Abstract
Objective. Loss of disc height is commonly associated with chronic low back pain (CLBP). Isolated lumbar extension (ILEX) exercise for the lumbar extensors is recommended to treat CLBP and is suggested such exercise might promote disc healing and regeneration. This study examined a 12-week ILEX intervention on indirect determination of disc height and shrinkage through seated stadiometry, strength, pain, and disability. Design. A quasi-experimental wait-list controlled design was used. Nine participants underwent pretesting (T1), a 12-week control period, retesting (T2), a 12-week intervention period, and finally posttesting (T3). Seated stadiometry, ILEX strength, pain, and disability were measured at each time point. Results. No significant repeated-measures effects for any seated stadiometry variables occurred. Significant improvement across the intervention period (T2 to T3) was found for strength (P <0.0001; effect size [ES] = 2.42). Change in pain was not significant for repeated effects (P = 0.064); however, ES for the intervention period (T2 to T3) was moderate (ES = −0.77). Change in disability was significant between time point T1 and T3 (P = 0.037) and ES for the intervention period (T2 to T3) was large (ES = −0.92). Pain and disability achieved minimal clinically important changes. Conclusions. This is apparently the first study to examine disc change in vivo after exercise in CLBP. Results of the present study, though supporting ILEX resistance training to improve strength, pain, and disability, did not find any effect on spinal height.
Keywords: disc, hydration, stadiometer, intervertebral disc cartilage
Introduction
Chronic low back pain (CLBP) is a highly prevalent,1-5 multifactorial condition,6,7 representing an enormous economic cost worldwide.8-10 The intervertebral discs have been suspected a potential source of painful symptoms in LBP for some time11 with considerable evidence regarding pain-causing mechanisms.12,13 Although it may be difficult to attribute specific disc pathologies to CLBP on an individual basis, there are consistent associations of more serious disc abnormalities in those who suffer from CLBP.14-16 Adams and Roughley12 suggest the presence of some degree of degeneration is a physiologic process associated with aging, whereas more severe degeneration and/or structural abnormality may be indicative of a pathological process or injury and more commonly present in those suffering from CLBP. Many studies support the contention that more severe degrees of degeneration and/or structural abnormality are more consistently apparent in participants with CLBP than those who are asymptomatic17-21 in a dose-dependent manner.22,23 Loss of disc hydration and disc height is also commonly considered indicative of degenerative processes as opposed to being age related.12,24 Even if not all disc abnormalities can be ascribed as the source of CLBP, any degenerative changes also heighten the risk for more severe disc degeneration or injury and thus pain and suffering.12,13 Thus it seems that, as a consistent finding in symptomatic participants, and a potential source of pain symptoms, disc degeneration or injury is a worthwhile factor to consider in treatment of CLBP.
Exercise is a common prescription for those with CLBP; however, the potential for it to specifically promote positive changes in the intervertebral discs is not often considered. It has been suggested that regular movement and exercise of the lumbar spine might counter and perhaps reverse loss in disc hydration.25-27 Nelson et al.28 reported that reduction in pain after isolated lumbar extension (ILEX) exercise was similar in all diagnosed conditions, including degenerative disc disease. Concerns have been expressed regarding the safety of using exercise such as ILEX when considering disc health.29 However, although disc degeneration can be affected negatively by loading, the potential for a “safe window” of disc loading that may stimulate optimal disc health does exist.30,31 Indeed the available animal model research appears to suggest its biological plausibility.32 A relatively high magnitude, low frequency and short duration dynamic loading may produce potentially regenerative effects on the intervertebral disc (including improvements in disc proteoglycan content, matrix gene expression, rate of cell apoptosis, and improved fluid flow and solute transport).33-37
ILEX exercise is suggested to be optimal in comparison to other modalities aimed at conditioning the lumbar extensors38 and provides significant and meaningful improvements in pain and disability.39 Moreover, as ILEX allows quantification of load and specific application to the lumbar spine it presents a suitable model for examining the effect of controlled loading on disc condition in CLBP participants. Indeed, strength produced through such exercise may affect the overall robustness of the spine to resist loading.40 ILEX has been shown to produce successful rehabilitation outcomes in participants diagnosed with degenerative discs28,41 in addition to participants undergoing lumbar discectomy for disc herniation.42 Furthermore, it has been applied in occupational settings with success in reducing both injury occurrence and costs associated with injury.43-46 However, no studies have quantified any change occurring in disc condition in vivo.
As noted, loss of disc hydration and disc height is a common disc abnormality. Disc hydration is often measured via magnetic resonance imaging (MRI),47 but indirect measurement can be obtained through measures of spinal height using stadiometry.48 As such, for researchers wishing to examine the effects of potential interventions on CLBP and associated symptoms such as disc hydration, as well as for clinicians examining changes in their patients, the use of stadiometry may be of value as an outcome measure. A recent study has reported that a custom-built seated stadiometer is reliable in measuring changes in spinal height variables, including spinal shrinkage.49 Thus, it might be a suitable outcome measure to examine the effect of disc loading through exercise on disc hydration. Therefore, the aim of the present study was to examine the potential effect of applied loading to the lumbar intervertebral discs through ILEX resistance exercise as measured using seated stadiometry.
Methods
Study Design
A quasi-experimental wait-list controlled design was adopted with all participants undergoing pretesting (T1) followed by an initial 12-week control period, before then being retested (T2) and then beginning the 12-week experimental period. Participants were posttested once the experimental period had finished (T3). The study was approved by the ethics committee at Southampton Solent University (SSU) and conducted within the Sport and Exercise Science Laboratories at SSU.
