During the rapid spread of HIV-1 in humans, the virus has evolved and diversified extensively. In this Opinion article, Eric Arts and colleagues discuss the potential impact of this diversification on viral fitness and spread, and speculate on whether HIV-1 is attenuating.
Supplementary information
The online version of this article (doi:10.1038/nrmicro1594) contains supplementary material, which is available to authorized users.
Abstract
During the rapid spread of HIV-1 in humans, the main (M) group of HIV-1 has evolved into ten distinct subtypes, undergone countless recombination events and diversified extensively. The impact of this extreme genetic diversity on the phenotype of HIV-1 has only recently become a research focus, but early findings indicate that the dominance of HIV-1 subtype C in the current epidemic might be related to the lower virulence of this subtype compared with other subtypes. Here, we explore whether HIV-1 has reached peak virulence or has already started the slow path to attenuation.
Supplementary information
The online version of this article (doi:10.1038/nrmicro1594) contains supplementary material, which is available to authorized users.
Main
There are four classified retroviruses that infect humans and cause symptomatic disease. Human T-cell lymphotropic virus (HTLV) types 1 and 2 are members of the Deltaretrovirus genus in the subfamily Orthoretrovirinae, show a low rate of evolution in the human population, and probably originated from a non-human primate millions of years ago1,2,3,4. The high frequency of HTLV-1 infections was localized in specific endemic areas (for example, southern Japan) until recent times, indicating a relatively slow spread in the human population compared with another human retrovirus, HIV-1.
HIV-1 was introduced into the human population just 60–80 years ago5 but an estimated 40 million individuals are currently infected with the virus6. The spread and expansion of HIV-1 across Africa and throughout the world has been accompanied by one of the most rapid evolutionary rates described for a human pathogen6, aside from hepatitis C virus. HIV-1 remains one of the most lethal pathogens (100% mortality) that currently infects humans, whereas infection by other human viruses that are often more feared, such as Ebola, severe acute respiratory syndrome (SARS), influenza H5NI and Lassa Fever, can have a mortality rate of <50% (Refs 7–9).
Whether or not a lethal pathogen evolves to become a relatively benign parasite or even a commensal organism has been a subject of great debate. The survival of Euroasian populations during the European bubonic plague (1347–1352) and the 1918 influenza epidemic is often perceived as evidence for pathogen attenuation. However, neither Yersinia pestis nor the H1N1 influenza virus seem to have attenuated during these relatively short human epidemics. Surviving the Y. pestis epidemic is thought to be more related to fractionation of the human population, the resistance of rodent ectoparasites and, possibly, the selection of resistant human hosts10,11.
Nonetheless, there are many examples of microparasite evolution and host selection leading to attenuation or resistance to disease. The best documented and controlled example is the rapid outgrowth of an attenuated myxoma virus following its introduction into Australia in 1950 to control the expanding rabbit population12,13,14. This virus might have attenuated as a result of altered immunomodulatory properties in addition to a reduction in the replication rate13,14. More recent analyses of archived reports from the fifteenth century suggest that the dramatic decrease in the severity of syphilis symptoms that took place over a period of less than 10 years was related to a reduction in virulence rather than the selection of resistant hosts15. Rapid (decades to centuries) versus slow (thousand to millions of years) attenuation might be related to the severity of disease, the rate of lethality and the transmission efficiency or transmission routes. The suggestion that the simian lentiviruses have attenuated in their non-human primate hosts during millions of years of co-evolution16 has been challenged by recent phylogenetic analyses that indicate the introduction of simian immunodeficiency virus (SIV) into African primates might be a relatively recent event that unfolded in the past hundreds or thousands of years, therefore indicating that primate lentiviruses might frequently jump between primate species and then rapidly adapt to the new host17,18.
Studies that have modelled the expansion and contraction of various epidemics have indicated that the inter-relationships between lethal pathogens and their hosts are complex and frequently not comparable19,20,21,22. These questions can only be answered by understanding pathogen evolution, dynamics and spread within the population at large and within infected hosts. The propensity for HIV-1 to evolve rapidly in response to various immune and other host pressures indicates that this virus is more likely to become attenuated through a reduction of virulence rather than selection of resistant hosts.
Defining pathogen fitness and virulence
The phylogenetic approaches that are commonly used to describe the evolution of pathogens do not examine how these genetic differences actually impact on the physical attributes, often termed the fitness, of the pathogen. This is understandable, given the complexities of measuring fitness, which can be defined as the adaptability or reproductive success of an organism in a specific environment. In the case of HIV-1 research, fitness is frequently misrepresented or over-interpreted23,24. For example, HIV-1 virions in which the reverse transcriptase (RT) harbours mutations that confer resistance to antiretroviral drugs (ARVs) such as 3TC (Lamivudine or Epivir) seem less fit than virions carrying the wild-type RT25,26. However, this reduced fitness in a reconstituted in vitro assay does not necessarily imply that the drug-resistant virus will be less fit in a human host receiving ARVs. Fitness in each environment can be inter-related but is not necessarily consequential. For the purposes of this Opinion article, HIV-1 fitness will be defined in a specific environment and only significant correlations between environments will be highlighted as being potentially consequential.
Fitness is not always synonymous with virulence, which is typically defined as the rate of host mortality as a consequence of infection27 but which can be further refined to include the reproduction rate and pathogenic potential of the pathogen28. These parameters also define the fitness of a pathogen within a host. Typically, virulence is difficult to measure for viruses such as HIV-1, given the long asymptomatic period after infection. Therefore, intermittent measures of HIV-1 replicative fitness during disease can often provide insights into the rates of disease progression29,30,31,32. Confusion between these principles of virulence and fitness is introduced when examining fitness within the host compared with examining fitness within a population24,33. Maynard, Ewald, Anderson, May, Novak and others22,33,34,35,36 suggest that pathogen strain 'A', with slow replicative fitness but high transmission efficiency, will be favoured in a host population over strain 'B', with increased replicative fitness. However, when the strains compete together in a single host, it is clear that strain 'B' is more virulent and would out-compete and, consequently, be fitter than, strain 'A'. In terms of a pathogen spreading through a host population, high pathogen fitness is then defined by a reduction in reproduction rate and virulence without a loss in transmission. This article will address the possible direct relationship between HIV-1 virulence and replicative fitness but it is important to note that attenuation of virulence is not always synonymous with lower replicative capacity. For example, the attenuation of myxoma virulence in the Australian rabbit population might be more related to changes in virus-mediated immunomodulation than to lower replicative fitness in rabbits13,14.
Expansion of HIV-1 in humans
HIV-1 was first introduced into the human population from the chimpanzee subspecies Pan troglodytes troglodytes37,38,39. Most recently, Keele et al. have discovered a region in southern Cameroon where chimpanzees carry SIV strains (SIVcpzptt) that are closely related to two distinct lineages of HIV-1, the main (M) and new (N) groups37 (Fig. 1). The origin of the outlier (O) group might be related to a jump from Gorilla gorilla40. SIVcpzptt was introduced into the human population multiple times, over decades to possibly centuries, but these transfer events probably started to increase in the 1920s to the 1950s as a result of human migration into this dense tropical region5,37,38,39. Similar to the pending introduction of influenza H5N1 from the avian to the human host41, a distinct but possibly rare SIVcpzptt strain might have had the capacity for efficient replication and sexual transmission in humans17,37. Following this founder event, human population density and contact had to be sufficiently high for subsequent transmission and spread. It is possible that a single transmission of HIV-1 group M spawned the >60–80 million infections that have taken place since the beginning of the epidemic37. By contrast, HIV-2 originated in humans from a cross-species transmission from sooty mangabeys in west Africa (near or in Guinea-Bissau) around 1930–1955 (Refs 42, 43). In the 1980s, the incidence of HIV-1 group M in central Africa and HIV-2 in west Africa expanded exponentially but clearly at different rates, as the prevalence of HIV-1 M infections (28 million) far exceeds that of HIV-2 infections (<1 million)6,44,45. In stark contrast, HIV-1 groups O and N are responsible for <25,000 infections, most of which are in Cameroon and Gabon6,46,47,48,49. The expansion and divergent evolution of HIV-1 group M into diverse subtypes (Fig. 1) has been dated to 1956–1976 and might have coincided with human emigration and the seeding of new regional epidemics in central Africa5,50,51.
Figure 1. Phylogenetic tree of human and simian lentiviruses.

The genetic similarity between different HIV and simian immunodeficiency virus (SIV) strains was compared by aligning the full genome sequences of 87 human and simian lentiviruses using ClustalX v.1.83 (the accession numbers are available on request). Phylogenetic trees based on nucleotide distance were constructed by neighbour-joining methods as implemented in ClustalX with 1,000 bootstrap resamplings (not presented) and schematically represented with the TreeView program. HIV-2 and HIV-1 share only 50–60% sequence identity and cluster at distinct locations on the phylogenetic tree whereas SIVcpz branches out from the root of the HIV-1 groups. The origins of these HIV-1 groups in southern Cameroon have recently been described and indicate two probable jumps from chimpanzee (groups M and N) and gorilla (group O) species. HIV-1 M subtypes probably evolved from a discrete introduction into the human population and then diverged into different subtypes. The subtypes defined as 'A-like' describe HIV-1 isolates with sequences that map phylogenetically more to subtype A than to any other subtype. For example, the recombinant form CRF02_AG (such as 02 AG.NG.IBNG in the HIV-1 group M A-like cluster) has longer genomic segments that are more related to subtype A than to subtype G. M, main; N, new.
