Abstract
Dengue fever and dengue hemorrhagic fever are mosquito-borne viral diseases. Dendritic cell–specific ICAM-3 grabbing nonintegrin (DC-SIGN1, encoded by CD209), an attachment receptor of dengue virus, is essential for productive infection of dendritic cells1,2. Here, we report strong association between a promoter variant of CD209, DCSIGN1-336, and risk of dengue fever compared with dengue hemorrhagic fever or population controls. The G allele of the variant DCSIGN1-336 was associated with strong protection against dengue fever in three independent cohorts from Thailand, with a carrier frequency of 4.7% in individuals with dengue fever compared with 22.4% in individuals with dengue hemorrhagic fever (odds ratio for risk of dengue hemorrhagic fever versus dengue fever: 5.84, P = 1.4 × 10−7) and 19.5% in controls (odds ratio for protection: 4.90, P = 2 × 10−6). This variant affects an Sp1-like binding site and transcriptional activity in vitro. These results indicate that CD209 has a crucial role in dengue pathogenesis, which discriminates between severe dengue fever and dengue hemorrhagic fever. This may have consequences for therapeutic and preventive strategies.
Supplementary information
The online version of this article (doi:10.1038/ng1550) contains supplementary material, which is available to authorized users.
Main
We recruited independent cohorts from three hospitals in Thailand, for a total of 606 individuals with dengue disease and 696 healthy population controls from the same hospitals. Viral diagnosis was confirmed by serologic tests, and affected individuals were classified in five groups according to World Health Organization criteria: dengue fever (no evidence of plasma leakage) or dengue hemorrhagic fever (evidence of plasma leakage) with increasing severity from grade I to grade IV. The study was restricted to hospitalized school-age children. For dengue fever, we restricted our study to the most symptomatic individuals with dengue fever (classical incapacitating dengue fever)3, excluding those whose only clinical manifestation was fever. Effectively, less severe cases may overlap with undifferentiated fever, which do not require hospitalization and represent, together with asymptomatic cases, most cases of dengue virus infection (∼90%) in endemic areas4. In this hospital-based recruitment, the frequency of dengue hemorrhagic fever was 74.9% overall, similar in each hospital; the sex distribution was unbiased; and there was no significant effect of age on disease severity (Supplementary Table 1 online). There was an increased risk of dengue hemorrhagic fever versus dengue fever in individuals who had secondary versus primary dengue infection (odds ratio (OR) = 3.30, P = 2 × 10−5; Supplementary Table 1 online), as previously reported5.
We screened the gene CD209 for polymorphisms, including all exons, part of the introns, 1,091 bp of sequence 5′ to the start codon and 369 bp 3′ to the gene, in 80 Thai individuals (affected individuals and controls). We identified 40 polymorphisms (Supplementary Table 2 online). There was significant linkage disequilibrium (LD) between several of these polymorphisms (Fig. 1), and three haplotypes accounted for 56% of all haplotypes.
Figure 1. LD between CD209 polymorphisms in the Thai population.
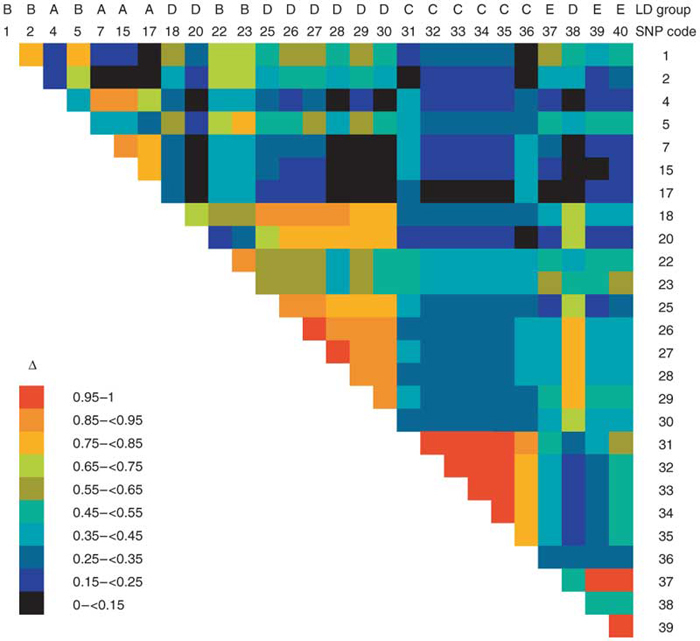
Δ values for SNPs with rare allele frequency ≥0.02.
We carried out tests for association with risk of disease (all individuals with dengue disease versus controls) and with severity of disease. Initially, we selected eight polymorphisms for association screening in the first cohort (cohort RA from Ramathibodi Hospital): polymorphisms representative of each LD group (groups defined as pairwise LD index Δ > 0.75) with rare allele frequency >0.02 or that affected the amino acid sequence. We obtained significant results for DCSIGN1-336, which affected disease severity among individuals with dengue classified in three groups (dengue fever, dengue hemorrhagic fever grade I and dengue hemorrhagic fever grades II–IV; P = 0.0022; data not shown). Because there was no heterogeneity between dengue hemorrhagic fever disease grades (grade I versus grades II–IV; P = 0.53; data not shown), we considered dengue hemorrhagic fever as a single group in subsequent analyses.
Genotypes GG and GA were rare in individuals with dengue fever (frequency = 2.0%) compared with controls (frequency = 18.9%; P = 0.0012) and were not associated with risk of disease overall (all individuals with dengue disease; Table 1). Genotypes GG and GA were strongly associated with risk of dengue hemorrhagic fever versus dengue fever (OR = 14.31; P = 2.3 × 10−4).
