Abstract
Paramyxovirus infections can be detected worldwide with some emerging zoonotic viruses and currently there are no specific therapeutic treatments or vaccines available for many of these diseases. Recent studies have demonstrated that peptides derived from the two heptad repeat regions (HR1 and HR2) of paramyxovirus fusion proteins could be used as inhibitors of virus fusion. The mechanism underlying this activity is in accordance with that of class I virus fusion proteins, of which human immunodeficiency virus (HIV) and influenza virus fusion proteins are members. For class I virus fusion proteins, the HR1 fragment binds to HR2 to form a six-helix bundle with three HR1 fragments forming the central coiled bundle surrounded by three coiled HR2 fragments in the post fusion conformational state (fusion core). It is hypothesized that the introduced exogenous HR1 or HR2 can compete against their endogenous counterparts, which results in fusion inhibition. Using Newcastle disease virus (NDV) as a model, we designed several protein inhibitors, denoted HR212 as well asHR121 and 5-Helix, which could bind the HR1 or HR2 region of fusion protein, respectively. All the proteins were expressed and purified using a GST-fusion expression system in Escherichia coli. The HR212 or GST-HR212 protein, which binds the HR1 peptide in vitro, displayed inhibitory activity against NDV-mediated cell fusion, while the HR121 and 5-Helix proteins, which bind the HR2 peptide in vitro, inhibited virus fusion from the avirulent NDV strain when added before the cleavage of the fusion protein. These results showed that the designed HR212, HR121 or 5-Helix protein could serve as specific antiviral agents. These data provide additional insight into the difference between the virulent and avirulent strains of NDV.
Keywords: inhibitors design, fusion, heptad repeat (HR), paramyxovirus, Newcastle disease virus (NDV)
Abbreviations used: DMEM, Dulbecco's modified Eagle's medium; FCS, fetal calf serum; NDV, Newcastle disease virus; HR, heptad repeat; GST, glutathione-S-transferase
Introduction
Like many enveloped viruses, paramyxovirus requires virus–cell fusion and mediates cell–cell fusion.1 At least two viral membrane glycoproteins of the paramyxovirus fusion protein (F) and attachment protein (HN, H or G, depending on the genus) participate in the fusion process. For many paramyxoviruses, such as Newcastle disease virus (NDV), the attachment proteins (HN for NDV) are absolutely required for F protein-mediated cell fusion,1 while other paramyxoviruses, such as simian parainfluenza virus 5 (SV5)2 and respiratory syncytial virus (RSV),3 fusion does not absolutely require attachment proteins. The mechanism underlying this difference is unclear. The F proteins of paramyxovirus contain conserved functional domains, which include the fusion peptide and two heptad repeat (HR) regions, called HR1 or HR-A and HR2 or HR-B.4 The fusion peptide, responsible for insertion of the F protein into the host cell membrane, is located immediately before the HR1 region in paramyxovirus. Biochemical and crystal structure studies demonstrated that the HR1 and HR2 could form a stable six-helix bundle, which is referred to as a trimer of HR1 and HR2 heterodimer or trimer of hairpins. This has been suggested to represent the core structure of the post-fusion F protein, known also as the fusion core.5, 6, 7, 8, 9, 10, 11 It has been shown that peptides derived from the HR regions could inhibit virus-mediated cell fusion, probably by disturbing the interaction of the HR regions during the conformational changes of the F protein.6
Like all the members in the Paramyxoviridae family, the NDV F protein is synthesized initially as a precursor, F0, and then cleaved into the disulfide-linked subunits F1 and F2 by a furin-like enzyme of the host cells.12 This cleavage of the F protein is necessary for protein maturation and fusion activation.13 The cleavability of the F0 protein is a key determinant for infectivity and pathogenicity of NDV.12 For avirulent NDV strains, the F protein cannot be cleaved by the furin-like enzyme in the cell culture and can exist as F0 only at the cell surface. Therefore, the fusion process cannot be fulfilled unless an exogenous protease such as trypsin is added to promote F0 cleavage into the fusion activation form. Previous studies14, 15, 16, 17 from our laboratory and others have shown that, similar to the HR2 peptides of the HIV-1 gp41 protein, the peptides derived from the NDV F protein HR2 regions could be used as inhibitors of F or virus-mediated cell fusion, but the inhibition activity of HR1 peptides was poorly characterized, likely due to their hydrophobicity, just as the HR1 peptides of the HIV-1 gp41 protein18, 19, 20. Interestingly, previous studies showed that peptides derived from the HR1 domain of the NDV F could inhibit F protein-mediated fusion only if added prior to the cleavage of the F0 protein in an in vitro transfection system.16 However, the mechanism involved was unclear. Our previous study showed that the free HR1 or glutathione-S-transferase (GST)-fusion HR1 could not block the fusion process mediated by the NDV virulent strain.17 One possibility for this negative result could be due to the instability and aggregation of the in vitro prepared HR1, rather than the difference between virulent and avirulent strains. Therefore, it requires further experimentation with more stable preparations to distinguish between these possibilities in order to better characterize the NDV fusion mechanism. In this study, we built upon our previous constructs9 and designed three new proteins called HR121, HR212 and 5-Helix. The HR121 and HR212 were the 2-Helix proteins,9 plus another HR1 at the C terminus or another HR2 at the N terminus. The 5-Helix was just the 6-Helix protein,9 lacking one HR2 at the C terminus. These constructs were predicted to be more stable than the free HR1 or HR2 peptides based on the formation of a six-helix bundle.9 At the same time, they should serve also as inhibitors, based on the current conformational change model, as they have or lack one free HR domain. The biochemical characters of the designed proteins were analyzed and the fusion inhibition activities were determined using NDV (virulent and avirulent strains) mediated cell fusion as a model. The probable mechanism of fusion inhibition is discussed.
Results
Design of protein inhibitors
As shown in Figure 1 , two heptad-repeat regions, HR1 and HR2, were determined in our study. The designed proteins, 5-Helix, HR121 and HR212, were engineered on the basis of our previous study.9 As shown in Figure 1(b), 5-Helix was similar to 6-Helix, but lacked one HR2 at the C terminus, while HR121 or HR212 were modified from 2-Helix that had an additional HR1 at the C terminus or one HR2 at the N terminus. All the genes of designed proteins were prepared by standard PCR methods and cloned into a GST fusion expression vector (see Materials and Methods).
Figure 1.
