Abstract
Feed intake is a major factor in maintaining the balance between ruminal fermentation and the microbial community of dairy cows. To explore the relationship among feed intake, microbial metabolism, and ruminal fermentation, we examined the combined signatures of the microbiome and metabolome in dairy cows with different feed intake levels. Eighteen dairy cows were allocated to high feed intake (HFI), medium feed intake (MFI), and low feed intake (LFI) groups according to their average daily feed intake. 16S rDNA sequencing results revealed that the relative abundance of Firmicutes in the HFI group was significantly higher than that in the MFI and LFI groups (P < 0.05). The ratio of Bacteroidetes to Firmicutes was significantly lower in the HFI group than in the MFI and LFI groups (P < 0.05). The relative abundance of Lachnospiraceae_unclassified, Veillonellaceae_unclassified, and Saccharofermentants was significantly higher in the HFI group than in the LFI and MFI groups (P < 0.05). The relative abundance of Erysipelotrichaceae_unclassified and Butyrivibrio was significantly higher in the HFI group than in the MFI and LFI groups (P < 0.05). Ultra high performance liquid chromatography-mass spectrometry revealed five key pathways, including the linoleic acid metabolism pathway, alpha-linolenic acid metabolism, arginine and proline metabolism, glutathione metabolism, and valine, leucine, and isoleucine biosynthesis, which are closely related to energy and amino acid metabolism. Linoleic acid, glutamate, alpha-linolenic acid, l-methionine, and l-valine levels were significantly lower in the HFI group than in the MFI and LFI groups (q < 0.05), while the relative content of glutamate was significantly lower in the MFI group than in the LFI group (q < 0.05). Stearic acid content was significantly higher in the HFI group than in the LFI group (q < 0.05). Our findings provide insight into the rumen microbiome of dairy cows with different feed intake and the metabolic pathways closely associated with feed intake in early-lactating cows. The candidates involved in these metabolic pathways may be useful for identifying variations in feed intake. The signatures of the rumen microbiome and metabolome in dairy cows may help make decisions regarding feeding.
Keywords: dairy cows, feed intake, metabolome, microbiome, ruminal fermentation
Introduction
Increased feed intake can improve the performance of dairy cows. Various factors affect feed intake, such as management, diet, weight, and milk production (Hayirli et al., 2002, Van De Stroet et al., 2016). Microbial communities are essential for the physiological function and the overall health of the host. Previous studies demonstrated that the ruminal microbial community is critical for milk production in dairy cows and average daily weight gain in steers (Jewell et al., 2015, Schären et al., 2018). Reportedly, fiber digestibility could be improved by increasing the metabolic activity of rumen microorganisms (Firkins and Yu, 2015) that, in turn, influenced feed intake (Durunna et al., 2011; Carberry et al., 2012; Hernandez-Sanabria et al., 2012). Additionally, recent studies have indicated that differences in dietary intake are primary factors influencing microbiota changes (Duncan et al., 2008; Jumpertz et al., 2011). Similarly, residual feed intake can lead to alterations in the microbiota composition and serum and urinary metabolic profiles in mice (Wu et al., 2016). Therefore, gut microbiota may critically modulate feed intake in dairy cows through a variety of mechanisms, including the secretion of metabolites and degraded products, which warrant in-depth research.
The understanding of the complex microbial activities, composition, and mechanism of microbial interactions is currently limited. Moreover, whether the mechanisms of microbial interaction affect host biology is unclear. We predicted that specific bacterial populations in the rumen affect feed intake, through microbial degradation byproducts (acetate, propionate, butyrate, etc.) that are produced during fermentation. Microbial ecology has been widely studied by 16S rDNA sequencing and metabolomics to reveal microbial interactions associated with digestibility, fermentation, and metabolites.
To explore the ruminal fluid signature of early-lactation Holstein dairy cows with different feed intake levels, the metabolic profile and microbial community were scrutinized using Illumina MiSeq and high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (HPLC-QTOF-MS), respectively. This study was aimed at determining whether the combined signatures of the microbiome and metabolome can be used to characterize dairy cows with different feed intake levels.
Materials and Methods
Procedures involving animals complied with the Animal Research Ethics Committee of Nanjing Agriculture University. A trial was conducted from October to January 2017 at the Huaian Dairy Farm of the WEIGANG Milk Industry Group Company of Nanjing, in Jiangsu Province, China.
Animals and management
Forty early-lactating Holstein dairy cows (mean ± SD; 59 ± 5 d in milk; body weight: 584 ± 10 kg; parity: 2 ± 0.9) without clinical signs of mastitis and other diseases were selected. The trial was conducted for 60 d. The dairy cows in the trial were managed in a tie-stall configuration with free access to water and individually fed with a total mixed ration three times per day (0700, 1330, and 1900 hours) (Tables 1 and 2).
Table 1.
Ingredient and chemical compositions of the total mixed ration
| Ingredient | g/kg of DM | Chemical composition | % of DM |
|---|---|---|---|
| Corn | 249.2 | NEL1, MJ/kg | 7.3 |
| Whole cottonseed | 48.4 | CP | 16 |
| Expanded soybean | 56.8 | NDF | 34 |
| Corn silage | 151.8 | ADF | 19.6 |
| Alfalfa hay | 207.6 | EE | 55.6 |
| Barley | 50.4 | ASH | 7.7 |
| Brewers grain | 29.1 | Ca | 6.5 |
| Caramel | 8.9 | P | 4.5 |
| Sugar beet meal | 55.2 | ||
| Soybean meal | 76.6 | ||
| Rapeseed meal | 11.5 | ||
| DDGS1 | 23.2 | ||
| Fatty acids calcium | 4.1 | ||
| Premix2 | 9.1 | ||
| CaHPO4 | 4.2 | ||
| Sodium bicarbonate | 6.4 | ||
| Salt | 4.2 | ||
| Vector | 2.5 | ||
| Mineral meal | 0.6 |
1Estimated according to the Hill et al. (2013) equations.
2Provided as per kilogram of diet: VA 5,130 IU, VD3 1,283 IU, VE 26 mg, Biotin 0.05 mg, Beta carotene 0.10 mg, Mn 12 mg, P 12 mg, S 0.85 mg, Zn 64 mg, Se 0.4 mg, and Co 0.19 mg.
Table 2.
Summary statistics for average daily dry matter intake and milk yield from dairy cows with different feed intake levels
| Items | LFI (n = 6)1 | MFI (n = 6) | HFI (n = 6) | SEM | P-value |
|---|---|---|---|---|---|
| Dry matter intake, kg/d | 21.19c | 23.30b | 25.76a | 0.47 | 0.00 |
| Milk yield, kg/d | 40.29c | 45.08b | 50.12a | 1.25 | 0.00 |
1 n = number of cows.
a–cDifferent superscript letters within rows suggest that means differ significantly (P < 0.05) among different feed intake groups.
Evaluation of feed intake and digestibility
Feed intake was calculated as the difference between the daily feed offered and refused (residual amount of feed ≥ 5%) by each cow with feed crates (1.0 m length, 0.5 m wide, and 0.65 m high) for 21 d in triplicate. At the end of the trial, the average daily feed intake of each cow ranged from 42.97 to 55.86 kg/day (SD = 3.32, n = 40) and was sorted in ascending order. Among the 40 cows, 18 were allocated to each of the three feed intake groups: low feed intake (LFI) group (mean feed intake = 44.15 kg/d, cows No. 1 to 6), medium feed intake (MFL) group (mean feed intake = 48.55 kg/d, cows No. 18 to 23), and high feed intake (HFL) group (mean feed intake = 53.67 kg/d, cows No. 35 to 40). On the last day of the trial, the total mixed ration and fecal samples were collected in sealed plastic bags at approximately 0200 hours before feeding. The dry matter content of the feed samples was determined by oven-drying at 65 °C for 48 h. Fecal samples were dried at 65 °C to a constant weight. The dried total mixed ration and fecal samples were ground, passed through a 1-mm sieve, and stored at −20 °C for subsequent chemical composition analyses. Acid-insoluble ash, which was used as an indigestible marker to evaluate the digestibility of the dietary components (Thiex et al., 2012), was obtained by a gravimetric method after acid hydrolysis with 3 M HCl followed by ashing at 550 °C. Ash was determined in a muffle furnace (Nabertherm, Bremen, Germany) at 550 °C for 4 h. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) digestibility (Mohd-Setapar et al., 2013) were analyzed using an Ankom 200 Fiber Analyzer (Ankom Technology, Fairport, NY, USA). The crude protein (CP) content was determined according to the Kjeldahl method (Thiex et al., 2002), using a Leco FP 528 N Analyzer (Leco Instruments, Ltd., Stockport, Cheshire, UK). The ether extract (EE) content was analyzed using a Soxtec instrument (Tecator, Höganäs, Sweden) with the soxhlet extraction method (Marques et al., 2015).
The contents of different nutrient compositions were calculated from the following equations:
where: A, sample weight; A1, bag tare weight; A2, weight of organic matter (OM) after extraction by neutral detergent; A3, weight of OM after extraction by acid detergent; B1, ash-corrected blank bag factor (a running average of the loss of weight after extraction of the blank bag/original blank bag). B2, ash-corrected blank bag factor (a running average of the loss of weight after extraction of the blank bag/original blank bag).
where A, dry sample weight; A1, filter paper weight before extraction; A2, filter paper weight after extraction.
