Cardiac sarcoidosis (CS) can occur in ≤25% of patients with sarcoidosis in other organ systems leading to life-threatening ventricular arrhythmias, heart block, heart failure, and death. An essential part of the innate immune system, the inflammasome is a macromolecular structure in the cell that responds to a danger signal by releasing IL (interleukin)-1β and amplifying the inflammatory response.1 IL-1β is indeed the prototypical proinflammatory cytokine processed within the inflammasome.1 A role for IL-1β in the pathogenesis of sarcoidosis has been proposed. IL-1β participates in the pathogenesis of granuloma formation in the mouse.2 The ratio of IL-1 receptor antagonist/IL-1β was a marker in predicting the persistence of pulmonary granulomatous lesions in patients.3 Importantly, the main mechanism of action of IL-1β is to activate the nuclear transcription factor NF-kB (nuclear factor-kappa B), also a target of glucocorticoids.
We hypothesized that CS would lead to the formation of the inflammasome. We studied cardiac pathology specimens from 3 patients with a diagnosis of CS based on Heart Rhythm Society 2014 Consensus Statement Criteria4 obtained from the left ventricle during total artificial heart implantation in 1 patient and left ventricular assist device implantation and subsequent orthotopic heart transplant in 2 patients. The regions of the heart to be sampled were chosen based on abnormalities upon macroscopic inspection. The study was approved by the Institutional Review Board of the Virginia Commonwealth University, Richmond, VA.
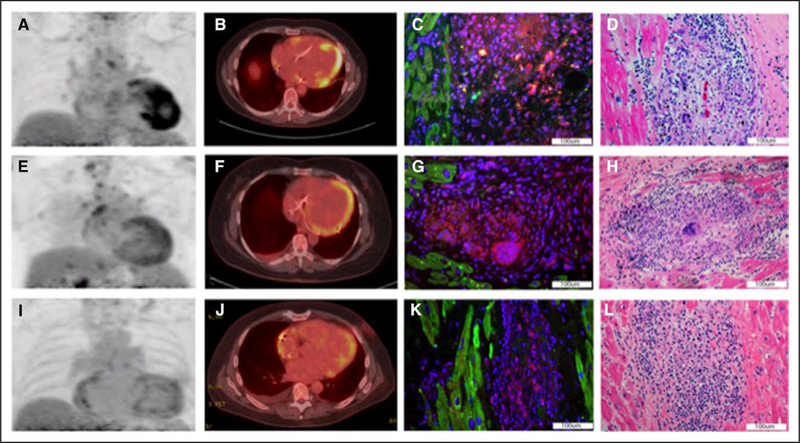
Patient No. 1 is a 59-year-old man with pulmonary sarcoidosis who presented with complete heart block, ventricular tachycardia, and left ventricular systolic dysfunction. The patient was treated with prednisone, mycophenolate mofetil, and hydroxychloroquine. Cardiac 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) 1 month before total artificial heart showed severe-intensity FDG uptake in the apical septum and inferior walls (Figure [A and B]). Because of progressive heart failure symptoms, he underwent total artificial heart followed 7 months later by orthotopic heart transplant.
Figure. Cardiac positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET) of patient No. 1 performed 1 mo before total artificial heart (TAH) showed severe-intensity FDG uptake in the apical septum and inferior walls.
A and B, An intense, positive staining for the ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) is shown in cardiac pathology tissue from the TAH surgery, which identifies the inflammasome (C). Classic hematoxylin and eosin (H&E) staining shows granulomas corresponding to inflammasome presence (D). FDG-PET of patient No. 2 performed 2 mo before left ventricular assist device (LVAD) showed moderate-intensity diffuse FDG uptake extending into the left ventricular apex (E and F). A moderate positivity for ASC in tissue from the LVAD apical core is displayed in G, and H&E staining shows a granuloma corresponding to inflammasome presence (H). The FDG-PET of patient No. 3 performed 1 mo before LVAD and 7 mo before orthotopic heart transplant showed mild-intensity patchy hypermetabolic activity in the left ventricle with mild activity in the distal lateral wall extending into the distal anterolateral wall and apex (I and J). A mildly positive staining for ASC of native cardiac tissue obtained during the transplant surgery is shown in K, and the corresponding H&E stain is shown in L.
Patient No. 2 is a 60-year-old woman with biopsy-proven pulmonary sarcoidosis who presented with complete atrioventricular block and left ventricular systolic dysfunction. FDG-PET performed 2 months before left ventricular assist device showed moderate-intensity diffuse FDG uptake extending into the left ventricular apex (Figure [E and F]). She then underwent Heartmate II implantation followed 3 months later by orthotopic heart transplant.
