Abstract
Olefin metathesis is now one of the most efficient ways to create new carbon – carbon bonds. While most efforts focused on the development of ever-more efficient catalysts, a particular attention has recently been devoted to developing latent metathesis catalysts, inactive species that need an external stimulus to become active. This furnishes an increased control over the reaction which is crucial for applications in materials science. Here, we report our work on the development of a new system to achieve visible-light-controlled metathesis by merging olefin metathesis and photoredox catalysis. The combination of a ruthenium metathesis catalyst bearing two N-heterocyclic carbenes with an oxidizing pyrylium photocatalyst affords excellent and spatial resolution using only visible light as stimulus. Applications of this system in synthesis, as well as in polymer patterning and photolithography with spatially-resolved ROMP, are described.
Graphical Abstract
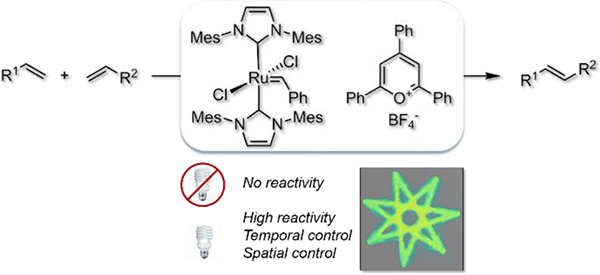
Olefin metathesis is one of the most attractive and powerful tools for the creation of carbon – carbon π bonds, finding numerous applications in synthetic chemistry, fine chemical synthesis and materials science.1,2 While most synthetic efforts have been devoted to the development of ever-more efficient catalysts, increased attention has been paid to the development of catalysts that can be activated/deactivated on demand.3 Such latent catalysts are dormant species under ambient conditions and require an external stimulus to become active. Increased control on reactions is crucial not only from an understanding viewpoint but also for applications in materials science for the production of new well-defined materials.4 Various stimuli have been exploited to achieve such control in metathesis reactions, including heat, light, ultrasound, acid and redox switches.5 Light is arguably the most convenient and attractive stimulus since it is non-invasive, can be easily manipulated and provides the opportunity for high temporal and spatial resolution (Figure 1a).6 As a consequence, several recent reports have described light-promoted olefin metathesis.5c,5d,7 While these have been important developments, they are dominated by UV light with most reports describing activation rather than gating control of alkene metathesis.
Figure 1.
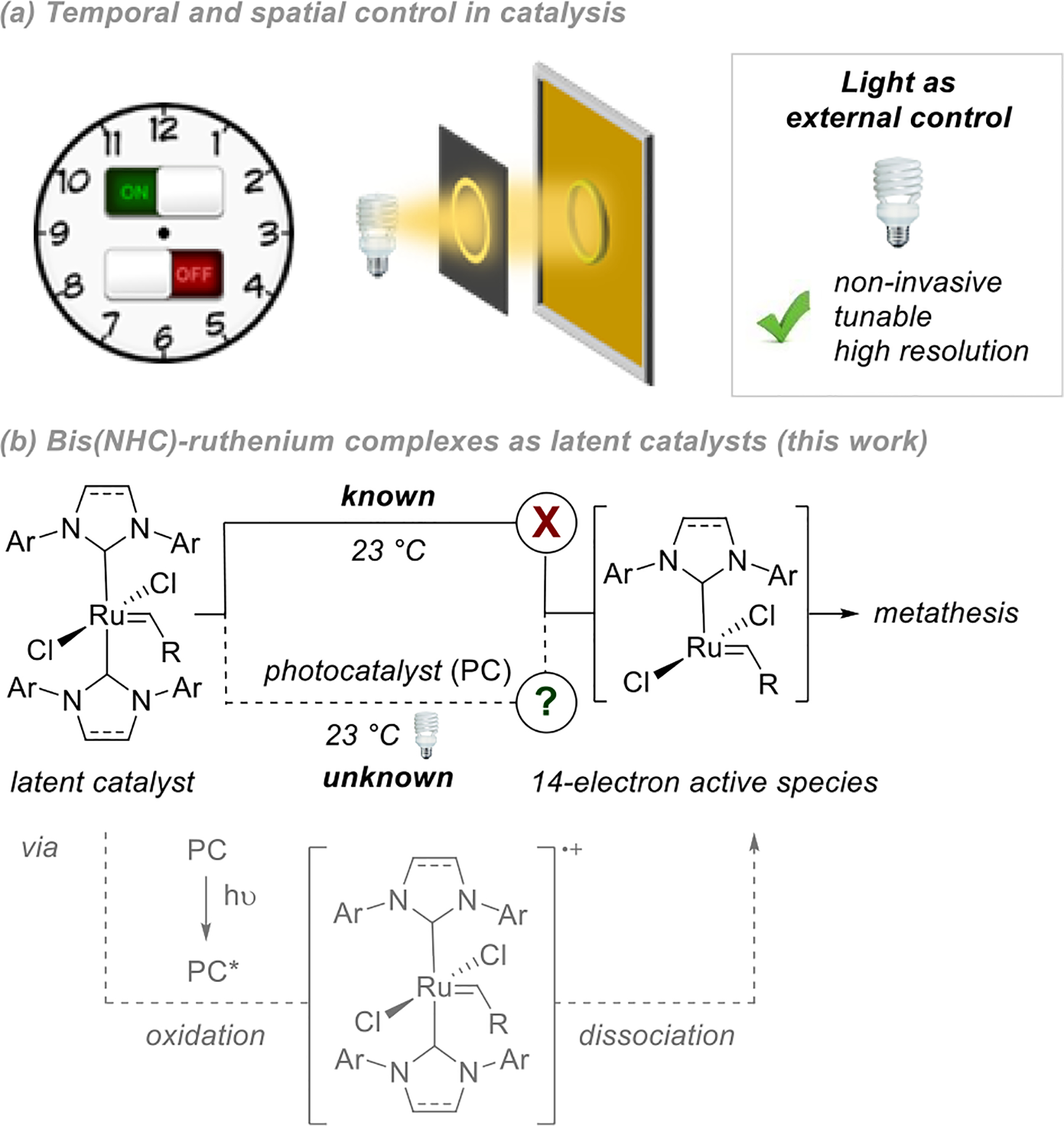
(a) Temporal and spatial control in catalysis. (b) Bis(NHC)-ruthenium complexes as latent catalysts using visible light (this work).
