Abstract
Backgrounds
Throughout the world, there exists a clear need for the maintenance of cancer statistics, forming an essential part of any rational programme of cancer control, health-care planning, etiological research, primary and secondary prevention, benefiting both individuals and society. The present work reports only on the prevalence of cancers in the Oncology Department of Jamhuriyat Hospital, Kabul, Afghanistan following several decades of war.
Materials and methods
A quantitative retrospective cross-sectional study was conducted using the medical records of patients diagnosed and treated from October 2015 to December 2017. Data includes information on gender, age, economic status, address and types of cancer diagnosed. The data was transferred to a customized form and analysed using Microsoft Excel program to classify cancer types.
Results
The total number of patients with completed documents were 1025. Of these, 403 (39.3%) were male and 622 (60.7%) female. Most of the patients were in the age range of 20–70 years old. The most common cancers in women were breast cancer (45.8%), followed by oesophagus (12.5%), colorectal (4.8%), Non-Hodgkin Lymphoma (4.7%), sarcoma (4.7%), ovary (3.8%), both stomach and liver (2.6%) and cervix uteri (1.9%). Contrarily to men, esophageal cancer was highest (21.8%), followed by stomach (12.2%), Non-Hodgkin Lymphoma (9.4%), sarcoma (8.9%), gastroesophageal junction (8.9%), colorectal (8.6%), Hodgkin lymphoma (4.7%), testis (4.2%), liver (3.2%), lung (2.7%) and Nonmelanoma skin squamous cell carcinoma 9 (2.2%).
Conclusion
Results showed that the most frequent cancers among Afghans were breast and oesophagus. The most common cancer in men was oesophagus and stomach at the age range of 50–70 years while in women, breast and oesophagus cancers were common and within the age range of 25–65 years old.
Keywords: Cancer research, Epidemiology, Public health, Oncology, Women's health, Afghanistan, Cancer prevalence, Kabul Jamhoriyat hospital
Cancer research; Epidemiology; Public health; Oncology; Women's health; Oncology; Afghanistan; Cancer prevalence; Kabul Jamhoriyat hospital.
1. Introduction
Around the world, cancers are one of the leading causes of death. According to WHO, globally the annual number of people dying because of cancer is around 8.2 million. In addition, nearly three in four cancer patients live in countries with low and middle incomes. Cancer survival rates in developing countries are lower than one-third of that in developed countries. According to WHO in 2012, about 15 thousand (8100 men and 7400 women) died because of cancers in Afghanistan.
WHO announced that life expectancy at birth in Afghanistan is 60 year, which is 58 year for male and 61 years for female. The total population of Afghanistan is estimated about 29825000, and age-standardized cancer mortality in 2012; in men, due to stomach cancer 24, oesophagus 15, lymphoma and multiple melanoma 13, mouth and oropharynx 12, and trachea, bronchus and lung 9 per 100 000, while for women, breast 25, stomach 11, cervix uteri and oesophagus each one 9, mouth and oropharynx 7 per 100 000. The most frequent new cases in men belong to the stomach and esophageal cancers, while in women breast and stomach were common [1].
Defining the distribution of various cancer in different populations and over time has been a highly effective way of developing hypotheses about causation and in quantifying the potential for preventive activities [2]. These play a critical role in the development and implementation of cancer control policy, be it through the identification of cancer problems, decisions on priorities for preventive and curative programs, outcome evaluation of programs of prevention, and early detection/screening and treatment concerning resource inputs.
Over several decades, a series of estimates of the global burden of cancer have been published on various international journals, as the International Journal of Cancer, as well as via organizations such as the WHO and IAEA. To build up a global picture, the various estimates essentially rely upon country-level best available data on cancer incidence and/or mortality. The results will be more or less accurate for different countries, depending upon the extent and accuracy of locally available data. Afghanistan is one of the countries in which evidence-based data is least available, a matter driving present interest in improving the situation.
