Abstract
Background
The IDEAL (Idea, Development, Evaluation, Assessment, Long‐term study) framework is a scheme of investigation for innovative surgical therapeutic interventions. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a procedure based on laparoscopy to deliver intraperitoneal chemotherapy for peritoneal metastases, introduced in 2011. The aim of this article was to review literature on PIPAC and assess whether development of the technique has followed the IDEAL framework.
Methods
A search of MEDLINE and Embase was carried out to identify scientific reports on PIPAC published between January 2000 and February 2019. The studies were categorized according to the IDEAL stages.
Results
Eighty‐six original research papers on PIPAC were identified. There were 23 stage 0, 18 stage 1, 25 stage 2a and six stage 2b studies. Protocol papers for stage 1, 2b and 3 studies, and trial registrations for stage 2a studies, were also identified. The number of centres publishing reports and the number of publications has increased each year. Overall, there has been progression through the IDEAL stages; however, about 60 per cent of clinical reports published in 2018 were stage 1 Idea‐type studies.
Conclusion
Since its introduction, studies investigating PIPAC have progressed in line with the IDEAL framework. However, the majority of studies reported recently were stage 0 and 1 studies.
This review assesses whether the introduction of pressurized intraperitoneal aerosol chemotherapy has followed the IDEAL (Innovation, Development, Evaluation, Assessment, Long‐term use) framework, and whether the problems with surgical innovation have been addressed.
Pipac and review
Antecedentes
El marco conceptual IDEAL (Idea, Desarrollo, Exploración, Evaluación y Estudio a largo plazo) es un esquema de investigación para intervenciones quirúrgicas innovadoras. La quimioterapia intraperitoneal presurizada con aerosol (Pressurised Intraperitoneal Aerosol Chemotherapy, PIPAC) es un procedimiento introducido en 2011 y basado en la laparoscopia para administrar quimioterapia intraperitoneal en las metástasis peritoneales. El objetivo de este manuscrito era revisar la literatura sobre PIPAC y evaluar si el desarrollo de la técnica se ha hecho siguiendo el marco IDEAL.
Métodos
Se realizó una búsqueda en Medline y Embase para identificar publicaciones científicas sobre PIPAC aparecidas entre enero de 2000 y febrero de 2019. Los estudios se clasificaron según las etapas IDEAL.
Resultados
Se identificaron 86 trabajos de investigación originales sobre PIPAC. Hubo 23 estudios de la etapa 0, 18 de la etapa 1, 25 de la etapa 2a y 6 de la etapa 2b. También se identificaron protocolos para estudios de las etapas 1, 2b y 3, así como registros de ensayos para estudios de la etapa 2a. El número de centros que publican trabajos y el número de publicaciones ha aumentado cada año. En general, ha habido una progresión a través de las etapas IDEAL; sin embargo, aproximadamente el 60% de los informes clínicos publicados en 2018 fueron estudios tipo “Idea” de etapa 1.
Conclusión
Desde su introducción, los estudios que investigan PIPAC han progresado en la línea del marco IDEAL. Sin embargo, la mayoría de los estudios publicados recientemente fueron estudios de las etapas 0 y 1.
Introduction
In 2008, the Society of University Surgeons defined an innovative procedure as one that ‘differs from currently accepted local practice, the outcomes of which have not been described, and which may entail risk to the patient’1. The IDEAL (Idea, Development, Evaluation, Assessment, Long‐term study) framework is a scheme of investigation for innovative surgical therapeutic interventions proposed by the Balliol Collaboration in 20092, 3, 4, 5 as a strategy to address concerns regarding shortcomings of research in surgery, with particular reference to novel procedures and practices (Table 1). The framework requires that surgical innovation should be carried out in a coordinated manner, with investigations progressing to a series of randomized trials. The results of the process should be audited using a clinical registry.
Table 1.
Summary of the stages of surgical innovation according to the IDEAL paradigm (adapted from references 2 and 5), with description of the interpretation of these stages in this review relating to published work on pressurized intraperitoneal aerosol chemotherapy
| Stage of innovation | Description | No. of patients | Proposed method of investigation | Studies in this stage included in this review |
|---|---|---|---|---|
| 0 – Idea | Preclinical work in vitro and in animals | None | Varied | Preclinical studies in animals (in vivo and post‐mortem models) and in vitro |
| 1 – Idea | First human applications: proof of concept and small safety studies | Very few | Structured case reports | Case reports and small case series. Occupational health and safety studies. Scientific studies of clinical samples Data presented relate to safety and/or initial feasibility/proof of concept Prospective or retrospective data collection |
| 2a – Development | Major technical details defined but technique remains experimental | Few, selected | Prospective development studies | Larger case series, and single‐arm non‐randomized studies. Scientific studies of clinical samples Prospective or retrospective data collection |
| 2b – Exploration | Individual learning curves progressing quickly, with resulting increase in patient accrual and broadening of indication. Effectiveness still not demonstrated formally | Many, mixed | Research database, explanatory or feasibility RCT | Large case series from a prospectively maintained database, and RCTs. Scientific studies of clinical samples. Prospective study relating to a new indication for the technique Primary outcomes are efficacy‐related. Prospective data collection |
| 3 – Assessment | Procedure is part of many surgeons' practice and becoming the standard of care | Many, variable | RCT | RCT with primary outcome relating to efficacy |
| 4 – Long‐term | Procedure is routine practice and long‐term outcomes and late/rare complications can be monitored | Almost all | Registry, rare case reports | Not applicable |
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases is a laparoscopic procedure used to assess the burden of the peritoneal disease, take biopsies, and deliver intraperitoneal chemotherapy as an aerosol, without increasing the intra‐abdominal pressure6. Intraperitoneal administration of chemotherapy for peritoneal metastases from ovarian cancer was developed in the 1970s7 and has subsequently been used for many different cancer types in the context of heated intraperitoneal chemotherapy (HIPEC) and early postoperative intraperitoneal chemotherapy (EPIC)8. Experimental work9, 10, 11 suggests that both increased temperature and increased pressure can enhance the effect of chemotherapy. The concept of PIPAC was first described in 200012, and was first performed in a patient in 201113.
