Abstract
Objective
The purpose of this study was to investigate whether a simple, biologically robust method for inducing calcification of degenerate intervertebral discs (IVD) could be developed to provide an alternative treatment for patients requiring spinal fusion.
Design
Nucleus pulposus (NP) cells isolated from 14 human IVDs were cultured in monolayer and exposed to osteogenic medium, 1,25-dihydroxyvitamin D3 (VitD3), parathyroid hormone (PTH), and bone morphogenic proteins (BMPs) 2/7 to determine if they could become osteogenic. Similarly explant cultures of IVDs from 11 patients were cultured in osteogenic media with and without prior exposure to VitD3 and BMP-2. Osteogenic differentiation was assessed by alkaline phosphatase activity and areas of calcification identified by alizarin red or von Kossa staining. Expression of osteogenic genes during monolayer culture was determined using polymerase chain reaction and explant tissues assessed for BMP inhibitors. Human bone marrow–derived mesenchymal stromal cells (MSCs) were used for comparison.
Results
Standard osteogenic media was optimum for promoting mineralization by human NP cells in monolayer. Some osteogenic differentiation was observed with 10 nM VitD3, but none following application of PTH or BMPs. Regions of calcification were detected in 2 of the eleven IVD tissue explants, one cultured in osteogenic media and one with the addition of VitD3 and BMP-2.
Conclusions
Human NP cells can become osteogenic in monolayer and calcification of the extracellular matrix can also occur, although not consistently. Inhibitory factors within either the cells or the extracellular matrix may hinder osteogenesis, indicating that a robust biological fusion at this time requires further optimization.
Keywords: intervertebral disc, calcification, nucleus pulposus, spinal fusion, in vitro
Introduction
Degeneration of the intervertebral disc (IVD) is a contributory factor to the back pain experienced by some patients1 and although conservative treatment will alleviate the symptoms for many, for some spinal fusion becomes their only option. Spinal fusion has been shown to be effective in approximately two-thirds of patients treated,2,3 and has been referred to as the “gold standard” for treating back pain.4,5 It involves the degenerate IVD being fused to its adjacent vertebrae, thus immobilizing the intervertebral space, reducing movement and alleviating the patient’s pain. The technique is both invasive and expensive and usually requires removal of disc material and replacement with autologous, allogeneic, xenogeneic, or synthetic bone and/or metal “cages” to replace the IVD,6 sometimes in combination with bone morphogenic proteins (BMPs) to stimulate new bone growth. Unfortunately, the procedure can still fail with nonfusion rates of up to 30% reported.7
Normally, the human IVD does not mineralize, but under certain conditions, calcification of the disc can occur, particularly in the thoracic spine of males, with increasing age and where there is loss of disc height.8 Calcification in the disc has long been associated with several pathological conditions such as myositis ossificans progressive, juvenile rheumatoid arthritis, and ankylosing spondylitis9 where spinal fusion occurs first via bony spurs between vertebrae, followed by calcification of the IVD. Calcification, which is reported to occur more commonly in the outer annulus fibrosus (AF) than the central nucleus pulposus (NP),10 has also been reported to occur following trauma; for example, 30% of children with calcification in their cervical discs had experienced previous severe trauma.11 In scoliotic patients, calcification has been identified in discs adjacent to calcified cartilage end-plates12 particularly in regions subjected to altered loading; calcium deposits have also been found in degenerate disc tissue.13
In previous work from our laboratory and others, the presence of local mesenchymal progenitor cells in IVD tissues has been highlighted within both the central NP and the outer AF of degenerate and nondegenerate human discs.14-18 They have shown that these cells can be driven down an osteogenic lineage by culturing in appropriate media. Nosikova et al.19 have also shown that bovine cells isolated from the AF are capable of mineralization while Haschtmann et al.20 induced ossification of the AF in lapine discs with BMP-2 and transforming growth factor–β.
For many years, research has focused on the biological repair or regeneration of the degenerate IVD, but little attention has been paid to facilitating a cell therapy to lead to spinal fusion for immobilizing the spine and reducing pain. The aim of this study was to determine the most successful way of inducing osteogenic differentiation of IVD cells, with the long-term aim of developing a minimally invasive, biological spinal fusion. Hence, we have investigated the potential of known osteogenic-promoting factors, including 1,25 dihydroxyvitamin D3 (VitD3), parathyroid hormone (PTH) and BMPs, on directing degenerate human NP cells, some of which have been shown to have stem cell-like properties,14 down an osteogenic pathway.
Methods
Sample Source
Following ethical approval from Shropshire Research Ethics Committee (04/02/RJH) and Isle of Wight, Portsmouth and South East Hampshire Research Ethics Committee (09/H0501/95) and written informed consent, degenerate human IVD samples (graded II-V on the Pfirrmann MRI score21) were obtained from 25 patients (14 males, 11 females, aged 41.0 ± 10.1 years) undergoing routine surgery for back and leg pain (16 with herniations and 9 with degenerative disc disease [DDD]; Table 1 ). Bone marrow from iliac crest and tibial bone chips was obtained from 10 patients (3 males, 7 females, aged 48.5 ± 13.9 years) undergoing knee arthroplasty to provide mesenchymal stromal cells (MSCs) for positive controls of cells with the capacity to go down an osteogenic lineage.
Table 1.
Patient Demographics.
|
NP |
MSCs |
|||
|---|---|---|---|---|
| No. of samples (male:female) | 25 (14:11) | 10 (3:7) | ||
| Location | No. | Location | No. | |
| Sample source | L2/3: | 1 | Iliac crest: | 8 |
| L3/4: | 1 | |||
| L4/5: | 17 | Tibial bone: | 2 | |
| L5/S1: | 6 | |||
| Disc degeneration grade | II | 1 | ||
| III | 9 | |||
| IV | 11 | |||
| V | 4 | |||
MSC = mesenchymal stromal cells; NP = nucleus pulposus.
Details of patients from whom intervertebral discs and bone marrow–derived MSCs were obtained for this study including a summary of the degenerative grade of the donated discs as assessed via T2-weighted magnetic resonance images and the Pfirrmann et al.21 grading algorithm.
