Abstract
Background
Antiplatelet antibodies are detected in multiple diseases including primary immune thrombocytopenia (ITP). Dynamics of how these antibodies change over time in ITP is unknown in dogs.
Hypothesis/Objectives
Antiplatelet antibodies (APA) will be detected in thrombocytopenic dogs with multiple etiologies and dynamics of APA in dogs with ITP can be used to evaluate response to treatment and relapse. Determine APA at the time of diagnosis in thrombocytopenic dogs and serially in primary ITP dogs.
Animals
Seventy‐nine thrombocytopenic dogs and 28 primary ITP dogs.
Methods
Direct flow cytometry was performed in thrombocytopenic dogs at initial evaluation and serially in suspected primary ITP dogs. In primary ITP dogs, a 2‐tailed Fisher's exact test was performed comparing survival to discharge between dogs with and without melena and to relate response to treatment and relapse to changes in APA and platelet count (repeated measures analysis, Spearman correlation).
Results
Twenty percent (16/79) of thrombocytopenic non‐ITP dogs with infectious, neoplastic, or other diseases and all primary ITP dogs were positive for APA. Melena at initial evaluation was associated with decreased survival to discharge (odds ratio 0.06; P = .01). Persistence of APA was not associated with response to treatment, but recurrence of antibodies was associated with relapse (odds ratio 205.0; P < .01). There was no difference in percentage of APA or platelet count at initial diagnosis between dogs that did or did not respond to treatment.
Conclusions and Clinical Importance
Serial monitoring of APA in dogs with primary ITP appeared beneficial for determining relapse of disease.
Keywords: autoimmune, flow cytometry, platelet, relapse
Abbreviations
- AGASACA
apocrine gland anal sac adenocarcinoma
- ANOVA
analysis of variance
- APA
antiplatelet antibodies
- IMHA
immune‐mediated hemolytic anemia
- IMN
immune‐mediated neutropenia
- ITP
immune thrombocytopenia
- MCT
mast cell tumor
- MPV
mean platelet volume
- OSA
osteosarcoma
- SD
standard deviation
- STS
soft tissue sarcoma
- TCC
transitional cell carcinoma
1. INTRODUCTION
Antiplatelet antibodies (APA) are detected in a number of dogs and humans with thrombocytopenia and concurrent infectious, toxic, inflammatory, and neoplastic diseases which suggests that immune responses could play a role in the pathogenesis of these conditions in some dogs (secondary immune thrombocytopenia [ITP]).1, 2 Primary or idiopathic ITP is diagnosed when all known other possible secondary causes of ITP have been excluded.1, 2 In historical studies with small numbers of cases, differentiation of primary or secondary ITP in dogs using APA detection methods has been considered to be inaccurate.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Antiplatelet antibodies are documented in approximately 60%‐70% of people diagnosed with primary ITP,2, 18 and it is theorized that non‐antibody‐dependent mechanisms such as direct cell‐mediated destruction contribute to this disease in some people.2, 19, 20 Some dogs with suspected idiopathic ITP lack antibodies.3 Recently, there was a call in human medicine to investigate these antibodies in patients diagnosed with primary ITP and to see if the presence of these antibodies relate to important clinical variables such as prognosis, response to treatment, or recurrence/relapse.21 There is a possible benefit of detecting APA in relationship to severity of disease such as bleeding risk, response to treatment, and recurrence of disease in people.20, 21, 22, 23, 24 To our knowledge, no such studies have been pursued in veterinary medicine. It is unknown how these antibodies change in dogs diagnosed with primary ITP over time and if these changes would be considered clinically relevant. Therefore, the objectives of our study were to evaluate thrombocytopenic dogs for the presence of APAs, to characterize the dynamics of these antibodies over time in dogs diagnosed with primary ITP, and to determine if APAs relate to response to treatment, relapse/recurrence, or both in dogs with primary ITP.
2. MATERIALS AND METHODS
2.1. Case selection and flow cytometry
This study was announced to veterinarians at the Veterinary Teaching Hospital at Colorado State University by email, didactic rounds, and in person during individual case consultation. In the first phase of the study, we performed a convenience sampling to specifically identify client‐owned dogs with concurrent conditions suspected or known to have been associated with secondary ITP such as infectious agents and neoplasia. In the second phase of the study, we specifically targeted client‐owned dogs suspected to have primary ITP. Antiplatelet antibody determination by flow cytometry was offered at no cost from the remaining blood sample previously used for a CBC. Thrombocytopenia was defined as a platelet count less than 60 000 platelets/μL for primary ITP dogs and less than 200 000 platelets/μL without many platelet clumps for thrombocytopenic dogs due to other causes. For all samples in the study, blood was collected in EDTA (BD Vacutainer K2 EDTA 3.6 mg 2.0 mL tubes, Franklin Lakes, New Jersey) and stored at 4°C until assayed by flow cytometry within 72 hours as previously described using FITC‐labeled rabbit anti‐dog IgG (Jackson ImmunoResearch Labs).25 Platelets from a healthy research Beagle served as a negative control at each time point. This was approved by the Institutional Animal Care and Use Committee, and the Beagle was housed in the institution's standard research facility. APA detected by flow cytometry were recorded as yes or no with regard to positivity in addition to percentages (mean of duplicate measures). All samples were analyzed using a flow cytometer instrument (Cyan ADP instrument, Beckman Coulter, Miami, Florida), and the generated data was analyzed using standard software (FlowJo software, Tree Star, Ashland, Oregon). Samples with APA ≤10% were categorized as negative and APA >10% were categorized as positive as described in the optimization of the assay.6, 15, 25
2.2. Client‐owned thrombocytopenic dogs with concurrent diseases
For this phase of the project, blood was collected from dogs evaluated during April 2016‐September 2018. After review of the medical record by 1 of the authors (S.S.), each of the dogs in this group was determined to have a possible secondary cause for thrombocytopenia such as neoplasia (diagnosed via cytology, histopathology, or both) or an infectious disease (diagnosed via serology, PCR, or both types of testing). To increase the number of samples positive for infectious agents, blood samples from thrombocytopenic dogs that were positive for Anaplasma spp., Ehrlichia spp., Bartonella spp., Babesia spp., or Leptospira spp. DNA via PCR (Fever of Unknown Origin RealPCR Panel [Comprehensive], IDEXX Laboratories Inc, Westbrook, Maine) for DNA were supplied by a collaborator and assayed within 72 hours of collection.