HIV-1 subtypes share 70–90% sequence identity, groups share <70%, and HIV-1 and HIV-2 can differ as much as 50% at the nucleotide level (Fig. 1). Given this extreme genetic diversity, is it reasonable to assume that not all HIV types, groups, subtypes and even isolates have evolved to maintain similar virulence? Although many research articles have described genotypic differences between subtypes, there are only a handful of studies that have examined potential phenotypic differences among the human lentiviruses52,53,54,55,56. No studies have modelled how phenotypic or 'fitness' differences among these viruses might affect disease progression in infected individuals or the general spread of the virus in the human population. This article can only provide predictions of temporal HIV-1 attenuation based on published studies and significant trends during the human epidemic. To understand how attenuation of virulence can occur, it is important to understand the population dynamics and fitness of HIV-1 in each infected host and during host-to-host transmission.
How HIV-1 infects, and evolves in, a host
In relation to other sexually transmitted pathogens, HIV-1 is transmitted with moderate efficiency by sexual contact and apparently greater efficiency by direct blood-to-blood contact (for example, needle sharing by intravenous drug users)57,58 or vertical mother-to-child perinatal transmission59. There are many cases of discordant couples in which the HIV-positive individual fails to transmit the virus to the uninfected partner despite frequent opportunity60. A schematic of transmission events and disease progression is provided in Fig. 2 and Box 1. An increased viral load in the donor and consequently in the inoculating dose increases the transmission efficiency61,62. An increased viral load in the donor also corresponds to greater genetic diversity of the inoculating HIV-1 population (termed the virus isolate)63 (Fig. 2), and yet few HIV-1 clones will establish an infection in the recipient64. This dramatic bottleneck might be related to accessing the initial target cells (cells of the dendritic lineage)65,66 and establishing a productive, systemic infection. Based on extensive studies on RNA viruses, it is clear that restrictive genetic bottlenecks invoked by various selection pressures will reduce replicative fitness67,68,69. Unfortunately, nearly all of these viral fitness studies have been done in tissue-culture infections and not in animal models or in defined donor–recipient transmission pairs. Greater HIV-1 genetic diversity during acute or early infection has been associated with more rapid disease progression70. In addition, the genetic diversity of HIV-1 seems to correlate directly with replicative fitness29.
Figure 2. A hypothetical example of changes in replicative fitness and viral load during HIV-1 disease progression.

An individual is typically infected by a few HIV-1 clones (depicted by a small green circle), which dramatically increase in copy number but not in genetic diversity during the first 1–2 months of infection. Following this acute infection period, viral load is reduced partly as a result of strong HIV-specific cell-mediated immunity. The virus population is thought to oscillate between expansion of HIV-1 populations owing to immune escape from existing HIV-specific cytotoxic T-lymphocyte (CTL) clones and contraction caused by new genetic bottlenecks that are induced by newly emerging CTL clones. The replicative fitness and genetic diversity of the HIV-1 population seem to track closely together and, following early disease (purple box), both increase at a relatively linear rate with the length of infection. This increase in replicative fitness correlates with increases in viral loads (right axis) and decreases in CD4+ T-cell counts (not shown). The scale for replicative fitness is arbitrary but is derived from the relative HIV-1 fitness values, that is, the ability of one HIV-1 isolate to out-compete another in ex vivo dual-virus competition experiments.
HIV is the obvious aetiological agent of AIDS, yet it is commonly assumed that the phenotypic characteristics and replication efficiency (the ex vivo fitness) of the infecting, wild-type HIV isolates have little impact on the rate of disease progression. Much attention has been focused on host correlates of disease progression, such as the strength of HIV-specific immune responses71,72,73,74,75,76,77 and host genetic polymorphisms that might affect HIV-1 replication78,79,80,81,82. RNA load remains the best predictor of HIV disease progression83 and the dramatic reductions in this load achieved by ARV treatment can delay disease progression84. It is important to note that a high viral load is not an absolute in relation to the severity of the disease. In non-human hosts such as sooty mangabeys, a high SIV load is not associated with clinical symptoms85. Recent studies indicate that the decline in CD4+ T-cell counts, and not the increase in viral load, is a better predictor of disease progression86.
The first evidence that specific viral genetic traits might affect virulence was the identification of long-term non-progressing individuals harbouring a defective HIV-1 isolate lacking a nef open reading frame87,88. Mutations or deletions in this accessory-protein-coding gene can significantly impair fitness in tissue-culture infections89. Even with this discovery 10 years ago, the effect of relative HIV-1 replicative fitness on disease progression has not been the subject of intense study owing to difficulties in accurately measuring HIV-1 fitness.
There have been only limited studies examining how virus fitness changes during natural infections31,32,90,91. The replication capacities of different HIV-1 isolates are commonly compared by measuring ex vivo growth kinetics in single virus infections31,32. The viruses used in many studies are not isolated from infected patients but rather are chimeric recombinant viruses containing gene(s) from the patient's virus within the genome of a laboratory HIV-1 strain32,92. Small environmental differences in separate single virus infections and the inherent variability of detection assays can prevent accurate estimates of viral fitness by these methods. By contrast, dual virus infections or competition assays involve multiple rounds of virus replication, are less affected by environmental changes and, as a result, can quantify small but significant differences in fitness29,93,94. In HIV research, multiple and single-cycle single virus infections, and competition experiments, have been used in studies that described impaired HIV-1 replicative fitness conferred by drug-resistance mutations95,96. Nevertheless, the impact of these drug resistance mutations in primary HIV-1 isolates (as opposed to chimeric viruses) has not been fully assessed.
Initial reports using HIV-1 competition assays in peripheral blood mononuclear cell (PBMC) cultures to measure fitness indicated that many long-term survivors harbour HIV-1 with an impaired replication capacity, whereas rapid progressors can be infected with more aggressive HIV-1 isolates30. Subsequent studies indicated that the genetic diversity and fitness of the intra-patient HIV-1 population continues to increase during disease and to diverge from the founder HIV-1 clones29,63,70 (Fig. 2). This diversity reaches an inflection point just before the inception of AIDS symptoms. During the asymptomatic period, the steady increase in HIV-1 diversity occurs concomitantly with increasing viral load and, more significantly, with increasing ex vivo replicative fitness of the infecting HIV-1 isolate29 (Fig. 2). Red Queen dynamics (in which the interaction between a parasite and its host leads to a constant evolutionary process of adaptation and counter-adaptation) predict that increasing replicative fitness must be associated with a continual expansion of the genetic breadth and size of a population97,98. Any strong selective pressure can result in the contraction of population size, the appearance of deleterious mutations and decreasing fitness99,100,101. Interestingly, the introduction of ARVs as a strong selective pressure during asymptomatic disease resulted in a dramatic decrease in HIV-1 load, genetic diversity and replicative fitness, even in the absence of drug resistance mutations29.
Following transmission, it is possible that the replicative fitness and genetic diversity of newly infecting HIV-1 isolates is lower than that of the inoculating virus from the donor (Box 1). Such a continual increase in HIV-1 diversity and fitness is in direct conflict with the idea that the HIV-specific acquired immune response can counter this viral expansion102. Discrepancies in these data might simply be due to the time period of sampling and analyses. HIV-1 genetic diversity and fitness only increases after the first one to two years of infection29 (Fig. 2). Immediately following acute infection, both host genetics and the HIV-specific immune response might have crucial roles in establishing a genetic diversity and fitness set point, which might also correspond with the well-documented viral-load set point83,103 (Fig. 2). Cell-mediated immunity could be the most dominant factor in specifically reducing viral load and, as a consequence, fitness and diversity104,105,106,107.
How can HIV-1 virulence be attenuated?
The genes at the human leukocyte antigen (HLA) loci are extremely variable. In humans in most geographical regions, it is typically rare to find a perfect match in the HLA A–G alleles that encode major histocompatibility complex (MHC) class I and in the six HLA D genes that encode MHC class II. Even in regions of specific ethnicities, there is considerable population diversity in HLA alleles, which encompass at least 21 major A, 35 B and 15 C alleles (see the dbMHC web site). Compared with Eurasian populations, HLA diversity is much greater in the main ethnic groups of sub-Saharan Africa; a study of >4,000 individuals characterized at the A, B and C loci found no preference for a single allele108. The least HLA diversity is often observed in more homogeneous Caucasian populations, most notably, the Caucasian Australian population (see the dbMHC web site)109.
