The single greatest threat to man’s continued existence on earth is the virus.
Joshua Ledeberg, Nobel Scientist
(Fig 1)
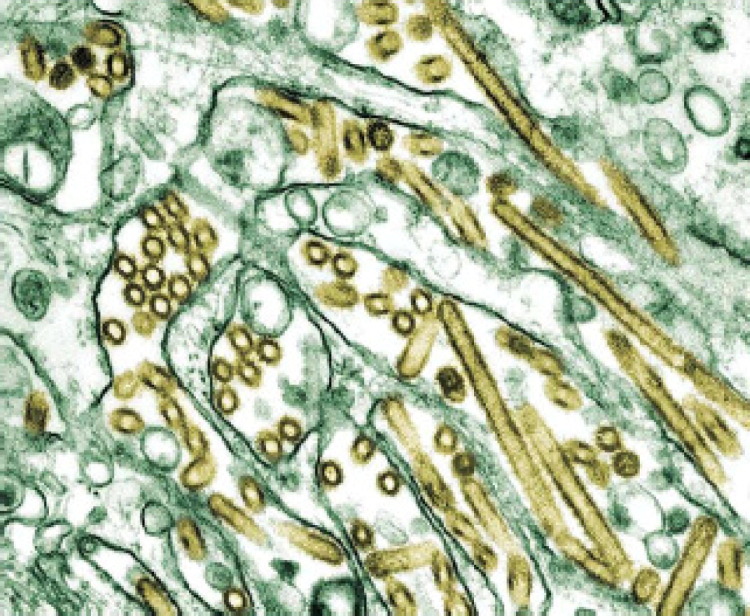
FIG 1.
Electron micrograph of avian flu virus. Transmission electron micrograph of avian influenza A H5N1 viruses (seen in gold) grown in MDCK cells (seen in green). (The Public Health Image Library, #1841.)
Introduction
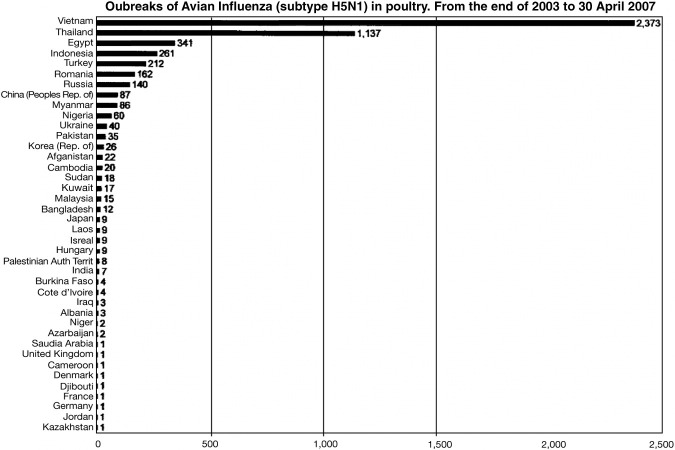
There have been three major influenza pandemics in the 20th century: the “Spanish Flu” (Influenza H1N1) in 1918, the “Asian Flu” (H2N2) in 1957, and the “Hong Kong Flu” (H3N2) in 1968.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 The latter two pandemics resulted in estimated worldwide deaths of 2 million in 1957 and 1 million in 1968. Although estimates vary on the total number of deaths credited with these pandemics, most agree that at least 50 million died from the Spanish Flu between 1918 and 1919, with deaths predominantly occurring within a few days of infection.1, 2, 3, 4, 5, 11 And, of all the deaths from the 1918 pandemic, over half the dead were young, healthy adults, an uncommon phenomenon with seasonal flu illness. Scientists concluded that origins of the strains of influenza virus causing the 3 pandemics were viruses containing combinations of genes from both a human influenza virus and an avian influenza virus.1, 4, 6, 7, 8 As an aside, in this era of bioterrorism preparedness, it is important to note that the reassortment of influenza genes could be employed for the creation of biological weapons.13, 14 Avian influenza therefore represents a likely pathogen to cause the next pandemic, according to the World Health Organization (WHO) and Centers for Disease Control (CDC). WHO issued a report in December 2004 in which the threat of an influenza pandemic occurring in the near future has greater likelihood with the recent appearance and wide spread of avian influenza H5N1.5 A pandemic is generally considered to be a global outbreak, ie, a pathogen that causes a multi-continent epidemic. H5N1 meets that criterion if you consider birds, given chickens, geese, pigeons, and other fowl have been afflicted from Asia to Europe. There have been several thousand outbreaks of H5N1 in poultry from 2003 to April 2007 worldwide, with most occurring in Vietnam (2,373) and Thailand (1,137), and the fewest in France, Germany, Jordan, the United Kingdom, Denmark, Kazakhstan, Djibouti, Cameroon, and Saudi Arabia each with 1 confirmed outbreak (Fig 2). WHO warns that the next influenza pandemic could result in the deaths of between 2 and 7 million people, with tens of millions requiring hospitalization, including treatment in intensive care units.11 This is a WHO “best case” scenario, modeled after the “milder” pandemics in the 20th century. WHO considers the pandemic of 1918 to be “extraordinary” and thus have chosen to model the 1957/1968 outbreaks. However, if one were to model the potential impact HPAI H5N1 could have if it were to become the causative agent of a pandemic, based on the 1918 pandemic, the death toll expected would be exponentially greater than the more conservative estimates given above.11 What makes the WHO 2004 report especially prescient, not long after it was published, in 2005, there occurred a significant increase in the spread of the avian influenza strain H5N1, resulting in millions of birds and hundreds of humans subsequently being affected (Table 1 and Fig 3). 15 Sporadic outbreaks of avian influenza continue to date with the most recent being in Egypt where several people have become infected and subsequently died.
FIG 2.
Worldwide Outbreaks of H5N1 (Source: http://www.WHO.int).
TABLE 1.
Cumulative number of confirmed human cases of Avian Influenza A/(H5N1) reported to WHO (11 April 2007)
| Country | 2003 |
2004 |
2005 |
2006 |
2007 |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | |
| Azarbaijan | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 5 | 0 | 0 | 8 | 5 |
| Cambodia | 0 | 0 | 0 | 0 | 4 | 4 | 2 | 2 | 1 | 1 | 7 | 7 |
| China | 1 | 1 | 0 | 0 | 8 | 5 | 13 | 8 | 3 | 2 | 25 | 16 |
| Djiboud | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Egypt | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 10 | 19 | 5 | 37 | 15 |
| Indonesia | 0 | 0 | 0 | 0 | 20 | 13 | 55 | 45 | 26 | 22 | 101 | 60 |
| Iraq | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 3 | 2 |
| Lao People’s Democratic Republic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 |
| Nigeria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Thailand | 0 | 0 | 17 | 12 | 5 | 2 | 3 | 3 | 0 | 0 | 25 | 17 |
| Turkey | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 4 | 0 | 0 | 12 | 4 |
| Viet Nam | 3 | 3 | 29 | 20 | 61 | 19 | 0 | 0 | 0 | 0 | 95 | 42 |
| Total | 4 | 4 | 46 | 32 | 98 | 43 | 115 | 79 | 54 | 33 | 317 | 191 |
Total number of cases includes number of deaths.
WHO reports only laboratory-confirmed cases.
All dates refer to onset of illness.
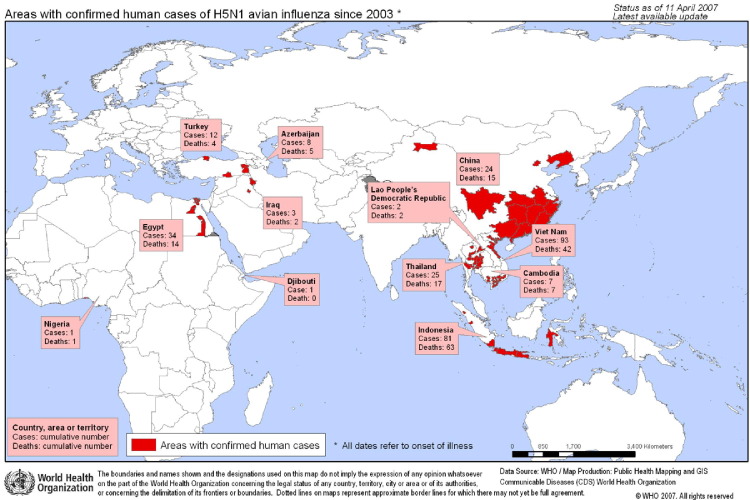
FIG 3.
Confirmed human cases avian flu: 2003 to present. World Health Organization. (Color version of figure is available online.)
Epidemiology: 1996 to April 2007
“Those who ignore history are condemned to repeat it.”
George Santayana
In 1996 a highly pathogenic avian influenza (HPAI) virus (H5N1) was isolated from a farmed goose in Guangdong Province, China.16 Soon thereafter outbreaks of HPAI H5N1 are reported in poultry farms and live animal markets in Hong Kong. Open air markets—important to local commerce and commonly frequented by tourists—are places of interaction and thus exposure risks between animals and humans. In 1997 several individuals became infected with what was soon to be recognized as a deadly emerging strain of avian influenza, H5N1, that was increasingly being associated with diseased chickens, resulting in the almost total destruction of Hong Kong poultry flocks.1, 2, 3, 4, 5, 9, 10, 17, 18, 19, 20, 21, 22, 23, 24, 25 Hong Kong, a British Crown Colony, enjoyed the financial and public health infrastructure that is credited with limiting the spread of avian influenza in 1997. Realizing the safety of Hong Kong, and potentially the region relied on poultry farmers being forthcoming with information about sick birds, with the full knowledge that the best way to contain the threat was to kill a farmers entire flock—something that poses an enormous burden to local communities—the government provided financial support to minimize the impact. As a result, widespread, intentional culling allowed the attenuation of the infection.17, 18, 19, 20, 21, 22, 23, 24, 25
This strategy worked well for several years, until in February 2003 two human cases of HPAI H5N1 infection, one of which died, were confirmed in a Hong Kong family that had recently traveled to Fujian Province, China.16
From 2003 to April 2007, a total of 291 human cases have been confirmed in 12 countries (Table 1 from Asia, the Middle East, and Africa.15, 26, 27, 28, 29, 30, 31, 32, 33 Of these cases, 172 have died, yielding a case fatality rate (CFR) overall of 59%. However, there is a wide range CFR of H5N1 depending on the country, time to treatment, patient’s underlying health, and other factors, with a high CFR of 100% among confirmed cases in Cambodia and a lower than average CFR of 33% among cases in Turkey.
Between December 2003 and January 2004 two tigers and two leopards died unexpectedly in a Thailand zoo.16 H5N1 similar to the strain killing poultry was identified as the lethal pathogen responsible for the deaths. These are the first reported cases of influenza causing disease and deaths in big cats. It was learned that they were fed on fresh chicken carcasses. In February 2004 a domestic cat in Thailand was infected after eating an infected pigeon.16
Although not surprising that an avian influenza would infect birds, widespread death and severe illness is not expected with typical strains of avian influenza viruses.2, 26 However, birds have been especially hard hit by this strain of influenza virus since 2003; experts suggest virtually all species are susceptible but poultry being among the most vulnerable.
Since 2003 HPAI H5N1 has reemerged, starting in Southeast Asia through Eurasia and into Europe,1, 2, 18, 20, 22, 25, 26, 27 leaving in its path over 140 million dead birds (some estimates go as high as 200 million).
