Abstract
Background
Study of human coronavirus and other virus-associated respiratory illnesses is needed to describe their clinical effects on chronically ill, older adults.
Methods
A prospective study during 2009 to 2013 clinically assessed acute respiratory illnesses soon after onset and 3 to 4 weeks later in patients aged ≥60 years with chronic lung and heart diseases (group 1, 100 subjects) and healthy adults aged 18 to 40 years (group 2, 101 subjects). Respiratory secretions were tested for nucleic acids of a panel of respiratory viruses. An increase in antibody titer was assessed for 4 coronavirus strains.
Results
Virus-associated illnesses (29 [39.1%] of 74 illnesses in group 1 and 59 [48.7%] of 121 illnesses in group 2) occurred in all calendar quarters, most commonly in the first and fourth quarters. Coronaviruses (group 1: 14 [18.9%] illnesses; group 2: 26 [21.5%] illnesses) and enteroviruses/rhinoviruses (group 1: 14 [18.9%] illnesses; group 2: 37 [30.6%] illnesses) were most common. Virus co-infections occurred in 10 illnesses. Illnesses with 9 to 11 symptoms were more common in group 1 (17 [23.0%]) than in group 2 (15 [12.4%]) (P < .05). Compared with group 2, more group 1 subjects reported dyspnea, more severe disease of longer duration, and treatment for acute illness with prednisone and antibiotics. Coronavirus-associated illnesses (percent of illnesses, group 1 vs group 2) were characterized by myalgias (21% vs 68%, P < .01), chills (50% vs 52%), dyspnea (71% vs 24%, P < .01), headache (64% vs 72%), malaise (64% vs 84%), cough (86% vs 68%), sputum production (86% vs 60%), sore throat (64% vs 80%), and nasal congestion (93% vs 96%).
Conclusions
Respiratory illnesses were commonly associated with coronaviruses and enteroviruses/rhinoviruses affecting chronically ill, older patients more than healthy, young adults.
Keywords: Cardiopulmonary disease, Coronavirus, Elderly, Epidemiology, Rhinovirus, Severity of illness, Upper respiratory infection
Clinical Significance.
-
•
Coronavirus and enterovirus/rhinovirus-related acute respiratory illness were common.
-
•
Older, chronically ill adults had more severe illnesses than young, healthy adults.
-
•
Dyspnea was more common in older, chronically ill than in young, healthy adults.
-
•
Respiratory illness symptom duration was longer in older, chronically ill adults.
-
•
Older, chronically ill adults were more likely to receive antibiotics and steroids.
Coronaviruses are enveloped, single-stranded, positive-sense RNA viruses and undergo RNA recombination and mutations facilitating adaptation from animals to humans.1, 2, 3, 4, 5 The identification of severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus as causes of human disease2, 3, 6, 7 has increased the clinical significance of coronaviruses. Human coronaviruses (HCoVs) cause the common cold and influenza-like illnesses. Coronaviruses and other respiratory viruses also are associated with a number of more serious acute respiratory illnesses, such as pneumonia, exacerbations of asthma and chronic obstructive pulmonary disease, croup, and bronchiolitis.8, 9, 10, 11, 12, 13
Two other coronaviruses, HCoV-NL63 and HCoV-HKU1, have a worldwide distribution and cause respiratory illness along with prototype strains, HCoV-229E and HCoV-OC43.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 In patients with chronic obstructive pulmonary disease studied during the 1998 to 1999 influenza season, 13.5% of illnesses were associated with HCoV-229E and HCoV-OC43 infection, with HCoV-OC43 being more common.30, 31, 32 Coronavirus-associated illness was less severe than influenza but was associated with multiple respiratory and systemic symptoms, and hospitalization.30 Walsh et al33 reported HCoV-229E and HCoV-OC43 infection rates of 2.8% to 26% in healthy young and elderly adults, high-risk adults, and hospitalized patients during the winters of 1999 to 2003 and as contributions to medical disease burden.33
In this multi-year, prospective study, our goal was to underscore the manifestations and importance of HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, and other respiratory infections throughout the calendar year in at-risk older patients with underlying chronic cardiopulmonary and other diseases compared with young healthy adults.
Materials and Methods
Study Design and Subjects
This was a prospective, observational study conducted from November 2009 to July 2013 to assess acute respiratory illness in patients aged ≥60 years with chronic lung or heart disease or both (group 1) and in healthy young adults aged 18 to 40 years (group 2). Group 1 patients were capable of attending outpatient clinics and complying with study procedures, but they were excluded if they had a life expectancy <3 years in the clinical judgment of the investigator, a febrile or respiratory illness within 15 days before enrollment, a significant bleeding disorder, asplenia, or a psychiatric condition that precluded compliance. Group 2 subjects were in good health shown by medical history and physical examination. Volunteers were excluded from group 2 if they had received immunosuppressive medications within 168 days, blood products within 120 days, or immunoglobulins within 60 days; were immunosuppressed; had a febrile or respiratory illness within 15 days before enrollment; or had a clinically significant medical condition. All patients gave written informed consent. Eligibility was confirmed by a study physician. The study received approvals by responsible institutional review boards and was conducted in accordance with the amended Declaration of Helsinki.
From November 2009 to July 2013, enrolled patients each participated for up to 2 years, received phone calls every 8 weeks to remind them to contact study personnel at the time of acute respiratory illness, were evaluated by a study physician and nurse in clinic when they had 3 symptoms or fever (body temperature ≥37.8°C) accompanied by 2 symptoms of acute respiratory illness, and kept a daily temperature and symptom diary during the illness. Nasal and oropharyngeal swab and serum specimens were obtained at the acute illness visit. Illness symptoms were reassessed, and a serum specimen was obtained 3 to 4 weeks after the onset of the illness. Clinical assessments were completed without knowledge of assay results on swab and serum specimens. Assays were performed near the end of the study.
Coronavirus-associated illness was the sudden onset of respiratory illness plus (1) a nasal and oropharyngeal swab specimen positive by reverse transcriptase polymerase chain reaction (RT-PCR) or (2) a >3-fold increase in the calculated titer of serum antibody to coronavirus (serologic change) by enzyme-linked immunosorbent assay comparing paired acute and convalescent sera assayed at the same time. Other virus-associated illness was the sudden onset of respiratory illness plus swab specimen positive for respiratory virus nucleic acid.
