Abstract
Background:
A “malignant” subphenotype of left ventricular hypertrophy (LVH) has been described, in which minimal elevations in cardiac biomarkers identify individuals with LVH at high risk for developing heart failure (HF). We tested the hypothesis that a higher prevalence of malignant LVH among blacks may contribute to racial disparities in HF risk.
Methods:
Participants (n=15, 710) without prevalent cardiovascular disease were pooled from three population-based cohort studies, the Atherosclerosis Risk in Communities Study (ARIC), the Dallas Heart Study (DHS), and the Multi-Ethnic Study of Atherosclerosis (MESA). Participants were classified into three groups: those without ECG-LVH, those with ECG-LVH and normal biomarkers (hs-cTnT < 6 ng/L and NT-proBNP < 100 pg/mL), and those with ECG-LVH and abnormal levels of either biomarker (malignant LVH). The outcome was incident HF.
Results:
Over the 10 year follow up period, HF occurred in 512 (3.3%) participants, with rates 5.2% among black men, 3.8% in white men, 3.2% in black women, and 2.2% in white women. The prevalence of malignant LVH was 3-fold higher among black men and women vs white men and women. Compared with participants without LVH, the adjusted hazard ratio for HF was 2.8 (95% CI 2.1 to 3.5) in those with malignant LVH and 0.9 (95% CI 0.6 to 1.5) in those with LVH and normal biomarkers, with similar findings in each race/sex subgroup. Mediation analyses indicated that 33% of excess hazard for HF among black men and 11% of the excess hazard among black women was explained by the higher prevalence of malignant LVH in blacks. Of black men who developed HF, 30.8% had malignant LVH at baseline, with a corresponding population attributable fraction (PAF) of 0.21. The proportion of HF cases occurring among those with malignant LVH, and the corresponding PAF, were intermediate and similar among black women and white men and lowest among white women.
Conclusions:
A higher prevalence of malignant LVH may in part explain the higher risk of heart failure among blacks vs whites. Strategies to prevent development or attenuate risk associated with malignant LVH should be investigated as a strategy to lower heart failure risk and mitigate racial disparities.
Keywords: LVH, troponin, BNP, heart failure, black race, racial disparities
Introduction
Left ventricular hypertrophy (LVH), which can be detected by cardiac imaging studies or electrocardiography (ECG), is an important pre-clinical abnormality that is associated with higher risk of developing HF and cardiovascular (CV) death. 1, 2 LVH is more common in blacks than other race/ethnic groups, 3 and associates strongly with the degree of African ancestry. 4 The natural history of LVH is heterogeneous with possible outcomes that include regression, stability, or progression to clinical heart failure. 5 Chronic cardiomyocyte injury and tissue fibrosis, 6 in addition to increased diastolic wall stress and neurohormonal activation,7 are hypothesized to play a role in the transition from asymptomatic LVH to HF.
A “malignant” subphenotype of LVH has recently been described, in which minimal elevations in biomarkers of cardiac injury or neurohormonal activation identified individuals with LVH who were at particularly high risk for progression to HF and CV death. 8-10 This malignant LVH subphenotype was associated with a very high absolute risk for progression to HF in black individuals enrolled in the Jackson Heart Study. 11 However, these prior studies were limited by insufficient numbers of clinical events to determine whether differences in the prevalence of malignant LVH contribute to higher risk for HF in black vs white individuals. Using pooled data from three biracial cohort studies, including two that were the basis of prior reports,8, 9 we tested the hypothesis that malignant LVH may contribute to racial disparities in HF risk.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population:
Participants were pooled from 3 population-based cohort studies, the Atherosclerosis Risk in Communities (ARIC) Study, the Dallas Heart Study (DHS) and the Multi-Ethnic Study of Atherosclerosis (MESA). ARIC is a prospective epidemiologic study that enrolled 15,792 adults aged 45-64 years from four US communities, including Forsyth County, North Carolina, Jackson, Mississippi, Minneapolis, Minnesota, and Washington County, Maryland. 12, 13 The DHS is a probability-based population cohort study of adults residing in Dallas County. The study included 3,557 subjects that completed a detailed in-home survey, laboratory testing, and imaging studies. 14 MESA is a population-based study that enrolled 6,815 adults aged 45-84 years, from 6 US communities, which included Baltimore, Maryland, Chicago, Illinois, Forsyth County, North Carolina, Los Angeles County, California, New York, New York, and St. Paul, Minnesota. 15
Each of the three cohorts is racially diverse, with approximately equal proportions of men and women. Detailed methods of each cohort’s study design, recruitment strategy, and visit protocols have been described previously. 12-15 For the present study, we excluded participants with prevalent cardiovascular disease (coronary artery disease, heart failure, and/or cerebrovascular disease), estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73m2, end-stage renal disease requiring dialysis, or race/ethnicity other than White or Black, yielding a final sample size of 15,710 in the pooled cohort (Supplementary Figure 1). Each study was approved by the Institutional Review Board (IRB) of the study coordinating center, and all participants provided written informed consent.
Clinical Covariates:
In all three study cohorts, the baseline examinations ascertained cardiovascular conditions and measured risk factors in all participants. Anthropometric parameters (including height and weight) as well as blood pressure were measured using standard protocols. Fasting blood samples were drawn, and measurements including lipids and serum creatinine levels were analyzed using standard techniques. Detailed methods regarding baseline assessments, clinical examinations, and blood analyses have been described previously for all three cohorts. 12, 14, 15 For the present study, the initial examination was used as the baseline for both DHS and MESA. The first ARIC exam occurred from 1987-1989, however, the second ARIC visit (1990-1992) was used as the baseline for the present study, as this was the first visit with available biomarker and LVH data.
Biomarker, ECG and Imaging Measurements:
High sensitivity cardiac troponin-T (hs-cTnT) levels and N-terminal pro-brain natriuretic peptide (NT-proBNP) were measured in ARIC and DHS using the Elecsys 2010 platform and in MESA with the Cobas e601 platform (Roche Diagnostics, Indianapolis, IN) as described previously. 16-20 Standard twelve-lead ECGs were performed in all three cohorts using a Marquette MAC-PC instrument (Marquette Electronics, Milwaukee, Wisconsin). 4, 12, 14, 15 Cardiac magnetic resonance imaging (MRI) studies were performed in DHS and MESA using 1.5-T MRI systems. Detailed methods outlining MRI measurements and interpretation have been described previously for both DHS 3 and MESA 21, 22. Participants with left ventricular hypertrophy (LVH) by MRI were further classified using the 4-tiered classification proposed by Khouri et al.23: indeterminate, dilated, thick, and both thick and dilated LVH.