2.3. Client‐owned primary ITP dogs
This phase of the study was approved by the institutional clinical review board, and all owners signed a client‐consent form at the time of enrollment. Blood samples were collected during September 2016‐September 2018. Each dog had to be negative for antibodies against Anaplasma spp., Borrelia burgdorferi, and Ehrlichia spp., antigens of Dirofilaria immitis, and DNA of Anaplasma spp., Babesia spp., Bartonella spp., Ehrlichia spp., the hemoplasmas, Neorickettsia spp., and Rickettsia spp. (Veterinary Diagnostic Laboratory, Colorado State University, Fort Collins, Colorado, and SNAP 4Dx Plus, IDEXX Laboratories Inc). There could be no evidence of other possible causes of thrombocytopenia such as other infectious agents, neoplasia, or other conditions based on other infectious disease screening, thoracic radiographs, or abdominal imaging (abdominal radiographs, ultrasound, or both). Immunosuppressive treatment at the time of study enrollment was not an exclusion criteria. Recheck appointments were determined at the discretion of the attending clinician. Antiplatelet antibodies detected by flow cytometry were recorded as percentages (mean of duplicate measures) and as yes or no as defined on all initial and recheck blood samples. The persistence of APA was defined as the presence of antibodies 4 weeks after initiation of treatment for primary ITP. Recurrence of antibodies was defined as the presence of antibodies after a dog had been previously documented to be negative for antibodies. Response to treatment (yes or no) and recurrence/relapse (yes or no) was also recorded for each dog. Response to treatment was defined as the platelet count returned to normal levels (defined as ≥200 000 platelets/μL) within 4 weeks of initial diagnosis. For statistical analysis, dogs that responded to treatment as defined by the study were referred to as responders and dogs that did not respond to treatment were referred to as nonresponders. To further evaluate trends in the percentage of APA and platelet count over time between responders and nonresponders, specific time points during the study were evaluated. Time points were defined as initial (sample collected at initial diagnosis), time 1 (sample collected within 1 week of initial diagnosis), time 2 (sample collected within 1‐2 weeks of initial diagnosis), time 3 (sample collected within 3‐4 weeks of initial diagnosis), and time 4 (sample collected within 5‐6 weeks of initial diagnosis). Relapse/recurrence of ITP was defined as a dog documented to have platelet counts return to normal (defined as ≥200 000 platelets/μL) after immunosuppressive treatment was initiated but on subsequent rechecks had a platelet count of less than or equal to 100 000 platelets/μL with few to no platelet clumps present.
2.4. Percentage binding for APA in dogs considered APA‐positive
In order to investigate whether the percentage of binding helped differentiate etiologies of thrombocytopenia in dogs that were considered positive for APA, the percentage of APA was evaluated between dogs in the different categories (idiopathic, infectious, neoplastic, other). For this comparison, only dogs that had not received any treatment for suspected ITP were included in order to eliminate the potential effect of previous drug administration.
2.5. Statistical analysis
A 2‐tailed Fisher's exact test was performed to compare survival to discharge (yes or no) between dogs with and without melena. Dogs were defined as having melena if melena was observed at presentation. A 2‐sample t‐test was performed to evaluate the association between mean percentage of APA at initial diagnosis and survival to discharge. A 2‐tailed Fischer's exact test was performed to compare response to treatment (yes or no) and persistence of APA (yes or no) and relapse (yes or no) and persistence or recurrence of APA (yes or no). A 2‐sample t‐test was performed to compare the mean percentage of APA and platelet count between responders and nonresponders at initial diagnosis. A repeated‐measures analysis (2‐way analysis of variance[ANOVA]) was performed to evaluate trends in the mean percentage of APA and platelet count between responders and nonresponders over specific time points during the study. A Spearman correlation coefficient (r) was used to assess the correlation between the mean percentage of APA and platelet count in responders and nonresponders at initial diagnosis and at 4 weeks after initial diagnosis. A 1‐way analysis (1‐way ANOVA) was performed to compare percentage binding for APA in dogs classified as APA‐positive in the different categories (infectious, neoplastic, other, idiopathic ITP). All statistical analysis with the exception of repeated‐measures analysis (2‐way ANOVA) were performed with commercially available software (GraphPad Prism 5.0, San Diego, California). For the repeated‐measures (2‐way ANOVA), analysis was performed using R 3.6.1 with the lme4 and emmeans packages. A mixed model was fit for each response variable (percent of APA or platelet count) separately. Fixed effects included group (responder or non‐responder) and time (initial, times 1, 2, 3, 4) and group×time interaction. Subject was included as a random effect to account for repeated measures on subjects. Square root transformation was used to help satisfy model assumptions. Comparisons of downstream time points versus initial were done using Dunnett's method. For all statistical analysis, a P < .05 was considered significant.
3. RESULTS
3.1. Client‐owned thrombocytopenic dogs with concurrent diseases
A total of 79 dogs with thrombocytopenia had concurrent diseases that could have been causative. These dogs were divided into the following categories: infectious (n = 17), neoplastic (n = 27), and other (n = 36). One dog was placed into the other category in 2016 and then was also included into the neoplastic category later in 2018, resulting in a total of 79 dogs rather than 80 dogs. The age range of all dogs was 9 weeks to 15 years.
3.2. Thrombocytopenia due to infectious diseases
The distribution of infectious agents' positive test results were Ehrlichia spp. (n = 6), Anaplasma spp. (n = 10), and Emmonsia spp. (n = 1). The signalment and case details for most of the dogs in the infectious category were unknown because the majority of samples were provided by IDEXX. The signalment was known for 2 of the 17 dogs: a 9‐week‐old FI Husky with acute ehrlichiosis and a 6‐year‐old MC German Shepherd with disseminated Emmonsia spp. The median platelet count for these 17 cases was 62 000 platelets/μL (range, 22 000‐180 000). Eight of the 17 dogs (8/17, 47%) were positive for APA; 6 were positive for Ehrlichia spp., 5 were positive for Anaplasma spp., 1 was positive for Emmonsia spp. For dogs in the infectious category; median APA was 17% (range, 0.8%‐68.0%).
