Abstract
Purpose
Antithrombin III (AT III) is an anticoagulant with anti-inflammatory properties. We assessed the benefits and harms of AT III in critically ill patients.
Methods
We searched from inception to 27 August 2015 in CENTRAL, MEDLINE, EMBASE, CAB, BIOSIS and CINAHL. We included randomized controlled trials (RCTs) irrespective of publication status, date of publication, blinding status, outcomes published or language.
Results
We included 30 RCTs with a total of 3933 participants. The majority of included trials were at high risk of bias. Combining all trials, regardless of bias, showed no statistically significant effect of AT III on mortality (RR 0.95, 95 % CI 0.88–1.03, I 2 = 0 %, fixed-effect model, 29 trials, 3882 participants). Among those with severe sepsis and disseminated intravascular coagulation (DIC), AT III showed no impact on mortality (RR 0.95, 95 % Cl 0.88–1.03, I 2 = 0 %, fixed-effect model, 12 trials, 2858 participants). We carried out multiple subgroup and sensitivity analyses to assess the benefits and harms of AT III and to examine the impact of risk of bias. AT III significantly increased bleeding events (RR 1.58, 95 % CI 1.35–1.84, I 2 = 0 %, fixed-effect model, 11 trials, 3019 participants). However, for all other outcome measures and analyses, the results did not reach statistical significance.
Conclusions
There is insufficient evidence to support AT III substitution in any category of critically ill participants including those with sepsis and DIC. AT III did not show an impact on mortality, but increased the risk of bleeding.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-016-4225-7) contains supplementary material, which is available to authorized users.
Keywords: Antithrombin III, Bleeding, DIC, Multi organ failure, Sepsis, Septic shock
Introduction
Despite advances in the medical field, growing numbers of patients are becoming critically ill. Each year, 5,700,000 people in the USA are admitted to intensive care units (ICUs) [1].
Critical illness is characterized by cellular immune dysfunction, vascular damage and uncontrolled hyperinflammation, even when the cause of illness is not infection. In critical illness, a systemic activation of coagulation may occur which, at its worst, results in a fulminant disseminated intravascular coagulation (DIC). DIC is characterized by simultaneous widespread microvascular thrombosis and profuse bleeding from various sites [2]. Sepsis resulting from a generalized inflammatory and procoagulant response to an infection is associated with a high risk of mortality. Twenty per cent of patients who develop severe sepsis will die during their hospitalization [3]. Septic shock is associated with the highest mortality, approaching 50 % [3]. This rate increases in the presence of circulatory shock despite aggressive antimicrobial therapy, adequate fluid resuscitation and optimal care [4], and it may reach as high as 70 % in patients with multiple organ dysfunction [5, 6].
Antithrombin III (AT III) is primarily a potent anticoagulant with independent anti-inflammatory properties. AT III irreversibly inhibits serine proteases (e.g. activated factor X and thrombin) in a one-to-one ratio, with the generation of protease–AT III complexes. Heparin prevents AT III from interacting with the endothelial cell surface by binding to sites on the AT III molecule, competing for the AT III binding site, and reducing AT III’s ability to interact with its cellular receptor. AT III’s anticoagulant effect is thus greatly accelerated (by a factor of 1000) by heparin; heparin reduces AT III’s anti-inflammatory properties, weakens vascular protection and increases bleeding events [7–9]
Heparin in patients with sepsis, septic shock or DIC associated with infection may be associated with decreased mortality [10]. However, the overall effect is still not clear. Major bleeding events related to heparin administration cannot be excluded [10] and safety outcomes have yet to be validated in a multicentre trial setting.
The objective of this review was to examine the effect of AT III on mortality in critically ill participants and the benefits and harms of AT III. We investigated complications specific and not specific to the trial intervention, bleeding events, the effect on sepsis and DIC and the length of stay in ICU and in hospital in general.
Methods
This systematic review was carried out in accordance with Cochrane Collaboration methodology, PRISMA and GRADE guidelines [11–13]. This publication is an update of the existing Cochrane review with a preapproved and published protocol [14]. For more detailed description of the search, methods, types of studies, participants, interventions, outcome measures, data collection, selection of studies, data extraction, primary and secondary outcomes, see Supplement.
Statistics
We used Review Manager 5 software [15] to calculate risk ratios (RRs) with 95 % confidence intervals (CIs) for dichotomous variables and mean difference (MD) with CI for continuous outcomes. We used the Χ 2 test to provide an indication of heterogeneity between studies, while the degree of heterogeneity observed in the results was quantified using the I 2 statistic. We used trial sequential analysis (TSA) to examine the impact of type 1 errors due to sparse data and repeated significance testing following updates with new trials [16]. For more detailed information on statistical analyses and data management, assessment of risk of bias, subgroup and sensitivity analyses, assessment of heterogeneity, assessment of reporting biases, and data synthesis including TSA, see Supplement.
Results
Through electronic searches (Supplement Table 1) and from reading the references of potentially relevant articles, we identified 11,287 publications (Fig. 1). After reading the abstracts, we could directly exclude 11,173 publications. We retrieved 65 relevant publications for further assessment. We included 30 trials [17–46] (Table 1), which randomized a total of 3933 participants. One trial [21] was only published as an abstract and the data were so inadequate that they could not be used for further processing. Our analyses include a total of 3882 participants. The sample size varied from 16 to 2314 participants. We excluded 24 publications for the reasons detailed in Supplement Table 2. We found one ongoing trial [47] but no data were provided for this trial.
Fig. 1.
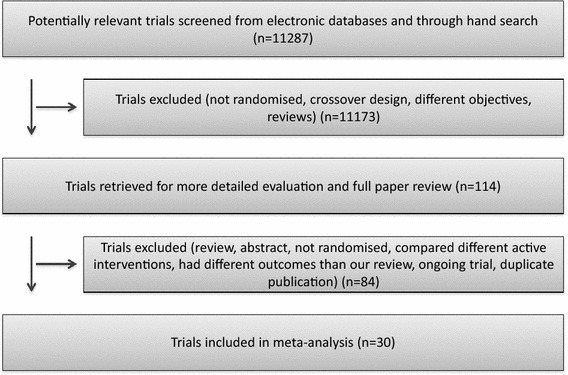
Flow chart in accordance with The Cochrane Collaboration guidelines. Three authors (FR, MA and AA) independently examined all potential primary studies and decided on their inclusion in the review
Table 1.
