Abstract
Airway inflammation is a key aspect of diseases such as asthma. Proinflammatory cytokines such as TNFα mediate the inflammatory response. In various diseases, inflammation leads to endoplasmic reticulum (ER) stress, the accumulation of unfolded proteins, which triggers homeostatic responses to restore normal cellular function. We hypothesized that TNFα triggers ER stress through an increase in reactive oxygen species generation in human airway smooth muscle (hASM) with a downstream effect on mitofusin 2 (Mfn2). In hASM cells isolated from lung specimens incidental to patient surgery, dose- and time-dependent effects of TNFα exposure were assessed. Exposure of hASM to tunicamycin was used as a positive control. Tempol (500 μM) was used as superoxide scavenger. Activation of three ER stress pathways were evaluated by Western blotting: 1) autophosphorylation of inositol-requiring enzyme1 (IRE1α) leading to splicing of X-box binding protein 1 (XBP1); 2) autophosphorylation of protein kinase RNA-like endoplasmic reticulum kinase (PERK) leading to phosphorylation of eukaryotic initiation factor 2α; and 3) translocation and cleavage of activating transcription factor 6 (ATF6). We found that exposure of hASM cells to tunicamycin activated all three ER stress pathways. In contrast, TNFα selectively activated the IRE1α/XBP1 pathway in a dose- and time-dependent fashion. Our results indicate that TNFα does not activate the PERK and ATF6 pathways. Exposure of hASM cells to TNFα also decreased Mfn2 protein expression. Concurrent exposure to TNFα and tempol reversed the effect of TNFα on IRE1α phosphorylation and Mfn2 protein expression. Selective activation of the IRE1α/XBP1 pathway in hASM cells after exposure to TNFα may reflect a unique homeostatic role of this pathway in the inflammatory response of hASM cells.
Keywords: airway smooth muscle, asthma, ER stress, inflammation, TNFα
INTRODUCTION
The accumulation of unfolded proteins in the endoplasmic reticulum (ER) triggers a homeostatic response to restore normal cellular function (4, 19, 26, 48). Within the ER, the molecular chaperone BiP/Grp78 serves as a sensor of protein unfolding. As unfolded proteins accumulate, more of the available BiP/Grp78 is required to bind to the exposed hydrophobic regions of these proteins, and as a result BiP/Grp78 dissociates from binding sites on three primary signaling proteins, which results in their activation. These ER stress signaling pathways include 1) autophosphorylation of inositol-requiring enzyme1 (IRE1α) with downstream splicing of the transcription factor X-box binding protein 1 (XBP1); 2) autophosphorylation of protein kinase RNA-like endoplasmic reticulum kinase (PERK), which mediates downstream phosphorylation of eukaryotic initiation factor 2α (eIF2α); and 3) translocation of activating transcription factor 6 (ATF6) to the Golgi, where it is cleaved by proteases to form an active 50 kDa transcription factor. Activation of the IRE1α/XBP1 pathway has been shown to be involved in cell survival, apoptosis, chaperone protein production, and inflammation. Activation of PERK/eIF2α and ATF6 pathways lead to inhibition of protein translation and transcription of chaperone proteins, respectively (10, 20, 46, 62).
In other cell types, TNFα has been shown to selectively activate one or more of the ER stress pathways (14, 29, 69). However, full characterization of a TNFα-mediated ER stress response in airway smooth muscle (ASM) cells is lacking. An association between increased TNFα and ER stress has been reported in mouse lungs following challenges with ovalbumin and/or lipopolysaccharide (34, 40). However, in these studies it was unclear which ER stress pathway was induced by TNFα. It was also reported that the proinflammatory cytokine IL-25 induces ER stress in human airway epithelial cells (70). However, no study to date has explored inflammation-mediated ER stress in ASM cells, despite the major role of inflammation in contributing to both exaggerated airway narrowing and cell proliferation (8, 30, 33, 52, 67, 68). Inflammation induces ER stress, most likely as a consequence of increased reactive oxygen species (ROS) formation (1, 5, 20), although it is unclear whether ROS is the only mechanism involved. Mitochondria are a major source of ROS formation, a by-product of oxidative phosphorylation. Because mitochondria interact both physiologically and functionally with the ER, perturbation in one organelle is believed to impact the other (9, 11, 16, 55, 65). The relationship between ER stress and mitofusin 2 (Mfn2), implicated in mitochondria morphology and ER-mitochondria junctions, is of particular interest and has been studied in many cell types but has not in hASM. Previous studies in other cell types have suggested that Mfn2 and altered mitochondrial dynamics are upstream to ER stress such that a reduction in Mfn2 triggers ER stress (7, 45, 47, 63). In asthmatic ASM, we found that Mfn2 expression was reduced and we suggested that inflammation may play a role in this effect, potentially leading to increased ROS formation (2).
In the present study, we hypothesized that exposure to TNFα induces ER stress in human ASM (hASM) by activating the IRE1α, PERK, and ATF6 pathways with downstream effect on Mfn2. To test this hypothesis, acutely dissociated hASM cells were exposed to tunicamycin as a positive control for ER stress followed by assessment of dose- and time-dependent effects of TNFα exposure.
METHODS
Isolation of hASM cells.
hASM cells were enzymatically dissociated from 3rd to 6th generation bronchi of lung samples incidental to patient surgery at the Mayo Clinic (approved and considered exempt by the Mayo Institutional Review Board). ASM tissue was dissected, and cells were dissociated using papain and collagenase with ovomucoid/albumin separation as per the manufacturer's instructions (Worthington Biochemical, Lakewood, NJ), previously described (53, 54). Cells were maintained at 37°C (5% CO2–95% air) in DMEM-10% FBS-1% ABAM medium (Invitrogen, Carlsbad, CA) until ~80% confluent. All experiments were performed in cells from passages 1–3 of subculture and serum-deprived at least for 24 h before all experiments. Assessment of hASM phenotype in acutely isolated cells and cells from passages 1–3 of subculture was confirmed as previously described (3, 31). Expression of smooth muscle actin and myosin, vimentin, and muscarinic receptor (M3 subtype), and the absence of fibroblast surface protein (FSP) expression was performed using Western blot analyses. In addition, immunofluorescence was used to estimate the number of cells expressing smooth muscle actin and the muscarinic receptor. The number of cells exhibiting a fusiform shape typical of hASM cells was also estimated before maintaining or using cells for experiments.
Exposure of hASM cells to TNFα and tunicamycin.
