Abstract
The NCCN Guidelines for Survivorship provide screening, evaluation, and treatment recommendations for consequences of cancer and cancer treatment to aid healthcare professionals who work with survivors of adult-onset cancer. Guidance is also provided to help promote physical activity, weight management, and proper immunizations in survivors and to facilitate care coordination to ensure that all needs are addressed. These NCCN Insights summarize some of the topics discussed by the NCCN Survivorship Panel during the 2019 update of the guidelines, including the survivorship population addressed, ways to improve care coordination, and pain management.
Overview
The number of cancer survivors in the United States increased from approximately 3 million in 1971 to nearly 15.5 million in 2016.1–3 These numbers are predicted to reach >20 million by 2026 and >26 million by 2040.1,2 This striking increase is generally attributed to increasing cancer incidence rates (mainly resulting from an aging population), earlier detection, and better treatment.
Unfortunately, many cancer survivors experience physical and psychosocial late and/or long-term effects of cancer and its treatment, which can be severe, debilitating, and sometimes permanent. Survivors may be discharged from the care of their oncologist and feel unsure about where to turn with cancer-related concerns. Furthermore, their primary care physicians (PCPs), who may now be responsible for their care, often do not know how best to address the specific concerns and needs of cancer survivors.4 ASCO’s statement, “Achieving High-Quality Cancer Survivorship Care,” cites a need for standardized, evidence-based practice guidelines for the management of treatment effects and health promotion of survivors.5 ASCO, NCCN, ACS, and other groups working in parallel hope to provide this guidance.6–9
The NCCN Survivorship Panel is comprised of a multidisciplinary panel of experts that includes at least one oncologist, bone marrow transplant clinician, gynecologist, urologist, infectious disease specialist, cardiologist, PCP, psychologist, nutrition scientist, nurse, epidemiologist, social worker, and patient advocate. The panel meets annually to discuss the latest data emerging in the field of survivorship and to decide on changes to the guidelines requested by panel members, other health professionals at NCCN Member Institutions, or outside individuals or groups. These NCCN Guidelines Insights summarize some of the issues discussed by the panel this year, with changes to the guidelines indicated in blue font within the figures.
Who Is a Cancer Survivor and to Whom Do These Guidelines Apply?
The NIH’s definition of a cancer survivor, which was adapted from the National Coalition for Cancer Survivorship, states, “An individual is considered a cancer survivor from the time of diagnosis, through the balance of his or her life. Family members, friends, and caregivers are also impacted by the survivorship experience and are therefore included in this definition.”10 The NCCN panel supports this definition but notes that the guidelines apply specifically to survivors of adult-onset cancer; family, friends, and caregivers are not currently addressed in the guidelines.
The panel discussed several requests for guideline modifications submitted by outside individuals (referred to as external comments or proposals). One proposal for the 2019 update of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Survivorship suggested that “survivorship” be defined as a phase beginning at posttreatment surveillance for recurrence of the primary cancer or even after this surveillance period is complete. Another proposal suggested that the guidelines should be renamed “Posttreatment Survivorship.” The panel discussed, however, the fact that “Survivorship” is not just for individuals in the posttreatment or postsurveillance setting, and felt strongly that many of the recommendations in the guidelines apply to those receiving active therapy, particularly those receiving treatment for many years. In fact, from the first version of these guidelines in 2013, the panel noted that the guideline recommendations pertain to patients who may be in remission, those for whom cancer has become a chronic disease, and those who are cured.
As part of this discussion, it was noted that the guidelines miss an opportunity to address the specific needs of the large and growing population of cancer survivors who are living with chronic cancer. This group includes survivors who have incurable disease that is controlled with systemic therapy and those who have treatable, slow-growing tumors who may be on treatment intermittently. Examples include patients with chronic myeloid leukemia who live for decades on tyrosine kinase inhibitors (TKIs) and those with chronic lymphocytic leukemia who live for long periods under observation and then for years on multiple treatments. Some of these patients can live with incurable cancer, fully functioning, for 10, 15, or even 20 years. Many issues faced by long-term survivors with chronic cancer are not addressed in these guidelines (eg, psychosocial issues related to living for years with a terminal diagnosis and uncertainty about the future; how to handle comorbid conditions and disease prevention, screening, and treatment in the setting of limited life expectancy; managing discussions regarding new drugs and early-stage clinical trials). However, survivors with chronic cancer and those in remission or who may be cured have many common concerns (eg, fatigue, anxiety, depression).11 The panel thus decided that it was important to emphasize more clearly that these guidelines may be used to guide the management of all cancer survivors—not just those who have completed treatment but also the population with chronic cancer.
