortality from 28 days after first stroke of different types
Plotted lines indicate the cumulative incidence, starting at the date of first stroke. Whiskers indicate 95% CIs. Deaths from any cause were treated as competing risks. Participants experiencing an event or death within 28 days from first stroke were excluded. IS=ischaemic stroke. ICH=intracerebral haemorrhagic stroke. SAH=subarachnoid haemorrhage.
Figure 3.
Estimated cumulative event rates of recurrent stroke, major vascular events, vascular mortality, and all-cause mortality from 28 days after first ischaemic stroke of different subtypes
Plotted lines show the cumulative incidence, starting with the date of first stroke. Deaths from any cause were treated as competing risks. Participants experiencing an event or death within 28 days following a first stroke were excluded. Only participants whose first stroke was ischaemic stroke were included. LACI=lacunar infarction.
For all types of first stroke, the cumulative recurrent stroke rate at 5 years was higher in men than women (except for subarachnoid haemorrhage) and in urban than rural areas (appendix p 14). All-cause mortality at 5 years was higher in men than women (except for subarachnoid haemorrhage), but lower in urban than rural areas for all stroke types (appendix p 15).
After a first ischaemic stroke, 91% of recurrent strokes were ischaemic stroke (urban 94%, rural 86%), 7% were intracerebral haemorrhage (urban 3%, rural 11%), and 2% were unspecified stroke type (urban 2%, rural 2%; appendix p 10). After a first intracerebral haemorrhage, 56% of recurrent strokes were intracerebral haemorrhage (urban 43%, rural 62%), 41% were ischaemic stroke (urban 53%, rural 35%), and 3% were unspecified stroke type (urban 3%, rural 3%). After a first subarachnoid haemorrhage, 43% of recurrent strokes were ischaemic stroke, 29% were intracerebral haemorrhage, and 27% were subarachnoid haemorrhage. After a stroke of unspecified type, 69% of recurrent strokes were ischaemic stroke, 22% were unspecified stroke, and 9% were intracerebral haemorrhage (appendix p 10).
After a first non-lacunar infarction, 80% of recurrent strokes were non-lacunar infarction (urban 78%, rural 82%), 10% were lacunar infarction, and 7% were intracerebral haemorrhage (appendix p 10). After a lacunar infarction, 47% of recurrent strokes were lacunar infarction (urban 50%, rural 23%), 48% were non-lacunar infarction (urban 47%, rural 64%), and 3% were intracerebral haemorrhage (urban 2%, rural 13%).
Compared with recurrent stroke (41%), the event rate of myocardial infarction at 5 years after first stroke was much lower, at about 2% (appendix p 16). The 5-year event rate for non-myocardial-infarction cardiac events was 15% after ischaemic stroke and 7% after intracerebral haemorrhage.
Among individuals who survived a first stroke at 28 days, the cumulative event rates for major vascular events (ie, non-fatal recurrent stroke, non-fatal myocardial infarction, or vascular death) were 18% at 1 year, 45% at 5 years, and 60% at 9 years (table 2). After combining both short-term (≤28 days) and long-term (>28 days) risks, the cumulative mortality after a first stroke was 15% at 1 year (ischaemic stroke 7%, intracerebral haemorrhage 53%) and 26% at 5 years (ischaemic stroke 19%, intracerebral haemorrhage 62%; appendix pp 8, 9).
After excluding individuals with stroke as secondary diagnosis, analyses restricted to 21 817 individuals with an adjudicated diagnosis of a non-fatal first stroke event as their main diagnosis showed similar 5-year cumulative event rates for recurrent stroke, major vascular events, vascular and all-cause mortality (appendix p 11). Further sensitivity analyses excluding individuals with recurrent stroke events who also had comorbidities only slightly reduced the overall 5-year recurrence rates (from 41% to 35%; appendix p 12).
Discussion
In this large community-based study in China, the incidence of stroke in the adult population was high, with around 9% of included participants experiencing a stroke during 9-year follow-up. Prognosis was also notably poor, with one in ten individuals dying within 28 days after onset of a first stroke, almost one in five dying by 5 years and more than 40% experiencing a recurrent stroke within 5 years of a first stroke. Among stroke types, the short-term and long-term prognosis for individuals with intracerebral haemorrhage was substantially worse than for those with ischaemic stroke, particularly in rural areas. After a first ischaemic stroke, although the 28-day mortality was low, the long-term risks of recurrent stroke and mortality were high. After a first ischaemic stroke, most of the incidences of recurrent stroke were also ischaemic stroke, whereas after a first intracerebral haemorrhage, a higher proportion of recurrent strokes were ischaemic stroke than were intracerebral haemorrhage.