It is worth mentioning that, this study is conducted in Jamhuriat Hospital of Kabul, which is the only available cancer centre in the country that people have access to (Referral Hospital). Majority of patients are referred from all parts of Afghanistan to this hospital to receive cancer treatment and services. Therefore, the result of this study could be regarded as an estimation of cancer distribution and profile for the whole country.
1.1. Background
The first known cancer study in Afghanistan was by Leslie Sobin in 1969 [3], a Visiting Professor from the US National Institute of Health working in the Pathology Department of Kabul University. His study, which included 895 cases, observed that among Afghans the common cancers in those geographical locations accessible to the study were skin cancer, cancers of the lymphoid and hematopoietic systems, soft tissues, eyes, breast and testis. More deeply located cancers included those of the stomach, oesophagus and ovary. In men, the relative prevalence of cancers was skin cancer (17.4%), lymphoma (12.3%), eye and soft tissue, both (7.2%). Most of the patients were within the age range of 35–64 years old. Common cancers among women were breast cancer (15.2%), skin (14.1%), soft tissues (9.6%) and ovary (6.8%). In 2014, WHO reported that Afghan women, with breast and stomach had the greatest mortality cancers while among men it was stomach and oesophagus [1].
Another study including 350 cancer patients was conducted by researchers from Batra Hospital and Medical Research Centre (New Delhi) and published as an abstract in J Clin Oncol in 2009. It was reported that the most common cancers among Afghans were oesophagus, lung and breast cancers [4]. In a study, published in 2016 by the Information Centre on Human Papillomavirus HPV of the Catalan Institute of Oncology, it was observed that in Afghanistan there were 8.77 million women of age greater than 15 years, a group acknowledged to be susceptible to cervical cancer. The study reported that annual crude incident rate of breast, cervix uteri, corpus uteri and stomach cancers in all ages to be 19.3, 5.3, 4.5 and 3.8 per 100 000 women per year respectively. In contrast, the crude mortality rate for breast, cervix uteri, corpus uteri and stomach cancers in all ages were 10.5, 3.5, 1.9 and 3.6 per 100 000 women per year respectively. In addition, about 862 new cervical cancer cases are diagnosed and about 570 cervical cancer deaths occur annually in Afghanistan. Age standardized incident and mortality rates for cervical cancer was 8.8 and 6.9 compared to the region's countries respectively (estimations for 2012) [5].
1.2. Objective
This study examines the distribution of different types of cancers diagnosed from October 2015 to December 2017 in the Cancer Centre of Jamhuriyat Hospital in Kabul, the first time since the Oncology Department had been re-established following decades of war. This is a hospital-based study and does not fully represent the cancer profile of the whole country.
2. Materials and methods
In this quantitative retrospective cross-sectional study, the information was derived from the medical records of patients registered, diagnosed and treated in the oncology ward of Jamhuriyat Hospital from October 2015 to December 2017. The information included demographic data and types of cancer diagnosed/recorded, socio-economic status, treatment consent letter, initial complains, present illness, history; drug, allergy and immunization history, personal, occupational and habitual history, family history; physical, imaging and pathological examinations. The medical document also includes chemotherapy and surgery protocols, medicine administration chart, consultation sheet, and nursing history. Microsoft Excel was used to sort the information according to the type of cancer, age, sex, and economic situation (based on the average income of middle-class people as a ‘fair income’) of patients. There was no specific system and formalities for determining the economic situation of people in Afghanistan. The three-level of good, fair and poor classification was based on the verbal declaration of the patients or their companions. The age distribution is designed by 5 years increment starting from 0 up to 100 years.
The study was hospital-based research and covers only statistics of those cancer patients who were referred and admitted to the Oncology department of Jumhuriyet Hospital and their medical history recorded. The data does not show the incidence or prevalence of cancers all over Afghanistan. Because a great but unknown number of patients go to India, Pakistan and Iran, and also to some other developed countries for their diagnosis and treatment.
