Abstract
We review findings and propose a model explaining why women's adaptation to traumatic stress might be different than men's, including the role of cycling hormones and sleep differences in the development of post-traumatic stress and other stress-related disorders. Women are diagnosed with stress-related mental health disorders at a higher frequency than men. Most mental health disorders involve sleep disturbances, which may contribute to these disorders. The mechanisms by which sleep contributes to the development of mental health disorders in women have not been addressed in basic research. Sleep features such as spindle density and rapid eye movement (REM) sleep theta power are important for the role of sleep in emotion and cognition. The effect of hormonal cycles on these and other critical sleep features is only beginning to be understood. We explore what sleep factors could confer resilience to mental health disorders and how they might be altered by hormonal cycles in women. We target a specific system at the nexus of arousal control, stress response, and memory consolidation processes that has not been explored at all in women or across the hormonal cycle in animal studies: the locus coeruleus noradrenergic (LC-NE) system.
Keywords: locus coeruleus, REM sleep, PTSD, estrogen, fear extinction, norepinephrine
Introduction
Sleep and mental health disorders in females
There is an increased vulnerability to stress-related mental health disorders in women.1 Sleep and mental health disorders are deeply intertwined. Sleep features are modified in arousal-related disorders such as insomnia, which occur more commonly in women.2 Conversely, mental health disorders are almost uniformly accompanied by sleep disruptions/changes.3–5 However, most animal studies of sleep, including those addressing the link to mental health disorders, have been conducted in males, which means not much is known about the influence of the hormonal cycle on sleep in females6 or about how sleep changes across the hormonal cycle might mitigate vulnerability to mental health disorders. One reason that more studies have not been conducted is the prevailing view that the hormonal cycle is too complicated to explore in conjunction with the still unknown interactions between sleep and mental health.
In this article we turn our attention to a particular mental health disorder that women are two to four times more likely to develop than men: post-traumatic stress disorder (PTSD) and propose a simplified model that invokes the hormonal cycle to explain the increased vulnerability of women to anxiety-related disorders involving memory. PTSD is characterized by a maladaptive response to trauma exposure and involves the physiological axes of stress, arousal, cognition, and mood. Anxiety, hypervigilance, and negative alterations in cognition/memory are also symptoms of PTSD. One critical feature of PTSD is impairment in extinction learning or its consolidation. In PTSD, previously fear-associated cues that should longer produce an activation of the stress/anxiety axis continue to do so.7,8 Disturbed sleep is a common symptom of PTSD and may play a role in establishing this disorder. Rapid eye movement (REM) sleep fragmentation, in particular, is commonly observed in objective sleep studies of those with PTSD.9 Insomnia is a common complaint and recent studies of those with insomnia also show REM sleep fragmentation and disruptions in the state leading to REM sleep.10 This transitional state is variously called ascending stage 2 in humans or transition to REM (TR) sleep and intermediate sleep in animals.9
As hormonal cycles in females have been shown to affect sleep features and architecture, we thought it prudent to assess the common sleep features that change in both PTSD and across the hormonal cycle. Such investigation could identify sleep factors that confer resilience to PTSD, particularly in women.
Estrous cycle changes sleep features relevant to memory
The varying levels of hormones over the menstrual cycle in women are known to contribute to alterations in sleep physiology.10–13 Sleep–wake features also vary across the estrous cycle in rodents.14,15 Work in rodents has revealed the role of estradiol and progesterone to alter amounts of REM sleep and non-REM (NREM) sleep in relation to wake.16,17 We have recently demonstrated that sleep features relevant to memory consolidation during sleep change across the hormonal cycle, including REM sleep amounts, theta power, and phase coherence in the delta and gamma bands during NREM sleep, as well as spindle density, power, and coherence in NREM/TR sleep (Tables 1 and 2).18
Table 1.
Identified Sleep Features Significantly Different from Other Menstrual Cycle Phases in Women
| Early follicular | Late follicular | Early luteal | Late luteal | |
|---|---|---|---|---|
| Sleep quality | −12 | |||
| REM amount | +13 | +13 | −11,12 | −11,12 |
| Spindle frequency | +12,13 | +12,13 | ||
| NREM/TR sleep amount | +13 | +13 |
Superscript numbers show publication references reporting the finding.