The digestibility coefficients were calculated by the indicator technique using the following formula (Stojanovic et al., 2014):
DNA extractions and 16S rDNA sequencing of rumen fluid samples
At the end of the trial, rumen fluid samples were collected 3 h after morning feeding. Approximately, 100 mL of ruminal sample was obtained from the esophageal tube and strained through four layers of cheesecloth. DNA was extracted from rumen fluid samples using the E.Z.N.A. Stool DNA Kit (D4015, Omega, Inc., USA) as per the method manufacturer’s instructions. The total DNA was eluted in 50 µL Elution buffer and stored at −80 °C until experimental use. The V3-V4 region of the bacterial small-subunit (16S) rRNA gene was amplified by applying primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5 ′-GGACTACHVGGGTWTCTAAT-3′) with rumen fluid DNA samples as templates (Fadrosh et al., 2014). The 5′ end of the primers in each sample was marked with specific barcodes and sequencing universal primers. Polymerase Chain Reaction (PCR) amplification of the bacterial V3-V4 region was performed using a Phusion Hot Start Flex 2X Master Mix PCR Kit (Shanghai Yitao Biological Instrument Co., Ltd., Shanghai, China). The 25-µL reaction mixture contained 50 ng template DNA, 12.5 µL PCR Premix, 2.5 µL each primer, and PCR-grade water to adjust the volume. PCR was performed as follows: initial denaturation at 98 °C for 30 s; 35 cycles of denaturation at 98 °C for 10 s, annealing at 54 °C/52 °C for 30 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 10 min. The PCR amplification products were purified by using AMPure XT beads (Beckman Coulter Genomics, Brea, CA, USA) and quantified with Qubit (Invitrogen Corporation, Carlsbad, CA, USA). The amplicon pools were prepared for sequencing. The amplicon library was quantified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and assessed with the Hieff NGSTM Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA, USA). Amplicon sequencing was performed on an Illumina MiSeq platform at Hangzhou Lianchuan Biological Technology Co., Ltd. (Hangzhou, China).
HPLC-QTOF/MS measurements
Methanol, acetonitrile (AC), isopropanol (IPA), and formic acid were provided by LC-Bio (Hangzhou, China). The collected ruminal fluid samples were thawed on ice, and then 50% methanol was added to 20 μL of the samples that were then vortexed for 1 min and incubated at 25 °C for 10 min. The extraction mixture was stored overnight at −20 °C. After centrifugation at 4,000 × g for 20 min, the supernatants were transferred into new 96-well plates and diluted in IPA/AC/water (2:1:1, V/V/V). The samples were stored at −80 °C prior to the LC-MS analysis. Additionally, pooled quality control samples were prepared by combining 10 μL of each extraction mixture. These samples were then placed among the other ruminal fluid samples (after every four samples). The analysis was performed using an ultra-performance liquid chromatography (UPLC) system (SCIEX, Framingham, MA, USA). Chromatographic separation was carried out on an ACQUITY UPLC BEH Amide column (100 × 2.1 mm, 1.7-μm particle size, Waters, Milford, MA, USA). The mobile phase was composed of 25 mM ammonium acetate containing 25 mM NH4H2O (A) and IPA/AC (V/V, 9:1) containing 0.1% formic acid (B). A gradient program was performed as follows: 95% B for 0 to 0.5 min, 95% to 65% B for 0.5 to 9.5 min, 65% to 40% B for 9.5 to 10.5 min, 40% B for 10.5 to 12 min, 40% to 95% B for 12 to 12.2 min, 95% B for 12.2 to 15 min. The flow rate was 0.4 mL/min and the column oven was maintained at 35 °C. The injection volume for each sample was 4 µL. MS was performed using an Agilent 6530 UHD and an Accurate-Mass Triple TOF 5600 plus (SCIEX) equipped with an electrospray ionization interface operating in either positive or negative ion mode. The parameters of the electrospray interface were optimized as follows: curtain gas 30 psi; temperature 650 °C; ion source sheath gas 60 psi; ion source auxiliary gas 60 psi; ion spray voltage (+) 5,000 V and ion spray voltage (−) −4,500 V. The mass spectrometer was set to perform information-dependent acquisition. The parameter for this step was a TOF mass range of 60 to 1,200 Da. Survey scans were acquired in 150 ms and as many as 12 product ion scans were collected if the threshold of 100 counts per second was exceeded (total cycle time: 0.56 s). To evaluate the stability of the LC-MS, a quality control sample (pool of all samples) was acquired after every four samples.
Evaluation of rumen fermentation
To evaluate rumen fermentation, 1 mL of 25% (w/v) metaphosphoric acid was added to 5 mL of ruminal fluid and stored at −80 °C for volatile fatty acid analysis. Another 5 mL of filtered ruminal fluid was added to 1 mL of 1% sulfuric acid and stored at −80 °C for NH3-N determination. Additionally, 5 mL of filtered ruminal fluid was stored at −80 °C to determine the microbial protein content. The pH was determined immediately with a pH meter (Model PHS-3C, Shanghai Precision Scientific Instrument Co., Ltd., Shanghai, China) as soon as the fluid samples were obtained. The microbial protein concentration and the concentrations of NH3-N were measured as described by Broderick and Kang (1980). To evaluate ruminal volatile fatty acid, the ruminal fluid samples were thawed and centrifuged at 12,000 × g for 15 min at 4 °C and quantified by gas chromatography (GC-14B; Shimadzu Corporation, Kyoto, Japan) with a capillary column (Agilent Technologies, Santa Clara, CA, USA; 30 m × 0.32 mm i.d., × 0.25 μm) and flame ionization detection. Crotonic acid was the internal standard. The carrier gas was hydrogen and column flow was 3 mL/min. The temperatures of the flame ionization detection, column, and vaporization were 220, 130, and 180 °C, respectively.
Statistical analysis
Samples were sequenced by 250-bp paired-end sequencing chemistry on an Illumina Miseq platform (Illumina, San Diego, CA, USA) according to the manufacturer’s recommendations provided by LC-Bio. Paired-end reads were first merged using FLASH (Magoč and Salzberg, 2011). Low-quality or ambiguous reads were excluded, including reads with any primer or barcode mismatch and ambiguous N character. Chimera removal was evaluated using VSEARCH software (v2.3.4) (Rognes et al., 2016). High-quality sequences with ≥97% homology were clustered to the same operational taxonomic units (OTUs) by VSEARCH (v2.3.4). Representative sequences were chosen for each OTU and taxonomically assigned using the Ribosomal Database Project (Cole et al., 2009) database. Alpha diversity was applied to analyze rarefaction curves and calculate richness estimators (Ace and Chao1) and diversity indices (Shannon and Simpson) based on the OTUs. All indices were calculated with QIIME (Caporaso et al., 2010). Beta diversity analysis was used to assess similarities in the microbial composition, which were calculated by principal coordinates analysis and cluster analysis using QIIME software (Version 1.8.0).
LC-MS raw data files were converted into mzXML format and processed with the XCMS, CAMERA metaX toolbox implemented with R software for preprocessing, which included peak picking, peak grouping, and retention time correction. The data were aligned and normalized based on total ion intensity. The ruminal compounds were identified by comparison with online the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Human Metabolome Database (HMDB) metabolome databases using exact m/z values and retention times. Wilcoxon tests were conducted to detect differences in metabolite concentrations between each group (HFI group vs. LFI group, HFI group vs. MFI group, and MFI group vs. LFI group). Principal component analysis, an unsupervised chemometric method, was to assess whether there was any clustering, trends, or outliers. The supervised partial least squares discriminant analysis was conducted using metaX to maximize the separation between feed intake groups of observations. R2X and Q2 values reflected the faithful representation and reliable predictive capacity of the model, respectively. Furthermore, model validity was assessed with rigorous permutation tests (n = 200). The variable importance projection value was calculated using a cutoff value of 1.0 to select relevant features.
The correlation network was evaluated using Spearman’s rank correlation coefficient on the differential taxonomy and metabolites in the R program, combined with a P-value of less than 0.01. To protect against type I error inflation, the R-P-adjust method in the R program were used to calculate false discovery rates, with a threshold of 0.05.
The effects of feed intake on fermentation parameters and apparent digestibility were assessed by one-way analysis of variance in SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). The data were analyzed according to the following model: Yij = a + Fi + C(F)ij + eij, where a is the overall mean, Fi is the feed intake group, C(F)ij is the cow within the group, and eij is the error term. The statistical model included the fixed effect of feed intake level and the random effect of a cow. Means were compared by using the least significant difference multiple-range test with statistical significance defined as P < 0.05.
Results
Analysis of variation in microbial community amplicon sequencing
We used high-throughput Illumina sequencing targeting the V3 and V4 regions of the 16S rRNA to detect and characterize the overall ruminal bacterial composition. A total of 408,704 raw reads were obtained from the Illumina MiSeq platform sequencing runs, averaging 22,706 sequencings per sample. A total of 359,423 clean sequences have remained after quality control, with a mean of 19,968 clean sequences per sample. The distributed range in length of all sequences was 400 to 500 bp. The clean sequences of the 18 rumen fluid samples were clustered based on their OTUs. A total of 6,058 OTUs were obtained by using a 3% threshold difference for OTU clustering.