Primary Exposures:
For the purposes of our study, an abnormal high-sensitivity cardiac troponin-T (hs-cTnT) was defined as hs-cTnT ≥ 6 ng/L. This threshold was selected apriori for the present study since this represents the limit of quantitation for this assay in clinical practice in the United States. An abnormal NT-proBNP level was prespecified as ≥ 100 pg/mL.
For our primary analyses, LVH was defined by 12-lead ECG, using the Sokolow-Lyon criteria, defined as the sum of the S-wave amplitude in lead V1 plus the maximum R-wave amplitude in V5 or V6 ≥ 3.5 mV (35 mm) or aVL R-wave amplitude ≥ 1.1 mV (11 mm). 24 Repolarization abnormalities (ECG-LVH with ST and T wave abnormalities) were adjudicated based on Novacode 6.1.1 in MESA but were not available in DHS or ARIC. In secondary analyses, LVH was defined by imaging measurements, using MRI definitions for DHS and MESA. Cardiac imaging studies were not available in visit 2 of ARIC. LVH was prespecified as LV mass/body surface area (BSA) ≥ 89 g/m2 in women and ≥112 g/m2 in men in DHS 3, and as LV mass/BSA ≥ 84.6 g/m2 in women and ≥ 106.2 g/m2 in men in MESA. 16
Participants were classified into prespecified phenotype exposure groups based on the presence or absence of ECG-LVH and evidence of abnormal levels of cardiac biomarkers. The reference group for all analyses was the group without ECG-LVH (ECG LVH−). Participants with ECG-LVH (ECG LVH+) were categorized further as ECG LVH+ biomarker– if both hs-cTnT and NT-proBNP were normal, or ECG LVH+ biomarker+ (malignant LVH) if either hs-cTnT or NT-proBNP was abnormal. In a secondary analysis, we further stratified participants without ECG-LVH into those with and without abnormal biomarkers.
Heart Failure Outcomes:
The primary outcome for this study was incident heart failure (HF). The HF definitions and adjudication procedures have been described previously for each individual study. 15, 17, 18 Briefly, in ARIC, continuous, retrospective surveillance of hospital discharges for HF were conducted for all residents from the four US communities included in the study. A hospitalization was considered eligible for confirmation as a HF event based on its International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) code. 17, 20 In the DHS, incident HF hospitalization was determined through a detailed annual health survey regarding interval CV events, and/or via quarterly tracking for hospital admissions using the Dallas-Fort Worth Hospital Council Data Initiative database. 18 In MESA, classification of HF events occurred from collection of death certificates, medical records from hospitalizations and outpatient visits, autopsy reports, and interviews with or questionnaires administered to participants, relatives, or physicians. 15 For our pooled analysis, we have included all HF events from the baseline visit through 10 years of follow up for each individual study cohort. DHS and ARIC included only hospitalized HF events while MESA included both hospitalizations and outpatient diagnoses of HF.
Statistical Analysis:
Participant characteristics and demographics across the 3 cohorts were compared for descriptive purposes. Primary participant level data for demographic information, risk factors, biomarkers, LVH, imaging data, and CV outcomes were pooled across the 3 cohorts. The cumulative incidence of heart failure among black and white men and women was estimated using cause-specific hazard functions that account for the competing risk of death.
The prevalence of malignant LVH was compared across race and sex groups using the Chi squared test. To assess the independent association of black race with malignant LVH, logistic regression analyses were performed separately in men and women, adjusting for age, systolic blood pressure, antihypertensive therapy, current smoking, diabetes, total and HDL cholesterol, and eGFR. Unadjusted proportions of heart failure in the three phenotype groups (ECG LVH−, ECG LVH+ biomarker- and malignant LVH) were estimated using cause-specific hazard functions and compared with the Log Rank test. Cox proportional hazard models were used to estimate the hazard of HF events in the ECG LVH+ biomarker- and malignant LVH groups, with ECG LVH− as referent. All models met assumptions for proportional hazards. Models included the same covariates as the logistic regression models described above. Cox models accounted for the competing risk of all-cause mortality, as well as different baseline hazards for each study via stratification, and robust standard errors were used to account for patients clustered within studies. In exploratory analyses, the dose effect of the number of abnormal biomarkers was evaluated by analyzing separately participants with LVH and elevations in one or both of the two biomarkers (hs-cTnT and NT-proBNP).
A series of analyses were performed to assess the influence of malignant LVH on racial differences in HF risk. Multiplicative interactions of malignant LVH with race and sex were evaluated. Analyses assessing the association of phenotype exposure groups with HF were stratified by race and sex. Next, the potential mediating effect of malignant LVH on racial differences in HF was assessed by constructing Cox proportional hazards models, stratified by sex. The first model included a term for race and the second included terms for race and malignant LVH. The change in hazard ratio after accounting for malignant LVH provides an assessment of the potential contribution of malignant LVH to racial differences in HF. 19, 25 To more formally assess causal mediation, we applied Accelerated Failure Time Outcome Regression, using a Weibull model and mediator logistic regression, which models controlled direct effects, natural direct effects, and natural indirect effects.26, 27
Sensitivity analyses were performed replacing ECG-LVH with MRI-defined LVH in DHS and MESA. We also performed subgroup analyses based on body mass index (BMI) categories (<25, 25-<30 and ≥ 30 kg/m2). All statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC) software version 9.4.
Results
A total of 9,363 participants from the ARIC study, 2,008 participants from DHS, and 4,339 participants from the MESA study met inclusion and exclusion criteria, resulting in 15,710 total participants in the pooled cohort (Supplementary Figure 1). Baseline characteristics in the three cohorts and the pooled cohort are presented in Supplementary Table 1. The median age of the pooled cohort was 57 years, with 56% female, 32% black, and 36% with hypertension. Participant characteristics stratified by sex and race are presented in Supplementary Table 2. Black men and women had higher rates of diabetes and hypertension, and higher blood pressure and BMI compared with white men and women.
ECG-LVH was present in 1,421 (9%) of participants in the pooled cohort, while 4,292 (27%) had an hs-cTnT concentration ≥ 6 ng/L (hs-cTnT+), and 3,314 (21%) had an NT-proBNP concentration ≥ 100 pg/mL (NT-proBNP+). Among those with LVH, 47% had no biomarker elevation, 37% had only one elevated biomarker, and 15% had both biomarkers elevated.
