Abstract
Aims
Green tea extract (GTE) can exert antiobesity and anti‐inflammatory effects. Our study determined whether the benefits of GTE are summative with exercise‐induced changes in anthropometric indices, and the levels of inflammatory cytokines, adiponectin and irisin in inactive overweight women.
Methods
Thirty overweight female participants were randomized to 3 groups: endurance training + placebo (ET + P); endurance training + GTE (ET + GTE); and Control (no exercise) + placebo (Control, n = 10). The exercise intervention consisted of an 8‐week endurance‐training programme of 3 sessions per week (aerobics, aerobic circuit training, and fast walking or jogging at a moderate intensity of 40–59% of the heart rate reserve). The dose of GTE used was 500 mg/day in the form of a green tea capsule.
Results
Body weight, body mass index, waist to hip ratio and body fat percentage were decreased in both ET + P and ET + GTE interventions (P < .001 for both interventions). The reduction of anthropometric values in the ET + GTE group was significantly higher than ET + P interventions (P < .001). Both exercise interventions also significantly (P < .001) increased adiponectin (ET + GTE = 5.28 mg/mL [95% confidence interval {CI}, 4.48 to 6.08] and ET + P = 3.34 mg/mL [95% CI, 2.76 to 3.92]) and decreased high‐sensitivity C‐reactive protein (hs‐CRP; ET + GTE = −0.95 mg/L [95% CI, −1.15 to −0.75] and ET + P = −0.35 mg/L [95% CI, −0.46 to −0.24]). Changes in adiponectin and hs‐CRP were greater (P < .05) in ET + GTE compared to ET + P. There were no significant differences in irisin, interleukin‐6 or tumour necrosis factor‐α between the 3 groups (P > .05).
Conclusions
GTE improves exercise‐induced body composition by further decreasing exercise‐induced changes in weight, body mass index, waist to hip ratio and body fat percentage. The combination of GTE and exercise also produced greater changes in anti‐inflammatory (increases in adiponectin) and metabolic (decreases in hs‐CRP) markers than exercise alone.
Keywords: adiponectin, exercise, green tea, inflammation, irisin, obesity
What is known about this subject
It is known that green tea extract can influence fat loss.
Endurance training lowers concentrations of proinflammatory biomarkers in humans.
What this study adds
This study furthers our understanding of the anti‐inflammatory effects of green tea extract by studyingmeasuring inflammatory cytokines, adiponectin and irisinlevels in inactive overweight women.
1. INTRODUCTION
The global epidemic of obesity is accompanied by a variety of health complications, in large part due to the accumulation of excessive adipose tissue that results in an imbalance in the expression of hormones, pro‐ and anti‐inflammatory adipokines and cytokines.1, 2 These specific fat‐related hormones mediate vascular changes in obesity, which is an important risk factor for diabetes mellitus, metabolic syndrome and cardiovascular diseases.3 Weight loss in overweight and/or obese people increases health span and quality of life while also improving clinical outcomes. Methods of weight loss include diet, physical activity, drug or herbal medicine therapy, and surgery.4 It has been claimed, albeit with little scientific evidence, that some medicinal plants are useful in the treatment of obesity.5
Green tea, made from the leaves of Camellia sinensis, contains catechins (such as epigallocatechin‐3‐gallate)6 as well as quercetin, thearubigins, theaflavins, theanine, caffeine, chlorogenic acid and gallic acid.7 The effect of green tea on obesity and body weight loss has been extensively described in nutritional research.8 Although considerable research has been conducted on the effects of green tea on obesity and its complications, the effectiveness of green tea on weight loss is unclear.8, 9
Multiple mechanisms have been proposed for the weight loss caused by green tea. These mechanisms include reducing food intake, interrupting lipid emulsification and absorption, suppressing adipogenesis and lipid synthesis and increasing energy expenditure via thermogenesis, fat oxidation and faecal lipid excretion.8 Additionally, previous clinical and laboratory studies suggest that dietary polyphenols have anti‐inflammatory effects.10 Green tea extract (GTE) exerts anti‐inflammatory effects by reducing nuclear factor‐κB expression and proinflammatory cytokine production.11 The protective effect of green tea have recently been proposed to be due mainly to the effects of polyphenol compounds such as catechins.12
Endurance training (ET) lowers concentrations of proinflammatory biomarkers in humans.13 The anti‐inflammatory effect of regular exercise may be mediated by reduced visceral fat mass (with a subsequent decreased release of adipokines from adipose tissue) and/or by the induction of an anti‐inflammatory environment.14 Regular exercise increases circulating levels of anti‐inflammatory adipokines and reduces levels of several circulating proinflammatory adipokines. Some studies suggest that the combination of ET with another anti‐inflammatory intervention such as calorie restriction may be more effective in reducing circulating levels of inflammatory biomarkers.15
Although the anti‐inflammatory effects of both ET and GTE are well known, the effect of the combination of these 2 interventions on inflammatory status is less well studied in humans. The objective of this study was to explore the effects of GTE supplementation on exercise induced changes in body composition, irisin, adipokines and proinflammatory cytokine in inactive overweight women.
2. METHODS
2.1. Participants
Thirty overweight sedentary women (age 38.36 ± 3.16 years) participated in this study. All participants were free of known pathologies and were otherwise healthy. None of the participants reported recent infections, joint and bone injuries, or symptoms of cardiovascular disease. All participants declared that they were not taking supplements (including vitamins, minerals, ergogenic aids), oral contraceptives or other medications during the 2 months prior to enrolling in the study. None of the participants reported ever consuming alcohol, and none of the participants engaged in any form of regular exercise for at least 6 months prior to the study. The participants provided written informed consent, and the ethical committee of Ferdowsi University of Mashhad, Mashhad, Iran, approved the protocol. The study was registered in the Iranian Registry of Clinical Trials (https://irct.ir/; IRCT20151025024699N3) and carried out in accordance with the Declaration of Helsinki.
