Abstract
Sporadic colon cancer accounts for ~80% of CRC, with high incidence in western societies strongly linked to dietary patterns. The only mouse model for sporadic CRC results from feeding mice a purified rodent western-style diet (NWD1), establishing mouse intake of several common nutrients that mimic for each its level consumed in western populations at higher risk for colon cancer (higher fat, lower vitamin D3, calcium, methyl donors and fiber). This causes sporadic colon and small intestinal tumors at an incidence and frequency similar to that of humans. NWD1 perturbs intestinal cell maturation and Wnt signaling throughout villi and colonic crypts before tumors are detected. Surprisingly, feeding NWD1 decreases mouse Lgr5hi intestinal stem cell contribution to homeostasis and tumorigenesis, associated with extensive Lgr5hi cell transcriptional reprogramming, with nutrient levels interactive in these effects. There is a key impact of the lower vitamin D3 in NWD1 and its signaling through the Vdr. The DNA mismatch repair pathway is elevated in Lgr5hi cells by lower vitamin D3 and/or calcium in NWD1, reducing accumulation of relevant somatic mutations detected by single cell exome sequencing. There are also alterations in metabolic pathways, including down-regulation of oxidative phosphorylation. In compensation for compromise of Lgr5hi cells, NWD1 also reprograms cells derived from the Bmi1+ population, defined as those cells marked in Bmi1creERT2, Rosa26tom mice following tamoxifen injection, and at least a portion of these cells then function and persist as stem-like cells in mucosal homeostasis and tumorigenesis.
The data establish a key role of the nutrient environment, and vitamin D signaling, in defining contribution of at least two different stem cell populations to mucosal homeostasis and tumorigenesis. This raises significant questions regarding impact of variable human diets on which and how multiple potential intestinal stem cell populations function in the human and give rise to tumors. Moreover, genetic and epigenetic changes in long-lived stem cells have important implications for understanding the effects of vitamin D and other nutrients on intestinal homeostasis and on intervention strategies for altering probability of tumor development.
Keywords: intestinal stem cells, nutritional environment, vitamin D, intestinal homeostasis, intestinal tumors
Introduction:
Continuous turnover of epithelial cells of the intestinal and colonic mucosa requires continuous production and differentiation of these cells to maintain mucosal homeostasis and normal functions. In the mouse, this is accomplished primarily by division of crypt base columnar (CBC) cells located at the bottom of the crypt that express a high level of the marker Lgr5 (Lgr5hi cells) [1–3]. However, there is extensive plasticity in the ability of other cell populations to take over this responsibility when Lgr5hi cells are damaged or ablated experimentally by radiation, chemicals, targeting of toxins, or genetic manipulation [1]. Here we summarize evidence that this plasticity can also be marshalled by changes in the nutritional environment, and in particular by the status of vitamin D signaling of the Lgr5hi cells. The implications of this for understanding the impact of environmental influence on the incidence of colorectal cancer (CRC), and for how this may inform intervention strategies to prevent CRC, are discussed.
1.1. Intestinal stem cells in homeostasis.
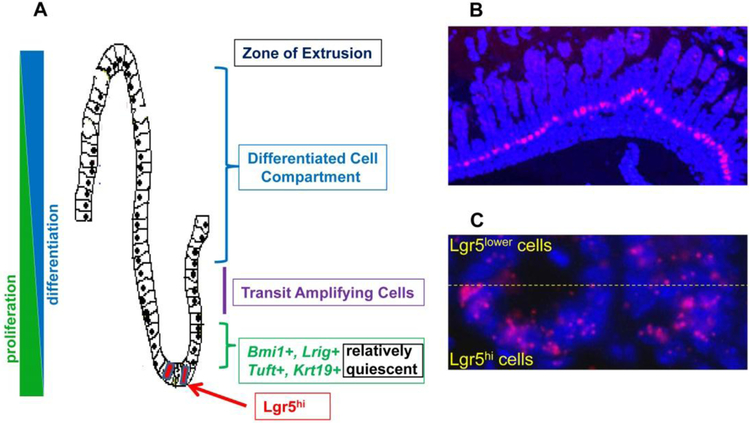
It was long appreciated that there must be a stem cell population underlying the constant turnover of the intestinal mucosa (reviewed in [4]). Two reports identifying specific cell populations with properties of stem cells were turning points in understanding how the mucosa constantly renewed itself. First, CBC cells at the crypt bottom of the mouse small intestine were identified as interdigitated with Paneth cells, and shown to be able to give rise to all mucosal epithelial cells [1–3] (Fig 1A–C). Soon thereafter, it was reported that cells at about the +4 position from the bottom of the mouse crypt, expressing the marker Bmi1, could also give rise to all cell lineages of the mucosa [5](Fig 1A). These reports initiated an explosion of research – not to be reviewed here – investigating these and other intestinal cell populations. A summary of the model that dominates the field is that the CBC Lgr5hi cells are normally responsible for maintaining the mucosa. A key feature of these cells is that they express high levels of the surface marker Lgr5, a receptor for the growth factor Rspondin that amplifies Wnt signals [6, 7] (Fig 1B,C). However, it is clear that when these Lgr5hi cells are ablated or damaged by radiation, chemicals or other experimental procedures, other cell types can “seamlessly take over maintenance of the mucosa” [1]. This is accomplished by mobilization of cells from other cell compartments to function as stem or progenitor-like cells. Several of the cell compartments that can contribute such cells are depicted in Fig 1A: Bmi1+ cells at the +4 position [8], although there is heterogeneity in this population in terms of mucosal distribution and co-expression with other stem cell markers [5, 8, 9]; differentiated cells that can revert to a more “stem- or progenitor-like” state [10, 11]; L-rig1+ cells [12]; Tuft cells expressing DCLK1 [13]; Krt19+ cells [14]; and Paneth cells [15–17]. Molecular analysis has also suggested that there may not be dedicated pre-existing cells that take over when Lgr5hi cells become non-functional as stem cells, but that a multi-potential progenitor cell is recruited as needed [18].
Fig 1.