Participants with malignant LVH were older, more likely to be male, with more diabetes, hypertension and higher systolic blood pressure in comparison with participants without LVH as well as those with LVH but without abnormal cardiac biomarkers (Table 1). Sokolow-Lyon ECG voltage, and cMRI defined LV mass and LV mass indexed to body surface area (BSA) were similar between participants with malignant LVH compared with those with LVH and negative biomarkers, but cMRI-defined LV wall thickness and LV end-diastolic volume were slightly higher. Moreover, pathological remodeling patterns, including dilated and thick and dilated hypertrophy categories, were significantly more common among participants with malignant LVH than those with LVH and normal biomarkers (Table 1). In a secondary analysis limited to MESA, ECG repolarization abnormalities consistent with strain pattern were more common among participants with malignant LVH vs those with LVH and negative biomarkers (p=0.03), but only 8.7% of those with malignant LVH had repolarization abnormalities (Table 1). The prevalence of malignant LVH increased across higher BMI categories, due to increasing prevalence of abnormal hs-cTnT, with minimal difference in the prevalence of abnormal NT-proBNP (Supplementary Table 3).
Table 1.
Baseline characteristics of the pooled cohort (MESA, ARIC, DHS) with no history of cardiovascular disease, stratified by presence of left ventricular hypertrophy (LVH) and abnormal biomarkers (High-sensitivity cardiac troponin-T (hs-cTnT) ≥ 6 and N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥ 100).
| Characteristics | LVH (−) (N = 14289) |
LVH (+) Biomarker (−) (N = 664) |
LVH (+) Biomarker (+) (Malignant LVH) (N = 757) |
|---|---|---|---|
| Age – years | 56.6 (51, 63) | 54.3 (50, 60)* | 60.6 (54, 66)*☨ |
| Men – n (%) | 6111 (42.8) | 325 (49)* | 447 (59.1)*☨ |
| Race/Ethnicity | |||
| Black – n (%) | 4127 (28.9) | 434 (65.4)* | 436 (57.6)*☨ |
| Hypertension – n (%) | 4826 (33.9) | 344 (52)* | 530 (70.1)*☨ |
| Systolic Blood Pressure (mm Hg) | 121.7 (109, 132) | 128.9 (116, 139.8)* | 139 (122, 153)*☨ |
| Diastolic Blood Pressure (mm Hg) | 72.4 (65.7, 79) | 77.4 (71, 83.3)* | 78.4 (70.7, 86)* |
| Anti-hypertensive therapy prescribed – n (%) | 4103 (28.7) | 248 (37.4)* | 427 (56.4)*☨ |
| Diabetes – n (%) | 1304 (9.1) | 76 (11.5)* | 123 (16.3)*☨ |
| Current Smoker – n (%) | 2733 (19.1) | 124 (18.7) | 123 (16.3)* |
| Estimated GFR (ml/min/1.73 m2) | 70.4 (56.8, 80) | 77.9 (62.5, 90.9)* | 72.2 (57.3, 81.3)☨ |
| Total Cholesterol (mg/dL) | 201.6 (176, 225) | 200.6 (172, 224) | 200.9 (174, 226) |
| HDL Cholesterol (mg/dL) | 50.9 (40, 60) | 50.7 (40, 58) | 49.8 (39, 59) |
| Statin therapy prescribed – n (%) | 1194 (8.4) | 45 (6.8) | 78 (10.3)☨ |
| Body Mass Index (BMI) (kg/m2) | 28.3 (24.4, 31.1) | 29.4 (25.4, 32.4)* | 29.4 (25.7, 32.4)* |
| Waist-hip ratio | 0.92 (0.86, 0.98) | 0.93 (0.88, 0.98)* | 0.94 (0.9, 0.99)*☨ |
| LV mass (gm) | 141.5 (111.6, 163.9) | 163.1 (126.1, 192.7)* | 168.9 (129.7, 196.9)* |
| LV mass/BSA (g/m2) | 74.3 (61.7, 83.5) | 83.6 (67.6, 95.7)* | 86.3 (68.7, 98.2)* |
| LV wall thickness (mm) | 10 (8.8, 11) | 10.8 (9.3, 12)* | 11.1 (9.6, 12.3)*☨ |
| LV end-diastolic volume (mL) | 101.2 (85.7, 115.9) | 113.7 (94.6, 129.3)* | 125.6 (97.5, 148.5)* |
| LV ejection fraction (%) | 65.7 (61.5, 70.1) | 65.4 (60.9, 70.4) | 62.3 (57.8, 68.5)*☨ |
| Sokolow Lyon ECG Voltage (mV) ± | 20.5 (16.2, 24.8) | 30.3 (23.1, 37.8)* | 31 (22.2, 37.9)* |
| ECG LVH with repolarization abnormality ⊥ | 11 (0.3) | 5 (3.1)* | 23 (8.7)*☨ |
| LV hypertrophy subtypes± | |||
| Indeterminate – n (%) | 96 (2.1) | 11 (4.7)* | 17 (6.9)*☨ |
| Dilated – n (%) | 179 (4) | 19 (8.1)* | 54 (21.8)*☨ |
| Thick walled – n (%) | 109 (2.4) | 22 (9.4)* | 23 (9.3)*☨ |
| Both Thick and Dilated – n (%) | 6 (0.1) | 0 | 8 (3.2)*☨ |
| High-sensitivity C-reactive protein (hsCRP) (mg/L) | 4.1 (0.98, 4.6) | 4.7 (1.1, 5.6)* | 4.5 (1.1, 5.4)* |
p < 0.05 versus no LVH group
p < 0.05 versus LVH+, biomarker- group
Data available from MESA only (total N = 4338)
Data available from DHS and MESA only (total N = 4960)
The prevalence of malignant LVH was 3-fold higher among black men and women vs white men and women, respectively (p < 0.0001 for each, Table 2 and Figure 1). Notably, the malignant LVH phenotype was observed more frequently among black women than white men (6.3% vs 4.1%, p<0.0001). In multivariable logistic regression analyses, the odds of malignant LVH remained significantly higher in black men (adjusted OR 2.84, 95% CI 2.26, 3.56) and black women (adjusted OR 2.46, 95% CI 1.88, 3.23) vs white men and women. Older age, higher average systolic blood pressure and use of antihypertensive medications were also significantly associated with higher odds of malignant LVH in men and women (Supplementary Table 4).
Table 2.
Prevalence of left ventricular hypertrophy (LVH) and elevated biomarkers (High-sensitivity cardiac troponin-T (hs-cTnT) ≥ 6 and N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥ 100), among men and women in the pooled cohort stratified by race/ethnicity.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Black (N = 2007) |
White (N = 4876) |
P value | Black (N = 2990) |
White (N = 5837) |
P value | |
|
LVH(+) Biomarker (+) (Malignant LVH) n (%) |
247 (12.3) | 200 (4.1) | <0.0001 | 189 (6.3) | 121 (2.1) | <0.0001 |
|
LVH (+) Biomarker (−) n (%) |
185 (9.2) | 140 (2.9) | <0.0001 | 249 (8.3) | 90 (1.5) | <0.0001 |
|
LVH (−) n (%) |
1575 (78.5) | 4536 (93) | <0.0001 | 2552 (85.4) | 5626 (96.4) | <0.0001 |
Figure 1.