2.2. Study design
Thirty participants were randomly assigned to 1 of 3 interventions for 8 weeks (Figure 1 ), and both the participants and researchers were blinded to placebo and GTE supplementations:
ET + placebo (P, n = 10)
ET + GTE (n = 10)
Control (no exercise) + P (Control, n = 10)
Figure 1.
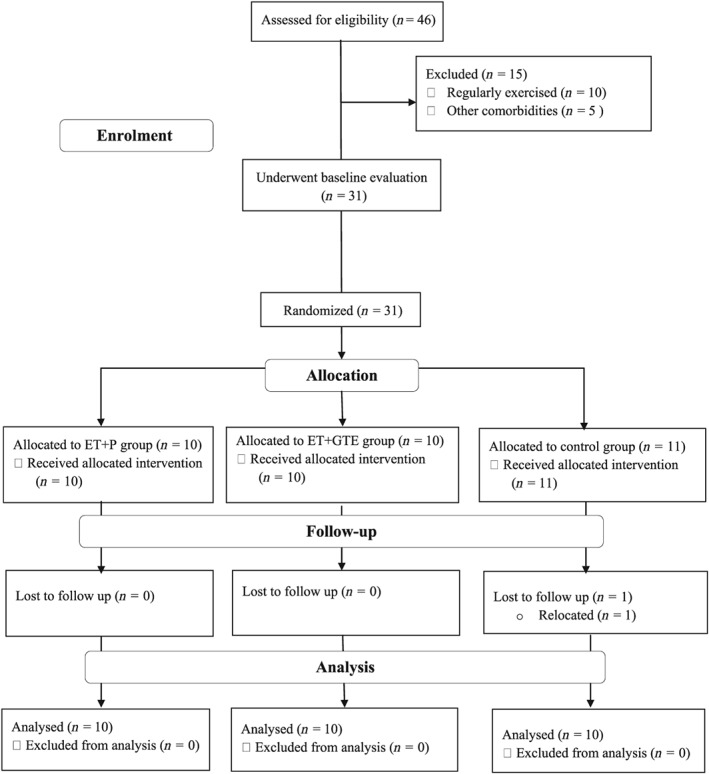
Participants flow diagram
Participant allocation was stratified by body mass index (BMI; ≤27.9 or ≥28 kg/m2) and age (˂40 or ≥40 years) before baseline measurements were made, Anthropometric and inflammatory markers were measured at baseline and after 8 weeks of intervention. All measurements were made during the early‐ to mid‐follicular phase of the menstrual cycle to minimize the potential effects of endogenous oestrogens on inflammatory markers.16 All measurements were made at the same time (±1 h) in the morning hours. The participants were instructed to fast for 12 h (an overnight fast, with at least 8 h of sleep) and to refrain from exercise for 48 h before the test.
2.3. Anthropometric measurements
Urine samples were collected before participants had their body weights measured on a digital scale (Lumbar, China) with a precision of 0.1 kg. The height of the participants was measured with a medical sampler (Race Industrialization, China) with a precision of 0.1 cm. BMI was recorded as kg/m2. Waist circumference was measured at the superior edge of the iliac crest. The waist to hip ratio (WHR) was calculated using the participant's waist circumference and height measurements. Body fat percentage (BFP) was evaluated using a multi‐frequency bioelectrical impedance device (In body 720, South Korea).
2.4. Assessment of inflammatory mediators
Fasting blood samples (5 mL) were obtained from the cubital vein ~48 h before baseline training sessions. Blood was allowed to clot at room temperature (1.5–2 h). Serum was frozen at −80°C until further analysis by enzyme‐linked immunosorbent assay. Interleukin 6 (IL‐6), tumour necrosis factor‐α (TNF‐α), irisin, adiponectin and high‐sensitivity C‐reactive protein (hs‐CRP) concentrations were measured by enzyme‐linked immunosorbent assay (Hangzhou Eastbiopharm Ltd, China) as per manufacturer instructions. Pre‐ and postintervention samples from the same participant were run on the same plate to minimize variability. The intra‐assay and interassay coefficient of variation for all inflammatory mediators were ˂10% and ˂12%, respectively.
2.5. Endurance training protocol
The exercise programme entailed supervised endurance training that included aerobics, aerobic circuit training, and fast walking or jogging. The training was performed 3 times/week with a moderate intensity (40–59% of the heart rate reserve [HRR]) according to the American College of Sports Medicine guidelines for overweight and obese individuals.17 A training intensity of 40–49% of HRR was used for weeks 1–4, which then progressed to 50–59% of HRR in weeks 5–8. Circuit training entailed 5 3‐min aerobic stations: fast walking or jogging on treadmill, rowing machine, bicycle ergometer, stair climber and elliptical. Subjects rested for 30 s between stations. This circuit was repeated 3 times per training session.18, 19 The duration of each workout was ~60 min.17, 20 The intensity of training was monitored by a wireless chest HR transmitter and a wrist monitor recorder (Polar Electro, Finland). Participants in the placebo group did not engage in any physical activity or change in lifestyle.