We considered that the merger of olefin metathesis with photoredox catalysis8 could lead to visible light control of alkene metathesis. Visible light photoredox catalysis has already proven successful for metal-free olefin metathesis polymerization via a radical mechanism.9 In particular, excitation of the appropriate photocatalyst by visible-light irradiation should permit the activation of a latent metathesis catalyst, most probably by inducing ligand dissociation,10 and therefore lead to the development of an on-demand metathesis system. Importantly, the use of visible light is more convenient than UV light while still providing high levels of temporal and spatial resolution. Overall, the development of such a system would open new perspectives in photolithography11,12 and in materials science for the design of new materials,13 as already illustrated by the impact of recent work reported for photo-controlled, living radical polymerizations.14
At the outset of these studies, we needed a ruthenium-based complex that is inactive at ambient temperature, and identified bis-NHC ligated Ru complexes first introduced by Herrmann.15 When substituted with aromatic groups on the nitrogen atoms, these catalysts lack activity for metathesis at room temperature, most probably because of the difficult dissociation of one NHC ligand to generate the corresponding 14-electron active catalyst.15,16 At higher temperatures, the activity of these catalysts is restored. In this regard, we surmised that the NHC dissociation event could be promoted at room temperature by using photoredox catalysis. A carefully chosen photocatalyst should be capable, after excitation upon irradiation with visible light, of activating these catalysts and therefore toggling them into their corresponding active species after dissociation of one NHC (Figure 1b).
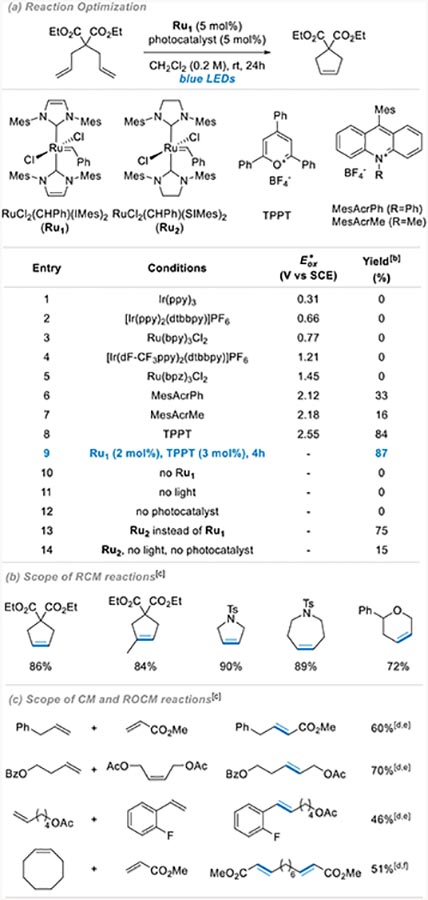
To test our hypothesis, we first evaluated the benchmark ring closing metathesis (RCM) of diethyl diallylmalonate using RuCl2(CHPh)(IMes)2 and RuCl2(CHPh)(SIMes)2, previously reported by Fogg17 and Grubbs,16 in the presence of different photocatalysts under visible-light irradiation. After screening several photocatalysts and reaction conditions (see Supporting Information for further details), we found that a combination of RuCl2(CHPh)(IMes)2 (Ru1) and 2,4,6-triphenylpyrylium tetrafluoroborate (TPPT) as photocatalyst gives the desired product in 87% yield after 4h of irradiation under blue LEDs at room temperature (Table 1, entry 9). While screening photocatalysts, we observed that only highly oxidizing ones such as acridinium and pyrylium derivatives provide some reactivity (entries 6–8), while no product is observed when switching to less oxidizing photocatalysts (entries 1–5). This is consistent with an activation mode involving oxidation of the Ru catalyst followed by dissociation of one NHC to generate the catalytically active species forming the corresponding radical cation.18 We indeed note that Ru1 has two distinct oxidation events as identified by cyclic voltammetry, with the first occurring at +0.44 V, likely corresponding to the generation of the radical cation by a metal-centered oxidation (see SI). While all photoredox catalysts should allow oxidation to the radical cation, the dissociation event might be caused by a second oxidation occurring at one NHC ligand that would only be promoted by highly oxidizing photocatalysts and explain that traditional Ir and Ru photocatalysts are not effective (see table 1, entries 1–5).19 Importantly, no reaction is observed in the absence of ruthenium, light or photocatalyst (entries 10–12). The lack of reactivity under light without photocatalyst also rules out a mechanism solely based on photo-induced dissociation of one NHC ligand and highlights the importance of the photoredox system. Finally, the use of RuCl2(CHPh)(SIMes)2 (RU2) delivers similar reactivity (entry 13). However, background reactivity is observed in the absence of light and photocatalyst (entry 14), indicating that dissociation of one NHC happens slowly at ambient temperature. RuCl2(CHPh)(IMes)2 (Ru1) was chosen as it displays optimal latent behavior.
Table 1.
