Abstract
BACKGROUND
The choice of drugs for patients with status epilepticus that is refractory to treatment with benzodiazepines has not been thoroughly studied.
METHODS
In a randomized, blinded, adaptive trial, we compared the efficacy and safety of three intravenous anticonvulsive agents — levetiracetam, fosphenytoin, and valproate — in children and adults with convulsive status epilepticus that was unresponsive to treatment with benzodiazepines. The primary outcome was absence of clinically evident seizures and improvement in the level of consciousness by 60 minutes after the start of drug infusion, without additional anticonvulsant medication. The posterior probabilities that each drug was the most or least effective were calculated. Safety outcomes included life-threatening hypotension or cardiac arrhythmia, endotracheal intubation, seizure recurrence, and death.
RESULTS
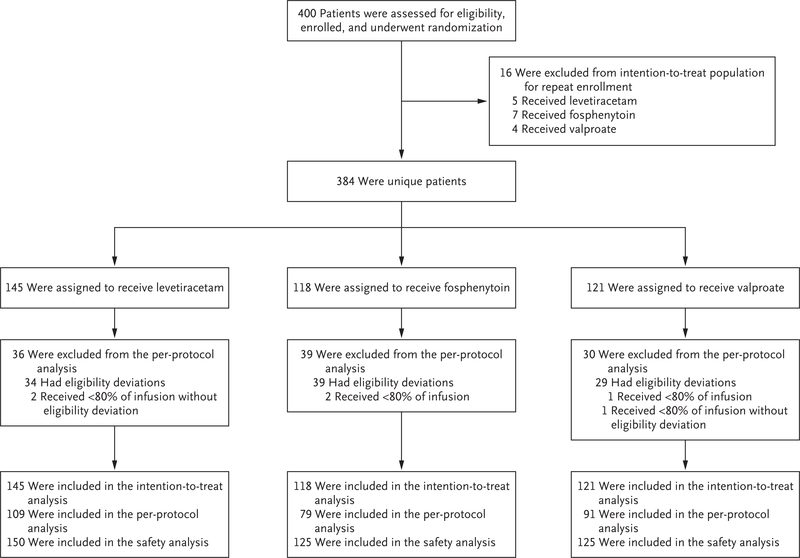
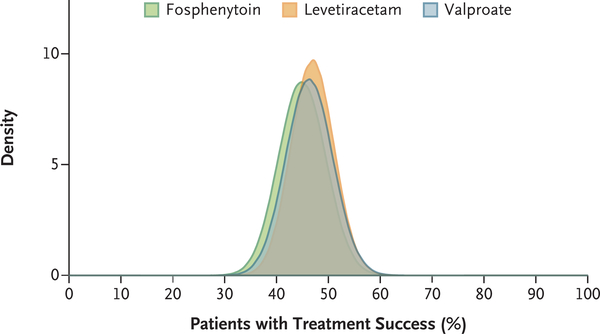
A total of 384 patients were enrolled and randomly assigned to receive levetiracetam (145 patients), fosphenytoin (118), or valproate (121). Reenrollment of patients with a second episode of status epilepticus accounted for 16 additional instances of randomization. In accordance with a prespecified stopping rule for futility of finding one drug to be superior or inferior, a planned interim analysis led to the trial being stopped. Of the enrolled patients, 10% were determined to have had psychogenic seizures. The primary outcome of cessation of status epilepticus and improvement in the level of consciousness at 60 minutes occurred in 68 patients assigned to levetiracetam (47%; 95% credible interval, 39 to 55), 53 patients assigned to fosphenytoin (45%; 95% credible interval, 36 to 54), and 56 patients assigned to valproate (46%; 95% credible interval, 38 to 55). The posterior probability that each drug was the most effective was 0.41, 0.24, and 0.35, respectively. Numerically more episodes of hypotension and intubation occurred in the fosphenytoin group and more deaths occurred in the levetiracetam group than in the other groups, but these differences were not significant.
CONCLUSIONS
In the context of benzodiazepine-refractory convulsive status epilepticus, the anticonvulsant drugs levetiracetam, fosphenytoin, and valproate each led to seizure cessation and improved alertness by 60 minutes in approximately half the patients, and the three drugs were associated with similar incidences of adverse events. (Funded by the National Institute of Neurological Disorders and Stroke; ESETT ClinicalTrials.gov number, NCT01960075.)
EVIDENCE SUPPORTS THE USE OF BENZOdiazepines as the initial treatment for status epilepticus1–3; however, seizures do not respond to benzodiazepines in up to a third of patients. The treatment for this type of benzodiazepine-refractory status epilepticus has not been well studied.1,3 Of the three medications most commonly used to treat benzodiazepine-refractory status epilepticus — levetiracetam, fosphenytoin, and valproate4–7 — only fosphenytoin is labeled by the Food and Drug Administration (FDA) for this indication in adults, and none has been approved for children.