The aim of this literature review was to assess whether the development and introduction of PIPAC has followed the IDEAL framework.
Methods
A literature search of the Ovid database was carried out using the terms ‘PIPAC’, ‘ePIPAC’, ‘aerosol$ adj3 chemotherapy’ and ‘pressuri$ adj3 chemotherapy’ on 28 February 2019. The first report describing the concept of a ‘therapeutic capnoperitoneum’ was published in 200012, so the search was limited from 1 January 2000 to February 2019. In addition, the reference lists of identified papers were screened, and http://researchgate.net was searched for the term PIPAC to identify other publications.
Conference abstracts, review articles, articles associated with videos, and book chapters were excluded, as were errata to articles. Only articles in English were reviewed. The full text was then obtained, and the studies were graded according to the stages of innovation set out in the IDEAL paradigm. To obtain an up‐to‐date picture of research activity, http://clinicaltrials.gov and the EU Clinical Trials Register (EudraCT) were searched to identify trials. The results were cross‐referenced with the identified publications. Trials that had not yet been reported in the literature were included and assigned a stage as published protocols. Criteria used to assign the stages are described in Table 1.
Results
PIPAC: a review of the literature in stages
The search strategy identified 287 articles after duplicates were removed (Fig. 1). Some 172 articles were excluded after review of the title and abstract, and a further 29 were excluded after reviewing the full text. This left 81 original research papers on PIPAC and/or related technology, and five trial registrations.
Figure 1.
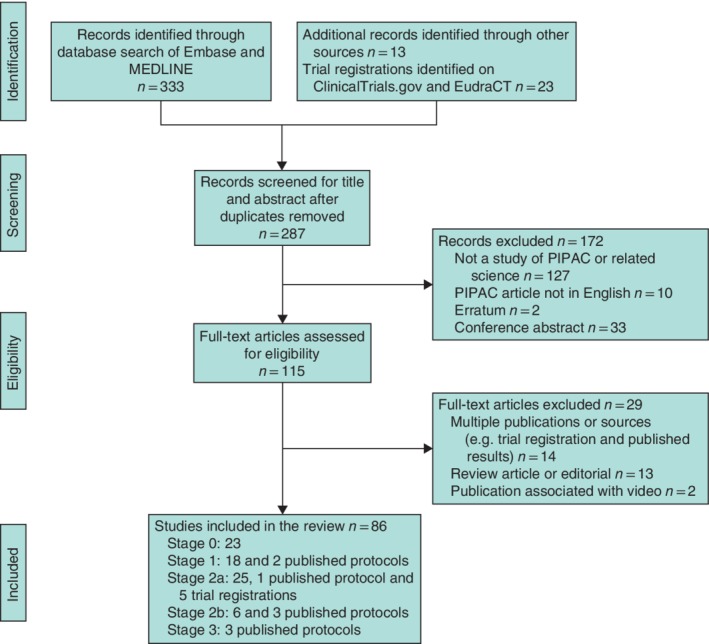
Flow diagram of the literature search and selection of articles for review Search of the EU Clinical Trials Register (EudraCT) and the US National Library of Medicine Trials Register (http://clinicaltrials.gov) is also included. PIPAC, pressurized intraperitoneal aerosol chemotherapy.
Overall, the search identified: 23 stage 0 studies6, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34; 18 stage 1 studies13, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 and two protocol papers52, 53 for stage 1 studies; 25 stage 2a studies54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, one protocol paper79 and five trial registrations (NCT01854255, NCT02735928, NCT03246321, NCT02604784 and NCT03124394) for stage 2a studies; six stage 2b studies80, 81, 82, 83, 84, 85 and three protocol papers86, 87, 88 for stage 2b studies; and three protocol papers89, 90, 91 for stage 3 studies. Fig. 2 shows the evolution of the literature and the geographical location of authors publishing in this field.
Figure 2.
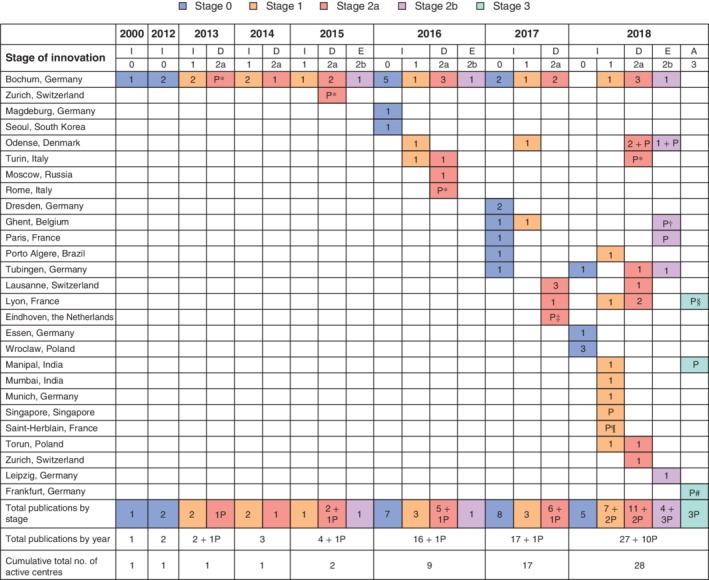
Adoption of pressurized intraperitoneal aerosol chemotherapy according to the IDEAL criteria Studies were identified using the search strategy described in Fig. 1. Included studies were then reviewed by a single author and assigned a stage of innovation according to the description of the stage and proposed method of investigation suggested by the IDEAL paradigm, as described in Table 1. Study centres are described by the city where the institution of the lead author was located. The number of studies published by each centre is shown, broken down by year and stage of innovation: I, Idea; D, Development; E, Exploration; A, Assessment; P, protocol paper. *Protocol from http://clinicaltrials.gov. †Protocol for multicentre study; other authors are from centres in Lausanne (Switzerland), Odense (Denmark), Paris (France), Tübingen (Germany) and Turin (Italy). ‡Multicentre study; other authors are from centres in Clermont Ferrand and Montpellier (France). §Protocol for multicentre study; other authors are from centres in Berlin, Bochum, Tübingen and Wiesbaden (Germany) and Rome (Italy). ¶Protocol for multicentre study; other authors are from centres in Lyon and Paris (France). #Protocol for multicentre study; other authors are from centres in Berlin, Bochum, Leipzig, Regensburg and Tübingen (Germany) and Geneva (Switzerland).