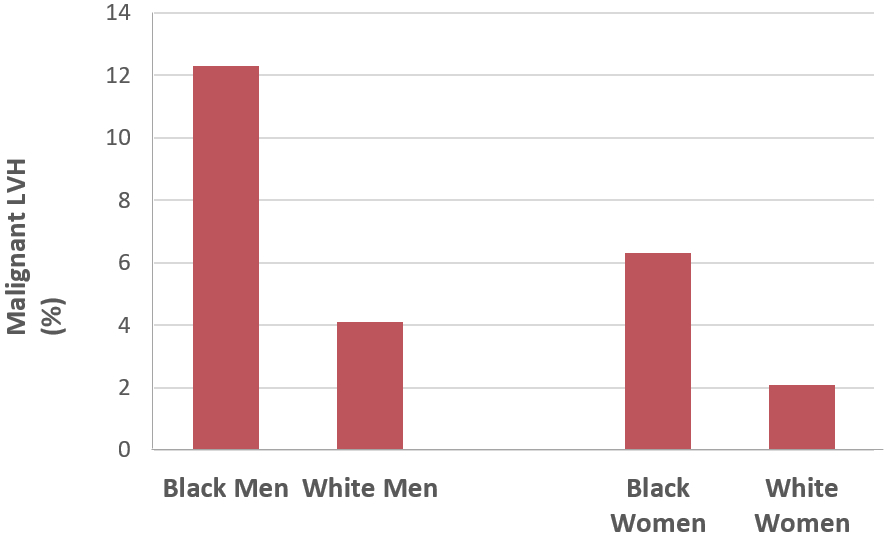
Prevalence of the malignant left ventricular hypertrophy (LVH) phenotype (evidence of LVH, with either high-sensitivity cardiac troponin-T (hs-cTnT) ≥ 6 and N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥ 100) among men and women stratified by race/ethnicity.
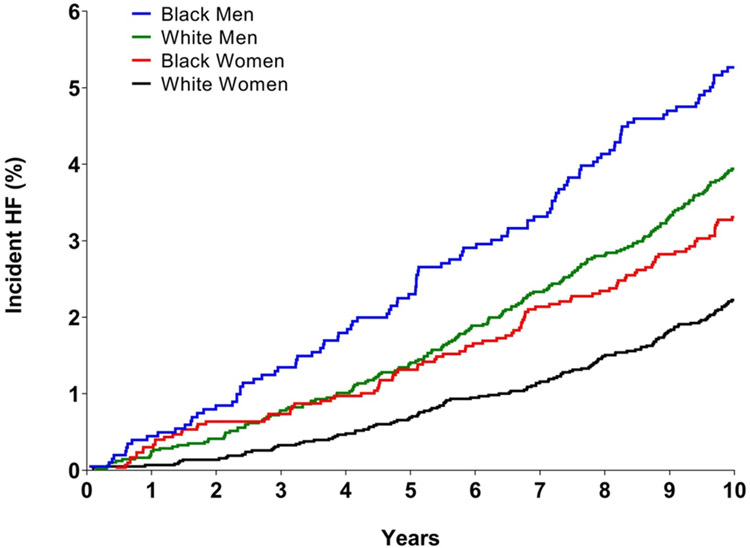
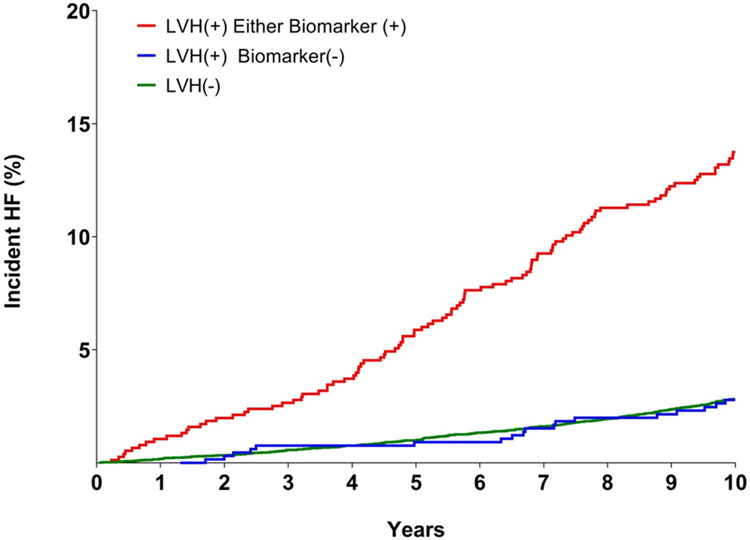
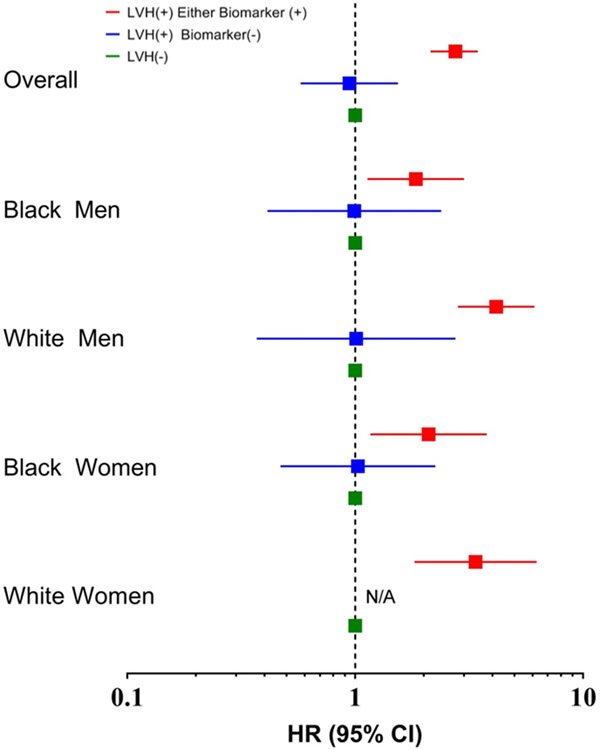
LVH with normal levels of biomarkers was also > 3-fold more common among black men and women than white men and women, respectively (p < 0.0001 for each, Table 2). Thus, the higher prevalence of malignant LVH among blacks is due to higher rates of overall LVH and not from a higher proportion of individuals with LVH who have abnormal biomarkers. Over the 10 year follow up period, HF occurred in 512 (3.3%) participants. Among those who developed HF, 56% were men and 39% were black. Kaplan Meier estimated rates of heart failure were highest among black men, intermediate (and similar) among white men and black women, and lowest among white women (Figure 2). Of the participants with malignant LVH, 13.6% developed HF, compared with 2.7% in the subgroup with LVH and normal biomarkers during the 10 year follow-up period (Figure 3). Compared with participants without LVH, the unadjusted hazard ratio (HR) for development of HF was 5.1 (95% CI 4.1 to 6.3) among participants with malignant LVH and 1.0 (95% CI 0.6 to 1.6) for those with LVH and negative biomarkers. After multivariable adjustment, the HR for HF was 2.8 (95% CI 2.1 to 3.5) in those with malignant LVH and 0.9 (95% CI 0.6 to 1.5) in those with LVH and normal biomarkers (Figure 4). No interaction of malignant LVH with BMI category was detected (Supplementary Table 5).
