Abstract
Zika virus (ZIKV) infection of pregnant women is associated with congenital Zika syndrome (CZS) and no vaccine is available, although several are being tested in clinical trials. We tested the efficacy of ZIKV DNA vaccine VRC5283 in a rhesus macaque model of congenital ZIKV infection. Most animal vaccine experiments have a set pathogen exposure several weeks or months after vaccination. In the real world, people encounter pathogens years or decades after vaccination, or may be repeatedly exposed if the virus is endemic. To more accurately mimic how this vaccine would be used, we immunized macaques prior to conception, and then exposed them repeatedly to ZIKV during early and mid-gestation. In comparison to unimmunized animals, vaccinated animals had a significant reduction in peak magnitude and duration of maternal viremia, early fetal loss, fetal infection, and placental and fetal brain pathology. Vaccine-induced neutralizing antibody titers on the day of first ZIKV exposure were negatively associated with the magnitude of maternal viremia, and the absence of prolonged viremia was associated with better fetal outcomes. These data support further clinical development of ZIKV vaccine strategies to protect against negative fetal outcomes.
One Sentence Summary:
A Zika virus vaccine administered to macaques prior to conception reduces maternal viremia and improves fetal outcomes.
Introduction
In 2016, the World Health Organization declared Zika virus (ZIKV) a Public Health Emergency of International Concern because of its rapid spread in Latin America and association with congenital abnormalities in infants of infected mothers. Although transmission occurs primarily via mosquitoes, ZIKV can also be transmitted sexually and through blood products (reviewed in (1)). ZIKV infection of healthy adults is generally asymptomatic and clinically benign. However, ZIKV infection during pregnancy is associated with a high risk of adverse fetal effects, including fetal death, microcephaly, and other neural and developmental abnormalities, which are collectively termed congenital Zika syndrome (CZS) (2, 3). Although the incidence of new ZIKV cases has declined substantially since 2016 (4), a high risk for sporadic outbreaks continues, especially with the expansion of mosquito territories and continued human travel to endemic areas. Accordingly, pregnant women will continue to be at risk. Ideally, a ZIKV vaccine can be developed to induce protective immunity in adolescent girls and women of child-bearing age prior to pregnancy and prevent CZS.
Nonhuman primates, especially rhesus macaques, have been shown to be a highly relevant animal model of ZIKV infection, because they recapitulate many of the features of human ZIKV infection, including the development of placental and fetal neurologic abnormalities and fetal loss (5–11). Preclinical studies in adult non-pregnant macaques have demonstrated the efficacy of several experimental ZIKV vaccine candidates (reviewed in (12)). This includes the ZIKV DNA vaccine VRC5283, which expresses pre-membrane and envelope proteins which form subviral particles (SVPs), with antigenic properties similar to infectious virions; protection against viremia correlated with serum neutralizing activity (13). VRC5283 DNA was immunogenic in healthy adults in a phase 1 clinical trial (14), and is currently being evaluated in a multi-site phase 2/2b clinical trial (15). These trials have provided valuable data on safety and immunogenicity. However, due to diminished incidence rates and difficulty predicting the location of future outbreaks, it is logistically challenging to achieve a clinical or virological endpoint and even more difficult to determine efficacy against CZS in randomized clinical trials (4, 16, 17). Therefore, understanding the basis for vaccine-induced protection in animal models will be essential for advanced development of candidate vaccines. In the current study, we evaluated the VRC5283 DNA vaccine in macaques that then became pregnant, as well as their offspring.
Results
Experimental design
We evaluated the VRC5283 DNA vaccine in a macaque model of ZIKV infection during pregnancy (Fig. 1A). Eighteen non-pregnant adult female rhesus macaques were immunized with VRC5283 delivered by needle-free injection using a Pharmajet device. Two doses of 1 mg were administered intramuscularly 4 weeks apart (Fig. 1B). Due to the initiation of the study late in the breeding season, time-mated breeding was initiated after the first immunization (see Materials and Methods). Thirteen vaccinated animals became pregnant, with a time interval between the 1st immunization and estimated conception ranging from 1 day to 1 year (table S1). The first 6 vaccinated animals that became pregnant conceived between the 1st and 2nd immunization, so that their first ZIKV inoculation occurred from 4 days to 28 days after the 2nd immunization (table S1, animals Vax-04 to Vax-28). The last 4 of these 13 conceptions were after treatment with the fertility drug clomiphene (see Materials and Methods). An additional 12 non-immunized female macaques served as ZIKV-inoculated control animals and were enrolled at time of pregnancy confirmation.
Fig. 1. Concept of Zika virus vaccine and experimental design for ZIKV DNA vaccine study in pregnant rhesus macaques.
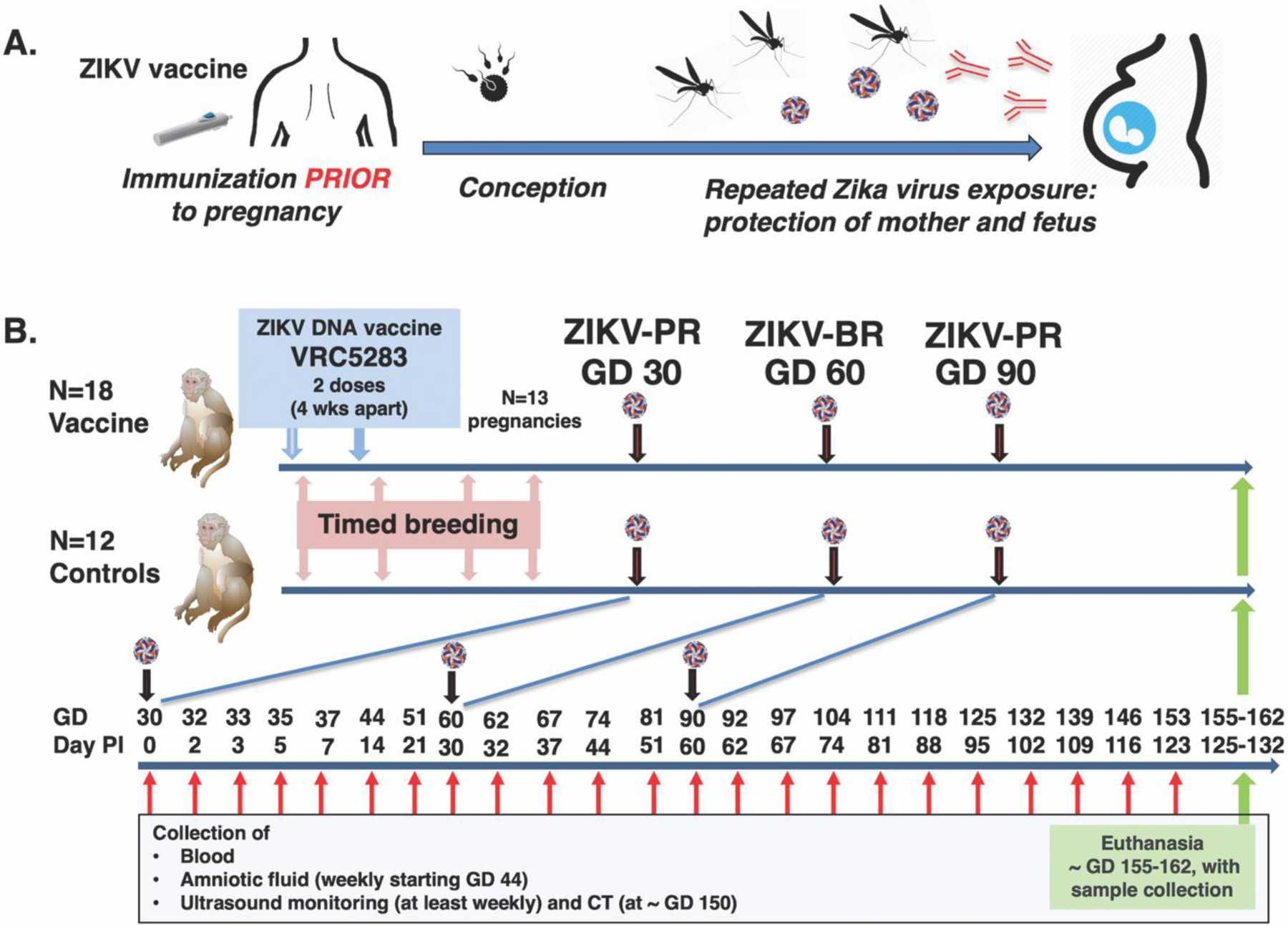
(A) Ideally, administration of a ZIKV vaccine regimen to adolescent girls and women of child-bearing age would be initiated prior to conception, and induce immunity (including neutralizing antibodies) that protects the mother against infection and also her fetus from transplacental infection to prevent congenital Zika syndrome. (B) Eighteen non-pregnant female rhesus macaques were immunized twice with VRC5283, a ZIKV DNA vaccine, 4 weeks apart. Timed breeding was initiated immediately after the first immunization; 13 animals became pregnant, of which 6 conceptions occurred between the 1st and 2nd immunization (see table S1), and consequently the first ZIKV challenge was in some cases only a few days after the 2nd vaccine dose. An additional 12 unimmunized pregnant animals at matched gestation days served as controls. The pregnant macaques were inoculated subcutaneously three times with ZIKV, alternating between Puerto Rican (PR) and Brazilian (BR) ZIKV isolates, at gestational days (GD) ~30, 60 and 90. PI is post-inoculation; CT is computed tomography.
Studies in humans suggest the highest risk of CZS is when maternal ZIKV infection occurs during the first and second trimesters of pregnancy (18, 19). To mimic repeated mosquito exposure, we challenged the pregnant macaques (which have a gestation of ~ 165 days) by subcutaneous inoculation 3 times during the first and second trimester, at gestational day (GD) 30, 60 and 90. Each inoculation consisted of 1,000 plaque forming units (PFU) of ZIKV; to mimic exposure to different variants that may be circulating in endemic areas, we alternated between two strains: a 2015 Puerto Rico isolate (PRVABC-59) and a 2015 Brazil isolate (SPH2015). Animals were monitored closely for health including ultrasound-monitoring of fetal development, with frequent collection of blood and amniotic fluid for virological and immunological analyses (2). At the end of gestation (GD 155–162), surviving fetuses were collected via hysterotomy for detailed tissue analysis.