2.6. Dietary assessment
Because of the importance of the timing of nutrition on exercise sessions, we controlled dietary intake during the hours prior (before and after) to training sessions. The participants consumed a banana (0.30–0.35 g carbohydrate/kg body weight) as a pre‐exercise snack ~1 h before commencing the training session. Additionally, dinner was consumed ~1.5–2 h after each training session and contained the same amount of carbohydrates (1.7 g/kg of body weight), protein (0.3 g/kg of body weight) and fats (0.4 g/kg of body weight). This was based on the recommendations by the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine for macronutrient distribution (55–65% of total calories from carbohydrates, ˂35% of total calories from fats and 10–15% of total calories from protein).21 Other than this minor equalization around the exercise sessions, the participants were instructed not to alter their dietary habits for the duration of the study. Each participant completed a questionnaire covering 3 days (2 weekdays and 1 day of the weekend) of food intake during the prior week and during the last week of the study. The first 3‐day diary was considered the standard diet and participants were asked to continue with this diet for the remainder of the study. Each food item was individually entered into Diet Analysis Plus (Version 10; Cengage, Boston, MA, USA) for determination of total energy consumed, and the amount of energy derived from proteins, fats, and carbohydrates.
2.7. GTE supplementation
Participants in the ET + GTE group received a daily dose of a 500 mg GTE capsule (VitaSage Green Tea Extract, USA) while those in the 2 other groups received an identical capsule but containing chickpea flour. Each GTE capsule contained a minimum of 75% polyphenol catechins, 15 mg of natural caffeine (about 1/6 of a cup of coffee) and a minimum of 45% EGCG, the key antioxidant responsible for the herb's many health benefits. Daily administration of capsules occurred in the evening; on training days this was ~1 h before each exercise training session for both ET + GTE and ET + P groups. The control group consumed capsules at the same time (±1 h) daily. The timing and dose of GTE were according to investigations other reports of improvements in body composition and metabolism after a combination of GTE and exercise.22, 23
2.8. Statistical analysis
We determined that 10 participants per group would provide 80% power (2‐sided α = 0.05) to detect 7% changes in adiponectin (our primary outcome) based on a previous report.24
The data were as confirmed for normality using the Shapiro–Wilk test. One‐way analysis of variance (ANOVA) was used to examine possible group differences at baseline. The effects of the interventions on all variables were evaluated by a 2 × 3 repeated‐measures ANOVA (time [baseline vs end] × group [ET + P vs ET + GTE vs control]). When a significant main effect was identified, paired t tests were used to determine within‐group differences from baseline. When a significant group‐by‐time interaction was found, we performed a Tukey posthoc test to determine differences between groups. Person's correlations were used to evaluate the relationships between changes in significant variables. A P‐value <.05 was considered significant. Effect size (Cohen's d) was determined via mean/standard deviation. All analyses were performed with SPSS 21.0 (SPSS Inc, Chicago, IL, USA).
3. RESULTS
Baseline anthropometric indices, adipokines, and irisin levels are presented in Table 1. Although baseline levels of body weight and height of participants were statistically different, there were no between‐group significant differences in either BMI or BFP (Figure 2A). There were also no significant differences in mean daily energy and macronutrient intake after each intervention (Table 2). Compliance to ET was 100% in both the ET + GTE and ET + P groups.
Table 1.
Baseline characteristics of participants in each study group
| Variables | ET + P | ET + GTE | Control | P‐value |
|---|---|---|---|---|
| Anthropometric characteristics | ||||
| Age (y) | 39.50 ± 4.17 | 37.60 ± 1.71 | 38.00 ± 3.12 | .38 |
| Weight (kg) | 67.23 ± 4.61 | 74.12 ± 4.91 | 70.01 ± 3.11 | .005 |
| Height (cm) | 158.60 ± 5.35 | 164.30 ± 3.19 | 159.40 ± 2.71 | .006 |
| BMI (kg/m 2 ) | 26.76 ± 2.07 | 27.49 ± 2.33 | 27.56 ± 1.40 | .61 |
| BFP (%) | 37.12 ± 5.47 | 39.39 ± 3.67 | 39.78 ± 3.65 | .35 |
| WHR (m) | 0.90 ± 0.04 | 0.91 ± 0.04 | 0.92 ± 0.04 | .60 |
All values are means ± standard deviation. ET: endurance training; P: placebo; GTE: green tea extract; BMI: body mass index; BFP: body fat percentage; WHR: waist to hip ratio.
Figure 2.