Participants
A convenience sample of 17 participants (males, n = 9; females, n = 8) were initially identified and recruited by posters, group email, and word of mouth from SSU and the surrounding locality. An a priori power analysis was conducted to determine participant numbers (n) in order to detect a moderate treatment effect size (ES), calculated using Cohen’s d,50 of 0.5. Participant numbers were calculated using G*Power. These calculations showed that 9 participants were required to meet the required power of 0.8 at an alpha value of P ≤ 0.05 for the statistical analyses proposed (see below).
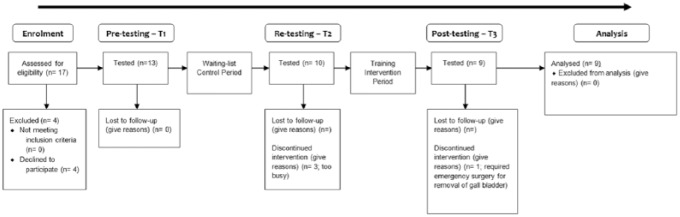
Inclusion criteria were as follows; participants suffering from nonspecific LBP having lasted longer than 12 weeks51 and have no medical condition for which resistance training would be contraindicated. Exclusion criteria included the following: participants must have no medical condition for which movement therapy would be contraindicated. These include acute (not reoccurring) low back injury occurring within the past 12 weeks, pregnancy, evidence of sciatic nerve root compression (sciatica), leg pain radiating to below the knee, paraesthesia (tingling or numbness), current tension sign, lower limb motor deficit, current disc herniation, previous vertebral fractures, or other major structural abnormalities. All participants were cleared to exercise prior to involvement in the study by either their general practitioner or the chiropractor in the research group. After pretesting, the participants underwent a 12-week control period where they were instructed to continue with their daily activities as normal and any treatment or intervention they were currently undertaking. After completion of this 12-week period, participants were retested and then underwent a 12-week ILEX exercise training intervention. Figure 1 shows the flow of participants through the study.
Figure 1.
Flow of participants through study.
Equipment
Participants’ standing stature (for demographic purposes) and seated stature (for determination of spinal height) were measured using a wall mounted stadiometer (Holtan Ltd, Crymych, Dyfed). Details of seated stature measures are below). Body mass was measured using scales (SECA, Germany) and body mass index (BMI) calculated. Isometric strength testing, range of motion (ROM) and training was performed using the MedX Lumbar Extension Machine (MedX Corporation, Ocala, FL). The ILEX machine has been shown to be reliable in assessing isometric strength at repeated angles in asymptomatic (test-retest correlation across angles tested, r = 0.81 to 0.97)52 and symptomatic participants (r = 0.57 to 0.93),53 and valid in measurement.54,55 Pain was measured using a 100-mm point visual analogue scale (VAS),56 and disability measured using the revised Oswestry disability index (ODI).57 A customized wooden seat in addition to custom-built wall-mounted adjustable postural rods ( Figure 2 ; SSU, Southampton) were used with the wall-mounted stadiometer for seated stature measurements in order to ensure participants adopted the same posture within the sagittal plane for each retest trial. The details and reliability of this setup has recently been reported elsewhere.49
Figure 2.
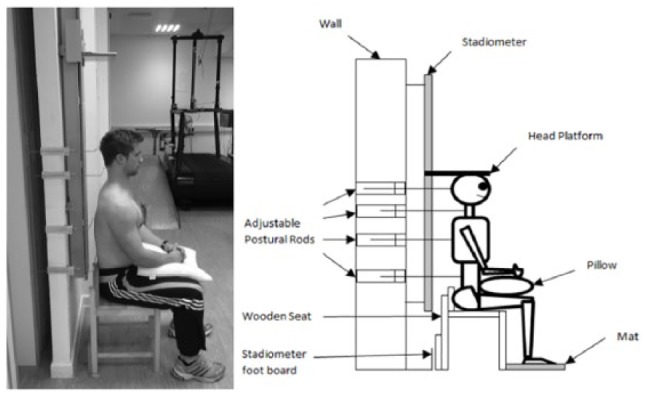
Schematic of seated stadiometry setup.
Participant Testing
All measurements were completed at the same time of day and participants were instructed to avoid heavy lifting for at least 2 days prior to testing.58 Participants underwent testing for seated stadiometry, and completed 2 isometric ILEX strength tests on separate days using the MedX Lumbar Extension Machine, at 3 points throughout the study (T1, T2, and T3). The ILEX test days were separated by at least 72 hours in order to avoid the effects of residual fatigue or soreness. Each test using the ILEX machine involved maximal voluntary isometric contractions at various angles through the participants full ROM in order to measure maximal isometric ILEX strength. The number of angles tested depended on the participants individual ROM. Participants where tested at as many of the following angles as they were able to achieve; 72°, 60°, 48°, 36°, 24°, 12°, and 0°. Details of the full test protocol using the ILEX machine and details of the restraint mechanisms have been documented previously elsewhere.52 At each time point, participants were also required to complete the VAS and ODI.