Early termination of convulsive status epilepticus decreases the risk of cardiac and respiratory complications2 and is associated with a reduced risk of admission to an intensive care unit (ICU)1,2 and decreased mortality among children.8 Convulsive and nonconvulsive status epilepticus are also associated with neuroimaging evidence of brain injury in humans and with neuronal loss in experimental models.9,10 Clinical guidelines emphasize the need for rapid control of benzodiazepine-refractory status epilepticus but do not provide guidance regarding the choice of medication on the basis of either efficacy or safety.6,7 We performed a randomized clinical trial to determine the superiority or inferiority of the three commonly used anticonvulsant medications with regard to treatment success among patients with status epilepticus in the emergency department.
METHODS
TRIAL OVERSIGHT
The Established Status Epilepticus Treatment Trial (ESETT) was an investigator-initiated, multicenter, randomized, blinded, comparative-effectiveness trial of levetiracetam, fosphenytoin, and valproate for the treatment of patients with established status epilepticus in the emergency department. The trial was developed through a program funded by the National Institutes of Health and the FDA and was conducted by the Neurological Emergencies Treatment Trials (NETT) Network and the Pediatric Emergency Care Applied Research Network (PECARN).11 The investigators were responsible for the trial design, data collection, and data analysis. The authors wrote the manuscript and vouch for the accuracy and completeness of the data and reporting of adverse events and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org. The trial was performed under an Investigational New Drug application with the FDA.
The trial was conducted under the exception from informed-consent requirements for emergency research (FDA regulation 21 CFR 50.2412). The institutional review boards for all participating institutions approved the protocol after consultation with the local community and public disclosure. Patients or their legally authorized representatives were notified about enrollment in the trial by the research team as soon as possible, usually while the patient was still in the emergency department, and were asked to provide written informed consent for continued data collection through the end of the trial.
Patients were enrolled at 57 hospital emergency departments across the United States. Sites included academic medical centers and community hospitals; 18 sites enrolled only children, 26 sites enrolled only adults, and 13 sites enrolled both. Emergency department clinical teams, including pharmacists, nurses, and physicians, were provided training in the trial protocol and continuing medical education in the management of seizures, and refresher protocol training was provided throughout the trial.
ELIGIBILITY CRITERIA
Patients were eligible for participation if they were 2 years of age or older, had been treated with a generally accepted cumulative dose of benzodiazepines for generalized convulsive seizures lasting more than 5 minutes, and continued to have persistent or recurrent convulsions in the emergency department at least 5 minutes after the last dose of benzodiazepine (to provide sufficient time for the drug at this dose to act) and no more than 30 minutes after the last dose of benzodiazepine (to avoid enrolling patients for whom readministration of benzodiazepines would have been appropriate). The seizure and its initial treatment with benzodiazepines could occur before the patient’s arrival in the emergency department.
The minimal adequate cumulative doses of benzodiazepines were defined as diazepam at a dose of 10 mg (administered intravenously or rectally), lorazepam at a dose of 4 mg (administered intravenously), or midazolam at a dose of 10 mg (administered intravenously or intramuscularly) for all adults and for children with a body weight of at least 32 kg; and diazepam at a dose of 0.3 mg per kilogram of body weight (administered intravenously or rectally), lorazepam at a dose of 0.1 mg per kilogram (administered intravenously), or midazolam at a dose of 0.3 mg of per kilogram (administered intramuscularly) or 0.2 mg per kilogram (administered intravenously) for children who weighed less than 32 kg. These drugs may have been administered in divided doses, including before the patient’s arrival in the emergency department.
Patients were excluded from the trial for the following reasons, as determined on arrival in the emergency department: the acute precipitant of seizure was major trauma, hypoglycemia, hyperglycemia, cardiac arrest, or postanoxia; the patient was pregnant or incarcerated; or the patient preemptively opted out of this trial by wearing a medical alert tag marked “ESETT declined” (these tags were made available by the trial when requested). Patients were also excluded if they had already been treated for the current episode of status epilepticus with anticonvulsant agents other than benzodiazepines or if the trachea was intubated. We excluded patients with known allergy or contraindications to any of the trial drugs, including known inborn metabolic disorder, liver disease, or severe renal impairment. The trial included patients who were taking anticonvulsants for the control of seizures. Those patients were randomly assigned to a treatment group without regard to the anticonvulsant medications they were using for long-term treatment.6,7
TRIAL TREATMENTS
After determining a patient’s eligibility in the emergency department, the clinical team accessed an age-stratified trial “use next” medication box in proximity to patient care areas in the emergency department. The medication box was opened, a protocol assist device was activated, and the assigned trial drug vial and administration set were used to prime an intravenous infusion line. The protocol assist device was a mobile electronic device that was used to automatically activate the research team, remind treatment teams about eligibility criteria and protocol interventions, provide timed alerts, obtain audio recordings of the clinical event, and facilitate unmasking of the trial drug if required for patient care. A body weight–based infusion rate was determined from an enclosed dose-administration chart with the use of a measured, stated, or estimated body weight. Alternatively, the infusion rate could be determined from an enclosed length-based weight-estimation tool for children for whom an accurate weight was not known. The trial drug was administered by an infusion pump programmed with a predetermined rate over a period of 10 minutes.