Figure 2.
Unadjusted Kaplan-Meier curves for incident heart failure (HF) stratified by race/ethnicity.
Figure 3.
Unadjusted Kaplan-Meier curves for incident heart failure (HF) in the overall pooled cohort.
Figure 4.
Adjusted associations between LVH and chronic myocardial injury-based categories and risk of heart failure stratified by gender and race/ethnicity. N/A: Insufficient number of events.
In a secondary analysis, participants without ECG-LVH were further stratified based on biomarkers status; the group with normal biomarkers and no ECG-LVH was the referent group. HF risk was significantly increased among individuals with abnormal biomarkers and no ECG-LVH and highest among those with abnormal biomarkers and ECG-LVH. In contrast, participants with ECG-LVH and normal biomarkers had similar HF risk vs those with no ECG-LVH and normal biomarkers (Supplementary Table 6).
In analyses evaluating abnormal hs-cTnT and NT-proBNP individually, the adjusted hazards for HF were significantly higher in the groups with ECG-LVH and abnormal hs-cTnT, and ECG-LVH and abnormal NT-proBNP, but were not increased in patients with ECG-LVH and normal biomarker levels (Supplementary Table 7). Graded associations with HF were seen among those with ECG-LVH based on the number of elevated biomarkers. The unadjusted HR was 10.3 (95% CI 7.6 to 13.8) for those with abnormal levels of both hs-cTnT and NT-proBNP, 3.1 (95% CI 2.3 to 4.3) for those with one abnormal biomarker and 1.0 (95% CI 0.6 to 1.6) for those that were LVH+ biomarker – (Supplementary Figure 2). After multivariable adjustment, the HR for those with both abnormal biomarkers was 4.2 (95% CI 2.9 to 5.9), 2.0 (95% CI 1.4 to 2.7) for those with one abnormal biomarker, and 0.9 (95% CI 0.6 to 1.5) for those that were LVH+ biomarker – (Supplementary Figure 2). In subgroup analyses, malignant LVH was more common across higher BMI categories.
A quantitative interaction was observed by race (p interaction = 0.012) but not sex (p interaction = 0.70) such that the hazard for HF associated with malignant LVH was modestly lower among black vs white participants (Figure 4). In analyses stratified by race/sex subgroups, malignant LVH was associated with significantly higher risk for HF in each race/sex subgroup in unadjusted (Supplementary Figure 3) and adjusted (Figure 4) analyses, whereas LVH without biomarker elevation was not associated with increased risk in any race/sex subgroup.
Among men, the unadjusted HR for HF associated with black race was 1.54 (95% CI 1.20-1.97), and after adjusting for the prevalence of malignant LVH was 1.20, (95% CI 0.93-1.56). Similarly, among women, the unadjusted HR associated with black race was 1.62 (95% CI 1.23-2.13) and after adjusting for malignant LVH was 1.40 (95% CI 1.05-1.86). In causal mediation analysis survival free from heart failure was 13% lower in black men and 15% lower in black woman, with malignant LVH mediating 33% of the association of race with heart failure in men (p=0.02) and 11% of the effect in women (p=0.003). No mediation was seen for benign LVH (p=0.53 in men and p=0.42 in women). Although only 4.8% of the study population manifested the malignant LVH phenotype, these participants accounted for 20% of all HF events, vs only 3.5% of HF events occurring among the 4.2% of the study population who were LVH+ biomarker-. Of black male participants that developed HF, 30.8% had the malignant LVH phenotype, with a corresponding population attributable fraction (PAF) of 0.21 (95% CI 0.11 to 0.30). The proportion of HF cases occurring among those with malignant LVH, and the corresponding PAF, were intermediate and similar among black women and white men, and lowest among white women (Table 3).
Table 3. Contribution of the malignant LVH phenotype to HF burden:
Heart failure (HF) cases (%) in individuals with the malignant left ventricular hypertrophy (LVH) phenotype, with Population Attributable Fraction (PAF) stratified by race/ethnicity.
| HF Cases (%) with Malignant LVH |
PAF, (95% CI) | |
|---|---|---|
| Black Men | 30.8% | 0.21 (0.11, 0.3) |
| White Men | 19.6% | 0.16 (0.1, 0.22) |
| Black Women | 20.8% | 0.16 (0.07, 0.24) |
| White Women | 10.9% | 0.09 (0.03, 0.14) |
Sensitivity analyses replacing ECG-LVH with imaging-LVH, using cardiac MRI in the DHS and MESA cohorts, displayed consistent findings, with higher rates of malignant LVH among black vs white participants and a more than three-fold higher unadjusted risk of HF observed among those with MRI-LVH biomarker+ compared with those with MRI-LVH biomarker– (Supplementary Table 8 and Supplementary Figure 4).
Discussion
In this large pooled biracial cohort of adults free from cardiovascular disease at baseline, we observed substantial heterogeneity in heart failure risk between black and white adults with LVH. Participants with LVH and evidence of subclinical myocardial injury or neurohormonal activation demonstrated by abnormal hs-cTnT and/or NT-proBNP levels had a significantly elevated risk for the development of clinical heart failure, consistent with other previously published studies. 8-11 In contrast, individuals with ECG-LVH with normal cardiac biomarkers display a clinical trajectory that is much more benign with regards to HF incidence. These participants were found to have a similarly low HF risk as individuals without ECG-LVH, including those with normal biomarkers and no ECG-LVH.
