Abstract
Acute and precise signal perception and transduction are essential for plant defense against insects. Insect elicitors—that is, the biologically active molecules from insects’ oral secretion (which contains regurgitant and saliva), frass, ovipositional fluids, and the endosymbionts—are recognized by plants and subsequently induce a local or systematic defense response. On the other hand, insects secrete various types of effectors to interfere with plant defense at multiple levels for better adaptation. Jasmonate is a main regulator involved in plant defense against insects and integrates with multiple pathways to make up the intricate defense network. Jasmonate signaling is strictly regulated in plants to avoid the hypersensitive defense response and seems to be vulnerable to assault by insect effectors at the same time. Here, we summarize recently identified elicitors, effectors, and their target proteins in plants and discuss their underlying molecular mechanisms.
Keywords: Plant defense, Insect herbivory, Jasmonate (JA), Elicitor, Effector
Introduction
There are about 1 million insects and over 300,000 plants on our planet, and plant–insect interactions are the driving force of biodiversity. With long-term co-evolution, plants and insects have developed sophisticated mechanisms for adaptation 1. In general, plants can recognize herbivore-/damage-/microbe-associated molecular patterns (HAMPs/DAMPs/MAMPs) and make the right defense. The early defense responses contain depolarization of the plasma transmembrane potential, changes of cytosolic Ca 2+, reactive oxygen species (ROS) burst, and mitogen-activated protein kinase (MAPK) 2, 3. Most of these reactions are able to activate jasmonate (JA)-mediated plant defense 4, 5. JA is a main regulator of plant defense and its synthesis and regulation have been extensively studied 6– 9. Recent studies reveal new insights in JA oxidative metabolism and their negative regulation in the JA pathway 10, 11. In most plants, JA-Ile is the active signal recognized by the COI1 and promotes JAZ–COI1 interaction leading to JAZ degradation. This relieves the JAZ-interacting transcription factors to activate downstream defense gene expressions 12– 16. However, in Marchantia polymorpha, MpCOI1 recognized OPDA-Ile instead of JA-Ile. That work revealed the ligand-receptor co-evolution of the JA signaling pathway in land plants 17. MYC2 is a well-studied transcription factor in JA signaling and can interact with both JAZ and MED25, the subunit of the mediator complex. The JAZ proteins recruit TOPLESS scaffold protein to inhibit gene transcription, whereas MED25 brings COI1 to MYC2 targeting promoters 18. In this model, COI1 is thought to be the nuclear receptor. JAT1, which localizes at the nuclear envelope and plasma membrane, is the transporter responsible for the influx of JA-Ile into nucleus 19. To balance the tradeoff between growth and defense, plants strictly regulate JA signaling to avoid a hypersensitive defense response 20, 21. Some development regulators, including SPLs and DELLAs, target JAZ or MYC transcription factors to modulate JA signaling output 22– 26. Interestingly, some insects use similar strategies to attenuate plant defense for fitness.
Herbivorous insects have different mouthparts and feeding habits. Active molecules from insects’ oral secretion (OS) (which contains regurgitant and saliva), frass, ovipositional fluids, and the endosymbionts of insects have a large impact on plant defense. Some of these molecules used by plants to trigger specialized defense are called elicitors, and those to weaken the plant defense response are defined as effectors. Plant–insect recognition is the first and also the key step of an effective defense in plants 27, 28. In this review, we discuss recent research advances in insect elicitors and effectors and their roles in plant–insect interactions.
Plant perceptions of insect herbivory
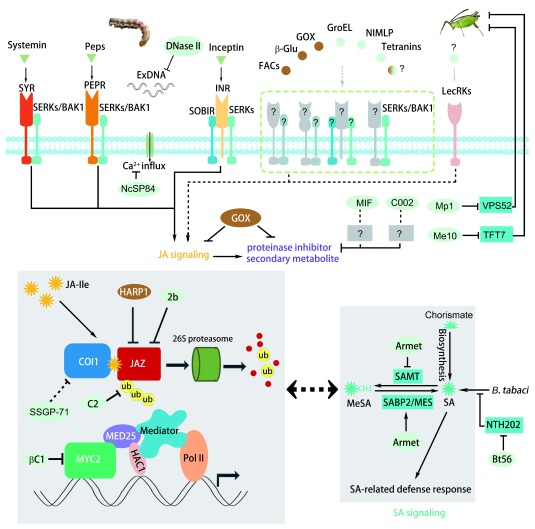
Plant perception of an insect attack is the first step of defense. Insect herbivory raised diverse active molecules such as damage-associated molecules, insect-derived elicitors, and the plant endogenous molecules activated by insect digestive enzymes ( Figure 1). The specific and efficient recognition of these active molecules guarantees the timely priming of plant defense 29, 30.
Figure 1. Schematic diagram of herbivory-associated elicitors and effectors manipulating plant defense.
Receptors (SYR, PEPR, INR, and LecRKs) located on the plant cell surface recognize small peptides (sytemine, inceptin, and Peps) and, together with the co-receptors (SERKs/BAK1 and SOBIR1), trigger downstream defense signaling. Also, elicitors derived from insects, including FACs, β-Glu, and GOX, are able to activate plant defense with the unknown mechanisms. On the other hand, insects secrete effectors to weaken plant defenses. Some effectors interfere with jasmonate (JA) signaling directly (HARP1, 2b, C2, βC1, and SSGP-71) or indirectly (Armet and Bt56) by enhancing salicylic acid (SA) accumulation to compromise JA signaling. Some effectors (Mp1 and Me10) target plant proteins (VPS52 and TFT7) that are directly involved in defense. The DNase II eliminates the extracellular DNA which is released by damaged cells to trigger plant defense. MIF and C002 from aphids are of benefit to insects living on the host plants, but the underlying mechanisms remain elusive. Notably, some elicitors/effectors are plant-specific. Here, the GOX from Helicoverpa zea acts as an effector, inhibiting nicotine accumulation in tobacco, and, on the other hand, acts as an elicitor specifically inducing plant response in tomato.