Dose-dependent effects of TNFα were assessed by exposing hASM cells to 5, 10, 20, 50, or 100 ng/mL TNFα for 12 h. Time-dependent of effects of TNFα (50 ng/mL) were assessed by exposing hASM cells for 3, 6, 12, or 24 h. Exposure to tunicamycin (2 µg/mL) for 3, 6, and 12 h was used as an ER stress-positive control. Untreated controls for each time point were performed. The superoxide scavenger tempol (100 µM) was used with concurrent TNFα exposure.
Western blotting.
Expression of key proteins involved in the ER stress pathways was evaluated by Western blotting. Cell samples were lysed in 1× LDH Assay Lysis Buffer containing protease and phosphatase inhibitors. Protein concentration was determined using a Lowry assay (Bio-Rad, Berkeley, CA). One hundred micrograms of protein was denatured in 1× Laemmli sample buffer with β-mercaptoethanol at 100°C for 3 min, separated by SDS-PAGE, and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Phosphorylated IRE1α (Abcam catalog no. ab124945, RRID:AB_11001365), XBP1 (Abcam catalog no. ab37152, RRID:AB_778939), phosphorylated eIF2 α (ab32157, RRID:AB_732117), and total eIF2α (ab157478) were detected with specific antibodies obtained from Abcam (Cambridge, UK). Total IRE1α (Cell Signaling Technology Cat no. 3294, RRID:AB_823545) and total PERK (Cell Signaling Technology Cat no. 3192, RRID:AB_2095847) were detected with specific antibodies obtained from Cell Signaling (Danvers, MA). ATF6 (Novus Cat no. NBP1–40256, RRID:AB_2058774) was detected with specific antibodies obtained from Novus Biologicals (Centennial, CO). Phosphorylated IRE1α antibody was validated in a previous study by Lee et al. (36), XBP1 antibody was validated in a previous study by Ming et al. (44), phosphorylated eIf2α antibody was validated in a previous study by Park et al. (49) and ATF6 antibody was validated in a previous study by Krawczyk et al. (35). It should be noted that we were not able to find an antibody for phosphorylated PERK that was previously validated or that we could validate. Mfn2 (Abcam Cat no. ab56889, RRID:AB_2142629) was detected with specific antibodies obtained from Abcam and was validated in a previous study by Aravamudan et al. (2). Ribosomal protein S16 (Abcam catalog no. ab26159, RRID:AB_470808) was used as a loading control. Antibodies were diluted 1:1,000 in 5% milk/TBS-T and visualized with chemiluminescent substrate SuperSignal West Dura Extended Duration Substrate (ThermoFisher Scientific, Rockford, IL). Bands were quantified with Molecular Imaging Software (Version 4.0.1, Kodak). Primary and secondary antibodies were removed with Restore Western Blot Stripping Buffer (ThermoFisher Scientific), and membranes were reprobed for specific ER stress targets.
Mitochondrial superoxide imaging.
ASM cells cultured in eight-well plates were loaded with Mitochondrial Superoxide Indicator MitoSOX Red (ThermoFisher Scientific) for 5 min at room temperature followed by extensive washing in dye-free solution. ASM cells were visualized using a Nikon Eclipse A1 laser-scanning confocal system (Nikon Instruments, Melville, NY) using a ×60/1.4 NA oil-immersion lens (2, 13).
Statistical analysis.
Experiments were performed using hASM cells obtained from a total of 12 patients. hASM cells from at least five patients (n = 5) were used for each set of experiments, with hASM cells from the same patient assigned to both untreated control and experimental groups. Statistical analyses were performed using JMP Pro software (JMP, RRID: SCR_014242). Results were analyzed using a two-way ANOVA. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Significance was considered at P < 0.05.
RESULTS
hASM phenotype.