The number of publications increased each year, from five or fewer before 2016, increasing to 17 publications in 2016, 18 in 2017, and 37 in 2018. The cumulative number of active centres has also increased. In general, there has been progression through the stages of innovation, with increasing numbers of Development and Evaluation studies as time passed.
The proportion of papers classified as either IDEAL stage 2a, 2b or 3 has increased over time, with 23 of 37 (62 per cent) of reports published in 2018 describing these types of studies. However, the majority of new centres began their programme of clinical research with a stage 1 Idea study.
The first publication from 11 of 17 centres (65 per cent) that reported clinical results described a stage 1 study, including six of nine centres (67 per cent) that published their first clinical study in 2018.
Stage 0 – Idea: preclinical work
The potential to deliver drugs by aerosolization into the pneumoperitoneum at laparoscopy was demonstrated initially in vivo in a pig model in 200012. Two hypotheses were proposed12 to support the technique. The first hypothesis was that intraperitoneal chemotherapy was superior to intravenous chemotherapy for the treatment of peritoneal metastases. The second was that delivery of intraperitoneal chemotherapy as an aerosolized solution to the pressurized pneumoperitoneum would confer pharmacological advantages over chemotherapy in the liquid phase, specifically better distribution around the abdominal cavity and improved penetration into the tissues.
The first studies6, 12 investigating the technique used methylene blue dye, allowing visual assessment of the distribution and penetration of the aerosol versus lavage in an in vivo pig model. Distribution of the dye was observed to be superior for aerosolization, although this was assessed by visual inspection. The second stage of preclinical testing involved an ex vivo tissue model to assess the penetration of a therapeutic substance, Dbait, into peritoneal tissue from a patient with metastatic endometrial cancer14. Dbait penetration was assessed using immunohistochemistry. Nodules treated with a pressurized aerosol had a more homogeneous drug uptake and deeper penetration than nodules treated by lavage.
Following these preclinical experiments, the same team progressed to human applications. Subsequent preclinical studies by other groups suggested that the first generation of PIPAC technology had limitations. Experiments using chemotherapeutic agents, where drug uptake can be measured objectively, found that drug distribution and penetration in ex vivo 18, 20 and post‐mortem animal21 models was heterogeneous. Although drug was detected in tissue that had not been exposed directly to the aerosol jet, the greatest deposition of the aerosol was opposite to the nebulizer.
Analysis of the aerosol has shown that the droplet size is heterogeneous23. One group24 has developed and brought to market a second device to enable PIPAC, but there is currently little information on the technical performance of this device, nor on its use outside Brazil.
Stage 1 – Idea: first human applications
The first human applications of PIPAC were carried out between 2011 and 2013, and the first reports were published in 201335 and 201413. PIPAC was delivered as an off‐label therapy to patients for whom ‘no satisfactory alternative therapy was available’ as a result of progression on systemic treatment or intolerance to systemic treatment. Patients were treated with doxorubicin 1·5 mg/m2 in 50 ml 0·9 per cent saline, and cisplatin 7·5 mg/m2 in 150 ml 0·9 per cent saline, the doses being set arbitrarily as 10 per cent of the HIPEC doses used at that institution. A formal dose escalation study was not done until 201848. The drugs were administered using the nebulizer device (MicroPump™, Reger Medizintechnik, Rottweil, Germany, until 2015; then CapnoPen®, Capnomed, Villingendorf, Germany, from 2015), and the pneumoperitoneum was then left in a steady state for 30 min. Regressive histological changes were observed in repeat biopsies from consecutive procedures, suggesting efficacy. Mild and moderate adverse events were reported, with patients experiencing fatigue, fever, pain and vomiting after surgery80. Pharmacokinetic data were collected; these determined that systemic absorption of the chemotherapy agents was low, although only doxorubicin was monitored13.
Data on the occupational health and safety aspects of the technique were collected, with no evidence of platinum contamination in the operating theatres36, 80. As centres across Europe started performing the procedure, verification of its occupational health and safety was repeated41, 43, 44, 45. Similarly, as shown in Fig. 2, small case series describing the initial experiences of new centres have been published40, 46, 47, 49, 51.
Stage 2a – Development: larger case series
The PIPAC programme at the original centre in Ruhr University Bochum, Germany, continued and further case series were published, including patients with colorectal cancer78, 81, primary peritoneal cancer81, gastric cancer58, 81 and malignant mesothelioma81. In gastrointestinal cancer78, oxaliplatin was used at a dose of 92 mg/m2. Again, this was an arbitrarily derived dose, and no formal dose‐finding study has been published. A larger series of patients with ovarian cancer included one patient who sustained a life‐threatening bowel perforation; however, this occurred when PIPAC was combined with cytoreductive surgery74.