NREM, non-REM; REM, rapid eye movement; TR, transition to REM, aka Stage 2.
Table 2.
Rodent Cycle Differences in Sleep Features Known to Be Important for Memory
| Sleep feature | Metestrus | Diestrus | Proestrus | Estrus |
|---|---|---|---|---|
| REM theta power | High18,58 | |||
| REM gamma coherence | High18 | |||
| Spindle sigma power | High18 | |||
| Spindle sigma coherence | High18 | |||
| Spindle density | High18 | |||
| NREM delta power | High18 | High18,58 | ||
| NREM delta coherence | High18 | |||
| Time in REM | Low14,16,18,55–59 | High16,18,55 | ||
| Time in NREM | Low18,59 |
Superscript numbers show publication references reporting the finding.
REM sleep
REM theta power increases in the neocortex during the last half of the dark phase of proestrus. Many studies have linked REM theta power with memory consolidation, most convincingly Boyce et al.19 Moreover, REM theta power is reduced in males and females who suffer from PTSD.20 Therefore, low points in REM theta power across the hormonal cycle, namely all cycle phases with low estradiol and progesterone, may be times wherein females are more vulnerable to PTSD. Conversely, higher theta power in the late proestrus phase could present a window of resilient sleep. Both slow and fast REM gamma coherence across brain areas also increases during the proestrus phase.18 Although there is not much research on gamma during sleep, gamma activity during wake in humans is shown to be important in working and long-term memory.21 Furthermore, theta–gamma coupling during REM sleep has been implicated in information processing.22,23 Interestingly, although theta power, gamma power, and coherence peak in late proestrus, females decrease time spent in REM through shorter REM bout lengths compared with other phases. REM sleep amounts rebound in the estrous phase although by then theta and gamma have returned to lower levels again.18
NREM sleep
Spindle (10–15 Hz sigma) power, density, and coherence increase across cortical areas during proestrus. Similarly, spindle density is correlated between cortical areas only during proestrus in females.18 Increases in the density of spindles are robustly linked with memory consolidation and in the integration of new information into existing knowledge in multiple studies.24–26 Delta power (1–4 Hz) and delta coherence also increase during the last half of proestrus across all brain areas measured (association, prefrontal, and hippocampus), persisting into the first part of estrous.18 Delta waves, and their hippocampal complement, sharp wave ripples, contain replay of memory sequences and have been associated with memory consolidation in multiple studies. In addition, their disruption is associated with impaired performance.27–29
Sex hormones and fear extinction
There have been numerous studies showing the ability of estradiol to improve memory and learning. Ovariectomized females lacking estrogens show deficits in learning that are reversed by administration of estrogens.30,31 Estradiol effects on learning may be due to direct action on memory-related synapses, or through indirectly modifying factors/states that are known to affect memory, that is, sleep. These hormonal effects extend to fear extinction learning and consolidation/recall. High circulating levels of estradiol enhance fear extinction recall while low levels impair extinction recall in naturally cycling rats.32–34 Women taking hormonal contraceptives that suppress synthesis of estradiol also show impaired fear extinction recall.34,35 Furthermore, one study demonstrates that estradiol supplements improve the efficacy of exposure therapy in women,36 whereas hormonal contraceptives show decreased efficacy of exposure therapy.37
Fear extinction memory consolidation is relevant to PTSD because the inability to suppress fear responses to cues that were once associated with trauma even after extinction training (exposure therapy) in safe contexts is a hallmark of PTSD. Many studies have combined to show that low estradiol levels contribute to PTSD vulnerability38 and systemically administering an estradiol receptor 2 agonist reduced vulnerability.34 Finally, women with low estradiol concentrations show higher autonomic signs of anxiety during fear extinction training,39 further supporting the idea that sex hormone levels can confer vulnerability to PTSD.