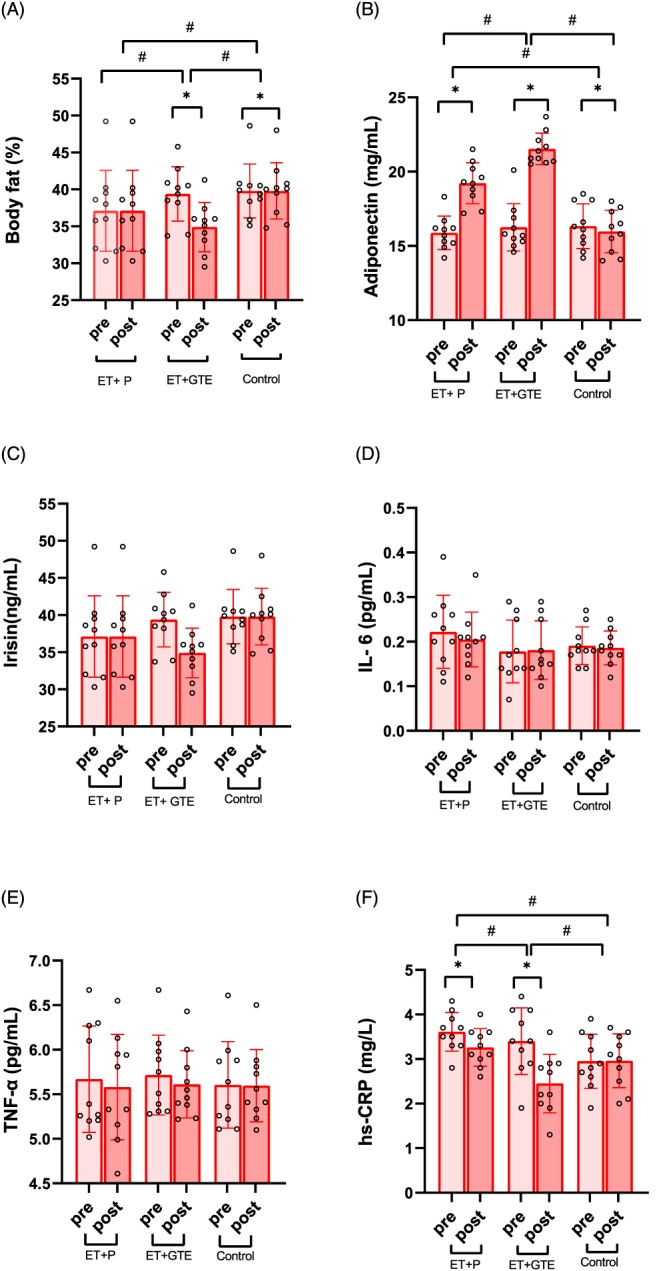
A, Body fat (%) by group pre‐ and post‐training. B, Serum adiponectin (mg/mL) levels by group pre and post training. C, Serum irisin (ng/mL) levels by group pre‐ and post‐training. D, Serum interleukin (IL)‐6 (pg/mL) levels by group pre and post training. E, Serum tumour necrosis factor (TNF)‐α (pg/mL) levels by group pre and post training. (F) Serum high‐sensitivity C‐reactive protein (hs‐CRP; mg/L) levels by group pre and post training. Error bars represent standard deviation. Individual data points as shown.
*: p < .01 difference from pre to post training;
#: p < .01 difference between groups
Table 2.
Inflammatory markers and mean daily macronutrient intake at baseline and following each intervention
| Variables | ET + P | ET + GTE | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | End | Δ | ES | Baseline | End | Δ | ES | Baseline | End | Δ | ES | |
| Weight (kg) | 67.2 ± 4.6 | 65.2 ± 4.4b , c | −1.6 ± 0.3 | 6.2 | 74.1 ± 4.9 | 71.9 ± 4.9b , e | −2.3 ± 0.4 | 5.9 | 70.0 ± 3.1 | 70.2 ± 3.3 | 0.1 ± 0.3 | −0.4 |
| BMI (kg/m 2 ) | 26.8 ± 2.1 | 26.1 ± 2.0b , c | −0.6 ± 0.1 | 6.4 | 27.5 ± 2.3 | 26.7 ± 2.3b , e | −0.8 ± 0.1 | 6 | 27.6 ± 1.4 | 27.6 ± 1.4 | 0.1 ± 0.1 | −0.4 |
| BFP (%) | 37.1 ± 5.5 | 34.4 ± 5.3b , c | −2.7 ± 0.4 | 7.8 | 39.4 ± 3.7 | 34.9 ± 3.3b , e | −4.5 ± 0.7 | 6.5 | 39.8 ± 3.7 | 39.8 ± 3.8 | 0.0 ± 1.2 | –0.1 |
| WHR | 0.90 ± 0.08 | 0.89 ± 0.04b , c | −0.01 ± 0.01 | 1.0 | 0.91 ± 0.04 | 0.89 ± 0.1b , e | −0.02 ± 0.01 | 2.0 | 0.92 ± 0.04 | 0.92 ± 0.04 | 0.0 ± 0.1 | –0.1 |
|
Irisin (ng/mL) |
138.0 ± 27.4 | 137.1 ± 26.8 | −0.9 ± 4.4 | 0.2 | 158.6 ± 35.9 | 159.0 ± 35.7 | 0.40 ± 6.1 | −0.1 | 136.2 ± 30.6 | 135.6 ± 29.4 | −0.6 ± 2.8 | 0.2 |
| IL6 (pg/mL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.1 | 0.5 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.1 | 0.5 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.1 | 0.5 |
| Adiponectin (mg/mL) | 15.9 ± 1.1 | 19.2 ± 1.4b , c | 3.3 ± 0.8 | −4.1 | 16.3 ± 1.6 | 21.5 ± 1.1b , d | 5.3 ± 1.1 | −4.7 | 16.3 ± 1.5 | 16.0 ± 1.4a | −0.4 ± 0.4 | 0.3 |
| TNF‐α (pg/mL) | 5.7 ± 0.6 | 5.6 ± 0.6 | −0.1 ± 0.3 | 0.3 | 5.7 ± 0.5 | 5.6 ± 0.4 | −0.1 ± 0.2 | 0.6 | 5.6 ± 0.5 | 5.6 ± 0.4 | 0.0 ± 0.1 | 0.1 |
| hs‐CRP (mg/L) | 3.6 ± 0.4 | 3.3 ± 0.4b , c | −0.4 ± 0.2 | 2.3 | 3.4 ± 0.7 | 2.5 ± 0.7b , d | −1.0 ± 0.3 | 3.5 | 3.0 ± 0.6 | 3.0 ± 0.6 | 0.0 ± 0.2 | −0.1 |
| Energy (kcal/day) | 1897 ± 270 | 1820 ± 318 | −87 ± 42 | 0.2 | 1926 ± 366 | 1815 ± 274 | −109 ± 52 | 0.3 | 1901 ± 470 | 1863 ± 407 | −37 ± 61 | 0.1 |
| Protein (g/day) | 58.5 ± 10.3 | 52.4 ± 12.2 | −6.1 ± 1.8 | 0.4 | 61.4 ± 17.4 | 55.5 ± 12.4 | −5.9 ± 1.4 | 0.4 | 51.1 ± 18.0 | 53.7 ± 15.8 | 2.6 ± 8.3 | 0.1 |
| Fat (g/day) | 60.1 ± 12.1 | 54.0 ± 14.8 | −5.2 ± 1.2 | 0.4 | 58.8 ± 17.1 | 50.1 ± 11.6 | −8.5 ± 2.0 | 0.5 | 55.9 ± 17.1 | 55.0 ± 13.9 | −0.9 ± 1.5 | 0.1 |
| Carbohydrate (g/day) | 280.4 ± 37.4 | 279 ± 49.0 | −1.0 ± 5.5 | 0.1 | 287.8 ± 46.7 | 285.3 ± 53.8 | −2.5 ± 4.2 | 0.1 | 297.1 ± 76.5 | 288.3 ± 65.3 | −8.9 ± 6.5 | 0.1 |
All values are means ± SD. n = 10 in each group. ET: endurance training; P: placebo; GTE: green tea extract; Δ: change; ES: effect size (Cohen's d); BMI: body mass index; BFP: body fat percentage; WHR: waist to hip ratio; IL‐6: interleukin 6; TNF‐α: tumour necrosis factor‐α; hs‐CRP: high sensitivity C‐ reactive protein.