In order to normalize spine height prior to stadiometry measurement, the participant was instructed to lie in the supine position for 10 minutes with his or her hands resting on the stomach, head in a neutral position and supported by a pillow, and legs uncrossed with a pillow under the knees for support. A custom setup (see Figure 2 ) was used in combination with the wall-mounted stadiometer used for standing measurements. Full details of the test protocol are detailed elsewhere.49 Ten repeated measurements were taken as close as possible to every 20 seconds over a period of approximately 3 to 3.5 minutes with the participant remaining in the stadiometer between measurements.59 From this spinal height for the first measurement, the average of the 10 measurements, total shrinkage (difference between first and last measurement), and the rate of shrinkage across the 10 measurements examined as the slope of the curve when a linear regression was fitted (standard error of measurement were 3.1 mm, 2.8 mm, 2.6 mm, and 0.212, respectively). Posttesting occurred 1 week after the final ILEX training session.
Participant Training
Training was conducted at a frequency of 1 time/week for a period of 12 weeks. This frequency of training has been shown to significantly improve ILEX strength and was chosen over more frequent training due to potential for overtraining when the lumbar extensor muscles are isolated.60 Also a second weekly training session offers no further improvements in symptomatic participants.61 Twelve weeks was the chosen duration as Carpenter et al.62 have demonstrated that strength improvement from ILEX training occurs largely within the first 12 weeks. Participants performed one set of variable resistance ILEX exercise through their full ROM. Resistance load was 80% of maximum recorded tested functional torque during maximal isometric testing for both groups and repetitions performed until momentary failure in order to control for intensity of effort.63 Repetitions were performed taking at least 2 seconds to complete the concentric phase, holding for 1 second in full extension and taking at least 4 seconds for the eccentric phase. Resistance load was increased by 5% in the next session once the participant was able to continue exercise for over 105 seconds using their current load before achieving failure. All training was supervised by the lead researcher.
Data Analysis
Nine participants’ data (males, n = 4; females, n = 5) were available after allowing for attrition. Isometric strength, recorded in units of torque, was measured as foot pounds (ft·lbs1) and converted to newton meters (N·m) using a correction of 1.356. Spinal height was calculated by subtracting the seat height (445 mm) from the stature recorded during seated stadiometry measurement. Because of individual differences between participants for lumbar ROM, ILEX strength data were averaged across all angles tested (ranging from 0° to 72°). Mauchly’s test for sphericity was used to determine equality of variance for data at P > 0.05. The independent variable to examine was the time point associated with the period (i.e., T1, T2, and T3) and dependent variables were ILEX strength, pain, disability, first measurement of each spinal height trial, average spinal height across the 10 measurements, total shrinkage defined as the difference between the last and first of the 10 measurements (i.e., a negative value represented loss of spinal height), and rate of shrinkage as the slope of the curve fitted using a linear regression model for time and spinal height (a higher value indicating a steeper slope and greater rate of shrinkage). Data with assumed sphericity for participant demographics and dependent variables were subjected to repeated-measures analysis of variance (ANOVA). Post hoc pairwise comparisons using a Bonferroni adjustment were conducted comparing T1 to T2 (encompassing the control period), T1 to T3 (encompassing both the control and intervention period), and T2 to T3 (encompassing the intervention period). Within participant effect sizes were calculated using Cohen’s d50 for absolute change in the independent variables across T1 to T2 and across T2 to T3 where an ES of 0.20 to 0.49 was considered as small, 0.50 to 0.79 as moderate, and ≥0.80 as large. In addition, changes in pain and disability were compared with consensus standards for minimal clinically important change (MCIC).64 Ostelo et al.64 propose the MCIC for VAS as 15 mm and for ODI 10 points. Statistical analysis was performed using SPSS statistics computer package (version 20) and P ≤ 0.05 set as the limit for statistical significance.
Results
Participants
Participant baseline demographics are shown in Table 1 .
Table 1.
Participant Baseline Demographics (Combined n = 9).
| Demographic | Mean (SD) |
|---|---|
| Age (years) | 51 (12) |
| Stature (cm) | 167.7 (6.9) |
| Body mass (kg) | 77.46 (13.94) |
| BMI (kg/m2) | 27.4 (3.2) |
| Symptom duration (years) | 15 (14) |
| ILEX strength (N·m) | 195.42 (109.99) |
| Lumbar ROM (degrees) | 65.7 (10.1) |
| VAS (mm) | 33.4 (23.3) |
| ODI (points) | 26.7 (11.2) |
BMI = body mass index; ILEX = isolated lumbar extension; ROM = range of motion; VAS = visual analogue scale; ODI = Oswestry disability index.
Seated Stadiometry
Table 2 shows spinal height results from seated stadiometry testing at each time point. No significant repeated-measures effects by time were found for any seated stadiometry variable (P = 0.542-0.713). ESs between T1 and T2 were 0.23, −0.29, −0.36, and −0.35 for first measure, average, shrinkage, and slope, respectively, with all being considered small. ESs between T2 and T3 were 0.07, 0.25, 0.15, and 0.11, respectively, with all being considered small or less than small.
Table 2.
Seated Stadiometry Result from Each Time Point.a
| T1 | T2 | T3 | |
|---|---|---|---|
| Seated stature: First measure (mm) | 864.2 (33.5) | 866.2 (37.4) | 867.1 (38.1) |
| Seated stature: Average (mm) | 863.6 (34.7) | 862.5 (37.0) | 864.6 (39.1) |
| Shrinkage: Total (mm) | −1.3 (3.3) | −5.0 (7.3) | −3.1 (6.3) |
| Rate of shrinkage (slope) | −0.193 | −0.471 | −0.329 |
Results are presented as mean (SD).