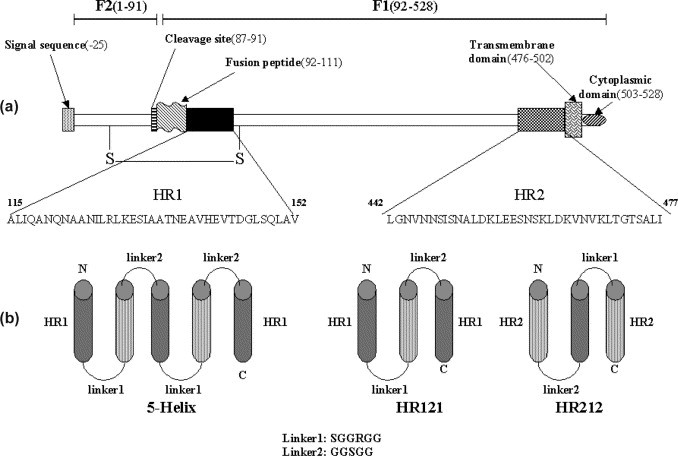
Heptad repeat regions of NDV F protein. (a) A diagram of the NDV F protein (F1 and F2) with the location of structurally significant domains. The sequences of HR1 (115–152) and HR2 (442–477) used in this study are indicated. S–S represents the disulfide bond between the F1 and F2 proteins. (b) Schematic constructs of 5-Helix, HR121 and HR212. The 5-Helix was produced by sequential linking of 3×HR1 and 2×HR2 with two linkers (SGGRGG and GGSGG). HR121 and HR212 were obtained by linking 3×HR1 and 1×HR2 or 3×HR2 and 1×HR1 with two amino acid linkers (SGGRGG and GGSGG) as shown.
All the designed proteins were soluble and could bind to their respective HR1 or HR2 peptides in vitro
The GST fusion 5-Helix, HR121 and HR212 were all expressed as soluble proteins in Escherichia coli at 37 °C or 30 °C and could be separated easily with a GST affinity column. Most of the GST fusion 5-Helix and HR121 proteins were soluble in the supernatants of the lysed E. coli cells when expressed at 30 °C, while GST-HR212 proteins were soluble when expressed at both 30 °C and 37 °C. For elution of GST-5-Helix and GST-HR121 proteins, 150 mM NaCl was added to the elution buffer and an increased elution volume was used in order to improve the results. This is likely due to the hydrophobicity of the HR1 region. The 5-Helix, HR121 and HR212 were purified after removing the cleaved GST and GST-3C (Figure 2 ). The GST pull-down assay showed that GST-5-Helix and GST-HR121 could bind especially well with HR2 or HR212, while GST-6-Helix did not show any convincing binding (Figure 3 ). GST-HR212 could bind with HR1 peptides (Figure 3(c)). It was further demonstrated by gel-filtration analysis that GST untagged 5-Helix, HR121 and HR212 could interact with HR2 or HR1 peptides, respectively. After 5-Helix, HR121 and HR212 were mixed with HR2 or HR1 peptides, their elution peaks shifted as compared to those of the controls. Furthermore, SDS-PAGE analysis demonstrated an association between the proteins and the peptides (Figure 4 ), which indicated a specific interaction of the peptide partners. These results suggested that the designed proteins could be potential fusion inhibitors of NDV F protein-mediated cell fusion.
Figure 2.
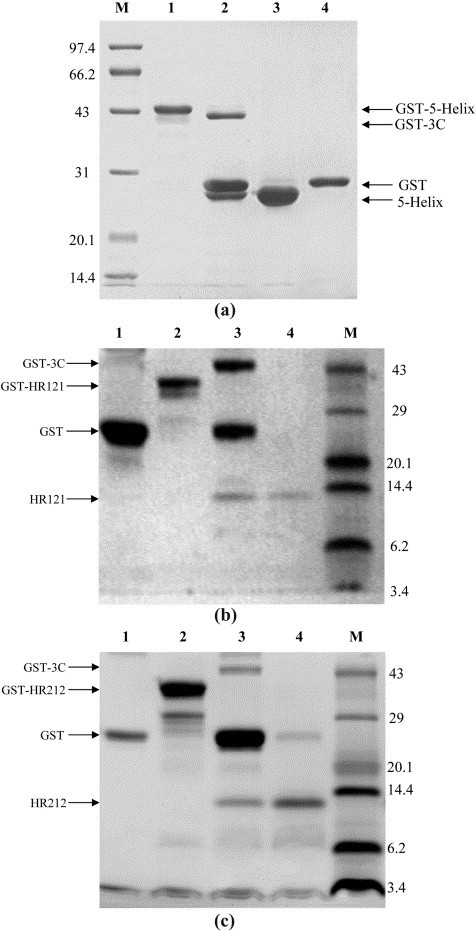
Expression and purification of 5-Helix, HR121 and HR212 in the GST fusion expression system. The resulting proteins were separated from the digestion product by running through a glutathione-Sepharose 4B column to remove GST and the GST-3C protease. (a) SDS-PAGE (12% (w/v) polyacrylamide gel) of 5-Helix. M, protein molecular mass markers (in kDa); lane 1, GST-5-Helix eluted from glutathione-Sepharose 4B by reduced glutathione; lane 2, GST-5-Helix after GST-3C protease digestion (16 h, 5 °C); lane 3, 5-Helix after removing GST and GST-3C protease by flowing through glutathione-Sepharose 4B; lane 4, purified GST as control. (b) Tris–tricine SDS-PAGE of HR121. M, protein molecular mass markers (in kDa). Lane 1, purified GST; lane 2, GST-HR121 eluted from glutathione-Sepharose 4B by reduced glutathione; lane 3, GST-HR121 after GST-3C protease digestion (16 h, 5 °C); lane 4, HR121 protein after removing GST and GST-3C protease by flowing through glutathione-Sepharose 4B. (c) Tris–tricine SDS-PAGE of HR212. Lane 1, purified GST; lane 2, GST-HR212 eluted from glutathione-Sepharose 4B by reduced glutathione; lane 3, GST-HR212 after GST-3C protease digestion (16 h, 5 °C); lane 4, HR212 protein after removing GST and GST-3C protease by flowing through glutathione-Sepharose 4B; M, protein molecular mass markers in (kDa).
Figure 3.
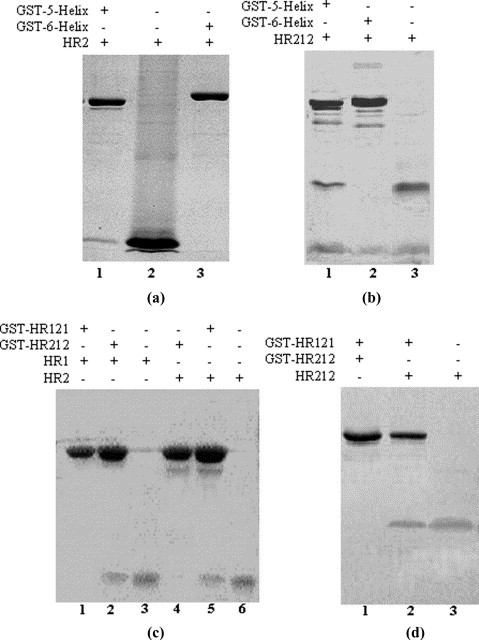
GST pull-down assays demonstrate the binding activities of 5-Helix, HR121 and HR212. The GST fusion proteins (10 mg/ml) were mixed with purified HR2, HR212 or HR1 for 30 min at 25 °C before glutathione-Sepharose 4B beads were added to precipitate the complexes containing GST fusion proteins. The beads were washed with PBS over ten bead volumes and eluted with 5 mM reduced glutathione. The eluted samples were run on Tris–tricine SDS-PAGE. (a) GST pull-down showed that the GST-5-Helix could interact with the HR2 peptide (lane 1), while the control GST-6-Helix protein did not show any clear binding band (lane 3). Lane 2, purified HR2 peptide. (b) GST pull-down showed that GST-5-Helix could interact with HR212 (lane 1), while the control GST-6-Helix protein did not show any clear binding band (lane 2). Lane 3, purified HR212. (c) GST pull-down showed that GST-HR212 could interact with HR1 peptide (lane 2), while the control GST-HR121 did not show any clear binding band (lane 1) and GST-HR121 could interact with HR2 (lane 5), while GST-HR212 did not show any clear binding band (lane 4). Lane 3, purified HR1 peptide; lane 6, purified HR2 peptide. (d) GST pull-down showed that GST-HR121 could interact with HR212 peptide (lane 2), while GST-HR212 did not show any clear binding band (lane 1). Lane 3, purified HR212.
