Abstract
Statins, a class of drugs that can effectively remove cholesterol from serum, are used to regulate plasma total cholesterol and reduce the risk of cardiovascular diseases, but it is still unclear whether the drug are modulated by gut microbiota or the structures of gut microbiota are shaped by statins. We investigated the interactions between statins and the human gut microbiota during the in vitro fermentation process by 16S rRNA gene sequencing, gas chromatography (GC), and high-performance liquid chromatography (HPLC) analyses. The presence of fluvastatin (FLU2) specifically promoted the growth of Escherichia/Shigella, Ruminococcaceae UCG 014, and Sutterella. However, the composition of the gut bacterial microbiota remained relatively static in samples treated with rosuvastatin (ROS), simvastatin (SIM), and atorvastatin (ATO). The PICRUSt program predicted moderate differences in the functional categories related to the biosynthesis of other secondary metabolites, cellular processes and signaling, and signal transduction in the FLU2 fermentation samples. Our study revealed substantial variation in the structure and function of microbiomes from the FLU2-treated samples. In addition, short-chain fatty acids (SCFAs) were also significantly decreased in FLU2-treated samples compared with the samples treated with other stains. Statins can be degraded by the human gut microbiota in vitro, and the degradation rate was approximately 7%–30% and 19%–48% after fermentation was allowed to proceed for 24 h and 48 h, respectively. Generally, FLU2 could largely shape the composition and function of human gut microbiota, which resulted in changes in the production of SCFAs. In turn, all statins could be degraded or modified by the gut microbiota. Our study paves the way for elucidating statin-gut microbiota interactions in vitro towards the improvement of the host health and personalized medicine.
Introduction
The gastrointestinal tract, the largest digestive and excretive organ in the human body, is colonized by a vast, complex, and dynamic consortium of microorganisms [1]. It becomes clear that the human gut microbiota and host mutually affect and depend on each other in an intimate relationship [2]. Gut microbiota compositions can influence many aspects of the host [3, 4], including metabolism, obesity, maturation, regulation of the immune system, and even brain function and decision-making. The dysbiosis of gut microbiota may have negative impacts on health and eventually lead to diseases.
To gain insight into the relationship among gut microbiota drugs and human health, it is necessary to disentangle the structure, diversity, and function of the gut microbiota. A previous study using [5] 16S rRNA gene profiling revealed that Bacteroidetes and the Firmicutes constitute over 90% of the known phylogenetic categories of the human gut bacterial microbiota [6]. Other studies showed that a substantial gut microbiome diversity exists between healthy individuals. However, there is a wide array of shared “core microbiome” and “core microbial species” among the sampled population [6, 7]. An increasing number of reports have shown that imbalances in the gut microbiota may cause intestinal dysfunctions and pathological states [8]. Furthermore, several studies on the impact of classes of drugs, xenobiotics, and dietary plant substances on the composition of the gut microbiota have been published [9]. Biocides, metals, and non-antibiotic chemicals with antibacterial properties also contributed to antimicrobial resistance via co-selection of resistant genes [10]. For example, Le Bastard et al. [11] thought that non-antibiotic prescription drugs have a notable impact on the gut microbiota composition. The disposition, efficacy, and toxicity of drugs that affect the gut microbiota could be explored by new sequencing and pyrotagging technologies [12]. Recent works showed that the gut microbiota could influence the pharmacokinetics of orally administered drugs and may have significant implications for their oral bioavailability [13].
Of the orally-administered lipid-lowering drugs developed to reduce the risk of cardiovascular disease, statins, which are 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, are most commonly used worldwide. However, the long-term use of statins can cause a series of diseases, such as cytotoxicity, liver injury or necrosis, kidney damage, and myopathy [14]. It has been reported that gut microbiota play roles in the mediation of lovastatin metabolism and consequent pharmacokinetics interactions [15]. The hypolipidemic effects of SIM [16], ROS [17], and ATO [18] were shown to be correlated with the compositions of mice gut microbiota. Statins have certain antibacterial functions and have been described as novel adjuvant antibiotics [10], but we know little about the statin effects on the human gut bacterial microbiota. Hence, we investigated the interactions of SIM, FLU, ROS, and ATO with gut microbiota using 16S rRNA gene high-throughput sequencing, GC, and HPLC analyses. Our aims were to discover if there are changes in the gut bacterial microbiota and KEGG pathways induced by different statins, statins are degraded or modified by gut microbiota, and whether the data would provide an explanation for the potential effects of statins on human health.
Material & methods
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Hunan University of Science and Engineering research committee and the 1964 Helsinki declaration (and its latest amendments) or comparable ethical standards.
Statins
FLU, ROS, SIM, and ATO (pharmaceutical secondary standard, certified reference material) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and used as received, without further purification.
Fecal sample collection
Fourteen healthy volunteers aged 18–22 years and living in Yongzhou city, Hunan province, China, were recruited for this study. Detailed information on these volunteers is shown in Table A in S1 Text. Participants did not receive antibiotics, probiotic, or prebiotic within the preceding three months. All donors provided informed consent, and the study was approved by the Ethics Committee of Hunan University of Science and Engineering (Yongzhou, China). Fecal samples were collected as soon as possible, and a fresh fecal sample (1 g) was immediately transferred to 10 mL of 0.1 M anaerobic PBS (pH 7.0) into glass beakers. Glass rods were then used to prepare a 10% (W/V) slurry, and then the solution was filtered through a four-layer gauze. The filtered slurries were used to inoculate the batch culture fermentation tubes; all of these steps were conducted in an anaerobic chamber except for fecal collection. The remainder was stored at -80°C for further analyses.
In vitro batch culture fermentation
Batch culture fermentation was carried out using the procedure described by Hu et al. [19], Rycroft et al. [20], and Lei et al. [21]. The VI growth medium was adjusted to pH 6.5 with 1 M HCl, and starch (8 g/L) was prepared and added according to our previous studies [12, 19, 22]. The liquid culture media was autoclaved at 121°C for 15 min and cooled to room temperature under anaerobic conditions (10% H2, 10% CO2, and 80% N2). Statin was dissolved in 50% DMSO-50% water (v/v) and added to the medium to obtain a final statin concentration of 20 μM or colon concentration [23]; controls were added with 50% DMSO only (all samples content 1% DMSO finally). The colon concentrations of ROS, SIM, FLU, and ATO were set at the dose of 10 mg, 20 mg, 80 mg, and 20 mg per day [24], which is about 30, 40, 240, and 64 μg/mL in vitro, and we detected the concentrations at 0 h to establish a baseline (28, 33, 265, and 82 μg/mL, respectively). The filtered fresh fecal slurry (500 μL) was added to the previously prepared fermentation culture medium (9.5 mL), within an anaerobic chamber, and then incubated at 37°C. Two-milliliter samples collected at 24 h and 48 h for further analyses.