ILEX Strength
Figure 3 shows ILEX strength measured at each time point. A significant repeated-measures effect by time was observed for ILEX strength, (F(2, 16) = 26.263, P < 0.0001). Post hoc pairwise comparisons revealed a significant difference between both T1 and T3 (P = 0.002) and T2 and T3 (P < 0.0001). ES between T1 and T2 was −0.34 and considered small. ES between T2 and T3 was 2.42 and considered large.
Figure 3.
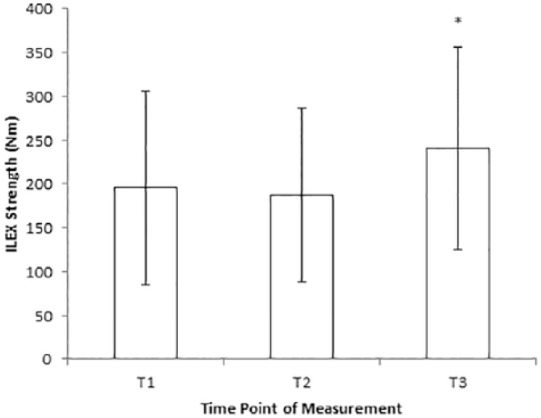
Isolated lumbar extension (ILEX) strength at each time point.
Oswestry Disability Index (ODI) and Visual Analogue Scale (VAS)
VAS and ODI measures for each time point are shown in Table 3 . ANOVA failed to achieve significance for repeated measures effect by time for VAS, (F(2, 16) = 3.281, P = 0.064). A significant repeated measures effect by time was observed for ODI, (F(2, 16) = 6.846, P = 0.007). Post hoc pairwise comparisons revealed a significant difference between T1 and T3 (P = 0.037) for ODI. Changes in VAS and ODI over the control period (between T1 and T2) did not achieve MCICs. Changes in VAS and ODI after the intervention period (between T2 and T3) both achieved MCICs (reduction of ~16 mm and ~12 points, respectively). ESs between T1 and T2 were 0.17 and 0.13 for VAS and ODI, respectively, and considered small. ESs between T2 and T3 were −0.77 and −0.92, respectively, and considered moderate and large respectively.
Table 3.
Change in Visual Analogue Scale (VAS) and Oswestry Disability Index (ODI).a
| T1 | T2 | T3 | |
|---|---|---|---|
| VAS (mm) | 33.4 (23.3) | 36.3 (22.8) | 20.1 (14.7) |
| ODI (points) | 26.7 (11.2) | 27.8 (9.4) | 16.0 (13.5)b |
Results are presented as mean (SD).
Indicates significant pairwise comparison between T1 and T3.
Discussion
The purpose this study was to examine the effects of a 12-week ILEX resistance training intervention in participants with CLBP on indirect determination of disc hydration through spinal height measured using seated stadiometry. To the authors’ knowledge, this is the first study to examine, albeit indirectly, whether positive changes in the discs measured in vivo result from exercise interventions in participants with CLBP.
Symptomatic degenerative discs show a number of abnormalities, including reduced glycosaminoglycans, dehydration, and reduced nucleus pulposus pH.65 Some have suggested that metabolic abnormalities in the intervertebral disc might be improved, thus potentially halting or reversing the degenerative process, through appropriate exercise of the lumbar spine.25-27 The exercise specifically considered by Mooney et al.27 and Mayer et al.26 was ILEX. Not all exercises are equally effective in conditioning the lumbar extensors and ILEX has been suggested recently as optimal for this purpose.38 Indeed, it has been hypothesized that such an exercise intervention might provide a suitable model for examining the potential for controlled loading to improving disc condition also.32
Some studies have suggested that continued compressive loading can contribute to harmful responses in the disc in a dose-dependent manner (i.e., magnitude and duration), which might further suggest cause for concern in employing ILEX resistance exercise for those with CLBP.66,67 However, this dose-dependent mechanism has important implications for ILEX resistance exercise, which is also typically employed in a dose-dependent manner. ILEX rehabilitation is normally employed using a resistance that allows only ~8 to 12 repetitions and exercise is performed to momentary failure using this resistance,39 which has been suggested as optimal for strength and hypertrophic adaptations68,69 in addition to improving pain and disability.39 An exercise frequency of once per week has also been identified as sufficient for improving lumbar extension strength, pain and disability.60,61 Thus, ILEX rehabilitation represents a relatively high loading on the disc though at a low frequency and volume. Walsh and Lotz33 report that, in comparison with higher frequency and lower load compression, lower frequency and higher load compression induces positive improvements in disc proteoglycan content, matrix gene expression and rate of cell apoptosis. Thus, there may be potential for ILEX rehabilitation to exert a similar adaptive effect. Indeed, Maclean et al.34,35 have also showed that anabolic and catabolic responses in the nucleus are dependent on load and frequency with anabolic genes being stimulated at low frequencies and catabolic genes being stimulated at higher frequencies. They also revealed that very low loading had no effect on gene expression suggesting that some degree of loading, though at a low frequency, is required to stimulate an adaptive anabolic response.
These studies have examined what might be considered regenerative processes, but as we have highlighted, a loss of disc hydration is also present in degenerative discs65 and so rehydration may also be an important consideration. Ferguson et al.36 have shown that loading increases fluid flow across the disc, which in turn also enhances transport of larger solutes into the intervertebral disc. Some authors have suggested ILEX rehabilitation may enhance pressure variance across the disc through its flexion-extension cycles and thus enhance interstitial fluid flow.26,27,61 The findings of Ferguson et al.36 would lend biological plausibility to this potential mechanism also. Furthermore, Wang et al.37 have presented that while static loading contributes to catabolic activity, dynamic compressive loading contrastingly promotes anabolic activity.