Table 1.
Association of DCSIGN1-336 in three independent cohorts from Thailand
| Genotype distribution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Frequency (%) | Cases versus controls a | DHF versus DF a | |||||||
| Cohort and group | GG | GA | AA | GG | GA | AA | OR | P b | OR (95% c.i.) | P b |
| RA cohort | ||||||||||
| Control | 5 | 51 | 240 | 1.7 | 17.2 | 81.1 | ||||
| DEN | 4 | 39 | 190 | 1.5 | 17.3 | 81.2 | 0.970 | 0.98 | ||
| DF | 0 | 1 | 50 | 0.0 | 2.0 | 98.0 | 0.090 | 0.0012 | 14.31 (3.34–61.23) | 2.3 × 10−4 |
| DHF | 4 | 38 | 140 | 2.2 | 20.9 | 76.9 | 1.286 | 0.33 | ||
| SI cohort | ||||||||||
| Control | 1 | 44 | 169 | 0.5 | 20.6 | 79.0 | ||||
| DEN | 0 | 49 | 190 | 0.0 | 20.5 | 79.5 | 0.968 | 0.98 | ||
| DF | 0 | 6 | 66 | 0.0 | 8.3 | 91.7 | 0.344 | 0.018 | 3.79 (1.62–8.87) | 0.0024 |
| DHF | 0 | 43 | 124 | 0.0 | 25.7 | 74.3 | 1.302 | 0.34 | ||
| KK cohort | ||||||||||
| Control | 3 | 31 | 149 | 1.6 | 16.9 | 81.4 | ||||
| DEN | 0 | 16 | 114 | 0.0 | 12.3 | 87.7 | 0.616 | 0.18 | ||
| DF | 0 | 0 | 27 | 0.0 | 0.0 | 100.0 | 0.008 | 0.012 | 99.75 (12.70–783.54) | 0.037 |
| DHF | 0 | 16 | 87 | 0.0 | 15.5 | 84.5 | 0.807 | 0.63 | ||
The SI and KK cohorts were replication cohorts. c.i., confidence interval; DEN, all individuals with dengue disease; DF, dengue fever; DHF, dengue hemorrhagic fever.
a The combined group of homozygotes with respect to the rare allele and heterozygotes was compared with the group of homozygotes with respect to the frequent allele.
bTwo-sided exact Fisher test.
To replicate these results and explore this genetic effect further, we studied these eight polymorphisms and three additional ones in LD with DCSIGN1-336, in this cohort (cohort RA) and two additional independent cohorts (cohorts SI and KK from Siriraj Hospital and Khon-Kaen Hospital, respectively). The results obtained for DCSIGN1-336 in cohort RA were replicated in cohorts SI and KK (Table 2): genotypes GG and GA had a lower frequency in individuals with dengue fever (frequency = 8.3% and 0.0%, P = 0.018 and 0.012, in cohorts SI and KK, respectively) and were associated with increased risk of dengue hemorrhagic fever versus dengue fever (P = 0.0024 and 0.037 in cohorts SI and KK, respectively; Table 1). There was no heterogeneity among affected individuals and among controls in the three cohorts or in the odds ratios observed between cohorts (P = 0.42). Therefore, the association between CD209 and clinical manifestation of dengue infection is replicated in the three independent cohorts from Thailand.
Table 2.
Association of DCSIGN1-336 in the three independent cohorts combined
| Genotype distribution | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymorphism | Number | Frequency (%) | Cases versus controls a | DHF versus DF a | ||||||||
| Code | Name | Group | 11 | 12 | 22 | 11 | 12 | 22 | OR | P b | OR (95% c.i.) | P b |
| 4 | DCSIGN1-336 | Control | 9 | 126 | 558 | 1.3 | 18.2 | 80.5 | ||||
| DEN | 4 | 104 | 494 | 0.7 | 17.3 | 82.3 | 0.904 | 0.52 | ||||
| DF | 0 | 7 | 143 | 0.0 | 4.7 | 95.3 | 0.204 | 2.0 × 10−6 | 5.84 (2.77–12.31) | 1.4 × 10−7 | ||
| DHF | 4 | 97 | 351 | 0.9 | 21.5 | 77.3 | 1.189 | 0.27 | ||||
| 5 | DCSIGN1-139 | Control | 62 | 310 | 322 | 8.9 | 44.7 | 46.4 | ||||
| DEN | 55 | 244 | 306 | 9.1 | 40.3 | 50.6 | 0.846 | 0.15 | ||||
| DF | 12 | 57 | 83 | 7.9 | 37.5 | 54.6 | 0.720 | 0.08 | 1.24 (0.86–1.79) | 1.0 | ||
| DHF | 43 | 187 | 223 | 9.5 | 41.3 | 49.2 | 0.893 | 0.38 | ||||
| 7 | DCSIGN1.in2+11 | Control | 7 | 101 | 585 | 1.0 | 14.6 | 84.4 | ||||
| DEN | 1 | 77 | 524 | 0.2 | 12.8 | 87.0 | 0.806 | 0.20 | ||||
| DF | 0 | 6 | 146 | 0.0 | 3.9 | 96.1 | 0.224 | 4.5 × 10−5 | 4.60 (2.07–10.22) | 5.4 × 10−5 | ||