Plant-derived signal molecules activated by herbivory
Wounding damage caused by insect herbivory will quickly trigger plant defense signaling. The first reported damage-related peptide signal was systemin, an 18–amino acid polypeptide cleaved from prosystemin (inactive form) in tomato upon wounding stimulus 31. Systemin promotes JA accumulation and activates the expression of genes encoding proteinase inhibitors which have insecticide activity 32. Whereas systemin had been reported long before, its receptor SYR1, a leucine-rich repeat receptor kinase (LRR-RK), was identified recently. The introgression line, which lacks SYR1 expression, is highly sensitive to Spodoptera littoralis 33. Besides systemin, other wound-induced peptides had been identified in plants, including Arabidopsis, maize, and rice. The application of synthetic 23–amino acid maize Peps could mimic the Spodoptera exigua attack, and similar Peps were found in rice recently 34, 35. In Arabidopsis, AtPeps, which is generated from PROPEPs under the catalyzation of the cysteine protease METACASPASE4 (MC4), acts as signals to trigger both JA and SA signaling pathways 36, 37. Like the systemin-SYR1 module, the reported receptors of AtPeps—AtPEPR1 and AtPEPR2—are also classified in the LRR-RK family 38, 39.
From Spodoptera frugiperda larval OSs, researchers isolated a disulfide-bridged peptide (+ICDINGVCVDA−), termed inceptin, that can induce the accumulation of defense hormones such as ethylene, JA, and SA in cowpea plants. Inceptin is the proteolytic fragment of chloroplastic ATP synthase γ-subunit of cowpea plants digested by S. frugiperda larvae 40, 41. Recently, on BioRxiv, it was reported that the receptor of inceptin in plants was a leucine-rich repeat receptor-like protein, INR, which is distinguished from LRR-RKs by lacking an intracellular kinase domain 42. These findings expand the paradigm of plant surface recognition of insect herbivory.
Elicitors secreted by insects
Besides plant signal molecules activated by insect feeding, a number of reported elicitors are derived from insects themselves and most of them belong to HAMPs 43. It had been reported that the OS, the oviposition and the honeydew of insects could induce a plant defense response, including the accumulation of JA and secondary metabolism 44. These insect-derived elicitors can be classified as fatty acid derivatives, enzymes, and some other proteins 43.
The first identified fatty acid–amino acid conjugate (FAC) elicitor was volicitin, which was isolated from S. exigua larval OSs. Volicitin can induce the emission of volatiles in maize to attract predators 45. After volicitin, other forms of FACs from various insect OSs had been found in succession 46, 47. In Nicotiana attenuate, FACs from Manduca sexta activate the MAPK pathway 48. Besides FACs, califerins, the sulfooxy fatty acids that exist in OSs of grasshopper ( Schistocerca americana) larvae, also have elicitor activity 49. Glucose oxidases (GOXs) and β-glucosidase are enzyme-like elicitors. GOX is identified from Helicoverpa zea and specifically activates defense response in tomato 50, 51. The β-glucosidase in Pieris brassicae larval OSs triggers the emission of volatiles from wounded cabbage leaves and this attracts predators such as parasitic wasp 52, 53. Lipase and phospholipase C are other types of salivary enzyme-like elicitors. Lipase of Schistocerca gregaria OS elevates oxylipin accumulation and defense response in Arabidopsis 54. Phospholipase C of Spodoptera frugiperda induces the accumulation of proteinase inhibitors in corn 55.
The above-mentioned elicitors are from chewing insects. The elicitors from the piercing-sucking insects are isolated largely from salivary glands. The mucin-like salivary protein (NlMLP) of planthopper ( Nilaparvata lugens) is a double-edged sword. On one hand, it contributes to the formation of salivary sheaths for successful feeding; on the other hand, it was used by plants to trigger a defense response, like Ca 2+ mobilization, the MEK2 MAPK cascades, and JA signaling transduction, thereby reducing the performance of N. lugens 56. Tetranins is another characterized elicitor identified from Tetranychus urticae. Tetranins increases the expression of defense genes and activates JA, salicylic acid (SA), and abscisic acid biosynthesis in plant. It also promotes volatile emission to attract predatory mites 57.
Some elicitors are from endosymbionts. MAMPs could be released through herbivory OSs and recognized by plants to induce pattern-triggered immunity (PTI) 58, 59. The chaperon GroEL from the endosymbionts Buchnera of potato aphids ( Macrosiphum euphorbiae) induces oxidative bursts and PTI in Arabidopsis 60. From the S. littoralis larval OSs, the porin-like proteins most likely of bacterial origin can induce the early response of plant defense 61. A recent report reveals that some elicitors are from honeydew-associated microbes in sucking arthropods 62.
Insect effectors twist plant defense
To adapt to their host plants, insects have developed multilayered means for fitness. Besides releasing elicitors, the insect releases effectors that disturb host plant defense response for successful feeding 63. The reported insect effectors are identified from both the herbivory itself and insect-related microbiomes ( Figure 1).
The first reported insect effector was GOX from the chewing insect, H. zea, which inhibits nicotine accumulation and elevates the SA-mediated PR-1a protein level in tobacco 64, 65. Notably, the same GOX protein induces plant response in tomato 50, 51, which we discussed in the ‘Elicitors secreted by insects’ section. This suggests that the same protein acts as the effector or as the elicitor depending on their interacted host plant. Another piece of evidence in support of insect effectors is that the S. littoralis larvae that fed on OS pretreated plants had a greater weight increase 66.