Therefore, the panel strengthened their wording around this important issue by adding to the definition of “survivor”: “an individual is considered a survivor from the time of diagnosis, during and immediately after treatment, and through the balance of his or her life” (see SURV-1, page 786). In addition, the panel moved the following note from a footnote into a more prominent position on the page: “These guidelines are applicable to survivors across the continuum of care, including those on endocrine therapy, with chronic cancers (eg, metastatic disease), and long-term survivors.” Finally, the panel modified the first statement in the General Principles of the Survivorship Guidelines to include “those who are enduring ongoing treatment” (see SURV-2, page 787).
Standards for Survivorship Care
In 2005, the Institute of Medicine (IOM) and the National Research Council compiled a report entitled, “From Cancer Patient to Cancer Survivor: Lost in Transition.”12 This report included essential components of survivorship care. In September 2011, the LIVESTRONG Foundation convened a meeting of experts and stakeholders to define updated essential elements of survivorship care. The NCCN Survivorship Panel has adapted these standards. Care of the cancer survivor should include:
Prevention of new and recurrent cancers and other late effects
Surveillance for cancer spread or recurrence, and screening for subsequent primary cancers
Assessment of late psychosocial and physical effects
Intervention for consequences of cancer and treatment (eg, medical problems, symptoms, psychologic distress, financial and social concerns)
Coordination of care between PCPs and specialists to ensure that all of the survivor’s health needs are met
Survivorship care planning
Implementation of these standards for survivorship care has been challenging, and reasons for the difficulties have been described.13 The NCCN Survivorship Panel hopes that these guidelines can help providers achieve these standards of care. At this year’s panel meeting, the panel discussed 2 elements of survivorship care: care coordination and survivorship care plans (SCPs).
Care Coordination
With the population of cancer survivors growing at a rapid pace, the demand for follow-up care is expected to increase. Primary care teams will likely perform an increasing proportion of this care. Studies have found that cancer survivors increase their number of consultations with primary care and have more chronic conditions compared with controls without cancer.14,15 In fact, approximately one-third of cancer-related visits to physicians’ offices are made to primary care.12
However, studies have shown that PCPs often do not know how best to care for the specific needs of cancer survivors.4,16–20 Furthermore, many survivors prefer oncologist-driven follow-up care over PCP follow-up care and feel that PCPs should only provide follow-up care if the responsibility is shared with the oncologist.21–23 Reasons commonly cited for this preference include the belief that PCPs lack the expertise to manage survivorship-specific issues and a desire for continuity of care. Importantly, however, 2 randomized trials comparing survivorship care administered by oncologists versus PCPs who were provided guidelines outlining appropriate follow-up care found no difference in disease-related outcomes, including survival.24,25
Survivorship Care Plans
Some data suggest that SCPs and treatment summaries improve outcomes, such as emotional concerns.26,27 However, a randomized controlled trial (RCT) of 408 breast cancer survivors found no differences in patient-reported outcomes, including cancer-specific distress, between patients who received a discharge visit and a care plan and those who received only a discharge visit.28,29 Criticisms of this trial, including the relevance of its outcome measures, have been published.30–32 Another trial randomly assigned 221 survivors of stage I–III colorectal cancer to usual care or usual care plus a SCP, educational materials, a needs assessment, an end-of-treatment session, and 3 follow-up telephone calls.33 No effects on distress, supportive care needs, or quality of life were seen, although survivors in the care plan group were more satisfied with their care. In addition, a trial in which 12 hospitals were randomly assigned to usual care or patient-tailored, automated SCPs found that receipt of a care plan was associated with an increase in symptoms, concern about illness, and emotional impact.34 No differences in satisfaction with information or care were evident.
More recent population-targeted RCTs are finding support for survivorship care planning. One tested the role of SCPs in 212 low-income, predominantly Latina survivors of stage 0–III breast cancer.35 The intervention group received the care plan with a treatment summary and a 1-hour counseling session with a trained, bilingual, bicultural nurse who encouraged patient empowerment; the care plan and treatment summary were also delivered to their healthcare providers. Results showed that patient-reported physician implementation of recommended survivorship care (eg, care for depression, hot flashes), the primary trial outcome, was greater in the intervention group compared with the usual care group (P=.003). Patient adherence to recommended survivorship care, the secondary outcome, was also greater for the intervention group, but did not reach statistical significance (P=.07). Although this trial provides support for the benefits of SCPs, it is impossible to separate the effects of the care plan and the intensive counseling session, and the applicability of the findings to other populations is unknown. Another RCT examined the mailing of a personalized SCP, which was designed with qualitative input of hematopoietic cell transplant survivors and briefly reviewed in a telehealth call by a trained nonprofessional.27 The study randomly assigned 458 hematopoietic cell transplant survivors 1 to 5 years after transplant to receive the SCP or delayed SCP. After 6 months, the SCP recipients reported reduced cancer-specific distress and improved general mental health, although they did not report higher levels of confidence in survivorship information when compared with the delayed care plan recipients as hypothesized. In this study, approximately two-thirds of survivors reported that they found the SCP useful in helping them understand their treatments and side effects and in managing their health.