3.3. Thrombocytopenia due to neoplastic causes
Of the 27 dogs in the neoplastic category, 15 were female spayed and 12 were male neutered. None of the dogs in this category were sexually intact. The median age was 10 years (range, 5‐15 years). Eighteen of the 27 (18/27, 67%) dogs were purebred and represented 12 breeds. There were 10 different neoplasms diagnosed. Four of the 27 (4/27, 15%) dogs had 2 concurrent neoplasms. Specifically, the concurrent neoplasms included mast cell tumor (MCT) and soft tissue sarcoma (STS); malignant pheochromocytoma with vascular invasion and maxillary osteosarcoma (OSA); lymphoma and metastatic appendicular OSA; and metastatic apocrine gland anal sac adenocarcinoma (AGASACA) and a myelodysplastic disorder. Neoplasms that were diagnosed in multiple dogs included lymphoma (n = 6), OSA (n = 4), hemangiosarcoma (n = 5), MCT (n = 3), transitional cell carcinoma (TCC) (n = 2), and STS (n = 2). Four of the 27 (4/27, 15%) dogs with neoplasia were positive for APA, and 23 of the 27 (23/27, 85%) dogs were negative for APA. Specific neoplasms that were associated with a positive APA result included MCT, disseminated carcinoma, concurrent lymphoma and OSA, and concurrent metastatic AGASACA and acute megakaryocytic leukemia. Median platelet count was 43 000 platelets/μL (range, 8000‐160 000 platelets/μL). Three of the four dogs that were positive for APA were tested for concurrent infectious disease via serology (Veterinary Diagnostic Laboratory, Colorado State University, and SNAP 4Dx Plus, IDEXX Laboratories Inc), PCR (Veterinary Diagnostic Laboratory, Colorado State University) or both serology and PCR and were negative. For dogs in the neoplastic category; median APA was 4.2% (range, 0.5%‐47.1%).
3.4. Thrombocytopenia in dogs due to other causes
Of the 36 dogs in the other category, 13 were female spayed, 2 were female intact, 18 were male neutered, and 3 were male intact. Median age was 6.5 years (range, 1‐12 years). Thirty‐one of the 36 (31/36, 86%) dogs were purebred and represented 26 breeds. Dogs in the other category had multiple final diagnoses but classifications with >1 representative included apparently healthy (n = 5), pancreatitis (n = 2), gastric ulceration (n = 2), pyelonephritis (n = 3), and rattlesnake bite (n = 3). It is note that dogs were classified as apparently healthy if no underlying disease was documented and dogs were otherwise healthy. Median platelet count was 96 500 platelets/μL (range, 2000‐198 000 platelets/μL). Four out of the 36 dogs (4/36, 11%) were positive for APA. Specific diseases associated with a positive APA included immune‐mediated hemolytic anemia (IMHA; n = 2), immune‐mediated neutropenia (IMN; n = 1), and bone marrow suppression from chlorambucil. For dogs in the other category; median APA was 1.8% (range 0.3%‐51.6%).
3.5. Client‐owned idiopathic/primary ITP dogs
3.5.1. Animals
Of the 28 dogs diagnosed with primary ITP, 15 were female spayed and 13 were male neutered dogs. Median age was 8 years (range 2‐13 years). Twenty‐four of the 28 dogs (24/28, 86%) were purebred dogs and represented 21 breeds. Median platelet count was 8000 platelets/μL (range 1000‐60 000 platelets/μL). All the dogs were positive for APA (median APA 37%; range, 11.5%‐93.8%).
3.5.2. Case presentation, survival to discharge, and follow‐up in idiopathic/primary ITP dogs
Clinical signs of bleeding was observed in most dogs (23/28, 82%) at presentation and included petechiation, ecchymoses, gingival bleeding, hematemesis, melena, and hematuria. However, thrombocytopenia was an incidental finding during a wellness examination in 5 dogs (5/28, 18%); median platelet count was 16 000 platelets/μL (range 12 000‐60 000 platelets/μL). Ten of the 28 dogs (10/28, 36%) presented with melena, and 5 of these dogs (5/10, 50%) were euthanized within 48 hours of presentation. Reasons for euthanasia included acute onset of a concurrent mesenteric torsion, financial constraints, and owner concern for suffering and quality of life. In total, 6 of the 28 (6/28, 21%) dogs were euthanized (n = 5) or died at home (n = 1) within 48 hours of presentation and 5 of these dogs (5/6, 83%) presented with melena. The presence of melena at initial evaluation was associated with a decreased probability for survival to discharge (P = .02). Overall, dogs that did not survive to discharge had lower percentages of APA (median 32%, range 14%‐44%), whereas survivors had variable percentages of APA at initial diagnosis (median 39%, range 11%‐94%). The initial mean percentage of APA at diagnosis was not statistically associated with survival to discharge (P = .06).
Twenty‐one of the 28 dogs (21/28, 75%) were evaluated for persistence of APA as defined by the study. Seven dogs could not be evaluated for persistence because they were not evaluated after 4 weeks into study due to being lost to follow‐up (n = 1), humane euthanasia (n = 5), or died at home (n = 1).
The remaining 21 dogs were followed serially for various periods of time (median 6 months; range, 2‐24 months) depending on the time of enrollment, clinician preference, and availability of for evaluation. Five additional dogs were deceased at the conclusion of the study; however, in 3 of the dogs, this was unrelated to ITP, progressive tracheal collapse, progressive TCC and urine obstruction, and generalized seizures. One dog had a relapse of ITP 10 months after initial diagnosis and owners chose to euthanize. Additionally, 1 dog was euthanized within 2 months of diagnosis due to suspected idiosyncratic mycophenolate toxicosis resulting in cutaneous lesions, increased serum activity of liver‐derived enzymes, and severe bone marrow suppression. Two of the 28 (2/28, 7%) dogs were lost to follow‐up at various time points after initial presentation (1 month and 2 months, respectively). Reasons for this included noncompliant owners and pet relinquishment to a shelter due to financial constraints.
