Abstract
Glucagon-like peptide-2 (GLP-2) is an intestinotrophic hormone that promotes intestinal growth and proliferation through downstream mediators, including epidermal growth factor (EGF) and insulin-like growth factor-1 (IGF-1). EGF synergistically enhances the proliferative actions of IGF-1 in intestinal cell lines, and both of these factors are known to be essential for the trophic effects of GLP-2 in vivo. However, whether EGF and IGF-1 interact to mediate the proliferative actions of GLP-2 in vivo remains unknown. Normal and knockout (KO) mice lacking the intestinal epithelial IGF-1 receptor (IE-IGF-1R) were therefore treated chronically with EGF and/or long-acting human hGly2GLP-2, followed by determination of intestinal growth parameters. Intestines from control and IE-IGF-1R KO mice were also used to generate organoids (which lack the GLP-2 receptor) and were treated with EGF and/or IGF-1. Combination treatment with EGF and hGly2GLP-2 increased small intestinal weight and crypt-villus height in C57Bl/6 mice in an additive manner, whereas only hGly2GLP-2 treatment increased crypt cell proliferation. However, although combination treatment also increased small intestinal weight and crypt-villus height in IE-IGF-1R KO mice, the proliferative responses to hGly2GLP-2 alone or with EGF were diminished in these animals. Finally, IGF-1 treatment of organoids undergoing EGF withdrawal was not additive to the effect of EGF replacement on proliferation, but could restore normal proliferation in the absence of EGF. Together, these findings demonstrate that the intestinal proliferative effects of hGly2GLP-2 are augmented by exogenous EGF in a manner that is partially dependent upon IE-IGF-1R signaling.
Keywords: EGF, GLP-2, growth, IGF-1, intestine, proliferation
Glucagon-like peptide-2 (GLP-2) is an enteroendocrine hormone that is secreted from the L-cell upon nutrient ingestion (1). The physiological actions of the endogenous hormone are not well defined, but include a small role in the maintenance of basal intestinal structure, in the small bowel regrowth that occurs upon feeding after a 24-hour fast, and in probiotic-induced preservation of barrier function in obesity (2–5). However, exogenous administration of either native GLP-2 or of long-acting GLP-2 receptor (GLP-2R) agonists, such as dipeptidylpeptidase IV–resistant human (h) (Gly2)GLP-2 (aka teduglutide), also promote nutrient digestion and absorption, crypt cell proliferation, epithelial cell survival, and intestinal blood flow (6–10). As a consequence of these beneficial actions on intestinal growth and function, teduglutide has been approved for the treatment of patients with intestinal failure associated with short bowel syndrome (11).
Despite what is known about the biological effects of GLP-2 administration, its mechanism of action has remained elusive. Glucagon-like peptide-2 receptor (GLP-2R) expression is known to be highest in the jejunum of the intestine, followed by the colon, and proximal gut (12). However, the initial report of GLP-2R expression in scattered enteroendocrine cells, but not in the proliferative crypt or absorptive epithelial cells, led to the suggestion that GLP-2 must exert its intestinal actions indirectly, through one or more intermediary factors (13). Subsequent discovery of GLP-2R expression in intestinal subepithelial myofibroblasts and enteric neurons but, again, not in the known target cells further established the requirement for downstream mediators (14, 15). These findings rapidly led to the discovery of multiple such factors including, most notably, keratinocyte growth factor for large intestinal growth, vasoactive intestinal polypeptide for inflammation-associated growth, and nitric oxide synthase for blood flow (10, 15, 16). However, greatest attention has been paid to the roles of 2 well-known intestinal growth factors, insulin-like growth factor-1 (IGF-1) and epidermal growth factor (EGF).
GLP-2 stimulates the production of IGF-1 by intestinal subepithelial myofibroblasts (17–19), and knockout (KO) of either IGF-1 or its intestinal epithelial receptor (IE-IGF-1R) profoundly reduces GLP-2-induced crypt cell proliferation, microvillus lengthening and barrier function (17, 20–22). Similarly, GLP-2 treatment increases expression of ErbB receptor ligands in the gut (4, 23), and inhibition of the ErbB family of receptors or KO of the intestinal epithelial EGF receptor (ErbB1) markedly impairs the ability of GLP-2 to stimulate intestinal proliferation (23, 24). However, the essential roles of both of these growth factors, and of their signaling pathways, in the proliferative actions of GLP-2 suggests the existence of redundancy—or possibly—additive or synergistic interactions downstream of GLP-2R activation.
Interestingly, pretreatment with EGF synergistically promotes IGF-1-induced proliferation of intestinal epithelial cells in vitro (25–27), while IGF-1 signaling can transactivate the EGFR, at least in keratinocytes (28). However, an early study utilizing CD1 mice reported no additive effects of EGF and hGly2GLP-2 treatment on small intestinal weight or crypt-villus height (29). Conversely, Kitchen et al (30) found that treatment of rats on total parenteral nutrition with both EGF and GLP-2 induced additive effects on small intestinal weight and crypt cell proliferation, and synergistic actions to increase crypt-villus height. Lim et al (31) also demonstrated synergistic effects of EGF and GLP-2 co-administration on bowel length, although no other interactions were noted in a piglet model of short bowel syndrome. However, no studies to date have examined the possibility of interactions between EGF and IGF-1 to mediate the trophic effects of GLP-2 on the gut. The overall aim of the present study was, therefore, to determine the proliferative effects of EGF and hGly2GLP-2 combination therapy on mice, and to establish whether these actions require IGF-1 signaling.
Materials and Methods
In vivo studies
Animals were housed in a 14/10-hour light/dark cycle room at the University of Toronto with ad libitum access to food and water. Mice included age- and sex-matched 6- to 8- week C57Bl/6 mice (Charles River) or IE-IGF-1R KO mice on a C57BL/6 background (B6;Cg-Igf1r<tm1.1Mhz> Tg(Vil1-cre/ERT2)23Syr/Plbk) (32)). In brief, KO mice were generated by crossing villin-cre ERT2/+ animals (from Dr S. Robine, France (33)) with Igf1rfl/fl mice (from Dr M. Holzenberger, France, via Dr R. Kulkarni, USA (34)), and have been maintained as a colony at the University of Toronto since 2008 (20). Hence, although only C57Bl/6 mice were used in the present study, multiple different lines of mice are represented. Mice were genotyped and treated with tamoxifen (100µl intraperitoneal (ip) injection of 10mg/mL in oil vehicle) for 5 days or vehicle alone (control), as previously described (20–22). Control animals included colony- or littermate villin-cre ERT2/+ and Igf1rfl/fl mice treated with tamoxifen or vehicle alone, as well as villin-cre ERT2/+;Igf1rfl/fl mice treated with vehicle. Animals were allowed to recover for a minimum of 7 days after the last injection of tamoxifen (or vehicle) if utilized for intestinal organoid generation (35).
Groups of 8 to 9 C57Bl/6 mice (4-5 male and 4-5 female) and 6 to 8 KO and control mice (3-4 males and 3-4 females) were treated for 10 days with subcutaneous (sc) injections of phosphate-buffered saline (PBS) (vehicle), 0.2 µg/g hGly2GLP-2 (American Peptide Company (20–22)) and/or 1 or 2 doses of 0.4 µg/g recombinant murine EGF (R&D Systems (23)) for 10 days, followed by a final treatment at 3 hours and an ip injection of EdU (100mg/kg; Invitrogen (36);) at 1 hour before sacrifice on day 11. After determination of body weight, the intestines were removed and cleared of luminal contents with PBS, and small intestinal and colonic weights and lengths (under constant tension) were measured. Sections (2 cm) of the jejunum were collected for mRNA, histological and proliferation analyses, or were fixed in 10% neutral-buffered formalin, embedded in paraffin and sectioned (Toronto General Hospital Pathology Laboratory). All animal protocols were approved by the University of Toronto Animal Care Committee and followed Canadian Council on Animal Care guidelines.