The direct interaction with JA signaling-related components is an efficient way for herbivory effectors to inhibit plant defense. In our recent work, we isolated a venom-like protein termed HARP1, which is identified from the OS of Helicoverpa armigera. HARP1 can interact with multiple JAZ proteins of Arabidopsis and cotton plants to prevent COI1-mediated JAZ degradation, thereby blocking the JA signaling output 67. SSGP-71 is an E3 ubiquitin ligase–mimicking protein in Hessian fly ( Mayetiola destructor). It allows the insect to hijack the plant proteasome and block the basal immunity 68. These studies fill in the gap of the working mechanism about how insects manipulate effectors to block plant defense for better adaptation.
Some insect effectors inhibit plant defense by interfering with the crosstalk between SA and JA. For example, Bt56 from the whitefly ( Bemisia tabaci) enhanced the performance of the whitefly on tobacco by decreasing JA signaling through the antagonism between JA and SA. Bt56 could directly interact with KNOTTED 1-like homeobox transcription factor NTH202 and eliminate the negative modulation of NTH202 on SA accumulation 69. Armet, the effector of pea aphid ( Acyrthosiphon pisum) protein, induced SA accumulation by blocking SA methylation and enhanced the pathogen resistance in plants, reflecting a novel tripartite interaction of insect–plant–pathogen 70, 71.
The extracellular DNA and hydrogen peroxide that are released by damaged cells can trigger plant defense 30. Therefore, some insects secrete effectors to eliminate the production of these DAMPs. The planthopper ( Laodelphax striatellus) secretes salivary DNase II, which acts as an effector by erasing extracellular DNA, and the Trichoplusia ni salivary catalase functions as an ROS scavenger to reduce hydrogen peroxide, thus inhibiting ROS burst and other plant defense responses 72, 73.
Moreover, some effectors were reported to target other defense-related proteins in plants. A set of saliva proteins in aphids were proven to have effector activity through proteomic combined RNA sequencing (RNA-seq) analysis 63, 74– 79. A macrophage migration inhibitory factor (MIF) from pea aphid saliva inhibits immune response in N. benthamiana and improves aphid performance. Interestingly, the MIFs in vertebrates are also involved in the immune pathway, suggesting the highly conserved function of MIF 80, 81. Vacuolar protein sorting-associated protein 52 (VPS52) in potato ( Solanum tuberosum) has negative impacts on green peach aphid ( Myzus persicae) infection. M. persicae saliva-secreted protein Mp1 targets the VPS52 as an effective virulence strategy 77, 82. Me10 from M. euphorbiae interacts with tomato TFT7, a 14-3-3 isoform involved in aphid resistance, and enhances aphid longevity and fecundity 83. Some effectors can target the host cell wall. Expansin-like protein (HaEXPB2) from the nematode ( Heterodera avenae) binds to cellulose of tobacco, thereby increasing nematode infectivity 84.
The effectors mentioned above are generated from the insect itself. Other effectors are also derived from insect-borne microbe. Although the exact effector components need to be explored, it was found that Colorado potato beetle ( Leptinotarsa decemlineata) larvae suppress tomato defense response by exploiting bacteria in their OSs and gut 85, 86. Besides bacteria, some active molecules from vector-borne pathogens are reported to interfere with plant defense and are of benefit for their insect vectors living on host plants 87. The phytoplasm protein SAP11 and SAP54 of aster yellows phytoplasma strain witches’ broom was proposed to promote aphid colonization and also interfere with plant development 88– 90. The βC1 of tomato yellow leaf curl China virus directly interacts with MYC2 protein to decrease the MYC2-regulated terpene synthase, thereby reducing plant resistance to the whitefly 91. The 2b protein of the aphid-borne cucumber mosaic virus (CMV) stabilizes JAZ proteins by direct interaction, thus blocking JA signaling output, and this benefits aphid ( M. persicae) performance on the host plant 92. The C2 protein of tomato yellow leaf curl virus can also compromise JA signaling in tobacco by interacting with plant ubiquitin to block JAZ1 protein degradation, thereby reducing plant resistance to the insect vector whitefly 93. These studies reveal the intricate interaction of plant–virus–insect vector. In Table 1, we summarize the reported insect-associated elicitors and effectors from different species and their probable roles.
Table 1. Herbivory-associated elicitors and effectors.