| DHF | 1 | 71 | 378 | 0.2 | 15.8 | 84.0 | 1.032 | 0.91 | ||||
| 12 | DCSIGN1.ex4SF | Control | 688 | 4 | 0 | 99.4 | 0.6 | 0.0 | ||||
| DEN | 594 | 9 | 0 | 98.5 | 1.5 | 0.0 | 2.588 | 0.17 | ||||
| DF | 151 | 1 | 0 | 99.3 | 0.7 | 0.0 | 1.181 | 1.0 | 2.61 (0.56–12.23) | 0.59 | ||
| DHF | 443 | 8 | 0 | 98.2 | 1.8 | 0.0 | 3.087 | 0.10 | ||||
| 13 | DCSIGN1.ex4RPT | Control | 0 | 8 | 685 | 0.0 | 1.2 | 98.8 | ||||
| DEN | 1 | 7 | 595 | 0.2 | 1.2 | 98.7 | 1.151 | 0.97 | ||||
| DF | 1 | 3 | 148 | 0.7 | 2.0 | 97.4 | 2.328 | 0.31 | 0.33 (0.09–1.16) | 0.23 | ||
| DHF | 0 | 4 | 447 | 0.0 | 0.9 | 99.1 | 0.771 | 0.91 | ||||
| 15 | DCSIGN1.in5-178 | Control | 6 | 96 | 586 | 0.9 | 14.0 | 85.2 | ||||
| DEN | 1 | 74 | 521 | 0.2 | 12.4 | 87.4 | 0.827 | 0.28 | ||||
| DF | 0 | 5 | 144 | 0.0 | 3.4 | 96.6 | 0.201 | 4.0 × 10−5 | 5.30 (2.25–12.46) | 3.0 × 10−5 | ||
| DHF | 1 | 69 | 377 | 0.2 | 15.4 | 84.3 | 1.067 | 0.76 | ||||
| 16 | DCSIGN1.ex6TI | Control | 0 | 7 | 679 | 0.0 | 1.0 | 99.0 | ||||
| DEN | 0 | 6 | 589 | 0.0 | 1.0 | 99.0 | 0.989 | 1.0 | ||||
| DF | 0 | 1 | 148 | 0.0 | 0.7 | 99.3 | 0.683 | 1.0 | 1.61 (0.32–8.08) | 1.0 | ||
| DHF | 0 | 5 | 441 | 0.0 | 1.1 | 98.9 | 1.103 | 1.0 | ||||
| 17 | DCSIGN1.in6-37 | Control | 6 | 74 | 598 | 0.9 | 10.9 | 88.2 | ||||
| DEN | 1 | 57 | 544 | 0.2 | 9.5 | 90.4 | 0.797 | 0.25 | ||||
| DF | 0 | 7 | 142 | 0.0 | 4.7 | 95.3 | 0.371 | 0.01 | 2.56 (1.19–5.52) | 0.02 | ||
| DHF | 1 | 50 | 402 | 0.2 | 11.0 | 88.7 | 0.949 | 0.86 | ||||
| 26 | DCSIGN1.2281 | Control | 287 | 330 | 72 | 41.7 | 47.9 | 10.4 | ||||
| DEN | 227 | 283 | 94 | 37.6 | 46.9 | 15.6 | 1.186 | 0.15 | ||||
| DF | 49 | 78 | 25 | 32.2 | 51.3 | 16.4 | 1.500 | 0.04 | 0.73 (0.50–1.08) | 0.14 | ||
| DHF | 178 | 205 | 69 | 39.4 | 45.4 | 15.3 | 1.099 | 0.48 | ||||
| 36 | DCSIGN1.3197 | Control | 11 | 138 | 510 | 1.7 | 20.9 | 77.4 | ||||
| DEN | 8 | 113 | 459 | 1.4 | 19.5 | 79.1 | 0.902 | 0.50 | ||||
| DF | 3 | 21 | 125 | 2.0 | 14.1 | 83.9 | 0.658 | 0.10 | 1.51 (0.93–2.45) | 0.12 | ||
| DHF | 5 | 92 | 334 | 1.2 | 21.3 | 77.5 | 0.994 | 1.0 | ||||
| 37 | DCSIGN1.3852 | Control | 353 | 258 | 47 | 53.6 | 39.2 | 7.1 | ||||
| DEN | 285 | 173 | 33 | 58.0 | 35.2 | 6.7 | 0.837 | 0.15 | ||||
| DF | 71 | 51 | 5 | 55.9 | 40.2 | 3.9 | 0.913 | 0.64 | 0.89 (0.59–1.33) | 0.64 | ||
| DHF | 214 | 122 | 28 | 58.8 | 33.5 | 7.7 | 0.811 | 0.13 | ||||
c.i., confidence interval; DEN, all individuals with dengue disease; DF, dengue fever; DHF, dengue hemorrhagic fever.
a The combined group of homozygotes with respect to the rare allele and heterozygotes was compared with the group of homozygotes with respect to the frequent allele.
bTwo-sided exact Fisher test.
Overall, we obtained significant association results for DCSIGN1-336, located in the CD209 promoter region, and three intronic polymorphisms in LD with it: DCSIGN1.in2+11, DCSIGN1.in5-178 and DCSIGN1.in6-37 (Δ = 0.92, 0.83 and 0.53, respectively), with the most significant results obtained for DCSIGN1-336 (Table 2). For DCSIGN1-336, genotypes GG and GA had a frequency of 4.7% in individuals with dengue fever and 22.4% in individuals with dengue hemorrhagic fever. The difference between dengue fever and dengue hemorrhagic fever was highly significant overall (OR = 5.84; P = 1.4 × 10−7). The frequency of genotypes GG and GA in the control population (19.5%) was similar to that in individuals with dengue hemorrhagic fever (22.4%; P = 0.27) and different from that in individuals with dengue fever (4.7%; P = 2 × 10−6). The P values remained highly significant after conservative correction for multiple testing. These genotype differences were similar for individuals with primary and secondary infections (Table 3).
Table 3.