Virus Nucleic Acid Detection Performed in Research Laboratory
RNA was purified from swab specimens using the QIAmp kit (Qiagen, Valencia, Calif) according to the manufacturer's procedures. Testing for HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, and other respiratory viruses (respiratory syncytial virus types A and B, influenza A and B viruses, parainfluenza viruses types 1 to 4, metapneumovirus, enterovirus/rhinovirus, adenovirus, and bocavirus) was done by multiplex RT-PCR using the xTAG Respiratory Viral Panel Fast and the manufacturer’s procedures (Luminex Molecular Diagnostics, Inc, Toronto, Ontario, Canada).34, 35
Coronavirus Antibody Enzyme-Linked Immunosorbent Assay
The 4 coronavirus antigens for the antibody assay were produced as described.30, 36, 37 Viral and mock antigens were used to coat flat-bottom 96-well Maxisorp Immuno-plates (Nalge Nunc International, Rochester, NY) followed by the sequence of serum in 8 serial 2-fold dilutions, mouse anti-human immunoglobulin-G conjugated with horseradish peroxidase (Accurate Chemical and Scientific, Westbury, NY), and peroxidase substrate (KPL, Gaithersburg, MD). Optical density was measured at 405 nm by use of a Tecan SLT400 spectrophotometer (Research Triangle Park, NC). The anti-coronavirus antibody titer was calculated using log-transformed data by the reference line least-squares-fit method, which has a low coefficient of variation.30, 38
Severity of Illness
The severity of illness was assessed in group 1 by a 6 symptom-based Chronic Lung Disease Severity Index that evaluates chronic lung disease functional status and wheezing, dyspnea, cough, and sputum production.39 The index ranges from 6 (best) to 27 (most severe). Severity of illness also was assessed in groups 1 and 2 by rating the severity of each of 11 symptoms and signs of acute respiratory illness (cough, dyspnea, sputum production, sore throat, nasal congestion, fever, chills, headache, myalgias, aches and pains, and malaise) as mild (symptom did not interfere with activity), moderate (symptom interfered with normal daily activities), or severe (incapacitating and prevented normal daily activities) and by 2 scores: a self-reported visual analogue scale of overall illness severity, ranging from 1 (mildest) to 10 (most severe), and a severity of influenza-like illness symptoms and signs score including 16 symptoms and signs that were each graded on a scale of 0 (absent) to 15 (most severe).40 All study subjects answered the 16 categories, and the total score reported is the sum of the 16 individual scores (maximum summed score = 240).
Statistical Methods
Descriptive statistics were used to analyze variables; 2 × 2 chi-square tests or Fisher exact tests were used to compare categoric variables, and the appropriate Student t test was used to compare continuous variables. Statistical analysis was done using SAS/STAT software, SAS version 9.2 (SAS Institute, Inc, Cary, NC).
Results
We enrolled 100 subjects in group 1 and 101 subjects in group 2 between November 2009 and August 2011 in St Louis, Missouri. There were greater proportions of men and subjects who ever smoked cigarettes in group 1 than group 2 (Table 1 ), and there were no gender differences in group 2 (data not shown). Heart disease was reported by 77.6% and lung disease was reported by 65.3% of group 1 patients.
Table 1.
Demographics, Medical History, and Smoking History of the 2 Patient Groups
| Group 1 (N = 100) | Group 2 (N = 101) | |
|---|---|---|
| Demographics | ||
| Age (y), Mean ± SD | 66.6 ± 20.3 | 30.6 ± 6.4 |
| Gender, No. (%) | ||
| Male | 90 (90.0)∗ | 44 (43.6)∗ |
| Female | 10 (10.0) | 57 (56.4) |
| Race (and ethnicity), No. (%) | ||
| Black/African | 28 (28.0) | 29 (28.7) |
| Hispanic/Latino | 0 | 1 (1.0) |
| Asian | 0 | 3 (3.0) |
| White | 71 (71.0) | 64 (63.3) |
| Hispanic/Latino | 0 | 2 (2.0) |
| Unknown (and Hispanic/Latino) | 1 (1.0) | 2 (2.0) |
| CLDSI,† baseline value, Mean ± SD | 14.4 ± 4.2 | NA |
| Medical History | ||
| Renal/kidney/bladder disease, No. (%) | 33 (33.7) | 1 (1.0) |
| Heart disease: Yes; No. (%) | 76 (77.6) | 0 |
| Congestive heart disease | 17 (17.4) | 0 |
| Ischemic/coronary artery disease | 62 (63.3) | 0 |
| Valvular disease | 15 (15.3) | 0 |
| Other heart disease | 12 (12.2) | 0 |
| Liver disease, No. (%) | 10 (10.2) | 1 (1.0) |
| Blood disease, No. (%) | 0 | 1 (1.0) |
| Lung disease:‡ Yes; No. (%) | 64 (65.3) | 0 |
| Chronic obstructive pulmonary disease | 37 (37.8) | 0 |
| Chronic bronchitis | 17 (17.4) | 0 |
| Emphysema | 10 (10.2) | 0 |
| Asthma | 16 (16.3) | 0 |
| Other lung disease | 10 (10.3) | 0 |
| Neurologic disease, No. (%) | 38 (38.8) | 7 (7.0) |
| Diabetes mellitus, No. (%) | 33 (33.7) | 0 |
| Cancer: Yes, No. (%) | 26 (26.5) | 1 (1.0) |
| Seasonal rhinitis, eg, hay fever: No. (%) | 60 (61.9) | 45 (44.6) |
| Otitis or sinusitis, No. (%) | 23 (24.0) | 21 (20.8) |
| Smoking History | ||
| Current cigarette smoker, No. (%) | 16 (16.5) | 19 (18.8) |
| Ever smoked cigarettes, No. (%) | 70 (72.9)§ | 31 (30.7)§ |
Group 1 patients were at least 60 years of age with underlying chronic lung or heart disease. Group 2 patients were healthy and aged 18-40 years. Group 2 patients could not have a medical history of underlying heart and lung disease. Comparisons of categoric variables by chi-square tests. Characteristics did not differ between male and female patients in group 2 (data not shown).