Cell Isolation and Maintenance
Nucleus pulposus was identified macroscopically and dissected from IVDs obtained from 14 patients (aged 37 ± 10 years), minced and digested for 16 hours at 37°C in 250 IU/mg collagenase (Type 2, Worthington Biochemical Corporation, Lakewood, NJ, USA).22 Cells were filtered, washed, and seeded at 5000 cells/cm2 and maintained in monolayer with “standard” culture medium (DMEM/F12 [Life Technologies, Paisley, UK] containing 10% [v/v] fetal bovine serum [FBS; Life Technologies], 0.5% [v/v] ascorbic acid [10 mg/mL solution in phosphate buffered saline, PBS; Sigma-Aldrich, St Louis, MO, USA], 0.5% [v/v] gentamicin [Life Technologies], 1% [v/v] penicillin and streptomycin [Life Technologies] and 0.05% [v/v] fungizone [Life Technologies]) at 5% CO2 in a humidified atmosphere. MSCs were also cultured in monolayer as previously described.14 All cells were passaged as standard by trypsinization on reaching 80% confluence.
Osteogenic Differentiation in Monolayer (2-Dimensional Culture)
Monolayer cultures of NP cells (at passages 1-4) and 100% confluency were treated with factors to induce osteogenic differentiation. For each experiment, cells from 3 different patients were used, and cells were seeded in triplicate in either 24- or 96-well plates for each patient and condition. All factors were compared with standard osteogenic medium, which contained 100 nM dexamethasone (Sigma Aldrich), 50 μM l-ascorbic acid-2-phosphate (Sigma Aldrich), and 10 mM β-glycerophosphate (Sigma Aldrich).23 Medium was replaced every 2 to 3 days for up to 21 days. We established both the effect of the individual components (and different combinations) of the osteogenic medium and also the following purported osteogenic inducers at concentrations previously found to promote osteogenesis in in vitro studies: (a) VitD3 (Sigma Aldrich) at 0.1, 1, or 10 nM24-26; (b) the 1-34 fragment of human PTH (Sigma Aldrich) at 1, 10, or 100 nM, either continuously or intermittently for 1 hour every 48 hours27-29 and (c) BMPs 2, 7, and 2/7 heterodimer (R&D systems, Minneapolis, MN, USA) at 10 or 100 ng/mL.30,31
Osteogenic Differentiation In Situ (3-Dimensional Culture)
Disc tissue, with the macroscopic appearance of NP, from 11 patients aged 44 ± 12 years was dissected into similar sized pieces (approximately 5 mm × 5 mm) and placed inside dialysis tubing (molecular weight cut off of 3.5kDa) and maintained in explant culture in standard or osteogenic culture medium for a minimum of 21 days, whereby swelling of the tissue was controlled by the inclusion of 10% polyethylene glycol (PEG, molecular weight 20kDa).32 Three samples were cultured for 8 weeks and maintained in osteogenic medium plus 50 ng/mL BMP-2 with each change of medium having an initial 15-minute transient treatment with 10 nM VitD3 to activate vitamin D receptors, as previously described by Chen et al.33
Assessment of Osteogenic Differentiation
In cultured cells, alkaline phosphatase activity was determined either histologically or via a colorimetric assay.34 For histological staining, cells were fixed for 10 minutes with 10% formalin, washed with PBS (Life Technologies) then incubated for 1 hour at room temperature (RT) with the stain solution: 25 mg naphthol AS-BI phosphate in 0.5 mL dimethylformamide and 50 mg Fast Red TR in 50 mL Tris-HCl buffer (pH 9; all Sigma Aldrich). Cells were washed with PBS and the percentage of cells stained were counted and the staining intensity semi-quantitatively assessed with scores ranging from 1 to 6, representing light to strong staining intensity. (All 3 wells for 1 patient’s cells per condition were viewed in their totality and ascribed an average score for both the number of cells [as a percentage of the total cells] and intensity in their staining.) Where appropriate, alkaline phosphatase activity was also determined via colorimetric assay in a 96-well format. For this, cells were lysed with 0.1% Triton X in PBS and 50 μL of lysate incubated for 1 hour at 37°C with 0.7% (v/v) 0.5 M MgCl2 and 1.24% (w/v) p-nitrophenol phosphate in 50 mM sodium carbonate and 50 mM Tris buffer (pH 9.5). Color change was read at 405 nm and background absorbance corrected with readings at 650 nm.
Three-dimensional explant cultures were assessed for calcification via von Kossa and alizarin red staining. Explants were snap-frozen in liquid nitrogen–cooled hexane and 7-μm thick cryosections were collected onto poly-l-lysine–coated slides and stored at −20°C. Tissue sections were fixed in 10% formalin for 10 minutes at RT then either alizarin red solution or 5% solution of silver nitrate (von Kossa) was added with these latter sections needing ultraviolet light to develop the stain. Adjacent sections were stained with Mayer’s hematoxylin and eosin.
Immunohistochemical Staining for BMP Inhibitors
Cryosections of explant cultures were also assessed for the presence of BMP inhibitors via immunohistochemistry (IHC). Slides were brought to RT and fixed in 4% paraformaldehyde for 15 minutes. All immunostaining steps were carried out at RT and PBS used for each wash unless stated otherwise. Sections were blocked for 1 hour with 10% normal horse serum, and then the following rabbit polyclonal antibodies applied overnight at 4°C: chordin (K-25, sc-130720, 2 µg/mL); gremlin (FL-184, sc-28873, 4 µg/ml); noggin (FL-232, sc-25656, 1 µg/Ml) all from Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA. Negative control sections were incubated with either (a) rabbit normal serum or rabbit IgG (sc-2027, Santa Cruz) at the appropriate concentration or (b) PBS. Sections were then incubated with secondary biotinylated goat anti-rabbit IgG (Vectastain Elite ABC, Vector Laboratories Ltd, Peterborough, UK) for 30 minutes before blocking for endogenous peroxidase activity with 0.3% hydrogen peroxide in methanol for 30 minutes. Immunolabeling was visualized using the streptavidin-biotin detection system with diaminobenzidine tetrachloride (Sigma Aldrich) as substrate. Sections were washed with distilled water and dehydrated using serial isopropyl alcohol solutions (70%-100%) before being cleared in xylene and mounted in Pertex (CellPath, Newtown, UK).