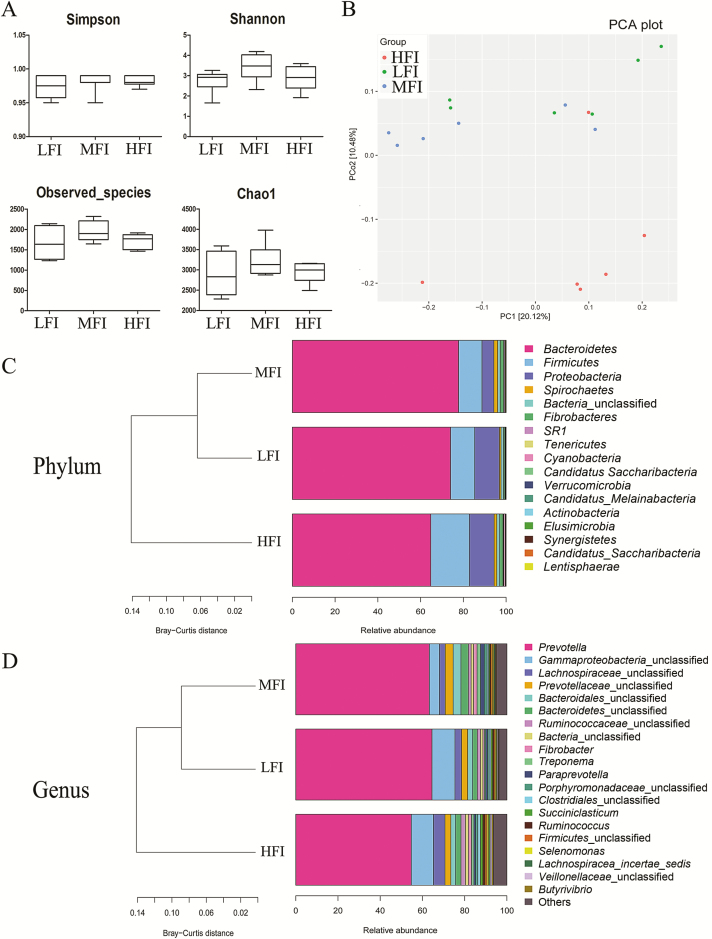
α-Diversity is measured within samples and based on the results of species annotation OTU. Our results revealed no significant difference in the Chao1 index, observed species, or Shannon and Simpson index (P > 0.05) (Figure 1A). Additionally, principal coordinates analysis revealed a better separation between the HFI group and MFI group and the HFI group and LFI group, indicating that feed intake was a crucial factor contributing to these differences.
Figure 1.
Boxplots of diversity variables of ruminal fluid associated bacteria in dairy cows from HFI group (n = 6), MFI group (n = 6), and LFI group (n = 6) (A). Principal coordinate (PC) analysis showing the relationship among different feed intake groups based on the bacteria OTU level (B). Colors represent different feed intake groups; green, LFI group; blue, MFI group; red, HFI group. Each point in the figure represents a rumen fluid sample per cow. Principal coordinate (PC) 1 and PC2 described 20.1% of the variance and PC2 10.5%. Percentage contribution of sequences belonging to each phylum (C) and genera (D) to the total number of sequences with cluster analysis based on Bray–Curtis distance, respectively.
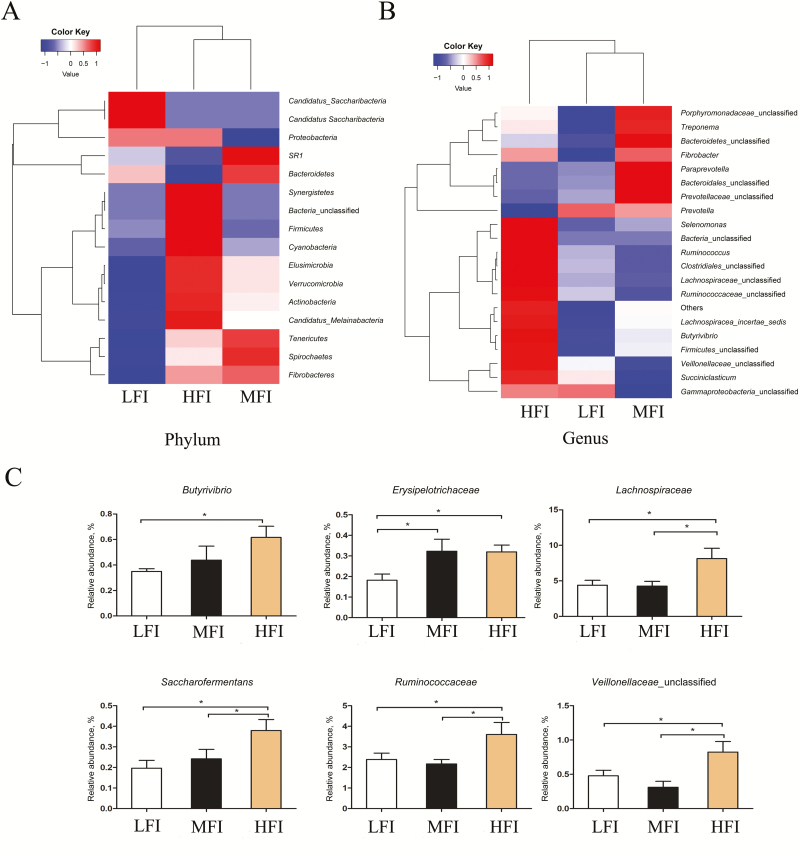
Bacteroidetes, Firmicutes, and Proteobacteria, with a total relative abundance of 96.88%, 94.27%, and 94.38% in the LFI group, MFI group, and HFI group, respectively, were identified as the dominant bacterial phyla by taxonomic assignment (Table 3). Less abundant phyla included unclassified bacteria, Fibrobacteres, SR1, Spirochaetes, and Tenericutes. As expected, we observed pronounced changes in bacterial phyla for animals among the feed intake groups. There were significantly higher proportions of Firmicutes and Tenericutes in the HFI group compared with the other feed intake groups (P < 0.05), while there was no significant difference between the LFI group and the MFI group. Additionally, the relative abundance of SR1 was significantly lower in the MFI group than in the LFI group and HFI group (P < 0.05), whereas there was no distinct difference between the LFI group and the HFI group (P > 0.05). For the Firmicutes/Bacteroidetes ratio in the rumen microbiota, we observed significant differences between the HFI group and MFI group (3.88 and 7.88, respectively) and the HFI group and MFI group (3.88 and 6.85, respectively). Notably, no significant differences were detected between the MFI group and the LFI group. The detected OTU sequences were assigned to 153 genera. In total, 19 of these genera represented more than 0.1% relative abundance in all samples from the different feed intake groups, as shown in Table 4. Prevotella was the most dominant among the 19 genera, accounting for 64.52%, 63.25%, and 54.73% in the LFI, MFI, and HFI groups, respectively. As graphically illustrated in Figure 2B and C, different feed intake levels exerted minimal effects on most of the dominant genera, except for Succiniclastium, unclassified Veillonellaceae, Butyrivibrio, and Saccharofermentans (P < 0.05). Additionally, at the family level, discrepant microbial species were observed among the feed intake groups, including Lachnospiraceae, Erysipelotrichaceae, and Ruminococcaceae. The families Lachnospiraceae and Ruminococcaceae showed a higher relative abundance in the HFI group than in the MFI group and LFI group (P < 0.05), while no significant difference was observed between the MFI group and LFI group. The family of Erysipelotrichaceae showed lower relative abundance in the LFI group relative to the MFI group and HFI group (P < 0.05), whereas there was no obvious change between the MFI group and HFI group. A significantly higher relative abundance of Butyrivibrio was observed in the HFI group compared with the LFI group (P < 0.05), but no distinct difference was observed among the other groups. The relative abundances of unclassified Veillonellaceae and Saccharofermentans were higher in the HFI group than in the MFI group and LFI group (P < 0.05), while there was no significant difference between the MFI group and LFI group. A significantly lower relative abundance of Succinicalastium was observed in the MFI group relative to the HFI group and LFI group (P < 0.05), but there was no distinct change between the HFI group and the LFI group.
Table 3.
In the phylum, the relative abundance of the bacterial communities from HFI, MFI, and LFI groups, %
| Item | LFI (n = 6)1 | MFI (n = 6) | HFI (n = 6) | SEM | P-value |
|---|---|---|---|---|---|
| Bacteroidetes | 74.01a | 77.76a | 64.76b | 2.52 | 0.09 |
| Firmicutes | 11.32b | 10.96b | 18.03a | 1.23 | 0.02 |
| Proteobacteria | 11.55 | 5.55 | 11.59 | 1.59 | 0.21 |
| Spirochaetes | 0.74 | 1.80 | 1.26 | 0.21 | 0.13 |
| Bacteria_unclassified | 1.16 | 1.16 | 1.40 | 0.09 | 0.45 |
| Fibrobacteres | 0.66 | 1.66 | 1.49 | 0.28 | 0.33 |
| SR1 | 0.18b | 0.51a | 0.14b | 0.06 | 0.01 |
| Tenericutes | 0.15b | 0.31a | 0.25a | 0.03 | 0.04 |
| Cyanobacteria | 0.03 | 0.05 | 0.71 | 0.22 | 1.03 |
| Candidatus Saccharibacteria | 0.09 | 0.08 | 0.08 | 0.01 | 0.30 |
| Verrucomicrobia | 0.03 | 0.05 | 0.07 | 0.01 | 1.21 |
| Candidatus melainabacteria | 0.02 | 0.04 | 0.08 | 0.02 | 1.60 |
| Actinobacteria | 0.03 | 0.04 | 0.05 | 0.00 | 1.62 |
| Elusimicrobia | 0.01 | 0.03 | 0.06 | 0.01 | 1.20 |
| Synergistetes | 0.01 | 0.02 | 0.04 | 0.00 | 6.31 |
| The ratio of Bacteroidetes/Firmicutes | 6.85a | 7.78a | 3.88b | 0.67 | 0.03 |
1 n = number of cows.
a,bDifferent superscript letters within rows suggest that means differ significantly (P < 0.05) among different feed intake groups.
