Abstract
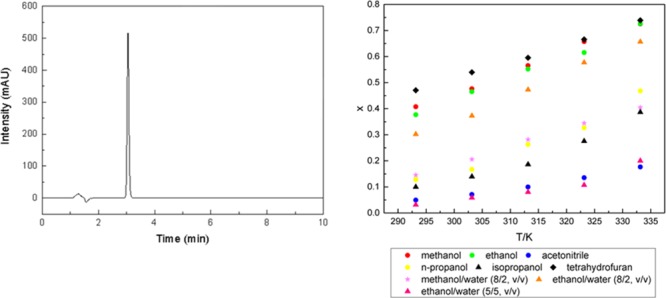
A precise determination method of azilsartan solubility between 293.15 and 333.15 K in several ordinary solvents and some of their aqueous mixtures was established by high-performance liquid chromatography. In all tested solvents, its solubility shows exponential growth with the increase in temperature. This trend is especially pronounced in methanol and ethanol. The order of solubility of azilsartan can be expressed as ethanol > tetrahydrofuran > ethanol/water (8/2, v/v) > methanol > methanol/water (8/2, v/v) > n-propanol > isopropanol > ethanol/Water (5/5, v/v) > acetonitrile. The solubility data of azilsartan were well correlated by the λh model. Moreover, the thermodynamic data including the dissolving enthalpy, entropy, and Gibbs free energy of azilsartan in each solvent were calculated which is crucial to its preparation technology study.
1. Introduction
Azilsartan (2-ethoxy-1-([2′-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl)-1H-benzimidazole-7-carboxylic acid) (Figure 1), as a hypotensive drug, is a novel angiotensin II receptor antagonist that competitively blocks the binding of angiotensin to the AT1 receptor.1,2 It was first synthesized in 1995 by Kohara and approved for sale in Japan in 2012.3 Because of its high efficiency and less adverse effects such as dry cough compared to the similar products, azilsartan is widely applied clinically4−7 in recent years. It is reported that there are four kinds of crystalline forms of this chemical,8−10 and the crystal form, size distribution, and polymorphism could influence its pharmaceutical quality and effectiveness.11 Despite sufficient studies on its polymorph,12 the solubility information of this drug has been ignored. Indeed, multiple solvents were applied in its crystallization and recrystallization process such as isopropanol, dimethylformamide, methanol, and acetonitrile. In addition, ethanol was used as the washing solvent in the synthesis process of azilsartan to achieve the desired purity.13,14
Figure 1.
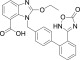
Chemical structure of azilsartan.
It is known that the choice of the solvent and its dissolving capacity have a huge impact on the drug manufacturing process efficiency.15 Technically, the solubility information of drugs is essential in all steps of drug discovery and development processes such as crystallization, separation, liquid extraction, and drug formulation.
In this study, the solubility of azilsartan in methanol, ethanol, acetonitrile, n-propanol, isopropanol, tetrahydrofuran, methanol/water (8/2, v/v), ethanol/water (8/2, v/v), and ethanol/water (5/5, v/v) was obtained based on the chromatographic method. The thermodynamic parameters, such as dissolved enthalpy, were calculated according to the van’t Hoff equation.
2. Results and Discussion
2.1. High-Performance Liquid Chromatography
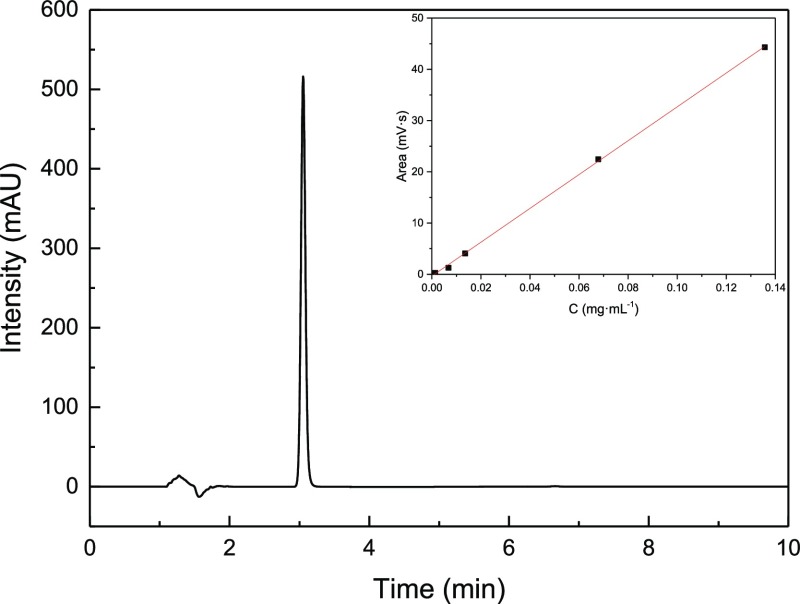
The purity of azilsartan was 99.84% determined by high-performance liquid chromatography (HPLC) with the optimized condition (Figure 2).