How might a human population that is diverse in terms of HLA type or other host polymorphisms influence HIV-1 evolution? Quantitative aspects of the HIV-specific immune response (for example, high levels of HIV-specific cytotoxic T lymphocytes (CTLs) versus low levels) are self-limiting because this immunity is not transferred between individuals. However, a qualitative difference in terms of what the immune system 'sees' in the infecting HIV-1 strain could have a significant impact on virulence. A mismatch of HLA genes between the donor and recipient of a transmission pair would result in the presentation of a different set of HIV-1 peptide epitopes to CD8+ T cells in the recipient that were not recognized in the donor110,111. With each new transmission, the infecting virus must then evolve to escape this pressure, which in turn seems to reduce replicative fitness112,113. In human populations with a low HLA diversity (for example, in Australia), CTL escape mutations might become fixed along with compensatory mutations that restore or maintain HIV-1 virulence109 (Box 1). By contrast, the constant passage of HIV-1 through humans of different HLA types (for example, in Africa) might prevent the accumulation of compensatory mutations, which could stabilize CTL escape mutations (Box 1). However, a model involving only HLA types is obviously too simplistic, considering the effects of other host factors. For example, polymorphisms in other host restriction factors, such as ABOBEC3G/3F and TRIM5α, could also alter disease progression114,115. Furthermore, host polymorphisms in the HIV-1 co-receptor CCR5 and its ligands (for example, CCL3L1) alter co-receptor or ligand expression levels and, as such, affect the ability of HIV-1 to use the co-receptor78,79,80,81,82. If these polymorphisms exist at high frequencies in the human population, they too could result in an oscillation between HIV-1 sequences that, in this example, reduce or increase co-receptor avidity, a correlate of replicative fitness.
In general, HIV-1 virulence might attenuate if, on average, the loss in fitness that occurs following transmission is greater than the gain in replicative fitness that occurs during asymptomatic disease progression (Box 1). Loss of fitness is probably due to the genetic bottleneck during transmission and/or escape from host restrictions or the host immune response during early disease. The continual introduction of new selective pressures and genetic bottlenecks would lead to the accumulation of deleterious mutations, which in turn would reduce replicative fitness or virulence. However, these hypotheses have not been empirically tested using HIV-1 transmission pairs.
Is there evidence for attenuation of HIV-1?
With the exceptions of anecdotes, a few case reports and one published study on a large cohort, there is no evidence that HIV-1 virulence has either increased or decreased during the past 20–30 years of the pandemic116,117,118,119,120,121. One of the main obstacles in comparing disease progression in the mid-1980s with the current day epidemic in the developed world is the significant medical advancements that have been made. In the developing world, until recently, few if any cohorts have been analysed for disease progression. Finally, owing to increased awareness and education about AIDS, patients are now diagnosed earlier, sometimes during acute infection, whereas in the 1980s many patients did not present to a clinic until the onset of AIDS. As a result, there is often the impression that HIV-1 infections are less aggressive now than they were at the beginning of the pandemic.
In a study published last year, >100 primary HIV-1 isolates were obtained from patient samples in Antwerp, Belgium, but only twelve could be matched from the periods 1985–1987 and 2002–2003 (Ref. 121). All 24 viruses from both time periods were competed against each other in PBMC from HIV-negative donors. Based on >500 head-to-head virus competition experiments, the recent HIV-1 isolates were significantly less fit than the historical isolates121. These findings indicate that HIV-1 might have attenuated in replicative fitness over the past 15–18 years. If the fitness of the infecting HIV-1 strain is a predictor of disease progression29,30,31,32, reduced ex vivo fitness in PBMC might be a strong correlate of decreased virulence. However, these results should be interpreted with caution owing to the limited sample size involved. Furthermore, the cross-sectional design of this study results in sampling of HIV-1 isolates at a single time point during disease progression, which was controlled for disease stage based on CD4+ T-cell count and viral load. Nonetheless, the increased fitness of the 1980 viruses versus the 2000 viruses was significant and greater than any increase in HIV-1 fitness observed during disease progression29,121. Future studies on large cohorts that span the epidemic temporally in various geographical regions are warranted but, as described below, these studies will again be limited to analyses of HIV-1 virulence (that is, replicative fitness) as rates of disease progression will not be available (see below).
Rates of disease progression have now been examined in a European HIV-infected cohort spanning the years 1986–2002 (Ref. 119). The slopes of CD4+ T-cell decline were calculated in antiretroviral-treatment-naive patients with confirmed dates of infection, at no fewer than nine time points over periods >1 year. Following this early period of the AIDS epidemic, there seems to be some evidence for slowing rates of disease progression from 1992–1998. Interestingly, the period following 1992 also corresponds to increasing use of combination ARV treatment, which could only be indirectly related to this attenuation as none of the patients received ARVs during the study period. It is possible that drug resistance mutations might have appeared at a higher frequency during sub-optimal treatment from 1992–1998, that is, before the widespread use of highly active antiretroviral therapy (HAART). These mutations have been associated with reduced fitness95,96 but their presence following a new transmission and in the absence of ARV treatment is low (<5–20%)122,123 and would not fully explain why the rates of disease progression might have decreased between 1992–1998.
The detection of fluctuations in the rate of CD4+ T-cell decline at the start of the HIV-1 epidemic in Europe is surprising given the preliminary data suggesting that replicative fitness might have been higher in the 1980s compared with the 2000s (Ref. 121). Slopes of CD4+ T-cell decline can be heavily influenced by the length of longitudinal follow-up, the follow-up period post-infection, and the number of data points124. For example, most patients in the 1980s presented to a clinic with advanced disease when the slopes of CD4+ T-cell decline are commonly lower than in early disease (especially once the level falls below 200 CD4+ T cells per μl)124,125. Therefore, 'slow' disease progression during 1986–1988 might be associated with the accrual of patients with more advanced disease. In general, natural history cohorts starting at early or acute infection and ending with AIDS-defining illnesses or morbidity (typically 3–10 years without ARV treatment) provide the best estimates of CD4+ T-cell decline and disease progression rates125. In the developed world, cohorts from the 1980s are not readily available owing to late diagnosis, whereas the current cohorts are difficult to assess for the natural history of disease due to ARV intervention. Finally, given the reduced HLA diversity and greater ethnic homogeneity in the Caucasian population, the attenuation rate of subtype B HIV-1 virulence might be less dramatic than the attenuation possible with non-B subtypes in sub-Saharan Africa.
In vivo and ex vivo fitness differences
Early observations on the rates of disease progression in west Africa showed that HIV-2 infections were less aggressive than HIV-1 infections126,127. HIV-2 is also transmitted less frequently than HIV-1 in human populations of similar demographics and similar opportunities for sexual contacts128,129. Whereas HIV-1 has spread across the globe, founder events of HIV-2 in areas other than west Africa did not result in regional epidemics (Fig. 3). Even HIV-1 infections are not evenly distributed among the different groups and subtypes: group N infections have only been characterized in five Cameroonians, whereas ∼30,000 group O infections are estimated in Cameroon and Gabon6,46.
Figure 3. HIV-1 diversity in the worldwide epidemic.
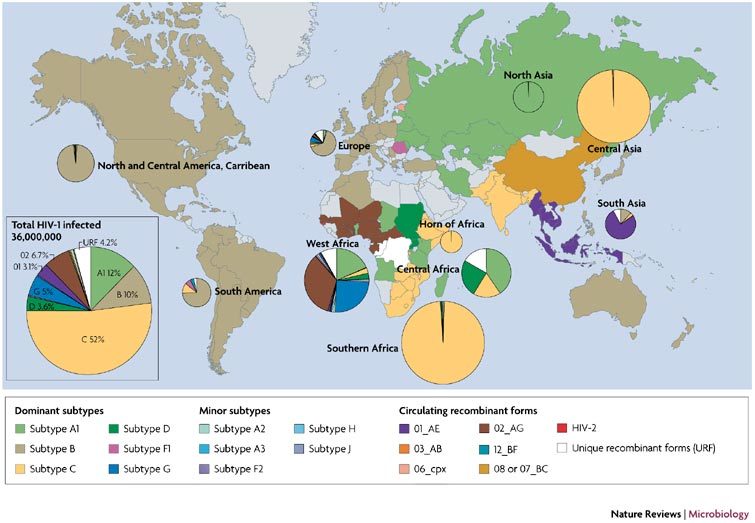
The frequency of each HIV-1 subtype and recombinant form was estimated in each country based on published findings. A complete breakdown of subtype prevalence per country and the countries present in each region are listed in the Supplementary information S1 (table). The countries are colour-coded based on the dominant HIV-1 group main (M) subtype. The countries coloured grey have a low level of HIV-1 prevalence or were not represented in the scientific literature related to HIV-1 subtype prevalence. The pie charts depict the proportion of each subtype or recombinant form in each geographical region. The size of the pies is proportional to the number of HIV-1 infected individuals in that particular region.
HIV-1 group M subtype C has spread more rapidly than any other M subtype, partly as a result of recent subtype C pandemics in southern Africa, south America and Asia130,131,132,133 (Figs 3,4a; and Supplementary information S1 (table)). 'Pure' HIV-1 subtype C or HIV-1 recombinant forms containing at least the envelope gene of subtype C are now responsible for >50% of the HIV-1 infections worldwide130. A founder event of subtype C or a C-containing recombinant form in many regions has also resulted in an apparent displacement of existing HIV-1 subtypes (such as subtypes B and CRF01_AE in south China131,132, many subtypes in Kinshasa, Democratic Republic of Congo134 and subtype B in southern Brazil133) (Figs 3,4A). This displacement could be due to the introduction of subtype C into a population by different transmission routes (heterosexual transmission versus intravenous drug use) or increased sexual activity (for example, commercial sex workers)135. A subtype might initially predominate in a specific transmission group but there is little supporting evidence to suggest that subtypes A, B, C, D and CRF01_AE (also referred to as E) are any more or less transmissible by a specific route, in a specific ethnic group or in specific cell types53,136,137,138,139,140,141. In addition, such initial predominance is generally lost during an epidemic. For example, in Thailand HIV-1 subtypes B and CRF01_AE were segregated to intravenous drug users and heterosexuals, respectively, in the late 1980s but are now more uniformly mixed in the Thai population142.