Model
The role of locus coeruleus (LC) in stress responses and sleep
One critical nucleus for adaptive stress response is the brainstem LC that responds to stressors and helps us quickly learn about them and consolidate them during sleep. Core symptoms of PTSD include heightened arousal states, which are associated with LC firing activity, and are directly linked with levels of norepinephrine (NE) released throughout the brain. It is generally accepted that the LC-NE system plays a role in the development or maintenance of PTSD, by overactivity of arousal networks following salient trauma cues and via overconsolidation of traumatic memories.
The LC exhibits relatively high levels of firing during wake compared with NREM and REM sleep. In males, the LC falls silent during REM sleep and 1 to 5 seconds before each sleep spindle. We have shown a critical role of LC silence during REM sleep to consolidating reversal memories that are dependent on the hippocampus.40 We showed that if the LC remains active, even at low rates, during REM sleep, reversal memory consolidation does not happen. Fear extinction, which is impaired in PTSD and other fear-related disorders, is similar to reversal learning in that previously consolidated associations between environmental cues and a behavior must be actively suppressed and a new competing association must be encoded. Sleep characteristics, especially theta power, spindle occurrence, and interregional electroencephalographic (EEG) coherence, are all impaired by even subarousal threshold level 2 Hz activation of the LC during sleep.
It is unknown whether the female LC falls silent during REM sleep, or whether there might be a hormone-related modulation of this silence. The LC is highly responsive to hormones that change across the estrous cycle, especially estradiol and progesterone.41–44 In addition, the LC is strongly inhibited by mu-opioid receptor activation, Curtis et al., and the concentration of mu-opioid receptors varies significantly across the estrous cycle, with highest levels during proestrus.41 Females have lower mu-opioid receptor function in the LC than males (ignoring the estrous cycle phase) that can render the LC overactive.45 If the expression profile change also occurs in the LC across the estrous cycle, then low hormonal phases would render the LC even more active. We posit that if the female LC does not fall silent during REM sleep at low hormonal phases, then sleep will not serve to consolidate reversal learning such as contextual extinction of fear.
Sleep is important in memory consolidation and in the flexible modification of memories.9,10,19,46 We propose that poor sleep in the period after a traumatic stressor is a critical junction point leading to maladaptive fear memory responses, and directly contributes to PTSD etiology. We summarize our proposed model in Figure 1. We address “good sleep” features leading to an adaptive post-trauma response and “poor sleep” features contributing to maladaptive responses to stress. Good sleep features would include those characteristics that have been associated with strong memory consolidation already mentioned, including fear extinction,7 which we find all peak at proestrus (Table 2).
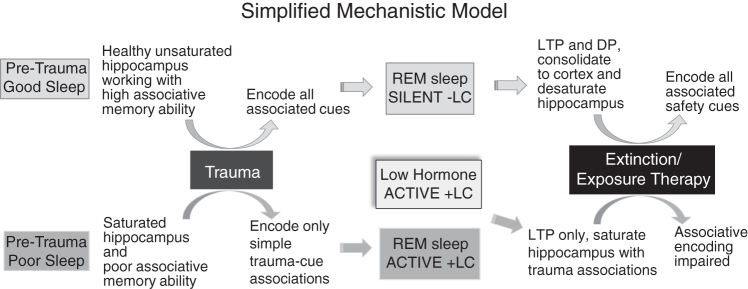
FIG. 1.
We propose that the ability to adaptively process a traumatic stressor is dependent on healthy sleep promoting both strengthening (LTP) and weakening (DP) of memory synapses. Weakening synapses requires silence of locus coeruleus (−LC), which normally occurs during REM sleep. DP avoids oversaturation of the hippocampus and allows proper encoding of all associated trauma cues. Conversely, poor sleep during the pretrauma period or maladaptive sleep (+LC) during the post-trauma period can contribute to conditions wherein hippocampal synapses are oversaturated and the person can only encode simple trauma–cue associations, leading to generalization of the fear response and contributing to PTSD development. Females at low hormone stages may be vulnerable to sleep-mediated hippocampal saturation effects if the LC continues firing during REM sleep. Effectiveness of extinction/exposure therapy in encoding cues previously associated with trauma to safety contexts may rely on LTP and DP efficiency, dependent on –LC condition. Hormonal phase and sleep efficiency should be considered in timing exposure therapies to maximize therapeutic outcomes. DP, depotentiation; LC, locus coeruleus; LTP, long term potentiation; PTSD, post-traumatic stress disorder; REM, rapid eye movement.