In vitro studies
Organoids were produced from the jejunum of 5- to 8-week-old mice as described (27). In brief, jejunal (10 cm) crypts were collected by trituration in cold PBS followed by incubation in 2 mM EDTA-PBS for 30 minutes with shaking, further trituration, centrifugation, and embedding in a 50 µL dome of Matrigel (Corning). Advanced DMEM/F12 media was supplemented with 100x GlutaMAX, 1 M Hepes, Pen-Strep 50x B27, 100x N2 (all from Gibco), 500 nM N-acetyl-L-cysteine (Sigma-Aldrich), 10% R-spondin-1 (v/v: conditioned media from 293T-HA-RspoI-Fc cells—a gift from Dr C. Kuo [Stanford University] through Dr C. O’Brien [University of Toronto]), 100 ng/µl noggin and 50 ng/µl EGF (both from PeproTech). Medium was replaced every 3 to 4 days and organoids were passaged every 7 days using medium containing 10 mM Y-27632 dihydrochloride (Sigma-Aldrich). Organoids were replated onto 8-well chamber slides (Falcon) for experimental purposes. Some organoids were treated with 1µg/µl EdU for 20 to 120 minutes and/or with 10 mM Y-27632 dihydrochloride (37) for 1 day to inhibit proliferation (38), followed by the addition of EdU for 20 minutes. Some organoids underwent EGF withdrawal for 5 days, or for 4 days followed by replacement of the EGF (50 ng/µl) with or without IGF-1 (100 ng/mL, Abcam) for 1 day, followed by the addition of EdU for 20 minutes. EGF-IGF-1 interaction studies were conducted using organoids from 1 female and 1 male KO mouse; controls included 1 vehicle-treated female villin-cre ERT2/+;Igf1rfl/fl, and 1 male and 1 female vehicle-treated Igf1rfl/fl mouse (villin-cre ERT2/+ organoids were not used due to poor survival post-passaging, with or without tamoxifen treatment of the mice followed by the 7-day washout period (35)). Organoids were washed with room temperature PBS, fixed in 4% paraformaldehyde for 30 minutes and stored at 4°C prior to staining.
Proliferation analyses
The Click-iT EdU Alexa Fluor 488 Imaging Kit (Invitrogen) was used to stain for EdU as per the manufacturer’s instructions. Vectashield Antifade Mounting Medium with DAPI (Vector Laboratories) was used for detection of nuclei in jejunal sections, with DAPI alone as the negative control (data not shown), whereas organoids were treated with 10 µg/mL DAPI (Roche) for 20 minutes, washed with 3% BSA in PBS and cover-slipped with Vectashield Antifade Mounting Medium without DAPI. Twenty cells on the right side of each jejunal section crypt were counted, starting at position 1 in the crypt base, and 20 crypts were counted per mouse. For each intestinal organoid, the percent EdU+ cells per crypt was counted in 1 to 4 crypts for each of 10 to 20 organoids per mouse. All cells were counted in a blinded fashion. Jejunal proliferation was calculated as the percent EdU+ cells per crypt cell position, and the area-under-the-curve (AUC) was determined for cell positions 1–3, 4–7, and 8–16 as indicators of the active stem zone, the reserved stem cell zone and the transit-amplifying zone, respectively, in C57Bl/6 mice (36), and that for crypt cell positions 13–16 for the transit-amplifying zone in the IR-IGF-1R KO (and control) animals.
Microscopy
Jejunal crypt depth and villus height were measured on hematoxylin and eosin-stained slides using a Zeiss microscope with AxioVision software (Zeiss Microscopy Canada); at least 40 crypts and 30 villi were measured for each mouse in the first experiment and at least 20 crypts and 20 villi were measured for each mouse in the second experiment, in a blinded fashion. For jejunal EdU+ cell counting, the Zeiss microscope was used with AxioVision software, while the 90 Nikon Eclipse T Swept Field microscope was used with NISA software for organoid EdU+ cell counting (Nikon Instruments, Inc.).
RNA extraction and analysis
RNA was isolated from jejunal mucosa scrapes and organoids using the RNeasy Plus Mini Kit (Qiagen Inc.) and converted to cDNA using 5x All-in-One RT Mastermix (Applied Biological Materials Inc.). Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed the Taqman Gene Expression Assay (Thermo Fisher) with the primers (Thermo-Fisher) listed in Table 1; 18S was used as internal control for the in vivo experiments (17) and H3a for the in vitro studies (27). Relative gene expression was quantified using the Livak method (39).
Table 1.
List of Primers Used for qRT-PCR
| Gene name | Primer ID Number |
|---|---|
| 18S | Hs99999901_s1 |
| Bax | Mm00432051_m1 |
| Bcl2 | Mm00477631_m1 |
| Egf | Mm00438696_m1 |
| Erbb1 | Mm01187858_m1 |
| Erbb2 | Mm00658541_m1 |
| Gcg | Mm00801712_m1 |
| Glp2r | Mm_01329477_m1 |
| H3a | Mm01612808_g1 |
| Igf1 | Mm00439559_m1 |
| Igf1r | Mm_00802837_m1 |
| Lgr5 | Mm01251805_m1 |
| Mki67 | Mm01278617_m1 |
Statistics
GraphPad Prism was used for statistical analyses. For the first mouse experiment and for the organoid studies, significance was determined by 1-way ANOVA followed by post hoc Sidak’s multiple comparisons test. For the KO animal studies, 3-way ANOVA was used followed by 2-way ANOVA and Holm-Sidak’s multiple comparisons tests for each genotype.
Results
Effects of hGly2GLP-2 and/or EGF treatment on the intestines of normal mice
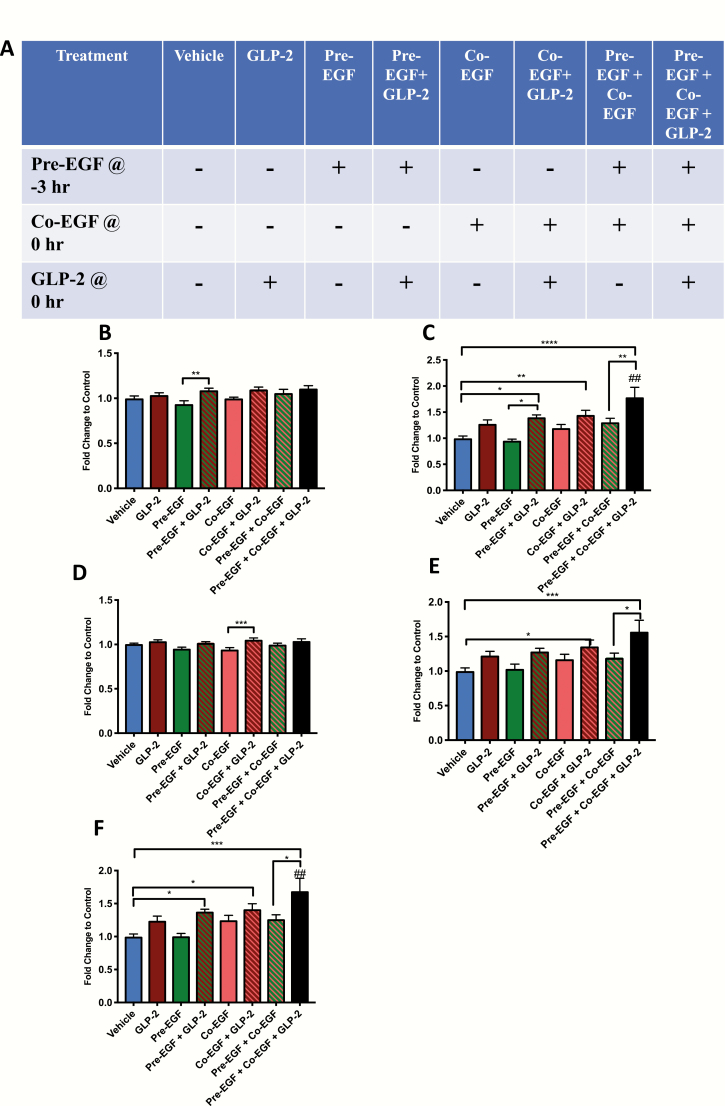
To determine the optimal timing for stimulation of intestinal proliferation by hGly2GLP-2 and EGF co-treatment, C57Bl/6 mice were subjected to 8 different treatments, daily for 11 days: vehicle at -3 and/or 0 hour, EGF at -3 hours (“pre-”), EGF at 0 hour (“co-”) or EGF at both time points (“pre-/co-”), each with either vehicle or hGly2GLP-2 at 0 hour (Fig. 1A). Although the average starting body weights were not different between the treatment groups, the male mice were significantly heavier than the female mice on day 1 (21.5 ± 0.2 vs 16.7 ± 0.2 g; n = 33 mice for each sex; P < 0.001). As body weight was used in some assessments, and no other sex differences were noted, all data were therefore normalized within each sex prior to combination within each treatment group.