A) the architectural organization of cell compartments of the intestinal crypt; B) low magnification of intestinal tissue showing Lgr5 expression (red) from in situ hybridization of RNAscope probes specific for Lgr5 mRNA at the crypt bottom; C) higher magnification depicting Lgr5hi cells at the crypt base, and Lgr5lower cells just above these. In B and C, the slides are counterstained with DAPI to reveal the position of nuclei.
In summary, these and other studies have established that there is tremendous plasticity of intestinal epithelial cells to serve as stem or progenitor cells, and that there may be a hierarchy of such cells that can be mobilized as needed. It is important, however, that although mechanisms have been dissected that can potentially mobilize different populations, there are key unanswered questions: how do the different cell populations “sense” that Lgr5hi cells are no longer functioning as stem cells; are signals transduced uniformly to each potential reserve population or are there conditions which specify which cell population is mobilized?
Stem cell populations and intestinal tumorigenesis.
The definition of what constitutes an adult tissue-specific stem cell population is somewhat fluid, especially with the evidence that multiple populations can acquire the characteristics of stem cells under different conditions. However, among important characteristics of stem cells are the ability to self-renew and to give rise to many or all of the differentiated cell types of a tissue. In the case of the intestine, Lgr5hi cells have been shown to divide, and then by a stochastic process, one of the daughter cells can become a self-replicating stem cell while the other begins the process of differentiation into the many epithelial cell types that comprise the intestinal mucosa [19, 20]. This distinction in the fate of daughter cells likely resides in the environment in which each finds itself. The Lgr5hi stem cell divides symmetrically, with the daughter cell remaining in the crypt – and thus exposed to stem cell niche signals – retaining functions of a stem cell, while the cell that begins to migrate up from the bottom of the crypt loses short-range Wnt signals and/or is exposed to other signals that initiate the process of differentiation [4]. These additional signals likely encompass important pathways that involve cell-cell contacts, such as Notch signaling [21–24], as well as the making and breaking of contacts with cells of the myofibroblast sheath and Paneth cells residing at the crypt bottom (reviewed in [4]).
The shift from a stem to a differentiating cell is rapid and complex: comparison of the Lgr5hi cell gene expression signature compared to that of the immediate daughter cells that are Lgr5lower (i.e. following the first symmetric cell division and movement out of the stem cell niche, as shown in Fig. 1C) identified 512 genes that differed in expression between the two states [9]. This demonstrates a considerable reprogramming that takes place immediately in the cells destined to give rise to all epithelial lineages.
In addition to the ability of stem cells to give rise to a plethora of differentiated cell types, stem cells are likely the cell of origin of tumors by virtue of their ability to continuously divide and self-renew. For example, both human and mouse intestinal tumors are most frequently initiated by inactivating mutations in the Apc gene, which removes a brake on Wnt signaling (reviewed in [25]). And indeed when inactivation of the Apc gene was targeted to Lgr5hi cells, intestinal tumors were initiated [26–28]. Moreover, in accord with the necessity of cells to have features of “stemness” in order to initiate tumors, targeting an Apc mutation to Bmi1+ cells also can give rise to intestinal tumors, as does targeting the mutation to other cell types when they are mobilized to take over for the canonical Lgr5hi stem cell when it is damaged. This included targeting the mutation to differentiated cells, but importantly, only when such cells had been induced to revert to a more stem cell-like state [10, 11]. Therefore, “stemness” seems to be a requirement for cells to be able to initiate tumor development once they acquire an initiating mutation or complement of mutations.
Dietary and vitamin D3 impact on intestinal stem cells and tumorigenesis
An aspect of our work on intestinal homeostasis and tumorigenesis has focused on the role of nutritional interactions in stem cell functions. This has made use of a novel dietary model of human sporadic colon cancer (sCRC) – the form of the disease that accounts for about 80% of all human CRC. To model conditions that may increase the probability for development of sCRC, Newmark and Lipkin developed the “western-style diet” (NWD1) based on the control purified diet AIN76A [29–33]. The principle of nutrient density was used to adjust the dietary formulation to generate intake for the mouse of several common nutrients each at its level consumed in western populations that are at higher risk for colonic cancer. The changes to NWD1 compared to AIN76A control are higher fat, lower vitamin D3, calcium, methyl donors (folate/methionine) and fiber, since there are epidemiological data associating each of these with elevated probability of developing sporadic human CRC. As a result of these nutritional changes, feeding NWD1 to mice is highly pro-tumorigenic: in mouse genetic models of intestinal tumor development, feeding NWD1 increased tumor number and caused more rapid tumor development than in mice fed control diet [34–37]. This was independent of the genetic driver(s) in each model that caused the tumors, or the aggressiveness of tumor development in each model when fed control diet. Therefore, there were common pro-tumorigenic effects of the diet on the intestinal mucosa independent of genetic etiology. Most important is that wild-type C57Bl/6 mice rarely - if ever - develop intestinal tumors. However, when fed the NWD1 from weaning, cohorts of these mice developed 1–2 intestinal tumors in the small and/or large intestine in 20% of the mice after 1–2 years, or about one-half to two thirds of their lifespan [33, 38, 39]. This is similar to the incidence, frequency and lag for tumor development found in the general US population undergoing routine screening by colonoscopy. Therefore, this is the only model of sporadic intestinal tumorigenesis, accounting for the majority of the human disease, and the form of the disease for which incidence is most influenced by long term nutritional patterns.
Mice fed NWD1 are generally healthy until tumors develop; they gain more weight, but do not become obese, and consistent with the higher fat content of NWD1, mice fed the diet shift to greater utilization of fat relative to carbohydrate as an energy source [40]. Despite the lower levels of vitamin D3 and calcium in NWD1, the mice do not suffer from rickets or altered bone mineral density [40]. Moreover, although the mucosa is “primed” to cause higher probability for tumor development, it appears histologically normal. This concept – that there are field effects in a tissue that alter the probability of eventual tumor development and progression – was proposed in 1953 [41], and is considered a milestone in chemoprevention research [42]. In the NWD1 fed mice, such alterations that precede tumor development by 1 or more years include expansion of the proliferative compartment, altered balance of lineage specific cell markers, a shift of cells towards glycolytic metabolism, ectopic expression of Paneth cell markers in the villi and crypts of the small and large intestine, and elevated Wnt signaling throughout the villi and crypts of the small and large intestine [38, 43].