| Name | Origin | Biofunction | References | ||
|---|---|---|---|---|---|
| Elicitors | Plant-derived | Systemin | Wounded tomato plants | Perceived by SYR1, induce accumulation
of proteinase inhibitor and ethylene, and induce oxidative bursts |
31, 33 |
| PEPs | Wounded plants (
Arabidopsis,
maize, rice) |
Induce defensin and burst of hydrogen
peroxide (H 2O 2) after perceiving by PEPRs |
36, 38, 39 | ||
| Inceptin | Degradation of cowpea ATP
synthase by Spodoptera frugiperda during herbivory |
Increase the concentration of JA and SA
by interacting with INR |
40, 42 | ||
| Derived from
insect |
Volicitin | Spodoptera exigua | Induce volatiles emission in corn | 45 | |
| Caeliferins | Schistocerca americana | 49 | |||
| GOX | Helicoverpa zea, Ostrinia nubilalis | Specifically promote defense response
in tomato |
50, 51 | ||
| β-glucosidase | Pieris brassicae | Increase volatile emission in cabbage | 52 | ||
| Lipase | Schistocerca gregaria | Elevate the oxylipins accumulation in
Arabidopsis |
54 | ||
| Phospholipase C | S. frugiperda | Trigger proteinase inhibitors
accumulation in corn |
55 | ||
| Bruchins | Bruchus pisorum | Induce neoplasms formation beneath the
insect egg in pea |
94, 95 | ||
| NlMLP | Nilaparvata lugens | Induce plant defense response in rice | 56 | ||
| Tetranins | Tetranychus urticae | Cytosolic calcium influx and membrane
depolarization induce biosynthesis of JA. SA and ABA in kidney bean |
57 | ||
| GroEL |
Buchnera in
Macrosiphum
euphorbiae |
Induce PTI and ROS accumulation in
Arabidopsis |
60 | ||
| Porin-like proteins | Bacteria in Spodoptera littoralis | Trigger membrane potential changes and
cytosolic Ca2 + elevations in Arabidopsis and Vicia faba |
61 | ||
| Unidentified | Gut-associated bacteria in H. zea | Increase salivary GOX to induce defense
in tomato |
96, 97 | ||
| Unidentified | Honeydew-associated microbes
N. lugens |
Induce accumulation of phytoalexins and
volatile emission in rice |
62, 98 | ||
| Effectors | Insect-derived | GOX | H. zea | Decrease nicotine accumulation in
tobacco |
64 |
| HARP1 | Helicoverpa armigera | Interact with and stabilize JAZs, depress
JA signaling in Arabidopsis |
67 | ||
| SSGP-71 | Mayetiola destructor | Interact with Skp, decrease plant
proteasome activity, thus block hormone signaling in wheat |
68 | ||
| Bt56 | Bemisia tabaci | Interact with NTH202 to increase SA
biosynthesis, thus decrease JA response in tobacco |
69 | ||
| Armet | Acyrthosiphon pisum | Help feeding of insect, induce SA
accumulation and pathogen response in N. benthamiana and Medicago truncatula |
70, 71 | ||
| DNase II | Laodelphax striatellus | Erase extracellular DNA released by
damaged cell in rice |
72 | ||
| Catalase | Trichoplusia ni | Reduce H 2O 2 in tomato | 73 | ||
| C002 | A. pisum, Myzus persicae | ApC002 and MpC002 help insect
foraging and feeding on fava bean and N. benthamiana, respectively |
63, 74 | ||
| MIF | A. pisum, M. persicae | Improve aphid performance, inhibit
immune response in N. benthamiana |
80 | ||
| Mp1 | M. persicae | Interact with VPS52 to relocalize to
vesicle-like structures and enhance insect virulence in Arabidopsis and potato |
82 | ||
| Me10 | M. euphorbiae | Interact with TFT7, enhance the longevity
and fecundity on tomato |
83 | ||
| Mp42, Mp55 Me23 |
M. persicae,
M. euphorbiae |
Increase aphid reproduction, suppress
N. benthamiana defenses |
99, 100 | ||
| HaEXPB2 | Heterodera avenae | Bind to cellulous and target cell wall
when parasitizing N. benthamiana |
84 | ||
| Phosphatase 2C | M. destructor | Interfere with the wheat signal
transduction pathway possibly by phosphatase ability |
101 | ||
| Endo-beta-1,4-
Glucanase (NIEG1) |
N. lugens | Degrade celluloses in plant cell wall,
enable insect stylet to reach the rice phloem |
102 | ||
| NcSP75 | Nephotettix cincticeps | Help successful ingestion from sieve
elements of rice |
103 | ||
| NcSP84 | N. cincticeps | Suppress accumulation of Ca2
+ and
H 2O 2 and sieve element clogging in rice |
104 | ||
| NlSEF1 | N. lugens | Help successful ingestion from sieve
elements of rice |
105 | ||
| Derived from
insect-borne microbe |
Unidentified | Gut and oral secretion–associated
bacteria in Colorado potato beetle |
Suppress tomato defense response
Bind and destabilize TCPs, reduce plant defense in Arabidopsis |
85, 86 | |
| SAP11 | Aster yellows witches’ broom in
Macrosteles quadrilineatus |
106 | |||
| SAP54 | Aster yellows witches’ broom in
M.
quadrilineatus |
Degrade MTFs through interacting with
RAD23, influence floral development in Arabidopsis |
90 | ||
| βC1 | Tomato yellow leaf curl China virus
in B. tabaci |
Interact with MYC2 and suppress
MYC2-regulated terpene synthesis in Arabidopsis |
91 | ||
| 2b | Cucumber mosaic virus (CMV) in
M. persicae |
Interact with and stable JAZ protein,
blocking JA signaling in Arabidopsis |
92 | ||
| C2 | Tomato yellow leaf curl virus in
B.