Unlike 1997, when the resources and global sensibilities of Great Britain were at play, Hong Kong was now under the control of China, a country not noted for openness or being forthcoming in terms of its handling of domestic challenges. There were numerous allegations that the Chinese Government was trying to suppress or withhold information about a potential reemergence of H5N1 infection, as well as engage in practices intended to curtail detection and contain the problem in China such as surreptitiously feeding poultry the antiviral medication amantadine. Unlike British procedures which halted the spread in 1997, Chinese government activities resulted in delayed culling of chickens, as well as delayed or nonpayment to farmers when their flocks were destroyed; these have all been suspected as contributing to the reemergence and spread of HPAI.34 Efforts by WHO, global reporting by numerous media organizations, and the collective cooperation and leadership among most of the Asian nations affected have contributed to vastly improved surveillance, laboratory capabilities, medical response, vaccine development, and other containment strategies.34, 35, 36, 37, 38, 39, 40, 41 It is worth remembering, we all live in a global world. The critical balance between sovereignty and global responsibility ceases to be an academic exercise in the face of widespread disease and death from a pathogen that won’t honor borders.34, 36, 37, 38 Infectious threats and resulting government actions in far-off lands can impact us in the United States. National self interest and a policy of secrecy—practices China is noted for—can imperil the world.34, 35, 36, 37, 38 One wonders had information about the second emergence of H5N1 been released in live time, how many lives—human and avian—could have been spared, had avian flu been as well contained in the early 2000s as it was in 1997?34, 35, 36, 37, 38, 39, 40, 41 Santayana was profound and his warning is worth remembering!
Influenza Viruses
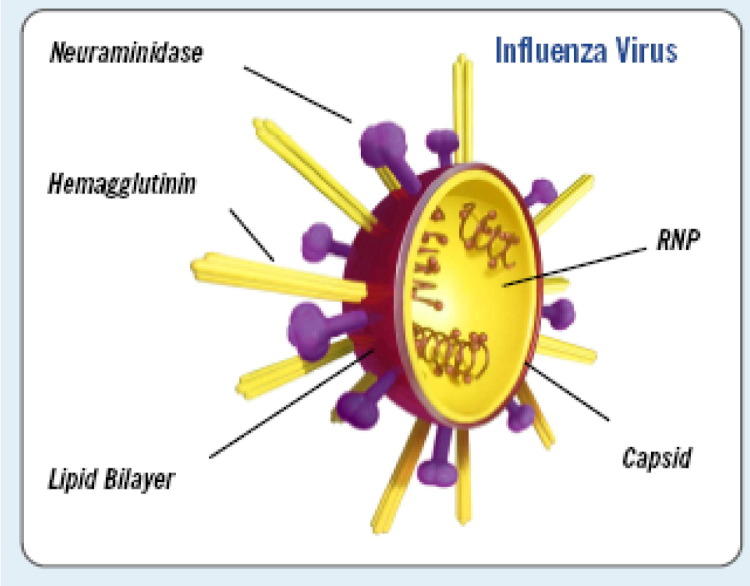
The term “influenza” describes an acute viral disease of the respiratory tract often referred to as “the flu” caused by viruses belonging to the orthomyxovirus family, which includes the genera of influenza virus A, B, and C as defined by the antigenicity of the nucleocapsid and matrix proteins (Fig 4). 1, 2, 17, 18, 20, 22, 26 Generally, A viruses are associated with more severe human illness, epidemics, and pandemics. Influenza A virus is a negative sense, single-stranded RNA virus, with an 8-segment genome that encodes for 10 proteins17, 20, 28 (Fig 2). They are further classified or sub-typed based on two surface proteins: haemagglutinin (H) which attaches the viral particle to the host cell for cell entry, and neuraminidase (N) which facilitates the spread of progeny virus. It is the latter which is a target for the class of antiviral therapy referred to as neuraminidase inhibitors.1, 2, 18, 22, 26, 28, 29 There are 16 H and 9 N subtypes making up all the subtypes of influenza A by various combinations.27
FIG 4.
Structure of influenza virus (with permission from BioCryst Corporation, http://www.biocryst.com/pdf/peramivirfacts.pdf).
The term “antigenic drift” refers to the various mutations and changes in surface antigenicity of these surface proteins as a response to host immunity. “Antigenic shift” is an event that can lead to the creation of a novel virus against which humans have little or no immunity. Because influenza has a segmented genome, shuffling of gene segments can occur if two different subtypes of influenza A virus co-infect the same cell. Conditions favorable for the emergence of antigenic shift have long been thought to involve humans living in proximity to farm animals, namely poultry and pigs. Pigs are susceptible to infection with both avian and mammalian virus. If a human influenza virus, such as H3N2, and an avian H5N1 virus co-infect a human or pig, it is possible a new virus H5N2 could emerge—a hybrid that could combine the high virulence of H5N1 with the efficiency of human to human transmission found in the “parent” human virus.19, 31
Studies suggest that this reassortment of genetic material is what happened in the 1957 and 1968 pandemics.1, 2, 3, 6, 7, 31 This reassortment could also be accomplished in a laboratory for bioterrorism.13, 14, 32 Therefore, bioterrorism preparedness, increased surveillance, and efforts to enhance physician training in emerging diseases are of significant value.13, 14, 32, 33
Avian Influenza
Avian influenza is an infectious disease of birds caused by influenza virus type A strains occurring worldwide. Avian influenza was first identified over 100 years ago in Italy.1 In 1955 studies revealed that all subtypes of influenza A viruses have been detected in more than 90 species of otherwise apparently healthy wild birds.1, 2, 8 The natural reservoir of avian influenza viruses are wild waterfowl, gulls, and shorebirds. Usually the gastrointestinal tract is involved, thus the risk of exposure when coming in contact with excreta. Historically, it is likely that these species of birds have carried avian influenza viruses without developing symptoms; such is an optimal condition of adaptation, especially virus to host. Given these birds are migratory, ie, highly mobile, they are known to carry viruses over great distances. These birds can excrete significant viral loads in their feces, yet remain healthy. Until recently, avian influenza viruses have been considered relatively nondangerous to birds and extremely rare findings in humans. When these infections did occur in humans, only mild illness occurred, usually conjunctivitis followed by full recovery. H5N1 has proven dramatically to be an exception, causing death to animals and humans. Of note, given that H5N1 is killing animals that normally are not affected by avian influenza viruses, this suggests that the virus has adapted and thus increased its virulence (a virus’ capacity to harm or kill). Since infected birds will excrete the virus, such commonness of bird feces promotes contamination of food, water, environment, and other animals, increasing the likelihood of human illness.2 The majority of the infections were the result of human to animal proximity in mostly agricultural countries with often substandard hygienic practices. It is thus easy to understand why environment and cultural cooking practices are of paramount concern. Eating raw eggs, poultry blood, or undercooked bird meat, playing “catch” with chicken heads, and domiciles shared with animals have been the primary routes of contamination for infected people.2
All bird species are thought to be susceptible to being infected, with variable degrees of symptoms possible, ranging from mild illness to contagious and rapidly fatal illness.8 HPAI H5N1 can kill within 48 hours. A total of 15 subtypes of influenza are known to infect birds, resulting in an extensive reservoir; this poses a significant challenge to governments and international public health efforts to contain an infection that can literally travel on the very winds the birds can fly! Of additional concern, low pathogenicity avian influenza (LPAI) can mutate into HPAI. An example of this was the H5N2 virus that circulated in the United States in 1983 to 1984. It began as an LPAI with a low mortality rate, but within 6 months it became an HPAI associated with a 90% mortality rate. Italy experienced a similar event; an H7N1 LPAI virus mutated within 9 months into an HPAI form, resulting in the death—disease or culling—of greater than 13 million birds.1, 8, 9
There are many genotypes of H5N1; the predominant one is “Z” which is associated with high virulence for a wide range of animals from poultry to tigers and cats.2, 19, 24 It appears to be stable in the environment for up to 6 days.2, 19, 20, 23
Although avian influenza has caused to date 191 deaths (Table 1),15 seasonal influenza has caused significant illness for centuries and remains a global public health problem, resulting in millions of cases of severe illness, as well as approximately 500,000 deaths worldwide, 36,000 deaths and over 200,000 hospitalizations in the United States (US) annually.1, 3, 6, 7, 12, 26, 27, 31, 42, 43, 44
Despite the efforts of the international health community to contain the avian influenza epidemic that emerged in Southeast Asia in 2003/2004, sporadic cases of human influenza A H5N1 infection are still reported and carry a high case fatality rate (Table 1).2, 15, 23, 24, 45
Scientists believe the influenza virus causing the pandemic of 1918 started off as an avian influenza strain. Unlike usual patterns of fatality associated with seasonal influenza, namely the very young and elderly, both the 1918 pandemic and what is currently occurring with H5N1 involved all age groups.
Never before has an avian influenza strain killed so many millions of birds, traveled so far so fast, and posed the potential to continue affecting Asia, Europe, and other continents.
Human Infection and Pandemic Potential
H5N1 is worrisome because it mutates rapidly and has the ability to acquire genes from viruses infecting other species. With an increased incidence in H5N1 in birds comes a concomitant risk for direct infection of humans. If humans are concurrently co-infected with a human influenza virus, human flu being contagious, the opportunity for human and avian strains to intermix resulting in a novel, highly contagious, and virulent strain becomes likely.1, 2, 9 Avian influenza infects people via the respiratory tract, which can be accomplished via fomites, inhalation, or hand contact to the mucosa (Fig 1). Fortunately people cannot get avian influenza from eating properly cooked poultry. Eating raw eggs, poultry blood, or undercooked bird meat are ways that one could become infected and are practices in countries where human avian influenza cases have been recorded.2, 17, 20
Three factors must be present in order for the emergence of a new influenza virus to result in an influenza pandemic1, 2, 17, 18, 19, 20, 25, 26, 27:
-
1
People have little or no immunity for the virus;
-
2
The virus spreads readily from person to person; and
-
3
No vaccine is readily available.
Although data suggest there have been a few cases resulting from human-to-human transmission, the infection stopped at the second person, usually a family member.2, 13, 17, 18, 20, 21, 31, 45 If avian influenza mutates into a more human-like influenza, it is likely to spread very rapidly in a sustained fashion across the globe and result in thousands, perhaps millions, of deaths,1, 2, 3, 17, 31 similar to the influenza pandemic of 1918 that resulted in 20 to 50 million deaths worldwide.1, 2, 3, 17, 18, 19, 20, 26, 27, 31, 45
Experts agree that the most likely mechanism pandemic influenza will arrive in the United States will be from human travel, migratory birds, the poultry trade, or a combination of the above. Illegal importation of sick animals is also a consideration; recall the monkeypox patient in 2003 that ultimately resulted from an illegally imported West African rodent.2, 14, 17, 19, 20, 46, 47, 48
Whereas much effort has been focused on hospital, public health, and government response2, 6, 18, 19, 20, 49, 50, 51 to a influenza pandemic, two vital responder communities—the emergency medical services (EMS) professional (EMT and paramedic) and the private physician—remain somewhat disenfranchised. Yet it is likely that 911 will be activated to send an ambulance to an acutely ill victim of avian influenza. In a recent online survey conducted by the Journal of Emergency Medical Services (JEMS), readers, most of whom are medics, emergency physicians, and EMS administrators, were asked if their EMS agency had a plan for operations during a pandemic flu outbreak, including stockpiles of N-95 masks. Only 17% of total respondents answered “yes,” whereas the majority stated “no” because they were focusing on other planning initiatives.52 This relative lack of preparedness may reflect lack of funding, inadequate training, or disenfranchisement from public health efforts. It is also just as likely that a community clinician may be called on to diagnose the sentinel case of avian flu in the United States, reminiscent of the diagnosis of the index case of inhalation anthrax in 2001.2 Most scenarios describe a significant number of people becoming quite ill over a relatively short period of time. It is important to realize that a sick individual may precede such an outbreak, underscoring the critical importance of providing advanced training to physicians in the recognition or suspicion of emerging pathogens, given that early diagnosis can save a life and alert the medical community of a developing threat.33, 53, 54, 55, 56 In a global world, anything from deadly animals used for exotic pets to infectious diseases can be imported.57, 58, 59, 60, 61 Primary care clinicians should be aware of these risks and convey information about them to their patients.
