Abstract
The high mannose patch (HMP) of the HIV envelope protein (Env) is the structure most frequently targeted by broadly neutralizing antibodies; therefore, many researchers have attempted to use mimics of this region as a vaccine immunogen. In our previous efforts, vaccinating rabbits with evolved HMP mimic glycopeptides containing Man9 resulted in an overall antibody response targeting the glycan core and linker rather than the full glycan or Manα1→2Man tips of Man9 glycans. A possible reason could be processing of our immunogen by host serum mannosidases. We sought to test whether more prolonged dosing could increase the antibody response to intact glycans, possibly by increasing the availability of intact Man9 to germinal centers. Here, we describe a study investigating the impact of immunization regimen on antibody response by testing immunogen delivery through bolus, an exponential series of mini doses, or a continuously infusing mini-osmotic pump. Our results indicate that, with our glycopeptide immunogens, standard bolus immunization elicited the strongest HIV Env-binding antibody response, even though higher overall titers to the glycopeptide were elicited by the exponential and pump regimens. Antibody selectivity for intact glycan was, if anything, slightly better in the bolus-immunized animals.
After decades of research, very little HIV vaccine efficacy has been observed in clinical trials.1−3 The challenges of HIV vaccine design include (1) the high mutation rate of HIV, which leads to vast phylogenetic and antigenic diversity, (2) the metastable nature of the HIV envelope protein (Env), a trimer of gp41/gp120 heterodimers that can adopt several functional conformational states, (3) the fact that gp120 can be shed from Env on the viral surface, (4) the low immunogenicity of the most conserved structural features on Env, leading to preferential formation of antibodies against strain-specific epitopes, and (5) the shielding of many conserved epitopes by the dense array of ∼70–80 N-glycans on the Env trimer. Most early generation HIV vaccine approaches utilizing recombinant protein or vectors encoding Env subunits (e.g., monomeric gp120) have elicited antibodies that bind misfolded Env or strain-specific Env structures and do not neutralize a broad range of viral strains.1 However, stable native-like Env trimers (SOSIP trimers) have been engineered that are correctly folded and elicit neutralizing antibodies to the strain used in the immunogen.4−8
Despite these challenges, continued optimism for design of a protective vaccine stems from the fact that broadly neutralizing antibodies (bnAbs) naturally arise in a surprisingly high fraction of infected individuals (∼20%).9 Although bnAbs typically arise too late after infection (usually >2 years) and viral diversification to be protective in the infected individual, several monoclonal bnAbs have been shown to confer protection if administered prior to encounter with virus.10−15 Hundreds of bnAb clones have now been isolated from patient cohorts, and their neutralizing breadth and binding epitopes have been characterized.16−18 These antibodies have stimulated vaccine design efforts because they are proof that an immunological solution exists to broad cross-reactivity with HIV Env; more specifically, structural studies of bnAbs in complex with Env have revealed which conserved motifs on Env are targeted in bnAb responses.19,20
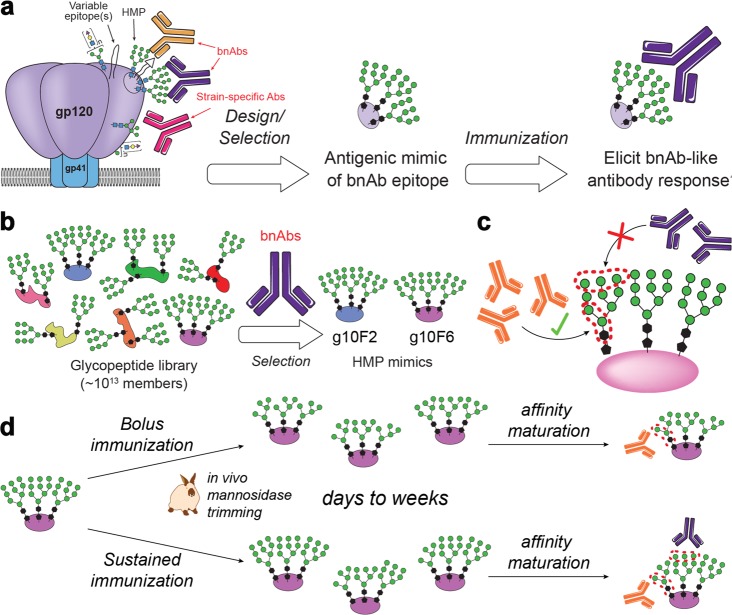
The Env region most commonly targeted21 by bnAbs (∼40%) is the high-mannose patch (HMP, Figure 1a), a region of gp120 containing a dense array of N-linked glycosylation sites (N332, N339, N392, N295, N262, N448, N363) largely populated by Man9/8GlcNAc2 glycans.22 bnAbs targeting this region bind to combinations of these glycans and conserved polypeptide residues (e.g., PGT121- and PGT128 bnAb families)23−25 or exclusively to glycans (e.g., bnAb 2G12).26,27 In all cases, these bnAb epitopes contain multiple glycans; thus, carbohydrate chemists have been very interested in the design and synthesis of carbohydrate clusters to mimic these epitopes.28−46 In these epitope-focused vaccine strategies, it is reasoned that a glycopeptide or carbohydrate mimic of the HMP might elicit antibodies that bind the HMP and are broadly neutralizing (Figure 1a).
Figure 1.
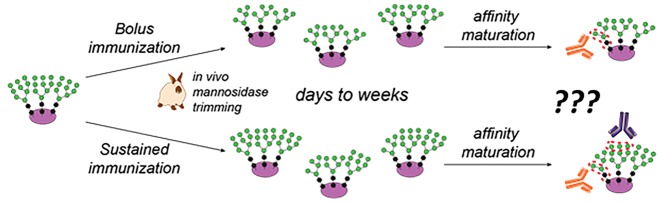
Glycopeptide mimics of HIV epitopes and the glycan trimming hypothesis. (a) The high-mannose patch (HMP) on HIV’s gp120 protein is a target of broadly neutralizing antibodies (bnAbs), and glycopeptides that mimic the HMP are attractive vaccine candidates. (b) Our method for in vitro selection of glycopeptides that mimic the HMP. (c) Antibodies elicited by glycopeptide HMP mimics have so far preferentially targeted the glycan core rather than the Manα1→2Man “tips” targeted by many bnAbs. (d) Immunization kinetics may influence glycan microspecificity: in bolus immunization, serum mannosidase trimming likely truncates most glycans before the bulk of affinity maturation; we hypothesize that, by contrast, continuous or repetitive immunization will provide fresh intact Man9 glycan, against which Manα1→2Man-specific antibodies could develop in germinal centers that were originally established by intact Man9 earlier in the immunization.