P < .05 different from baseline,
P < .01 different from baseline,
P < .01 different from control group,
P < .05 different from ET + P and control groups,
P < .01 different from ET + P and control groups.
The effects of GTE and exercise on anthropometric measurements and plasma levels of adiponectin, irisin, IL‐6, TNF‐α and hs‐CRP are shown in in Table 2 and Figure 2. Due to the between group differences in baseline levels of weight and height existed, all variables were adjusted for weight and height. Both exercise interventions (ET + GTE and ET + P) significantly (P < .001) decreased body weight (ET + GTE = −2.27 kg [95% confidence interval {CI}, −2.54 to −2.00] and ET + P = −1.61 kg [95% CI, −1.80 to −1.42]), BMI (ET + GTE = −0.84 kg/m2 [95% CI, −0.95 to −0.74] and ET + P = −0.64 kg/m2 [95% CI, −0.71 to −0.57]), BFP (Figure 2A, ET + GTE = −4.48% [95% CI, −4.98 to −3.98] and ET + P = −2.73% [95% CI, −2.98 to −2.48]) and WHR (ET + GTE = −0.02 [95% CI, −0.03 to −0.01] and ET + P = −0.01 [95% CI, −0.02 to −0.01]). These reductions in the ET + GTE group were significantly (P < .001) greater than in the ET + P and control groups (Table 2). Both exercise interventions also significantly (P < .001) increased in adiponectin as shown in Figure 2B (ET + GTE = 5.28 mg/mL [95% CI, 4.48 to 6.08] and ET + P = 3.34 mg/mL [95% CI, 2.76 to 3.92]) and decreased hs‐CRP as shown in Figure 2F (ET + GTE = −0.95 mg/L [95% CI, −1.15 to −0.75] and ET + P = −0.35 mg/L [95% CI, −0.46 to −0.24]). Changes in adiponectin and hs‐CRP were greater (P < .05) in ET + GTE compared to ET + P and control groups. In addition, there were significant reductions (P < .05) in adiponectin levels in the control group (−0.36 mg/mL [95% CI, −0.67 to −0.05]) at the end of the intervention, while there no significant changes in the plasma levels of irisin (Figure 2C), IL‐6 (Figure 2D) and TNF‐α (Figure 2E) in any of the 3 groups. There was a correlation (r = .75, P < .001) between changes in body fat with adiponectin levels (Figure 3A) and a correlation (r = .55, P < .001) between changes in body fat (Figure 3B) with hs‐CRP levels.
Figure 3.
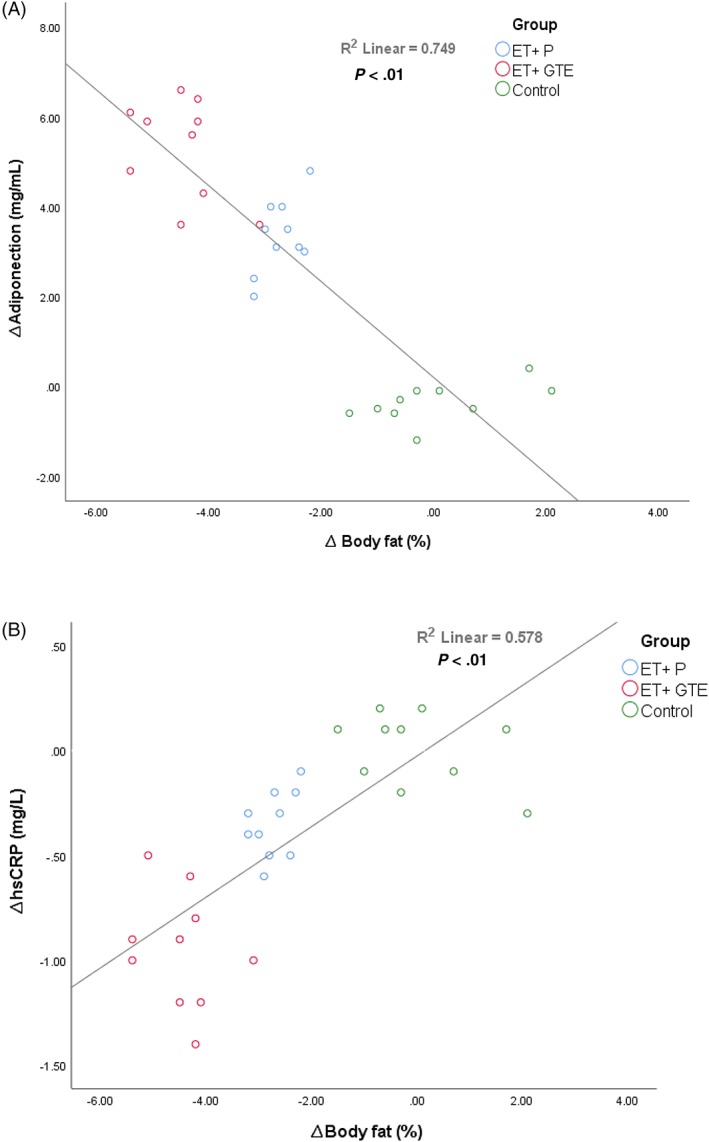
Relationship between changes in body fat with (A) adiponectin and (B) high‐sensitivity C‐reactive protein (hs‐CRP) levels
4. DISCUSSION
This double‐blind placebo‐controlled study investigated the effects of 8 weeks GTE supplementation on exercise‐induced changes in body composition and plasma levels of irisin, adiponectin and proinflammatory cytokines in inactive overweight women. There is a growing body of evidence showing that intake of GTE improves anthropometric indices by reducing BMI, body weight, WHR and BFP.12 The main effects of green tea on body composition is proposed to be due to catechins.25 In addition to catechins, green tea also contains caffeine.26 Potential mechanisms for the effect of green tea on body weight and BFP involve inhibition of adipocyte differentiation and proliferation, reduced fat absorption, inhibition of catechol‐o‐methyl‐transferase, increased energy expenditure, increased utilization of fat, and increased energy expenditure and thermogenesis.27
During moderate‐intensity exercise, energy expenditure is several times greater than at rest, and absolute rates of both lipolysis and fat oxidation are also increased.28 Our anthropometric assessment indicates a 39% increase in BFP loss when GTE was added to ET, which suggests that GTE potentiated, at least to a certain extent, the elevation of fat metabolism during our moderate‐intensity exercise protocol. Indeed, a study by Venables et al. observed a 17% higher contribution by fat to total energy expenditure during exercise when GTE was added.29 Lipolysis during low‐intensity exercise of this nature is not thought to limit fat oxidation,30 and it is possible that GTE has additional effects on lipid metabolism, as proposed by Ichinose et al. who evaluated the additional effect of GTE on endurance training changes in whole‐body fat utilization during exercise16. They observed that GTE increased the proportion of whole‐body fat utilization throughout the exercise period, even when there were no was no fat oxidation occurring during exercise alone.23 There are no reports of studies on humans or animals evaluating the molecular mechanism by which GTE augments exercise‐induce fat utilization. Feeding animals with GTE increases the gene expression of fatty acid translocase/CD36 and medium‐chain acyl‐CoA dehydrogenase, proteins involved in lipid transport and oxidation.31 In addition, GTE reduces the content of malonyl CoA to increase the activity of carnitine palmitoyltransferase I,32 which is involved in fatty‐acid metabolism.33
