Abstract
American Indian adults are at an increased risk for cardiovascular disease compared with non-Hispanic white adults. Scant research exists examining the underlying physiological and psychological mechanisms associated with these risks. This study aimed to examine possible psychological and physiological stress-related mechanisms related to cardiovascular disease risk in healthy American Indian and non-Hispanic white adults. Forty American Indian (60% female, Mean age = 19.93, SD = 2.08 years) and 45 non-Hispanic white (70% female, Mean age = 20.18, SD = 2.22 years) participants attended an in-person laboratory session. Salivary cortisol and cardiovascular activity were measured before (baseline), during, and after exposure to a 10-minute mental arithmetic task. Compared to non-Hispanic white participants, American Indian had diminished salivary cortisol (p < .001), blood pressure (p’s < .001), and heart rate (p = .041) responses to acute psychological stress. These effects could not be accounted for by differences in task performance or self-reported engagement. Previous research has shown that exaggerated responses to stress are associated with increased risk of cardiovascular disease. However, diminished responses to stress are associated with early childhood stress and future adverse behaviors (e.g., addiction, obesity). Diminished reactivity may influence behaviors that can impact future development of cardiovascular disease in American Indian populations.
Keywords: Acute psychological stress, cardiovascular activity, cortisol, American Indian
1. Introduction
The indigenous people of the United States, or American Indians (AIs) and Alaska Natives (ANs), are a diverse population, comprised of over 550 federally recognized tribal nations. Each of these tribal nations have distinct, cultures, communities, values and languages. As a people, both AIs and ANs suffered tremendously during the period of European colonialism because of disease, war, and genocide. In addition, the culture of the indigenous people was under further attack during the boarding school era, during which the federal government removed children from their homes to place them in boarding schools, with the goal of eradicating tribal languages and practices. As a result of these adversities, exposures and traumas, the population declined from an estimated 10 million people to fewer than a million people.
Since then, there has been a steady increase in population for both AIs and Ans. In the period from 1990-2009, while there was a significant decrease in all-cause mortality for Whites, this change was not observed for AI/AN persons (Espey et al., 2014). Public health issues have changed dramatically for this population. As recently as 40 years ago, the rates of the risk factors for cardiovascular disease were low in these communities. However, several recent studies have shown that incidence of cardiovascular disease (CVD), and its risk factors, including obesity and diabetes, are increasing in this growing population (Denny et al., 2003; Galloway, 2005; Jernigan et al., 2010).
Previous research aiming to elucidate physiological pathways that may contribute to or protect from cardiovascular disease have focused on the magnitude of individuals’ cardiovascular responses to acute psychological stress. The reactivity hypothesis proposes that large magnitude cardiovascular reactions to acute psychological stress may play a role in the development of cardiovascular disease (Chida and Steptoe, 2010; Light, 1981; Manuck et al., 1990). Cardiovascular reactivity in response to psychological stress, differs from cardiovascular responses to physical exertion. The latter are closely tied to the metabolic demands associated with physical exertion, while the former are not (Balanos et al.,2010; Carroll et al., 2009). Evidence from a number of cross-sectional and prospective investigations highlight a positive relationship between the magnitude of cardiovascular responses to acute psychological stress and future resting blood pressure and hypertension (Carroll et al., 2011; Carroll et al., 2003; Carroll et al., 1995; Carroll et al., 2001; Markovitz et al., 1998; Matthews et al., 1993; Newman et al., 1999). Further, individuals who show exaggerated cardiovascular responses to stress are more likely to develop atherosclerosis (Barnett et al., 1997) and are at increased risk for cardiovascular disease mortality (Carroll et al., 2012).
A separate body of literature indicates that diminished cardiovascular reactions to acute psychological stress are also associated with adverse health outcomes, the majority of which are behavioral (Carroll et al., 2017; Phillips et al., 2013). As an example, diminished cardiovascular reactivity has previously associated with reduced behavioral perseverance (Ginty et al., 2015) and higher levels of depressive symptomology (Brindle et al., 2013; Phillips et al., 2011; Schiweck et al., 2019). Diminished reactivity to stress has also been observed in individuals with type 2 diabetes (Steptoe et al., 2014). Interestingly, blunted cardiovascular reactivity has also been related to adverse cardiovascular outcomes such as mortality in heart failure patients (Sherwood et al., 2017) and greater intima-media thickness in an adult population (Ginty et al., 2016). It has been proposed that blunted reactivity may lead to an increase in adverse behaviors which in turn lead to increased risk for cardiovascular disease (Ginty et al., 2016).
A parallel body of work focuses on changes in levels of the hormone cortisol in response to stress. This line of research provides evidence that comparatively blunted cortisol reactions associate with adverse health outcomes. For example. blunted cortisol reactivity in response to acute psychological stress associates with smoking (al’Absi, 2006; al’Absi et al., 2005; Phillips et al., 2009), alcohol and substance use addictions (Brenner and Beauchaine, 2011; Lovallo, 2005; Panknin et al., 2002). In addition, this pattern of cortisol response associates with obesity (De Rooij, 2013), depression (Brinkmann et al, 2009), disordered eating (Ginty et al., 2012), and poor self-rated health (De Rooij and Roseboom, 2010). Exaggerated cortisol responses to stress have been associated with higher coronary artery calcification (Hamer et al., 2010; Hamer, Endrighi, Venuaraju, Lahiri, & Steptoe, 2012; Hamer & Steptoe, 2012).
Diabetes and CVD are two conditions which disproportionately affect AI and AN communities (Espey et al., 2014) and previous research indicates that patterns of cardiovascular and cortisol responses to psychological stress associate with these conditions and their risk factors (Steptoe, Hackett, Lazzarino, Bostock, La Marca, Carvalho, & Hamer, 2014; Chida and Steptoe, 2010). However, to date, no research has investigated cardiovascular and cortisol responses to acute psychological stress in AI/AN populations. Given that blunted reactivity is related to Type II Diabetes and exaggerated reactions are related to CVD, it is possible that two distinct physiological stress response patterns may contribute to the high incidence of these conditions in AI and AN populations. The purpose of this study was to investigate the pattern of cardiovascular and cortisol responses to acute psychological stress among AI/AN college students, and compare these responses to non-Hispanic White college students.