3.5.3. Treatment
At initial evaluation, IV (n = 16) or PO (n = 10) glucocorticoids (range, 0.8‐2.6 mg/kg/day) were administered in 26 of the 28 dogs (26/28, 93%). Two dogs were initially not administered glucocorticoids due to concurrent conditions that could be exacerbated by glucocorticoids such as diabetes mellitus and urethral sphincter mechanism incompetence. Fifteen dogs (15/28, 54%) were administered vincristine (0.02 mg/kg IV once) at initial evaluation. Nine dogs (9/28, 32%) were administered ≥1 blood transfusions; 6 dogs received 1 transfusion, 2 dogs received 2 transfusions, and 1 dog received 3 transfusions. A second immunomodulatory drug in addition to glucocorticoids was started at initial evaluation in 21 dogs (21/28, 75%). Specifically, 16 dogs (16/21, 76%) were administered either IV (n = 2) or PO (n = 14) mycophenolate (range 8.8‐28.1 mg/kg/day); 2 dogs (2/21, 10%) were administered cyclosporine (range 4.5‐12.1 mg/kg/day); 1 dog (1/21, 5%) was administered cyclosporine (4.5 mg/kg/day) and azathioprine (1.1 mg/kg/day); 1 dog (1/21, 5%) was administered azathioprine (2.3 mg/kg/day); and 1 dog (1/21, 5%) was administered leflunomide (0.89 mg/kg/day). Twelve dogs (12/28, 43%) were administered doxycycline (range 5.1‐8 mg/kg twice daily or 10.1‐11.7 mg/kg once daily) for 14‐28 days.
Two dogs that were administered mycophenolate developed adverse effects; 1 dog developed severe diarrhea necessitating discontinuation of the medication and 1 dog presented with a hemoabdomen and was suspected to have suffered a severe idiosyncratic reaction from the drug resulting in cutaneous lesions (patchy erythema), increased serum liver enzyme activities, and severe bone marrow suppression. There were no reported adverse effects from cyclosporine administration. In the 1 dog that was administered leflunomide, severe diarrhea was observed within 12 hours post‐administration of this medication resulting in discontinuation; however, it was later reinstituted with no diarrhea observed; therefore, it is suspected that the diarrhea was not related to leflunomide.
3.5.4. Response to treatment
Twenty‐two dogs were evaluated for response to treatment as defined by the study (defined as occurring within 4 weeks of diagnosis); 18 dogs (18/22, 82%) were classified as having a response to treatment, whereas 4 dogs (4/22, 18%) did not. Four of the 18 dogs (4/18, 22%) that had a response to treatment remained positive for APA during this time period despite having normal platelet counts. Figure 1 illustrates dynamics of APA and platelet count in these 4 dogs. Two of the 4 dogs (2/4, 50%) that were classified as not having a response to treatment during this time frame remained positive for APA the entire time. Figure 2 illustrates the dynamics of APA and platelet count in these 4 dogs over time. There was no statistical difference in response to treatment between dogs with or without persistent antibodies. There was no statistical difference in the percent of APA between responders (n = 18, mean = 44.4, standard deviation [SD] = 24.2) and nonresponders (n = 4, mean = 56, SD = 32.6) at initial diagnosis (P = .42). There was no statistical difference in the platelet count between responders (n = 18, mean = 12.6, SD = 13.7) and nonresponders (n = 4, mean = 14.5, SD = 6.2) at initial diagnosis (P = 0.79). In dogs that had a response to treatment and were able to evaluated for a change in APA levels (n = 17), dogs became negative for APA within 3 to 67 days from initial diagnosis (mean approximately 18 days). In responders, the percent of APA at initial diagnosis was statistically higher than at time 2 (difference = −3.23, P = .0001), time 3 (−4.15, P < .0001), and time 4 (−3.62, P < .0001), and the platelet count at initial diagnosis was statistically lower than at all measured time points: time 1 (difference = 10.21, P = .0001), time 2 (14.74, P < .0001), time 3 (16.48, P < .0001), and time 4 (16.44, P < .0001). In nonresponders, the percent of APA and platelet count at initial diagnosis was not statistically different from any time points (time 1‐4): specifically, for the percent APA, time 1 (difference = −2.23, P = 0.42), time 2 (−2.93, 0.07), time 3 (−1.75, 0.78), and time 4 (−1.12, 0.85); for the platelet count: time 1 (difference = 2.53, P = 0.91), time 2 (7.16, 0.28), time 3 (6.58, 0.71), and time 4 (0.25, 0.99). When comparing responders to nonresponders, the percent APA was not statistically different at initial diagnosis (difference = 0.69, P = .66) or at time 1 (−0.52, .78), time 2 (0.99, P = .54), time 3 (3.09, P = .19) or time 4 (difference = 3.19, P = .09). When comparing responders to nonresponders, the platelet count was not statistically different at initial diagnosis (difference = 0.45, P = .89) or at time 1 (−7.23, P = .07), time 2 (−7.14, P = .05), or time 3 (−9.46, P = .14) but was statistically different at time 4 (−15.74, P = .001) where responders had higher platelet counts compared to nonresponders. There was no correlation between the mean percentage of APA and platelet count at initial diagnosis (r = .35, P = .11) or at 4 weeks after initial diagnosis (r = −0.29, P = 0.32) in responders and nonresponders.
Figure 1.
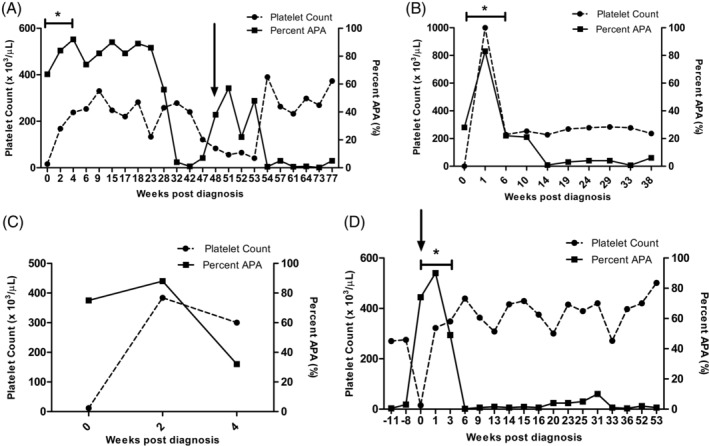
The platelet count and antiplatelet antibodies (shown as percent APA) at multiple recheck time points during study enrollment in 4 (4/18) client‐owned dogs that were diagnosed with idiopathic/primary immune thrombocytopenia (ITP) and that demonstrated a response to treatment and remained positive for APA during this time period (within 4 weeks of initial diagnosis) despite having normal platelet counts. A percent APA ≤10% is considered negative. A, Dog that demonstrated a response to treatment and also experienced a relapse. B, Dog that demonstrated a response to treatment but did not experience a relapse. C, Dog that demonstrated a response to treatment but was lost to follow‐up after 4 weeks. D, Dog with historical ITP that experienced a relapse while on treatment (was negative for APA at 8 and 11 weeks, respectively, before relapse) and then subsequently showed a response to treatment.*Indicates response to treatment. Response to treatment was defined as the platelet count returned ≥200 000 platelets/μL within 4 weeks of initial diagnosis. Black arrows indicates the time of relapse. Relapse/recurrence of ITP was defined as a platelet count dropping to ≤100 000 platelets/μL with few to no platelet clumping present
Figure 2.