Pathogenesis of Avian Influenza
H5N1, like the 1918 pandemic flu strain, has the ability to kill directly. Unlike seasonal influenza, which destroys the cells that line the upper respiratory tract, facilitating development of bacterial pneumonia especially in immune-compromised patients, avian and Spanish flu attack within the lung and initiate a severe immune response causing tissue necrosis and hemorrhage. H5N1 induces pro-inflammatory cytokines,62, 63, 64, 65 such as interferon gamma inducible protein and tumor necrosis factor (TNF) alpha, in human macrophage cells which may lead to a cytokine storm and death without extra-pulmonary viral dissemination.2, 17, 62, 63, 64, 65, 66 The haemagglutinin of H5N1 may also attach to respiratory epithelial cells causing inhibition of epithelial sodium channels leading to pulmonary edema, alveolar flooding, and early acute respiratory failure—events that rarely accompany seasonal influenza.2, 10, 62, 63, 64, 65, 66
Clinical Presentation
Unlike seasonal influenza, the severity of H5N1 is reflected by the early presenting symptoms that are similar to acute, rapidly progressive pneumonia: rapid onset of high, persistent fever, impaired consciousness, respiratory distress, and/or multiorgan dysfunction. The fever spike is ≥38°C or ≥101°F and often >39°C. Nearly universal is dyspnea. Significant difficulty breathing early in the illness warrants aggressive intervention.2, 10, 17, 18, 20, 23, 25 However, the early absence of severe respiratory symptoms in the presence of risk factors and other symptoms consistent with avian influenza does not rule out the illness; pulmonary involvement can develop rapidly, and the astute clinician should be alert to respiratory changes. As noted earlier, avian influenza is capable of direct pulmonary injury to any age group, including young healthy adults, resulting in a variety of manifestations on chest radiographs, respiratory and fluid derangements, unlike seasonal influenza which causes severe illness or death usually as a result in secondary bacterial pneumonia that tends to be limited to immunocompromised, elderly, very young, or ill patients.66 It is worth noting that other family members or coworkers may manifest similar symptoms with varying degrees of severity and development of illness.
There are numerous aggressive respiratory infections that may present with significant illness, both commonly found in the United States and emerging pathogens. Included in the differential diagnosis of rapidly progressive febrile illness with pulmonary involvement include Legionella pneumonia (Legionaires disease), other gram negative pneumonias, mycoplasma, Chlamydia, tuberculosis, HIV, Leptospira, and Severe Acute Respiratory Syndrome (SARS).
Avian flu illness is not subtle. By the time patients seek medical care, it is unlikely to present like the common cold or the average case of seasonal flu (Table 2). Analysis of human H5N1 infections in Hong Kong, Vietnam, Thailand, and Cambodia revealed that fever and cough were the most common presenting initial symptoms.2, 70, 73, 74, 75, 76, 77 Of those presenting for medical attention, the illness is rapidly progressive with patients often complaining of chest pain and dyspnea, which is rarely associated with seasonal influenza, especially in young, otherwise healthy patients (Table 2). Dyspnea under most circumstances is worrisome and should be properly evaluated, but in the context of a rapid rising fever should raise an alarm for avian influenza or other serious infection.2, 22, 23, 29, 31, 32, 48, 49, 50, 54, 55, 76
TABLE 2.
“BIODROME” key differentiating symptoms: avian influenza compared with seasonal flu and influenza-like illnesses/upper respiratory infections
| Is it? Number of “+” indicates strength of association | Avian Flu (H5N1) | Seasonal Influenza (Flu) | Upper Respiratory Infection | Common Cold |
|---|---|---|---|---|
| Elevated temp | +++/++++ | ++ | ++ | +/− |
| Fever/chills | ++++ | ++++ | ++++ | |
| Cough | +++ | +++ | +++ | +++ |
| Shortness of breath | ++++ | +/− | +/− | |
| Chest discomfort | +++ | ++ | ++ | |
| Sore throat | +/− | +++ | +++ | ++ |
| Vomiting/nausea | ++ | + | + | − |
| Diarrhea | ++ | + (young children) | +/− | − |
| CNS/encephalopathy/seizures | ++ | − | − | − |
| Malaise/fatigue | +++ | +++ | + | +/− |
| Runny nose/watery eyes | +/− | + | ++ | +++ |
| Headache/muscle ache | +++ | +++ | ++ | +/− |
| Young healthy at risk for serious illness | +++ | +/− | +/− | − |
Also, avian influenza can have an extra-pulmonary impact, including the central nervous system, causing seizures or encephalitis. Of note, whereas GI symptoms may be more common among children with seasonal influenza, young adults and other age groups may experience abdominal pain, nausea, vomiting, or diarrhea before or during the development of respiratory symptoms resulting from H5N1, again symptoms not common with seasonal influenza in this age group.2, 19, 20, 21, 22, 23, 24, 25, 29, 48, 49, 50, 51, 53, 54 Unlike seasonal influenza deaths, which often result from secondary infections such as bacterial pneumonia, avian influenza seems to have some direct pulmonary effects that can cause non-cardiogenic pulmonary edema as well as viral pneumonia.2, 20, 31, 32, 62, 63, 64, 65, 66, 67, 68, 69
Diagnostic Considerations
History
Early detection of human cases is critical to contain a potential outbreak. This rests largely upon maintaining a high index of suspicion for emerging pathogens, unusually aggressive infections, and global events especially as pertain to highly virulent pathogens.72 Diagnosing a potential case of avian influenza is based on clinical findings; of paramount importance is a thorough history, including even seemingly unrelated symptoms over the last several days of illness, patient travel (within 14 to 16 days), especially to countries suspected of having H5N1 disease (Fig 1), and occupation that may pose additional risk: veterinarian, commercial interests in animals, laboratorian, Peace Corps, poultry farming, especially in at-risk regions.20, 22, 25, 50, 70 It is important to ascertain whether coworkers, family members, or fellow travelers developed similar symptoms, especially rapidly progressive fevers and respiratory complaints. Whether influenza season or as a result of WHO or CDC alerts that additional avian flu cases are occurring, it is important to train intake personnel, receptionists, and triage nurses about key questions and symptoms to look for in order to rapidly implement infection control measures and alert appropriate personnel to diagnose and manage potential contagious patients. Laboratory testing may be helpful to confirm H5N1 but should not delay initiation of infection control or other critical interventions.
Clinical Testing
Chest Radiography
Any patient presenting with shortness of breath or other significant respiratory symptoms warrants additional and immediate attention. Radiographic investigation; usually a chest x-ray is an appropriate study especially in the setting of a potential avian influenza patient. Again, exercise precautions including infection control measures including PPE, communicating concerns about potential contagious illness to health care team, laboratory personnel, and testing technicians. Major findings on chest radiograph (CXR) associated with H5N1 include extensive infiltration bilaterally, lobar collapse, focal consolidation, and less commonly interstitial lung infiltrates (Fig 5). Experts at the University of Oxford have studied 98 chest x-rays of 14 patients admitted to Ho Chi Minh City Hospital in Vietnam after testing positive for avian influenza.77 Of the 14 patients studied, 9 died and 5 survived. The most common abnormality found was multifocal consolidation, considered representative of pus and infection. They further suggested that the findings were similar to chest x-rays seen in SARS patients: multiple accumulations. They also found enlarged lymph nodes, cavities forming in the lung tissue, and fluid in the space surrounding the lungs, not usually associated with SARS CXR. The researchers suggest that the severity of chest x-rays may be a good predictor of prognosis; clinical deterioration associated with these is common.2, 19, 20, 22, 25, 27, 77
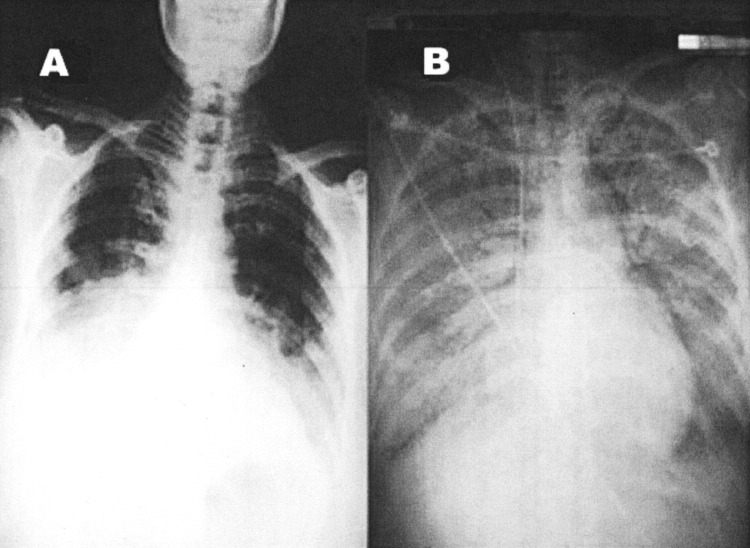
FIG 5.
Chest x-ray findings avian influenza. (A) Chest radiograph on hospital day 5 at referring hospital shows patchy infiltration at bilateral lower lung fields. (B) Chest radiograph upon admission to our hospital (24 hours later) shows rapidly progressive pneumonia in both lung fields, compatible with adult respiratory distress syndrome. Reprinted from Apisarnthanarak A, Kitphati R, Thongphubeth K, Patoomanunt P, Anthanont P, Auwanit W, et al. Atypical avian influenza (H5N1). Emerg Infect Dis [serial on the Internet]. 2004 Jul [date cited]. Available from: http://www.cdc.gov/ncidod/ElD/vol10no7/04-0415.htm
Computed tomography (CT) scans were obtained from three of the five survivors after discharge. Some abnormalities such as scar tissue formation were observed.77 Whether these were related to avian influenza or incidental findings is unknown, and the study sample size is too small to make definitive conclusions. Nevertheless, it is worth remembering that avian influenza can cause direct damage to lung tissue.
Viral Identification
If H5N1 is suspected based on symptoms, travel, and exposure history, collect respiratory samples such as a nasopharyngeal swab or aspirate. Alert your local health department, which should be able to provide the most current information on sample collection, packaging, and transportation and have access to the state laboratory or Laboratory Response Network (LRN)—a federally sponsored initiative to provide advanced testing capabilities throughout the United States.2, 70, 74 If you cannot access your local health official, then contact the CDC Director’s Emergency Operation Center at 770-488-7100.