A PIPAC training programme was developed, and sales of the device required to deliver PIPAC were limited to clinicians who had been certified. This programme is now overseen by the International Society for the Study of Pleura and Peritoneum92. In addition, clinicians were asked to agree to submit data from all cases to an international registry, managed independently by the University of Magdeburg (NCT03210298).
Stage 2b – Exploration: expanding the indications
As experience of PIPAC has increased, there has been evolution in the perioperative management of these patients, and in the indications for surgery. The duration of hospital stay has decreased, and in one centre selected patients undergo PIPAC as a day‐case procedure75. Additional technology has been applied to the technique by some users. Electrostatic PIPAC (ePIPAC) involves the application of electrostatic precipitation to the abdominal cavity, with the aim of increasing drug deposition and adsorption. In preclinical testing, its use during PIPAC increased the penetration of drug17. The use of PIPAC in other scenarios, for example in combination with systemic chemotherapy, is under investigation57, 86. Although the majority of procedures are still performed where disease has progressed despite conventional treatment, one centre87 has proposed a randomized trial that will use PIPAC as adjuvant therapy after resection of high‐risk colorectal cancer. The potential of PIPAC as a downstaging treatment to enable cytoreductive surgery was noted in some of the larger retrospective case series82, 93, and will be evaluated further in an upcoming RCT86.
The future: Moving to stage 3
As documented in Fig. 2, there has been rapid expansion in the published literature since the first human report of PIPAC less than a decade ago, and an increasing number of centres are participating. PIPAC is now in widespread use worldwide. There are a number of RCTs proposed or ongoing86, 89, 90, 91, focusing in particular on survival and aiming to determine whether PIPAC could become part of standard care in the treatment of peritoneal disease.
Discussion
The introduction of PIPAC has, in principle, followed the pattern of investigation advocated by the IDEAL collaboration. However, this review of the development of PIPAC has highlighted some of the difficulties in research in surgical practice. Despite hundreds of reported cases in the literature, the true efficacy of the procedure is still unclear.
In many instances, PIPAC has been initiated outside of a trial. Although centres have published case series of prospectively collected data, there have been multiple publications of data from overlapping time periods, for example in 2014 and 201513, 38, 55, 74 and 201640, 73. Formally registered programmes of research have also been conducted, usually consisting of small studies of safety and feasibility initially, followed by larger cohort studies. However, this pattern has been repeated several times across different centres in different countries. This may have been unnecessary in cases where the same drug doses and procedural steps were used, and may have delayed the progression to larger phase II/III studies.
The difficulty of overcoming the technical learning curve associated with a new procedure has been described as a potential barrier to effective research in surgery in the past3, 4. In the case of PIPAC, there may also be issues relating to patient selection and perioperative care. Other reasons for the duplication of safety studies may relate to individual regulatory requirements in the different countries, or because studies were conceived before the results of earlier trials had been published.
The IDEAL framework recommends that trial protocols are registered publicly, and advocates the reporting of the results from new procedures on online registers available to all surgeons2. This recommendation has not been followed by all PIPAC centres. Although 67 clinical studies were published, only 23 of them were registered on http://clinicaltrials.gov and EudraCT.
There is also the issue of the limited funds available for surgical studies. This can result in the duplication of smaller, less powerful, studies, without assessing the effect of the treatment on longer‐term outcomes.
Ethical concerns have been raised in the past about surgical innovation94. Three systematic reviews95, 96, 97 have summarized the likelihood of adverse effects after PIPAC, although the lack of efficacy data resulting from controlled trials makes a risk versus benefit discussion challenging. There may also be rare complications not identified during early development of the technique, such as the cases of peritoneal sclerosis after PIPAC with oxaliplatin65 and severe hypersensitivity reactions to platinum‐based chemotherapy during PIPAC69. The IDEAL Collaboration suggest that clinicians use all available data from previous studies to counsel patients and disclose the possibility of unknown or unanticipated side‐effects and complications5.
There may be ethical concerns relating to the involvement of the innovator or manufacturer of a new device in ongoing research. In the case of PIPAC, the sale of the MicroPump™/CapnoPen® device was initially limited to clinicians who had been trained by the developer, and a contribution to an independently managed international registry (NCT03210298) was expected. An annual symposium was organized, and latterly this platform for sharing experience and update training has been formalized with the foundation of the International Society for the Study of Pleura and Peritoneum92, contributing to standardization of the technique98. This may prove useful for the conduct of multicentre trials in the future.
Disclosure
The authors declare no conflict of interest.