Extraction of bacterial genomic DNA
The genomic DNA was extracted using the QIAamp DNA Stool Mini Kit according to the manufacturer’s instructions, and the lysis temperature was increased to 95°C to promote gram-positive bacterial lysis (QIAGEN, Hilden, Germany). The concentration of extracted DNA was quantified by a spectrophotometer (Colibri Microvolume Spectrometer, Titertek Berthold, Pforzheim, Germany).
16S rRNA gene analysis of gut microbiota
The V3–V4 region of bacterial 16S rRNA genes was amplified using the primers 338F (5’-ACT CCT ACG GGA GGC AGC AG-3’) and 806R (5’-GGA CTA CHV GGG TWT CTA AT-3’) [25]. The amplicons were sequenced on the Illumina MiSeq 300PE platform by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The resulting reads were analyzed using the Quantitative Insights Into Microbial Ecology (QIIME 2) pipeline. Then high-quality sequences were taxonomically classified in defining operational taxonomic units (OTUs) using Mothur at 97% sequence similarity [26]. The representative sequences were chosen and classified using the RDP classifier method [27, 28] and aligned against the SILVA database (https://www.arb-silva.de/documentation/release-123/). Good’s coverage, α-diversity, Simpson and Shannon index, and richness (number of OTUs) were analyzed using Visual Genomics–AS software (http://amplicon.vgenomics.cn:9000/). The data have been deposited in the sequence read archive (SRA) of NCBI as GenBank Accession Number PRJNA562063. Basic data on 16S rRNA gene high-throughput sequencing are listed in Table B in S1 Text.
PICRUSt [29] software was used in this study to predict the metagenome functional profiling by using the bacterial 16S rRNA gene sequences. The distribution of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of these samples predicted by PICRUSt software was further used for statistical analysis.
Short-chain fatty acids analysis
SCFAs were determined by GC, as previously described [30, 31]. Briefly, 1 mL of the fermentation products were mixed with 0.2 mL 25% (w/v) of metaphosphoric acid, prior to mixing, the metaphosphoric acid solution was added croconic acid as an internal standard (final concentration 10 mmol/L). The samples were frozen at -20°C overnight, and then subsequently centrifuged (14,000 g for 20 min). The supernatant was used for SCFAs analysis using a GC-2010 Plus (Shimadzu Corporation, Japan) equipped with a flame ionization detector and an InertCap FFAP (0.25 mm ×30 m × 0.25 μm) column. The peaks were integrated using GC Solution software, and SCFA content was quantified using the single-point internal standard method. Peak identity and internal response factors were determined using a 20-mM calibration cocktail that included acetic, propionic, isobutyric, butyric, isovaleric, and valeric acids. The chromatograms of these SCFA mixtures are shown in S1 Fig.
Statin concentration analysis
Statins were analyzed by the Shimadzu Black 20 AT system (Shimadzu, Kyoto, Japan) as previously described [32, 33] with some modifications. Briefly, statin fermentation samples were just centrifuged (14,000 g for 20 min), and the supernatant was used for statin analysis by HPLC. Chromatographic software Lab Solution was used for data collection and processing. Statins were detected by a UV-vis diode array detector at 238 nm. A Vertex C18 analytical column (4.6×250 mm, 5.0 μm, Vertex, USA) was used for the HPLC separation of statins. The injection volume was 10 μL, and the column oven temperature was kept at 30°C. The binary mobile phase, which is composed of acetonitrile and 0.1% formic acid (65:35), was pumped at a flow rate of 1.0 mL/min. The chromatograms and standard curves of stains are shown in S2 Fig.
Statistical analysis
SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA) and the Tamhane’s T2 (M) test were used to perform statistical analyses. The p values were then used to compute the false discovery rate (FDR) to account for multiple hypothesis testing using Microsoft Excel. P values and FDR less than 0.05 were considered statistically significant. Plot cladograms and significantly different bacterial taxa were analyzed using LEfSe Software (https://bitbucket.org/biobakery/biobakery/wiki/lefse) with default parameters and statistical methods.
Results
16S rRNA gene sequencing and diversity analysis
In summary, 245,914,669 and 2,011,409,834 valid reads were obtained from 14 original fecal samples and 126 fermented samples. Reads that could be classified as bacteria (97% sequence identity) were used for the subsequent analysis. The rarefaction curves, Shannon-Wiener curves, specaccum analysis, and rank-abundance distribution curve were used to evaluate the sequence quality (S3 Fig), which indicated that the number of sequences tended toward saturation as a function of sequence depth, suggesting that most of the bacterial sequences were captured in all samples.
In this study, a total of 1,212 OTUs from 140 samples were obtained based on 97% similarity. For the original fecal samples, 146–276 OTUs were identified, 69–511, and 98–372 OTUs were identified in the statins and DMSO fermented samples, respectively. The indexes of Ace, Chao, Shannon, and Simpson were calculated for fully illustrating the α-diversity of the bacterial communities. The α-diversity indexes of Ace, Chao, and Shannon were slightly different among the original fecal, DMSO, ROS, ATO, FLU and SIM samples (Table B in S1 Text and S3 Fig).