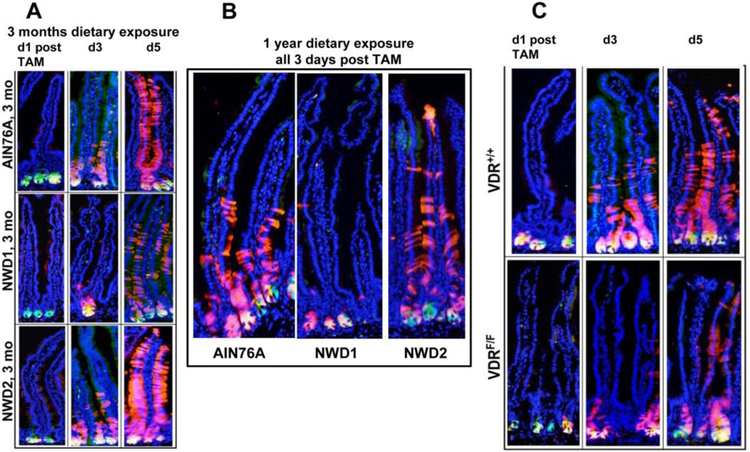
These distinct alterations in the normal appearing and functioning intestinal mucosa, especially perturbed maturation of epithelial cells and elevated Wnt signaling, prompted investigation of whether NWD1 had altered the function of intestinal stem cells. The first approach investigated lineage tracing of the progeny of the Lgr5hi stem cells at the bottom of the crypt [44]. This used a mouse model in which Lgrhi stem cells expressed a green fluorescent marker as long as they were at the crypt bottom, but that their progeny were induced to permanently express a red fluorescent marker, and thus could be tracked for their subsequent fate. In mice fed control AIN76A diet for 3 months from weaning, the results recapitulated that published for mice fed other control diets: from 1 to 5 days, “red” progeny emerged from the “green” Lgr5hi cells at the crypt base, and progressively made their way up the villi, populating the mucosa with newly generated epithelial cells (Fig 2A-top row). However, when fed NWD1 for 3 months, many of these red cells remained at the bottom of the crypt: Lgr5hi cells which were now green and red appeared yellow at the crypt bottom, with many fewer red cells migrating into, and then up, the villi (Fig 2A-middle row). The same was true for the mice fed NWD1 vs AIN76A for 1 year (Fig 2B). However, when a rescue diet was fed, in which vitamin D3 and calcium were elevated in the NWD1 (ie, NWD2), normal lineage tracing of the Lgr5hi cell progeny was present (Fig 2A-bottom row; 2B).
Fig 2: Dietary and genetic effects on lineage tracing from Lgr5 cells.
Lgr5EGFP-creERT2, Rosa26tom mice were fed either AIN76A, NWD1 or NWD2 (NWD1+ higher vitamin D and calcium) for 3 months from weaning, injected with tamoxifen, and sacrificed 1–5 days thereafter. Frozen sections of the intestine were observed for fluorescence. B) Mice of the same strain were maintained on diets for 1 year from weaning, injected with tamoxifen and sections examined 3 days post Tamoxifen. C) The same strain of mice in A and B, but in the bottom panel also Vdrflox/flox, were fed control AIN76A diet for 3 months from weaning, injected with tamoxifen, and the sacrificed 1, 3 or 5 days later. All data are reprinted from [44].
Therefore, Lgr5hi cell stem cell function was dependent on higher levels of vitamin D3 and calcium in the diet fed the mice. In reviewing the literature, it was noted that the Lgr5hi cell stem cell signature had identified 512 genes altered in expression in comparing Lgr5hi cells to their immediate Lgr5lower progeny [9]. Thirty-three of these were altered most robustly, and one of these encoded the vitamin D receptor (Vdr). The Vdr gene was expressed significantly at both the RNA and protein level in Lgr5hi CBC cells but was reduced in the Lgr5lower immediate daughter cells that were exiting the stem cell niche [9]. This strongly suggested that Vdr signaling was necessary for Lgr5hi cells to function as stem cells, but not for cells that were no longer undergoing self-renewal. Therefore, in retrospect, the reduction in lineage tracing from Lgr5hi cells in mice fed NWD1 in which vitamin D3 was greatly reduced was predicted by this Lgr5hi stem cell signature [44–46].
To pursue this, a mouse was bred in which induction of the red fluorescence in the Lgr5hi cells and their progeny was accompanied by genetic inactivation of the Vdr gene, eliminating vitamin D signaling specifically in these same Lgr5hi cells. In these mice, there was a reproduction of the NWD1 phenotype: the Lgr5hi cell progeny remained at the crypt bottom, even though these mice were fed control AIN76A diet (Fig 2C-bottom row). Thus, the ability of Lgr5hi cells to function as stem cells to populate the mucosa is dependent on vitamin D signaling, which can be compromised either by lower dietary vitamin D3 or genetic inactivation of its receptor.