Research thus far has been conducted using in vitro animal models. This study is apparently the first to attempt to examine the chronic effects of specific loading on the disc in vivo. Because of suggestions from other authors regarding use of ILEX to “rehydrate” the discs25,26 and that loading increases fluid flow, enhancing transport of larger solutes into the intervertebral disc,36 it was considered that ILEX may create pressure variance across the disc through flexion-extension cycles and thus enhance interstitial fluid flow. Thus, it was hypothesized that a 12-week ILEX resistance training intervention in CLBP participants would improve disc hydration as measured indirectly through spinal height measures using seated stadiometry.
However, the results of the present study suggested that although the 12-week intervention improved ILEX strength, pain, and disability, there was no change in any of the seated stadiometry variables measured. Seated stature measures did not achieve significance, ESs were all small or less than small, and were also within the between-day range of error determined for the custom seated stadiometry setup used.49 Our sample estimate was based on the detection of an ES of at least 0.5 and so the lack of change may be the result of a type II error. As no other study has examined the effects of an intervention on chronic adaptation in the discs in vivo it is not possible to discern whether these results truly reflect a lack of change from the intervention or whether they stem from the testing utilized.
Acute studies of stature changes from various loading conditions reveal a wide range of changes some of which the current setup used may have been sensitive enough to detect; ~0.5 mm,70 ~3 mm,71 ~5 mm,48 ~7.5 to 10 mm,72 and ~6 to 7 mm.73 Considering the possible magnitudes of acute differences detected by some of these studies, it may be that the ILEX intervention merely did not induce any change in hydration of the discs, or at least not of a sufficient magnitude to be detected. MRI is more sensitive in detecting changes in disc hydration, in particular due to the ability to examine individual discs, as opposed to the cumulative total of their height, including the vertebral bodies and other oseoligamentous structures, when using seated stadiometry. Kourtis et al.48 report an error when using MRI of ~0.5 mm, which is considerably lower than the error within seated stadiometry, including our custom seated stadiometry setup (3.1 mm). Further study should examine whether changes in disc hydration occur from exercise based interventions when tested using MRI. Whether or not such small changes in disc hydration, if indeed they occur as a result of ILEX resistance training, are meaningful or not is yet to be determined. However, loss of hydration is only one aspect of a range of possible factors indicating disc condition12 and so, though there may not be a change in disc hydration after exercise interventions, the potential mechanisms of adaptation might impart positive adaptation in other features of the disc. Additional categorization of disc condition would be a further benefit of follow-up study utilizing MRI.
A further aspect examined in the present study was the time-dependent loss of stature, or shrinkage, related to spinal loading. This is considered an indicator of spinal “creep” due to its viscoelastic properties and may reflect the potential for spinal structures to experience time related changes in biomechanical stresses.72,74 Indeed stature shrinkage from constant static loading differs between asymptomatic controls and participants with CLBP75 and prior work has found a relationship between trunk extension strength and stature loss.40 This study examined change in spinal height and rate of shrinkage due to the participants own upper body mass over a 3- to 3.5-minute test where the participant remained seated in the stadiometer. The between-day reliability of this variable in our custom setup49 was similar to that reported by others.76 However, as with measurements of stature, there was no significant change in shrinkage or rate of shrinkage after the ILEX intervention and ESs were small or less than smallsuggesting there was no chronic change in the viscoelastic properties of the spine.
Despite absence of changes in seated stadiometry variables in response to the intervention, changes were observed for ILEX strength, pain, and disability. No changes in any variables were found over the 12-week control period. However, ILEX strength increased significantly over the intervention period and to a similar degree (~34%) as other studies utilizing the same intervention.61,77 These results also indicated the ILEX intervention period resulted in a significant reduction in disability measured using the ODI between baseline (T1) and retest after the intervention period (T3). Though change in pain and disability over the intervention period did not achieve significance they were similar to other studies utilizing the same ILEX intervention in CLBP participants61,77 and thus likely reflect the studies small sample size and thus a type II error. Indeed, despite this, change in pain and disability across the intervention period using VAS and ODI did both achieve MCICs (reduction of ~16 mm and ~12 points, respectively), ESs were moderate to large, and therefore can be considered meaningful.
One limitation of the present study was the relatively high average age of the sample population. This may have meant that age related changes were present in the discs which are suggested to be more difficult to reverse than producing healing of degenerated discs.13 Thus, future studies, in addition to considering utilization of MRI to detect in vivo changes in disc condition, should also utilize a larger sample size of younger adults. Furthermore, the duration of the intervention (12 weeks), though sufficient for inducing changes in tissues such as muscle, may be insufficient for inducing changes in the disc due to the particularly slow turnover rates of collagen and proteoglycans.78,79 Additional work in this area might thus consider the investigation of interventions of longer duration.
The utility of the intervention should also be considered in context. A minimal approach such as ILEX also offers the benefit of time efficiency. ILEX sessions require at least ~50% less time compared with regular physical therapy.80 Recent analysis suggested greater benefit may occur with a greater frequency of exercise sessions (an additional 8 sessions required to improve VAS scores by 1 mm compared with controls81). ILEX specifically, however, is highly effective using only a single weekly session with no further benefit from additional sessions.61 It seems that ILEX is also as effective as either part of a multifaceted intervention or as a standalone approach39 and that the benefits can occur from as little as one session per week taking approximately 10 to 15 minutes with only 1 to 2 minutes of that comprising exercise. As one of the biggest economic losses through CLBP is due to work hours lost, both through treatment and absenteeism, a workplace strengthening program43-46 using ILEX could be a promising occupational approach.
