Key Points
Question
Which brain changes are associated with sleep-disordered breathing in aging?
Findings
In this cross-sectional study of 127 community-dwelling older individuals who were cognitively unimpaired, the presence of sleep-disordered breathing was associated with greater amyloid burden, gray matter volume, metabolism, and perfusion in the posterior cingulate cortex and precuneus. There was no association with cognitive performance, self-reported cognitive or sleep difficulties, or excessive daytime sleepiness.
Meaning
Sleep-disordered breathing–associated changes include amyloid deposition in brain regions typically involved in Alzheimer disease, which might explain why sleep-disordered breathing is associated with an increased risk for developing Alzheimer clinical syndrome at a younger age.
This cross-sectional study seeks to determine which brain changes, including changes in amyloid deposition, gray matter volume, perfusion, and glucose metabolism, are associated with the presence of sleep-disordered breathing in older individuals who are cognitively unimpaired.
Abstract
Importance
Increasing evidence suggests that sleep-disordered breathing (SDB) increases the risk of developing Alzheimer clinical syndrome. However, the brain mechanisms underlying the link between SDB and Alzheimer disease are still unclear.
Objective
To determine which brain changes are associated with the presence of SDB in older individuals who are cognitively unimpaired, including changes in amyloid deposition, gray matter volume, perfusion, and glucose metabolism.
Design, Setting, and Participants
This cross-sectional study was conducted using data from the Age-Well randomized clinical trial of the Medit-Ageing European project, acquired between 2016 and 2018 at Cyceron Center in Caen, France. Community-dwelling older adults were assessed for eligibility and were enrolled in the Age-Well clinical trial if they did not meet medical or cognitive exclusion criteria and were willing to participate. Participants who completed a detailed neuropsychological assessment, polysomnography, a magnetic resonance imaging, and florbetapir and fluorodeoxyglucose positron emission tomography scans were included in the analyses.
Main Outcomes and Measures
Based on an apnea-hypopnea index cutoff of 15 events per hour, participants were classified as having SDB or not. Voxelwise between-group comparisons were performed for each neuroimaging modality, and secondary analyses aimed at identifying which SDB parameter (sleep fragmentation, hypoxia severity, or frequency of respiratory disturbances) best explained the observed brain changes and assessing whether SDB severity and/or SDB-associated brain changes are associated with cognitive and behavioral changes.
Results
Of 157 participants initially assessed, 137 were enrolled in the Age-Well clinical trial, and 127 were analyzed in this study. The mean (SD) age of the 127 participants was 69.1 (3.9) years, and 80 (63.0%) were women. Participants with SDB showed greater amyloid burden (t114 = 4.51; familywise error–corrected P = .04; Cohen d, 0.83), gray matter volume (t119 = 4.12; familywise error–corrected P = .04; Cohen d, 0.75), perfusion (t116 = 4.62; familywise error–corrected P = .001; Cohen d, 0.86), and metabolism (t79 = 4.63; familywise error–corrected P = .001; Cohen d, 1.04), overlapping mainly over the posterior cingulate cortex and precuneus. No association was found with cognition, self-reported cognitive and sleep difficulties, or excessive daytime sleepiness symptoms.
Conclusions and Relevance
The SDB-associated brain changes in older adults who are cognitively unimpaired include greater amyloid deposition and neuronal activity in Alzheimer disease–sensitive brain regions, notably the posterior cingulate cortex and precuneus. These results support the need to screen and treat for SDB, especially in asymptomatic older populations, to reduce Alzheimer disease risk.
Trial Registration
ClinicalTrials.gov Identifier: NCT02977819
Introduction
Sleep-disordered breathing (SDB) is a respiratory disorder characterized by recurrent upper airway collapse during sleep, associated with intermittent hypoxia and sleep fragmentation.1,2 It affects 30% to 80% of older adults who are cognitively unimpaired, depending on the SDB definition criteria.3,4 Patients with a clinical diagnosis of Alzheimer disease (AD) are even more likely to suffer from SDB,5 and untreated SDB is associated with cognitive decline and conversion to the Alzheimer clinical syndrome at a younger age.6,7,8
To clarify the mechanisms underlying the association between SDB and dementia risk, growing efforts have been deployed to better characterize the brain changes associated with SDB. Yet, previous studies have provided heterogeneous results, showing both SDB-associated decreases9,10,11 and increases12,13 in gray matter (GM) volume or cortical thickness in various brain areas including frontal, temporal, and parietal regions. Similarly, both decreased perfusion14,15,16 and increased perfusion15,17 have been reported in patients with SDB compared with control participants. Finally, SDB has been associated with increased amyloid and tau levels in the blood and cerebrospinal fluid, both cross sectionally and longitudinally.18,19,20,21 However, cross-sectional positron emission tomography (PET) studies have reported inconsistent results, some showing greater amyloid burden in association with SDB,22,23 while others do not report significant associations.21,24 These discrepancies may be explained by the characteristics of patients with SDB (eg, recruited from sleep clinics vs from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, and/or the lack of controls for possibly biasing covariates.
To our knowledge, no previous study has used a multimodal neuroimaging approach to highlight early brain changes associated with the presence of untreated SDB within a large sample of older participants who are cognitively unimpaired and are recruited from the community, with no or few sleep symptoms. Therefore, our main objective was to provide a comprehensive picture of structural, functional, and molecular brain changes associated with untreated SDB in aging, to provide substantial advances in the understanding of early brain mechanisms underlying the associations between SDB and AD. Secondary objectives aimed at identifying which aspect of SDB severity (ie, sleep fragmentation, hypoxia severity, or frequency of respiratory events) is most associated to brain changes. Lastly, we investigated the associations between SDB severity and/or SDB-associated brain changes and cognitive performance, self-reported cognitive and sleep difficulties, or symptoms of sleepiness.