In our attempts to mimic the HMP, we have developed directed evolution methods that enable us to select multivalent Man9 clusters that bind bnAb 2G12 from extraordinarily diverse libraries of up to 1013 glycopeptides40,47 or glycoDNAs.41−43,48 Antibody 2G12 binds our evolved glycopeptides with nanomolar to subnanomolar affinity, at least as tightly as it binds to gp120, and in a glycan-dependent manner (Figure 1b). As conjugates to CRM197 carrier protein, our glycopeptides exhibited strong immunogenicity, eliciting robust binding titers (ELISA EC50 ≈ 20 000) against autologous glycopeptide.38 Encouragingly, two of these glycopeptide immunogens (g10F6 and g10F2) elicited detectable HIV binding or neutralizing antibodies; however, the anti-HIV titers were very weak, suggesting modest binding to gp120 or its high-mannose glycans. Glycan microspecificity studies indicated that the glycan-dependent antibodies were primarily directed at two core mannose residues of the glycan, and its hydrophobic linker, rather than the Manα1→2Man tips (Figure 1c). Mass spectrometric studies then showed that incubation of immunogen in serum readily trims mannose residues from the glycans, suggesting that mannosidase activity49−52in vivo may interfere with development of antibodies against these structures.
Serum mannosidase trimming of glycopeptides could also influence the specificity of the antibody response, depending on the relative kinetics of vaccination and immunogen degradation. In natural HIV infection, high-mannose-binding bnAbs arise after months of affinity maturation in the presence of continuously produced viral glycoprotein.53 By contrast, immunizations are traditionally administered in several bolus doses spaced weeks apart. As we have shown that glycans of relatively dense glycopeptides can be trimmed by serum mannosidase on a time scale of days, germinal center formation and affinity maturation likely occurs primarily in the presence of truncated glycans (Figure 1d). Thus, one might ask, could the glycan microspecificity of vaccine-elicited antibodies be modulated by altering the kinetics of the vaccine dose, with prolonged administration of intact immunogen? In fact, for the antibody response to HIV Env protein, it has been shown that sustained delivery of antigen not only increases the overall antibody titer but also improves selectivity for intact versus misfolded or degraded HIV Env.54
Results
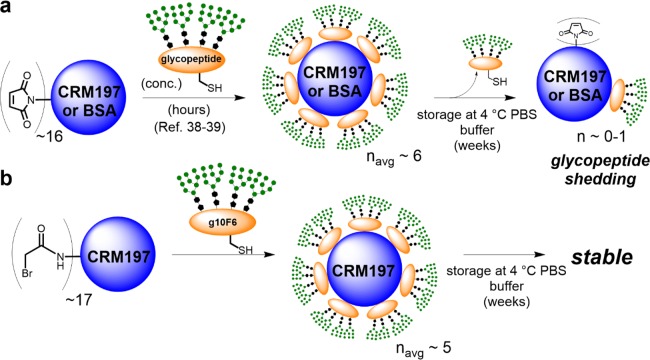
To test the effect of antigen delivery kinetics on the anti-glycan response, we conducted rabbit immunizations with our g10F6 glycopeptide conjugated to CRM197 carrier protein,38−40 administered in three different regimens (Figure 2). In the control group, a standard bolus dose was tested at 4-week intervals. In the second group, we tested an exponentially increasing series of minidoses, designed to mimic the kinetics of antigen produced by a replicating pathogen, as recently described.54 In the third group, we tested a continuous two-week infusion of half the glycopeptide conjugate via a subcutaneous mini-osmotic pump, followed by the second half of the dose as a bolus at the end of the 2 weeks.54−57 Each type of immunization regimen was repeated at t = 4 and 8 weeks, and all groups of animals received the same total dose of conjugate containing 50 μg of glycopeptide, together with 50 μg of QS-21 saponin/cholesterol liposomes (SI Figure S3). For this study, g10F6 glycopeptide was conjugated to CRM197 through cysteine–bromoacetamide substitution rather than the cysteine–maleimide addition used in our previous immunogenicity studies,38 as we found that our original cysteine–maleimide conjugates shed glycopeptide upon storage at 4 °C in water after 1 week (Figure 3a and SI Figure S4). The reversibility of the cysteine–maleimide linkage is well-known,58−61 but we found it to be especially evident in the case of our bulky (∼12.5 kDa) glycopeptides conjugated to CRM197. Although this shedding could be prevented by storage of the conjugates in lyophilized form, the use of a nonreversible linkage would rule out shedding after redissolution. The use of potentially labile maleimide conjugates was out of the question for this study because the conjugates would be held at body temperature in the osmotic pump reservoir for 2 weeks in one of our rabbit groups. The cysteine–bromoacetamide CRM197 conjugations proceeded with good efficiency (median loading of 5), and the resulting thioether linkages lack the capacity for a reverse reaction to shed glycopeptide thiol (Figure 3b and SI Figures S1 and S2).62,63
Figure 2.
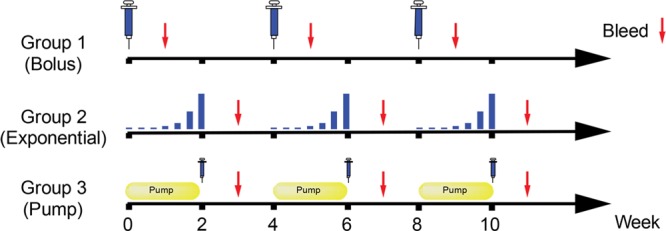
Standard vs sustained immunization regimens. Groups of six rabbits were immunized with the g10F6–CRM197 conjugate by three different regimens. In addition to the group that received standard bolus immunizations, one group (“exponential”) received seven exponentially increasing small doses over 2 weeks, and another group (“pump”) received half of the dose continuously over 2 weeks by mini-osmotic pump, followed by the second half dose at the end of the 2 weeks. All groups received the same total 50 μg of antigen, and the cycle of immunization was repeated at four-week intervals for all groups. Blood was collected 1 week after the conclusion of each immunization cycle.
Figure 3.
Shedding of glycopeptides from maleimide conjugates versus stability of thioether conjugates.
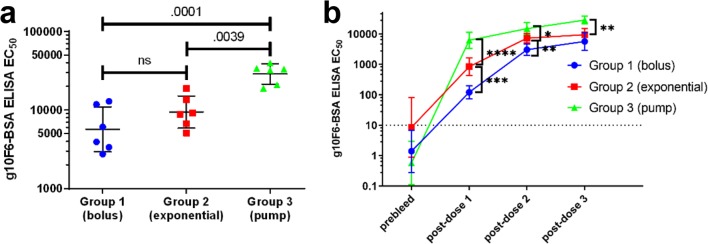
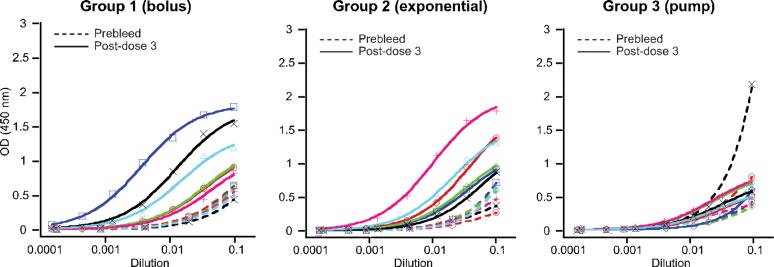
Rabbit polyclonal sera were collected 1 week after each immunization and assayed by ELISA to assess IgG binding to test antigens. Consistent with prior studies of sustained immunization that used SOSIP trimeric HIV gp140 Env proteins,54−57 we observed the strongest antibody binding titers against glycopeptide using the most sustained delivery method, with ELISA EC50 titers for glycopeptide–BSA increasing in the order of bolus < exponential < pump (Figure 4a). Differences were greatest after the first dose, with the bolus group partially catching up to the other two groups by the postdose 3 titer measurement (Figure 4b). For all groups, the IgG titers to glycopeptide–BSA were higher than to either peptide–BSA or CRM carrier (Figure 5), consistent with our previous studies.38 Although significant titers to CRM carrier were observed, measurement of glycopeptide-binding antibodies with glycopeptide–BSA ensured that carrier-independent antibodies were detected. Moreover, our previous studies had shown that antibodies elicited by CRM alone did not cross-react to either glycopeptides or HIV Env.38
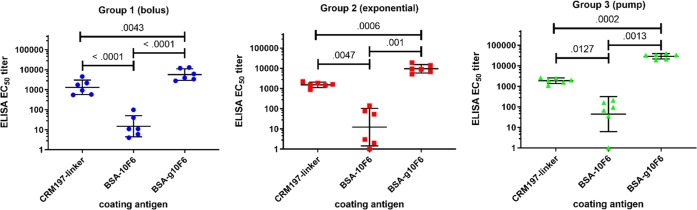
Figure 4.