Characteristics of the included trials
| Trial | Number of participants | Population/trial description | Intervention/characteristics | Heparin (both groups) | Follow-up (days) | ITT/bias risk |
|---|---|---|---|---|---|---|
| Albert et al. [17] | 32 | ICU patients with <70 % AT III level, primary endpoint: mortality. No sample size calculation | Infusion twice daily as long as AT III <90 %. Total amount: 3500–17,000 IU. AT III before treatment unknown. No placebo | Yes | 90 | No/high |
| Balk et al. [18] | 34 | Adults patients with sepsis, cared for in a tertiary care centre. Trial only published as an abstract | AT III (Kybernin) and placebo. No details stated. Use of heparin not stated. Control: no placebo. First dose: 2000–4000 IU, total amount: 3500–17,000 IU. Control: no placebo | NA | 28 | No/high |
| Baudo et al. [19] | 29 | Liver transplantation in cirrhotic patients. Primary outcome: coagulatory variables and bleeding. No sample size calculation | Substitution preoperatively until AT III >100 %, infusion 1000 IU/h during entire surgery. Total amount: 1000–4000 IU before surgery, 1740–12,000 IU during surgery. No placebo | No | NA | Yes/high |
| Baudo et al. [20] | 120 | ICU patients with <70 % AT III. Primary outcomes: mortality, 30 days survival, MOF score, FFP, and PC requirements. Sample size calculated | Fixed dose of 4000 AT III and 2000 IU/12 h, 5 days. Total amount: 24,000 IU. AT III (%) before treatment: 52.9 (SD 14.5) IU in placebo group, 52.8 (15.5) IU in AT III group. Placebo: albumin | No | 30 | No/low |
| Blauhut et al. [21] | 51 | Patients with DIC and septic shock. AT III activity <70 % and at least 3 of the following: platelet count <100, thrombin time >24 s, thrombin coagulase time >22 s, fibrinogen level <150 mg/dl, ethanol gelatin test positive | Group 1: Heparin: iv heparin 3000 IU followed by continuous infusion of 250 IU/h. Intervention group 1: AT III substitution with the aim to keep AT III activity constantly around 100 %. Intervention group 2: same AT III substitution as group 1 with heparin 1000 IU iv and continuous infusion of 100 IU/h | NA | NA | No/high |
| Diaz-Cremades et al. [22] | 36 | ICU patients with <70 % AT III, no DIC. Primary outcome: mortality. No sample size calculation | Initial dose 60 U/kg + 10 U/kg every 6 h. Total amount: 11.165 (SD 5.980) IU. AT III (%) before treatment: 48.3 (SD 12.2) IU in placebo group: 52 (11.7) IU in AT III group. Placebo: albumin | Yes | NA | No/high |
| Eisele et al. [23] | 42 | Septic and critically ill patients. Primary outcome: 30 days mortality. No sample size calculation | Loading dose: 3000 IU + 1500 IU/12 h, 5 days. Total amount: 18,000 IU. AT III (%) before treatment: 49.0 (SD 19.1) IU in placebo group, 45.7 (14.4) in AT III group. Placebo: unknown | No | 30 | Yes/high |
| Fourrier et al. [24] | 35 | Critically ill with septic shock and DIC. Primary outcome: 28 days mortality. Sample size calculated | Loading dose over 3 h (3 ml/kg) + 3 ml/kg over 21 h, then 90–120 IU/kg/day for 3 days. Total amount: average 6000 IU. AT III (%) before treatment: 44 (SD 16) IU in placebo group, 52 (20) IU in AT III group. Placebo: albumin | No | 28 | Yes/low |
| Fulia et al. [25] | 60 | Infants, gestational age <30 weeks, <40 % AT III. Primary outcome: mortality and intraventricular haemorrhage. No sample size calculation | Loading dose: 2 ml/kg (100 U/kg), then 1 ml/kg (50 U/kg)/8 h for 48 h. Total amount: unknown. AT III (%) before treatment (mg/dl): 7.93 (SD 0.59) in placebo group, 8.22 (0.62) in AT III group. Placebo: glucose | Yes | 8 | Yes/low |
| Gando et al. [26] | 60 | DIC patients with sepsis and antithrombin levels of 50 to 80 %. Primary outcome: recovery from DIC on day 3. Secondary outcome: 28-day all-cause mortality | Immediately after the patients met the inclusion criteria, they were randomly assigned to either a group receiving antithrombin at a dose of 30 IU/kg (given over 60 min) per day for 3 days, or to the control group with no intervention | No | 28 | Yes/high |
| Grenander et al. [27] | 28 | Traumatic brain injury patients. Primary outcome: coagulatory variables, 90 days mortality. No sample size calculation outcome: coagulatory variables, 90 days mortality. No sample size calculation | 60 IU/kg initially. 20 IU/kg 8 and 16 h later, total 100 IU/kg during 24 h (adjusted to nearest 500 IU). Total dose: 8269 (SD 1562) IU. AT III (%) before treatment: 0.87 (0.12) in control, 1.06 (0.46) in AT III group. No placebo | Yes | 90 | No/high |
| Haire et al. [28] | 49 | Patients with malignant disease admitted for HSCT. Primary outcome: mortality, severity of illness score, length of hospital stay. Sample size calculated | 70 IU/kg <24 h of organ dysfunction detection + 50 IU/kg 8, 16, 48, and 72 h later. Mean total dose: 20,520 IU. No values of AT III before treatment. Placebo: albumin | No | 41 | Yes/low |
| Harper et al. [29] | 93 | ICU population with <70 % AT III. Primary outcome: mortality, coagulatory parameters. No sample size calculation | Aim: AT III >120. AT III until discharge from ICU, twice daily. No information on total amount or values before treatment. No placebo | No | 10 | Yes/high |
| Inthorn et al. [30] | 40 | Severe sepsis, ICU population. Primary outcome: 14 and 90 days mortality, hospital discharge. Sample size calculated | Continuous AT III infusion over 14 days. Aim: AT III >120 %. Total amount: 6000 IU on first day and 4000 IU on subsequent days. AT III (%) before treatment: 58 (SD 11) in control, 50.5 (3.2) in AT III group. No placebo | Yes | 90 | Yes/high |
| Kobayashi et al. [31] | 29 | Severe pre-eclamptic shock, gestational age 24–36 weeks. Primary outcome: mortality, week of delivery, coagulatory parameters, gestosis index improvement. Sample size calculated | 1500 U/day once daily for 7 consecutive days. Total amount: 10,500 IU. No values of AT III before treatment. Placebo: unknown | Yes | 90 | Yes/low |
| Langley et al. [32] | 25 | Hepatic coma with sepsis or risk of organ failure. Primary outcome: mortality, AT III activity. Sample size calculated | Initial dose: 3000 IU + 1000 IU/6 h unless normal AT III levels. Total amount: 3000–23,000 IU (mean 7000 (SD 5000 U). AT III (%) before treatment: 26 (4) in control, 26 (3) in AT III group. No placebo | No | 15 | Yes/high |
| Lavrentieva et al. [33] | 31 | Patients with severe burn injury. Primary outcome: Diagnosis of DIC. Secondary outcome: 28 days mortality. No sample size calculation | AT administration was started from the first post-burn day and continued for the next three consecutive days at a dose of 64.9 ± 11.4 U/kg/day | NA | 28 | No/high |
| Maki et al. [34] | 146 | Severe pre-eclampsia (gestational age 24–35 weeks). Primary outcomes: mortality, gestosis index improvement, IUGR, coagulatory parameters. No sample size calculation | 3000 IU once daily for 7 consecutive days. Total amount: 21,000 IU. AT III (%) before treatment: 82.3 (SD 19.4) in placebo group, 72.3 (25.7) in AT III group. Placebo: albumin | No | 60 | Yes/low |
| Mitchell et al. [35] | 85 | Children with ALL. Primary outcome: prevalence of thrombotic events, bleeding events. Sample size calculated | Once weekly for 4 weeks. Aim: AT III levels between 3.0–4.0 units/ml. No data on total amount or values of AT III before treatment. No placebo | Yes | 28 | No/low |
| Muntean and Rossegger [36] | 98 | Preterm neonates. Outcomes: Patients received artificial ventilation. Duration of artificial ventilation. Intraventricular haemorrhage and mortality. No sample size calculation | Intervention group: n = 45. Single bolus AT III immediately after birth. Birth weight under 1500 g was given 100 U, over 1500 g was given 200 U. Control group: n = 53, standard treatment. No details | NA | NA | No/high |
| Neporada et al. [37] | 43 | Patients with DIC and AT activity ≤70 %. Outcomes: respiratory function, organ failure assessment, DIC, JAAM score, bleeding complications, allergy and all-cause 30-day mortality | Intervention group 1: 15 patients received AT III 500–1000 IU/day. Control group: n = 15 received FFP (10–17 ml/kg). Intervention group 2 (initiated at a later stage): n = 13, same intervention as group 1 and control group combined | Yes | 30 | No/high |
| Nishiyama et al. [38] | 16 | Patients with DIC. Outcome: Mortality over 28 days. No sample size calculation | Intervention group: received as a 1500-unit infusion for 30 min/day for 5 days. Control group: received gabexate mesilate as a 2000-mg infusion for 24 h/day for 5 days | NA | 28 | No/high |
| Palareti et al. [39] | 59 | Sepsis and/or post surgical complications requiring haemodynamic or respiratory support. AT III <70 %. No sample size calculation | AT III: loading dose 4000 U followed by 2000 U/12 h by continuous dose over 5 days. Control: placebo (no details described) | NA | 7 | No/high |