Osteogenic Gene Expression
To determine individual osteogenic genes, which could be altered following treatment of monolayer cells for osteogenic differentiation, RNA was extracted using the RNeasy Mini Kit (Qiagen, Manchester, UK). cDNA was synthesized from extracted RNA using the RT2 First strand kit (Qiagen) and RT-Q PCR performed using an ABI 7500 RT-PCR system (Applied Biosystems) for Runt-related transcription factor 2 (RUNX2), osteocalcin, and osteopontin with GAPDH as the reference gene (Qiagen). Fold changes greater than ±2 were noted and the reference gene expression checked to ensure it was constant throughout.
Statistical Analyses
Statistical analyses were performed using Microscoft Excel and Analyse-It (Analyse-it Software Ltd, Leeds, UK). Distribution was determined using the Anderson-Darling test for normality and data sets compared using Student t- test or 1-way analysis of variance with a Bonferroni post hoc test. Data are presented as mean ± standard deviation and significance set at P ≤ 0.05.
Results
Optimal Media Conditions for Osteogenic Differentiation of NP Cells
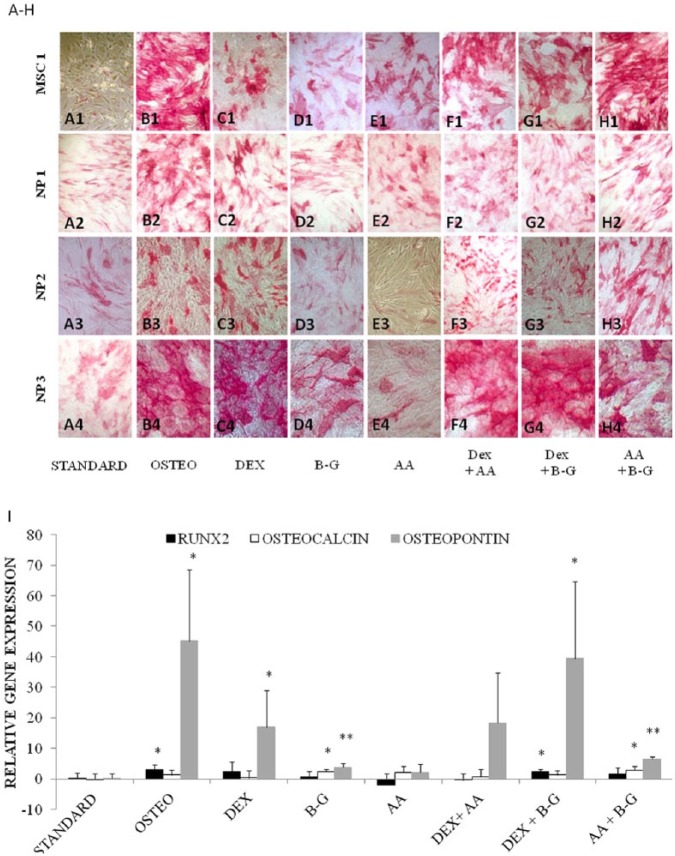
Positive staining for alkaline phosphatase activity was seen in all monolayer NP cells treated with osteogenic medium for 21 days, although the extent of staining varied considerably, between patients, both in number (from 10% to 80% of cells) and intensity ( Figs. 1B2-B4 , 2B2-B4 , and 2H1-H3 ). The effect of each component of the osteogenic medium (dexamethasone, β-glycerophosphate, and l-ascorbic acid-2-phosphate) was also assessed either singularly ( Fig. 1C1-C4 , 1D1-D4 , and 1E1-E4 , respectively) or in different combinations ( Fig. 1F1-F4 , 1G1-G4 , and 1H1-H4 ). Maximal alkaline phosphatase activity was observed in cells treated with complete osteogenic media, with 57% ± 23% of cells having strong positive staining. In particular, dexamethasone and β-glycerophosphate, either individually or in combination increased alkaline phosphatase activity. No relationship between degenerative grade and intensity and percentage cells stained for alkaline phosphatase was observed in this study. As expected, MSCs treated with osteogenic induction medium ( Fig. 1B1 ) stained positively for alkaline phosphatase (75% ± 7%) though surprisingly, in NP cells maintained in standard culture medium, a small amount of alkaline phosphatase staining was also observed ( Fig. 1A2-A4 ). PCR analysis demonstrated an increase in osteopontin expression, with cells cultured in osteogenic medium to be greatest (45-fold; Fig. 1I ) compared with cells maintained in standard culture medium (P < 0.01), followed by dexamethasone either in combination with β-glycerophosphate (39-fold, P < 0.05), or on its own (17-fold, P < 0.05). Osteopontin expression also increased in response to β-glycerophosphate in combination with l-ascorbic acid-2-phosphate (6.5-fold, P < 0.0005) and on its own (3.9-fold, P < 0.005). At 21 days, 2-to 3-fold significant increase in RUNX2 expression was observed when NP cells were cultured with osteogenic or dexamethasone in combination with β-glycerophosphate and also in osteocalcin expression when cultured with β-glycerophosphate alone or in combination with l-ascorbic acid-2-phosphate (all P < 0.05). In case any change in RUNX2 expression had been missed by 21 days, the experiment was repeated with an earlier endpoint of 7 days. A 5-fold increase in RUNX2 expression confirmed our suspicions that this transcription factor is expressed earlier than 21 days in response to osteogenic medium.
Figure 1.
(A-H) Alkaline phosphatase staining of cultured mesenchymal stromal cells (MSCs) and human nucleus pulposus (NP) cells maintained in monolayer for 21 days with osteogenic medium (osteo) (B1-B4) and its components, dexamethasone (DEX) (C1-C4), β-glycerophosphate (B-G) (D1-D4), l-ascorbic acid-2-phosphate (AA) (E1-E4) and in combination (F1-H4), in comparison with the control of “standard” medium (A1-A4). (I) Expression of osteocalcin, osteopontin, and RUNX2 genes (relative to GAPDH) for each corresponding culture condition at 21 days. Results show the mean values of NP cell cultures from 3 different patients and the bar represents the standard deviation. Significant differences are denoted by *P < 0.05 and **P < 0.005.