In June 2006 the CDC released updated guidelines that provide more details on when to test a patient for the H5N1 avian influenza virus as well as greater information on laboratory testing. These new guidelines recommend lab testing for a patient whose illness is associated with all of the following:
-
1
Hospitalization or death;
-
2
A fever of 38°C (100.4°F) or higher;
-
3Radiographically confirmed; and
-
apneumonia, or
-
bacute respiratory distress syndrome, or
-
cother severe respiratory illness; and
-
a
-
4
Potential exposure* within 10 days of symptom onset.
*The CDC defines “potential exposure” as any of the following:
-
a
History of travel to a country with documented H5N1 in poultry, wild birds, or people and, during travel, at least one potential exposure (eg, contact with sick or dead domestic poultry, consumptions of incompletely cooked poultry, or close contact with a person who was hospitalized with a severe, unexplained respiratory illness).
-
b
Close contact (within about 3 feet) of a sick person who has confirmed or suspected to have H5N1.
-
c
Working with live influenza H5N1 in a laboratory.
The guidelines also recommend considering testing for a patient with:
-
1
Mild or atypical disease, eg, respiratory illness and fever that does not require hospitalization or significant neurologic or gastrointestinal symptoms in the absence of respiratory disease) and one of the exposures in the list above, or
-
2
Severe or fatal respiratory disease whose epidemiologic information is uncertain, unavailable, or otherwise suspicious.
Based on the 1997 experiences, Real Time Polymerase Chain Reaction (RT-PCR) has superior sensitivity and specificity compared to antigen detection and can aid in rapid diagnosis.2, 10, 19, 20, 70, 72, 74, 78, 79, 80, 81, 82 Influenza H5N1-specific RT-PCR testing conducted under biosafety level 2 conditions is the preferred diagnostic method. Rapid diagnosis tests have low sensitivity.10 Commercial immunochromatographic membrane enzyme immunoassay tests are not specific for H5 and only have 70% specificity compared with viral culture.2 Nasopharyngeal aspirate or bronchial alveolar lavage (BAL) followed by nasopharyngeal swab or throat swab placed in viral transport medium (VTM) should be collected with airborne precautions in patients suspected of having avian influenza. A stool or rectal swab also placed in VTM should be considered. If you suspect H5N1, alert your laboratory to take proper precautions.
According to the latest CDC recommendations, oropharyngeal swab specimens and lower respiratory tract specimens (BAL or tracheal aspirates) are preferred over nasal or nasopharyngeal swab specimens. Detection of H5N1 is more likely from specimens collected within 3 days of illness onset. Appropriate precautions should be taken including gloves, gown, goggles, and fit tested respirator when obtaining BAL; it should be noted it is an aerosol-generating procedure. When using swabs to obtain specimens, make certain that they have a Dacron® top on an aluminum or plastic shaft.
On February 3, 2006 the Food and Drug Administration (FDA) approved a new laboratory test to diagnose patients suspected of being infected with avian influenza A/H5 viruses.2, 25, 27, 31, 71, 74, 75 The test is referred to as Influenza A/H5 (Asian Lineage) Virus Real-time RT-PCR primer and Probe Set. This test can provide preliminary results on suspected H5 Influenza samples within 4 hours once sample testing begins at the lab. This is a major advance given previous technology required 2 to 3 days for similar results. If the H5 strain is identified, further testing is conducted to determine the specific subtype such as N1, etc. The test will be distributed nationwide to LRN designated laboratories in order to enhance surveillance and diagnostic capabilities. There are approximately 140 LRN laboratories throughout the United States. The CDC recommends if a clinician suspects a patient may be infected with avian influenza, it is important to contact the local or state health department for assistance in accessing the LRN capabilities.31 It cannot be overemphasized that clinical findings and history of exposure may be the most helpful in the early, albeit presumptive identification of patients with H5N1.1, 2, 10, 19, 20, 21, 26, 27, 28, 29, 31
Hematology and Blood Chemistry Testing
In comparison to the 1997 Hong Kong avian influenza cases, several patients in the past year have demonstrated lower total peripheral white blood cell counts, that are more often lymphopenic and associated with fatality.2, 4, 20, 21, 22, 24, 74 Laboratory findings of patients with severe infection from influenza H5N1 presenting for medical care with pneumonia had impaired liver function represented by abnormal liver function tests aspartate aminotransferase–alanine aminotransferase, alkaline phosphatase, lactase dehydrogenase, also prolonged clotting times, prothrombin and partial thromboplastin times, alterations in white blood cells resulting in leukopenia, lymphopenia, and renal impairment, abnormal blood urea nitrogen, and creatinine levels.2, 4, 10, 20, 21, 22, 25, 26, 27, 28, 29, 30
In one cohort of patients, diarrhea was present in 70% of patients along with lower respiratory symptoms.10, 25 Owing to the potential severity or duration of gastrointestinal (GI) symptoms, which have been associated with a significant percentage of avian flu patients, which may be prolonged—diarrhea and vomiting—the astute clinician will aggressively monitor and manage fluid and electrolyte derangements that may result.2, 10, 25, 26, 27, 28, 29, 30
Transmission of Influenza Virus
To date the most important route of acquisition for H5N1 infection is through contact with infected birds or their excreta. However, hospital-acquired infection was also demonstrated in a retrospective study. Healthcare workers (HCW) exposed to patients with H5N1 infection were more likely to be seropositive, and this was not attributable to animal exposure.2, 17, 18, 19, 20, 25, 51, 55, 70, 72, 83 It is reasonable to assume that the route of infection for avian influenza patients, like most influenza patients, can be from inhalation of infective respiratory secretions and/or contact with virus-laden secretions and subsequent transference contact with mucous membranes (Fig 6). 33, 83 Studies suggest that airborne transmission of influenza is possible, which would explain the sometimes numerically explosive nature of flu epidemics.2, 66, 70, 72, 73, 83 Although the true epidemiology is unknown, it is possible that there is asymptomatic or mildly symptomatic infection by H5N1; perhaps patients presenting for care are representative of the most severely infected. The actual transmission rate per person is unknown; the basic reproductive number for influenza (the number of secondary cases produced by one primary case) varies from 1.68 to 20. Viral shedding starts within 24 hours before the onset of symptoms and peaks within 48 hours afterwards.2, 45, 12, 48, 65, 70, 72, 84 Therefore, CDC recommends treating potential H5N1 patients as a contagion risk: respiratory/airborne precautions in addition to droplet, contact, and standard precautions as infection control practices for HCW and healthcare facilities.2, 22, 31, 32, 33, 49, 85 Although H5N1 is inefficient at person-to-person transmission, it is likely to acquire this capability in the advent of a pandemic.2, 19, 22, 23 Although not studied fully in all populations, the period of communicability of H5N1 can last for up to 3 weeks in children.
FIG 6.
Respiratory infections. (Color version of figure is available online.)
Treatment Options
General Management
Avian influenza patients can deteriorate rapidly. Initiating antiviral therapy as soon as possible, along with airway management and respiratory support, which may include intubation, ventilator, and intensive care, has the greatest likelihood of patient survival when provided early. Because H5N1 can cause pulmonary and extrapulmonary involvement, aggressive symptomatic and supportive care, including fluid management, is critical.
Current Antivirals
There are two classes of antivirals available to treat influenza virus: the neuraminidase inhibitors oseltamivir (Tamiflu®; Roche, Nutley, NJ) (Fig 7) and zanamivir (Relenza®; GlaxoSmithKline, Research Triangle Park, NC), and the M blockers amantadine (Symmetrel®; Endo Pharmaceuticals, Chadds Ford, PA) and rimantadine (Flumadine®; Forest Pharmaceuticals, St. Louis, MO); the former class is approved for influenza A and B viruses.2, 12, 19, 20, 22 Each class is designed to take advantage of influenza viral structure. Both classes can treat influenza viruses. However, the present circulating H5N1 genotype “Z” confers a residue on the M2 protein, making this avian flu intrinsically resistant to the M blockers.2, 17, 19, 31 The neuraminidase inhibitors remain effective against seasonal and avian influenza but should be administered early, ideally within 48 hours of illness onset.2, 12, 18, 20, 21, 22, 31, 32, 33, 43, 44 Oseltamivir when administered for seasonal influenza is usually given at a dosage of 75 mg by mouth twice daily for 5 days. A higher dose, 150 mg orally given twice daily, has been recommended in clinical trials and associated with a larger reduction in viral load and shorter duration of illness. Whether a higher dose given over a longer duration would confer benefit in avian influenza remains to be further evaluated but should be considered in patients with significant pulmonary and GI symptoms. Children older than 1 year of age can receive twice daily oral dosing based on weight: 30 mg per dose if 15 kg or less, 45 mg if 15–23 kg, 60 mg for 23–40 kg, and 75 mg for those over 40 kg. Resistance to oseltamivir is emerging.2, 12, 20, 22, 31, 32, 33, 43, 44, 83, 86, 88
FIG 7.
Antiviral therapy: Neuraminidase inhibiting medications. http://www.biocryst.com/pdf/peramivirfacts.pdf. (Color version of figure is available online.)
Oseltamivir can be used as a prophylactic chemotherapy for persons exposed to avian influenza. WHO recommends healthcare workers exposed to H5N1 receive 75 mg orally once a day for at least 7 days.20, 22, 25, 30 Vaccination against seasonal influenza is recommended if the HCW has not been immunized.22, 87, 88
Zanamivir (Relenza®) is an inhaled neuraminidase inhibitor and has little systemic absorption; it may not be useful if extrapulmonary disease occurs. Data are lacking in terms of the effectiveness of Zanamivir against H5N1 either for acute treatment or as chemoprophylaxis, although experts consider it of value given the class effect of neuraminidase inhibitors.
Newer Treatments
Studies are underway evaluating a new neuraminidase inhibitor: Peramivir® (BioCryst Pharmaceuticals, Birmingham, AL). When compared with other neuraminidase inhibitors, it showed promise against influenza including avian influenza12, 29, 90, 91 (Fig 8). The US Department of Health and Human Services (HHS) has awarded a $102.6 million, 4-year contract to BioCryst Pharmaceuticals, Inc. for advanced development of their drug Peramivir.90 Peramivir is being studied as a drug that can be administered parenterally, in contrast to the other neuraminidase inhibitors which are administered by oral or inhalation routes. Parenteral administration may be especially advantageous in the hospital setting given the potential for rapidly achieving high levels of drug associated with this route. Clearly, this route of administration provides an advantage for patients too ill to take medications by mouth.90, 91 Given the potential for an influenza pandemic, additional antivirals effective against avian and seasonal influenza viruses are necessary.
FIG 8.
Oseltamivir (Tamiflu): Oral medication for use with seasonal or avian influenza. (Color version of figure is available online.)
Other drugs being investigated to treat both seasonal and H5N1 include long-acting neuraminidase inhibitors, the antiviral ribavirin, and interferon alpha.12, 43, 44, 65, 92
Other Medical Treatments
It is important to avoid aspirin-containing products as a precaution against Reyes Syndrome, especially in patients younger than 16 years of age.2, 3, 21, 50, 59 Reyes Syndrome has also been reported, albeit rarely, in adults. In addition to early administration of antiviral therapy, respiratory support and intensive care are critical during the acute stage of H5N1 pneumonic illness.