Figure 1.
Treatments with EGF and/or hGly2GLP-2 increase small intestinal weight in C57Bl/6 mice. (A) Male (n = 4–5) and female (n = 4–5) 6–8 wk C57Bl/6 mice were treated for 11 days with various combinations of vehicle, EGF, and/or hGly2GLP-2. (B) Body weight, (C) Small intestinal weight, (D) Small intestinal length, (E) Small intestinal weight/body weight and (F) Small intestinal weight/small intestinal length. All data were normalized by sex prior to combination within each treatment group to make n = 8–9. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated; ##P < 0.01 vs hGly2GLP-2 alone.
Normalized body weights on day 11 were relatively uniform across all treatment groups (Fig. 1B). Small intestinal weight increased significantly in the pre-EGF+co-EGF+ hGly2GLP-2 group, and was higher than in mice treated with vehicle, hGly2GLP-2 alone, and pre-EGF+co-EGF alone (P < 0.01–0.001) (Fig. 1C). Pre-EGF+ hGly2GLP-2 treatment also had significant effects on small intestinal weight compared with vehicle (P < 0.01) and pre-EGF alone (P < 0.01), but not in comparison with hGly2GLP-2 alone, whereas pre-EGF+co-EGF differed only from vehicle alone (P < 0.01). Small intestinal lengths did not differ markedly between the 8 treatment groups (Fig. 1D).
When small intestinal weight was normalized to body weight, the pre-EGF+co-EGF+ hGly2GLP-2 treatment group was significantly greater than the vehicle (P < 0.001) and pre-EGF+co-EGF groups (P < 0.05), but not as compared with hGly2GLP-2 alone (Fig. 1E). However, when small intestinal weight was normalized to small intestinal length, the pre-EGF+co-EGF+ hGly2GLP-2 mice demonstrated a significantly greater response than found for vehicle-, pre-EGF+co-EGF alone, and hGly2GLP-2-treated animals (P < 0.05–0.001) (Fig. 1F). Interestingly, the sum of the mean response in small intestinal weight/small intestinal length to hGly2GLP-2 plus that to pre-EGF+co-EGF was similar to that of the combined effect of pre-EGF+co-EGF+ hGly2GLP-2 treatment (0.014 vs 0.019, respectively), suggesting additive effects of pre-EGF+co-EGF and hGly2GLP-2 on this growth parameter.
Colonic weights of both the pre-EGF+co-EGF group and the pre-EGF+co-EGF+ hGly2GLP-2 group increased significantly compared with vehicle (P < 0.01), while the pre-EGF+ hGly2GLP-2 group demonstrated a significant increase compared with its respective pre-EGF, control group (P < 0.05) (Fig. 2A). Colonic lengths did not differ between the groups (Fig. 2B). No profound differences were observed when colonic weight was normalized to body weight (Fig. 2C). Furthermore, although colonic weight per length demonstrated significant increases for the pre-EGF+co-EGF+ hGly2GLP-2 group as compared with both the vehicle controls and the hGly2GLP-2 treated mice alone (P < 0.001), no differences were found as compared with the pre-EGF+co-EGF treatment alone (Fig. 2D).
Figure 2.
Treatments with EGF and/or hGly2GLP-2 increase colonic weight in C57Bl/6 mice. Male (n = 4–5) and female (n = 4–5) 6- to 8-week C57Bl/6 mice were treated for 11 days with various combinations of vehicle, EGF, and/or hGly2GLP-2. (A) Colonic weight, (B) Colonic length, (C) Colonic weight/body weight and (D) Colonic weight/small intestinal length. All data were normalized by sex prior to combination within each treatment group to make n = 8–9. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated; ###P < 0.001 vs hGly2GLP-2 alone.
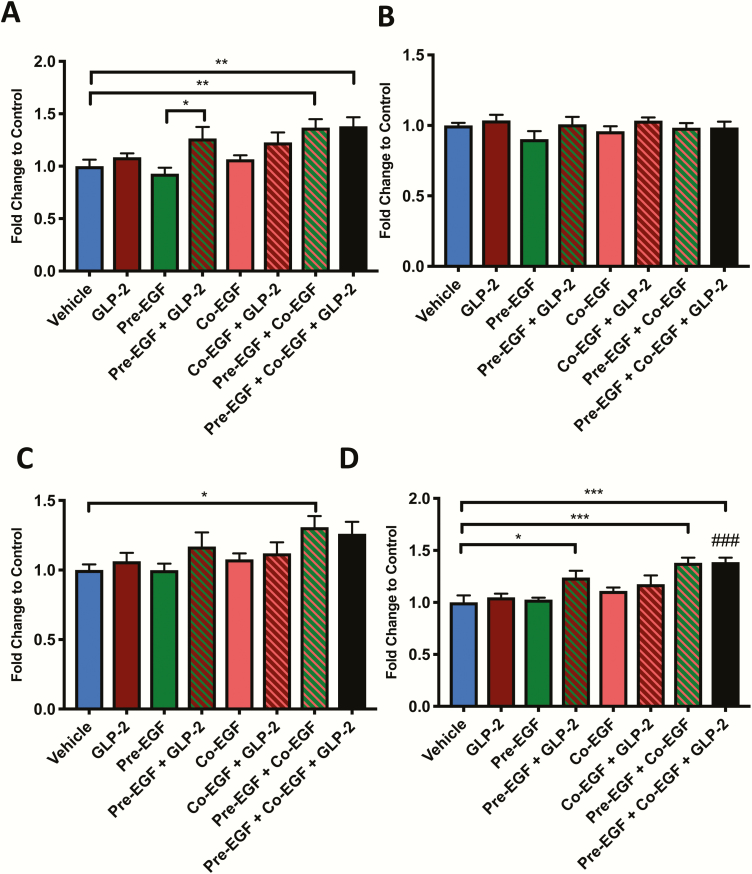
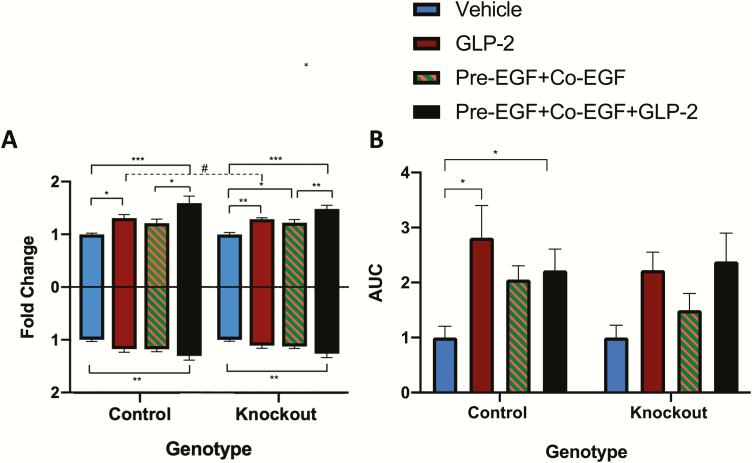
As the increased small intestinal weight with several of the treatment paradigms was independent of differences in unit length, jejunal crypt-villus height measurements were taken in order to examine one well-established mechanism for mucosal growth responses to both hGly2GLP-2 and EGF (17, 20, 22, 23). Treatment with hGly2GLP-2 alone increased villus height (P < 0.01) (Fig. 3A). The pre-EGF+co-EGF treated mice also demonstrated a significant increase in villus height compared with vehicle (P < 0.01), whereas neither of the single EGF treatments (i.e. pre-EGF and co-EGF alone) were effective. However, all combination groups with EGF plus hGly2GLP-2 exhibited significant increases in villus height relative to vehicle, to hGly2GLP-2 alone, and to their respective EGF treatments alone (P < 0.05–0.001). Furthermore, although all treatments that included hGly2GLP-2 also increased crypt depth as compared with vehicle (P < 0.05–0.001), only the co-EGF+ hGly2GLP-2 group demonstrated an increase in crypt depth compared with vehicle, hGly2GLP-2, and the respective EGF treatment alone (P < 0.05–0.001). In addition, as found for the small intestinal weight per small intestinal length, the sum of the responses in crypt-villus height to hGly2GLP-2 plus that to pre-EGF+co-EGF did not appear to differ from that found for the combined pre-EGF+co-EGF+ hGly2GLP-2 treatment (164.0 vs 169.1, respectively), again consistent with additive effects.