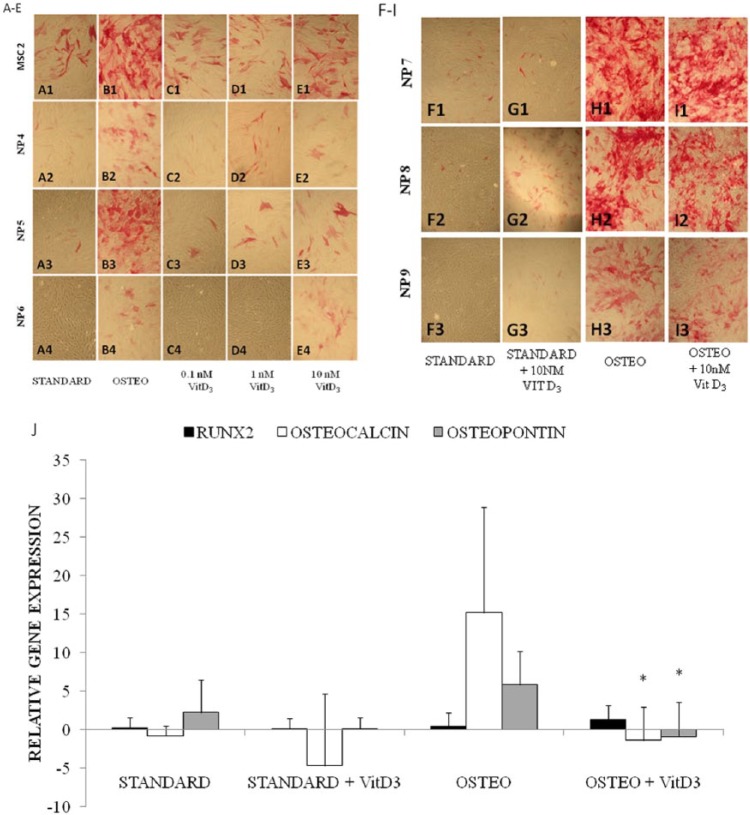
Figure 2.
(A-E) Alkaline phosphatase staining of monolayer cultured human nucleus pulposus (NP) cells from 3 patients and one population of mesenchymal stromal cells (MSCs) maintained with osteogenic medium (B1-B4) and 3 doses of VitD3 (0.1, 1, and 10 nM; C1-4, D1-4, and E1-4, respectively) in comparison to standard medium (A1-A4) and (F-I) cultured human NP cells from 3 patients exposed to standard and osteogenic medium ± 10 nM VitD3, all at 21 days. (J) Expression of osteocalcin, osteopontin, and RUNX2 genes (relative to GAPDH) for each culture condition in (F-I). Results show the mean values of NP cells cultured from n = 3 patients with bars representing the standard deviation. Significant differences between osteogenic media with and without VitD3 are denoted by *P < 0.01.
Vit D3 Induces Osteogenic Differentiation
Little or no osteogenic differentiation was seen at 21 days in cells treated with 0.1 nM VitD3 for 21 days ( Fig. 2C2-C4 ) with faint alkaline phosphatase staining being present in only approximately 4% of cells. With 1 or 10 nM VitD3, however, some alkaline phosphatase staining was seen in both the NP cells (10%) and MSCs (25%-40%; Fig. 2D1-D4 and 2E1-E4 ). Cells treated with complete osteogenic medium were run as a positive control and showed the greatest staining (~45% in NP cells and 80% in MSCs) for alkaline phosphatase ( Fig. 2B1-B4 ). When 10 nM VitD3 was added to standard or osteogenic media it did not augment the alkaline phosphatase activity ( Fig. 2G1-G3 and I1-I3 , respectively). Interestingly, PCR analysis detected significantly decreased osteocalcin and osteopontin expression in NP cells maintained in osteogenic medium with 10 nM VitD3 compared to osteogenic medium without VitD3 ( Fig. 2J ).
Lack of Osteogenic Differentiation Following Application of pTH or BMPs
Neither continuous nor intermittent doses of PTH over the concentration range of 1 to 100 nM increased alkaline phosphatase (assessed both via histological staining or activity assay) in the NP cells or MSCs (data not shown). In addition, there was no observed increase in alkaline phosphatase as assessed by activity assay in NP cells or MSCs treated with BMP-2, -7, or -2/7 heterodimer at concentrations between 10 and 100 ng/mL (data not shown). In fact the application of BMPs, in particular BMP-2/7, seemed to reduce alkaline phosphatase activity in human NP cells compared with those maintained in standard culture.