Figure 4.
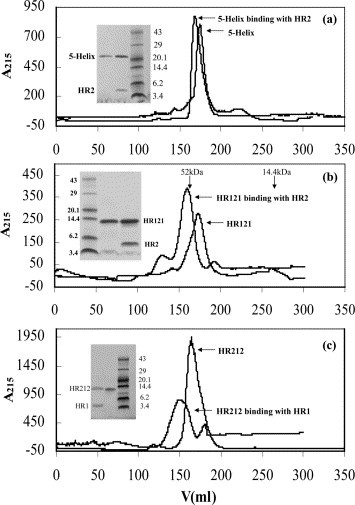
Gel-filtration analysis of 5-Helix, HR121 and HR212 proteins. (a) The 5-Helix proteins or mixtures of 5-Helix and HR2 peptides subjected to Superdex G75 gel-filtration chromatography. The inset is the protein from the peaks run on Tris–tricine SDS-PAGE, which indicates the interaction of 5-Helix and HR2. The elution curve showed that the peak of 5-Helix shifted after binding with HR2 peptides. (b) HR121 proteins or the mixtures of HR121 and HR2 peptides subjected to Superdex G75 gel-filtration chromatography. The inset is the protein from the peaks run on Tris–tricine SDS-PAGE, which indicates the interaction of HR121 with HR2. The elution curve showed that the peak of HR121 shifted after binding with HR2 peptides. (c) HR212 proteins or the mixtures of HR212 and HR1 peptides subjected to Superdex G75 gel-filtration chromatography. The inset is the protein from the peaks run on Tris–tricine SDS-PAGE, which indicates the interaction of HR212 and HR1. The elution curve showed that the peak of HR212 shifted after binding with HR1 peptides. The eluted peak positions of the standard proteins (52 kDa and 14.4 kDa) run on the same column are shown in (b). The 5-Helix, HR121 and HR212 proteins are all eluted from the column between the eluted volumes corresponding to 52 kDa and 14.4 kDa protein standards, which indicate that the 5-Helix protein is a monomer, while HR121 and HR212 proteins could form trimers. Protein molecular mass markers are shown (in kDa) in all SDS-PAGE figures. Note, none of the proteins has measurable absorbance at 280 nm, therefore the absorbance was recorded at 215 nm. Protein concentrations used in these experiments were 10 mg/ml for all preparations.
Both HR121 and HR212 proteins could form trimers in vitro
In order to determine the actual molecular mass and assembling character of HR121 and HR212 proteins, gel-filtration and chemical cross-linking analysis were used as described in Materials and Methods. In the gel-filtration assay, the elution peaks of both HR121 and HR212 proteins were just after the peak position corresponding to 52 kDa (Figure 4(b) and (c)), whereas the calculated molecular mass of HR121 and HR212 is 12.87 kDa and 12.78 kDa, respectively. This indicated that both HR121 and HR212 could form multimers, probably trimers. Subsequently, chemical cross-linking of HR121 and HR212 preparations showed that both dimers and trimers could be seen on a reducing gel (Figure 5 (a) and (b)). Monomers/dimers tended to fade as the EGS cross-linker concentrations were increased, whereas the trimer bands became more prominent. These data implicated protein trimers, although the monomer/dimer bands were still visible, even in high concentrations of the cross-linker. It was also demonstrated by gel-filtration peak shifting that one trimer of HR121 or HR212 probably could bind three HR2 or HR1 molecules (Figure 4(b) and (c)).
Figure 5.
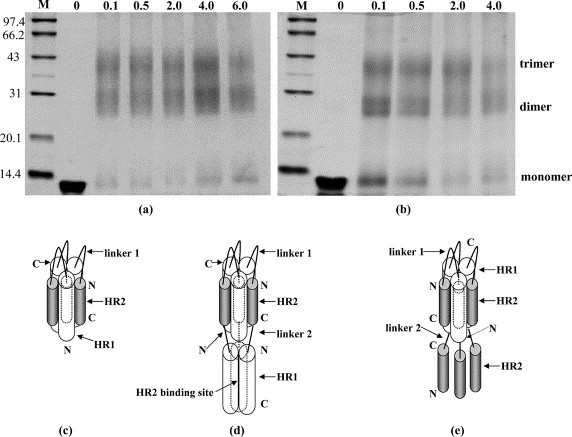
Chemical crosslinking of HR121 and HR212 proteins. (a) Chemical crosslinking of HR121. (b) Chemical crosslinking of HR212; crosslinked products were separated by SDS-PAGE (14% (w/v) polyacrylamide gel) followed by staining with Commassie brilliant blue. Protein mass markers are shown (in kDa). The numbers 0, 0.1, 0.5, 2.0, 4.0, and 6.0 indicate the concentration of ethyleneglycol bis(-succinimidylsuccinate) (EGS) used (in mM). Bands corresponding to monomer, dimer and trimer are indicated. (c) The HR1/HR2 six-helix bundle complex drawing according to the crystal structure of NDV F 2-Helix (HR1–HR2) proteins (unpublished results). (d) A possible schematic structure model of the designed HR121 protein, which displays the trimer formation and HR2 binding sites. (e) A possible schematic structure model of the designed HR212 protein, which displays the trimer formation and the dissociative HR2 peptides.
All the designed proteins were α-helical and thermostable
CD spectra of all the designed proteins display the characteristic signature of α-helical proteins with minima at 208 nm and at 222 nm (Figure 6 (a), (c) and (d)). Thermodynamic stability analysis demonstrated that the 5-Helix, HR121 and HR212 proteins were all thermostable in PBS buffer (Figure 6(e), (g)and (h)), even in PBS containing 4 M GuHCl (Figure 6(e)). According to the melting temperatures (T m), 79 °C, 77 °C, 90 °C and 91 °C for 5-Helix, HR121, HR212 and 6-Helix (Figure 6(f)), respectively, the HR212 protein was almost as stable as the 6-Helix protein, and was most stable among the three designed proteins, consistent with its highest solubility. These results showed that the designed proteins retained the characteristics of the 2-Helix and 6-Helix proteins as described,9 and were more stable than either single HR1 or HR2 peptides.17 The CD results provided evidence for the specific interaction of 5-Helix and HR121 with HR2 peptides. After the addition of HR2 peptides into 5-Helix or HR121, their CD spectra were increased, especially at 208 nm and 222 nm (Figure 6(a) and (c)), which indicated an increase of α-helical content. In contrast, there were no changes in the CD spectra curves for the mixtures of 6-Helix or HR212 with HR2 peptides (Figure 6(b) and (d)). These results demonstrated that 5-Helix and HR121 proteins could interact with HR2 peptides in an α-helical mode.