Figure 2.
HPLC chromatogram of azilsartan. Inset: linear relationship between the chromatogram peak area (Y) and the concentration (X) of azilsartan in methanol.
A calibration curve for the concentration of azilsartan was prepared by using a standard solution which was prepared from 1.357 × 10–3 to 1.357 × 10–1 mg·mL–1 in methanol. All experiments were repeated three times at each temperature. The peak area was considered the average measured by HPLC. The calibration curve was obtained based on the chromatogram (Figure 2).
The linear equation between the mass concentration and the peak area of azilsartan sample solution is Y = 330.2X – 0.3387, while correlation coefficient R2 = 0.9998. The linearity range is from 4.1 × 10–4 to 1.4 × 10–1 mg·mL–1.
2.2. Correlation Data of Azilsartan
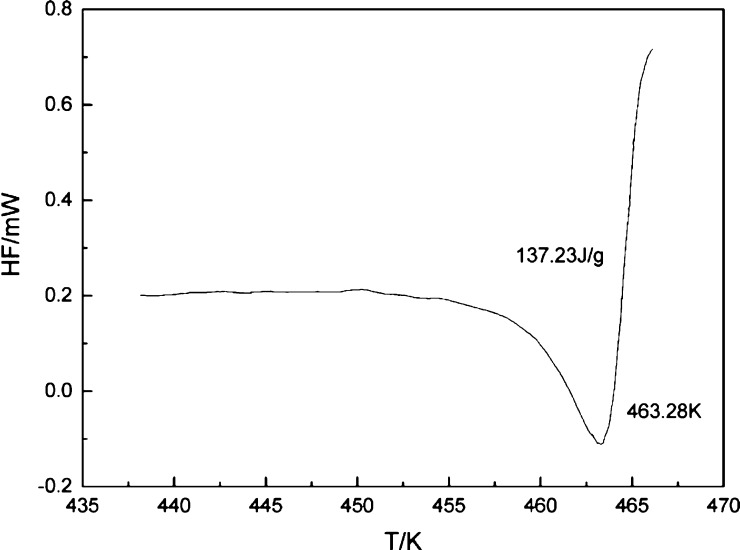
The melting point of azilsartan was obtained by the differential scanning calorimetry (DSC) study shown in Figure 3. The abscissa and ordinate of the graph is the absolute temperature and the heat flow, respectively. It can be observed that the melting point of this solute is 463.28 K, and the melting enthalpy is 137.23 J/g.
Figure 3.
The DSC curve of azilsartan.
The solubility of azilsartan in the calibration curve is expressed by the mass concentration detected by HPLC. The mole fraction of the solute can be calculated by eq 1.
| 1 |
wherein xA is the mole fraction of the solute azilsartan; m0 is the mass of the solution; mA is the mass of the solute; MA is the molar mass of the solute azilsartan; and MB is the molar mass of the solvent. At 95% confidence level, the experimental measurement uncertainty is approximately 5.0%.
The solid–liquid equilibrium was described by the λh model which is one of the thermodynamic models proposed by Buchowski et al.,16 presented as eq 2.
| 2 |
Here, x is the mole fraction of the solute; T is the experimental temperature corresponding to x; Tm is the melting point of the solute; λ and h are the parameters of the equation.