CLDSI = Chronic Lung Disease Severity Index; NA = not available; SD = standard deviation.
P < .0001, greater proportion of male patients in group 1 than group 2.
The CLDSI score was determined only in group 1 patients.
Patients with underlying lung disease could have >1 pulmonary diagnosis based on reported history and medical record review.
P < .0001, greater proportion of patients who ever smoked cigarettes in group 1 than in group 2.
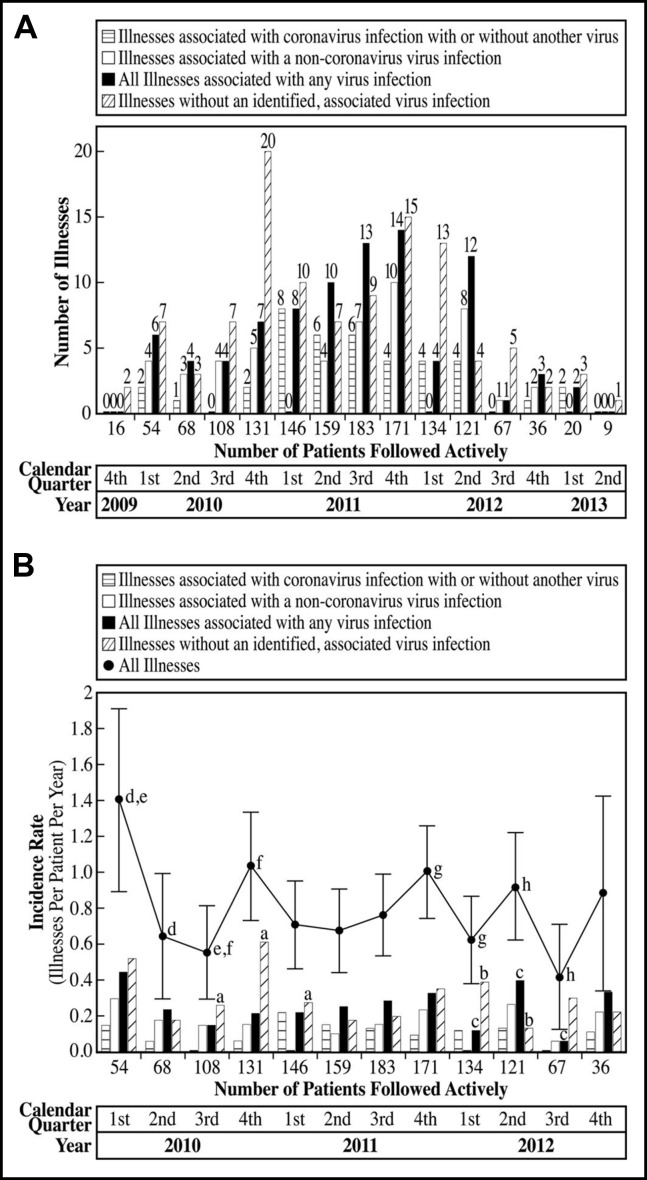
Illnesses were reported and assessed in all calendar quarters, and the largest numbers of illnesses occurred between the fourth quarter of 2010 and the second quarter of 2012, when the numbers of patients being followed were highest (Figure 1A ). Incidence rates for all illnesses were highest in the first quarter of 2010, fourth quarters of 2010 and 2011, and second quarter of 2012. Incidence rates overall were lowest in the second and third quarters of 2010 and third quarter of 2012 (Figure 1B). Coronavirus-associated illnesses were not detected in the third quarter of 2010 and 2012 (Figure 1B). The decrease in the number of illnesses during the last 4 quarters of the study was most likely due to the fewer number of patients being actively followed.
Figure 1.
Acute respiratory illnesses evaluated by calendar quarter. (A) Shown are the numbers of enrolled study subjects who were being actively followed in the study for acute respiratory illnesses by calendar quarter and the numbers of illnesses, in association with viral infection or not, with onset during each calendar quarter. (B) Shown are the numbers of enrolled subjects who were being actively followed in the study for acute respiratory illnesses by calendar quarter when more than 20 subjects were actively enrolled. The incidence rates of acute respiratory illness, calculated as illnesses per patient per year, are shown by calendar quarter and association with viral infection or not. Comparisons of categoric variables were by 2-sided Fisher exact test. The “All Illnesses” line includes the 95% confidence interval of the incidence rate for each calendar quarter. aP < .05, incidence of illnesses not associated with a virus infection was highest in the fourth quarter of 2010 compared with the third quarter of 2010 and first quarter of 2011. bP < .05, incidence of illnesses not associated with a virus infection was higher in the first quarter of 2012 compared with the second quarter of 2012. cP < .05, incidence of illnesses associated with all virus infections was highest in the second quarter of 2012 compared with the first and third quarters of 2012. dP < .05, eP < .05, incidence of all illnesses was higher in the first quarter of 2010 compared with the second and third quarters of 2010. fP < .05, incidence of all illnesses was higher in the fourth quarter of 2010 compared with the third quarter of 2010. gP < .05, incidence of all illnesses was higher in the fourth quarter of 2011 compared with the first quarter of 2012. hP < .05, incidence of all illnesses was higher in the second quarter of 2012 compared with the third quarter of 2012.