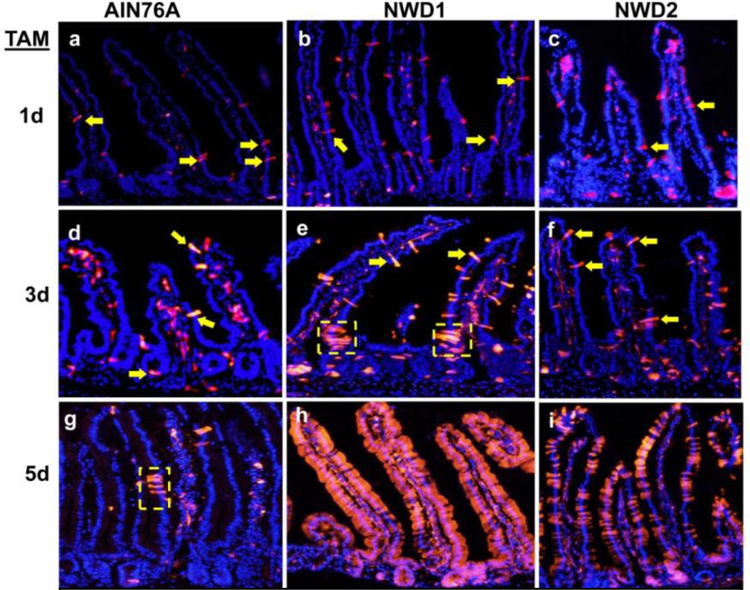
Since the mice fed NWD1 were healthy and lived to 2–3 years of age, it was then asked whether other cells had replaced Lgr5hi cells in functioning as stem cells. The first investigation was with a genetic strain in which the progeny of Bmi1+ cells could be tracked. When these mice were fed control diet, there were occasional bursts of lineage tracing distributed throughout the mucosa, but these were limited in number and extent (Fig 3a,d,g). However, when mice were fed NWD1, the number of such tracks of lineage tracing increased substantially, and by 5 days, many crypt-villi were populated by the progeny of these cells (FIG 3b,e,h). Moreover, this was greatly reduced when the mice were fed the “rescue” diet – NWD1 with elevated vitamin D3 and calcium (Fig 3c,f,i). Therefore, these progeny of Bmi1+ cells populated the mucosa when the diet suppressed Lgr5hi cell stem cell function. Moreover, the ability of a cell population expressing Bmi1creERT2 to do this persisted out to at least 60 days following marking of the cells, as long as the mice were fed NWD1, but was reduced by 30 days after switching the mice back to the control diet [46]. The conclusion is that the nutritional environment of the cell can influence relative contribution of cells from different compartments to maintain the mucosa.
Fig. 3: Mobilization of Bmi1+ cells in mice fed different diets.
Bmi1creERT2, Rosa26tom mice were fed AIN76A, NWD1 or NWD2 diets for 3 months from weaning, injected with tamoxifen, and then sacrificed 1, 3 or 5 days later. Yellow arrows: examples of red fluorescently marked epithelial cells; Yellow dotted boxes: examples of short bursts of lineage tracing of marked cells. All data are reprinted from [46].
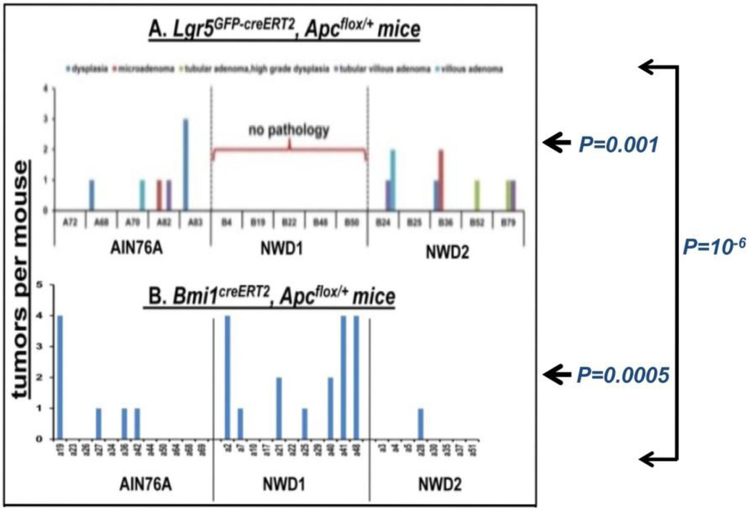
The ability of diet to influence stem cell contribution to tumor development was then investigated. When an Apc mutation was targeted to Lgr5hi cells, tumors developed in mice fed AIN76A or the rescue diet – that is, when the Lgr5hi cells were functioning efficiently as stem cells – but not when fed NWD1 (Fig 4A). However, when the Apc mutation was targeted using Bmi1creERT2, most efficient tumor development was with NWD1 – when the Bmi1 marked cells had been mobilized to function as stem cells (Fig 4B). Therefore, there is a direct correspondence between the ability of cells from each of these two compartments to function as stem cells and their efficiency in causing tumors when an initiating mutation is introduced.
Fig 4: Apc initiated tumor development as a function of diet.
Apcloxp/+ mice that were also Lgr5EGFPcreERT2 (A-top) or Bmi1creERT2 (B–bottom) were fed different diets from weaning for 3 months, then received a single injection of tamoxifen, and each mouse then continued on its respective diet until 9 months of age. On sacrifice, swiss rolls were prepared, and formalin fixed, H and E stained sections of these were used to score tumor incidence. Reprinted from [46].
Mechanisms impacted by nutritional changes.
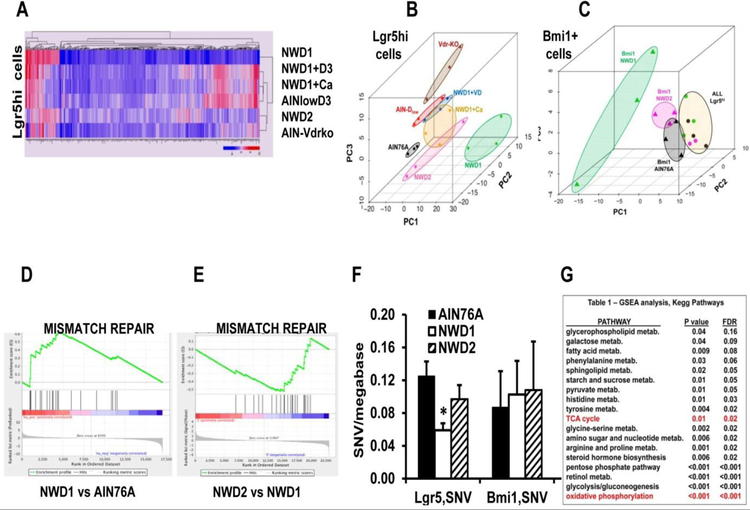
RNAseq analysis of both Lgr5hi and Bmi1+ intestinal cells from mice fed different diets provided insight into mechanisms underlying the dietary induced altered contribution of the two stem cell populations to homeostasis. Moreover, to gain insight into potential nutrient interactions, the diets interrogated were: AIN76A control diet, NWD1, NWD1 + elevated vitamin D3 and calcium = NWD2), NWD1+ elevated vitamin D3 or calcium; and AIN76A with only lower vitamin D3 (Fig 5A). Clustering of the data from each dietary group based on principal components analysis showed that in each cell type, there was a major shift in profile of gene expression when comparing either Lgr5hi or Bmi1 + cells from mice fed NWD1 compared to those from mice fed control AIN76A (Fig 5B,C). Feeding NWD2 – in which both vitamin D3 and calcium were elevated – caused the NWD1 gene expression pattern for each cell type to shift back towards that of the cells from AIN76A fed mice (Fig 5B,C). However, adding back either vitamin D3 or calcium to NWD1, or lowering only vitamin D3 in AIN76A, all produced distinguishable results, suggesting that changes in transcriptional programming of the cells was a function of interaction among nutrient levels [46].
