Abstract
Hydrogen sulfide has emerged as an important gaseous signaling molecule and a regulator of critical biological processes. However, the physiological significance of hydrogen sulfide metabolites such as persulfides, polysulfides, and other reactive sulfur species (RSS) has only recently been appreciated. Emerging evidence suggests that these RSS molecules may have similar or divergent regulatory roles compared with hydrogen sulfide in various biological activities. However, the chemical nature of persulfides and polysulfides is complex and remains poorly understood within cardiovascular and other pathophysiological conditions. Recent reports suggest that RSS can be produced endogenously, with different forms having unique chemical properties and biological implications involving diverse cellular responses such as protein biosynthesis, cell-cell barrier functions, and mitochondrial bioenergetics. Enzymes of the transsulfuration pathway, CBS (cystathionine beta-synthase) and CSE (cystathionine gamma-lyase), may also produce RSS metabolites besides hydrogen sulfide. Moreover, CARSs (cysteinyl-tRNA synthetase) are also able to generate protein persulfides via cysteine persulfide (CysSSH) incorporation into nascently formed polypeptides suggesting a new biologically relevant amino acid. This brief review discusses the biochemical nature and potential roles of RSS, associated oxidative stress redox signaling, and future research opportunities in cardiovascular disease.
Keywords: cardiovascular diseases, gases, ischemia, sulfur, vascular remodeling
Highlights.
Biochemical nature of sulfide and its metabolites include polysulfide/reactive sulfur species.
Synthesis and metabolism of reactive sulfur species may occur through different enzymatic pathways.
Reactive sulfur species/polysulfides participate in cardiovascular pathophysiology.
Genetic polymorphisms of CSE (cystathionine gamma-lyase; or CTH) implicated in cardiovascular disease.
Nearly 2 decades later, hydrogen sulfide (H2S) is now established as a crucial signaling molecule and a key regulator of biological functions. While much has been revealed regarding the importance of H2S, its molecular signaling, synthesis, and metabolism pathways are still much unclear.1–5 As a byproduct of the transsulfuration pathway, H2S is known for its pathophysiological roles in various tissues and organs, including the regulation of neuronal and vascular systems, cardioprotection, protection from ischemic insult and wound repair, and insulin release. However, the chemical nature of H2S is purported to translate to many biological and biochemical associations reported in the literature. It is likely an oversimplification to conclude that this single molecule in its primary biochemical state alone mediates all the reported signaling and biological effects. Thus, it is prudent to consider the hypothesis that the presence of other chemical intermediates or derivatives of sulfide may mediate many biological functions. Indeed, it is now appreciated that a wide range of sulfide metabolites may be produced via the transsulfuration enzymes CBS (cystathionine beta-synthase) and CSE (cystathionine gamma-lyase) besides H2S. These metabolites including persulfides (RSSH), polysulfides (RSS(n)H), and other oxidized sulfide species represent a group of reactive molecules called reactive sulfur species (RSS) and have been identified as signaling agents themselves associated with many biological activities.6 Therefore, H2S represents only a portion of sulfur-containing molecules that contribute to bioavailable RSS with other molecules contained in acid-labile sulfide (eg, iron-sulfur clusters, not a focus of this review) and bound sulfane sulfur (BSS; eg, persulfides, polysulfides, and others; Figure 1A).
Figure 1.
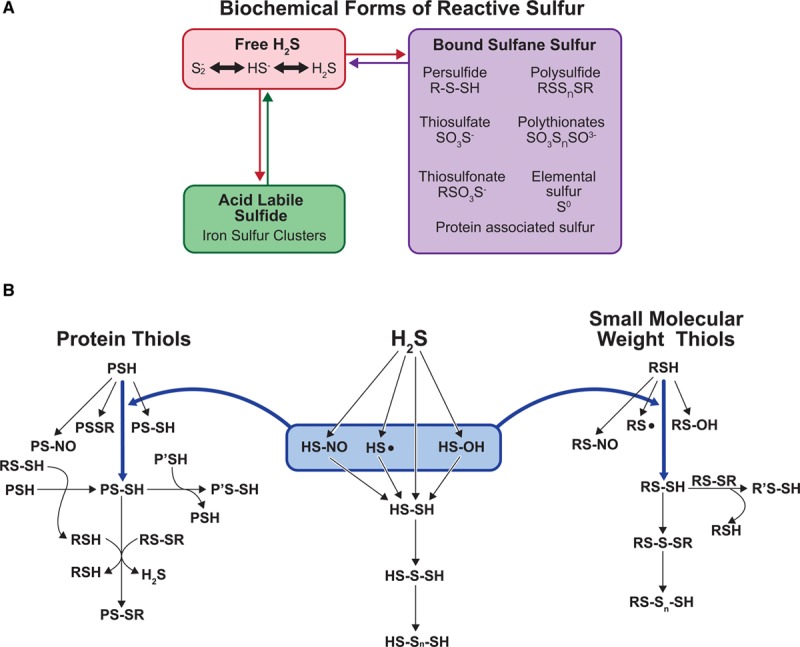
Biochemical nature and species of reactive sulfur species (RSS). A, Various biochemical forms of reactive sulfur including free hydrogen sulfide (H2S), acid-labile sulfide (eg, iron-sulfur clusters), and bound sulfane sulfur. B, Various sulfide species associated with different biochemical sulfur-containing molecules. Three categories of sulfur-containing molecules involve protein thiols, H2S itself, and small-molecular-weight thiols (R designates molecules such as GSH or cysteine).
Please see www.ahajournals.org/atvb/atvb-focus for all articles published in this series.
RSS: a New Cousin to Reactive Oxygen Species and Reactive Nitrogen Species
Reactive oxygen species (ROS) and reactive nitrogen species can contribute to redox signaling, mediating changes in pathophysiological responses at cellular and tissue level. Reactive nitrogen species include various NO-derived compounds that play a crucial role in the regulation of many pathophysiological conditions via posttranslational modifications and interactions with ROS.7,8 This includes regulation of cell injury and death and pathological events including neurological and cardiovascular pathologies and metabolic and inflammatory diseases. However, the role of RSS beyond cellular antioxidant systems only recently has gained attention but remains ambiguous. Sulfide metabolites comprising RSS may exist in different chemical forms (Figure 1A). Our group and others have shown that sulfide may be available in biochemical pools including free sulfide, acid-labile sulfide, and BSS.9,10 Moreover, RSSs encompass sulfur-containing reactive biomolecules from small molecules to proteins (Figure 1B).11,12 Sulfane sulfurs are small-molecular-weight thiols or protein thiols bound with sulfur atoms having an oxidation state 0 or −1. Representative RSSs include H2S, protein thiols (PSH), small-molecular-weight thiols (RSH), hydrogen persulfide/polysulfide (H2Sn; n≥2), small-molecular-weight thiol persulfide (RSSH), protein persulfide (PS-SH), various polysulfides (RSS(n)H, RSS(n)R, and H2Sn; n>1), sulfenic acids (RSOH), nitrosothiols (RSNO), and various sulfide bridge forms (PS-SR, RS-S-SR, and RS-Sn-SH).12,13 Most RSSs are known to be unstable and reactive. This is reflected by the fact that the sulfur atom (S) can accept or donate electrons and exist in a wide range of oxidation states between −2 and +6, which plays an import role in many biochemical and chemical biological processes.12,14 Evidence indicates that polysulfides, rather than H2S itself, mediate protein sulfuration (aka sulfhydration) of reduced protein thiol (RSH) target proteins resulting in the formation of protein persulfide (RSSH) as the sulfur in both H2S and reduced thiol (RSH) have the same valency state and cannot react with one another.15–17 On the other hand, H2S can lead to the formation of polysulfide via reaction with sulfenic acid (RSOH) or RSNO resulting in the formation of the persulfide (RSSH) moiety. These 2 pathways alone hint at the significant degree of chemical complexity associated with RSS while highlighting their ability to react with both ROS and reactive nitrogen species metabolites.