Reaction optimization and scope of RCM, CM, and ROCM reactions.
|
All optimization reactions were conducted on a 0.1 mmol scale.
Determined by 1H NMR spectroscopy using 1,2-dibromoethane as an internal standard.
Conditions: substrate (0.2 mmol), RuCl2(CHPh)(IM es)2 (2 mol%), TPPT (3 mol%), CH2Cl2 (0.2M), rt, blue LEDs, 4h.
4 mol% of TPPT.
Left substrate (0.2 mmol), right substrate (0.4 mmol).
Left substrate (0.2 mmol), right substrate (0.6 mmol).
For additional samples, see SI.
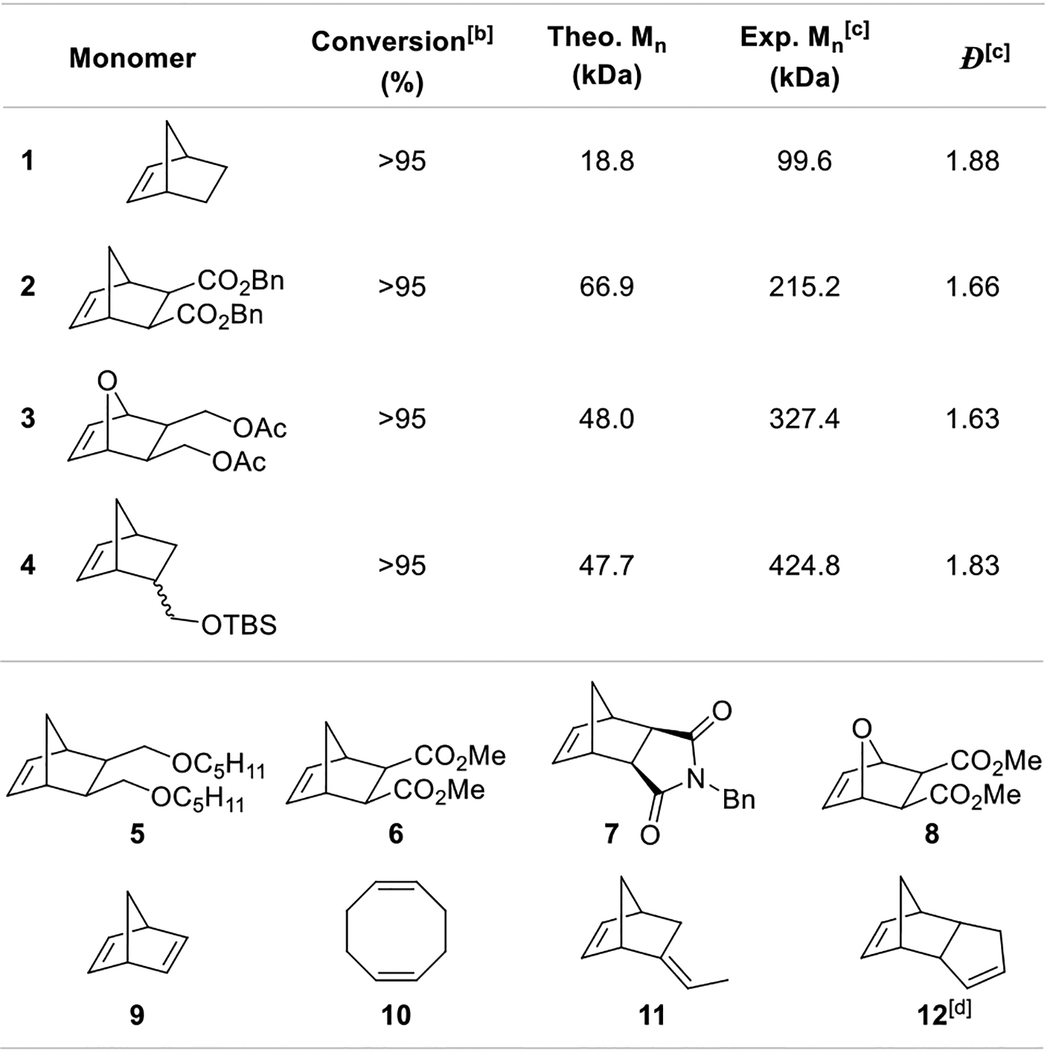
With an efficient system in hand, we first explored its ability to promote different types of metathesis reactions. While standard metathesis reactions can be readily promoted using this photoredox catalytic system, as illustrated with representative examples in Table 1 and SI, we were more interested in interrogating ring-opening metathesis polymerization (ROMP) applications. To this end, several monomers such as norbornene derivatives 1–8,11, norbornadiene 9, 1,5-cyclooctadiene 10 and dicyclopentadiene 12 could be readily polymerized within 1h under blue LED irradiation in the presence of RuCl2(CHPh)(IMes)2 and TPPT (Table 2). Molecular weights (Mn) obtained after polymerization of monomers 1–4 are significantly higher than the expected values which suggests that polymerization is faster than catalyst initiation. Dispersities were found in the range of 1.63 to 1.88. Monomers 5–10 are also smoothly polymerized within an hour of irradiation but lead to insoluble polymers, which precludes GPC analysis. Finally, cross-linking monomers 11 and 12 could also be efficiently polymerized to afford complete gelation within an hour, the latter only requiring 0.01 mol% of Ru1, 0.05 mol% of TPPT and 15 minutes of irradiation. Importantly, the latency is successfully maintained with dicyclopentadiene 12 since, in the absence of light, less than 5% polymerization is observed after 24h (5% after 3 days, 9% after a week). When stopped after 90 seconds under light, 16% polymerization is observed. The rate of polymerization under visible light can therefore be estimated to be 12,000 times faster than in the dark.