ECG voltage and MRI-determined LV mass were not significantly different between individuals with benign and malignant LVH, and only modest differences were seen in other measures of LV size. However, MRI remodeling patterns most strongly associated with increased risk for heart failure, 28 including dilated hypertrophy and thick and dilated hypertrophy, were more common among participants with malignant LVH. These findings support the hypothesis that it is pathological remodeling associated with LVH, in particular LV dilation, and not increased LV mass per se, that is most strongly associated with HF risk, and that abnormal biomarkers characterize such pathological remodeling. It is reasonable to conclude based on these findings that ECG-LVH with abnormal hs-cTnT and/or NT-proBNP represents a malignant LVH subphenotype, while LVH in the absence of subclinical myocardial injury appears to be relatively benign. The latter point is particularly relevant as almost half of ECG-LVH can be sub classified as benign.
Strengths of our study include the large sample size with sufficient numbers of black participants to evaluate racial differences in phenotype prevalence and outcomes, as well as the comprehensive phenotyping performed in the participants. In addition, our findings using ECG-LVH were confirmed in sensitivity analyses with MRI-LVH. Finally, associations with heart failure risk were similar across BMI categories.
Previously published data has indicated that the malignant LVH subphenotype is associated with a very high absolute risk for progression to HF in black individuals. 11 However, prior research in this area has been limited by insufficient numbers of clinical events to determine whether differences in the prevalence of malignant LVH contribute to higher risk for HF in black vs white individuals. A major contribution of this pooled cohort study is the demonstration of important racial differences in the prevalence of the malignant LVH phenotype, which may play a role in racial disparities in HF incidence. Incident HF was notably higher among black vs white participants in our study; black men had the highest incidence and, strikingly, black women had a similar incidence of HF as white men. The population risk attributable to malignant LVH was higher in black men and women than white men and women, due to a markedly higher prevalence of malignant LVH, rather than from a higher risk of incident HF associated with malignant LVH. Indeed, we found a slightly lower hazard associated with malignant LVH in blacks versus whites. Mediation analyses suggest that a modest but significant proportion of the excess risk for heart failure among black men and women may be explained by the higher prevalence of malignant LVH in blacks. Taken together, these findings suggest that malignant LVH may contribute to racial disparities in HF risk among black individuals in the general population.
In our study, racial differences in malignant LVH reflected higher rates of ECG-LVH among blacks, with a similar proportion of benign vs malignant LVH in whites and blacks. Previous studies have demonstrated higher rates of LVH among blacks than whites, with larger racial differences observed for ECG vs imaging-defined LVH 3, 29, findings also seen in the present study. Moreover, community-based data from the Atherosclerosis Risk in Communities (ARIC) study reported that pathological hypertrophy and remodeling in response to arterial elastance was augmented in black individuals, suggesting a greater sensitivity to afterload stress. 30 In our study, participants with malignant LVH had more diabetes and hypertension and worse BP control than those without LVH or LVH and normal biomarkers, adverse risk factor profiles also observed to be more common among black vs white participants. Greater sensitivity to stimuli for hypertrophy, combined with a higher prevalence and worse control of risk factors such as hypertension and obesity, 3 likely contribute to racial differences in the prevalence of LVH overall, as well as the malignant LVH subphenotype. Importantly, as we only assessed risk factors at the time of presentation, our adjusted analyses likely underestimate the contribution of poor blood pressure control to racial differences in malignant LVH.
Abnormal hs-cTnT and NT-proBNP have been associated with myocardial fibrosis in MESA, predominantly in a non-ischemic pattern. 31, 32 Moreover, nonischemic patterns of myocardial fibrosis detected by MRI have been associated with increased heart failure risk in the community. 33 Additional study is needed to determine whether the burden and distribution of nonischemic myocardial fibrosis differ between individuals with benign and malignant LVH, and whether fibrosis may be a mechanism contributing to the increased risk of heart failure among these high-risk individuals.
In a recent analysis from the Dallas Heart Study, 4 in which self-reported race/ethnicity and the proportion of African ancestry were modeled together, African ancestry, but not self-reported race, was associated with ECG voltage, LVH, and concentric remodeling, findings that suggest genetic contributions to racial differences in LVH. Relatively little is known regarding which specific genetic traits may contribute to excess LVH in blacks. Genetic variants in APOL1 that are over-represented among individuals of African descent associate with excess nephropathy in blacks, but not with LVH. 34 In contrast, genetic variation in the natriuretic peptide system may play a role. Previous studies have noted that after accounting for known determinants of NP levels, black individuals have lower NT-proBNP levels than whites, and genetic admixture studies indicate that this correlates with the degree of African ancestry. 35-37 Variants in Corin, the enzyme that converts pro-BNP to the active BNP hormone, associate with hypertension and LVH in blacks. 38, 39 These findings raise the possibility that a “natriuretic peptide handicap” may contribute to excess LVH and possibly malignant LVH in blacks. 40 Such a finding may be clinically relevant, given the emergence of neprilysin inhibitors as safe, effective and clinically available therapies that raise BNP and improve HF outcomes
In addition to genetic factors, it is likely that environmental, psychosocial, and behavioral factors play important roles in the observed racial differences in the prevalence of malignant LVH. In previous studies, perceptions of neighborhood cohesion, physical environment, and violence have been shown to associate with obesity in black individuals. 41 In addition, it has also been shown that lower neighborhood socioeconomic level is associated with weight gain, incident hypertension, and LVH. 42-45 Future studies should evaluate whether psychosocial stress and neighborhood environmental factors potentially contribute to race/ethnic differences in malignant LVH prevalence through unhealthy behaviors and poor risk factor control.
Limitations
Minor differences in the HF definition were present across studies, with DHS and ARIC including only hospitalized HF and MESA also including outpatient diagnoses. ECG repolarization abnormalities were not considered in the definition of malignant LVH as they were only adjudicated and available in MESA. However, the low prevalence of ECG-LVH with repolarization abnormalities overall (<1% of MESA participants), and among those with malignant LVH (<9% of participants), argues that there is likely minimal impact from not including repolarization abnormalities in our definition of malignant LVH. Risk factors were assessed and adjusted for only at the time of presentation. Thus, it is not possible to determine the influence of cumulative differences in risk factor burden and control over the lifespan on the observed racial differences in prevalence of malignant LVH.
Clinical Implications
Our findings may have important future implications regarding assessment of heart failure risk among individuals with LVH , especially among blacks who are at highest risk of all race/ethnic groups. When LVH is detected by ECG or cardiac imaging, measuring hs-cTnT and NT-proBNP levels, may help to distinguish those in whom risk for HF is favorable from those at much higher risk, in whom targeted preventive interventions, including aggressive risk factor control, may be warranted. Specifically, application of more aggressive blood pressure targets should be considered in individuals with malignant LVH, given the association of malignant LVH with prior hypertension and subsequent HF, and the strong benefit seen in SPRINT on incident HF. 46 Moreover, given emerging data suggesting that sodium-glucose cotransporter-2 (SGLT2) inhibitors may favorably effect LVH and incident HF among individuals without prior CVD, it is plausible that these agents would reduce HF risk among individuals with malignant LVH. 47, 48 Finally, secondary testing with hs-cTnT and NT-proBNP may be useful when ECG-LVH is detected among athletes, particularly black athletes, to help determine if LVH is pathological or physiological. These hypotheses warrant additional study before clinical implementation.