2. Methods
2.1. Participants
Undergraduate students (N = 85) identifying as non-Hispanic white (n = 45) or American Indian (n = 40) were recruited from a university in Montana. Data was unavailable from five participants in the non-Hispanic white group due to signal acquisition problems (n = 3) and failure to complete questionnaires (n = 2), resulting in a final sample of 80 participants (age, M = 20.11, SD = 2.18, range = 18 – 30 years, 63.7% female). We measured subjective socioeconomic status using the MacArthur’s scale of subjective socioeconomic status (Adler, Epel, Castellazo & Ickovics, 2000). Participants were asked to place an “x” on a ten-rung ladder to indicate their perceived socioeconomic status (SES) relative to the rest of the United States. We asked American Indian participants whether they previously lived on a tribal reservation and whether they were enrolled in a tribe. All demographic information for both American Indian and non-Hispanic white participants is reported in Table 1. Exclusion criteria included history of cardiovascular, neuroendocrine, and/or immune disorders. All participation was voluntary and all participants provided informed consent. Participants were compensated $30.00. The study received institutional review board approval and was conducted in accordance with the Declaration of Helsinki.
Table 1.
Demographics and descriptive statistics for American Indian and Non-Hispanic white participants.
| American Indian Mean (SD) | Non-Hispanic white Mean (SD) | |
|---|---|---|
| Age (years) | 20.18 (2.22) | 20.11 (2.18) |
| Gender (% female) | 60 | 67.5 |
| Subjective | 3.81(2.04) | 4.63(2.70) |
| Socioeconomic Status (1-0) | ||
| % who previously lived on reservation | 52.5% | --- |
| % enrolled in a tribe | 40% | --- |
2.2. Control and Confounding Variables
2.2.1. Anthropometric Measures
Height was measured with a stadiometer and weight measurements were taken with participants standing in the center of a scale. Body mass index was derived from these measurements. Waist and Hip were measured following the World Health Organization’s protocol (World Health Organization, 2011). Waist:Hip ratio for each individual participant was computed after data collection as: Waist/Hip.
2.2.2. Depression and Anxiety
Symptoms of depression and anxiety were measured using the Hospital Anxiety Depression Scale (HADS; Zigmond and Snaith, 1983). The HADS consists of fourteen items, seven items for the depression subscale and 7 items of the anxiety subscale. Example items for the anxiety subscale include, “I get sudden feelings of panic,” “Worrying thoughts go through my mind.” Example items for the depression subscale include, “I feel as if I am slowed down,” “I still enjoy things I used to enjoy (reverse scored).” Items are scored on a 4-point scale, 0-3; the higher the score the greater the depression/anxiety. The HADS has good validity (Bjelland et al., 2002; Bramley et al., 1988) and good test-retest reliability (Herrmann, 1997; Moorey et al., 1991). In the present sample, the HADS demonstrated good reliability with a Cronbach alpha coefficient of .81 for the depression subscale and .71 the anxiety subscale.
2.2.3. Early life Adversity
The Risky Families Questionnaire was used to assess participants’ exposure to physical, mental, and emotional neglect or adversity and the presence or absence of warmth during their childhood and adolescent years (Repetti, Taylor & Seeman, 2002). In the RFQ, participants indicate how frequently certain events or situations occurred in their homes during the ages of 5-15 years old utilizing a 5-Point Likert Scale (1= Not at All and 5= Very Often). Example questions from this questionnaire include “How often did a parent or other adult in the household make you feel that you were loved, supported, and cared for?” and “How often would you say there was quarreling, arguing, or shouting between your parents?” Items measuring the presence of positive qualities in the family environment are reverse scored and all 10 items are summed to capture the overall level risk in the early family environment. In this sample, Cronbach’s alpha=.79 for this questionnaire.
2.2.4. Psychological stress task questionnaire
To examine potential group differences in perceptions of the stress task, participants were asked to rate how stressed they were before, immediately following the practice portion, and after completion of the task on a 7-item Likert scale (1= not at all stressed, 7 = extremely stressed). After completion of the task, participants were asked how well they thought they performed on the task on a 7-item Likert Scale (1= very poorly, 7= very well).
2.3. Salivary cortisol measurements
Saliva samples were obtained using stimulated salivettes (Salimetrics, USA) 7 minutes into the baseline period and 25 minutes after the beginning of the acute psychological stress task. For each sample, participants placed the salivette in their mouths and gently chewed for 2 minutes. Participants returned the swab to the salivette tube and all samples were frozen at −80 C within 4 hours of collection. Samples were thawed on the day of analyses and centrifuged at 1500 x g for 15 minutes. All salivary cortisol samples were processed using a High Sensitivity ELISA (Salimetrics). The inter-assay and intra-assay coefficient were below 8%.
2.4. Cardiovascular measurements
There were three periods during the laboratory session: 10 min adaptation, 10 min baseline, and 10 min of stress task exposure. During the baseline and stress task exposure periods, systolic (SBP) and Diastolic (DBP) blood pressure and heart rate (HR) were measured discontinuously every two minutes during the baseline and stress protocol using a standard blood pressure cuff positioned over the brachial artery on the non-dominant arm, and a semi-automatic sphygmomanometer (GE Dinamap v100, Milwaukee, WI).
2.5. Acute psychological stress task
The acute psychological stress task was the paced auditory serial addition test (PASAT) (Gronwall, 1977). The PASAT consistently increases blood pressure and heart rate (Ring et al., 2002; Ginty et al., 2012; Trotman et al. 2019) as well as salivary cortisol (Ginty et al., 2012). Additionally, the task demonstrates good test retest reliability (Willemsen et al., 1998; Ginty et al. 2013). The PASAT was presented via speakers in the laboratory. The task involves attention and memory, social evaluation, and simple addition. Participants are presented a series of single digit numbers and required, in each case, to add any given number to the number previously presented and say the answer out loud. The intervals between the numbers were 4.5 s for the first 2 min and shortened by .5 s every subsequent two min. Participants were required to look at themselves in a mirror placed approximately .5 m away throughout the task. Participants were also videotaped throughout the PASAT and told the tape would be assessed by “body language experts” Participants were also told that they were in direct competition with their peers and a leader board was placed prominently in the room. Participants were awarded 1000 points at the start at the task, but lost five points for every incorrect answer or omission. The final points served as a performance score. Additionally, an experimenter stood directly next to the participant to score their performance and provide a loud burst of noise every block of 10 numbers. Participants thought they were receiving the burst every time they hesitated, however, all participants received the same number of bursts.