Of 74 illnesses in 38 subjects in group 1 and 121 illnesses in 59 subjects in group 2, 29 (39.1%) and 59 (48.7%) were virus associated, respectively. Coronaviruses were identified by serologic change or nucleic acid detection in 14 (48.3%) of 29 virus-associated (18.9% of 74 total) illnesses in group 1 and 26 (44.1%) of 59 virus-associated (21.5% of 121 total) illnesses in group 2 without gender differences. HCoV-OC43 was the most common. Of the 40 coronavirus-associated illnesses in groups 1 and 2, 13 had a >3-fold increase in antibody titer (serologic change) to more than 1 coronavirus strain. In group 1, 9 illnesses had a serologic change to only 1 coronavirus strain: 3 to HCoV-229E, 3 to HCoV-OC43, 2 to HCoV-NL63, and 1 to HCoV-HKU1. In group 1, 5 illnesses had a serologic change to more than 1 coronavirus strain: 2 to both HCoV-OC43 and HCoV-HKU1, which were also HCoV-OC43 RT-PCR positive; 1 to both HCoV-OC43 and HCoV-NL63; 1 to HCoV-NL63 and HCoV-HKU1, which was also HCoV-OC43 RT-PCR positive; and 1 to HCoV-OC43, HCoV-NL63, and HCoV-HKU1. In group 2, 3 illnesses were HCoV-OC43 RT-PCR positive without a serologic change, and 18 illnesses had a serologic change to only 1 coronavirus strain: 3 to HCoV-229E, 7 to HCoV-OC43, 5 to HCoV-NL63, and 3 to HCoV-HKU1. In group 2, 8 illnesses had a serologic change to more than 1 coronavirus strain: 3 to both HCoV-OC43 and HCoV-HKU1; 2 to both HCoV-NL63 and HCoV-HKU1, of which 1 was also HCoV-HKU1 RT-PCR positive; 1 to HCoV-229E and HCoV-HKU1; and 2 to HCoV-229E, HCoV-NL63, and HCoV-HKU1.
There were co-infections in 10 illnesses, 9 with and 1 without a coronavirus detected. In addition to coronavirus, another virus was associated with 2 illnesses in group 1 (both enterovirus/rhinovirus) and 7 illnesses in group 2 (1 with parainfluenza virus and 6 with enterovirus/rhinovirus). Among illnesses not associated with coronavirus in group 1 patients, enterovirus/rhinovirus was detected in 12 illnesses, and respiratory syncytial virus, metapneumovirus, and adenovirus were each detected in 1 illness (total illnesses = 15). Among illnesses not associated with coronavirus in group 2 patients, enterovirus/rhinovirus was detected in 30 illnesses, parainfluenza and enterovirus/rhinovirus were detected in 1 illness, and parainfluenza and adenovirus were separately detected in 1 illness each (total illnesses = 33) (Table 2 ). Thus, enterovirus/rhinoviruses were detected in the majority of illnesses with virus infections implicated that were not associated with coronaviruses.
Table 2.
Illnesses by Cause and Number of Illness Symptoms at Onset of Acute Respiratory Illness
| No. of Illnesses (% of Total Illnesses) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: Aged ≥60 Y N = 74 Total Illnesses |
Group 2: Aged 18-40 Y N = 121 Total Illnesses (Among Male Patients, N = 37, and Female Patients, N = 84) |
|||||||||
| Coronavirus | Coronavirus with Another Virus | Other Virus(es) | No Virus | All Illnesses | Coronavirus | Coronavirus with Another Virus | Other Virus(es) | No Virus | All Illnesses | |
| Illnesses by Category | 12 (16.2) | 2 (2.7) | 15 (20.2) | 45 (60.8) | 74 (100) | 19 (15.7) | 7 (5.8) | 33 (27.3) | 62 (51.2) | 121 (100) |
| No. of Illness Symptoms | ||||||||||
| 3-5 | 6 (8.1) | 1 (1.4) | 3 (4.1) | 13 (17.6) | 23 (31.1) | 7 (5.8) | 2 (1.7) | 10 (8.3) | 28 (23.1) | 47 (38.8) |
| 6-8 | 4 (5.4) | 1 (1.4) | 9 (12.2) | 20 (27) | 34 (45.9) | 9 (7.4) | 4 (3.3) | 20 (16.5) | 26 (21.5) | 59 (48.8) |
| 9-11 | 2 (2.7) | 0 | 3 (4.1) | 12 (16.2)∗ | 17 (23.0)∗ | 3 (2.5) | 1 (0.8) | 3 (2.5) | 8 (6.6)∗ | 15 (12.4)∗ |
“Coronavirus with another virus” refers to illnesses that were associated with coronavirus and a second virus. The “Other Virus(es)” category refers to illnesses that were not coronavirus associated but were associated with ≥1 other viruses. Comparisons of categoric variables by 2-sided Fisher exact test. The percent of illnesses in each illness category did not differ between male and female patients in group 2 (data not shown).
P < .05, greater proportion of group 1 patients who had 9 to 11 symptoms at onset of illness for illnesses without a virus identified and for all illnesses (virus identified or not), respectively, than group 2 patients.
At illness onset, the majority of patients reported at least 6 symptoms of respiratory illness (group 1: 51 [68.9%] and group 2: 74 [61.2%] illnesses). The 6 most common symptoms at onset in both groups were nasal congestion, cough, sore throat, headache, malaise, and sputum production. Group 1 patients were significantly more likely to experience 9 to 11 symptoms at illness onset than the younger subjects, but male and female patients did not differ within group 2 (Table 2).