Elevated inflammatory biomarkers occur in several chronic diseases. CRP is a marker of inflammation, and elevated basal levels of CRP have been linked to increased risk of diabetes, hypertension and cardiovascular disease.34 Physical activity has anti‐inflammatory effects and reduces the risk of inflammatory‐related diseases,35 as shown in studies reporting an inverse relationship between physical activity and levels of proinflammatory markers such as IL‐6, TNF‐α and CRP.36 Our study shows that ET with or without GTE decreased plasma levels of hs‐CRP but not IL‐6 and TNF‐α. Furthermore, GTE improved exercise‐induced anti‐inflammatory effects by reducing hs‐CRP concentrations with training alone. This modulatory effect of GTE on CRP levels may be a protective mechanism against cardiovascular disease.37 Green tea has anti‐inflammatory effects and the production of proinflammatory mediators.38 We detected no changes in IL‐6 and TNF‐α levels due to GTE, in contrast to other reports that GTE reduced TNF‐α and IL‐6 levels.39 Several factors such as differences in GTE dosage, duration of study interventions and/or differences in participant's characteristics such as age, sex and health status could explain this discrepancy. Body fat is related to the levels of inflammatory markers such as IL‐6, TNF‐α, CRP and adiponectin.40 GTE intake increases expression of lipases, reduces adipose fat mass, and in parallel increases inflammatory molecules and cytokines.41 This may explain the positive relationship between changes in hs‐CRP concentrations and BFP that we observed (Figure 2B). Furthermore, the anti‐inflammatory effects of GTE can reduce the levels of proinflammatory cytokines by suppression of nuclear factor‐κB.42, 43 A recent report suggests that irisin suppresses the production of proinflammatory cytokines.44 We assumed that the anti‐inflammatory effects of ET with or without GTE can be mediated by irisin; however, we recorded no changes in plasma irisin levels in any of the experimental groups.
Physical activity increases plasma adiponectin concentrations in humans,45, 46 and there is a negative association between adiponectin and BMI as well as body fat.47 Our results demonstrate a correlation between changes in adiponectin and BFP (Figure 2A), suggesting that participants with greater increases in adiponectin had larger decreases in BFP. Although it is not possible to infer causality from correlation (i.e. whether the increase in adiponectin leads to the declined in BFP or vice versa), our findings are consistent with the model proposed by Steiger et al. in which an enlarged visceral adipose tissue could explain low adiponectin concentrations.48 As high molecular weight adiponectin is preferentially produced by visceral fat, it appears that altered levels of adiponectin and related proteins may be a pathway by which excess fat promotes systemic inflammation.48
We show significant increases in adiponectin levels after 8 weeks of ET, which was further enhanced by GTE. Although it has been shown that GTE increases the mRNA and serum levels of adiponectin,49 the combined effect of GTE and exercise intervention have not been previously reported. We report that adiponectin levels were increased by 17% in the ET + P and by 25% in the ET + GTE groups (Table 2). The potentiated changes in adiponectin levels could at least in part account for the greater decreases in BFP in the ET + GTE group. Surprisingly, the levels of adiponectin were decrease in the control group, possibly reflecting time related changes.