Pretrauma good sleep properly encodes and consolidates salient cues after traumatic stress, appropriately assigning a danger tag to aspects of the contextual environment directly linked with danger and safety tags to those aspects within the environmental context that are not dangerous. This requires that the novelty encoding networks of the hippocampus not be oversaturated (see Figure 1), and that the stress axis is not overstimulated. In contrast, oversaturated hippocampal circuits and excessive stress activation during the sleep period after traumatic stress can lead to incorrect or under contextualization of danger and safety cues, and thus poor consolidation of emotional memories, leading to PTSD. LC firing during REM sleep may directly contribute to saturation of hippocampal novelty encoding circuits.46 Depotentiation processes leading to renewal of hippocampal circuits only occur in the absence of norepinephrine,47–49 and in males the LC noradrenergic neurons are turned off for the necessary length of time to allow depotentiation only in the seconds before each sleep spindle and during REM sleep. If the LC remains active across sleep in females at low hormonal phases, then desaturation of synapses in these novelty encoding circuits cannot occur.
Exposure therapy inducing the extinction of fear also needs an unsaturated hippocampus to encode the safety context associations. Sleep is necessary for the consolidation of contextual fear extinction learning.7,50 We posit that maladaptive sleep, that is, sleep that includes LC firing in TR and REM sleep, will not serve this purpose because LC firing reduces sleep spindles, delta power, REM theta power, and interregional coherence features,39 all found to be important to the incorporation of new information into existing schema. Low hormonal phases of naturally cycling gonadal hormones in females likely impair those adaptive sleep features important in the consolidation of fear extinction, and this effect may be due to the burden of maintained LC activity.
Summary and critical unanswered questions
A more complete understanding of the details of female physiology across sleep is needed to better understand the increased propensity for mental health disorders such as PTSD. We have outlined several critical brain activity measures across the hormonal cycle that are relevant to memory, and memory processes are critical to cognitive and emotional function. The locus coeruleus, centrally equipped in its role as a modulator of arousal and memory via noradrenergic influence, has been implicated as a vital player in PTSD etiology.51–53 Overactivity of the LC during the pre- and post-trauma period, particularly during REM sleep, may overwhelm memory circuits and mechanisms contributing to resilient adaptation after stress. Estrogen and progesterone act on the LC, but it is not known whether cycling sex hormones change NE signaling in emotional processing and learning centers as a result. In addition, it is unknown whether the LC falls silent during REM sleep at all phases of the hormonal cycle in females. REM sleep theta and absence of NE as a result of pauses in LC activity allow for flexible modification of memories, which is relevant for the effectiveness of treatments such as exposure therapy. Thus exposure therapy may be most effective at high hormonal phases or be enhanced by sex hormone modifications such as adjuvant estradiol.36,54 The consideration of sex hormones and vulnerability to PTSD may be relevant to questions of trauma responses in postmenopausal women. Our model suggests that postmenopausal women, an understudied group in post-traumatic stress research, are more vulnerable to PTSD.
Sleep changes across the hormonal cycle and after a stressful event affect responses to trauma and recovery and can affect a broad variety of anxiety-related disorders. Hormonal stage and sleep tracking in studies of PTSD will aid in developing effective treatment strategies for this and possibly other stress disorders.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was funded by National Institute of Mental Health R01-60670 and the Department of Integrative Biology and Physiology to GRP. YC has a Cota-Robles Fellowship.