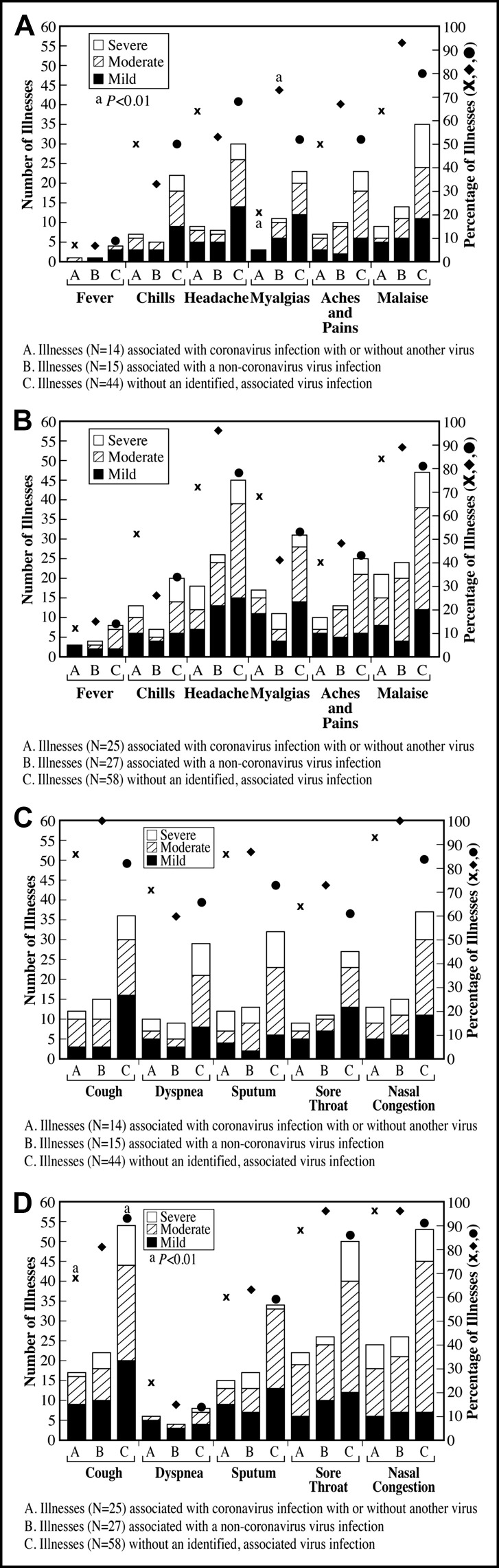
The maximum symptom severity was often mild to moderate; however, the more common the symptom, the greater the proportion of illnesses with a moderate to severe grade (Figure 2 ). The frequency of symptoms and distribution of severity grades were similar for illnesses associated with coronavirus only compared with a grouping combining illnesses with a coronavirus alone and coronavirus with another co-infecting virus. Dyspnea, including moderate to severe grades, was more common in group 1 compared with group 2 for coronavirus-associated infections (10 [71.4%] of 14 vs 6 [24%] of 25 illnesses, P < .01), noncoronavirus viral infections (9 [60%] of 15 vs 4 [14.8%] of 27 illnesses, P < .01), and illnesses without an identified virus infection (29 [65.9%] of 44 vs 8 [13.8%] of 58, P < .0001) (Figure 2A, B). Among group 1 patients, myalgia was less common in patients with a coronavirus-associated illness than in patients with a noncoronavirus viral infection (Figure 2A). Myalgia was also less common in group 1 than group 2 patients with coronavirus-associated illness (3 [21%] of 14 vs 17 [68%] of 25 illnesses, P < .01). Fever was uncommon in both groups. Nasal congestion was reported by 84% to 100% of patients. Among group 2 patients, cough was more frequent during illnesses without an identified virus infection than among coronavirus-associated illnesses (Figure 2). Sore throat and headache were more common in group 2 patients compared with group 1 patients with noncoronavirus viral infections (sore throat, 26 [96.3%] of 27 vs 11 [73.3%] of 15 illnesses, P < .05; headache, 26 [96.3%] of 27 vs 8 [53.3%] of 15 illnesses, P < .01) (Figure 2).
Figure 2.
The number of illnesses with each symptom and the maximum symptom severity during each illness are shown by severity grade (mild, moderate or severe), subject age group, and association of illness with viral infection or not. Data for maximum severity were not available for 1 illness in group 1 and 11 illnesses in group 2. The height of each vertical bar is the number of illnesses with that symptom. The percentage of illnesses with that symptom is plotted with each vertical bar, respectively. Comparisons of categoric variables were by 2-sided Fisher exact test. (A) Maximum systemic illness symptoms in group 1, chronically ill, older adults aged ≥60 years. aP < .01, myalgias were more common in patients with viral infection other than coronaviruses compared with illnesses with a coronavirus infection. (B) Maximum systemic illness symptoms in group 2 healthy, young adults aged 18 to 40 years. (C) Maximum local/respiratory illness symptoms in group 1 chronically ill, older adults, aged ≥60 years. (D) Maximum local/respiratory illness symptoms in group 1 healthy, young adults aged 18 to 40 years. aP < .01, cough was more common among patients with an illness not associated with a virus infection compared with those associated with coronavirus infection.
Among group 1 patients, the mean Chronic Lung Disease Severity Index indicated less severe symptoms at the enrollment visit than at the acute illness visits considering all illnesses (14.4 ± 4.2 vs 17.1 ± 4.9, P < .0001) and did not improve at the follow-up illness visit (17.2 ± 4.6). The mean index was higher at the acute than the follow-up illness visit for coronavirus-associated illnesses (18.0 ± 4.8 and 15.9 ± 4.3). The mean symptoms and signs scores at the acute illness visits were significantly greater than at the follow-up illness visits in both groups 1 and 2 for both virus- and nonvirus-associated illnesses. The mean symptoms and signs score and the mean visual analogue scale of overall illness severity were significantly higher at the follow-up illness visit for group 1 than group 2 (Table 3 ). Mean symptoms and signs scores at the acute illness visit for group 2 female patients were higher than for male patients, but statistical significance was achieved only in the case of mean scores for all illnesses (Table 3). The duration of illness is not available because this was not an outcome measure. Especially in the group 1 patients, underlying respiratory symptoms at baseline changed during acute illness but did not resolve, so that end of acute illness was difficult to pinpoint. The symptom scores suggest that illnesses in group 1 patients as a group had not returned to baseline at the convalescent 3- to 4-week follow-up illness visit.
Table 3.