The event rate of first stroke in the present study population was considerably higher than the incidence reported in a recent national representative survey (345·1 per 100 000 person-years).3 The discrepant results in the present study might reflect the older mean age of the CKB study participants or the inclusion of areas with a high incidence of stroke (eg, Harbin and Hunan).11 Because the present study was not designed to be nationally representative, it is not appropriate to make direct comparisons of stroke incidence in CKB with those in representative national surveys. Nevertheless, the observed north-to-south gradients and rural-urban differences in stroke incidence observed in CKB were generally consistent with those reported in the most recent representative national survey.3
In the present study, 28-day mortality of intracerebral haemorrhage was considerably higher than that of ischaemic stroke (47% vs 3%), which is expected and could reflect differences in both pathology and effects of treatment.17 A systematic review of 8145 individuals with intracerebral haemorrhage from 36 studies in 21 countries also reported a similar 28-day mortality of 40%,18 and a previous study involving 16 031 Chinese adults who were enrolled between 1991 and 2000 reported 28-day mortality of 49·4% for intracerebral haemorrhage.19 Similar studies in western populations have reported lower 28-day mortality of intracerebral haemorrhage (eg, 29·6% in France20 and 30·7% in Sweden21). The higher mortality of intracerebral haemorrhage in China might reflect differences in stroke severity (due to poor detection and control of hypertension in China),11, 22 or delays in access to hospital care (including access to neurosurgery), particularly in rural areas.11 Similarly, differences in lifestyle factors might also account for the higher mortality observed in rural areas than urban areas in CKB. Unexpectedly, the present study showed lower mortality for subarachnoid haemorrhage than for intracerebral haemorrhage. Because subarachnoid haemorrhage is relatively rare among stroke types, the possibility of misclassification of subarachnoid haemorrhage as intracerebral haemorrhage for some individuals cannot be entirely excluded. The lower mortality of subarachnoid haemorrhage in Asian countries (Japan and China) than in other regions was also reported in a recent meta-analysis of subarachnoid haemorrhage involving 33 studies in 19 countries recruited after 1995.23 In contrast with intracerebral haemorrhage, the 28-day mortality for ischaemic stroke observed in the present study and in previous studies in China were considerably lower than those reported in western populations,21, 24, 25, 26 possibly reflecting differences in the incidence of ischaemic stroke subtypes (eg, a higher proportion of individuals with small vessel ischaemic stroke) or better detection of less severe stroke types.27, 28
Few previous studies in China have examined the long-term prognosis after the onset of first stroke. In the China National Stroke Registry study, which involved 11 560 individuals with ischaemic stroke recruited in 2007–08 from major hospitals, 1-year cumulative mortality after a first ischaemic stroke was twice as high as that reported in the present study (14% vs 7%),29 but the stroke recurrence rate at 1 year was comparable to that observed in the present study (17% vs 16%). Less evidence is available on the long-term prognosis after first intracerebral haemorrhage. In a meta-analysis of 2408 individuals with intracerebral haemorrhage from 122 studies in different countries,30 the overall cumulative mortality was 54% at 1 year and 71% at 5 years. The results of the present study were similar to those observed in a recent Swedish study, which reported mortality rates of 42% at 1 year and 62% at 5 years.21 A meta-analysis involving 1102 individuals with intracerebral haemorrhage enrolled between 1981 and 2007 reported higher stroke recurrence rates than the present study (46% vs 23%), but the differences might reflect improvements in health-care during the period between the studies.30
The poor long-term prognosis after a first stroke in China might also reflect the inadequate use of established medications (eg, antiplatelet therapy, blood pressure-lowering, and lipid-lowering treatments), as shown in a previous report from the CKB study population.22 Other community-based studies in China have reported that only 10% of individuals with ischaemic stroke used aspirin, 2% used LDL cholesterol-lowering therapy, and around 35% used antihypertensive medication.11, 31 The scarce use of such treatments with proven efficacy might reflect inadequate reimbursement policies, poor access to primary health care services,32 or poor awareness among patients and their doctors of the need for long-term use of such treatments for stroke survivors.11, 33
The present study showed that nearly all incidences of recurrent stroke after ischaemic stroke were also ischaemic stroke, which was consistent with previous studies done in western populations.34 In contrast with previous reports that suggested that lacunar infarction and non-lacunar infarction have a distinct pathology,28, 35 the present study showed that subsequent stroke can be both lacunar infarction and non-lacunar infarction after first lacunar infarction or non-lacunar infarction indicating overlapping causes underlying lacunar infarction and non-lacunar infarction. Few large studies have specifically examined the type of recurrent stroke events after intracerebral haemorrhage. A systematic review of ten studies, involving 1880 individuals with intracerebral haemorrhage recorded between 1982 and 2000, reported that 75% of the individuals who had recurrent strokes had intracerebral haemorrhage.36 By contrast, a meta-analysis involving 2408 individuals with intracerebral haemorrhage reported similar proportions of recurrent ischaemic stroke and recurrent intracerebral haemorrhage after first intracerebral haemorrhage.30 The present study, involving three-times as many individuals with intracerebral haemorrhage as the previous meta-analysis,30 also reported that more than 40% of recurrent strokes after intracerebral haemorrhage were ischaemic stroke.