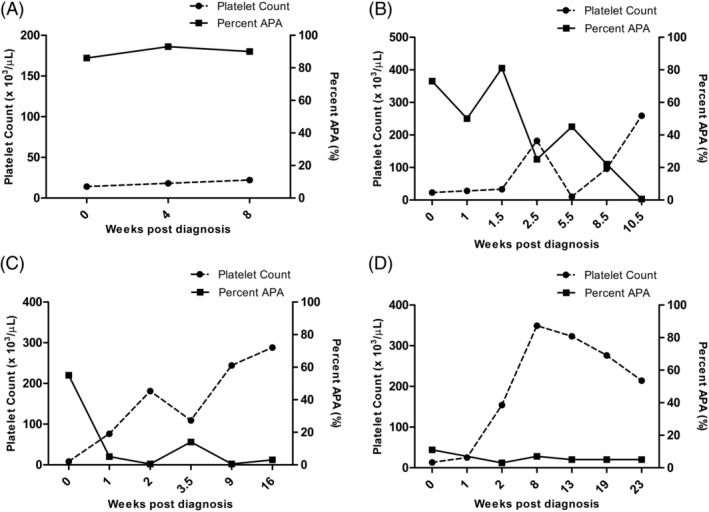
The platelet count and antiplatelet antibodies (shown as percent APA) at multiple recheck time points during study enrollment in 4 (4/4) client‐owned dogs that did not demonstrate a response to treatment and were diagnosed with idiopathic/primary immune thrombocytopenia (ITP). Response to treatment was defined as the platelet count returned ≥200 000 platelets/μL within 4 weeks of initial diagnosis. A percent APA ≤10% is considered negative. A, Dog that remained positive but was lost to follow‐up due to pet relinquishment. B, Dog that remained positive for >8 weeks following initial diagnosis. C, Dog that was intermittently positive during the study period. D, Dog that became quickly negative and remained negative throughout the study period
3.5.5. Relapse
Relapse was diagnosed in 2 of the 22 dogs (2/22, 9%) evaluated as defined by the study. Both dogs that relapsed were historical idiopathic ITP dogs that had already experienced a previous relapse. In 1 dog, 2 relapses occurred during the course of the study. The first relapse occurred 17 months after the initial diagnosis of idiopathic ITP, the second relapse occurred exactly 1 year after the first relapse, and the third relapse occurred exactly 1 year after the second relapse. In the second dog that relapsed, the first relapse occurred 10 months after the initial diagnosis of idiopathic ITP and the second relapse occurred 11 months after the first relapse. In the 2 dogs that relapsed, both had been previously negative for APA but were positive for APA (recurrence of antibodies) at the time of relapse. Relapse of ITP was associated with a recurrence of antibodies (P < .01).
3.5.6. Percentage binding for APAs in dogs considered APA positive
The percentage of APA varied greatly and had varying amounts of overlap between the different categories (idiopathic, infectious, neoplastic, other) and was not statistically significant (P = .49) (Figure 3).
Figure 3.
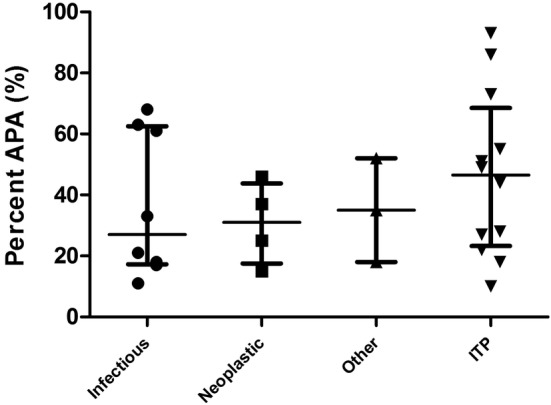
The percentage of antiplatelet antibodies (APA) were recorded for dogs that were divided into 4 categories: infectious, neoplastic, other, and immune thrombocytopenia (ITP). Only dogs that were classified as positive (APA >10%) for APAs are depicted graphically. The percentage of APA varied greatly and had varying amounts of overlap between the categories. Median and interquartile range are shown
4. DISCUSSION
In this study, APAs were documented in 100% of dogs diagnosed with primary/idiopathic ITP but were also documented in 20% of thrombocytopenic dogs with infectious, neoplastic, or other immune‐mediated conditions. All dogs diagnosed with idiopathic ITP were positive for APAs. In dogs with idiopathic ITP, the response to treatment was not associated with persistence of antibodies; however, relapse of ITP was associated with recurrence of antibodies. Serial evaluation of antibodies in conjunction with platelet count in dogs with idiopathic ITP could be beneficial for determining relapse.
Antiplatelet antibodies were documented in thrombocytopenic dogs with evidence of a number of different primary causes including idiopathic ITP which is in accordance with previous studies3, 5, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 further emphasizing the fact that these antibodies should not be used to differentiate primary from secondary ITP in dogs. For infectious disease etiologies, dogs with ehrlichiosis and anaplasmosis can be positive for APA, but to our knowledge, this is the first time this has been reported in a dog with disseminated Emmonsia spp. infection.8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Dogs with a variety of neoplasms can be positive for APA, but our study also documented antibodies in dogs with MCT and disseminated carcinoma which has not been previously documented.5, 8, 14, 16
For thrombocytopenic dogs in the other category, only a small number were documented to be positive for APA. Previous studies have documented these antibodies in IMHA and IMN which was in accordance with our study.5, 6 Additionally, antibodies have been detected in dogs with suspected secondary ITP from trimethoprim/sulfadiazine drug administration,8 but to our knowledge, this has not been documented with chlorambucil. In humans, a large number of drugs have been implicated in drug‐induced thrombocytopenia and can be associated with the formation of immunoglobulin‐associated platelets. The mechanisms behind drug‐induced thrombocytopenia are complex but include processes such as hapten, neoepitope, and autoantibody mechanisms.26, 27 As mentioned previously, only a small number of thrombocytopenic dogs in the other category were positive for APA. None of these dogs were subsequently diagnosed with idiopathic ITP or required immunosuppression. Thrombocytopenia in dogs lacking APAs is unlikely to be related to a humoral primary or secondary immune‐mediated process. Anecdotally, it has been theorized that the lower the platelet count, the more likely the process is to be immune‐mediated and potentially a primary immune‐mediated process. Although the median platelet count for the dogs in the other category was numerically higher than the median platelet count in dogs with idiopathic ITP, it is important to note that several of these dogs had severe thrombocytopenia and were initially thought to be idiopathic ITP. This misclassification could have resulted in additional financial burden to clients, the use of unnecessary medications, and potential negative outcomes if aggressive immunosuppression had been pursued.