Table 4.
In the genus level, the relative abundance of the bacterial communities from HFI, MFI, and LFI groups, %
| Items | LFI(n = 6)1 | MFI (n = 6) | HFI (n = 6) | SEM | P-value |
|---|---|---|---|---|---|
| Prevotella | 64.52 | 63.25 | 54.73 | 2.11 | 0.12 |
| Gammaproteobacteria_unclassified | 10.90 | 4.79 | 10.50 | 1.56 | 0.21 |
| Lachnospiraceae_unclassified | 3.12b | 2.73b | 5.46a | 0.47 | 0.03 |
| Prevotellaceae_unclassified | 2.92 | 3.77 | 2.80 | 0.23 | 0.19 |
| Bacteroidales_unclassified | 2.35b | 3.69a | 2.26b | 0.28 | 0.06 |
| Bacteroidetes_unclassified | 2.18 | 3.51 | 2.53 | 0.31 | 0.20 |
| Ruminococcaceae_unclassified | 1.51 | 1.32 | 2.11 | 0.18 | 0.18 |
| Fibrobacter | 0.66 | 1.66 | 1.49 | 0.28 | 0.33 |
| Treponema | 0.68 | 1.65 | 1.15 | 0.20 | 0.15 |
| Paraprevotella | 0.79b | 1.73a | 0.75b | 0.20 | 0.06 |
| Porphyromonadaceae_unclassified | 0.81 | 1.35 | 1.07 | 0.13 | 0.27 |
| Clostridiales_unclassified | 0.86 | 0.77 | 1.25 | 0.12 | 0.21 |
| Succiniclasticum | 0.81a | 0.49b | 1.17a | 0.09 | 0.00 |
| Ruminococcus | 0.63 | 0.55 | 1.02 | 0.10 | 0.13 |
| Firmicutes_unclassified | 0.57 | 0.70 | 0.92 | 0.09 | 0.26 |
| Selenomonas | 0.63 | 0.65 | 0.83 | 0.11 | 0.72 |
| Veillonellaceae_unclassified | 0.48b | 0.31b | 0.83a | 0.08 | 0.02 |
| Butyrivibrio | 0.35b | 0.44b | 0.62a | 0.05 | 0.09 |
| Erysipelotrichaceae_unclassified | 0.18b | 0.32a | 0.32a | 0.03 | 0.04 |
| Saccharofermentants | 0.20b | 0.24b | 0.38a | 0.03 | 0.03 |
1 n = number of cows.
a,bDifferent superscript letters within rows suggest that means differ significantly (P < 0.05).
Figure 2.
Heat map analysis associated bacteria in dairy cows from HFI group (n = 6), MFI group (n = 6), and LFI group (n=6), at phylum level (A). Relative abundance of bacteria at phylum level in HFI group (n = 6), MFI group (n = 6), and LFI group (n = 6), with cluster analysis based on Bray–Curtis distance (B). Discrepant microbial results confirmed by 16S rDNA in the LFI group (n = 6), MFI group (n = 6), and HFI group (n = 6) (C). *indicates a statistical difference (P < 0.05).
Discrepancy of metabolites in dairy cows with different feed intake levels
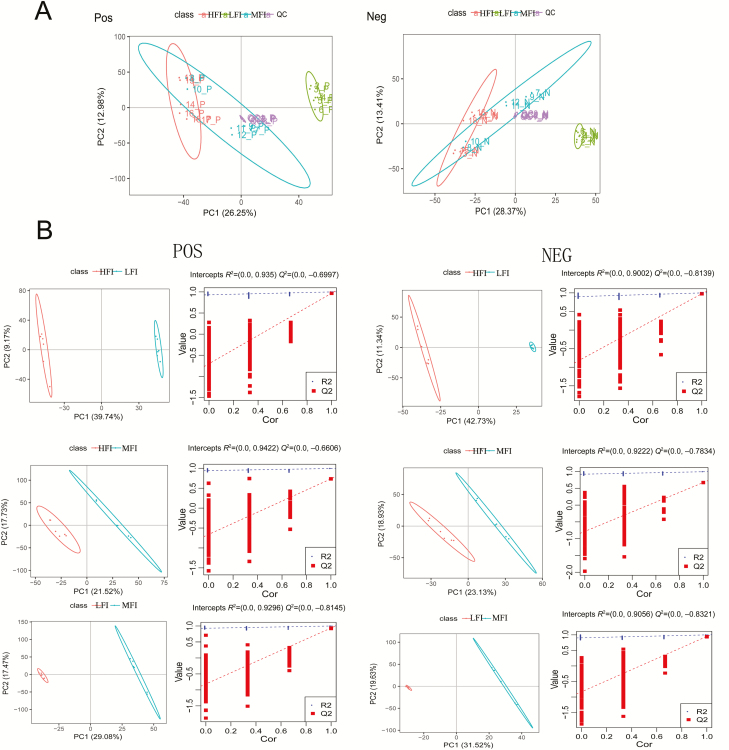
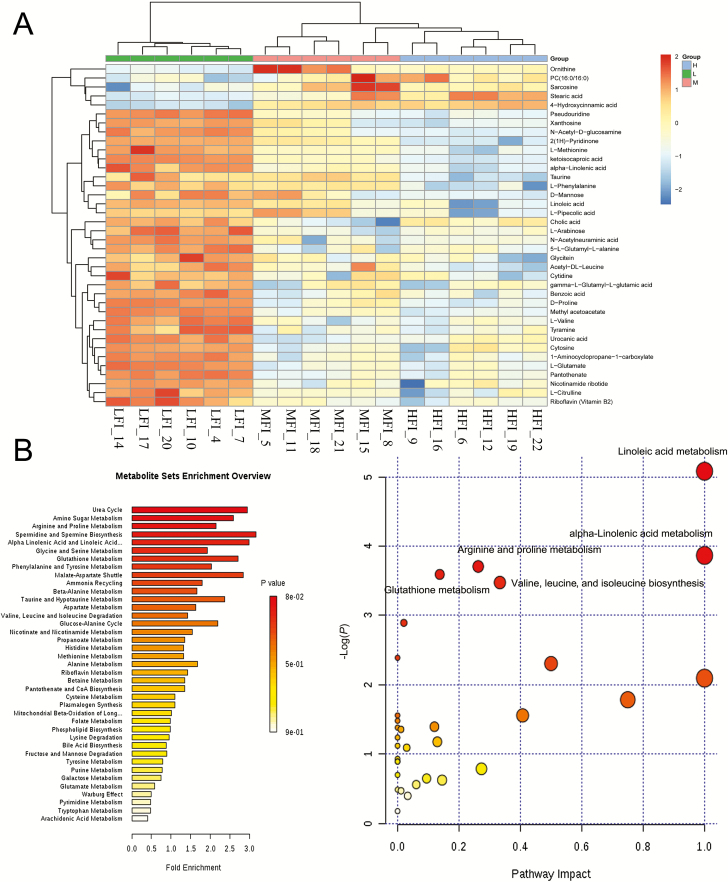
MS data for ruminal fluid metabolites from the HFI, MFI, and LFI groups were analyzed by UPLC-Q-TOF-MS. The principal component analysis showed a primary unsupervised separation among groups (Figure 3A). To further augment the separation to identify discrepant metabolites, we used a supervised partial least squares-discriminant analysis model among the feed intake groups (Figure 3B). The score plot displayed a clear separation between HFI and LFI groups, HFI and MFI groups, and between MFI and LFI groups. Therefore, we further evaluated these three sets of data to detect differential metabolites among the groups. A total of 122 differential metabolites were obtained between the HFI group and the LFI group; 8 differential metabolites were observed between the HFI group and the MFI group; 66 differential metabolites were detected between the MFI group and LFI group. Finally, 38 metabolites with a variable importance projection value above 1.0 were presented in Table 5, among the feed intake groups. Notably, these discrepant metabolites were mainly amino acids, organic acids, aromatic compounds, lipid compounds, nucleic acids, and base derivatives. According to key metabolic pathway analysis using the KEGG database in positive and negative ion modes, metabolic pathways with P-value < 0.05 were selected as the main impact pathways and shown in Table 6, among the feed intake groups. There were five key pathways in total: linoleic acid metabolism; alpha-linolenic acid metabolism; arginine and proline metabolism; glutathione metabolism; and valine, leucine, and isoleucine biosynthesis, which are primarily involved in amino acid metabolism and energy metabolism (Figure 4B). Moreover, with respect to the impact values of linoleic acid metabolism and alpha-linolenic acid metabolism showing values of 1, both parameters were considered as more important pathways. As shown in Table 5 and graphically represented in Figure 5, we also found discriminated metabolites, including stearic acid, linoleic acid, l-glutamate, urocanic acid, l-methionine, l-proline, l-valine, and alpha-linolenic acid, which were involved in five key metabolic pathways. UPLC-MS analyses revealed lower ruminal fluid excretion of l-methionine, linoleic acid, alpha-linolenic, l-glutamate, urocanic acid, and d-proline in the HFI group relative to the LFI group. The level of stearic acid was higher in the HFI group relative to the LFI group. Compared with the LFI group results, the levels of l-glutamate, l-methionine, and l-valine were significantly lower in the MFI group (q < 0.05), whereas there were no significant differences for these metabolic compounds between the HFI group and MFI group.