Cells used for experiments and subculture overwhelmingly (~98%) exhibited a fusiform shape typical of hASM cells. Cells also showed sustained expression of smooth muscle actin and myosin, vimentin, and muscarinic receptor (M3 subtype). By contrast, FSP expression was negligible. Using immunofluorescence, we estimated that ~95% of cells expressed smooth muscle actin and the muscarinic receptor.
Tunicamycin activated the IRE1α/XBP1, PERK/eIF2α, and ATF6 ER stress pathways.
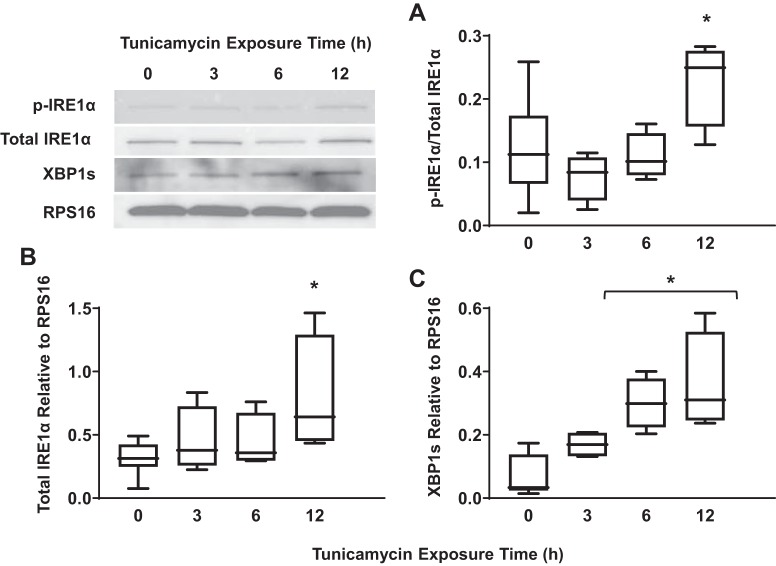
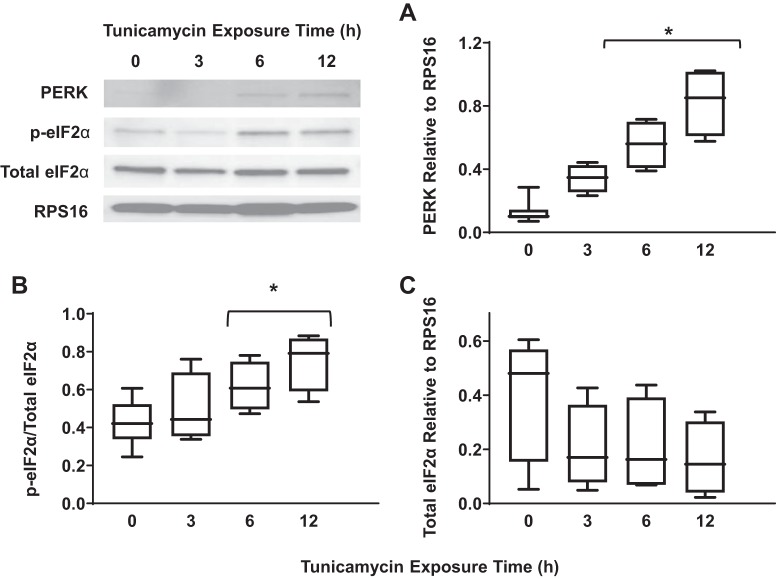
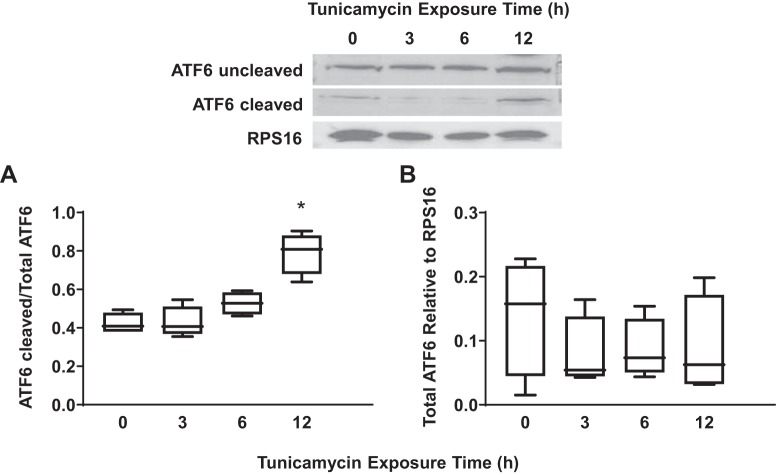
Exposure of hASM cells to tunicamycin was used as a positive control for ER stress as indicated by a significant increase in IRE1α phosphorylation (Fig. 1A) (n = 5, P < 0.05), total IRE1α expression (Fig. 1B) (n = 5, P < 0.05), and XBP1 splicing (Fig. 1C) (n = 5, P < 0.05) after 12 h. Total PERK expression (Fig. 2A) (n = 5, P < 0.05) and the ratio of phosphorylated to total eIF2α (Fig. 2B) (n = 5, P < 0.05) increased significantly after exposure to tunicamycin for 6 h and peaked after 12 h of exposure. However, we could not validate PERK phosphorylation using commercially available antibodies. Finally, tunicamycin exposure significantly increased ATF6 cleavage after 12 h (Fig. 3A) (n = 5, P < 0.05).
Fig. 1.
Exposure to tunicamycin activates the inositol-requiring enzyme1 (IRE1α)/X-box binding protein 1 (XBP1) pathway in airway smooth muscle (ASM) cells. A: 12-h exposure to tunicamycin significantly increased the ratio of phosphorylated to total IRE1α in ASM cells compared with untreated controls. Representative Western blots are shown. B: 12-h exposure to tunicamycin also significantly increased total IRE1α expression [relative to ribosomal protein S16 (RPS16)] in ASM cells compared with untreated controls. Representative Western blots are shown. C: splicing of XBP1 (relative to RPS16) increased significantly after exposure to tunicamycin for 6 and 12 h in ASM cells compared with untreated controls. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. Untreated controls for each time point were performed. *Significant difference (P < 0.05) compared with untreated control (n = 5 patients).
Fig. 2.
Exposure to tunicamycin activates the protein kinase RNA-like endoplasmic reticulum kinase (PERK)/ eukaryotic initiation factor 2α (eIF2α) pathway in airway smooth muscle (ASM) cells. A: 12-h exposure to tunicamycin significantly increased total PERK expression [relative to ribosomal protein S16 (RPS16)] in ASM cells compared with untreated controls. Representative Western blots are shown. B: 12-h exposure to tunicamycin significantly increased the ratio of phosphorylated to total eIF2α in ASM cells compared with untreated controls. Representative Western blots are shown. C: exposure to tunicamycin did not significantly change total eIF2α expression (relative to RPS16) in ASM cells compared with untreated controls. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. Untreated controls for each time point were performed. *Significant difference (P < 0.05) compared with untreated control (n = 5 patients).
Fig. 3.
Exposure to tunicamycin activates the activating transcription factor 6 (ATF6) pathway in airway smooth muscle (ASM) cells. A: 12-h exposure to tunicamycin significantly increased the ratio of cleaved to uncleaved ATF6 in ASM cells compared with untreated controls. Representative Western blots are shown. B: exposure to tunicamycin did not significantly change total ATF6 expression [relative to ribosomal protein S16 (RPS16)] in ASM cells compared with untreated controls. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. Untreated controls for each time point were performed. *Significant difference (P < 0.05) compared with untreated control (n = 5 patients).
TNFα activated the IRE1α/XBP1 ER stress pathway.