Trial-drug vials contained levetiracetam (50 mg per milliliter), fosphenytoin (16.66 mg phenytoin equivalents [mgPE] per milliliter), or valproate (33.33 mg per milliliter). The weight-based infusion rate provided levetiracetam at a dose of 60 mg per kilogram (maximum, 4500 mg), fosphenytoin at a dose of 20 mgPE per kilogram (maximum, 1500 mgPE), or valproate at a dose of 40 mg per kilogram (maximum, 3000 mg). Medication vials were produced, packaged, and labeled by the University of California at Davis Good Manufacturing Practice facility. The purity, concentration, sterility, and stability of drugs were determined by ARL Bio Pharma. Trial drugs were identical in appearance, formulation, packaging, and administration, including the total volume in the vial and duration of infusion. After 10 minutes, the infusion of the trial drug was discontinued. Rescue therapy was given as clinically determined by the care team for persistent or recurrent seizures after 20 minutes from the start of trial-drug infusion. Unmasking of the trial drug for purposes of patient care, after determination of the primary outcome at 60 minutes, was allowed, but emergency unblinding (before 60 minutes) was considered a protocol deviation.
OUTCOMES
The primary outcome was an absence of clinically apparent seizures and improving responsiveness at 60 minutes after the start of trial-drug infusion, without additional anticonvulsant medication, including medication used for endotracheal intubation. Clinically apparent seizure was determined by the treating emergency department physician and was defined as visually observed focal or generalized tonic–clonic movements, nystagmoid or rhythmic eye movements, or generalized or segmental myoclonus. Improvement in responsiveness was also determined by the treating physician and was defined as purposeful responses to noxious stimuli, the ability to follow commands, or verbalization. The primary outcome as determined by the attending physician was communicated to the research team, a member of which was present at the bedside in the emergency department (Table S5 in the Supplementary Appendix, available at NEJM.org).
Secondary efficacy outcomes included time to termination of seizures, as determined in the subgroup of patients with audio recordings that made accurate determination of times possible; admission to the ICU; and the length of ICU and hospital stays. The time to termination of seizures was defined as the interval from the start of infusion of the trial drug to the cessation of clinically apparent seizures. A central clinical phenomenology core of four neurologists adjudicated from the medical records the time to seizure cessation, the time in status epilepticus before trial-drug initiation, and the cause of the seizure. For each enrollment, two neurologists from this core group conducted independent initial reviews and then determined a consensus or consulted a third adjudicator, as needed. The primary outcome was also adjudicated for use in a secondary analysis. Adjudicators were unaware of the treatment assignments and made determinations by medical record review.
The primary safety outcome was the composite of life-threatening hypotension or cardiac arrhythmia within 60 minutes after the start of trial-drug infusion. Data on serious adverse events were collected through the end of participation in the trial (hospital discharge or 30 days, whichever came first) for every patient. Data on adverse events were collected through the first 24 hours after enrollment. Life-threatening hypotension was defined as systolic blood pressure remaining below the age-specified thresholds on two consecutive readings at least 10 minutes apart and remaining below the age-specified thresholds for more than 10 minutes after reduction of the rate of trial-drug infusion (or its termination) and an intravenous fluid challenge. Life-threatening cardiac arrhythmia was defined as any arrhythmia that persisted despite reducing the rate of infusion of the trial drug and that led to intervention with chest compressions, pacing, defibrillation, or the use of an antiarrhythmic agent or procedure. Additional safety outcomes included death before the end of participation in the trial, endotracheal intubation within 60 minutes after the start of trial-drug infusion, acute seizure recurrence more than 60 minutes after the start of trial-drug infusion, and acute anaphylaxis. Acute seizure recurrence was defined as convulsive or electroencephalographic seizure activity triggering further anticonvulsant therapy occurring between 60 minutes and 12 hours after the start of trial-drug infusion. The definition of acute seizure recurrence excluded patients who were given additional anticonvulsants as prophylaxis or as treatment for vague or uncertain clinical findings or nondiagnostic findings on electroencephalography. Electroencephalography was not performed for this trial, but electroencephalographic data were collected if it was performed as part of clinical care.