Severity of Influenza-like Illness Symptoms and Signs Score and Visual Analogue Scale of Illness Severity Comparing Age Groups by Cause and Comparing Acute Illness and 3- to 4-Week Illness Follow-up Time Points
| Severity of Illness Parameter | Time Point | Mean ± SD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1: Aged ≥60 Y |
Group 2: Aged 18-40 Y (Male Only; Female Only) |
||||||||
| Coronavirus | Other Virus(es) | No Virus | All Illnesses | Coronavirus | Other Virus(es) | No Virus | All Illnesses | ||
| Symptoms and Signs Score | Acute | 39.8 ± 18∗ | 48.9 ± 23† | 47.6 ± 23‡ | 47.1 ± 22‡ | 42.0 ± 17‡ (32.6 ± 13; 45.6 ± 18) | 43.5 ± 21‡ (40.3 ± 25; 45.1 ± 20) | 39.7 ± 17‡ (33.3 ± 17; 42.1 ± 17) | 41.5 ± 18‡ (35.2 ± 20††; 44.3 ± 18††) |
| 3- to 4-wk follow-up | 18.8 ± 11∗§ | 20.9 ± 11†§ | 24.9 ± 18‡§ | 22.9 ± 16‡§ | 4.9 ± 7‡§ (3.1 ± 7; 6.0 ± 6) | 5.2 ± 9‡§ (8.1 ± 11; 3.8 ± 7) | 7.5 ± 11‡§ (7.8 ± 18; 7.4 ± 7) | 6.4 ± 10‡§ (6.8 ± 15; 6.3 ± 7) | |
| Visual Analogue Scale | Acute | 5.1 ± 1‖ | 5.3 ± 2‖ | 5.0 ± 2¶ | 5.1 ± 2‖ | 5.0 ± 2‖ (5.0 ± 1; 5.0 ± 2) | 4.6 ± 2‖ (4.8 ± 2; 4.5 ± 1) | 4.8 ± 2‖ (4.9 ± 2; 4.9 ± 2) | 4.8 ± 2‖ (4.7 ± 2; 4.8 ± 2) |
| 3- to 4-wk follow-up | 2.3 ± 1‖ | 3.5 ± 1‖# | 3.6 ± 2¶∗∗ | 3.3 ± 2‖∗∗ | 2.1 ± 1‖ (1.9 ± 1; 2.3 ± 1) | 2.3 ± 2‖# (2.0 ± 1; 2.4 ± 2) | 2.5 ± 2‖∗∗ (3.3 ± 2; 2.6 ± 2) | 2.4 ± 2‖∗∗ (2.1 ± 1; 2.5 ± 2) | |
Comparisons of continuous variables by Student t test.
P < .05, †P < .01, mean score was higher at acute than follow-up visit.
P < .0001, mean score was higher at acute than follow-up visit comparing acute illness with follow-up time points within each age and etiologic group, respectively.
P < .0001, mean score at follow-up visit was higher in group 1 than group 2, comparing group 1 with group 2 for each illness cause category, respectively.
P < .0001, ¶P < .05, mean score at acute illness visit was higher than at follow-up visit, comparing acute illness with follow-up time points within each age and illness etiologic group, respectively.
P < .05, **P < .001, mean score was higher at follow-up visit in group 1 than group 2, respectively.
P < .05, mean score at acute illness visit in group 2 was higher in female than male patients.
Among group 1 patients, 1 was hospitalized for respiratory symptoms approximately 2 weeks after an acute respiratory illness during which no virus was detected, 1 was hospitalized for pneumonia but did not undergo study evaluation at that time, and 1 died without recent acute respiratory illness reported. One group 2 patient died in a motor vehicle accident.
Proportions of illnesses for which 1 or more new medications were taken as treatment were similar in group 1 (47.9% of illnesses) and group 2 (46.3% of all illnesses; male patients: 15 [41.7%] of 36, female patients: 41 [49.4%] of 83) (Table 4 ). Prednisone was more commonly prescribed for illnesses in group 1 than group 2 patients, particularly with coronavirus and other virus-associated illnesses, and antibiotics were more commonly prescribed in group 1 than group 2 patients during illnesses, whether virus associated or not (Table 4), without differences by gender in group 2. Group 1 patients who were prescribed prednisone had underlying chronic obstructive pulmonary disease, emphysema, or asthma, but the clinician’s reason for prescribing prednisone was not recorded in the study database. Only 1 patient received an antiviral (oseltamivir).
Table 4.
New Medications Taken to Treat the Acute Respiratory Illnesses
| New medication | No. of Illnesses (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: Aged ≥60 Y |
Group 2: Aged 18-40 Y |
|||||||||
| Coronavirus (N = 12) | Coronavirus With Another Virus (N = 2) | Other virus(es) (N = 14) | No Virus (N = 43) | All Illnesses (N = 71) | Coronavirus (N = 19) | Coronavirus With Another Virus (N = 7) | Other Virus(es) (N = 33) | No Virus (N = 62) | All Illnesses (N = 121) | |
| 1 new medication | 3 (25.0) | 0 (0) | 5 (35.7) | 8 (18.6) | 16 (22.5) | 5 (26.3) | 4 (57.1) | 5 (15.2) | 14 (22.6) | 28 (23.1) |
| 2 new medications | 4 (33.3) | 1 (50) | 3 (21.4) | 5 (11.6) | 13 (18.3) | 2 (10.5) | 0 (0) | 6 (18.2) | 8 (13.3) | 16 (13.2) |
| ≥3 new medications | 0 (0) | 0 (0) | 1 (7.1) | 4 (9.3) | 5 (7.0) | 3 (15.8) | 0 (0) | 2 (6.1) | 7 (11.7) | 12 (9.9) |
| Antibiotic | 5 (41.7) | 1 (50) | 6 (42.9)∗ | 11 (25.6)† | 23 (32.4)‡ | 2 (10.5) | 2 (28.6) | 4 (12.1)∗ | 4 (6.5)† | 12 (9.9)‡ |
| Acetaminophen or NSAID | 0 (0) | 0 (0) | 0 (0) | 3 (7.0) | 3 (4.2) | 3 (15.8) | 1 (14.3) | 7 (21.2) | 10 (16.1) | 21 (17.4) |
| Combination cough/cold medication | 2 (16.7) | 1 (50) | 4 (28.6) | 7 (16.3) | 14 (19.7) | 7 (36.8) | 1 (14.3) | 9 (27.3) | 18 (29.0) | 35 (28.9) |
| Antihistamine/decongestant | 1 (8.3) | 0 (0) | 0 (0) | 2 (4.6) | 3 (4.2) | 2 (10.5) | 0 (0) | 1 (3.0) | 10 (16.1) | 13 (10.7) |
| Prednisone | 3 (25)§ | 0 (0) | 3 (21.4)§ | 3 (7.0) | 9 (12.7)‖ | 0 (0)§ | 0 (0) | 0 (0)§ | 2 (3.2) | 2 (1.7)‖ |
New medication data were not available for all illnesses. Comparisons of categoric variables by 2-sided Fisher exact test. “Coronavirus with another Virus” refers to illnesses that were associated with coronavirus and a second virus.
NSAID = nonsteroidal anti-inflammatory drug.
P < .05, †P < .01, ‡P < .0001, proportion of respective illnesses for which antibiotics were given was higher in group 1 than group 2 patients.