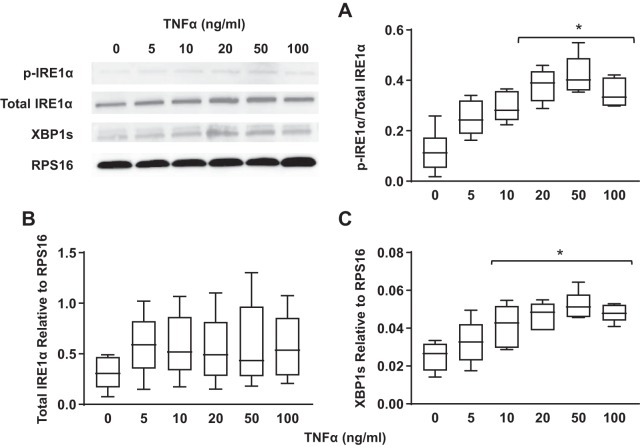
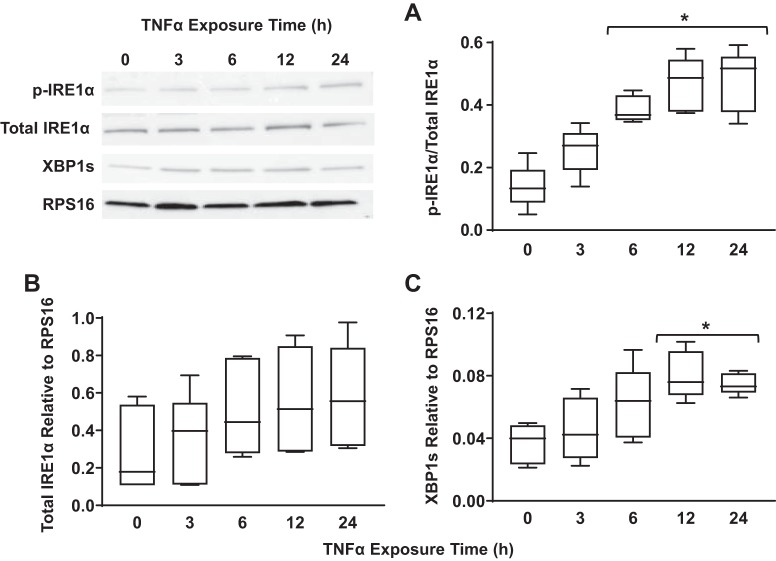
Concentration- and time-dependent activation of the IRE1α/XBP1 pathway in hASM cells in response to TNFα was assessed by measuring the ratio of phosphorylated to total IRE1α, total IRE1α expression, and expression of spliced XBP1, a downstream target of IRE1α. Exposing hASM cells to TNFα at 20, 50, or 100 ng/mL for 12 h increased the ratio of phosphorylated to total IRE1α. There was a concentration dependency of this ER stress response to TNFα with a significant increase in IRE1α phosphorylation at 20 ng/mL, which peaked at 50 ng/mL (Fig. 4A) (n = 5, P < 0.05). In addition, we found that the ER stress response induced by TNFα was time dependent. Exposure to 50 ng/mL TNFα induced a significant increase in IRE1α phosphorylation after 6 h, which peaked after 24 h (Fig. 5A) (n = 5, P < 0.05).
Fig. 4.
TNFα activates the inositol-requiring enzyme1 (IRE1α)/X-box binding protein 1 (XBP1) pathway in airway smooth muscle (ASM) cells in a concentration-dependent fashion. A: exposure to TNFα at 20, 50, and 100 ng/mL (12-h) significantly increased the ratio of phosphorylated (p) to total IRE1α in ASM cells compared with untreated controls. Representative Western blots are shown. B: exposure to TNFα did not change total IRE1α expression [relative to ribosomal protein S16 (RPS16)} in ASM cells compared with untreated controls. Representative Western blots are shown. C: splicing of XBP1 (relative to RPS16) increased significantly after exposure to TNFα at 50 and 100 ng/mL in ASM cells compared with untreated controls. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. *Significant difference (P < 0.05) compared with untreated control (n = 5 patients).
Fig. 5.
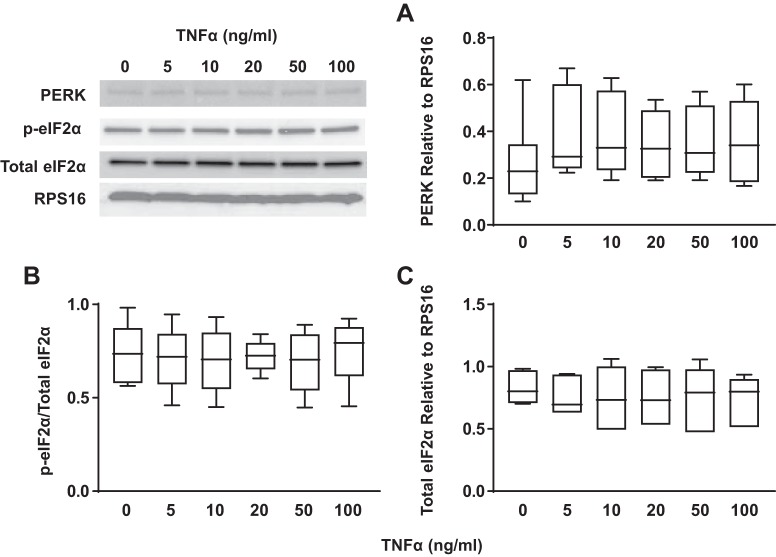
TNFα did not activate the protein kinase RNA-like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2α (eIF2α) pathway in airway smooth muscle (ASM) cells in a concentration-dependent fashion. A: exposure to different concentrations of TNFα did not significantly change total PERK expression [relative to ribosomal protein S16 (RPS16)} in ASM cells compared with untreated controls. B: exposure to different concentrations of TNFα did not significantly change the ratio of phosphorylated to total eIF2α in ASM cells compared with untreated controls. Representative Western blots are shown. C: exposure to different concentrations of TNFα did not significantly change total eIF2α expression (relative to RPS16) in ASM cells compared with untreated controls. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. Untreated controls for each time point were performed. *Significant difference (P < 0.05) compared with untreated control (n = 5 patients).
The TNFα-induced increase in IRE1α phosphorylation was associated with a concentration- and time-dependent increase in the expression of spliced XBP1 in hASM cells. The expression of spliced XBP1 relative to ribosomal protein S16 (RPS16) significantly increased after 12-h exposure to 20 ng/mL TNFα and peaked at 50 ng/mL (Fig. 4C) (n = 5, P < 0.05). Similar to IRE1α phosphorylation, exposing hASM cells to 50 ng/mL TNFα induced a significant increase in the expression of spliced XBP1 relative to RPS16, with this response peaking at 12 h (Fig. 5C) (n = 5, P < 0.05).
TNFα does not activate the PERK/eIF2α ER stress pathway.
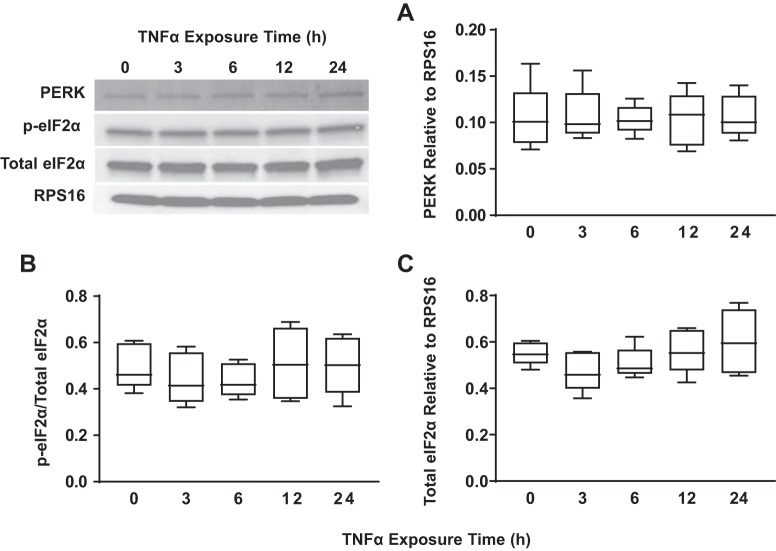
Concentration- and time-dependent activation of the PERK/eIF2α ER stress pathway in hASM cells in response to TNFα was assessed by measuring total PERK expression and phosphorylation of eIF2α, a downstream target of PERK. Exposing hASM cells to TNFα (concentrations ranging from 5 to100 ng/mL) for 12 h did not affect expression of total PERK relative to RPS16 (Fig. 6A) (n = 5), nor did it affect the ratio of phosphorylated to total eIF2α (Fig. 6B) (n = 5) or expression of total eIF2α relative to RPS16 (Fig. 6C) (n = 5) compared with untreated control hASM cells. Similarly, exposing hASM cells to 50 ng/mL TNFα for periods ranging from 3 to 24 h did not affect the expression of PERK relative to RPS16 (Fig. 7A) (n = 5), the ratio of phosphorylated to total eIF2α (Fig. 7B) (n = 5) or expression of total eIF2α relative to RPS16 (Fig. 7C) (n = 5).