Figure 3.
Treatments with EGF and/or hGly2GLP-2 increase crypt-villus growth in C57Bl/6 mice. Male (n = 4–5) and female (n = 4–5) 6- to 8-week C57Bl/6 mice were treated for 11 days with various combinations of vehicle, EGF, and/or hGly2GLP-2. (A) Jejunal villus height and crypt depth, (B) Proliferative index in the jejunal transit amplifying zone and (C) Bax:Bcl2 ratio in jejunal mucosal scrapes. All data were normalized by sex prior to combination within each treatment group to make n = 8–9. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs hGly2GLP-2 alone.
As chronic treatment with either hGly2GLP-2 or EGF is known to increase crypt cell proliferation in the mouse (17, 20–23, 36), EdU incorporation into jejunal crypt cells was determined in all mice. Proliferation in the active and reserve stem zones did not show any significant differences between the groups (data not shown). However, in the transient amplifying zone, all animals receiving hGly2GLP-2, either alone or with EGF, demonstrated increased proliferation compared with vehicle-treated mice (P < 0.001) (Fig. 3B). Unexpectedly, EGF treatment alone (ie, pre-EGF, co-EGF, and pre-EGF+co-EGF) did not increase proliferation in the transit amplifying zone. As this finding did not correlate with the increased crypt-villus height observed in the same animals (Fig. 3A), qRT-PCR of the jejunal mucosa was used to determine the Bax:Bcl2 ratio as a marker of apoptosis (40). All mice treated with co-EGF, either alone or with pre-EGF and/or hGly2GLP-2, demonstrated a significant reduction in the Bax:Bcl2 ratio (Fig. 3C).
To determine whether the changes in intestinal growth between the treatment groups could be consequent to differential gene expression for the growth factors or their receptors, jejunal mucosal scrapes were analyzed by qRT-PCR. No significant differences were observed for proglucagon (the prohormone for GLP-2) and the GLP-2R, for IGF-1 and its receptor, or for EGF and its 2 receptors, ErbB1 (aka EGFR) and ErbB2 (Fig. 4).
Figure 4.
Treatments with EGF and/or hGly2GLP-2 do not affect expression of growth factor ligands or their receptors in C57Bl/6 mice. Male (n = 4–5) and female (n = 4–5) 6- to 8-week C57Bl/6 mice were treated for 11 days with various combinations of vehicle, EGF and/or hGly2GLP-2. Jejunal mucosal scrapes were analysed by qRT-PCR for (A) proglucagon (GCG), (B) GLP-2R, (C) IGF-1, (D) IGF-1R, (E) EGF, (F) ErbB1, and (G) ErbB2 transcripts, as normalized to expression of 18S rRNA. All data were normalized by sex prior to combination within each treatment group to make n = 8–9.
Collectively, these findings indicated that pre-EGF+coEGF+ hGly2GLP-2 treatment for 11 days was the most effective treatment for increasing small intestinal mucosal growth in normal mice. This treatment paradigm was therefore selected for further study.
Effects of hGly2GLP-2 and/or EGF treatment on the intestines of IE-IGR-1R KO mice
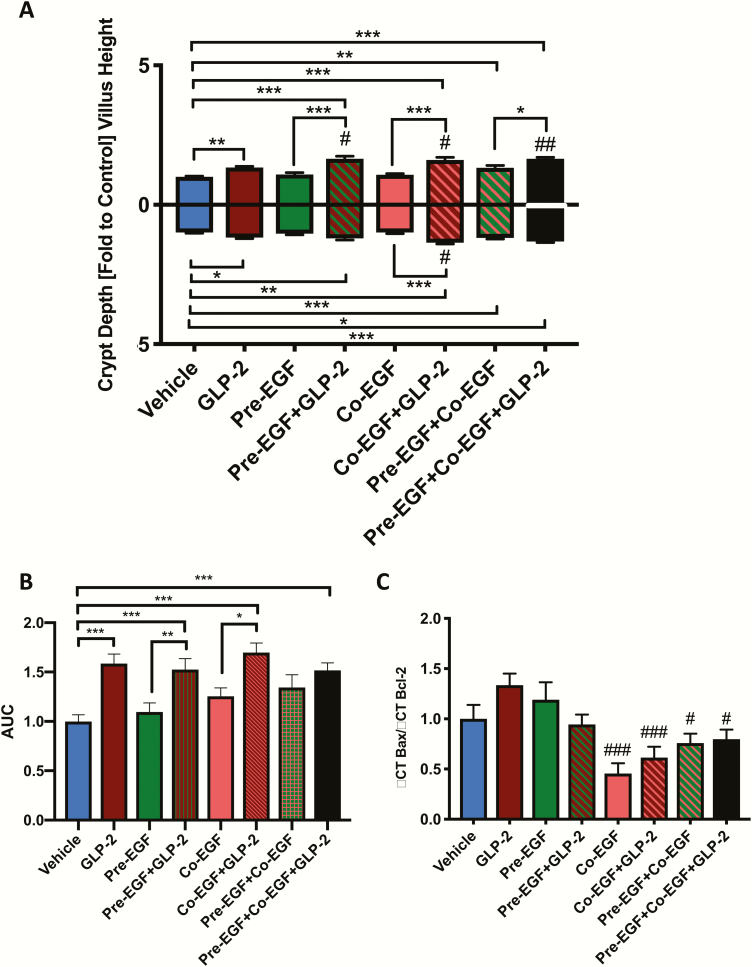
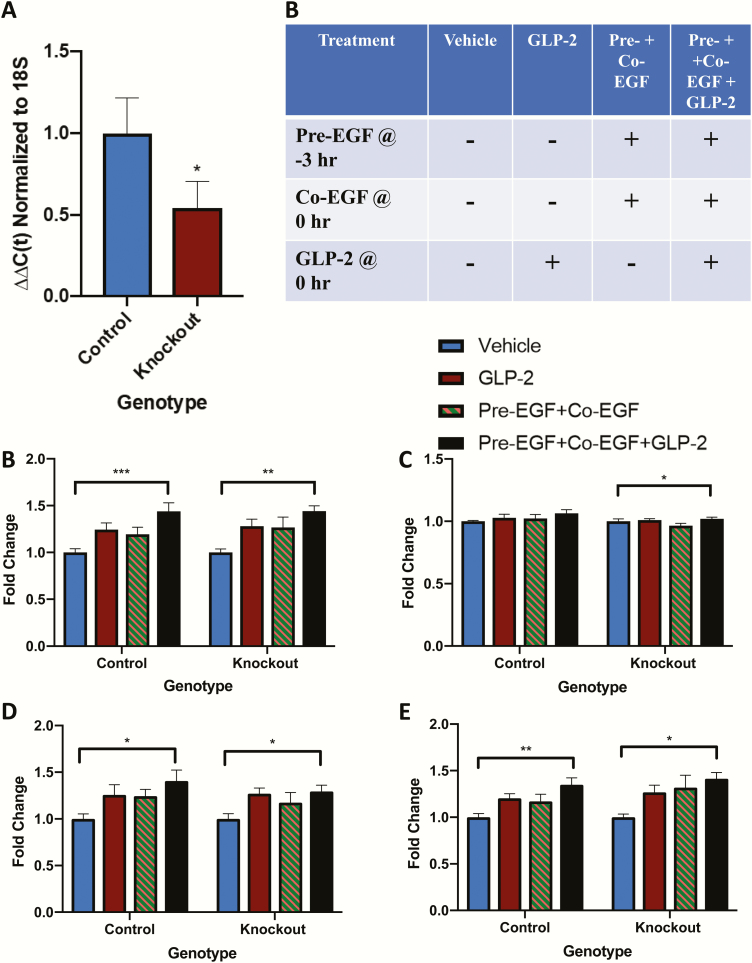
Villin-cre ERT2/+, Igf1rfl/fl and villin-cre ERT2/+;Igf1rfl/fl mice were treated with either vehicle or tamoxifen, followed by a 7-day wash-out period. qRT-PCR for Igf1r demonstrated a 46% reduction in jejunal mucosal transcript expression (P < 0.05) (Fig. 5A), consistent with loss of the transcript in the epithelial cells but maintenance of expression in other mucosal cell types (20–22).