Three-Dimensional Culture of NP Tissue and Presence of BMP Inhibitors
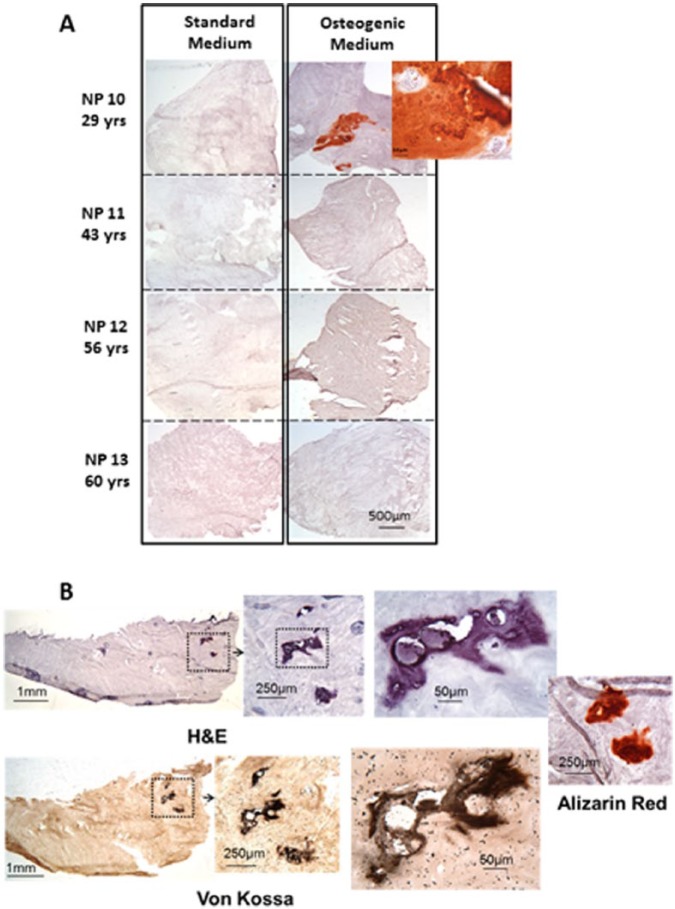
Calcification was identified in two of the herniated human NP tissues maintained in explant culture ( Fig. 3A and B ). Calcification was detected via alizarin red staining in disc tissue isolated from a 29-year-old female (L4/5) and maintained in osteogenic media for 21 days and also in disc tissue from a 44-year-old male (L5/S1) maintained in osteogenic media with the addition of VitD3 followed by BMP2 for 8 weeks. All other discs grown as explant cultures showed no induction of mineralization regardless of culture conditions or time in culture. As in monolayer, degenerate grade did not appear to be associated with calcification of the explant tissues.
Figure 3.
(A) Four representative disc explants cultured in standard and osteogenic culture medium for 21 days and stained with alizarin red. Only the explant from the youngest patient (NP10) showed calcification. (B) Explant cultures of disc from a 44 year old male cultured in Vit D3 for 15 minutes prior to bone morphogenic protein (BMP) osteogenic medium for 8 weeks, showing ossification in sections stained with hematoxylin and eosin, alizarin red, and von Kossa.
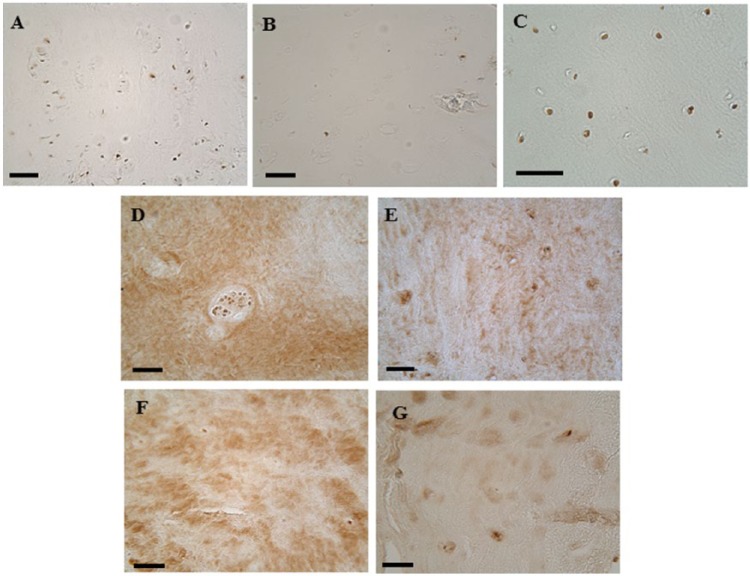
All 5 of the IVD explants assessed for the presence of the BMP inhibiting molecules chordin, gremlin, and noggin within the cells and extracellular matrix were found to be positive for gremlin ( Fig. 4D-G ), but the number of cells and intensity of staining varied from few and pale to numerous and dark. Cells were positive for chordin in 3 of 5 samples while noggin was detected in 4 of the samples. Neither chordin nor noggin were clearly detected in any the extracellular matrix of the explants. No clear relationship between the presence of these BMP inhibitors and calcification of the extracellular matrix was observed.
Figure 4.
Immunohistochemical of staining for the bone morphogenic protein (BMP) inhibitors chordin, gremlin, and noggin. Human osteoarthritic cartilage was used as a positive control for chordin and gremlin (A and B, respectively) and bovine cartilage for noggin (C). Images D-G show positive gremlin staining in human degenerative explant tissues cultured in standard medium (D), osteogenic medium (E and F) and osteogenic with VitD3 and BMP2 (G). (D and E) and (F and G) are from the same intervertebral disc (IVD) explants with the sample in (E) being positive for calcification (NP10 from Fig. 3A ). Scale bar = 50 micron.
Discussion
We have shown that human degenerate IVD cells are capable of becoming osteogenic in both monolayer culture and in situ within NP tissue. In monolayer culture, cells from human degenerate NPs, maintained in osteogenic media, were consistently capable of osteogenic differentiation. Some NP cells stained positive for alkaline phosphatase even under standard conditions, indicating a “natural” predilection for, or tendency to becoming osteogenic. This may reflect the presence of progenitor cells that have previously been identified in disc tissue.14-17,35 We have shown that 11% to 20% of cells within degenerate discs have positive staining for the putative stem cell markers, Notch-1 and cytokeratins 8 and 19, although there is a huge range between individual patients’ discs (e.g., from 0% to 96% for Notch-1).14 Recent work by Tekari et al.36 identified approximately 9% of bovine NP cells as being positive for the angiopoietin-1 receptor (Tie2). Cells positive for Tie2 have been shown to have progenitor-like multipotency and in human discs their incidence decreases with both age and degeneration.35
Cells express different markers at different stages of osteogenesis. RUNX2 is one of the initial markers prior to alkaline phosphatase production, followed by osteopontin and then osteocalcin.37 RUNX2 is a key transcription factor essential for osteoblastic differentiation and is usually expressed early by committed osteoprogenitor cells or immature osteoblasts. The expression of RUNX2 in our experiments was found to be increased at 7 days but by 21 days this upregulation had ceased, typical of its early production in osteogenesis.19 The later markers, osteocalcin and osteopontin, are both secreted by mature osteoblasts, but osteopontin is also produced in other tissues. Both these molecules are involved in the regulation of hydroxyapatite formation with osteopontin, in particular, having a role in inhibiting crystal growth.38 At 21 days, osteocalcin was significantly upregulated (up to 3-fold) when β-glycerophosphate, either alone or with l-ascorbic acid-2-phosphate, was added to the monolayer cultures. Osteopontin was significantly expressed at high levels during culture with osteogenic media and all culture conditions with dexamethasone except when VitD3 was added.