Dependence of g10F6-binding antibodies on immunization regimen. (a) ELISA IgG EC50 titers for rabbit serum binding to g10F6–BSA after three cycles of immunization with g10F6–CRM. Geometric means and standard deviations are shown. Numbers displayed above data are p-values determined by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, using log-transformed data; ns denotes p > 0.1. (b) Titers as in panle a but plotted versus dose. All data except the prebleed are for sera collected 1 week after the dose: * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001, **** denotes p < 0.0001. The dotted line indicates the lowest serum dilution tested in the ELISA.
Figure 5.
Rabbit serum binding to glycopeptide versus peptide versus carrier. ELISA IgG EC50 titers are shown for postdose 3 sera. Glycopeptide g10F6 and peptide 10F6 are both conjugated to BSA in the coating antigen. CRM197 used as coating antigen is functionalized with the linker for conjugation. Numbers displayed above data are p-values determined by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, using log-transformed data.
We then investigated the ability of the antisera to bind to native-like trimeric HIV Env (BG505T332N SOSIP.664 gp140 trimers).4 Bolus group sera showed the most binding to HIV Env, with three animals out of six exhibiting EC50 titers substantially above preimmunization baseline levels (Figure 6). Exponential group sera showed somewhat less Env binding, and pump group sera exhibited negligible binding above preimmunization baseline. TZM-bl neutralization assays using an HIV strain sensitive to HMP-directed antibodies showed just one serum from group 1 animals was weakly neutralizing (SI Table S2). Although elicitation of even weak HIV binding or neutralizing sera without using HIV Env immunogens is encouraging, the greater HIV binding activity among bolus-immunized animals is surprising, as it is the reverse of the trend seen in overall titers to the glycopeptide (cf. Figure 4a); moreover, we had hypothesized that sustained immunization regimens would lead to more antibodies against the intact glycan structures present in the HMP of Env, compared with bolus immunization.
Figure 6.
Postdose 3 serum binding to trimeric HIV Env. Data are ELISA for rabbit IgG binding to plates coated with 200 ng/well BG505T332N SOSIP trimers. Solid lines are postdose 3 data, and dotted lines are prebleed data. One animal in Group 3 exhibited a high ELISA absorbance in the preimmunization bleed, at the highest serum concentration (right graph, black dotted line). Because that animal showed no SOSIP binding after glycopeptide immunizations (right graph, black solid line), the prebleed was not investigated further.
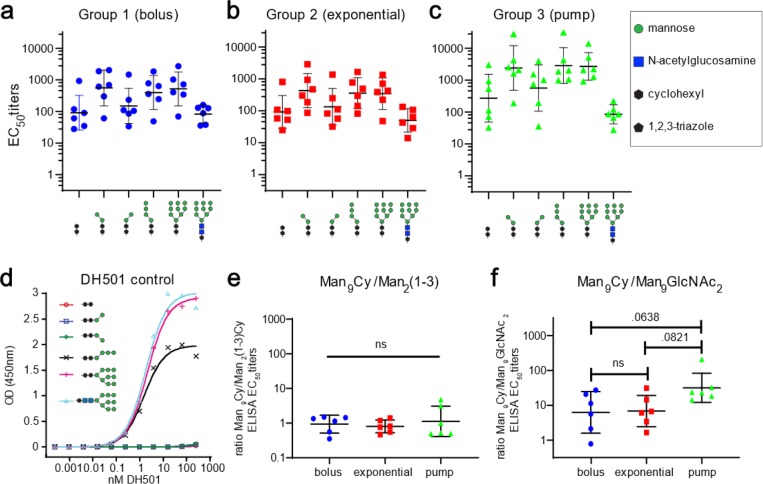
To examine glycan specificity in more detail, we assayed the sera by ELISA against a panel of truncated or altered glycans clustered on a different peptide, 10F12M (Figure 7a–c and SI Figure S6).38 An antibody response that targets primarily Manα1→2Man tips of the Man9 structure should bind more strongly in ELISA to Man9-Cy (Cy = cyclohexyl) than to Man2(1→3)-Cy conjugates, which are truncated to remove Manα1→2Man linkages. As observed in our previous immunizations with g10F6 conjugate, within each rabbit group, sera bound similarly to Man9-Cy and Man2(1→3)-Cy, but more weakly to the Cy linker alone, suggesting that these core two mannose residues together with the cyclohexyl linker are sufficient to account for the bulk of serum reactivity. Moreover, the rabbit antibodies generally bound much better to Man9-Cy than to Man9-GlcNAc2 structures, suggesting that the core and linker of the glycan was most important and that the Manα1→2Man motif was not the major determinant of binding, as we observed in our previous study.38 By contrast, a monoclonal macaque antibody DH501 with crystallographically observed Manα1→2Man binding specificity64 exhibited identical low nanomolar binding EC50 values to Man9-Cy, Man4-Cy, and Man9GlcNAc2 but did not bind at all to either the (1→3) or (1→6) isomer of Man2-Cy (Figure 7d). When the ratio of binding EC50 values for Man9/Man2(1→3) was calculated for each vaccinated rabbit, no significant difference was observed between groups (Figure 7e). Nearly all animals exhibited binding selectivity for Man9-Cy vs Man9GlcNAc2, with apparently the strongest selectivity among pump-immunized animals (Figure 7f). Although the difference between this group and the others was of borderline statistical significance (p = 0.06 and 0.08 for comparison with bolus and exponential groups, respectively), it suggests that the most gradual release of the glycoconjugate is, if anything, detrimental to the development of (Manα1→2Man)-focused antibodies, in the context of these immunizations.
Figure 7.