At this time, definitive data supporting the benefits of SCPs are still insufficient.36 However, a survey that included 1,020 PCPs found that they were 9 times more likely (95% CI, 5.74–14.82) to have survivorship discussions with survivors if they received a written care plan.37 Furthermore, the Commission on Cancer (CoC) accreditation standards include the provision of an SCP at the completion of treatment, as recommended in the IOM report.12,38 The NCCN panel therefore recommends providing a care plan that includes:
Summary of treatment received
Information regarding follow-up care, surveillance, and screening recommendations
Information on posttreatment needs, including information regarding treatment-related effects and health risks when possible
Delineation regarding roles of oncologists, PCPs, and subspecialty care physicians in long-term care and the timing of transfer of care if appropriate
Healthy behavior recommendations
Panel Discussion Regarding Care Coordination and Care Plans
The panel discussed ways in which these guidelines could help facilitate care coordination to ensure that all of a survivor’s needs are adequately addressed. The panel noted that, although these guidelines are intended for use by oncologists and PCPs, most PCPs are not aware of the guidelines. The panel discussed ways that they could raise awareness of these guidelines within the primary care community, including possibly partnering with other professional organizations to reach a primary care audience. The latter could be accomplished by having NCCN representation at primary care national meetings and collaboration on joint consensus statements. It was noted that outreach should also include advance practice providers because they often see survivors and share the information with their physician partners. The idea of writing review articles on the topic of cancer survivorship for primary care journals was also discussed. The panel noted that there have been more survivorship reviews in the PCP literature lately and that awareness of survivorship issues is increasing within the PCP community. However, PCPs need to be up-to-date on many aspects of care (eg, diabetes, cardiovascular health, infectious disease, gun violence), so these guidelines may not be impactful unless the recommendations can be integrated into the PCP’s workflow. The panel then discussed ways to accomplish this, including adding a link to the NCCN Guidelines for Survivorship on SCPs, documenting follow-up recommendations within oncology notes, and educating survivors so that they know what to ask their PCPs. Panel members agreed that these are measures they can work on within their own institutions.
The panel also discussed whether adding additional online resources for SCP creation to the guidelines would promote their use within the oncology community. However, the panel noted that there are several plan generators, and panel members agreed that many reasonable options are easy to find online. In addition, some institutions have SCPs embedded in their electronic health record systems. Panel consensus was that most care plans are too long and that PCPs prefer shorter ones. Furthermore, it was noted that the reading level is often too high for most survivors, many of whom want to have the same information their clinicians have. In general, the panel consensus was that these online survivorship care planning resources can be helpful, but that there was no reason to add additional links in the guidelines. The guidelines already include links to ASCO Cancer Treatment and Survivorship Care Plans (http://www.cancer.net/survivorship/follow-care-after-cancer-treatment/asco-cancer-treatment-and-survivorship-care-plans) and Journey Forward (http://www.journeyforward.org/). In addition, in the absence of evidence, the panel does not recommend a specific format or process for care plan delivery, but rather encourages oncology practices to develop and incorporate survivorship care planning into their routine oncology care delivery.
Pain
More than one-third of posttreatment cancer survivors experience chronic pain, which often leads to psychologic distress; decreased activity, motivation, and personal interactions; and an overall poor quality of life.39–43 However, pain in survivors is often ineffectively managed.44,45 Barriers to optimal pain management in cancer survivors include healthcare providers’ lack of training, fear of side effects and addiction, reimbursement issues, and patient difficulty accessing prescriptions.46,47 When discussing updates to the pain section of the NCCN Guidelines for Survivorship, the panel maintained a focus on recommending items that oncologists or PCPs could use with survivors and on recommending referral for refractory pain and more specialized interventions.