Fig. 5: Transcriptional and mutational profiles of Lgr5hi and Bmi1+ cells are profoundly influenced by diet.
Lgr5EGFP-creERT2 or Bmi1creERT2, Rosa26tom mice were randomized to different diets at weaning, the Bmi1 group given a single injection of TAM at 3 months, and all mice sacrificed 2 days later. Crypts were purified from each mouse, and single cell suspensions of each used for isolation of Lgr5hi or Bmi1creERT2 marked cells by FACS. RNAs from these cells were then used for RNAseq analysis. An additional group was RNAseq analysis for Lgr5hi cells from Lgr5EGFP,creERT2, Vdrloxp/loxp mice fed AIN76A control diet from weaning and given a single injection of Tamoxifen to genetically inactivate the Vdr gene in Lgr5hi cells 3 days before sacrifice at 3 months of age. A) heat map of transcript expression of Lgr5hi cells as a function of diet. B and C) Principal Component Analysis of Lgr5hi or Bmi1creERT2 marked cells. D,E) GSEAof the DNA mismatch repair pathway comparing mice fed NWD1 to AIN76A (P=0.003, FDR=0.021), or NWD2 to NWD1 (P=0.02, FDR=0.06). F) Single nucleotide variants (i.e. point mutations) determined for Lgr5hi and Bmi1creERT2 marked cells from mice fed different diets for 3 months from weaning, determined by single cell whole exome sequencing, (* P=0.01). G) Metabolic pathways significantly altered by feeding NWD1 compared to AIN76A for 3 months, determined by GSEA. All data are from [46].
For the Lgr5hi cells, interrogation of the expression data by gene set enrichment analysis (GSEA) showed many pathways altered in these cells by the dietary change to NWD1. High on the list was an elevation in DNA mismatch repair (MMR) in Lgr5hi cells from mice fed NWD1 (Fig 5D), but not in Bmi1+ cells (not shown). However, the MMR pathway was significantly decreased in comparing Lgr5hi cells from mice fed NWD2 to those fed NWD1 (Fig 5E), thus reversing the NWD1 induced increase. Because MMR is a major contributor to the extent of DNA mutation accumulation [47], we then determined how mutations accumulated in the Lgr5hi and Bmil1+ derived cells under different dietary conditions. This required single cell analysis, since the goal was to identify mutation accumulation in the presumably unselected cell populations, along with development of a rigorous bioinformatics pipeline to unambiguously identify these mutations and to distinguish them from potential germ line variants [46]. Coincident with the elevation of the MMR pathway in Lgr5hi cells by feeding NWD1, there was decreased accumulation of mutations, but no change in the Bmi1+ cells, in which the MMR pathway was not altered (Fig 5F). The combination of fewer mutations per Lgr5hi cell and fewer of these cells indicated the mutational burden presented by Lgr5hi cells was decreased by feeding NWD1. In contrast, although there was no change in the mutations per Bmi1+ derived cell, the vastly increased number of these cells in NWD1 fed mice indicates that the mutational burden presented by the cells derived from Bmi1+ cells greatly increased under these dietary conditions. Moreover, analysis of the mutational signature showed the mutations that accumulated were relevant to those that accumulate in intestinal epithelial cells [46].
GSEA also showed that many metabolic pathways were altered in Lgr5hi cells by feeding the NWD1, including a major reduction in mitochondrial oxidative phosphorylation and the TCA cycle (Fig 5G). Extensive experiments are underway to determine the role this plays in potentially reducing the contribution of Lgr5hi cells to maintaining the intestinal mucosa and in reducing their ability to form tumors on targeted introduction of an Apc mutation. This is important since it has been reported that mitochondrial oxidative phosphorylation is necessary for Lgr5hi cells to function as stem cells [48, 49].
Implications of these studies for understanding intestinal homeostasis, tumorigenesis and chemoprevention.
There are key unanswered questions regarding the impact of the western diet on intestinal stem cells.
A principle biological question is the nature of the Bmi1+ cells mobilized by feeding the NWD1. Bmi1+ derived intestinal epithelial cells are heterogeneous both in biochemical/molecular identity and in their distribution throughout the crypt-villus architecture. Indeed, Lgr5hi CBC cells are known to also express Bmi1, and the Lgr5hi population can, under some experimental conditions of abrogating Lgr5hi cell stem cell function, be replenished from the Bmi1+ cell population. This may not be a mechanism involved in response to NWD1, since with uninterrupted feeding of this diet, the Lgr5hi cells would be continuously suppressed in giving rise to progeny. However, there could be a subpopulation of Lgr5hi cells at the crypt base that can function as stem cells under the nutrient conditions established by feeding NWD1, including lower vitamin D availability. There is some precedent for this idea, although the data are indirect : random drift is responsible for development of crypt stem cell clonality, but some mutations can impart selective pressure for a given population [50]. It can therefore be envisioned that during long-term feeding of NWD1, genetic or epigenetic changes could select for Lgr5hi cells that robustly function as stem cells under the nutritional conditions conferred by feeding NWD1. However, since the reduction of ability for Lgr5hi progeny to lineage trace extends to at least a year of feeding NWD1 ([44], and Fig 2B), this may not be likely. Therefore, a major effort underway uses single cell RNAseq to identify the subset of cells that function as stem cells in mice fed the NWD1, and their reprogramming that permits this.