Methods
Participants
We included 127 older adults who were cognitively unimpaired from the baseline visit of the Age-Well randomized clinical trial of the Medit-Ageing European Project25 (flow diagram in Figure 1), sponsored by the French National Institute of Health and Medical Research (INSERM). Participants were recruited from the general population, older than 65 years, native French speakers, retired for at least 1 year, educated for at least 7 years, and able to perform within the normal range on standardized cognitive tests. The main exclusion criteria were safety concerns associated with magnetic resonance image (MRI) or PET scanning, evidence of a major neurological or psychiatric disorder (including alcohol or drug abuse), history of cerebrovascular disease, presence of a chronic disease or acute unstable illness, and current or recent medication usage that may interfere with cognitive functioning.
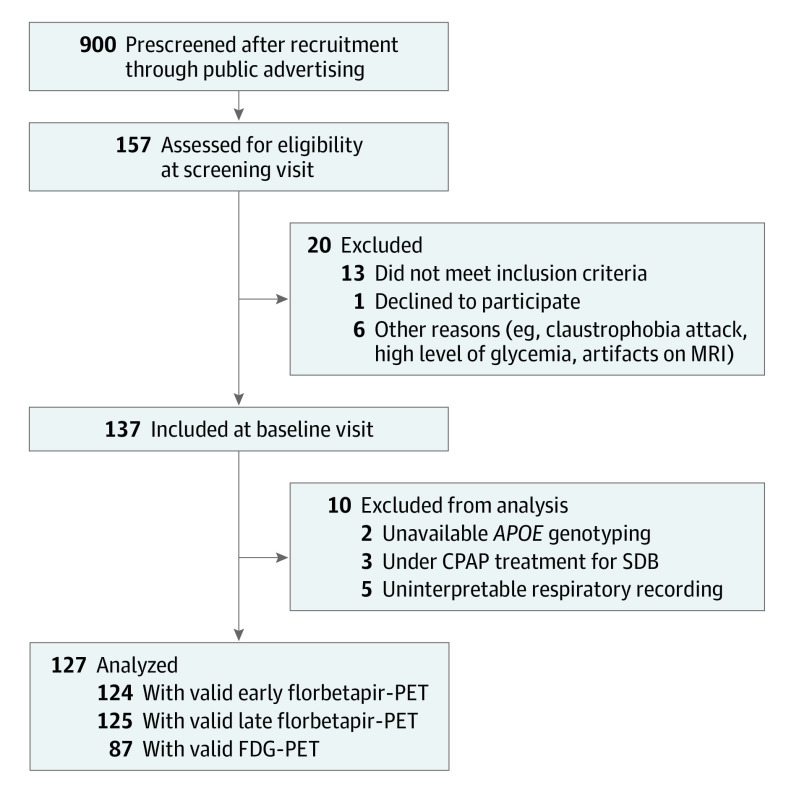
Figure 1. Flow Diagram of the Inclusion Process.
APOE indicates apolipoprotein E; CPAP, continuous positive airway pressure; FDG, 18F-fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography; SDB, sleep-disordered breathing.
Participants meeting inclusion criteria performed a detailed cognitive assessment, a polysomnography, structural MRI, fluroine 18–labeled (18F) florbetapir PET and 18F-fluorodeoxyglucose (FDG) PET scans, and apolipoprotein E ε4 (APOE4) genotyping within a maximum period of 3 months. All participants gave their written informed consent prior to the examinations, and the Age-Well randomized clinical trial was approved by the ethics committee (Comité de Protection des Personnes Nord-Ouest III, Caen, France; trial registration number: EudraCT: 2016-002441-36; IDRCB: 2016-A01767-44; ClinicalTrials.gov Identifier: NCT02977819).
Cognitive and Behavioral Assessment
The detailed neuropsychological evaluation encompassed global cognitive functioning, processing speed, attention, working memory, executive functions, and episodic memory.25 For each cognitive domain, a composite score was computed (eAppendix in the Supplement). In addition, self-reported cognitive difficulties were assessed using the Cognitive Difficulties Scale.26 Moreover, self-reported sleep quality was assessed using the Pittsburgh Sleep Quality Index,27 and excessive daytime sleepiness symptoms were measured using the Epworth Sleepiness Scale.28
Neuroimaging Examinations
All participants underwent high-resolution T1-weighted anatomical imaging to measure GM volume and a florbetapir-PET scan with a 10-minute early acquisition that began immediately after the intravenous injection and reflected brain perfusion and a 10-minute late acquisition (beginning 50 minutes after injection) that reflected amyloid burden. A subset of participants (n = 87) also underwent an FDG-PET scan to measure brain glucose metabolism. All participants were scanned at Cyceron Center (Caen, France) on the same MRI scanner (Philips Achieva 3.0T) and PET scanner (Discovery RX VCT 64 PET-CT; General Electric Healthcare). The detailed acquisition and preprocessing procedures25 are available in the eAppendix in the Supplement. The PET analyses were performed on images corrected for partial volume effects (PVE) using the 3-compartmental voxelwise Müller-Gartner method.29
SDB Characterization
Participants underwent polysomnography using a portable home device (Siesta; Compumedics). Acquisition parameters are described in the eAppendix in the Supplement. Sixty-eight percent of the participants (86 of 127 individuals) underwent 2 polysomnography recordings, including a habituation night, which was not included in the analyses. The remaining 39 participants only had 1 polysomnography recording. Recordings were visually scored in 30-second epochs following the recommended standard criteria of the American Academy of Sleep Medicine.1 Sleep apnea was defined by a 90% or greater drop of nasal pressure for at least 10 seconds, whereas sleep hypopnea was characterized by a 30% or greater drop of nasal pressure for a minimum of 10 seconds, associated with an arousal or a 3% or greater oxygen desaturation.