Alternative Treatments
The physician should also be aware of and concerned about alternative and unproven methods to prevent or “cure” avian influenza. The Internet is full of advertisements extolling the virtues of herbal remedies containing natural “antiviral” properties. Whether these products are referred to as botanicals, supplements, nutriceuticals, or other preparations, they are not approved by FDA or other globally recognized health regulatory entities, nor are they recommended by CDC or WHO. The offers and advertisements emanate from their respective companies which have corporate titles that sound like quasi pharmaceutical entities, which of course creates the image that these are authoritative, ie, scientific and rigorously regulated companies. The reality, to date, these claims are anecdotal, the products are generally untested, and likely create a false sense of security to patients, in addition to posing a risk for adverse effects. This is not to say there aren’t botanicals that don’t or won’t exhibit antiviral properties given some of our widely known and prescribed FDA medications had origins as botanicals, such as digitalis, from the cardiac glycoside foxglove. Nevertheless, health care professionals should routinely query patients about the current or planned use of alternative therapies and products, enhancing the therapeutic dialogue, not only in an era of emerging pathogens but to encourage such discussions during flu season or routine visits is essential. Increasingly patients are taking greater ownership in their health as seen in the increase in sales of complementary and alternative therapies. However, it is important for patients to have a realistic expectation of the values, risks, benefits, and likely outcomes of such practices.
Vaccine Strategies
Vaccination remains one of the most effective ways to reduce the spread of infections. There are, however, limitations associated with vaccinations; levels of immune response may not be robust among the elderly or immune compromised patients.92 Nevertheless, vaccines remain a critical component to enhance health and control contagious diseases. Healthy People 2010 goals to improve influenza vaccination rates among institutionalized elderly, high-risk persons and the general public, if met, would significantly reduce the current mortality as well as the enormous burden faced by patients and the healthcare system.2, 22, 46, 49, 50, 88
Nationwide influenza immunization rates are disappointing, especially among children and HCW.2, 42, 43, 44, 46, 50 National Health Interview Survey (NHIS) data show only 36% of HCW are immunized against influenza each year.53 Medical literature suggests that unimmunized HCW are a serious problem and can be a potential cause of influenza outbreaks in a variety of healthcare settings. Primary care physicians need to lead by example. HCW immunization not only reduces the risk of outbreaks, but has been shown to reduce morbidity and mortality among geriatric patients in long-term facilities. It is important to encourage patients to obtain influenza vaccines every year, regardless of the presence or absence of avian influenza illnesses. It is well recognized that children can be a source of influenza infection to adults. Children can safely receive influenza vaccine. Patients often take their cue from their physicians and healthcare providers. Stocking adequate supplies and encouraging staff, colleagues, and patients to obtain either the injected or inhaled vaccine is an essential component to well patient, preventive care.
Even during years when the concordance between the influenza vaccine and circulating virus isn’t high, there remains significant value to receiving it; promoting vaccines as an essential “stay healthy” practice that all healthcare providers should encourage as well as partake in. Seasonal influenza vaccines can be provided to persons over the age of 6 months. Occupations placing individuals among crowds, such as fire-rescue, law enforcement, health care and emergency medical services, along with caregivers—especially those assisting the elderly or with chronic illnesses—should be especially encouraged to obtain annual vaccinations. Although the seasonal vaccine won’t confer direct protection against H5N1, reducing influenza prevalence may decrease the likelihood of both viruses intermixing if an individual is exposed to seasonal and avian influenza.87, 88, 89 This may decrease the chance of viral genetic assortment and the emergence of new avian influenza capable of human-to-human transmission. Moreover, it is the right thing to do as a HCP.
There are currently two forms of seasonal influenza vaccines31, 32, 33, 48, 93: the “flu shot” which contains killed virus, administered via a syringe/needle and approved for use in people older than 6 months of age; and the nasal spray influenza vaccine “Live Attenuated Influenza Vaccine,” or LAIV, which is made with live but weakened viruses. It is approved for persons aged 5 to 49 years who are NOT pregnant. Most people tolerate the influenza shot very well with only minor soreness, redness, or swelling at the injection site, possibly a low-grade fever and some aches often lasting 1-2 days at most. There are few contraindications, including severe allergy to chicken eggs, severe reaction to influenza vaccine in the past, which includes Guillain-Barre Syndrome (GBS) within 6 weeks of receiving an influenza vaccine. Although rare, transmission of vaccine viruses via LAIV to close contacts has been reported. Side effects associated with LAIV are usually mild and include runny nose and headache. In children, fever and vomiting have been reported along with muscle aches. In adults, sore throat and cough may occur. These are all usually of short duration. If these effects last more than a couple days, the vaccinee should be reevaluated.
Each vaccine contains two influenza “A” and one influenza “B” virus: currently A H3N2 and A H1N1. Influenza vaccines change yearly based on international surveillance; the most virulent strains expected to predominate globally are selected. It is important for patients to have realistic expectations of vaccine response; it takes approximately 2–3 weeks after receiving influenza vaccine to produce sufficient antibodies to adequately protect against the flu, although some protection may develop earlier.
On April 17, 2007 the Food and Drug Administration (FDA) approved the first US vaccine for use in humans against the avian influenza virus H5N1.94 The vaccine, manufactured by Sanofi-Pasteur, Inc (Swiftwater, PA), was obtained from a human strain. It is intended for adults 18-64 years of age who could be at increased risk of exposure to H5N1. Clinical testing involved 103 healthy adults. The vaccine was well tolerated with side effects similar to other injected immunizations: pain at the injection site and headache. In addition, some patients complained of general ill feeling and muscle pain. Additional information is being collected on safety and effectiveness in other age groups and is expected to be reported to the FDA in the near future. The vaccine immunization consists of 2 intramuscular injections given 1 month apart. The vaccine will not be available commercially; it was purchased by the US Government to be included in the National Stockpile. The vaccine will be distributed by public health officials if needed.31, 94
Other companies and nations are conducting research and developing vaccines against avian influenza. Sinovac received a reassortant influenza strain for developing a Pandemic Influenza Vaccine (H5N1) from the British National Biological Standard and Control (NIBSC) in March 2004 and is working in collaboration with the Chinese Centre of Disease Control and Prevention.95 Novartis announced in February 2007 it has received a positive opinion supporting European Union (EU) regulatory approval of the human vaccine Focetria for use in the event of an H5N1/influenza pandemic.96 Focetria would be manufactured to contain the pandemic influenza strain declared at the time of a pandemic along with the proprietary adjuvant MF 59 developed by Novartis. MF 59 could boost the body’s immune response to the vaccine’s active constituent. WHO collaborating centers and reference labs have developed several H5N1 prototype vaccine strains, including A/Vietnam/1194/04, A/Vietnam/12030/04, and A/Hongkong/213/03 according to the requirements of pharmaceutical companies for possible vaccine production. WHO will make prototype strains available to vaccine manufacturers, as part of a global effort to develop and ultimately provide adequate supplies to even the most economically disadvantaged nations.97 Supply and demand represent interrelated and interdependent market forces; the WHO, like CDC believes promoting seasonal vaccine use can increase manufacturing capacity for both seasonal and pandemic vaccines. Although this makes good business sense, encouraging wider use of vaccines is also a powerful medical intervention to reduce suffering from vaccine preventable diseases.
Economic Impact of Avian Influenza
The CDC estimates that the next pandemic could result in over 200,000 deaths and 734,000 hospitalizations in the United States alone. The estimated economic impact could exceed $160 billion.4, 91 From a global perspective, poultry constitutes an important industry for many Asian nations. Thailand is the world’s fourth largest exporter of poultry meat with over $1 billion exported annually.98 Whereas attempts to model the economic impact of infection outbreaks can be confounded by numerous influencers and often difficult to obtain data, the recent SARS outbreak can provide valuable insight. The Asian Development Bank estimated that the impact of the SARS epidemic resulted in $59 billion in business losses. Canada lost millions due to conference cancellations and loss of tourism during their SARS experience. An avian flu pandemic would create incalculable losses in life, animal and human, economic burdens ranging from lost tourism to severe deprivation resulting from the impact on food supplies, commerce, trade, and worker availability.91, 98
Potential Magnitude of a Pandemic
Although estimates vary, in the worst case scenario, modeled after the severity of the 1918 pandemic, an influenza pandemic could sicken upwards of 90 million people in the United States, including over 1/3 of the healthcare workforce. Federal officials are concerned that 10 million influenza patients could require hospitalization at least for 1 night including almost 1.5 million requiring intensive care and possibly 750,000 needing ventilator assistance. If the next pandemic is similar to the 1957 or 1968 outbreaks, it could result in 200,000 deaths and 734,000 hospitalizations.4, 91 These numerical estimates represent human lives; they should not be taken lightly nor should the “best case” scenarios be received with relief compared with worst case estimates, especially given the nationwide lack of surge capacity at our overburdened healthcare facilities.2, 14, 55, 85
Preparing Our Offices and Practices (Table 3)
TABLE 3.
Guidelines for infection prevention and control in the physician’s office, 2004 http://www2.worksafebc.com/PDFs/Healthcare/Backgrounder_drs_infection_control_guidelines.pdf
| Routine Infection Control Practices |
Routine infection control practices are to be used with all patients, at all times, regardless of presumed infections status or diagnosis. Routine Infection Control Practices include:
|
| Preventing Transmission |
Preventing the transmission of infectious diseases spread by either airborne or droplet routes poses a significant challenge in the outpatient setting. Special arrangements for patients with a suspected respiratory infection can reduce this risk. These include:
|
Preventing the transmission of infectious diseases spread by direct contact such as antibiotic resistant organisms (e.g. MRSA, VRE) require special attention to decrease the likelihood of spread. Patients may harbour resistant bacteria as part of their respiratory or gastrointestinal tract flora for an extended period. Precautions include:
|
Though beyond the scope of this article, it is important to remember that most of our healthcare facilities are overcrowded, understaffed, and not designed to function as isolation or quarantine facilities. The number of negative pressure rooms, intensive care units, and isolation capabilities varies widely across regions and healthcare centers. The world of competing demands and financial cut-backs is an omnipresent reality for healthcare professionals today. Yet the number of emerging pathogens, changes in virulence patterns among known infections such as tuberculosis (TB) evolving beyond simply being multidrug resistant towards extremely drug resistant TB (XDR TB) are today’s reality. In the event of a SARS or avian influenza outbreak, we must be prepared to handle large numbers of potentially contagious patients (FIG 9, FIG 10). This requires having a well thought out plan in place that utilizes input from all major stakeholders, has been practiced and buttressed by the knowledge that participants have appropriate tools to carry out the plan—equipment, training, personnel, countermeasures (medicines)—and are trained in the appropriate use of such materials. Having appropriate personal protective equipment (PPE) such as n-95 respirators (Fig 11) isn’t enough. Personnel must know how to wear PPE appropriately and select it according to the situation based on well-accepted guidelines.4, 5, 8, 9 Knowing how to cohort patients, convert space in the HCF to adapt to contagious patients, the number of avian or SARS patients or other type of deadly pathogen in greater than one or two patients, including professional expertise needed, equipment, and other countermeasures is critical in the interpandemic phase. Developing mutual aid agreements with HCF or organizations, perhaps in different regions, that may have greater resources or equipment, is one option to strengthening response capabilities. Realizing the continuum of care starts in the community, increased communications with prehospital professions; emergency medicine as well as community stakeholders can increase the likelihood that the response to a contagion can be managed effectively.