Carbohydrate selectivity of rabbit serum IgGs elicited by g10F6–CRM. (a–c) ELISA EC50 IgG titers to different glycans displayed on peptide 10F12M–BSA for animals immunized by (a) group 1 (bolus), (b) group 2 (exponential), and (c) group 3 (pump) regimens. (d) Control ELISA of monoclonal macaque antibody DH501 binding to sugar conjugates from panels a–c. DH501 binds to (Manα1→2Man) termini of glycans, and unlike g10F6–CRM-elicited sera, binds identically to Man9Cy and Man9GlcNAc2 conjugates. (e, f) For each rabbit, ratio of serum binding to full vs truncated glycan and full Man9 glycan with vaccine-derived cyclohexyl linker vs GlcNAc2. Numbers displayed above data are p-values determined by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, using log-transformed data; ns denotes p > 0.1. For background binding of rabbit sera to BSA and linker, see Figure S5.
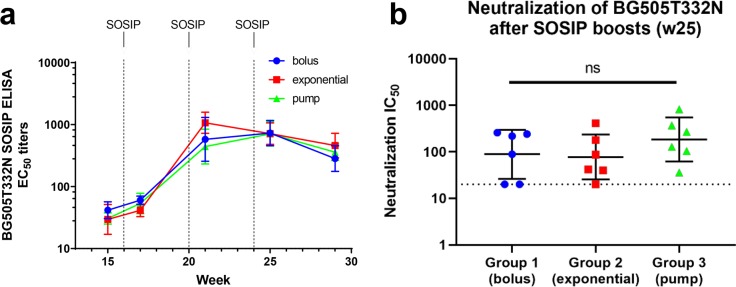
We next assessed whether these three glycopeptide immunization regimens might differentially prime responses to an HIV Env boost. The minimal epitope prime/SOSIP protein boost strategy has recently shown some promise in HIV vaccine approaches to focus the antibody response on bnAb epitopes.46,65 Thus, all groups were boosted with three bolus doses of native-like trimeric BG505T332N SOSIP.664 gp140 protein. Consistent with previous rabbit immunization studies using this trimeric Env,7 all animals developed binding to this Env (ELISA EC50 titers of 400–1500) and most developed BG505T332N neutralizing activity (IC50 titers of 40–800, Figure 8 and SI Table S3). However, no statistically significant differences were observed between groups. In our previous work,38 the SOSIP boost immunizations did not increase titers against the g10F6 glycopeptide, and they elicited similar SOSIP binding titers in animals primed with either CRM–g10F6 or CRM alone; therefore, these effects were not investigated in the present study.
Figure 8.
HIV binding and neutralization after SOSIP boosts. (a) Rabbit serum ELISA EC50 IgG titers to BG505T332N SOSIP gp140 trimeric HIV Env protein (12 ng/well), the same strain used for the boost immunizations. Dotted lines indicate the time points of the boost immunizations. (b) TZM-bl neutralization assays against BG505T332N pseudovirus. The dotted line denotes the lowest (20:1) dilution tested in the assay. The three data points on this line represent less than half-maximal inhibition at this dilution. ns denotes p > 0.1 in one-way ANOVA.
Discussion and Conclusions
This study was designed to test the hypothesis that slow release immunization regimens could be used to increase the selectivity of vaccine elicited antibodies for the nonreducing “tips” (Manα1→2Man moieties) of oligomannose glycans. Because these glycans are trimmed by serum mannosidase on a time scale (hours to days) competitive with germinal center formation (days) and affinity maturation (weeks), it was reasoned that a constant supply of fresh immunogen would increase the exposure of germinal centers to intact glycans, thereby resulting in a stronger antibody response to the Manα1→2Man tips of the Man9. Instead, we observed similar results from standard bolus immunizations versus exponential and continuous immunization, with, if anything, more response to intact glycans in the bolus-immunized animals. A better understanding of this result would require more detailed data about the relative kinetics of (1) mannosidase trimming and (2) activation of the B cells that lead to this antibody response. Although we have observed38 ∼50% cleavage of our immunogen glycans from Man9 to Man8/7/6 within ∼17 h in serum ex vivo at a high concentration (100 μL/mL), we do not know the rates in vivo and at relevant concentrations; moreover, it is not known what concentrations might saturate the mannosidase activity in vivo. If the bolus dose were to saturate mannosidase activity, intact oligomannose would be more likely to encounter B cells; by contrast, the low rate of immunogen release in pump immunizations might allow for more extensive mannosidase trimming prior to B cell encounter. Future dose or kinetics studies might address these questions, although a more straightforward route to vaccine optimization would be to eliminate the degradation of the glycan, using mannosidase inhibitors or chemical modification of the glycans.
Independent of immunization regimen, the lack of antibody response to the Manα1→2Man termini may also be due to a failure to engage appropriate germline precursors of Manα1→2Man-binding antibodies. A great deal of HIV vaccine research is currently devoted to approaches that utilize immunogens designed to bind to inferred germline precursors of bnAbs, under the supposition that vaccine-elicited bnAbs with the same specificity are more likely to arise from the same germline Ab lineage.66 To address this question experimentally would require the design of immunogens that bind to the human germline precursors of 2G12 or other HMP bnAbs and to test them in germline bnAb knock-in animals.67−69
Another potential barrier to elicitation of Manα1→2Man-binding Abs or bnAbs could be insufficient somatic hypermutation. Nearly all HIV bnAbs are highly somatically mutated. This includes the high-mannose-patch antibodies, which generally bear ∼20% nucleotide mutation in the heavy chain.70 In contrast, antibodies from vaccination have been reported to average ∼6% nucleotide mutation.71,72 DH501, which binds oligomannose but neutralizes only kifunensine-treated virus (bearing exclusively Man8/9 glycans), is also highly mutated (23% of amino acids in the heavy chain).73 DH501 arose during a four-year course of 17 immunizations using both Env protein and Env DNA in monkeys, which is the only vaccine regimen to date that has definitively elicited antibodies that bind to the Manα1→2Man motif.64 To recapitulate and improve on those findings with a more practical immunization regimen remains an important goal.
Methods
Detailed information for all methods used can be found in the Supporting Information.
Acknowledgments
I.J.K. gratefully acknowledges the support of the NIH (R01 AI090745, R01 AI113737, and R03 AI136720) and Brandeis’ SPROUT program. I.A.W. acknowledges HIVRAD P01 AI110657, CHAVD (UM1 AI144462), and the IAVI/Scripps CAVD (OPP119635) supported by the Bill and Melinda Gates Foundation. The Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including P41 GM103393). A.A.R. acknowledges the Brandeis National Science Foundation Materials Research Science Engineering Center, Bioinspired Soft Materials (NSF-DMR 1420382). Neutralizing antibody assays were supported by Contract No. HHSN27201100016C (to D.C.M.) from NIAID/NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS, NIAID, or NIH. K. Saunders (Duke University) is gratefully acknowledged for providing the DH501 antibody. A. Lees and Fina Biosolutions (Rockville, MD) are acknowledged for the supply of CRM197.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.0c00053.