DCSIGN1-336 in primary and secondary dengue virus infections
| Primary infection Genotype |
Secondary infection Genotype |
|||||
|---|---|---|---|---|---|---|
| Number | Frequency (%) | Number | Frequency (%) | |||
| GG + GA | AA | GG + GA | GG + GA | AA | GG + GA | |
| DF | 0 | 33 | 0.0 | 7 | 108 | 6.1 |
| DHF | 9 | 26 | 25.7 | 90 | 318 | 22.1 |
DF, dengue fever; DHF, dengue hemorrhagic fever.
Frequency estimates of haplotypes constructed from the five SNPs in significant LD with DCSIGN1-336 showed that all three haplotypes with a G at DCSIGN1-336 had a lower frequency in individuals with dengue fever than in controls or individuals with dengue hemorrhagic fever (Supplementary Table 3 online). After accounting for DCSIGN1-336, the other polymorphisms were not significantly correlated with disease status. In contrast, the association with DCSIGN1-336 was always significant, even after adjusting for other SNPs in a logistic regression (Table 4).
Table 4.
Genetic mapping of the variant responsible for CD209 association with dengue to DCSIGN1-336
| Second variant | Residual effect of DCSIGN1-336a |
Residual effect of second variantb |
|---|---|---|
| DCSIGN1-139 | 5 × 10−8 | 0.44 |
| DCSIGN1.in2+11 | 0.0006 | 0.67 |
| DCSIGN1.ex4SF | 5 × 10−8 | 0.31 |
| DCSIGN1.ex4RPT | 7 × 10−8 | 0.20 |
| DCSIGN1.in5-178 | 0.002 | 0.93 |
| DCSIGN1.ex6TI | 2 × 10−8 | 0.61 |
| DCSIGN1.in6-37 | 6 × 10−7 | 0.54 |
| DCSIGN1.2281 | 9 × 10−8 | 0.62 |
| DCSIGN1.3197 | 1 × 10−8 | 0.21 |
| DCSIGN1.3852 | 4 × 10−8 | 0.11 |
Statistical analysis was done with a likelihood ratio test using logistic regression.
a P value testing for residual effect of DCSIGN1-336 when accounting for the effect of the second variant.
bP value testing for residual effect of the second variant when accounting for the effect of DCSIGN1-336.
We also investigated whether the observed association could be due to LD between CD209 SNPs and a functional variant located in one of the flanking genes. The two closest flanking genes are CLEC4M (also called L-SIGN or CD209L, liver/lymph node-specific ICAM3-grabbing nonintegrin), located 15.7 kb proximal to CD209, and CLEC4G (also called LSECtin, liver and lymph node sinusoidal endothelial cell C-type lectin), 7.9 kb distal to CD209 (ref. 6). We sequenced the exons and promoter regions of these two genes in 80 Thai individuals and found limited LD between CD209 and both genes, with Δ values <0.75, except for one SNP located 3′ to CLEC4M that showed strong association with four SNPs in CD209 (Δ > 0.95) that were not associated with disease (Supplementary Fig. 1 online). All Δ values of CLEC4M and CLEC4G SNPs with DCSIGN1-336 were <0.27 (Supplementary Table 4 online). Therefore, it is unlikely that this effect is related to variants in these two flanking genes. Overall, these results support the idea that DCSIGN1-336 is responsible for the observed association with severity of dengue disease.
DCSIGN1-336 is located in the promoter region of CD209, 212 bp 5′ of the major transcription start site7. This site affects multiple predicted transcription factor binding sites for Sp1/GATA1/CACCC- and CAC-binding transcription factors, of the type GGGTGGG, with allele G (underlined) associated with the presence of binding sites, and allele A with its absence. This sequence was conserved in the homologous gene CD209L, with a G at the corresponding position7. We carried out electrophoretic mobility shift assays (EMSAs) by incubating HeLa nuclear extracts with two alternative 22-nucleotide probes differing at the DCSIGN1-336 position (Fig. 2a,b). The G variant showed increased Sp1 binding capacity compared with the A variant, which was effectively competed by the Sp1 consensus oligonucleotide. This suggests that the DCSIGN1-336 variant affects the binding of Sp1 and possibly other transcription factors that modulate transcriptional activity, supporting the idea that is has a functional role in the transcriptional regulation of CD209. It probably does not affect the tissue specificity of expression, which is controlled by sequences outside of the promoter7.
Figure 2. Effects of the DCSIGN1-336 polymorphism on transcriptional activity.