In order to verify the uncertainty of the data, the relative deviation (δ) was introduced according to eq 3. Moreover, the deviation between xAcalc and xA was estimated by mean deviation (MD), measuring the correlation degree of the mathematical model.
| 3 |
where xA is the mole fraction of solute azilsartan; xAcalc can be calculated from eq 2.
| 4 |
here N is the number of experimental data; xA and xAcalc are the experimental and calculated values of solubility, respectively.
The solubility data of azilsartan in methanol, ethanol, acetonitrile, n-propanol, isopropanol, tetrahydrofuran, and mixed solvents in the temperature range from 293.15 to 333.15 K are listed in Table 1 and graphically presented in Figure 4.
Table 1. Solubility of Azilsartan as a Function of Temperature in Different Solvents under 0.1 MPaa.
| solvent | T/K | xA | xAcalc | 100δ |
|---|---|---|---|---|
| methanol | 293.15 | 0.4074 | 0.3963 | 2.73 |
| 303.15 | 0.4761 | 0.4871 | –2.32 | |
| 313.15 | 0.5655 | 0.5739 | –1.48 | |
| 323.15 | 0.6575 | 0.6523 | 0.79 | |
| 333.15 | 0.7251 | 0.7203 | 0.66 | |
| ethanol | 293.15 | 0.3771 | 0.3721 | 1.34 |
| 303.15 | 0.465 | 0.4644 | 0.12 | |
| 313.15 | 0.552 | 0.5540 | –0.37 | |
| 323.15 | 0.6153 | 0.6360 | –3.37 | |
| 333.15 | 0.7268 | 0.7076 | 2.65 | |
| acetonitrile | 293.15 | 0.0493 | 0.05345 | –8.43 |
| 303.15 | 0.0709 | 0.0760 | –7.21 | |
| 313.15 | 0.0997 | 0.1050 | –5.34 | |
| 323.15 | 0.135 | 0.1412 | –4.59 | |
| 333.15 | 0.1764 | 0.1849 | –4.81 | |
| n-propanol | 293.15 | 0.1285 | 0.1135 | 11.67 |
| 303.15 | 0.167 | 0.1757 | –5.21 | |
| 313.15 | 0.2629 | 0.2559 | 2.64 | |
| 323.15 | 0.3268 | 0.3506 | –7.30 | |
| 333.15 | 0.4683 | 0.4527 | 3.32 | |
| isopropanol | 293.15 | 0.0998 | 0.0858 | 13.99 |
| 303.15 | 0.1394 | 0.1351 | 3.10 | |
| 313.15 | 0.1862 | 0.2011 | –8.03 | |
| 323.15 | 0.2755 | 0.2831 | –2.77 | |
| 333.15 | 0.3868 | 0.3768 | 2.58 | |
| tetrahydrofuran | 293.15 | 0.4706 | 0.4644 | 1.32 |
| 303.15 | 0.5395 | 0.5390 | 0.10 | |
| 313.15 | 0.5956 | 0.6084 | –2.15 | |
| 323.15 | 0.6658 | 0.6711 | –0.79 | |
| 333.15 | 0.7390 | 0.7262 | 1.73 | |
| methanol/water (8/2, v/v) | 293.15 | 0.1454 | 0.1537 | –5.69 |
| 303.15 | 0.2056 | 0.2075 | –0.93 | |
| 313.15 | 0.2819 | 0.2701 | 4.18 | |
| 323.15 | 0.3444 | 0.3395 | 1.41 | |
| 333.15 | 0.4047 | 0.4130 | –2.06 | |
| ethanol/water (8/2, v/v) | 293.15 | 0.3022 | 0.2912 | 3.62 |
| 303.15 | 0.3729 | 0.3836 | –2.88 | |
| 313.15 | 0.4728 | 0.4793 | –1.38 | |
| 323.15 | 0.5772 | 0.5714 | 1.01 | |
| 333.15 | 0.6568 | 0.6547 | 0.32 | |
| ethanol/water (5/5, v/v) | 293.15 | 0.0315 | 0.0273 | 13.28 |
| 303.15 | 0.0586 | 0.0478 | 18.37 | |
| 313.15 | 0.0801 | 0.0797 | 0.43 | |
| 323.15 | 0.1072 | 0.1264 | –17.94 | |
| 333.15 | 0.1999 | 0.1902 | 4.86 |
Standard uncertainties u are u(T) = 0.1 K, u(p) = 0.05 MPa, and related standard uncertainty ur(x) = 0.05 (0.95 level of confidence).