Table 2.
Scope of ROMP reactions.
|
Conditions: monomer (0.2 mmol), RuCl2(CHPh)(IMes)2 (0.5 mol%), TPPT (1 mol %), CD2Cl2 (0.2 M), rt, blue LEDs, 1h.
Determined by 1H NMR spectroscopy using mesitylene as internal standard.
Determined by GPC.
Using IMes2RuCl2 CHPh (0.01 mol%), TPPT (0.05 mol%) for 15 min under blue LEDs.
Further experiments were conducted to examine the influence of light and to probe our ability to exert temporal and spatial controls over the reaction. First, temporal control was evaluated by conducting on/off experiments with alternating periods of irradiation and darkness for the ring closing metathesis of diethyl diallylmalonate. The ability to exert temporal control over a reaction is of great interest for the design of orthogonal multicomponent reactions, as well as for the development of new systems designed to produce new highly functionalized materials. As can be seen in Figure 2, temporal control can be achieved since maximal reactivity was obtained during irradiation whereas darkness only afforded minimal increases in yields (from 0 to 3%).
Figure 2.
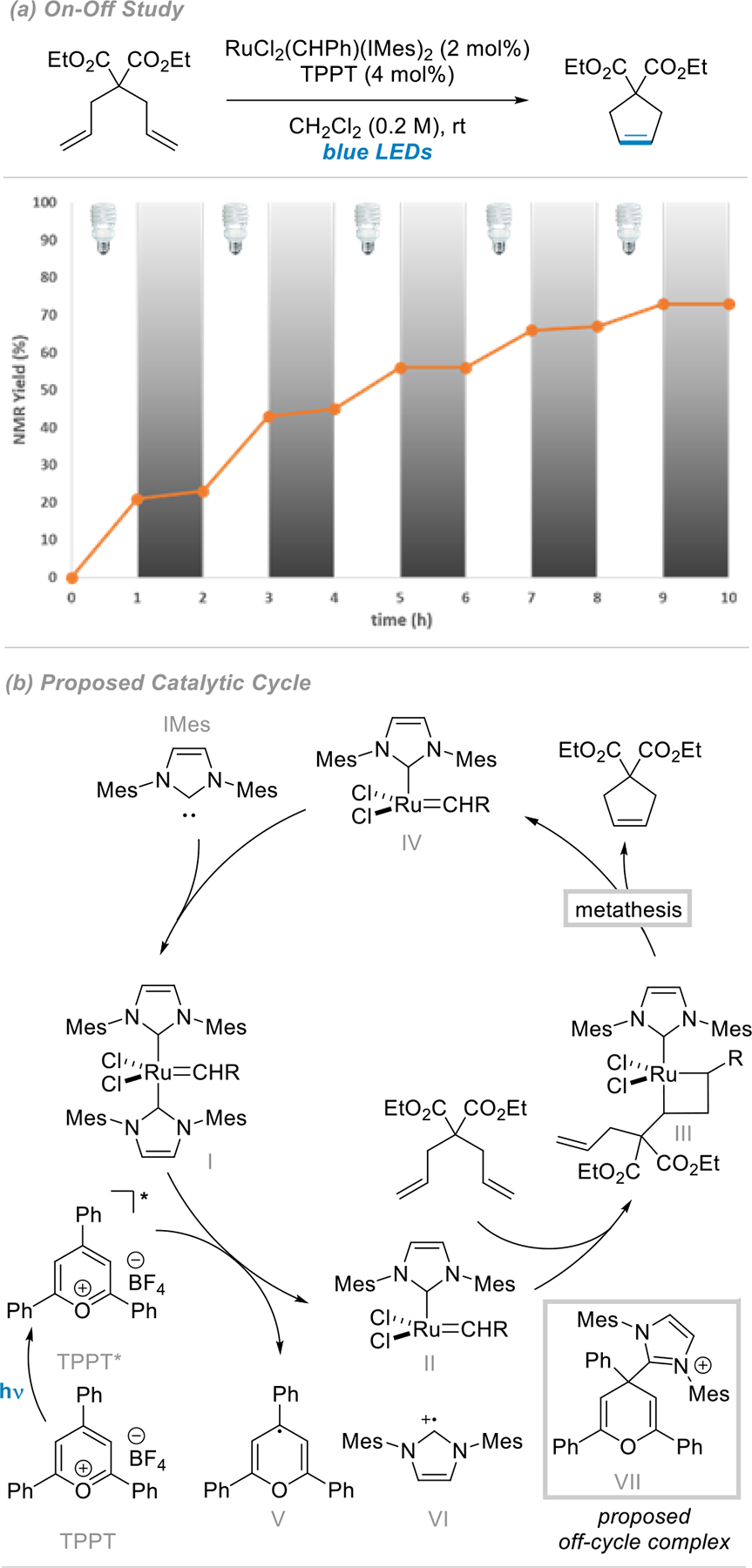
Temporal control over the RCM of diethyl diallylmalonate and corresponding proposed mechanism.