Figure 4. Relationship between the increasing prevalence of HIV-1 subtype C and its low pathogenic fitness.

The prevalence of HIV-1 subtype C or subtype C-containing recombinant forms has increased in proportion to other HIV-1 subtypes in Rio do Sul, Brazil (from 35% in 1996 to 52% 2002)133, in Kinshasa and Mbuji-Mayi, Democratic Republic of Congo (DRC) (from 2.1% and 16.3% in 1997 to 9.7% and 25% in 2002, respectively)134 and in Yunnan, China (from 5.1% in 1992 to 90% in 2002)162,163. By contrast, subtype C is dominant in South Africa164 and did not increase in proportion to subtypes A and D or recombinant forms in Kenya165. The number of HIV-1 cases increased in all of these regional epidemics (except in Kinshasa)6. a | The number of HIV-1 infections in specific years is subdivided into subtype C (yellow) and non-subtype-C (maroon) HIV-1 infections. b | A schematic representation of the pathogenic fitness of human lentiviruses, derived from >3,000 pair-wise dual HIV-1 competition experiments26,27,49,50,108,120,123,125,165. The coloured ovals plot the fitness of primary HIV-1 isolates competed against isolates of the same type, group or subtype (y axis; mean fitness of 1 or equal fitness) compared with the fitness of primary HIV-1 isolates competed against isolates of different types, groups or subtypes. Each oval encompasses the fitness (x and y fitness values) of at least 10 to 20 primary HIV-1 isolates. M, main; O, other.
Recent studies have attempted to establish the ex vivo 'pathogenic' and 'transmission' fitness of different HIV types, groups and subtypes52. Ex vivo 'pathogenic fitness' has been previously described as the relative replication capacity of HIV-1 in primary blood cells (CD4+ T cells and PBMCs), which in turn, might be related to HIV-1 virulence30,53. By contrast, ex vivo 'transmission fitness' is measured by competing primary HIV-1 isolates in cells of the dendritic lineage, which are derived from blood monocytes or human skin53,143. Future dual HIV-1 competition experiments using vaginal or cervical biopsy explants might be the best model for transmission fitness65,66. To first establish the 'pathogenic fitness' (or virulence) of HIV, pair-wise competitions were done with eight HIV-2, six HIV-1 group O and 15 HIV-1 group M isolates (2 subtype A, 5 subtype B, 4 subtype C, 2 subtype D and 2 subtype CRF01_AE) in PBMCs144 (Fig. 4b). These viruses were classified as using either the CCR5 (R5) or the CXCR4 (X4) co-receptor for entry. When HIV isolates of the same phenotype (X4 or R5) were competed against each other, the following fitness order (based on >2,000 competitions) was observed (Fig. 4b): HIV-1 group M (subtype A, B, D and CRF01_AE) had a greater fitness than subtype C, which in turn was greater than HIV-2, and HIV-1 group O showed the lowest fitness.
With the exception of subtype C, this order reflects the prevalence of these human lentiviruses in the human population and the proposed rates of transmission efficiency128,129. The R5 HIV-1 subtype C isolates were at least 100-fold less fit than HIV-1 isolates of any other group M subtype53. Aside from subtype C, no other HIV-1 subtype has shown a significant fitness difference in group M53,144. Subtype C HIV-1 isolates are preferentially CCR5-tropic and non-syncytium-inducing (NSI) throughout disease, whereas infections with HIV-1 isolates of other subtypes result in a switch from CCR5 to CXCR4-tropic virus in approximately 50% of late disease cases. A few rare X4 subtype C isolates have been obtained from infected Zimbabweans late in disease145 but even the switch from R5 to X4 did not restore fitness, compared with other group M isolates (Nankya, I. et al, unpublished data).
What causes differential spread?
Differential spread of human lentiviruses in the host population is controlled by both transmission efficiency and opportunity, which is collectively referred to as R0 (Ref. 22). Several models of microparasite expansion in humans have been proposed20,22,33,146,147but most assume that during the early stages of the epidemic, the rate of infection (dY/dt) simply increases as a function of the number of infected individuals (Y) during an infectious period (v = rate of moving into a non-infectious state), the probability of infecting a new host (β) and the frequency of sexual contact (c) (Ref. 22).
The opportunities for HIV-1 transmission relate to the number of sexual contacts, which are more frequent during acute infection and in the asymptomatic period of disease than during AIDS. An infected individual (without general knowledge of the transmission routes or infection status) could engage in several sexual encounters during the acute or early phase (approximately 3 months)148, the period of highest HIV-1 load61,62. Therefore, the exponential growth of HIV-1 infections in the early 1980s could have been primarily the result of transmission from acutely infected men having sex with men or from intravenous drug users148,149,150 (Box 1). However, in heterosexual populations with a reduced frequency of sexual contacts, a donor might transmit HIV-1 to more recipients during a long asymptomatic period (2–5 years) of infection, with moderate viral load, than during the acute or early infection stage (1–3 months), with high viral load. Low HIV-1 virulence, which could lead to longer asymptomatic periods, could also increase the opportunity for transmission (Box 1).
Similarly, poor fitness of HIV-1 group O and HIV-2 compared with HIV-1 group M isolates was found when competitions were carried out in both PBMCs (ex vivo pathogenic fitness) and in dendritic–T-cell cultures (ex vivo transmission fitness)144. In relation to this ex vivo model, poor transmission efficiency has been well documented for HIV-2 compared with HIV-1 in the human populations in which both circulate128,129. By contrast, subtype C HIV-1 isolates had similar ex vivo transmission fitness53 compared with group M subtype B isolates in Langerhans cells even though subtype C isolates are at least 100-fold less fit in PBMC cultures53. In vivo observations indicate that subtype C HIV-1 is transmitted as efficiently as other HIV-1 group M isolates in human cohorts135,137 and in rhesus macaques, through the use of SIV–HIV env chimeric virus (SHIV-MJ4)151. These ex vivo and in vivo observations imply that HIV-1 subtype C is efficiently transmitted but is less virulent than other HIV-1 group M isolates.
HIV-2 and HIV-1 groups O and N might have had limited expansion in the human population due to poor host adaptation and transmission efficiencies. By contrast, HIV-1 group M seems to be more virulent as well as more transmissible. Therefore, the progenitor of the HIV-1 group M lineage might have been more 'fit' for human infection, promoting further adaptation through rapid evolution and passage through the human population. New founder events in segregated human populations in central Africa might have led to subsequent divergence into subtypes. HIV-1 subtypes probably evolved and diverged under similar selection pressures, unless infecting a human population with distinct genetic polymorphisms that had an effect on virulence. Adaptation to the human population followed by attenuation of lethal virulence might, however, be occurring at different rates for divergent HIV-1 clades. If diversity in HLA types has a significant role in HIV-1 evolution, subtypes predominating in Africa, such as subtype C, might attenuate virulence at a faster rate than HIV-1 subtype B, which infects the Caucasian population of lower HLA diversity in the developed world.
Prevalence and reduced virulence
HIV-1 subtype C might be in a more advanced stage of attenuation than other HIV-1 subtypes53,96,144. If it is less virulent than other subtypes, subtype C infections might result in slower disease progression, longer periods of asymptomatic infection and more opportunities for transmission; that is, it would be associated with an increase in R0 (see equation [1]; Box 1). To support a model of disproportionate expansion of HIV-1 subtype C in the human population (Figs 3,4a), the transmission efficiency of subtype C must be greater than, or comparable to, other HIV-1 group M strains. Preliminary data suggest that subtype C is transmitted as efficiently as other HIV-1 group M subtypes53,135,137,151. Therefore, decreased virulence could have a key role in the spread of subtype C by increasing the opportunity for transmission33. So far, comparisons of cohorts infected with subtype C and other subtypes show similar or higher viral loads in the subtype C-infected individuals152,153. These initial observations would argue against the possible subtype C attenuation and would support the more popular assumption, that subtype C is more virulent.