Vaccine-induced development of neutralizing antibodies
Neutralizing antibody responses were measured by an established reporter virus particle assay(13). Serum antibody responses peaked 2–4 weeks after the 2nd immunization (Fig. 2). A slow decline of serum neutralizing activity over time can be observed in animals that did not immediately become pregnant and thus had delayed ZIKV challenge. Since ZIKV challenge of pregnant animals at GD 30 occurred at variable times (4 to 369 days) after the 2nd immunization, there was a wide range of neutralizing activity at the time of ZIKV exposure (EC50 titers: 1:161 to 1:1,518) reflecting a real-world scenario where time is variable between vaccination and exposure, and providing an opportunity to assess correlates of protective immunity.
Fig. 2. Neutralizing antibody responses following ZIKV DNA immunization.
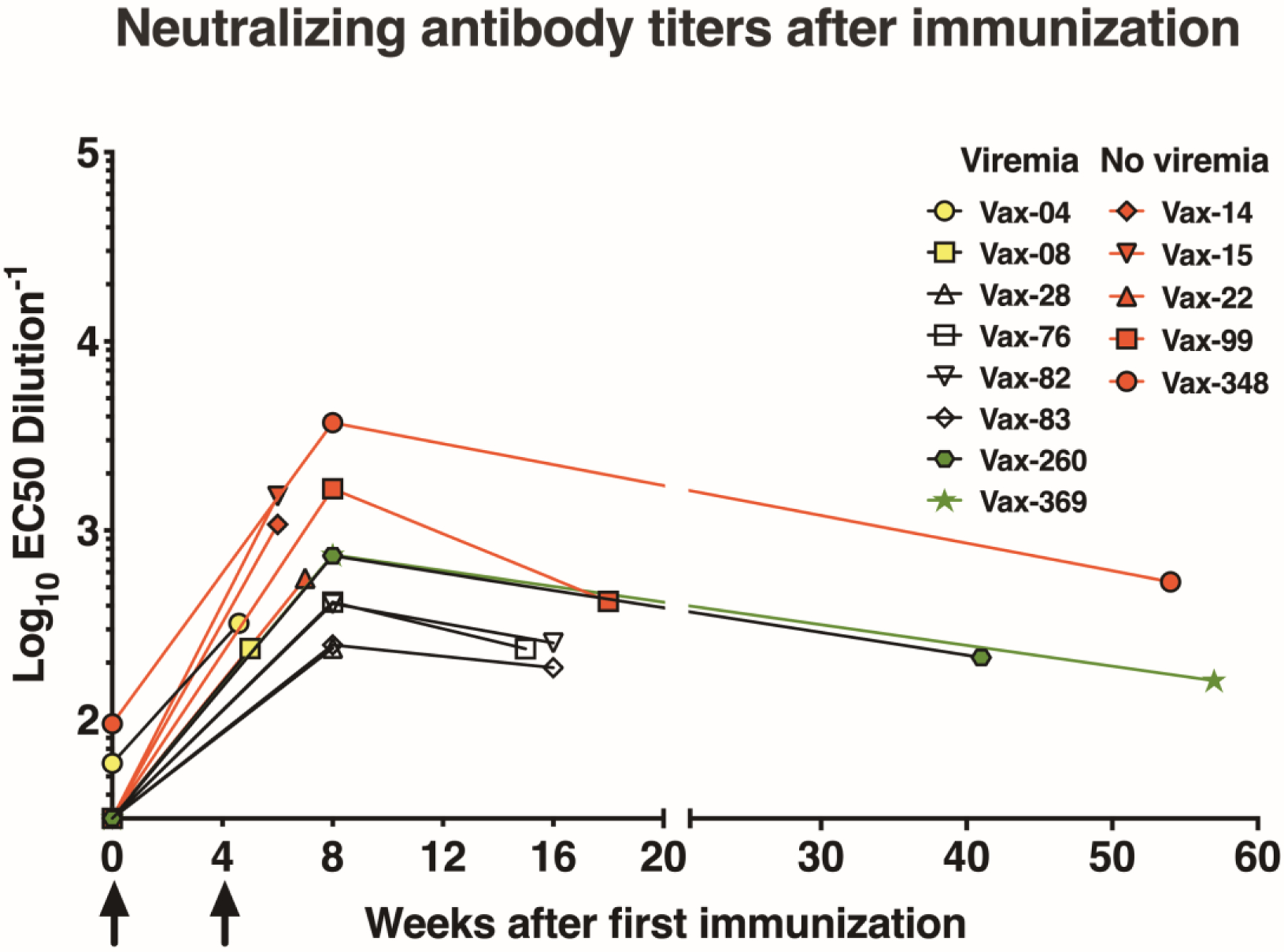
Neutralizing antibody responses in vaccinated animals (n=13) after two DNA immunizations (at weeks 0 and 4; arrows) and prior to ZIKV challenge. Neutralizing activity, expressed as reciprocal EC50 dilution, was assessed on the day of challenge and at week 8 if challenge occurred after week 8. The last data point for each animal represents the time of first ZIKV exposure.
Durability of vaccine efficacy in non-pregnant animals
Five vaccinated animals did not become pregnant despite multiple breeding attempts for a year. All animals had detectable neutralizing activity at 13 months post-vaccination. They were challenged with a single dose of ZIKV (1,000 PFU) 13 months after the last DNA immunization and had either no detectable viremia or transient viremia (Fig. S1). An IC50 serum neutralizing titer of greater than ~1:300 on day of ZIKV exposure protected against viremia.
Reduced viremia in vaccinated pregnant animals after ZIKV exposure
Following the first ZIKV inoculation at GD 30, all pregnant control animals reached peak viremia (range 3.5 to 6, mean 4.8 log10 viral RNA (vRNA) copies/ml plasma) within 2–5 days (Fig. 3A). Two dams had early fetal losses at day 5 and day 30 after the first ZIKV exposure (GD 35 and GD 60, respectively). Most unvaccinated control animals displayed prolonged viremia during pregnancy, with 8/11, 4/10, and 2/10 still viremic at day 21 (GD 51), day 60 (GD 90) and day 90 (GD 120) after the first virus inoculation, respectively, with the decrease in denominator due to early pregnancy loss. Vaccinated pregnant animals had reduced virus replication after ZIKV exposure. When viremia occurred in vaccinated animals, it was transient, as none of the vaccinated animals had viremia beyond 14 days after the first ZIKV inoculation (Fig. 3B). Five of the thirteen vaccinated pregnant animals had no detectable vRNA in plasma, and the remaining 8 animals had a 100-fold reduction in mean peak viremia compared to unvaccinated pregnant controls (range 1.5 to 5.4; mean 2.9 log10 vRNA copies/ml)(Fig. 3B). Of the 8 viremic animals, 2 animals were challenged within 4–8 days of the 2nd DNA immunization prior to peak antibody titers, and 2 animals were challenged 260–369 days after the 2nd DNA immunization when antibody responses had waned (table S1). The vaccinated animal with highest peak viremia (animal ID Vax-369, 5.4 log10 vRNA/ml) was challenged about one year after vaccination.. DNA vaccination significantly reduced maternal peak viremia and overall magnitude of viremia as measured by area-under-the-curve (AUC) analyses (p<0.001; Fig. 3C–D). For all control and vaccinated animals, once viremia was controlled, the 2nd and 3rd ZIKV inoculations (at GD 60 and 90 respectively) did not result in detectable viremia, consistent with earlier observations in nonpregnant macaques that a primary ZIKV infection induces protective immunity against re-infection (20, 21). These data demonstrate that DNA vaccination prior to pregnancy is able to prevent or reduce viremia in pregnant dams exposed to ZIKV.
Fig. 3. Viremia of pregnant rhesus macaques after ZIKV inoculation.