Figure 4.
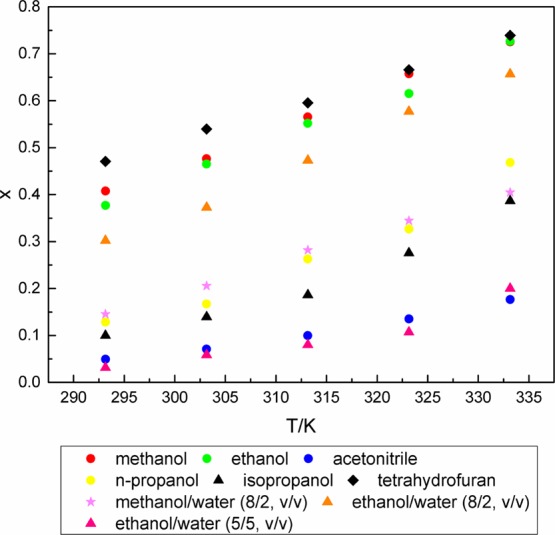
Temperature dependence of mole fraction of azilsartan in several solvents.
The data of molar fraction (xA) of the solubility are close to the theoretical molar fraction xAcalc, indicating that the solubility experimental data have good reliability and particularly instructive.
The equation parameters, the determination coefficient R2, and the MD values obtained by fitting with the λh model are listed in Table 2.
Table 2. Parameters of λh Model in Different Solvents.
| solvent | λ | h | R2 | MD |
|---|---|---|---|---|
| methanol | 36.07 | 89.05 | 0.9927 | 1.596 |
| ethanol | 37.35 | 88.90 | 0.9848 | 1.568 |
| acetonitrile | 3.365 | 991.2 | 0.9998 | 6.077 |
| n-propanol | 36.22 | 124.5 | 0.9789 | 6.029 |
| isopropanol | 26.27 | 171.3 | 0.9850 | 6.094 |
| tetrahydrofuran | 19.68 | 128.4 | 0.9881 | 1.218 |
| methanol/water (8/2, v/v) | 9.574 | 332.2 | 0.9906 | 2.856 |
| ethanol/water (8/2, v/v) | 39.00 | 93.40 | 0.9950 | 1.843 |
| ethanol/water (5/5, v/v) | 17.93 | 287.6 | 0.9524 | 10.98 |
This shows that the λh model fitted the data well because the value of determination coefficient R2 was between 0.9524 and 0.9998, while the value of MD was between 1.218 and 10.98. Besides the λh model, polynomial empirical equation, Apelblat model, and Wilson model were also used to correlate the solubility of azilsartan. However, when the polynomial empirical equation was used for fitting, the calculated solubility was significantly different from the experimental value. Moreover, when the Apelblat model and the Wilson model were used for fitting, the determination coefficient R2 is negative, which means the fitting cannot converge.
The solid phase of azilsartan in equilibrium with the saturated solutions was also characterized by X-ray powder diffraction analysis, and significant differences were observed among the diffraction patterns. According to the characteristic diffraction peak, three kinds of crystalline forms were definitely distinguished. Six strong diffraction peaks between 9.183°–23.808° were observed in the X-ray powder diffractograms obtained in methanol, ethanol, acetonitrile, n-propanol, methanol/water (8/2, v/v), ethanol/water (8/2, v/v), and ethanol/water (5/5, v/v), which was defined as crystalline form I, while 10 strong diffraction peaks together with the characteristic diffraction peak at 2θ = 7.834° in isopropanol as crystalline form II and seven strong diffraction peaks together with the characteristic diffraction peak at 2θ = 22.420° in tetrahydrofuran as crystalline form III (Supporting Information).