Based on the apnea-hypopnea index (AHI) value (ie, the sum of apnea and hypopnea events per hour of sleep), participants were classified into 2 groups with a cutoff value of 15, independent of clinical symptoms, in accordance with the recommendations of the third edition of the International Classification of Sleep Disorders.30 Thus, we obtained a group without SDB (AHI <15 events per hour; n = 31) and a group with SDB (AHI ≥15 events per hour; n = 96).6,20,31,32 The subsample of 87 participants who also had an FDG-PET scan was composed of 69 participants with SDB and 18 participants without SDB, who did not differ from the main sample regarding the proportion of participants with vs without SDB (χ21 = 0.26; P = .61) or demographic, sleep, and cognitive variables (data not shown).
Lastly, to address secondary objectives, we computed 2 composite scores reflecting sleep fragmentation and hypoxia severity. This composite score approach, also used in a previous study,12 was preferred to a single-item approach, to minimize the issue of multiple statistical testing. The sleep fragmentation composite score corresponded to the mean of the z scores of the respiratory arousal index, the number of shifts to non–rapid eye movement sleep stage 1 per hour, and the number of nocturnal awakenings per hour. The hypoxia composite score corresponded to the mean of the z scores of the oxygen desaturation index, the proportion of total sleep time with oxygen desaturation of 90% or less, and the minimal oxygen saturation.
Statistical Analyses
Between-Group Differences
Between-group differences for demographical, behavioral, sleep, and cognitive variables were assessed using t tests for continuous variables and χ2 statistics for categorical variables, with statistical significance set to P < .05. Voxelwise group differences in amyloid burden, GM volume, perfusion, and glucose metabolism were explored using analyses of covariance in SPM12 (Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology), controlling for age, sex, education, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), sleep medication use, and APOE4 status. Voxelwise analyses were performed using MRI and PVE-corrected PET data and considered significant at a voxel-level threshold of P < .005 and a cluster-level threshold of P < .05, corrected for familywise errors (FWE).
Stepwise Regression Analyses
As a second step, we aimed at further assessing which aspect of SDB severity (ie, the frequency of respiratory disturbances, associated sleep fragmentation, and/or hypoxia severity) was more specifically involved in the SDB-associated brain changes highlighted in the previous analysis. For this purpose, amyloid burden, GM volume, perfusion, and glucose metabolism signal values were extracted from the significant clusters obtained in the voxelwise group comparisons. Then, stepwise regression analyses were performed on the whole sample of participants, with 9 measures entered in the model as independent variables (ie, the AHI value, sleep fragmentation composite score, hypoxia composite score, age, sex, education, BMI, sleep medication use, and APOE4 status), while dependent variables were neuroimaging signal values, each modality being tested separately.
Partial Correlation Analyses
Finally, partial correlations were performed on the whole sample of participants to assess potential associations between (1) the 3 measures of SDB severity and (2) SDB-associated brain changes with cognitive and behavioral variables. These analyses were controlled for age, sex, education, BMI, sleep medication use, and APOE4 status, and considered significant at a P < .05 threshold, after applying a Bonferroni correction for multiple comparisons. Thus, the threshold for significance was set to P ≤ (0.05/number of comparisons).
Results
Participants’ Characteristics
Participants’ characteristics, including demographical and behavioral variables, neuropsychological scores, and sleep parameters, as well as corresponding between-group differences, are provided in Table 1. Participants with vs without SDB did not differ in age, sex, education, depression and anxiety scores, current use of sleep medication, and the proportion of individuals carrying APOE4. As expected, participants with SDB had significantly higher BMI values (t125 = 2.25; mean difference, 1.96 [95% CI, 0.23-3.69]; P = .03), AHI values (t125 = 8.64; mean difference, 20.91 [95% CI, 16.12-25.70]; P < .001), levels of sleep fragmentation (t125 = 5.96; mean difference, 0.90 [95% CI, 0.60-1.20]; P < .001), and hypoxia (t116 = 3.68; mean difference, 0.59 [95% CI, 0.27-0.91]; P < .001). Interestingly, no between-group difference was observed for subjective sleep quality, daytime sleepiness symptoms, objectively measured total sleep time, sleep efficiency, and sleep onset latency on the polysomnography night. Moreover, cognitive performance and self-reported cognitive difficulties were comparable between the 2 groups.
Table 1. Participant Characteristics and Between-Group Differences.