References
- 1. Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 2019;44:111–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suh S, Cho N, Zhang J. Sex differences in insomnia: from epidemiology and etiology to intervention. Curr Psychiatry Rep 2018;20:69. [DOI] [PubMed] [Google Scholar]
- 3. Nutt DJ, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci 2008;10:329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cox RC, Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord 2016;37:104–129 [DOI] [PubMed] [Google Scholar]
- 5. Krystal AD. Psychiatric disorders and sleep. Neurol Clin 2012;30:1389–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parry BL, Martínez LF, Maurer EL, López AM, Sorenson D, Meliska CJ. Sleep, rhythms and women's mood. Part I. Menstrual cycle, pregnancy and postpartum. Sleep Med Rev 2006;10:129–144 [DOI] [PubMed] [Google Scholar]
- 7. Pace-Schott EF, Germain A, Milad MR. Sleep and REM sleep disturbance in the pathophysiology of PTSD: The role of extinction memory. Biol Mood Anxiety Disord 2015;5:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pitman RK, Rasmusson AM, Koenen KC, et al. . Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012;13:769–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanderheyden WM, Poe GR, Liberzon I. Trauma exposure and sleep: Using a rodent model to understand sleep function in PTSD. Exp Brain Res 2014;232:1575–1584 [DOI] [PubMed] [Google Scholar]
- 10. Wassing R, Lakbila-Kamal O, Ramautar J, Stoffers D, Schalkwijk F, Someren E. Restless REM sleep impedes overnight amygdala adaptation. Curr Biol 2019;29:2351–2358.e4. [DOI] [PubMed] [Google Scholar]
- 11. Baker FC, Lee KA. Menstrual cycle effects on sleep. Sleep Med Clin 2018;13:283–294 [DOI] [PubMed] [Google Scholar]
- 12. Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med 2007;8:613–622 [DOI] [PubMed] [Google Scholar]
- 13. Driver HS, Dijk DJ, Werth E, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab 1996;81:728–735 [DOI] [PubMed] [Google Scholar]
- 14. Fang J, Fishbein W. Sex differences in paradoxical sleep: Influences of estrus cycle and ovariectomy. Brain Res 1996;734:275–285 [PubMed] [Google Scholar]
- 15. Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep 2006;29:1211–1223 [DOI] [PubMed] [Google Scholar]
- 16. Colvin GB, Whitmoyer DI, Sawyer CH. Circadian sleep wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol 1969;616425:616–625 [DOI] [PubMed] [Google Scholar]
- 17. Deurveilher S, Rusak B, Semba K. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep 2011;34:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swift K, Keus K, Gonzalez C, et al. . Sex differences within sleep in gonadally-intact rats. Sleep 2019;pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 2016;352:812–816 [DOI] [PubMed] [Google Scholar]
- 20. Cowdin N, Kobayashi I, Mellman TA. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res 2014;232:1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 2007;30:317–324 [DOI] [PubMed] [Google Scholar]
- 22. Montgomery S, Sirota A, Buzsáki G. Theta and gamma coordination of hippocampal networks during waking and REM sleep. J Neurosci 2008;28:6731–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bandarabadi M, Boyce R, Herrera CG, et al. . Dynamical modulation of theta-gamma coupling during REM sleep. Sleep 2017;41:169656 [Google Scholar]
- 24. Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci 2010;30:14356–14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mednick SC, McDevitt EA, Walsh JK, et al. . The critical role of sleep spindles in hippocampal-dependent memory: A pharmacology study. J Neurosci 2013;33:4494–4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vermeulen MCM, Van der Heijden KB, Swaab H, Van Someren EJW. Sleep spindle characteristics and sleep architecture are associated with learning of executive functions in school-age children. J Sleep Res 2019;28:e12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 2009;12:1222–1223 [DOI] [PubMed] [Google Scholar]
- 28. Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science 1994;265:676–679 [DOI] [PubMed] [Google Scholar]
- 29. Genzel L, Kroes MCW, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: A question of global versus local processes? Trends Neurosci 2014;37:10–19 [DOI] [PubMed] [Google Scholar]
- 30. Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 2003;144:2836–44 [DOI] [PubMed] [Google Scholar]
- 31. Duarte-Guterman P, Yagi S, Chow C, Galea LAM. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm Behav 2015;74:37–52 [DOI] [PubMed] [Google Scholar]