| Schmidt et al. [40] | 122 | Premature infants with RDS in neonatal ICU. Primary outcome: mortality. Sample size calculated | Loading dose 100 U/kg followed by 50 U/kg every 6 h for 48 h. No data on total amount of AT III. AT III (%) before treatment: 32 (SD 8) in placebo group, 33 (8) in AT III group. Placebo: albumin | Yes | 90 | Yes/low |
| Schorr et al. [41] | 50 | Patients with secondary peritonitis, surgical population in ICU. Primary outcome: 90 days mortality. No sample size calculation |
AT III: continuous IV AT III and 2 intraperitoneal installations of fresh frozen serum A calculated bolus was given in 1 h followed by a continuous infusion of AT III (200–800 IU per hour) depending on the 6-hourly measurements of plasma AT III The first FFS was supplemented with 1500 IU AT III to equalize the lack of AT III in FFS. Mean AT III administered was 26,196 (±299 SEM) IU Control: No placebo |
No | 90 | Yes/high |
| Schuster et al. [42] | 45 | Patients with sepsis without DIC. Primary outcome: Mortality. Secondary outcomes: number of days at ICU, mechanical ventilation, organ function scores, side effects, organ failure and function, bleeding and transfusions, AT III activity |
Intervention group: allocated to AT III supplementation (loading dose of 3000 IU followed by 500 IU every 4 h for 7 days, total dose 17,000 IU) Control: placebo (albumin) |
Yes | NA | No/high |
| Smith-Erichsen et al. [43] | 83 | Critically ill and trauma patients in ICU. Primary outcome: plasma protease changes, mortality, days in ICU and hospital. No sample size calculation | Aim: AT III activity of 100 % (SD 10 %) for 3 consecutive days, max 14 days treatment. No data on total amount or values of AT III before treatment. No placebo | No | 34 | Yes/high |
| Vorobyeva et al. [44] | 38 | Patients with DIC. Primary outcome: Mortality. Other outcomes are not described in the paper. No sample size calculation |
Intervention group (AT III): (100 % minus measured AT III %) × kg body weight, 1000 IU/h, max 1500 IU/day Control group (FFP): 10–17 ml/kg, max 1000 ml/day |
No | NA | No/high |
| Warren et al. [45] | 2314 | Critically ill population of ICU patients with severe sepsis and septic shock. Primary outcome: all-cause mortality at 28 days (subgroup 28- and 90-day survival for patients not receiving heparin). Sample size calculation reported |
AT III: loading dose of 6000 IU given over 30 min, followed by a continuous iv infusion of 6000 IU per day for 4 days, total of 30,000 IU Control: equivalent volume of placebo solution (1 % of human albumin) |
Allowed, but not all received | 90 | Yes/low |
| Waydhas et al. [46] | 40 | Trauma patients in ICU. Primary outcome: incidence and severity of multiple organ dysfunction, mortality, incidence of respiratory failure, severity of organ failure, duration of mechanical ventilation and length of stay in the ICU and hospital. No sample size calculation |
AT III: a total of 20,000 IU (16,125–22,875), Additional AT III or placebo was substituted to keep the AT III concentration at 140 % of normal. In addition, on the next 2 days the test substance was administered once daily in the morning Control: placebo, 20 % human albumin in corresponding doses and volume |
Yes | NA | Yes/low |
ALL acute lymphatic leukaemia, APACHE acute physiology and chronic health evaluation, APGAR appearance, pulse, grimace, activity, and respiration, APTT activated partial thromboplastin time, ARDS acute respiratory distress syndrome, AT III antithrombin III, BP blood pressure, CABG coronary artery bypass graft, CI confidence interval, CNS central nervous system, COPD chronic obstructive pulmonary disease, CPP cerebral perfusion pressure, CRP C-reactive protein, DIC disseminated intravascular coagulation, DVT deep vein thrombosis, FFP fresh frozen plasma, GCS Glasgow coma scale, HELLP haemolysis, elevated liver enzymes, and low platelet count, HIV human immunodeficiency virus, ICP intracranial pressure, INR international normalized ratio, ISS injury severity score, ITT intention-to-treat, IU international unit, IUGR intrauterine growth retardation, JAAM Japanese Association for Acute Medicine, LMWH low molecular weight heparin, MODS multiorgan dysfunction syndrome, MOFS multiorgan failure score, MPI Mannheimer peritonitis index, NA no assessment, NSAID nonsteroidal anti-inflammatory drug, NYHA New York Heart Association functional classification, OFS organ failure score, OSFS organ system failure scoring, RDS respiratory distress syndrome, SAPS simplified acute physiology score, SBP systolic blood pressure, SD standard deviation, sys systolic, TAT thrombin–antithrombin complex, TISS therapeutic intervention scoring system, Tp temperature
We classified two trials as obstetric studies [31, 34], four trials as paediatric trials [25, 35, 36, 40] and a further two trials as trauma studies [27, 46]. The remaining trials consisted of mixed populations of critically ill participants, mainly with sepsis.
The duration of the intervention varied from less than 24 h to 4 weeks. Three trials had a median duration of AT III intervention that was longer than 1 week [30, 35, 43]. Follow-up ranged from 7 to 90 days.
The 30 included trials were published between 1985 and 2013. Five trials of AT III were multicentre trials [20, 23, 26, 35, 45]. Five trials of AT III were multinational trials including Germany, Belgium, the Netherlands, Canada and the USA [19, 23, 26, 35, 45]. One trial was carried out in 19 countries [45]. Two trials did not state the location [21, 39]. The 30 included trials involved a total of 3933 participants. The details of the included studies are provided in Table 1.
Fourteen trials recruited more male than female participants [20, 22–27, 30, 33, 35, 38, 43, 45, 46]. One trial recruited only men [19], two trials recruited only women [31, 34], six did not report the gender of the participants [21, 29, 37, 39, 42, 44] and two studies had more female than male participants [18, 28]. The age of the participants included extends from the premature infant to the elderly intensive care participant. It therefore makes little sense to calculate the average age of the participants included. One trial, however, excluded participants older than 75 [37].
Eighteen trials used an initial loading dose either based on weight (U/kg) or as a fixed dose [17, 19, 22–25, 27–30, 32, 39–45]. All trials except two [18, 21] stated the use of a maintenance dose. Nine trials used albumin as the control intervention [20, 22, 24, 28, 34, 40, 42, 45, 46], two trials used fresh frozen plasma as the control intervention [37, 44] and three trials only stated the use of an unknown placebo [18, 23, 31]. Nine trials used no placebo [17, 19, 27, 29, 30, 32, 35, 41, 43]. Four trials did not state which control they applied [20, 26, 33, 39].
Risk of bias in included studies
Allocation
Generation of allocation sequence was adequately reported in 15 trials [17, 20, 24–28, 31, 33–35, 37, 40, 45, 46] (Fig. 2). Allocation concealment was adequately reported in 14 trials [17, 20, 24–28, 31, 34, 35, 37, 40, 45, 46] (Fig. 2).
Fig. 2.
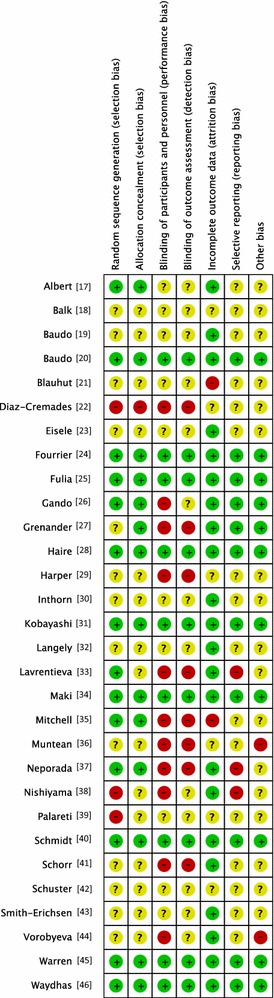
Risk of bias summary. All trials were evaluated for major potential sources across the various bias domains in accordance with The Cochrane Collaboration’s tool for risk of bias assessment
Blinding
Nine trials provided sufficient data to be categorized as double-blinded [20, 24, 25, 28, 31, 34, 40, 45, 46]. The remaining 21 trials were either open label or did not provide sufficient data on how the double-blinding was achieved (Fig. 2).
Incomplete outcome data
Two trials did not provide sufficient data (high risk) on follow-up [21, 35]. Six trials did not provide any data on follow-up (unclear risk) [18, 22, 29, 36, 39, 42]. Twenty-two trials had adequate follow-up (low risk) [17, 19, 20, 23–28, 30–34, 37, 38, 40, 41, 43–46] (Fig. 2).
Selective reporting
Ten trials provided adequate information to be classified as low-risk trials [20, 24–27, 31, 34, 40, 45, 46]. This was often due to supplementary information provided based on online registration, protocol availability or authors providing supplementary information while responding to our questions (Fig. 2).
Other potential sources of bias
Fourteen trials performed analysis according to the intention-to-treat (ITT) method or provided sufficient data for us to perform ITT analyses [19, 23–25, 28–32, 34, 40, 41, 45, 46]. Eight trials reported sample size calculations [20, 24, 28, 30, 31, 35, 40, 45]. Three trials [27, 32, 45] reported receiving pharmaceutical company funding (Fig. 2).
Overall quality of evidence
We rank the quality of findings from moderate to very low quality of evidence across the different outcomes. The main limiting factors were high risk of bias and small and poorly described trials.