2.6. Procedure
All participants attended a 90-minute laboratory session between the hours of 10 a.m. and 12 p.m. Participants were required to not: engage in vigorous exercise or consume alcohol 12 h before the laboratory session, consume caffeine within 2h of the testing session, or consume food and drink other than water within 1 h of the testing session. After providing informed consent, participants had their height and weight measured, and BMI was calculated. Participants then completed demographic questionnaires. During the adaptation phase, participants were fitted with the blood pressure cuff and sat quietly for 10 minutes. This was followed by a formal 10-min resting baseline period. Participants then received instructions regarding the task and completed a brief practice. Participants then completed the 10 min PASAT stress task. Immediately afterwards, they rated the impact of their stress. This was followed by a period of rest until the final saliva sample was obtained.
2.7. Data reduction and analysis
For the cardiovascular data, averages of each phase (baseline and task) were computed. Group differences in anthropometric measures, mood, childhood history, integration, stress task performance, and stress task ratings were explored using univariate ANOVAs. Analyses of group differences in cardiovascular and cortisol activity were examined using group (American Indian, Non-Hispanic white) x Time (baseline, stress) ANOVA. ANCOVAs were run to determine if any group, group x time, and time differences withstood adjustment for potential confounding variables i.e., a priori variables known to be related to cardiovascular and cortisol reactivity. Data was processed using IBM SPSS Statistics Version 25.
3. Results
3.1. Differences between groups on demographic, anthropometric, and psychological variables
As noted previously, demographics for AIs and Non-Hispanic Whites in this sample are presented in Table 1. There were no significant differences between groups on age (p = .800) or gender distribution (p = .417). Additionally, there were no significant group differences on HADS anxiety or depression scores. There were no significant group differences on BMI, however, the American Indian group had a significantly higher waist:hip ratio. There were no statistically significant differences between groups in early childhood adversity, although there was a trend for those in the American Indian group to have experienced higher levels of adversity. Table 2 displays means and standard deviations for each group and the results of the one-way ANOVAs examining differences between groups.
Table 2.
Anthropometric, and psychological characteristics of the American Indian and Non-Hispanic white groups.
| American Indian Mean (SD) | Non-Hispanic white Mean (SD) | F | p | peta2 | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 26.80 (5.29) | 25.65 (5.88) | 0.837 | .363 | .011 |
| Waist:Hip Ratio* | 0.90 (0.10) | 0.80 (0.64) | 26.13 | <.001 | .253 |
| HADS depression | 5.23 (2.52) | 5.28 (2.46) | 0.008 | .928 | <.001 |
| HADS anxiety | 4.38 (1.98) | 4.70 (1.94) | 0.550 | .461 | .007 |
| Risky Family Questionnaire | 24.05 (7.26) | 20.98 (7.39) | 3.525 | .064 | .043 |
3.2. Stress task performance and post-task ratings
There were no significant differences between PASAT performance scores or self-report performance. Additionally, there were no differences between groups on how stressed they felt about the upcoming task or how stressed they felt during the task. See Table 3 for group means and standard deviations and the results of the one-way ANOVAs examining differences between groups.
Table 3.
Task performance and reported task impact for American Indian and Non-Hispanic white groups
| American Indian Mean (SD) | Non-Hispanic white Mean (SD) | F | p | peta2 | |
|---|---|---|---|---|---|
| PASAT performance | 532.95 (192.65) | 541.08 (167.30) | 0.180 | .673 | .002 |
| Perceived performance | 2.65 (1.21) | 2.85 (1.27) | 0.519 | .473 | .007 |
| Pre-task stressfulness | 3.70 (1.38) | 3.63 (1.71) | 0.047 | .829 | .001 |
| Task stressfulness | 3.78 (1.39) | 4.23 (1.95) | 1.41 | .239 | .018 |
3.3. Cardiovascular reactions to acute psychological stress
Repeated measures ANOVA on SBP indicated a significant main effect for time, F (1,78) = 172.92, p < .001, peta2 = .689. There was no significant main effect for group, but there was a significant group x time interaction, F (1,78) = 16.25, p < .001, peta2 = .172. This interaction is illustrated in Figure 1a, which demonstrates the American Indian showed a blunted SBP response to stress. Repeated measures ANOVA on HR indicated a significant main effect for time, F (1,78) = 128.09, p < .001, peta2 = .622. There was no significant main effect for group, but there was a significant group x time interaction, F (1,78) = 4.29, p = .042, peta2 = .052 (see Figure 1b). Post-hoc analyses indicated that the American Indian group had slightly elevated baseline HR, however, the difference was not statistically significant. The American Indian group had significantly blunted HR responses to stress compared to baseline, while the non-Hispanic white group had a larger response to stress. A repeated measures ANOVA on DBP revealed a significant main effect for time, F (1,78) = 228.69, p < .001, peta2 = .746 and a significant main effect for group, F (1,78) = 4.11, p < .046, peta2 = .050. There was also a significant group x time interaction, F (1,78) = 12.83, p < .001, peta2 = .141. Post-hoc analyses demonstrated that the American Indian group had a blunted response to stress compared to the non-Hispanic white group (see Figure 1c).
Figure 1.