Materials and methods, including preparation and mass spectral data of glycopeptide conjugates, adjuvant preparation and characterization, immunization procedures, ELISA protocol, neutralization assay protocol and data, supplementary ELISA data, and data on glycoconjugate stability (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- del Moral-Sánchez I.; Sliepen K. (2019) Strategies for inducing effective neutralizing antibody responses against HIV-1. Expert Rev. Vaccines 18, 1127–1143. 10.1080/14760584.2019.1690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. (2017) Protection against HIV Acquisition in the RV144 Trial. J. Virol. 91, e00905-17. 10.1128/JVI.00905-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S.; Pitisuttithum P.; Nitayaphan S.; Kaewkungwal J.; Chiu J.; Paris R.; Premsri N.; Namwat C.; de Souza M.; Adams E.; Benenson M.; Gurunathan S.; Tartaglia J.; McNeil J. G.; Francis D. P.; Stablein D.; Birx D. L.; Chunsuttiwat S.; Khamboonruang C.; Thongcharoen P.; Robb M. L.; Michael N. L.; Kunasol P.; Kim J. H. (2009) Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 361, 2209–2220. 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Sanders R. W.; Derking R.; Cupo A.; Julien J. P.; Yasmeen A.; de Val N.; Kim H. J.; Blattner C.; de la Pena A. T.; Korzun J.; Golabek M.; de los Reyes K.; Ketas T. J.; van Gils M. J.; King C. R.; Wilson I. A.; Ward A. B.; Klasse P. J.; Moore J. P. (2013) A Next-Generation Cleaved, Soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, Expresses Multiple Epitopes for Broadly Neutralizing but Not Non-Neutralizing Antibodies. PLoS Pathog. 9, e1003618 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J.-P.; Cupo A.; Sok D.; Stanfield R. L.; Lyumkis D.; Deller M. C.; Klasse P.-J.; Burton D. R.; Sanders R. W.; Moore J. P.; Ward A. B.; Wilson I. A. (2013) Crystal Structure of a Soluble Cleaved HIV-1 Envelope Trimer. Science 342, 1477–1483. 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyumkis D.; Julien J.-P.; de Val N.; Cupo A.; Potter C. S.; Klasse P.-J.; Burton D. R.; Sanders R. W.; Moore J. P.; Carragher B.; Wilson I. A.; Ward A. B. (2013) Cryo-EM Structure of a Fully Glycosylated Soluble Cleaved HIV-1 Envelope Trimer. Science 342, 1484–1490. 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. W.; van Gils M. J.; Derking R.; Sok D.; Ketas T. J.; Burger J. A.; Ozorowski G.; Cupo A.; Simonich C.; Goo L.; Arendt H.; Kim H. J.; Lee J. H.; Pugach P.; Williams M.; Debnath G.; Moldt B.; van Breemen M. J.; Isik G.; Medina-Ramírez M.; Back J. W.; Koff W. C.; Julien J.-P.; Rakasz E. G.; Seaman M. S.; Guttman M.; Lee K. K.; Klasse P. J.; LaBranche C.; Schief W. R.; Wilson I. A.; Overbaugh J.; Burton D. R.; Ward A. B.; Montefiori D. C.; Dean H.; Moore J. P. (2015) HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349, aac4223. 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. W.; Moore J. P. (2017) Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol. Rev. 275, 161–182. 10.1111/imr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D.; Burton D. R. (2018) Recent progress in broadly neutralizing antibodies to HIV. Nat. Immunol. 19, 1179–1188. 10.1038/s41590-018-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselinovic M.; Neff C. P.; Mulder L. R.; Akkina R. (2012) Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology 432, 505–510. 10.1016/j.virol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A. J.; Rakasz E. G.; Poignard P.; Hangartner L.; Landucci G.; Forthal D. N.; Koff W. C.; Watkins D. I.; Burton D. R. (2009) Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5, e1000433 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A. J.; Poignard P.; Hunter M.; Hangartner L.; Tehrani D. M.; Bleeker W. K.; Parren P.; Marx P. A.; Burton D. R. (2009) Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15, 951–U155. 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. M.; Burton D. R. (2018) Passive immunotherapy of viral infections: ’super-antibodies’ enter the fray. Nat. Rev. Immunol. 18, 297–308. 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai M.; Donau O. K.; Plishka R. J.; Buckler-White A.; Mascola J. R.; Nabel G. J.; Nason M. C.; Montefiori D.; Moldt B.; Poignard P. (2014) Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 211, 2061. 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julg B.; Tartaglia L. J.; Keele B. F.; Wagh K.; Pegu A.; Sok D.; Abbink P.; Schmidt S. D.; Wang K.; Chen X.; Joyce M. G.; Georgiev I. S.; Choe M.; Kwong P. D.; Doria-Rose N. A.; Le K.; Louder M. K.; Bailer R. T.; Moore P. L.; Korber B.; Seaman M. S.; Abdool Karim S. S.; Morris L.; Koup R. A.; Mascola J. R.; Burton D. R.; Barouch D. H. (2017) Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci. Transl. Med. 9, eaal1321. 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy L. E.; Burton D. R. (2017) Identification and specificity of broadly neutralizing antibodies against HIV. Immunol. Rev. 275, 11–20. 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J. R.; Haynes B. F. (2013) HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol. Rev. 254, 225–244. 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F.; Mouquet H.; Dosenovic P.; Scheid J. F.; Scharf L.; Nussenzweig M. C. (2013) Antibodies in HIV-1 vaccine development and therapy. Science 341, 1199. 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. B.; Wilson I. A. (2017) The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol. Rev. 275, 21–32. 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murin C. D.; Wilson I. A.; Ward A. B. (2019) Antibody responses to viral infections: a structural perspective across three different enveloped viruses. Nat. Microbiol. 4, 734–747. 10.1038/s41564-019-0392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landais E.; Huang X.; Havenar-Daughton C.; Murrell B.; Price M. A.; Wickramasinghe L.; Ramos A.; Bian C. B.; Simek M.; Allen S.; Karita E.; Kilembe W.; Lakhi S.; Inambao M.; Kamali A.; Sanders E. J.; Anzala O.; Edward V.; Bekker L.-G.; Tang J.; Gilmour J.; Kosakovsky-Pond S. L.; Phung P.; Wrin T.; Crotty S.; Godzik A.; Poignard P. (2016) Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort. PLoS Pathog. 