Nine trials were reported as being at completely low risk of bias [20, 24, 25, 28, 31, 34, 40, 45, 46] (Fig. 2). Of 30 included trials, 16 were at high risk or unclear risk of bias in random sequence generation (selection bias) [18, 19, 21–23, 27, 29, 30, 32, 36, 38, 39, 41–44]. Only one trial was registered on an available trial database [26].
The five trials [18, 21, 36, 39, 42] only published as abstracts lack a great amount of valuable information with regard to methodology and outcomes, and we consequently rate them at high risk of bias.
Effects of primary outcomes
Combining all trials showed no statistically significant effect of AT III on mortality, with a risk ratio (RR) of 0.95 (95 % CI 0.88–1.03, I 2 statistic = 0 %, P value = 0.91), based on data from 3882 participants in 29 trials. Results were analysed using a fixed-effect model because heterogeneity was low. We downgraded the outcome from high to moderate quality of evidence because of 20 trials with high risk of bias. However, TSA led us to upgrade the overall assessment. Equally, for trials with only low risk of bias [20, 24, 25, 28, 31, 34, 40, 45, 46] we found no statistically significant effect, RR 0.96 (95 % CI 0.88–1.04, I 2 statistic = 0 %, fixed-effect model, 9 trials, 2915 participants). Trials with only high risk of bias [17–19, 22, 23, 26, 27, 29, 30, 32, 33, 35–39, 41–44] had a non-significant RR of 0.94 (95 % CI 0.77–1.14, I 2 statistic = 0 %, fixed-effect model, 20 trials, 967 participants) (Table 2; Fig. 3).
Table 2.
Main results. Subgroup and sensitivity analyses were conducted in regard to our primary outcome
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate | Heterogeneity |
|---|---|---|---|---|---|
| Mortality (subgroup analysis on bias risk) | 29 | 3882 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.03) | I 2 = 0 % |
| Trials with low bias risk | 9 | 2915 | Risk ratio (M–H, fixed, 95 % CI) | 0.96 (0.88 to 1.04) | I 2 = 0 % |
| Trials with high bias risk | 20 | 967 | Risk ratio (M–H, fixed, 95 % CI) | 0.94 (0.77 to 1.14) | I 2 = 0 % |
| Overall mortality (subgroup analysis on median follow-up) | 28 | 3848 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.03) | I 2 = 0 % |
| Mortality in trials with follow-up less than median of all trials | 18 | 1024 | Risk ratio (M–H, fixed, 95 % CI) | 0.93 (0.77 to 1.13) | I 2 = 0 % |
| Mortality in trials with follow-up longer than median of all trials | 10 | 2824 | Risk ratio (M–H, fixed, 95 % CI) | 0.96 (0.88 to 1.04) | I 2 = 0 % |
| Overall mortality (subgroup analysis on duration of intervention) | 28 | 3848 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.03) | I 2 = 0 % |
| Median duration of AT III intervention equal to or less than 1 week | 25 | 3640 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88, 1.03) | I 2 = 0 % |
| Median duration of AT III intervention longer than 1 week | 3 | 208 | Risk ratio (M–H, fixed, 95 % CI) | 0.89 (0.59 to 1.34) | I 2 = 0 % |
| Overall mortality (trauma) | 2 | 68 | Risk ratio (M–H, fixed, 95 % CI) | 2.15 (0.81 to 5.72) | I 2 = 0 % |
| Trials with high bias risk | 1 | 28 | Risk ratio (M–H, fixed, 95 % CI) | 3.43 (0.15 to 77.58) | Not applicable |
| Trials with low bias risk | 1 | 40 | Risk ratio (M–H, fixed, 95 % CI) | 2.00 (0.72 to 5.59) | Not applicable |
| Overall mortality (obstetrics) | 2 | 332 | Risk ratio (M–H, fixed, 95 % CI) | 1.03 (0.33 to 3.21) | I 2 = 0 % |
| Overall maternal mortality, trials with low bias risk | 2 | 174 | Risk ratio (M–H, fixed, 95 % CI) | Not estimable | Not applicable |
| Overall fetal and neonatal mortality, trials with low bias risk | 2 | 158 | Risk ratio (M–H, fixed, 95 % CI) | 1.03 (0.33 to 3.21) | I 2 = 0 % |
| Overall mortality (paediatrics) | 4 | 365 | Risk ratio (M–H, fixed, 95 % CI) | 1.44 (0.73 to 2.83) | I 2 = 0 % |
| Trials with low bias risk | 2 | 182 | Risk ratio (M–H, fixed, 95 % CI) | 1.60 (0.54 to 4.72) | I 2 = 21 % |
| Trials with high bias risk | 2 | 183 | Risk ratio (M–H, fixed, 95 % CI) | 1.32 (0.56 to 3.15) | Not applicable |
| Overall mortality (heparin, Warren et al. as a trial with adjuvant heparin therapy) | 26 | 3779 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.03) | I 2 = 0 % |
| Trials with complete or partially adjuvant heparin therapy | 16 | 3121 | Risk ratio (M–H, fixed, 95 % CI) | 0.96 (0.88 to 1.04) | I 2 = 0 % |
| Trials without adjuvant heparin | 10 | 658 | Risk ratio (M–H, fixed, 95 % CI) | 0.93 (0.71 to 1.23) | I 2 = 0 % |
| Overall mortality (heparin, Warren et al. as a trial without adjuvant heparin therapy) | 26 | 3779 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.03) | I 2 = 0 % |
| Trials with complete or partially adjuvant heparin therapy | 15 | 807 | Risk ratio (M–H, fixed, 95 % CI) | 0.96 (0.79 to 1.17) | I 2 = 0 % |
| Trials without adjuvant heparin | 11 | 2972 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.03) | I 2 = 0 % |
| Overall mortality (heparin, Warren et al. data split based on heparin administration) | 26 | 3779 | Risk ratio (M–H, random, 95 % CI) | 0.95 (0.88 to 1.02) | I 2 = 0 % |
| Trials with complete or partially adjuvant heparin therapy | 16 | 2423 | Risk ratio (M–H, random, 95 % CI) | 0.98 (0.90 to 1.08) | I 2 = 0 % |
| Trials without adjuvant heparin | 11 | 1356 | Risk ratio (M–H, random, 95 % CI) | 0.89 (0.78 to 1.01) | I 2 = 0 % |
| Overall mortality among patients with severe sepsis and DIC | 12 | 2858 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.03) | I 2 = 0 % |
| Trials with low bias risk | 4 | 2529 | Risk ratio (M–H, fixed, 95 % CI) | 0.95 (0.88 to 1.04) | I 2 = 0 % |
| Trials with high bias risk | 8 | 329 | Risk ratio (M–H, fixed, 95 % CI) | 0.87 (0.64 to 1.20) | I 2 = 0 % |
| Complications during the in-patient stay specific to the trial intervention | 3 | 2454 | Risk ratio (M–H, random, 95 % CI) | 1.26 (0.83 to 1.92) | I 2 = 9 % |
| Trials with low bias risk | 2 | 2429 | Risk ratio (M–H, random, 95 % CI) | 1.62 (0.96 to 2.73) | I 2 = 0 % |
| Trials with high bias risk | 1 | 25 | Risk ratio (M–H, random, 95 % CI) | 0.92 (0.51 to 1.66) | Not applicable |
| Complications during the in-patient stay not specific to the trial intervention | 2 | 65 | Risk ratio (M–H, random, 95 % CI) | 0.71 (0.08 to 6.11) | I 2 = 28 % |
| Trials with low bias risk | 1 | 40 | Risk ratio (M–H, random, 95 % CI) | 3.00 (0.13 to 69.52) | Not applicable |
| Trials with high bias risk | 1 | 25 | Risk ratio (M–H, random, 95 % CI) | 0.31 (0.04 to 2.57) | Not applicable |
| Complication specific to the trial intervention other than bleeding | 3 | 187 | Risk ratio (M–H, fixed, 95 % CI) | 0.72 (0.42 to 1.25) | I 2 = 0 % |