2.3. Thermodynamic Properties for Azilsartan Dissolution
In order to have an insight into the azilsartan dissolve process in each solvents, the Gibbs energy (ΔsolG°), the dissolving enthalpy (ΔsolH°), and the dissolving entropy (ΔsolS°) were further studied.
The chemical potential of the solute azilsartan in the solid and liquid phases is equivalent when the dissolution equilibrium is achieved.17 It can be expressed as eq 5.
| 5 |
here, μΑ° is the chemical potential of solute azilsartan when the atmospheric pressure is 0.1 MPa; αΑ is the activity of the real solution, which is defined as αΑ = γΑxΑ; γA is the activity coefficient of the solute; and xA is the mole fraction of solute azilsartan.
Therefore, the relationship between the mole fraction of solute azilsartan xA and the Gibbs free energy change in the dissolution process was established when the two phases reached equilibrium at any certain temperature (T) as eq 6.
| 6 |
where ΔsolGΑ°(T) is the Gibbs energy of dissolution of the solute azilsartan at temperature T.
Generally, the Gibbs energy of dissolution at temperature T is given by eq 7.
| 7 |
Therefore, the mole fraction of solute azilsartan xA can be associated with the changes of entropy and enthalpy in the dissolution process as eq 8.
| 8 |
The activity coefficient (γA) goes to 1 when the mole fraction of the solute (xA) goes to zero in an ideal dilute solution. Equation 8 was simplified as eq 9. Moreover, the relative contributions of enthalpy % ζH and entropy % ζTS in the dissolution process were introduced to measure the contribution of enthalpy and entropy to the change of the Gibbs free energy during the dissolution process, as eq 10 and 11.
| 9 |
| 10 |
| 11 |
The dissolving thermodynamic data of azilsartan obtained are shown in Table 3.
Table 3. Dissolving Thermodynamic Data of Azilsartan in Different Solvents.
| solvent | ΔsolH° (kJ·mol–1) | ΔsolS° (J·K–1·mol–1) | ΔsolG° (kJ·mol–1) | % ζH | % ζTS |
|---|---|---|---|---|---|
| methanol | 12.5957 | 35.297 | 1.565 | 53.312 | 46.688 |
| ethanol | 12.9615 | 36.254 | 1.632 | 53.359 | 46.641 |
| acetonitrile | 26.3828 | 64.913 | 6.097 | 56.532 | 43.468 |
| n-propanol | 26.4377 | 72.866 | 3.666 | 53.725 | 46.275 |
| isopropanol | 27.4736 | 74.287 | 4.258 | 54.200 | 45.800 |
| tetrahydrofuran | 8.6881 | 23.489 | 1.348 | 54.204 | 45.796 |
| methanol/water (8/2, v/v) | 20.913 | 55.701 | 3.506 | 54.574 | 45.426 |
| ethanol/water (8/2, v/v) | 16.194 | 45.329 | 2.028 | 53.340 | 46.660 |
| ethanol/water (5/5, v/v) | 34.8772 | 90.548 | 6.580 | 55.208 | 44.792 |
The dissolution of azilsartan in the selected solvents was an endothermic and entropic increase process. In the aqueous solution, the heat absorption and entropy increased with the increase in the water ratio during the dissolution process.
3. Conclusions
In this study, the liquid chromatographic method was introduced to measure the solubility of azilsartan in methanol, ethanol, acetonitrile, n-propanol, isopropanol, tetrahydrofuran, methanol/water (8/2, v/v), ethanol/water (8/2, v/v), and ethanol/water (5/5, v/v). In the single organic solvents, the molar fraction of azilsartan in methanol, ethanol, and tetrahydrofuran is much greater than it in acetonitrile, n-propanol, and isopropanol. In the mixed solvents, azilsartan has the highest solubility in ethanol/water (8/2, v/v) aqueous solutions. Moreover, its solubility decreased when the proportion of water in the mixed solvents increased. The solubility of azilsartan in nine solvents definitely increases with the increasing temperature, and the largest solubility change happened in methanol and ethanol, which could provide the theoretical basis in its recrystallization process.