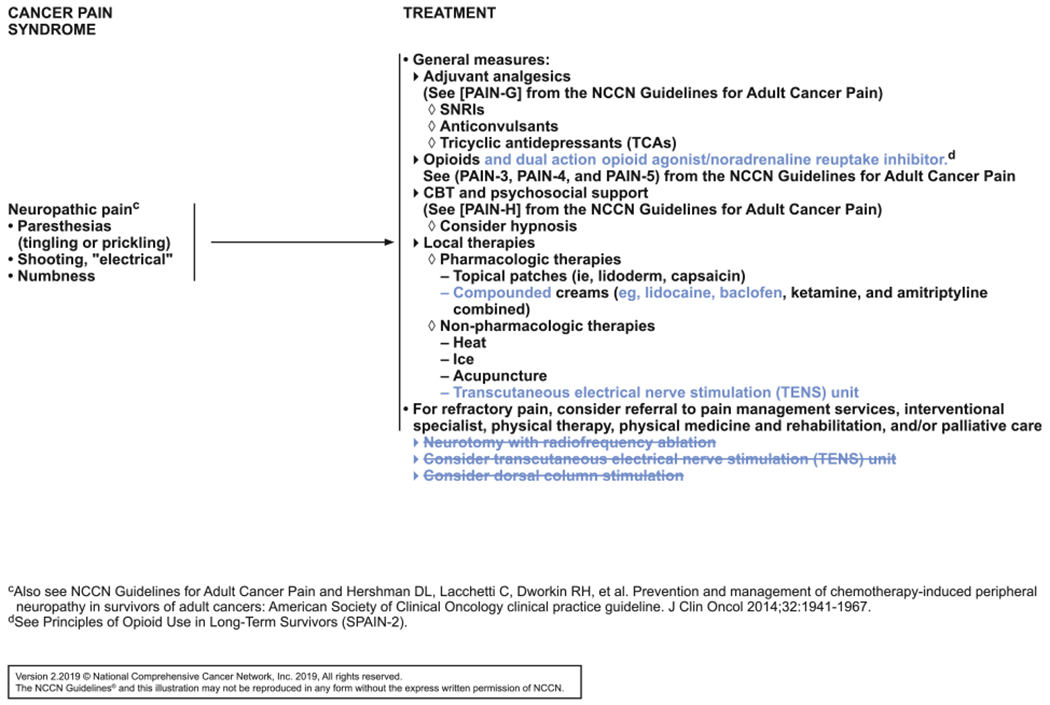
Based on an external request, the panel discussed whether they should add tramadol and tapentadol as additional options for the treatment of neuropathic pain (see SPAIN-4, page 788). Tramadol is an opioid pain medication, and opioids were already included as an option for neuropathic pain. A meta-analysis and systematic Cochrane review found that the quality of evidence supporting tramadol for the management of neuropathic pain was low or very low.48 The panel therefore decided not to specifically list tramadol as an opioid recommendation.
Tapentadol is an opioid analgesic with a dual mechanism of action as both a mu-opioid agonist and a noradrenaline reuptake inhibitor.49 Two separate RCTs in patients with painful diabetic peripheral neuropathy (N=588 and N=358) showed that tapentadol improved pain intensity compared with placebo.50,51 Two other RCTs in patients with chronic malignant tumor–related pain (N=325 and N=236) also showed improvements in pain intensity with tapentadol compared with placebo.52,53 No studies in cancer survivors could be identified. The panel concluded that tapentadol was a reasonable option to add for the treatment of neuropathic pain in survivors based on available data. Because the other recommendations in this setting are listed as drug classes, the panel added it as “opioids and dual action opioid agonist/noradrenaline reuptake inhibitor’ (see SPAIN-4, page 788). The panel also pointed out that the NCCN Guidelines for Adult Cancer Pain have more information on tapentadol and other opioid options for neuropathic pain (to view the most recent version, visit NCCN.org).
An external comment noted that the language in the guidelines surrounding creams for neuropathic pain (see SPAIN-4, page 788) made it seem like the only recommended option was “ketamine and amitriptyline combined.” The panel agreed that the language was misleading and that several compounded creams are appropriate. In fact, data supporting cream with ketamine and amitriptyline combined are limited, with a mix of positive and negative studies.54–61 The panel discussed that the evidence for compounded topical gel containing baclofen, amitriptyline, and ketamine is stronger. In an RCT of 208 participants with chemotherapy-induced peripheral neuropathy, the compounded gel group showed a trend toward improvements in the sensory and motor subscales of the EORTC QLQ-CIPN20 compared with the placebo group.62 The greatest improvements were seen in tingling, cramping, shooting/burning pain in the hands, and difficulty holding a pen. The panel noted that this is just a single trial, but that compounded creams are reasonable to try. This conclusion is consistent with that of an ASCO clinical practice guideline panel.63 The panel also discussed data for a cream form of lidocaine, which was already included in the guidelines as a patch.64 In one small randomized trial, lidocaine cream resulted in a small improvement in pain intensity compared with amitriptyline or placebo cream.57 Compounded cream containing lidocaine and ketamine has also been studied in a retrospective chart review, in which 8 of 11 patients benefited.65 The panel added lidocaine and baclofen after “compounded creams” and added “eg” to show that these are just examples of agents that can be used in various combinations.
Other external comments led the panel to consider including peripheral neurolysis and intrathecal opioid therapy as additional options for neuropathic pain in survivors (see SPAIN-4, page 788). The panel discussed that both of these options would fall under referral to pain management services, which the panel lists for refractory pain. Panelists felt that oncologists and PCPs lack the expertise to perform these procedures. As part of this discussion, the panel decided that they did not have the appropriate expertise to even list the options that would be considered by pain specialists. They therefore removed the listed items. The panel believes that transcutaneous electrical nerve stimulation (TENS), however, is available more broadly and that many oncologists and PCPs would be comfortable prescribing this therapy. TENS is a noninvasive procedure in which electrodes are placed on or around the painful area using a small device.41 Although data supporting the efficacy of TENS for reducing cancer-related pain are inconclusive, the panel believes it should still be an option for some survivors,66,67 especially for institutions that do not have pain services readily available. The panel therefore included TENS as a recommended option under non-pharmacologic therapies (see SPAIN-4, page 788).