Figure 6.
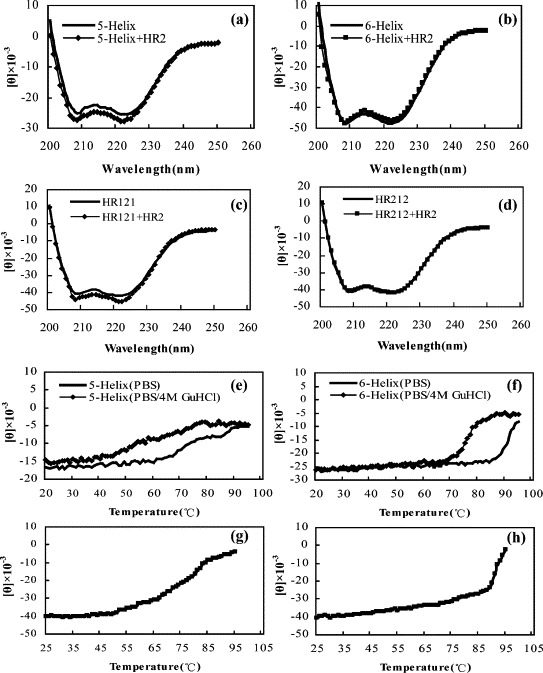
The CD spectra and thermal stability measurement of 5-Helix, HR121 and HR212 proteins. All of the 5-Helix, HR121 and HR212 proteins (10 μg/ml used) show (a), (c) and (d) a typical α-helix secondary structure, and (e), (g) and (h) thermal stability in PBS and even (e) and (f) in PBS containing 4 M GuHCl. (f) The 6-Helix protein seems more stable than the 5-Helix, HR121 and HR212 proteins, and (h) HR212 seems slightly more stable than (g) HR121 protein. The mixing of 5-Helix and HR2, and the mixing of HR121 and HR2, led to (a) and (c) the increase in ellipticity at 208 nm and 222 nm, while there were no changes after (b) the mixing of 6-Helix and HR2 or (d) the mixing of HR212 and HR2, which indicates the interactions between 5-Helix and HR2, and between HR121 and HR2. Thermal denaturation curves were recorded at 222 nm with a scan rate of 1 deg. C/min.
Only HR212 proteins could inhibit NDV virulent strain-mediated cell fusion and plaque formation
To investigate the fusion-inhibition activity of the designed proteins, the virus-induced cell fusion and plaque formation inhibition assay were performed as described in Materials and Methods. As shown in Figure 7 , only HR212 or GST-HR212 proteins could block the NDV virulent strain (F48E9) mediated cell fusion and plaque formation, while 5-Helix and HR121 had no inhibition activity. The HR212 and GST-HR212 could completely inhibit virus-induced syncytia or plaque formation at high concentrations (>25 μM) (Figure 7(a) C and (b) C). Dose–response curves of cell-fusion inhibition (Figure 7(c) A) showed that the half-inhibition concentrations (IC50) of HR212 and GST-HR212 were 2.76 μM and 3.10 μM, respectively. Dose–response curves of plaque reduction (Figure 7(c) B) showed that the IC50 of HR212 was 3.62 μM. In the fusion inhibition assay, all the protein samples were added at the time of virus adsorption, i.e. to block virus infection, but similar results were obtained if they were added into the culture medium after the infection of the cells. These results indicated that HR212 could be an inhibitor of NDV virulent strain-mediated cell fusion, while 5-Helix and HR121 could not.
Figure 7.
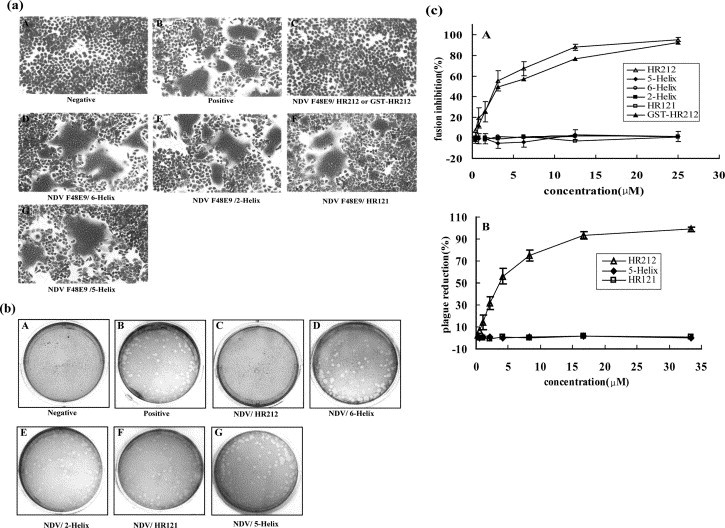
Inhibition of NDV virulent strain (F48E9) mediated cell fusion or plaque formation. (a) Purified 5-Helix, HR121, HR212 or GST-HR212 proteins were added at the time of virus adsorption to HeLa T4 cells at 37 °C for 1 h. A, negative control of HeLa T4 cells; B, positive control cells infected by NDV without protein treatment; C, infected cells treated with 25 μM HR212 or GST-HR212; D, infected cells treated with 25 μM 6-Helix as a control; E, infected cells treated with 25 μM 2-Helix as a control; F, infected cells treated with 30 μM HR121; G, infected cells treated with 30 μM 5-Helix. (b) Inhibition of plaque formation. Purified 5-Helix, HR121 and HR212 proteins were added at the time of virus adsorption to chicken embryo blast cells at 37 °C for 1 h. A, negative control cells; B, positive control cells infected by NDV without protein treatment; C, infected cells treated with 40 μM HR212; D, infected cells treated with 40 μM 6-Helix as a control; E, infected cells treated with 40 μM 2-Helix as a control; F, infected cells treated with 40 μM HR121; G, infected cells treated with 40 μM 5-Helix. (c) Inhibition of (A) cell fusion and (B) plaque reduction. HR212 or GST-HR212 had inhibition activity, while HR121 and 5-Helix proteins had no inhibition effect.
All the designed proteins could inhibit NDV avirulent strain-mediated cell fusion only if added before trypsin digestion of the infected cells
NDV avirulent strain-infected cells could not form syncytia (Figure 8 (a), A) because of the uncleavability of F0 protien, but after trypsin digestion obvious syncytia were observable (Figure 8(a), B). To determine the fusion inhibition activity of the designed proteins added before or after trypsin digestion, cell fusion inhibition tests were performed as described in Materials and Methods. The results showed that all the designed proteins, including 5-Helix, HR121 and HR212 could inhibit syncytia formation if added before trypsin digestion (Figure 8(a) C and (b) A). The IC50 values were 2.67 μM, 2.51 μM and 3.01 μM for 5-Helix, HR121 and HR212 respectively. However, the 5-Helix and H121 proteins lost the inhibition activity if added after trypsin digestion and, similarly, the HR212 proteins showed a much lower inhibitory activity (Figure 8(a) D and (b) B). These results showed that the 5-Helix and HR121 could interact only with the uncleaved F0 protein and could not interact with the mature F0 protein, while HR212 could interact with either F0 or cleaved F protein (i.e. the activated form of the F1/F2 complex).