Figure 5.
Treatment with EGF and hGly2GLP-2 increases small intestinal weight in IE-IGF-1R KO mice. Male (n = 2–4) and female (n = 3–6) 6- to 10-week control and IE-IGF-1R KO mice were treated for 11 days with various combinations of vehicle, EGF, and/or hGly2GLP-2. (A) Small intestinal weight, (B) Small intestinal length, (C) Small intestinal weight/body weight, and (D) Small intestinal weight/small intestinal length. All data were normalized by sex prior to combination within each treatment group to make n = 6–8. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated.
IE-IGF-1R KO and control mice were then treated for 11 days with: vehicle at 0 hour, hGly2GLP-2 alone at 0 hour, pre-EGF+co-EGF at -3 and 0 hours, respectively, and a combination of hGly2GLP-2 and the EGF treatments (Fig. 5B). Body weight was not affected by treatment in control mice, although a small increase was noted with the combined treatment in the KO animals (P < 0.05; data not shown). Small intestinal weight was increased by the combined treatment in both groups of mice, and length was increased in the KO animals (P < 0.05–0.001) (Fig. 5B and 5C). Small intestinal weight normalized to body weight and to small intestinal length were increased in control mice (P < 0.05–0.01) (Fig. 5D and 5E). The response in the KO mice was not significantly different from that found in controls for both parameters. Finally, although none of the individual treatments alone reached significance in these animals, the changes in response to each agent alone compared with the combined treatment were consistent with additive effects of hGly2GLP-2 in combination with pre-EGF+co-EGF.
Control mice demonstrated a significant increase in villus height in response to hGly2GLP-2 as well as to combined EGF+ hGly2GLP-2 treatment (P < 0.05–0.001) (Fig. 6A). As previously reported (20–22), IE-IGF-1R KO mice demonstrated a reduced response to the effects of hGly2GLP-2 on villus height (P < 0.05 vs control mice, P < 0.01 vs KO vehicle). However, unexpectedly, villus height was increased in the KO animals by treatment with either pre-EGF+co-EGF alone, and this was increased in an apparent additive manner by co-administration of hGly2GLP-2 (P < 0.05–0.001). Control and KO mice also responded in a similar fashion to the combination treatment with respect to stimulation of crypt depth (P < 0.001). Finally, crypt cell proliferation in the transit amplifying zone was increased by hGly2GLP-2 alone as well as by combined EGF+ hGly2GLP-2 treatment in control mice (P < 0.05); however, interestingly, neither of these parameters were significantly increased in the KO animals (Fig. 6B).
Figure 6.
Treatments with EGF and/or hGly2GLP-2 increase crypt-villus growth abnormally in IE-IGF-1R KO mice. Male (n = 2–4) and female (n = 3–6) 6- to 10-week control and IE-IGF-1R KO mice were treated for 11 days with various combinations of vehicle, EGF, and/or hGly2GLP-2. (A) Jejunal villus height and crypt depth and (B) Proliferative index in the jejunal transit amplifying zone. All data were normalized by sex prior to combination within each treatment group to make n = 6–8. *P < 0.05, **P < 0.01, ***P < 0.001, # P < 0.05 as indicated.