Another external proposal was for the panel to consider the addition of scrambler therapy for chronic pain syndrome (amputation, neck dissection, mastectomy, thoracotomy). Scrambler therapy is a device for noninvasive electrocutaneous nerve stimulation. Studies in patients with cancer pain show that it appears safe and may be effective at reducing pain.68–73 However, the panel noted that this therapy would require referral to a pain specialist, so they did not add it to the guidelines, instead leaving it to the discretion of pain specialists.
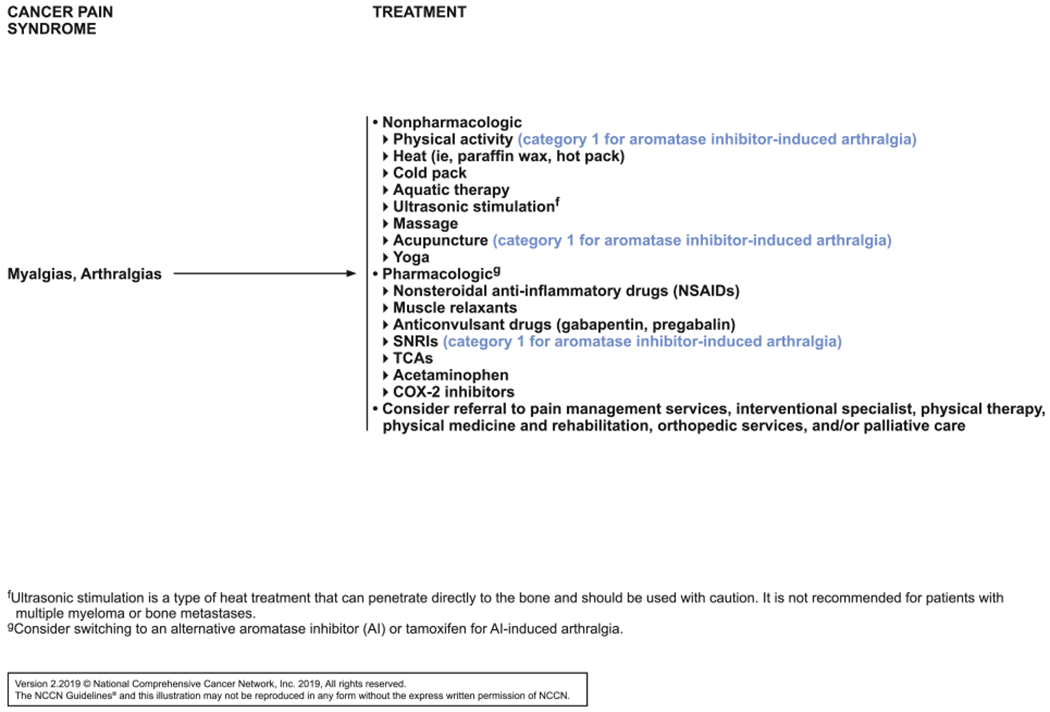
An external proposal included a request for a section specifically addressing aromatase inhibitor (AI)–induced arthralgias based on new data and the large number of survivors affected by this syndrome. A double-blind phase III RCT, which included 299 postmenopausal survivors of early-stage breast cancer with joint pain, showed that duloxetine improved average joint pain score, worst pain, joint stiffness, pain interference, and functioning at 12 weeks.74 Another trial randomized 226 postmenopausal women with early-stage breast cancer and AI-induced joint pain 2:1:1 to acupuncture, sham acupuncture, or waitlist.75 The acupuncture group experienced a small but statistically significant reduction in joint pain at 6 weeks. The panel discussed these trials and agreed that a separate section on AI-induced arthralgias was not needed because the section on arthralgia and myalgias covered the topic sufficiently. The panel agreed that the trials represented high-quality data, so they added category 1 designations for this subset of patients after both serotonin and norepinephrine reuptake inhibitors and acupuncture (see SPAIN-6, page 789). The panel then noted that data on physical activity for management of AI-induced arthralgias are similarly strong, particularly a trial in which breast cancer survivors with AI-induced arthralgia randomized to an exercise arm (150 min/wk of aerobic exercise plus supervised strength training twice per week) experienced greater improvements in worst joint pain scores, pain severity, and pain interference than those in the usual care arm (all P<.001).76 The panel thus added the category 1 designation to physical activity for this subset of patients.
Conclusions
The definition of survivorship starts at a patient’s diagnosis and encompasses all phases of care, including those living with a chronic cancer diagnosis. The survivorship guidelines are meant to be a tool to help oncologists and PCPs better address the needs of survivors who are undergoing surveillance and those on chronic treatments. Current updates to the guidelines included broadening the focus and audience of the guidelines, reflecting on the evolving role of survivorship care planning, and revising the Pain algorithms in response to the data. With multidisciplinary care coordination, facilitated by SCPs, oncologists and PCPs can work together to improve the lives of the survivors. This update helps clarify consensus reached at the 2019 meeting and reflects the panels’ position on important changes made to the survivorship guidelines.