tabaci |
Interact with plant ubiquitin, blocking JA
signaling in tobacco |
93 | ||
Prospects
JA is a conserved defense regulator in the plant kingdom. On one hand, various elicitors can be recognized by plants to trigger JA signaling. On the other hand, the JA pathway tends to be targeted by a diverse range of attackers for fitness ( Figure 1). Some insect effectors have a mechanism similar to that of the virus proteins in blocking JA signaling 67, 91– 93. It would be interesting to study whether there are relationships between the phylogeny of insect effectors and viral proteins. Although numerous elicitors and effectors were identified, their target proteins, the underlying mechanisms, and the transportation mechanisms of the effectors entering plant cells are largely unknown and deserve further investigation. In plants, JA is integrated with multiple signaling to form a complex and flexible defense network. Recent research has revealed the intricate defense network shaped by insect herbivory 69, 107– 109. Studies have also shown that insects can use plant defense metabolites to find their host plants and to fend off predators 110, 111; this gives new insight into plant–insect interactions. Further investigations will greatly enrich our knowledge of the complex and flexible interactions between plants and insects and will also be helpful for breeding insect-proof crops 112, 113.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Gregg Howe, MSU-DOE Plant Research Laboratory, Michigan State University, East Lansing, MI, 48824, USA
Gary Felton, Department of Entomology, Pennsylvania State University, University Park, PA, USA
Funding Statement
This work was supported by Ministry of Science and Technology of China grant 2016YFA0500803, the Ministry of Agriculture of China grant 2016ZX08009001-009, National Natural Sciences of China grants 31772177 and 31788103, and Chinese Academy of Sciences grant QYZDY-SSW-SMC026.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Wu J, Baldwin IT: New insights into plant responses to the attack from insect herbivores. Annu Rev Genet. 2010;44:1–24. 10.1146/annurev-genet-102209-163500 [DOI] [PubMed] [Google Scholar]
- 2. Li J, Liu X, Wang Q, et al. : A Group D MAPK Protects Plants from Autotoxicity by Suppressing Herbivore-Induced Defense Signaling. Plant Physiol. 2019;179(4):1386–401. 10.1104/pp.18.01411 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Ye M, Glauser G, Lou Y, et al. : Molecular Dissection of Early Defense Signaling Underlying Volatile-Mediated Defense Regulation and Herbivore Resistance in Rice. Plant Cell. 2019;31(3):687–98. 10.1105/tpc.18.00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zebelo SA, Maffei ME: Role of early signalling events in plant-insect interactions. J Exp Bot. 2015;66(2):435–48. 10.1093/jxb/eru480 [DOI] [PubMed] [Google Scholar]
- 5. Erb M, Meldau S, Howe GA: Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012;17(5):250–9. 10.1016/j.tplants.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howe GA, Major IT, Koo AJ: Modularity in Jasmonate Signaling for Multistress Resilience. Annu Rev Plant Biol. 2018;69:387–415. 10.1146/annurev-arplant-042817-040047 [DOI] [PubMed] [Google Scholar]
- 7. Howe GA: Plant hormones: Metabolic end run to jasmonate. Nat Chem Biol. 2018;14(2):109–10. 10.1038/nchembio.2553 [DOI] [PubMed] [Google Scholar]
- 8. Huang H, Liu B, Liu L, et al. : Jasmonate action in plant growth and development. J Exp Bot. 2017;68(6):1349–59. 10.1093/jxb/erw495 [DOI] [PubMed] [Google Scholar]
- 9. Browse J: Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205. 10.1146/annurev.arplant.043008.092007 [DOI] [PubMed] [Google Scholar]
- 10. Smirnova E, Marquis V, Poirier L, et al. : Jasmonic Acid Oxidase 2 Hydroxylates Jasmonic Acid and Represses Basal Defense and Resistance Responses against Botrytis cinerea Infection. Mol Plant. 2017;10(9):1159–73. 10.1016/j.molp.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 11. Caarls L, Elberse J, Awwanah M, et al. : Arabidopsis JASMONATE-INDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc Natl Acad Sci U S A. 2017;114(24):6388–93. 10.1073/pnas.1701101114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Chini A, Gimenez-Ibanez S, Goossens A, et al. : Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol. 2016;33:147–56. 10.1016/j.pbi.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 13. Xie DX, Feys BF, James S, et al. : COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280(5366):1091–4. 10.1126/science.280.5366.1091 [DOI] [PubMed] [Google Scholar]
- 14. Sheard LB, Tan X, Mao H, et al. : Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468(7322):400–5. 10.1038/nature09430 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Chini A, Fonseca S, Fernández G, et al. : The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448(7154):666–71. 10.1038/nature06006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Thines B, Katsir L, Melotto M, et al. : JAZ repressor proteins are targets of the SCF COI1 complex during jasmonate signalling. Nature. 2007;448(7154):661–5. 10.1038/nature05960 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Monte I, Ishida S, Zamarreño AM, et al. : Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat Chem Biol. 2018;14(5):480–8. 10.1038/s41589-018-0033-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. An C, Li L, Zhai Q, et al. : Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc Natl Acad Sci U S A. 2017;114(42):E8930–E8939. 10.1073/pnas.1710885114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Q, Zheng J, Li S, et al. : Transporter-Mediated Nuclear Entry of Jasmonoyl-Isoleucine Is Essential for Jasmonate Signaling. Mol Plant. 2017;10(5):695–708. 10.1016/j.molp.2017.01.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Guo Q, Yoshida Y, Major IT, et al. : JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc Natl Acad Sci U S A. 2018;115(45):E10768–E10777. 10.1073/pnas.1811828115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Guo Q, Major IT, Howe GA: Resolution of growth-defense conflict: mechanistic insights from jasmonate signaling. Curr Opin Plant Biol. 2018;44:72–81. 10.1016/j.pbi.2018.02.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Hou X, Lee LY, Xia K, et al. : DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell. 2010;19(6):884–94. 10.1016/j.devcel.2010.10.024 [DOI] [PubMed] [Google Scholar]