When taken together, these findings indicate that exogenous EGF is sufficient to bypass lack of the IE-IGF-1R with respect to stimulation of small intestinal weight and jejunal villus growth, but that crypt cell proliferation in response to hGly2GLP-2, as well as to both EGF and combined treatment with hGly2GLP-2 and EGF is slightly impaired by IE-IGF-1R KO.
Effects of EGF and IGF-1 treatment on proliferation of organoids from IE-IGR-1R KO mice
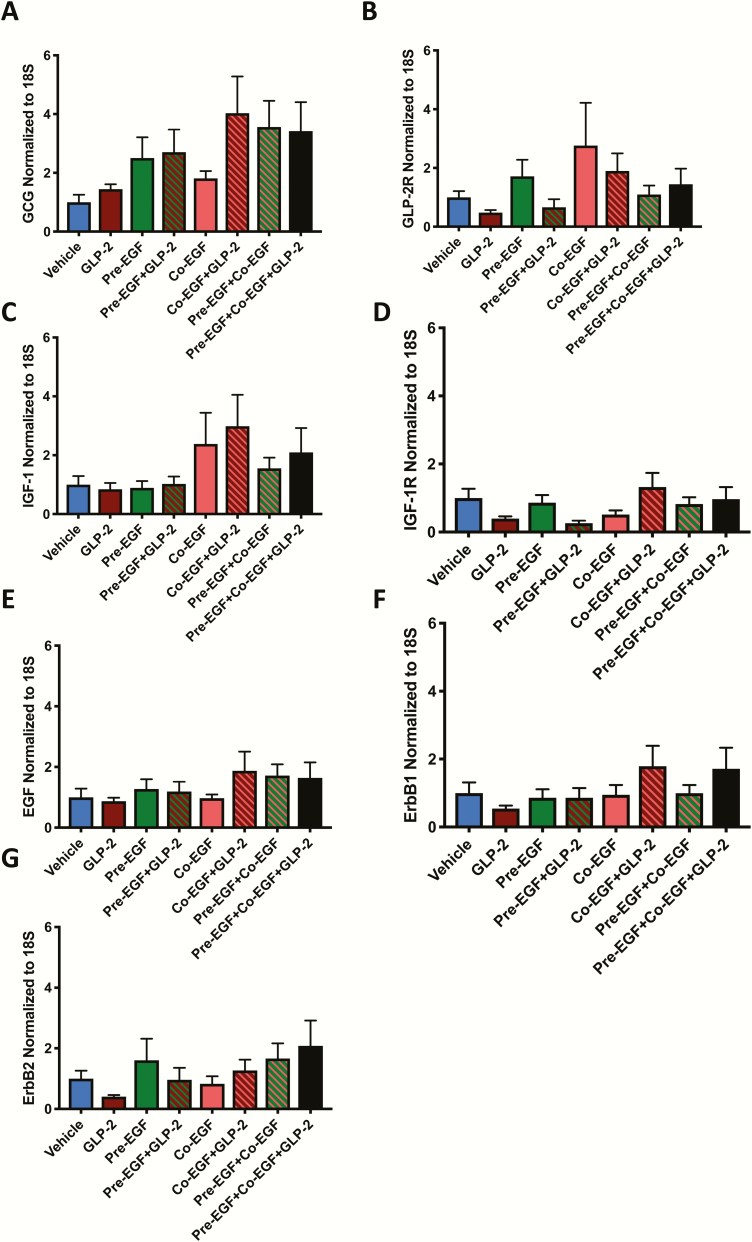
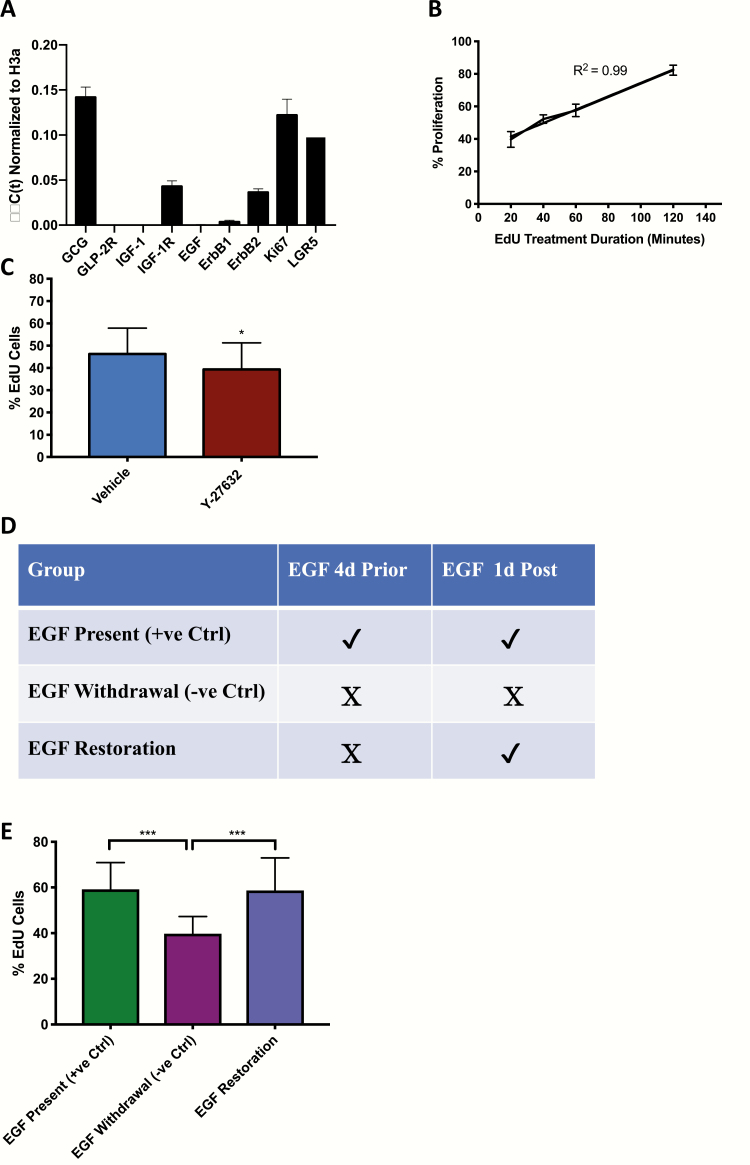
To determine whether EGF and IGF-1 interact in the stimulation of crypt cell proliferation in vitro, organoid cultures were prepared from control and IE-IGF-1R KO mice. qRT-PCR revealed expression of proglucagon, IGF-1R, and both ErbB1 and ErbB2, but not of the GLP-2R, or of either IGF-1 or EGF, as expected (41–43) (Fig. 7A). After pilot studies to determine the incubation period for EdU (selected as 20 min) (Fig. 7B), organoid crypt cell proliferation was shown to be responsive to inhibition, by treatment with Y-27632, which reduced EdU uptake into crypt cells by 15% (P < 0.05), and by withdrawal of EGF, which decreased the number of EdU-positive cells by 32% (P < 0.001), as well as to stimulation, by replacement of EGF after withdrawal, which restored EdU labeling to control levels (Fig. 7C–E).
Figure 7.
Proliferation in organoids from C57Bl/6 mice is responsive to both inhibition and stimulation. (A) qRT-PCR of organoids from C57Bl/6 mice for growth factors, their receptors and markers of both proliferation and stem cells. (B) Organoids were treated with EdU for 20–120 min followed by determination of the percent labeled crypt cells (n = 5–9). (C) Organoids were treated with vehicle or Y-27632 for 1 day followed by treatment with EdU for 20 min and determination of the percent labeled crypt cells (*P < 0.05 vs vehicle; n = 25–27). (D-E) Organoids were treated with or without EGF for 5 d, or with EGF for 4 d followed by EGF withdrawal for 1 d (D), and were then treated with EdU for 20 min and the percent labeled crypt cells determined (E). (n = 1 mouse per group, n = 10–20 organoids per mouse, n = 1–4 crypts per organoids) *** P < 0.001 as indicated.
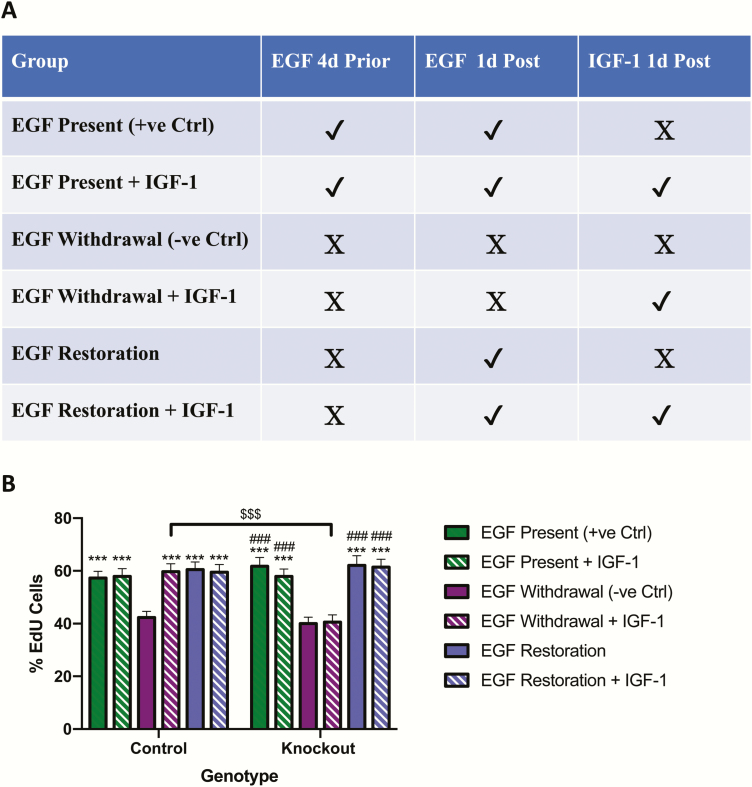
Finally, to determine EGF-IGF-1 interactions in regulating crypt cell proliferation, organoids were subjected to continuous EGF treatment for 5 days (positive control, equivalent to “pre” treatment), withdrawal of EGF for 5 days (negative control), or withdrawal of EGF for 4 days followed by replacement for 1 day (equivalent to “co” treatment), all with and without IGF-1 treatment on the last day (Fig. 8A). Organoids from control mice demonstrated a 26% reduction in proliferation with EGF withdrawal, and a return to baseline levels following EGF replacement (P < 0.001) (Fig. 8B). Unexpectedly, IGF-1 treatment did not affect proliferation in the presence of EGF (ie, with and without EGF withdrawal); however, in the absence of EGF, IGF-1 stimulated proliferation to the same extent as EGF replacement alone (P < 0.001). Basal levels of proliferation as well as the responses to both EGF withdrawal and EGF replacement were not different between organoids from IE-IGF-1R KO mice and organoids from control animals. However, functional KO of the IGF-1R was confirmed by the inability of IGF-1 to stimulate proliferation in these cells (P < 0.001 for the response to IGF-1 alone in EGF-deprived control vs IE-IGF-1R KO organoids).