However, there are few studies on the natural history and progression of patients infected by any HIV-1 subtype other than subtype B154,155,156,157. Two studies have described faster disease progression in individuals infected with subtype D compared with subtype A- or subtype C-infected individuals in Uganda and Tanzania154,155,156. The possibility of greater subtype D virulence has been attributed to a higher propensity to switch from the NSI/R5 phenotype to the more aggressive syncytium-inducing (SI)/X4 phenotype158. By contrast, subtype C rarely switches from the NSI/R5 to the SI/X4 phenotype. At a recent presentation at the sixteenth International AIDS Conference in Toronto, Canada159, interim analyses of disease progression were described in a cohort of 256 subtype A-, C- and D-infected women, recruited at acute or early infection and followed for a mean of 36 months. The mean slope of CD4+ T-cell decline in the subtype C-infected women from Zimbabwe was 2.3-fold slower than in subtype A- and D-infected women from Uganda (p<0.008). However, there were no differences in the mean viral loads at 3 and 12 months between the countries or between different subtypes. Slower disease progression, as described by slower declines in CD4+ T-cell counts, was not attributable to any other factors (such as opportunistic infections, sexual activity, diet, age and weight) apart from being infected with subtype C versus subtype A or D. In the Bantu corridor extending from Zimbabwe to Uganda, the frequency of HLA-A, -B and -C alleles and other host polymorphisms (for example, CCR5 deletions or promoter polymorphisms) are similar and could rule out obvious human genetic differences skewing HIV-1 evolution108.
Summary
The attenuation of infectious pathogens remains contentious and many doubt that the spread of HIV-1 through the human population will lead to decreased virulence as HIV-1 isolates actually gain replicative (or pathogenic) fitness during infection of human hosts. However, this gain might not be sufficient to reverse the loss in pathogenic fitness that occurs during transmission and early disease. The extreme genetic bottleneck following transmission reduces the HIV-1 population size and diversity by at least 10,000-fold. In vitro studies indicate that this genetic reduction correlates with a significant fitness loss. The few HIV-1 clones establishing infection must evade the immune system, resulting in yet another series of genetic bottlenecks reducing fitness. Therefore, attenuation of virulence might occur if the net fitness loss during transmission or early disease is greater than the increase in pathogenic fitness during disease progression and between transmission events. Greater diversity in HLA alleles in African populations compared with Caucasian populations might result in stronger immune pressure in the human population and possibly faster rates of HIV-1 attenuation. Is the global dominance of HIV-1 subtype C related to an attenuation of virulence? Does subtype C infection lead to slower disease progression and more opportunities for new transmission events? These questions might hold the key to the future of the HIV-1 epidemic and could provide a new focus for ARV treatment and vaccine design.
Box 1 | A model for HIV-1 attenuation.
The figure shows a model for HIV-1 attenuation in a human population with diverse and limited genetic polymorphisms that are associated with HIV-1 disease progression. In this illustration, patient A is the founder who will infect patient B approximately 4 years into the infection of A. Before transmission from patient A to patient B, the replicative (or pathogenic) fitness of the virus has increased from 4 to 7 (arbitrary units, see Fig. 2 legend). Although the replicative fitness increases in patient A, the transmission from patient A to patient B results in a genetic bottleneck that resets the fitness to a lower baseline or set level (dotted line). As patients A and B are not an HLA match, the cell-mediated immune response (or CTL response) of patient B recognizes a different set of HIV-1 epitopes than in patient A. In patient B, mutations in this new set of epitopes are necessary for CTL escape and for the virus to expand in the face of this anti-viral response. However, this 'escape' from the CTL response comes at a fitness cost and reduces the replicative fitness in the first 1–2 years of infection. Following this escape, the infecting HIV-1 isolates seem to continually increase in number (that is, the viral load increases)83 and gain replicative fitness29 (Fig. 2). This process is repeated with each transmission event in patients with diverse HLA alleles.
Patient B transmits to patient C and patient C transmits to patient D during acute infection. Increased transmission efficiency is associated with higher viral loads, which are highest during acute infection. However, the short time interval of acute infection provides limited opportunity for transmission. As the disease progresses and viral load increases, the efficiency of transmission will increase even though opportunities for transmission can remain constant. Consequently, more transmission events might result from prolonged, compared with rapid, disease progression (for example, transmission from patient D to E) even when accounting for the high transmission efficiencies during acute infection149,150. In this model, owing to the fitness bottlenecks at transmission and the subsequent loss in fitness caused by CTL escape111,112,113, the circulating HIV-1 isolate will start to lose virulence. This loss in virulence would decrease pathogenesis, prolong the time to AIDS or even result in a non-symptomatic infection. The complete clearance of HIV-1 is unlikely because of the stable integration of the provirus into memory T cells with long half lives and possible re-activation of virus replication in these cells160,161.
In patients with limited genetic diversity and possible HLA matches, loss of fitness might be associated with the genetic bottleneck following transmission but the infecting HIV-1 isolate could be more 'resistant' to the breadth of possible CTL responses. For example, patient A transmits HIV-1 to patient X 1.5 years into the infection. After 1.5 years, the HIV-1 isolate in patient A has mutated the dominant HIV-1 epitopes and escaped CTL activity. When patient A infects patient X (who is a match at some or all of the HLA alleles), the virus from patient A cannot be efficiently recognized by the CTL response of patient X and so could result in a more rapid disease progression. An HIV-1 isolate passing through patients of limited HLA diversity might actually increase in virulence. CTL, cytotoxic T lymphocyte; HLA, human leukocyte antigen.
Supplementary information
(XLS 116 kb)
Acknowledgements
We thank Miguel Quinones-Mateu, Luc Kestens, Robert Colebunders and Guido van der Groen for their helpful suggestions in the development of these attenuation hypotheses. We also thank Aslam Syed and Lora Angelova in the Arts laboratory for their contributions to the supplementary data.
Biographies
Kevin K. Ariën obtained his Ph.D. from the University of Antwerpen, Belgium (2005) for his work at the Institute of Tropical Medicine on HIV replicative fitness under the direction of Guido Vanham. During his thesis research, he worked on several occasions in the laboratory of Eric Arts at the Case Western Reserve University, Cleveland, USA. In close collaboration with Arts, Ariën and Vanham published ground breaking but controversial studies on the replicative fitness of human lentiviruses and the impact of this fitness on temporal and global evolution of the HIV-1 epidemic. Ariën is currently a Postdoctoral Fellow of the Research Foundation-Flanders at Ghent University, Ghent, Belgium.
Guido Vanham graduated from the University of Leuven in 1980 (M.D.) and with a Ph.D. in 1994. In 1997, he joined the faculty in the Unit of Immunology, Microbiology Departments at the Institute of Tropical Medicine (ITM) in Antwerp, Belgium. He currently heads the HIV and Retrovirology Research Unit at the ITM and is a Professor in tropical infectious diseases, Department of Biomedical Sciences, University of Antwerp. He is renowned for his research on ex vivo models of HIV-1 transmission, in development of anti-HIV-1 microbicides, and in lentiviral pathogenesis/ fitness. His research has involved several international collaborations and the establishment of laboratories in Uganda, Rwanda and Peru.
Eric J. Arts received his Ph.D. degree (1994) from McGill University, Montreal, Canada, in the laboratory of Mark Wainberg and then did a post-doctoral fellowship with Stuart Le Grice at Case Western Reserve University (Case), Cleveland, Ohio, through a fellowship with Health Canada. Currently, Arts is an Associate Professor at Case, serves on several journal editorial boards, and is a permanent member of an NIH AIDS study section. Based on his experience in molecular virology and enzyme biochemistry, Arts has developed new techniques for HIV-1 cloning and competitive replicative assays, which have been exported to laboratories around the world. His research focuses on the relationship between HIV-1 fitness with disease progression, global evolution within the epidemic, emergence of HIV-1 drug resistance, and immune escape. He directs laboratories at Case Medical School and at the Joint Clinical Research Center, Kampala, Uganda, as well as collaborates with researchers in Uganda, Zimbabwe, Cameroon, Canada, Belgium, UK, France and Argentina.
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Competing interests
The authors declare no competing financial interests.
References
- 1.Lemey P, Pybus OG, Van DS, Vandamme AM. A Bayesian statistical analysis of human T-cell lymphotropic virus evolutionary rates. Infect. Genet. Evol. 2005;5:291–298. doi: 10.1016/j.meegid.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Van DS, Salemi M, Vandamme AM. Dating the origin of the African human T-cell lymphotropic virus type-i (HTLV-I) subtypes. Mol. Biol. Evol. 2001;18:661–671. doi: 10.1093/oxfordjournals.molbev.a003846. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida M. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene. 2005;24:5931–5937. doi: 10.1038/sj.onc.1208981. [DOI] [PubMed] [Google Scholar]
- 4.Coulthart MB, Posada D, Crandall KA, Dekaban GA. On the phylogenetic placement of human T cell leukemia virus type 1 sequences associated with an Andean mummy. Infect. Genet. Evol. 2006;6:91–96. doi: 10.1016/j.meegid.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korber B, et al. Timing the ancestor of the HIV-1 pandemic strains. Science. 2000;288:1789–1796. doi: 10.1126/science.288.5472.1789. [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS/WHO. AIDS Epidemic Update 2006. [online], (UNAIDS/WHO, Geneva, 2006).