- 23. Yang DL, Yao J, Mei CS, et al. : Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci U S A. 2012;109(19):E1192–200. 10.1073/pnas.1201616109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Hong GJ, Xue XY, Mao YB, et al. : Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell. 2012;24(6):2635–48. 10.1105/tpc.112.098749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wild M, Davière JM, Cheminant S, et al. : The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. Plant Cell. 2012;24(8):3307–19. 10.1105/tpc.112.101428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao YB, Liu YQ, Chen DY, et al. : Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat Commun. 2017;8: 13925. 10.1038/ncomms13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howe GA, Jander G: Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. 10.1146/annurev.arplant.59.032607.092825 [DOI] [PubMed] [Google Scholar]
- 28. Aljbory Z, Chen MS: Indirect plant defense against insect herbivores: a review. Insect Sci. 2018;25(1):2–23. 10.1111/1744-7917.12436 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Erb M, Reymond P: Molecular Interactions Between Plants and Insect Herbivores. Annu Rev Plant Biol. 2019;70:527–57. 10.1146/annurev-arplant-050718-095910 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Gust AA, Pruitt R, Nürnberger T: Sensing Danger: Key to Activating Plant Immunity. Trends Plant Sci. 2017;22(9):779–91. 10.1016/j.tplants.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 31. Pearce G, Strydom D, Johnson S, et al. : A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991;253(5022):895–7. 10.1126/science.253.5022.895 [DOI] [PubMed] [Google Scholar]
- 32. Orozco-Cardenas M, McGurl B, Ryan CA, et al. : Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc Natl Acad Sci U S A. 1993;90(17):8273–6. 10.1073/pnas.90.17.8273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Einig E, Almeida-Trapp M, et al. : The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat Plants. 2018;4(3):152–6. 10.1038/s41477-018-0106-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Shinya T, Yasuda S, Hyodo K, et al. : Integration of danger peptide signals with herbivore-associated molecular pattern signaling amplifies anti-herbivore defense responses in rice. Plant J. 2018;94(4):626–37. 10.1111/tpj.13883 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Huffaker A, Dafoe NJ, Schmelz EA: ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol. 2011;155(3):1325–38. 10.1104/pp.110.166710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huffaker A, Pearce G, Ryan CA: An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci U S A. 2006;103(26):10098–103. 10.1073/pnas.0603727103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hander T, Fernández-Fernández ÁD, Kumpf RP, et al. : Damage on plants activates Ca 2+-dependent metacaspases for release of immunomodulatory peptides. Science. 2019;363(6433): pii: eaar7486. 10.1126/science.aar7486 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Yamaguchi Y, Pearce G, Ryan CA: The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci U S A. 2006;103(26):10104–9. 10.1073/pnas.0603729103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamaguchi Y, Huffaker A, Bryan AC, et al. : PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22(2):508–22. 10.1105/tpc.109.068874 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Schmelz EA, Carroll MJ, LeClere S, et al. : Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci U S A. 2006;103(23):8894–9. 10.1073/pnas.0602328103 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Schmelz EA, LeClere S, Carroll MJ, et al. : Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol. 2007;144(2):793–805. 10.1104/pp.107.097154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steinbrenner AD, Muñoz-Amatriaín M, Aguilar Venegas JM, et al. : A receptor for herbivore-associated molecular patterns mediates plant immunity. bioRxiv. 2019;679803 10.1101/679803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonaventure G, VanDoorn A, Baldwin IT: Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011;16(6):294–9. 10.1016/j.tplants.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 44. Stam JM, Kroes A, Li Y, et al. : Plant interactions with multiple insect herbivores: from community to genes. Annu Rev Plant Biol. 2014;65:689–713. 10.1146/annurev-arplant-050213-035937 [DOI] [PubMed] [Google Scholar]
- 45. Alborn HT, Turlings TCJ, Jones TH, et al. : An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276(5314):945–9. 10.1126/science.276.5314.945 [DOI] [Google Scholar]
- 46. Halitschke R, Schittko U, Pohnert G, et al. : Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125(2):711–7. 10.1104/pp.125.2.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoshinaga N, Aboshi T, Ishikawa C, et al. : Fatty acid amides, previously identified in caterpillars, found in the cricket Teleogryllus taiwanemma and fruit fly Drosophila melanogaster larvae. J Chem Ecol. 2007;33(7):1376–81. 10.1007/s10886-007-9321-2 [DOI] [PubMed] [Google Scholar]
- 48. Wu J, Hettenhausen C, Meldau S, et al. : Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell. 2007;19(3):1096–122. 10.1105/tpc.106.049353 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Alborn HT, Hansen TV, Jones TH, et al. : Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci U S A. 2007;104(32):12976–81. 10.1073/pnas.0705947104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian D, Peiffer M, Shoemaker E, et al. : Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS One. 2012;7(4):e36168. 10.1371/journal.pone.0036168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Louis J, Peiffer M, Ray S, et al. : Host-specific salivary elicitor(s) of European corn borer induce defenses in tomato and maize. New Phytol. 2013;199(1):66–73. 10.1111/nph.12308 [DOI] [PubMed] [Google Scholar]
- 52. Mattiacci L, Dicke M, Posthumus MA: beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci U S A. 1995;92(6):2036–40. 10.1073/pnas.92.6.2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hopke J, Donath J, Blechert S, et al. : Herbivore-induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a beta-glucosidase and jasmonic acid. FEBS Lett. 1994;352(2):146–50. 10.1016/0014-5793(94)00948-1 [DOI] [PubMed] [Google Scholar]