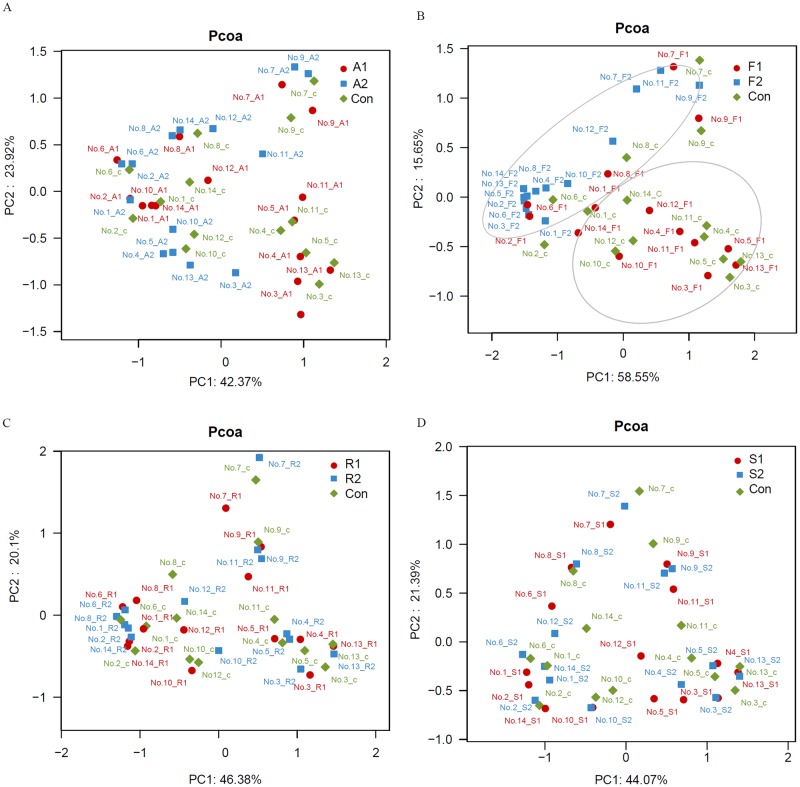
As illustrated in Fig 1, β-diversity analysis was employed to elucidate the similarity of the samples used in this study. The results showed that the gut microbiota was most substantially affected by FLU2, followed by ATO2, SIM, and ROS. Most of the samples from groups FLU2 were clustered together, and separated from FLU1 and control samples.
Fig 1. Principal coordinates analysis (PCoA) plot.
PCoA plot of the gut microbiota based on the unweighted UniFrac metric. Basic analysis of sequenced data. Con, A1, A2, F1, F2, R1, R2, S1, and S2 represent the gut microbiota of fermented samples using the media plus DMSO and different statins at 20 μM and colon concentration. The p value of A, F, R, and S is 0.951, 0.006, 1, and 0.999, respectively, which was obtained by permanova.
Effects of statin on the composition of human gut bacterial communities
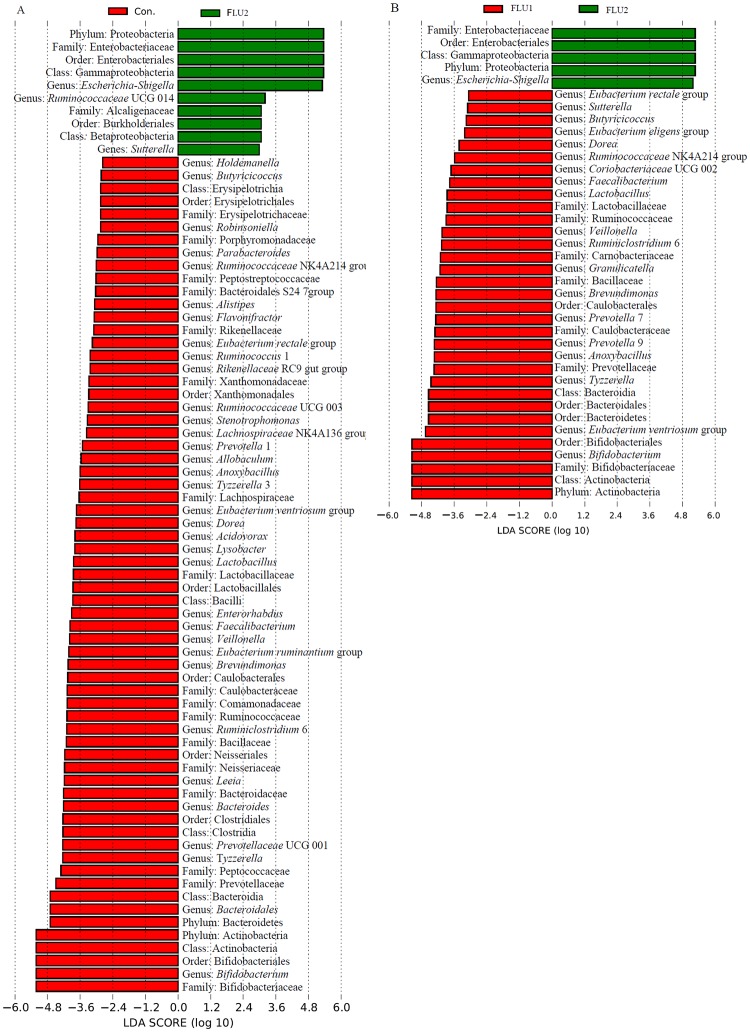
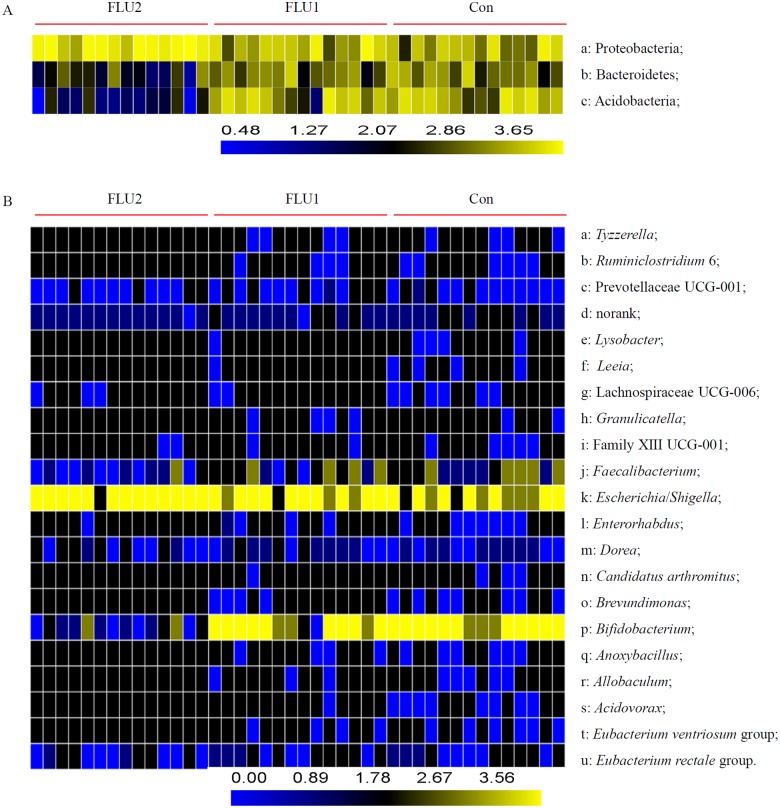
After classification, 19 bacterial phyla and 286 bacterial genera were identified from all samples (Table B in S1 Text). For the specific bacterial taxa among statin fermentation samples, we employed the linear discriminant analysis effect size (LEfSe) method and identified the significantly different bacteria across all taxa levels. The results showed that the addition of FLU2 and ATO2 statins significantly altered the structures of the gut bacterial microbiota. The phylum Proteobacteria, Bacteroidetes, and Actinobacteria were significantly affected by FLU2 when compared with groups FLU1 and DMSO (Figs 2 & 3A). The Actinobacteria were significantly affected by ATO2 compared with DMSO (S4 Fig).