Interestingly, factors which are commonly used as osteogenic agents in the clinic (VitD3, PTH, and BMPs) had less effect on disc cells and MSCs than osteogenic culture medium traditionally used in in vitro systems. In vivo, the active form of vitamin D, VitD3, maintains the homeostasis of calcium and phosphate ions and reduces osteoporotic risk.39 In osteoblasts, 2 receptors, 1 nuclear (vitamin D receptor [VDR])40 and 1 in the plasma membrane (protein disulfide isomerase family A member 3 [Pdia3]),41,42 have been identified for VitD3. We found that the lower concentration (0.1 nM) of VitD3 did not stimulate upregulation of alkaline phosphatase in human degenerate disc cells, but at higher concentrations, such as 1 or 10 nM, alkaline phosphatase activity was increased. A similar response to 10 nM VitD3 has been observed in other studies using MSCs obtained from vertebral bodies whereby this concentration promoted osteogenesis.24,25 At a concentration of 10 nM, VitD3 increased alkaline phosphatase staining in NP cells and MSCs, but did not augment the effect of osteogenic medium, perhaps due to dexamethasone interfering, as has been shown to occur in human osteoblasts.43 In contrast, in response to the addition of VitD3 to osteogenic medium, both osteocalcin and osteopontin expression were significantly decreased in NP cells (compared with osteogenic medium alone) and yet alkaline phosphatase staining remained the same.
In vivo, PTH acts to increase the presence of calcium ions by binding to osteoblasts and increasing RANKL expression but inhibiting osteoprotegerin (OPG) production. RANKL is then free to bind to RANK instead of OPG, thus stimulating osteoclast precursors to fuse and form new osteoclasts which break down bone and release calcium ions from the bone matrix. However, the presence of both intermittent28 and continuous27 application of 1-45 PTH has been shown to induce alkaline phosphatase in rodent MSCs, although Yang et al.27 found that intermittent application reduced alkaline phosphatase levels in their rat MSCs to lower than controls and Fujita et al.44 found continuous PTH decreased alkaline phosphatase in mice osteoblasts. Neither intermittent nor continuous application of PTH in this study was found to affect the presence of alkaline phosphatase in human degenerate NP cells. Disc cells may behave differently from MSCs, since Madiraju et al.45 applied PTH to degenerate discs cells and found that it reduced alkaline phosphatase activity in human NP cells (but not AF cells).
BMP-2 and -7 have long been known to be involved in inducing bone and cartilage formation and in osteoblast differentiation. They have also been reported to have an anabolic effect on IVD cells and tissues, by increasing proteoglycan and matrix production. Kim et al.46 have shown that human NP cells produce alkaline phosphatase and at a higher level by cells from less degenerate discs than more degenerate ones. Injections of BMP-7 into canine discs have been shown to stimulate the production of peridiscal bone.47 However, the dose of BMP injected into these discs was more than 1000-fold of that used in the present study where we found no response. It is also known that disc cells are capable of producing inhibitors to osteogenic factors such as BMPs; these include noggin, gremlin, and chordin. Chan et al.48 have shown that human disc cells express the genes for these antagonists and co-culture of disc cells reduced osteogenesis in MSCs. In addition, expression of GREM1 was higher in NP cells than AF cells. The same group have shown reversal of the disc’s inhibitory effect on MSCs with the addition of a BMP2 variant (L51P).49 Immunohistochemical staining for the BMP inhibitors in our study demonstrated that they were present in some disc samples and to varying degrees.
There are conflicting reports in the literature about the propensity of NP cells and tissues to mineralize, both in vitro and in vivo. For example, Nosikova et al.19 actually used bovine NP cells as a negative control in their in vitro study on mineralization of AF cells (and our laboratory has found a similar resistance of bovine NP cells to become osteogenic (unpublished results)). There are many pathological syndromes, however, where ectopic calcification of discs occurs naturally in canines, sheep,50 and humans. The etiology of the dystrophic calcification is not known and indeed the calcification process itself is complex such that there are numerous factors that can influence the many pathways involved. The samples used for explant culture were from both degenerate (n = 5) and herniated discs (n = 6) but only 2 herniated discs demonstrated calcification following culture under osteogenic conditions. These findings concur with others that found herniated discs more prone to calcification than normal discs.51 Other reasons for the variation of human degenerate IVDs to calcify are that perhaps some discs may have a higher number of progenitor cells (and so be more likely to calcify). This may explain the calcification observed in the NP of the 29-year-old in Figure 3A while other samples, from older patients, may have lower numbers of progenitor cells. Alternatively, some discs may have higher levels of molecules that are known to inhibit mineralization, such as gremlin, noggin, and chordin, which have been investigated here. Other factors that are known to influence the likelihood of calcification are pH and levels of phosphate, calcium ions, alkaline phosphatase activity, and pro-inflammatory cytokines, but these have not been examined in the current study. Variability between individual patients in all these factors could explain the lack of a clear pattern of calcification in our samples as well, perhaps, as explain why some patients are resistant to BMP-2 treatment for spinal fusion.
Not studying all the factors known to influence calcification may be considered as a limitation of this study. A further limitation of the study is the lack of adequate cells available from the limited surgical waste material and being unable to provide enough at an early passage as was preferred for all the replicate tests performed; hence cells were assessed in monolayer at passages varying between P1 and P4. Although the explant culture model enables maintenance of an environment much more akin to in vivo than monolayer cultures, it is still not perfect. For example, whilst it restricts swelling to some extent, it may not completely mimic the physical restriction nor reflect the limited nutrient supply that the disc is exposed to in vivo.