Figure 8.
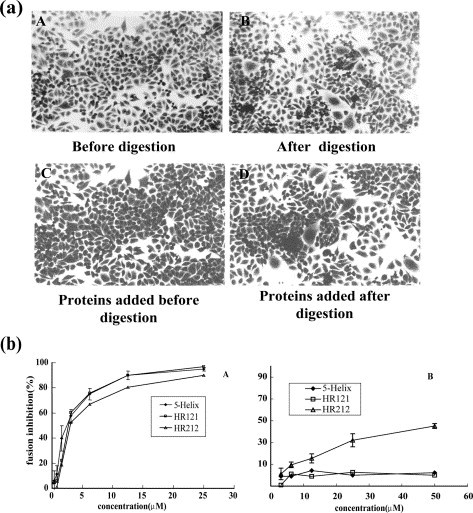
Inhibition of NDV avirulent strain (clone30) mediated cell fusion NDV clone30 infected HeLa T4 cells (a) before trypsin digestion and (b) after trypsin digestion. Obvious syncytia were formed after digestion. Infected cells were treated with proteins (HR212, HR121 or 5-Helix) until trypsin digestion (at room temperature for 10 min) or treated with proteins during the time of incubation for 12 h after digestion. (c) Syncytia formation could be inhibited if the proteins were added before trypsin digestion, while (d) it could not be inhibited if added after digestion. (b) Inhibition curves of NDV avirulent strain (clone30) mediated cell fusion. All the proteins including 5-Helix, HR121 and HR212 had inhibition activities if added (a) before trypsin digestion, while (b) 5-Helix and HR121 had no and HR212 had slight inhibition effect if added after digestion.
Indication of the inhibition mechanism of HR212 proteins
In order to determine how the HR212 proteins performed their inhibition activity, the HR212 and 5-Helix proteins were mixed in different proportions before determining the inhibition activity of the NDV virulent strain-mediated cell fusion. It was found that the inhibition activity of HR212 was neutralized by 5-Helix when they were mixed at equal molar concentrations, and this effect was decreased with a reduction in the concentration of 5-Helix (Figure 9 ). These results, as well as the GST pull-down assay (Figure 3), indicated that HR212 proteins performed their fusion inhibition activity through binding to the HR1 domain of F protein, but it was still unclear at which stage this occurred. To address this, a high concentration of HR212 proteins was added at different times after adsorption of the NDV virulent strain to HeLa cells. Strikingly, the HR212 proteins could inhibit cell fusion only at a very early stage of virus adsorption, i.e. before 2 min (Figure 10 ), which suggested that HR212 bound to the F protein at the early stage of conformational change.
Figure 9.
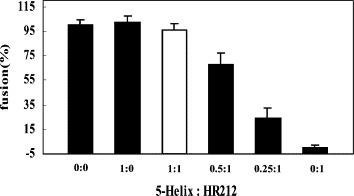
The fusion inhibition of the mixtures of 5-Helix and HR212. The 5-Helix and HR212 proteins mixed in different proportions (0:0, 1:0, 1:1, 0.5:1, 0.25:1, 0:1) were added at the time of NDV F48E9 adsorption to HeLa T4 cells at 37 °C for 1 h. It was revealed that there was no inhibition effect when the 5-Helix and HR212 proteins were mixed at equimolar concentrations (25 μM). This demonstrated that the interaction between 5-Helix and HR212 disturbed the inhibition activity of HR212, and HR212 proteins exerted their fusion inhibition activity, possibly through binding with the HR1 domain of F protein.
Figure 10.
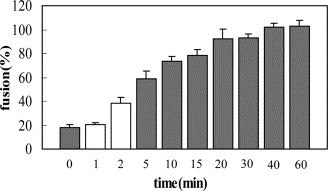
The fusion inhibition effect of HR212 added at different times of NDV absorption. HR212 proteins (20 μM) were added at the time of 0 min, 1 min, 2 min, 5 min, 10 min, 15 min, 20 min, 30 min, 40 min, and 60 min after NDV F48E9 adsorption to HeLa T4 cells and incubated for another 30 min before being replaced by DMEM containing 2% FCS. It showed that the HR212 proteins had little inhibition effect if added after virus adsorption for more than 5 min. The results indicated that the virus adsorption and conformational changes of NDV F protein occurred in 1–2 min, and the conformational changes were irreversible, and HR212 could bind with F protein only before or at the time of conformational change.
Discussion
Preventing virus entry has become a new therapeutic strategy to prevent virus infection, and virus fusion inhibition is currently one of the most promising approaches. Great efforts have been made to elucidate the molecular mechanism of enveloped virus entry and fusion, with an increasing number of studies showing that most enveloped viruses have a similar fusion process. Therefore, similar studies on fusion inhibition have been conducted in recent years.4, 6, 21 Peptides derived from the heptad repeat regions of virus fusion proteins have been demonstrated to be fusion inhibitors for many enveloped viruses (class I), such as retrovirus and paramyxovirus. For HIV, a peptide called T-20 or Enfuvirtide©, derived from the HR2 (or C-peptide) domain of gp41 has currently finished the third clinical trial in USA as an anti-HIV drug.22 However, the high cost of peptide synthesis and instability limit the broad use of this drug. Studies showed that peptides corresponding to the N-terminal heptad repeat (HR1 or N-peptide) of gp41 were weak inhibitors of HIV fusion, likely due to their tendency to aggregate.19, 20 Much effort has been devoted to the design of more stable and effective protein inhibitors of HIV fusion, including N-peptides fused with trimeric coiled-coils,18 a chimeric protein termed NCCG-gp41,23 and a protein called 5-Helix.24 They were all demonstrated to prevent HIV fusion. In this study, we designed several protein inhibitors, including 5-Helix, on the basis of our previous studies of paramyxovius fusion,9 and the results showed that all the proteins could inhibit NDV-mediated cell fusion. This study showed also that all the proteins, including HR121 and HR212, were very stable and could be obtained easily by expression and purification. This design strategy should be tested for other enveloped viruses, especially HIV.