All the dogs that were diagnosed with idiopathic ITP in this study were positive for APA. This is in contrast to previous studies where some dogs diagnosed with idiopathic ITP were documented to be negative for antibodies.3, 16, 28 Possibilities for this include differences in study design, assay methodology, or non‐antibody‐mediated mechanisms for thrombocytopenia. For example, only 60% to 70% of people are documented to have antibodies potentially indicating that non‐antibody‐dependent mechanisms are responsible for platelet destruction.2, 18 When considering platelet destruction in humans with ITP who lack antibodies, CD8+ T cells result in direct platelet destruction and are also involved in subsequent platelet desialylation.2, 18, 29, 30, 31, 32 It is unknown if these mechanisms occur in dogs.18
When considering factors such as survival to discharge in dogs with idiopathic ITP, melena is a poor prognostic indicator.33 Possible reasons include the need for blood transfusions and longer hospitalizations so dogs may be euthanized due to financial constraints. Additionally, dogs with melena can often appear much more clinically ill than other dogs affected by ITP which can also lead to humane euthanasia if owners feel that the quality of life is not acceptable. However, a possible confounding factor in our study was that 3 dogs were receiving concurrent nonsteroidal anti‐inflammatory drugs (NSAIDs—meloxicam or aspirin). Nonsteroidal anti‐inflammatory drugs affect platelet function and are associated with gastrointestinal bleeding in dogs.34, 35 Therefore, it is suspected that concurrent NSAID use could have contributed to the observed melena and overall decreased survival to discharge and underlines the importance of thorough history taking upon initial evaluation. However, the presence of melena could be associated with a worse outcome and decreased survival to discharge. Additionally, in our study, there was no association between the initial mean percentage of APA at diagnosis and survival to discharge. Future studies investigating the relationship between APA percentage at initial diagnosis and survival to discharge in dogs with idiopathic ITP could be performed.
There was no difference in the percentage of APA or platelet count at initial diagnosis between dogs that did or did not respond to treatment. In other words, there was no evidence of an association between the magnitude of APA positivity or degree of thrombocytopenia and response. Additionally, there was no statistical difference in trends of percent of APA (at any time point) or in platelet count between responders and nonresponders until approximately within 5‐6 weeks following initial diagnosis. From our correlation analysis, the percent of APA and platelet count did not correlate at either time point in responders or nonresponders. For example, if a dog (responder or nonresponder) was severely thrombocytopenic, the percent of APA could be lower (eg, 17%) or higher (eg, 77%) and thus varied.
Persistence of APAs was not associated with lack of response to treatment as defined in this study. In humans, there have been contradictory results but lack of antibodies at baseline is associated with a decreased response to rituximab which is considered a common second‐line treatment in humans with ITP and works by depleting B cells.24 Additionally, the persistence of these antibodies in people after treatment for ITP can indicate more severe disease.20
In contrast to assessing response to treatment, the dynamics of APA did appear to be clinically relevant because the recurrence of antibodies was associated with relapse. Specifically, 1 of the dogs that relapsed had initially remained persistently positive for APA for months but had become negative and was doing clinically well. However, over time, the dog became positive again with a gradually decreasing platelet count. Of interest, peak cyclosporine levels had been evaluated (Clinical Pharmacology Laboratory, Auburn University, College of Veterinary Medicine) in addition to an assay evaluating activated T‐cell mRNA IL‐2 expression (Pharmacodynamic Laboratory, Mississippi State University, College of Veterinary Medicine) to assess appropriate immunosuppression on cyclosporine treatment. The peak cyclosporine level was at the high end of the recommended range, and the IL‐2 assay was consistent with maximal immunosuppression. Despite these results, the dog relapsed on cyclosporine treatment alone and glucocorticoids were reinstituted which resulted in a rapid rebound in platelet count (527 000 platelets/μL) and the APA became negative.
The documentation of recurrence of antibodies was helpful in both dogs that relapsed because it helped to confirm that an active immune process was likely contributing to the thrombocytopenia and that it seemed unlikely to be related to benign causes such as method of venipuncture or only exuberant platelet clumping. However, questionable thrombocytopenic samples should always be evaluated by a clinical pathologist or simply repeated via clean venipuncture by a skilled individual with or without fresh blood smear evaluation. In clinical practice, dogs with idiopathic ITP are typically monitored by evaluating trends in platelet counts but it can be difficult to discern if a relapse is occurring in some scenarios. In addition to platelet count, mean platelet volume (MPV) can be monitored; however, multiple mechanisms other than immune processes can result in an increased MPV so it is unlikely this can be used for determining relapse in dogs with ITP.36, 37, 38, 39 Additionally, there are variable results as to whether dogs with idiopathic ITP consistently have an increased MPV.16, 40 It is important to determine if a relapse is occurring because not only can life‐threatening bleeding events occur but it is important for appropriate case management and client communication. Therefore, when monitoring dogs diagnosed with idiopathic ITP over time, dynamics of antibodies can be clinically useful when determining if a relapse has occurred or possibly if there is concurrent active disease (such as infectious or neoplastic causes).