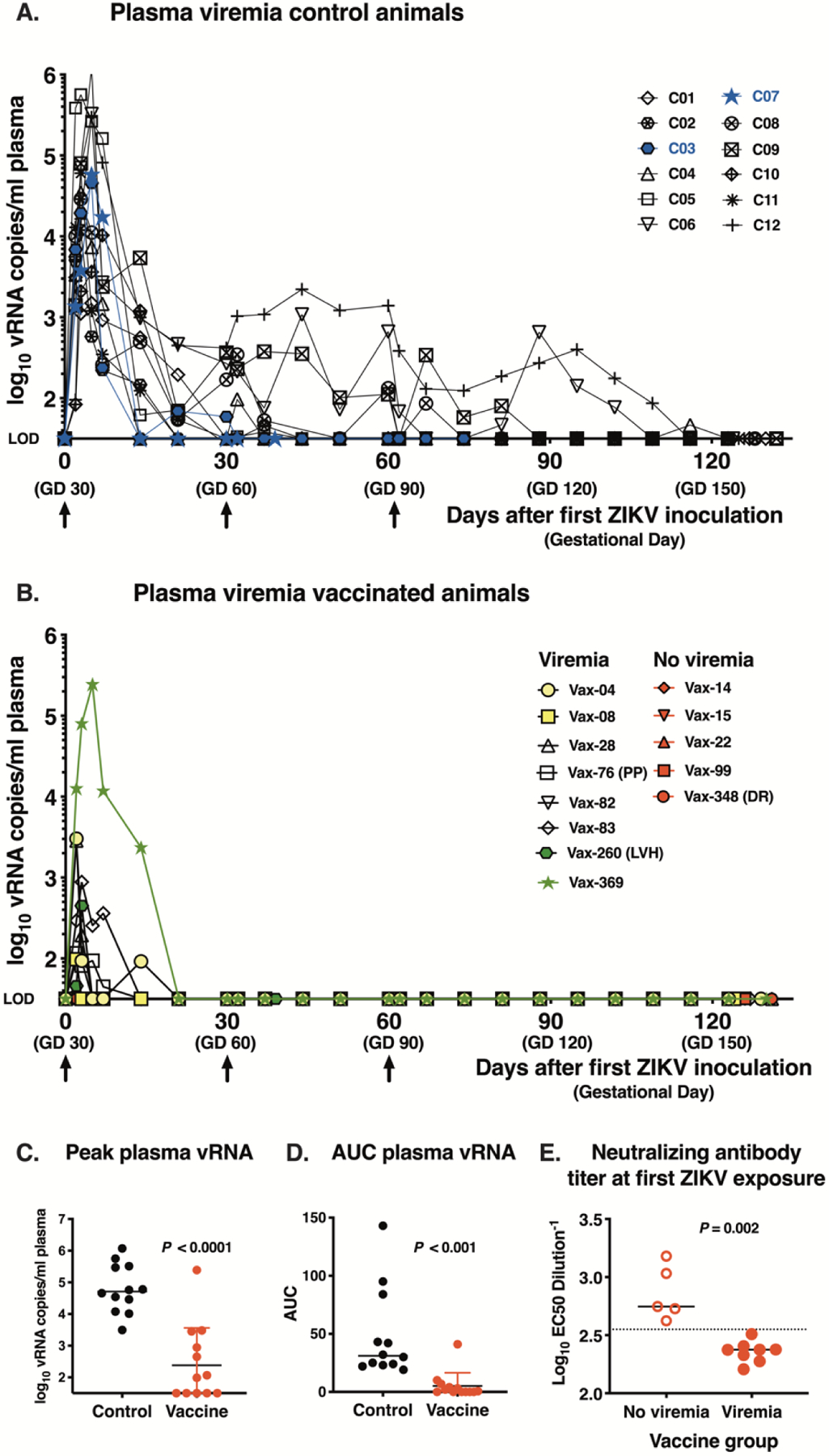
(A) Plasma ZIKV RNA (vRNA) in unimmunized control animals (n=12). Arrows show 3 ZIKV inoculations and blue shows the 2 animals with early fetal death. Each viremia value reported represents the mean of triplicate qRT-PCR measurements; the assay limit of detection (LOD) of ~1.5 log10 vRNA copies/ml is used as baseline for the Y-axis. (B) Plasma vRNA in vaccinated pregnant macaques (n=13). Yellow (n=2) and green (n=2) symbols indicate viremic animals for which the first ZIKV-inoculation occurred either very early (4–8 days) or late (260–369 days) after the 2nd DNA immunization, respectively. Red symbols are 5 vaccinated dams that after challenge had undetectable viremia. The three vaccinated animals with late fetal death are indicated: PP= placenta previa; LVH= left ventricular hypertrophy, DR=decidual reaction. (C) and (D). Peak viremia and area under the curve of log10-transformed plasma ZIKV RNA levels over time, respectively (both comparisons with two-tailed Mann Whitney test). (E) Neutralizing antibody titers (with indication of median) on day of first ZIKV challenge and viremia status of vaccinated pregnant animals (n=13) after ZIKV inoculation. The dotted line, drawn between the ‘not viremic’ and ‘viremic’ animals that were vaccinated is at EC50 dilution value of ~ 2.55 log10 (or titer of 1:355).
Whether a vaccinated animal had no viremia or transient viremia corresponded strongly with the anti-ZIKV neutralizing activity in plasma on the day of first ZIKV exposure. Aviremic animals had higher neutralizing activity than those with transient viremia (p=0.002), and a serum IC50 titer of ~ 1:355 was the apparent threshold level that prevented viremia (Fig. 3E). This level of vaccine-induced neutralizing activity is similar to the mean IC50 titer of 1:304 achieved at the dose regimen used in the phase 2b efficacy trial of VRC5283 using the same assay (14). Neutralizing activity on the day of first challenge correlated negatively with both the magnitude and duration of viremia (AUC) (Fig. S2).
Augmented neutralizing antibody responses after ZIKV exposure
After the first ZIKV challenge, all vaccinated animals, including the aviremic ones, mounted rapid anamnestic antibody responses, which declined again towards the end of gestation (Fig. 4A). This suggests that the absence of detectable viremia was not a sign of sterilizing immunity against the inoculum. This rapid anamnestic antibody response in which all vaccinated animals reached neutralizing antibody titers > 1:2,000 likely explains the absence of viremia following the 2nd and 3rd ZIKV inoculation. The control animals mounted rapid primary antibody responses after ZIKV inoculation (Fig. 4B). Towards the end of gestation, the control animals had higher neutralizing activity than the vaccinated animals, which may be explained by the increased antigenic stimulation associated with the prolonged viremia; in fact, the 4 control animals with the most prolonged viremia were also among the ones with the highest neutralizing antibody titers at the end of gestation (Fig. 4C). Titers of neutralizing antibodies in fetal cord blood were similar to those in their dams, reflecting transplacental antibody transfer towards the end of gestation (Fig. 4D).
Fig. 4. Neutralizing antibody responses in pregnant macaques after ZIKV inoculation.
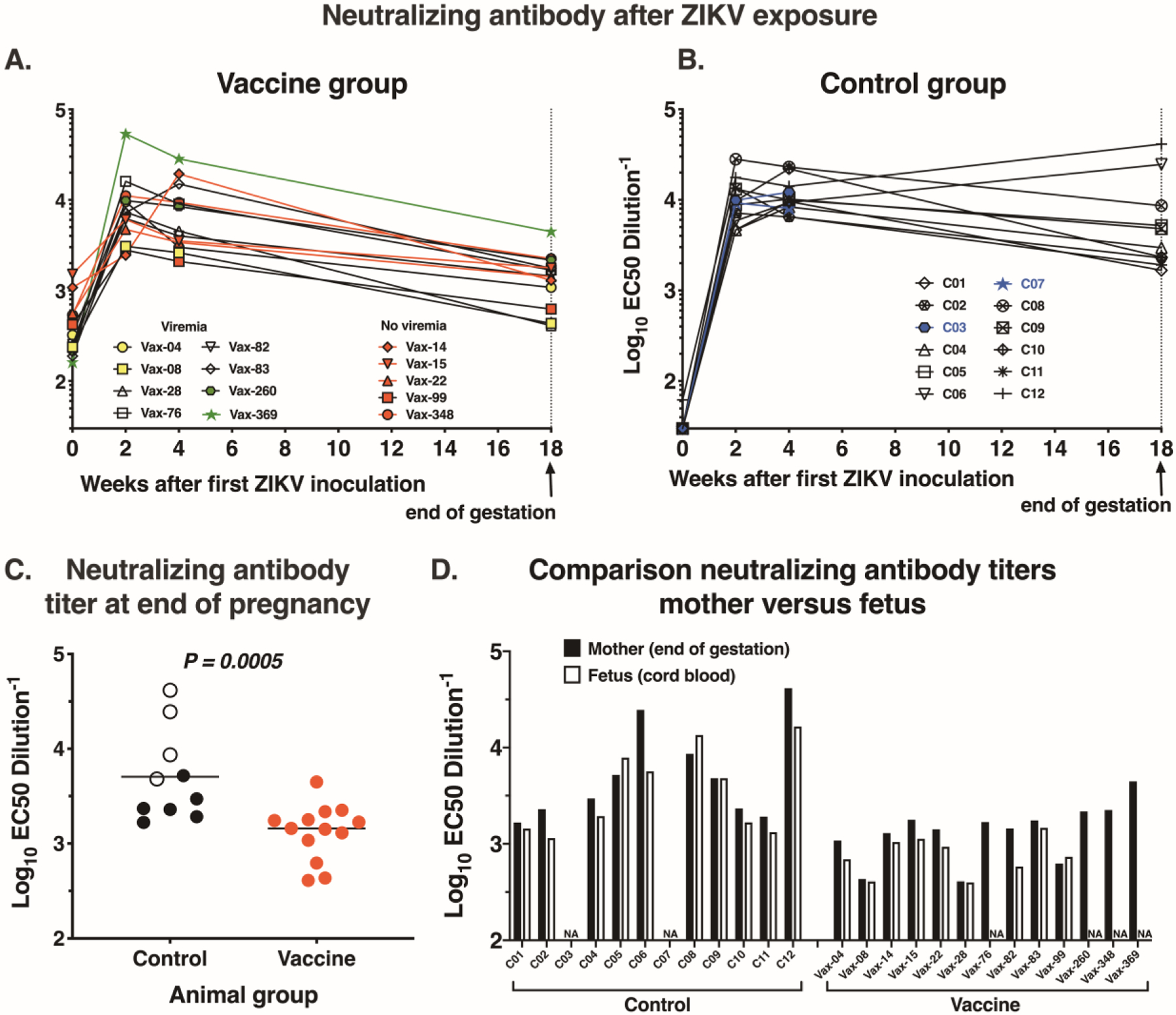
(A) and (B) Neutralizing antibody responses in vaccinated pregnant animals (n=13) and control pregnant animals (n=12) after ZIKV inoculation. (C) Comparison of neutralizing antibody titers (with median, indicated by horizontal lines) between 10 control animals (after exclusion of 2 animals with early fetal loss) and 13 vaccinated animals at end of gestation (two-tailed Mann-Whitney test). Open circles indicate the 4 control animals with most prolonged viremia (> 60 days after first ZIKV inoculation). (D) Comparison of neutralizing antibody titers in serum from the pregnant animals at end of gestation and fetal cord blood; NA indicates not available.
Development of virus-binding IgG antibodies and association with viremia
Virus-binding IgG antibody responses, measured by a whole-virion ELISA, followed similar patterns as the neutralizing antibodies. Vaccine-induced virion-binding IgG titers peaked shortly after the second DNA immunization, declined for animals with delayed challenge, and increased after ZIKV challenge to reach levels similar to those of the ZIKV-infected control pregnant macaques (Fig. S3A–C). For the vaccinated animals, although virion-binding IgG titers on the day of challenge correlated with presence or absence of viremia, they did not correlate with the magnitude of viremia (Fig. S3D–F). Thus, neutralizing activity was a better predictor of viremia than virion-binding IgG titers.
Vaccine-induced ZIKV-specific T cell responses
Following immunization, the vaccinated animals mounted detectable CD4+ T cell responses to Zika virus envelope protein E as measured by intracellular cytokine staining; no significant CD8+ T cell responses were detected (Fig. 5). The CD4+ T cell responses were significantly different from baseline (i.e., pre-immunization) at day of first ZIKV challenge (P=0.01) and one week after challenge (P=0.036). In contrast, the control animals had almost no detectable CD4+ T cell responses one week after challenge. Indeed, these responses were statistically different between groups at this time point (P=0.013). These results indicate that vaccinated animals had increased antiviral T cell responses early after challenge relative to the controls.