P < .05, ‖P < .01, proportions of respective illnesses for which prednisone was given were higher in group 1 than group 2 patients.
Discussion
The clinical significance of coronavirus infections and other respiratory illnesses was studied in older adults with chronic cardiopulmonary conditions compared with healthy younger adults by active surveillance and recording signs and symptoms and their severity, including numeric gauges of severity of illness. This multi-year study involved prospective interactions with study subjects throughout the calendar year, not solely during winter seasons. A weakness is that it was a single-center study in 1 geographic region. Coronaviruses and enteroviruses/rhinoviruses contributed to morbidity in both subject groups and illnesses qualifying for clinical assessment. Patients were assessed for all 4 commonly circulating coronaviruses rather than for 1 or 2, as has been the more typical report in the literature. They are alphacoronaviruses (HCoV-229E and HCoV-NL63) and betacoronaviruses lineage A (HCoV-OC43 and HCoV-HKU1). The related Middle East respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus are betacoronaviruses, but of the lineages B and C, respectively. More severe illness and higher mortality rates are associated with Middle East respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus, although a spectrum of mild to moderate disease also occurs.2, 3, 6, 7
Respiratory illnesses occurred throughout the calendar year, but most commonly in the first and fourth calendar quarters and least commonly in the third calendar quarter. In both age groups, coronaviruses accounted for approximately 20% of illnesses and 44% to 48% of illnesses with which a virus was associated. Because coronavirus infections were the focus of this study, we were more likely to identify coronavirus-associated illnesses due to serologic diagnosis of coronavirus infection in addition to the multiplex RT-PCR assay that detected fewer infections. Enteroviruses/rhinoviruses were as likely to be identified as coronaviruses, using only RT-PCR the results of which clinically could be available sooner than acute and convalescent serology results. Viral co-infections occurred as in other reports.28, 33 The overall incidence of coronavirus-associated illnesses (considering all 4 strains in our study) was higher than that reported previously when testing for 2 strains (HCoV-229E and HCoV-OC43).30, 33 HCoV-OC43 was most frequent, and seroconversion to more than 1 coronavirus strain occurred in one third of illnesses, perhaps due to antigen cross-reactivity. The sensitivity of the multiplex may be less than real-time polymerase chain reaction and can be affected by viral load in the specimen and the virus strain.34, 35 The study was conducted in the years when widespread influenza epidemics were not reported, and fever, which is a hallmark of influenza, was uncommon and consistent with the lack of influenza viruses detected.
Illness symptoms were more frequent in the older patients. Among respiratory symptoms, dyspnea was more common in older patients than in the younger group, and this was true for coronavirus-associated illnesses too. Cough, sputum, sore throat, and nasal congestion were common in both age groups, but in the younger age group cough was less common in coronavirus-associated illnesses than in nonvirus illnesses. Sore throat and malaise were more common in the younger age group than in older patients with coronaviruses. Among older patients, myalgias were less common in association with coronaviruses compared with noncoronavirus illnesses and younger patients with coronavirus-associated illnesses.
The severity of illness scores helped quantify morbidity. The Chronic Lung Disease Severity Index indicated worse symptoms at the acute illness compared with baseline in older patients. The symptoms and signs score and the visual analogue scale of illness severity indicated more severe illness at the acute illness visit than at the 3- to 4-week follow-up visit for both age groups, but slower recovery during convalescence among the older patients and perhaps more severe symptoms among female than male patients at illness onset in the younger patients.
New medications were taken during approximately 50% of illnesses in both age groups. Prednisone and antibiotics were prescribed more frequently for the illness in older patients, indicating possible effects on their underlying cardiopulmonary status. Antibiotics should be used judiciously in this clinical setting.
Walsh et al33 reported high rates of nasal congestion with coronavirus-associated illnesses, which is consistent with our findings now and previously,30 but dyspnea and sputum production were more prominent symptoms in our chronically ill patients and may have contributed to antibiotic use in the older patients.30 Other coronavirus studies, such as in younger adults by Lu et al,28 reported high rates of fever, sore throat, and headache, but lower rates of cough, sputum production, and nasal congestion compared with our patients. Rhinovirus and coronavirus infection–associated hospitalizations among older adults have been associated with symptoms of cough, dyspnea, nasal congestion, and sputum production, and steroid, bronchodilator, and antibiotic treatment.10 Rhinoviruses (32%) followed by coronaviruses (17%) were the 2 most common viruses associated with respiratory illness in elderly, community-dwelling adults in a study reported by Graat et al,11 with a clinical picture of lower respiratory tract and systemic symptoms, restriction of activity, and illness-associated medication use.
Conclusions
Our study describes the symptoms, greater severity, medical burden, and seasonality of acute respiratory illness in older adults with underlying cardiopulmonary diseases compared with young healthy adults. Coronavirus and enteroviruses/rhinoviruses were the most common viruses associated with illness. The most common symptoms associated with but not unique to coronaviruses were chills, headache, malaise, cough, sputum production, sore throat, and nasal congestion; dyspnea was more common in the older group, and myalgia was more common in the younger group. Noncoronavirus, virus-associated illnesses, which were predominantly enterovirus/rhinovirus infection, commonly manifested headache, myalgias, malaise, cough, sputum production, sore throat, and nasal congestion. Older, chronically ill patients experienced more severe and prolonged disease, and were more likely to receive treatment with antibiotics and prednisone with coronavirus and other virus-associated illnesses. The results of more sensitive virus nucleic acid detection techniques could help clinicians with diagnosis and encourage the judicious use of antimicrobials.
Acknowledgments
The authors thank the patients who participated in the study, Carolyn Novotny and Kiana Wilder for secretarial assistance, and the Center for Vaccine Development at Saint Louis University.
Footnotes
Funding: This work was supported by VA Research, Department of Veterans Affairs Office of Research and Development.
Conflict of Interest: None.
Authorship: All authors had access to the data and played a role in writing this manuscript.