There were several limitations to this study not previously mentioned. This study focused on whether a dog was considered positive or negative based on our cutoff values25 in order to investigate variables such as response to treatment with persistence or recurrence of antibodies. Analyses utilizing the actual percentages of APA were also pursued but there are some limitations that should be discussed. One limitation is that the percent of APA can vary within the previously validated 72‐hour period and samples for our study were not all collected at a single designated time point but rather analyzed within 72 hours.25 However, our study objective was to evaluate the dynamics of APA (ie, whether a dog is positive or negative) versus evaluating the trends of the actual percentages (ie, whether a dog decreases from 80% to 40%). However, this study could be pursued in the future and could help better characterize APA in dogs with ITP. How the dynamics of antibodies were related to specific treatment protocols could have been investigated but the study design did not dictate treatment protocols. This was not pursued because the aim of the study was to evaluate these antibodies over time in dogs diagnosed with idiopathic ITP regardless of treatment protocol. A second limitation includes that the dogs were not monitored at specific follow‐up times. Therefore, it is unknown exactly when individual dogs became positive or negative for APA during the course of treatment. However, unless the dogs were sampled daily for months, this would also have been true even if weekly evaluations had been performed in all dogs. Our study evaluated dogs for persistence or recurrence of antibodies over time at each recheck appointment and was not designed to determine the specific time it takes to shift from positive to negative or vice versa. Power and sample size calculations were not performed, which is a limitation of this study, and results should be evaluated accordingly. Additionally, because of this limitation, there is the possibility of a type II error and future studies with sample size calculations should be pursued. Another limitation of the study could include the smaller number of dogs enrolled that were diagnosed with idiopathic ITP. However, most of the dogs were evaluated for prolonged periods of time which garnered additional data for evaluation. An additional limitation to consider was the definition of what was considered a response to treatment for dogs with idiopathic ITP. If this definition had been less stringent or categorized dogs as having a partial or full response, the results could have been different and is a consideration for future studies in dogs when evaluating the association between response and APA percentage.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the Colorado State University Institutional Clinical Review Board.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Breed, final diagnosis, and platelet count in thrombocytopenic dogs due to causes other than idiopathic/primary, infectious, or neoplastic.
ACKNOWLEDGMENTS
We thank the Center for Companion Animal Studies for providing the funding for this study, Dr. Christian Leutenegger for his help in providing samples from IDEXX, Dr. Ann Hess for her guidance for the statistical analysis, the staff in Clinical Pathology at CSU for helping to collect our samples, Drs. Allison Bradley, Meredith Sherrill, and Cynthia Panek to help recruit outside cases, the interns, faculty, and internal medicine residents for recruiting internal cases, the internal medicine nurses for ensuring appropriate sample collection, and Jonathan Coy for his help in setting up the flow protocol.
Shropshire S, Dow S, Lappin M. Detection and dynamics of anti‐platelet antibodies in thrombocytopenic dogs with and without idiopathic immune thrombocytopenia. J Vet Intern Med. 2020;34:700–709. 10.1111/jvim.15737
Funding information Center for Companion Animal Studies
REFERENCES
- 1. Neel JA, Birkenheuer AJ, Grindem CB. Thrombocytopenia In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XV. St. Louis, MO: Saunders Elsevier; 2014:280‐291. [Google Scholar]
- 2. Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16:620‐632. [DOI] [PubMed] [Google Scholar]
- 3. Bachman DE, Forman MA, Hostutler RA, Corn S, Lin J, Kociba GJ. Prospective diagnostic accuracy evaluation and clinical utilization of a modified assay for platelet‐associated immunoglobulin in thrombocytopenic dogs. Vet Clin Pathol. 2015;44:355‐368. [DOI] [PubMed] [Google Scholar]
- 4. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168‐187. [DOI] [PubMed] [Google Scholar]
- 5. Kristensen AT, Weiss DJ, Klausner JS, Laber J, Christie DJ. Detection of antiplatelet antibody with a platelet immunofluorescence assay. J Vet Intern Med. 1994;8:36‐39. [DOI] [PubMed] [Google Scholar]
- 6. Kristensen AT, Weiss DJ, Klausner JS, Laber J, Christie DJ. Comparison of microscopic and flow cytometric detection of platelet antibody in dogs suspected of having immune‐mediated thrombocytopenia. Am J Vet Res. 1994;55:1111‐1114. [PubMed] [Google Scholar]
- 7. Lewis DC, McVey DS, Shuman WS, Muller WB. Development and characterization of a flow cytometric assay for detection of platelet‐bound immunoglobulin G in dogs. Am J Vet Res. 1995;56:1555‐1558. [PubMed] [Google Scholar]
- 8. Lewis DC, Meyers KM, Callan MB, Bücheler J, Giger U. Detection of platelet‐bound and serum platelet‐bindable antibodies for diagnosis of idiopathic thrombocytopenic purpura in dogs. J Am Vet Med Assoc. 1995;206:47‐52. [PubMed] [Google Scholar]
- 9. Scott MA, Kaiser L, Davis JM, Schwartz KA. Development of a sensitive immunoradiometric assay for detection of platelet surface‐associated immunoglobulins in thrombocytopenic dogs. Am J Vet Res. 2002;63:124‐129. [DOI] [PubMed] [Google Scholar]
- 10. Waner T, Harrus S, Weiss DJ, Bark H, Keysary A. Demonstration of serum antiplatelet antibodies in experimental acute ehrlichiosis. Vet Immunol Immunopathol. 1995;48:177‐182. [DOI] [PubMed] [Google Scholar]
- 11. Harrus S, Waner T, Weiss DJ, Keysary A, Bark H. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol. 1996;51:13‐20. [DOI] [PubMed] [Google Scholar]
- 12. Grindem CB, Breitschwerdt EB, Perkins PC, Cullins LD, Thomas TJ, Hegarty BC. Platelet‐associated immunoglobulin (antiplatelet antibody) in canine Rocky Mountain spotted fever and ehrlichiosis. J Am Anim Hosp Assoc. 1999;35:56‐61. [DOI] [PubMed] [Google Scholar]