12, e1005369 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. N., and Saunders K. O. (2019) Antibody responses to the HIV-1 envelope high mannose patch, in Advances in Immunology (Alt F., Ed.), Chapter 2, pp 11–73, Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.; Lee J. H.; Doores K. J.; Murin C. D.; Julien J.-P.; McBride R.; Liu Y.; Marozsan A.; Cupo A.; Klasse P.-J.; Hoffenberg S.; Caulfield M.; King C. R.; Hua Y.; Le K. M.; Khayat R.; Deller M. C.; Clayton T.; Tien H.; Feizi T.; Sanders R. W.; Paulson J. C.; Moore J. P.; Stanfield R. L.; Burton D. R.; Ward A. B.; Wilson I. A. (2013) Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 20, 796–803. 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R.; Doores K. J.; Walker L. M.; Khayat R.; Huang P.-S.; Wang S.-K.; Stanfield R. L.; Julien J.-P.; Ramos A.; Crispin M.; Depetris R.; Katpally U.; Marozsan A.; Cupo A.; Maloveste S.; Liu Y.; McBride R.; Ito Y.; Sanders R. W.; Ogohara C.; Paulson J. C.; Feizi T.; Scanlan C. N.; Wong C.-H.; Moore J. P.; Olson W. C.; Ward A. B.; Poignard P.; Schief W. R.; Burton D. R.; Wilson I. A. (2011) A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield. Science 334, 1097–1103. 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.; Torrents de la Peña A.; Deller M. C.; Garces F.; Sliepen K.; Hua Y.; Stanfield R. L.; Sanders R. W.; Wilson I. A. (2015) Complete epitopes for vaccine design derived from a crystal structure of the broadly neutralizing antibodies PGT128 and 8ANC195 in complex with an HIV-1 Env trimer. Acta Crystallogr., Sect. D: Biol. Crystallogr. 71, 2099. 10.1107/S1399004715013917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murin C. D.; Julien J.-P.; Sok D.; Stanfield R. L.; Khayat R.; Cupo A.; Moore J. P.; Burton D. R.; Wilson I. A.; Ward A. B. (2014) Structure of 2G12 Fab2 in Complex with Soluble and Fully Glycosylated HIV-1 Env by Negative-Stain Single-Particle Electron Microscopy. J. Virol. 88, 10177–10188. 10.1128/JVI.01229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarese D. A.; Scanlan C. N.; Zwick M. B.; Deechongkit S.; Mimura Y.; Kunert R.; Zhu P.; Wormald M. R.; Stanfield R. L.; Roux K. H.; Kelly J. W.; Rudd P. M.; Dwek R. A.; Katinger H.; Burton D. R.; Wilson I. A. (2003) Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300, 2065–2071. 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- Horiya S.; MacPherson I. S.; Krauss I. J. (2014) Recent strategies targeting HIV glycans in vaccine design. Nat. Chem. Biol. 10, 990–999. 10.1038/nchembio.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Zhang R. S.; Orwenyo J.; Giddens J.; Yang Q.; Labranche C. C.; Montefiori D. C.; Wang L. X. (2018) Synthetic HIV V3 Glycopeptide Immunogen Carrying a N334 N-Glycan Induces Glycan-Dependent Antibodies with Promiscuous Site Recognition. J. Med. Chem. 61, 10116–10125. 10.1021/acs.jmedchem.8b01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Zhang R.; Orwenyo J.; Giddens J.; Yang Q.; Labranche C. C.; Montefiori D. C.; Wang L. X. (2018) Multivalent Antigen Presentation Enhances the Immunogenicity of a Synthetic Three-Component HIV-1 V3 Glycopeptide Vaccine. ACS Cent. Sci. 4, 582–589. 10.1021/acscentsci.8b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwenyo J.; Cai H.; Giddens J.; Amin M. N.; Toonstra C.; Wang L. X. (2017) Systematic Synthesis and Binding Study of HIV V3 Glycopeptides Reveal the Fine Epitopes of Several Broadly Neutralizing Antibodies. ACS Chem. Biol. 12, 1566–1575. 10.1021/acschembio.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Orwenyo J.; Guenaga J.; Giddens J.; Toonstra C.; Wyatt R. T.; Wang L. X. (2017) Synthetic multivalent V3 glycopeptides display enhanced recognition by glycan-dependent HIV-1 broadly neutralizing antibodies. Chem. Commun. 53, 5453–5456. 10.1039/C7CC02059G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Orwenyo J.; Giddens J. P.; Yang Q.; Zhang R. S.; LaBranche C. C.; Montefiori D. C.; Wang L. X. (2017) Synthetic Three-Component HIV-1 V3 Glycopeptide Immunogens Induce Glycan-Dependent Antibody Responses. Cell. Chem. Biol. 24, 1513–1522. 10.1016/j.chembiol.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trattnig N.; Mayrhofer P.; Kunert R.; Mach L.; Pantophlet R.; Kosma P. (2019) Comparative Antigenicity of Thiourea and Adipic Amide Linked Neoglycoconjugates Containing Modified Oligomannose Epitopes for the Carbohydrate-Specific anti-HIV Antibody 2G12. Bioconjugate Chem. 30, 70–82. 10.1021/acs.bioconjchem.8b00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trattnig N.; Blaukopf M.; Bruxelle J. F.; Pantophlet R.; Kosma P. (2019) Synthesis of an Undecasaccharide Featuring an Oligomannosidic Heptasaccharide and a Bacterial Kdo-lipid A Backbone for Eliciting Neutralizing Antibodies to Mammalian Oligomannose on the HIV-1 Envelope Spike. J. Am. Chem. Soc. 141, 7946–7954. 10.1021/jacs.9b02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R.; Trattnig N.; Murrell S.; Lu N.; Chau D.; Rempel C.; Wilson I. A.; Kosma P. (2017) Bacterially derived synthetic mimetics of mammalian oligomannose prime antibody responses that neutralize HIV infectivity. Nat. Commun. 8, 1601. 10.1038/s41467-017-01640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield R. L.; De Castro C.; Marzaioli A. M.; Wilson I. A.; Pantophlet R. (2015) Crystal structure of the HIV neutralizing antibody 2G12 in complex with a bacterial oligosaccharide analog of mammalian oligomannose. Glycobiology 25, 412–419. 10.1093/glycob/cwu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D. N.; Xu B.; Stanfield R. L.; Bailey J. K.; Horiya S.; Temme J. S.; Leon D. R.; LaBranche C. C.; Montefiori D. C.; Costello C. E.; Wilson I. A.; Krauss I. J. (2019) Oligomannose Glycopeptide Conjugates Elicit Antibodies Targeting the Glycan Core Rather than Its Extremities. ACS Cent. Sci. 5, 237–249. 10.1021/acscentsci.8b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. K.; Nguyen D. N.; Horiya S.; Krauss I. J. (2016) Synthesis of multivalent glycopeptide conjugates that mimic an HIV epitope. Tetrahedron 72, 6091–6098. 10.1016/j.tet.2016.