| Trials with low bias risk | 1 | 60 | Risk ratio (M–H, fixed, 95 % CI) | 0.75 (0.18 to 3.07) | Not applicable |
| Trials with high bias risk | 2 | 127 | Risk ratio (M–H, fixed, 95 % CI) | 0.72 (0.40 to 1.30) | I 2 = 0 % |
| Bleeding events | 11 | 3019 | Risk ratio (M–H, fixed, 95 % CI) | 1.58 (1.35 to 1.84) | I 2 = 0 % |
| Trials with low bias risk | 6 | 2791 | Risk ratio (M–H, fixed, 95 % CI) | 1.58 (1.35 to 1.85) | I 2 = 37 % |
| Trials with high bias risk | 5 | 228 | Risk ratio (M–H, fixed, 95 % CI) | 1.57 (0.71 to 3.49) | I 2 = 0 % |
| Amount of red blood cells administered | 4 | 137 | Mean difference (IV, random, 95 % CI) | 138.49 (−391.35 to 668.34) | I 2 = 88 % |
| Trials with low bias risk | 1 | 35 | Mean difference (IV, random, 95 % CI) | −600.00 (−899.18 to −300.82) | Not applicable |
| Trials with high bias risk | 3 | 102 | Mean difference (IV, random, 95 % CI) | 595.10 (−287.14 to 1477.34) | I 2 = 82 % |
| Incidence of surgical intervention | 3 | 103 | Risk ratio (M–H, fixed, 95 % CI) | 1.04 (0.85 to 1.27) | I 2 = 0 % |
| Trials with low bias risk | 3 | 103 | Risk ratio (M–H, fixed, 95 % CI) | 1.04 (0.85 to 1.27) | I 2 = 0 % |
| Severity of sepsis I | 3 | 156 | Mean difference (IV, random, 95 % CI) | −1.24 (−2.18 to −0.29) | I 2 = 48 % |
| Final MOF score among survivors, trials with low bias risk | 1 | 88 | Mean difference (IV, random, 95 % CI) | −0.70 (−1.22, to −0.18) | Not applicable |
| Final MOF score among survivors, trials with high bias risk | 2 | 68 | Mean difference (IV, Random, 95 % CI) | −1.92 (−3.05, to −0.78) | I 2 = 0 % |
| Severity of sepsis II | 3 | 102 | Mean difference (IV, fixed, 95 % CI) | −2.18 (−4.36 to −0.00) | I 2 = 0 % |
| Final APACHE I and II scores among survivors, trials with high bias risk | 3 | 102 | Mean difference (IV, fixed, 95 % CI) | −2.18 (−4.36 to −0.00) | I 2 = 0 % |
| Incidence of respiratory failure not present at admission | 6 | 2591 | Risk ratio (M–H, random, 95 % CI) | 0.93 (0.76 to 1.14) | I 2 = 32 % |
| Trials with low bias risk | 5 | 2564 | Risk ratio (M–H, random, 95 % CI) | 0.97 (0.77 to 1.22) | I 2 = 40 % |
| Trials with high bias risk | 1 | 27 | Risk ratio (M–H, random, 95 % CI) | 0.73 (0.45 to 1.18) | Not applicable |
| Duration of mechanical ventilation | 3 | 190 | Mean difference (IV, fixed, 95 % CI) | 2.20 (−1.21 to 5.60) | I 2 = 0 % |
| Trials with low bias risk | 2 | 162 | Mean difference (IV, fixed, 95 % CI) | 2.26 (−1.69 to 6.22) | I 2 = 0 % |
| Trials with high bias risk | 1 | 28 | Mean difference (IV, fixed, 95 % CI) | 2.00 (−4.68 to 8.68) | Not applicable |
| Length of stay in hospital | 4 | 202 | Mean difference (IV, random, 95 % CI) | 1.10 (−7.16 to 9.36) | I 2 = 74 % |
| Trials with low bias risk | 2 | 89 | Mean difference (IV, random, 95 % CI) | −5.67 (−16.24 to 4.90) | I 2 = 53 % |
| Trials with high bias risk | 2 | 113 | Mean difference (IV, random, 95 % CI) | 7.17 (2.75 to 11.59) | I 2 = 0 % |
| Mean length of stay in ICU | 7 | 376 | Mean difference (IV, fixed, 95 % CI) | 0.24 (−1.34 to 1.83) | I 2 = 0 % |
| Trials with low bias risk | 3 | 195 | Mean difference (IV, fixed, 95 % CI) | −0.73 (−3.41 to 1.95) | I 2 = 3 % |
| Trials with high bias risk | 4 | 181 | Mean difference (IV, fixed, 95 % CI) | 0.77 (−1.20 to 2.74) | I 2 = 0 % |
APACHE acute physiology and chronic health evaluation, CI confidence interval, ICU intensive care unit, M–H Mantel–Haenszel, MOFS multiorgan failure score
Fig. 3.
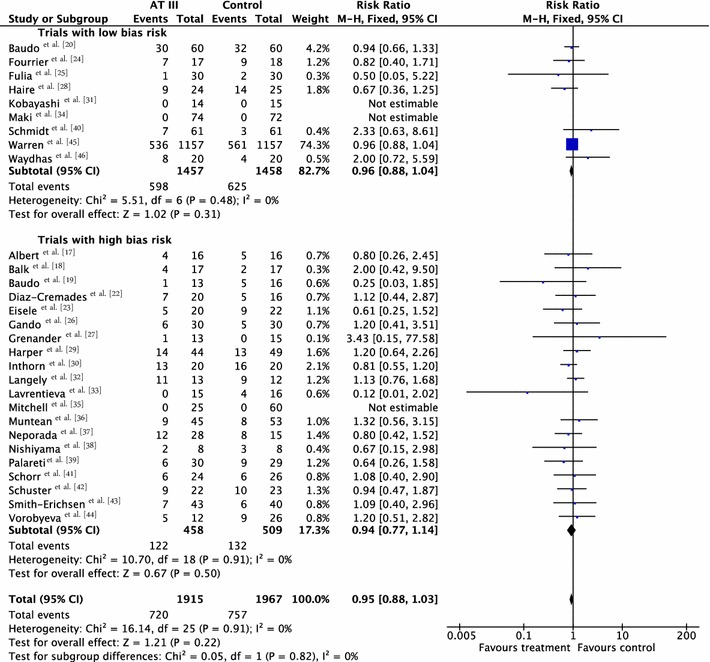
Forest plot of overall mortality with subgroup analysis based on the overall methodological quality of the included trials (trials with low risk of bias versus trials with high risk of bias). CI confidence interval, M–H Mantel–Haenszel
We conducted subgroup analyses (Table 2) and carried out 15 subgroup and sensitivity analyses in regard to our primary outcome (Table 2 and Supplement Table 3).
Effects of secondary outcomes
Two trials with low risk of bias [40, 45] and one trial with high risk of bias [32] demonstrated a statistically significant increase in complications specific to the trial intervention: RR 1.26 (95 % CI 0.83–1.92, I 2 statistic = 9 %, P value = 0.33), based on data from 2454 participants in the three trials (Table 2). We analysed results using a random-effects model. We downgraded the outcome from high to very low quality because of the small number of trials.
Two trials [32, 46] did not reach statistical significance assessing complications not specific to the trial intervention: RR 0.71 (95 % CI 0.08–6.11, I 2 statistic = 28 %, P value = 0.24) (Table 2), based on data from 65 participants. We analysed results using a random-effects model. We downgraded the outcome from high to very low quality because of the small number of trials.
Three trials, one with low risk of bias [25] and two with high risk of bias [23, 35], examined complications specific to the trial intervention other than bleeding: RR 0.72 (95 % CI 0.42–1.25, I 2 statistic = 0 %, P value = 0.95), based on data from 187 participants in the three trials (Table 2). We analysed results using a fixed-effect model. We downgraded the outcome from high to very low quality because of the small number of trials.
Six trials with low risk of bias [20, 25, 31, 34, 40, 45] and five with high risk of bias [26, 27, 32, 35, 37] demonstrated a statistically significant increase in bleeding events in the intervention group compared to the control group, with an RR of 1.58 (95 % CI 1.35–1.84, I 2 statistic = 0 %, P value = 0.57), based on data from 3019 participants in the 11 trials. We analysed results using a fixed-effect model (Table 2 and Supplement Fig. 1). We downgraded the outcome from high to moderate quality because of the proportion of trials with high risk of bias.
Four trials referred to the amount of red blood cells administered, one with low risk of bias [24] and three with high risk of bias [19, 21, 30] with a mean difference (MD) of 138.49 (95 % CI −391.35 to 668.34, I 2 statistic = 88 %, P value = 0.0001) (Table 2), based on data from 137 participants. We analysed results using a random-effects model (Table 2). We downgraded the outcome from high to very low quality because of the small number of trials, three of them with high risk of bias.
Three trials referred to the incidence of surgical intervention, all with low risk of bias [24, 31, 46] with an RR of 1.04 (95 % CI 0.85–1.27, I 2 statistic = 0 %, P value = 0.61), based on data from 103 participants. We analysed results using a fixed-effect model (Table 2). We downgraded the outcome from high to very low quality because of the small number of trials with few participants.