Conclusions
Among a diverse group of individuals without underlying cardiovascular disease, the combination of LVH and subclinical myocardial injury or neurohormonal stress identifies a malignant subclinical HF phenotype with a significantly increased absolute risk of HF. Individuals with LVH and normal hs-cTnT and NT-proBNP levels exhibit a much lower HF risk, thus displaying a more benign clinical course. A 3-fold higher prevalence of the malignant LVH subphenotype is seen in black individuals, a finding that may account for some of the disparity in heart failure incidence observed among blacks. The underlying mechanisms that contribute to the development of malignant LVH as well as the transitions to clinical HF are not currently well understood. Additional study is needed to determine whether targeted screening to identify malignant LVH among asymptomatic but at-risk black adults, followed by aggressive risk factor modification, may benefit individuals found to have this malignant subphenotype, and reduce racial disparities in HF.
Supplementary Material
Clinical Perspective.
What is new?
Using pooled data from 3 large multi-ethnic cohorts, we characterize a “malignant” subphenotype of left ventricular hypertrophy (LVH), defined by ECG-LVH with abnormal levels of hs-cTnT or NT-proBNP, that is associated with increased risk of heart failure. In contrast, the prognosis for individuals with ECG-LVH and normal biomarker levels appears benign.
Malignant LVH is 3 times more common in black vs white individuals, a disparity that may account for some of the excess risk for heart failure seen among blacks.
What are the clinical implications?
When LVH is detected by ECG or cardiac imaging, measuring hs-cTnT and NT-proBNP levels may help to distinguish those in whom risk for heart failure is favorable from those at much higher risk.
Additional research is needed to identify strategies to prevent the transition to malignant LVH, and to reduce cardiac injury and neurohormonal activation when malignant LVH is present.
Acknowledgements
The authors thank the staff and participants of the ARIC, MESA and DHS studies for their important contributions.
Funding Sources:
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Veterans Affairs System or National Heart, Lung, and Blood Institute; the National Institute on Minority Health and Health Disparities; the National Institutes of Health; or the US Department of Health and Human Services.
Reagents for the hs-cTnT and NTproBNP assays were donated by the Roche Diagnostics Corporation.
The Dallas Heart Study was supported by a grant from the Donald W. Reynolds Foundation and by a grant from the National Center for Advancing Translational Sciences (UL1TR001105). The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
MESA was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Disclosures:
Dr. Neeland has received honoraria, consulting and speaker’s bureau fees, and travel support from Boehringer-Ingelheim/Lilly Alliance (significant), has received a research grant from
Novo Nordisk (significant), and has been a member of the scientific advisory board of AMRA Medical (modest). Dr. Ballantyne is a consultant for Abbott and Roche, and is named on provisional patent no. 61721475 entitled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine and Roche. Dr. Nambi is an investigator on a provisional patent (patent no. 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Roche and Baylor College of Medicine. Dr. Powell-Wiley is funded by the Division of Intramural Research of the National Heart, Lung, and Blood Institute and the Intramural Research Program of the National Institute on Minority Health and Health Disparities. Dr. Berry discloses research support from Abbott. He is also a national coordinator for the STRENGTH trial. Dr. Seliger receives grant support from Roche Diagnostics. Dr. DeFilippi has received consulting, honorarium, and royalties from Siemens Healthcare Diagnostics, Alere/Abbott Diagnostics, FujiRebio, Radiometer, Roche Diagnostis, Ortho Diagnostics, Metabolomics, Quintiles, WebMD and UpToDate. He has also received research grants from Siemens Healthcare Diagnostics, Roche Diagnostics, Abbott Diagnostics, Ortho Diagnostics and FujiRebio. Dr. de Lemos reports grant support from Roche Diagnostics and Abbott Diagnostics, consulting fees from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Quidel Cardiovascular, Inc, Novo Nordisc, Amgen, Regeneron, and Esperion. Dr’s Seliger, DeFilippi and de Lemos have a patent pending (US Patent Application Number: 15/309,754) entitled: “Methods for Assessing Differential Risk for Developing Heart Failure.” All the other authors have no relationships to disclose.
Non-standard Abbreviations and Acronyms:
- ARIC
Atherosclerosis Risk in Communities
- BMI
Body Mass Index
- BSA
Body Surface Area
- cMRI
Cardiac Magnetic Resonance Imaging
- CV
Cardiovascular
- DHS
Dallas Heart Study
- ECG
Electrocardiography
- eGFR
Estimated Glomerular Filtration Rate
- HF
Heart Failure
- Hs-cTnT
High-sensitivity Cardiac Troponin-T
- ICD-9-CM
International Classification of Disease, 9th Revision, Clinical Modification
- IRB
Institutional Review Board
- LVH
Left Ventricular Hypertrophy
- MESA
Multi-Ethnic Study of Atherosclerosis
- NT-proBNP
N-terminal pro-Brain Natriuretic Peptide
- PAF
Population Attributable Fraction
- SGLT2
Sodium-Glucose Cotransporter-2
References
- 1.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL and Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB and Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 3.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D and Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. [DOI] [PubMed] [Google Scholar]
- 4.Alame AJ, Garg S, Kozlitina J, Ayers C, Peshock RM, Matulevicius SA and Drazner MH. Association of African Ancestry With Electrocardiographic Voltage and Concentric Left Ventricular Hypertrophy: The Dallas Heart Study. JAMA Cardiol. 2018;3(12):1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JA and Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG and Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. [DOI] [PubMed] [Google Scholar]
- 7.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr., Abraham WT, Smart FW, Stevenson LW, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. [DOI] [PubMed] [Google Scholar]
- 8.Neeland IJ, Drazner MH, Berry JD, Ayers CR, Defilippi C, Seliger SL, Nambi V, McGuire DK, Omland T and de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters MN, Seliger SL, Christenson RH, Hong-Zohlman SN, Daniels LB, Lima JAC, de Lemos JA, Neeland IJ and deFilippi CR. “Malignant” Left Ventricular Hypertrophy Identifies Subjects at High Risk for Progression to Asymptomatic Left Ventricular Dysfunction, Heart Failure, and Death: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American Heart Association. 2018;7:e006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seliger SL, de Lemos J, Neeland IJ, Christenson R, Gottdiener J, Drazner MH, Berry J, Sorkin J and deFilippi C. Older Adults, “Malignant” Left Ventricular Hypertrophy, and Associated Cardiac-Specific Biomarker Phenotypes to Identify the Differential Risk of New-Onset Reduced Versus Preserved Ejection Fraction Heart Failure: CHS (Cardiovascular Health Study). JACC Heart failure. 2015;3:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Keshvani N, Ayers C, Correa A, Drazner MH, Lewis A, Rodriguez CJ, Hall ME, Fox ER, Mentz RJ, et al. Association of Cardiac Injury and Malignant Left Ventricular Hypertrophy With Risk of Heart Failure in African Americans: The Jackson Heart Study. JAMA Cardiol. 2019;4(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M and Nieto FJ. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. [DOI] [PubMed] [Google Scholar]