| Characteristic | Mean (SD) | P value for between-group differencesa | |
|---|---|---|---|
| Without SDB (n = 31) | With SDB (n = 96) | ||
| Demographic | |||
| Age, y | 69.19 (3.53) | 69.00 (3.98) | .81 |
| Female, No. (%) | 24 (77.4) | 56 (58.3) | .06 |
| Education, y | 13.45 (2.94) | 12.90 (3.12) | .38 |
| Geriatric Depression Scale score | 1.55 (1.89) | 1.21 (1.70) | .35 |
| State-Trait Inventory form B total score | 36.13 (7.35) | 34.05 (6.88) | .15 |
| BMI | 24.77 (5.07) | 26.73 (3.92) | .03 |
| Current sleep medication use, No. (%)b | 2 (6.45) | 10 (10.42) | .51 |
| Florbetapir standard uptake value ratioc | 0.91 (0.13) | 0.99 (0.23) | .07 |
| Amyloid positive, % | 10 | 25 | .08 |
| Carrying APOE4, No. (%) | 9 (29.03) | 26 (27.08) | .83 |
| Sleep | |||
| Pittsburgh Sleep Quality Index score | 4.39 (2.67) | 5.17 (3.16) | .22 |
| Epworth Sleepiness Scale score | 4.77 (3.04) | 5.22 (3.45) | .52 |
| Total sleep time, min | 358.63 (59.13) | 362.81 (67.62) | .76 |
| Sleep efficiency, % | 76.39 (10.24) | 77.36 (9.89) | .64 |
| Sleep onset latency, min | 18.73 (12.41) | 21.12 (12.49) | .35 |
| Apnea-hypopnea index events, No. per h | 9.63 (3.91) | 30.54 (13.26) | <.001 |
| Fragmentation composite score | −0.68 (0.41) | 0.22 (0.81) | <.001 |
| Respiratory arousals index, No. per h | 7.15 (3.34) | 25.28 (11.30) | <.001 |
| Shifts to non–rapid eye movement sleep stage 1, No. per h | 5.81 (2.08) | 8.39 (3.53) | .001 |
| Awakenings, No. per h | 3.29 (1.12) | 4.11 (1.72) | .02 |
| Hypoxia composite scored | −0.44 (0.39) | 0.15 (0.85) | <.001 |
| Oxygen desaturation ≥3% index, No. per h | 5.50 (3.96) | 16.50 (11.21) | <.001 |
| Total sleep time with oxygen saturation ≤90%, % | 0.50 (1.08) | 2.88 (9.69) | .18 |
| Minimal oxygen saturation, % | 88.27 (3.82) | 85.89 (5.05) | .02 |
| Cognition scores | |||
| Mini-Mental State Examination | 28.81 (1.08) | 29.06 (1.04) | .24 |
| Mattis Dementia Rating Scale | 140.61 (2.89) | 141.17 (2.64) | .32 |
| Processing speed composite | 0.05 (0.85) | −0.02 (0.70) | .66 |
| Attention composite | −0.04 (0.66) | 0.04 (0.63) | .58 |
| Executive functioning composite | 0.12 (0.70) | −0.04 (0.67) | .26 |
| Working memory composite | 0.04 (1.03) | −0.03 (0.85) | .72 |
| Episodic memory composite | 0.16 (0.81) | −0.05 (0.69) | .17 |
| Cognitive Difficulties Scale | 33.84 (14.27) | 34.18 (15.59) | .91 |
Abbreviations: APOE, apolipoprotein E; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); SDB, sleep-disordered breathing.
Between-group differences were assessed using t tests for continuous variables and χ2 statistics for categorical variables. Statistical significance was set to P < .05.
Use of sleep medication on a regular basis (>1/week), excluding phytotherapy and homeopathy.
Thirty control participants with valid florbetapir–positron emission tomography scan. The threshold for amyloid positivity was defined as more than 0.99 and corresponded to the 99.9th percentile of the neocortical standard uptake value ratio distribution among 45 healthy young individuals younger than 40 years.
Thirty participants without SDB and 88 participants with SDB with valid oxygen saturation data.
Brain Changes Associated With the Presence of SDB
Results of between-group comparisons for MRI and PVE-corrected PET data are presented in Figure 2. Cluster peak statistics and coordinates are detailed in eTable 1 in the Supplement.
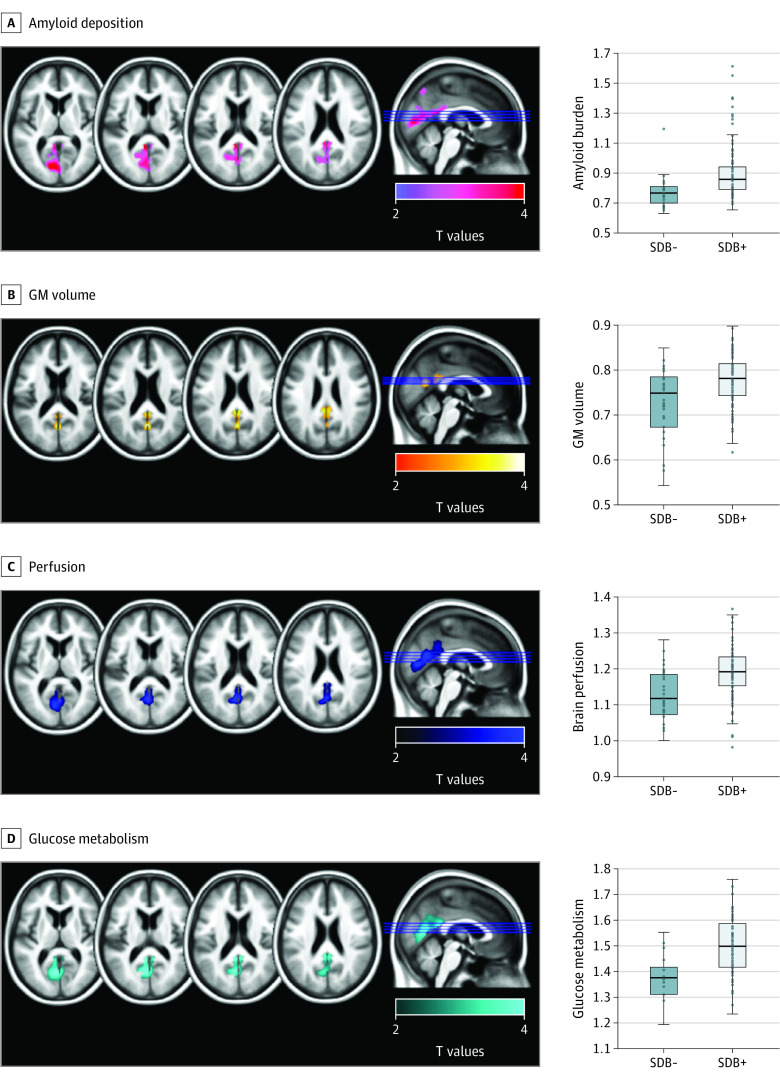
Figure 2. Neuroimaging Pattern of the Significant Differences Between Participants With vs Without Sleep-Disordered Breathing in Amyloid Deposition, Gray Matter Volume, Perfusion, and Glucose Metabolism.