Funding information
No funding
Presented as a poster to the Annual Meeting of the Association of Coloproctology of Great Britain and Ireland, Dublin, Ireland, July 2019
References
- 1. Biffl WL, Spain DA, Reitsma AM, Minter RM, Upperman J, Wilson M et al; Society of University Surgeons Surgical Innovations Project Team. Responsible development and application of surgical innovations: a position statement of the Society of University Surgeons. J Am Coll Surg 2008; 206: 1204–1209. [DOI] [PubMed] [Google Scholar]
- 2. McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC et al No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009; 374: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 3. Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien PA, Reeves BC et al Challenges in evaluating surgical innovation. Lancet 2009; 374: 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM, Altman DG et al; Balliol Collaboration . Evaluation and stages of surgical innovations. Lancet 2009; 374: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 5. Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J et al No surgical innovation without evaluation: evolution and further development of the ideal framework and recommendations. Ann Surg 2019; 269: 211–220. [DOI] [PubMed] [Google Scholar]
- 6. Solaß W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 2012; 26: 1849–1855. [DOI] [PubMed] [Google Scholar]
- 7. Jones RB, Myers CE, Guarino AM, Dedrick RL, Hubbard SM, DeVita VT. High volume intraperitoneal chemotherapy (‘belly bath’) for ovarian cancer. Pharmacologic basis and early results. Cancer Chemother Pharmacol 1978; 1: 161–166. [DOI] [PubMed] [Google Scholar]
- 8. Goodman MD, McPartland S, Detelich D, Saif MW. Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post‐operative intraperitoneal chemotherapy. J Gastrointest Oncol 2016; 7: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Facy O, Al Samman S, Magnin G, Ghiringhelli F, Ladoire S, Chauffert B et al High pressure enhances the effect of hyperthermia in intraperitoneal chemotherapy with oxaliplatin: an experimental study. Ann Surg 2012; 256: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 10. Jacquet P, Stuart OA, Chang D, Sugarbaker PH. Effects of intra‐abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs 1996; 7: 596–603. [DOI] [PubMed] [Google Scholar]
- 11. van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW et al Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin–DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer 1998; 34: 148–154. [DOI] [PubMed] [Google Scholar]
- 12. Reymond MA, Hu B, Garcia A, Reck T, Köckerling F, Hess J et al Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg Endosc 2000; 14: 51. [DOI] [PubMed] [Google Scholar]
- 13. Solass W, Kerb R, Mürdter T, Giger‐Pabst U, Strumberg D, Tempfer C et al Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014; 21: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solass W, Herbette A, Schwarz T, Hetzel A, Sun JS, Dutreix M et al Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc 2012; 26: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Minnaert AK, Dakwar GR, Benito JM, García Fernández JM, Ceelen W, De Smedt SC et al High‐pressure nebulization as application route for the peritoneal administration of siRNA complexes. Macromol Biosci 2017; 17: 1700024. I. [DOI] [PubMed] [Google Scholar]
- 16. Jung do H, Son SY, Oo AM, Park YS, Shin DJ, Ahn SH et al Feasibility of hyperthermic pressurized intraperitoneal aerosol chemotherapy in a porcine model. Surg Endosc 2016; 30: 4258–4264. [DOI] [PubMed] [Google Scholar]
- 17. Kakchekeeva T, Demtröder C, Herath NI, Griffiths D, Torkington J, Solaß W et al In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann Surg Oncol 2016; 23(Suppl 5): 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khosrawipour V, Khosrawipour T, Diaz‐Carballo D, Förster E, Zieren J, Giger‐Pabst U. Exploring the spatial drug distribution pattern of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol 2016; 23: 1220–1224. [DOI] [PubMed] [Google Scholar]
- 19. Khosrawipour V, Bellendorf A, Khosrawipour C, Hedayat‐Pour Y, Diaz‐Carballo D, Förster E et al Irradiation does not increase the penetration depth of doxorubicin in normal tissue after pressurized intra‐peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. In Vivo 2016; 30: 593–597. [PubMed] [Google Scholar]
- 20. Khosrawipour V, Khosrawipour T, Falkenstein TA, Diaz‐Carballo D, Förster E, Osma A et al Evaluating the effect of MicroPump© position, internal pressure and doxorubicin dosage on efficacy of pressurized intra‐peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. Anticancer Res 2016; 36: 4595–4600. [DOI] [PubMed] [Google Scholar]
- 21. Khosrawipour V, Khosrawipour T, Kern AJ, Osma A, Kabakci B, Diaz‐Carballo D et al Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol 2016; 142: 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khosrawipour V, Giger‐Pabst U, Khosrawipour T, Pour YH, Diaz‐Carballo D, Förster E et al Effect of irradiation on tissue penetration depth of doxorubicin after pressurized intra‐peritoneal aerosol chemotherapy (PIPAC) in a novel ex‐vivo model. J Cancer 2016; 7: 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Göhler D, Khosrawipour V, Khosrawipour T, Diaz‐Carballo D, Falkenstein TA, Zieren J et al Technical description of the microinjection pump (MIP®) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg Endosc 2017; 31: 1778–1784. [DOI] [PubMed] [Google Scholar]
- 24. Seitenfus R, Ferreira PRW, Santos GOD, Alves RJV, Kalil AN, Barros ED et al A prototype single‐port device for pressurized intraperitoneal aerosol chemotherapy. Technical feasibility and local drug distribution. Acta Cir Bras 2017; 32: 1056–1063. [DOI] [PubMed] [Google Scholar]