Current guidelines recommend use of antiplatelet therapy and statins for ischaemic stroke, but not for intracerebral haemorrhage.37 However, most of the available evidence about the use of such treatment in intracerebral haemorrhage is based on studies done in western populations in which the incidence of intracerebral haemorrhage was low. Given the high incidence of intracerebral haemorrhage in China, large randomised trials are needed to assess the efficacy and safety of long-term antiplatelet and cholesterol-lowering therapy for secondary prevention of ischaemic stroke. A previous estimate suggested that the benefits of LDL cholesterol-lowering treatments on ischaemic stroke and ischaemic heart disease would exceed the adverse effects on intracerebral haemorrhage by more than five times in Chinese adults.38
This study had several strengths, including the prospective study design, the large number of participants, detailed data on stroke types, and complete long-term follow-up for both fatal and non-fatal incidences of stroke and other vascular diseases. Of note, more than 90% of the reported first strokes were confirmed by brain imaging. However, the study also had several limitations. Firstly, the CKB cohort was established mainly to investigate the determinants of common chronic diseases. Therefore, the study design focused on diversity rather than representativeness of study populations. As such, the findings may not be directly generalisable to the overall Chinese population. Secondly, recurrent strokes were defined as any reported stroke occurring more than 24 h after a first incident stroke that led to new hospitalisation.35 It is possible that some of the recurrent stroke cases might not be new stroke events, but were recorded as new cases for various reasons (including transfers between different hospitals). However, most of the recurrent strokes occurred many months or years after their initial event, frequently involving a different stroke pathological type, suggesting that they were unlikely to be readmissions for the same stroke or an evolving stroke after a first event. Thirdly, stroke survivors admitted to hospitals subsequently for reasons other than a new stroke could still have a stroke diagnosis recorded in their medical records, leading to false positive diagnoses for recurrent strokes. However, sensitivity analyses using recurrent stroke as the primary diagnosis or excluding those that also had comorbidities recorded at hospital admission did not notably alter the high rates of stroke recurrence and mortality. Nevertheless, the possibility of overestimation or underestimation of stroke recurrence after a first stroke cannot be entirely excluded and the verification of at least some recurrent stroke events in future studies would help to clarify this.
This study showed a high incidence of stroke in Chinese adults, and a surprisingly poor long-term prognosis after a first stroke of any type, particularly for individuals living in rural areas. The findings highlight the need for improved primary prevention of stroke through lifestyle modification, and for urgent improvements to the delivery of effective secondary prevention treatments for stroke (eg, anti-platelet, lipid-lowering, and blood pressure-lowering medication) in both urban and rural areas in China and in other low-income and middle-income countries.
Contributors
YC, LL, RCl, and ZC had full access to the data. All authors were involved in study design, conduct, long-term follow-up, analysis of data, interpretation, and writing of the report, and had final responsibility for the decision to submit for publication.
Declaration of interests
We declare no competing interests.
Acknowledgments
The baseline survey was supported by the Kadoorie Charitable Foundation, Hong Kong. Long-term follow-up was supported by grants from the UK Wellcome Trust (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), National Natural Science Foundation of China (81390540, 81390541, 81390544), and the National Key Research and Development Program of China (2016YFC 0900500, 0900501, 0900504, 1303904). The British Heart Foundation, UK Medical Research Council, and Cancer Research UK provided core support to the Clinical Trial Service Unit. We thank the participants, the project staff, and the China National Centre for Disease Control and Prevention and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provided electronic linkage to all hospitalised individuals.
Contributor Information
Yiping Chen, Email: yiping.chen@ndph.ox.ac.uk.
Zhengming Chen, Email: zhengming.chen@ndph.ox.ac.uk.