There are some limitations in our study. We did not include a group consuming GTE alone (without exercise); however, others have reported that GTE improves body composition and increases anti‐inflammatory markers.12, 42, 43 We included different training modalities (e.g. aerobics, circuit training, and fast walking or jogging) to avoid monotony and increase exercise training compliance. However, all participants in the training groups completed these sessions and this minimized variations in total exercise performed. Another limitation is the short duration of our intervention, and further investigations with longer interventions are needed to understand the relationship between the combination of GTE and ET and changes in body composition and anti‐inflammatory parameters.
In summary, this study demonstrates that GTE improves exercise‐induced body composition changes by decreasing weight, BMI, WHR and BFP. Moreover, GTE potentiated the anti‐inflammatory effects of ET by decreasing hs‐CRP levels. Our results show that GTE further increased ET induced changes in plasma adiponectin levels. Because overweight women are at an increased risk of cardio metabolic disease and dysfunction, our findings may have potential clinical implications for overweight populations. Additionally, GTE could be a useful addition to exercise programmes tailored to improve body fatness and inflammation levels. This work was financially supported by a grant (CMRC‐9412) fromthe Vice Chancellor for Research Affairs, Ferdowsi University ofMashhad, Iran.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
A.R., R.B. and H.Z. conceived and designed research. A.R., R.B., D.A.L., M.A. and M.S.M. conducted the experiment. A.R., R.B. and A.W. analysed data. A.R., R.B., A.W., I.L. and H.Z. wrote the manuscript. All authors read and approved the manuscript.
ACKNOWLEDGEMENTS
We thank the Ferdowsi University for the financial support and all participants in this research project.
Bagheri R, Rashidlamir A, Ashtary‐Larky D, et al. Does green tea extract enhance the anti‐inflammatory effects of exercise on fat loss? Br J Clin Pharmacol. 2020;86:753–762. 10.1111/bcp.14176
Prof. H. Zouhal and Dr. A. Rashidlamir have contributed equally to the work.
The authors confirm that the Principal Investigator for this paper is Dr Reza BAGHERI and that he had direct clinical responsibility for patients.
Contributor Information
Amir Rashidlamir, Email: rashidlamir@um.ac.ir.
Hassane Zouhal, Email: hassane.zouhal@univ-rennes2.fr.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hosseini SA, Ghaedi E, Zakerkish M, et al. Effects of ginseng extract on chemerin, apelin and glycemic biomarkers in type 2 diabetic patients. Indian J Physiol Pharmacol. 2017;61(2):152‐158. [Google Scholar]
- 3. Dolezelova E, Stein E, Derosa G, Maffioli P, Nachtigal P, Sahebkar A. Effect of ezetimibe on plasma adipokines: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2017;83(7):1380‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashtary‐Larky D, Ghanavati M, Lamuchi‐Deli N, et al. Rapid weight loss vs. slow weight loss: which is more effective on body composition and metabolic risk factors? International Journal of Endocrinology and Metabolism. 2017;15(3):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasani‐Ranjbar S, Jouyandeh Z, Abdollahi M. A systematic review of anti‐obesity medicinal plants‐an update. J Diabetes Metab Disord. 2013;12(1):10‐15, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cercato LM, White PA, Nampo FK, Santos MR, Camargo EA. A systematic review of medicinal plants used for weight loss in Brazil: is there potential for obesity treatment? J Ethnopharmacol. 2015;176:286‐296. [DOI] [PubMed] [Google Scholar]
- 8. Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. The anti‐obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 2014;68(10):1075‐1087. [DOI] [PubMed] [Google Scholar]
- 9. Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst Rev. 2012;12:1‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Medina‐Remón A, Casas R, Tressserra‐Rimbau A, et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol. 2017;83(1):114‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti‐inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alipour M, Malihi R, Hosseini SA, et al. The effects of catechins on related risk factors with type 2 diabetes: a review. Progress in Nutrition. 2018;20(1):12‐20. [Google Scholar]
- 13. Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411(11–12):785‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti‐inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607‐615. [DOI] [PubMed] [Google Scholar]
- 15. You T, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of exercise training on chronic inflammation in obesity. Sports Med. 2013;43(4):243‐256. [DOI] [PubMed] [Google Scholar]
- 16. Au A, Feher A, McPhee L, Jessa A, Oh S, Einstein G. Estrogens, inflammation and cognition. Front Neuroendocrinol. 2016;40:87‐100. [DOI] [PubMed] [Google Scholar]
- 17. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM's recommendations for exercise preparticipation health screening. 2015. [DOI] [PubMed]
- 18. Paoli A, Pacelli QF, Moro T, et al. Effects of high‐intensity circuit training, low‐intensity circuit training and endurance training on blood pressure and lipoproteins in middle‐aged overweight men. Lipids Health Dis. 2013;12(1):1‐9, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mosher PE, Nash MS, Perry AC, LaPerriere AR, Goldberg RB. Aerobic circuit exercise training: effect on adolescents with well‐controlled insulin‐dependent diabetes mellitus. Arch Phys Med Rehabil. 1998;79(6):652‐657. [DOI] [PubMed] [Google Scholar]