3. Results
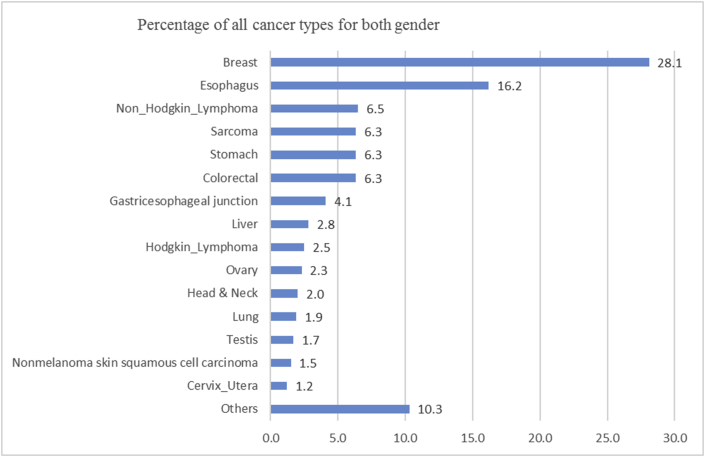
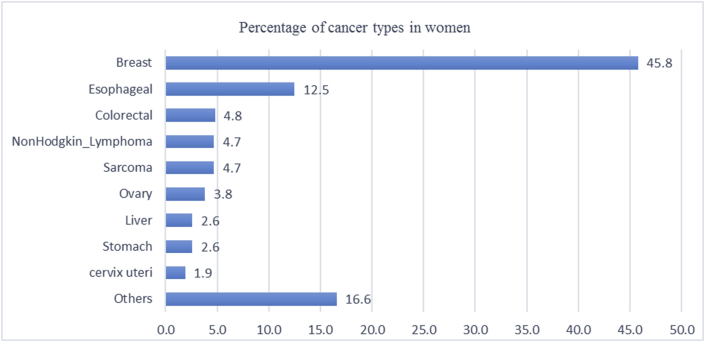
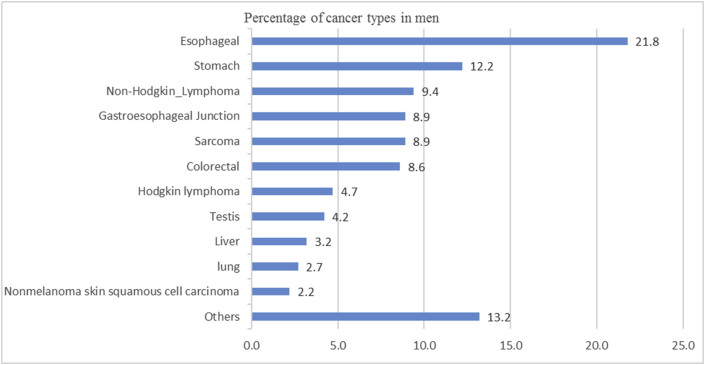
Initially, a total of 1254 cases were included for pre-evaluation and data collection. During the extraction of information from medical records and data registration, it was found that due to shortage of a standardized medical recording system and less experienced staff, some of the documents did not contain necessary information required for the study. Patients records with missing information such as; age, sex, diagnosis and economical status (229 cases) were excluded. With the remaining 1025 cases, 403 (39.3%) were males and 622 (60.7%) being female. Age distribution showed that the age range of female patients was from 2 to 100 years, with a mode age range of 46–50 years old, while for male, the corresponding age range was from 10 months to 100 years, with a mode age range of 56–60 years old. The median age for all cancer was 50 year, but for breast cancer 45.5 year, 58 year for oesophagus and 30 year for sarcoma. The most frequent cancers recorded were breast 288 (28.1%), oesophagus 166 (16.2%), Non-Hodgkin Lymphoma 67 (6.5%), colorectal, stomach and sarcoma each one 65 (6.3%) (Figure 1). It was determined that in women, the most frequent cancer was breast cancer 285 (45.8%), followed by oesophagus 78 (12.5%), colorectal 30 (4.8%), Non-Hodgkin Lymphoma 29 (4.7%), sarcoma 29 (4.7%), ovary 24 (3.8%), both stomach and liver 16 (2.6%) and cervix uteri 12 (1.9%) (Figure 2). Also, the most frequent cancer in men was oesophagus 88 (21.8%), followed by stomach 49 (12.2%), Non Hodgkin Lymphoma 38 (9.4%), sarcoma 36 (8.9%), gastroesophageal junction 36 (8.9%), colorectal 35 (8.6%), Hodgkin lymphoma 19 (4.7%), testis 17 (4.2%), liver 13 (3.2%), lung 11 (2.7%), and Nonmelanoma skin squamous cell carcinoma 9 (2.2%) (Figure 3).