China Kadoorie Biobank Collaborative Group:
Yiping Chen, Neil Wright, Yu Guo, Iain Turnbull, Christiana Kartsonaki, Ling Yang, Zheng Bian, Pei Pei, Dongxia Pan, Yidan Zhang, Haiqiang Qin, Yilong Wang, Jun Lv, Ming Liu, Zilong Hao, Yongjun Wang, Canqing Yu, Richard Peto, Rory Collins, Liming Li, Robert Clarke, Zhengming Chen, Junshi Chen, Robin Walters, Daniel Avery, Derrick Bennett, Ruth Boxall, Fiona Bragg, Yumei Chang, Huaidong Du, Simon Gilbert, Alex Hacker, Michael Holmes, Rene Kerosi, Garry Lancaster, Kuang Lin, John McDonnell, Iona Millwood, Qunhua Nie, Paul Ryder, Sam Sansome, Dan Schmidt, Rajani Sohoni, Jenny Wang, Lin Wang, Xiaoming Yang, Xiao Han, Can Hou, Biao Jing, Chao Liu, Zengchang Pang, Ruqin Gao, Shanpeng Li, Shaojie Wang, Yongmei Liu, Ranran Du, Yajing Zang, Liang Cheng, Xiaocao Tian, Hua Zhang, Yaoming Zhai, Feng Ning, Xiaohui Sun, Feifei Li, Silu Lv, Junzheng Wang, Wei Hou, Mingyuan Zeng, Ge Jiang, Xue Zhou, Liqiu Yang, Hui He, Bo Yu, Yanjie Li, Qinai Xu, Quan Kang, Ziyan Guo, Dan Wang, Ximin Hu, Hongmei Wang, Jinyan Chen, Yan Fu, Zhenwang Fu, Xiaohuan Wang, Min Weng, Zhendong Guo, Shukuan Wu, Yilei Li, Huimei Li, Zhifang Fu, Ming Wu, Yonglin Zhou, Jinyi Zhou, Ran Tao, Jie Yang, Jian Su, Fang Liu, Jun Zhang, Yihe Hu, Yan Lu, Liangcai Ma, Aiyu Tang, Shuo Zhang, Jianrong Jin, Jingchao Liu, Zhenzhu Tang, Naying Chen, Ying Huang, Mingqiang Li, Jinhuai Meng, Rong Pan, Qilian Jiang, Jian Lan, Yun Liu, Liuping Wei, Ningyu Chen, Ping Wang, Fanwen Meng, Yulu Qin, Sisi Wang, Xianping Wu, Ningmei Zhang, Xiaofang Chen, Weiwei Zhou, Guojin Luo, Jianguo Li, Xunfu Zhong, Jiaqiu Liu, Qiang Sun, Pengfei Ge, Xiaolan Ren, Caixia Dong, Hui Zhang, Enke Mao, Xiaoping Wang, Tao Wang, Xi Zhang, Ding Zhang, Gang Zhou, Shixian Feng, Liang Chang, Lei Fan, Yulian Gao, Tianyou He, Huarong Sun, Pan He, Chen Hu, Xukui Zhang, Huifang Wu, Min Yu, Ruying Hu, Hao Wang, Yijian Qian, Chunmei Wang, Kaixu Xie, Lingli Chen, Zhongxi Fu, Qiaohua Xu, Xin Xu, Hao Zhang, Huajun Long, Xianzhi Li, Libo Zhang, and Zhe Qiu
Supplementary Material
References
- 1.Feigin VL, Krishnamurthi RV, Parmar P. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. 2015;45:161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zhu J. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Jiang B, Sun H. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Wang S, Yi X. Estimate of ischemic stroke prevalence according to a novel 4-tiered classification of left ventricular hypertrophy: insights from the general Chinese population. Ann Med. 2018;50:519–528. doi: 10.1080/07853890.2018.1500702. [DOI] [PubMed] [Google Scholar]
- 5.Tsai CF, Anderson N, Thomas B, Sudlow CL. Risk factors for ischemic stroke and its subtypes in Chinese vs. Caucasians: systematic review and meta-analysis. Int J Stroke. 2015;10:485–493. doi: 10.1111/ijs.12508. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Yang G, Offer A. Body mass index and mortality in China: a 15-year prospective study of 220 000 men. Int J Epidemiol. 2012;41:472–481. doi: 10.1093/ije/dyr208. [DOI] [PubMed] [Google Scholar]
- 7.Mok VC, Lau AY, Wong A. Long-term prognosis of Chinese patients with a lacunar infarct associated with small vessel disease: a five-year longitudinal study. Int J Stroke. 2009;4:81–88. doi: 10.1111/j.1747-4949.2009.00262.x. [DOI] [PubMed] [Google Scholar]
- 8.Fang XH, Wang WH, Zhang XQ. Incidence and survival of symptomatic lacunar infarction in a Beijing population: a 6-year prospective study. Eur J Neurol. 2012;19:1114–1120. doi: 10.1111/j.1468-1331.2012.03709.x. [DOI] [PubMed] [Google Scholar]
- 9.Amarenco P, Lavallée PC, Monteiro Tavares L. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182–2190. doi: 10.1056/NEJMoa1802712. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell MJ, Chin SL, Rangarajan S. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 11.Wu S, Wu B, Liu M. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Lee L, Chen J. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Chen J, Collins R. China Kadoorie Biobank of 0·5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang GH, Stroup DF, Thacker SB. National public health surveillance in China: implications for public health in China and the United States. Biomed Environ Sci. 1997;10:1–13. [PubMed] [Google Scholar]