Fig 2. Analysis of the different abundant bacterial taxa using LEfSe.
The bacterial percentage of the fermented samples was used for LEfSe analysis. The P-value <0.05 was identified as being significantly different between groups. Significantly enriched bacterial phyla, classes, orders, families, and genera are listed next to the histogram. Con, FLU1, and FLU2 represent samples collected after cultured using VI media with DMSO, 20 μM, and colon concentration of FLU. A Significantly different bacteria between FLU1 and FLU2. B Significantly different bacteria between groups of FLU2 and DMSO.
Fig 3. Relative abundance of individual gut microbiota composition at different taxonomic rank.
Heatmap visualizes logarithm (base-10) of ratios (p<0.05). A Phyla found to be statistically different among the three groups. B Genera found to be statistically different among the three groups.
At the genus level, the relative abundance of gut bacterial microbiota among all samples is shown in S5 Fig. Escherichia/Shigella, Bifidobacterium, Megamonas, Prevotella 9, Megasphaera, Bacteroides, Klebsiella, Faecalibacterium, Lactobacillus, and Citrobacter are the top 10 genera. Escherichia/Shigella, Ruminococcaceae UCG 014, and Sutterella were significantly enriched in groups FLU2 compared with DMSO, and Bifidobacterium, Bacteroidales, Tyzzerella, Prevotellaceae UCG 001, Bacteroides, Leeia, Ruminiclostridium 6, Brevundimonas, Eubacterium ventriosum group, Veillonella, Enterorhabdus, Lysobacter, Acidovorax, among others, were enriched in DMSO (Fig 2A). Only Escherichia/Shigella were enriched in FLU2 compared with FLU1, and Bifidobacterium, Eubacterium ventriosum group, Tyzzerella, Anoxybacillus, Prevotella 9, Prevotella 7, Brevundimonas, Granulicatella, Ruminiclostridium 6, among others, were enriched in FLU1 (Fig 2B). Some genera, such as Bifidobacterium, Tyzzerella, and Lactobacillus, were also decreased significantly in the ATO2 groups compared with DMSO groups (S4 Fig). However, those genera were not statistically affected by the colon concentration of other statins compared with DMSO or 20 μM statin.
Predictive metagenome functional profiling of gut microbiota
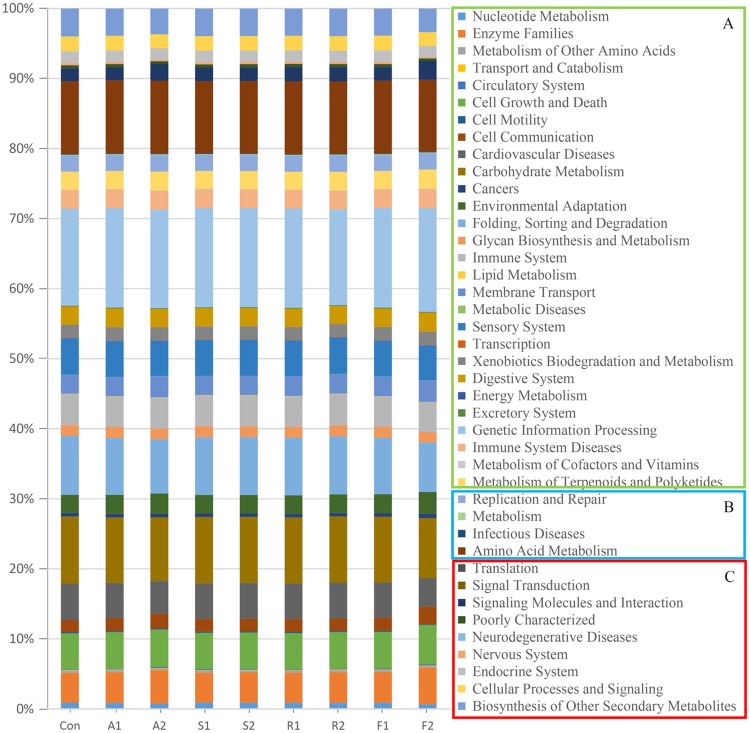
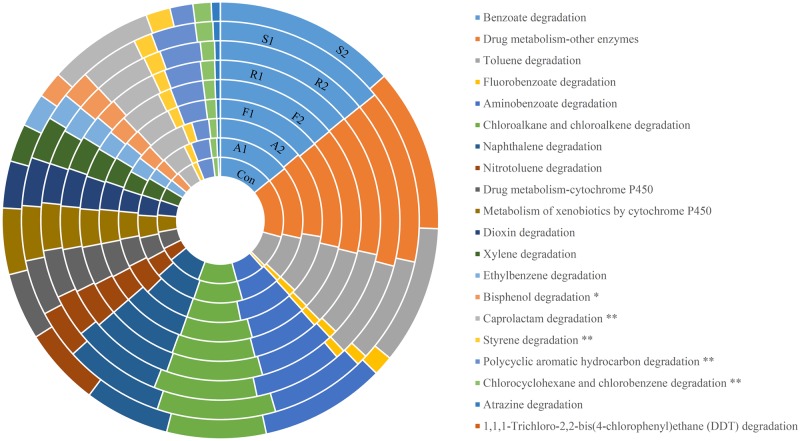
For function annotation, PICRUSt [29] was used to predict the metagenome functional contents based on the 16S rRNA gene sequences of the gut bacterial microbiota. These results showed that statins dramatically altered the abundance of many proteins encoded by the gut microbiota, particularly in the FLU2 samples. The functions of the genes that were changed more significantly (FDR<0.05) in FLU2 than in other statins, except for ATO2, as showed in the rectangular box C. For instance, the KEGG pathways of cellular processes and signaling, signal transduction, and neurodegenerative diseases, were significantly increased in FLU2; the pathways of biosynthesis of other secondary metabolites, endocrine system, nervous system, signaling molecules and interaction, were significantly decreased in FLU2. In box B, these pathways were altered more significantly (FDR<0.1) in FLU2 than others except for ATO2, the pathway of replication and repair was not significantly different between FLU2 and the control when considering FDR. In box A, it can be seen that some pathways were not affected by some statins or were affected but with an FDR>0.1 (Fig 4). Moreover, the xenobiotics biodegradation and metabolism pathway were further analyzed. The degradation of caprolactam, styrene, polycyclic aromatic hydrocarbon, chlorocyclohexane and chlorobenzene were found to be significantly affected by FLU2 when compared with other statins and the control (Fig 5).