A series of experiments (described in the SI) lead us to suggest the following mechanism for the on/off behavior enabled by photoredox catalysis and light irradiation.9a It is commonly accepted that Ru catalysts mediate olefin metathesis via a coordinatively unsaturated Ru(II) intermediate such as II (Figure 2). Given that only highly oxidizing excited state photocatalysts provide appreciable yield (Table 1), we propose that ligand loss occurs at ambient temperature from an oxidized Ru intermediate, potentially at the IMes moiety to give active metathesis catalyst II and reduced pyrylium V as well as VI. The latter two can combine to form VII, analogous to adducts reported lacking substitution at the 4 position.20 Release of the IMes provides a pool of free ligand which can coordinate IV and arrest catalysis.21
We also interrogated our ability to exert spatial control over metathesis with this system, due to the potential applications in materials science with polymer patterning, 3D printing and photolithography. In this regard, the development of a system controlled by visible light appears especially attractive and convenient. To this end, dicyclopentadiene 12, and some other monomers,22 were first irradiated with visible light (blue Kessil lamp, 40 W) in the presence of RuCl2(CHPh)(IMes)2 and TPPT through different photomasks in order to produce macroscopic polymers with controlled geometric patterns. Removal of the masks and unreacted monomers nicely affords the corresponding patterned polymers in short irradiation times (15–60 minutes) and with minimal bleeding in the unexposed areas (Figure 3a–c).23 Interestingly, the thickness of these patterned polymers can be easily controlled by tuning the irradiation time (see SI). Finally, an important feature of this system is its practicality and user-friendliness. While the excited state TPPT* is modestly sensitive to oxygen, the photomask patterning experiments can be performed with minimal precautions of placing the monomer/catalyst mixture under a blanket of inert gas.
Figure 3.
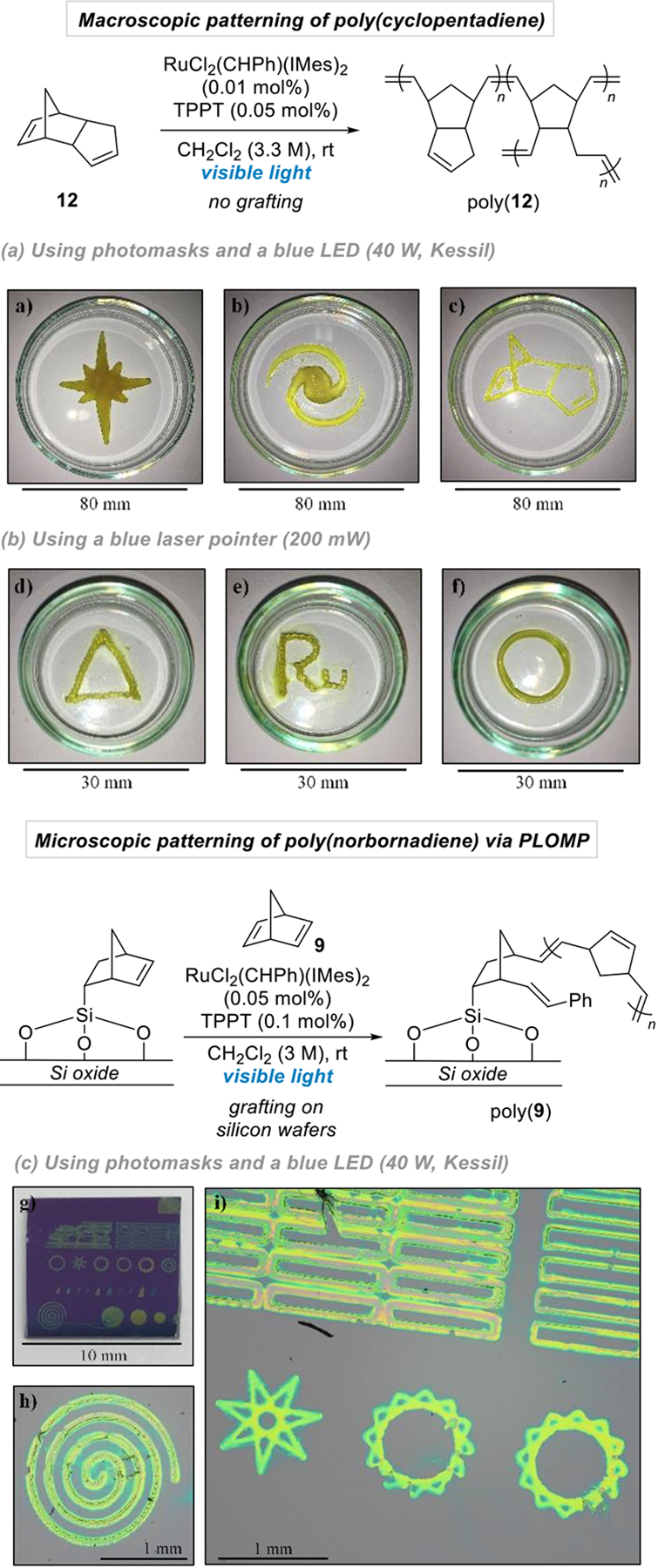
Polymer patterning and photolithographic olefin metathesis polymerization (PLOMP) using visible light.
Higher resolutions are required in order to apply this visible-light-controlled system for applications in PhotoLithographic Olefin Metathesis Polymerization (PLOMP). While most photolithographic techniques are based on the use of high resolution photomasks, an attractive alternative is the use of high resolution light sources, such as lasers, which should provide a straightforward way to reach pinpoint resolution and find new applications in photolithography.24 As proof of concept, we could successfully induce similar patterning from dicyclopentadiene solutions using a simple blue laser pointer (200 mV). In these cases, the patterns are directly and conveniently “drawn” from the bulk solution in a few minutes, either manually (Figure 3d,e) or using an orbital shaker providing constant movement (Figure 3f).
The two afore-described techniques allow the convenient fabrication of macroscopic patterned polymers through spatially-resolved ROMP promoted by visible light and without the need for grafting of the monomers. As for microscopic patterning, we also demonstrate the efficiency of our system for PLOMP applications.12 Although photolithography is now a commonly used technique in microfabrication, such systems based on olefin metathesis are still rare. To this end, 1 cm × 1 cm silicon wafers were first pre-functionalized with a norbornene unit to ensure grafting of the growing polymer onto the surface.12a Those pre-functionalized silicon wafers were then used as support to perform the spatially-resolved polymerization of norbornadiene on a microscale by simply irradiating a solution of the monomer, RuCl2(CHPh)(IMes)2 and TPPT in dichloromethane with a regular blue LED light bulb (blue Kessil lamp, 40 W) through high resolution photomasks (Figure 3g).