STATISTICAL ANALYSIS
We used a response-adaptive comparative-effectiveness design. Patients were randomly assigned to receive one of the three trial drugs, initially in a 1:1:1 ratio.13 After 300 patients were assigned to a treatment group, response-adaptive randomization was initiated on the basis of previously defined decision rules, with the goal of maximizing the likelihood of identifying the most effective treatment. Interim analyses were planned after the enrollment of 400, 500, 600, and 700 patients, at which times the trial could be stopped early for success or futility, the rules for which are contained in the protocol. At each interim analysis, the randomization assignment probabilities were updated. The maximum sample was 795 patients. Randomization was stratified according to age category (2 to 17 years, 18 to 65 years, and >65 years) at the targeted assignment probabilities.13
Before assessment of the trial results, all three drugs were considered to be equally likely to be the most effective or least effective treatment. Response rates in each of the treatment groups were modeled independently with the use of Bayesian analysis. The percentage of patients with treatment success in each group was calculated starting with a uniform(0,1) prior probability distribution (which allows the treatment success to take any value between 0 and 100%) and was updated on the basis of the observed binomial data with the use of a conjugate beta-binomial model. From these three posterior distributions, the probability that each treatment was the most or least effective treatment was calculated as described previously.11 We randomly and repeatedly (106 iterations) drew from these three posterior probabilities to calculate the probability that a given treatment was better than the other two. The same approach was taken for the potentially worst treatment. The criterion for declaring a most or least effective treatment was a probability greater than 0.975. The threshold of 0.975 was chosen by convention (analogous to an alpha of 0.025 in a one-sided comparison) and because a simulation study showed that with this threshold and trial design, the type I error rate was controlled. Unlike a trial in which success can be achieved in a number of different ways (e.g., multiple treatments vs. a control), only one treatment could be identified as best. A maximum sample of 720 unique patients from 795 enrollments provided 90% power to identify the most effective treatment when one treatment group had a true response rate of 65% and the true response rate was 50% in the other two groups (an absolute difference of 15 percentage points).
We report the percentage response in each treatment group with 95% credible intervals. The primary analysis was based on the intention-to-treat population and included all unique patients who underwent randomization, regardless of the amount of treatment that was actually received. Patients who enrolled more than once for separate episodes of status epilepticus had only their first enrollment included in the primary efficacy analysis, but both enrollments were included in the safety analysis. At each planned interim analysis, the predictive probability of identifying either a most effective or a least effective treatment at the maximum sample size was calculated. If the predictive probability was greater than 0.975, then the trial would be stopped for success; if less than 0.05, it would be stopped for futility.
Secondary sensitivity analyses of the primary outcome included a per-protocol analysis (excluding patients who had eligibility deviations or who did not receive the intervention) and an analysis of the adjudicated primary outcome. Binary outcomes were compared by first testing, in a chi-square test (or Fisher’s exact test, depending on the frequency of events), the null hypothesis that the percentages of responses in all three treatment groups were equal. If the three-way null hypothesis was rejected, then all pairwise comparisons would be performed as two-sample tests of proportions. Baseline covariates of age group (<18 years or ≥18 years), weight group (<75 kg or ≥75 kg), final diagnosis, time from seizure onset to enrollment, sex, race (black, white, or other), and ethnic group (Hispanic or non-Hispanic) were evaluated individually in logistic-regression models that included treatment group, the main effect of the covariate, and interaction terms with treatment. There was no planned adjustment for multiple comparisons of secondary outcomes, and these results are presented as point estimates and interquartile ranges from which no conclusions can be drawn.
RESULTS
PATIENTS
A total of 400 enrollments of 384 unique patients occurred from November 3, 2015, to October 31, 2017 (Fig. 1). Sixteen patients were enrolled twice, and their second enrollment was not included in the intention-to-treat analysis. In November 2017, enrollment was discontinued at the recommendation of the data and safety monitoring board after the trial met the predefined futility criterion in a planned interim analysis, since there was a 1% chance of showing a most effective or least effective treatment if the trial were to continue to the maximum sample size. Computations for the futility analysis are given in Table S6. A predefined analysis did not exclude an interaction with age, so it was decided to continue enrollment in the pediatric subcohort to enrich a planned secondary subgroup analysis according to age, which has not been performed.
Figure 1. Randomization, Group Assignments, and Analyses.
The safety analysis included all enrollments (including patients who enrolled more than once). The intention-to-treat analysis included all unique patients but did not include repeat enrollments of the same patient. The per-protocol analysis excluded repeat enrollments, enrollments in which there were eligibility deviations, and enrollments in which patients did not receive the assigned drug dose.