Figure 1.
Prevalence of cancers diagnosed at Jamhuriyat Hospital.
Figure 2.
Prevalence of cancers in women diagnosed at Jamhuriyat Hospital.
Figure 3.
Prevalence of cancers in men diagnosed at Jamhuriyat Hospital.
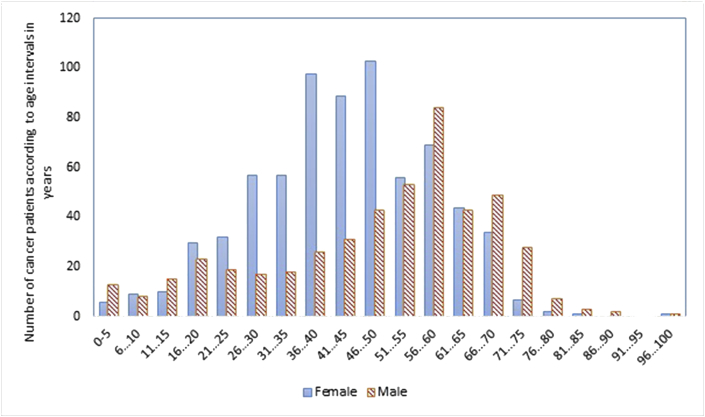
Based on the verbal declaration of the patients or their relatives 20.69% of them had a good economic situation followed by 17.93% having a middle level of income and 61.38% being poor. The number of women with cancer peaks in their 40s, while for men, it was in their 60's (Figure 4). No exact data about the stages of cancer exist, but as the in-charge doctor stated, at the time of admission, roughly 70% of patients were in the advanced stage and the rest were in the earlier stages.
Figure 4.
Patients' age distribution.
4. Discussion
The study by Sobin in 1968 showed that the most commonly observed cancers amongst Afghan men were of the skin, followed by lymphoma, eye and soft tissue, with most patients being in the age of 35–64 years old. Conversely among women, the most prevalent cancers were of the breast, skin, soft tissues and ovary, with patient age ranging between 35-64 years old [3]. However, based on the present study, the most common cancer among Afghans reporting at Jamhuriyat Hospital was breast cancer (28.0%), followed by oesophageal cancer (16.2%), Non-Hodgkin Lymphoma (6.5%), colorectal and sarcoma (6.3%). The most reported cancers in men was oesophagus (21.8%), stomach (12.2%), Non-Hodgkin Lymphoma (9.4%), sarcoma (8.9%), gastroesophageal junction (8.9%), colorectal (8.6%), Hodgkin lymphoma (4.7%), testis (4.2%), liver (3.2%), lung (2.7%) and Nonmelanoma skin squamous cell carcinoma (2.2%).
In women, the highest common cancers was: breast cancer (45.8%), followed by oesophagus (12.5%), colorectal (4.8%), sarcoma (4.7%), Non-Hodgkin Lymphoma (4.7%), ovary (3.8%), both stomach and liver (2.6%) and cervix uteri (1.9%). While present studies show the most common cancer among Afghan women to remain breast cancer, for men present results differ from that found in the 1968 study, as summarised above. Studies agree that the common cancers amongst Afghan people are of the breast and oesophagus, which is followed by lymphoma, stomach, colorectal and sarcoma. Amori et. al investigated the most common cancers in Iran during 2004–2008 and showed that the most common cancers in males were skin, stomach, bladder, prostate and colorectal, while in females breast, skin, colorectal, stomach and haematocyte were common [6].