A second biological question is what the signal is that notifies other epithelial cells in the mucosa that Lgr5hi cells are compromised, triggering a compensatory response. These signals can be biochemically transmitted between cells in an exocrine or endocrine manner, but can also include mechanical signals since the generation of cells at the bottom of the crypt likely provides mechanical force to the crypt-villus structure as cells are produced and migrate. Further, since many different cell populations can respond [1], are there multiple signals that integrate to favor the response of one cell population over another? In this context, adaptive radiation – the rapid expansion of species and higher taxa to occupy new niches – is thought to be driven principally by ability of organisms to adapt to and utilize new sources of food [51]. Therefore, an interesting hypothesis is that the plasticity of cells to function as stem cells may have arisen to provide flexibility to organisms to function in new ecosystems.
The data also raise clinically important issues. We have discussed that standard rodent diets used in stem cell research differ considerably in the levels of key nutrients they provide compared to levels that characterize the human population, especially that of populations at higher risk for sporadic colon cancer. A prime example is the level of 25(OH)vitamin D. There is a broad range of exposure to this vitamin in the human population that varies somewhat with sex, age and demographics, but with a maximum of about 100nmol/L serum. In mice fed standard chow diets, the level reaches ~125 nmol/L and with purified control AIN76A about 100nmol/L. Therefore, serum levels of 25(OH)D in the laboratory mouse fed standard control diets are well above levels for almost 100% of the US population (data shown and discussed in [44, 46, 52]. Since the data demonstrate that Lgr5hi cells require Vdr signaling to function as stem cells, these cells may be considerably reduced in this function in the population, and especially for those with lower vitamin D levels that are at elevated risk for CRC [53–55]. Therefore, there may be greater heterogeneity in which and how intestinal cells are functioning as stem cells in the human than is currently appreciated. This may also be in flux for an individual as a function of their daily vitamin D, and/or other, nutrient exposures.
This raises important questions regarding which and how stem cells initiate tumor development, with fundamental implications for early detection, prevention, prognosis and therapy. Are tumors heterogeneous, depending on which stem cell population harbors the initiating mutation(s) giving rise to the tumor, and does this influence efficacy of prevention, treatment and prognosis? Moreover, as regards prevention, we established that the nutritional environment extensively alters expression profile of both Lgr5hi and Bmi1+ intestinal cells [46]. The extent to which these changes are relatively stable, or if they can be reversed by altering vitamin D levels and levels of other nutrients, is under investigation. This is important since the epidemiological evidence is strong that low vitamin D levels are related to higher incidence of colon tumors [53–55], but relatively short-term intervention studies with vitamin D supplements later in life has been ineffective in preventing tumor recurrence or development. However, if there are stable alterations in long-lived stem cells that can initiate tumorigenesis, the study design of intervening later in life for too short a period - largely dictated by the cost and logistics of such trials - may be “too little, too late”. For example, in familial adenomatous polyposis (FAP), individuals inherit a mutant APC allele but only develop tumors when the wild-type allele is also mutated or lost [25]. In carriers of the germ line mutation, inactivation or loss of the wild-type allele and tumor initiation seems to take at least 10–15 years, based on the age at which multiple tumors are detected. This would therefore seem to be a minimum length of time before one might expect an intervention that targets initiation to show beneficial effects, and perhaps longer for patients that do not inherit a mutant allele. However, a recent meta-analysis that determined there was no effect of vitamin D supplementation on cancer incidence included trials with follow-up ranging from about 4 to 7 years [56]. Moreover, in the very large and well-conducted Vital trial, reported to have shown no effect of vitamin D supplementation on incidence of colorectal or other cancer [54], this was at 6 years of follow-up. Thus, it is too early in any of this work to conclude there is no benefit to vitamin D, or of potentially other nutritional interventions that may be epidemiologically linked to lower incidence of tumors.
The caution regarding intervention studies that are conducted in adult subjects at or beyond mid-life is reinforced by recent studies regarding intestinal stem cells, which are long-lived and likely the cell of origin of CRC. Stem cells in a single crypt take 2 to 3 years to reach “clonality” in the human [50]. This is usually through a process of “neutral drift” in which there is no selective pressure for the any of the 5–10 stem cells in each crypt to eventually become clonal [19, 20]. However, it was demonstrated that mutations in key oncogenic genes/pathways can impose selective pressure for a stem cell to outcompete the others in the crypt [50]. Therefore, individuals at mid- to late adulthood may already harbor many crypts that are clonal as regards their stem cells, all of which share mutation(s) that may support tumor development and be refractory to nutritional intervention. Thus again, study design of intervening later in life may have a high probability of failing.
The stakes in this are high: data from migrant populations, and shifts in cancer incidence in countries as their populations alter long-term dietary patterns, suggest that a considerable amount of cancer might be delayed or avoided altogether if we understood long-term impact and mechanisms better, leading to a greater imperative to change dietary habits. This is not simple to achieve, especially when the shorter-term trials not only confuses the public, but leads to premature conclusions in the scientific community that there is not significant value in such interventions. This is perhaps unavoidable. However, understanding fundamental mechanisms by which vitamin D and other nutrients alter stem cell functions and mucosal homeostasis can be important in pursuing these public health goals, and in potentially reducing the cancer burden in a population.
Highlights:
In mice fed control diets, Lgr5hi crypt base columnar cells serve as the principle intestinal stem cell in continually regenerating the intestinal mucosa as cells differentiate and are lost
There is remarkable plasticity among intestinal epithelial cell populations to serve as stem-like cells when Lgr5hi cells are damaged or inhibited in functioning as stem cells
Nutritional alterations can also marshal this plasticity, especially reduction in level of vitamin D, effects that are recapitulated by genetic inactivation of the gene that encodes the vitamin D receptor. This raises fundamental questions regarding which and how cells in the intestinal mucosa contribute to stem cell functions in homoeostasis and tumorigenesis in human populations in which there are widely varying exposures to different nutrients and to level of vitamin D. This may contribute to the biological and clinical heterogeneity of tumors.
The potential that epigenetic and genetic changes persist in long-lived stem cells suggests that intervention trials of vitamin D and other nutrients to reduce incidence of colon cancer have been much too short to be conclusive.