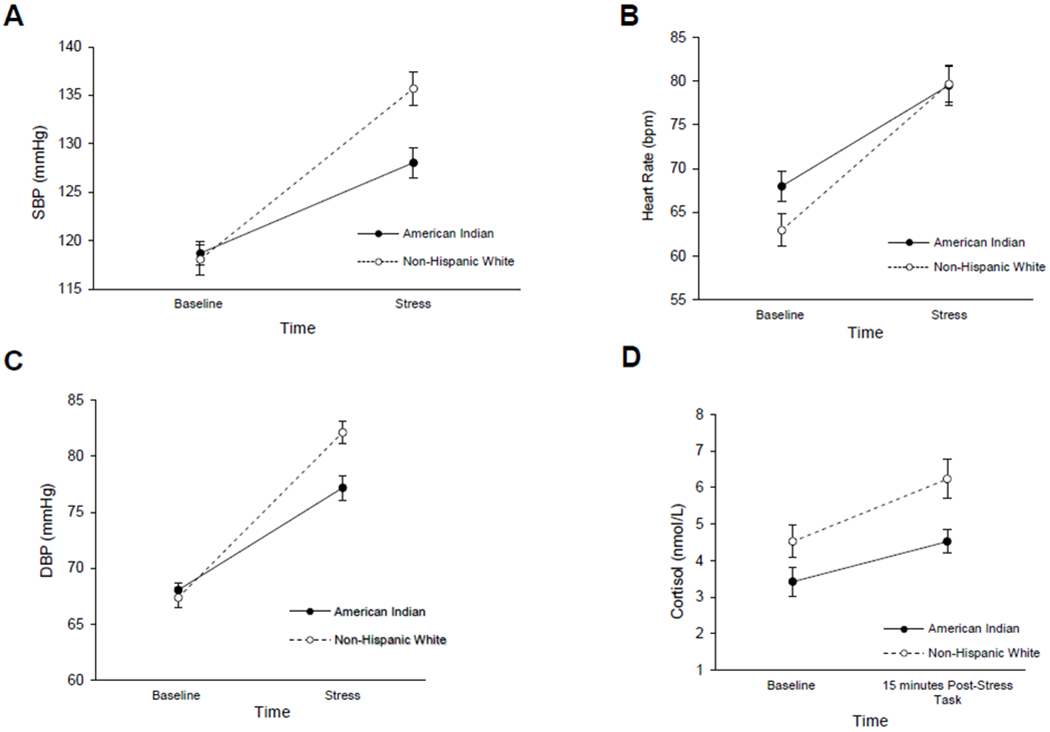
(a) Mean (SE) systolic blood pressure at baseline and during the acute stress task in the AI/AN and Non-Hispanic White groups. (b) Mean (SE) heart rate at baseline and during the acute stress task in the AI/AN and Non-Hispanic White groups. (c) Mean (SE) diastolic blood pressure at baseline and during the acute stress task in the AI/AN and Non-Hispanic White groups. (d) Mean (SE) salivary cortisol at baseline and 15 minutes after the acute stress task in the AI/AN and Non-Hispanic White groups.
3.4. Salivary cortisol reactions to acute psychological stress
Repeated measures ANOVA on cortisol indicated a main effect for time F (1,78) = 91.62 p < .001, peta2 = .540, but no main effect for group (p = .153). There was a significant group x time interaction, F (1,78) = 18.96 p < .001, peta2 = .195. Post-hoc analyses demonstrated that both groups had a significant increase in cortisol levels between baseline and post-stress, however, the increase was less in the American Indian group compared to the non-Hispanic white group (see Figure 1d).
3.5. Covariance analyses
Given that waist:hip ratio, BMI, depression, and early childhood adversity have all been associated with cardiovascular reactivity (Carroll et al., 2008; Phillips et al., 2012; Singh and Shen, 2013; Laederach-Hoffman et al., 2000; Barnes et al., 1998; Steptoe and Wardle, 2005; Schiwek et al., 2018; Waldstein et al., 1999; Cărnuţă et al., 2015; Heleniak et al., 2016; Kuras et al., 2017; Voellmin et al., 2015; Ginty et al., 2015) all cortisol and cardiovascular analyses were revisited adjusting for these potential confounding variables. One participant was lost in these analyses due to not having a measurement of waist:hip ratio. Results were similar for salivary cortisol, there was a main effect of time, F(1,77) = 90.06, p < .001, peta2 = .539, a significant group x time interaction, F(1,77) = 17.48, p < .001, peta2 = .185, but no main effect of group (p =.170). For SBP, there was still a significant main of time, F(1,77) = 167. 65, p < .001, peta2 = .685 and a significant group x time interaction (F(1,77) = 15.23, p < .001, peta2 = .165. DBP still revealed a significant main effect for time, F (1,77) = 222.18, p < .001, peta2 = .743, a significant main effect for group, F (1,77) = 4.02, p < .048, peta2 = .050, and a significant group x time interaction, F (1,77) = 12.29, p =.001, peta2 = .138. Similarly, for HR, there was still a significant main effect of time, F (1,77) = 124.40, p < .001, peta2 = .618 and a significant group x time interaction, , F (1,77) = 4.09, p =.047, peta2 = .050, but no main effect of group (p = .391).
4. Discussion
To our knowledge, this research provides the first data on cardiovascular and cortisol responses to acute psychological stress in AI/AN college students. We found that across SBP, DBP, HR, and cortisol, AI/AN college students had significantly blunted responses to acute psychological stress compared to non-Hispanic White students. Our analyses also indicate that there were no group differences in task performance or perceptions of stress going into the task. This is important as it suggests that the observed differences are not a function of task engagement or differences in psychological stress associated with task. There were also no differences between groups in age or gender distribution. Similarly, there were no differences between groups on depressive symptomology, anxiety, body mass index, or childhood adversity. AI/AN college students had significantly higher waist:hip ratio than non-Hispanic White students. However, analyses controlling for a priori potential confounding variables (i.e., BMI, depression, waist:hip ratio, and early life adversity) did not change the statistical significance of any results. These initial findings provide an impetus for future investigations in order to better understand whether this pattern of cardiovascular and cortisol responses to psychological stress persists across different types of stressors and in larger samples and how such patterns may contribute to high levels of cardiovascular disease and diabetes seen in AI/AN populations.