For paramyxovirus, virus fusion involves at least two envelope glycoproteins, an attachment protein and fusion protein, which are responsible for receptor binding and cell fusion, respectively. F protein is believed to mediate the fusion between the cellular membrane and the virus membrane through a series of conformational changes.1 In the current model,6 there are at least three conformational states of the F protein, i.e. a pre-fusion native state, pre-hairpin intermediate state and post-fusion state. In the post-fusion state, the two conserved HR regions bind each other in an anti-parallel fashion as a coiled-coil structure and form the so-called 6-Helix trimer.6 The two HR regions are highly conserved in all sequenced paramyxoviruses. Like HIV, many studies, including ours, showed that HR2 peptides could inhibit virus-mediated or F protein-mediated cell fusion in many paramyxoviruses, including NDV.15, 16, 17 However, details of the inhibition activity of HR1 peptides had not been clear so far. Our previous study showed that HR1 peptides derived from the NDV F protein had no fusion inhibition ability against NDV-mediated cell fusion,17 while the other studies showed HR1 peptides could inhibit F protein-mediated cell fusion if added before the F0 protein was cleaved.16 Like the HR1 of HIV gp41, the HR1 peptides of paramyxovirus fusion proteins are hydrophobic and tend to aggregate, so it is necessary to design more stable protein inhibitors that have the same function as HR1. We previously designed a construct called 2-Helix, which was a construct connecting HR1 and HR2 as a single chain (HR1–HR2) and the results showed that the 2-Helix protein could form a stable six-helix bundle trimer.9, 10, 11 In this study, on the basis of the 2-Helix construct, another HR2 or HR1 was added at the N terminus or C terminus of 2-Helix, i.e. HR2–HR1–HR2 (HR212) and HR1–HR2–HR1 (HR121), to form two new constructs. A 5-Helix protein was constructed according to a recent HIV study.24 It was demonstrated that all the proteins were α-helical and thermostable, similar to 2-Helix.9 The 5-Helix and HR121 have the same binding character as HR1, and they are more stable than the single HR1 peptide. The HR212 has the same characteristics as HR2. Both fusion and plaque formation inhibition tests showed that only HR212 could inhibit NDV virulent strain-mediated cell fusion, while 5-Helix and HR121 could not, which was consistent with the results of our previous study.17 It indicated also that the fusion inhibition of HR212 was mediated through its binding activity to HR1 peptides. This suggests that HR212 might inhibit virus fusion through interacting with the HR1 domain of the intact F protein. All the designed proteins, including 5-Helix and HR121, could inhibit the fusion of the NDV avirulent strain if added before F0 was cleaved. This suggested that 5-Helix and HR121 probably interact with the corresponding domain of the F0 protein, not the cleaved F1/F2 complex. The paramyxovirus F protein is synthesized initially as a precursor, F0, which is cleaved into disulfide-linked F2 and F1 by the furin-like enzyme of the host cell. For the F0 protein of the NDV avirulent strain, the furin-like enzyme cannot cleave it into F1 and F2, so it cannot be activated to promote cell fusion. Additional enzymes are required to cleave the F0 protein to promote the infection with the avirulent stain. Using this system, it was convenient to detect the inhibition activity of the peptides that were added before F0 cleavage. On the basis of our results, peptides corresponding to the HR1 and HR2 domains of the NDV F protein can inhibit virus-mediated cell fusion, but the inhibition probably occurs through a different mechanism, e.g. at different stages of F protein conformational changes.
In our study, we showed that HR212 could inhibit NDV-mediated cell fusion if added both before and after F0 cleavage. This was in contrast to the results of others,16 which showed that both HR1 and HR2 could inhibit cell fusion only when added prior to cleavage activation. As a further characterization, we detected the inhibition of HR212 added at different stages of virus attachment. The results showed that HR212 could exhibit high fusion inhibition only when added as early as 2 min after virus attachment. There was almost no inhibition detected if added after 10 min after the attachment. This result implied that the conformational changes of the F protein occurred very quickly after receptor binding to the HN protein, and that this change was irreversible (i.e. HR212 could interact with the F protein only at the very early stage of fusion). This may explain why the inhibition activity was much lower if added after cleavage activation. However, all these results are still consistent with the idea that HR2 peptides could interact both with cleaved and uncleaved F protein, while the HR1 peptides could interact only with the F0 protein to inhibit virus fusion.
The fusion mediated by the NDV F protein absolutely requires the participation of the HN protein,1 while other paramyxoviruses, such as SV52 and RSV,3 do not. Previous studies have shown that HR1 peptides derived from the paramyxoviruses whose fusion absolutely requires HN proteins (or its homologue G or H) appear to not have any fusion inhibition,17, 25, 26 whereas the HR1 peptides derived from the paramyxoviruses whose fusion does not absolutely require the attachment protein show the fusion inhibition effect.14, 27 Therefore, we assumed that the interaction of the HN and F proteins plays an important role in the HR1/HR2 domain exposure during conformational changes of the F protein. It had been accepted that the conformational change of the F protein was promoted by the HN protein binding to the receptor,4 and several studies showed that the HN protein was associated with the F protein.25, 28, 29 Co-expression of the HN and F proteins does alter the conformation of cell-surface F protein.30 In addition, a recent report demonstrated that the HN protein of NDV could interact with the HR2 domain of the F protein,31 indicating that this type of interaction may affect the fusion inhibition of HR1 peptides. According to this result, we supposed that the HR2 domain of the mature F protein was hidden by the interaction with the HN protein, and this interaction continues with the conformational change of the F protein. This may be the reason why HR1 peptides cannot bind to block fusion. However, the HR2 domain of the immature F0 protein was not covered by the HN protein and can be exposed to external HR1 peptides. Additional studies are required to clarify the detailed interaction of HN/F along with associated conformational changes.
This study provides a useful design strategy for the creation of viral fusion inhibitors. The designed proteins may serve also as drug targets and vaccine candidates, especially for HIV and other severe viral diseases. Recent studies have shown that coronavirus likely employ a fusion mechanism similar to those of other enveloped viruses.32 Our studies on SARS coronavirus showed that peptides derived from the HR regions of the spike protein could also inhibit SARS virus infection in vitro.33 This indicates that a similar design strategy may yield new SARS coronavirus fusion inhibitors as well. Our study has further characterized the molecular mechanism of NDV fusion.
Materials and Methods
Cells and virus
NDV strain F48E9 (virulent strain, kindly provided by China Institute of Veterinary Drug Control) and NDV strain Clone30 (avirulent strain, kindly provided by Professor Zaishi Wang, China Institute of Veterinary Drug Control) were used in this study. HeLa T4 cells were obtained from American Type Culture Collection (ATCC).
Vector construction
The NDV F gene was cloned from the Chinese virulent isolate F48E9 (Gene Bank accession no. AF079172). The HR1 region used was derived from amino acid residues 115 to 152 and HR2 from 442 to 477 (See Figure 1). Three different constructs were made by PCR: 5-Helix, HR121 and HR212, as shown in Figure 1. The 5-Helix construct was engineered by linking 3×HR1 and 2×HR2 with two amino acid linkers (SGGRGG and GGSGG, single-letter amino acid abbreviations used here). The HR121 and HR212 constructs were generated by sequential linking of the HR1, HR2 and HR1 or HR2, HR1 and HR2 using two different linkers (linker 1 as SGGRGG or linker 2 as GGSGG) (Figure 1(b)). All the constructs were cloned into the BamH I and Xho I sites of the GST fusion expression vector pGEX-6p-1 (Pharmacia), in which there is a rhinovirus 3C protease cleavage site for the fusion protein (the same as the commercial PreScission™ protease cleavage site). The positive plasmids were verified by direct DNA sequencing.