Figure 8.
EGF and IGF-1 increase proliferation independently in organoids from control and IE-IGF-1R KO mice. (A-B) Organoids from control and IE-IGF-1R KO mice were treated with EGF for 5 days (positive control), without EGF for 5 days (negative control) or without EGF for 4 days followed by EGF replacement for 1 day, all with and without IGF-1 treatment on the last day (A), and were then treated with EdU for 20 min and the percent labeled crypt cells determined (B). (n = 2–3 mice per group, 10–20 organoids per mouse and 1–4 crypts per organoid) ***P < 0.001 vs EGF withdrawal group in paired genotype group; ###P < 0.001 vs EGF withdrawal + IGF-1 in paired genotype group; $$$P < 0.001 as indicated.
Discussion
Due to the known lack of expression of the GLP-2R by both the intestinal epithelial and crypt stem cells (13–15, 41–43), as also confirmed in the present study, the trophic actions of this enteroendocrine hormone have an obligatory requirement for downstream signaling by ErbB1 and the IGF-1R (4, 17–24). While EGF pretreatment has been demonstrated to interact synergistically with IGF-1 to enhance the proliferation of intestinal cell lines in vitro (25–27), whether such effects are preserved in physiological systems has remained unclear. The results of the present study indicate that, while exogenous administration of hGly2GLP-2 and EGF stimulates intestinal growth, the combination of these trophic agents exerts additive effects that are only partially dependent on IE-IGF-1R signaling.
To allow for possible synergistic effects when given in combination, various combinations of EGF with hGly2GLP-2 were assessed to ensure submaximal intestinal growth. Chronic treatment with single doses of EGF alone (ie, pre- or co-EGF) did not result in any marked effects on intestinal weight, length, crypt-villus height, proliferation, or apoptosis in C57Bl/6 mice. Of note, these findings stand in contrast to findings of enhanced small intestinal weight and crypt-villus height in CD1 mice with chronic EGF treatment (23), suggesting strain-dependent actions. Indeed, early studies demonstrated that CD1 EGFR KO mice survive for up to 3 weeks postnatally, whereas C57 mice die after only 8 days (44). Furthermore, the findings do not provide support for a synergistic effect of exogenous EGF pre-treatment alone on the IGF-1-dependent GLP-2 signaling pathway in vivo. However, administration of 2 doses of EGF (pre- plus co-EGF) did enhance small intestinal crypt-villus height, as well as colonic weight and length, indicating dose-dependent actions of this growth factor, as well as suboptimal effects when compared with other treatment regimens tested. Similarly, when a dose of hGly2GLP-2 known to be intestinotrophic in CD1 mice was administered (29), both small intestinal crypt-villus height and TA zone proliferation were increased, with only small, nonsignificant effects to enhance overall intestinal weight and length detected, indicating a suboptimal dose in the C57Bl/6 strain. Nonetheless, when administered in combination, EGF and hGly2GLP-2 treatment additively increased small intestinal weight and crypt-villus height, both as compared with vehicle alone and relative to the effects of each growth factor alone. The additive nature of these effects stands in contrast to previous findings in normal CD1 mice, in which hGly2GLP-2 and EGF were reported to demonstrate no interactions, or were additive or synergistic effects in animal models of intestinal insufficiency (29, 31, 45). When taken together, these findings indicate that the intestinotrophic actions of exogenous EGF and hGly2GLP-2 are highly strain-, dose- and model-dependent. It is also acknowledged that the current study utilized only a single time period for peptide administration (11 days). Further studies using additional doses of each agent, alone and together, as well as testing additional durations of administration, will be useful in determining the exact dose-relationships between these intestinotrophic peptides.
As reported previously (20–22), IE-IGF-1R KO mice demonstrated small impairments in the enhancement of both villus height and TA zone proliferation by hGly2GLP-2 treatment. However, unlike the control animals in this study, a significant villus response to EGF treatment was noted in the KO mice, which was further increased by co-treatment with hGly2GLP-2, suggestive of adaptive responses despite the rather acute loss of IGF-1R signaling in the intestinal epithelium (5 days induction; 11 days treatment). Nonetheless, although the proliferative response of the TA zone to hGly2GLP-2 alone or with EGF was dampened in the IE-IGF-1R KO animals, there remained a trend to an increase for both conditions, suggesting that the induction of crypt cell proliferation is regulated by the actions of multiple growth factors.
To examine the relationship between EGF and IGF-1 signaling in the regulation of crypt cell proliferation at the cellular level, organoids lacking these growth factors were derived from control and IE-IGF-1 KO mice. Consistent with a previous report (46), crypt cell proliferation in control organoids was impaired by 4 days of EGF withdrawal and restored by 24 hours of EGF replacement. However, no indication of either additive or synergistic effects of EGF and IGF-1 could be detected in this model. Furthermore, although similar responses to EGF withdrawal and replacement were observed in organoids from the KO animals, IGF-1 treatment in the absence of EGF did not have any effect on proliferation, as expected, whereas IGF-1 unexpectedly replaced the loss of EGF in the control organoids. A previous study from the Lund group has demonstrated that IGF-1 stimulates the proliferation of the 2 stem cell populations in vivo, Sox9-eGFPhigh and Sox9-eGFPlow, which are putatively the position 4 “reserve” and Lgr5+ “crypt base columnar” stem cells, respectively (47). However, in organoids, IGF-1 treatment stimulated proliferation only in the Sox9-eGFPhigh cells. The present findings therefore raise the intriguing possibility that the lack of effect of EGF and IGF-1 combination treatment on crypt cell proliferation in the current models may have been masked by differential responses between the 2 stem cell populations.
In conclusion, the results of the present study indicate that, in C57Bl/6 mice, exogenous EGF and hGly2GLP-2 enhance intestinal growth in an additive manner that is, at least partially, dependent upon the intestinal epithelial IGF-1R. However, whether many of the other known subepithelial myofibroblast growth factors play a role in this signaling pathway remains unknown (48). The finding that EGF and IGF-1 act independently to induce crypt cell proliferation in vitro further suggests that different stem cell populations may be involved in the intestinotrophic actions of these growth factors, both of which are known to act downstream of GLP-2R signaling.
Glossary
Abbreviations
- EGF
epidermal growth factor
- ErbB1
intestinal epithelial EGF receptor
- GLP-2
glucagon-like peptide-2
- GLP-2R
glucagon-like peptide-2 receptor
- IE-IGF-1R
intestinal epithelial IGF-1 receptor
- IGF-1
insulin-like growth factor 1
- KO
knockout
Acknowledgments
The authors are grateful to Drs C. Kuo (Stanford University) and C. O’Brien (University of Toronto) for the gift of HEK293-Rspo1 cells; and to Ms. J.A. Chalmers, Ms. Y. Nahaei, and Dr A. Hardy for technical assistance. P.L.B. was supported by the Canada Research Chairs program.
Financial Support: This study was supported by an operating grant from the Canadian Institutes of Health Research (PJT-14853), and some of the equipment was supported by the 3D (Diet, Digestive Tract and Disease) Centre funded by the Canadian Foundation for Innovation and Ontario Research Fund (project numbers 19442 and 30961).
Author Contributions: Z.F. and P.L.B. designed experiments and wrote the paper; Z.F. and E.M. collected and analyzed data.
Additional Information
Disclosure Summary: The authors have no relevant conflicts of interest to disclose.