Conclusions
In conclusion, the results of the present study, though further supporting the use of ILEX resistance training to improve ILEX strength, pain and disability, did not find any effect on spinal height or shrinkage measures using seated stadiometry. Thus, despite its impact on other aspects of the multifactorial nature of LBP, suggestion that ILEX exercise improves disc condition in CLBP participants is presently not supported and remains a hypothesis requiring further study.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from Southampton Solent University Research Ethics Committee (FBSE-STEELE01).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
References
- 1. World Health Organization. The World Health Report 1998: Life in the 21st century: a vision for all. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 2. Office for National Statistics. Social trends 30. London, UK: The Stationery Office; 2000. [Google Scholar]
- 3. Waddell G, Burton AK. 2000. Occupational health guidelines for the management of low back pain at work: evidence review. Occup Med. 2000;51:126-35. [DOI] [PubMed] [Google Scholar]
- 4. Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13:205-17. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Clinical Excellence. Low back pain: early management of persistent non-specific low back pain. London, UK: Royal College of General Practitioners; 2009. [PubMed] [Google Scholar]
- 6. National Research Council. Work-related musculoskeletal disorders: a review of the evidence. Washington, DC: National Academy Press; 1998. [Google Scholar]
- 7. National Research Council, Institute of Medicine. Musculoskeletal disorders and the workplace: low back and upper extremities. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 8. Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Back pain exacerbations and lost productive time costs in United States workers. Spine. 2006;31:3052-60. [DOI] [PubMed] [Google Scholar]
- 9. Katz JN. Lumbar disc disorders and low back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21-4. [DOI] [PubMed] [Google Scholar]
- 10. Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-5. [Google Scholar]
- 12. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151-61. [DOI] [PubMed] [Google Scholar]
- 13. Adams MA, Stefanakis M, Dolan P. Healing of a painful intervertebral disc should not be confused with reversing disc degeneration: implications for physical therapies for discogenic back pain. Clin Biomech. 2010;25:961-71. [DOI] [PubMed] [Google Scholar]
- 14. Endean A, Palmer KT, Coggon D. Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies. Spine. 2011;36:160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNee P, Shambrook J, Harris EC, Sampson M, Palmer KT, Coggon D. Predictors of long-term pain and disability in patients with low back pain investigated by magnetic resonance imaging: a longitudinal study. BMC Musculoskelet Disord. 2011;12:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shambrook J, McNee P, Harris EC, Kim M, Sampson M, Palmer KT, et al. Clinical presentation of low back pain and association with risk factors according to findings on magnetic resonance imaging. Pain. 2011;152:1659-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73. [DOI] [PubMed] [Google Scholar]
- 18. Weishaupt D, Zanetti M, Hodler J, Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661-9. [DOI] [PubMed] [Google Scholar]
- 19. Holt EP., Jr. The question of lumbar discography. J Bone Joint Surg Am. 1968;50:720-6. [DOI] [PubMed] [Google Scholar]
- 20. Walsh TR, Weinstein JN, Spratt KF, Lehmann TR, Aprill C, Sayre H. Lumbar discography in normal subjects. A controlled, prospective study. J Bone Joint Surg Am. 1990;72:1081-8. [PubMed] [Google Scholar]
- 21. Kjaer P, Leboeuf-Yde C, Kowsholm L, Sorenson JS, Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic study of 40 year old men and women. Spine. 2005;30:1173-80. [DOI] [PubMed] [Google Scholar]
- 22. Cheung KM, Karppinen J, Chan D, Ho DW, Song YQ, Sham P, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34:934-40. [DOI] [PubMed] [Google Scholar]
- 23. de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, et al. The association between lumbar disc degeneration and low back pain. Spine. 2010;35:531-6. [DOI] [PubMed] [Google Scholar]
- 24. Griffith JF, Wang YJ, Antonio GE, Choi KC, Yu A, Ahuja AT, et al. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine. 2007;32:E708-12. [DOI] [PubMed] [Google Scholar]
- 25. Norris CM. Back stability: integrating science and therapy. Champaign, IL: Human Kinetics; 2008. [Google Scholar]
- 26. Mayer J, Mooney V, Dagenais S. Evidence informed management of chronic low back pain with lumbar extensor strengthening exercises. Spine J. 2008;8:96-113. [DOI] [PubMed] [Google Scholar]
- 27. Mooney V, Verna J, Morris C. Clinical management of chronic, disabling low back syndromes. In: Morris C, editor. Rehabilitation of the spine: a practitioner’s manual. New York, NY: McGraw-Hill; 2006. [Google Scholar]