Several functional similarities exist between ROS and RSS that are beginning to reveal a rich and previously unappreciated biochemical relationship. It has even been proposed that there could be a misinterpretation of RSS-mediated functions equated to that of ROS and vice versa, as reviewed elsewhere.18 The presence of both RSS and redox reaction mechanisms dates back to the origin of life over 4 billion years ago. For nearly the first 2 billion years of our planetary history, the environment was dominated by ferruginous- and then euxinic (H2S)-rich oceans.19 During this time, the evolution of early eukaryotic cells and ancient biochemical enzyme reaction pathways came into being followed by the Great Oxidation Event. The free radical and redox biology fields have long proposed that existing oxidant-antioxidant relationships came about in response to the Great Oxidation Event as a way for organismal adaptation and survival. While this may be true, it is also clear that RSSs were present before an abundance of oxygen and recent evidence reveals that ancient enzymes involved in antioxidant actions, such as catalase and superoxide dismutase, are also potent oxidoreductases for RSS.20,21 As such, RSSs likely serve as important biological redox mediators through participation in nucleophilic-electrophilic reactions, with thiols (RSH) being nucleophilic, disulfides (RSSR; aka oxidized thiol) being electrophilic, and persulfides (RSSH) being either electrophilic or nucleophilic upon deprotonation (RSS−). Their significant abundance further suggests the biological importance of these molecules with RSS (eg, persulfide/polysulfide) having been reported in the low-to-mid micromolar range, depending on the tissue and cell type studied.5,22 Unfortunately, our understanding of these RSS metabolites and how they contribute to cardiovascular pathophysiology remains essentially unknown, thereby requiring much more study.
Chemical Nature of Thiol, Sulfide, and RSS
Chemically, all acid-base and redox reactions can be determined in terms of electrophiles and nucleophiles. Electrophiles are neutral or charged atoms that are electron deficient. While nucleophiles are electron rich that will react to decrease their electron density, they can be neutral or charged and tend to have negative charges. However, there are neutral molecules that are both electrophiles and nucleophiles. Chemically, H2S has reductive and nucleophilic properties that contribute to its physiological actions. Importantly, as indicated above, persulfides can exhibit a dual nature, as a nucleophile and an electrophile. While persulfides can have enhanced nucleophilicity with the corresponding thiol, the inner and outer sulfurs can both act as electrophiles.
Thiols (RSH) play critical roles in regulating redox signaling, cellular functions, and in improving protein structure and stability.16 Thiols are also the redox currency of the major intracellular redox buffer, glutathione, and a cadre of redox enzymes, such as peroxiredoxins, Trx (thioredoxins), Trx reductases, glutaredoxins, and glutathione reductases, are used to maintain this balance. Renewed interests have recently led the research community to study thiol chemistry and biological implications including (1) thiol role to generate H2S, which is now an established regulator of biological functions, and (2) identification of various thiol/sulfide compounds that may have potential biological significance. Importantly, thiols can regulate cellular processes through protein modifications, including S-sulfhydration/sulfuration (PS-SH), S-glutathionylation (PS-SG), and cysteinylation (PS-S-Cys).11,23
RSS Formation in Biology
H2S exists at physiological pH and 37°C in various forms of gas H2S (≈20%), H2S ion (HS−, ≈80%), and sulfide ion (S2−, <0.1%). H2S is predominantly produced by CBS in the nervous system and CSE in the vascular system using homocysteine, cystathionine, or L-cysteine as substrates (Figure 2).24–26 Importantly, CBS and CSE can also use cystine (CysSSCys) as a substrate forming cysteine persulfide (CysSSH), which is biologically relevant.22 Per- and polysulfides are not only formed enzymatically but can also be carried by proteins such as plasma albumin, which has the ability to bind `and transport sulfane sulfur.27 There are several biologically relevant oxidants that can support oxidation of H2S. H2S can be oxidized by 1- or 2-electron oxidant pathway with varied rate constants described in Figure 2. For 2-electron oxidation, the bimolecular rate constant between hydrogen peroxide (H2O2) and sulfide is 0.73 M−1 s−1. For the 1-electron oxidation, the sulfhydryl radical (HS·) can be produced, following a series of radical chain reactions that results in persulfide formation. Additionally, MST (3-mercaptosulfotransferase) has the ability to generate per/polysulfides apart from H2S.28 MST obtains sulfur from 3-mercaptopyruvate to produce MST polysulfide, which can be reduced by Trx to release H2Sn, such as H2S, H2S2, H2S3, and others. MST can transfer the sulfur atom from 3-mercaptopyruvate to its catalytic site (Cys248) to form MST persulfide (MST-SSH) or form H2S3.29,30 Moreover, SQR can catalyze the oxidation of H2S to sulfane sulfur, with this sulfane sulfur metabolized to sulfite leading to the formation of thiosulfate or glutathione persulfide.31,32 GSSH may also react with glutathione disulfide (GSSG) to produce the glutathione trisulfide (GSSSG) or GSSSSG. Importantly, all of these oxidized glutathione species can be reduced by glutathione reductase to form glutathione per/polysulfide.33–35
Figure 2.