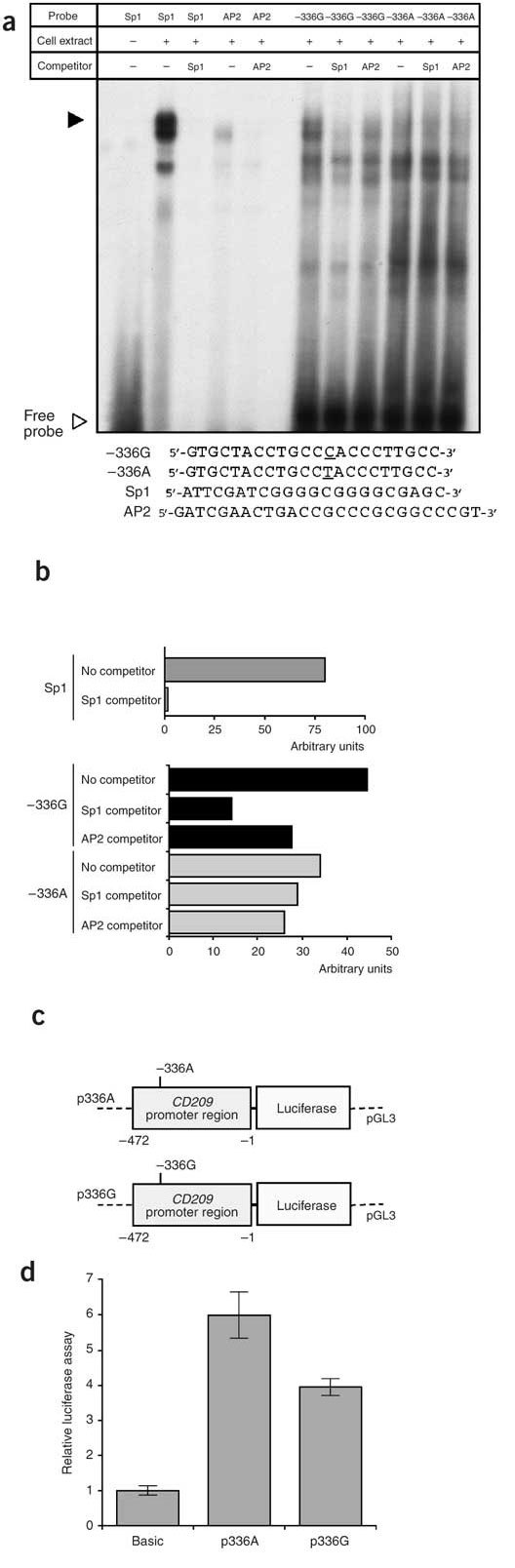
(a) EMSAs with 32P-labeled probes containing the −336G variant, the −336A variant, the Sp1 consensus site or the AP2 consensus site and HeLa cell nuclear extract. Competition experiments were done in the presence of a tenfold excess of unlabeled Sp1 or AP2 probe. (b) Quantification of the major oligonucleotide-nuclear protein complex (filled arrowhead in panel a). Competition with unlabeled Sp1 consensus probe showed a threefold decrease in binding to the −336G probe, but no significant decrease in binding to the −336A probe. The AP2 consensus probe showed slight and similar competition to both probes. (c,d) Luciferase expression of reporter gene constructs expressing sequence from −472 to −1 in transfected HeLa cells. (c) Schematic representation of reporter gene constructs containing the CD209 promoter region with the DCSIGN1-336 polymorphism. (d) Relative luciferase expression from pGL3-Basic, the parental vector without promoter. Luciferase expression from the CD209 reporters relative to this value is shown.
We then tested the effect of the DCSIGN1-336 variant on transcription, using two luciferase reporter gene constructs spanning the promoter region from −472 to −1 (CD209 start codon at +1), with either A or G at position −336, and an invariant nucleotide (G) at position −139 (Fig. 2a,b). Transient transfections of HeLa cells showed that these reporters had promoter function (Fig. 2c,d), confirming previous results7. The activity of the −336G reporter was significantly lower than that of the −336A reporter (−336A/G ratio = 1.5, P = 10−5; Fig. 2c,d), suggesting that the DCSIGN1-336 variant affects promoter activity.
We show that a promoter variant in CD209, DCSIGN1-336, is strongly associated with the risk of developing dengue fever versus dengue hemorrhagic fever and controls. DCSIGN1-336 affects an Sp1 binding site and the level of transcription of CD209 in vitro, which supports the idea that this variant has a functional role. Polymorphisms in Sp1 binding sites have been hypothesized to modify phenotypes or susceptibility to diseases such as bone density and osteoporosis8,9, estrogen response10 and acute myeloid leukemia11.
On the basis of these results, we propose that the G allele at DCSIGN1-336 is associated with a dominant protection against dengue fever (OR = 4.90; P = 2 × 10−6) but not against dengue hemorrhagic fever. Among individuals with dengue, genotypes GG and GA strongly increased the risk of contracting dengue hemorrhagic fever versus dengue fever (OR = 5.84; P = 1.4 × 10−7). Therefore, there is strong evidence of genetic heterogeneity between individuals with dengue fever and dengue hemorrhagic fever.
Our genetic results suggest that dengue fever and dengue hemorrhagic fever, two severe forms of dengue disease, may involve pathophysiological processes that are at least partially distinct, with different levels of involvement of CD209 and possibly other host and viral factors. These findings bring new insight to the classical view of a continuum of disease severity between dengue fever and dengue hemorrhagic fever12. The expected decrease in expression of CD209 carrying the G allele at DCSIGN1-336 may result in a lower susceptibility of dendritic cells to dengue virus in the early stages of infection1,2, which would protect against dengue fever, whereas other pathophysiological processes would be prevalent in dengue hemorrhagic fever. It is notable that major disturbances of immune responses are observed in dengue hemorrhagic fever compared with dengue fever13.
We propose two possible hypotheses to explain the selective protection of the CD209 variant against dengue fever but not dengue hemorrhagic fever. First, there may be differential interaction between dengue virus and CD209, depending on the genetic variability of the dengue virus strain, which affects both the level of infectivity of CD209-expressing dendritic cells2 and the viral virulence14. Second, in dengue hemorrhagic fever, there may be an alternative pathway to the CD209-mediated dengue virus entry to dendritic cells. The high viral load observed in dengue hemorrhagic fever may be caused by the antibody-enhanced dengue virus infection of monocytes through Fcγ receptors in individuals with pre-existing dengue virus antibodies (antibody-dependant enhancement). Previous studies have reported some association of polymorphisms in HLA class I, TNFα and Fcγ receptor IIA genes with dengue hemorrhagic fever15,16,17,18,19,20. Notably, some HLA alleles were specifically associated with the risk of dengue fever rather than dengue hemorrhagic fever18. Overall, the risk of infection and disease severity probably results from complex interactions between CD209 variability and other host and viral factors, whose elucidation requires further detailed investigations.