Protein expression and purification
E. coli strain BL21(DE3) transformed with the recombinant pGEX-6p-1 plasmid was grown at 37 °C in 2×YTA medium to an absorbance of 0.8–1.0 at 590 nm before induction with 1 mM IPTG for 4 h at 37 °C (30 °C for 5-Helix and HR121 expression). Bacterial cells were harvested and lysed by sonication in PBS (10 mM sodium phosphate (pH 7.3), 150 mM NaCl). Triton X-100 was then added to a final concentration of 1% (v/v), and the lysate was incubated for 30 min at 0 °C and subsequently clarified by centrifugation at 12,000 g for 30 min at 4 °C. The clarified supernatants were passed over a glutathione-Sepharose 4B column (equalibrated with PBS). The GST fusion protein-bound column was washed with ten column volumes of PBS and eluted with three column volumes of 10 mM reduced glutathione (an extra 150 mM NaCl was added for elution of 5-Helix and HR121 fusion proteins). The GST fusion proteins were then cleaved by GST-fusion rhinovirus 3C protease (kindly provided by Drs K. Hudson and J. Heath) at 5 °C for 16 h in cleavage buffer (50 mM Tris–HCl (pH 7.0), 150 mM NaCl, 1 mM DTT, 1 mM EDTA (pH 8.0)). The free GST and the GST-3C protease were removed by passage through the glutathione-Sepharose 4B column again. The resulting proteins, 5-Helix, HR121 and HR212, were dialysed against PBS and concentrated by ultrafiltration and stored at −70 °C. Proteins were analyzed by Tris–tricine PAGE or SDS-PAGE (12% polyacrylamide gel). Protein concentrations were determined by the BCA protein determination assay (Pierce Biochemicals). GST-6-Helix, 2-Helix and GST- or free HR1 and HR2 proteins were prepared as described.9, 17
GST pull-down assay
Purified GST fusion proteins, GST-5-Helix, GST-HR121 and GST-HR212, were mixed with purified HR2,17 HR212 or HR117 for 30 min at 25 °C before glutathione-Sepharose 4B beads were added to precipitate the complexes containing GST fusion proteins. The beads were washed with ten column volumes of PBS and eluted with 10 mM reduced glutathione. The eluted samples were run on Tris–tricine SDS-PAGE. Purified GST-6-Helix protein was used as a control.9 The concentrations of all protein preparations used in this experiment were 10 mg/ml.
Gel-filtration chromatography
The GST column-purified 5-Helix, HR121 and HR212 proteins were further purified by gel-filtration chromatography using the Hiload Superdex™ G75 column with an Akta FPLC system (Pharmacia). The gel-filtration-purified 5-Helix and HR121 were mixed with equimolar HR2 peptides separately and loaded onto the Hiload Superdex™ G75 column. The gel-filtration-purified HR212 was mixed with equimolar HR1 peptides and loaded onto the same column. The fractions of the peak were collected and run on Tris–tricine SDS-PAGE. The peak molecular mass was estimated by comparison with protein standards (Pharmacia) running on the same column. Protein concentrations used in these experiments were 10 mg/ml for all the protein preparations.
Chemical cross-linking
The gel-filtration-purified HR121 and HR212 proteins were dialysed against cross-linking buffer (50 mM Hepes (pH 8.3), 100 mM NaCl) and concentrated to about 2 mg/ml by ultrafiltration (103 kDa cut-off). Proteins were cross-linked with ethyleneglycol bis(-succinimidylsuccinate) (EGS) (Sigma). The reactions were incubated for 1 h on ice at concentrations of 0, 0.1 mM, 0.5 mM, 2.0 mM, 4.0 mM, and 6.0 mM EGS and stopped by 50 mM glycine. Cross-linked products were analyzed under reducing conditions by SDS-PAGE (14% polyacrylamide gel).
CD spectroscopy
CD spectra were performed on a Jasco J-715 spectrophotometer in PBS (10 mM sodium phosphate (pH 7.3), 150 mM NaCl). Wavelength spectra were recorded at 25 °C using a 0.1 cm path-length cuvette. Thermodynamic stability was measured at 222 nm by recording the CD signals in the temperature range of 25–95 °C with a scan rate of 1 deg. C/min.
Cell fusion and fusion inhibition assay
Virus-induced cell fusion assays were used to detect the fusion inhibition activity of the designed proteins. HeLa T4 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) containing 10% (v/v) fetal calf serum (FCS). Dilutions of the purified protein samples in DMEM without FCS were added into the cell monolayers in 48-well plates at the time of the adsorption of NDV strain F48E9 at 100 pfu/well. The 2-Helix and 6-Helix proteins9 were used as negative controls. After incubation for 1 h at 37 °C, the inoculum was replaced by DMEM with 2% FCS. The monolayers were fixed and stained by 0.5% (w/v) crystal violet in 100% methanol after incubation for 12 h at 37 °C in a 5% CO2 incubator. Numbers of syncytia in each well were counted and a reduction of syncytia formation in comparison with an infected cell control without adding proteins is indicative of fusion-inhibited activity. For NDV avirulent strain (clone 30) mediated cell fusion assay, HeLa T4 cell monolayers in 24-well plates were infected by NDV strain clone 30 at 1000 EID50. After 12 h of infection, the monolayers were washed three times with DMEM and then digested by 10 μg/ml of acetylated trypsin (Sigma) in DMEM at room temperature for 10 min, followed with washing three times with 20 μg/ml of trypsin inhibitor in DMEM and the monolayers were incubated in DMEM containing 2% FCS for another 12 h before staining with crystal violet. To detect the fusion inhibition ability, protein samples were added to the medium during virus infection before trypsin digestion or just after digestion. The medium containing protein samples was replaced every 3 h. Fusion-inhibited activity of protein samples was evaluated as described above.
Plaque reduction assay
Monolayers of chicken embryo fibroblast cell were prepared according to standard methods. Dilutions of purified proteins, 5-Helix, HR121 or HR212, in DMEM without FCS were added to the cell monolayers in six-well plates at the time of the adsorption of NDV strain F48E9 at 30 pfu/well. The 2-Helix and 6-Helix proteins were used as negative controls.9 After incubation for 1 h at 37 °C, the inoculum was removed and the monolayers were overlaid with 2% (w/v) white agar mixed with an equal volume of 2×DMEM containing 4% FCS. The plates were inverted and incubated at 37 °C in a 5% CO2 incubator for 72 h before staining with 0.1% (w/v) neutral red in PBS. The numbers of plaques in each well were counted, and plaque reduction in comparison with an infected cell control without adding proteins is indicative of inhibition of plaque formation.
Acknowledgements
This work was supported by grants from the National Frontier Research Program (Project 973) and the 10th-5-Year-Plan Key Project of the Ministry of Science and Technology of the People's Republic of China (grant nos 2001CB510001, 2005CB523000 and 2004BA519A29), the National Natural Sciences Foundation of China (grant no. 30228025) and the CAS President Fund (to G.F.G.). We thank Professor Zaishi Wang of the China Institute of Veterinary Drug Control for the NDV Clone 30. We are grateful to Dr Justin L. Merritt from UCLA School of Dentistry for his critical reading of the manuscript.
Edited by J. Karn
Contributor Information
Po Tien, Email: tienpo@sun.im.ac.cn.