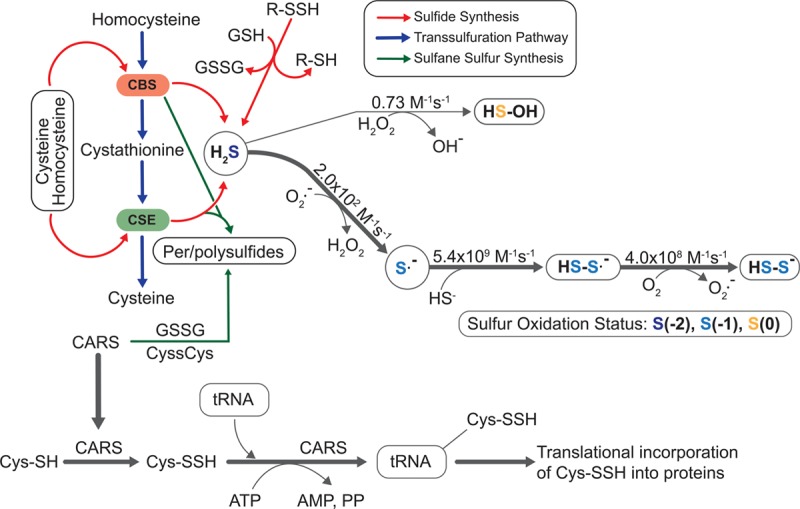
Formation and metabolism of reactive sulfur species. Transsulfuration enzymes CBS (cystathionine beta-synthase) and CSE (cystathionine gamma-lyase) use substrates homocysteine, cystathionine, or cysteine to generate hydrogen sulfide (H2S). H2S may subsequently react with reactive oxygen species (eg, superoxide) resulting in sulfide radical formation leading to persulfide formation. Rate constants (M−1 s−1) are shown for each reaction indicating that H2S reacts more quickly with superoxide vs hydrogen peroxide. CARS (cysteinyl-tRNA synthetase) enzyme activity also contributes to cysteine persulfide formation that may be translationally incorporated into nascent polypeptide formation. PP indicates pyrophosphate; and tRNA, transfer RNA.
Recently, the CARS (cysteinyl-tRNA synthetases) group of enzymes have been identified to generate per/polysulfides and RSS, which can regulate different protein functions. Cysteine persulfide and polysulfide are produced in cells and are abundant in low-molecular-weight proteins and protein fractions. However, the physiological functions of cysteine persulfides/polysulfides produced in cells remain poorly understood. Results from Akaike et al have established CARS (CARS-1 and 2) as novel enzymes that can synthesize persulfides. CARS catalyzes the transfer of sulfur from one cysteine to another cysteine forming a cysteine persulfide and polysulfide.15 CARS2 being the mitochondrial isoform of CARS predominantly produces RSS that contributes to mitochondrial function.15,36 However, how these reactive sulfur molecules are formed, or what role they play within cells and tissues, has not been well defined. Additionally, the bioavailable concentrations of different RSS metabolites including per/polysulfides, their intracellular localization, and chemical reactivity with other molecules remain unknown under healthy versus pathological cardiovascular conditions.
Among nonenzymatic reactions, H2S can form persulfide and polysulfide through various reactions involving ROS such as superoxide (Figures 1B and 2). Superoxide can oxidize H2S resulting in thiyl radical formation that can react with hydrosulfide (HS−) anion to generate perthiyl radical leading to further polysulfide formation. H2S in its gaseous form does not readily react with oxygen but under aqueous conditions can be oxidized to hydrogen thioperoxide (HSOH), sulfurous acid (H2SO3), thiosulfuric acid (H2S2O3), and other small oxoacids of sulfur including sulfenic (HSOH), sulfoxylic (HS[O]OH), and thiosulfoxylic acids (H2S2O2).37 HSSH can react with other sulfane sulfur to produce hydrogen polysulfide (HSSnSH; n>1). As mentioned earlier, sulfenic acid (RSOH) and S-nitrosothiols (RSNO) can also react with H2S forming persulfide.38 Lastly, some metal centers can oxidize H2S to form sulfhydryl radical (HS·), which can react with free thiols, ultimately generating persulfide and polysulfide.39,40
Bioavailability and Biological Significance of RSS
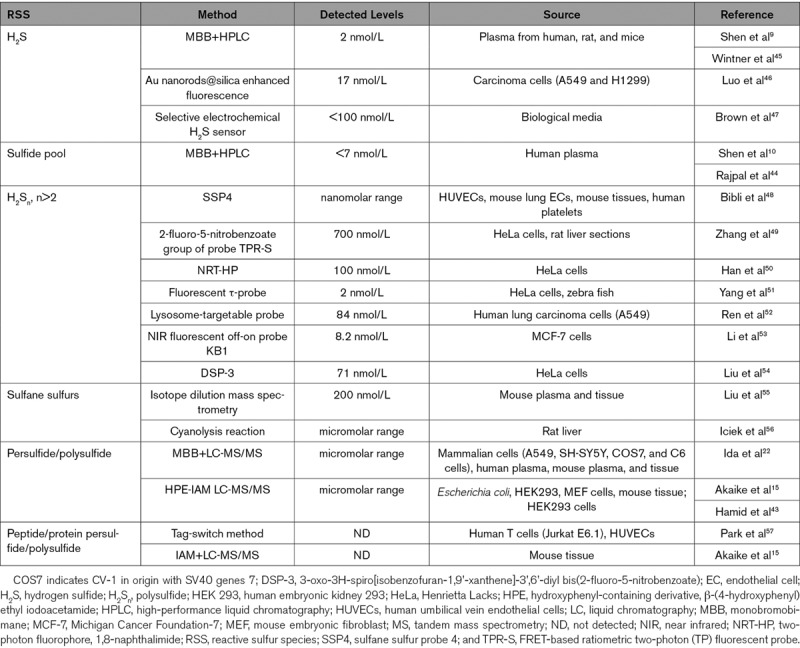
RSS can be formed as byproduct of major thiols or as a result of oxidation of sulfite or sulfate molecules. Per/polysulfides have been identified in mammalian and other biological systems with possible involvement in various cellular functions. Intracellular cysteine persulfide and polysulfide exist in abundance in both low-molecular-weight proteins and protein fractions. Recent works focused on detection techniques have determined the levels of per/polysulfides levels in biological systems.12,15,16,36,41–43 Various assays including monobromobimane LC-MS/MS, polarographic electrode, and chemiluminescence/fluorescent probes have revealed per/polysulfides of cell culture and tissue lysates to range from low micromolar to nano/picomolar concentrations (Table).
Table.
RSS Measurement and Bioavailability
BSS pools predominantly include per- and polysulfides, which can subsequently release H2S under reducing conditions. The clinical relevance of sulfide pools, including BSS, has recently been demonstrated by our group showing that plasma H2S metabolite bioavailability can be a predictive indicator for cardiovascular disease (CVD).44 In this study, we have reported that the BSS levels were significantly reduced in subjects with CVD compared with those without disease. This underscores the importance of cellular redox state for regulating H2S bioavailability and also suggests a role for BSS in cardiovascular health.