- 7.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nature Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 8.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nature Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 9.Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nature Rev. Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gage KL, Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu. Rev. Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 11.Drancourt M, Houhamdi L, Raoult D. Yersinia pestis as a telluric, human ectoparasite-borne organism. Lancet Infect. Dis. 2006;6:234–241. doi: 10.1016/S1473-3099(06)70438-8. [DOI] [PubMed] [Google Scholar]
- 12.Fenner F, Radcliffe FN. Myxomatosis. 1965. [Google Scholar]
- 13.Zuniga MC. A pox on thee! Manipulation of the host immune system by myxoma virus and implications for viral–host co-adaptation. Virus Res. 2002;88:17–33. doi: 10.1016/s0168-1702(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 14.Best SM, Kerr PJ. Coevolution of host and virus: the pathogenesis of virulent and attenuated strains of myxoma virus in resistant and susceptible European rabbits. Virology. 2000;267:36–48. doi: 10.1006/viro.1999.0104. [DOI] [PubMed] [Google Scholar]
- 15.Knell RJ. Syphilis in renaissance Europe: rapid evolution of an introduced sexually transmitted disease? Proc. Biol. Sci. 2004;271:S174–S176. doi: 10.1098/rsbl.2003.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin MJ, et al. Mosaic genome structure of simian immunodeficiency virus from west African green monkeys. EMBO J. 1994;13:2935–2947. doi: 10.1002/j.1460-2075.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apetrei C, Robertson DL, Marx PA. The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front. Biosci. 2004;9:225–254. doi: 10.2741/1154. [DOI] [PubMed] [Google Scholar]
- 18.Courgnaud V, et al. Identification of a new simian immunodeficiency virus lineage with a vpu gene present among different cercopithecus monkeys (C. mona, C. cephus, and C. nictitans) from Cameroon. J. Virol. 2003;77:12523–12534. doi: 10.1128/JVI.77.23.12523-12534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassel MP. The Spatial and Temporal Dynamics of Host-Parasitoid Interactions. 2000. [Google Scholar]
- 20.Diekmann O, Hessterbeek O. Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis, and Interpretation. 2006. [Google Scholar]
- 21.Dronamraju KR. Infectious Diseases and Host-Pathogen Evolution. 2004. [Google Scholar]
- 22.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. 1991. [Google Scholar]
- 23.Arts EJ, Quinones-Mateu ME. Sorting out the complexities of HIV-1 fitness. AIDS. 2003;17:780–781. doi: 10.1097/00002030-200303280-00026. [DOI] [PubMed] [Google Scholar]
- 24.Quinones-Mateu ME, Arts EJ. HIV-1 Fitness: Implications for Drug Resistance, Disease Progression, and Global Epidemic Evolution. 2001. [Google Scholar]
- 25.Feng JY, Anderson KS. Mechanistic studies examining the efficiency and fidelity of DNA synthesis by the 3TC-resistant mutant (184V) of HIV-1 reverse transcriptase. Biochemistry. 1999;38:9440–9448. doi: 10.1021/bi990709m. [DOI] [PubMed] [Google Scholar]
- 26.Deval J, et al. Mechanistic basis for reduced viral and enzymatic fitness of HIV-1 reverse transcriptase containing both K65R and M184V mutations. J. Biol. Chem. 2004;279:509–516. doi: 10.1074/jbc.M308806200. [DOI] [PubMed] [Google Scholar]
- 27.Bull JJ. Virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 28.Bremermann HJ, Pickering J. A game-theoretical model of parasite virulence. J. Theor. Biol. 1983;100:411–426. doi: 10.1016/0022-5193(83)90438-1. [DOI] [PubMed] [Google Scholar]
- 29.Troyer RM, et al. Changes in human immunodeficiency virus type 1 fitness and genetic diversity during disease progression. J. Virol. 2005;79:9006–9018. doi: 10.1128/JVI.79.14.9006-9018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinones-Mateu ME, et al. A dual infection/competition assay shows a correlation between ex vivo human immunodeficiency virus type 1 fitness and disease progression. J. Virol. 2000;74:9222–9233. doi: 10.1128/jvi.74.19.9222-9233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaak H, Brouwer M, Ran LJ, de Wolf F, Schuitemaker H. In vitro replication kinetics of human immunodeficiency virus type 1 (HIV-1) variants in relation to virus load in long-term survivors of HIV-1 infection. J. Infect. Dis. 1998;177:600–610. doi: 10.1086/514219. [DOI] [PubMed] [Google Scholar]
- 32.Barbour JD, et al. Higher CD4+ T cell counts associated with low viral pol replication capacity among treatment-naive adults in early HIV-1 infection. J. Infect. Dis. 2004;190:251–256. doi: 10.1086/422036. [DOI] [PubMed] [Google Scholar]
- 33.Ewald PW. Evolution of Infectious Disease. 1994. [Google Scholar]
- 34.Nowak MA, May RM. Virus Dynamics: Mathematical Principles of Immunology and Virology. 2000. [Google Scholar]
- 35.Maynard SJ. Group selection and kin selection. Nature. 1964;201:1145–1147. [Google Scholar]
- 36.Szathmary E, Maynard SJ. From replicators to reproducers: the first major transitions leading to life. J. Theor. Biol. 1997;187:555–571. doi: 10.1006/jtbi.1996.0389. [DOI] [PubMed] [Google Scholar]
- 37.Keele BF, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao F, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 39.Santiago ML, et al. SIVcpz in wild chimpanzees. Science. 2002;295:465. doi: 10.1126/science.295.5554.465. [DOI] [PubMed] [Google Scholar]
- 40.Van Heuverswyn F, et al. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- 41.Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nature Rev. Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 43.Lemey P, et al. Tracing the origin and history of the HIV-2 epidemic. Proc. Natl Acad. Sci. USA. 2003;100:6588–6592. doi: 10.1073/pnas.0936469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Loeff MF, Aaby P. Towards a better understanding of the epidemiology of HIV-2. AIDS. 1999;13:S69–S84. [PubMed] [Google Scholar]
- 45.van der Loeff MF, et al. Sixteen years of HIV surveillance in a West African research clinic reveals divergent epidemic trends of HIV-1 and HIV-2. Int. J. Epidemiol. 2006;35:1322–1328. doi: 10.1093/ije/dyl037. [DOI] [PubMed] [Google Scholar]
- 46.Ayouba A, et al. HIV-1 group O infection in Cameroon, 1986 to 1998. Emerg. Infect. Dis. 2001;7:466–467. doi: 10.3201/eid0703.010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi J, et al. HIV infections in northwestern Cameroon: identification of HIV type 1 group O and dual HIV type 1 group M and group O infections. AIDS Res. Hum. Retroviruses. 2004;20:944–957. doi: 10.1089/aid.2004.20.944. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi J, et al. HIV-1 Group N: evidence of ongoing transmission in Cameroon. AIDS Res. Hum. Retroviruses. 2006;22:453–457. doi: 10.1089/aid.2006.22.453. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi J, et al. Identification of HIV type 1 group N infections in a husband and wife in Cameroon: viral genome sequences provide evidence for horizontal transmission. AIDS Res. Hum. Retroviruses. 2006;22:83–92. doi: 10.1089/aid.2006.22.83. [DOI] [PubMed] [Google Scholar]
- 50.Salemi M, et al. Dating the common ancestor of SIVcpz and HIV-1 group M and the origin of HIV-1 subtypes using a new method to uncover clock-like molecular evolution. FASEB J. 2001;15:276–278. doi: 10.1096/fj.00-0449fje. [DOI] [PubMed] [Google Scholar]
- 51.Travers SA, et al. Timing and reconstruction of the most recent common ancestor of the subtype C clade of human immunodeficiency virus type 1. J. Virol. 2004;78:10501–10506. doi: 10.1128/JVI.78.19.10501-10506.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arien KK, et al. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J. Virol. 2005;79:8979–8990. doi: 10.1128/JVI.79.14.8979-8990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ball SC, et al. Comparing the ex vivo fitness of CCR5-tropic human immunodeficiency virus type 1 isolates of subtypes B and C. J. Virol. 2003;77:1021–1038. doi: 10.1128/JVI.77.2.1021-1038.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnett SW, Quiroga M, Werner A, Dina D, Levy JA. Distinguishing features of an infectious molecular clone of the highly divergent and noncytopathic human immunodeficiency virus type 2 UC1 strain. J. Virol. 1993;67:1006–1014. doi: 10.1128/jvi.67.2.1006-1014.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talbott R, Kraus G, Looney D, Wong-Staal F. Mapping the determinants of human immunodeficiency virus 2 for infectivity, replication efficiency, and cytopathicity. Proc. Natl Acad. Sci. USA. 1993;90:4226–4230. doi: 10.1073/pnas.90.9.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pollakis G, et al. Phenotypic and genotypic comparisons of CCR5- and CXCR4-tropic human immunodeficiency virus type 1 biological clones isolated from subtype C-infected individuals. J. Virol. 2004;78:2841–2852. doi: 10.1128/JVI.78.6.2841-2852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curran JW, et al. Epidemiology of HIV infection and AIDS in the United States. Science. 1988;239:610–616. doi: 10.1126/science.3340847. [DOI] [PubMed] [Google Scholar]
- 58.Parazzini F, et al. Number of sexual partners, condom use and risk of human immunodeficiency virus infection. Int. J. Epidemiol. 1995;24:1197–1203. doi: 10.1093/ije/24.6.1197. [DOI] [PubMed] [Google Scholar]
- 59.Ryder RW, Behets F. Reasons for the wide variation in reported rates of mother-to-child transmission of HIV-1. AIDS. 1994;8:1495–1497. doi: 10.1097/00002030-199410000-00019. [DOI] [PubMed] [Google Scholar]
