Abstract
Antibody-drug conjugates (ADCs) are becoming an increasingly important sub-class of antibody-related therapeutics. Two ADCs, brentuximab vedotin (Adcetris®) and ado-trastuzumab emtansine (Kadcyla®), were recently approved for marketing both by the US Food and Drug Administration (FDA) and the European Medicine Agency (EMA). Brentuximab vedotin is marketed as therapy for hematological malignancies (Hodgkin lymphoma, systemic anaplastic large cell lymphoma), while ado-trastuzumab emtansine is marketed for treatment of a solid tumor (breast cancer). The approvals of these two ADCs followed the mitigated success of gemtuzumab ozogamicin (Mylotarg®), which was withdrawn from the US market in 2010, ten years after approval by the FDA
Antibody-drug conjugates (ADCs) are becoming an increasingly important sub-class of antibody-related therapeutics. Two ADCs, brentuximab vedotin (Adcetris®) and ado-trastuzumab emtansine (Kadcyla®), were recently approved for marketing both by the US Food and Drug Administration (FDA) and the European Medicine Agency (EMA). Brentuximab vedotin is marketed as therapy for hematological malignancies (Hodgkin lymphoma, systemic anaplastic large cell lymphoma), while ado-trastuzumab emtansine is marketed for treatment of a solid tumor (breast cancer). The approvals of these two ADCs followed the mitigated success of gemtuzumab ozogamicin (Mylotarg®), which was withdrawn from the US market in 2010, ten years after approval by the FDA.1
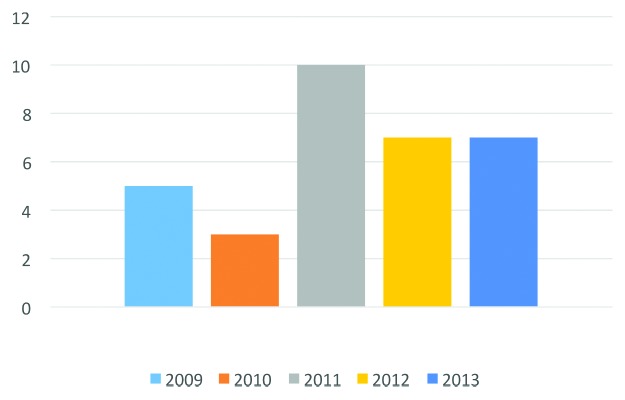
The approval successes of brentuximab vedotin and ado-trastuzumab emtansine may be just the first two of (potentially) many to come. Based on information available as of November 2013, 35 novel ADCs are currently being investigated in clinical studies as treatments for a variety of solid and liquid tumors, which is a sign of dynamism in the pipeline. Of the 35 ADCs, nearly 70% entered clinical study in the past three years (Fig. 1). Allowing for an average clinical development period of ~6 y for ADCs, the number of these drugs undergoing evaluation in pivotal studies and regulatory review should substantially increase in the next 3?5 y. One ADC, inotuzumab ozogamicin, is in Phase 3 studies as of November 2013.2
Figure 1. Novel antibody-drug conjugates entering clinical study during 2009?2013. Data available as of November 2013.
The ADCs in the clinical pipeline are directed against a plethora of different antigenic targets, but based on a limited number of cytotoxic drugs, such as calicheamicin, auristatins, maytansinoids, and more recently duocarmycins and pyrolidobenzodiazepines (PBDs). This indicates how difficult it is to find suitable drugs for ADCs that are highly potent to support an average of drug-to-antibody ratio (DAR) of 2 to 4, not too hydrophobic, ?linkable,? and accessible by simple synthetic pathways, to name a few of mandatory properties.
Recent Major Investments in ADCs
In the past few months, the major biopharmaceutical firms Novartis, Lilly, Roche and AstraZeneca have all announced investments in ADC research and development (R&D). Novartis and Lilly entered into licensing agreements with ImmunoGen giving the companies rights to use ImmunoGen?s ADC technology to develop therapeutics for specific targets. In mid-October 2013, Roche announced plans to invest 800 million Swiss francs within its global manufacturing network to increase production capabilities for its biologic medicines over the next five years. In addition, construction of an ADC production facility is planned in Basel through an investment of over 190 million Swiss francs, which is expected to create 50 jobs. This investment will provide additional capacity and flexibility to support Roche?s Kadcyla product and a further eight ADCs in clinical development. In December 2013, Roche announced an alliance with Molecular Partners AG to discover, develop, and commercialize DARPin®-drug conjugates for the treatment of cancer. The DARPins, which are small, non-antibody-based targeted proteins, will be conjugated to toxic agents developed at Roche.
AstraZeneca announced that MedImmune, its global biologics R&D arm, acquired Spirogen, a privately-held biotech company focused on ADCs based on PBD-based warheads. MedImmune has also entered into a collaboration agreement with ADC Therapeutics (ADCT) to jointly develop two new programs that are in preclinical development. MedImmune will also make an investment in ADC Therapeutics, which has an existing licensing agreement with Spirogen. MedImmune will acquire 100% of Spirogen?s shares, which comprises an initial consideration of $200 million, plus up to $240 million potential milestones. ADCT is an oncology drug development company that specializes in the development of proprietary ADCs targeting cancers, such as breast, lung, prostate, renal and blood. The company was launched in 2012 with a $50 million commitment. ADCT has access to warhead and linker chemistries via existing agreements with Spirogen. It operates a virtual business model based in Lausanne, Switzerland.