Our conclusion that the CD209 variant DCSIGN1-336 has a role in disease orientation (dengue fever or dengue hemorrhagic fever) may have consequences for the development of CD209-based prophylaxis and therapy against infections by dengue virus and a wide variety of pathogens of medical relevance. In addition to its role in infection by arboviruses such as dengue virus21,22, CD209 is an attachment factor for many other pathogens: viruses with glycosylated envelope proteins, including human immunodeficiency virus-1, hepatitis C virus, cytomegalovirus, Ebola virus and SARS-coV; bacteria such as Mycobacterium tuberculosis; and parasites such as Leishmania and Schistosoma mansoni22,23,24,25. DCSIGN1-336 (and possibly other genetic variants of CD209) may therefore have a role in the susceptibility to other infectious diseases, as suggested for susceptibility to parenteral HIV-1 infection26. Host response to medical vaccines and drugs in terms of efficiency and safety might depend on genotype with respect to CD209 polymorphisms, which should be taken into account to optimize the therapeutic designs.
Methods
Patients and controls.
We recruited individuals with dengue disease and controls independently at three medical centers in Thailand: two in Bangkok, Ramathibodi Hospital (cohort RA) and Siriraj Hospital (cohort SI), Mahidol University, and one in the northeast region of Thailand, Khon-Kaen Hospital (cohort KK). Diagnosis was established by expert clinicians in each center, following World Health Organization criteria as follows. Individuals suspected to have dengue viral infection on the basis of clinical features including high fever, severe headache, retro-orbital pain, myalgia, arthalgia, nausea and vomiting, and rash were admitted to the hospitals for clinical observation and treatment. The diagnosis of dengue virus infection was later confirmed by a comparable dengue-specific IgG and IgM enzyme-linked immunosorbent assay titer on a late acute or convalescent sera27. Differential diagnosis of dengue fever and dengue hemorrhagic fever was established on the basis of the absence (dengue fever) or presence (dengue hemorrhagic fever) of evidence for increased vascular permeability manifested by hemoconcentration or pleural effusion. Specifically, the diagnosis of dengue hemorrhagic fever was made on the basis of the four following characteristics: (i) high continuous fever lasting 2–7 d, (ii) hemorrhagic tendency such as a positive tourniquet test, petechii, purpura or hematemesis, (iii) thrombocytopenia (platelet count ≥100,000 μl−1and (iv) evidence of plasma leakage due to increased vascular permeability manifested by hemoconcentration (an increased in hematocrit of 20% or more) or pleural effusion. The severity of dengue hemorrhagic fever was categorized in four grades according to World Health Organization criteria3. Grades III and IV were dengue hemorrhagic fever with narrowing pulse pressure with a characteristically elevated diastolic pressure to profound shock. In the three cohorts, clinical and biological data were recorded during their hospitalization and stored into a database.
In addition to the common criteria used for diagnosis, there were specific features in each center. At Ramathibodi Hospital, but not in other centers, a chest X-Ray in right lateral decubitus was done in individuals without evidence of hemoconcentration to refine the differential diagnosis of dengue hemorrhagic fever versus dengue fever on the basis of pleural effusion. At Ramathibodi Hospital, affected individuals were requested to come back to the hospital 2–3 weeks after discharge, and a follow-up hematocrit and platelet measure was done, allowing for better evaluation of increase in hematocrit during previous admission. At Khon-Kaen Hospital, clinical and biological recording was more systematic and was computerized from the start of the study, and this detailed information was used to complement the dengue status established by clinicians. In cohort KK, hematocrit measurements were done at least four times a day, and up to every hour during the critical period of defeverescence. Because of the detailed biological data collected in this cohort, we made the differential diagnosis of dengue hemorrhagic fever grade I versus dengue fever on the basis of evidence of plasma leakage (increased hematocrit of 20% or more), whereas dengue hemorrhagic fever grades II–IV was established by the clinicians. Secondary infection was defined as a dengue-specific IgM/IgG ratio <1.8.
To preserve homogeneity, we included only school-age children (between the ages of 5 and 15 years, inclusive). To limit heterogeneity among individuals with dengue fever, we excluded from our study those presenting with fever as the only clinical manifestation. In practice, only children with dengue fever manifesting at least one of the following diagnosis criteria, in addition to fever, were included in the dengue fever group: rash, bleeding manifestations (including petechiae) or hepatomegaly. These most closely reflect classical incapacitating dengue fever3.
We studied a total of 606 affected individuals: 235, 241 and 130 in cohorts RA, SI and KK, respectively; 301 males and 305 females.
Controls consisted of a total of 696 healthy blood donors, 296, 216 and 184 recruited from Ramathibodi Hospital, Siriraj Hospital and Khon-Kaen Hospital, respectively (50% female). For Ramathibodi Hospital and Siriraj Hospital, both cases and control groups came from Bangkok and the central part of Thailand; for Khon-Kaen Hospital, both came from the northeast region of Thailand. We collected whole blood samples on EDTA from affected individuals and controls and extracted DNA using a standard phenol-chloroform method.
We obtained signed informed consent from all participants or their tutors, and ethical approvals were granted by the ethical committees from the Faculty of Medicine, Ramathibodi Hospital, Mahidol University; the Faculty of Medicine, Siriraj Hospital, Mahidol University; the Khon Kaen Hospital and the Ministry of Public Health.
Polymorphism identification and genotyping.