Studies on exogenous H2S delivery have suggested that oxidized sulfur species, including sulfane sulfur, may mediate physiological functions that were thought to be solely H2S driven.18,58 Although H2S is a short-lived molecule, numerous studies demonstrated its prolonged biological effects in mammalian systems. Apart from the exogenous formation of inorganic polysulfides in a solution of NaHS, the existence of endogenous inorganic polysulfides has also been demonstrated.59–61
Atherosclerosis is a chronic progressive disease manifesting in clinical CVD. Atherosclerosis is a complex process involving endothelial dysfunction and vascular inflammation, among others. Interrelation between H2S and atherosclerotic progression has recently been appreciated.62–64 Wang et al63 demonstrated that CSE/H2S pathway was disturbed in the vasculature of apoE (apolipoprotein E)−/− mice. A significant decrease in H2S bioavailability was observed in the plasma and aorta of apoE−/− mice but a higher CSE expression in aorta. However, exogenous NaHS therapy inhibits proatherogenic and inflammatory effects in apoE−/− via IkB degradation and thus NF-κB (nuclear factor-kappa B) signaling pathway. Further, Mani et al62 have demonstrated that decreased endogenous H2S bioavailability leads to early development of atherosclerosis. They observed that CSE-knockout mice fed with high-fat diet have increased proatherogenic symptoms including elevated cholesterol-rich lipoproteins, aortic lesions, enhanced aortic intimal proliferation, and proinflammatory signaling. Additionally, CSE/apoE double-knockout mice have further exacerbated atherosclerosis development than single-gene knockouts.65 CSE deficiency can increase neointima formation in ligated carotid arteries, which was attenuated by sulfide therapy. The proatherogenic effects were significantly reduced either by exogenous sulfide treatment or global CSE overexpression.62,66 These studies indicate that CSE/sulfide metabolite signaling regulates atherogenesis via inflammatory signaling.62–64 Elevated homocysteinemia can also lead to impairment in macrophage CSE/polysulfide production that eventually causes vascular inflammation in mice mediated by elevated proinflammatory cytokines TNF-α (tumor necrosis factor-α) and IL-1β (interleukin-1β).67 Additionally, homocysteinemia can also elevate DNA hypermethylation eventually repressing CSE transcription.
Work from our group has identified a unique relationship between shear flow pattern-specific CSE expression and endothelial cell phenotype.68 In this study, Yuan et al reported that laminar shear significantly reduced CSE protein expression and polysulfide production, whereas disturbed flow regions show elevated CSE in endothelial cells. Concurrently, in vivo model showed higher CSE expression where aortic curvature is lesser compared with the greater curvature. Change in CSE expression under disturbed flow protects against inward vascular remodeling. Additionally, CSE−/− mice showed reduced inward remodeling where it remains dilated under reduced shear indicating compromised regulation in vascular remodeling after partial carotid ligation. These observations indicate that CSE protects proatherogenic condition by promoting flow-mediated vascular remodeling and reduced endothelial activation (Figure 3A). Lack of CSE also limits disturbed flow-induced proinflammatory signaling including ICAM-1 (intercellular adhesion molecule-1) and VCAM-1 (vascular cell adhesion molecule-1) gene expressions and monocyte infiltration mediated by NF-κB.68 Recently, work from Bibli et al69 confirmed our observations revealing that expression of CSE was higher in the lesser curvature and arterial bifurcations. CSE critically maintains low arterial CD62E levels to minimize monocyte adhesion at sites of low or disturbed flow to minimize monocyte-mediated inflammation. Exogenous polysulfide through SG1002 or CSE overexpression restored sulfhydration of HuR (human antigen R) and reduced CD62E (E-selectin) protein expression to attenuate monocyte adherence (Figure 3B). However, in inflammatory conditions, this protective mechanism was lost due to phosphorylation (on Ser377) and inactivation of CSE.69 These studies suggest that defective CSE/polysulfide signaling can lead to accelerated development of endothelial dysfunction and atherosclerosis, which can be rectified via CSE/polysulfide therapy. Furthermore, Bibli et al65 reported a paradigm in which endothelial CSE may prevent atherosclerosis development and redox regulation via a loss of Prx6 (peroxiredoxin 6) sulfhydration. Interestingly, the polysulfide donor SG1002 could restore Prx6 sulfhydration in CSE-deficient ECs attributing the beneficial effects of polysulfide to attenuate atherosclerosis development. However, much uncertainty remains regarding the distinct roles of CSE and other sulfur species in shear stress and vascular remodeling that warrants additional studies to better understand specific mechanistic pathways.
Figure 3.
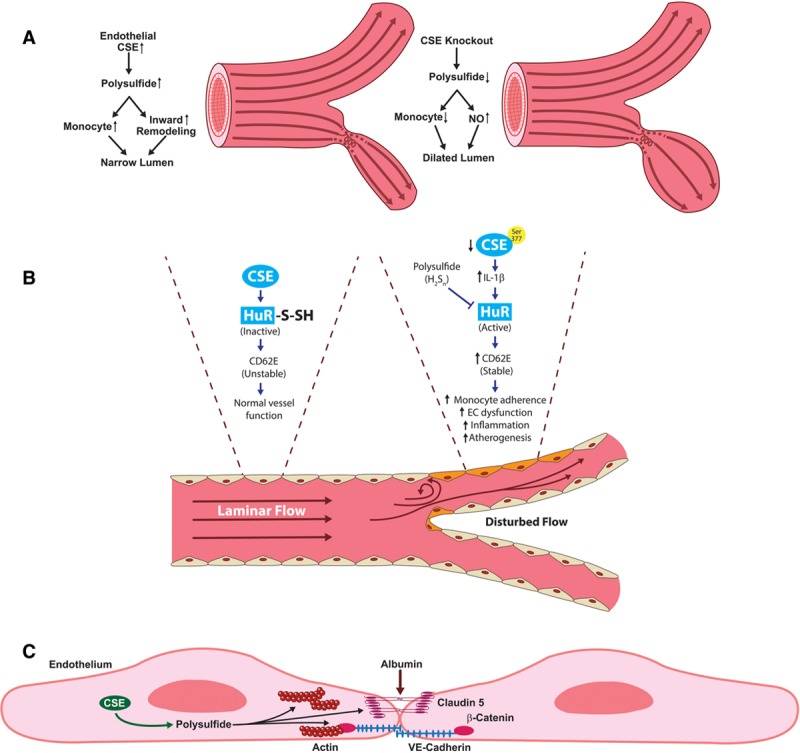
CSE (cystathionine gamma-lyase)/polysulfide modulates vascular remodeling responses. A, CSE expression and sulfane sulfur production are enhanced by disturbed flow in conduit vessels. Enhanced CSE increases macrophage recruitment in areas of disturbed flow that induces flow-mediated vascular remodeling. CSE knockout conditions have reduced polysulfide following partial carotid artery ligation, leading to defective inward remodeling, and a dilated vascular phenotype due to elevated NO bioavailability in CSE knockout carotid arteries. B, CSE-derived polysulfide inactivates HuR (human antigen R) via S-sulfhydration and attenuates CD62E expression that consequently regulates vascular inflammation and atherogenesis. Defective CSE/polysulfide leads to activation of HuR and subsequent CD62E stability that induces EC dysfunction and atherogenesis. C, CSE-derived sulfur species increase endothelial solute permeability via regulation of endothelial junction proteins claudin 5 and VE-cadherin and enhanced actin stress fiber formation. CD62E indicates E-selectin; EC, endothelial cells; IL-1β, interleukin-1β; and VE-Cadherin, vascular endothelial cadherin.