- 54. Schäfer M, Fischer C, Meldau S, et al. : Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiol. 2011;156(3):1520–34. 10.1104/pp.111.173567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Acevedo FE, Peiffer M, Ray S, et al. : Intraspecific differences in plant defense induction by fall armyworm strains. New Phytol. 2018;218(1):310–21. 10.1111/nph.14981 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Shangguan X, Zhang J, Liu B, et al. : A Mucin-Like Protein of Planthopper Is Required for Feeding and Induces Immunity Response in Plants. Plant Physiol. 2018;176(1):552–65. 10.1104/pp.17.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Iida J, Desaki Y, Hata K, et al. : Tetranins: new putative spider mite elicitors of host plant defense. New Phytol. 2019;224(2):875–85. 10.1111/nph.15813 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Kaloshian I: Gene-for-gene disease resistance: bridging insect pest and pathogen defense. J Chem Ecol. 2004;30(12):2419–38. 10.1007/s10886-004-7943-1 [DOI] [PubMed] [Google Scholar]
- 59. Noman A, Aqeel M, Qasim M, et al. : Plant-insect-microbe interaction: A love triangle between enemies in ecosystem. Sci Total Environ. 2020;699:134181. 10.1016/j.scitotenv.2019.134181 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Chaudhary R, Atamian HS, Shen Z, et al. : GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc Natl Acad Sci U S A. 2014;111(24):8919–24. 10.1073/pnas.1407687111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guo H, Wielsch N, Hafke JB, et al. : A porin-like protein from oral secretions of Spodoptera littoralis larvae induces defense-related early events in plant leaves. Insect Biochem Mol Biol. 2013;43(9):849–58. 10.1016/j.ibmb.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 62. Wari D, Kabir MA, Mujiono K, et al. : Honeydew-associated microbes elicit defense responses against brown planthopper in rice. J Exp Bot. 2019;70(5):1683–96. 10.1093/jxb/erz041 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Bos JIB, Prince D, Pitino M, et al. : A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010;6(11):e1001216. 10.1371/journal.pgen.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Musser RO, Hum-Musser SM, Eichenseer H, et al. : Herbivory: caterpillar saliva beats plant defences. Nature. 2002;416(6881):599–600. 10.1038/416599a [DOI] [PubMed] [Google Scholar]
- 65. Musser RO, Cipollini DF, Hum-Musser SM, et al. : Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in solanaceous plants. Arch Insect Biochem Physiol. 2005;58(2):128–37. 10.1002/arch.20039 [DOI] [PubMed] [Google Scholar]
- 66. Consales F, Schweizer F, Erb M, et al. : Insect oral secretions suppress wound-induced responses in Arabidopsis. J Exp Bot. 2012;63(2):727–37. 10.1093/jxb/err308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen CY, Liu YQ, Song WM, et al. : An effector from cotton bollworm oral secretion impairs host plant defense signaling. Proc Natl Acad Sci U S A. 2019;116(28):14331–8. 10.1073/pnas.1905471116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao C, Escalante LN, Chen H, et al. : A massive expansion of effector genes underlies gall-formation in the wheat pest Mayetiola destructor. Curr Biol. 2015;25(5):613–20. 10.1016/j.cub.2014.12.057 [DOI] [PubMed] [Google Scholar]
- 69. Xu HX, Qian LX, Wang XW, et al. : A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc Natl Acad Sci U S A. 2019;116(2):490–5. 10.1073/pnas.1714990116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Cui N, Lu H, Wang T, et al. : Armet, an aphid effector protein, induces pathogen resistance in plants by promoting the accumulation of salicylic acid. Philos Trans R Soc Lond B Biol Sci. 2019;374(1767): 20180314. 10.1098/rstb.2018.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Wang W, Dai H, Zhang Y, et al. : Armet is an effector protein mediating aphid-plant interactions. FASEB J. 2015;29(5):2032–45. 10.1096/fj.14-266023 [DOI] [PubMed] [Google Scholar]
- 72. Huang HJ, Cui JR, Xia X, et al. : Salivary DNase II from Laodelphax striatellus acts as an effector that suppresses plant defence. New Phytol. 2019;224(2):860–74. 10.1111/nph.15792 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Rivera-Vega LJ, Stanley BA, Stanley A, et al. : Proteomic analysis of labial saliva of the generalist cabbage looper ( Trichoplusia ni) and its role in interactions with host plants. J Insect Physiol. 2018;107:97–103. 10.1016/j.jinsphys.2018.03.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Mutti NS, Louis J, Pappan LK, et al. : A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc Natl Acad Sci U S A. 2008;105(29):9965–9. 10.1073/pnas.0708958105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mutti NS, Park Y, Reese JC, et al. : RNAi knockdown of a salivary transcript leading to lethality in the pea aphid, Acyrthosiphon pisum. J Insect Sci. 2006;6:1–7. 10.1673/031.006.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pitino M, Coleman AD, Maffei ME, et al. : Silencing of aphid genes by dsRNA feeding from plants. PLoS One. 2011;6(10):e25709. 10.1371/journal.pone.0025709 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Pitino M, Hogenhout SA: Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol Plant Microbe Interact. 2013;26(1):130–9. 10.1094/MPMI-07-12-0172-FI [DOI] [PubMed] [Google Scholar]
- 78. Ollivier M, Legeai F, Rispe C: Comparative analysis of the Acyrthosiphon pisum genome and expressed sequence tag-based gene sets from other aphid species. Insect Mol Biol. 2010;19(Suppl 2):33–45. 10.1111/j.1365-2583.2009.00976.x [DOI] [PubMed] [Google Scholar]
- 79. Ji R, Wang Y, Cheng Y, et al. : Transcriptome Analysis of Green Peach Aphid ( Myzus persicae): Insight into Developmental Regulation and Inter-Species Divergence. Front Plant Sci. 2016;7:1562. 10.3389/fpls.2016.01562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Naessens E, Dubreuil G, Giordanengo P, et al. : A Secreted MIF Cytokine Enables Aphid Feeding and Represses Plant Immune Responses. Curr Biol. 2015;25(14):1898–903. 10.1016/j.cub.2015.05.047 [DOI] [PubMed] [Google Scholar]