Figure 3.
Principal component analysis (PCA) score plots of metabolic profiling in positive and negative ion mode (A). Colors represent different feed intake groups and quality control (QC) group; green, LFI group (n = 6); blue, MFI group (n = 6); red, HFI group (n = 6); purple, QC group (n = 5). Principal coordinate (PC) A1 and PCA2 elucidated 26.25% and 12.98% of the variance, respectively, in the positive ion mode, PCA1 described 28.37% of the variance and PCA2 13.41% in the negative ion mode. PLS-DA score plots and corresponding permutation test model validation plot of PLS-DA of ruminal fluid samples from dairy cows in LFI group, MFI group, and HFI group, in the positive and negative ion mode, respectively (B).
Table 5.
Identification of significantly different metabolites among different feed intake groups analyzed using UPLC-Q-TOF-MS
| Chemical compound | MFI group vs. LFI group | HFI group vs. MFI group | HFI group vs. LFI group | Rtmed3 | Mzmed4 | Chemical structure |
|---|---|---|---|---|---|---|
| Ketoisocaproic acid | ↓ Down1 | ↓ Down2 | ↓ Down | 62.10 | 129.05 | C6H10O3 |
| l-Methionine | ↓ Down | None | ↓ Down | 317.46 | 148.04 | C5H11NO2S |
| Cytosine | ↓ Down | None | ↓ Down | 195.22 | 112.05 | C4H5N3O |
| Linoleic acid | None | None | ↓ Down | 35.89 | 279.23 | C18H32O2 |
| Stearic acid | None | None | ↑ Up | 35.83 | 283.26 | C18H36O2 |
| n-Acetylneuraminic acid | ↓ Down | None | ↓ Down | 299.90 | 290.08 | C11H19NO9 |
| Nicotinamide ribotide | ↓ Down | None | ↓ Down | 402.70 | 333.06 | C11H16N2O5 |
| l-Pipecolic acid | None | None | ↓ Down | 275.60 | 130.09 | C6H10NO2 |
| Sarcosine | ↑ Up | None | ↑ Up | 310.73 | 131.08 | C3H7NO2 |
| l-Glutamate | ↓ Down | None | ↓ Down | 402.67 | 148.06 | C5H9NO4 |
| 2(1H)-Pyridinone | ↓ Down | ↓ Down | ↓ Down | 106.21 | 113.07 | C7H9NO |
| Pseudouridine | ↓ Down | None | ↓ Down | 254.08 | 245.08 | C9H12N2O6 |
| Pantothenate | ↓ Down | None | ↓ Down | 272.59 | 220.12 | C9H17NO5 |
| Cytidine | None | None | ↓ Down | 240.15 | 244.09 | C9H13N3O5 |
| Alpha-Linolenic acid | None | None | ↓ Down | 66.81 | 279.23 | C18H30O2 |
| Riboflavin | ↓ Down | None | ↓ Down | 218.00 | 377.14 | C17H20N4O6 |
| 1-Aminocyclopropane-1-carboxylate | ↓ Down | None | ↓ Down | 402.91 | 84.04 | C4H7NO2 |
| Tyramine | ↓ Down | None | ↓ Down | 277.28 | 120.08 | C8H12NO |
| Urocanic acid | None | None | ↓ Down | 291.69 | 139.05 | C6H6N2O2 |
| l-Arabinose | ↓ Down | None | ↓ Down | 141.45 | 173.04 | C5H10O5 |
| Methylacetoacetate | ↓ Down | None | ↓ Down | 150.02 | 117.05 | C5H8O3 |
| l-Phenylalanine | None | ↓ Down | ↓ Down | 275.10 | 166.08 | C9H11NO2 |
| Acetyl-dl-Leucine | None | None | ↓ Down | 195.61 | 172.10 | C8H15NO3 |
| l-Citrulline | ↓ Down | None | ↓ Down | 402.67 | 174.09 | C6H13N3O3 |
| Ornithine | None | None | ↑ Up | 313.89 | 174.12 | C5H12N2O2 |
| Taurine | None | ↓ Down | ↓ Down | 298.48 | 124.00 | C2H7NO3S |
| l-Valine | ↓ Down | None | ↓ Down | 334.11 | 116.07 | C5H11NO2 |
| Benzoic acid | None | None | ↓ Down | 33.82 | 121.03 | C6H5COOH |
| 5-l-Glutamyl-l-alanine | ↓ Down | None | ↓ Down | 415.94 | 217.09 | C8H14N2O5 |
| Cholic acid | ↓ Down | None | None | 225.45 | 407.28 | C24H40O5 |
| gamma-l-Glutamyl-l-glutamic acid | None | None | ↓ Down | 464.96 | 277.10 | C10H16N2O7 |
| n-Acetyl-d-glucosamine | ↓ Down | None | ↓ Down | 273.58 | 186.08 | C8H15NO6 |
| PC(16:0/16:0) | None | None | ↑ Up | 161.80 | 756.55 | C40H80NO8P |
| d-Mannose | None | None | ↓ Down | 300.58 | 198.10 | C12H24O12 |
| Glycitein | None | None | ↓ Down | 37.80 | 285.08 | C16H12O5 |
| 4-Hydroxycinnamic acid | None | None | ↑ Up | 318.18 | 327.08 | C9H8O3 |
| Xanthosine | None | None | ↓ Down | 255.69 | 283.07 | C10H12N4O6 |
| d-Proline | None | None | ↓ Down | 311.49 | 116.07 | C10H16NO4 |
1The“↓”means that relative peak area of metabolites in the MFI group was significantly lower compared with LFI group and that relative peak area of metabolites in the HFI group was significantly lower compared with LFI or MFI group. “None” means that there is no significant difference between MFI group and LFI group, between HFI group and LFI group, and between HFI group and MFI group.
2“Down” and “Up” indicate P < 0.05, which is adjusted by Bonferroni correction.
3Rtmed, median of m/z.
4Mzmed, median of retention time.
Table 6.
The results of metabolic pathway affected the important metabolites detected among feed intake groups based on the MetaboAnalyst software 3.0
| Metabolic pathway name | Total compounds1 | Hits2 | Raw P-Value3 | Impact |
|---|---|---|---|---|
| Linoleic acid metabolism | 5 | 2 | 0.01 | 1.00 |
| Alpha-Linolenic acid metabolism | 9 | 2 | 0.02 | 1.00 |
| Arginine and proline metabolism | 44 | 4 | 0.02 | 0.26275 |
| Glutathione metabolism | 26 | 3 | 0.03 | 0.1374 |
| Valine, leucine, and isoleucine biosynthesis | 11 | 2 | 0.03 | 0.33333 |
1Total compounds indicate the number of compounds in the pathway.
2The Hits indicates the actual matched number from the user uploaded data.
3The Raw P-Value is the original P-value calculated from enrichment analysis.
Figure 4.
Hierarchical clustering analysis for identified differential metabolites in the LFI group (n = 6), MFI group (n = 6), and HFI (n = 6) (A). Green squares represent LFI group, pink squares represent MFI group, and blue squares represent HFI group. Each row represents one metabolite; each column represents one sample. Cells are colored based on the signal intensity measured from LC-MS. Dark brown represents high ruminal levels, blue shows low signal intensity, and gray cells show the intermediate level (see color scale on the right of heat map). Analysis of metabolism pathways as visualized using a bubble plot, through MetPA software (B). The bubble size is proportional to the impact of each pathway and the bubble color presents the significance, from highest in red to lowest in white; P-value is less than 0.05 and pathway impact factor is more than 0.5 indicate that the pathway is greatly influenced.
Figure 5.
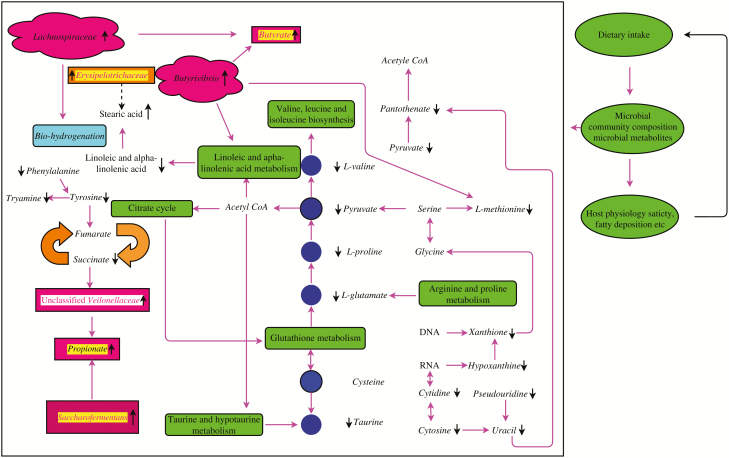
Network of key pathways and their related to metabolites from LFI group and HFI group. Showing the functions of the significantly altered microbial communities and differential expression of metabolites. Black arrows revealed decreased or increased levels in the HFI group. The metabolic pathways were generated using the reference map by KEGG.