We identified polymorphisms in CD209 by direct sequencing of PCR-amplified genomic DNA from 80 Thai individuals: 48 individuals with dengue (25 with dengue fever and 23 with dengue hemorrhagic fever) and 32 controls. Primers used for sequencing CD209 exons, part of introns, and 5′ and 3′ regions are listed in Supplementary Table 5 online. Similarly, we identified polymorphisms in CLEC4M and CLEC4G in 48 individuals with dengue and 32 controls using primers shown in Supplementary Table 5 online. We analyzed sequencing results by Genalys Software28. We also carried out a direct investigation of a repeated region in exon 4 (ref. 29) in 96 cases and 96 controls, which extended the identification of DCSIGN1.ex4RPT and DCSIGN1.ex4SF polymorphisms. Only three rare variants (<2%) affected the coding regions of the protein: two amino acid substitutions and one variation in the number of repeats of a 23-amino-acid unit located in the predicted extracellular domain29 (Supplementary Table 2 online). The other polymorphisms were located in introns (8), in the promoter or 5′ region of the gene (6), in the 3′ untranslated region (21) or 3′ to the gene (2). Sixteen of these polymorphisms were located in repeat sequences. Twenty-seven of these polymorphisms had a rare allele frequency >0.02. We genotyped DCSIGN1-336, DCSIGN1-139, DCSIGN1.in2+11, DCSIGN1.ex4SF and DCSIGN1.2281 using PCR-RFLP assays and DCSIGN1.ex4RPT by PCR and agarose gel electrophoresis (Supplementary Table 6 online). We genotyped DCSIGN1-336, DCSIGN1.in5-178, DCSIGN1.ex6TI, DCSIGN1.in6-37, DCSIGN1.3197 and DCSIGN1.3852 by TaqMan assay (Supplementary Table 7 online) using ABI Prism 7000 Sequence Detection System, with recommended protocols. We genotyped DCSIGN1-336 in duplicate, using both methods, with identical results. We tested deviation from Hardy-Weinberg equilibrium in individuals with dengue disease and in controls, in each cohort and overall, and found that it was not statistically significant for any SNP. We carried out heterogeneity testing for the origin of cohort for each polymorphism in individuals with dengue disease and in controls and found that it was not significant.
Statistical analyses.
We carried out tests for association and LD using χ2 or two-sided Fisher exact tests. We estimated haplotype frequencies using the EM algorithm, as implemented in the HAPLO program (S. Heath, unpublished data). We calculated the LD index (Δ) using all polymorphisms identified in up to 80 Thai individuals with dengue disease and controls (25 with dengue fever, 23 with dengue hemorrhagic fever and 32 controls), using the ldmax program, as implemented in the GOLD package30. We carried out fine mapping of the responsible variant using logistic regression, comparing likelihoods of one-variant models to two-variants models, using STATVIEW statistic package.
Transcription factor binding sites prediction.
We used the TESS interface to predict and compare binding sites in alternative alleles.
EMSA.
We detected the DNA-protein complexes by EMSA using the Gel Shift Assay System kit (Promega). We end-labeled the double-stranded DNA probes corresponding to the A and G alleles of variant DCSIGN1-336 (−336A probe and −336G probe, respectively) with (γ-32P) ATP, incubated them with HeLa nuclear extract (Promega) and separated them on a 4% nondenaturing acrylamide gel. We established the specificity of the DNA binding site by preincubating the HeLa nuclear extract with an excess of unlabeled competitor probes corresponding to the consensus Sp1 and AP2 sites. All DNA probes sequences are shown in Figure 2a. We analyzed band intensity with PhosphoImager (Molecular Dynamics).
Reporter plasmid construction, plasmid transfection and reporter gene assays.
To construct CD209 promoter reporters, we amplified fragments encompassing nucleotides −472 to −1 (relative to the ATG start codon at +1) by PCR from genomic DNA of two individuals homozygous with respect to the corresponding genotypes, using primers tailed with BglII and HindIII restriction sites, and subcloned them into the BglII and HindIII sites of the pGL3-Basic expression vector (Promega). We verified all recombinant clones by DNA sequencing. We cultured HeLa cells on 12-well culture plates (2 × 105 cells per well). After 1 d of culture, cell monolayers were transfected for 40 h with 4 μg of plasmid per well in the presence of FuGene 6 transfection reagent (Roche Molecular Biochemicals). After the transfection period, we lysed cells with 0.5 ml of reporter lysis buffer (Promega), incubated them on ice for 10 min and subjected the lysates to centrifugation at 6,000 r.p.m. for 3 min. We assayed 10 μl of supernatant for luciferase activity using the Promega luciferase assay system. We carried out luciferase assays in quadruplicate in at least three independent transfection experiments.
URL.
The TESS interface is available at http://www.cbil.upenn.edu/tess/.
Note: Supplementary information is available on the Nature Genetics website.
Supplementary information
Linkage disequilibrium between SNPs in CD209 and flanking genes CLEC4M (CD209L) and CLEC4G (LSECtin). (PDF 385 kb)
Populations studied. (PDF 53 kb)
Description of CD209 polymorphisms. (PDF 98 kb)
Haplotypes constructed from the five SNPs in LD with DCSIGN1-336 and their frequencies. (PDF 46 kb)
Description of CLEC4G (LSECtin) and CLEC4M (CD209L) polymorphisms. (PDF 90 kb)
Sequencing primers for CD209, CLEC4M (CD209L) and CLEC4G (LSECtin). (PDF 80 kb)
PCR-RFLP genotyping and PCR gel electrophoresis assays. (PDF 49 kb)
TaqMan genotyping assays. (PDF 31 kb)
Acknowledgements
We thank A. Chunharas, P. Kitpoka and A. Chairunsri for help in recruiting affected individuals and controls and L. Damrikarnlerd and S. Swasdiworn for managing the clinical database. This work was supported by the Senior Research Scholarship Program of the Thailand Research Fund (P.M.), the Thailand Tropical Disease Research Program T2 (P.M.), the Thailand National Center for Genetic Engineering and Biotechnology (P.M., P.Y. and N.T.), Mahidol University (A.S.), the Thailand SNP Discovery Program (T.L.), the Direction Générale pour l'Armement (P.D.), the Medical Scholar Program, Mahidol University and the Split Mode PhD program (S.M.K.), and the Royal Golden Jubilee Program, the Thailand Research Fund and the French Embassy of Thailand (C.T.).