Fig 4. KEGG categories present in the human gut microbiota.
The KEGG pathways in box A were not affected by FLU2; the KEGG pathways in boxes B and C were affected significantly by FLU2 compared with other statins (Box A p>0.05; Box B: p<0.05, 0.05<FDR<0.1; Box C: p<0.05, FDR<0.05).
Fig 5. Functional analysis of the proteins involved in biosynthesis of xenobiotics biodegradation and metabolism.
** means significantly different between F2 and some other samples except for A2 (p<0.05; FDR<0.05); * means significantly different between F2 and some other samples except for A2 (p<0.05; 0.05<FDR<0.1).
Concentration of SCFAs
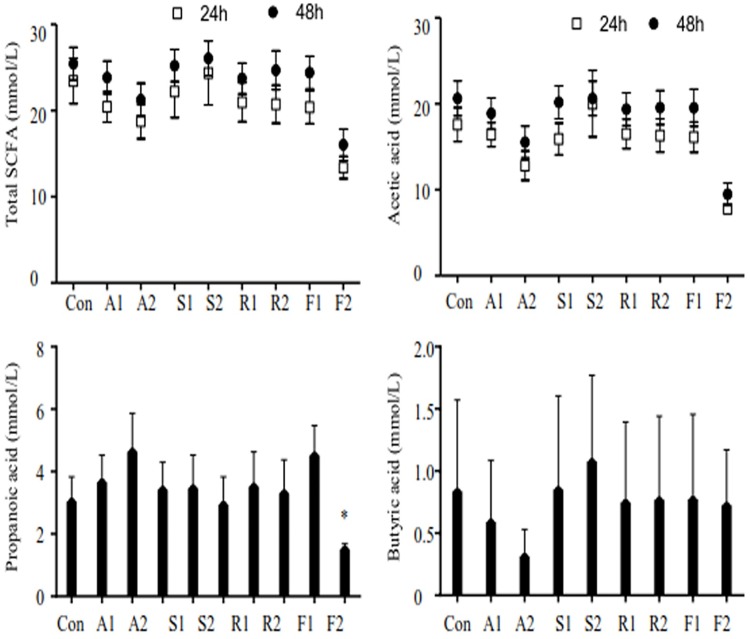
After 24 h and 48 h fermentation, the concentration of total SCFAs, acetic, propionic, butyric acid in the fermentation samples were not significantly different, except for the acetic and propionic acid in FLU2 samples (p<0.05). The concentration of propionic and butyric acid was affected more in the ATO2 groups than in other groups (Fig 6).
Fig 6. Effect of statins on SCFA production after fermentation.
Acetic, propionic, isobutyric, butyric, isovaleric, and valeric acid were detected using GC in this paper. Each sample was measured in triplicate. Figures were generated using GraphPad Prism version 5.01. The figure shows total SCFA, acetic, propionic, and butyric acid concentration of the following samples: (1: 20 μM; 2: colon concentration; A: ATO; S: SIM; R: ROS; F: FLU).
Degradation or modification of statin by human gut microbiota
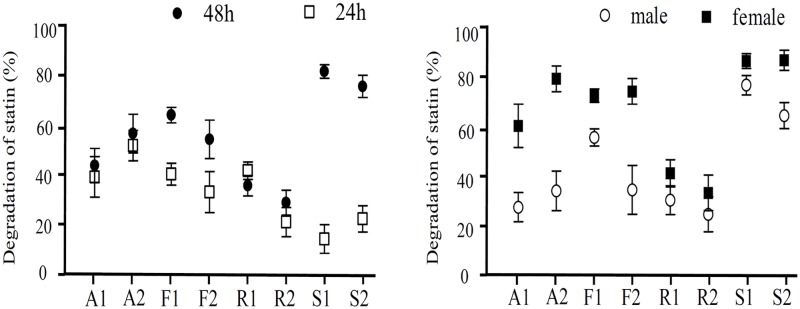
To evaluate whether the detected concentration of stains was different from the expected calculation concentration, the concentrations of ROS, SIM, FLU, and ATO at 0 h were used as a baseline for the calculation of the degradation ratio. After fermenting for 24 h and 48 h, the degradation or modification rates of ATO by the human gut microbiota were a little higher in the colon concentration groups than in the 20 μM groups (Fig 7). The degradation or modification rates of SIM were up to 77.1%, and the minimum degradation rate of ROS was about 32.7%. However, 13.4%–24.1% of statins were precipitated in the culture medium or absorbed by the gut microbiota after high-speed centrifugation (data not shown); the real degradation or modification was approximately 7–30% and 19%-48% after fermentation for 24 h and 48 h, respectively. Compared with the male samples, the degradation rates of ATO and FLU were higher in the female samples.
Fig 7. Degradation of statins.
Different statins were detected by HPLC, and the degradation was obtained by the original percentage subtracted measurable percentage. Each statin sample was measured in duplicate.
Discussion
The human body hosts a complex microbial ecosystem that facilitates host metabolism and adiposity by expanding nutrient sources, producing essential vitamins, and carrying out xenobiotic metabolism [34]. Moreover, xenobiotics, including therapeutic drugs, can potentially alter the gut microbiota community structures and associated functions [35]. However, relatively little is known about how the gut microbiota interacts with xenobiotics during long-term exposures [17]. Statins, the most effective lipid-lowering drugs, which are widely used for the treatment of hypercholesterolemia owing to their inhibition of HMGR protein [36], also have off-target effects including anti-inflammation function [37, 38]. The antibacterial activities of these drugs have been widely reported but little is known about their effects on gut microbiota. Our experiment showed that Bacteroidetes decreased significantly in FLU2 fermentation samples, and numerous studies [3, 39, 40] found that the obesity is associated with changes in the relative abundance of the Bacteroidetes, not only in mice but also in human. For instance, previous research with germ-free mice showed that obesity decreased the relative abundance of Bacteroidetes [41]. Other studies [42, 43] have suggested that a significant reduction of the phylum Bacteroidetes occurs in Crohn’s disease/ulcerative colitis patients compared with controls. The meta-analysis also showed a significant lowering of LDL, and increases in height and weight with statin administration [44]. All of these data might imply that a high dose of FLU may cause obesity, disease, or intestinal microbiota disorders, at least partially.