Fig. 5. T cell responses to Zika virus E protein in vaccine and control groups measured by intracellular cytokine staining.
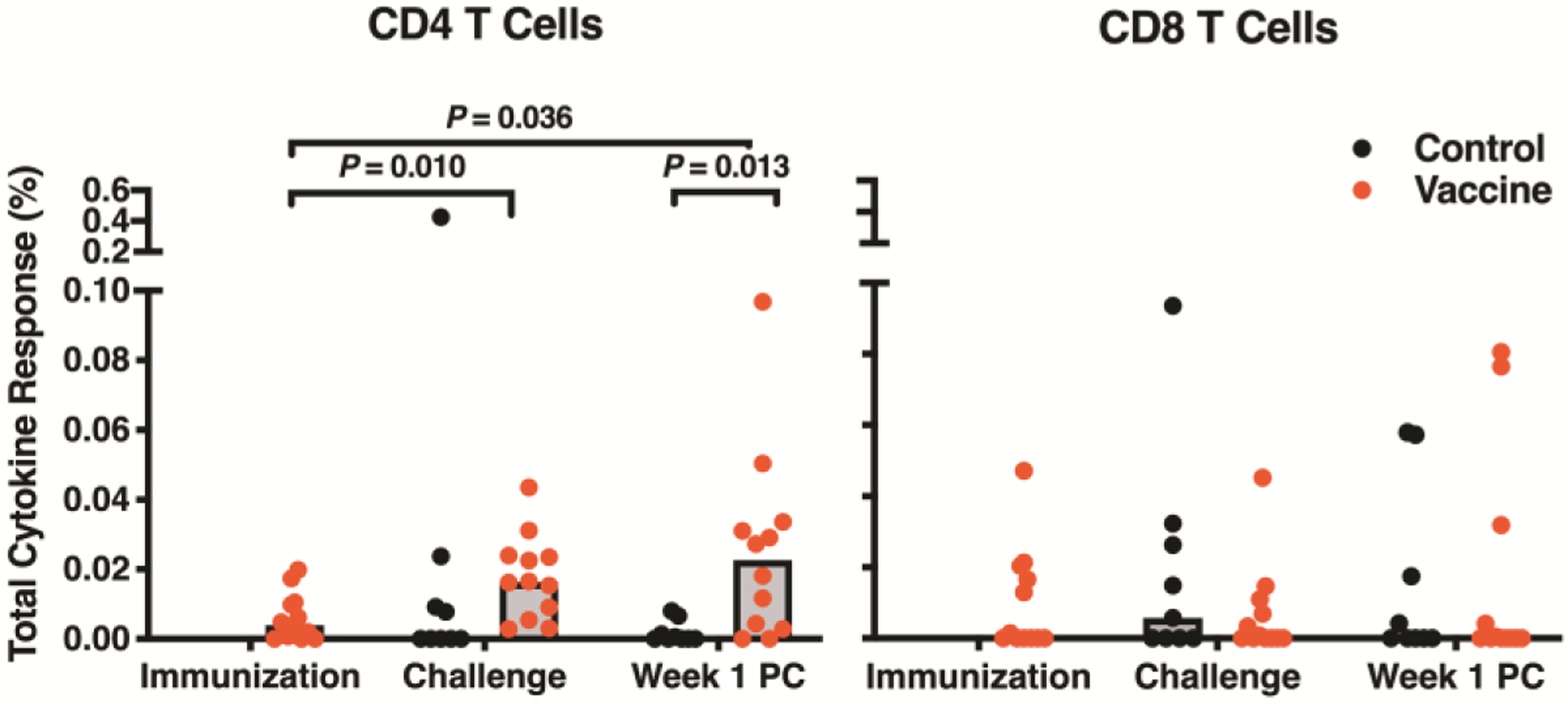
The frequencies of total CD4 and CD8 T cells producing interleukin 2, interferon γ, and tumor necrosis factor α, or a combination of these cytokines, were measured at time of immunization (vaccinated animals only), day of first ZIKV challenge, and week one post-challenge (PC). Boolean gating was performed, responses were background subtracted, and all cytokine positive gates were summed using SPICE software to calculate the total frequency of cytokine-positive T cells responding to the E peptide pool. Samples with less than 35,000 events and 35% viability were excluded from analysis; therefore, twelve vaccinated animals and nine control animals are depicted. Statistical significance was calculated by Student’s t-test. Bars are drawn at the median.
Effect of vaccine on fetal clinical outcomes
Two control pregnant animals (C07 and C03) had early fetal death (detected as an absence of fetal heartbeat by ultrasound) at 5 days and 30 days after the first ZIKV inoculation (i.e., GD 35 and 60). Both dead fetuses were removed via hysterotomy and had abundant vRNA in fetal-placental tissues (Fig. 6), and evidence of deciduitis (table S2–3). These observations are consistent with a growing body of evidence of increased fetal loss in ZIKV-infected pregnant nonhuman primates (5) and humans (18, 19). Although both these control animals had detectable viremia on the day of hysterotomy, viremia was undetectable at the next time point of blood collection (2 to 7 days later); this is consistent with earlier data from nonhuman primate studies and human studies, that the likely source of the prolonged maternal viremia is the placental-fetal compartment (6, 11, 22).
Fig. 6. Detection of ZIKV RNA in fetal macaques from control or vaccine group pregnancies.
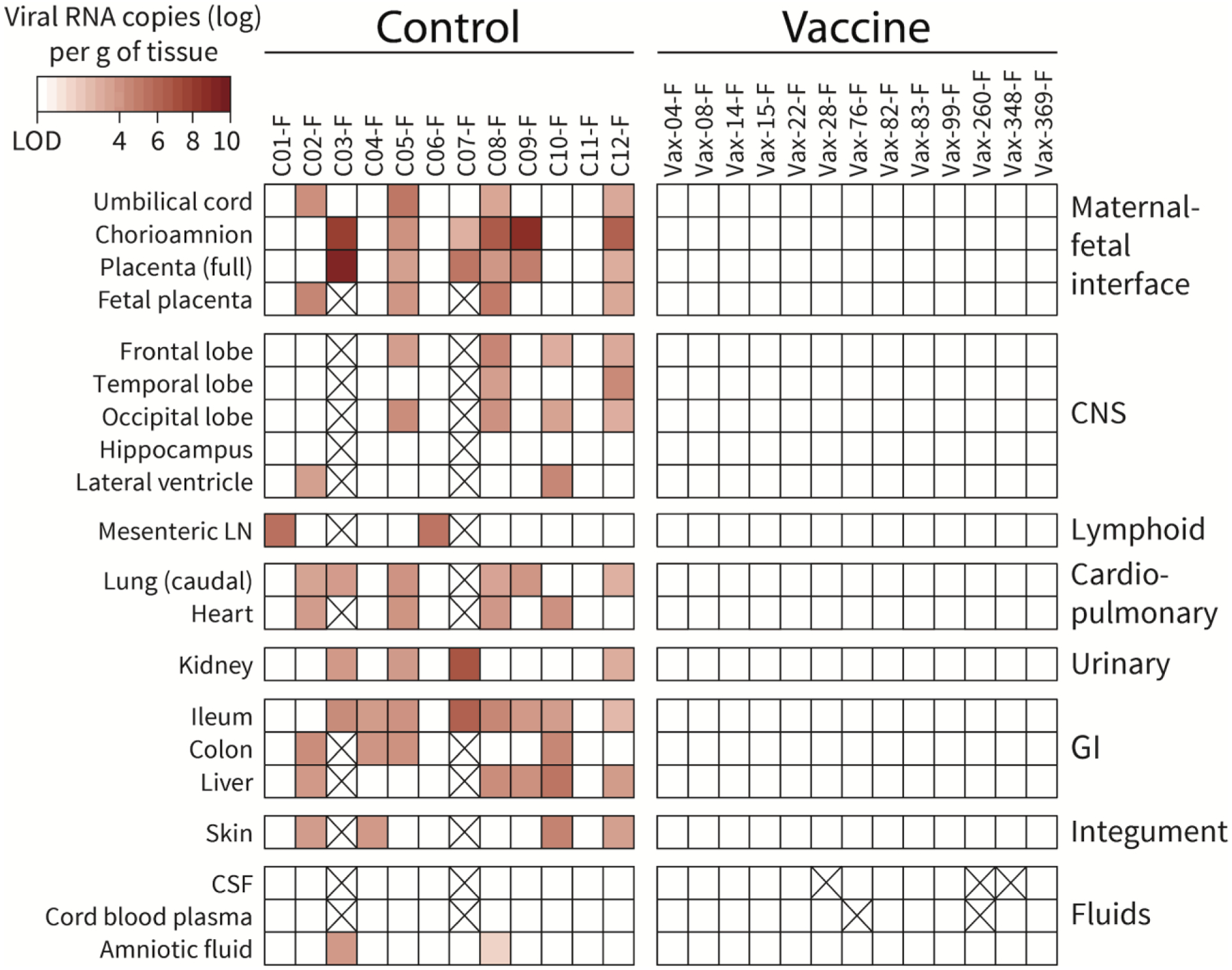
Tissues are categorized per system; CNS= central nervous system; GI=gastrointestinal. Full placenta refers to the full thickness of the primary placental disc (i.e., chorioamnion, villous placenta, basal plate and decidua); fetal placenta is the villous placenta and chorionic plate. Viral RNA was detected by qRT-PCR. The intensity of red highlights the quantity of viral RNA detected. Samples that were not available are crossed out.
In contrast, none of the 13 vaccinated pregnant animals had early fetal loss. There were three complications late in pregnancy (≥ GD 146), which based on the available evidence (including onset of detection, predisposing factors, lack of detectable vRNA in fetal or maternal tissue, and pathological findings) were considered unrelated to ZIKV infection: animal Vax-76, placenta previa with placental disruption, stunted fetal growth and death; Vax-260, left ventricular hypertrophy with cardiac arrest of the dam (23); Vax-348: marked decidual reaction with placental insufficiency.