Fig. 6.
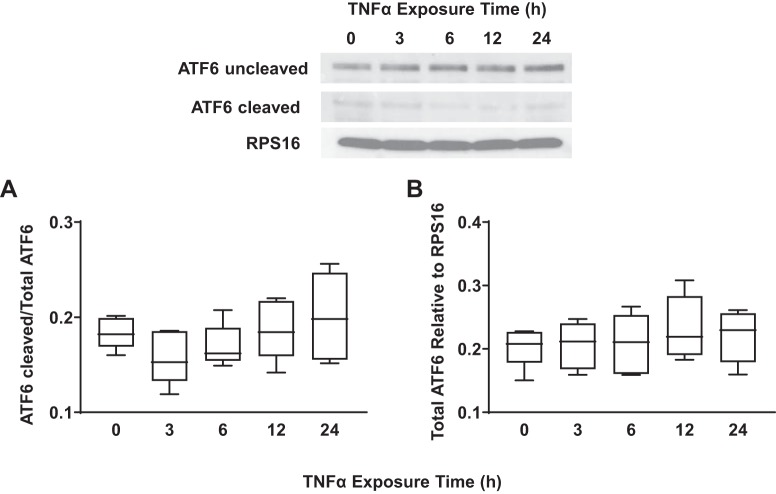
TNFα did not activate the activating transcription factor 6 (ATF6) pathway in airway smooth muscle (ASM) cells in a concentration-dependent fashion. A: exposure to different concentrations of TNFα did not significantly change the ratio of cleaved to uncleaved ATF6 in ASM cells compared with untreated controls. Representative Western blots are shown. B: exposure to different concentrations of TNFα did not significantly change total ATF6 expression [relative to ribosomal protein S16 (RPS16)] in ASM cells compared with untreated controls. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA.
Fig. 7.
TNFα activates the inositol-requiring enzyme1 (IRE1α)/X-box binding protein 1 (XBP1) pathway in airway smooth muscle (ASM) cells in a time-dependent fashion. A: exposure to TNFα for 6, 12, and 24 h (50 ng/mL) significantly increased the ratio of phosphorylated to total IRE1α in ASM cells compared with untreated controls. Representative Western blots are shown. B: exposure to TNFα at different times did not significantly change total IRE1α expression [relative to ribosomal protein S16 (RPS16)} in ASM cells compared with untreated controls. Representative Western blots are shown. C: splicing of XBP1 (relative to RPS16) increased significantly after exposure to TNFα at 12 and 24 h (50 ng/mL) in ASM cells compared with untreated controls. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. Untreated controls for each time point were performed.
TNFα does not activate the ATF6 ER stress pathway.
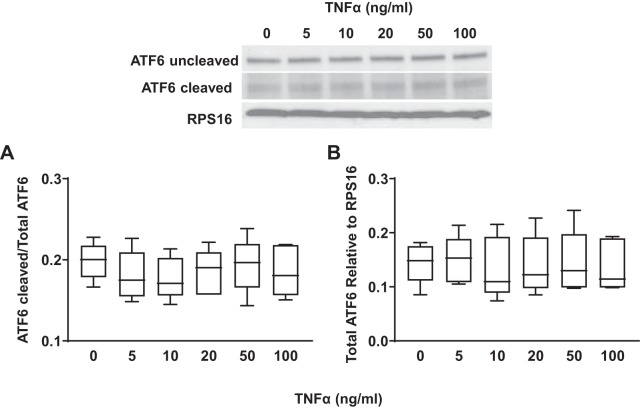
Concentration- and time-dependent activation of the ATF6 ER stress pathway in hASM cells in response to TNFα was assessed by measuring the cleavage of ATF6 from its inactive (90 kDa) to active (50 kDa) form. Exposing hASM cells to TNFα (concentrations ranging from 5 to100 ng/mL) for 12 h did not affect the expression of the cleaved form of ATF6 (Fig. 8A) (n = 5) nor expression of total ATF6 relative to RPS16 (Fig. 8B) (n = 5) compared with untreated control hASM cells. Exposing hASM cells to 50 ng/mL TNFα for 3 to 24 h did not affect the expression of the cleaved form of ATF6 (Fig. 9A) (n = 5) nor the expression of total ATF6 relative to RPS16 (Fig. 9B) (n = 5).
Fig. 8.
TNFα did not activate the protein kinase RNA-like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2α (eIF2α) pathway in airway smooth muscle (ASM) cells in a time-dependent fashion. A: exposure to TNFα at different time points did not significantly change total PERK expression [relative to ribosomal protein S16 (RPS16)] in ASM cells compared with untreated controls. B: exposure to TNFα at different time points did not significantly change the ratio of phosphorylated to total eIF2α in ASM cells compared with untreated controls. Representative Western blots are shown. C: exposure to TNFα at different time points did not significantly change total eIF2α expression (relative to RPS16) in ASM cells compared with untreated controls. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA.
Fig. 9.
TNFα did not activate the activating transcription factor 6 (ATF6) pathway in airway smooth muscle (ASM) cells in a time-dependent fashion. A: exposure to TNFα at different time points did not significantly change the ratio of cleaved to uncleaved ATF6 in ASM cells compared with untreated controls. Representative Western blots are shown. B: exposure to TNFα at different time points did not significantly change total ATF6 expression [relative to ribosomal protein S16 (RPS16)] in ASM cells compared with untreated controls. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. Untreated controls for each time point were performed.