- 25. Khosrawipour V, Diaz‐Carballo D, Acikelli AH, Khosrawipour T, Falkenstein TA, Wu D et al Cytotoxic effect of different treatment parameters in pressurized intraperitoneal aerosol chemotherapy (PIPAC) on the in vitro proliferation of human colonic cancer cells. World J Surg Oncol 2017; 15: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eveno C, Haidara A, Ali I, Pimpie C, Mirshahi M, Pocard M. Experimental pharmacokinetics evaluation of chemotherapy delivery by PIPAC for colon cancer: first evidence for efficacy. Pleura Peritoneum 2017; 2: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schnelle D, Weinreich FJ, Kibat J, Reymond MA. A new ex vivo model for optimizing distribution of therapeutic aerosols: the (inverted) bovine urinary bladder. Pleura Peritoneum 2017; 2: 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khosrawipour V, Khosrawipour T, Hedayat‐Pour Y, Diaz‐Carballo D, Bellendorf A, Böse‐Ribeiro H et al Effect of whole‐abdominal irradiation on penetration depth of doxorubicin in normal tissue after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a post‐mortem swine model. Anticancer Res 2017; 37: 1677–1680. [DOI] [PubMed] [Google Scholar]
- 29. Mikolajczyk A, Khosrawipour V, Schubert J, Chaudhry H, Pigazzi A, Khosrawipour T. Particle stability during pressurized intra‐peritoneal aerosol chemotherapy (PIPAC). Anticancer Res 2018; 38: 4645–4649. [DOI] [PubMed] [Google Scholar]
- 30. Bellendorf A, Khosrawipour V, Khosrawipour T, Siebigteroth S, Cohnen J, Diaz‐Carballo D et al Scintigraphic peritoneography reveals a non‐uniform 99mTc‐pertechnetat aerosol distribution pattern for pressurized intra‐peritoneal aerosol chemotherapy (PIPAC) in a swine model. Surg Endosc 2018; 32: 166–174. [DOI] [PubMed] [Google Scholar]
- 31. Weinreich J, Struller F, Sautkin I, Giuashvili S, Reymond M, Königsrainer A et al Chemosensitivity of various peritoneal cancer cell lines to HIPEC and PIPAC: comparison of an experimental duplex drug to standard drug regimens in vitro Invest New Drugs 2019: 37; 415–423. [DOI] [PubMed] [Google Scholar]
- 32. Mikolajczyk A, Khosrawipour V, Schubert J, Grzesiak J, Chaudhry H, Pigazzi A et al Effect of liposomal doxorubicin in pressurized intra‐peritoneal aerosol chemotherapy (PIPAC). J Cancer 2018; 9: 4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khosrawipour V, Mikolajczyk A, Schubert J, Khosrawipour T. Pressurized intra‐peritoneal aerosol chemotherapy (PIPAC) via endoscopical microcatheter system. Anticancer Res 2018; 38: 3447–3452. [DOI] [PubMed] [Google Scholar]
- 34. Göhler D, Große S, Bellendorf A, Falkenstein TA, Ouaissi M, Zieren J et al Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an improved approach for pressurised intraperitoneal aerosol chemotherapy (PIPAC): technical description, experimental validation and first proof of concept. Beilstein J Nanotechnol 2017; 8: 2729–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blanco A, Giger‐Pabst U, Solass W, Zieren J, Reymond MA. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol 2013; 20: 2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Solass W, Giger‐Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol 2013; 20: 3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tempfer CB, Solass W, Buerkle B, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in a woman with pseudomyxoma peritonei: a case report. Gynecol Oncol Rep 2014; 10: 32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giger‐Pabst U, Solass W, Buerkle B, Reymond MA, Tempfer CB. Low‐dose pressurized intraperitoneal aerosol chemotherapy (PIPAC) as an alternative therapy for ovarian cancer in an octogenarian patient. Anticancer Res 2015; 35: 2309–2314. [PubMed] [Google Scholar]
- 39. Reymond M, Demtroeder C, Solass W, Winnekendonk G, Tempfer C. Electrostatic precipitation pressurized intraperitoneal aerosol chemotherapy (ePIPAC): first in‐human application. Pleura Peritoneum 2016; 1: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vaira M, Robella M, Borsano A, De Simone M. Single‐port access for pressurized intraperitoneal aerosol chemotherapy (PIPAC): technique, feasibility and safety. Pleura Peritoneum 2016; 1: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Graversen M, Pedersen PB, Mortensen MB. Environmental safety during the administration of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2016; 1: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tempfer CB, Hartmann F, Hilal Z, Rezniczek GA. Intraperitoneal cisplatin and doxorubicin as maintenance chemotherapy for unresectable ovarian cancer: a case report. BMC Cancer 2017; 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Willaert W, Sessink P, Ceelen W. Occupational safety of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2017; 2: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ndaw S, Hanser O, Kenepekian V, Vidal M, Melczer M, Remy A et al Occupational exposure to platinum drugs during intraperitoneal chemotherapy. Biomonitoring and surface contamination. Toxicol Lett 2018; 298: 171–176. [DOI] [PubMed] [Google Scholar]
- 45. Ametsbichler P, Böhlandt A, Nowak D, Schierl R. Occupational exposure to cisplatin/oxaliplatin during pressurized intraperitoneal aerosol chemotherapy (PIPAC)? Eur J Surg Oncol 2018; 44: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 46. Nowacki M, Grzanka D, Zegarski W. Pressurized intraperitoneal aerosol chemotherapy after misdiagnosed gastric cancer: case report and review of the literature. World J Gastroenterol 2018; 24: 2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Somashekhar S, Rajagopal AK, Zaveri SS, Chandrashekhar RK, Rauthan A, Rakshit SH. First Indian study on pressurized intraperitoneal aerosol chemotherapy (PIPAC) procedure for advanced peritoneal carcinomatosis secondary to epithelial ovarian cancer. Indian J Gynecol Oncol 2018; 16: 25. [Google Scholar]
- 48. Tempfer CB, Giger‐Pabst U, Seebacher V, Petersen M, Dogan A, Rezniczek GA. A phase I, single‐arm, open‐label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol 2018; 150: 23–30. [DOI] [PubMed] [Google Scholar]
- 49. Solanki SL, Kumar PP, DeSouza A, Saklani AP. Perioperative concerns and management of pressurised intraperitoneal aerosolised chemotherapy: report of two cases. Indian J Anaesth 2018; 62: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Graversen M, Detlefsen S, Bjerregaard JK, Pfeiffer P, Mortensen MB. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Clin Exp Metastasis 2017; 34: 309–314. [DOI] [PubMed] [Google Scholar]