In conclusion, this work has shown that cells from degenerate human IVDs have the capability to be driven down an osteogenic pathway, such that they can produce calcium and phosphate salts when grown in monolayer and in some disc tissue itself. Unfortunately, this study has not shown that factors commonly used in the clinic for inducing bone formation and mineralization will reliably do this within discs, perhaps due to the complexity of the factors that influence the calcification process. Further developing our understanding of these control points, and being better able to manipulate them, could lead to a relatively simple and minimally invasive approach for fusing the spine in back pain patients in the future.
Footnotes
Acknowledgments and Funding: The authors would like to thank the patients and surgeons (Messrs M. Ockendon, S. Chitgopkar, J. Trivedi, D. Jaffray and S. Ahmed) for provision of the surgical samples, Professor Stephen Eisenstein for helpful discussions and Mrs Annie Kerr for obtaining patient consents. The work was undertaken in the Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust, Oswestry. Grant support was obtained from the Orthopaedic Institute Ltd, Oswestry and the Garfield Weston Foundation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from the Shropshire Research Ethics Committee (04/02/RJH) and Isle of Wight, Portsmouth, and South East Hampshire Research Ethics Committee (09/H0501/95).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
References
- 1. Brinjikji W, Diehn FE, Jarvik JG, Carr CM, Kallmes DF, Murad MH, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36:2394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner JA, Ersek M, Herron L, Haselkorn J, Kent D, Ciol MA, et al. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907-11. [PubMed] [Google Scholar]
- 3. Stromqvist B, Fritzell P, Hagg O, Jonsson B. The Swedish Spine Register: development, design and utility. Eur Spine J. 2009;18(suppl 3):294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fritzell P, Hagg O, Wessberg P, Nordwall A. 2001 Volvo Award Winner in Clinical Studies. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26:2521-32. [DOI] [PubMed] [Google Scholar]
- 5. Carreon LY, Glassman SD, Howard J. Fusion and nonsurgical treatment for symptomatic lumbar degenerative disease: a systematic review of Oswestry Disability Index and MOS Short Form-36 outcomes. Spine J. 2008;8:747-55. [DOI] [PubMed] [Google Scholar]
- 6. Babu MA, Coumans JV, Carter BS, Taylor WR, Kasper EM, Roitberg BZ, et al. A review of lumbar spinal instrumentation: evidence and controversy. J Neurol Neurosurg Psychiatry. 2011;82:948-51. [DOI] [PubMed] [Google Scholar]
- 7. Watkins R, Watkins R, III, Hanna R. Non-union rate with stand-alone lateral lumbar interbody fusion. Medicine (Baltimore). 2014;93:e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chanchairujira K, Chung CB, Kim JY, Papakonstantinou O, Lee MH, Clopton P, et al. Intervertebral disk calcification of the spine in an elderly population: radiographic prevalence, location, and distribution and correlation with spinal degeneration. Radiology. 2004;230:499-503. [DOI] [PubMed] [Google Scholar]
- 9. Dussault RG, Kaye JJ. Intervertebral disk classification associated with spine fusion. Radiology. 1977;125:57-61. [DOI] [PubMed] [Google Scholar]
- 10. Kerns S, Pope TL, Jr, de Lange EE, Fechner RE, Keats TE, Cimmino C. Annulus fibrosus calcification in the cervical spine: radiologic-pathologic correlation. Skeletal Radiol. 1986;15:605-9. [DOI] [PubMed] [Google Scholar]
- 11. Sonnabend DH, Taylor TKF, Chapman GK. Intervertebral disc calcification syndromes in children. J Bone Jt Surg. 1982;64-B:25-31. [DOI] [PubMed] [Google Scholar]
- 12. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11:747-57. [DOI] [PubMed] [Google Scholar]
- 13. Hristova GI, Jarzem P, Ouellet JA, Roughley PJ, Epure LM, Antoniou J, et al. Calcification in human intervertebral disc degeneration and scoliosis. J Orthop Res. 2011;29:1888-95. [DOI] [PubMed] [Google Scholar]
- 14. Turner S, Balain B, Caterson B, Morgan C, Roberts S. Viability, growth kinetics and stem cell markers of single and clustered cells in human intervertebral discs: implications for regenerative therapies. Eur Spine J. 2014;23:2462-72. [DOI] [PubMed] [Google Scholar]
- 15. Henriksson H, Thornemo M, Karlsson C, Hagg O, Junevik K, Lindahl A, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976). 2009;34:2278-87. [DOI] [PubMed] [Google Scholar]
- 16. Feng G, Yang X, Shang H, Marks IW, Shen FH, Katz A, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92:675-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brisby H, Papadimitriou N, Brantsing C, Bergh P, Lindahl A, Barreto HH. The presence of local mesenchymal progenitor cells in human degenerated intervertebral discs and possibilities to influence these in vitro: a descriptive study in humans. Stem Cells Dev. 2013;22:804-14. [DOI] [PubMed] [Google Scholar]
- 18. Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 Annual ORS meeting. J Orthop Res. 2015;33:283-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nosikova Y, Santerre JP, Grynpas MD, Kandel RA. Annulus fibrosus cells can induce mineralization: an in vitro study. Spine J. 2013;13:443-53. [DOI] [PubMed] [Google Scholar]
- 20. Haschtmann D, Ferguson SJ, Stoyanov JV. BMP-2 and TGF-β3 do not prevent spontaneous degeneration in rabbit disc explants but induce ossification of the annulus fibrosus. Eur Spine J. 2012;21:1724-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873-8. [DOI] [PubMed] [Google Scholar]
- 22. Garcia J, Mennan C, McCarthy HS, Roberts S, Richardson JB, Wright KT. Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow, infrapatellar fat pad, and subcutaneous fat. Stem Cells Int. 2016;2016:6969726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295-312. [PubMed] [Google Scholar]