In a study conducted in Afghanistan, investigating epidemiological profile of oesophageal cancer among patients in northern parts of the country, they reported that 92 cases out of 364 people had oesophageal cancer. In this study male to female ratio was 1.8:1 [7]. The prevalence of oesophageal cancer is very low in developed and western countries in compared to Asian countries [8]. The high prevalence of oesophageal cancer in the country indicates that Afghanistan (especially northern parts) lies in the Central Asian Esophageal Cancer Belt [9, 10]. In a study conducted in Iran showed higher incidence and prevalence rates of oesophageal cancer was common in western, northwest, northern and northeast provinces of Iran, which was also more common among males [11].
Breast cancer was the most common cancer among females; both in Afghanistan and worldwide [12, 13]. The prevalence of this cancer is higher in developed countries than developing ones, but it is increasing in developing countries as well. For example, in Iran 76% of female cancers are breast cancer [6, 14, 15]. According to this study, 45.8% of female cancers were breast cancer.
Recent studies have reported that lifestyle of Afghans is an important predisposing factor such as; Afghans drink lots of hot tea, which is a known risk factor for oesophageal cancer [16, 17, 18], eat more dried meat processed by salt which is kept up to 6 months to one year, lots of oily food, which are all known risk factors for cancers [19, 20, 21]. They also use hookah and cigarettes and they are so commonly exposed to the smoke of burning woods and animal faeces [22, 23]. Furthermore, majority of Afghan men and some women use snuff (dried tobacco + ash). Again Afghan women, due to household chores might have a lack of vitamin D intake and sun exposures which are known risk factors for breast cancer and other cancers [24, 25, 26].
In comparison, Maryam Taiyebi et al. study in Babulsar City in the north of Iran, showed that breast cancer in women account for 41.4% of all cancer cases in that city, followed by oesophageal cancer (8.0%). Whiles in men, oesophageal cancer was the commonest likely to be associated with lifestyle [27]. Bhurgri also has reported that cancer distribution in Pakistan was as follows: for males: Mouth/pharynx, lung, larynx, bladder, prostate, lymphoma and colorectal; and for females: breast, mouth/pharynx, cervix, oesophagus, ovary, lymphoma and gall bladder [28].
It is worth mentioning that, this study is conducted in Jamhuriat Hospital of Kabul, which is the only available cancer centre in the country that people have access to (Referral Hospital). Majority of patients are referred from all parts of Afghanistan to this hospital to receive cancer treatment services. Therefore, the result of this study could be regarded as an estimation of cancer distribution and profile for the whole country. However, it does not show a complete true map of cancers in Afghanistan, because a great but unknown number of patients go to India, Pakistan and Iran, and also to some other developed countries for their diagnosis and treatment.
5. Conclusion
The results of this research showed that the age range of cancer patients visiting Kabul Jamhuriyat hospital from 2015- 2017 was 10 months–100 years old, with majority between 20-70 years of age. Overall, around 50 types of cancers were diagnosed and received available treatment in the oncology ward, the most common of which were breast, oesophagus, lymphoma, stomach, colorectal and sarcoma.
The importance of cancer registry relies on its completeness and accuracy, which can be used for cancer control, epidemiological research, public health program planning and patient care programs. This research certainly reflects some notable shortcomings, including lack of standardized data recording system, the relatively low number of cases contained within the survey itself, also innately highlighting the lacking in health provisions in standard diagnostic facilities, lack of a sizeable expert oncology team and with all these, the potential for low accuracy of data regarding age and economic situations. Nevertheless, it does provide for the first time an estimated profile of cancer spread in present-day Afghanistan, which can also allow a degree of guidance towards designing cancer control programs and further comprehensive research about cancer prevalence and risk factors in the country.
Fortunately, Afghanistan's national cancer registry system has been opened recently and started registry of patients dispute all the short falls. The aim is to change the country national cancer registry centre to a developed and standardized database centre of cancer profile for the whole country. The information gathers in this information bank will help the country's health management system to draw a clear perspective for the future management and burden of cancer.
Declarations
Author contribution statement
Musa Joya: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zabihullah Stanikzai: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Isa Akbarzadeh: Analyzed and interpreted the data; Wrote the paper.