- 60.Gray RH, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 61.Quinn TC, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N. Engl. J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 62.Garcia PM, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N. Engl. J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 63.Shankarappa R, et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 1999;73:10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu T, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 65.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature Rev. Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 66.Shattock RJ, Griffin GE, Gorodeski GI. In vitro models of mucosal HIV transmission. Nature Med. 2000;6:607–608. doi: 10.1038/76138. [DOI] [PubMed] [Google Scholar]
- 67.Bergstrom CT, McElhany P, Real LA. Transmission bottlenecks as determinants of virulence in rapidly evolving pathogens. Proc. Natl Acad. Sci. USA. 1999;96:5095–5100. doi: 10.1073/pnas.96.9.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lazaro E, Escarmis C, Perez-Mercader J, Manrubia SC, Domingo E. Resistance of virus to extinction on bottleneck passages: study of a decaying and fluctuating pattern of fitness loss. Proc. Natl Acad. Sci. USA. 2003;100:10830–10835. doi: 10.1073/pnas.1332668100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elena SF, et al. Evolution of fitness in experimental populations of vesicular stomatitis virus. Genetics. 1996;142:673–679. doi: 10.1093/genetics/142.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sagar M, et al. Infection with multiple human immunodeficiency virus type 1 variants is associated with faster disease progression. J. Virol. 2003;77:12921–12926. doi: 10.1128/JVI.77.23.12921-12926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pontesilli O, et al. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J. Infect. Dis. 1998;178:1008–1018. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 72.Dyer WB, et al. Strong human immunodeficiency virus (HIV)-specific cytotoxic T- lymphocyte activity in Sydney blood bank cohort patients infected with nef-defective HIV type 1. J. Virol. 1999;73:436–443. doi: 10.1128/jvi.73.1.436-443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Eng. J. Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 74.Pantaleo G, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N. Eng. J. Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 75.Montefiori DC, et al. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J. Infect. Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 76.Carotenuto P, Looij D, Keldermans L, de Wolf F, Goudsmit J. Neutralizing antibodies are positively associated with CD4+ T-cell counts and T-cell function in long-term AIDS-free infection. AIDS. 1998;12:1591–1600. doi: 10.1097/00002030-199813000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg ES, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 78.Dean M, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 79.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez E, et al. Global survey of genetic variation in CCR5, RANTES, and MIP-1α: impact on the epidemiology of the HIV-1 pandemic. Proc. Natl Acad. Sci. USA. 2001;98:5199–5204. doi: 10.1073/pnas.091056898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez E, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 82.McDermott DH, et al. CCR5 promoter polymorphism and HIV-1 disease progression. Multicenter AIDS Cohort Study (MACS) Lancet. 1998;352:866–870. doi: 10.1016/s0140-6736(98)04158-0. [DOI] [PubMed] [Google Scholar]
- 83.Mellors JW, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 84.Collier AC, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N. Engl. J. Med. 1996;334:1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 85.Silvestri G, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez B, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 87.Deacon NJ, et al. Genomic structure of an attenuated quasispecies of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 88.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Eng. J. Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 89.Huang Y, Zhang l, Ho DD. Biological characterization of nef in long-term survivors of human immunodeficiency virus type 1 infection. J. Virol. 1995;69:8142–8146. doi: 10.1128/jvi.69.12.8142-8146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leroux C, Issel CJ, Montelaro RC. Novel and dynamic evolution of equine infectious anemia virus genomic quasispecies associated with sequential disease cycles in an experimentally infected pony. J. Virol. 1997;71:9627–9639. doi: 10.1128/jvi.71.12.9627-9639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Plagemann PG, Chen Z, Li K. Replication competition between lactate dehydrogenase-elevating virus quasispecies in mice. Implications for quasispecies selection and evolution. Arch. Virol. 2001;146:1283–1296. doi: 10.1007/s007050170091. [DOI] [PubMed] [Google Scholar]
- 92.Lu J, Kuritzkes DR. A novel recombinant marker virus assay for comparing the relative fitness of hiv-1 reverse transcriptase variants. J. Acquir. Immune. Defic. Syndr. 2001;27:7–13. doi: 10.1097/00126334-200105010-00002. [DOI] [PubMed] [Google Scholar]
- 93.Holland JJ, de la Torre JC, Clarke DK, Duarte E. Quantitation of relative fitness and great adaptability of clonal populations of RNA viruses. J. Virol. 1991;65:2960–2967. doi: 10.1128/jvi.65.6.2960-2967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Domingo E, Escarmis C, Menendez-Arias L, Holland J. Origin And Evolution of Viruses. 1999. pp. 141–161. [Google Scholar]
- 95.Harrigan PR, Bloor S, Larder BA. Relative replicative fitness of zidovudine-resistant human immunodeficiency virus type 1 isolates in vitro. J. Virol. 1998;72:3773–3778. doi: 10.1128/jvi.72.5.3773-3778.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quinones-Mateu ME, Arts EJ. Virus fitness: concept, quantification, and application to HIV population dynamics. Curr. Top. Microbiol. Immunol. 2006;299:83–140. doi: 10.1007/3-540-26397-7_4. [DOI] [PubMed] [Google Scholar]
- 97.Clarke DK, et al. The red queen reigns in the kingdom of RNA viruses. Proc. Natl Acad. Sci. USA. 1994;91:4821–4824. doi: 10.1073/pnas.91.11.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Novella IS, et al. Exponential increases of RNA virus fitness during large population transmissions. Proc. Natl Acad. Sci. USA. 1995;92:5841–5844. doi: 10.1073/pnas.92.13.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chao L. Fitness of RNA virus decreased by Muller's ratchet. Nature. 1990;348:454–455. doi: 10.1038/348454a0. [DOI] [PubMed] [Google Scholar]
- 100.Domingo E, Holland JJ. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 1997;51:151–178. doi: 10.1146/annurev.micro.51.1.151. [DOI] [PubMed] [Google Scholar]
- 101.Yuste E, Sanchez-Palomino S, Casado C, Domingo E, Lopez-Galindez C. Drastic fitness loss in human immunodeficiency virus type 1 upon serial bottleneck events. J. Virol. 1999;73:2745–2751. doi: 10.1128/jvi.73.4.2745-2751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mullins JI, Jensen MA. Evolutionary dynamics of HIV-1 and the control of AIDS. Curr. Top. Microbiol. Immunol. 2006;299:171–192. doi: 10.1007/3-540-26397-7_6. [DOI] [PubMed] [Google Scholar]
- 103.Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 104.Betts MR, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosenberg ES, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 106.Goulder PJ, et al. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nature Rev. Immunol. 2004;4:630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- 108.Cao K, et al. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens. 2004;63:293–325. doi: 10.1111/j.0001-2815.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 109.Moore CB, et al. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 110.Goulder PJ, et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 2001;412:334–338. doi: 10.1038/35085576. [DOI] [PubMed] [Google Scholar]
- 111.Leslie AJ, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nature Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 112.Martinez-Picado J, et al. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 2006;80:3617–3623. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Troyer, R. M. et al. The fitness cost of CTL escape: not a terrible hardship on HIV-1? [online], (XVI International AIDS Conference, Toronto, Canada 13–18 August, 2006)
- 114.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 115.Stremlau M, et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 116.Markowitz M, et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report. Lancet. 2005;365:1031–1038. doi: 10.1016/S0140-6736(05)71139-9. [DOI] [PubMed] [Google Scholar]
- 117.Van de PP. Viral and host determinants of HIV-1 pathogenesis. AIDS. 2006;20:933–934. doi: 10.1097/01.aids.0000218560.67155.1c. [DOI] [PubMed] [Google Scholar]
- 118.Muller V, De Boer RJ. The integration hypothesis: an evolutionary pathway to benign SIV infection. PLoS Pathog. 2006;2:e15. doi: 10.1371/journal.ppat.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muller V, et al. Stable virulence levels in the HIV epidemic of Switzerland over two decades. AIDS. 2006;20:889–894. doi: 10.1097/01.aids.0000218553.51908.6b. [DOI] [PubMed] [Google Scholar]