Results of the voxelwise analyses of covariance exhibiting between-group differences in amyloid deposition (A), gray matter (GM) volume (B), perfusion (C), and glucose metabolism (D), using magnetic resonance imaging and partial volume effects–corrected positron emission tomography data. Analyses were adjusted for age, sex, education, body mass index, sleep medication use, and APOE4 status. Results were obtained at a P < .005 (uncorrected) threshold, and only clusters surviving a familywise error–cluster-level correction are reported. APOE indicates apolipoprotein E; SDB−, negative for sleep-disordered breathing; SDB+, positive for sleep-disordered breathing.
Participants with SDB presented greater amyloid burden in the left precuneus, posterior cingulate, calcarine, and cuneus regions (Figure 2A; t117 = 4.51; FWE-corrected P = .04; Cohen d, 0.83), compared with participants without SDB. They also showed greater GM volume in the precuneus and posterior cingulate cortex, bilaterally (Figure 2B; t119 = 4.12; FWE-corrected P = .04; Cohen d, 0.75), and greater perfusion in parietooccipital regions, including the precuneus, posterior cingulate, calcarine, and lingual areas (Figure 2C; t116 = 4.62; FWE-corrected P = .001; Cohen d, 0.86). Finally, participants with SDB presented greater glucose metabolism in the precuneus, posterior cingulate, and lingual areas, bilaterally (Figure 2D; t79 = 4.63; FWE-corrected P = .001; Cohen d, 1.04). Additionally, we compared brain changes between participants with moderate SDB (ie, 15≤ AHI values <30) vs severe SDB (AHI values ≥30) and found no significant differences at the P < .005 level, combined with an FWE cluster-level correction (data not shown). This suggests that brain changes associated with SDB are detectable from the moderate stage and are not exacerbated in participants with severe SDB.
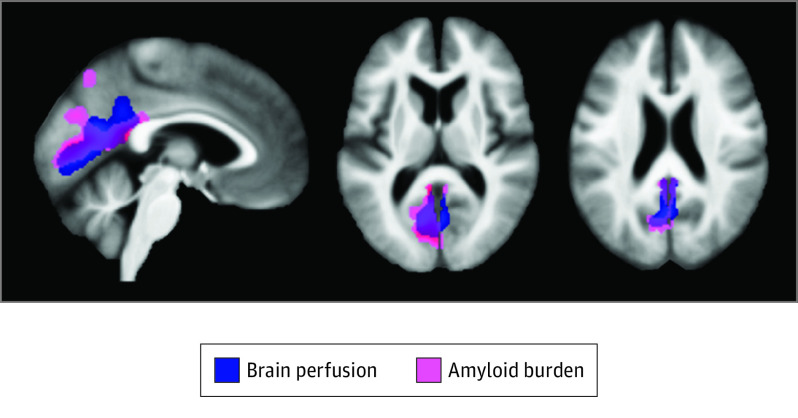
Interestingly, there was an overlap between brain changes observed in all 4 neuroimaging modalities over the posterior cingulate cortex, the precuneus, and the cuneus (Figure 3). Perfusion, metabolism, and GM volume signal values extracted from significant clusters showed strong intercorrelations (brain perfusion and glucose metabolism: Pearson r, 0.70 [95% CI, 0.58-0.80]; P < .001; brain perfusion and GM volume: Pearson r, 0.59 [95% CI, 0.46-0.69]; P < .001; metabolism and GM volume: Pearson r, 0.40 [95% CI, 0.21-0.56]; P < .001), and amyloid deposition was significantly correlated with GM volume (Pearson r, 0.21 [95% CI, 0.03-0.37]; P = .02) and perfusion (Pearson r, 0.34 [95% CI, 0.18-0.49]; P < .001; eTable 2 in the Supplement).
Figure 3. Overlap of Sleep-Disordered Breathing–Associated Brain Changes Across Neuroimaging Modalities.
Representation of the overlap between sleep-disordered breathing–associated patterns of greater perfusion (blue) and amyloid deposition (pink), obtained at a P < .005 (uncorrected) threshold combined with a familywise error–cluster-level correction.
Lastly, for the sake of completeness, region of interest–based complementary analyses were performed. The signal was extracted from PVE-corrected and uncorrected FDG, early and late florbetapir PET images, in regions of interest from the Automated Anatomical Labeling Atlas33 overlapping with the significant clusters obtained in the voxelwise analyses. The results are summarized in eTable 3 in the Supplement and are consistent overall with the findings from the voxelwise analyses. Only results obtained with PVE-uncorrected amyloid PET images were less significant, with between-group comparisons being significant for 3 regions of interest when using uncorrected PET images (right cuneus, F, 4.21; P = .04; partial η2, 0.04; left precuneus, F, 4.03; P = .05, partial η2, 0.03; left lingual, F, 4.26; P = .04; partial η2, 0.04), compared with 9 regions of interest with corrected PET images (eTable3 in the Supplement), which likely reflect the between-group differences in GM volume highlighted in the main analyses.
Links Between SDB-Associated Brain Changes and Measures of SDB Severity
Forward stepwise regressions were then performed to determine which aspect of SDB severity is the most closely associated with SDB-associated brain changes (Table 2). The factors most strongly associated with amyloid burden were the hypoxia composite score, explaining 8% of the variance (unstandardized β, 0.06 [95% CI, 0.02-0.10]; P = .002), followed by APOE4 status, explaining 4% of the variance (unstandardized β, 0.07 [95% CI, 0.001-0.14]; P = .05). No other variable entered the model. The AHI value was the only variable associated with GM volume, explaining 4% of the variance (unstandardized β, 0.01; P = .04). No variable was significantly associated with brain perfusion or metabolism.