After developing in dichloromethane, patterns of poly(norbomadiene) with resolutions down to 30–40 microns could be successfully printed over silicon wafers within 10 minutes of irradiation (Figure 3h,i). These results are accomplished using readily available visible light sources, and are complementary to Fourkas’ positive photoresist,12a as well as Grubbs’ negative photoresist system,12b both of which use UV light, while giving access to similar resolutions.
In conclusion, we have reported the development of an efficient and user-friendly system for olefin metathesis controlled by visible light. The combination of a latent bis(NHC)-ruthenium complex and an oxidizing pyrylium photocatalyst efficiently promotes olefin metathesis under visible light, providing high levels of temporal and spatial resolution. In particular, applications in polymer patterning and photolithographic olefin metathesis polymerization are also demonstrated. Further studies to broaden the scope and applications of this system, as well as to gain more insights into its mechanism, are currently ongoing in our group.
Supplementary Material
ACKNOWLEDGMENT
Our work was supported by the National Institute of General Medical Sciences (GM125206). Cédric Theunissen acknowledges the Belgian American Educational Foundation (B.A.E.F) for postdoctoral fellowship. We thank Prof. Deryn Fogg (Ottawa) for helpful discussions. We thank Natalia Gadjieva and Prof. Colin Nuckolls (Columbia) for insightful discussions and their help on the PLOMP experiments.
Footnotes
Supporting Information
Experimental procedures, characterization, copies of 1H and 13C NMR spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
A provisional patent has been filed on this work.
REFERENCES
- (1).(a) Grela K Olefin Metathesis: Theory and Practice; Wiley: Hoboken, NJ, 2014; [Google Scholar]; (b) Grubbs RH; Wenzel AG Handbook of Metathesis, 2nd ed.; Wiley-VHC: Weinheim, 2015; [Google Scholar]; (c) Trnka TM, Grubbs RH The Development of L2X2Ru=CHR Olefin Metathesis Catalysts: An Organometallic Success Story Acc. Chem. Res 2001, 34, 18–29; [DOI] [PubMed] [Google Scholar]; (d) Hoveyda AH; Zhugralin AR The remarkable metal-catalysed olefin metathesis reaction Nature 2007, 450, 243–250. [DOI] [PubMed] [Google Scholar]; (e) Ogba OM; Warner NC; O’Leary DJ; Grubbs RH Recent advances in ruthenium-based olefin metathesis Chem. Soc. Rev 2018, 47, 4510–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Katz TJ Olefin Metathesis and Related Reaction Initiated by Carebene Derivatives of Metals in Low Oxidation States. Angew. Chem. Int. Ed 2005, 44, 3010–3019. [DOI] [PubMed] [Google Scholar]
- (2).Higman CS; Lummiss JAM; Fogg DE Olefin Metathesis at the Dawn of Implementation in Pharmaceuticals and Specialty-Chemicals Manufacturing. Angew. Chem. Int. Ed 2016, 55, 3552–3565. [DOI] [PubMed] [Google Scholar]
- (3).(a) Blanco V; Leigh DA; Marcos V Artificial switchable catalysts. Chem. Soc. Rev 2015, 44, 5341–5370; [DOI] [PubMed] [Google Scholar]; (b) Choudhury J Recent developments on artificial switchable catalysis. Tetrahedron Lett 2018, 59, 487–495. [Google Scholar]
- (4).(a) For recent reviews on externally regulated polymerizations:Leibfarth FA; Mattson KM; Fors BP; Collins HA; Hawker CJ External Regulation of Controlled Polymerizations. Angew. Chem. Int. Ed 2013, 52, 199–210; [DOI] [PubMed] [Google Scholar]; (b) Teator AJ; Lastovickova DN; Bielawski CW Switchable Polymerization Catalysts. Chem. Rev 2016, 116, 1969–1992; [DOI] [PubMed] [Google Scholar]; (c) Ogawa KA; Goetz AE; Boydston AJ Developments in Externally Regulated Ring-Opening Metathesis Polymerization. Synlett 2016, 27, 203–214; [Google Scholar]; (d) Teator AJ; Bielawski CW Remote Control Grubbs Catalysts That Modulate Ring-Opening Metathesis Polymerizations. J. Polym. Sci. Pol. Chem 2017, 55, 2949–2960. [Google Scholar]
- (5).(a) For reviews on latent olefin metathesis catalysis:Szadkowska A; Grela K Initiation at Snail’s Pace: Design and Applications of Latent Olefin Metathesis Catalysts Featuring Chelating Alkylidene Ligands. Curr. Org. Chem 2008, 12, 1631–1647; [Google Scholar]; (b) Monsaert S; Lozano Vila A; Drozdzak R; Van Der Voort P; Verpoort F Latent olefin metathesis catalysts. Chem. Soc. Rev 2009, 38, 3360–3372. [DOI] [PubMed] [Google Scholar]; (c) For recent reviews on light-promoted olefin metathesis, see:Vidavsky Y Lemcoff NG Light-induced olefin metathesis. Beilstein J. Org. Chem 2010, 6, 1106–1119; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Eivgi O; Lemcoff NG Turning the Light On: Recent Developments in Photoinduced Olefin Metathesis. Synthesis 2018, 50, 49–63. [Google Scholar]