- 51. Seitenfus R, Kalil AN, Barros ED, Fedrizzi G. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) through a single port: alternative delivery for the control of peritoneal metastases. Rev Col Bras Cir 2018; 45: e1909. [DOI] [PubMed] [Google Scholar]
- 52. Dumont F, Senellart H, Pein F, Campion L, Glehen O, Goere D et al Phase I/II study of oxaliplatin dose escalation via a laparoscopic approach using pressurized aerosol intraperitoneal chemotherapy (PIPOX trial) for nonresectable peritoneal metastases of digestive cancers (stomach, small bowel and colorectal): rationale and design. Pleura Peritoneum 2018; 3: 20180120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim G, Tan HL, Chen E, Teo SC, Jang CJM, Ho J et al Study protocol: phase 1 dose escalating study of pressurized intra‐peritoneal aerosol chemotherapy (PIPAC) with oxaliplatin in peritoneal metastasis. Pleura Peritoneum 2018; 3: 20180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger‐Pabst U, Zieren J et al Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol 2015; 137: 223–228. [DOI] [PubMed] [Google Scholar]
- 55. Tempfer CB, Rezniczek GA, Ende P, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin in women with peritoneal carcinomatosis: a cohort study. Anticancer Res 2015; 35: 6723–6729. [DOI] [PubMed] [Google Scholar]
- 56. Rezniczek GA, Jüngst F, Jütte H, Tannapfel A, Hilal Z, Hefler LA et al Dynamic changes of tumor gene expression during repeated pressurized intraperitoneal aerosol chemotherapy (PIPAC) in women with peritoneal cancer. BMC Cancer 2016; 16: 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khomyakov V, Ryabov A, Ivanov A, Bolotina L, Utkina A, Volchenko N et al Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low‐dose cisplatin and doxorubicin administered as a pressurized aerosol: an open‐label, phase‐2 study (PIPAC‐GA2). Pleura Peritoneum 2016; 1: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nadiradze G, Giger‐Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low‐dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 2016; 20: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hilal Z, Rezniczek GA, Klenke R, Dogan A, Tempfer CB. Nutritional status, cachexia, and anorexia in women with peritoneal metastasis and intraperitoneal chemotherapy: a longitudinal analysis. J Gynecol Oncol 2017; 28: e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khosrawipour T, Khosrawipour V, Giger‐Pabst U. Pressurized intra peritoneal aerosol chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS One 2017; 12: e0186709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alyami M, Gagniere J, Sgarbura O, Cabelguenne D, Villeneuve L, Pezet D et al Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol 2017; 43: 2178–2183. [DOI] [PubMed] [Google Scholar]
- 62. Hübner M, Grass F, Teixeira‐Farinha H, Pache B, Mathevet P, Demartines N. Pressurized intraperitoneal aerosol chemotherapy – practical aspects. Eur J Surg Oncol 2017; 43: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 63. Teixeira Farinha H, Grass F, Kefleyesus A, Achtari C, Romain B, Montemurro M et al Impact of pressurized intraperitoneal aerosol chemotherapy on quality of life and symptoms in patients with peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract 2017; 2017: 4596176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hübner M, Teixeira Farinha H, Grass F, Wolfer A, Mathevet P, Hahnloser D et al Feasibility and safety of pressurized intraperitoneal aerosol chemotherapy for peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract 2017; 2017: 6852749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Graversen M, Detlefsen S, Pfeiffer P, Lundell L, Mortensen MB. Severe peritoneal sclerosis after repeated pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC OX): report of two cases and literature survey. Clin Exp Metastasis 2018; 35: 103–108. [DOI] [PubMed] [Google Scholar]
- 66. Larbre V, Alyami M, Mercier F, Vantard N, Bonnefoy I, Opsomer MA et al No renal toxicity after repeated treatment with pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with unresectable peritoneal metastasis. Anticancer Res 2018; 38: 6869–6875. [DOI] [PubMed] [Google Scholar]
- 67. Horvath P, Beckert S, Struller F, Königsrainer A, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases of pancreas and biliary tract cancer. Clin Exp Metastasis 2018; 35: 635–640. [DOI] [PubMed] [Google Scholar]
- 68. Giger‐Pabst U, Demtröder C, Falkenstein TA, Ouaissi M, Götze TO , Rezniczek GA et al Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer 2018; 18: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siebert M, Alyami M, Mercier F, Gallice C, Villeneuve L, Bérard F et al Severe hypersensitivity reactions to platinum compounds post‐pressurized intraperitoneal aerosol chemotherapy (PIPAC): first literature report. Cancer Chemother Pharmacol 2019; 83: 425–430. [DOI] [PubMed] [Google Scholar]
- 70. Tempfer CB, Hilal Z, Dogan A, Petersen M, Rezniczek GA. Concentrations of cisplatin and doxorubicin in ascites and peritoneal tumor nodules before and after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peritoneal metastasis. Eur J Surg Oncol 2018; 44: 1112–1117. [DOI] [PubMed] [Google Scholar]
- 71. Falkenstein TA, Götze TO , Ouaissi M, Tempfer CB, Giger‐Pabst U, Demtröder C. First clinical data of pressurized intraperitoneal aerosol chemotherapy (PIPAC) as salvage therapy for peritoneal metastatic biliary tract cancer. Anticancer Res 2018; 38: 373–378. [DOI] [PubMed] [Google Scholar]
- 72. Teixeira Farinha H, Grass F, Labgaa I, Pache B, Demartines N, Hübner M. Inflammatory response and toxicity after pressurized intraperitoneal aerosol chemotherapy. J Cancer 2018; 9: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol 2016; 14: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tempfer CB, Celik I, Solass W, Buerkle B, Pabst UG, Zieren J et al Activity of pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum‐resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol 2014; 132: 307–311. [DOI] [PubMed] [Google Scholar]