4. Experimental Section
4.1. Materials
Azilsartan used in this work was provided by Shandong Xinhua Pharmaceutical Co., Ltd. Methanol, ethanol, acetonitrile, and tetrahydrofuran (chromatographic grade) were purchased from the Beijing Bellingway Technology Co., Ltd. without further purification. n-Propanol and isopropanol were obtained from Beijing Guangtong Fine Chemical Company without further processing. Deionized water (18.25 MΩ·cm–1) was obtained from a Millipore Mili-Q Plus water system. All solution was filtered through 0.22 μm membranes before use.
4.2. Liquid Chromatographic Conditions
The purity and content analysis of azilsartan were performed on an UltiMate 3000 HPLC and UHPLC system (America). The stationary and mobile phase were TechMate C18 ST-II (4.6 × 150 mm, 5 μm, 100 Å) and acetonitrile/water (57/43, v/v, 1 wt % glacial acetic acid), respectively. The detection wavelength was 251 nm; the flow rate was 1.0 mL·min–1; and the injection volume was 10 μL.
4.3. Measurement of Azilsartan Solubility
An excess of azilsartan was taken in a glass vial and mixed with 10 mL of methanol, ethanol, acetonitrile, n-propanol, isopropanol, tetrahydrofuran, methanol/water (8/2, v/v), ethanol/water (8/2, v/v), and ethanol/water (5/5, v/v) to get a supersaturated solutions, respectively. Sequentially, the vial was incubated in temperature thermostatic water bath stirring at 293.15, 303.15, 313.15, 323.15, and 333.15 K for 12 h until the dissolution equilibrium was obtained, respectively. The temperature was determined by a pure solvent bottle with a thermometer inside. The uncertainty of the temperature was ±0.1 K. After another 12 h standing at the corresponding temperature,18,19 aliquots of 1.0 mL of supernatant of each vial was withdrawn by a syringe with a 0.22 μm membrane. The solution was transferred to a dried, weighed double dish, and the dish was weighed quickly to determine the mass of the solution (m0) with an uncertainty of ±0.1 mg. After the solution was completely dried under nitrogen, the residue was dissolved in methanol and exactly diluted to 10 mL. Then 10 μL of the reconstituted samples was taken for HPLC analysis. All of the experiments were carried out three times simultaneously and analyzed by HPLC.
Meanwhile, the solid phase in the equilibrium with the saturated solutions was characterized by the X-ray powder diffraction at 296(2) K under Moka ray (λ = 0.71073 Å) and ω-scanning method.
Acknowledgments
We express our profound thanks to the instrumental analysis center of Beijing Institute of Technology for their kind help.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00156.
X-ray powder diffractogram of azilsartan in methanol, ethanol, acetonitrile, n-propanol, isopropanol, tetrahydrofuran, methanol/water (8/2, v/v), ethanol/water (8/2, v/v), and ethanol/water (5/5, v/v) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Dargad R. R.; Parekh J. D.; Dargad R. R.; Kukrety S. Azilsartan: Novel Angiotensin Receptor Blocker. J. Assoc. Physicians India 2016, 64, 96. [PubMed] [Google Scholar]
- Perry C. M. Azilsartan medoxomil: a review of its use in hypertension. Clin. Drug Invest. 2012, 32, 621–639. 10.1007/bf03261917. [DOI] [PubMed] [Google Scholar]
- Kohara Y.; Imamiya E.; Kubo K.; Wada T.; Inada Y.; Naka T. A new class of angiotensin II receptor antagonists with a novel acidic bioisostere. Bioorg. Med. Chem. Lett. 1995, 5, 1903–1908. 10.1016/0960-894x(95)00319-o. [DOI] [Google Scholar]
- Lam S. Azilsartan: a newly approved angiotensin II receptor blocker. Cardiol. Rev. 2011, 19, 300–304. 10.1097/crd.0b013e31822e9ba3. [DOI] [PubMed] [Google Scholar]
- Bakris G. L.; Sica D.; Weber M.; White W. B.; Roberts A.; Perez A.; Cao C.; Kupfer S. The Comparative Effects of Azilsartan Medoxomil and Olmesartan on Ambulatory and Clinic Blood Pressure. J. Clin. Hypertens. 2011, 13, 81–88. 10.1111/j.1751-7176.2010.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakugi H.; Enya K.; Sugiura K.; Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I–II essential hypertension: a randomized, double-blind clinical study. Hypertens. Res. 2012, 35, 552–558. 10.1038/hr.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönner G.; Bakris G. L.; Sica D.; Weber M. A.; White W. B.; Perez A.; Cao C.; Handley A.; Kupfer S. Antihypertensive efficacy of the angiotensin receptor blocker azilsartan medoxomil compared with the angiotensin-converting enzyme inhibitor Ramipril. J. Hum. Hypertens. 2013, 27, 479–486. 10.1038/jhh.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Zhou H.; Liu W.; He G.. Two crystal forms of azilsartan and preparation method thereof, CN 103319473 A, 2013.