Small- to medium-sized enterprises are also investing in ADCs. In mid-November 2013, Sorrento Therapeutics, Inc. announced the entry into a definitive agreement to acquire San Diego-based Concortis Biosystems, Corp. in a deal that provides Sorrento with a comprehensive technology platform for next-generation ADCs. This acquisition will enable Sorrento to utilize the antibodies identified from its G-MAB® library along with Concortis' conjugation technologies and novel toxins to create a new generation of ADCs. Concortis' proprietary conjugation chemistries enable site-specific conjugation of toxins to the antibody, which will produce homogeneous ADCs with well-defined DARs. Contract manufacturing organizations such as Lonza, Piramal/ Fuji, Carbogen Amicis and SAFC have also made additional investments in the production of ADCs.3,4
Next-Generation Site-Specific ADCs
Conventional conjugation methods of the ADCs currently on the market and in clinical studies result in heterogeneous mixtures with different molar ratios of conjugated species comprising antibody linked to drugs at different sites.5 The drug payloads may be, for example, randomly attached to surface-exposed lysine residues distributed on both light and heavy chains (average of 80 to 95 per IgG), as illustrated by ado-trastuzumab emtansine. Alternatively, site-specific coupling to two or more of the eight cysteine residues involved in inter-chain disulfide bridges of chimeric, humanized or human IgG1 after mild reduction may be used, as illustrated in the case of brentuximab vedotin.
To create more homogeneously loaded ADCs that are better suited to clinical development, antibodies with engineered cysteines to enable thiol conjugation at these specific sites (THIOMABs) have been created.6,7 Various site-specific conjugation strategies are currently used for the production of ADCs, including use of other engineered cysteine residues, unnatural amino acids, and enzymatic conjugation through glycotransferases and transglutaminases as reviewed in the present issue of mAbs by Junutula8 and colleagues and by Behrens and Liu.9
Improvement of Analytical and Structural Methods for ADC Extensive Characterization
Conjugation of drugs to mAbs increases the structural complexity of the resulting molecule, which triggers the need for improved characterization methods.10 In the past decade, hundreds of papers have been published on the analytical and structural characterization of mAbs and related products, and the trend was amplified the last two years.11 Multiple and complementary liquid chromatography, electrophoresis, and mass spectrometry methods are used at all stages of drugs, mAbs and ADCs discovery and preclinical and clinical development. Importantly, the early use in the R&D process of MS methods also helps one to optimize the structure of next-generation ADCs from a pharmaceutical perspective, allowing the development of candidates with reduced chemistry manufacturing and control (CMC) liabilities and better drug-like properties (e.g., OptimADCs). Such improved methods have been recently described by Heck et al.,12 as well as by Wagner-Rousset et al. in the present issue of mAbs.3
Growth in ADC Information Exchange
The first global ADC Summit was organized in Boston in October 2010. Since 2011, two ADC Summits are held every year in Frankfurt and San Fransciso, respectively. These events are now established as the largest meetings fully devoted to ADC topics, attracting up to 500 attendees interested in the topic for the latest one held mid-October 2013.14,15 The trend toward increased coverage of ADC topics is observed in more general antibody meetings, such as the European Antibody Congress. Up to 2011, ADCs represented only a half-day sub-session in the congress,16-18 but by the end of 2011 the number of talks dedicated to ADCs comprised the largest part compared with naked antibodies and other related products such as bispecific antibodies, radio-immunoconjugates, immunotoxins, Fc-fusion proteins and peptides, and scaffolds of biosimilar antibodies.19,20 The same trend is also observed in other antibody meetings such as the PEGS meetings in Boston21 and CLARA?s meetings.22 As events in 2014 unfold, we look forward to publishing additional research reports, meeting reports and commentary on ADC development in mAbs.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Beck A, Haeuw JF, Wurch T, Goetsch L, Bailly C, Corvaïa N. . The next generation of antibody-drug conjugates comes of age. Discov Med 2010; 10:329 - 39; PMID: 21034674 [PubMed] [Google Scholar]
- 2.Reichert JM. . Antibodies to watch in 2014. MAbs 2013; 6; In press PMID: 24284914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner-Rousset E, Janin-Bussat MC, Colas O, Excoffier M, Haeuw JF, Rilatt I, Perez M, Corvaïa N, Beck A. . Antibody Drug Conjugate model fast characterization by LC-MS following IdeS proteolytic digestion. MAbs 2013; 6; Forthcoming 2013 PMID: 24135617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck A. Review of Antibody-Drug Conjugates, Methods in Molecular Biology series: A book edited by Laurent Ducry, mAbs 2014; 6: in press. [Google Scholar]