Similar to previous ZIKV studies in pregnant macaques (6, 10, 11), no development of gross microcephaly was detected in fetuses in the current study. Fetal growth was monitored via weekly ultrasound monitoring, and a computed tomography (CT) scan at the end of gestation. In addition, at the time of experimental hysterotomy and fetal euthanasia, measurements of head, brain and body development were collected. With exception of the fetus with placenta previa and stunted fetal growth, no differences could be detected between the control and vaccine groups, and available historical control data (Fig. S4–S5); an exception was that the ZIKV-infected control group had slightly lower placental weights (Fig. S5A), but as placental weight did not correlate with placental histopathology scores, its biological significance is unclear.
Detection of ZIKV RNA in fetal and maternal tissues
Six control animals had detectable ZIKV RNA in at least 1 amniotic fluid sample. In contrast, none of the vaccinated animals had detectable vRNA in amniotic fluid samples (Fig. S6). Consistent with earlier observations in fetal macaques (6), none of the fetuses in the current study that were carried to term, including the control fetuses, had detectable vRNA in cerebrospinal fluid or cord blood plasma, suggesting the limited value of these fluid samples in diagnosing congenital ZIKV infection (Fig. 6).
Fetal tissues from the maternal-fetal interface, CNS, lymphoid, cardiopulmonary, genitourinary, gastrointestinal and integument system were tested for ZIKV RNA using qRT-PCR. A caveat of any tissue analysis is that it represents a single time point. Except for the cases of early fetal losses, tissue harvesting was done approximately 4 months after maternal ZIKV inoculation; thus, an absence of detectable ZIKV RNA does not preclude the possibility of an earlier infection or an infection below the limit of detection of the assay. We designated a fetus as ZIKV-infected if at least 1 tissue had detectable vRNA. Eleven of the 12 control fetuses (including the 2 early fetal losses) were diagnosed as being ZIKV-infected with an average of 7 tissues per fetus having vRNA (Fig. 6, Fig. S7). Strikingly, none of the 13 vaccine group fetuses had a tissue with detectable viral RNA. Thus, the vaccine significantly reduced fetal infection rates from 91% to 0% (P<0.0001; 2-sided Fisher’s Exact test). Previous studies have observed detectable vRNA in lymphoid tissues of pregnant macaques (6), so viral load analysis was also performed on five maternal tissues: spleen, mesenteric lymph node (LN), obturator LN, inguinal LN, and uterus. Whereas all 12 control dams still had viral RNA in lymphoid tissues, only 2 vaccinated dams that had early viremia had detectable vRNA, each in one lymph node (Fig. S8). Thus, tissue analysis demonstrated that ZIKV DNA vaccination decreased the presence of vRNA in maternal lymphoid tissues and prevented detection of vRNA in fetal tissues.
Evaluation of histopathology
Histopathology was evaluated and scored by pathologists blinded to the study groups. Six of the 12 control fetuses had moderate-to-severe pathology scores in brain, placenta or amnion (Fig. 7A). Brain and placental lesions were similar to those described earlier in ZIKV-infected fetuses (5–11, 22), including segmental loss of ependymal cell lining, calcification and gliosis; trophoblastic cell loss, necrosis, and neutrophilic inflammation of the fetal placenta (Fig. S9–11). The control fetus with highest pathology score (C08-F) was also among the ones with highest vRNA detection in fetal and placental tissues, further supporting a direct role of ZIKV infection. On the other hand, concentrations of cytokines and chemokines in amniotic fluid did not correlate with placental pathology (Fig. S12). In contrast to the control fetuses, only 2 fetuses of the vaccine group had a pathology score of moderate-to-severe for any tissue (Fig. 7A). One of these fetuses was from animal Vax-348, which was aviremic, but had decidual reaction with placental insufficiency (Table S2–4); its fetal brain lesions were consistent with hypoxia rather than typical ZIKV-induced lesions. The second vaccine group fetus with a severe brain pathology score, and lesions consistent with ZIKV infection, was Vax-82F. Its dam had only a single time point of detectable viremia (1.5 log10 vRNA copies/ml at day 3 after the first ZIKV inoculation), but no vRNA could be detected in fetal tissues; although it is possible that this fetus may have experienced an undetected early ZIKV infection. Overall, vaccine group fetuses had lower brain pathology scores than the control group, although this was not stastically significant (Fig. 7B). For the control fetuses, a higher fetal pathology score was associated with the persistence of maternal viremia (Fig. 7C), which supports the contention that the placental-fetal compartment is the source; such association between maternal viremia and fetal pathology score was not observed for the vaccine group fetuses (Fig. 7D–E). Consistent with previous ZIKV studies (6, 24), there was no ZIKV-attributable pathology in maternal tissues (Table S5).
Fig. 7. Pathology scores are lower in fetuses from vaccinated versus control dams.
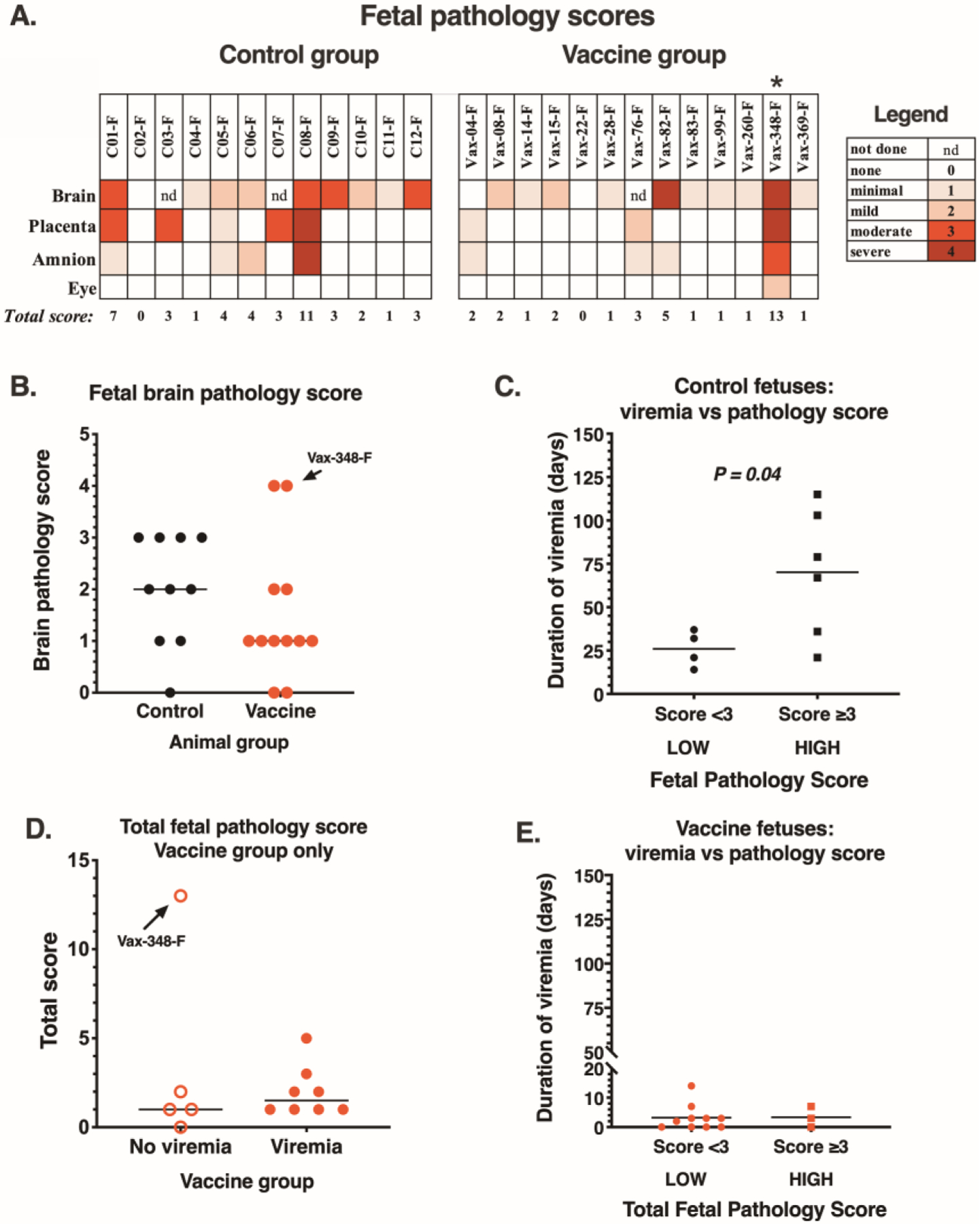
(A) Fetal brain (13–20 sections/animal), placenta (1–2 sections/animal), amnion (1–2 sections/animal) and eye (1 section/animal) were evaluated blindly by 2 pathologists and were assigned a score from 0 to 4 based on severity (see tables S2–3 for more details, including the scoring system). nd indicates not done (due to fetal death with autolysis). * indicates Vax348-F that was removed via hysterotomy on GD 148 due to fetal distress. (B) Comparison of fetal brain pathology scores between control (n=10) and vaccine group (n=12) fetuses (P = 0.10; one-sided Mann-Whitney). Horizontal lines represent median value. Vax-348-F (with placental insufficiency/brain hypoxia) is indicated by an arrow. (C) Comparison of the duration of maternal viremia with pathology score in the 10 control fetuses that survived to the end of gestation (P = 0.04; one-tailed Mann-Whitney). . (D) Comparison of total fetal pathology score between vaccinated dams with or without viremia; P =0.37 and 0.16 (one-tailed Mann-Whitney), with and without inclusion of Vax-348-F with placental insufficiency, respectively. (E) Duration of maternal viremia in the 13 vaccinated dams in comparison to fetal pathology score (P = 0.46; one-tailed Mann-Whitney).
Discussion
The current study demonstrates the efficacy of a ZIKV DNA vaccine expressing the pre-membrane and envelope proteins of ZIKV, initiated prior to pregnancy, in a nonhuman primate pregnancy model. In comparison to unimmunized animals, vaccinated pregnant animals had reduced viremia and improved fetal outcomes.