Our data indicate that physiological responses to stress (i.e. cardiovascular and cortisol) in AI/AN college students are blunted rather than exaggerated. The existing literature on blunted reactivity provides evidence that this pattern of response associates with addiction, depression, and obesity (Carroll et al., 2017; Phillips et al., 2013; Lovallo, 2011). The incidence of these outcomes is disproportionately high in AI/AN communities. As such, the blunted physiological responses observed here in AI/AN college could be a physiological pathway that may contribute to the high rates of these adverse outcomes in these populations. Carroll et al. (2017) proposed a model in which blunted physiological responses to stress are regarded as a product of dysregulation in brain areas central to motivation, autonomic and endocrine control. Currently, research suggests blunted reactivity is a risk marker of behavioral and health outcomes. Further research is needed to determine if blunted reactivity is a cause or consequence of these behaviors (Carroll et al., 2017). Behaviors associated with blunted physiological reactivity (e.g., smoking, depression) may over time contribute to increase risk of cardiovascular disease. Indeed, a study in a European population demonstrated that blunted cardiovascular reactions to acute psychological stress were associated with higher levels of intima media thickness and this was mediated through smoking status and high BMI (Ginty et al., 2016). The indirect relationship between diminished reactivity and adverse cardiovascular outcomes through increases in adverse behaviors may partially explain a pathway through which a blunted physiological response may possibly predict increased risk for both CVD and Type II diabetes in AI/AN populations (Steptoe et al., 2014). However, this is speculative and future research is needed in AI/AN populations to examine the longitudinal relationships between physiological reactivity, behavioral patterns, and the risk factors and diagnoses of CVD and diabetes.
Further, previous work indicates that early childhood environments can lead to blunted reactivity (Cărnuţă et al., 2015; Heleniak et al., 2016; Kuras et al., 2017; Voellmin et al., 2015). Given high rates of early life adversity in AI/AN communities, subsequent research should investigate whether exposures to adversity in childhood predict blunted physiological responses in this population. As noted, there were no differences in exposure to early life adversity in this sample using the Risky Family Questionnaire (Repetti, Taylor & Seeman, 2003), however it is possible that a more sensitive measure with subscales of trauma such as the Childhood Trauma Questionnaire (Bernstein et al., 1994), could reveal differences between groups. Further, in addition to early life adversity, AI/AN communities are affected by historical trauma, or the legacy of numerous traumatic events, the consequences of which are transmitted intergenerationally (Brave Heart, 1999; Evans-Campbell, 2008). As such, historical trauma may by itself, or in conjunction with early life and current trauma inform reactivity patterns for AI/AN communities, however this remains unexplored. As noted by Whitbeck and Colleagues (2004a, 2004b), the loss and trauma experienced by AI/AN people are ongoing and not necessarily limited to a single time period.
In other racial and ethnic groups, the magnitude of cardiovascular responses to stress is moderated by psychosocial factors. For example, social support moderated cardiovascular reactivity particularly for individuals who had experienced a high number of life events in the preceding 12 months (Roy, Steptoe, Kirschbaum, 1998). Separately, personality was found to moderate the relationship between life event stress and cardiovascular reactivity (Gallagher et al., 2018). Further, an individual’s perception of their social integration (i.e. loneliness) and their social participation associate with patterns of cardiovascular responses to acute psychological stress (Brown et al., 2019; John-Henderson et al., 2019). The current study did not measure these possible moderators. However, based on the findings of this study, future research should aim to elucidate moderators of the patterns observed in this investigation. Longitudinal and daily life studies could elucidate the pathways through which blunted physiological reactivity to acute stress might contribute to high rates of diabetes and cardiovascular disease. For example, as noted previously, blunted cardiovascular reactivity to acute psychological stress associated with lower reports of social participation in a previous study (John-Henderson et al., 2019). In line with this, it is possible that blunted reactivity to acute stress may affect rates of these conditions by shaping the nature and frequency of social interactions.
There are important limitations of this research. First, the sample size was relatively small and drawn from a college sample. However, the sample size was similar to other studies examining differences in cardiovascular responses to stress based on categorical groups (e.g., Stoney et al., 2002). Future work should aim for more robust sample size and also draw from a more representative sample of AI/AN individuals (i.e. age, education). Second, the study was cross-sectional, so causation cannot be inferred (Christenfeld, 2004). A longitudinal study design could provide more insights into how changes in environment and experiences may influence reactivity. While this study measured reactivity in both the autonomic and HPA axis, future work should include reactivity across other systems, such as the inflammatory system (Marsland et al., 2017; Steptoe et al., 2014). Lastly, blunted cardiovascular and cortisol responses to stress could be due to lack of engagement in the task. However, the were no differences between groups in self-reported engagement or task performance. This is in line with previous work suggesting that blunted reactivity is likely not due to disinterest or disengagement with the task (Brindle et al., 2017).
Overall, this investigation represents an initial step towards the understanding of physiological mechanisms and/or pathways that may contribute to high rates of diabetes and cardiovascular disease in AI/AN communities. Future work should extend upon these initial findings and identify factors that precede and follow the observed pattern of findings, as well as identify factors that may moderate the pattern of findings, and potentially reduce incidence of these diseases in these at-risk communities.