George F. Gao, Email: gaof@im.ac.cn.
References
- 1.Lamb R.A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 2.Horvath C.M., Lamb R.A. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J. Virol. 1992;66:2443–2455. doi: 10.1128/jvi.66.4.2443-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn J.S., Schnell M.J., Buonocore L., Rose J.K. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology. 1999;254:81–91. doi: 10.1006/viro.1998.9535. [DOI] [PubMed] [Google Scholar]
- 4.Morrison T.G. Structure and function of a paramyxovirus fusion protein. Biochim. Biophys. Acta. 2003;1614:73–84. doi: 10.1016/s0005-2736(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 5.Baker K.A., Dutch R.E., Lamb R.A., Jardetzky T.S. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- 6.Eckert D.M., Kim P.S. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 7.Lamb R.A., Joshi S.B., Dutch R.E. The paramyxovirus fusion protein forms an extremely stable core trimer: structural parallels to influenza virus haemagglutinin and HIV-1 gp41. Mol. Membr. Biol. 1999;16:1–19. doi: 10.1080/096876899294715. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X., Singh M., Malashkevich V.N., Kim P.S. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc. Natl Acad. Sci. USA. 2000;97:14172–14177. doi: 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J., Li P., Wu T., Gao F., Ding Y., Zhang C.W.-H. Design and analysis of post-fusion 6-helix bundle of heptad repeat regions from Newcastle disease virus F protein. Protein Eng. 2003;16:373–379. doi: 10.1093/protein/gzg041. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J., Zhang C.W.-H., Qi Y., Tien P., Gao G.F. The fusion protein core of measles virus forms stable coiled-coil trimer. Biochem. Biophys. Res. Commun. 2002;299:897–902. doi: 10.1016/s0006-291x(02)02761-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J.Q., Zhang C.W.-H., Rao Z., Tien P., Gao G.F. Biochemical and biophysical analysis of heptad repeat regions from the fusion protein of Menangle virus, a newly emergent paramyxovirus. Arch. Virol. 2003;148:1301–1316. doi: 10.1007/s00705-003-0105-x. [DOI] [PubMed] [Google Scholar]
- 12.Nagai Y., Klenk H.D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976;72:494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- 13.Nagai Y., Inocencio N.M., Gotoh B. Paramyxovirus tropism dependent on host proteases activating the viral fusion glycoprotein. Behring Inst. Mitt. 1991:35–45. [PubMed] [Google Scholar]
- 14.Wang E., Sun X., Qian Y., Zhao L., Tien P., Gao G.F. Both heptad repeats of human respiratory syncytial virus fusion protein are potent inhibitors of viral fusion. Biochem. Biophys. Res. Commun. 2003;302:469–475. doi: 10.1016/s0006-291x(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 15.Young J.K., Hicks R.P., Wright G.E., Morrison T.G. Analysis of a peptide inhibitor of paramyxovirus (NDV) fusion using biological assays, NMR, and molecular modeling. Virology. 1997;238:291–304. doi: 10.1006/viro.1997.8834. [DOI] [PubMed] [Google Scholar]
- 16.Young J.K., Li D., Abramowitz M.C., Morrison T.G. Interaction of peptides with sequences from the Newcastle disease virus fusion protein heptad repeat regions. J. Virol. 1999;73:5945–5956. doi: 10.1128/jvi.73.7.5945-5956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M., Wang E., Liu Y., Cao D., Jin N., Zhang C.W. Six-helix bundle assembly and characterization of heptad repeat regions from the F protein of Newcastle disease virus. J. Gen. Virol. 2002;83:623–629. doi: 10.1099/0022-1317-83-3-623. [DOI] [PubMed] [Google Scholar]
- 18.Eckert D.M., Kim P.S. Design of potent inhibitors of HIV-1 entry from the gp41 N-peptide region. Proc. Natl Acad. Sci. USA. 2001;98:11187–11192. doi: 10.1073/pnas.201392898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu M., Blacklow S.C., Kim P.S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nature Struct. Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 20.Wild C., Oas T., McDanal C., Bolognesi D., Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peisajovich S.G., Shai Y. New insights into the mechanism of virus-induced membrane fusion. Trends Biochem. Sci. 2002;27:183–190. doi: 10.1016/s0968-0004(01)02050-3. [DOI] [PubMed] [Google Scholar]
- 22.Lalezari J.P., Henry K., O'Hearn M., Montaner J.S., Piliero P.J., Trottier B. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 2003;348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 23.Louis J.M., Bewley C.A., Clore G.M. Design and properties of N(CCG)-gp41, a chimeric gp41 molecule with nanomolar HIV fusion inhibitory activity. J. Biol. Chem. 2001;276:29485–29489. doi: 10.1074/jbc.C100317200. [DOI] [PubMed] [Google Scholar]
- 24.Root M.J., Kay M.S., Kim P.S. Protein design of an HIV-1 entry inhibitor. Science. 2001;291:884–888. doi: 10.1126/science.1057453. [DOI] [PubMed] [Google Scholar]
- 25.Wild T.F., Buckland R. Inhibition of measles virus infection and fusion with peptides corresponding to the leucine zipper region of the fusion protein. J. Gen. Virol. 1997;78:107–111. doi: 10.1099/0022-1317-78-1-107. [DOI] [PubMed] [Google Scholar]
- 26.Tong S., Compans R.W. Alternative mechanisms of interaction between homotypic and heterotypic parainfluenza virus HN and F proteins. J. Gen. Virol. 1999;80:107–115. doi: 10.1099/0022-1317-80-1-107. [DOI] [PubMed] [Google Scholar]
- 27.Joshi S.B., Dutch R.E., Lamb R.A. A core trimer of the paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp41. Virology. 1998;248:20–34. doi: 10.1006/viro.1998.9242. [DOI] [PubMed] [Google Scholar]
- 28.Stone-Hulslander J., Morrison T.G. Detection of an interaction between the HN and F proteins in Newcastle disease virus-infected cells. J. Virol. 1997;71:6287–6295. doi: 10.1128/jvi.71.9.6287-6295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plemper R.K., Hammond A.L., Gerlier D., Fielding A.K., Cattaneo R. Strength of envelope protein interaction modulates cytopathicity of measles virus. J. Virol. 2002;76:5051–5061. doi: 10.1128/JVI.76.10.5051-5061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGinnes L.W., Gravel K., Morrison T.G. Newcastle disease virus HN protein alters the conformation of the F protein at cell surfaces. J. Virol. 2002;76:12622–12633. doi: 10.1128/JVI.76.24.12622-12633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravel K.A., Morrison T.G. Interacting domains of the HN and F proteins of Newcastle disease virus. J. Virol. 2003;77:11040–11049. doi: 10.1128/JVI.77.20.11040-11049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosch B.J., van der Z.R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J., Xiao G., Xu Y., Yuan F., Zheng C., Liu Y. Following the rule: formation of the 6-helix bundle of the fusion core from severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein and identification of potent peptide inhibitors. Biochem. Biophys. Res. Commun. 2004;319:15–20. doi: 10.1016/j.bbrc.2004.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]