We then further investigated the driving forces of microbial metabolites by measuring the levels of acetate, propionate, butyrate, N-NH3, and microbial CP content. We assessed the effects of different feed intake levels on rumen fermentation parameters (Table 7). There were no significant differences among the feed intake groups in the N-NH3 and microbial CP, acetate, volatile fatty acid, or acetate/propionate ratio. Notably, a significantly lower pH was observed in the LFI group, relative to the MFI group and HFI group (P < 0.05), whereas there was no pronounced change between the MFI group and the HFI group. Additionally, there were significantly lower propionate and butyrate concentrations in the MFI group compared with the HFI group and LFI group (P < 0.05), while no noticeable difference was observed between the MFI group and HFI group. To determine the effects of different feed intake levels on apparent digestibility, we utilized the acid-insoluble ash method to measure fecal samples from 18 dairy cows. Digestibility coefficients measured among the different feed intake groups are presented in Table 7. The similarity in digestibility coefficients detected among the different feed intake groups was reflected in OM, CP, EE, NDF, and hemicellulose (HCEL) (P > 0.05). Significant differences were identified among these groups for acid detergent fiber and the cellulose (CEL) digestibility coefficient. The acid detergent fiber and CEL digestibility coefficient were significantly increased in the HFI group compared with the MFI group and LFI group (P < 0.05), whereas there was no significant difference between the MFI group and HFI group (P > 0.05).
Table 7.
Apparent digestibility of nutrients and ruminal fermentation parameters including volatile fatty acid (VFA) concentration for HFI, MFI, and LFI groups
| Item | LFI (n = 6)1 | MFI (n = 6) | HFI (n = 6) | SEM | P-value |
|---|---|---|---|---|---|
| Apparent digestibility, g/kg | |||||
| OM | 70.44 | 71.86 | 72.99 | 0.79 | 0.44 |
| CP | 69.04 | 70.27 | 69.48 | 0.67 | 0.77 |
| EE | 81.54 | 84.75 | 85.10 | 1.37 | 0.53 |
| NDF | 47.83 | 50.16 | 51.95 | 1.30 | 0.46 |
| ADF | 36.90b | 45.30a | 44.78a | 1.62 | 0.05 |
| HCEL | 56.78 | 58.54 | 62.43 | 1.57 | 0.34 |
| CEL | 48.83b | 58.52a | 57.48a | 1.61 | 0.02 |
| pH | 6.43b | 6.8a | 6.68a | 0.05 | 0.00 |
| N-NH3, mg/d | 6.68 | 5.77 | 5.62 | 0.54 | 0.71 |
| Microbial CP, mg/mL | 0.31 | 0.35 | 0.45 | 0.04 | 0.41 |
| VFA, mol/100 mol | |||||
| Acetate | 59.39 | 59.57 | 62.67 | 1.47 | 0.62 |
| Propionate | 24.66b | 27.80b | 30.03a | 0.87 | 0.03 |
| Butyrate | 10.96b | 11.73b | 13.80a | 0.50 | 0.04 |
| Total VFA | 98.18 | 102.88 | 110.67 | 2.43 | 0.10 |
| Acetate/Propionate ratio | 2.42 | 2.42 | 2.11 | 0.07 | 0.17 |
1 n = number of cows.
a,bDifferent superscript letters within rows suggest that means differ significantly (P < 0.05) among different feed intake groups.
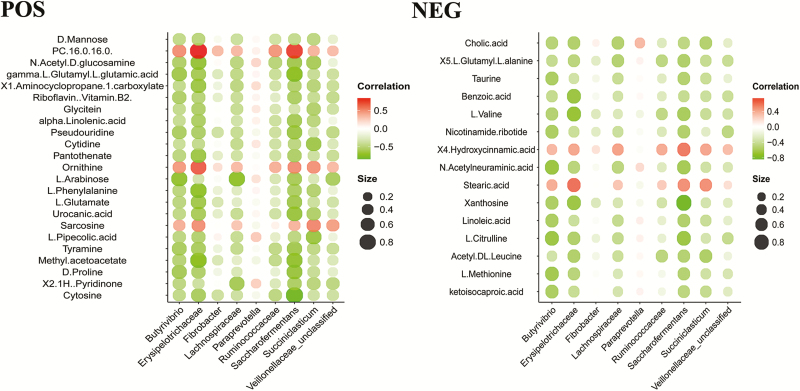
To further study the correlation between ruminal fluid metabolites perturbations and microbiome changes, we examined the correlation of nine microbial taxa at the family and genera levels and 38 discrepant metabolites using the Spearman’s rank coefficient correlation method (Figure 6). Correlations were evaluated to define the relationships between discrepant metabolites and the perturbed rumen microbiome. Positive associations were observed between Erysipelotrichaceae and PC (16:0/16:0) or stearic acid and between Fibrobacter and ornithine. In contrast, Erysipelotrichaceae was negatively associated (r > 0.45, P < 0.05) with 27 discrepant metabolites. Saccharofermentans was negatively related (r > 0.45, P < 0.05) to 16 differential metabolites. Butyrivibrio was negatively associated (r > 0.45, P < 0.05) with six discrepant metabolites. Succiniclasticum was negatively related (r > 0.45, P < 0.05) to three differential metabolites. Lachnospiraceae was negatively associated (r > 0.45, P < 0.05) with three discrepant metabolites. We did not examine the causes of these biological interactions for significant metabolites associated with the microbial community structure. The correlation between metabolites and microbes may reflect a combination of ruminal signatures.
Figure 6.
Heatmap of Spearman’s rank correlation coefficients of the relative abundances of different microbial communities at the family and genera level and ruminal fluid metabolites in the LFI group (n = 6), MFI group (n = 6), and HFI group (n = 6). Circle sizes and color intensity represent the magnitude of correlation. Red circles = positive correlation; green circles = negative correlation.
Discussion
The microbiome is closely associated with appetite regulation (Million et al., 2012; Evans et al., 2013), which may be the basis for an effective strategy for distinguishing dairy cows with HFI from those with LFI. We utilized high-throughput Illumina sequencing targeting the V3-V4 region of the 16S rDNA to evaluate the overall ruminal bacterial composition and UPLC-Q-TOF-MS to characterize the ruminal fluid metabolome. This is the first tentative model to associate the signatures of feed intake with integrative analysis of the microbiome and metabolome.
Some alterations in the rumen microbiome may be useful for characterizing dairy cows with different feed intake levels. In animals with HFI, Butyrivibrio and Erysipelotrichaceae were enriched (Elolimy et al., 2018; Kubasova et al., 2018), which agreed with our results. Interestingly, Erysipelotrichaceae has been associated with increased dietary fat intake, body weight, and fat deposition in mice (Fleissner et al., 2010). It is generally acknowledged that Ruminococcaceae is comprised of known fibrolytic members, including two dominant ruminal cellulolytic species, Ruminococcaceae albus and Ruminococcaceae flavefaciens. Our study revealed that dairy cows with HFI had a higher abundance of Ruminococcaceae, corresponding to higher digestibility of ADF and CEL. Sugar-fermenting bacteria such as Saccharofermentans and Butyrivibrio were richer in CEL digestate. Our results revealed that dairy cows with HFI had higher digestibility of CEL corresponding to more abundant Saccharofermentans. Besides, many members of Lachnospiraceae were characterized by their CEL-decomposing activity and were linked to other CEL-degrading bacteria (Flint et al., 2008). Thus, Ruminococcaceae, Saccharofermentans, and Lachnospiraceae may be closely associated with increased feed intake, possibly because of the higher digestibility of ADF and CEL. We also found that the Firmicutes/Bacteroidetes ratio was depleted in dairy cows with HFI. A previous study revealed that abatement of the Firmicutes/Bacteroidetes ratio is closely related to fat deposition, which may be attributed to augmentation of feed intake (Ley et al., 2006). Further, dairy cows with HFI correspond to the abatement of the Firmicutes/Bacteroidetes ratio, allowing them to obtain more energy from the diet to meet their maintenance and production needs, which is stored as fat because of excessive energy intake. In summary, upregulation of Butyrivibrio, Erysipelotrichaceae, Saccharofermentans, Ruminococcaceae, and Lachnospiraceae and downregulation of the Firmicutes/Bacteroidetes ratio may be the signatures of dairy cows with HFI. In addition, our study showed a greater abundance of Prevotella in the dairy cows; this may be altered under different circumstances, such as diets and feeding patterns (Li et al., 2014; Smith et al., 2018).