- 120.Kannangara S, DeSimone JA, Pomerantz RJ. Attenuation of HIV-1 infection by other microbial agents. J. Infect. Dis. 2005;192:1003–1009. doi: 10.1086/432767. [DOI] [PubMed] [Google Scholar]
- 121.Arien KK, et al. Replicative fitness of historical and recent HIV-1 isolates suggests HIV-1 attenuation over time. AIDS. 2005;19:1555–1564. doi: 10.1097/01.aids.0000185989.16477.91. [DOI] [PubMed] [Google Scholar]
- 122.Yerly S, et al. Transmission of antiretroviral-drug-resistant HIV-1 variants. Lancet. 1999;354:729–733. doi: 10.1016/S0140-6736(98)12262-6. [DOI] [PubMed] [Google Scholar]
- 123.Turner D, Wainberg MA. HIV transmission and primary drug resistance. AIDS Rev. 2006;8:17–23. [PubMed] [Google Scholar]
- 124.Eyster ME. Test may predict which patients with HIV infection will develop AIDS. Am. Fam. Physician. 1989;39:276. [PubMed] [Google Scholar]
- 125.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 126.Marlink R, et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265:1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 127.Matheron S, et al. Factors associated with clinical progression in HIV-2 infected-patients: The French ANRS cohort. AIDS. 2003;17:2593–2601. doi: 10.1097/00002030-200312050-00006. [DOI] [PubMed] [Google Scholar]
- 128.Gilbert PB, et al. Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal. Stat. Med. 2003;22:573–593. doi: 10.1002/sim.1342. [DOI] [PubMed] [Google Scholar]
- 129.Kanki PJ, et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343:943–946. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 130.Essex M. Human immunodeficiency viruses in the developing world. Adv. Virus Res. 1999;53:71–88. doi: 10.1016/s0065-3527(08)60343-7. [DOI] [PubMed] [Google Scholar]
- 131.Luo CC, et al. HIV-1 subtype C in China. Lancet. 1995;345:1051–1052. doi: 10.1016/s0140-6736(95)90792-0. [DOI] [PubMed] [Google Scholar]
- 132.Piyasirisilp S, et al. A recent outbreak of human immunodeficiency virus type 1 infection in southern China was initiated by two highly homogeneous, geographically separated strains, circulating recombinant form AE and a novel BC recombinant. J. Virol. 2000;74:11286–11295. doi: 10.1128/jvi.74.23.11286-11295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soares EA, et al. HIV-1 subtype C dissemination in southern Brazil. AIDS. 2005;19:S81–S86. doi: 10.1097/01.aids.0000191497.00928.e4. [DOI] [PubMed] [Google Scholar]
- 134.Vidal N, et al. Distribution of HIV-1 variants in the Democratic Republic of Congo suggests increase of subtype C in Kinshasa between 1997 and 2002. J. Acquir. Immune. Defic. Syndr. 2005;40:456–462. doi: 10.1097/01.qai.0000159670.18326.94. [DOI] [PubMed] [Google Scholar]
- 135.Walker PR, Pybus OG, Rambaut A, Holmes EC. Comparative population dynamics of HIV-1 subtypes B and C: subtype-specific differences in patterns of epidemic growth. Infect. Genet. Evol. 2005;5:199–208. doi: 10.1016/j.meegid.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 136.Renjifo B, et al. Differences in perinatal transmission among human immunodeficiency virus type 1 genotypes. J. Hum. Virol. 2001;4:16–25. [PubMed] [Google Scholar]
- 137.Renjifo B, et al. Preferential in utero transmission of HIV-1 subtype C as compared to HIV-1 subtype A or D. AIDS. 2004;18:1629–1636. doi: 10.1097/01.aids.0000131392.68597.34. [DOI] [PubMed] [Google Scholar]
- 138.Yang C, et al. Genetic diversity of HIV-1 in western Kenya: subtype-specific differences in mother-to-child transmission. AIDS. 2003;17:1667–1674. doi: 10.1097/01.aids.0000060412.18106.d4. [DOI] [PubMed] [Google Scholar]
- 139.Eshleman SH, et al. Comparison of mother-to-child transmission rates in Ugandan women with subtype A versus D HIV-1 who received single-dose nevirapine prophylaxis. HIV Network For Prevention Trials 012. J. Acquir. Immune. Defic. Syndr. 2005;39:593–597. [PubMed] [Google Scholar]
- 140.Pope M, et al. Human immunodeficiency virus type 1 strains of subtypes B and E replicate in cutaneous dendritic cell-T-cell mixtures without displaying subtype-specific tropism. J. Virol. 1997;71:8001–8007. doi: 10.1128/jvi.71.10.8001-8007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dittmar MT, et al. Langerhans cell tropism of human immunodeficiency virus type 1 subtype A through F isolates derived from different transmission groups. J. Virol. 1997;71:8008–8013. doi: 10.1128/jvi.71.10.8008-8013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tovanabutra S, et al. The changing molecular epidemiology of HIV type 1 among northern Thai drug users, 1999 to 2002. AIDS Res. Hum. Retroviruses. 2004;20:465–475. doi: 10.1089/088922204323087705. [DOI] [PubMed] [Google Scholar]
- 143.Zaitseva M, et al. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nature Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 144.Arien KK, et al. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J. Virol. 2005;79:8979–8990. doi: 10.1128/JVI.79.14.8979-8990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johnston ER, et al. High frequency of syncytium-inducing and CXCR4-tropic viruses among human immunodeficiency virus type 1 subtype C-infected patients receiving antiretroviral treatment. J. Virol. 2003;77:7682–7688. doi: 10.1128/JVI.77.13.7682-7688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gupta S, Anderson RM, May RM. Networks of sexual contacts: implications for the pattern of spread of HIV. AIDS. 1989;3:807–817. [PubMed] [Google Scholar]
- 147.Anderson RM, Ng TW, Boily MC, May RM. The influence of different sexual-contact patterns between age classes on the predicted demographic impact of AIDS in developing countries. Ann. N. Y. Acad. Sci. 1989;569:240–274. doi: 10.1111/j.1749-6632.1989.tb27374.x. [DOI] [PubMed] [Google Scholar]
- 148.Pilcher CD, Eron JJ, Jr, Galvin S, Gay C, Cohen MS. Acute HIV revisited: new opportunities for treatment and prevention. J. Clin. Invest. 2004;113:937–945. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jacquez JA, Koopman JS, Simon CP, Longini IM., Jr. Role of the primary infection in epidemics of HIV infection in gay cohorts. J. Acquir. Immune. Defic. Syndr. 1994;7:1169–1184. [PubMed] [Google Scholar]
- 150.Koopman JS, et al. The role of early HIV infection in the spread of HIV through populations. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1997;14:249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- 151.Ndung'u T, et al. Infectious simian/human immunodeficiency virus with human immunodeficiency virus type 1 subtype C from an African isolate: rhesus macaque model. J. Virol. 2001;75:11417–11425. doi: 10.1128/JVI.75.23.11417-11425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gray CM, et al. Viral dynamics and CD4+ T cell counts in subtype C human immunodeficiency virus type 1-infected individuals from southern Africa. AIDS Res. Hum. Retroviruses. 2005;21:285–291. doi: 10.1089/aid.2005.21.285. [DOI] [PubMed] [Google Scholar]
- 153.Neilson JR, et al. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J. Virol. 1999;73:4393–4403. doi: 10.1128/jvi.73.5.4393-4403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaleebu P, et al. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J. Infect. Dis. 2002;185:1244–1250. doi: 10.1086/340130. [DOI] [PubMed] [Google Scholar]
- 155.Vasan A, et al. Different rates of disease progression of HIV type 1 infection in Tanzania based on infecting subtype. Clin. Infect. Dis. 2006;42:843–852. doi: 10.1086/499952. [DOI] [PubMed] [Google Scholar]
- 156.Kaleebu P, et al. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Ugandan cohort. AIDS. 2001;15:293–299. doi: 10.1097/00002030-200102160-00001. [DOI] [PubMed] [Google Scholar]
- 157.Choge I, et al. Genotypic and phenotypic characterization of viral isolates from HIV-1 subtype C-infected children with slow and rapid disease progression. AIDS Res. Hum. Retroviruses. 2006;22:458–465. doi: 10.1089/aid.2006.22.458. [DOI] [PubMed] [Google Scholar]
- 158.Senkaali D, et al. The relationship between HIV type 1 disease progression and V3 serotype in a rural Ugandan cohort. AIDS Res. Hum. Retroviruses. 2004;20:932–937. doi: 10.1089/aid.2004.20.932. [DOI] [PubMed] [Google Scholar]
- 159.Arts, E. J. et al. Infection with subtype C HIV-1 of lower replicative fitness as compared to subtypes A and D leads to slower disease progression in Zimbabwean and Ugandan women. [online], (XVI International AIDS Conference, Toronto, Canada 13–18 August, 2006).
- 160.Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu. Rev. Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 161.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 162.Li DQ, Zheng XW, Zhang GY. Study on the distribution HIV-1 C subtype in Ruili and other counties, Yunnan, China (Translation) Zhonghua Liu Xing. Bing. Xue. Za Zhi. 1996;17:337–339. [PubMed] [Google Scholar]
- 163.Li XJ, et al. Molecular epidemiology of the heterosexual HIV-1 transmission in Kunming, Yunnan Province of China suggests origin from the local IDU epidemic. AIDS Res. Hum. Retroviruses. 2005;21:977–980. doi: 10.1089/aid.2005.21.977. [DOI] [PubMed] [Google Scholar]
- 164.Van Harmelen JH, et al. A predominantly HIV type 1 subtype C-restricted epidemic in South African urban populations. AIDS Res. Hum. Retroviruses. 1999;15:395–398. doi: 10.1089/088922299311376. [DOI] [PubMed] [Google Scholar]
- 165.Rainwater S, et al. No evidence for rapid subtype c spread within an epidemic in which multiple subtypes and intersubtype recombinants circulate. AIDS Res. Hum. Retroviruses. 2005;21:1060–1065. doi: 10.1089/aid.2005.21.1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS 116 kb)