Acknowledgments
This work was supported by the National Cancer Institute, National Institutes of Health grants R01CA174432, R01CA229216, R01CA214625 and P30-13330; American Institute for Cancer Research grant 314707; New York State Stem Cell Science grant C029154; and support from the Jane A. and Myles P. Dempsey fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited:
- [1].Mills JC, Sansom OJ, Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract, Science signaling, 8 (2017) [doi: 10.1126/scisignal.aaa7540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barker N, Clevers H, Tracking down the stem cells of the intestine: strategies to identify adult stem cells, Gastroenterology, 133 (2007) 1755–1760. [DOI] [PubMed] [Google Scholar]
- [3].Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H, Identification of stem cells in small intestine and colon by marker gene Lgr5, Nature, 449 (2007) 1003–1007. [DOI] [PubMed] [Google Scholar]
- [4].Clevers H, The intestinal crypt, a prototype stem cell compartment, Cell, 154 (2013) 274–284. [DOI] [PubMed] [Google Scholar]
- [5].Sangiorgi E, Capecchi MR, Bmi1 is expressed in vivo in intestinal stem cells, Nat Genet, 40 (2008) 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es J, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H, Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling, Nature, 476 (2011) 293–297. [DOI] [PubMed] [Google Scholar]
- [7].Koo BK, Clevers H, Stem cells marked by the R-spondin receptor LGR5, Gastroenterology, 147 (2014) 289–302. [DOI] [PubMed] [Google Scholar]
- [8].Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ, A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable, Nature, 478 (2011) 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ, Clevers H, The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers, EMBO J, 31 (2012) 3079–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR, Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties, Cell, 152 (2013) 25–38. [DOI] [PubMed] [Google Scholar]
- [11].Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H, Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters, Cell Stem Cell, 18 (2016) 203–213. [DOI] [PubMed] [Google Scholar]
- [12].Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ, The Pan-ErbB Negative Regulator Lrig1 Is an Intestinal Stem Cell Marker that Functions as a Tumor Suppressor, Cell, 149 (2012) 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, Chen X, May R, Houchen CW, Fox JG, Gershon MD, Quante M, Wang TC, Long-lived intestinal tuft cells serve as colon cancer-initiating cells, J Clin Invest, 124 (2014) 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, Westphalen CB, von Burstin J, Mastracci TL, Worthley DL, Guha C, Quante M, Rustgi AK, Wang TC, Krt19(+)/Lgr5(−) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine, Cell Stem Cell, 16 (2015) 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roth S, Franken P, Sacchetti A, Kremer A, Anderson K, Sansom O, Fodde R, Paneth cells in intestinal homeostasis and tissue injury, PLoS One, 7 (2012) e38965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jones JC, Brindley CD, Elder NH, Myers MG Jr., Rajala MW, Dekaney CM, McNamee EN, Frey MR, Shroyer NF, Dempsey PJ, Cellular Plasticity of Defa4(Cre)-Expressing Paneth Cells in Response to Notch Activation and Intestinal Injury, Cellular and molecular gastroenterology and hepatology, 7 (2019) 533–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yu S, Tong K, Zhao Y, Balasubramanian I, Yap GS, Ferraris RP, Bonder EM, Verzi MP, Gao N, Paneth Cell Multipotency Induced by Notch Activation following Injury, Cell Stem Cell, 23 (2018) 46–59 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jadhav U, Saxena M, O’Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, Shivdasani RA, Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory Precursors into Lgr5+ Intestinal Stem Cells, Cell Stem Cell, 21 (2017) 65–77 e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lopez-Garcia C, Klein AM, Simons BD, Winton DJ, Intestinal stem cell replacement follows a pattern of neutral drift, Science, 330 (2010) 822–825. [DOI] [PubMed] [Google Scholar]
- [20].Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H, Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells, Cell, 143 (2010) 134–144. [DOI] [PubMed] [Google Scholar]
- [21].Vooijs M, Liu Z, Kopan R, Notch: architect, landscaper, and guardian of the intestine, Gastroenterology, 141 (2011) 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guruharsha KG, Kankel MW, Artavanis-Tsakonas S, The Notch signalling system: recent insights into the complexity of a conserved pathway, Nat Rev Genet, 13 (2012) 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ueo T, Imayoshi I, Kobayashi T, Ohtsuka T, Seno H, Nakase H, Chiba T, Kageyama R, The role of Hes genes in intestinal development, homeostasis and tumor formation, Develop, 139 (2012) 1071–1082. [DOI] [PubMed] [Google Scholar]
- [24].VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud A, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC, Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells, Develop, 139 (2012) 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clevers H, Nusse R, Wnt/beta-catenin signaling and disease, Cell, 149 (2012) 1192–1205. [DOI] [PubMed] [Google Scholar]
- [26].Reya T, Morrison SJ, Clarke MF, Weissman IL, Stem cells, cancer, and cancer stem cells, Nature, 414 (2001) 105–111. [DOI] [PubMed] [Google Scholar]
- [27].Stange DE, Clevers H, Concise review: the yin and yang of intestinal (cancer) stem cells and their progenitors, Stem Cells, 31 (2013) 2287–2295. [DOI] [PubMed] [Google Scholar]
- [28].Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H, Crypt stem cells as the cells-of-origin of intestinal cancer, Nature, 457 (2009) 608–611. [DOI] [PubMed] [Google Scholar]
- [29].Newmark HL, Nutrient density: an important and useful tool for laboratory animal studies, Carcinogenesis, 8 (1987) 871–873. [DOI] [PubMed] [Google Scholar]
- [30].Newmark HL, Lipkin M, Maheshwari N, Colonic hyperplasia and hyperproliferation induced by a nutritional stress diet with four components of westen-style diet, J. Nat. Cancer Inst, 82 (1990) 491–496. [DOI] [PubMed] [Google Scholar]
- [31].Newmark HL, Lipkin M, Maheshwari N, Colonic hyperproliferation induced in rats and mice by nutritional-stress diets containing four components of a human western-style diet (series 2), Am. J. Clin. Nutr, 54 (1991) 209S–214S. [DOI] [PubMed] [Google Scholar]