Table 2. Results of Forward Stepwise Regressions Showing the Variables Most Strongly Associated With SDB-Associated Brain Changes.
| Factor | Unstandardized coefficient (95% CI) | Standardized coefficient | R2 | P value |
|---|---|---|---|---|
| Amyloid burden | ||||
| Step 1 | ||||
| Intercept | 0.88 (0.85-0.91) | NA | NA | <.001 |
| Hypoxia composite | 0.06 (0.02-0.10) | 0.28 | 0.08 | .002 |
| Step 2 | ||||
| Intercept | 0.86 (0.82-0.90) | NA | NA | <.001 |
| Hypoxia composite | 0.06 (0.02-0.10) | 0.26 | NA | .004 |
| APOE4 status | 0.07 (0.001-0.14) | 0.18 | 0.11 | .05 |
| Full model | NA | NA | 0.16 | .02 |
| Gray matter volume | ||||
| Step 1 | ||||
| Intercept | 0.77 (0.76-0.78) | NA | NA | <.001 |
| Apnea-hypopnea index | 0.01 (5.718 × 10−4-0.02) | 0.19 | 0.04 | .04 |
| Full model | NA | NA | 0.11 | .20 |
Abbreviations: APOE4, apolipoprotein E; NA, not applicable.
To ensure that these findings were not biased by a possible circularity issue, we replicated stepwise regression analyses with neuroimaging values that were independent from the previous between-group comparison. For this purpose, amyloid deposition was more globally measured through the global neocortical standardized uptake value ratio, and GM volume was extracted from the composite regions of interest of posterior regions of the Automated Anatomical Labeling Atlas described above (eTable 4 in the Supplement). Briefly, results were similar for amyloid burden, with hypoxia severity being the variable most strongly associated with the global neocortical amyloid standardized uptake value ratio (unstandardized β, 0.06 [95% CI, 0.01-0.11]; P = .01), followed by APOE4 status (unstandardized β, 0.09 [95% CI, 0.01-0.17]; P = .04). Moreover, the AHI value remained the SDB-associated variable most strongly associated with GM volume, although it was preceded by the BMI, sex, and age.
Links With Cognition, Self-reported Cognitive Difficulties, and Sleep Difficulties
Lastly, we aimed at exploring the cognitive and behavioral correlates of SDB severity and associated brain changes. No association survived the Bonferroni correction for multiple comparisons (eTable 5 in the Supplement). Neither measures of SDB severity nor measures of SDB-associated brain changes were associated with cognitive performance, self-reported cognitive and sleep difficulties, or symptoms of sleepiness.
Discussion
The main goal of the present study was to provide a comprehensive overview of brain changes associated with untreated SDB in community-dwelling older participants who had few self-reported sleep difficulties. Our results show that participants with SDB presented greater amyloid burden, GM volume, metabolism, and perfusion in parietooccipital regions, including the precuneus and posterior cingulate cortex. Interestingly, greater amyloid burden was robustly associated with the severity of hypoxia. Neither SDB severity nor SDB-associated brain changes were associated with cognitive performance, self-reported cognitive and sleep difficulties, and symptoms of sleepiness.
The association between SDB and greater amyloid deposition is in line with previous studies showing that SDB is associated with lower serum and cerebrospinal fluid amyloid levels.18,20 Furthermore, these results characterize the regional pattern of amyloid deposition in individuals with SDB who are cognitively unimpaired, extending the findings of Yun and colleagues23 to a larger sample of older individuals. The association between amyloid deposition and hypoxia severity is also consistent with animal studies, showing that hypoxia promotes the cleavage of the amyloid precursor protein by the β-secretase and γ-secretase, leading to increased β-amyloid levels.34,35,36 Moreover, it may partly explain why this specific aspect of SDB, rather than the AHI value or sleep fragmentation, is significantly associated with cognitive decline and conversion to Alzheimer clinical syndrome in older adults.6,37
Interestingly, participants with SDB also presented greater GM volume, perfusion, and metabolism, in line with the findings of several other studies.12,15,17,38,39 Nevertheless, other groups have also reported GM atrophy, hypoperfusion, and hypometabolism in participants with SDB.9,10,11,14,16 Discrepancies across studies may be attributable, at least in part, to methodological differences, because most studies have been performed on smaller samples of young and middle-aged participants with severe SDB (AHI >30 events per hour). Alternatively, it is possible that studies including participants with less severe SDB (ie, from the moderate stage, corresponding to an AHI >15 events per hour) and few symptoms, as in the present study and in others,12,15 may be more able to capture earlier brain changes associated with the presence of SDB.
Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater GM volume, perfusion, and metabolism in older participants with SDB who are cognitively unimpaired.38,40,41,42 We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms. Indeed, it has been demonstrated that higher neuronal activity is associated with increased β-amyloid production.43,44,45,46 In addition, several studies47 have shown that neuroinflammatory processes play a central role in AD progression and are associated with higher levels of amyloid deposition. Thus, SDB-associated neuroinflammatory processes and associated neuronal hyperactivity are likely to promote amyloid deposition in the same area. Furthermore, greater GM volume, perfusion, and metabolism colocalizing with amyloid deposition may precede the development of neuronal injury, such as hypometabolism and atrophy.48,49 Alternatively, greater GM volume, perfusion, and metabolism could also reflect higher brain reserve,50 helping to cope with amyloid pathology and maintain cognitive performance.