- 24. D’Ippolito G, Schiller PC, Perez-stable C, Balkan W, Roos BA, Howard GA. Cooperative actions of hepatocyte growth factor and 1,25-dihydroxyvitamin D3 in osteoblastic differentiation of human vertebral bone marrow stromal cells. Bone. 2002;31:269-75. [DOI] [PubMed] [Google Scholar]
- 25. Chen K, Aenlle KK, Curtis KM, Roos BA, Howard GA. Hepatocyte growth factor (HGF) and 1,25-dihydroxyvitamin D together stimulate human bone marrow-derived stem cells toward the osteogenic phenotype by HGF-induced up-regulation of VDR. Bone. 2012;51:69-77. [DOI] [PubMed] [Google Scholar]
- 26. De Kok IJ, Hicok KC, Padilla RJ, Young RG, Cooper LF. Effect of vitamin D pretreatment of human mesenchymal stem cells on ectopic bone formation. J Oral Implantol. 2006;32:103-9. [DOI] [PubMed] [Google Scholar]
- 27. Yang C, Frei H, Burt HM, Rossi F. Effects of continuous and pulsatile PTH treatments on rat bone marrow stromal cells. Biochem Biophys Res Commun. 2009;380:791-6. [DOI] [PubMed] [Google Scholar]
- 28. Rey A, Manen D, Rizzoli R, Ferrari SL, Caverzasio J. Evidences for a role of p38 MAP kinase in the stimulation of alkaline phosphatase and matrix mineralization induced by parathyroid hormone in osteoblastic cells. Bone. 2007;41:59-67. [DOI] [PubMed] [Google Scholar]
- 29. Rickard DJ, Wang FL, Rodriguez-Rojas AM, Wu Z, Trice WJ, Hoffman SJ, et al. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39:1361-72. [DOI] [PubMed] [Google Scholar]
- 30. Laflamme C, Rouabhia M. Effect of BMP-2 and BMP-7 homodimers and a mixture of BMP-2/BMP-7 homodimers on osteoblast adhesion and growth following culture on a collagen scaffold. Biomed Mater. 2008;3:015008. [DOI] [PubMed] [Google Scholar]
- 31. Song I, Kim BS, Kim CS, Im GI. Effects of BMP-2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochem Biophys Res Commun. 2011;408:126-31. [DOI] [PubMed] [Google Scholar]
- 32. Bayliss MT, Urban JPG, Johnstone B, Holm S. In vitro method for measuring synthesis rates in the intervertebral disc. J Orthop Res. 1986;4:10-7. [DOI] [PubMed] [Google Scholar]
- 33. Chen J, Dosier CR, Park JH, De S, Guldberg RE, Boyan BD, et al. Mineralization of three-dimensional osteoblast cultures is enhanced by the interaction of 1a,25-dihydroxyvitamin D3 and BMP2 via two specific vitamin D receptors. J Tissue Eng Regen Med. 2016;10:40-51. doi: 10.1002/term.1770. [DOI] [PubMed] [Google Scholar]
- 34. McCarthy HS. The Regulation of Osteoprotegerin and Dickkopf-1 in Osteoblastic Cells [dissertation]. Chester, UK: University of Chester; 2012. [Google Scholar]
- 35. Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tekari A, Chan SC, Sakai D, Grad S, Gantenbein B. Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res Ther. 2016;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wagner ER, Luther G, Zhu G, Luo Q, Shi Q, Kim SH, et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma. 2011;2011:325238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010-8. [DOI] [PubMed] [Google Scholar]
- 40. Walters MR, Rosen DM, Norman AW, Luben RA. 1,25-Dihydroxyvitamin D receptors in an established bone cell line. Correlation with biochemical responses. J Biol Chem. 1982;257:7481-4. [PubMed] [Google Scholar]
- 41. Boyan BD, Bonewald LF, Sylvia VL, Nemere I, Larsson D, Norman AW, et al. Evidence for distinct membrane receptors for 1 α,25-(OH)2D3 and 24R,25-(OH)2D3 in osteoblasts. Steroids. 2002;67:235-46. [DOI] [PubMed] [Google Scholar]
- 42. Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem. 2010;285:37041-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jorgensen NR, Henriksen Z, Sorensen OH, Civitelli R. Dexamethasone, BMP-2, and 1,25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids. 2004;69:219-26. [DOI] [PubMed] [Google Scholar]
- 44. Fujita T, Fukuyama R, Izumo N, Hirai T, Meguro T, Nakamuta H, et al. Transactivation of core binding factor alpha1 as a basic mechanism to trigger parathyroid hormone-induced osteogenesis. Jpn J Pharmacol. 2001;86:405-16. [DOI] [PubMed] [Google Scholar]
- 45. Madiraju P, Gawri R, Wang H, Antoniou J, Mwale F. Mechanism of parathyroid hormone-mediated suppression of calcification markers in human intervertebral disc cells. Eur Cell Mater. 2013;25:268-83. [DOI] [PubMed] [Google Scholar]
- 46. Kim SH, Kuh SU, Kim KN, Park JY, Cho KH, Chin DK, et al. Biologic response of degenerative living human nucleus pulposus cells to treatment with cytokines. Yonsei Med J. 2015;56:277-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Willems N, Bach FC, Plomp SG, van Rijen MH, Wolfswinkel J, Grinwis GC, et al. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res Ther. 2015;17:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chan SC, Tekari A, Benneker LM, Heini PF, Gantenbein B. Osteogenic differentiation of bone marrow stromal cells is hindered by the presence of intervertebral disc cells. Arthritis Res Ther. 2015;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tekari A, May RD, Frauchiger DA, Chan SC, Benneker LM, Gantenbein B. The BMP2 variant L51P restores the osteogenic differentiation of human mesenchymal stromal cells in the presence of intervertebral disc cells. Eur Cell Mater. 2017;33:197-210. [DOI] [PubMed] [Google Scholar]
- 50. Melrose J, Burkhardt D, Taylor TK, Dillon CT, Read R, Cake M, et al. Calcification in the ovine intervertebral disc: a model of hydroxyapatite deposition disease. Eur Spine J. 2009;18:479-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karamouzian S, Eskandary H, Faramarzee M, Saba M, Safizade H, Ghadipasha M, et al. Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine. 2010;35:881-6. [DOI] [PubMed] [Google Scholar]