We have previously demonstrated that endogenous BSS regulates endothelial barrier functions.61 Our data revealed that endothelial solute permeability is critically regulated via exogenous and endogenous sulfide bioavailability with a dominant role of polysulfides. Polysulfides induced endothelial junction disorganization leading to increased vascular permeability.61 Additionally, we found that CSE regulates endothelial barrier integrity through regulation of endogenous polysulfide production. Genetic deficiency of CSE in endothelial cells significantly reduced the BSS pool compared with wild-type cells, which was associated with decreased basal endothelial permeability (ie, tighter barrier function).61 In comparison with exogenous sulfide donors such as NaHS, the polysulfides including Na2S2, Na2S3, and Na2S4 elicited a much stronger increase in endothelial solute permeability and loss of endothelial barrier function (Figure 3C). In another study, the Pluth Laboratory reported that the polysulfide diallyl trisulfide and synthetic polysulfides regulate cell proliferation of a murine brain endothelial cell line.64 Their work demonstrated trisulfide and tetrasulfide release H2S via thiol-mediated reduction in the presence of cysteine or reduced glutathione associated with bEnd.3 cell viability.64 These studies highlight that rather than just H2S, per- and polysulfides are equally capable of regulating various endothelial cell functions.
Works from the Akaike Laboratory have shown that the enzyme CARS, which generates cysteine persulfides, can regulate cellular functions including mitochondria function and bioenergetics.36,43,70 Activation of TRPA1 in rat astrocytes has been induced by polysulfide donors more effectively than exogenous H2S donors.71,72 Similarly, induction of Keap1/Nrf2 (Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2) activation has been observed in neuronal cells by polysulfide donors.71–73 Moreover, garlic-derived polysulfides have long been well known to possess antitumor effects.74 However, further study is needed to elucidate the antitumor mechanisms of inorganic polysulfides and to differentiate their role from exogenous H2S. Lastly, recent work from Zhang et al75 has observed that cellular polysulfides may play a role in the regulation of inflammatory signaling and can be a potential target for inflammatory disorders. This group demonstrated that polysulfides desensitize macrophages to TLR4 (Toll-like receptor 4) and negatively regulate TLR4-mediated proinflammatory signaling. However, these observations require further study in CVD and inflammatory model systems.
Lefer et al have provided insights into cardioprotective role of sulfide and polysulfide in various models of CVD and cardiovascular injury. Exogenous H2S delivery or endogenous CSE overexpression and H2S modulation has been therapeutically beneficial for ischemic heart failure.76–79 Modulation of endogenous H2S through cardiac-specific CSE overexpression has cardioprotective effects.77 In a mouse model of myocardial ischemia-reperfusion, exogenous H2S delivery during reperfusion inhibited myocardial inflammation, reduced infarct size, and preserved left ventricular function. There was a significant reduction in myocardial injury and cytoprotection in the model of myocardial ischemia-reperfusion in mice with cardiac-specific CSE overexpression. Exogenous donor diallyl trisulfide rescued from myocardial injury in a murine model of myocardial ischemia/reperfusion.78 Diallyl trisulfide therapy not only reduced myocardial infarct size but also improved myocardial contractile function, preserving mitochondria function. These events were mediated via extended endogenous H2S release, increased eNOS activity, and NO metabolites.78 Similarly, Goodchild and et al79 demonstrated cardio- and vasoprotective effects of sulfide prodrug, SG1002, in a porcine model of peripheral arterial disease (PAD). Sulfide prodrug preserved vessel density and improved endothelial-dependent coronary artery vasorelaxation in critical limb ischemia model via H2S/NO metabolite signaling. These observations are in conjunction with our clinical study that reported a significant decrease in the levels of total, acid-labile, and bound sulfane sulfide in plasma samples of subjects of vascular disease including CVD and PAD.44 This implicates the association of polysulfide to CVD, which needs extensive studies.
Genetic polymorphisms of CSE/CTH may also be implicated in various disease conditions including CVD.80 As discussed earlier, we observed variations in H2S metabolite levels in CVD.44 Interestingly, we found polymorphisms in CSE/CTH gene were associated with the risk of CVD. A significant increase in CSE (CTH) 1364 G-T allele frequency was observed in patients with CVD compared with controls. These findings were concurrent with reduced plasma H2S/BSS bioavailability changes in CVD.44 However, future clinical studies are needed to identify the significance and relevance of polysulfide as a biomarker of any pathology as different ethnic populations may not be similarly influenced by genetic polymorphisms.81 A case-controlled GWAS in Greek population has investigated previously documented 9 SNPs82 and compared composite genetic risk scores associated with CHD that would impact high-risk alleles.83 Changes in genetic risk scores have only modestly improved CHD risk prediction; however, there was a lack of distinction between ischemia and hemorrhagic stroke for their relevance into CHD-specific risk alleles. Moreover, few of the CHD-associated SNPs that were identified previously were not found in Greek populations. These observations suggest that ethnic differences could play a substantial role in CVD-associated genetic variations, which warrants further studies based on ethnic populations.
Conclusions
While H2S is considered a signaling molecule and regulator of many biological functions, it is clear that RSSs have similar properties. Moreover, specific chemical forms that are associated with biological functions remain inconclusive, as do specific stimuli mediating their formation and tissue and cellular distribution. Understanding bioavailable levels of endogenous persulfide/polysulfide will shed important light into developing strategies for various cardiovascular pathological conditions. Other pertinent questions also remain such as What biological chemistry conditions trigger the enzymatic release of per/polysulfide? What factors mediate their release and balance with H2S bioavailability? How does the redox status of cellular microenvironments influence the generation and bioavailability of per/polysulfide? What is the differential role and importance of per/polysulfide formation through redox reactions versus enzymatic synthesis (eg, CARS)? Additionally, genomics and metabolomics approaches may also reveal new mechanisms of per/polysulfide formation and metabolism impacting cellular signaling and function. In summary, it is imperative to understand the pathophysiological importance of RSS per- and polysulfides, which will provide new insight into sulfide biology while identifying novel therapeutic strategies for CVD.
Sources of Funding
This work was supported by an Institutional Development Award from the National Institutes of General Medical Sciences of the National Institutes of Health under grant No. P20GM121307 to C.G. Kevil.
Disclosures
C.G. Kevil and X. Shen have provisional patents regarding measurement and use of sulfides. C.G. Kevil is a cofounder of Innolyzer, LLC. The other author reports no conflicts.