Disclosure of Relevant Financial Relationships.
The NCCN staff listed below discloses no relevant financial relationships:
Kerrin M. Rosenthal, MA; Kimberly Callan, MS; Genevieve Emberger Hartzman, MA; Erin Hesler; Kristina M. Gregory, RN, MSN, OCN; Rashmi Kumar, PhD; Karen Kanefield; and Kathy Smith.
Individuals Who Provided Content Development and/or Authorship Assistance:
Tara Sanft, MD, Vice Chair, has disclosed that she has no relevant financial relationships.
Crystal S. Denlinger, MD, Panel Chair, has disclosed that she receives grant/research support from Amgen Inc, Bristol-Myers Squibb Company, Sanofi-aventis US LLC, BeiGene, Ltd, MacroGenics, Inc, Lycera Corp, Eli Lilly and Company, AstraZeneca Pharmaceuticals LP, Array BioPharma Inc, Agios Pharmaceuticals, and MedImmune, LLC; and that she receives consulting fees/honoraria from Bayer AG, Astellas US LLC, Bristol-Myers Squibb Company, and BeiGene, Ltd.
Melissa Hudson, MD, Panel Member, has disclosed that she has no relevant financial relationships.
Lindsay Peterson, MD, Panel Member, has disclosed that she has no relevant financial relationships.
Karen L. Syrjala, PhD, Panel Member, has disclosed that she has no relevant financial relationships.
Nicole R. McMillian, MS, CHES, Guidelines Coordinator, NCCN, has disclosed that she has no relevant financial relationships.
Deborah A. Freedman-Cass, PhD, Oncology Scientist/Senior Medical Writer, NCCN, has disclosed that she has no relevant financial relationships.
To view all of the conflicts of interest for the NCCN Guidelines panel, go to NCCN.org/disclosures/guidelinepanellisting.aspx.
NCCN CATEGORIES OF EVIDENCE AND CONSENSUS
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management of any patient with cancer is in a clinical trial. Participation in clinical trials is especially encouraged.
PLEASE NOTE
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of evidence and consensus of the authors regarding their views of currently accepted approaches to treatment. The NCCN Guidelines Insights highlight important changes in the NCCN Guidelines recommendations from previous versions. Colored markings in the algorithm show changes and the discussion aims to further the understanding of these changes by summarizing salient portions of the panel’s discussion, including the literature reviewed.
The NCCN Guidelines Insights do not represent the full NCCN Guidelines; further, the National Comprehensive Cancer Network® (NCCN®) makes no representations or warranties of any kind regarding their content, use, or application of the NCCN Guidelines and NCCN Guidelines Insights and disclaims any responsibility for their application or use in any way.
The complete and most recent version of these NCCN Guidelines is available free of charge at NCCN.org.
© National Comprehensive Cancer Network, Inc. 2019. All rights reserved. TheNCCNGuidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.

SURV-1

SURV-2
SPAIN-4
SPAIN-6
Acknowledgments
This activity is supported by educational grants from AstraZeneca, Celgene Corporation, Clovis Oncology, Eisai, Genentech, Genomic Health, Inc., Novartis, Taiho Oncology, Inc., and TESARO. This activity is supported by an independent educational grant from AbbVie. This activity is supported by educational funding provided by Amgen. This activity is supported by an unrestricted educational grant from Gilead Sciences, Medical Affairs.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep 2011;60:269–272. [PubMed] [Google Scholar]
- 4.Nekhlyudov L, Aziz NM, Lerro C, et al. Oncologists’ and primary care physicians’ awareness of late and long-term effects of chemotherapy: implications for care of the growing population of survivors. J Oncol Pract 2014;10:e29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol 2013;31:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin 2015;65:428–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resnick MJ, Lacchetti C, Penson DF. Prostate cancer survivorship care guidelines: American Society of Clinical Oncology practice guideline endorsement. J Oncol Pract 2015;11:e445–449. [DOI] [PubMed] [Google Scholar]
- 8.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 2016;34:611–635. [DOI] [PubMed] [Google Scholar]
- 9.Cohen EE, LaMonte SJ, Erb NL,et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin 2016;66: 203–239. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Survivorship definitions. Available at: http://cancercontrol.cancer.gov/ocs/statistics/definitions.html Accessed July 10, 2018.