Highlights:
We measured cardiovascular and cortisol reactivity to acute psychological stress
We compared responses between American Indian and Non-Hispanic White college students
American Indian college students exhibited comparably blunted cortisol, blood pressure and heart rate responses
These effects were not accounted for by differences in task performance or task engagement
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103474. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: None
References
- 1.al’Absi M, 2006. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol 59, 218–227. 10.1016/j.ijpsycho.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 2.al’Absi M, Hatsukami D, Davis GL, 2005. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 181, 107–117. 10.1007/s00213-005-2225-3 [DOI] [PubMed] [Google Scholar]
- 3.Balanos GM, Phillips AC, Frenneaux MP, McIntyre D, Lykidis C, Griffin HS, Carroll D, 2010. Metabolically exaggerated cardiac reactions to acute psychological stress: the effects of resting blood pressure status and possible underlying mechanisms. Biol Psychol 85, 104–111. 10.1016/j.biopsycho.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 4.Barnes VA, Treiber FA, Davis H, Kelley TR, Strong WB, 1998. Central adiposity and hemodynamic functioning at rest and during stress in adolescents. Int. J. Obes. Relat. Metab. Disord 22, 1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett PA, Spence JD, Manuck SB, Jennings JR, 1997. Psychological stress and the progression of carotid artery disease. J. Hypertens 15, 49–55. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151, 1132–1136. 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- 7.Bjelland I, Dahl AA, Haug TT, Neckelmann D, 2002. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52, 69–77. [DOI] [PubMed] [Google Scholar]
- 8.Bramley PN, Easton AM, Morley S, Snaith RP, 1988. The differentiation of anxiety and depression by rating scales. Acta Psychiatr Scand 77, 133–138. [DOI] [PubMed] [Google Scholar]
- 9.Brave Heart MY, 1999. Gender differences in the historical trauma response among the Lakota. J Health Soc Policy 10, 1–21. 10.1300/J045v10n04_01 [DOI] [PubMed] [Google Scholar]
- 10.Brenner SL, Beauchaine TP, 2011. Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: a pilot study. Psychophysiology 48, 1588–1596. 10.1111/j.1469-8986.2011.01230.x [DOI] [PubMed] [Google Scholar]
- 11.Brindle RC, Ginty AT, Conklin SM, 2013. Is the association between depression and blunted cardiovascular stress reactions mediated by perceptions of stress? Int J Psychophysiol 90, 66–72. 10.1016/j.ijpsycho.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Brindle RC, Whittaker AC, Bibbey A, Carroll D, Ginty AT, 2017. Exploring the possible mechanisms of blunted cardiac reactivity to acute psychological stress. Int J Psychophysiol 113, 1–7. 10.1016/j.ijpsycho.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann K, Schüpbach L, Joye IA, Gendolla GHE, 2009. Anhedonia and effort mobilization in dysphoria: reduced cardiovascular response to reward and punishment. Int J Psychophysiol 74, 250–258. 10.1016/j.ijpsycho.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 14.Brown EG, Creaven A-M, Gallagher S, 2019. Loneliness and cardiovascular reactivity to acute stress in younger adults. Int J Psychophysiol 135, 121–125. 10.1016/j.ijpsycho.2018.07.471 [DOI] [PubMed] [Google Scholar]
- 15.Cărnuţă M, Crişan LG, Vulturar R, Opre A, Miu AC, 2015. Emotional non-acceptance links early life stress and blunted cortisol reactivity to social threat. Psychoneuroendocrinology 51, 176–187. 10.1016/j.psyneuen.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 16.Carroll D, Ginty AT, Der G, Hunt K, Benzeval M, Phillips AC, 2012. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology 49, 1444–1448. 10.1111/j.1469-8986.2012.01463.x [DOI] [PubMed] [Google Scholar]
- 17.Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR, 2017. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci Biobehav Rev 77, 74–86. 10.1016/j.neubiorev.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll D, Phillips AC, Balanos GM, 2009. Metabolically exaggerated cardiac reactions to acute psychological stress revisited. Psychophysiology 46, 270–275. 10.1111/j.1469-8986.2008.00762.x [DOI] [PubMed] [Google Scholar]
- 19.Carroll D, Phillips AC, Der G, 2008. Body mass index, abdominal adiposity, obesity, and cardiovascular reactions to psychological stress in a large community sample. Psychosom Med 70, 653–660. 10.1097/PSY.0b013e31817b9382 [DOI] [PubMed] [Google Scholar]
- 20.Carroll D, Phillips AC, Der G, Hunt K, Benzeval M, 2011. Blood pressure reactions to acute mental stress and future blood pressure status: data from the 12-year follow-up of the West of Scotland Study. Psychosom Med 73, 737–742. 10.1097/PSY.0b013e3182359808 [DOI] [PubMed] [Google Scholar]
- 21.Carroll D, Ring C, Hunt K, Ford G, Macintyre S, 2003. Blood pressure reactions to stress and the prediction of future blood pressure: effects of sex, age, and socioeconomic position. Psychosom Med 65, 1058–1064. [DOI] [PubMed] [Google Scholar]
- 22.Carroll D, Smith GD, Sheffield D, Shipley MJ, Marmot MG, 1995. Pressor reactions to psychological stress and prediction of future blood pressure: data from the Whitehall II Study. BMJ 310, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG, 2001. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med 63, 737–743. [DOI] [PubMed] [Google Scholar]
- 24.Chida Y, Steptoe A, 2010. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 55, 1026–1032. 10.1161/HYPERTENSIONAHA.109.146621 [DOI] [PubMed] [Google Scholar]
- 25.Christenfeld NJS, Sloan RP, Carroll D, Greenland S, 2004. Risk factors, confounding, and the illusion of statistical control. Psychosom Med 66, 868–875. 10.1097/01.psy.0000l40008.70959.41 [DOI] [PubMed] [Google Scholar]
- 26.De Rooij S, 2013. Blunted cardiovascular and cortisol reactivity to acute psychological stress: A summary of results from the Dutch Famine Birth Cohort Study. Int J Psychophsiol 90(1), 21–27. [DOI] [PubMed] [Google Scholar]
- 27.De Rooij SR, Roseboom TJ, 2010. Further evidence for an association between self-reported health and cardiovascular as well as cortisol reactions to acute psychological stress. Psychophysiology 47, 1172–1175. 10.1111/j.1469-8986.2010.01023.x [DOI] [PubMed] [Google Scholar]