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiya S.; Bailey J. K.; Temme J. S.; Guillen Schlippe Y. V.; Krauss I. J. (2014) Directed evolution of multivalent glycopeptides tightly recognized by HIV antibody 2G12. J. Am. Chem. Soc. 136, 5407–5415. 10.1021/ja500678v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme J. S.; MacPherson I. S.; DeCourcey J. F.; Krauss I. J. (2014) High Temperature SELMA: Evolution of DNA-Supported Oligomannose Clusters Which Are Tightly Recognized by HIV bnAb 2G12. J. Am. Chem. Soc. 136, 1726–1729. 10.1021/ja411212q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme J. S.; Drzyzga M. G.; MacPherson I. S.; Krauss I. J. (2013) Directed evolution of 2G12-targeted nonamannose glycoclusters by SELMA. Chem. - Eur. J. 19, 17291–17295. 10.1002/chem.201303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson I. S.; Temme J. S.; Habeshian S.; Felczak K.; Pankiewicz K.; Hedstrom L.; Krauss I. J. (2011) Multivalent glycocluster design through directed evolution. Angew. Chem., Int. Ed. 50, 11238–11242. 10.1002/anie.201105555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S. M.; Aussedat B.; Vohra Y.; Meyerhoff R. R.; Cale E. M.; Walkowicz W. E.; Radakovich N. A.; Anasti K.; Armand L.; Parks R.; Sutherland L.; Scearce R.; Joyce M. G.; Pancera M.; Druz A.; Georgiev I. S.; Von Holle T.; Eaton A.; Fox C.; Reed S. G.; Louder M.; Bailer R. T.; Morris L.; Abdool-Karim S. S.; Cohen M.; Liao H.-X.; Montefiori D. C.; Park P. K.; Fernández-Tejada A.; Wiehe K.; Santra S.; Kepler T. B.; Saunders K. O.; Sodroski J.; Kwong P. D.; Mascola J. R.; Bonsignori M.; Moody M. A.; Danishefsky S.; Haynes B. F. (2017) Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide. Sci. Transl. Med. 9, eaai7521. 10.1126/scitranslmed.aai7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera D.; Lee M. S.; Wiehe K.; Meyerhoff R. R.; Piai A.; Bonsignori M.; Aussedat B.; Walkowicz W. E.; Ton T.; Zhou J. O.; Danishefsky S.; Haynes B. F.; Harrison S. C. (2018) HIV envelope V3 region mimic embodies key features of a broadly neutralizing antibody lineage epitope. Nat. Commun. 9, 1111. 10.1038/s41467-018-03565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francica J. R.; Laga R.; Lynn G. M.; Mužíková G.; Androvič L.; Aussedat B.; Walkowicz W. E.; Padhan K.; Ramirez-Valdez R. A.; Parks R.; Schmidt S. D.; Flynn B. J.; Tsybovsky Y.; Stewart-Jones G. B. E.; Saunders K. O.; Baharom F.; Petrovas C.; Haynes B. F.; Seder R. A. (2019) Star nanoparticles delivering HIV-1 peptide minimal immunogens elicit near-native envelope antibody responses in nonhuman primates. PLoS Biol. 17, e3000328 10.1371/journal.pbio.3000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiya S.; Bailey J. K.; Krauss I. J. (2017) Directed Evolution of Glycopeptides Using mRNA Display. Methods Enzymol. 597, 83–141. 10.1016/bs.mie.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme J. S.; Krauss I. J. (2015) SELMA: Selection with Modified Aptamers. Curr. Protoc Chem. Biol. 7, 73–92. 10.1002/9780470559277.ch140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Brown D.; Reed C.; Chung S.; Lutman J.; Stefanich E.; Wong A.; Stephan J.-P.; Bayer R. (2012) Production, characterization and pharmacokinetic properties of antibodies with N-linked Mannose-5 glycans. mAbs 4, 475–487. 10.4161/mabs.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Liu Y. D.; Flynn G. C. (2008) The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 19, 240–249. 10.1093/glycob/cwn120. [DOI] [PubMed] [Google Scholar]
- Porwoll S.; Fuchs H.; Tauber R. (1999) Characterization of a soluble class I α-mannosidase in human serum. FEBS Lett. 449, 175–178. 10.1016/S0014-5793(99)00422-6. [DOI] [PubMed] [Google Scholar]
- Higel F.; Seidl A.; Demelbauer U.; Viertlboeck-Schudy M.; Koppenburg V.; Kronthaler U.; Sörgel F.; Frieß W. (2015) N-glycan PK Profiling Using a High Sensitivity nanoLCMS Work-Flow with Heavy Stable Isotope Labeled Internal Standard and Application to a Preclinical Study of an IgG1 Biopharmaceutical. Pharm. Res. 32, 3649–3659. 10.1007/s11095-015-1724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. L.; Gray E. S.; Wibmer C. K.; Bhiman J. N.; Nonyane M.; Sheward D. J.; Hermanus T.; Bajimaya S.; Tumba N. L.; Abrahams M. R.; Lambson B. E.; Ranchobe N.; Ping L. H.; Ngandu N.; Karim Q. A.; Karim S. S. A.; Swanstrom R. I.; Seaman M. S.; Williamson C.; Morris L. (2012) Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat. Med. 18, 1688–1692. 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam H. H.; Melo M. B.; Kang M.; Pelet J. M.; Ruda V. M.; Foley M. H.; Hu J. K.; Kumari S.; Crampton J.; Baldeon A. D.; Sanders R. W.; Moore J. P.; Crotty S.; Langer R.; Anderson D. G.; Chakraborty A. K.; Irvine D. J. (2016) Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. U. S. A. 113, E6639–E6648. 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. K.; Crampton J. C.; Cupo A.; Ketas T.; van Gils M. J.; Sliepen K.; de Taeye S. W.; Sok D.; Ozorowski G.; Deresa I.; Stanfield R.; Ward A. B.; Burton D. R.; Klasse P. J.; Sanders R. W.; Moore J. P.; Crotty S. (2015) Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity. J. Virol. 89, 10383–10398. 10.1128/JVI.01653-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner M.; Havenar-Daughton C.; Sok D.; Nkolola J. P.; Bastidas R.; Boopathy A. V.; Carnathan D. G.; Chandrashekar A.; Cirelli K. M.; Cottrell C. A.; Eroshkin A. M.; Guenaga J.; Kaushik K.; Kulp D. W.; Liu J.; McCoy L. E.; Oom A. L.; Ozorowski G.; Post K. W.; Sharma S. K.; Steichen J. M.; de Taeye S. W.; Tokatlian T.; Torrents de la Peña A.; Butera S. T.; LaBranche C. C.; Montefiori D. C.; Silvestri G.; Wilson I. A.; Irvine D. J.; Sanders R. W.; Schief W. R.; Ward A. B.; Wyatt R. T.; Barouch D. H.; Crotty S.; Burton D. R. (2017) Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 46, 1073–1088. 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli K. M.; Carnathan D. G.; Nogal B.; Martin J. T.; Rodriguez O. L.; Upadhyay A. A.; Enemuo C. A.; Gebru E. H.; Choe Y.; Viviano F.; Nakao C.; Pauthner M. G.; Reiss S.; Cottrell C. A.; Smith M. L.; Bastidas R.; Gibson W.; Wolabaugh A. N.; Melo M. B.; Cossette B.; Kumar V.; Patel N. B.; Tokatlian T.; Menis S.; Kulp D. W.; Burton D. R.; Murrell B.; Schief W. R.; Bosinger S. E.; Ward A. B.; Watson C. T.; Silvestri G.; Irvine D. J.; Crotty S. (2019) Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 177, 1153–1171. 10.1016/j.cell.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine S. D.; Reid R.; Robinson L.; Ashley G. W.; Santi D. V. (2015) Long-Term Stabilization of Maleimide–Thiol Conjugates. Bioconjugate Chem. 26, 145–152. 10.1021/bc5005262. [DOI] [PubMed] [Google Scholar]