Only one analysis reached statistical significance, with an MD of −1.24 (95 % CI −2.18 to −0.29, I 2 statistic = 48 %, P value = 0.015, random-effects model, 3 trials, 156 participants) (Table 2) [20, 23, 30] when examining the effect of AT III on various illness scores (severity of sepsis). Six trials provided data [20, 22, 23, 28, 30, 41]. However, the trials that did provide data adequate for meta-analysis were quite heterogenous in their application of various scores and their choice of time points.
Six trials examined the effect of AT III on the incidence of respiratory failure (not present at admission) [23, 24, 31, 34, 45, 46]. There was no statistically significant difference, with an RR of 0.93 (95 % CI 0.76–1.14, I 2 statistic = 32 %, P value = 0.22) (Table 2), based on data from 2591 participants in six trials. We analysed results using a random-effects model. We downgraded the outcome from high to moderate quality because one trial contributed with the majority of patients [45]. It is considered to skew the finding; however, it is rated as low risk of bias and contributes a weighting of only 30.7 %. A sensitivity analysis removing it from the plot eliminates all heterogeneity in that subgroup.
Three trials examined the effect of the trial intervention on duration of mechanical ventilation [27, 40, 46]. There was no statistically significant difference, with an MD of 2.20 (95 % CI −1.21 to 5.60, I 2 statistic = 0 %, P value = 0.89), based on data from 190 participants (Table 2). We analysed results using a fixed-effect model. We downgraded the outcome from high to very low quality of evidence because of the small number of trials, few participants and imprecision of results with a wide confidence interval. The mean duration of mechanical ventilation in the intervention group was 2.2 days more.
Four trials examined the intervention effect on the length of stay in hospital [28, 37, 43, 46] with an MD of 1.10 (95 % CI −7.16 to 9.36, I 2 statistic = 74 %, P value = 0.009), based on data from 202 participants (Table 2). We analysed results using a random-effects model (Table 2). We downgraded the outcome from high to very low quality of evidence because of the small number of trials, few participants and imprecision of results with a wide confidence interval. The mean length of stay in hospital in the intervention group was 1.1 days more.
Three trials with low risk of bias [20, 24, 46] and four trials with high risk of bias [17, 22, 37, 43] examined the intervention effect on length of stay in the ICU. There was insufficient evidence to support any beneficial effect of the intervention, with an MD of 0.24 (95 % CI −1.34 to 1.83, I 2 statistic = 0 %, P value = 0.70), based on data from 376 participants. We analysed results using a fixed-effect model (Table 2). We downgraded the outcome from high to very low quality of evidence because of the small number of trials, most of them with high risk of bias. The mean length of stay in ICU in the intervention group was 0.24 days more.
Twelve trials examined the intervention effect on mortality among participants with severe sepsis and disseminated intravascular coagulation (DIC) [18, 20, 23, 24, 26, 30, 37–39, 41, 42, 45]. The trials did not demonstrate a statistically significant decrease in mortality in favour of the trial intervention: RR 0.95 (95 % CI 0.88–1.03, I 2 statistic = 0 %, P value = 0.98), based on data from 2858 participants. We analysed results using a fixed-effect model (Table 2; Fig. 4). We downgraded the outcome from high to very low quality of evidence, because of numerous trials with high risk of bias.
Fig. 4.
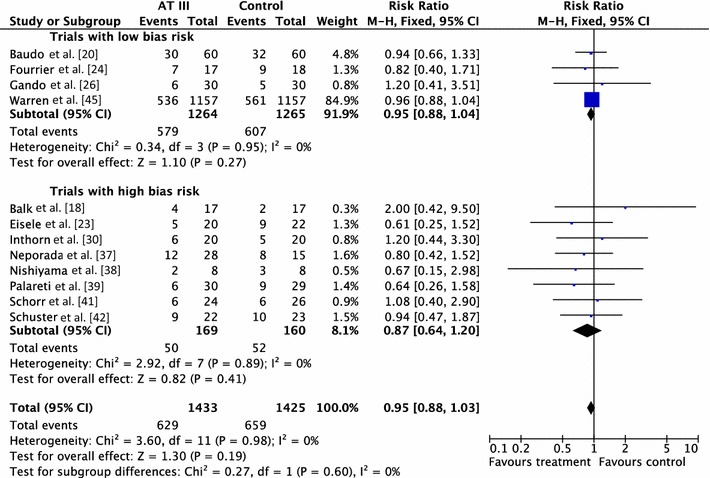
Forest plot of overall mortality among patients with severe sepsis and disseminated intravascular coagulation (DIC) with subgroup analysis based on the overall methodological quality of the included trials (trials with low risk of bias versus trials with high risk of bias). CI confidence interval, M–H Mantel–Haenszel
Only one trial examined the intervention’s effect on quality of life (Rublee et al. [9] based on data from Warren et al. [45]. There was an objective assessment of physical performance and dependency, and a subjective overall quality-of-life assessment analysis. Neither assessment supported intervention with AT III, with an MD of −2.00, (95 % CI −4.49 to 0.49, fixed-effect model, 897 participants) and an MD of −2.00, (95 % CI −5.01 to 1.01, fixed-effect model, 897 participants) respectively, both rated at very low quality (Table 2).
The heparin issue
A detrimental interaction between AT III and heparin was suspected before the Warren et al. trial [45] and we predefined use of AT III with and without heparin in the protocol for secondary analyses. However, the participants were not stratified according to heparin administration and the protocol allowed concomitant use of heparin by indication, after randomization to AT III or placebo. Even if the baseline comparison of participants allocated to AT III and placebo, in the subgroup without heparin, showed similar characteristics, the randomization is violated in the subgroup analysis.
Pooling all trials with and without concomitant use of heparin, with the Warren et al. trial [45] as either a trial with concomitant use of heparin or as a trial without use of heparin, does not provide evidence of a statistically significant intervention effect of AT III. Even when splitting the 39 trials into two ‘separate trials’, with and without concomitant use of heparin, and pooling these results with the other trials, we found no statistically significant intervention effect of AT III in the subgroup of trials without adjuvant heparin administration (RR 0.95, 95 % CI 0.88–1.03, I 2 statistic = 0 %, fixed-effects model (Table 2). However, splitting the Warren et al. trial [45] violates the randomization procedure.
Trial sequential analysis
We conducted TSA of AT III versus control on longest follow-up mortality (Fig. 3, Supplement Fig. 2). The TSA-adjusted confidence interval for the meta-analysis of the primary outcome with continuity correction for zero events trials (0.001 event in each arm) in a fixed-effect model results in an RR of 0.95 (95 % CI 0.88–1.03, I 2 statistic = 0 %, diversity D 2 = 0 %). The point estimate of the potential intervention effect as suggested by the low risk of bias trials in the meta-analysis of the effect of AT III on mortality is a relative risk reduction (RRR) of 5 % and the low-bias heterogeneity-adjusted information size (LBHIS) calculated based on this intervention effect (with 80 % power and alpha 0.05, assuming a double-sided type I risk of 5 % and a type II risk of 20 %) is 23,634 participants (Supplement Fig. 3). With an accrued information size of 3882 participants and no boundaries crossed so far, only 16.43 % of the required information size is actually available at this stage to reject or accept a 4 % RRR for overall mortality.
However, solid evidence may be obtained with fewer participants if eventually the cumulative meta-analysis Z curve crosses the trial sequential monitoring boundary constructed for a required information size of 23,634 randomized participants.
On the other hand, to demonstrate or reject an a priori anticipated intervention effect of an RRR of 10 %, 5037 should be randomized. In this analysis, the cumulative Z curve breaks through the boundary for futility (non-superiority) (Fig. 5). As 3882 participants are included in the present meta-analyses on mortality without the meta-analysis becoming statistically significant and since the futility boundary is crossed, an intervention effect of 10 % RRR or more on mortality is unlikely.
Fig. 5.