Although ZIKV infection of pregnant macaques recapitulates many features of ZIKV infection in pregnant women, this animal model and our experimental design have inherent limitations. Due to financial and logistical constraints, animal group sizes are necessarily small. We inoculated animals at gestational day 30, shortly after pregnancy was confirmed via ultrasound; thus, our study design does not reflect women who are exposed to ZIKV earlier in gestation, when infection may be more harmful to fetal development. In addition, to make optimal use of the vaccinated animals, we aimed for high pregnancy rates, by repeated time-mated breedings for up to a year. Humans and animals that have difficulty conceiving have higher risks for developing pregnancy complications (25). Thus, vaccinated animals that became pregnant late (such as Vax-348, that had early evidence of abnormal placental development leading to progressive placental insufficiency and late fetal loss) had inherently higher risks of complications. In contrast, the control pregnant animals were enrolled as soon they became available from our breeding colony, which may have created a bias for healthy control pregnancies at the onset. Despite this potential bias, our study demonstrates that vaccinated animals fared better than the control animals. Other limitations of our study design are that the many experimental procedures (including sedations and amniocentesis) performed during pregnancy may have affected placental and fetal development, as reflected in minor pathological lesions even in animals that appeared to be uninfected. Finally, in order to acquire timely data, we tested the durability of protection only up to 1 year after immunization, and we terminated pregnancies at the end of gestation (to allow access to placental and fetal tissues for analysis), rather than monitoring infants after birth for normal development into adulthood.
Despite these limitations, this study provides further insights in the pathogenesis of ZIKV infection in this nonhuman primate pregnancy model. Our data suggest that vertical transmission is most likely to occur during primary infection, which is thus the critical target for preventive interventions. Maternal VRC5283 immunization initiated prior to pregnancy protected against fetal ZIKV infection. Vaccinated animals had fewer early fetal losses and had reduced placental and fetal pathology. Vaccinated animals also had reduced peak viremia and were protected against persistent ZIKV viremia which was associated with fetal infection in control animals. This study suggests that sterilizing immunity in the mother is not required for protection against CZS and that prevention of persistent viremia in pregnant women may be an important endpoint to consider in future clinical trials.
In this study, vaccine protection correlated with serum neutralizing activity and increased antiviral T cell responses early after challenge. Although the antibody responses were detectable for at least one year, they gradually waned, and animals exposed to ZIKV late after immunization were more likely to experience transient viremia. A study in nonpregnant macaques comparing different ZIKV vaccine platforms found that some vaccines had better durability of neutralizing antibody responses one year after immunization, and this was associated with protection against viremia (26). Thus, more studies are needed understand the immunological basis of preventing viremia and persistent viremia in pregnant women, with the goal of developing ZIKV vaccine modalities that induce effective and durable immunity.
In conclusion, the current study highlights the usefulness of the nonhuman primate pregnancy model to gain further insights into the pathogenesis of vertical ZIKV transmission and to explore intervention strategies. By demonstrating the efficacy of VRC5283 in this animal model, these findings will help guide the clinical development of future ZIKV vaccine strategies.
Materials and Methods
Study design
To evaluate the efficacy of the VRC5283 DNA vaccine in a pregnant macaque model, one group of 18 macaques was immunized with VRC5283 prior to time-mated breeding, with the goal of having at least 12 pregnancies. A second group consisted of 12 control pregnant animals enrolled at time of pregnancy confirmation. Group sizes were determined using power analysis: assuming control animals would have a 75% fetal infection rate, a group size of 12 animals would have 95% power to detect a 80% reduction in fetal infection rate in the vaccine group (one-sided p=0.05). Pregnant animals were inoculated three times with ZIKV, and monitored closely. At the end of gestation (GD 155–162), surviving fetuses were collected via hysterotomy for detailed tissue analysis. All pregnancies, including those with complications that may be unrelated to ZIKV, were included in the analysis. Immunized animals that did not become pregnant were ZIKV-inoculated once ~ 13 months after the last immunization, and euthanized ~2 weeks later. Morphometry, virology, serology and pathology analyses were performed blinded. Primary data are reported in data file S1.
Animals and care
All adult female rhesus macaques (Macaca mulatta) in the study were born and raised in the conventional (i.e., not specific pathogen free) breeding colony at the California National Primate Research Center (CNPRC). Whereas none of the animals were positive for type D retrovirus, SIV or simian lymphocyte tropic virus type 1, some animals were seropositive for West Nile virus due to prior outdoor housing. All animals had prior successful pregnancies (range 1–7).
The CNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal care was performed in compliance with the 2011 Guide for the Care and Use of Laboratory Animals provided by the Institute for Laboratory Animal Research. Macaques were housed indoor in stainless steel cages (Lab Product, Inc.) whose sizing was scaled to the size of each animal, as per national standards, and were exposed to a 12-hour light/dark cycle, 64–84°F, and 30–70% room humidity. Animals had free access to water and received commercial chow (high protein diet, Ralston Purina Co.) and fresh produce supplements. The study was approved by the Institutional Animal Care and Use Committee of the University of California, Davis (protocol number 19695).
Immunizations with VRC5283 vaccine
The DNA vaccine plasmid VRC5283 was based on the H/PF/2013 French Polynesian virus isolate (GenBank accession AHZ13508.1) and has been previously described (13). Briefly, the DNA plasmid encodes the ZIKV prM and E proteins under the control of the CMV immediate early promoter and uses the Japanese encephalitis virus (JEV) prM signal sequence previously used for a WNV DNA vaccine (27). Non-pregnant adult rhesus macaques were immunized with 2 doses (1 mg each, in a volume of 0.5 ml) of VRC5283, administered 4 weeks apart in the quadriceps muscle region (right side for the 1st, and left side for the 2nd immunization) via the same needle-free Stratis injection device (Pharmajet) as that used in the previously described NHP studies (13) and clinical trials (14).
Time-mated breeding and pregnancy selection
For time-mated breeding, the female macaques were monitored for reproductive cycle and at the time of optimal receptiveness, temporarily housed with breeding males to induce pregnancy. Rhesus macaques are seasonal breeders; by the time the first immunization started (February 2017), it was already late in the breeding season, which is known to be associated with a lower rate of successful conception. Therefore, instead of waiting until after both immunizations, we decided to start breeding immediately after the first immunization. Nine animals became pregnant within 80 days of the first immunization. Animals that were not pregnant 80 days after the first immunization were started on treatment with the fertility drug clomiphene (oral, 25 mg/dose) administered once daily for 4 days from days 5 to 8 of each menstrual cycle (28); an additional 4 vaccinated animals became pregnant. Unimmunized control pregnant animals were selected from the CNPRC’s timed-breeding colony as they became available (without clomiphene treatment). Gestational ages were determined from the menstrual cycle of the dam and the fetus length at initial ultrasound compared to growth data in the CNPRC rhesus macaque colony. Fetal health and viability were rechecked via ultrasound immediately before the first ZIKV inoculation (~ GD 30) and regularly thereafter.
Virus inoculations
Each pregnant animal was inoculated with virus 3 times. The normal gestation of rhesus macaques is 165 days; inoculations occurred at approximately GD 30, 60 and 90, corresponding to first and second trimester of human gestation. The GD 30 and GD 90 inoculations were done with a 2015 Puerto Rico isolate (PRVABC-59; GenBank KU501215), whereas the GD 60 inoculation was done with a 2015 Brazil isolate (strain Zika virus/H.sapiens-tc/BRA/2015/Brazil_SPH2015; GenBank KU321639.1), the same strain as tested earlier in pregnant and non-pregnant animals (6, 24). The use of two strains was intended to mimic an endemic area where different variants may circulate. Aliquots of both virus stocks were kept frozen in liquid nitrogen, and a new vial was thawed shortly before each inoculation. For each inoculation, the inoculum was adjusted to 1,000 PFU in 0.5 ml of RPMI-1640 medium, then kept on wet ice and injected subcutaneously to simulate the route of mosquito feeding. This dose is higher than the typical dose received from a mosquito bite, which in laboratory experiments was ~30 PFU for both the ZIKV isolates used in this study (29).
Sample collection and clinical observations and monitoring
Macaques were evaluated twice daily for clinical signs of disease including poor appetence, stool quality, dehydration, diarrhea, and inactivity. When necessary, macaques were immobilized with ketamine hydrochloride (Parke-Davis) at 10 mg/kg and injected intramuscularly after overnight fasting. Animals in both the ZIKV treated and placebo cohorts were sedated at days 0 (time of first virus inoculation; approximately GD 30), 2, 3, 5, 7, 14, 21, 30 (2nd ZIKV inoculation at GD60), 32, 37, 44, 51, 60 (3rd ZIKV inoculation; GD90), 62, 67, and then weekly until time of euthanasia between GD155–162, for sample collection and ultrasound monitoring of fetal health. EDTA-anti-coagulated blood was collected using venipuncture at every time point for complete blood counts (with differential count), and a separate aliquot of blood was centrifuged for 10 min at 800 g to separate plasma from cells. The plasma was spun an additional 10 min at 800 g to further remove cells, and aliquots were immediately frozen at −80°C. The cellular blood fraction was diluted with Dulbecco’s phosphate buffered saline (DPBS) and layered on lymphocyte separation medium (MP Biomedicals) and spun for 30 m at 800 g to isolate peripheral blood mononuclear cells that were washed and then cryopreserved for later analyses. At selected time points, blood was collected without anti-coagulant to provide serum.
Ultrasound guided amniocentesis was conducted starting at day 14 after inoculation (GD44), and then at all time points listed above with exception of days 32 and 62 after initial infection. The amniocentesis was conducted using sterile techniques by inserting a 22 gauge, 1.5 inch spinal needle into the amniotic sac. The fetal heart rate was obtained before and after amniocentesis. The area of umbilical entry through the amniotic sac was always avoided to prevent damage to the umbilical arteries and vein. Whenever possible, placental tissue was avoided during the collection of amniotic fluid. Amniotic fluid was spun to remove cellular debris, and the supernatant was aliquoted and immediately cryopreserved at −80°C for viral RNA assays.