- 11.Langbaum T, Smith TJ. Time to study metastatic-cancer survivorship. N Engl J Med 2019;380:1300–1302. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition Committee on Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 13.Earle CC, Ganz PA. Cancer survivorship care: don’t let the perfect be the enemy of the good. J Clin Oncol 2012;30:3764–3768. [DOI] [PubMed] [Google Scholar]
- 14.Heins M, Schellevis F, Rijken M, et al. Determinants of increased primary health care use in cancer survivors. J Clin Oncol 2012;30:4155–4160. [DOI] [PubMed] [Google Scholar]
- 15.Snyder CF, Frick KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med 2009;24:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luctkar-Flude M, Aiken A, McColl MA, et al. Are primary care providers implementing evidence-based care for breast cancer survivors? Can Fam Physician 2015;61:978–984. [PMC free article] [PubMed] [Google Scholar]
- 17.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med 2011;26:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virgo KS, Lerro CC, Klabunde CN, et al. Barriers to breast and colorectal cancer survivorship care: perceptions of primary care physicians and medical oncologists in the United States. J Clin Oncol 2013;31: 2322–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter FM, Usher-Smith JA, Yadlapalli S, et al. Caring for people living with, and beyond, cancer: an online survey of GPs in England. Br J Gen Pract 2015;65:e761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinstein EB, Miller WL, Hudson SV, et al. Cancer survivorship care in advanced primary care practices: a qualitative study of challenges and opportunities. JAMA Intern Med 2017;177:1726–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson SV, Miller SM, Hemler J, et al. Adult cancer survivors discuss follow-up in primary care: ‘not what I want, but maybe what I need’. Ann Fam Med 2012;10:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer EL, Gropper AB, Neville BA, et al. Breast cancer survivors’ perceptions of survivorship care options. J Clin Oncol 2012;30:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan NF, Evans J, Rose PW. A qualitative study of unmet needs and interactions with primary care among cancer survivors. Br J Cancer 2011; 105(Suppl 1)S46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol 2006;24:848–855. [DOI] [PubMed] [Google Scholar]
- 25.Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer 2006;94:1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rechis R, Beckjord EB, Nutt S. Potential benefits of treatment summaries for survivors’ health and information needs: results from a LIVESTRONG survey. J Oncol Pract 2014;10:75–78. [DOI] [PubMed] [Google Scholar]
- 27.Majhail NS, Murphy E, Laud P, et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica 2019;104: 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunfeld E, Julian JA, Pond G, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol 2011;29:4755–4762. [DOI] [PubMed] [Google Scholar]
- 29.Boekhout AH, Maunsell E, Pond GR, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. J Cancer Surviv 2015;9:683–691. [DOI] [PubMed] [Google Scholar]
- 30.Jefford M, Schofield P, Emery J. Improving survivorship care. J Clin Oncol 2012;30:1391–1392. [DOI] [PubMed] [Google Scholar]
- 31.Smith TJ, Snyder C. Is it time for (survivorship care) plan B? J Clin Oncol 2011;29:4740–4742. [DOI] [PubMed] [Google Scholar]
- 32.Stricker CT, Jacobs LA, Palmer SC. Survivorship care plans: an argument for evidence over common sense. J Clin Oncol 2012;30:1392–1393. [DOI] [PubMed] [Google Scholar]
- 33.Jefford M, Gough K, Drosdowsky A, et al. A randomized controlled trial of a nurse-led supportive care package (SurvivorCare) for survivors of colorectal cancer. Oncologist 2016;21:1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolaije KA, Ezendam NP, Vos MC, et al. Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol 2015;33:3550–3559. [DOI] [PubMed] [Google Scholar]
- 35.Maly RC, Liang LJ, Liu Y, et al. Randomized controlled trial of survivorship care plans among low-income, predominantly Latina breast cancer survivors. J Clin Oncol 2017;35:1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobsen PB, DeRosa AP, Henderson TO, et al. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol 2018;36:2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol 2014;32:1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care. Available at: https://www.facs.org/quality-programs/cancer/coc/standards Accessed July 10, 2018. [Google Scholar]
- 39.Pachman DR, Barton DL, Swetz KM, et al. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol 2012;30:3687–3696. [DOI] [PubMed] [Google Scholar]
- 40.Paice JA, Ferrell B. The management of cancer pain. CA Cancer J Clin 2011;61:157–182. [DOI] [PubMed] [Google Scholar]
- 41.Raphael J, Hester J, Ahmedzai S, et al. Cancer pain: part 2: physical, interventional and complimentary therapies; management in the community; acute, treatment-related and complex cancer pain: a perspective from the British Pain Society endorsed by the UK Association of Palliative Medicine and the Royal College of General Practitioners. Pain Med 2010; 11:872–896. [DOI] [PubMed] [Google Scholar]
- 42.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–1449. [DOI] [PubMed] [Google Scholar]
- 43.Meretoja TJ, Leidenius MHK, Tasmuth T, et al. Pain at 12 months after surgery for breast cancer. JAMA 2014;311:90–92. [DOI] [PubMed] [Google Scholar]
- 44.Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 2014;32:4149–4154. [DOI] [PubMed] [Google Scholar]
- 45.Improving the quality of pain management through measurement and action. Available at: https://www.npcnow.org/system/files/research/download/Improving-the-Quality-of-Pain-Management-Through-Measurement-and-Action.pdf Accessed April 26, 2019.