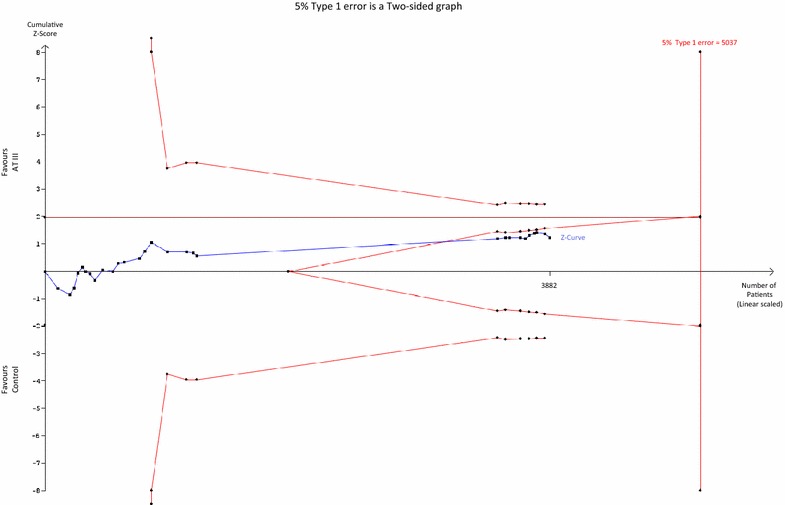
Trial sequential analysis (TSA) of all trials of the effect of AT III on mortality. Cumulative Z curve in blue does not cross the boundary constructed for an information size of 5037 in the meta-analysis (full red line with diamonds) with a relative risk reduction (RRR) of 10 % (α = 0.05) and a power of 80 % (β = 0.20). However, the cumulative Z curve breaks through the boundary for futility (non-superiority). The analysis therefor led to rejection of an intervention effect of an RRR of 10 % with a power of 80 % in 30 randomized trials with a total number of accrued participants of 3882
When carrying out the same TSA analyses as above for trials of sepsis and DIC only (Fig. 4) with an anticipated RRR of 10 %, the required information size is 3794 participants without the meta-analysis becoming statistically significant, and with the boundary for futility being crossed, thus indicating that an RRR of 10 % is to be rejected.
TSA analysis based on a potential RRR of 5 % as indicated by the meta-analysis for studies on sepsis and DIC (Fig. 5) yields an LBHIS of 21,657 participants and with an accrued information size of 2992 participants and no boundaries being crossed so far, only 13.82 % of the required information size is actually available (Supplement Fig. 4).
Discussion
In this systematic review of 30 trials with 3933 participants we found no significant beneficial effect of AT III on mortality (Fig. 3).
On the basis of follow-up less than or longer than the median of all trials, we undertook a subgroup analysis to examine the intervention effect on mortality. However, there was no statistically significant association between follow-up and mortality. The median follow-up time was 32 days.
We also examined the intervention effect based on the median duration of intervention being less than or longer than 1 week. Only three trials with a total of 208 participants had a median duration of intervention longer than 1 week [30, 35, 43]. The current evidence does not support a longer duration of intervention.
Additionally, on the basis of the existing data, we have to conclude that there is insufficient data to help us support or refute the use of AT III intervention among trauma, obstetric or paediatric populations.
Very few trials met our requirements in terms of trial intervention effect on various illness scores (severity of sepsis). We accepted the various definitions provided by the authors and undertook four different meta-analyses. The participant numbers in these analyses ranged from 28 to 156, and only one meta-analysis reached statistical significance. The meta-analyses examining the overall mortality in the septic population, based on 2918 participants, also failed to demonstrate a statistically significant reduction of mortality.
We examined a potential detrimental interaction of AT III with heparin by carrying out three separate analyses (Table 2) pooling mortality data from trials with concomitant heparin use against those without, while examining the impact of data from Warren et al. [45]. The latter trial was either defined as a trial with or without heparin use and finally we chose to split data from Warren et al. [45] in order to examine the hypothesis. As such, this is to be considered a post hoc analysis violating the randomization procedure. However, none of these analyses demonstrated any statistically significant interaction effects.
Our systematic review has several potential limitations. As for all systematic reviews, our findings and interpretations are limited by the quality and quantity of the available evidence on the effects of AT III on mortality. We assessed the risk of bias of the included trials by using the published data, which ultimately may not reflect the truth. We tried to contact all authors but only a few responded and provided further information. Three trials with 260 participants reported zero mortality in both trial groups [31, 34, 35].
We included five trials submitted only as abstracts in this updated review [18, 21, 36, 39, 42]. These abstracts lack important information. Nevertheless, a sensitivity analysis on the mortality data of only these trials yielded an RR of 0.91 (95 % CI 0.55–1.52, I 2 statistic = 0 %, fixed-effect model).
As a result of a lack of convincing evidence in favour of AT III in settings without heparin, we chose to implement TSA results, since the hypothesis of a beneficial effect of AT III in critically ill people still generates much attention.
Although there was minimal heterogeneity among trial results on mortality, we are aware that we pooled very heterogeneous trials in terms of participants, settings and treatment regimens. However, all the included conditions cause low levels of AT III, can result in DIC and have similar inflammatory pathways. We therefore think that there is a biologically plausible reason to perform an inclusive meta-analysis, which also considerably increases the generalizability and usefulness of the review. Furthermore, a broad meta-analysis increases power, reduces the risk of erroneous conclusions and facilitates exploratory analyses which can generate hypotheses for future research (e.g. adjuvant heparin) [48].
We have adhered to Cochrane methodology and applied additional statistical methods, such as TSA, to strengthen our conclusions and reduce the risk of random error.
Conclusions
There is insufficient evidence to support AT III substitution in any category of critically ill people. We did not find a statistically significant effect of AT III on mortality, but AT III increased the risk of bleeding events. Subgroup analyses performed according to duration of intervention, length of follow-up, different patient groups and use of adjuvant heparin did not show differences in the estimates of intervention effects. Serious methodological shortcomings of the included studies are, however, likely to have influenced the overall intervention effect (Supplement Table 4)
TSA showed that there is sufficient evidence to reject a beneficial effect of more than 10 % RRR (4 % absolute risk reduction) on overall mortality and for trials including participants with sepsis and DIC. There also remains the possibility that the use of AT III may be harmful.
The GRADE approach only reaffirmed our interpretation of the level of evidence, and we are confident that at this stage the quality of evidence in regard to our primary outcomes is moderate, despite the fact that many of the trials have high risk of bias.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
Mikkel Allingstrup, Frederikke B. Ravn, Ann Merete Møller and Arash Afshari declare that there are no conflicts of interest. Jørn Wetterslev declares that he is a member of the task force on TSA at Copenhagen Trial Unit developing and programming TSA.
Footnotes
Take-home message: There is insufficient evidence to support Antithrombin III substitution in any category of critically ill people. We did not find a statistically significant effect of Antithrombin III on mortality, but the use increased the risk of bleeding.