- 28. Nelson BW, O’Reilly E, Miller M. The clinical effects of intensive, specific exercise on low back pain: a controlled study of 895 consecutive patients with a one year follow up. Orthopedics. 1995;18:971-81. [DOI] [PubMed] [Google Scholar]
- 29. McGill SM. Low back disorders: evidence-based rehabilitation and prevention. 2nd ed. Champaign, IL: Human Kinetics; 2007. [Google Scholar]
- 30. Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29:2724-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan SC, Ferguson SJ, Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur Spine J. 2011;20:1796-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steele J, Bruce-Low S, Smith D, Osborne N, Thorkeldsen A. Can specific loading through exercise impart healing or regeneration of the intervertebral disc? Spine J. 2015;15:2117-21. [DOI] [PubMed] [Google Scholar]
- 33. Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329-37. [DOI] [PubMed] [Google Scholar]
- 34. Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193-200. [DOI] [PubMed] [Google Scholar]
- 35. Maclean JJ, Lee CR, Alini M, Iatridis JC. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120-7. [DOI] [PubMed] [Google Scholar]
- 36. Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37:213-21. [DOI] [PubMed] [Google Scholar]
- 37. Wang DL, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine. 2007;32:2521-8. [DOI] [PubMed] [Google Scholar]
- 38. Steele J, Bruce-Low S, Smith D. A review of the specificity of exercises designed for conditioning the lumbar extensors. Br J Sports Med. 2015;49:291-7. [DOI] [PubMed] [Google Scholar]
- 39. Steele J, Bruce-Low S, Smith D. A review of the clinical value of isolated lumbar extension resistance training for chronic low back pain. PM R. 2015;7:169-87. [DOI] [PubMed] [Google Scholar]
- 40. Wilby J, Linge K, Reilly T, Troup JD. Spinal shrinkage in females: circadian variation and the effects of circuit weight-training. Ergonomics. 1987;30:47-54. [DOI] [PubMed] [Google Scholar]
- 41. Highland TR, Dreisinger TE. Degenerative disc disease in a collegiate volleyball player. Med Sci Sports Exerc. 1992;24:S163. [Google Scholar]
- 42. Choi G, Raiturker PP, Kim M, Jin CD, Chae Y, Lee S. The effect of early isolated lumbar extension exercise program for patients with herniated disc undergoing lumbar discectomy. Neurosurgery. 2005;57:764-72. [DOI] [PubMed] [Google Scholar]
- 43. Mooney V, Matheson L, Holmes D, Leggett S, Grant J, Negri S, et al. Effect of focused strength training after low back injury. Paper presented at: North American Spine Society Annual Meeting, 1993, San Diego, CA. [Google Scholar]
- 44. Mooney V, Kron M, Rummerfield P, Holmes B. The effect of workplace based strengthening on low back injury rates: a case study in the strip mining industry. J Occup Rehabil. 1995;5:157-67. [DOI] [PubMed] [Google Scholar]
- 45. Matheson L, Mooney V. Employment screening and functional capacity evaluation to determine safe return to work. In: Liebenson C, editor. Rehabilitation of the spine: a practitioner’s manual. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. p. 276-292. [Google Scholar]
- 46. Dreisinger TE. Does prevention work. In: San Diego Comprehensive Care Symposium, 2000, San Diego, CA. [Google Scholar]
- 47. Paajanen H, Lehto I, Alanen A, Erkintalo M, Komu M. Diurnal fluid changes of lumbar discs measured indirectly by magnetic resonance imaging. J Orthop Res. 1994;12:509-14. [DOI] [PubMed] [Google Scholar]
- 48. Kourtis D, Magnusson ML, Smith F, Hadhipavlou A, Pope M. Spine height and disc height changes as the effect of hyperextension using stadiometry and MRI. Iowa Orthop J. 2004;24:65-71. [PMC free article] [PubMed] [Google Scholar]
- 49. Steele J, Bruce-Low S, Smith D, Jessop D, Osborne N. Determining the reliability of a custom built seated stadiometry set-up for measuring spinal height in participants with chronic low back pain. Appl Ergon. 2016;53:203-8. [DOI] [PubMed] [Google Scholar]
- 50. Cohen J. A power primer. Psychol Bull. 1992;112:155-9. [DOI] [PubMed] [Google Scholar]
- 51. Frymoyer J. Back pain and sciatica. N Engl J Med. 1988;318:291-300. [DOI] [PubMed] [Google Scholar]
- 52. Graves JE, Pollock ML, Carpenter DM, Leggett SH, Jones A, MacMillan M, et al. Quantitative assessment of full range of motion isometric lumbar extension strength. Spine. 1990;15:289-94. [DOI] [PubMed] [Google Scholar]
- 53. Robinson ME, Greene AF, O’Connor P, Graves JE, MacMillan M. Reliability of lumbar isometric torque in patients with chronic low back pain. Phys Ther. 1992;72:186-90. [DOI] [PubMed] [Google Scholar]
- 54. Pollock ML, Graves JE, Leggett SH, Young WG, Gazzarella Z, Carpenter DM, et al. Accuracy of counter weighting to account for upper body mass in testing lumbar extension strength. Med Sci Sports Exerc. 1991;23:66. [Google Scholar]
- 55. Inanami H. Iwai Orthopedic Hospital rehabilitation program. Paper presented at: International Spinal Rehabilitation Update 1991 Symposium, 1991, Daytona, FL. [Google Scholar]