- 15.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 16.Choudhury JB. Non-parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Stat Med. 2002;21:1129–1144. doi: 10.1002/sim.1070. [DOI] [PubMed] [Google Scholar]
- 17.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LF, Yang J, Hong Z. Proportion of different subtypes of stroke in China. Stroke. 2003;34:2091–2096. doi: 10.1161/01.STR.0000087149.42294.8C. [DOI] [PubMed] [Google Scholar]
- 20.Béjot Y, Grelat M, Delpont B. Temporal trends in early case-fatality rates in patients with intracerebral hemorrhage. Neurology. 2017;88:985–990. doi: 10.1212/WNL.0000000000003681. [DOI] [PubMed] [Google Scholar]
- 21.Sennfält S, Norrving B, Petersson J, Ullberg T. Long-term survival and function after stroke. Stroke. 2019;50:53–61. doi: 10.1161/STROKEAHA.118.022913. [DOI] [PubMed] [Google Scholar]
- 22.Lewington S, Lacey B, Clarke R. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524–532. doi: 10.1001/jamainternmed.2016.0190. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 24.Ekker MS, Verhoeven JI, Vaartjes I, Jolink WMT, Klijn CJM, de Leeuw FE. Association of stroke among adults aged 18 to 49 years with long-term mortality. JAMA. 2019;321:2113–2123. doi: 10.1001/jama.2019.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Yiin GS, Geraghty OC. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14:903–913. doi: 10.1016/S1474-4422(15)00132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothwell PM, Coull AJ, Giles MF. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 27.Wardlaw JM, Doubal F, Armitage P. Lacunar stroke is associated with diffuse blood–brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 28.Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lewis SC, Sudlow CL. Differences between ischemic stroke subtypes in vascular outcomes support a distinct lacunar ischemic stroke arteriopathy: a prospective, hospital-based study. Stroke. 2009;40:3679–3684. doi: 10.1161/STROKEAHA.109.558221. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Li J, Wang C. Gender differences in 1-year clinical characteristics and outcomes after stroke: results from the China National Stroke Registry. PLoS One. 2013;8:e56459. doi: 10.1371/journal.pone.0056459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–667. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Li L, Zhang Q. Use of drug treatment for secondary prevention of cardiovascular disease in urban and rural communities of China: China Kadoorie Biobank Study of 0·5 million people. Int J Cardiol. 2014;172:88–95. doi: 10.1016/j.ijcard.2013.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng Q, Xu L, Zhang Y. Trends in access to health services and financial protection in China between 2003 and 2011: a cross-sectional study. Lancet. 2012;379:805–814. doi: 10.1016/S0140-6736(12)60278-5. [DOI] [PubMed] [Google Scholar]
- 33.Yusuf S, Islam S, Chow CK. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 34.Toni D, Di Angelantonio E, Di Mascio MT, Vinisko R, Bath PM. Types of stroke recurrence in patients with ischemic stroke: a substudy from the PRoFESS trial. Int J Stroke. 2014;9:873–878. doi: 10.1111/ijs.12150. [DOI] [PubMed] [Google Scholar]
- 35.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005;128:2507–2517. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology. 2001;56:773–777. doi: 10.1212/wnl.56.6.773. [DOI] [PubMed] [Google Scholar]
- 37.Hemphill JC, 3rd, Greenberg SM, Anderson CS. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 38.Sun L, Clarke R, Bennett D. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. 2019;25:569–574. doi: 10.1038/s41591-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.