Considering that the VI medium could simulate the human gut microbiota in vitro with higher than 80% similarity with the original fecal bacterial community [22], VI medium was used in this study. After fermentation in vitro, the high throughput sequencing results showed that the FLU2 samples were enriched for the microbial groups of Escherichia/Shigella, Ruminococcaceae UCG 014, and Sutterella. Given the higher relative abundance of Escherichia/Shigella, the gut microbiota would be more susceptible to colorectal cancer [45]. However, some particular drugs exert their therapeutic effects through selectively altering the structure of the gut microbiota, for example, increasing the abundance of Escherichia [46]. Sutterella is an opportunistic bacterium of the phylum Proteobacteria; human who have higher levels of the genera of Sutterella would have a lower concentration and percentage of butyric acid [47]. In particular, there is evidence that the Sutterella species is associated with autism, Down’s syndrome, and inflammatory disease [48, 49]. Menni and collaborators [50] found that arterial stiffness is negatively correlated with the abundance of the family Ruminococcaceae. It is assumed that the effects of statins are largely individual-dependent. Specifically, the family Ruminococcaceae was enriched differently by statins. However, Scheperjans et al. [51] found that the Ruminococcaceae population was increased significantly in Parkinson’s disease (PD) compared with healthy controls. Based on those analyses, our research could explain why the use of statin is associated with a higher risk of PD [52]. Notably, the family Ruminococcaceae was also significantly increased in patients with constipation [53].
After treating the gut microbiota with statins, there was a significant reduction of several clusters, including the genus Bifidobacterium in the FLU2 fermentation samples compared with the other samples. These differences between FLU2 and other statins could be explained by the high doses and low lipid-lowering effect of FLU. Taken together, patients that need to reduce LDL-C levels more than 35% might choose ROS first, then ATO and SIM, and should not be given FLU. The patient’s statin-sensitive responses are linked to the gut microbiota, and the gut microbiota may guide the statin dosage adjustments [54]. There are indeed several reports showing that statins influence the diversity of the human gut microbiota [18, 55–57]. In addition, although the research on the antibacterial activity of FLU has been scarce and more studies are needed [58], it seems that its antifungal activity may be higher than that of other statins [59]. In another study, administration of ROS to mice randomly in the diet for 28 days significantly influenced the structure of the gut microbiota [17]. Some researchers found that ATO and SIM may have a more potent antibacterial activity than ROS, but the clinical strains were less sensitive to statin compared with the standard bacteria [60]. Thus, during the in vitro fermentation, the gut microbiota was not significantly affected by ROS, ATO, and SIM at a suitable dose. Hence, the differences between FLU2 and other samples might further support the findings that a high dose of statins is not adequate for Chinese people [61].
We did not observe significant differences in bacterial diversity between two concentrations of the same statins (except for FLU), but differences were detected for different statins. This suggested that the gut microbiota was influenced differently by different statins and that a variation of the microbiota occurs much like the ecological successions associated with the genetics, social environment, diet, metabolic disorders, and antibiotic treatment [62]. However, some research works provide evidence that there is little “stability” within the resident clonal populations of the common gut bacterial family of Enterobacteriaceae. Given that clones can vary substantially in genome content and that evolutionary processes operate at the population level, the biological relevance of our results may require the demonstration of stability at lower taxonomic levels [63].
PICRUSt allowed us to know more about the overall microbiome metabolism and its functional specialization. We also performed a statistical analysis of differences in all samples. Interestingly, about 50.91% KEGG pathways were significantly changed in FLU2 compared with controls, and 10.67% KEGG pathways were significantly affected in ATO2, but there were no statistical differences in ROS and SIM when compared with controls. Consistently, the content of predictive metagenome functionalities was remarkably influenced by FLU2 when compared with other statins. FLU2 only slightly affected xenobiotics biodegradation and metabolism, but the effect was statistically different in some specific substances. For example, caprolactam degradation and styrene degradation were significantly overrepresented in the FLU2 metagenome. Those pathways may be related to FLU degradation, and further studies are required for elucidating the pharmacological or toxicological properties of the gut microbial metabolites and metabolites contribution during statin treatment.
From another perspective, very few studies have examined how the human gut microbiota degrades statins. We are aware of only two studies that have considered the degradation of statin by gut microbiota. One study, based on the fecal samples from nine healthy volunteers, found that β-hydroxy acid form lovastatin was degraded by the gut microbiota in vitro [64]. Likewise, a study reported the degradation of SIM by the human gut microbiota, and implicated the pathway of colonic microbial metabolism in this process [65]. However, the characteristics of the gut microbiota-driven degradation of statins have not yet been fully investigated, and a more thorough investigation is needed to understand the association between statins, the gut microbiota, and the host. Additional time points, metabolites of statins, and culture assays to determine if bacteria change in relative abundance need to be reported. The currently underdeveloped studies on the concentration change of statins after incubation with the human gut microbiota could become an exciting area of pharmacological research.
Conclusion
We observed that FLU2 significantly influenced the composition of the gut bacterial microbiota and the functional profiles, and that it changed SCFA production correspondingly. In turn, the statins were also degraded or modified by gut microbiota. The results may provide support for the microbial mediation of the therapeutic effects of FLU through short-chain fatty acid production, as well as for potential microbiota-mediated mechanisms behind the known intestinal adverse effects in the form of a relative increase in abundance of Escherichia/Shigella, Ruminococcaceae, and Sutterella. It is unclear whether the statistically significant alterations in gut microbiota have the potential for promoting key physiological adaptations and lipid-lowering effects. Further studies are needed to evaluate whether the gut microbiota alters the disposition, toxicity, efficacy of statins, and the analyses on interactions among drugs, gut microbiota, SCFA, and host should be extended to more populations and cover longer periods. In this myriad of untapped interactions, one may find strategies for personalized medicine to improve health and longevity.
Supporting information
Table A. Pharmacokinetic parameters of used statins. Table B. Basic statistical results for high-throughput 16S rRNA gene sequencing.
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Data Availability
The data have been deposited in the sequence read archive (SRA) of NCBI as GenBank Accession Number PRJNA562063. (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA562063).
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31741109) to HC and the Hunan Natural Science Foundation (No. 2018JJ3200) to YY, and the construct program of applied characteristic discipline in Hunan University of Science and Engineering to YY.