Footnotes
Nonstandard Abbreviations and Acronyms
- apoE
- apolipoprotein E
- BSS
- bound sulfane sulfur
- CARS
- cysteinyl-tRNA synthetase
- CBS
- cystathionine beta-synthase
- CSE/CTH
- cystathionine gamma-lyase
- CVD
- cardiovascular disease
- H2S
- hydrogen sulfide
- HuR
- human antigen R
- ICAM-1
- intercellular adhesion molecule-1
- IL-1β
- interleukin-1β
- MST
- 3-mercaptosulfotransferase
- NF-κB
- nuclear factor-kappa B
- Prx6
- peroxiredoxin 6
- ROS
- reactive oxygen species
- RSS
- reactive sulfur species
- TLR4
- Toll-like receptor 4
- TNF-α
- tumor necrosis factor-α
- Trx
- thioredoxin
- VCAM-1
- vascular cell adhesion molecule-1
For Sources of Funding and Disclosures, see page 882.
References
- 1.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose P, Moore PK, Zhu YZ. H2S biosynthesis and catabolism: new insights from molecular studies. Cell Mol Life Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie ZZ, Liu Y, Bian JS. Hydrogen sulfide and cellular redox homeostasis. Oxid Med Cell Longev. 2016;2016:6043038. doi: 10.1155/2016/6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan S, Patel RP, Kevil CG. Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol. 2015;308:L403–L415. doi: 10.1152/ajplung.00327.2014. doi: 10.1152/ajplung.00327.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura H. Physiological roles of hydrogen sulfide and polysulfides. Handb Exp Pharmacol. 2015;230:61–81. doi: 10.1007/978-3-319-18144-8_3. doi: 10.1007/978-3-319-18144-8_3. [DOI] [PubMed] [Google Scholar]
- 7.Adams L, Franco MC, Estevez AG. Reactive nitrogen species in cellular signaling. Exp Biol Med (Maywood) 2015;240:711–717. doi: 10.1177/1535370215581314. doi: 10.1177/1535370215581314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez MC, Andriantsitohaina R. Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxid Redox Signal. 2009;11:669–702. doi: 10.1089/ars.2007.1993. doi: 10.1089/ars.2007.1993. [DOI] [PubMed] [Google Scholar]
- 9.Shen X, Kolluru GK, Yuan S, Kevil CG. Measurement of H2S in vivo and in vitro by the monobromobimane method. Methods Enzymol. 2015;554:31–45. doi: 10.1016/bs.mie.2014.11.039. doi: 10.1016/bs.mie.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen X, Peter EA, Bir S, Wang R, Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med. 2012;52:2276–2283. doi: 10.1016/j.freeradbiomed.2012.04.007. doi: 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iciek M, Kowalczyk-Pachel D, Bilska-Wilkosz A, Kwiecie I, Gorny M, Wlodek L. S-sulfhydration as a cellular redox regulation. Biosci Rep. 2015;36:e00304. doi: 10.1042/BSR20150147. doi: 10.1042/BSR20150147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan S, Shen X, Kevil CG. Beyond a gasotransmitter: hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid Redox Signal. 2017;27:634–653. doi: 10.1089/ars.2017.7096. doi: 10.1089/ars.2017.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J, Xu S, Radford MN, Zhang W, Kelly SS, Day JJ, Xian M. O→S relay deprotection: a general approach to controllable donors of reactive sulfur species. Angew Chem Int Ed Engl. 2018;57:5893–5897. doi: 10.1002/anie.201802845. doi: 10.1002/anie.201802845. [DOI] [PubMed] [Google Scholar]
- 14.Lau N, Pluth MD. Reactive sulfur species (RSS): persulfides, polysulfides, potential, and problems. Curr Opin Chem Biol. 2019;49:1–8. doi: 10.1016/j.cbpa.2018.08.012. doi: 10.1016/j.cbpa.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Akaike T, Ida T, Wei FY, Nishida M, Kumagai Y, Alam MM, Ihara H, Sawa T, Matsunaga T, Kasamatsu S, et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuto JM, Ignarro LJ, Nagy P, Wink DA, Kevil CG, Feelisch M, Cortese-Krott MM, Bianco CL, Kumagai Y, Hobbs AJ, et al. Biological hydropersulfides and related polysulfides - a new concept and perspective in redox biology. FEBS Lett. 2018;592:2140–2152. doi: 10.1002/1873-3468.13090. doi: 10.1002/1873-3468.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLeon ER, Gao Y, Huang E, Arif M, Arora N, Divietro A, Patel S, Olson KR. A case of mistaken identity: are reactive oxygen species actually reactive sulfide species ? Am J Physiol Regul Integr Comp Physiol. 2016;310:R549–R560. doi: 10.1152/ajpregu.00455.2015. doi: 10.1152/ajpregu.00455.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson KR, Straub KD. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology (Bethesda) 2016;31:60–72. doi: 10.1152/physiol.00024.2015. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
- 20.Kevil CG. Catalase as a regulator of reactive sulfur metabolism; a new interpretation beyond hydrogen peroxide. Redox Biol. 2017;12:528–529. doi: 10.1016/j.redox.2017.03.018. doi: 10.1016/j.redox.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson KR, Gao Y, DeLeon ER, Arif M, Arif F, Arora N, Straub KD. Catalase as a sulfide-sulfur oxido-reductase: an ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol. 2017;12:325–339. doi: 10.1016/j.redox.2017.02.021. doi: 10.1016/j.redox.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci USA. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee R. Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism. J Biol Chem. 2012;287:4397–4402. doi: 10.1074/jbc.R111.287995. doi: 10.1074/jbc.R111.287995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 26.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toohey JI. Sulphane sulphur in biological systems: a possible regulatory role. Biochem J. 1989;264:625–632. doi: 10.1042/bj2640625. doi: 10.1042/bj2640625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagahara N, Koike S, Nirasawa T, Kimura H, Ogasawara Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem Biophys Res Commun. 2018;496:648–653. doi: 10.1016/j.bbrc.2018.01.056. doi: 10.1016/j.bbrc.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 29.Kimura Y, Toyofuku Y, Koike S, Shibuya N, Nagahara N, Lefer D, Ogasawara Y, Kimura H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci Rep. 2015;5:14774. doi: 10.1038/srep14774. doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav PK, Yamada K, Chiku T, Koutmos M, Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem. 2013;288:20002–20013. doi: 10.1074/jbc.M113.466177. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson MR, Melideo SL, Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51:6804–6815. doi: 10.1021/bi300778t. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 32.Libiad M, Yadav PK, Vitvitsky V, Martinov M, Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J Biol Chem. 2014;289:30901–30910. doi: 10.1074/jbc.M114.602664. doi: 10.1074/jbc.M114.602664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasamatsu S, Nishimura A, Morita M, Matsunaga T, Abdul Hamid H, Akaike T. Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules. 2016;21:E1721. doi: 10.3390/molecules21121721. doi: 10.3390/molecules21121721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishida M, Kumagai Y, Ihara H, Fujii S, Motohashi H, Akaike T. Redox signaling regulated by electrophiles and reactive sulfur species. J Clin Biochem Nutr. 2016;58:91–98. doi: 10.3164/jcbn.15-111. doi: 10.3164/jcbn.15-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono K, Akaike T, Sawa T, Kumagai Y, Wink DA, Tantillo DJ, Hobbs AJ, Nagy P, Xian M, Lin J, et al. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic Biol Med. 2014;77:82–94. doi: 10.1016/j.freeradbiomed.2014.09.007. doi: 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii S, Sawa T, Motohashi H, Akaike T. Persulfide synthases that are functionally coupled with translation mediate sulfur respiration in mammalian cells. Br J Pharmacol. 2019;176:607–615. doi: 10.1111/bph.14356. doi: 10.1111/bph.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar MR, Farmer PJ. Chemical trapping and characterization of small oxoacids of sulfur (SOS) generated in aqueous oxidations of H2S. Redox Biol. 2018;14:485–491. doi: 10.1016/j.redox.2017.10.012. doi: 10.1016/j.redox.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talipov MR, Timerghazin QK. Protein control of S-nitrosothiol reactivity: interplay of antagonistic resonance structures. J Phys Chem B. 2013;117:1827–1837. doi: 10.1021/jp310664z. doi: 10.1021/jp310664z. [DOI] [PubMed] [Google Scholar]
- 39.Miljkovic JLj, Kenkel I, Ivanović-Burmazović I, Filipovic MR. Generation of HNO and HSNO from nitrite by heme-iron-catalyzed metabolism with H2S. Angew Chem Int Ed Engl. 2013;52:12061–12064. doi: 10.1002/anie.201305669. doi: 10.1002/anie.201305669. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Macinkovic I, Devarie-Baez NO, Pan J, Park CM, Carroll KS, Filipovic MR, Xian M. Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl. 2014;53:575–581. doi: 10.1002/anie.201305876. doi: 10.1002/anie.201305876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogdándi V, Ida T, Sutton TR, Bianco C, Ditrói T, Koster G, Henthorn HA, Minnion M, Toscano JP, van der Vliet A, et al. Speciation of reactive sulfur species and their reactions with alkylating agents: do we have any clue about what is present inside the cell? Br J Pharmacol. 2019;176:646–670. doi: 10.1111/bph.14394. doi: 10.1111/bph.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dóka É, Arnér ESJ, Schmidt EE, Nagy P. ProPerDP: a protein persulfide detection protocol. Methods Mol Biol. 2019;2007:51–77. doi: 10.1007/978-1-4939-9528-8_5. doi: 10.1007/978-1-4939-9528-8_5. [DOI] [PubMed] [Google Scholar]
- 43.Hamid HA, Tanaka A, Ida T, Nishimura A, Matsunaga T, Fujii S, Morita M, Sawa T, Fukuto JM, Nagy P, et al. Polysulfide stabilization by tyrosine and hydroxyphenyl-containing derivatives that is important for a reactive sulfur metabolomics analysis. Redox Biol. 2019;21:101096. doi: 10.1016/j.redox.2019.101096. doi: 10.1016/j.redox.2019.101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajpal S, Katikaneni P, Deshotels M, Pardue S, Glawe J, Shen X, Akkus N, Modi K, Bhandari R, Dominic P, et al. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biol. 2018;15:480–489. doi: 10.1016/j.redox.2018.01.007. doi: 10.1016/j.redox.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol. 2010;160:941–957. doi: 10.1111/j.1476-5381.2010.00704.x. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y, Song Y, Zhu C, Li S, Xian M, Wai CM, Lin Y, Du D. Visualization of endogenous hydrogen sulfide in living cells based on Au nanorods@silica enhanced fluorescence. Anal Chim Acta. 2019;1053:81–88. doi: 10.1016/j.aca.2018.12.008. doi: 10.1016/j.aca.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Brown MD, Hall JR, Schoenfisch MH. A direct and selective electrochemical hydrogen sulfide sensor. Anal Chim Acta. 2019;1045:67–76. doi: 10.1016/j.aca.2018.08.054. doi: 10.1016/j.aca.2018.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bibli SI, Luck B, Zukunft S, Wittig J, Chen W, Xian M, Papapetropoulos A, Hu J, Fleming I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 2018;18:295–304. doi: 10.1016/j.redox.2018.07.016. doi: 10.1016/j.redox.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Zhu XY, Hu XX, Liu HW, Li J, Feng LL, Yin X, Zhang XB, Tan W. Ratiometric two-photon fluorescent probe for in vivo hydrogen polysulfides detection and imaging during lipopolysaccharide-induced acute organs injury. Anal Chem. 2016;88:11892–11899. doi: 10.1021/acs.analchem.6b03702. doi: 10.1021/acs.analchem.6b03702. [DOI] [PubMed] [Google Scholar]
- 50.Han Q, Mou Z, Wang H, Tang X, Dong Z, Wang L, Dong X, Liu W. Highly selective and sensitive one- and two-photon ratiometric fluorescent probe for intracellular hydrogen polysulfide sensing. Anal Chem. 2016;88:7206–7212. doi: 10.1021/acs.analchem.6b01391. doi: 10.1021/acs.analchem.6b01391. [DOI] [PubMed] [Google Scholar]
- 51.Yang F, Gao H, Li SS, An RB, Sun XY, Kang B, Xu JJ, Chen HY. A fluorescent τ-probe: quantitative imaging of ultra-trace endogenous hydrogen polysulfide in cells and in vivo. Chem Sci. 2018;9:5556–5563. doi: 10.1039/c8sc01879k. doi: 10.1039/c8sc01879k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren Y, Zhang L, Zhou Z, Luo Y, Wang S, Yuan S, Gu Y, Xu Y, Zha X. A new lysosome-targetable fluorescent probe with a large Stokes shift for detection of endogenous hydrogen polysulfides in living cells. Anal Chim Acta. 2019;1056:117–124. doi: 10.1016/j.aca.2018.12.051. doi: 10.1016/j.aca.2018.12.051. [DOI] [PubMed] [Google Scholar]
- 53.Li KB, Chen FZ, Yin QH, Zhang S, Shi W, Han DM. A colorimetric and near-infrared fluorescent probe for hydrogen polysulfides and its application in living cells. Sens Actuators B Chem. 2018;254:222–226. [Google Scholar]
- 54.Liu C, Chen W, Shi W, Peng B, Zhao Y, Ma H, Xian M. Rational design and bioimaging applications of highly selective fluorescence probes for hydrogen polysulfides. J Am Chem Soc. 2014;136:7257–7260. doi: 10.1021/ja502968x. doi: 10.1021/ja502968x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Zhang F, Munske G, Zhang H, Xian M. Isotope dilution mass spectrometry for the quantification of sulfane sulfurs. Free Radic Biol Med. 2014;76:200–207. doi: 10.1016/j.freeradbiomed.2014.08.003. doi: 10.1016/j.freeradbiomed.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iciek M, Górny M, Bilska-Wilkosz A, Kowalczyk-Pachel D. Is aldehyde dehydrogenase inhibited by sulfur compounds? In vitro and in vivo studies. Acta Biochim Pol. 2018;65:125–132. doi: 10.18388/abp.2017_2324. doi: 10.18388/abp.2017_2324. [DOI] [PubMed] [Google Scholar]