Somayyeh Babaloui: Analyzed and interpreted the data.
David.A. Bradley, Shakardokht M. Jafari: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at Mendeley Data, https://doi.org/10.17632/jvpfk7y8f9.
The clinical trial described in this paper was registered at Mendeley Data.
Acknowledgements
The first author would like to thanks Mr Abdullah Mayhan the Oncology Project Manager for his help and assistance.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.WHO . 2014. Cancer Country Profiles.https://www.who.int/cancer/country-profiles/afg_en.pdf?ua=1 Retrieved April 27, 2016. [Google Scholar]
- 2.Tomatis L., Aitio A. Oxford University Press; 1990. Cancer: Causes, Occurrence, and Control. [Google Scholar]
- 3.Sobin L.H. Cancer in Afghanistan. Cancer. 1969;23(3):678–688. doi: 10.1002/1097-0142(196903)23:3<678::aid-cncr2820230322>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Pendharkar D., Salim-Ul-Zaman H. Epidemiology of cancer in Afghanistan. J. Clin. Oncol. 2009;27(15S) e22200-e. [Google Scholar]
- 5.Bruni L., Barrionuevo-Rosas L. 2016. ICO information centre on HPV and cancer (HPV information centre). Human Papillomavirus and related diseases in Afghanistan; pp. 1–64. Summary Report. [Google Scholar]
- 6.Amori N., Aghajani M., Asgarian F.S., Jazayeri M. Epidemiology and trend of common cancers in Iran (2004–2008) Eur. J. Canc. Care. 2017;26(5) doi: 10.1111/ecc.12449. [DOI] [PubMed] [Google Scholar]
- 7.Hamrah M.S., Hamrah M.H., Rabi M., Wu H.X., Hao C.N., Harun-Or-Rashid M. Prevalence of oesophagal cancer in the northern part of Afghanistan. Asian Pac. J. Cancer Prev. APJCP. 2014;15(24):10981–10984. doi: 10.7314/apjcp.2014.15.24.10981. [DOI] [PubMed] [Google Scholar]
- 8.Bloomfeld R.S., Bridgers D.I., Pineau B.C. The sensitivity of upper endoscopy in diagnosing oesophagal cancer. Dysphagia. 2005;20(4):278–282. doi: 10.1007/s00455-005-0025-x. [DOI] [PubMed] [Google Scholar]
- 9.Mir M.M., Dar N.A. Esophageal cancer in Kashmir (India): an enigma for researchers. Int. J. Health Sci. 2009;3(1):71. [PMC free article] [PubMed] [Google Scholar]
- 10.Mansour-Ghanaei F., Heidarzadeh A., Naghipour M.R., Joukar F., Valeshabad A.K., Fallah M.-S. A 10-year study of oesophagal cancer in Guilan Province, Iran: the Guilan cancer registry study (GCRS) Asian Pac. J. Cancer Prev. APJCP. 2012;13(12):6277–6283. doi: 10.7314/apjcp.2012.13.12.6277. [DOI] [PubMed] [Google Scholar]
- 11.Khazaei S., Ayubi E., Mansori K., Gholamaliee B., Khazaei S., Shadmani F.K. Geographic, sex and age distribution of oesophagal cancer incidence in Iran: a population-based study. Middle East J. Canc. 2017;8(2):103–108. [Google Scholar]
- 12.Amoori N., Mirzaei M., Cheraghi M. Incidence of cancers in Khuzestan province of Iran: trend from 2004 to 2008. Asian Pac. J. Cancer Prev. APJCP. 2014;15(19):8345–8349. doi: 10.7314/apjcp.2014.15.19.8345. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. Cancer statistics. Ca - Cancer J. Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. 2009. [DOI] [PubMed] [Google Scholar]
- 14.Enayatrad M., Amoori N., Salehiniya H. Epidemiology and trends in breast cancer mortality in Iran. Iran. J. Public Health. 2015;44(3):430. [PMC free article] [PubMed] [Google Scholar]
- 15.Karami K., Cheraghi M., Amori N., Pedram M., Sobhani A. Common cancers in Khuzestan province, south-west of Iran, during 2005-2011. Asian Pac. J. Cancer Prev. APJCP: Asian Pac. J. Cancer Prev. APJCP. 2014;15(21):9475–9478. doi: 10.7314/apjcp.2014.15.21.9475. [DOI] [PubMed] [Google Scholar]