- [32].Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M, Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer, Carcinogenesis, 30 (2009) 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H, A western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl//6 mice, Carcinogenesis, 22 (2001) 1871–1875. [DOI] [PubMed] [Google Scholar]
- [34].Yang WC, Mathew J, Velcich A, Edelmann W, Kucherlapati R, Lipkin M, Yang K, Augenlicht LH, Targeted inactivation of the p21 WAF1/cip1 gene enhances Apc initiated tumor formation and the tumor promoting activity of a Western-style high risk diet by altering cell maturation in the intestinal mucosa, Cancer Res, 61 (2001) 565–569. [PubMed] [Google Scholar]
- [35].Yang W, Bancroft L, Nicholas C, Lozonschi I, Augenlicht LH, Targeted inactivation of p27kip1 is sufficient for large and small intestinal tumorigenesis in the mouse, which can be augmented by a western-style high-risk diet, Cancer Res, 63 (2003) 4990–4996. [PubMed] [Google Scholar]
- [36].Yang W, Velcich A, Lozonschi I, Liang J, Nicholas C, Zhuang M, Bancroft L, Augenlicht LH, Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2-/- mice, Am J Pathol, 166 (2005) 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang K, Popova N, Yang W, Lozonschi I, Tadesse S, Kent S, Bancroft L, Matise I, Cormier R, Scherer S, Edelmann W, Lipkin M, Augenlicht L, Velcich A, Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: potential role of chronic inflammation, Cancer Res, 68 (2008) 7313–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W, Velcich A, Lipkin M, Augenlicht L, Dietary induction of colonic tumors in a mouse model of sporadic colon cancer, Cancer Res, 68 (2008) 7803–7810. [DOI] [PubMed] [Google Scholar]
- [39].Aslam MN, Paruchuri T, Bhagavathula N, Varani J, A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet, Integr Cancer Ther, 9 (2010) 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bastie C, Gaffney-Stomberg E, Ting-Wen L, Dhima E, Pessin J, Augenlicht L, Dietary cholecalcifero and calcium levels in a western-style defined rodent diet alter energy metabolism and inflammatory response in mice, J. Nutrition, 142 (2012) 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Slaughter DP, Southwick HW, Smejkal W, Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin, Cancer, 6 (1953) 963–968. [DOI] [PubMed] [Google Scholar]
- [42].Lochhead P, Chan AT, Nishihara R, Fuchs CS, Beck AH, Giovannucci E, Ogino S, Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression, Mod Pathol, 28 (2015) 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang D, Peregrina K, Dhima E, Lin EY, Mariadason JM, Augenlicht LH, Paneth cell marker expression in intestinal villi and colon crypts characterizes dietary induced risk for mouse sporadic intestinal cancer, Proc Natl Acad Sci U S A, 108 (2011) 10272–10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peregrina K, Houston M, Daroqui C, Dhima E, Sellers RS, Augenlicht LH, Vitamin D is a determinant of mouse intestinal Lgr5 stem cell function, Carcinogenesis, 36 (2015) 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Augenlicht L, Environmental Impact on Intestinal Stem Cell Functions in Mucosal Homeostasis and Tumorigenesis, Journal of Cellular Biochemistry, available online, 11 january 2017 (2017). [DOI] [PMC free article] [PubMed]
- [46].Li W, Zimmerman S, Peregrina K, Houston M, Mayoral J, Zhang J, Maqbool SB, Zhang Z, Cai Y, Ye Q, Augenlicht L, The nutritional environment determines which and how intestinal stem cells contribute to homeostasis and tumorigenesis, Carcinogenesis, 40 (2019) 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Supek F, Lehner B, Differential DNA mismatch repair underlies mutation rate variation across the human genome, Nature, 521 (2015) 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rodriguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM, Interplay between metabolic identities in the intestinal crypt supports stem cell function, Nature, 543 (2017) 424–427. [DOI] [PubMed] [Google Scholar]
- [49].Mihaylova MM, Cheng CW, Cao AQ, Tripathi S, Mana MD, Bauer-Rowe KE, Abu-Remaileh M, Clavain L, Erdemir A, Lewis CA, Freinkman E, Dickey AS, La Spada AR, Huang Y, Bell GW, Deshpande V, Carmeliet P, Katajisto P, Sabatini DM, Yilmaz OH, Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging, Cell Stem Cell, 22 (2018) 769–778 e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nicholson AM, Olpe C, Hoyle A, Thorsen AS, Rus T, Colombe M, Brunton-Sim R, Kemp R, Marks K, Quirke P, Malhotra S, Ten Hoopen R, Ibrahim A, Lindskog C, Myers MB, Parsons B, Tavare S, Wilkinson M, Morrissey E, Winton DJ, Fixation and Spread of Somatic Mutations in Adult Human Colonic Epithelium, Cell Stem Cell, 22 (2018) 909–918 e908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Neige P, Events of Increased Biodiversity, Science Direct 2015, pp. 47–64.
- [52].Augenlicht LH, Environmental Impact on Intestinal Stem Cell Functions in Mucosal Homeostasis and Tumorigenesis, J Cell Biochem, 118 (2017) 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ, The role of vitamin D in reducing cancer risk and progression, Nat Rev Cancer, 14 (2014) 342–357. [DOI] [PubMed] [Google Scholar]
- [54].Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, V.R. Group, Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease, N Engl J Med, (2018). [DOI] [PubMed]
- [55].McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR, Eliassen AH, Zeleniuch-Jacquotte A, Agnoli C, Albanes D, Barnett MJ, Buring JE, Campbell PT, Clendenen TV, Freedman ND, Gapstur SM, Giovannucci EL, Goodman GG, Haiman CA, Ho GYF, Horst RL, Hou T, Huang WY, Jenab M, Jones ME, Joshu CE, Krogh V, Lee IM, Lee JE, Mannisto S, Le Marchand L, Mondul AM, Neuhouser ML, Platz EA, Purdue MP, Riboli E, Robsahm TE, Rohan TE, Sasazuki S, Schoemaker MJ, Sieri S, Stampfer MJ, Swerdlow AJ, Thomson CA, Tretli S, Tsugane S, Ursin G, Visvanathan K, White KK, Wu K, Yaun SS, Zhang X, Willett WC, Gail MH, Ziegler RG, Smith-Warner SA, Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts, J Natl Cancer Inst, (2018). [DOI] [PMC free article] [PubMed]
- [56].Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E, Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials, Annals of oncology : official journal of the European Society for Medical Oncology, 30 (2019) 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]