Competing interests
The authors declare no competing financial interests.
References
- 1.Tassaneetrithep B, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro-Sanchez E, et al. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:1–6. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control (World Health Organization, Geneva, 1997).
- 4.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 5.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.239.4839.476. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 2004;279:18748–18758. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Yu W, Liou LY, Rice AP. Isolation and characterization of the human DC-SIGN and DC-SIGNR promoters. Gene. 2003;313:149–159. doi: 10.1016/S0378-1119(03)00674-7. [DOI] [PubMed] [Google Scholar]
- 8.Grant SF, et al. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat. Genet. 1996;14:203–205. doi: 10.1038/ng1096-203. [DOI] [PubMed] [Google Scholar]
- 9.Mann V, et al. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J. Clin. Invest. 2001;107:899–907. doi: 10.1172/JCI10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harendza S, et al. Linked common polymorphisms in the gelatinase a promoter are associated with diminished transcriptional response to estrogen and genetic fitness. J. Biol. Chem. 2003;278:20490–20499. doi: 10.1074/jbc.M211536200. [DOI] [PubMed] [Google Scholar]
- 11.Sibley K, et al. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003;63:4327–4330. [PubMed] [Google Scholar]
- 12.Rothman AL. Immunology and immunopathogenesis of dengue disease. Adv. Virus Res. 2003;60:397–419. doi: 10.1016/S0065-3527(03)60010-2. [DOI] [PubMed] [Google Scholar]
- 13.Mongkolsapaya J, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 14.Vaughn DW, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 15.Loke H, et al. Strong HLA class I–restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J. Infect. Dis. 2001;184:1369–1373. doi: 10.1086/324320. [DOI] [PubMed] [Google Scholar]
- 16.Chiewsilp P, Scott RM, Bhamarapravati N. Histocompatibility antigens and dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1981;30:1100–1105. doi: 10.4269/ajtmh.1981.30.1100. [DOI] [PubMed] [Google Scholar]
- 17.Paradoa Perez ML, Trujillo Y, Basanta P. Association of dengue hemorrhagic fever with the HLA system. Haematologia (Budap.) 1987;20:83–87. [PubMed] [Google Scholar]
- 18.Stephens HA, et al. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens. 2002;60:309–318. doi: 10.1034/j.1399-0039.2002.600405.x. [DOI] [PubMed] [Google Scholar]
- 19.Loke H, et al. Susceptibility to dengue hemorrhagic fever in vietnam: evidence of an association with variation in the vitamin d receptor and Fc gamma receptor IIa genes. Am. J. Trop. Med. Hyg. 2002;67:102–106. doi: 10.4269/ajtmh.2002.67.102. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Mestre MT, Gendzekhadze K, Rivas-Vetencourt P, Layrisse Z. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens. 2004;64:469–472. doi: 10.1111/j.1399-0039.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 21.Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Kooyk Y, Appelmelk B, Geijtenbeek TB. A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance. Trends Mol. Med. 2003;9:153–159. doi: 10.1016/S1471-4914(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 23.Halary F, et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–664. doi: 10.1016/S1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- 24.Tailleux L, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang ZY, et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin MP, et al. Association of DC-SIGN promoter polymorphism with increased risk for parenteral, but not mucosal, acquisition of human immunodeficiency virus type 1 infection. J. Virol. 2004;78:14053–14056. doi: 10.1128/JVI.78.24.14053-14056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innis BL, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M, Matsuda F, Margetic N, Lathrop M. Automated identification of single nucleotide polymorphisms from sequencing data. J. Bioinform. Comput. Biol. 2003;1:253–265. doi: 10.1142/S021972000300006X. [DOI] [PubMed] [Google Scholar]
- 29.Mummidi S, et al. Extensive repertoire of membrane-bound and soluble dendritic cell-specific ICAM-3-grabbing nonintegrin 1 (DC-SIGN1) and DC-SIGN2 isoforms. Inter-individual variation in expression of DC-SIGN transcripts. J. Biol. Chem. 2001;276:33196–33212. doi: 10.1074/jbc.M009807200. [DOI] [PubMed] [Google Scholar]
- 30.Abecasis GR, Cookson WO. GOLD-graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linkage disequilibrium between SNPs in CD209 and flanking genes CLEC4M (CD209L) and CLEC4G (LSECtin). (PDF 385 kb)
Populations studied. (PDF 53 kb)
Description of CD209 polymorphisms. (PDF 98 kb)
Haplotypes constructed from the five SNPs in LD with DCSIGN1-336 and their frequencies. (PDF 46 kb)
Description of CLEC4G (LSECtin) and CLEC4M (CD209L) polymorphisms. (PDF 90 kb)
Sequencing primers for CD209, CLEC4M (CD209L) and CLEC4G (LSECtin). (PDF 80 kb)
PCR-RFLP genotyping and PCR gel electrophoresis assays. (PDF 49 kb)
TaqMan genotyping assays. (PDF 31 kb)