- 75. Graversen M, Detlefsen S, Bjerregaard JK, Fristrup CW, Pfeiffer P, Mortensen MB. Prospective, single‐center implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol 2018; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nowacki M, Zegarski W. The scientific report from the first pressurized intraperitoneal aerosol chemotherapy (PIPAC) procedures performed in the eastern part of central Europe. J Int Med Res 2018; 46: 3748–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kuchen N, Cereser T, Hailemariam S, Schoeb O. Safety and efficacy of pressurized intraperitoneal/intrathoracic aerosol chemotherapy (PIPAC/PITAC) in patients with peritoneal and/or pleural carcinomatosis: a preliminary experience. J Med Therap 2018; 2: 2–6. [Google Scholar]
- 78. Demtröder C, Solass W, Zieren J, Strumberg D, Giger‐Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 2016; 18: 364–371. [DOI] [PubMed] [Google Scholar]
- 79. Graversen M, Detlefsen S, Asmussen J, Mahdi B, Fristrup C, Pfeiffer P et al Treatment of peritoneal carcinomatosis with pressurized intraperitoneal aerosol chemotherapy – PIPAC‐OPC2. Pleura Peritoneum 2018; 3: 20180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kurtz F, Struller F, Horvath P, Solass W, Bösmüller H, Königsrainer A et al Feasibility, safety, and efficacy of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis: a registry study. Gastroenterol Res Pract 2018; 2018: 2743985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Odendahl K, Solass W, Demtröder C, Giger‐Pabst U, Zieren J, Tempfer C et al Quality of life of patients with end‐stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur J Surg Oncol 2015; 41: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 82. Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2016; 14: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Graversen M, Lundell L, Fristrup C, Pfeiffer P, Mortensen MB. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as an outpatient procedure. Pleura Peritoneum 2018; 3: 20180128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Giger‐Pabst U, Tempfer CB. How to perform safe and technically optimized pressurized intraperitoneal aerosol chemotherapy (PIPAC): experience after a consecutive series of 1200 procedures. J Gastrointest Surg 2018; 22: 2187–2193. [DOI] [PubMed] [Google Scholar]
- 85. Gockel I, Jansen‐Winkeln B, Haase L, Rhode P, Mehdorn M, Niebisch S et al Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in gastric cancer patients with peritoneal metastasis (PM): results of a single‐center experience and register study. J Gastric Cancer 2018; 18: 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Eveno C, Jouvin I, Pocard M. PIPAC EstoK 01: pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: a randomized and multicenter phase II study. Pleura Peritoneum 2018; 3: 20180116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Graversen M, Detlefsen S, Fristrup C, Pfeiffer P, Mortensen MB. Adjuvant pressurized intraperitoneal aerosol chemotherapy (PIPAC) in resected high‐risk colon cancer patients – study protocol for the PIPAC‐OPC3 trial. A prospective, controlled phase 2 study. Pleura Peritoneum 2018; 3: 20180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Van De Sande L, Graversen M, Hubner M, Pocard M, Reymond M, Vaira M et al Intraperitoneal aerosolization of albumin‐stabilized paclitaxel nanoparticles (Abraxane™) for peritoneal carcinomatosis – a phase I first‐in‐human study. Pleura Peritoneum 2018; 3: 20180112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bakrin N, Tempfer C, Scambia G, De Simone M, Gabriel B, Grischke EM et al PIPAC‐OV3: a multicenter, open‐label, randomized, two‐arm phase III trial of the effect on progression‐free survival of cisplatin and doxorubicin as pressurized intra‐peritoneal aerosol chemotherapy (PIPAC) vs. chemotherapy alone in patients with platinum‐resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer. Pleura Peritoneum 2018; 3: 20180114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Somashekhar SP, Ashwin KR, Rauthan CA, Rohit KC. Randomized control trial comparing quality of life of patients with end‐stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) and intravenous chemotherapy. Pleura Peritoneum 2018; 3: 20180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oliver Goetze T, Al‐Batran SE, Pabst U, Reymond M, Tempfer C, Bechstein WO et al Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in combination with standard of care chemotherapy in primarily untreated chemo naïve upper GI‐adenocarcinomas with peritoneal seeding – a phase II/III trial of the AIO/CAOGI/ACO. Pleura Peritoneum 2018; 3: 20180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reymond MA. Founding of the International Society for the Study of Pleura and Peritoneum (ISSPP). Pleura Peritoneum 2018; 3: 20180125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Alyami M, Mercier F, Siebert M, Bbonnot PE, Bonnefoy I, Villeneuve L et al Pressurized intraperitoneal aerosol chemotherapy (PIPAC) before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for nonresectable peritoneal metastasis. Eur J Surg Oncol 2019; 45: e16–e17. [Google Scholar]
- 94. Johnson J, Rogers W, Lotz M, Townley C, Meyerson D, Tomossy G. Ethical challenges of innovative surgery: a response to the IDEAL recommendations. Lancet 2010; 376: 1113–1115. [DOI] [PubMed] [Google Scholar]
- 95. Grass F, Vuagniaux A, Teixeira‐Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 2017; 104: 669–678. [DOI] [PubMed] [Google Scholar]
- 96. Tempfer C, Giger‐Pabst U, Hilal Z, Dogan A, Rezniczek GA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch Gynecol Obstet 2018; 298: 243–257. [DOI] [PubMed] [Google Scholar]
- 97. Garg PK, Jara M, Alberto M, Rau B. The role of pressurized intraperitoneal aerosol chemotherapy in the management of gastric cancer: a systematic review. Pleura Peritoneum 2019; 4: 20180127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nowacki M, Alyami M, Villeneuve L, Mercier F, Hubner M, Willaert W et al Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: an international survey study. Eur J Surg Oncol 2018; 44: 991–996. [DOI] [PubMed] [Google Scholar]