- 32. Chang Y, Yang C, Liang Y, Yeh C. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor b. Hippocampus 2009;1150:1142–1150 [DOI] [PubMed] [Google Scholar]
- 33. Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 2009;164:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry 2013;73:371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hwang MJ, Zsido RG, Song H, et al. . Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry 2015;15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Graham BM, Scott E. Effects of systemic estradiol on fear extinction in female rats are dependent on interactions between dose, estrous phase, and endogenous estradiol levels. Horm Behav 2018;97:67–74 [DOI] [PubMed] [Google Scholar]
- 37. Li S, Graham BM. Estradiol is associated with altered cognitive and physiological responses during fear conditioning and extinction in healthy and spider phobic women. Behav Neurosci 2016;130:614–623 [DOI] [PubMed] [Google Scholar]
- 38. Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biol Mood Anxiety Disord 2012;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem 2014;116:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swift KM, Gross BA, Frazer MA, et al. . Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr Biol 2018;28:3599–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Curtis AL, Leiser SC, Snyder K, Valentino RJ. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology 2012;62:1737–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huhn AS, Berry MS, Dunn KE. Systematic review of sex-based differences in opioid-based effects. Int Rev Psychiatry 2018;30:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bangassar DA, Wiersielis KR, Khantsis S. Sex differences in the locus coeruleus -norepinephrine system and its regulation by stress. Brain Res 2016;1641(pt B):177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Helena CV, de Ovliveda PM, Sanvito GL, et al. . Changes in alpha estradiol receptor progesterone receptor expression in the locus coeruleus and preoptic area throughout the rat estrous cycle. J Endocrinol 2006;188:155–165 [DOI] [PubMed] [Google Scholar]
- 45. Guajardo HM, Snyder K, Ho A, Valentino RJ. Sex differences in μ-opioid receptor regulation of the rat locus coeruleus and their cognitive consequences. Neuropsychopharmacology 2017;42:1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poe GR. Sleep is for forgetting. J Neurosci 2017;37:464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. Activity dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 1996;17:475–482 [DOI] [PubMed] [Google Scholar]
- 48. Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol 1997;77:3013–3020 [DOI] [PubMed] [Google Scholar]
- 49. O'Dell TJ, Connor SA, Guglietta R, Nguyen PV. Beta-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem 2015;22:461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Genzel L, Spoormaker V, Konrad B, Dressler M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiol Learn Mem 2015;122:110–121 [DOI] [PubMed] [Google Scholar]
- 51. Javanbakht A, Poe GR. Behavioral neuroscience of circuits involved in arousal regulation. In: Ressler K, Liberzon I, eds. The neurobiology of PTSD. New York, NY: Oxford University Press, 2016:130–147 [Google Scholar]
- 52. Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry 1995;38:174–179 [DOI] [PubMed] [Google Scholar]
- 53. Geracioti TD Jr., Baker DG, Kasckow JW, et al. . Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology 2008;33:416–424 [DOI] [PubMed] [Google Scholar]
- 54. Graham BM, Daher M. Estradiol and progesterone have opposing roles in the regulation of fear extinction in female rats. Neuropsychopharmacology 2016;41:774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yokoyama A, Ramirez VD, Sawyer CH. Sleep and wakefulness in female rats under various hormonal and physiological conditions. Gen Comp Endocrinol 1966;7:10–17 [Google Scholar]
- 56. Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res 1968;7:173–81 [DOI] [PubMed] [Google Scholar]
- 57. Koehl M, Battle SE, Turek FW. Sleep in female mice: A strain comparison across the estrous cycle. Sleep 2003;26:267–272 [DOI] [PubMed] [Google Scholar]
- 58. Schwierin B, Borbély AA, Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res 1998;811:96–104 [DOI] [PubMed] [Google Scholar]
- 59. Zhang SQ, Kimura M, Inoué S. Sleep patterns in cyclic and pseudopregnant rats. Neurosci Lett 1995;193:125–128 [DOI] [PubMed] [Google Scholar]