- 20. Rokling‐Andersen MH, Reseland JE, Veierød MB, et al. Effects of long‐term exercise and diet intervention on plasma adipokine concentrations. Am J Clin Nutr. 2007;86(5):1293‐1301. [DOI] [PubMed] [Google Scholar]
- 21. Thomas DT, Erdman KA, Burke LM. Position of the academy of nutrition and dietetics, dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet. 2016;116(3):501‐528. [DOI] [PubMed] [Google Scholar]
- 22. Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 23. Ichinose T, Nomura S, Someya Y, Akimoto S, Tachiyashiki K, Imaizumi K. Effect of endurance training supplemented with green tea extract on substrate metabolism during exercise in humans. Scand J Med Sci Sports. 2011;21(4):598‐605. [DOI] [PubMed] [Google Scholar]
- 24. Hosseini SRA, Fathei M, Mir E. The effect of combination exercise training on cardiovascular risk factors (adiponectin, interleukin‐6 and homocysteine) in sedentary middle aged men. International Journal of Applied Exercise Physiology. 2016;5(4):55‐63. [Google Scholar]
- 25. McKay DL, Blumberg JB. Roles for epigallocatechin gallate in cardiovascular disease and obesity: an introduction. J am Coll Nutr. 2007;26(4):362S‐365S. [Google Scholar]
- 26. Goto T, Yoshida Y, Kiso M, Nagashima H. Simultaneous analysis of individual catechins and caffeine in green tea. J Chromatogr a. 1996;749(1–2):295‐299. [Google Scholar]
- 27. Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta‐analysis. Am J Clin Nutr. 2009;91(1):73‐81. [DOI] [PubMed] [Google Scholar]
- 28. Henderson GC, Fattor JA, Horning MA, et al. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol. 2007;584(3):963‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Venables MC, Hulston CJ, Cox HR, Jeukendrup AE. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87(3):778‐784. [DOI] [PubMed] [Google Scholar]
- 30. Horowitz JF, Mora‐Rodriguez R, Byerley LO, Coyle EF. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. American Journal of Physiology‐Endocrinology and Metabolism. 1997;273(4):E768‐E775. [DOI] [PubMed] [Google Scholar]
- 31. Murase T, Haramizu S, Shimotoyodome A, Nagasawa A, Tokimitsu I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology. 2005;288(3):R708‐R715. [DOI] [PubMed] [Google Scholar]
- 32. Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I, Hase T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology. 2006;290(6):R1550‐R1556. [DOI] [PubMed] [Google Scholar]
- 33. Lu K‐L, Xu W‐N, Wang L‐N, Zhang D‐D, Zhang C‐N, Liu W‐B. Hepatic β‐oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PloS One. 2014;9(3):1‐12, e93135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Derosa G, Maffioli P, Sahebkar A. Improvement of plasma adiponectin, leptin and C‐reactive protein concentrations by orlistat: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;81(5):819‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashtary‐Larky D, Lamuchi‐Deli N, Milajerdi A, et al. Inflammatory and biochemical biomarkers in response to high intensity resistance training in trained and untrained men. Asian J Sports Med. 2017;8(2):1A‐1A. [Google Scholar]
- 36. Ghafourian M, Ashtary‐Larky D, Chinipardaz R, Eskandary N, Mehavaran M. Inflammatory biomarkers' response to two different intensities of a single bout exercise among soccer players. Iran Red Crescent Med J. 2016;18(2):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pang J, Zhang Z, Zheng T, et al. Green tea consumption and risk of cardiovascular and ischemic related diseases: a meta‐analysis. Int J Cardiol. 2016;202:967‐974. [DOI] [PubMed] [Google Scholar]
- 38. Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y. Anti‐inflammatory action of green tea. Anti‐Inflammatory & Anti‐Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry‐Anti‐Inflammatory and Anti‐Allergy Agents). 2016;15(2):74‐90. [DOI] [PubMed] [Google Scholar]
- 39. Sae‐Tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res. 2011;64(2):146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity. 2010;18(12):2354‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cunha CA, Lira FS, Rosa Neto JC, et al. Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low‐grade inflammation in mice fed a high‐fat diet. Mediators Inflamm. 2013;2013:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Babu A, Pon V, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15(18):1840‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh R, Akhtar N, Haqqi TM. Green tea polyphenol epigallocatechi3‐gallate: inflammation and arthritis. Life Sci. 2010;86(25–26):907‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shao L, Meng D, Yang F, Song H, Tang D. Irisin‐mediated protective effect on LPS‐induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun. 2017;487(2):194‐200. [DOI] [PubMed] [Google Scholar]
- 45. Tsukinoki R, Morimoto K, Nakayama K. Association between lifestyle factors and plasma adiponectin levels in Japanese men. Lipids Health Dis. 2005;4(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simpson KA, Singh MAF. Effects of exercise on adiponectin: a systematic review. Obesity. 2008;16(2):241‐256. [DOI] [PubMed] [Google Scholar]
- 47. Lubkowska A, Radecka A, Bryczkowska I, Rotter I, Laszczyńska M, Dudzińska W. Serum adiponectin and leptin concentrations in relation to body fat distribution, hematological indices and lipid profile in humans. Int J Environ Res Public Health. 2015;12(9):11528‐11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Staiger H, Tschritter O, Machann J, et al. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes Res. 2003;11(3):368‐376. [DOI] [PubMed] [Google Scholar]
- 49. Rocha A, Bolin AP, Cardoso CAL, Otton R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur J Nutr. 2016;55(7):2231‐2244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