- Wang X.; Shui Q.; Tang C.; Liu S.; Ouyang Q.; Shi H.. Azilsartan crystal and preparation method and application thereof, CN 103880829 A, 2012.
- Chen Q.; Zhu Y.; Zhang L.; Ding L.. Polycrystalline Substance of Azilsartan and Preparation Method Thereof, CN 102766139 B, 2012.
- Ma J.; Yang Y.; Sun Y.; Sun J. Optimization, characterization and in vitro/vivo, evaluation of azilsartan nanocrystals. Asian J. Pharm. Sci. 2017, 12, 344–352. 10.1016/j.ajps.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y.; Li T.; Cheng J. Crystal Type I of Azilsartan Polymorphs: Preparation and Analysis. J. Cryst. Process Technol. 2016, 06, 1–10. 10.4236/jcpt.2016.61001. [DOI] [Google Scholar]
- Kohara Y.; Kubo K.; Imamiya E.; Wada T.; Inada Y.; Naka T. Synthesis and Angiotensin II Receptor Antagonistic Activities of Benzimidazole Derivatives Bearing Acidic Heterocycles as Novel Tetrazole Bioisosteres. J. Med. Chem. 1996, 39, 5228–5235. 10.1021/jm960547h. [DOI] [PubMed] [Google Scholar]
- Garaga S.; Misra N. C.; Raghava Reddy A. V.; Prabahar K. J.; Takshinamoorthy C.; Sanasi P. D.; Babu K. R. Commercial synthesis of Azilsartan Kamedoxomil: an angiotensin II receptor blocker. Org. Process Res. Dev. 2015, 19, 514–519. 10.1021/op500357r. [DOI] [Google Scholar]
- Sardari F.; Jouyban A. Solubility of Nifedipine in Ethanol + Water and Propylene Glycol + Water Mixtures at 293.2 to 313.2 K. Ind. Eng. Chem. Res. 2013, 52, 14353–14358. 10.1021/ie402588t. [DOI] [Google Scholar]
- Buchowski H.; Ksiazczak A.; Pietrzyk S. Solvent activity along a saturation line and solubility of hydrogen-bonding solids. J. Phys. Chem. 1980, 84, 975–979. 10.1021/j100446a008. [DOI] [Google Scholar]
- Silbey R. J.; Alberty R. A.. Fundamental Equations of Thermodynamics. In Physical Chemistry, 4th ed.; Brennan D., Ed.; John Wiley & Sons, Inc: New York, 2004; p 102. [Google Scholar]
- Liu X.; Hu Y.; Liang M.; Li Y.; Yin J.; Yang W. Measurement and correlation of the solubility of maleic anhydride in different organic solvents. Fluid Phase Equilib. 2014, 367, 1–6. 10.1016/j.fluid.2014.01.008. [DOI] [Google Scholar]
- Luning Prak D. J.; O’Sullivan D. W. Solubility of 4-Nitrotoluene, 2,6-Dinitrotoluene, 2,3-Dinitrotoluene, and 1,3,5-Trinitrobenzene in Pure Water and Seawater. J. Chem. Eng. Data 2007, 52, 2446–2450. 10.1021/je700374j. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.