TNFα induces superoxide generation.
Exposure of hASM cells to tunicamycin (2 µg/mL) or TNFα (20 ng/mL) for 12 h significantly increased superoxide generation compared with untreated control hASM cells (Fig. 10) (n = 5, P < 0.05). The tunicamycin-induced increase in superoxide was significantly higher than for TNFα-exposed hASM cells (Fig. 10) (n = 5, P < 0.05).
Fig. 10.
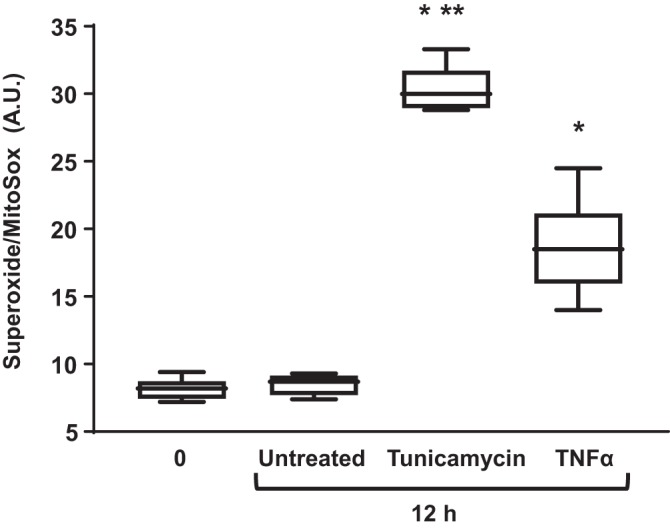
TNFα increases superoxide generation in airway smooth muscle (ASM) cells. Exposure to TNFα (50 ng/mL) or tunicamycin (2 µg/mL) for 12 h significantly increased superoxide generation in ASM cells compared with ASM cell control or untreated time-matched control ASM cells. *Significant difference (P < 0.05) compared with untreated control (n = 5 patients). **Significant difference (P < 0.05) compared with TNFα (n = 5 patients).
Tempol reduces the effect of TNFα on IRE1α phosphorylation.
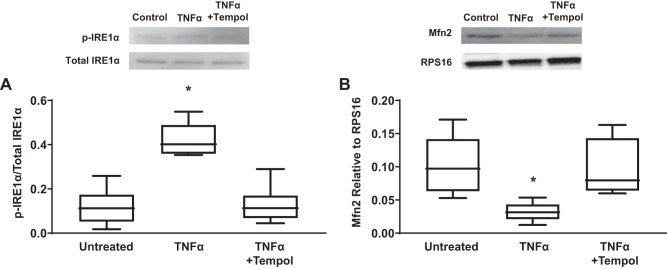
The TNFα-induced increase in IRE1α phosphorylation (20 ng/mL, 12 h) was blunted by concurrent exposure of hASM cells to the superoxide scavenger tempol (500 µM, 12 h) (Fig. 11A) (n = 5, P < 0.05).
Fig. 11.
Tempol reduces TNFα-induced increase in inositol-requiring enzyme1 (IRE1α) phosphorylation and reduction in mitofusin 2 (Mfn2) expression in airway smooth muscle (ASM) cells compared with untreated control ASM cells. A: exposure to TNFα for 12 h (50 ng/mL) significantly increased the ratio of phosphorylated to total IRE1α in ASM cells compared with untreated controls, while concomitant exposure with antioxidant tempol reduced the effect of TNFα. Representative Western blots are shown. B: exposure to TNFα for 12 h (50 ng/mL) significantly decreased Mfn2 expression [relative to ribosomal protein S16 (RPS16)] in ASM cells compared with untreated controls, while concomitant exposure with antioxidant tempol reduced the effect of TNFα. Representative Western blots are shown. Data are presented as medians and interquartile range (IQR) represented as a box-and-whisker plot. Results were analyzed using a 2-way ANOVA. *Significant difference (P < 0.05) compared with untreated control (n = 5 patients).
TNFα reduces Mfn2 expression and this effect is blunted by tempol.
Exposure of hASM cells to TNFα (20 ng/mL) for 12 h significantly reduces Mfn2 expression (Fig. 11B) (n = 5, P < 0.05). This effect was blunted by concurrent exposure of hASM cells to the superoxide scavenger tempol (500 µM, 12 h) (Fig. 11B) (n = 5, P < 0.05).
DISCUSSION
The results of the present study showed that exposing hASM cells to TNFα selectively activates the IRE1α/XBP1 ER stress pathway in both a concentration- and time-dependent fashion. Activation of the IRE1α/XBP1 pathway in hASM cells was evidenced by an increase in IRE1α phosphorylation and expression of spliced XBP1. We found that both IRE1α phosphorylation and expression of spliced XBP1 peaked in response to 50 ng/mL TNFα and that the response to 50 ng/mL TNFα peaked after 24-h exposure. Importantly, TNFα induced a selective IRE1α/XBP1 ER stress response and did not activate either the PERK/eIF2α or ATF6 ER stress pathways. As a positive control, we confirmed that all three ER stress pathways were activated in hASM cells by tunicamycin in a time-dependent fashion. We also found that TNFα induced superoxide generation, and the superoxide scavenger tempol reversed the effect of TNFα on IRE1α phosphorylation. Importantly, TNFα reduced Mfn2 expression, and this was also blunted by tempol.
Selective ER stress response.
The ER stress response is ubiquitous across a variety of cell types and plays an important homeostatic role in responding to inflammation (29, 37, 71). Importantly, we found that exposing hASM cells to tunicamycin activated all three ER stress signaling pathways. However, exposing hASM cells to TNFα activated only the IRE1α/XBP1 pathway, suggesting that there is selective activation of ER stress pathways that depends on the stimulus.