The baseline characteristics of the patients were similar in the three treatment groups (Table 1). A total of 55% of the patients who were enrolled were male, 43% were black, and 16% were Hispanic; 39% were children and adolescents (up to 17 years of age), 48% were younger adults (18 to 65 years of age), and 13% were older adults (>65 years of age). Most of the enrolled patients had a final diagnosis of status epilepticus (87%), as determined retrospectively by the clinical phenomenology core, and 10% had psychogenic nonepileptic seizures. The adjudicated causes of status epilepticus are shown in Table S3.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Levetiracetam (N = 145) | Fosphenytoin (N = 118) | Valproate (N = 121) |
|---|---|---|---|
| Age — yr | |||
| Mean | 33.3±26.0 | 32.8±25.4 | 32.2±25.4 |
| Range | 1–94 | 1–84 | 1–85 |
| Age group — no. (%) | |||
| 0–5 Yr | 30 (20.7) | 24 (20.3) | 28 (23.1) |
| 6–10 Yr | 17 (11.7) | 15 (12.7) | 7 (5.8) |
| 11–20 Yr | 9 (6.2) | 10 (8.5) | 18 (14.9) |
| 21–40 Yr | 31 (21.4) | 20 (16.9) | 19 (15.7) |
| 41–60 Yr | 34 (23.4) | 26 (22.0) | 26 (21.5) |
| ≥61 Yr | 24 (16.6) | 23 (19.5) | 23 (19.0) |
| Male sex — no. (%) | 77 (53.1) | 71 (60.2) | 65 (53.7) |
| Race — no. (%)† | |||
| Black | 62 (42.8) | 49 (41.5) | 54 (44.6) |
| White | 62 (42.8) | 49 (41.5) | 49 (40.5) |
| Other, more than one, or unknown | 21 (14.5) | 20 (16.9) | 18 (14.9) |
| Hispanic ethnic group — no. (%)† | 23 (15.9) | 18 (15.3) | 22 (18.2) |
| History of epilepsy — no. (%)‡ | 97 (66.9) | 80 (67.8) | 83 (68.6) |
| Final diagnosis — no. (%)‡ | |||
| Seizure or status epilepticus | 128 (88.3) | 104 (88.1) | 102 (84.3) |
| Nonepileptic spell | 13 (9.0) | 11 (9.3) | 13 (10.7) |
| Unable to adjudicate | 4 (2.8) | 3 (2.5) | 6 (5.0) |
| Median lorazepam dose equivalents (IQR)§ | |||
| Dose for patients weighing ≥32 kg — mg | 4.7 (4.0–6.0) | 4.9 (4.0–6.0) | 5.0 (4.0–7.0) |
| Dose for patients weighing <32 kg — mg/kg | 0.2 (0.2–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.2) |
| Median duration of seizure at enrollment (IQR) — min‡¶ | 62.0 (43.0–85.0) | 59.0 (43.0–94.0) | 61.5 (38.5–86.5) |
| Benzodiazepines given before arrival — no. (%) | 89 (61.4) | 68 (57.6) | 62 (51.2) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. IQR denotes interquartile range.
Information on race and ethnic group was obtained from the medical record.
The determination of this characteristic was based on adjudication.
Data include all 400 enrollments (i.e., including the patients who enrolled more than once).
The duration of seizure at enrollment could not be determined for 39 enrollments.
Because of the emergency setting of the trial, deviations from the eligibility criteria occurred in 108 enrollments (27%). These patients were followed and included in the primary analysis. Deviations were due to benzodiazepines having been administered too long before or too proximate to enrollment (50 patients), inadequate cumulative doses of benzodiazepines having been administered before enrollment (26 patients), and enrollment of patients without status epilepticus (33 patients), including patients with psychogenic nonepileptic seizures. Unblinding of investigators and treating clinicians to the assigned trial drug in situations in which it was considered necessary for patient care occurred in 200 of 400 enrollments; most of these instances of unblinding occurred after the primary outcome had been determined at 60 minutes (154 patients). Unblinding occurred before 60 minutes in 46 patients, but only after a criterion for failure with regard to the primary outcome had already been met. These unblinding events were performed by treating clinicians to inform the choice of an additional dose of levetiracetam, fosphenytoin, or valproate in patients with persistent seizures.
Electroencephalography was performed as part of standard care within 24 hours after seizure onset in 60% of enrollments. Continuous or prolonged recordings were obtained for 157 of the 238 patients (66%) with electroencephalographic data, and routine 30-minute recordings were obtained for 81 patients (34%).
EFFICACY ANALYSIS
In the intention-to-treat analysis, an absence of seizures and improvement in responsiveness without additional anticonvulsant medications at 60 minutes after trial-drug administration was found in 68 of 145 patients (47%) in the levetiracetam group, 53 of 118 (45%) in the fosphenytoin group, and 56 of 121 (46%) in the valproate group (Table 2 and Fig. 2). The posterior probability that levetiracetam was better than fosphenytoin and valproate was 0.41, the probability that fosphenytoin was better than levetiracetam and valproate was 0.24, and the probability that valproate was better than levetiracetam and fosphenytoin was 0.35. The results were similar in the per-protocol and adjudicated-outcome analyses (Table 2). Pairwise treatment-group differences are shown in Table S2. There was no interaction between treatment group and age group or any other interaction with a baseline covariate or trial site (Table S4). Among the 207 patients in whom the primary outcome was not achieved, 144 (70%) were treated with additional anticonvulsant medications (Table S5). Another 52 of these patients (25%) did not receive additional medication and had not had a clinically apparent seizure at 60 minutes, but they did not have improving responsiveness at 60 minutes.