FIG 9.
Patient flow: how do potentially sick patients get to you the physician? (Reprinted with permission from Threat Science.) (Color version of figure is available online.)
FIG 10.
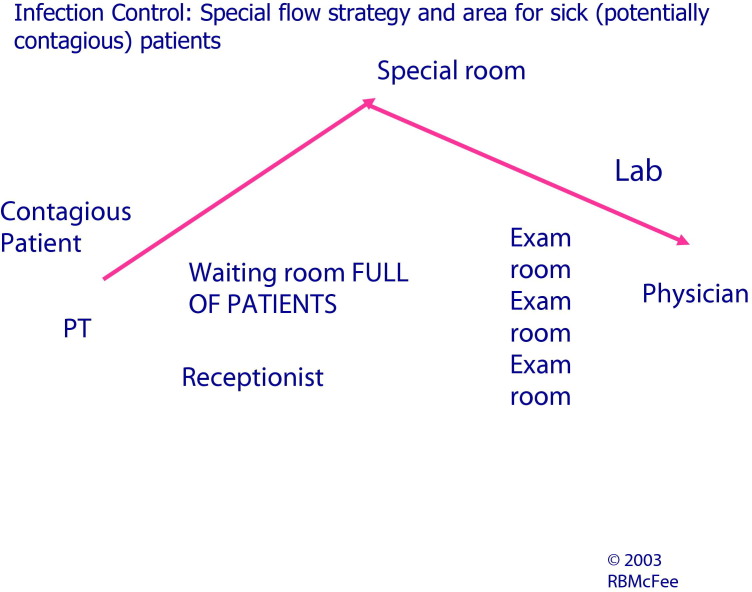
Infection control: special flow strategy and area for sick (potentially contagious) patients. (Reprinted with permission from Threat Science.) (Color version of figure is available online.)
FIG 11.
Example of an “n95 Respirator Mask” (not an endorsement of brand; there are many high-quality n95 masks available commercially. The key is wearing it appropriately and being fit-tested). (Color version of figure is available online.)
Physicians and healthcare facilities should remain vigilant for severe respiratory illnesses; SARS may reappear in addition to avian influenza, or other emerging threats.
Clearly early diagnosis and rapid implementation of chemotherapy is a mainstay of containment.1, 2, 8, 13, 20, 34, 35, 36, 50, 72, 83, 99, 100, 101, 104, 105
WHO established a set of guidelines for physicians to utilize (Table 3).48, 50, 72, 106 These WHO containment strategies and guidelines rest largely upon early diagnosis with efforts to reduce transmission to HCW and other patients. Their strategy depends on clinicians maintaining a high index of suspicion, identifying patients who have traveled to affected areas, alerting the proper authorities, and initiating appropriate treatments, including antiviral medications, in a timely manner.48, 50, 61, 68, 71, 103 WHO also put forth five essential action strategies to reduce the risk of a pandemic (Table 4).
TABLE 4.
World Health Organization—Recommended containment strategies for avian influenza (www.who.org)
|
Studies evaluating why patients consult their primary care physician demonstrate they often come to our offices as “worried well” seeking information. One study showed that unnecessary visits could be prevented by offering patients information about effective interventions including self-care2, 4, 49, 88, 100 one pagers that can be downloaded from CDC concerning avian influenza, obtaining flu shots, and respiratory hygiene.2, 20, 33, 46, 50, 94, 101
It is important to ask patients if they are concerned about avian influenza. This is an opportunity to offer flu vaccine, teach about the critical differences in symptoms associated with influenza, avian influenza, other severe respiratory infections, and the common cold (Table 2) and preventive strategies, including respiratory hygiene, to ward off infection in general.2, 4, 99, 101, 102
Although most patients present to our offices with symptoms, there may be the “worried well” coming during the first wave of avian influenza. How many people does a potentially contagious person have to go through in a healthcare facility or your office practice before being placed in a private area? The potential for spreading or receiving unwanted “germs” is impressive from patient to patient (FIG 7, FIG 8). Therefore, preventive strategies should be implemented early. Given the high case fatality rate (CFR) of avian influenza as well as pneumonia in high-risk patients, a combination of contact, droplet, and airborne precautions are recommended. Whenever possible, separate well from sick patients in the waiting room.2, 53, 83, 99, 101, 102 Intake staff should use reception prompts that identify potentially contagious patients and place them into an exam room rapidly to cut down on waiting room times (FIG 7, FIG 8).4, 33, 48, 50, 99, 100, 101, 102, 103 Sanitizing gels, tissues, and disposable masks, as well as appropriate information about influenza and other timely materials should be available in your (frequently cleaned) waiting room. Whereas there is limited prospective research evaluating the practice of separating well from sick patients, emerging studies in aerobiology suggest the preventive measures practiced during the 1960s and 1970s whereupon pediatricians encouraged parents to bring sick kids with rashes and high fevers to the back door directly to an exam room instead of the general waiting room has merit99, 100, 101, 102 (Fig 8). Instances of measles transmission, not from direct contact but by the persistent virus left behind from an infected child, have been well documented.2, 83, 100 As an aside, it is worth noting that measles has not been eradicated. Measles kills an estimated 600,000 people worldwide annually; however, the actual number may be greater.106, 107 Cases and outbreaks can occur as a result of inadequate immunization within communities domestically and from foreign visitors who are ill. In the summer of 2006, a computer programmer from India arrived in Boston. Several days after he arrived, he developed symptoms of measles; soon thereafter several people became infected, causing the first such outbreak in Massachusetts since 1999.108 Most of the cases involved people in their 30s and 40s who were vaccinated in the 1960s with an ineffective vaccine. This episode underscores our vulnerability to global threats, especially if our patients are unvaccinated or inadequately vaccinated.
Study data reveal that patients would be willing to wear a mask in a (HCF) waiting area if one was offered to them. Those studies also reveal that, although a mask would reduce the risk they posed to others, few patients were offered such preventive measures when a likely respiratory illness was identified. Reducing contact between persons using airborne and other precautions will be critical until adequate vaccine and antiviral supplies are available.
Hand washing is of proven value in preventing the transmission of infectious agents.2, 101 Hands should be washed before and after contact with patients, body fluids, dirty materials, between procedures, invasive or otherwise, after glove removal, and use of restrooms. Gloves should always be readily available.
Promote respiratory hygiene practices. Stock annual influenza vaccines and encourage staff, colleagues, and patients to obtain either the flu shot or inhaled vaccine.
Travel Recommendations
Patients planning to travel overseas for work, recreation, or humanitarian outreach should be able to do so relatively safely if precautions are taken.4, 19, 20, 58, 61, 68 Encourage your patients to allow as much advance preparation time as possible.4, 5, 8, 9, 50, 61, 68, 100, 103 Vaccines against diseases endemic to the new host region being visited may need weeks to evoke an immune response. Special precautions should be taken when visiting countries where H5N1 infections are reported in birds, other animals, or people. This includes avoiding crowded places and farms, marketplaces that have poultry and/or kill chickens on demand, and changing clothes if visiting any of the above. Frequent hand-washing and respiratory hygiene should be stressed. Referral to a travel medicine clinic or physician who specializes in travel medicine may be appropriate. The United States Department of State provides important information about most countries with timely alerts on emerging threats, political instability, or other risks for US citizens. The CDC publishes the Yellow Book, a valuable travel health resource. Also, recommendations about vaccines appropriate to foreign destinations can be obtained through CDC.4, 33, 48
Although the incidence of imported infectious disease presenting to HCF is not well defined, it is well known that significant numbers of patients present to medical facilities upon return from traveling with a variety of complaints, including respiratory infections. Studies suggest clinicians do a poor job of obtaining a travel history, including a general lack of awareness by physicians concerning the potential for non-endemic disease in the population that they attend.61, 68 One such study evaluating whether a travel history was recorded in patients, a travel history was recorded in only 2% of all patients presenting to this emergency department (ED), although among total number of patients presenting to the ED, 5.3% actually had the potential for a travel-related illness.68 Thus, there remains the risk that imported diseases such as avian influenza may be undiagnosed in the acute setting, leading to possible spread before containment can be effected and delayed treatment.
Discussion
Chronic diseases such as coronary disease and diabetes have replaced acute infections as the leading cause of mortality in persons older than 65 (Table 5) in the United States (US) with outbreaks of contagious infectious diseases remaining uncommon.109 With such rarity comes lack of familiarity with or concern about illnesses that haven’t caused widespread illness in the US since the early 20th century but persist in many other parts of the world today (Table 6). 109, 110, 111
TABLE 5.
Top 5 causes of death for persons 65 years of age and older in the US109
| Whites | Blacks | American Indians | Asian or Pacific Islanders | Hispanics |
|---|---|---|---|---|
| 1. Heart disease | Heart disease | Heart diease | Heart disease | Heart disease |
| 2. Cancer | Cancer | Cancer | Cancer | Cancer |
| 3. Stroke | Stroke | Stroke | Stroke | |
| 4. COPD | Diabetes | Stroke | Pneumonia/influenza | COPD |
| 5. Pneumonia/influenza | Pneumonia/influenza | COPD | COPD | Pneumonia/influenza |
TABLE 6.
Leading causes of death 1900 & 1997 US, 1992 Peru110
| Major Causes of Death (Attributable) | 1900 United States | Peru 1992 | 1997 United States |
|---|---|---|---|
| 1 | Respiratory disease | Respiratory infections | Heart disease |
| 2 | Tuberculosis | Cancer | Cancer |
| 3 | Gastrointestinal disease | Gastrointestinal disease | Cerebrovascular disease |
| 4 | Heart disease | Heart disease | Pulmonary disease |
| 5 | Infectious/parasitic diseases | Tuberculosis | Accidents |
| 6 | Kidney diseases | Cerebrovascular disease | Pneumonia/influenza |
| 7 | Early infancy diseases | Urinary system disease | Diabetes |
| 8 | Cerebrovascular disease | Nutritional deficiencies | Suicide |
| 9 | Cancer | Early infancy | Homicide |
| 10 | Liver disease | HIV AIDS |
The dramatic changes in the top leading causes of death from infection related to chronic disease could give the false sense of “victory” that we have conquered pathogens in the United States. However, we remain vulnerable since many nations still endure poverty, overcrowding, or unsanitary conditions that include sleeping near livestock such as poultry or swine, lack of immunizations or antivirals. “Victory” is better exchanged for the term “stalemate” with infections, possible only as long as we practice the sound infection control practices that lead to the changes in mortality from 1900 to 2000 (Table 6).109, 110, 111 Inattention to these practices may account for the death rate from infectious diseases rising 58% between 1980 and 1992 (making them in the aggregate the third leading cause of death in the United States). Influenza and pneumonia remain responsible for 5.5% of the deaths of people 65 and older6, 40, 92 in 1997, with an increase in infection-related deaths among older persons from 1980 to 1992.109, 110, 111 And, the combined death rate from influenza and pneumonia for all age-race-sex groups has increased.110, 111 Evidence suggests hand hygiene can reduce healthcare-associated infection rates, whether using soap and water or waterless interventions including alcohol-based hand rubs. Failure to perform appropriate hand hygiene is a leading cause of healthcare-associated infections, the spread of multi-drug resistant organisms, and contributes to outbreaks. Approximately 1 in 20 patients contracts an infection in hospitals across the US. About 2 million patients acquire nosocomial infections each year in US hospitals; the war against infectious diseases is clearly not over.60, 90
Overcrowded waiting rooms, healthcare facilities lacking surge capacity and filled to overflowing give less time for proper sanitation. Healthcare workers not adhering to good hygiene practices contribute to the rising infection problem. Overcrowding in the absence of an epidemic only portends a system-wide failure in the presence of a highly transmissible virus.14, 55 Recent ED closures in the face of increased patient volume, lack of affordable resources for uninsured persons contribute to diversions and overcrowding. Lack of hospital beds exacerbates the problem. Given that influenza occurs seasonally and predictably, exerting profound effects on the population causing over 36,000 deaths annually and 200,000 hospitalizations, widespread influenza activity in a region can severely strain already overburdened HCF. How will we provide routine care to chronically ill patients while responding to a pandemic? Physicians and HCF need to prepare now and identify solutions in anticipation of an epidemic: H5N1, SARS, or other emerging pathogen.