References
- 1.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annual Review of Nutrition. 2002; 22(1): 283–307. [DOI] [PubMed] [Google Scholar]
- 2.Guchte MVD, Blottière HM, Doré J. Humans as holobionts: Implications for prevention and therapy. Microbiome. 2018; 6(1): 81 10.1186/s40168-018-0466-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006; 444: 1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 4.Allen AP, Dinan TG, Clarke G, Cryan JF. A psychology of the human brain-gut-microbiome axis. Social & Personality Psychology Compass. 2017; 11(4): e12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Applied and Environmental Microbiology. 1998; 64(10): 3854–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010; 464: 59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009; 457: 480–484. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, et al. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Research. 2010; 20(10): 1411–1419. 10.1101/gr.107987.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jourova L, Anzenbacher P, Anzenbacherova E. Human gut microbiota plays a role in the metabolism of drugs. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc. 2016; 160 (3). [DOI] [PubMed] [Google Scholar]
- 10.Ko HHT, Lareu RR, Dix BR, Hughes JD. Statins: Antimicrobial resistance breakers or makers? Peer J. 2017; 5: e3952 10.7717/peerj.3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Bastard Q, Al-Ghalith GA, Grégoire M, Chapelet G, Javaudin F, Dailly E, et al. Systematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Alimentary Pharmacology & Therapeutics. 2018; 47(3): 332–345. [DOI] [PubMed] [Google Scholar]
- 12.Saad R, Rizkallah MR, Aziz RK. Gut pharmacomicrobiomics: The tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathogens. 2012; 4(1): 16 10.1186/1757-4749-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ireland A, Priddle JD, Jewell DP. Acetylation of 5-aminosalicylic acid by isolated human colonic epithelial cells. Clinical Science. 1990; 78(1): 105–111. 10.1042/cs0780105 [DOI] [PubMed] [Google Scholar]
- 14.Liu A, Wu Q, Guo J, Ares I, Rodríguez JL, Martínez-Larrañaga MR, et al. Statins: Adverse reactions, oxidative stress and metabolic interactions. Pharmacology & Therapeutics. 2019; 195: 54–84. [DOI] [PubMed] [Google Scholar]
- 15.Yoo DH, Kim IS, Van Le TK, Jung IH, Yoo HH, Kim DH. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metabolism and Disposition. 2014; 42(9): 1508–1513. 10.1124/dmd.114.058354 [DOI] [PubMed] [Google Scholar]
- 16.He X, Zheng N, He J, Liu C, Feng J, Jia W, et al. Gut microbiota modulation attenuated the hypolipidemic effect of simvastatin in high-fat/cholesterol-diet fed mice. Journal of Proteome Research. 2017; 16(5): 1900–1910. 10.1021/acs.jproteome.6b00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolan JA, Skuse P, Govindarajan K, Patterson E, Konstantinidou N, Casey PG, et al. The influence of rosuvastatin on the gastrointestinal microbiota and host gene expression profiles. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2017; 312(5): 488–497. [DOI] [PubMed] [Google Scholar]
- 18.Caparrós-Martín JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017; 5(1): 95 10.1186/s40168-017-0312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Chen H, Li P, Li B, Cao L, Zhao C, et al. Analysis of interactions between endobiotics and human gut microbiota using in vitro bath fermentation systems. JoVE. 2019; e59725. [DOI] [PubMed] [Google Scholar]
- 20.Rycroft CE, Jones MR, Gibson GR, Rastall RA. Fermentation properties of gentio-oligosaccharides. Letters in Applied Microbiology. 2001; 32(3): 156–161. 10.1046/j.1472-765x.2001.00875.x [DOI] [PubMed] [Google Scholar]
- 21.Lei F, Yin Y, Wang Y, Deng B, Yu HD, Li L, et al. Higher-level production of volatile fatty acids in vitro by chicken gut microbiotas than by human gut microbiotas as determined by functional analyses. Applied and Environmental Microbiology. 2012; 78(16): 5763–5772. 10.1128/AEM.00327-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Y, Fan B, Liu W, Ren R, Chen H, Bai S, et al. Investigation into the stability and culturability of Chinese enterotypes. Scientific Reports. 2017; 7: 7947 10.1038/s41598-017-08478-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018; 555: 623 10.1038/nature25979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mach F, Senouf D, Fontana P, Boehlen F, Reber G, Daali Y, et al. Not all statins interfere with clopidogrel during antiplatelet therapy. European Journal of Clinical Investigation. 2005; 35(8): 476–481. 10.1111/j.1365-2362.2005.01522.x [DOI] [PubMed] [Google Scholar]
- 25.Dennis KL, Wang Y, Blatner NR, Wang S, Saadalla A, Trudeau E, et al. Adenomatous polyps are driven by microbe-instigated focal inflammation and are controlled by IL-10-producing T cells. Cancer Research. 2013; 73(19): 5905–5913. 10.1158/0008-5472.CAN-13-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schloss PD, Westcott SL, Thomas R, Hall JR, Martin H, Hollister EB, et al. Introducing mothur: Open-source, platform-Independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology. 2009; 75(23): 7537 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The ribosomal database project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Research. 2009; 37: D141–D145. 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007; 73(16): 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology. 2013; 31: 814 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Bian G, Su Y, Zhu W. Comparison of faecal microbial community of lantang, bama, erhualian, meishan, xiaomeishan, duroc, landrace, and yorkshire sows. Asian-Australasian Journal of Animal Sciences. 2014; 27(6): 898–906. 10.5713/ajas.2013.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Zhou Y, Qin Y, Li Y, Yu L, Li R, et al. Sex-dependent effects of PM2.5 maternal exposure and quercetin intervention on offspring’s short chain fatty acids. International Journal of Environmental Research and Public Health. 2019; 16: 4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nováková L, Lopéz SA, Solichová D, Šatínský D, Kulichová B, Horna A, et al. Comparison of UV and charged aerosol detection approach in pharmaceutical analysis of statins. Talanta. 2009; 78(3): 834–839. 10.1016/j.talanta.2008.12.057 [DOI] [PubMed] [Google Scholar]
- 33.Fukiwake T, Hasegawa T, Takahashi K, Saijo M, Hamana M. Simultaneous determination of statins in dietary supplements by ultra-performance liquid chromatography. Shokuhin eiseigaku zasshi Journal of the Food Hygienic Society of Japan. 2014; 55(2): 94–102. 10.3358/shokueishi.55.94 [DOI] [PubMed] [Google Scholar]
- 34.Sommer F, Bäckhed F. The gut microbiota-masters of host development and physiology. Nature Reviews Microbiology. 2013; 11: 227–238. 10.1038/nrmicro2974 [DOI] [PubMed] [Google Scholar]