Stimulus-specific activation of ER stress responses has been shown in other cell types (17, 69). For example, in a study of mouse fibrosarcoma cells, tunicamycin and TNFα induced activation of all three ER stress pathways whereas arsenite induced only eIF2α phosphorylation (69). In addition, while TNFα induced ER stress in fibrosarcoma cells, TNFα did not induce ER stress in mouse embryonic fibroblasts (69), suggesting that selective ER stress activation is also dependent on cell type. Xue and colleagues (69) speculated that selectivity of ER stress was dependent on production of different ROS. They reported that TNFα primarily induced production of hydrogen peroxide, whereas arsenite primarily induced production of the superoxide anion. In a separate study, these authors found that TNFα did not induce ROS production in mouse embryonic fibroblasts (61). The results of these studies suggest that different ROS may activate different ER stress pathways. In our study, we found that TNFα selectively activated the IRE1α/XBP1 pathway. If ROS were mediating the selective activation of the IRE1α/XBP1 pathway, then we might speculate that ROS such as hydrogen peroxide or the superoxide anion trigger ER stress in a cell-specific manner. Others have also shown that ER stress activation is ROS dependent (5, 20, 27, 41, 43). In one study, lipopolysaccharide (LPS) treatment in macrophages induced selective activation of the IRE1α/XBP1 pathway through the toll-like receptors (TLRs) (43). In macrophages, TLRs have been shown to induce ROS through NADPH oxidase 2 (NOX2) (23, 43). NOX proteins transfer electrons from NADPH to oxygen to generate the superoxide anion (23, 66). In macrophages, the antioxidant apocynin mitigated IRE1α/XBP1 activation in response to LPS (43). If the superoxide anion triggered selective activation of the IRE1α/XBP1 pathway in macrophages, but not in fibrosarcoma cells, this would further suggest that specific ROS may trigger ER stress in a cell-specific manner. It is possible that one role of ER stress activation may be to inhibit ROS production.
Even if the selective activation of the IRE1α/XBP1 ER stress pathway was mediated by ROS, the mechanism underlying selective activation remains unclear. Under basal conditions, IRE1α resides on the ER membrane and its luminal domain is associated with BiP/Grp78 (38, 73). With ER stress, IRE1α disassociates from BiP/Grp78 and becomes activated via autophosphorylation (71). BiP/Grp78 dissociation is known to regulate not only IRE1α but also the other two stress sensors, PERK and ATF6 (6, 51). However, the three ER stress signaling pathways have been shown to display different sensitivities to different ER stress inducers (tunicamycin, thapsigargin, and dithiothreitol) (17). The difference in sensitivity of the three ER stress signaling pathways may reflect differences in BiP/Grp78 binding affinity to the protein sensor (i.e., IRE1α, PERK, or ATF6) and how the specific stressor affects BiP/Grp78 binding.
Selective activation of the IRE1α/XBP1 pathway.
The selective activation of the IRE1α/XBP1 pathway suggests that this pathway may mediate other downstream responses to TNFα. The transcription factor XBP1 targets many genes involved in multiple cellular functions and this response varies with cell type and condition (28, 32, 56, 57). Cell-specific XBP1 targets that have been identified include interleukin-6 (IL-6) in plasma cells, CCAAT-enhancer-binding proteins (C/EBPs) in adipocytes, lipogenic genes in hepatocytes, proinflammatory cytokines in macrophages, and the transcription factor MIST-1 (muscle, intestine and stomach expression 1) in myocytes (28). Although the IRE1α/XBP1 pathway has been well documented in many cell types, the IRE1α/XBP1 pathway is only beginning to be explored in different types of airway cells (39, 57, 58, 72). In human airway epithelial cells and human airway macrophages, XBP1 has been shown to induce secretion of interleukin-8 (IL-8) and TNFα, respectively, in cystic fibrosis airways (39, 42). However, the role of IRE1α/XBP1 in hASM cells has been largely ignored. In hASM cells, XBP1 may target genes involved in the inflammatory response to TNFα, such as hASM cell proliferation and increased hASM contractility (3, 15).
Downstream targets of selective activation of the IRE1α/XBP1 pathway.
One possible way XBP1 may mediate the effects of TNFα in hASM cells is by decreasing mitochondrial activity and thereby reducing ROS formation. Others have reported that ER stress reduces expression of mitochondria-associated ER membrane (MAM) proteins that are involved in tethering mitochondria to the ER (11, 22, 55). One MAM protein that is a potential target of XBP1 is mitofusin 2 (Mfn2), which is involved in tethering mitochondria to the ER (18, 25, 50), thereby facilitating mitochondrial Ca2+ influx through the mitochondrial Ca2+ uniporter (MCU) (21, 24, 59, 60). Mfn2 is also involved in mitochondrial fusion and, together with dynamin-related protein 1 (DRP1), which mediates mitochondrial fission, these proteins mediate dynamic mitochondrial remodeling (2, 11, 64). In a recent study, we found that exposing hASM cells to cigarette smoke reduces Mfn2 expression, resulting in mitochondrial fragmentation (2). We also reported that Mfn2 expression is reduced in asthmatic hASM (2). In the present study, we show that TNFα exposure reduces Mfn2 expression in hASM cells. Consistent with reduced Mfn2 expression, we previously found that TNFα exposure reduces mitochondrial Ca2+ influx in hASM cells in response to agonist stimulation (11, 12). However, the relationship between reduced Mfn2 expression, disrupted mitochondrial tethering to the ER, and reduced mitochondrial Ca2+ influx still needs to be clearly demonstrated. A disrupted mitochondrial tethering to the ER would likely affect mitochondrial function and affect ROS formation. Indeed, we show in this study that 24-h TNFα exposure increases superoxide generation in hASM. Importantly, the superoxide scavenger tempol blunted the effects of TNFα on both IRE1α phosphorylation and reduced Mfn2 expression. These results suggest that reduced Mfn2 expression induced by TNFα is a potential downstream target of the IRE1α/XBP1 pathway.
Summary and conclusions.
Our study is the first to characterize ER stress in hASM. We found that TNFα activated the IRE1α/XBP1 ER stress pathway, suggesting this pathway’s role in the response to TNFα on hASM: enhanced contractility, ASM cell proliferation, and enhanced cytosolic calcium, etc. The exact mechanism or mechanisms by which pathways activated ER stress are still unknown and illustrate the complexity of ER stress signaling cross talk. Although the IRE1α/XBP1 pathway may contribute to the inflammatory responses generated by TNFα, the underlying mechanism remains to be established. Future studies may focus on IRE1α/XBP1 pathway involvement in mitochondrial dysfunction, including disruption of mitochondrion-ER coupling, decreased mitochondrial Ca2+ buffering, mitochondrial fragmentation, and increased cell proliferation, all of which could contribute to the pathology of asthma.
GRANTS
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute Grants RO1HL126451 and RO1HL150890 to G. C. Sieck.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.Y., X.C., P.F.D., and G.C.S. conceived and designed research; J.Y. and X.C. performed experiments; J.Y. and X.C. analyzed data; J.Y., P.F.D., and G.C.S. interpreted results of experiments; J.Y. prepared figures; J.Y. drafted manuscript; J.Y., P.F.D., and G.C.S. edited and revised manuscript; J.Y., X.C., P.F.D., and G.C.S. approved final version of manuscript.