- 5.Beck A, Wurch T, Bailly C, Corvaïa N. . Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 2010; 10:345 - 52; http://dx.doi.org/ 10.1038/nri2747; PMID: 20414207 [DOI] [PubMed] [Google Scholar]
- 6.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, et al. . Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 2008; 26:925 - 32; http://dx.doi.org/ 10.1038/nbt.1480; PMID: 18641636 [DOI] [PubMed] [Google Scholar]
- 7.Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, Parsons-Reponte KL, Tien J, Yu SF, Mai E, et al. . Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol 2012; 30:184 - 9; http://dx.doi.org/ 10.1038/nbt.2108; PMID: 22267010 [DOI] [PubMed] [Google Scholar]
- 8.Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR. . Site-specific antibody drug conjugates for cancer therapy. MAbs 2014; 6; Forthcoming 2013 http://dx.doi.org/ 10.4161/mabs.27022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrens CR, Liu B. . Methods for site-specific drug conjugation to antibodies. MAbs 2013; 6; In press PMID: 24135651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakankar A, Chen Y, Gokarn Y, Jacobson FS. . Analytical methods for physicochemical characterization of antibody drug conjugates. MAbs 2011; 3:161 - 72; http://dx.doi.org/ 10.4161/mabs.3.2.14960; PMID: 21441786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck A, Wagner-Rousset E, Ayoub D, Van Dorsselaer A, Sanglier-Cianférani S. . Characterization of therapeutic antibodies and related products. Anal Chem 2013; 85:715 - 36; http://dx.doi.org/ 10.1021/ac3032355; PMID: 23134362 [DOI] [PubMed] [Google Scholar]
- 12.Rosati S, van den Bremer ET, Schuurman J, Parren PW, Kamerling JP, Heck AJ. . In-depth qualitative and quantitative analysis of composite glycosylation profiles and other micro-heterogeneity on intact monoclonal antibodies by high-resolution native mass spectrometry using a modified Orbitrap. MAbs 2013; 5:917 - 24; http://dx.doi.org/ 10.4161/mabs.26282; PMID: 23995615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck A, Senter P, Chari R. . World Antibody Drug Conjugate Summit Europe: February 21-23, 2011; Frankfurt, Germany. MAbs 2011; 3:331 - 7; http://dx.doi.org/ 10.4161/mabs.3.4.16612; PMID: 21691144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck A, Lambert J, Sun M, Lin K. . Fourth World Antibody-Drug Conjugate Summit: February 29-March 1, 2012, Frankfurt, Germany. MAbs 2012; 4:637 - 47; http://dx.doi.org/ 10.4161/mabs.21697; PMID: 22909934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klinguer-Hamour C, Strop P, Shah DK, Ducry L, Xu A, Beck A. . World Antibody-Drug Conjugate Summit, October 15-16, 2013, San Francisco, CA. MAbs 2014; 6 Forthcoming 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck A, Hanala S, Reichert JM. . 4th European Antibody Congress 2008: December 1-3, 2008, Geneva, Switzerland. MAbs 2009; 1:93 - 103; http://dx.doi.org/ 10.4161/mabs.1.2.7890; PMID: 20061813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck A, Reichert JM, Wurch T. . 5th European Antibody Congress 2009: November 30?December 2, 2009, Geneva, Switzerland. MAbs 2010; 2:108 - 28; http://dx.doi.org/ 10.4161/mabs.2.2.11302; PMID: 20179425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck A, Wurch T, Reichert JM. . 6th Annual European Antibody Congress 2010: November 29-December 1, 2010, Geneva, Switzerland. MAbs 2011; 3:111 - 32; http://dx.doi.org/ 10.4161/mabs.3.2.14788; PMID: 21441785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugovskoy AA, Reichert JM, Beck A. . 7th Annual European Antibody Congress 2011: November 29-December 1, 2011, Geneva, Switzerland. MAbs 2012; 4:134 - 52; http://dx.doi.org/ 10.4161/mabs.4.2.19426; PMID: 22453093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck A, Carter PJ, Gerber HP, Lugowskoy AA, Wurch T, Junutula JR, Kontermann K, Mabry R. . 8th Annual European Antibody Congress 2012: November 27-28, 2012, Geneva, Switzerland. MAbs 2013; 5:339 - 57; http://dx.doi.org/ 10.4161/mabs.24105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauwels PJ, Dumontet C, Reichert JM, Beck A, Goetsch L, Corvaia N, Klein C, Coiffier B, Teicher B. . 7th cancer scientific forum of theCancéropôle Lyon Auvergne Rhône-Alpes: March 20-21, 2012, Lyon, France. MAbs 2012; 4:434 - 44; http://dx.doi.org/ 10.4161/mabs.20869; PMID: 22684281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho M, Royston I, Beck A. . 2nd PEGS Annual Symposium on Antibodies for Cancer Therapy: April 30-May 1, 2012, Boston, USA. MAbs 2012; 4:562 - 70; http://dx.doi.org/ 10.4161/mabs.21521; PMID: 22864478 [DOI] [PMC free article] [PubMed] [Google Scholar]