Fetal Measurements
Fetal measurements were collected during pregnancy after dams were sedated with ketamine hydrochloride (10 mg/kg) for sonographic assessments and amniocentesis. The biparietal diameter (BPD) was measured from ultrasound (US) images collected by veterinary staff. Images were analyzed by a researcher blind to the group assignment and ZIKV infection status of each animal. BPD was drawn in ImageJ and rescaled into metric units using the scale available on each image that was produced by the US machine. The CNPRC colony BPD mean was derived from historical data (30). Computerized tomography (CT) scans were performed on a GE Medical Systems machine (Discovery 610), using a helical head sequenced acquisition protocol. After a localizer scan, a scan with the following parameters was deployed: 10.0 s 99.38 mm 0.62 mm 20.0 mm 1.0 1 X-ray source. CT X-ray source parameters: 1 120.0 kV 280.0 mA 280.0 mA 2 s; CT Dose 100.79 mGycm IEC Head Dosimetry Phantom 1007.88 mGycm. CT images were analyzed by a researcher blind to the group assignment and infection status of each animal. Average skull volumes were computed in Osirix on all three imaging orientations (axial, sagittal, and coronal) and then averaged. Length of the clavicles was computed from the CT images and used to create a skull-to-shoulder ratio. At necropsy of the fetus, fetal and organ weights were measured with a scale, and crown-rump length, biparietal diameter, head height, head length, and femur length, were derived from caliper measurements.
Necropsy and tissue collection
All necropsies were performed by a board-certified anatomic pathologist and a pathology technician. For animals that had early fetal loss, the procedures described below were performed to the best extent possible based on fetal size. Hysterotomy was performed by a veterinary surgeon on the pregnant macaques under inhalation anesthesia. After collection of amniotic fluid and cord blood, the fetus was euthanized with an overdose of sodium pentobarbital (≥120 mg/kg), and a detailed tissue dissection was performed. Except for some animals that had early fetal loss and were maintained several weeks after removal of the fetus, all other dams were euthanized shortly after their fetus with an overdose of sodium pentobarbital for tissue collection.
Each tissue was grossly evaluated in situ, and then excised, with further dissection with separate forceps and razor blades for each tissue to minimize risks for cross-contamination. Tissues were collected for viral analyses in RNAlater (according to manufacturer’s instructions, Thermo Fisher); extra available samples were snap-frozen and stored at −70°C. Tissues were also preserved in 10% neutral buffered formalin and routinely paraffin-embedded and slides were created and routinely stained with hematoxylin and eosin (H&E).
Isolation and quantitation of viral RNA from fluids and tissues for determination of infection status
ZIKV RNA was isolated from samples and measured in triplicate by qRT-PCR according to methods described previously (24) modified to increase the initial volume of sample tested from 140 to 300 μl (when available) to increase sensitivity. According to the volume available, the limit of detection for plasma and amniotic fluid ranged from 1.5 to 2.3 log10 viral RNA copies per ml fluid. RNAlater-preserved tissues were homogenized to a liquid state with glass beads (Fisher Scientific) or a 5 mm steel ball (Qiagen). For tissues, the limit of detection (LOD) varied depending on the weight of tissue sampled with a mean of 3.53 log10 RNA copies/g of tissue, determined according to the calculations described in (31).
For maternal plasma, amniotic fluid, fetal cord blood and fetal cerebrospinal fluid, all samples from both control and vaccine group animals that could be collected were tested. For the maternal tissue samples, as maternal infection was not the main focus of this study, a limited selection of lymphoid tissues most likely to have viral RNA based on prior studies was tested (6, 24), with addition of uterus due to proximity to the placenta and fetus. For the analysis of fetal tissue samples for viral RNA, we started our analysis with the control fetuses, and tested 26 fetal and maternal-fetal-interface (MFI) tissues, consisting of tissues most likely to contain viral RNA based on our prior study (6). Next we generated a heatmap and selected a list of 17 tissues consisting of those most likely to be infected and diagnosing the most infections in the control fetuses, with addition of brain regions most likely to have histological lesions in the current study (such as hippocampus), or found to be infected in human fetuses affected by CZS (32). The criteria to define infection were used systematically for both control and vaccine group fetuses: a fetus was considered ZIKV-infected if at least one tissue had a consistent qRT-PCR signal (3/3 replicates positive for viral RNA); this situation applied to 11 of the 12 control fetuses. In a rare case, when a fetus only had a sample with inconsistent qRT-PCR signal (1 out of 3 replicates positive) while all other tissues were negative, retesting was performed, generally on a different aliquot. If the retest result was negative (3/3 replicates), the sample was considered not infected, and the fetus was considered not infected; one control fetus (C11-F) and 2 vaccine group fetuses met these criteria and were therefore considered uninfected.
Detection of neutralizing antibodies in macaque serum
Reporter virus particles (RVPs) incorporating the structural proteins of ZIKV strain H/PF/2013 were produced by complementation of a sub-genomic GFP-expressing replicon derived from a lineage II strain of WNV as previously described (13, 33). Briefly, HEK-293T cells were transfected with plasmids encoding the replicon and structural genes at a 1:3 ratio by mass using Lipofectamine 300 (Invitrogen), followed by incubation at 30°C. RVP-containing supernatant was harvested from cells at days 3–6 post-transfection, filtered through a 0.22 μm filter, and stored at −80°C. To determine virus titer, two-fold dilutions of RVPs were used to infect Raji cells that express the flavivirus attachment factor DC-SIGNR (Raji-DCSIGNR) {Davis, 2006 #6394} in duplicate technical replicates at 37°C. GFP-positive infected cells were detected by flow cytometry 2 days later. In subsequent neutralization assays, RVPs were sufficiently diluted to within the linear range of the virus-infectivity dose-response curve to ensure antibody excess at informative points.
For detection of neutralizing antibodies, ZIKV RVPs were mixed with serial dilutions of heat-inactivated macaque serum for 1 h at 37°C, followed by infection of Raji-DCSIGNR cells in duplicate technical replicates. Infections were carried out at 37°C and GFP-positive infected cells quantified by flow cytometry 2 days later. Results were analyzed by non-linear regression analysis to estimate the dilution of serum required to inhibit 50% of infection (EC50). Serum samples were initially tested at a starting dilution of 1:60 (based on the final volume of cells, virus, and serum per well), which was designated as the limit of detection. EC50 titers estimated as <60 by non-linear regression analysis were reported as half the limit of detection (a titer of 30).
Detection of ZIKV-specific binding antibodies in macaque plasma
High-binding 96-well ELISA plates (Greiner) were coated with 30 ng/well of 4G2 monoclonal antibody, produced in a mouse hybridoma cell line (D1-4G2-4-15, ATCC), diluted in 0.1M carbonate buffer (pH 9.6) and incubated overnight at 4°C. Plates were blocked in 1X Tris-buffered saline containing 0.05% Tween-20 and 5% normal goat serum for 1 hour at 37°C, followed by an incubation with a 1:5 dilution of ZIKV (strain PRVABC59, BEI Resources) for 1 hour at 37°C. Plasma samples were tested at a dilution series of 1:12.5–204,800 with 4-fold dilutions and incubated for 1 hour at 37°C. A Zika-specific monoclonal antibody, H24 (10 μg/mL, isolated from a Zika-infected rhesus macaque; unpublished data) and rhesus hyperimmunoglobulin (purified from ZIKV-infected rhesus macaque serum) were used as positive controls. An anti-horseradish peroxidase (HRP)-conjugated goat anti-monkey IgG secondary antibody (AbCam) was used at a 1:2,500 dilution and incubated at 37°C for 1 hour, followed by the addition of SureBlue Reserve TMB Substrate (KPL). Reactions were stopped by Stop Solution (KPL) after a 5-minute incubation per plate in the dark. Optical density (OD) was detected at 450 nm on a Victor X Multilabel plate reader (PerkinElmer). Binding was considered detectable if the sample OD value at the lowest dilution was greater than 0.986, the mean OD of 16 seronegative monkeys at the lowest dilution plus two standard deviations (SD). For samples above this threshold, the OD values for the serial dilution were entered into Prism v8 (GraphPad Software) to determine the 50% effective dilution (ED50). The ED50 was calculated by log transforming the dilution series and then analyzing using a sigmoidal dose-response regression model. Samples with an ED50 below the limit of detection were assigned an ED50 value of 12.5, the lowest dilution tested. IgG Zika-specific binding was reported in Log10 ED50.
Measurement of ZIKV-specific T cell responses via intracellular cytokine staining (ICS)
Cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed by using Thawsome adapter (34). The cells were washed in pre-warmed R10 [RPMI 1640 (BioWhittaker), 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin] with 50 U/ml Benzonase (Novagen). Cells were then resuspended at 1–2 million cells/ml in R10 and rested overnight in a 37C/5% CO2 incubator. The following morning, cells were stimulated at 1–3 million cells/well in a 96 well v-bottom plate with an overlapping peptide pool of the ZIKV E protein (15-mers overlapping by 11 amino acids; final concentration of 2 μg/ml) in the presence of GolgiPlug (at a final concentration of 10 μg/ml; BD Biosciences) for 6 h. Negative controls received an equal concentration of DMSO instead of peptides. ICS was performed as outlined (35). The following monoclonal antibodies were used: CD4-BV421 (clone OKT4; BioLegend), CD8- BUV395 (clone RPA-T8; BD Biosciences), CD69-ECD (clone TP1.55.3; Beckman Coulter), CD3-Cy7APC (clone SP34.2; BD Biosciences), IFNγ-APC (clone B27; BD Biosciences), IL-2-PE (clone MQ1–17H12; BD Biosciences), and TNF-FITC (clone Mab11; BD Biosciences). Aqua LIVE/DEAD kit (Invitrogen, Carlsbad, CA) was used to exclude dead cells. All antibodies were previously titrated to determine the optimal concentration. Samples were acquired on an BD Symphony flow cytometer and analyzed using FlowJo version 9.9.6 (Treestar, Inc.) and graphed using SPICE software (NIAID).
Cytokine and chemokine measurement in amniotic fluid
Cytokine and chemokine concentrations in amniotic fluid were determined by Luminex technology using the Non-Human Primate MILLIPLEX MAP (Millipore) according to the manufacturer’s instructions.
Histopathology
Sections of fixed and embedded maternal and fetal tissues were stained with H&E, and evaluated blindly by a board-certified anatomic pathologist. Scoring of critical fetal tissues was done by 2 pathologists according to established criteria, and based on reading 13–20 sections for brain, 1–2 sections for placenta, and 1 section for amnion, umbilical cord and eye.