- (6).(a) Stoll RA; Hecht S Artificial Light-Gated Catalyst Systems Angew. Chem. Int. Ed 2010, 49, 5054–5075; [DOI] [PubMed] [Google Scholar]; (b) Neilson BN; Bielawski CW Illuminating Photoswitchable Catalysis. ACS Catal. 2013, 3, 1874–1885; [Google Scholar]; (c) Göstl R; Senf A; Hecht S Remote-controlling chemical reactions by light: Towards chemistry with high spatio-temporal resolution. Chem. Soc. Rev 2014, 43, 1982–1996. [DOI] [PubMed] [Google Scholar]
- (7).(a) For recent representative examples using ruthenium catalysts, see:Wang D; Wurst K; Knolle W; Decker U; Prager L; Naumov S; Buchmeiser MR Cationic RuII Complexes with N-Heterocyclic Carbene Ligands for UV-Induced Ring-Opening Metathesis Polymerization. Angew. Chem. Int. Ed 2008, 47, 3267–3270; [DOI] [PubMed] [Google Scholar]; (b) Wang D; Wurst K; Buchemeiser MR Cationic versus Neutral RuII N-heterocyclic Carbene Complexes as Latent Precatalysts for the UV-Induced Ring-Opening Metathesis Polymerization. Chem. Eur. J 2010, 16, 12928–12934; [DOI] [PubMed] [Google Scholar]; (c) Keitz BK; Grubbs RH A Tandem Approach to Photoactivated Olefin Metathesis: Combining a Photoacid Generator with an Acid Activated Catalyst. J. Am. Chem. Soc 2009, 131, 2038–2039; [DOI] [PubMed] [Google Scholar]; (d) Khalimon AY; Leitao EM; Piers WE Photogeneration of a Phosphonium Alkylidene Olefin Metathesis Catalyst. Organometallics 2012, 31, 5634–5637; [Google Scholar]; (e) Ben-Asuly A; Aharoni A; Diesendruck CE; Vidavsky Y; Goldberg I; Straub BF; Lemcoff NG Photoactivation of Ruthenium Olefin Metathesis Initiators. Organometallics 2009, 28, 4652–4655; [Google Scholar]; (f) Levin E; Mavila S; Eivgi O; Tzur E; Lemcoff NG Regioselective Chromatic Orthogonality with Light-Activated Metathesis Catalysts. Angew. Chem. Int. Ed 2015, 54, 12384–12388; [DOI] [PubMed] [Google Scholar]; (g) Sutar RL; Levin E; Butilkov D; Goldberg I; Reany O; Lemcoff NG A Light-Activated Olefin Metathesis Catalyst Equipped with a Chromatic Orthogonal Self-Destruct Function. Angew. Chem. Int. Ed 2016, 55, 764–767; [DOI] [PubMed] [Google Scholar]; (h) Teator AJ; Shao H; Lu G; Liu P; Bielawski CW A Photoswitchable Olefin Metathesis Catalyst. Organometallics 2017, 36, 490–497. [Google Scholar]
- (8).(a) For reviews on photoredox catalysis, see:Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322–5363; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tellis JC; Kelly CB; Primer DN; Jouffroy M; Patel NR; Molander GA Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3-sp2 Cross-Coupling. Acc. Chem. Res 2016, 49, 1429–1439; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]; (d) Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev 2016, 116, 10035–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Ogawa KA; Goetz AE; Boydston AJ Metal-Free Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc 2015, 137, 1400–1403; [DOI] [PubMed] [Google Scholar]; (b) Goetz AE; Boydston AJ Metal-Free Preparation of Linear and Cross-Linked Polydicyclopentadiene. J. Am. Chem. Soc 2015, 137, 7572–7575; [DOI] [PubMed] [Google Scholar]; (c) Goetz AE; Pascual LMM; Dunford DG; Ogawa KA; Knorr DB Jr.; Boydston AJ Expanded Functionality of Polymers Prepared Using Metal-Free Ring-Opening Metathesis Polymerization. ACS Macro Lett. 2016, 5, 579–582. [DOI] [PubMed] [Google Scholar]
- (10).(a) For our previous work on controlling cobalt-catalyzed cycloadditions with photoredox catalysis, see:Ruhl KE; Rovis T Visible Light-Gated Cobalt Catalysis for a Spatially and Temporally Resolved [2+2+2] Cycloaddition. J. Am. Chem. Soc 2015, 138, 15527–15530; [DOI] [PubMed] [Google Scholar]; (b) Ravetz BD; Ruhl KE; Rovis T External Regulation of Cobalt-Catalyzed Cycloaddition Polymerization with Visible Light. ACS Catal. 2018, 8, 5323–5327; [Google Scholar]; (c) Ravetz BD; Wang JY; Ruhl KE; Rovis T Photoinduced Ligand-to-Metal Charge Transfer Enables Photocatalyst-Independent Light-Gated Activation of Co(II). ACS Catal. 2019, 9, 200–204. [Google Scholar]
- (11).(a) For general references on photolithography, see:Bratton D; Yang D; Dai JY; Ober CK Recent progress in high resolution lithography. Polym. Adv. Technol 2006, 17, 94–103; [Google Scholar]; (b) Madou MJ Fundamentals of Microfabrication and Nanotechnology, 3rd ed., CRC Press: Boca Raton, FL, 2011; ISBN 9780849331800; [Google Scholar]; (c) Xu H; Kosma V; Giannelis EP; Ober CK In pursuit of Moore’s Law: Polymer Chemistry in Action. Polym. J 2018, 50, 45–55. [Google Scholar]