This review is an abridged version of a Cochrane Review previously published in the Cochrane Database of Systematic Reviews: Allingstrup M, Wetterslev J, Ravn FB, Møller A , Afshari A. Antithrombin III for critically ill patients. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No.: CD005370. DOI:10.1002/14651858.CD005370.pub3 (see http://www.cochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
References
- 1.Wunsch H, Angus D, Harrison D, et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36:2787–2793. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, De Jonge E, Van der Poll T. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Ann Med. 2004;36:41–49. doi: 10.1080/07853890310017251. [DOI] [PubMed] [Google Scholar]
- 3.Mayr F, Yende S, Angus D. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Periti P. Current treatment of sepsis and endotoxaemia. Expert Opin Pharmacother. 2000;1:1203–1217. doi: 10.1517/14656566.1.6.1203. [DOI] [PubMed] [Google Scholar]
- 5.Polderman KH, Girbes ARJ. Drug intervention trials in sepsis: divergent results. Lancet. 2004;363:1721–1723. doi: 10.1016/S0140-6736(04)16259-4. [DOI] [PubMed] [Google Scholar]
- 6.Becker BF, Heindl B, Kupatt C, Zahler S. Endothelial function and hemostasis. Z Kardiol. 2000;89:160–167. doi: 10.1007/pl00007320. [DOI] [PubMed] [Google Scholar]
- 7.Diaz R, Moffett BS, Karabinas S, et al. Antithrombin concentrate use in children receiving unfractionated heparin for acute thrombosis. J Pediatr. 2015;167:645–649. doi: 10.1016/j.jpeds.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Opal SM, Kessler CM, Roemisch J, Knaub S. Antithrombin, heparin, and heparan sulfate. Crit Care Med. 2002;30:325–331. doi: 10.1097/00003246-200205001-00024. [DOI] [PubMed] [Google Scholar]
- 9.Rublee D, Opal SM, Schramm W, et al. Quality of life effects of antithrombin III in sepsis survivors: results from the KyberSept trial. Crit Care. 2002;6:349–356. doi: 10.1186/cc1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarychanski R, Abou-Setta AM, Kanji S, et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43:511–518. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration. http://www.cochrane-handbook.org. Accessed March 2011
- 14.Afshari A, Wetterslev J, Brok J, Møller A. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2008;16(3):5370. doi: 10.1002/14651858.CD005370.pub2. [DOI] [PubMed] [Google Scholar]
- 15.The Cochrane Collaboration (2014) Review Manager (RevMan), version 5.3.5. The Nordic Cochrane Centre, Copenhagen
- 16.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C (2011) User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, pp 1–115. http://www.ctu.dk/tsa/files/tsa_manual.pdf
- 17.Albert J, Blomqvist H, Gardlund B, et al. Effect of antithrombin concentrate on haemostatic variables in critically ill patients. Acta Anaesthesiol Scand. 1992;36:745–752. doi: 10.1111/j.1399-6576.1992.tb03557.x. [DOI] [PubMed] [Google Scholar]
- 18.Balk RA, Bedrosian C, McCormick L, et al. Prospective double blind placebo-controlled trial of ATIII substitution in sepsis. Intensive Care Med. 1995;21:17. [Google Scholar]
- 19.Baudo F, DeGasperi A, De Cataldo F, et al. Antithrombin III supplementation during orthotopic liver transplantation in cirrhotic patients: a randomized trial. Thromb Res. 1992;68:409–416. doi: 10.1016/0049-3848(92)90099-V. [DOI] [PubMed] [Google Scholar]
- 20.Baudo F, Caimi TM, De Cataldo F, et al. Antithrombin III (ATIII) replacement therapy in patients with severe sepsis and/or postsurgical complications: a controlled double-blind, randomized, multicenter study. Intensive Care Med. 1998;24:336–342. doi: 10.1007/s001340050576. [DOI] [PubMed] [Google Scholar]
- 21.Blauhut B, Kramar H, Vinazzer H, Bergmann H. Substitution of antithrombin III in shock and DIC: a randomized study. Thromb Res. 1985;39:81–89. doi: 10.1016/0049-3848(85)90123-9. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Cremades JM, Lorenzo R, Sánchez M, et al. Use of antithrombin III in critical patients. Intensive Care Med. 1994;20:577–580. doi: 10.1007/BF01705725. [DOI] [PubMed] [Google Scholar]
- 23.Eisele B, Lamy M, Thijs LG, et al. Antithrombin III in patients with severe sepsis. A randomized, placebo-controlled, double-blind multicenter trial plus a meta-analysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis. Intensive Care Med. 1998;24:663–672. doi: 10.1007/s001340050642. [DOI] [PubMed] [Google Scholar]
- 24.Fourrier F, Chopin C, Huart JJ, et al. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest. 1993;104:882–888. doi: 10.1378/chest.104.3.882. [DOI] [PubMed] [Google Scholar]
- 25.Fulia F, Cordaro S, Meo P, et al. Can the administration of antithrombin III decrease the risk of cerebral hemorrhage in premature infants. Biol Neonate. 2003;83:1–5. doi: 10.1159/000067005. [DOI] [PubMed] [Google Scholar]
- 26.Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17:297. doi: 10.1186/cc13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grenander A, Bredbacka S, Rydvall A, et al. Antithrombin treatment in patients with traumatic brain injury: a pilot study. J Neurosurg Anesthesiol. 2001;13:49–56. doi: 10.1097/00008506-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Haire WD, Ruby EI, Stephens LC, et al. A prospective randomized double-blind trial of antithrombin III concentrate in the treatment of multiple-organ dysfunction syndrome during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 1998;4:142–150. doi: 10.1016/S1083-8791(98)50003-1. [DOI] [PubMed] [Google Scholar]
- 29.Harper PL, Williamson L, Park G, et al. A pilot study of antithrombin replacement in intensive care management: the effects on mortality, coagulation and renal function. Transfus Med. 1991;1:121–128. doi: 10.1111/j.1365-3148.1991.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 30.Inthorn D, Hoffmann JN, Hartl WH, et al. Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction. Shock. 1997;8:328–334. doi: 10.1097/00024382-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Terao T, Ikenoue T, et al. Treatment of severe preeclampsia with antithrombin concentrate: results of a prospective feasibility study. Semin Thromb Hemost. 2003;29:645–652. doi: 10.1055/s-2004-815632. [DOI] [PubMed] [Google Scholar]
- 32.Langley PG, Hughes RD, Forbes A, et al. Controlled trial of antithrombin III supplementation in fulminant hepatic failure. J Hepatol. 1993;17:326–331. doi: 10.1016/S0168-8278(05)80213-2. [DOI] [PubMed] [Google Scholar]
- 33.Lavrentieva A, Kontakiotis T, Bitzani M, et al. The efficacy of antithrombin administration in the acute phase of burn injury. Thromb Haemost. 2008;100:286–290. [PubMed] [Google Scholar]
- 34.Maki M, Kobayashi T, Terao T, et al. Antithrombin therapy for severe preeclampsia: results of a double-blind, randomized, placebo-controlled trial. Thromb Haemost. 2000;84:583–590. [PubMed] [Google Scholar]
- 35.Mitchell L, Andrew M, Hanna K, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thromb Haemost. 2003;90:235–244. doi: 10.1160/TH02-11-0283. [DOI] [PubMed] [Google Scholar]
- 36.Muntean W, Rossegger H. Antithrombin III concentrate in preterm infants with IRDS: an open, controlled, randomized clinical trial (abstract) Thromb Haemost. 1989;62:288. [Google Scholar]
- 37.Neporada E, Vorobyeva N, Nedashkovsky E. Antithrombin III deficiency correction in patients with disseminated intravascular coagulation. Obshaya Reanimatol. 2008;5:49–54. doi: 10.15360/1813-9779-2008-5-49. [DOI] [Google Scholar]
- 38.Nishiyama T, Kohno Y, Koishi K. Effects of antithrombin and gabexate mesilate on disseminated intravascular coagulation: a preliminary study. Am J Emerg Med. 2012;30:1219–1223. doi: 10.1016/j.ajem.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Palareti G, Legnani C, Coccheri S, et al. Laboratory effects of antithrombin-III (ATIII) replacement in patients with sepsis and or postsurgical complications requiring hemodynamic and or respiratory support—a controlled, double-blind, randomized multicenter study. Thromb Haemost. 1995;73:1251. [Google Scholar]
- 40.Schmidt B, Gillie P, Mitchell L, et al. A placebo-controlled randomized trial of antithrombin therapy in neonatal respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:470–476. doi: 10.1164/ajrccm.158.2.9712116. [DOI] [PubMed] [Google Scholar]
- 41.Schorr M, Siebeck M, Zugel N, et al. Antithrombin III and local serum application: adjuvant therapy in peritonitis. Eur J Clin Invest. 2000;30:359–366. doi: 10.1046/j.1365-2362.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 42.Schuster HP, Eisele B, Keinecke HO, et al. S-AT III study: antithrombin III in patients with sepsis. Intensive Care Med. 1997;23(Suppl 1):76. doi: 10.1007/s001340050642. [DOI] [PubMed] [Google Scholar]
- 43.Smith-Erichsen N, Aasen AO, Kongsgaard UE, et al. The effect of antithrombin III substitution therapy on components of the plasma protease systems in surgical patients. Clin Intensive Care. 1996;7:291–296. doi: 10.3109/tcic.7.6.291.296. [DOI] [Google Scholar]
- 44.Vorobyeva N, Neporada E, Turundaevskaia O, Mel’nikova G. Place of antithrombin III concentrate in the intensive care of disseminated intravascular coagulation. Anesteziol Reanimatol. 2007;2:42–44. [PubMed] [Google Scholar]
- 45.Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis, a randomized controlled trial. JAMA. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 46.Waydhas C, Nast-Kolb D, Gippner-Steppert C, et al. High-dose antithrombin III treatment of severely injured patients: results of a prospective study. J Trauma. 1998;45:931–940. doi: 10.1097/00005373-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 47.D’Angelo A, Valsecchi L (2015) High-dose antithrombin supplementation in early pre-eclampsia: a double-blind, placebo controlled study (on behalf of the AT III-EPAS study group). http://abstract.mci-group.com/cgi-bin/mc/printabs.pl?APP=ISTH2009ABS-abstract&TEMPLATE=&keyf=0771&showHide=show&client. Accessed 21 April 2015
- 48.Gotzsche PC. Why we need a broad perspective on meta-analysis. It may be crucially important for patients. BMJ. 2000;321:585–586. doi: 10.1136/bmj.321.7261.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