In our study, SDB-associated brain changes were not associated with cognitive performance, self-reported cognitive and sleep difficulties, or symptoms of excessive daytime sleepiness. We believe that this finding indicates that greater amyloid deposition, GM volume, perfusion, and metabolism may represent early and asymptomatic brain changes associated with SDB. Studies exploring the associations between SDB and cognitive performance using cross-sectional designs have provided mixed results,7,51,52,53 but longitudinal studies showed that SDB is associated with conversion to Alzheimer clinical syndrome and cognitive decline over time.6,7,8 Therefore, older adults with SDB may exhibit silent brain changes, including increased amyloid deposition, which may propagate with time and explain why they are more at risk of developing Alzheimer clinical syndrome. The main strengths of the present study are, first, the multimodal assessment of bain integrity, which allowed us to reveal the overlap of brain changes, and second, the detailed cognitive assessment, on a large sample of older individuals who are cognitively unimpaired.
Limitations
However, the cross-sectional design of the analyses does not allow for the assessment of the causal associations between SDB and brain changes. Longitudinal studies are needed to investigate whether these early SDB-associated brain changes will progress to neurodegeneration and cognitive deficits.
Conclusions
Taken together, community-dwelling older individuals with untreated SDB presented greater amyloid deposition, GM volume, perfusion, and metabolism mainly over the posterior cingulate, cuneus, and precuneus areas, which are typically altered in AD. However, no association with cognitive performance, self-reported cognitive or sleep difficulties, or sleepiness symptoms was observed. Early neuroinflammatory and neuronal hyperactivity processes promoting amyloid deposition could represent the underlying mechanisms increasing the susceptibility to AD at an asymptomatic stage of SDB. Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations.
eAppendix. Methods
eTable 1. Detailed statistics of significant neuroimaging clusters.
eTable 2. Results of inter-modality correlations.
eTable 3. Results of between-group comparisons using a ROI approach for PET data.
eTable 4. Results of complementary forward stepwise regression analyses.
eTable 5. Results of partial correlation analyses between SDB parameters and SDB-related brain changes with cognitive and behavioural scores.
eReferences.
References
- 1.Berry RB, Brooks R, Gamaldo C, et al. . AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med. 2017;13(5):665-666. doi: 10.5664/jcsm.6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237-245. doi: 10.1016/S0140-6736(02)09464-3 [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Kripke DF, Mason W, Messin S. Comparisons of home sleep recordings and polysomnograms in older adults with sleep disorders. Sleep. 1981;4(3):283-291. doi: 10.1093/sleep/4.3.283 [DOI] [PubMed] [Google Scholar]
- 4.Senaratna CV, Perret JL, Lodge CJ, et al. . Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70-81. doi: 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 5.Emamian F, Khazaie H, Tahmasian M, et al. . The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci. 2016;8:78. doi: 10.3389/fnagi.2016.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe K, Laffan AM, Harrison SL, et al. . Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613-619. doi: 10.1001/jama.2011.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 2017;74(10):1237-1245. doi: 10.1001/jamaneurol.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osorio RS, Gumb T, Pirraglia E, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964-1971. doi: 10.1212/WNL.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Tang S, Lyu X, Yang C, Chen X. Structural and functional brain alterations in obstructive sleep apnea: a multimodal meta-analysis. Sleep Med. 2019;54:195-204. doi: 10.1016/j.sleep.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Chen L, Chen T, et al. . A meta-analysis of voxel-based brain morphometry studies in obstructive sleep apnea. Sci Rep. 2017;7(1):10095. doi: 10.1038/s41598-017-09319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahmasian M, Rosenzweig I, Eickhoff SB, et al. . Structural and functional neural adaptations in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Neurosci Biobehav Rev. 2016;65:142-156. doi: 10.1016/j.neubiorev.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baril A-A, Gagnon K, Brayet P, et al. . Gray Matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med. 2017;195(11):1509-1518. doi: 10.1164/rccm.201606-1271OC [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig I, Kempton MJ, Crum WR, et al. . Hippocampal hypertrophy and sleep apnea: a role for the ischemic preconditioning? PLoS One. 2013;8(12):e83173. doi: 10.1371/journal.pone.0083173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innes CRH, Kelly PT, Hlavac M, Melzer TR, Jones RD. Decreased regional cerebral perfusion in moderate-severe obstructive sleep apnoea during wakefulness. Sleep. 2015;38(5):699-706. doi: 10.5665/sleep.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baril A-A, Gagnon K, Arbour C, et al. . Regional cerebral blood flow during wakeful rest in older subjects with mild to severe obstructive sleep apnea. Sleep. 2015;38(9):1439-1449. doi: 10.5665/sleep.4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JS, Seo JH, Kang M-R, et al. . Effect of continuous positive airway pressure on regional cerebral blood flow in patients with severe obstructive sleep apnea syndrome. Sleep Med. 2017;32:122-128. doi: 10.1016/j.sleep.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 17.Nie S, Peng D-C, Gong H-H, Li H-J, Chen L-T, Ye C-L. Resting cerebral blood flow alteration in severe obstructive sleep apnoea: an arterial spin labelling perfusion fMRI study. Sleep Breath. 2017;21(2):487-495. doi: 10.1007/s11325-017-1474-9 [DOI] [PubMed] [Google Scholar]
- 18.Bu X-L, Liu Y-H, Wang Q-H, et al. . Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep. 2015;5(1):13917. doi: 10.1038/srep13917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bubu OM, Pirraglia E, Andrade AG, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep. 2019;42(6):zsz048. doi: 10.1093/sleep/zsz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liguori C, Mercuri NB, Izzi F, et al. . Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5). doi: 10.1093/sleep/zsx011 [DOI] [PubMed] [Google Scholar]