Table 2.
Efficacy Analyses.*
| Outcome and Population | Levetiracetam (N = 145) | Fosphenytoin (N = 118) | Valproate (N = 121) |
|---|---|---|---|
| Primary efficacy outcome: cessation of seizures and improvement in consciousness at 60 min without other anticonvulsant medications | |||
| Intention-to-treat population | |||
| No. with outcome | 68 | 53 | 56 |
| Percent of patients with outcome (95% credible interval) | 47 (39–55) | 45 (36–54) | 46 (38–55) |
| Probability that treatment is the most effective | 0.41 | 0.24 | 0.35 |
| Probability that treatment is the least effective | 0.24 | 0.45 | 0.31 |
| Per-protocol population | |||
| No. with outcome/total no. | 51/109 | 37/79 | 43/91 |
| Percent of patients with outcome (95% credible interval) | 47 (38–56) | 47 (36–58) | 47 (37–57) |
| Probability that treatment is the most effective | 0.31 | 0.34 | 0.36 |
| Probability that treatment is the least effective | 0.34 | 0.35 | 0.31 |
| Adjudicated-outcomes population | |||
| No. with outcome | 67 | 57 | 60 |
| Percent with outcome (95% credible interval) | 46 (38–54) | 48 (39–57) | 50 (41–58) |
| Probability that treatment is the most effective | 0.17 | 0.35 | 0.48 |
| Probability that treatment is the least effective | 0.51 | 0.29 | 0.20 |
| Secondary efficacy outcomes | |||
| Admission to ICU — no. (%) | 87 (60.0) | 70 (59.3) | 71 (58.7) |
| Median length of ICU stay (IQR) — days | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| Median length of hospital stay (IQR) — days | 3 (1–7) | 3 (1–6) | 3 (2–6) |
| Median time from start of trial-drug infusion to termination of seizures for patients with treatment success (IQR) — min† | 10.5 (5.7–15.5) | 11.7 (7.5–20.9) | 7.0 (4.6–14.9) |
ICU denotes intensive care unit.
Data were available for 14 patients in the levetiracetam group, 15 patients in the fosphenytoin group, and 10 patients in the valproate group.
Figure 2. Posterior Probabilities of Success According to Treatment Group for the Primary Outcome of Cessation of Status Epilepticus at 60 Minutes.
The relative posterior probabilities of treatment success with regard to the primary outcome for each drug are shown. The percentage of patients with treatment success was 47% (95% credible interval, 39 to 55) in the levetiracetam group, 45% (95% credible interval, 36 to 54) in the fosphenytoin group, and 46% (95% credible interval, 38 to 55) in the valproate group.
The median time from the start of trial-drug infusion to seizure termination among the 39 patients who met the primary outcome criteria for treatment success and for whom an audio recording was available was 10.5 minutes (interquartile range, 5.7 to 15.5) in the levetiracetam group, 11.7 minutes (interquartile range, 7.5 to 20.9) in the fosphenytoin group, and 7.0 minutes (interquartile range, 4.6 to 14.9) in the valproate group. There was also no significant difference among the three groups in a post hoc analysis of seizure cessation within 20 minutes after trial-drug initiation in patients with treatment success (Table S7). The percentage of patients with acute seizure recurrence (i.e., seizure activity triggering further anticonvulsant therapy occurring between 60 minutes and 12 hours after the start of trial-drug infusion) was 10.7%, 11.2%, and 11.2%, respectively.
SAFETY ANALYSIS
The frequency of life-threatening hypotension (0.7% in the levetiracetam group, 3.2% in the fosphenytoin group, and 1.6% in the valproate group), arrhythmia (0.7% in the levetiracetam group and no cases in either of the other two groups), endotracheal intubation (20.0%, 26.4%, and 16.8%, respectively), and other safety outcomes did not differ significantly among the treatment groups (Table 3). We did not detect a significant difference in the frequency of the composite safety outcome of life-threatening hypotension or cardiac arrhythmia among the treatment groups (1.3% in the levetiracetam group, 3.2% in the fosphenytoin group, and 1.6% in the valproate group).
Table 3.