- 28.Denny CH, Holtzman D, Cobb N, 2003. Surveillance for health behaviors of American Indians and Alaska Natives. Findings from the Behavioral Risk Factor Surveillance System, 1997-2000. MMWR Surveill Summ 52, 1–13. [PubMed] [Google Scholar]
- 29.Denny CH, Holtzman D, Goins RT, Croft JB, 2005. Disparities in Chronic Disease Risk Factors and Health Status Between American Indian/Alaska Native and White Elders: Findings From a Telephone Survey, 2001 and 2002. Am J Public Health 95, 825–827. 10.2105/AJPH.2004.043489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espey DK, Jim MA, Cobb N, Bartholomew M, Becker T, Haverkamp D, Plescia M, 2014. Leading causes of death and all-cause mortality in American Indians and Alaska Natives. Am J Public Health 104 Suppl 3, S303–311. 10.2105/AJPH.2013.301798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans-Campbell T, 2008. Historical trauma in American Indian/Native Alaska communities: a multilevel framework for exploring impacts on individuals, families, and communities. J Interpers Violence 23, 316–338. 10.1177/0886260507312290 [DOI] [PubMed] [Google Scholar]
- 32.Gallagher S, O’Riordan A, McMahon G, Creaven A-M, 2018. Evaluating personality as a moderator of the association between life events stress and cardiovascular reactivity to acute stress. Int J Psychophysiol 126, 52–59. 10.1016/j.ijpsycho.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 33.Galloway JM, 2005. Cardiovascular health among American Indians and Alaska Natives: successes, challenges, and potentials. Am J Prev Med 29, 11–17. 10.1016/j.amepre.2005.07.023 [DOI] [PubMed] [Google Scholar]
- 34.Ginty AT, Brindle RC, Carroll D, 2015. Cardiac stress reactions and perseverance: Diminished reactivity is associated with study non-completion. Biol Psychol 109, 200–205. 10.1016/j.biopsycho.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 35.Ginty AT, Gianaros PJ, Derbyshire SWG, Phillips AC, Carroll D, 2013. Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiology 50, 219–229. 10.1111/psyp.12017 [DOI] [PubMed] [Google Scholar]
- 36.Ginty AT, Masters NA, Nelson EB, Kaye KT, & Conklin S (2016). Cardiovascular reactions to psychological stress and abuse history: the role of occurrence, frequency, and type of abuse. Stress, Anxiety, & Coping, 30, 755–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginty AT, Phillips AC, Higgs S, Heaney JLJ, Carroll D, 2012. Disordered eating behaviour is associated with blunted cortisol and cardiovascular reactions to acute psychological stress. Psychoneuroendocrinology 37, 715–724. 10.1016/j.psyneuen.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 38.Ginty AT, Williams SE, Jones A, Roseboom TJ, Phillips AC, Painter RC, Carroll D, de Rooij SR, 2016. Diminished heart rate reactivity to acute psychological stress is associated with enhanced carotid intima-media thickness through adverse health behaviors. Psychophysiology 53, 769–775. 10.1111/psyp.12640 [DOI] [PubMed] [Google Scholar]
- 39.Gronwall DM, 1977. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills 44, 367–373. 10.2466/pms.1977.44.2.367 [DOI] [PubMed] [Google Scholar]
- 40.Hamer M, Endrighi R, Venuraju SM, Lahiri A, Steptoe A, 2012. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS ONE 7, e31356 10.1371/journal.pone.0031356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamer M, O’Donnell K, Lahiri A, Steptoe A, 2010. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. Eur. Heart J 31, 424–429. 10.1093/eurheartj/ehp386 [DOI] [PubMed] [Google Scholar]
- 42.Hamer M, Steptoe A, 2012. Cortisol responses to mental stress and incident hypertension in healthy men and women. J. Clin. Endocrinol. Metab 97, E29–34. 10.1210/jc.2011-2132 [DOI] [PubMed] [Google Scholar]
- 43.Heleniak C, McLaughlin KA, Ormel J, Riese H, 2016. Cardiovascular reactivity as a mechanism linking child trauma to adolescent psychopathology. Biol Psychol 120, 108–119. 10.1016/j.biopsycho.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrmann C, 1997. International experiences with the Hospital Anxiety and Depression Scale-A review of validation data and clinical results. Journal of Psychosomatic Research 42, 17–41. 10.1016/S0022-3999(96)00216-4 [DOI] [PubMed] [Google Scholar]
- 45.Jernigan VBB, Duran B, Ahn D, Winkleby M, 2010. Changing patterns in health behaviors and risk factors related to cardiovascular disease among American Indians and Alaska Natives. Am J Public Health 100, 677–683. 10.2105/AJPH.2009.164285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jernigan VBB, Wetherill M, Hearod J, Jacob T, Salvatore AL, Cannady T, Grammar M, Standridge J, Fox J, Spiegel J, Wiley A, Noonan C, Buchwald D, 2017. Cardiovascular Disease Risk Factors and Health Outcomes Among American Indians in Oklahoma: the THRIVE Study. J Racial Ethn Health Disparities 4, 1061–1068. 10.1007/s40615-016-0310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John-Henderson NA, Counts CJ, Sanders CS, Ginty AT, 2019. Diminished cardiovascular stress reactivity is associated with lower levels of social participation. J Psychosom Res 118, 12–16. 10.1016/j.ipsychores.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuras YI, Assaf N, Thoma MV, Gianferante D, Hanlin L, Chen X, Fiksdal A, Rohleder N, 2017. Blunted Diurnal Cortisol Activity in Healthy Adults with Childhood Adversity. Front Hum Neurosci 11, 574 10.3389/fnhum.2017.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laederach-Hoffmann K, Mussgay L, Rúddel H, 2000. Autonomic cardiovascular regulation in obesity. J. Endocrinol 164, 59–66. 10.1677/joe.0.1640059 [DOI] [PubMed] [Google Scholar]
- 50.Light KC, 1981. Cardiovascular Responses to Effortful Active Coping: Implications for the Role of Stress in Hypertension Development. Psychophysiology 18, 216–225. 10.1111/j.1469-8986.1981.tb03021.x [DOI] [PubMed] [Google Scholar]
- 51.Lovallo WR, 2011. Do low levels of stress reactivity signal poor states of health? Biol Psychol 86 121–128. 10.1016/j.biopsycho.20l0.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lovallo WR, 2005. Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. Int J Psychophysiol 58, 119–132. 10.1016/j.ijpsycho.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 53.Manuck SB, Kasprowicz AL, Muldoon MF, 1990. Behaviorally-Evoked Cardiovascular Reactivity and Hypertension: Conceptual Issues and Potential Associations. Ann Behav Med 12, 17–29. 10.1207/sl5324796abm1201_2 [DOI] [Google Scholar]