- 14.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AC, Gjesdal O, Almeida A, Nacif M, Wu C, Bluemke DA, Brumback L and Lima JA. Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Echocardiography. 2014;31:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G and Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Lemos JA, Ayers CR, Levine BD, deFilippi CR, Wang TJ, Hundley WG, Berry JD, Seliger SL, McGuire DK, Ouyang P, et al. Multimodality Strategy for Cardiovascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation. 2017;135:2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora S, Cook N, Buring JE, Ridker PM and Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loehr LR, Rosamond WD, Chang PP, Folsom AR and Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 21.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA and Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. [DOI] [PubMed] [Google Scholar]
- 22.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL and Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khouri MG, Peshock RM, Ayers CR, de Lemos JA and Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3:164–171. [DOI] [PubMed] [Google Scholar]
- 24.Sokolow M and Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. [DOI] [PubMed] [Google Scholar]
- 25.Zhao D, Post WS, Blasco-Colmenares E, Cheng A, Zhang Y, Deo R, Pastor-Barriuso R, Michos ED, Sotoodehnia N and Guallar E. Racial Differences in Sudden Cardiac Death. Circulation. 2019;139:1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valeri L and VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26:e23–4. [DOI] [PubMed] [Google Scholar]
- 28.Garg S, de Lemos JA, Ayers C, Khouri MG, Pandey A, Berry JD, Peshock RM and Drazner MH. Association of a 4-Tiered Classification of LV Hypertrophy With Adverse CV Outcomes in the General Population. JACC Cardiovasc Imaging. 2015;8:1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DK, Marantz PR, Devereux RB, Kligfield P and Alderman MH. Left ventricular hypertrophy in black and white hypertensives. Standard electrocardiographic criteria overestimate racial differences in prevalence. JAMA. 1992;267:3294–3299. [PubMed] [Google Scholar]
- 30.Fernandes-Silva MM, Shah AM, Hegde S, Goncalves A, Claggett B, Cheng S, Nadruz W, Kitzman DW, Konety SH, Matsushita K, et al. Race-Related Differences in Left Ventricular Structural and Functional Remodeling in Response to Increased Afterload: The ARIC Study. JACC Heart Fail. 2017;5:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JAC, de Lemos JA, Bertoni A and deFilippi CR. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation. 2017;135:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CY, Heckbert SR, Lai S, Ambale-Venkatesh B, Ostovaneh MR, McClelland RL, Lima JAC and Bluemke DA. Association of Elevated NT-proBNP With Myocardial Fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2017;70:3102–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanbhag SM, Greve AM, Aspelund T, Schelbert EB, Cao JJ, Danielsen R, Thornorgeirsson G, Sigurethsson S, Eiriksdottir G, Harris TB, et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur Heart J. 2019;40:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez OM, Limou S, Lin F, Peralta CA, Kramer HJ, Carr JJ, Bibbins-Domingo K, Winkler CA, Lewis CE and Kopp JB. APOL1 nephropathy risk variants do not associate with subclinical atherosclerosis or left ventricular mass in middle-aged black adults. Kidney Int. 2018;93:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta DK, de Lemos JA, Ayers CR, Berry JD and Wang TJ. Racial Differences in Natriuretic Peptide Levels: The Dallas Heart Study. JACC Heart Fail. 2015;3:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta DK, Claggett B, Wells Q, Cheng S, Li M, Maruthur N, Selvin E, Coresh J, Konety S, Butler KR, et al. Racial differences in circulating natriuretic peptide levels: the atherosclerosis risk in communities study. J Am Heart Assoc. 2015;4:e001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta DK, Daniels LB, Cheng S, deFilippi CR, Criqui MH, Maisel AS, Lima JA, Bahrami H, Greenland P, Cushman M, et al. Differences in Natriuretic Peptide Levels by Race/Ethnicity (From the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2017;120:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. [DOI] [PubMed] [Google Scholar]
- 39.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS and Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. [DOI] [PubMed] [Google Scholar]
- 40.de Lemos JA, McGuire DK and Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. [DOI] [PubMed] [Google Scholar]
- 41.Powell-Wiley TM, Ayers CR, de Lemos JA, Lakoski SG, Vega GL, Grundy S, Das SR, Banks-Richard K and Albert MA. Relationship between perceptions about neighborhood environment and prevalent obesity: data from the Dallas Heart Study. Obesity (Silver Spring). 2013;21:E14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell-Wiley TM, Ayers C, Agyemang P, Leonard T, Berrigan D, Ballard-Barbash R, Lian M, Das SR and Hoehner CM. Neighborhood-level socioeconomic deprivation predicts weight gain in a multi-ethnic population: longitudinal data from the Dallas Heart Study. Prev Med. 2014;66:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell-Wiley TM, Cooper-McCann R, Ayers C, Berrigan D, Lian M, McClurkin M, Ballard-Barbash R, Das SR, Hoehner CM and Leonard T. Change in Neighborhood Socioeconomic Status and Weight Gain: Dallas Heart Study. Am J Prev Med. 2015;49:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deere B, Griswold M, Lirette S, Fox E and Sims M. Life Course Socioeconomic Position and Subclinical Disease: The Jackson Heart Study. Ethn Dis. 2016;26:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claudel SE, Adu-Brimpong J, Banks A, Ayers C, Albert MA, Das SR, de Lemos JA, Leonard T, Neeland IJ, Rivers JP and Powell-Wiley TM. Association between neighborhood-level socioeconomic deprivation and incident hypertension: A longitudinal analysis of data from the Dallas heart study. Am Heart J. 2018;204:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 48.Verma S, Mazer CD, Yan AT, et al. EMPA-HEART Cardiolink-6 Trial: A Randomized Trial Evaluating the Effect of Empagliflozin on Left Ventricular Structure, Function and Biomarkers in People With Type 2 Diabetes (T2D) and Coronary Heart Disease American Heart Association Scientific Sessions 2019. Chicago, IL: 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.