References
- 1.Lau S.K.P., Lee P., Tsang A.K.L. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J Virol. 2011;85:11325–11337. doi: 10.1128/JVI.05512-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Boheemen S., de Graaf M., Lauber C. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3:e00473–e00512. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyrc K., Berkhout B., van der Hoek L. The novel human coronaviruses NL63 and HKU1. J Virol. 2007;81:3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo P.C.Y., Lau S.K.P., Huang Y., Tsoi H.-W., Chan K.H., Yuen K.-Y. Phylogenetic and recombination analysis of coronavirus HKU1, a novel coronavirus from patients with pneumonia. Arch Virol. 2005;150:2299–2311. doi: 10.1007/s00705-005-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel Coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 7.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 8.Fagon J.-Y., Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Semin Respir Infect. 1996;11:109–118. [PubMed] [Google Scholar]
- 9.Greenberg S.B., Allen M., Wilson J., Atmar R.L. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 10.Falsey A.R., Walsh E.E., Hayden F.G. Rhinovirus and Coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graat J.M., Schouten E.G., Heijnen M.-L.A. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infections. J Clin Epidemiol. 2003;56:1218–1223. doi: 10.1016/S0895-4356(03)00171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vabret A., Mourez T., Gouarin S., Petitjean J., Freymuth F. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis. 2003;36:985–989. doi: 10.1086/374222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Elden L.J.R., van Loon A.M., van Alphen F. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel, real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Hoek L., Pyrc K., Jebbink M.F. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouchier R.A.M., Hartwig N.G., Bestebroer T.M. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vabret A., Mourez T., Dina J. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11:1225–1229. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Hoek L., Pyrc K., Berkhout B. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol Rev. 2006;30:760–773. doi: 10.1111/j.1574-6976.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastien N., Anderson K., Hart L. Human coronavirus NL63 infection in Canada. J Infect Dis. 2005;191:503–506. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arden K.E., Nissen M.D., Sloots T.P., Mackay I.M. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75:455–462. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo P.C.Y., Lau S.K.P., Chu C. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vabret A., Dina J., Gouarin S., Petitjean J., Corbet S., Freymuth F. Detection of the new human coronavirus HKU1: a report of 6 cases. Clin Infect Dis. 2006;42:634–639. doi: 10.1086/500136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo P.C., Lau S.K., Yip C.C. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J Virol. 2006;80:7136–7145. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esper F., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Coronavirus HKU1 infection in the United States. Emerg Infect Dis. 2006;12:775–779. doi: 10.3201/eid1205.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo P.C., Lau S.K., Tsoi H.-W. Clinical and molecular epidemiological features of coronavirus HKU-1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau S.K., Woo P.C., Yip C.C. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garbino J., Crespo S., Aubert J.-D. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (non-SARS)-related human coronavirus infection. Clin Infect Dis. 2006;43:1009–1015. doi: 10.1086/507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaunt E.R., Hardie A., Claas E.C., Simmonds P., Templeton K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010;48:2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu R., Yu X., Wang W. Characterization of human coronavirus etiology in Chinese adults with acute upper respiratory tract infection by real-time RT-PCR assays. PLoS One. 2012;7:e38638. doi: 10.1371/journal.pone.0038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan K.H., Cheng V.C.C., Woo P.C.Y. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin Diagn Lab Immunol. 2005;12:1317–1321. doi: 10.1128/CDLI.12.11.1317-1321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorse G.J., O'Connor T.Z., Hall S.L., Vitale J.N., Nichol K.L. Human coronaviruses and acute respiratory illnesses in older patients with chronic obstructive pulmonary disease. J Infect Dis. 2009;199:847–857. doi: 10.1086/597122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorse G.J., O'Connor T.Z., Young S.L. Impact of a winter respiratory virus season on patients with COPD and association with influenza vaccination. Chest. 2006;130:1109–1116. doi: 10.1378/chest.130.4.1109. [DOI] [PubMed] [Google Scholar]
- 32.Gorse G.J., O'Connor T.Z., Young S.L. Efficacy trial of live, cold-adapted and inactivated influenza virus vaccines in older adults with chronic obstructive pulmonary disease: a VA cooperative study. Vaccine. 2003;21:2133–2144. doi: 10.1016/s0264-410x(02)00748-x. [DOI] [PubMed] [Google Scholar]
- 33.Walsh E.E., Shin J.H., Falsey A.R. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis. 2013;208:1634–1642. doi: 10.1093/infdis/jit393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadsby N.J., Hardie A., Claas E.C.J., Templeton K.E. Comparison of the Luminex respiratory virus panel fast assay with in-house real-time PCR for respiratory viral infection diagnosis. J Clin Microbiol. 2010;48:2213–2216. doi: 10.1128/JCM.02446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pabbaraju K., Tokaryk K.L., Wong S., Fox J.D. Comparison of the Luminex xTAG respiratory viral panel with in-house nucleic acid amplification tests for diagnosis of respiratory virus infections. J Clin Microbiol. 2008;46:3056–3062. doi: 10.1128/JCM.00878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson C.V., Riggs J.L., Lennette E.H. Photochemical inactivation of DNA and RNA viruses by psoralen derivatives. J Gen Virol. 1978;40:345–358. doi: 10.1099/0022-1317-40-2-345. [DOI] [PubMed] [Google Scholar]
- 37.Redfield D.C., Richman D.D., Oxman M.N., Kronenberg L.H. Psoralen inactivation of influenza and herpes simplex viruses and of virus-infected cells. Infect Immun. 1981;32:1216–1226. doi: 10.1128/iai.32.3.1216-1226.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reizenstein E., Hallander H.-O., Blackwelder W.C., Kühn I., Ljungman M., Möllby R. Comparison of five calculation modes for antibody ELISA procedures using pertussis serology as a model. J Immunol Methods. 1995;183:279–290. doi: 10.1016/0022-1759(95)00067-k. [DOI] [PubMed] [Google Scholar]
- 39.Selim A.J., Ren X.S., Fincke G., Rogers W., Lee A., Kazis L. A symptom-based measure of the severity of chronic lung disease. Results from the Veterans Health Study. Chest. 1997;111:1607–1614. doi: 10.1378/chest.111.6.1607. [DOI] [PubMed] [Google Scholar]
- 40.Arden N.H., Patriarca P.A., Fasano M.B. The roles of vaccination and amantadine prophylaxis in controlling an outbreak of influenza A (H3N2) in a nursing home. Arch Intern Med. 1988;148:865–868. [PubMed] [Google Scholar]