- 81. Calandra T, Roger T: Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. 10.1038/nri1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rodriguez PA, Escudero-Martinez C, Bos JI: An Aphid Effector Targets Trafficking Protein VPS52 in a Host-Specific Manner to Promote Virulence. Plant Physiol. 2017;173(3):1892–903. 10.1104/pp.16.01458 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Chaudhary R, Peng HC, He J, et al. : Aphid effector Me10 interacts with tomato TFT7, a 14-3-3 isoform involved in aphid resistance. New Phytol. 2019;221(3):1518–28. 10.1111/nph.15475 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Liu J, Peng H, Cui J, et al. : Molecular Characterization of A Novel Effector Expansin-like Protein from Heterodera avenae that Induces Cell Death in Nicotiana benthamiana. Sci Rep. 2016;6: 35677. 10.1038/srep35677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chung SH, Rosa C, Scully ED, et al. : Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci U S A. 2013;110(39):15728–33. 10.1073/pnas.1308867110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Chung SH, Scully ED, Peiffer M, et al. : Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Sci Rep. 2017;7: 39690. 10.1038/srep39690 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Jiang Y, Zhang CX, Chen R, et al. : Challenging battles of plants with phloem-feeding insects and prokaryotic pathogens. Proc Natl Acad Sci U S A. 2019;116(47):23390–7. 10.1073/pnas.1915396116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Pecher P, Moro G, Canale MC, et al. : Phytoplasma SAP11 effector destabilization of TCP transcription factors differentially impact development and defence of Arabidopsis versus maize. PLoS Pathog. 2019;15(9):e1008035. 10.1371/journal.ppat.1008035 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Sugio A, Kingdom HN, MacLean AM, et al. : Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc Natl Acad Sci U S A. 2011;108(48):E1254–63. 10.1073/pnas.1105664108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. MacLean AM, Orlovskis Z, Kowitwanich K, et al. : Phytoplasma effector SAP54 hijacks plant reproduction by degrading MADS-box proteins and promotes insect colonization in a RAD23-dependent manner. PLoS Biol. 2014;12(4):e1001835. 10.1371/journal.pbio.1001835 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Li R, Weldegergis BT, Li J, et al. : Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell. 2014;26(12):4991–5008. 10.1105/tpc.114.133181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu D, Qi T, Li WX, et al. : Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res. 2017;27(3):402–15. 10.1038/cr.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Li P, Liu C, Deng WH, et al. : Plant begomoviruses subvert ubiquitination to suppress plant defenses against insect vectors. PLoS Pathog. 2019;15(2):e1007607. 10.1371/journal.ppat.1007607 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Hilker M, Meiners T: Early herbivore alert: insect eggs induce plant defense. J Chem Ecol. 2006;32(7):1379–97. 10.1007/s10886-006-9057-4 [DOI] [PubMed] [Google Scholar]
- 95. Hilker M, Fatouros NE: Resisting the onset of herbivore attack: plants perceive and respond to insect eggs. Curr Opin Plant Biol. 2016;32:9–16. 10.1016/j.pbi.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 96. Wang J, Peiffer M, Hoover K, et al. : Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New Phytol. 2017;214(3):1294–306. 10.1111/nph.14429 [DOI] [PubMed] [Google Scholar]
- 97. Wang J, Yang M, Song Y, et al. : Gut-Associated Bacteria of Helicoverpa zea Indirectly Trigger Plant Defenses in Maize. J Chem Ecol. 2018;44(7–8):690–9. 10.1007/s10886-018-0970-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Wari D, Alamgir KM, Mujiono K, et al. : Brown planthopper honeydew-associated symbiotic microbes elicit momilactones in rice. Plant Signal Behav. 2019;14(11):1655335. 10.1080/15592324.2019.1655335 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Elzinga DA, de Vos M, Jander G: Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol Plant Microbe Interact. 2014;27(7):747–56. 10.1094/MPMI-01-14-0018-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Atamian HS, Chaudhary R, Cin VD, et al. : In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol Plant Microbe Interact. 2013;26(1):67–74. 10.1094/MPMI-06-12-0144-FI [DOI] [PubMed] [Google Scholar]
- 101. Zhao C, Shukle R, Navarro-Escalante L, et al. : Avirulence gene mapping in the Hessian fly ( Mayetiola destructor) reveals a protein phosphatase 2C effector gene family. J Insect Physiol. 2016;84:22–31. 10.1016/j.jinsphys.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 102. Ji R, Ye W, Chen H, et al. : A Salivary Endo-β-1,4-Glucanase Acts as an Effector That Enables the Brown Planthopper to Feed on Rice. Plant Physiol. 2017;173(3):1920–32. 10.1104/pp.16.01493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Matsumoto Y, Hattori M: The green rice leafhopper, Nephotettix cincticeps (Hemiptera: Cicadellidae), salivary protein NcSP75 is a key effector for successful phloem ingestion. PLoS One. 2018;13(9):e0202492. 10.1371/journal.pone.0202492 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 104. Hattori M, Nakamura M, Komatsu S, et al. : Molecular cloning of a novel calcium-binding protein in the secreted saliva of the green rice leafhopper Nephotettix cincticeps. Insect Biochem Mol Biol. 2012;42(1):1–9. 10.1016/j.ibmb.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 105. Ye W, Yu H, Jian Y, et al. : A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci Rep. 2017;7:40498. 10.1038/srep40498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sugio A, MacLean AM, Hogenhout SA: The small phytoplasma virulence effector SAP11 contains distinct domains required for nuclear targeting and CIN-TCP binding and destabilization. New Phytol. 2014;202(3):838–48. 10.1111/nph.12721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ma F, Yang X, Shi Z, et al. : Novel crosstalk between ethylene- and jasmonic acid-pathway responses to a piercing-sucking insect in rice. New Phytol. 2019;225(1):474–87. 10.1111/nph.16111 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 108. Liu L, Sonbol FM, Huot B, et al. : Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat Commun. 2016;7:13099. 10.1038/ncomms13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li R, Llorca LC, Schuman MC, et al. : ZEITLUPE in the Roots of Wild Tobacco Regulates Jasmonate-Mediated Nicotine Biosynthesis and Resistance to a Generalist Herbivore. Plant Physiol. 2018;177(2):833–46. 10.1104/pp.18.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ye M, Veyrat N, Xu H, et al. : An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci Adv. 2018;4(5):eaar4767. 10.1126/sciadv.aar4767 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Hu L, Mateo P, Ye M, et al. : Plant iron acquisition strategy exploited by an insect herbivore. Science. 2018;361(6403):694–7. 10.1126/science.aat4082 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Zhao Y, Huang J, Wang Z, et al. : Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc Natl Acad Sci U S A. 2016;113(45):12850–5. 10.1073/pnas.1614862113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu Y, Wu H, Chen H, et al. : A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat Biotechnol. 2015;33(3):301–5. 10.1038/nbt.3069 [DOI] [PubMed] [Google Scholar]