- 35.Maurice Corinne F, Haiser Henry J, Turnbaugh Peter J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013; 152(1–2): 39–50. 10.1016/j.cell.2012.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Istvan ES. Structural mechanism for statin inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. American Heart Journal. 2002; 144(6): S27–S32. [DOI] [PubMed] [Google Scholar]
- 37.Nenseter MS, Aukrust P, Ose L, Holven KB. Low level of inflammatory marker in hyperhomocysteinemic patients on statin therapy. Scandinavian Journal of Clinical and Laboratory Investigation. 2014; 74(1): 1–7. 10.3109/00365513.2013.854926 [DOI] [PubMed] [Google Scholar]
- 38.Strandberg TE, Vanhanen H, Tikkanen MJ. Effect of statins on C-reactive protein in patients with coronary artery disease. The Lancet. 1999; 353(9147): 118–119. [DOI] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444(7122): 1027–1031. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 40.Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L, Savastano S, et al. Gut microbiota: A new path to treat obesity. International Journal of Obesity Supplements. 2019; 9: 10–19. 10.1038/s41367-019-0011-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007; 104(3): 979–984. 10.1073/pnas.0605374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004; 53(5): 685 10.1136/gut.2003.025403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences. 2007; 104(34): 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Gorman CS, Mary FH, Michael BON. Systematic review and metaanalysis of statins for heterozygous familial hypercholesterolemia in children: Evaluation of cholesterol changes and side effects. Pediatric Cardiology. 2009; 30(4): 482–489. 10.1007/s00246-008-9364-3 [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. The ISME Journal. 2012; 6(2): 320–329. 10.1038/ismej.2011.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pryor R, Norvaisas P, Marinos G, Best L, Thingholm LB, Quintaneiro LM, et al. Host-microbe-drug-nutrient screen identifies bacterial effectors of metformin therapy. Cell. 2019; 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjørkhaug ST, Aanes H, Neupane SP, Bramness JG, Malvik S, Henriksen C, et al. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes. 2019; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio. 2012; 3(1): e00261–00211. 10.1128/mBio.00261-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang LV, Christophersen Claus T, Sorich Michael J, Gerber Jacobus P, Angley Manya T, Conlon Michael A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Molecular Autism. 2013; 4(1): 42 10.1186/2040-2392-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menni C, Lin C, Cecelja M, Mangino M, Matey-Hernandez ML, Keehn L, et al. Gut microbial diversity is associated with lower arterial stiffness in women. European Heart Journal. 2018; 39(25): 2390–2397. 10.1093/eurheartj/ehy226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Movement Disorders. 2015; 30(3): 350–358. 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- 52.Liu G, Sterling NW, Kong L, Lewis MM, Mailman RB, Chen H, et al. Statins may facilitate Parkinson’s disease: Insight gained from a large, national claims database. Movement Disorders. 2017; 32(6): 913–917. 10.1002/mds.27006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu L, Liu W, Alkhouri R, Baker R, Bard J, Quigley E, et al. Structural changes in the gut microbiome of constipated patients. Physiological Genomics. 2014; 46(18): 679–686. 10.1152/physiolgenomics.00082.2014 [DOI] [PubMed] [Google Scholar]
- 54.Sun B, Li L, Zhou X. Comparative analysis of the gut microbiota in distinct statin response patients in East China. Journal of Microbiology. 2018; 56(12): 886–892. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Song X, Zhou H, Zhou X, Xia Y, Dong X, et al. Gut microbiome associates with lipid-lowering effect of Rosuvastatin in vivo. Frontiers in Microbiology. 2018; 9: 530 10.3389/fmicb.2018.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Medicine. 2017; 9(1): 39 10.1186/s13073-017-0428-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan TJ, Ahmed YM, Zamzami MA, Siddiqui AM, Khan I, Baothman OAS, et al. Atorvastatin treatment modulates the gut microbiota of the hypercholesterolemic patients. OMICS a Journal of Integrative Biology. 2018; 22(2): 154–163. 10.1089/omi.2017.0130 [DOI] [PubMed] [Google Scholar]
- 58.Hennessy E, Adams C, Reen FJ, Gara F. Is there potential for repurposing statins as novel antimicrobials? Antimicrobial Agents and Chemotherapy. 2016; 60(9): 5111 10.1128/AAC.00192-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lima WG, Alves-Nascimento LA, Andrade JT, Vieira L, de Azambuja Ribeiro RIM, Thomé RG, et al. Are the statins promising antifungal agents against invasive candidiasis? Biomedicine & Pharmacotherapy. 2019; 111: 270–281. [DOI] [PubMed] [Google Scholar]
- 60.Masadeh M, Mhaidat N, Alzoubi K, Al-Azzam S, Alnasser Z. Antibacterial activity of statins: A comparative study of Atorvastatin, Simvastatin, and Rosuvastatin. Annals of Clinical Microbiology and Antimicrobials. 2012; 11(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao SP, Yu BL, Peng DQ, Huo Y. The effect of moderate-dose versus double-dose statins on patients with acute coronary syndrome in China: Results of the CHILLAS trial. Atherosclerosis. 2014; 233(2): 707–712. 10.1016/j.atherosclerosis.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 62.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012; 489: 220–230. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinson JNV, Pinkham NV, Peters GW, Cho H, Heng J, Rauch M, et al. Rethinking gut microbiome residency and the Enterobacteriaceae in healthy human adults. The ISME Journal. 2019; 13: 2306–2318. 10.1038/s41396-019-0435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beltrán D, Frutos-Lisón MD, Espín JC, García-Villalba R. Re-examining the role of the gut microbiota in the conversion of the lipid-lowering statin monacolin K (lovastatin) into its active β-hydroxy acid metabolite. Food & Function. 2019; 10(4): 1787–1791. [DOI] [PubMed] [Google Scholar]
- 65.Aura AM, Mattila I, Hyötyläinen T, Gopalacharyulu P, Bounsaythip C, Orešič M, et al. Drug metabolome of the simvastatin formed by human intestinal microbiota in vitro. Molecular BioSystems. 2011; 7(2): 437–430. 10.1039/c0mb00023j [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A. Pharmacokinetic parameters of used statins. Table B. Basic statistical results for high-throughput 16S rRNA gene sequencing.
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
The data have been deposited in the sequence read archive (SRA) of NCBI as GenBank Accession Number PRJNA562063. (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA562063).