Statistical analyses
Statistical analyses were performed using Prism version 8 (GraphPad), with selection of the test as outlined in the results. P values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments:
We thank Vanessa Bakula, Miles Christensen, Irma Cazares, Wilhelm von Morgenland, Ramya Immareddy, Pablo Badra, Sarah Lockwood, Katherine Olstad, Veterinary staff, Pathology Unit, Colony Research Services, Clinical Laboratories and Multimodal Imaging Core of CNPRC for their expert technical assistance; T. Friedrich (Wisconsin National Primate Research Center) for providing the ZIKV PRVABC-59 stock.
Funding: This work was supported by R21AI129479-S (KKAVR); the Office of Research Infrastructure Program/OD (P510D11107; CNPRC), R21NS104692 (EBM), R21HD090856 and R01HD098389 (SGK), NIH T32 CA009111 (TS) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH (KMM, BMF, KEB, ATN, KAD, KEF, TCP, BSG).
Footnotes
Competing interests: BSG, KAD and TCP are inventors on patents applications describing the VRC5283 DNA vaccine (US patent application number 16/334,099 and PCT/2018/018809). Other authors have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or Supplementary Materials. Sharing of materials is dependent on availability of materials and may require material transfer agreements.
References and Notes
- 1.Musso D, Gubler DJ, Zika Virus. Clin Microbiol Rev 29, 487–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR, Zika Virus and Birth Defects - Reviewing the Evidence for Causality. N Engl J Med, 1981–1987 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Krauer F, Riesen M, Reveiz L, Oladapo OT, Martinez-Vega R, Porgo TV, Haefliger A, Broutet NJ, Low N, W. H. O. Z. C. W. Group, Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barre Syndrome: Systematic Review. PLoS Med 14, e1002203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J, Where has all the Zika gone? Science 357, 631–632 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Dudley DM, Van Rompay KK, Coffey LL, Ardeshir A, Keesler RI, Bliss-Moreau E, Grigsby PL, Steinbach RJ, Hirsch AJ, MacAllister RP, Pecoraro HL, Colgin LM, Hodge T, Streblow DN, Tardif S, Patterson JL, Tamhankar M, Seferovic M, Aagaard KM, Martin CS, Chiu CY, Panganiban AT, Veazey RS, Wang X, Maness NJ, Gilbert MH, Bohm RP, Adams Waldorf KM, Gale M Jr., Rajagopal L, Hotchkiss CE, Mohr EL, Capuano SV 3rd, Simmons HA, Mejia A, Friedrich TC, Golos TG, O’Connor DH, Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat Med 24, 1104–1107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffey LL, Keesler RI, Pesavento PA, Woolard K, Singapuri A, Watanabe J, Cruzen C, Christe KL, Usachenko J, Yee J, Heng VA, Bliss-Moreau E, Reader JR, von Morgenland W, Gibbons AM, Jackson K, Ardeshir A, Heimsath H, Permar S, Senthamaraikannan P, Presicce P, Kallapur SG, Linnen JM, Gao K, Orr R, MacGill T, McClure M, McFarland R, Morrison JH, Van Rompay KKA, Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat Commun 9, 2414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinot AJ, Abbink P, Afacan O, Prohl AK, Bronson R, Hecht JL, Borducchi EN, Larocca RA, Peterson RL, Rinaldi W, Ferguson M, Didier PJ, Weiss D, Lewis MG, De La Barrera RA, Yang E, Warfield SK, Barouch DH, Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell 173, 1111–1122 e1110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M Jr., Rajagopal L, Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 22, 1256–1259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, Lo JO, Liu Z, Kroenke CD, Smith JL, Kelleher M, Broeckel R, Kreklywich CN, Parkins CJ, Denton M, Smith P, DeFilippis V, Messer W, Nelson JA, Hennebold JD, Grafe M, Colgin L, Lewis A, Ducore R, Swanson T, Legasse AW, Axthelm MK, MacAllister R, Moses AV, Morgan TK, Frias AE, Streblow DN, Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun 9, 263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams Waldorf KM, Nelson BR, Stencel-Baerenwald JE, Studholme C, Kapur RP, Armistead B, Walker CL, Merillat S, Vornhagen J, Tisoncik-Go J, Baldessari A, Coleman M, Dighe MK, Shaw DWW, Roby JA, Santana-Ufret V, Boldenow E, Li J, Gao X, Davis MA, Swanstrom JA, Jensen K, Widman DG, Baric RS, Medwid JT, Hanley KA, Ogle J, Gough GM, Lee W, English C, Durning WM, Thiel J, Gatenby C, Dewey EC, Fairgrieve MR, Hodge RD, Grant RF, Kuller L, Dobyns WB, Hevner RF, Gale M Jr., Rajagopal L, Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med 24, 368–374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S 3rd, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG, Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 13, e1006378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbink P, Stephenson KE, Barouch DH, Zika virus vaccines. Nat Rev Microbiol 16, 594–600 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, Gordon DN, Gallagher JR, Chen X, Todd JP, Tsybovsky Y, Harris A, Huang YS, Higgs S, Vanlandingham DL, Andersen H, Lewis MG, De La Barrera R, Eckels KH, Jarman RG, Nason MC, Barouch DH, Roederer M, Kong WP, Mascola JR, Pierson TC, Graham BS, Rapid development of a DNA vaccine for Zika virus. Science 354, 237–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, Berkowitz N, Mendoza F, Saunders JG, Novik L, Hendel CS, Holman LA, Gordon IJ, Cox JH, Edupuganti S, McArthur MA, Rouphael NG, Lyke KE, Cummings GE, Sitar S, Bailer RT, Foreman BM, Burgomaster K, Pelc RS, Gordon DN, DeMaso CR, Dowd KA, Laurencot C, Schwartz RM, Mascola JR, Graham BS, Pierson TC, Ledgerwood JE, Chen GL, Vrc, V. R. C. s. teams, Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet 391, 552–562 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett ADT, Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. NPJ Vaccines 3, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J, Steep drop in Zika cases undermines vaccine trial. Science 361, 1055–1056 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Vannice KS, Cassetti MC, Eisinger RW, Hombach J, Knezevic I, Marston HD, Wilder-Smith A, Cavaleri M, Krause PR, Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine 37, 863–868 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabie A, Callier C, Carles G, Cassadou S, Cesaire R, Douine M, Herrmann-Storck C, Kadhel P, Laouenan C, Madec Y, Monthieux A, Nacher M, Najioullah F, Rousset D, Ryan C, Schepers K, Stegmann-Planchard S, Tressieres B, Volumenie JL, Yassinguezo S, Janky E, Fontanet A, Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N Engl J Med 378, 985–994 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baiao AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K, Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med 375, 2321–2334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O’Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O’Connor DH, A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7, 12204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM, Barry GL, Weisgrau KL, Eudailey JA, Rakasz EG, Vosler LJ, Post J, Capuano S 3rd, Golos TG, Permar SR, Osorio JE, Friedrich TC, O’Connor SL, O’Connor DH, Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLoS neglected tropical diseases 10, e0005168 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O, Zika virus Infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374, 2142–2151 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Reader JR, Canfield DR, Lane JF, Kanthaswamy S, Ardeshir A, Allen AM, Tarara RP, Left Ventricular Hypertrophy in Rhesus Macaques (Macaca mulatta) at the California National Primate Research Center (1992–2014). Comp Med 66, 162–169 (2016). [PMC free article] [PubMed] [Google Scholar]
- 24.Coffey LL, Pesavento PA, Keesler RI, Singapuri A, Watanabe J, Watanabe R, Yee J, Bliss-Moreau E, Cruzen C, Christe KL, Reader JR, von Morgenland W, Gibbons AM, Allen AM, Linnen J, Gao K, Delwart E, Simmons G, Stone M, Lanteri M, Bakkour S, Busch M, Morrison J, Van Rompay KK, Zika Virus Tissue and Blood Compartmentalization in Acute Infection of Rhesus Macaques. PLoS One 12, e0171148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F, Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update 22, 104–115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, Kirilova M, Peterson R, Li Z, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Chandrashekar A, Jetton D, Mojta S, Gandhi P, LeSuer J, Khatiwada S, Lewis MG, Modjarrad K, Jarman RG, Eckels KH, Thomas SJ, Michael NL, Barouch DH, Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci Transl Med 9, eaao4163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML, West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol 75, 4040–4047 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bialas KM, Tanaka T, Tran D, Varner V, Cisneros De La Rosa E, Chiuppesi F, Wussow F, Kattenhorn L, Macri S, Kunz EL, Estroff JA, Kirchherr J, Yue Y, Fan Q, Lauck M, O’Connor DH, Hall AH, Xavier A, Diamond DJ, Barry PA, Kaur A, Permar SR, Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc Natl Acad Sci U S A 112, 13645–13650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Main BJ, Nicholson J, Winokur OC, Steiner C, Riemersma KK, Stuart J, Takeshita R, Krasnec M, Barker CM, Coffey LL, Vector competence of Aedes aegypti, Culex tarsalis, and Culex quinquefasciatus from California for Zika virus. PLoS neglected tropical diseases 12, e0006524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarantal AF, in The Laboratory Primate, Wolfe-Coote S, Ed. (Elsevier, London, UK, 2005), pp. 317–352. [Google Scholar]
- 31.Forootan A, Sjoback R, Bjorkman J, Sjogreen B, Linz L, Kubista M, Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol Detect Quantif 12, 1–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azevedo RSS, Araujo MT, Oliveira CS, Filho AJM, Nunes BTD, Henriques DF, Silva EVP, Carvalho VL, Chiang JO, Martins LC, Vasconcelos BCB, Sousa JR, Araujo FMC, Ribeiro EM, Castro ARP, de Queiroz MGL, Verotti MP, Nunes MRT, Cruz ACR, Rodrigues SG, Shi PY, Quaresma JAS, Tesh RB, Vasconcelos PFC, Zika Virus Epidemic in Brazil. II. Post-Mortem Analyses of Neonates with Microcephaly, Stillbirths, and Miscarriage. J Clin Med 7, 496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith AR, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, Diamond MS, Ledgerwood JE, Pierson TC, Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep 16, 1485–1491 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beddall M, Chattopadhyay PK, Kao SF, Foulds K, Roederer M, A simple tube adapter to expedite and automate thawing of viably frozen cells. J Immunol Methods 439, 74–78 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Foulds KE, Donaldson M, Roederer M, OMIP-005: Quality and phenotype of antigen-responsive rhesus macaque T cells. Cytometry A 81, 360–361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