Safety Analyses.*
| Outcome | Levetiracetam (N = 150) | Fosphenytoin (N = 125) | Valproate (N = 125) |
|---|---|---|---|
| number of patients (percent) | |||
| Life-threatening hypotension within 60 min after start of trial-drug infusion | 1 (0.7) | 4 (3.2) | 2 (16) |
| Life-threatening cardiac arrhythmia within 60 min after start of trial-drug infusion | 1 (07) | 0 | 0 |
| Endotracheal intubation within 60 min after start of trial-drug infusion | 30 (20.0) | 33 (26.4) | 21 (16.8) |
| Acute seizure recurrence 60 min to 12 hr after start of trial-drug infusion | 16 (10.7) | 14 (11.2) | 14 (11.2) |
| Acute anaphylaxis | 0 | 0 | 0 |
| Acute respiratory depression | 12 (8.0) | 16 (12.8) | 10 (8.0) |
| Hepatic aminotransferase or ammonia elevations | 1 (0.7) | 0 | 1 (0.8) |
| Purple glove syndrome | 0 | 0 | 0 |
| Death | 7 (4.7) | 3 (2.4) | 2 (1.6) |
No significant differences among the groups were detected for safety outcomes.
Serious adverse events are summarized in Table S1. A total of 248 serious adverse events occurred in 42% of patients. The most frequent serious adverse events were convulsions after 60 minutes, a depressed level of consciousness, and respiratory distress.
DISCUSSION
In this prospective, randomized, double-blind, adaptive comparative-effectiveness trial involving patients with benzodiazepine-refractory status epilepticus, we found no significant difference in the percentage of patients with seizure cessation among the levetiracetam group, fosphenytoin group, and valproate group. The results of a planned interim analysis performed at the time that 400 patients had been enrolled met a predefined futility criterion for stopping the trial. Status epilepticus stopped in approximately 50% of patients in each treatment group. Hypotension and endotracheal intubation were more frequent with fosphenytoin than with the other two drugs, and deaths were more frequent with levetiracetam, but these differences were not significant. The differences in the time to cessation of seizures after the start of the trial-drug infusion numerically favored valproate but were not subject to formal analysis because of the limited number of patients for whom audio recordings were available to corroborate the time of seizure cessation.
The results of this trial contrast with those of previous, mostly observational studies that used varying definitions of cessation of status epilepticus.14–19 In a retrospective review involving 279 adult patients with benzodiazepine-refractory status epilepticus who were not randomly assigned to a drug treatment, the percentage of patients in whom seizures were stopped was 51.7% with levetiracetam, and the percentages with valproate and phenytoin (74.6% and 59.6%, respectively) were higher than those in the current trial.14 A meta-analysis of 22 studies showed higher effectiveness with levetiracetam (68.5%) and valproate (75.7%) than was seen in our trial but similar effectiveness with phenytoin (50.2%).15
The strengths of our trial include the relatively large sample of 400 enrollments, which provided adequate power to detect a difference between treatment groups, and the use of weight-based dosing. We also used an adaptive statistical design to increase the chance of finding a difference if a true difference existed.
Limitations of this trial included the need for unblinding in some instances in order to choose a second anticonvulsant to treat ongoing seizures (occurring after the determination of the primary outcome in most patients) and the fact that 10% of the patients enrolled had psychogenic nonepileptic seizures. Clinical rather than electroencephalographic criteria were used to determine the primary outcome of seizure cessation. Without electrographic confirmation, it is not possible to distinguish postictal or benzodiazepine-related sedation from continued nonconvulsive status epilepticus as the cause of treatment failure in the 52 patients who had resolution of clinically evident seizure without additional anticonvulsant medications but did not have improving consciousness at 60 minutes. We chose doses of trial drugs from published experience in status epilepticus, but other doses may have different efficacy. Fosphenytoin has more restrictions on the maximal rate of infusion than the other agents; the constraint of a 10-minute infusion limited the maximal dose to 1500 mgPE, which may be a submaximal dose in patients with a body weight greater than 75 kg. All serious adverse events were recorded; however, data on nonserious adverse events occurring more than 24 hours after enrollment were not collected, and therefore events such as rashes or self-limited liver-enzyme elevations with delayed presentations may have been missed. Finally, a relatively high percentage of enrolled patients had eligibility deviations related to benzodiazepine dosing. However, the results of the per-protocol analyses were concordant with those of the primary analysis.
In conclusion, fosphenytoin, valproate, and levetiracetam were effective in approximately half the patients with benzodiazepine-refractory status epilepticus, and they did not differ significantly with regard to effectiveness and safety.
Supplementary Material
Acknowledgments
Supported by grants (U01NS088034, U01NS088023, U01NS056975, U01NS059041, and U01NS073476) from the National Institute of Neurological Disorders and Stroke (NINDS).
Dr. Cloyd reports holding patent US9629797B2 on intravenous carbamazepine and holding intellectual property on intravenous topiramate, licensed to Ligand; Dr. Lowenstein, serving on an advisory board for Bloom Science; Dr. Shinnar, receiving fees for serving on data and safety monitoring boards from Eisai, INSYS Therapeutics, and UCB Biosciences; Dr. Cock, receiving consulting fees from BIAL Pharma UK and Sage Therapeutics; and Dr. Fountain, receiving grant support, paid to the University of Virginia Rectors and Visitors, from Cerebral Therapeutics, GW Pharmaceuticals, Medtronic, Neurelis, NeuroPace, SK Life Science, Takeda California, and UCB Biosciences. No other potential conflict of interest relevant to this article was reported.