- 16.Rasouli M., Ghadimi M.R., Mahmoodi M., Mohammad K., Zeraati H., Hosseini M. Survival analysis of patients with oesophagal cancer using parametric cure model. Asian Pac. J. Cancer Prev. APJCP. 2011;12(9):2359–2363. [PubMed] [Google Scholar]
- 17.Chen Y., Tong Y., Yang C., Gan Y., Sun H., Bi H. Consumption of hot beverages and foods and the risk of oesophagal cancer: a meta-analysis of observational studies. BMC Canc. 2015;15(1) doi: 10.1186/s12885-015-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middleton D.R.S., Menya D., Kigen N., Oduor M., Maina S.K., Some F. Hot beverages and oesophageal cancer risk in western Kenya: findings from the ESCCAPE case-control study. Int. J. Canc. 2019;144(11):2669–2676. doi: 10.1002/ijc.32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiechle M., Friese K., Felberbaum R. Lack of exercise, unhealthy diet and overweight: avoidable risk factors for the development and prognosis of gynaecological cancers. Gynäkologe. 2019;52(7):480–481. [Google Scholar]
- 20.Esposito K., Ciardiello F., Giugliano D. Unhealthy diets: a common soil for the association of metabolic syndrome and cancer. Endocrine. 2014;46(1):39–42. doi: 10.1007/s12020-013-0151-4. [DOI] [PubMed] [Google Scholar]
- 21.Behrens G., Gredner T., Stock C., Leitzmann M.F., Brenner H., Mons U. Cancer due to overweight, low physical activity and unhealthy diet. Dtsch Arztebl Inter. 2018;115(35-36):578–585. doi: 10.3238/arztebl.2018.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C.H., Lee Y.C.A., Hung R.J., McNallan S.R., Cote M.L., Lim W.Y. Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the International Lung Cancer Consortium (ILCCO) Int. J. Canc. 2014;135(8):1918–1930. doi: 10.1002/ijc.28835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatta D.N., Hiatt R.A., Van Loon K., Glantz S.A. Exposure to household tobacco smoke and risk of cancer morbidity and mortality: analysis of data from the Afghanistan Demographic and Health Survey 2015. Prev. Med. 2019;123:217–224. doi: 10.1016/j.ypmed.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Trowbridge R., Mittal S.K., Agrawal D.K. Vitamin D and the epidemiology of upper gastrointestinal cancers: a critical analysis of the current evidence. Cancer Epidemiol. Biomark. Prev. 2013;22(6):1007–1014. doi: 10.1158/1055-9965.EPI-13-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan Q.J., Kimler B.F., Fabian C.J. The relationship between vitamin D and breast cancer incidence and natural history. Curr. Oncol. Rep. 2010;12(2):136–142. doi: 10.1007/s11912-010-0081-8. [DOI] [PubMed] [Google Scholar]
- 26.Bidgoli S.A., Azarshab H. Role of vitamin D deficiency and lack of sun exposure in the incidence of premenopausal breast cancer: a case-control study in Sabzevar, Iran. Asian Pac. J. Cancer Prev. APJCP. 2014;15(8):3391–3396. doi: 10.7314/apjcp.2014.15.8.3391. [DOI] [PubMed] [Google Scholar]
- 27.Tayebi M., Shabestani M.A., Moslemi D. 2012. A 10 Year Survey of Cancer in Patients Who Referred to Shahid Rajai Radiotherapy Center in North of Iran (2000-2009) [Google Scholar]
- 28.Bhurgri Y. Karachi cancer registry data-implications for the national cancer control program of Pakistan. Asian Pac. J. Cancer Prev. APJCP. 2004;5(1):77–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.