- 13. Waner T, Leykin I, Shinitsky M, et al. Detection of platelet‐bound antibodies in beagle dogs after artificial infection with Ehrlichia canis. Vet Immunol Immunopathol. 2000;77:145‐150. [DOI] [PubMed] [Google Scholar]
- 14. Kohn B, Engelbrecht R, Leibold W, Giger U. Clinical findings, diagnostics and treatment results in primary and secondary immune‐mediated thrombocytopenia in the dog. Kleintierpraxis. 2000;45:893‐907. [Google Scholar]
- 15. Terrazzano G, Cortese L, Piantedosi D, et al. Presence of anti‐platelet IgM and IgG antibodies in dogs naturally infected by Leishmania infantum. Vet Immunol Immunopathol. 2006;110:331‐337. [DOI] [PubMed] [Google Scholar]
- 16. Dircks BH, Schuberth HJ, Mischke R. Underlying diseases and clinicopathologic variables of thrombocytopenic dogs with and without platelet‐bound antibodies detected by use of a flow cytometric assay: 83 cases (2004–2006). J Am Vet Med Assoc. 2009;235:960‐966. [DOI] [PubMed] [Google Scholar]
- 17. Cortese L, Terrazzano G, Piantedosi D, et al. Prevalence of anti‐platelet antibodies in dogs naturally co‐infected by Leishmania infantum and Ehrlichia canis. Vet J. 2011;188:118‐121. [DOI] [PubMed] [Google Scholar]
- 18. Chow L, Aslam R, Speck ER, et al. A murine model of severe immune thrombocytopenia is induced by antibody‐ and CD8+ T cell‐mediated responses that are differentially sensitive to therapy. Blood. 2010;115:1247‐1253. [DOI] [PubMed] [Google Scholar]
- 19. Xu XR, Zhang D, Oswald BE, et al. Platelets are versatile cells: New disoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53:409‐430. [DOI] [PubMed] [Google Scholar]
- 20. Arnold DM, Vrbensky JR, Karim N, et al. The effect of rituximab on anti‐platelet autoantibody levels in patients with immune thrombocytopenia. Br J Haematol. 2017;178:302‐307. [DOI] [PubMed] [Google Scholar]
- 21. Arnold DM, Santoso S, Greinacher A. Platelet Immunology Scientific Subcommittee of the ISTH. Recommendations for the implementation of platelet autoantibody testing in clinical trials of immune thrombocytopenia. J Thromb Haemost. 2012;10:695‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sikorska A, Konopka L, Maślanka K. The effect of platelet autoantibodies on the course of the disease and clinical response of patients with idiopathic thrombocytopenic purpura. Int J Lab Hematol. 2008;30:58‐64. [DOI] [PubMed] [Google Scholar]
- 23. Fabris F, Scandellari R, Ruzzon E, Randi ML, Luzzatto G, Girolami A. Platelet‐associated autoantibodies as detected by a solid‐phase modified antigen capture ELISA test (MACE) are a useful prognostic factor in idiopathic thrombocytopenic purpura. Blood. 2004;103:4562‐4564. [DOI] [PubMed] [Google Scholar]
- 24. Porcelijn L, Huiskes E, Schipperus M, van der Holt B, de Haas M, Zwaginga JJ. Dutch HOVON 64 Study Group. Lack of detectable platelet autoantibodies is correlated with nonresponsiveness to rituximab treatment in ITP patients. Blood. 2017;129:3389‐3391. [DOI] [PubMed] [Google Scholar]
- 25. Shropshire SB, Dow S, Lappin M. Validation of a clinically applicable flow cytometric assay for the detection of immunoglobulin associated platelets in dogs. Vet Immunol Immunopathol. 2018;202:109‐114. [DOI] [PubMed] [Google Scholar]
- 26. Visentin GP, Liu CY. Drug‐induced thrombocytopenia. Hematol Oncol Clin North Am. 2007;21:685‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curtis BR. Drug‐induced immune thrombocytopenia: incidence, clinical features, laboratory testing, and pathogenic mechanisms. Immunohematology. 2014;30:55‐65. [PubMed] [Google Scholar]
- 28. Wilkerson MJ, Shuman W, Swist S, Harkin K, Meinkoth J, Kocan AA. Platelet size, platelet surface‐associated IgG, and reticulated platelets in dogs with immune‐mediated thrombocytopenia. Vet Clin Pathol. 2001;30:141‐149. [DOI] [PubMed] [Google Scholar]
- 29. Olsson B, Andersson PO, Jernås M, et al. T‐cell‐mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123‐1124. [DOI] [PubMed] [Google Scholar]
- 30. Zhang F, Chu X, Wang L, et al. Cell‐mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2006;76:427‐431. [DOI] [PubMed] [Google Scholar]
- 31. Zhou H, Qiu JH, Wang T, et al. Interleukin 27 inhibits cytotoxic T‐lymphocyte‐mediated platelet destruction in primary immune thrombocytopenia. Blood. 2014;124:3316‐3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiu J, Liu X, Li X, et al. CD8+ T cells induce platelet clearance in the liver via platelet desialylation in immune thrombocytopenia. Sci Rep. 2016;6:27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346‐352. [DOI] [PubMed] [Google Scholar]
- 34. Khan SA, McLean MK. Toxicology of frequently encountered nonsteroidal anti‐inflammatory drugs in dogs and cats. Vet Clin North Am Small Anim Pract. 2012;42:289‐306. [DOI] [PubMed] [Google Scholar]
- 35. Mullins KB, Thomason JM, Lunsford KV, et al. Effects of carprofen, meloxicam and deracoxib on platelet function in dogs. Vet Anaesth Analg. 2012;39:206‐217. [DOI] [PubMed] [Google Scholar]
- 36. Handagama P, Feldman B, Kono C, Farver T. Mean platelet volume artifacts: the effect of anticoagulants and temperature on canine platelets. Vet Clin Pathol. 1986;15:13‐17. [DOI] [PubMed] [Google Scholar]
- 37. Sullivan PS, Manning KL, McDonald TP. Association of mean platelet volume and bone marrow megakaryocytopoiesis in thrombocytopenic dogs: 60 cases (1984‐1993). J Am Vet Med Assoc. 1995;206:332‐334. [PubMed] [Google Scholar]
- 38. Bommer NX, Shaw DJ, Milne EM, Ridyard AE. Platelet distribution width and mean platelet volume in the interpretation of thrombocytopenia in dogs. J Small Anim Pract. 2008;49:518‐524. [DOI] [PubMed] [Google Scholar]
- 39. Llewellyn EA, Todd JM, Sharkey LC, Rendahl A. A pilot study evaluating the prognostic utility of platelet indices in dogs with septic peritonitis. J Vet Emerg Crit Care (San Antonio). 2017;27:569‐578. [DOI] [PubMed] [Google Scholar]
- 40. Schwartz D, Sharkey L, Armstrong PJ, Knudson C, Kelley J. Platelet volume and plateletcrit in dogs with presumed primary immune‐mediated thrombocytopenia. J Vet Intern Med. 2014;28:1575‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Breed, final diagnosis, and platelet count in thrombocytopenic dogs due to causes other than idiopathic/primary, infectious, or neoplastic.