- 46.Sun V, Borneman T, Piper B, et al. Barriers to pain assessment and management in cancer survivorship. J Cancer Surviv 2008;2:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Key findings summary: opioid access research project. Available at: https://www.fightcancer.org/sites/default/files/ACS%20CAN%20PQLC%20Opioid%20Research%20Project%20Key%20Findings%20Summary%20Memo%20FINAL.pdf Accessed April 26, 2019.
- 48.Duehmke RM, Derry S, Wiffen PJ, et al. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev 2017;6:CD003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nucynta [package insert]. Newark, CA: Depomed, Inc.; 2018. [Google Scholar]
- 50.Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin 2011;27:151–162. [DOI] [PubMed] [Google Scholar]
- 51.Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care 2014;37:2302–2309. [DOI] [PubMed] [Google Scholar]
- 52.Imanaka K, Tominaga Y, Etropolski M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr Med Res Opin 2013;29:1399–1409. [DOI] [PubMed] [Google Scholar]
- 53.Kress HG, Koch ED, Kosturski H, et al. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician 2014;17:329–343. [PubMed] [Google Scholar]
- 54.Argoff CE. Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc 2013;88:195–205. [DOI] [PubMed] [Google Scholar]
- 55.Barros GA, Miot HA, Braz AM, et al. Topical (S)-ketamine for pain management of postherpetic neuralgia. An Bras Dermatol 2012;87:504–505. [DOI] [PubMed] [Google Scholar]
- 56.Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med 2005;2:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho KY, Huh BK, White WD, et al. Topical amitriptyline versus lidocaine in the treatment of neuropathic pain. Clin J Pain 2008;24:51–55. [DOI] [PubMed] [Google Scholar]
- 58.Lin PL, Fan SZ, Huang CH, et al. Analgesic effect of lidocaine patch 5% in the treatment of acute herpes zoster: a double-blind and vehicle-controlled study. Reg Anesth Pain Med 2008;33:320–325. [DOI] [PubMed] [Google Scholar]
- 59.Lynch ME, Clark AJ, Sawynok J, et al. Topical amitriptyline and ketamine in neuropathic pain syndromes: an open-label study. J Pain 2005;6:644–649. [DOI] [PubMed] [Google Scholar]
- 60.Lynch ME, Clark AJ, Sawynok J, et al. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes: a randomized, double-blind, placebo-controlled trial. Anesthesiology 2005;103:140–146. [DOI] [PubMed] [Google Scholar]
- 61.Gewandter JS, Mohile SG, Heckler CE, et al. A phase III randomized, placebo-controlled study of topical amitriptyline and ketamine for chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study of 462 cancer survivors. Support Care Cancer 2014;22:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barton DL,Wos EJ, Qin R, et al. Adouble-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer 2011;19:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:1941–1967. [DOI] [PubMed] [Google Scholar]
- 64.Derry S, Wiffen PJ, Moore RA, et al. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev 2014:CD010958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tam E, Furlan AD.Transdermal lidocaine and ketamine for neuropathic pain: a study of effectiveness and tolerability. Open Neurol J 2012;6:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurlow A, Bennett MI, Robb KA, et al. Transcutaneous electric nerve stimulation (TENS) for cancer pain in adults. Cochrane Database Syst Rev 2012;3:CD006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev Neurother 2011;11:735–753. [DOI] [PubMed] [Google Scholar]
- 68.Coyne PJ, Wan W, Dodson P, et al. Atrial of scramblertherapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother 2013;27:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pachman DR, Weisbrod BL, Seisler DK, et al. Pilot evaluation of scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer 2015;23:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith T, Cheville AL, Loprinzi CL,et al. Scramblertherapy for the treatment of chronic post-mastectomy pain (cPMP). Cureus 2017;9:e1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ricci M, Fabbri L, Pirotti S, et al. Scrambler therapy: what’s new after 15 years? The results from 219 patients treated for chronic pain. Medicine (Baltimore) 2019;98:e13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith TJ, Razzak AR, Blackford AL, et al. A pilot randomized sham-controlled trial of MC5-A scrambler therapy in the treatment of chronic chemotherapy-induced peripheral neuropathy(CIPN).J Palliat Care 2019: 825859719827589. [DOI] [PubMed] [Google Scholar]
- 73.Loprinzi CL, Le-Rademacher J, Majithia N, et al. Scrambler therapy for established chemotherapy-induced neuropathy: a randomized phase II trial [abstract]. J Clin Oncol 2018;36(Suppl):Abstract 10016. [Google Scholar]
- 74.Henry NL, Unger JM, Schott AF, et al. Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol 2018;36:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA 2018;320:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors.J Clin Oncol 2015;33:1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]