- 21.Sharma RA, Varga AW, Bubu OM, et al. . Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: a longitudinal study. Am J Respir Crit Care Med. 2018;197(7):933-943. doi: 10.1164/rccm.201704-0704OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias A, Cummins T, Tyrrell R, et al. . Risk of Alzheimer’s disease in obstructive sleep apnea syndrome: amyloid-β and tau imaging. J Alzheimers Dis. 2018;66(2):733-741. doi: 10.3233/JAD-180640 [DOI] [PubMed] [Google Scholar]
- 23.Yun C-H, Lee H-Y, Lee SK, et al. . Amyloid burden in obstructive sleep apnea. J Alzheimers Dis. 2017;59(1):21-29. doi: 10.3233/JAD-161047 [DOI] [PubMed] [Google Scholar]
- 24.Spira AP, Yager C, Brandt J, et al. . Objectively measured sleep and β-amyloid burden in older adults: a pilot study. SAGE Open Med. 2014;2. doi: 10.1177/2050312114546520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poisnel G, Arenaza-Urquijo E, Collette F, et al. ; Medit-Ageing Research Group . The Age-Well randomized controlled trial of the Medit-Ageing European Project: effect of meditation or foreign language training on brain and mental health in older adults. Alzheimers Dement (N Y). 2018;4:714-723. doi: 10.1016/j.trci.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNair DM, Kahn RJ, Crook T, Ferris S, Bartus R. Assessment in Geriatric Psychopharmacology. Mark Powley Associates; 1983. [Google Scholar]
- 27.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 29.Müller-Gärtner HW, Links JM, Prince JL, et al. . Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12(4):571-583. doi: 10.1038/jcbfm.1992.81 [DOI] [PubMed] [Google Scholar]
- 30.Sateia MJ. International classification of sleep disorders–third edition: highlights and modifications. Chest. 2014;146(5):1387-1394. doi: 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 31.Ju Y-ES, Finn MB, Sutphen CL, et al. . Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol. 2016;80(1):154-159. doi: 10.1002/ana.24672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osorio RS, Ayappa I, Mantua J, et al. . Interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35(6):1318-1324. doi: 10.1016/j.neurobiolaging.2013.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. . Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273-289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 34.Sun X, He G, Qing H, et al. . Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103(49):18727-18732. doi: 10.1073/pnas.0606298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2009;30(7):1091-1098. doi: 10.1016/j.neurobiolaging.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 36.Shiota S, Takekawa H, Matsumoto S-E, et al. . Chronic intermittent hypoxia/reoxygenation facilitate amyloid-β generation in mice. J Alzheimers Dis. 2013;37(2):325-333. doi: 10.3233/JAD-130419 [DOI] [PubMed] [Google Scholar]
- 37.Blackwell T, Yaffe K, Laffan A, et al. ; Osteoporotic Fractures in Men Study Group . Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63(3):453-461. doi: 10.1111/jgs.13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenzweig I, Williams SCR, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Curr Opin Pulm Med. 2014;20(6):565-571. doi: 10.1097/MCP.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 39.Cross NE, Memarian N, Duffy SL, et al. . Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. Eur Respir J. 2018;52(1):1800740. doi: 10.1183/13993003.00740-2018 [DOI] [PubMed] [Google Scholar]
- 40.Aviles-Reyes RX, Angelo MF, Villarreal A, Rios H, Lazarowski A, Ramos AJ. Intermittent hypoxia during sleep induces reactive gliosis and limited neuronal death in rats: implications for sleep apnea. J Neurochem. 2010;112(4):854-869. doi: 10.1111/j.1471-4159.2009.06535.x [DOI] [PubMed] [Google Scholar]
- 41.Li K, Zhang J, Qin Y, Wei Y-X. Association between serum homocysteine level and obstructive sleep apnea: a meta-analysis. Biomed Res Int. 2017;2017:7234528. doi: 10.1155/2017/7234528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daulatzai MA. Death by a thousand cuts in Alzheimer’s disease: hypoxia—the prodrome. Neurotox Res. 2013;24(2):216-243. doi: 10.1007/s12640-013-9379-2 [DOI] [PubMed] [Google Scholar]
- 43.Bero AW, Yan P, Roh JH, et al. . Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14(6):750-756. doi: 10.1038/nn.2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676-682. doi: 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckner RL, Sepulcre J, Talukdar T, et al. . Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860-1873. doi: 10.1523/JNEUROSCI.5062-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cirrito JR, Yamada KA, Finn MB, et al. . Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48(6):913-922. doi: 10.1016/j.neuron.2005.10.028 [DOI] [PubMed] [Google Scholar]
- 47.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4:575-590. doi: 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fortea J, Vilaplana E, Alcolea D, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Cerebrospinal fluid β-amyloid and phospho-tau biomarker interactions affecting brain structure in preclinical Alzheimer disease. Ann Neurol. 2014;76(2):223-230. doi: 10.1002/ana.24186 [DOI] [PubMed] [Google Scholar]
- 49.Pegueroles J, Vilaplana E, Montal V, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Longitudinal brain structural changes in preclinical Alzheimer’s disease. Alzheimers Dement. 2017;13(5):499-509. doi: 10.1016/j.jalz.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 50.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. ; Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup . Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018;pii:S1552-5260(18)33491-5. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev. 2018;38:39-49. doi: 10.1016/j.smrv.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 52.Boland LL, Shahar E, Iber C, Knopman DS, Kuo TF, Nieto FJ; Sleep Heart Health Study (SHHS) Investigators . Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res. 2002;11(3):265-272. https://www.ncbi.nlm.nih.gov/pubmed/12220323. doi: 10.1046/j.1365-2869.2002.00308.x [DOI] [PubMed] [Google Scholar]
- 53.Sforza E, Roche F, Thomas-Anterion C, et al. . Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33(4):515-521. doi: 10.1093/sleep/33.4.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eTable 1. Detailed statistics of significant neuroimaging clusters.
eTable 2. Results of inter-modality correlations.
eTable 3. Results of between-group comparisons using a ROI approach for PET data.
eTable 4. Results of complementary forward stepwise regression analyses.
eTable 5. Results of partial correlation analyses between SDB parameters and SDB-related brain changes with cognitive and behavioural scores.
eReferences.