- (12).(a) For two examples of photolithographic ring opening metathesis polymerization, see:Harris RF; Ricci MJ; Farrer RA; Praino J; Miller SJ; Saleh BEA; Teich MC; Fourkas JT Photolithographic Patterning of Ring-Opening Metathesis Catalysts on Silicon. Adv. Mater 2005, 17, 39–42; [Google Scholar]; (b) Weitekamp RA; Atwater HA; Grubbs RH Photolithographic Olefin Metathesis Polymerization. J. Am. Chem. Soc 2013, 135, 16817–16820. [DOI] [PubMed] [Google Scholar]
- (13).For a review on switchable polymerization catalysts, see:Teator AJ; Lastovickova DN; Bielawski CW Switchable Polymerization Catalysts. Chem. Rev 2016, 116, 1969–1992. [DOI] [PubMed] [Google Scholar]
- (14).(a) For a recent review, see:Chen M; Zhong M; Johnson JA Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev 2016, 116, 10167–10211. [DOI] [PubMed] [Google Scholar]; For selected examples, see:; (b) Fors BP; Hawker CJ Control of a Living Radical Polymerization of Methacrylates by Light. Angew. Chem. Int. Ed 2012, 51, 8850–8853; [DOI] [PubMed] [Google Scholar]; (c) Anastasaki A; Nikolaou V; Zhang Q; Burns J; Samanta SR; Waldron C; Haddleton AJ; McHale R; Fox D; Percec V; Wilson P; Haddleton DM Copper(II)/Tertiary Amine Synergy in Photoinduced Living Radical Polymerization: Accelerated Synthesis of ω-Functional and α,ω-Heterofunctional Poly(acrylates). J. Am. Chem. Soc 2014, 136, 1141–1149; [DOI] [PubMed] [Google Scholar]; (d) Treat NJ; Sprafke H; Kramer JW; Clark PG; Barton BE; Read de Alaniz J; Fors BP; Hawker CJ Metal-Free Atom Transfer Radical Polymerization. J. Am. Chem. Soc 2014, 136, 16096–16101; [DOI] [PubMed] [Google Scholar]; (e) Pan X; Malhotra N; Simakova A; Wang Z; Konkolewicz D; Matyjaszewski K Photoinduced Atom Transfer Radical Polymerization with ppm-Level Cu Catalyst by Visible Light in Aqueous Media. J. Am. Chem. Soc 2015, 137, 15430–15433. [DOI] [PubMed] [Google Scholar]
- (15).Weskamp T; Schattenmann MS; Herrmann WA A Novel Class of Ruthenium Catalysts for Olefin Metathesis. Angew. Chem. Int. Ed 1998, 37, 2490–2492. [DOI] [PubMed] [Google Scholar]
- (16).Trnka TM; Morgan JP; Sanford MS; Wilhelm TE; Scholl M; Choi T-L; Ding S; Day MW; Grubbs RH Synthesis and Activity of Ruthenium Alkylidene Complexes Coordinated with Phosphine and N-Heterocyclic Carbene Ligands. J. Am. Chem. Soc 2003, 125, 2546–2558. [DOI] [PubMed] [Google Scholar]
- (17).Conrad JC; Yap GPA; Fogg DE Concise Route to Highly Reactive Ruthenium Metathesis Catalysts Containing a Labile Donor and an N-Heterocyclic Carbene (NHC) Ligand. Organometallics 2003, 22, 1986–1988. [Google Scholar]
- (18).(a) Eelman MD; Blacquiere JM; Moriarty MM; Fogg DE Shining New Light on an Old Problem: Retooling MALDI Mass Spectrometry for Organotransition-Metal Catalysis. Angew. Chem. Int. Ed 2008, 47, 303–306; [DOI] [PubMed] [Google Scholar]; (b) Bailey GA; Fogg DE Confronting Neutrality: Maximizing Success in the Analysis of Transition-Metal Catalysts by MALDI Mass Spectrometry. ACS Catal. 2016, 6, 4962–4971. [Google Scholar]
- (19).For a study on the reaction of NHCs with one-electron oxidants, see:Ramnial T; McKenzie I; Gorodetsky B; Tsang EMW; Clyburne JAC Reactions of N-Heterocyclic carbenes (NHCs) with one-electron oxidants: possible formation of a carbene cation radical. Chem. Commun 2004, 1054–1055. [DOI] [PubMed] [Google Scholar]
- (20).(a) Antoni PW; Hansmann MM Pyrylenes: A New Class of Tunable, Redox-Switchable, Photoexcitable Pyrylium-Carbene Hybrids with Three Stable Redox-States. J. Am. Chem. Soc 2018, 140, 14823–14835. [DOI] [PubMed] [Google Scholar]; (b) For radical addition to the 4-possition of TPPT, see:Branchi B; Bietti M; Ercolani G; Izquierdo MA; Miranda MA; Stella L The Role of Aromatic Radical Cations and Benzylic Cations in the 2,4,6-Triphenylpyrylium Tetrafluoroborate Photosensitized Oxidation of Ring-Methoxylated Benzyl Alcohols in CH2Cl2 Solution. J. Org. Chem 2004, 69, 8874–8885. [DOI] [PubMed] [Google Scholar]
- (21).At this time, we cannot discount potential pathways due to catalyst decomposition.
- (22).Similar patterning could be obtained with: norbornadiene 9, 1,5-cyclooctadiene 10 and 5-ethylidene-2-norbornene 11; see Supplementary Information for details.
- (23).The amount of monomer consumed that is not present in the final patterned polymer was estimated at 7% by analysis of the wash using an external reference.
- (24).Rühe J And There Was Light: Prospects for the Creation of Micro- and Nanostructures through Maskless Photolithography. ACS Nano 2017, 11, 8537–8541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