Footnotes
A list of the NETT and PECARN investigators is provided in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Gerhard Bauer and Brian Fury of the University of California at Davis Good Manufacturing Practice Laboratory for manufacturing of the investigational drug products; Jessica Munson of ARL Bio Pharma for testing the quality and stability of the investigational drugs; Henry Wang and Edward Jauch for serving as independent medical safety monitors; the members of the data and safety monitoring board (Barbara Dworetzky [chair], Gail Anderson, Jeffrey Buchhalter, Elizabeth Sugar, Alexis Topjian, and Peter Gilbert [NINDS liaison]); and especially all our patients and the emergency department nurses, pharmacists, and physicians who made the trial possible.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
CLINICAL TRIAL REGISTRATION
The Journal requires investigators to register their clinical trials in a public trials registry. The members of the International Committee of Medical Journal Editors (ICMJE) will consider most reports of clinical trials for publication only if the trials have been registered. Current information on requirements and appropriate registries is available at www.icmje.org/about-icmje/faqs/.
Contributor Information
Jaideep Kapur, Department of Neurology, University of Virginia, Charlottesville
Jordan Elm, Data Coordination Unit, Department of Public Health Sciences, Medical University of South Carolina, Charleston
James M. Chamberlain, Division of Emergency Medicine, Children’s National Medical Center, Washington, DC
William Barsan, Department of Emergency Medicine, University of Michigan, Ann Arbor
James Cloyd, College of Pharmacy, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis
Daniel Lowenstein, Department of Neurology, University of California, San Francisco, San Francisco
Shlomo Shinnar, Department of Neurology and Pediatrics, Albert Einstein College of Medicine, Montefiore Medical Center, New York
Robin Conwit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD
Caitlyn Meinzer, Data Coordination Unit, Department of Public Health Sciences, Medical University of South Carolina, Charleston
Hannah Cock, St. George’s University of London and St. George’s University Hospitals NHS Foundation Trust, London
Nathan Fountain, Department of Neurology, University of Virginia, Charlottesville
Jason T. Connor, ConfluenceStat, the University of Central Florida College of Medicine — both in Orlando
Robert Silbergleit, Department of Emergency Medicine, University of Michigan, Ann Arbor
REFERENCES
- 1.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012; 366: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001; 345:631–7. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain JM, Okada P, Holsti M, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA 2014; 311: 1652–60. [DOI] [PubMed] [Google Scholar]
- 4.Cook AM, Castle A, Green A, et al. Practice variations in the management of status epilepticus. Neurocrit Care 2012; 17: 24–30. [DOI] [PubMed] [Google Scholar]
- 5.Riviello JJ Jr, Claassen J, LaRoche SM, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care 2013; 18: 193–200. [DOI] [PubMed] [Google Scholar]
- 6.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17: 3–23. [DOI] [PubMed] [Google Scholar]
- 7.Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaínza-Lein M, Sánchez Fernández I, Jackson M, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol 2018; 75: 410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avdic U, Ahl M, Chugh D, et al. Nonconvulsive status epilepticus in rats leads to brain pathology. Epilepsia 2018; 59:945–58. [DOI] [PubMed] [Google Scholar]
- 10.Shinnar S, Bello JA, Chan S, et al. MRI abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology 2012; 79:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor JT, Elm JJ, Broglio KR. Bayesian adaptive trials offer advantages in comparative effectiveness trials: an example in status epilepticus. J Clin Epidemiol 2013;66: Suppl: S130–S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CFR — Code of Federal Regulations: 21CFR50.24. Silver Spring, MD: Food and Drug Administration, 2018. (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=50.24). [Google Scholar]
- 13.Zhao W, Ciolino J, Palesch Y. Step-forward randomization in multicenter emergency treatment clinical trials. Acad Emerg Med 2010;17:659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez V, Januel J-M, Burnand B, Rossetti AO. Second-line status epilepticus treatment: comparison of phenytoin, valproate, and levetiracetam. Epilepsia 2011;52:1292–6. [DOI] [PubMed] [Google Scholar]
- 15.Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure 2014;23:167–74. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi M, Vibha D, Choudhary N, et al. Management of refractory status epilepticus at a tertiary care centre in a developing country. Seizure 2010;19:109–11. [DOI] [PubMed] [Google Scholar]
- 17.Dalziel SR, Borland ML, Furyk J, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet 2019; 393: 2135–45. [DOI] [PubMed] [Google Scholar]
- 18.Lyttle MD, Rainford NEA, Gamble C, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet 2019; 393: 2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure 2007;16:527–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.