Conclusion
Although no cases of human avian influenza are reported in the United States, being alert for this or other emerging threats is good medical practice and may facilitate early intervention of other potentially dangerous illnesses imported through immigrants, visitors, or travelers. Concern about H5N1 provides opportunities to discuss with patients good respiratory hygiene practices and influenza vaccinations.
If and, perhaps more reasonably, when the first human cases of avian influenza arrive in the United States, the primary care physician may be in the position to identify the index case, initiate proper antiviral treatment, and alert authorities, setting off a cascade of events including public health containment strategies.
The healthcare community is concerned with its ability to handle a pandemic. The high case-fatality rate over 50% in selected countries of avian influenza H5N1 is worrisome.4, 5, 8, 24 The ability of H5N1 to rapidly overcome species barriers, sicken birds normally not overcome by avian influenza viruses, travel West quickly coupled with the propensity of influenza viruses to undergo genetic reassortment, adapt, and mutate, argue for this strain of avian influenza to be a likely candidate for the next human pandemic.
Rapid diagnosis and early treatment are critical to containing and preventing an outbreak from becoming an epidemic of global proportions. All treatments must be given early in the course of illness. Of four antivirals known to treat influenza virus A infection, only two are potentially useful and commercially available at the present time as chemotherapy and chemoprophylaxis against H5N1: Oseltamivir and Zanamivir.2, 4, 5, 8, 24 The former has been approved for both indications, but resistance is emerging. Peramivir holds promise as a new neuraminidase inhibitor, but it is still in clinical trials. The recently approved avian influenza vaccine is not commercially available but is part of the US Government response stockpile. Continuing research is being conducted internationally to develop processes that facilitate quicker integration of current strains into vaccines especially if there is low concordance between circulating virus and available vaccines.
Healthcare professionals are encouraged to remain aware of emerging global threats, coordinate with preparedness agencies within the community, obtain updates regularly, and provide appropriate counseling and preventive measures to patients, especially annual influenza vaccines. Whether diagnosing the index case in the United States or being called upon to perform under increasingly challenging circumstances in the face of an epidemic, the healthcare professional of the 21st century will face emerging infections, possible bioterrorism, and persistent nosocomial infections. As such, healthcare professionals have an important role in limiting the spread of an outbreak, and, moreover can contribute to local preparedness efforts, provide appropriate educational information and medical care to patients, and set the stage for recovery.
References
- 1.Ligon B.L. Avian influenza virus H5N1: a review of its history and information regarding its potential to cause the next pandemic. Semin Pediatr Infect Dis. 2005;16:326–335. doi: 10.1053/j.spid.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.McFee R.B., Bush L.M., Boehm K.M. Avian influenza: critical considerations for the primary care physician. Johns Hopkins Adv Stud Med. 2006;6(10):431–440. [Google Scholar]
- 3.Tuabenberger J.K., Reid A.H., Lourens R.M. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;4437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Information about influenza pandemics. Available at: http://www.cdc.gov/flu/avian/gen-info/pandemics.htm.
- 5.WHO. Estimating the impact of the next influenza pandemic: enhancing preparedness. Available at: http://www.who.int/csr/disease/influenza/preparedness2004_12_08/en/index.html.
- 6.Tumpey T.M., Basler C.F., Aguilar P.V. Characterization of the reconstructed 1918 Spanish Influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 7.Belshe R.B. The origins of pandemic influenza: lessons from the 1918 virus. N Engl J Med. 2005;353(21):2209–2211. doi: 10.1056/NEJMp058281. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Avian influenza: assessing the pandemic threat. Available at: http://www.who/cds/2005.29.
- 9.WHO. Avian influenza (“Bird flu”) and the significance of transmission to humans. Available at: http://www.who.int/mediacentre/factsheets/avian_influenza/en.
- 10.Apisarnthanarak A., Kitphati R., Thongphubeth P. Atypical avian influenza (H5N1) Emerging Infect Dis. 2004;10(7):1321–1324. doi: 10.3201/eid1007.040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO: Ten things you need to know about pandemic influenza. Available at: http://www.who.int/csr/disease/influenza/pandemic10things/en/index.html. [PubMed]
- 12.Sidwell R.W., Smee D.F. Peramivir (BCX-1812, RWJ-270201): potential new therapy for influenza: Expert Opinion. Invest Drug. 2002;11:859–869. doi: 10.1517/13543784.11.6.859. [DOI] [PubMed] [Google Scholar]
- 13.Krug R.M. The potential use of influenza virus as an agent for bioterrorism. Antiviral Res. 2003;57:147–150. doi: 10.1016/s0166-3542(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 14.McCaughey B. Saving lives and the bottom line. Modern Healthcare Online. January 30, 2006. Available at: http://www.modernhealthcare.org. Accessed October 31, 2006. [PubMed]
- 15.WHO. Confirmed cases of avian influenza to 4/11/07. Available at: http://www.wpro.who.int/sites/csr/data/data_Tables.htm.
- 16.WHO. H5N1 avian influenza: timeline of major events. Available at: http://www.who.int/csr/disease/influenza/pandemictimeline/en/index.html.
- 17.Bridges C.B., Lim W., Hu-Primmer J. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong 1997-1998. J Infect Dis. 2002;185:1005–1010. doi: 10.1086/340044. [DOI] [PubMed] [Google Scholar]
- 18.Saw T.A., Lim W., Shortridge K. Isolation of avian influenza A (H5N1) viruses from humans-Hong Kong. MMWR Morb Mortal Wkly Rep. 1997;46:1204–1207. [PubMed] [Google Scholar]
- 19.Yuen K.Y., Wong S.S.Y. Human infection by avian influenza A H5N1. Hong Kong Med J. 2005;11:189–199. [PubMed] [Google Scholar]
- 20.Beigel J.H., Farrar J., Han A.M. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services. PandemicFlu.gov Web site. Available at: http://www.flu.gov/vaccine. Accessed March 7, 2006.
- 22.WHO. WHO interim guidelines on clinical management of humans infected by influenza A (H5N1). Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/Guidelines_Clinical%20Management_H5N1_rev.pdf. Accessed October 31, 2006.
- 23.Ungchusak K., Auerwarakul P., Dowell S.F. Probable person to person transmission of avian influenza A H5N1. N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 24.Gupta N.E. Everything you should know about virulent avian influenza. Cortlandt Forum. 2005:26–34. [Google Scholar]
- 25.Tran T.H., Nguyen T.L., Nguyen T.D., World Health Organization International Avian Influenza Investigative team Avian influenza A H5N1 in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 26.Peiris J.S., Yu W.C., Leung C.W. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chotpitayasunondh T., Ungchusak K., Hanshaoworakul W. Human disease from influenza A (H5N1), Thailand, 2005. Emerg Infect Dis. 2005;11:201–209. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiensin T., Chaitaweesub P., Songserm T. A highly pathogenic avian influenza H5N1, Thailand, 2004. Emerg Infect Dis. 2005;11:1664–1672. doi: 10.3201/eid1111.050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.deJong M.D., Bach V.C., Phan T.Q. Fatal avian influenza A H5N1 in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Epidemiology of WHO-confirmed human cases of avian influenza A (H5N1) infection. Wkly Epidemiol Rec. 2006;81:249–257. [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration. Flu information. Available at: http://www.fda.gov/oc/opacom/hottopics/flu.html.
- 32.National Institutes of Health. Avian flu/pandemic flu. Available at: http://www.nih.gov.
- 33.Centers for Disease Control and Prevention. Avian influenza information for physicians. Available at: http://www.cdc.gov/flu.
- 34.Heymann D.L. SARS and emerging infectious diseases: a challenge to place global solidarity above national sovereignty. Ann Acad Med Singapore. 2006;35:350–353. [PubMed] [Google Scholar]
- 35.Heymann D.L., Rodier G. Global surveillance, national surveillance and SARS. Emerg Infect Dis. 2004;10:173–175. doi: 10.3201/eid1002.031038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Department of Defense Global Emerging Infections Surveillance and Response System (DoD-GEIS). Available at: http://www.geis.fhp.osd.mil/aboutGEIS.asp.
- 37.Sims L.D., Ellis T.M., Liu K.K. Avian influenza in Hong Kong 1997–2002. Avian Dis. 2003;47(3):832–838. doi: 10.1637/0005-2086-47.s3.832. (suppl) [DOI] [PubMed] [Google Scholar]
- 38.Department of Health, Hong Kong. Letter to doctors on two cases of H5N1 infection in 2004. Available at: http://www.info.gov.hk.dh/useful/ltod/h5n12003.htm.
- 39.Snacken R., Kendal A.P., Haaheim L., Wood J.M. The next influenza pandemic: lessons from Hong Kong. 1997. Emerg Infect Dis. 1999;5:195–203. doi: 10.3201/eid0502.990202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wester R., Hulse D. Controlling avian flu at the source. Nature. 2005;435:415–416. doi: 10.1038/435415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou N.N., Shortridge K.F., Claas E.C.J. Rapid evolution of H5N1 influenza viruses in chickens in Hong Kong. J Virol. 1999;73:3366–3374. doi: 10.1128/jvi.73.4.3366-3374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fouchier R.A., Munster V., Wallensten A. Characterization of a novel influenza A virus hemagglutinin subtype H16 obtained from black headed gulls. J Virol. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govarkova E.A., Leneva I.A., Galaubeva O.G. Comparison of efficacies of RWJ 270201, zanamivir and oseltamivir against H5N1, H9N2 and other avian influenza viruses. Antimicrob Agents Chemother. 2001;45:2723–2732. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madren L.K., Shipman C., Jr, Hayden F.G. In vitro inhibitory effects of combinations of anti-influenza agents. Antivir Chem Chemother. 1995;6:109–113. [Google Scholar]
- 45.Taisuke H., Kawaaka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;5:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. Key facts about influenza vaccine. Available at: http://www.cdc.gov/flu/ protect/keyfacts.htm.
- 47.Brown H. Nations set out a global plan for influenza action. Lancet. 2005;12:1684–1685. doi: 10.1016/S0140-6736(05)67679-9. [DOI] [PubMed] [Google Scholar]
- 48.National Foundation for Infectious Diseases. Improving influenza vaccination rates in health-care workers: strategies to increase protection for workers and patients. Available at: http://www.nfid.org/publications/hcwmonograph.pdf.