- Szijj P. A.; Bahou C.; Chudasama V. (2018) Minireview: Addressing the retro-Michael instability of maleimide bioconjugates. Drug Discovery Today: Technol. 30, 27–34. 10.1016/j.ddtec.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Alley S. C.; Benjamin D. R.; Jeffrey S. C.; Okeley N. M.; Meyer D. L.; Sanderson R. J.; Senter P. D. (2008) Contribution of Linker Stability to the Activities of Anticancer Immunoconjugates. Bioconjugate Chem. 19, 759–765. 10.1021/bc7004329. [DOI] [PubMed] [Google Scholar]
- Tumey L. N.; Charati M.; He T.; Sousa E.; Ma D.; Han X.; Clark T.; Casavant J.; Loganzo F.; Barletta F.; Lucas J.; Graziani E. I. (2014) Mild Method for Succinimide Hydrolysis on ADCs: Impact on ADC Potency, Stability, Exposure, and Efficacy. Bioconjugate Chem. 25, 1871–1880. 10.1021/bc500357n. [DOI] [PubMed] [Google Scholar]
- Inman J. K. (1993) Syntheses of Macromolecular Immunomodulators and Conjugates Employing Haloacetyl Reagents. Ann. N. Y. Acad. Sci. 685, 347–350. 10.1111/j.1749-6632.1993.tb35887.x. [DOI] [PubMed] [Google Scholar]
- Rector E. S.; Schwenk R. J.; Tse K. S.; Sehon A. H. (1978) A method for the preparation of protein-protein conjugates of predetermined composition. J. Immunol. Methods 24, 321–336. 10.1016/0022-1759(78)90135-7. [DOI] [PubMed] [Google Scholar]
- Saunders K. O.; Nicely N. I.; Wiehe K.; Bonsignori M.; Meyerhoff R. R.; Parks R.; Walkowicz W. E.; Aussedat B.; Wu N. R.; Cai F.; Vohra Y.; Park P. K.; Eaton A.; Go E. P.; Sutherland L. L.; Scearce R. M.; Barouch D. H.; Zhang R.; Von Holle T.; Overman R. G.; Anasti K.; Sanders R. W.; Moody M. A.; Kepler T. B.; Korber B.; Desaire H.; Santra S.; Letvin N. L.; Nabel G. J.; Montefiori D. C.; Tomaras G. D.; Liao H.-X.; Alam S. M.; Danishefsky S. J.; Haynes B. F. (2017) Vaccine Elicitation of High Mannose-Dependent Neutralizing Antibodies against the V3-Glycan Broadly Neutralizing Epitope in Nonhuman Primates. Cell Rep. 18, 2175–2188. 10.1016/j.celrep.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K.; Acharya P.; Kong R.; Cheng C.; Chuang G.-Y.; Liu K.; Louder M. K.; O’Dell S.; Rawi R.; Sastry M.; Shen C.-H.; Zhang B.; Zhou T.; Asokan M.; Bailer R. T.; Chambers M.; Chen X.; Choi C. W.; Dandey V. P.; Doria-Rose N. A.; Druz A.; Eng E. T.; Farney S. K.; Foulds K. E.; Geng H.; Georgiev I. S.; Gorman J.; Hill K. R.; Jafari A. J.; Kwon Y. D.; Lai Y.-T.; Lemmin T.; McKee K.; Ohr T. Y.; Ou L.; Peng D.; Rowshan A. P.; Sheng Z.; Todd J.-P.; Tsybovsky Y.; Viox E. G.; Wang Y.; Wei H.; Yang Y.; Zhou A. F.; Chen R.; Yang L.; Scorpio D. G.; McDermott A. B.; Shapiro L.; Carragher B.; Potter C. S.; Mascola J. R.; Kwong P. D. (2018) Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat. Med. 24, 857–867. 10.1038/s41591-018-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L.; Pancera M.; McGuire A. T. (2017) Germline-targeting immunogens. Immunol. Rev. 275, 203–216. 10.1111/imr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steichen J. M.; Kulp D. W.; Tokatlian T.; Escolano A.; Dosenovic P.; Stanfield R. L.; McCoy L. E.; Ozorowski G.; Hu X.; Kalyuzhniy O.; Briney B.; Schiffner T.; Garces F.; Freund N. T.; Gitlin A. D.; Menis S.; Georgeson E.; Kubitz M.; Adachi Y.; Jones M.; Mutafyan A. A.; Yun D. S.; Mayer C. T.; Ward A. B.; Burton D. R.; Wilson I. A.; Irvine D. J.; Nussenzweig M. C.; Schief W. R. (2016) HIV Vaccine Design to Target Germline Precursors of Glycan-Dependent Broadly Neutralizing Antibodies. Immunity 45, 483–496. 10.1016/j.immuni.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine J. G.; Kulp D. W.; Havenar-Daughton C.; Sarkar A.; Briney B.; Sok D.; Sesterhenn F.; Ereño-Orbea J.; Kalyuzhniy O.; Deresa I.; Hu X.; Spencer S.; Jones M.; Georgeson E.; Adachi Y.; Kubitz M.; deCamp A. C.; Julien J.-P.; Wilson I. A.; Burton D. R.; Crotty S.; Schief W. R. (2016) HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351, 1458–1463. 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine J. G.; Ota T.; Sok D.; Pauthner M.; Kulp D. W.; Kalyuzhniy O.; Skog P. D.; Thinnes T. C.; Bhullar D.; Briney B.; Menis S.; Jones M.; Kubitz M.; Spencer S.; Adachi Y.; Burton D. R.; Schief W. R.; Nemazee D. (2015) Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 349, 156–161. 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N. S.; Sutton M. S.; Shiakolas A. R.; Guenaga J.; Jarosinski M. C.; Georgiev I. S.; McKee K.; Bailer R. T.; Louder M. K.; O'Dell S.; Connors M.; Wyatt R. T.; Mascola J. R.; Doria-Rose N. A. (2016) Multiple Antibody Lineages in One Donor Target the Glycan-V3 Supersite of the HIV-1 Envelope Glycoprotein and Display a Preference for Quaternary Binding. J. Virol. 90, 10574. 10.1128/JVI.01012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D.; Suguitan A. L. Jr.; Pinna D.; Silacci C.; Fernandez-Rodriguez B. M.; Vanzetta F.; Santos C.; Luke C. J.; Torres-Velez F. J.; Temperton N. J.; Weiss R. A.; Sallusto F.; Subbarao K.; Lanzavecchia A. (2010) Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120, 1663–1673. 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölich D.; Giesecke C.; Mei H. E.; Reiter K.; Daridon C.; Lipsky P. E.; Dörner T. (2010) Secondary Immunization Generates Clonally Related Antigen-Specific Plasma Cells and Memory B Cells. J. Immunol. 185, 3103. 10.4049/jimmunol.1000911. [DOI] [PubMed] [Google Scholar]
- Wu N. R.; Nicely N. I.; Lee E. M.; Reed R. K.; Watts B. E.; Cai F.; Walkowicz W. E.; Aussedat B.; Jones J. A.; Eaton A.; Trama A. M.; Alam S. M.; Montefiori D. C.; Haynes B. F.; Saunders K. O. (2019) Cooperation between somatic mutation and germline-encoded residues enables antibody recognition of HIV-1 envelope glycans. PLoS Pathog. 15, e1008165 10.1371/journal.ppat.1008165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.