- 57.Park CM, Macinkovic I, Filipovic MR, Xian M. Use of the “tag-switch” method for the detection of protein S-sulfhydration. Methods Enzymol. 2015;555:39–56. doi: 10.1016/bs.mie.2014.11.033. doi: 10.1016/bs.mie.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 58.Wen YD, Wang H, Zhu YZ. The drug developments of hydrogen sulfide on cardiovascular disease. Oxid Med Cell Longev. 2018;2018:4010395. doi: 10.1155/2018/4010395. doi: 10.1155/2018/4010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 60.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan S, Pardue S, Shen X, Alexander JS, Orr AW, Kevil CG. Hydrogen sulfide metabolism regulates endothelial solute barrier function. Redox Biol. 2016;9:157–166. doi: 10.1016/j.redox.2016.08.004. doi: 10.1016/j.redox.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 64.Bolton SG, Cerda MM, Gilbert AK, Pluth MD. Effects of sulfane sulfur content in benzyl polysulfides on thiol-triggered H2S release and cell proliferation. Free Radic Biol Med. 2019;131:393–398. doi: 10.1016/j.freeradbiomed.2018.12.025. doi: 10.1016/j.freeradbiomed.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bibli SI, Hu J, Leisegang MS, Wittig J, Zukunft S, Kapasakalidi A, Fisslthaler B, Tsilimigras D, Zografos G, Filis K, et al. Shear stress regulates cystathionine γ lyase expression to preserve endothelial redox balance and reduce membrane lipid peroxidation. Redox Biol. 2020;28:101379. doi: 10.1016/j.redox.2019.101379. doi: 10.1016/j.redox.2019.101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung SH, Kwok WK, To KF, Lau JY. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (CSE) gene. PLoS One. 2014;9:e113038. doi: 10.1371/journal.pone.0113038. doi: 10.1371/journal.pone.0113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li JJ, Li Q, Du HP, Wang YL, You SJ, Wang F, Xu XS, Cheng J, Cao YJ, Liu CF, et al. Homocysteine triggers inflammatory responses in macrophages through inhibiting CSE-H2S signaling via DNA hypermethylation of CSE promoter. Int J Mol Sci. 2015;16:12560–12577. doi: 10.3390/ijms160612560. doi: 10.3390/ijms160612560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan S, Yurdagul A, Jr, Peretik JM, Alfaidi M, Al Yafeai Z, Pardue S, Kevil CG, Orr AW. Cystathionine γ-lyase modulates flow-dependent vascular remodeling. Arterioscler Thromb Vasc Biol. 2018;38:2126–2136. doi: 10.1161/ATVBAHA.118.311402. doi: 10.1161/ATVBAHA.118.311402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bibli SI, Hu J, Sigala F, Wittig I, Heidler J, Zukunft S, Tsilimigras DI, Randriamboavonjy V, Wittig J, Kojonazarov B, et al. Cystathionine γ lyase sulfhydrates the RNA binding protein human antigen R to preserve endothelial cell function and delay atherogenesis. Circulation. 2019;139:101–114. doi: 10.1161/CIRCULATIONAHA.118.034757. doi: 10.1161/CIRCULATIONAHA.118.034757. [DOI] [PubMed] [Google Scholar]
- 70.Ikeda M, Ishima Y, Chuang VTG, Sakai M, Osafune H, Ando H, Shimizu T, Okuhira K, Watanabe H, Maruyama T, et al. Distribution of polysulfide in human biological fluids and their association with amylase and sperm activities. Molecules. 2019;24:E1689. doi: 10.3390/molecules24091689. doi: 10.3390/molecules24091689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem Int. 2013;63:492–497. doi: 10.1016/j.neuint.2013.09.003. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Miyamoto R, Koike S, Takano Y, Shibuya N, Kimura Y, Hanaoka K, Urano Y, Ogasawara Y, Kimura H. Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci Rep. 2017;7:45995. doi: 10.1038/srep45995. doi: 10.1038/srep45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao X, Nie X, Xiong S, Cao L, Wu Z, Moore PK, Bian JS. Renal protective effect of polysulfide in cisplatin-induced nephrotoxicity. Redox Biol. 2018;15:513–521. doi: 10.1016/j.redox.2018.01.012. doi: 10.1016/j.redox.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munday R, Munday JS, Munday CM. Comparative effects of mono-, di-, tri-, and tetrasulfides derived from plants of the allium family: redox cycling in vitro and hemolytic activity and Phase 2 enzyme induction in vivo. Free Radic Biol Med. 2003;34:1200–1211. doi: 10.1016/s0891-5849(03)00144-8. doi: 10.1016/s0891-5849(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 75.Zhang T, Ono K, Tsutsuki H, Ihara H, Islam W, Akaike T, Sawa T. Enhanced cellular polysulfides negatively regulate tlr4 signaling and mitigate lethal endotoxin shock. Cell Chem Biol. 2019;26:686–698.e4. doi: 10.1016/j.chembiol.2019.02.003. doi: 10.1016/j.chembiol.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–H2418. doi: 10.1152/ajpheart.00044.2012. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rushing AM, Donnarumma E, Polhemus DJ, Au KR, Victoria SE, Schumacher JD, Li Z, Jenkins JS, Lefer DJ, Goodchild TT. Effects of a novel hydrogen sulfide prodrug in a porcine model of acute limb ischemia. J Vasc Surg. 2019;69:1924, 1935. doi: 10.1016/j.jvs.2018.08.172. doi: 10.1016/j.jvs.2018.08.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajendran S, Shen X, Glawe J, Kolluru GK, Kevil CG. Nitric oxide and hydrogen sulfide regulation of ischemic vascular growth and remodeling. Compr Physiol. 2019;9:1213, 1247. doi: 10.1002/cphy.c180026. doi: 10.1002/cphy.c180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giannakopoulou E, Konstantinou F, Ragia G, Gerontitis Z, Tavridou A, Papapetropoulos A, Mikroulis D, Manolopoulos VG. Association study of the CTH 1364 g>t polymorphism with coronary artery disease in the greek population. Drug Metab Pers Ther. 2019;34 doi: 10.1515/dmpt-2018-0033. doi: 10.1515/dmpt-2018-0033. [DOI] [PubMed] [Google Scholar]
- 82.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, et al. WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yiannakouris N, Katsoulis M, Dilis V, Parnell LD, Trichopoulos D, Ordovas JM, Trichopoulou A. Genetic predisposition to coronary heart disease and stroke using an additive genetic risk score: a population-based study in Greece. Atherosclerosis. 2012;222:175–179. doi: 10.1016/j.atherosclerosis.2012.02.033. doi: 10.1016/j.atherosclerosis.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