- 54.Markovitz JH, Raczynski JM, Wallace D, Chettur V, Chesney MA, 1998. Cardiovascular reactivity to video game predicts subsequent blood pressure increases in young men: The CARDIA study. Psychosom Med 60, 186–191. [DOI] [PubMed] [Google Scholar]
- 55.Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun 64, 208–219. 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Lassila HC, Wolfson SK, 1998. Stress-induced pulse pressure change predicts women’s carotid atherosclerosis. Stroke 29, 1525–1530. [DOI] [PubMed] [Google Scholar]
- 57.Matthews KA, Woodall KL, Allen MT, 1993. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension 22, 479–485. [DOI] [PubMed] [Google Scholar]
- 58.Moorey S, Greer S, Watson M, Gorman C, Rowden L, Tunmore R, Robertson B, Bliss J, 1991. The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. Br J Psychiatry 158, 255–259. 10.1192/bjp.158.2.255 [DOI] [PubMed] [Google Scholar]
- 59.Newman JD, McGarvey ST, Steele MS, 1999. Longitudinal association of cardiovascular reactivity and blood pressure in Samoan adolescents. Psychosom Med 61, 243–249. [DOI] [PubMed] [Google Scholar]
- 60.Panknin TL, Dickensheets SL, Nixon SJ, Lovallo WR, 2002. Attenuated heart rate responses to public speaking in individuals with alcohol dependence. Alcohol. Clin. Exp. Res 26, 841–847. [PubMed] [Google Scholar]
- 61.Phillips AC, Der G, Hunt K, Carroll D, 2009. Haemodynamic reactions to acute psychological stress and smoking status in a large community sample. Int J Psychophysiol 73, 273–278. 10.1016/j.ijpsycho.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips AC, Ginty AT, Hughes BM, 2013. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int J Psychophysiol 90, 1–7. 10.1016/j.ijpsycho.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 63.Phillips AC, Hunt K, Der G, Carroll D, 2011. Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: evidence from a large community study. Psychophysiology 48, 142–148. 10.1111/j.1469-8986.2010.01045.x [DOI] [PubMed] [Google Scholar]
- 64.Phillips AC, Roseboom TJ, Carroll D, de Rooij SR, 2012. Cardiovascular and cortisol reactions to acute psychological stress and adiposity: cross-sectional and prospective associations in the Dutch Famine Birth Cohort Study. Psychosom Med 74, 699–710. 10.1097/PSY.0b013e31825e3b91 [DOI] [PubMed] [Google Scholar]
- 65.Ring C, Burns VE, Carroll D, 2002. Shifting hemodynamics of blood pressure control during prolonged mental stress. Psychophysiology 39, 585–590. https://doi.org/10.1017.S0048577202011320 [DOI] [PubMed] [Google Scholar]
- 66.Roy MP, Steptoe A, Kirschbaum C, 1998. Life events and social support as moderators of individual differences in cardiovascular and cortisol reactivity. J Pers Soc Psychol 75, 1273–1281. [DOI] [PubMed] [Google Scholar]
- 67.Schiweck C, Piette D, Berckmans D, Claes S, Vrieze E, 2019. Heart rate and high frequency heart rate variability during stress as biomarker for clinical depression. A systematic review. Psychol Med 49, 200–211. 10.1017/S0033291718001988 [DOI] [PubMed] [Google Scholar]
- 68.Sherwood A, Hill LK, Blumenthal JA, Adams KF, Paine NJ, Koch GG, O’Connor CM, Johnson KS, Hinderliter AL, 2017. Blood pressure reactivity to psychological stress is associated with clinical outcomes in patients with heart failure. Am. Heart J 191, 82–90. 10.1016/j.ahj.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh K, Shen B-J, 2013. Abdominal obesity and chronic stress interact to predict blunted cardiovascular reactivity. Int J Psychophysiol 90, 73–79. 10.1016/j.ijpsycho.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 70.Steptoe A, Hackett RA, Lazzarino AI, Bostock S, La Marca R, Carvalho LA, Hamer M, 2014a. Disruption of multisystem responses to stress in type 2 diabetes: investigating the dynamics ofallostatic load. Proc. Natl. Acad. Sci. U.S.A, 111, 15693–15698. 10.1073/pnas.1410401111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steptoe A, Hackett RA, Lazzarino AI, Bostock S, La Marca R, Carvalho LA, Hamer M, 2014b. Disruption of multisystem responses to stress in type 2 diabetes: investigating the dynamics ofallostatic load. Proc. Natl. Acad. Sci. U.S.A, 111, 15693–15698. 10.1073/pnas.1410401111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steptoe A, Wardle J, 2005. Cardiovascular stress responsivity, body mass and abdominal adiposity. Int J Obes (Lond) 29, 1329–1337. 10.1038/sj.ijo.0803011 [DOI] [PubMed] [Google Scholar]
- 73.Stoney CM, Hughes JW, Kuntz KK, West SG, Thornton LM, 2002. Cardiovascular stress responses among Asian Indian and European American women and men. Ann Behav Med 24, 113–121. 10.1207/S15324796ABM2402_08 [DOI] [PubMed] [Google Scholar]
- 74.Trotman GP, Gianaros PJ, Veldhuijzen van Zanten JJCS, Williams SE, Ginty AT, 2019. Increased stressor-evoked cardiovascular reactivity is associated with reduced amygdala and hippocampus volume. Psychophysiology 56, e13277 10.1111/psyp.13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voellmin A, Winzeler K, Hug E, Wilhelm FH, Schaefer V, Gaab J, La Marca R, Pruessner JC, Bader K, 2015. Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology 51, 58–67. 10.1016/j.psyneuen.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 76.Waldstein SR, Burns HO, Toth MJ, Poehlman ET, 1999. Cardiovascular reactivity and central adiposity in older African Americans. Health Psychol 18, 221–228. [DOI] [PubMed] [Google Scholar]
- 77.Whitbeck LB, Chen X, Hoyt DR, Adams GW (2004a). Discrimination, historical loss and enculturation: culturally specific risk and resiliency factors for alcohol abuse among American Indians. Journal of the Study of Alcohol 65, 409–418. [DOI] [PubMed] [Google Scholar]
- 78.Whitbeck LB, Adams GW, Hoyt DR, Chen X (2004b). Conceptualizing and measuring historical trauma among American Indian people. American Journal of Community Psychology 33,119–130. [DOI] [PubMed] [Google Scholar]
- 79.Willemsen G, Ring C, Carroll D, Evans P, Clow A, Hucklebridge F, 1998. Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic and cold pressor. Psychophysiology 35, 252–259. [PubMed] [Google Scholar]
- 80.Winkleby MA, Cubbin C, 2004. Changing patterns in health behaviors and risk factors related to chronic diseases, 1990–2000. Am J Health Promot 19, 19–27. 10.4278/0890-1171-19.1.19 [DOI] [PubMed] [Google Scholar]
- 81.World Health organization, 2011. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. World Health organization, Geneva. [Google Scholar]
- 82.Zigmond AS, Snaith RP, 1983. The hospital anxiety and depression scale. Acta Psychiatr Scand 67, 361–370. [DOI] [PubMed] [Google Scholar]