Data Availability. All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Brubaker PL. Glucagon-like peptide-2 and the regulation of intestinal growth and function. Compr Physiol. 2018;8(3):1185–1210. [DOI] [PubMed] [Google Scholar]
- 2. Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology. 2005;128(5):1340–1353. [DOI] [PubMed] [Google Scholar]
- 3. Wismann P, Barkholt P, Secher T, et al. . The endogenous preproglucagon system is not essential for gut growth homeostasis in mice. Mol Metab. 2017;6(7):681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology. 2010;138(7):2447–2456. [DOI] [PubMed] [Google Scholar]
- 5. Cani PD, Possemiers S, Van de Wiele T, et al. . Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93(15):7911–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai CH, Hill M, Asa SL, Brubaker PL, Drucker DJ. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol. 1997;273(1 Pt 1):E77–E84. [DOI] [PubMed] [Google Scholar]
- 8. Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol. 1997;273(6):R1965–R1971. [DOI] [PubMed] [Google Scholar]
- 9. Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272(6 Pt 1):E1050–E1058. [DOI] [PubMed] [Google Scholar]
- 10. Guan X, Stoll B, Lu X, et al. . GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125(1):136–147. [DOI] [PubMed] [Google Scholar]
- 11. Jeppesen PB. Gut hormones in the treatment of short-bowel syndrome and intestinal failure. Curr Opin Endocrinol Diabetes Obes. 2015;22(1):14–20. [DOI] [PubMed] [Google Scholar]
- 12. Munroe DG, Gupta AK, Kooshesh F, et al. . Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1999;96(4):1569–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yusta B, Huang L, Munroe D, et al. . Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119(3):744–755. [DOI] [PubMed] [Google Scholar]
- 14. Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci U S A. 2001;98(22):12497–12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124(1-3):105–112. [DOI] [PubMed] [Google Scholar]
- 16. Sigalet DL, Wallace LE, Holst JJ, et al. . Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G211–G221. [DOI] [PubMed] [Google Scholar]
- 17. Dubé PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology. 2006;131(2):589–605. [DOI] [PubMed] [Google Scholar]
- 18. Leen JL, Izzo A, Upadhyay C, et al. . Mechanism of action of glucagon-like peptide-2 to increase IGF-I mRNA in intestinal subepithelial fibroblasts. Endocrinology. 2011;152(2):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shawe-Taylor M, Kumar JD, Holden W, et al. . Glucagon-like petide-2 acts on colon cancer myofibroblasts to stimulate proliferation, migration and invasion of both myofibroblasts and cancer cells via the IGF pathway. Peptides. 2017;91:49–57. [DOI] [PubMed] [Google Scholar]
- 20. Rowland KJ, Trivedi S, Lee D, et al. . Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology. 2011;141(6):2166–2175.e7. [DOI] [PubMed] [Google Scholar]
- 21. Markovic MA, Brubaker PL. The roles of glucagon-like peptide-2 and the intestinal epithelial insulin-like growth factor-1 receptor in regulating microvillus length. Sci Rep. 2019;9(1):13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong CX, Zhao W, Solomon C, et al. . The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology. 2014;155(2):370–379. [DOI] [PubMed] [Google Scholar]
- 23. Yusta B, Holland D, Koehler JA, et al. . ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology. 2009;137(3):986–996. [DOI] [PubMed] [Google Scholar]
- 24. Feng Y, Demehri FR, Xiao W, et al. . Interdependency of EGF and GLP-2 signaling in attenuating mucosal atrophy in a mouse model of parenteral nutrition. Cell Mol Gastroenterol Hepatol. 2017;3(3):447–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duncan MD, Korman LY, Bass BL. Epidermal growth factor primes intestinal epithelial cells for proliferative effect of insulin-like growth factor I. Dig Dis Sci. 1994;39(10):2197–2201. [DOI] [PubMed] [Google Scholar]
- 26. Simmons JG, Hoyt EC, Westwick JK, Brenner DA, Pucilowska JB, Lund PK. Insulin-like growth factor-I and epidermal growth factor interact to regulate growth and gene expression in IEC-6 intestinal epithelial cells. Mol Endocrinol. 1995;9(9):1157–1165. [DOI] [PubMed] [Google Scholar]
- 27. Austin K, Tsang D, Chalmers JA, Maalouf MF, Brubaker PL. Insulin-like growth factor-binding protein-4 inhibits epithelial growth and proliferation in the rodent intestine. Am J Physiol Gastrointest Liver Physiol. 2018;315(2):G206–G219. [DOI] [PubMed] [Google Scholar]
- 28. Krane JF, Murphy DP, Carter DM, Krueger JG. Synergistic effects of epidermal growth factor (EGF) and insulin-like growth factor I/somatomedin C (IGF-I) on keratinocyte proliferation may be mediated by IGF-I transmodulation of the EGF receptor. J Invest Dermatol. 1991;96(4):419–424. [DOI] [PubMed] [Google Scholar]
- 29. Drucker DJ, DeForest L, Brubaker PL. Intestinal response to growth factors administered alone or in combination with human [Gly2]glucagon-like peptide 2. Am J Physiol. 1997;273(6):G1252–G1262. [DOI] [PubMed] [Google Scholar]
- 30. Kitchen PA, Fitzgerald AJ, Goodlad RA, et al. . Glucagon-like peptide-2 increases sucrase-isomaltase but not caudal-related homeobox protein-2 gene expression. Am J Physiol Gastrointest Liver Physiol. 2000;278(3):G425–G428. [DOI] [PubMed] [Google Scholar]
- 31. Lim DW, Levesque CL, Vine DF, et al. . Synergy of glucagon-like peptide-2 and epidermal growth factor coadministration on intestinal adaptation in neonatal piglets with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2017;312(4):G390–G404. [DOI] [PubMed] [Google Scholar]
- 32. RRID:MGI:6377558.
- 33. el Marjou F, Janssen KP, Chang BH, et al. . Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186–193. [DOI] [PubMed] [Google Scholar]
- 34. Kappeler L, De Magalhaes Filho C, Dupont J, et al. . Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. Plos Biol. 2008;6(10):e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bohin N, Carlson EA, Samuelson LC. Genome toxicity and impaired stem cell function after conditional activation of CreERT2 in the INTESTINE. Stem Cell Reports. 2018;11(6):1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smither BR, Pang HY, Brubaker PL. Glucagon-like peptide-2 requires a full complement of Bmi-1 for its proliferative effects in the murine small intestine. Endocrinology. 2016;157(7):2660–2670. [DOI] [PubMed] [Google Scholar]
- 37. Mahe MM, Aihara E, Schumacher MA, et al. . Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol. 2013;3(4):217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269(5228):1270–1272. [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 40. Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124(1-2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grün D, Lyubimova A, Kester L, et al. . Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525(7568):251–255. [DOI] [PubMed] [Google Scholar]
- 42. Kim TH, Saadatpour A, Guo G, et al. . Single-cell transcript profiles reveal multilineage priming in early progenitors derived from Lgr5(+) intestinal stem cells. Cell Rep. 2016;16(8):2053–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yan KS, Gevaert O, Zheng GXY, et al. . Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. 2017;21(1):78–90.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong RW. Transgenic and knock-out mice for deciphering the roles of EGFR ligands. Cell Mol Life Sci. 2003;60(1):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kitchen PA, Goodlad RA, FitzGerald AJ, et al. . Intestinal growth in parenterally-fed rats induced by the combined effects of glucagon-like peptide 2 and epidermal growth factor. JPEN J Parenter Enteral Nutr. 2005;29(4):248–254. [DOI] [PubMed] [Google Scholar]
- 46. Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell. 2017;20:177–190 e174. [DOI] [PubMed] [Google Scholar]
- 47. Van Landeghem L, Santoro MA, Mah AT, et al. . IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J. 2015;29(7):2828–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277(2):C183–C201. [DOI] [PubMed] [Google Scholar]