To obtain a deeper understanding of the underlying physiological mechanisms contributing to variations in feed intake, we performed UPLC-Q-TOF-MS analysis combined with univariate and multivariate statistical methods to characterize dairy cows with different feed intake levels. Our results showed that these discrepant ruminal fluid metabolites mainly consist of phospholipids, organic acids and derivatives, amino acids, fatty acids, glycerides, cholesterol esters, nucleosides, organooxygen compounds, organoheterocyclic compounds, and biogenic amines, which agrees with previous results of bovine ruminal fluid (O’Callaghan et al., 2018). Linoleic acid and alpha-linolenic acid are common unsaturated essential fatty acids. A previous study confirmed that unsaturated fatty acids are toxic to ruminal bacteria, in which Butyrivibrio and related strains were sensitive to unsaturated fatty acids such as linoleic acid and alpha-linolenic acid. Further, the accumulation of unsaturated fatty acids may decrease feed intake by inhibiting the growth of Butyrivibrio and related strains. Our study also showed that dairy cows with HFI had lower contents of linoleic acid and alpha-linolenic acid. Recent evidence confirmed the existence of glutamatergic innervation of Neuropeptide Y (NPY)-expressing neurons in the rat hypothalamic arcuate nucleus (Chitravanshi et al., 2016). Moreover, glutamate can activate NPY/AgRP-expressing neurons through ionotropic glutamate receptors (Yang et al., 2011). Our study revealed that dairy cows with HFI had lower levels of glutamate, indicating that higher levels of glutamate are activated and release NPY in the hypothalamic arcuate nucleus. l-Arabinose and d-mannose are components of HCEL. We observed lower levels of l-arabinose and d-mannose in dairy cows with HFI, suggesting that dairy cows with HFI rapidly utilize these degradation products to meet their energy requirements based on the higher abundance of HCEL-degrading bacteria. A recent study revealed that the feed intake of animals is distinctly increased when consuming a high-methionine diet (Li et al., 2016). Our study showed that dairy cows with HFI had lower levels of l-methionine, indicating higher utilization of l-methionine for high-yielding milk and energy requirements. We also detected biogenic amines such as tyramine and histamine, which were derived from the decomposition of tyrosine and histidine. These compounds are commonly produced as a result of the activities of the amino acid decarboxylases of bacteria. The presence of tyramine and histamine may pose a risk to host health (del Rio et al., 2017). Moreover, these compounds may affect the appetite of animals. Lingaas and Tveit (1992) confirmed that injection of putrescine (100 g/d) in vivo reduced the intake and milk production of cows (). Similarly, amines negatively impact the mean rate of ingestion (g DM/min), which tended to be the lowest in the amines group (Van Os et al., 1996). Therefore, we predicted that biogenic amines such as tyramine and histamine disturbed the appetite of dairy cows with HFI when biogenic amine levels were low. Notably, taurine levels were lower in dairy cows with HFI. Dairy cows with HFI appeared to be prone to fatty deposition, suggesting decreased lipolysis in vivo. Humer et al. (2016) also observed that dairy cows with HFI had lower lipolysis levels, corresponding to lower levels of taurine. In our study, two branched-chain amino acids were detected, including valine and acetyl-dl-leucine, which showed lower levels in dairy cows with HFI. These results agree with those of Trottier and Easter (1995) who suggested that branched-chain amino acid supplementation decreased voluntary intake in lactating sows. We also detected degradation products of bacterial nucleic acids, such as cytosine, xanthine, and pseudouracil among the feed intake groups, which was supported by a study by Sutton et al. (1975), who demonstrated that bacterial nucleic acids (DNA or RNA) incubated with rumen fluid were rapidly converted into xanthine and hypoxanthine (Noel et al., 2017). The lower levels of xanthine and hypoxanthine may be linked with microbial activity, which increased the feed intake. l-Pipecolic acid is a primary metabolic intermediate of l-lysine oral. Oral and intracerebroventricular administration of l-pipecolic acid may suppress feed intake regardless of the species (Takahama et al., 1982). Our study also showed that dairy cows with HFI had lower levels of l-pipecolic acid. Interestingly, reduced feed intake following the administration of l-phenylalanine was associated with a greater sensation of fullness (Rogers et al., 1991; Ballinger and Clark, 1994), indicating that dairy cows with HFI had lower levels of l-phenylalanine. Ruminal fluid metabolites may be intermediaries of interchange between the rumen microbiota and host. Our study revealed that some ruminal fluid metabolites were associated with differential levels of the microbiota. In dairy cows with HFI, three metabolites involved in energy production were enriched (alpha-linolenic acid, linoleic acid, and stearic acid) and may increase proneness to fatty deposition. Oomura et al. (1975) suggested that neurons sense fatty acids and that hypothalamic fatty acid-sensing plays a vital role in regulating feed intake. Indeed, our study also showed that alpha-linolenic acid and linoleic acid metabolism were essential in the feed intake groups. Moreover, Butyrivibrio and related strains, which metabolized linoleic acid and showed accumulation of stearic acid, strains belonging to Ruminococcaceae, and uncultured members of Lachnospiraceae may be involved in the biohydrogenation process (Enjalbert et al., 2017). Briefly, the discrepant ruminal fluid metabolites for dairy cows with HFI may involve alpha-linolenic acid and linoleic acid metabolism.
In conclusion, our findings provide not only novel insights into the rumen microbiome of dairy cows with different feed intake levels, altered for enriched bacterial taxa but also reveal metabolic pathways that are closely linked to feeding intake in early-lactating cows. These novel candidates may be useful in practice for identifying variations in feed intake.
The signatures in the rumen microbiome and metabolome of dairy cows may be useful for guiding decisions when changing feed.
Acknowledgments
The study was financially supported by the Jiangsu Agricultural Industry Technology System (project no. JATS (2019)434). We gratefully thank the coworkers at the Institute of Dairy Science.
Glossary
Abbreviations
- AC
acetonitrile
- ADF
acid detergent fiber
- CEL
cellulose
- CP
crude protein
- DDGS
distillers dried grains with soluble
- DM
dry matter
- EE
ether extract
- HCEL
hemicelluloses
- HFI
high feed intake
- HPLC
high-performance liquid chromatography
- IPA
isopropanol
- LFI
low feed intake
- MFI
medium feed intake
- MS
mass spectrometry
- NDF
neutral detergent fiber
- NEL
net energy of lactation
- OM
organic matter
- OTU
operational taxonomic unit
- QTOF
quadrupole time-of-flight
- UPLC
ultra-performance liquid chromatography
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Ballinger A. B., and Clark M. L.. . 1994. l-Phenylalanine releases cholecystokinin (CCK) and is associated with reduced food intake in humans: evidence for a physiological role of CCK in control of eating. Metabolism 43:735–738. doi: 10.1016/0026-0495(94)90123-6 [DOI] [PubMed] [Google Scholar]
- Broderick G. A., and Kang J. H.. . 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8 [DOI] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., . et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. methods. 7:335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carberry C. A., Kenny D. A., Han S., Mccabe M. S. and Waters S. M.. . 2012. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl. Environ. Microbiol. 78:4949–4958. doi: 10.1128/AEM.07759-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitravanshi V. C., Kawabe K., and Sapru H. N.. . 2016. Stimulation of the hypothalamic arcuate nucleus increases brown adipose tissue nerve activity via hypothalamic paraventricular and dorsomedial nuclei. Am. J. Physio. Heart Circ. Physiol. 311:H433–H444. doi: 10.1152/ajpheart.00176.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. R., Wang Q., Cardenas E., Fish J., Chai B., Farris R. J., Kulam-Syed-Mohideen A. S., Mcgarrell D. M., Marsh T., Garrity G. M., . et al. 2009. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37:D141–D145. doi: 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio B., Redruello B., Linares D. M., Ladero V., Fernandez M., Martin M. C., Ruas-Madiedo P., and Alvarez M. A.. . 2017. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 218:249–255. doi: 10.1016/j.foodchem.2016.09.046 [DOI] [PubMed] [Google Scholar]
- Duncan S. H., Lobley G. E., Holtrop G., Ince J., Johnstone A. M., Louis P., and Flint H. J.. 2008. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. (Lond). 32:1720–1724. doi: 10.1038/ijo.2008.155 [DOI] [PubMed] [Google Scholar]
- Durunna O. N., Mujibi F. D. N., Goonewardene L., Okine E. K., Basarab J. A., Wang Z., and Moore S. S.. . 2011. Feed efficiency differences and reranking in beef steers fed grower and finisher diets1. J Anim Sci. 89:158–167. doi: 10.2527/jas.2009-2514 [DOI] [PubMed] [Google Scholar]