- 49.Centers for Disease Control and Prevention Experiences with influenza-like illness and attitudes regarding influenza prevention-United States 2003-2004 influenza season. MMWR Morb Mortal Wkly Rep. 2004;53:1156–1158. [PubMed] [Google Scholar]
- 50.Bridges C.B., Kuehnert M.J., Hall C.B. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37:1094–1111. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 51.Li K.S., Guan Y., Wang J. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 52.JEMS. Available at http://www.JEMS.com.
- 53.Centers for Disease Control and Prevention. 2004 National immunization survey. Available at: http://www.dshs. state.tx.us/immunize/coverage/nis.shtm.
- 54.Liem N.T., Lim W. Lack of H5N1 avian influenza transmission to hospital employees. Emerg Infect Dis. 2005;11:210–215. doi: 10.3201/eid1102.041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zigmund J. No more room; overcrowding blamed for ambulance diversions. Modernhealthcare.com. Available at: http://www.modernhealthcare.com/printwindow.cms?articleId=38689&pageType=article. [PubMed]
- 56.Webster R.G. Wet markets-a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bush L.M., Abrams B.H., Beall A. Index case of fatal inhalation anthrax due to bioterrorism in the United States. N Engl J Med. 2001;345:1607–1610. doi: 10.1056/NEJMoa012948. [DOI] [PubMed] [Google Scholar]
- 58.Smith S.M. Where have you been?: The potential to overlook imported disease in the acute setting. Eur J Emerg Med. 2005;12:230–233. doi: 10.1097/00063110-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Osterholm MT. Emerging infectious diseases: a real public health crisis? Postgraduate Medicine online. Available at: http://www.postgradmed.com/issues/1996/11_96/ed_nov.htm. [DOI] [PubMed]
- 60.Lederberg J., Shope R.E., Oaks S.C. Emerging Infections: Microbial Threats to Health in the United States. National Academy Press; Washington, DC: 1992. [PubMed] [Google Scholar]
- 61.Stienlauf S., Segal G., Sidi Y. Epidemiology of travel-related hospitalization. J Travel Med. 2005;12:136–141. doi: 10.2310/7060.2005.12308. [DOI] [PubMed] [Google Scholar]
- 62.Chan M.C., Cheung C.Y., Chui W.H. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheung C.Y., Poon L.I., Lau A.S. Induction of proinflammatory cytokines in human macrophages by influenza A H5N1 viruses; a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 64.Kunzelmann K., Beesley A.H., King N.H. Influenza inhibits amiloride-sensitive NA+ channels in respiratory epithelia. Proc Natl Acad Sci USA. 2000;97:10282–10287. doi: 10.1073/pnas.160041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baron S., Isaacs A. Absence of interferon in lungs from fatal cases of influenza. Br Med J. 1962;5270:18–20. doi: 10.1136/bmj.1.5270.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X.J., Set S., Yue G. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2004;287:L366–L373. doi: 10.1152/ajplung.00011.2004. [DOI] [PubMed] [Google Scholar]
- 67.Li K.S., Guan Y., Wang J. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 68.Ver Herck K., Van Damme P., Castelli F. Knowledge, attitudes and practices in travel-related infectious diseases: the European airport survey. J Travel Med. 2004;11(1):3–8. doi: 10.2310/7060.2004.13609. [DOI] [PubMed] [Google Scholar]
- 69.Chotpitayasunondh T., Lochindarat S., Srisan P. Cases of influenza A (H5N1): Thailand 2004. MMWR Morb Mortal Wkly Rep. 2004;53:100–103. [PubMed] [Google Scholar]
- 70.Goldmann D.A. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19(10):97S–102S. doi: 10.1097/00006454-200010001-00002. (suppl) [DOI] [PubMed] [Google Scholar]
- 71.WHO. Responding to the avian influenza pandemic threat. Communicable Disease Surveillance and Response Global Influenza Programme. Available at: http://www.who.int.
- 72.Goldmann D.A. Epidemiology and prevention of pediatric viral respiratory infections in health care institutions. Emerg Infect Dis. 2001;7:249–253. doi: 10.3201/eid0702.010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsieh Y., Wu T., Liu D. Influenza pandemics: past, present and future. J Formos Med Assoc. 2006;105:1–6. doi: 10.1016/S0929-6646(09)60102-9. [DOI] [PubMed] [Google Scholar]
- 74.Yuen K.Y., Chan P.K.S., Peiris M. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 75.Chan P.K.S. Outbreak of avian influenza A (H5N1) virus infection in Hong Kong in 1997. Clin Infect Dis. 2002;34:S58–S64. doi: 10.1086/338820. [DOI] [PubMed] [Google Scholar]
- 76.Reed K.D., Melski J.W., Graham M.B. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 77.Qureshi N. Proceedings of the 2005 annual meeting of the Radiological Society of North America. Available at: http://english.people.com.cn/200512/03eng20051203_225411.html.
- 78.Nicholson K.G., Wood J.M., Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Webster R. Predictions for future human influenza pandemics. J Infect Dis. 1997;176(suppl 1):S14–S19. doi: 10.1086/514168. [DOI] [PubMed] [Google Scholar]
- 80.Spackman E., Senne D.A., Myers T.J. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munch M., Nielsen L.P., Handberg K.J. Detection and subtyping (H5 and H7) of avian type A influenza virus by reverse transcription PCR and PCR Elisa. ArchVirol. 2001;146:87–97. doi: 10.1007/s007050170193. [DOI] [PubMed] [Google Scholar]
- 82.Lee M.S., Change P.C., Shien J.H. Identification and subtyping of avian influenza viruses by reverse transcription-PCR. J Virol Methods. 2001;97:13–22. doi: 10.1016/s0166-0934(01)00301-9. [DOI] [PubMed] [Google Scholar]
- 83.Langmuir A.D. Changing concepts of airborne infection of acute contagious diseases; a reconsideration of classic epidemiologic theories. In: Kundsin R.B., editor. Airborne Contagion. Annals of the New York Academy of Sciences; New York, NY: 1980. pp. 35–44. [DOI] [PubMed] [Google Scholar]
- 84.van Riel D., Munster V.J., deWit E. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 85.McFee R.B. Preparing for an era of weapons of mass destruction (WMD): are we there yet?: Why we should all be concerned. Part I. Vet Human Tox. 2002;44(4):193–199. [PubMed] [Google Scholar]
- 86.deJong M.D., Thanh T.T., Khanh T.H. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 87.Canada communicable disease report. June 15, 2006; Volume 32:Issue ACS-7. Available at: http://www.medscape.com/ medline/publicationbrowser/4220/32_ACS-7/dt_06152006/limit_0. Accessed October 31, 2006.
- 88.Centers for Disease Control and Prevention. Interim guidance for protection of persons involved in U.S. avian influenza outbreak disease control and eradication activities. Available at: http://www.cdc.gov/flu/avian/professional/protectguid.htm. Accessed October 31, 2006.
- 89.L’vov D.K., Fediakina I.T., Shchelkanov M.I. In vitro effects of antiviral drugs on the reproduction of highly pathogenic influenza A/H5N1 virus strains that induce epizooty among poultry in the summer of 2005. Vopr Virusol. 2006;51:20–22. [PubMed] [Google Scholar]
- 90.HHS Pursues Advance Development of New Influenza Antiviral Drug. Available at: http://www.hhs.gov/news/press/2007pres/20070104.html.
- 91.Peramivir–BioCryst Pharmaceuticals. Available at: http://www.biocryst.com/pdf/peramivirfacts.pdf.
- 92.Knight V., Gilbert B.E. Ribavirin aerosol treatment of influenza. Infect Dis Clin North Am. 1987;1:441–457. [PubMed] [Google Scholar]
- 93.U.S. Food and Drug Administration. Influenza virus vaccine. Available at: http://www.fda.gov/cder/flu/flu.htm.
- 94.FDA News 4/16/07: FDA Approves First US Vaccine for Humans Against Avian Influenza Virus H5N1. Available at: http://www.fda.gov/bbs/topics/NEWS/2007/NEW01611.html.
- 95.Medical News Today: Sinovac’s Avian Flu Vaccine Development 3 Mar 2007. Available at: http://www.medicalnewstoday.com/printerfriendlynews.php?newsid=20650.
- 96.Medical News Today: Focetria Pandemic Influenza Vaccine Receives Positive European Union Regulatory Agency Opinion Supporting Approval. 25 Feb 2007. Available at: http://www.medicalnewstoday.com/printerfriendlynews.pho?newsid=63829.
- 97.WHO. Availability of H5N1 prototype strains for influenza pandemic vaccine development. February 2005. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/avian_influenza_prototype_strain.
- 98.Verbiest JP, Castillo CN. Avian flu: an economic assessment for selected developing countries in Asia. ERD Policy Brief No. 24, March 2004. Office of External Relations, Asian Development Bank. Manila, Philippines.
- 99.MMWR . Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. 2002. Guideline for Hand hygiene in Health-Care Settings. Vol 51/RR-16 A publication of the CDC. [Google Scholar]
- 100.Imported Measles with Subsequent Airborne Transmission in a Pediatrician’s Office, Michigan. MMWR. 1983;32(31):401–403. [PubMed] [Google Scholar]
- 101.Bischoff W.E., Reynolds T.M., Sessler C.N. Handwashing compliance by health care workers. Arch Intern Med. 2000;160:1017–1021. doi: 10.1001/archinte.160.7.1017. [DOI] [PubMed] [Google Scholar]
- 102.Hellekson K. Am Fam Physician. 2001. American Association of Pediatricians (AAP) Issues Recommendations on Infection Control in Physicians Offices. Available at: http://www.aafp.org/afp/20010215/practice.html. [PubMed] [Google Scholar]
- 103.Influenza A (H5N1): WHO interim infection control guidelines for health care facilities 2004. World Health Organization. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/infectioncontrol1/en/.
- 104.Marshall S.J. Governments in a dilemma over bird flu. Bull World Health Organ. 2005;83:325–326. [PMC free article] [PubMed] [Google Scholar]
- 105.Infectious Diseases Society of America. Available at: http://www.idsociety.org.
- 106.Otten M. Public health impact of accelerated measles control in the WHO African Region 2000–2003. Lancet. 2005;366:832–839. doi: 10.1016/S0140-6736(05)67216-9. [DOI] [PubMed] [Google Scholar]
- 107.Parker A.A., Staggs W., Dayan G.H. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med. 2006;355:447–455. doi: 10.1056/NEJMoa060775. [DOI] [PubMed] [Google Scholar]
- 108.Smith S. Measles outbreak shows a global threat; worker from India tied to Hub cases. Boston Globe, June 10, 2006. Available at: http://www.boston.com/news/local/articles/2006/06/10/measles_outbreak_shows_a_global.
- 109.Trends in Causes of Death Among the Elderly. Aging Trends No. 1. March 2001. Publication of the Centers for Disease Control and Prevention. National Center for Health Statistics, Atlanta, Georgia.
- 110.2001 Population Reference Bureau. United States Government.
- 111.NCHS-FASTATS. Leading causes of death. (Data are for U.S. for year indicated). Number of deaths for leading causes of death. Available at: http://www.cdc.gov/nchs/fastats/lcod.htm. Last accessed May 28, 2006.