- 56. Ogon M, Krismer M, Sollner W, Kantner-Rumplmair W, Lampe A. Chronic low back pain measurement with visual analogue scales in different settings. Pain. 1996;64:425-8. [DOI] [PubMed] [Google Scholar]
- 57. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271-3. [PubMed] [Google Scholar]
- 58. McGill SM, van Wijk MJ, Axler CT, Gletsu M. Studies of spinal shrinkage to evaluate low-back loading in the workplace. Ergonomics. 1996;39:92-102. [DOI] [PubMed] [Google Scholar]
- 59. Stothart JP, McGill SM. Stadiometry: on measurement technique to reduce variability in spine shrinkage measurement. Clin Biomech. 2000;15:546-8. [DOI] [PubMed] [Google Scholar]
- 60. Graves JE, Pollock ML, Foster D, Leggett SH, Carpenter DM, Vuoso R, et al. Effect of training frequency and specificity on isometric lumbar extension strength. Spine. 1990;15:504-9. [DOI] [PubMed] [Google Scholar]
- 61. Bruce-Low S, Smith D, Burnet S, Fisher J, Bissell G, Webster L. One lumbar extension training session per week is sufficient for gains and reductions in pain in patients with chronic low back pain. Ergonomics. 2012;55:500-7. [DOI] [PubMed] [Google Scholar]
- 62. Carpenter DM, Graves JE, Pollock ML, Leggett SH, Foster D, Holmes B, et al. Effect of 12 and 20 weeks of resistance training on lumbar extension torque production. Phys Ther. 1991;71:580-8. [DOI] [PubMed] [Google Scholar]
- 63. Steele J. Intensity; in-ten-si-ty; noun. 1. Often used ambiguously within resistance training. 2. Is it time to drop the term altogether? Br J Sports Med. 2014;48:1586-8. [DOI] [PubMed] [Google Scholar]
- 64. Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33:90-4. [DOI] [PubMed] [Google Scholar]
- 65. Kitano T, Zerwekh JE, Usui Y, Edwards ML, Flicker PL, Mooney V. Biochemical changes associated with the symptomatic human intervertebral disk. Clin Orthop Relat Res. 1993;293:372-7. [PubMed] [Google Scholar]
- 66. Lotz JC, Colliou OK, Chin JR, Duncan NA, Lienbenberg E. Compression-induced degeneration of the 16 intervertebral disc: and in vivo mouse model and finite-element study. Spine. 1998;23:2493-506. [DOI] [PubMed] [Google Scholar]
- 67. Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, et al. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27:2684-90. [DOI] [PubMed] [Google Scholar]
- 68. Fisher J, Steele J, Smith D. Evidence based resistance training recommendations for muscular hypertrophy. Med Sport. 2013;17:217-35. [Google Scholar]
- 69. Fisher J, Steele J, Bruce-Low S, Smith D. Evidence based resistance training recommendations. Med Sport. 2011;15:147-62. [Google Scholar]
- 70. Healey EL, Fowler NE, Burden AM, McEwan IM. The influence of different unloading positions upon stature recovery and paraspinal muscle activity. Clin Biomech. 2004;20:365-71. [DOI] [PubMed] [Google Scholar]
- 71. Owens SC, Brismee J, Penell PN, Dedrick GS, Sizer PS, James CR. Changes in spinal height following sustained lumbar flexion and extension postures: a clinical measure of intervertebral disc hydration using stadiometry. J Manipulative Physiol Ther. 2009;32:358-63. [DOI] [PubMed] [Google Scholar]
- 72. Magnusson ML, Aleksiev AR, Spratt KF, Lakes RS, Pope MH. Hyperextension and spine height changes. Spine. 1996;21:2670-5. [DOI] [PubMed] [Google Scholar]
- 73. Rodacki ALF, Weidle CM, Fowler NE, Rodacki CLN, Persch LN. Changes in stature during and after spinal traction in young male subjects. Rev Bras Fisioter Sao Carlos. 2007;11:63-71. [Google Scholar]
- 74. Van Dieen JH, Toussaint HM. Spinal shrinkage as a parameter of functional load. Spine. 1993;18:1504-14. [PubMed] [Google Scholar]
- 75. Kanlayanaphotporn R, Trott P, Williams M, Fulton I. Effects of chronic low back pain, age and gender on vertical spinal creep. Ergonomics. 2003;46:561-73. [DOI] [PubMed] [Google Scholar]
- 76. Kanlayanaphotporn R, Williams M, Fulton I, Trott P. Reliability of the vertical spinal creep response measured in sitting (asymptomatic and low back-pain subjects). Ergonomics. 2002;45:240-7. [DOI] [PubMed] [Google Scholar]
- 77. Smith D, Bissell G, Bruce-Low S, Wakefield C. The effect of lumbar extension training with and without pelvic stabilization on lumbar strength and low back pain. J Back Musculoskelet Rehabil. 2011;24:1-9. [DOI] [PubMed] [Google Scholar]
- 78. Sivan SS, Hayes AJ, Wachtel E, Caterson B, Merkher Y, Maroudas A, et al. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur Spine J. 2014;23(Suppl 3):S344-53. [DOI] [PubMed] [Google Scholar]
- 79. Sivan SS, Wachtel E, Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim Biophys Acta. 2014;1840:3181-9. [DOI] [PubMed] [Google Scholar]
- 80. Helmhout PH, Harts CC, Viechtbauer W, de Bie RA, Staal JB. Isolated lumbar extensor strengthening versus regular physical therapy in an army working population with nonacute low back pain. Arch Phys Med Rehabil. 2008;89:1675-85. [DOI] [PubMed] [Google Scholar]
- 81. Ferreira ML, Smeets RJ, Kamper SJ, Ferreira PK, Machado LA. Can we explain heterogeneity among randomized clinical trials of exercise for chronic back pain? A meta-regression analysis of randomized controlled trials. Phys Ther. 2010;90:1383-403. [DOI] [PubMed] [Google Scholar]