- Elolimy A. A., Arroyo J. M., Batistel F., Iakiviak M. A., and Loor J. J.. . 2018. Association of residual feed intake with abundance of ruminal bacteria and biopolymer hydrolyzing enzyme activities during the peripartal period and early lactation in Holstein dairy cows. J. Anim. Sci. Biotechnol. 9:43–43. doi: 10.2527/jas.2009-2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert F., Combes S., Zened A., and Meynadier A.. . 2017. Rumen microbiota and dietary fat: a mutual shaping. J. Appl. Microbiol. 123:782–797. doi: 10.1111/jam.13501 [DOI] [PubMed] [Google Scholar]
- Evans J., Morris L., and Marchesi J.. . 2013. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J. Endocrinol. 218:R307–318. doi: 10.1530/JOE-13-0131 [DOI] [PubMed] [Google Scholar]
- Fadrosh D. W., Ma B., Gajer P., Sengamalay N., Ott S., Brotman R. M., and Ravel J.. . 2014. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2:6–6. doi: 10.1186/2049-2618-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firkins J. L., and Yu Z.. . 2015. RUMINANT NUTRITION SYMPOSIUM: how to use data on the rumen microbiome to improve our understanding of ruminant nutrition. J. Anim. Sci. 93:1450–1470. doi: 10.2527/jas.2014-8754 [DOI] [PubMed] [Google Scholar]
- Fleissner C. K., Huebel N., Abd El-Bary M. M., Loh G., Klaus S., and Blaut M.. . 2010. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 104:919–929. doi: 10.1017/S0007114510001303 [DOI] [PubMed] [Google Scholar]
- Flint H. J., Bayer E. A., Rincon M. T., Lamed R., and White B. A.. . 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6:21–131. doi: 10.1038/nrmicro1817 [DOI] [PubMed] [Google Scholar]
- Hayirli A., Grummer R. R., Nordheim E. V., and Crump P. M.. . 2002. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J. Dairy Sci. 85:430–3443. doi: 10.3168/jds.S0022-0302(02)74431-7 [DOI] [PubMed] [Google Scholar]
- Hernandez-Sanabria E., Goonewardene L. A., Wang Z., Durunna O. N., Moore S. S., and Guan L. L.. . 2012. Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl. Environ. Microbiol. 78:1203–1214. doi: 10.1128/AEM.05114-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T., Bateman H., Quigley J., Aldrich J., Schlotterbeck R. L., and Heinrichs A.. 2013. REVIEW: New information on the protein requirements and diet formulation for dairy calves and heifers since the Dairy NRC 20011. Professional Animal Scientist 29:199–207. [Google Scholar]
- Humer E., Khol-Parisini A., Metzler-Zebeli B. U., Gruber L., and Zebeli Q.. . 2016. Alterations of the lipid metabolome in dairy cows experiencing excessive lipolysis early postpartum. PLoS One. 11:e0158633–e0158633. doi: 10.1371/journal.pone.0158633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell K. A., Mccormick C. A., Odt C. L., Weimer P. J., and Suen G.. . 2015. Ruminal bacterial community composition in dairy cows is dynamic over the course of two lactations and correlates with feed efficiency. Appl. Environ. Microbiol. 81:4697–4710. doi: 10.1128/AEM.00720-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumpertz R., Le D. S., Turnbaugh P. J., Trinidad C., Bogardus C., Gordon J. I., and Krakoff J.. . 2011. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 94:58–65. doi: 10.3945/ajcn.110.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasova T., Davidova-Gerzova L., Babak V., Cejkova D., Montagne L., Le-Floc’H N., and Rychlik I.. . 2018. Effects of host genetics and environmental conditions on fecal microbiota composition of pigs. PLoS One. 13:e0201901–e0201901. doi: 10.1371/journal.pone.0201901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. E., Turnbaugh P. J., Klein S., and Gordon J. I.. . 2006. Human gut microbes associated with obesity. Nature 444:1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Li Z., Wright A.-D., Liu H., Bao K., Zhang T., Wang K., Cui X., Yang F., Zhang Z., and Li G.. . 2014. Bacterial community composition and fermentation patterns in the rumen of sika deer (Cervus nippon) fed three different diets. Microb. Ecol. 69:307–318. doi: 10.1007/s00248-014-0497-z [DOI] [PubMed] [Google Scholar]
- Li M., Zhai L., and Wei W.. . 2016. High-methionine diet attenuates severity of arthritis and modulates IGF-I related gene expressions in an adjuvant arthritis rats model. Mediators Inflamm. 2016:9280529–9280529. doi: 10.1155/2016/9280529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingaas F., and Tveit B.. . 1992. Etiology of acetonemia in Norwegian cattle. 2. Effect of butyric acid, valeric acid, and putrescine. J. Dairy Sci. 75:2433–2439. doi: 10.3168/jds.S0022-0302(92)78004-7 [DOI] [PubMed] [Google Scholar]
- Magoč T. and Salzberg S. L.. . 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques R., Chagas L., Owens F., and Santos F.. . 2015. Effects of various roughage levels with whole flint corn grain on performance of finishing cattle. J. Anim. Sci. 94:339–348. doi: 10.2527/jas2015-9758 [DOI] [PubMed] [Google Scholar]
- Million M., Maraninchi M., Henry M., Armougom F., Richet H., Carrieri P., Valero R., Raccah D., Vialettes B., and Raoult D.. . 2012. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes (Lond). 36:817–825. doi: 10.1038/ijo.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mohd-Setapar S., Talib N., and Aziz R.. . 2013. Silage from Malaysia rice straw treated with rice bran, coconut pulp, molasses and effective microorganisms. J. Biobased Mater. Bioenergy 7:295–299. doi: 10.1166/jbmb.2013.1317 [DOI] [Google Scholar]
- Noel S. J., Attwood G. T., Rakonjac J., Moon C. D., Waghorn G. C., and Janssen P. H.. . 2017. Seasonal changes in the digesta-adherent rumen bacterial communities of dairy cattle grazing pasture. PLoS One. 12:e0173819–e0173819. doi: 10.1371/journal.pone.0173819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’callaghan T. F., Vázquez-Fresno R., Serra-Cayuela A., Dong E., Mandal R., Hennessy D., Mcauliffe S., Dillon P., Wishart D. S., Stanton C., . et al. 2018. Pasture feeding changes the bovine rumen and milk metabolome. Metabolites 8:27. doi: 10.3390/metabo8020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Nakamura T., Sugimori M., and Yamada Y.. 1975. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol. behav. 14:483–486. [DOI] [PubMed] [Google Scholar]
- Rogers P., Keedwell P., and Blundell J.. . 1991. Further analysis of the short-term inhibition of food intake in humans by the dipeptide l-aspartyl-l-phenylalanine methyl ester (aspartame). Physiol. Behav. 49:739–743. doi: 10.1016/0031-9384(91)90312-C [DOI] [PubMed] [Google Scholar]
- Rognes T., Flouri T., Nichols B., Quince C., and Mah F.. . 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 4:e2584–e2584. doi: 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schären M., Frahm J., Kersten S., Meyer U., Hummel J., Breves G., and DäNicke S.. . 2018. Interrelations between the rumen microbiota and production, behavioral, rumen fermentation, metabolic, and immunological attributes of dairy cows. J. Dairy Sci. 101:4615–4637. doi: 10.3168/jds.2017-13736 [DOI] [PubMed] [Google Scholar]
- Smith S., Saldinger L., Barlow J., Alvez J., Roman J., and Kraft J.. . 2018. Alteration of Rumen bacteria and protozoa through grazing regime as a tool to enhance the bioactive fatty acid content of bovine milk. Front. Microbiol. 9:904. doi: 10.3389/fmicb.2018.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic B., Grubić G., Djordjevic N., Božičković A., Ivetić A., and Davidović V.. . 2014. Effect of physical effectiveness on digestibility of ration for cows in early lactation. J Anim Physiol. Anim Nutr (Berl). 98:714–721. doi: 10.1111/jpn.12129 [DOI] [PubMed] [Google Scholar]
- Sutton J. D., Smith R. H., Mcallan A. B., Storry J. E., and Corse D. A.. . 1975. Effect of variations in dietary protein and of supplements of cod-liver oil on energy digestion and microbial synthesis in the rumen of sheep fed hay and concentrates. J. Agric. Sci. 84(02):317. doi: 10.1017/S0021859600052461 [DOI] [Google Scholar]
- Takahama K., Miyata T., Hashimoto T., Okano Y., Hitoshi T., and Kase’ Y.. . 1982. Pipecolic acid: a new type of α-amino acid possessing bicuculline-sensiti action in the mammalian brain. Brain Res. 239:294–298. doi: 10.1016/0006-8993(82)90855-1 [DOI] [PubMed] [Google Scholar]
- Thiex N., Manson H., Andersson S., and Persson J-Å.. . 2002. Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: collaborative study. J. AOAC Int. 85:309–317. doi: 10.1093/jaoac/85.2.309 [DOI] [PubMed] [Google Scholar]
- Thiex N., Novotny L., and Crawford A.. . 2012. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 95:1392–1397. doi: 10.5740/jaoacint.12-129 [DOI] [PubMed] [Google Scholar]
- Trottier N. L., and Easter R. A.. . 1995. Dietary and plasma branched-chain amino acids in relation to tryptophan: effect on voluntary feed intake and lactation metabolism in the primiparous sow. J. Anim. Sci. 73:1086–1092. doi: 10.2527/1995.7341086x [DOI] [PubMed] [Google Scholar]
- Van de Stroet D. L., Calderón Díaz J. A., Stalder K. J., Heinrichs A. J., and Decho C. D.. . 2016. Association of calf growth traits with production characteristics in dairy cattle. J Dairy Sci. 99:8347–8355. doi: 10.3168/jds.2015-10738 [DOI] [PubMed] [Google Scholar]
- Van Os M., Jailler M., and Dulphy J. P.. . 1996. The influence of ammonia, biogenic amines and γ-aminobutyric acid on grass silage intake in sheep. Br. J. Nutr. 76:347–358. doi: 10.1079/BJN19960041 [DOI] [PubMed] [Google Scholar]
- Wu J., Yang L., Li S., Huang P., Liu Y., Wang Y., and Tang H.. . 2016. Metabolomics insights into the modulatory effects of long-term low calorie intake in mice. J. Proteome Res. 15:2299–2308. doi: 10.1021/acs.jproteome.6b00336 [DOI] [PubMed] [Google Scholar]
- Yang Y., Atasoy D., Su H. H., and Sternson S. M.. . 2011. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146:992–1003. doi: 10.1016/j.cell.2011.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]