Patient No. 3 is a 64-year-old male with sinus node dysfunction and nonischemic cardiomyopathy. Cardiac PET showed FDG uptake concerning for CS and hilar and mediastinal lymphadenopathy. Carinal lymph node biopsy showed noncaseating granulomas. He was treated with prednisone and methotrexate. FDG-PET performed showed mild-intensity patchy hypermetabolic activity in the left ventricle with mild activity in the distal lateral wall extending into the distal anterolateral wall and apex (Figure [I and J]). He underwent Heartmate II implantation 1 month later and underwent orthotopic heart transplant after 6 months.
The presence of the inflammasome was detected by immunostaining for ASC (apoptosis speck-like protein containing a caspase-1 recruiting domain) in formalin-fixed paraffin-embedded heart tissue sections. After antigen retrieval, slides were incubated with a rabbit primary antibody against human ASC (1:100; Sigma-Aldrich) overnight at 4°C. Anti-rabbit AlexaFluor 594-conjugated secondary antibody (1:100) was applied. Counterstaining was performed with 4′,6-diamidino-2-phenylindole. Stainings for cleaved caspase-1 and NLRP3 (NACHT, LRR, and PYD domains–containing protein 3) were achieved by immunohistochemistry. Following antigen retrieval, endogenous peroxidases were inactivated by a solution of hydrogen peroxide. Primary antibody for caspase-1 (cleaved Asp210; 1:50; ThermoFisher Scientific) and NLRP3 (1:100; Novus Biologicals) was used, and an antirabbit IgG, HRP (horseradish peroxidase)-linked secondary antibody (1:100) was then applied, followed by VECTOR NovaRED Peroxidase Substrate (Vector Laboratories, Burlingame, CA) and then stained with hematoxylin. Images were acquired with an IX70 microscope and cellSens Dimension digital imaging software (Olympus, Central Valley, PA) using a 40× objective (×400 magnification) for immunofluorescence and Zeiss Axio Imager.A1 using 10× objective for immunohistochemistry. The authors declare that all supporting data are available within the article.
Surgical cardiac specimens from the 3 patients with CS all showed inflammasome aggregates in correspondence of the sarcoid granulomas (100%; Figure [C, G, and K], respectively), and none of the heart samples from 5 individuals who died of noncardiac causes (controls; P=0.018 at Fisher exact test). Intense staining for the NLRP3 and cleaved caspase-1 was seen in the corresponding regions of intense ASC staining in the same samples. Classic histopathology with hematoxylin and eosin staining showed granulomas in the sections used for assessment of the inflammasome in all 3 patients (Figure [D, H, and L], respectively). Appropriate controls showed lack of nonspecific immunofluorescence.
In conclusion, we showed that patients with clinically active CS and evidence of active inflammation on FDG-PET scans 1 to 2 months before initial cardiac surgery displayed the activation of the inflammasome in granulomatous lesions. The presence of the inflammasome within the granulomas in the hearts of CS patients provides additional support for a role of IL-1 in the pathogenesis of CS, thus raising the possibility of IL-1–targeted therapies to treat CS.5 While the current findings are not evidence that patients with CS would have clinical benefit from IL-1β blockade, this novel discovery can be used to explore new pathways for treating this challenging disease.
Acknowledgments
Dr Kron is supported by the VCU Wright Center for Clinical and Translational Research.
Footnotes
Disclosures
None.
Contributor Information
Jordana Kron, Pauley Heart Center, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
Adolfo Gabriele Mauro, Pauley Heart Center, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
Aldo Bonaventura, Pauley Heart Center, Department of Internal Medicine, Virginia Commonwealth University, Richmond; First Clinic of Internal Medicine, Department of Internal Medicine, University of Genoa, Italy.
Stefano Toldo, Pauley Heart Center, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
Fadi N. Salloum, Pauley Heart Center, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
Kenneth A. Ellenbogen, Pauley Heart Center, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
Antonio Abbate, Pauley Heart Center, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
REFERENCES
- 1.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161 [DOI] [PubMed] [Google Scholar]
- 2.Mikuniya T, Nagai S, Takeuchi M, Mio T, Hoshino Y, Miki H, Shigematsu M, Hamada K, Izumi T. Significance of the interleukin-1 receptor antagonist/interleukin-1 beta ratio as a prognostic factor in patients with pulmonary sarcoidosis. Respiration. 2000;67:389–396. doi: 10.1159/000029536 [DOI] [PubMed] [Google Scholar]
- 3.Kasahara K, Kobayashi K, Shikama Y, Yoneya I, Soezima K, Ide H, Takahashi T. Direct evidence for granuloma-inducing activity of interleukin-1. Induction of experimental pulmonary granuloma formation in mice by interleukin-1-coupled beads. Am J Pathol. 1988;130:629–638. [PMC free article] [PubMed] [Google Scholar]
- 4.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 5.Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–2069. doi: 10.1093/eurheartj/ehy128 [DOI] [PubMed] [Google Scholar]