REFERENCES
- 1.Adolph TE, Niederreiter L, Blumberg RS, Kaser A. Endoplasmic reticulum stress and inflammation. Dig Dis 30: 341–346, 2012. doi: 10.1159/000338121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravamudan B, Kiel A, Freeman M, Delmotte P, Thompson M, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 306: L840–L854, 2014. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med 16: 812–823, 2012. doi: 10.1111/j.1582-4934.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291: H1411–H1420, 2006. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baban B, Liu JY, Mozaffari MS. Endoplasmic reticulum stress response and inflammatory cytokines in type 2 diabetic nephropathy: role of indoleamine 2,3-dioxygenase and programmed death-1. Exp Mol Pathol 94: 343–351, 2013. doi: 10.1016/j.yexmp.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332, 2000. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari P, Song M, Dorn GW II. Dissociation of mitochondrial from sarcoplasmic reticular stress in Drosophila cardiomyopathy induced by molecularly distinct mitochondrial fusion defects. J Mol Cell Cardiol 80: 71–80, 2015. doi: 10.1016/j.yjmcc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black JL, Panettieri RA Jr, Banerjee A, Berger P. Airway smooth muscle in asthma: just a target for bronchodilation? Clin Chest Med 33: 543–558, 2012. doi: 10.1016/j.ccm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AF, Rothermel BA, Lavandero S. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol 44: 16–20, 2012. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF, Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol 301: 215–290, 2013. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmotte P, Sieck GC. Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca2+ regulation in airway smooth muscle (ASM). Can J Physiol Pharmacol 93: 97–110, 2015. doi: 10.1139/cjpp-2014-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmotte P, Yang B, Thompson MA, Pabelick CM, Prakash YS, Sieck GC. Inflammation alters regional mitochondrial Ca2+ in human airway smooth muscle cells. Am J Physiol Cell Physiol 303: C244–C256, 2012. doi: 10.1152/ajpcell.00414.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmotte P, Zavaletta VA, Thompson MA, Prakash YS, Sieck GC. TNF-α decreases mitochondrial movement in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 313: L166–L176, 2017. doi: 10.1152/ajplung.00538.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denis RG, Arruda AP, Romanatto T, Milanski M, Coope A, Solon C, Razolli DS, Velloso LA. TNF-α transiently induces endoplasmic reticulum stress and an incomplete unfolded protein response in the hypothalamus. Neuroscience 170: 1035–1044, 2010. doi: 10.1016/j.neuroscience.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Dogan M, Han YS, Delmotte P, Sieck GC. TNF-α enhances force generation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 312: L994–L1002, 2017. doi: 10.1152/ajplung.00550.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorn GW II, Maack C. SR and mitochondria: calcium cross-talk between kissing cousins. J Mol Cell Cardiol 55: 42–49, 2013. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 17.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell 17: 3095–3107, 2006. doi: 10.1091/mbc.e06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filadi R, Theurey P, Pizzo P. The endoplasmic reticulum-mitochondria coupling in health and disease: molecules, functions and significance. Cell Calcium 62: 1–15, 2017. doi: 10.1016/j.ceca.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Forman MS, Lee VM, Trojanowski JQ. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci 26: 407–410, 2003. doi: 10.1016/S0166-2236(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 20.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med 18: 589–598, 2012. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38: 280–290, 2010. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Gilady SY, Bui M, Lynes EM, Benson MD, Watts R, Vance JE, Simmen T. Ero1alpha requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM). Cell Stress Chaperones 15: 619–629, 2010. doi: 10.1007/s12192-010-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandvaux N, Soucy-Faulkner A, Fink K. Innate host defense: Nox and Duox on phox’s tail. Biochimie 89: 1113–1122, 2007. doi: 10.1016/j.biochi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium 28: 285–296, 2000. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 25.Hajnóczky G, Csordás G, Yi M. Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium 32: 363–377, 2002. doi: 10.1016/S0143416002001872. [DOI] [PubMed] [Google Scholar]
- 26.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163, 2001. doi: 10.1016/S1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 27.Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, McDonald V, Florin TH, McGuckin MA. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 144: 357–368.e9, 2013. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Sun S, Sha H, Liu Z, Yang L, Xue Z, Chen H, Qi L. Emerging roles for XBP1, a sUPeR transcription factor. Gene Expr 15: 13–25, 2010. doi: 10.3727/105221610X12819686555051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89–102, 2012. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 30.James A. Remodelling of airway smooth muscle in asthma: what sort do you have? Clin Exp Allergy 35: 703–707, 2005. doi: 10.1111/j.1365-2222.2005.02270.x. [DOI] [PubMed] [Google Scholar]
- 31.Jia L, Delmotte P, Aravamudan B, Pabelick CM, Prakash YS, Sieck GC. Effects of the inflammatory cytokines TNF-α and IL-13 on stromal interaction molecule-1 aggregation in human airway smooth muscle intracellular Ca2+ regulation. Am J Respir Cell Mol Biol 49: 601–608, 2013. doi: 10.1165/rcmb.2013-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang D, Niwa M, Koong AC. Targeting the IRE1α-XBP1 branch of the unfolded protein response in human diseases. Semin Cancer Biol 33: 48–56, 2015. doi: 10.1016/j.semcancer.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joubert P, Hamid Q. Role of airway smooth muscle in airway remodeling. J Allergy Clin Immunol 116: 713–716, 2005. doi: 10.1016/j.jaci.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 34.Kim SR, Kim DI, Kang MR, Lee KS, Park SY, Jeong JS, Lee YC. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor κB activation. J Allergy Clin Immunol 132: 1397–1408e11, 2013. doi: 10.1016/j.jaci.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 35.Krawczyk KK, Ekman M, Rippe C, Grossi M, Nilsson BO, Albinsson S, Uvelius B, Swärd K. Assessing the contribution of thrombospondin-4 induction and ATF6α activation to endoplasmic reticulum expansion and phenotypic modulation in bladder outlet obstruction. Sci Rep 6: 32449, 2016. doi: 10.1038/srep32449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Noh JY, Oh Y, Kim Y, Chang JW, Chung CW, Lee ST, Kim M, Ryu H, Jung YK. IRE1 plays an essential role in ER stress-mediated aggregation of mutant huntingtin via the inhibition of autophagy flux. Hum Mol Genet 21: 101–114, 2012. doi: 10.1093/hmg/ddr445. [DOI] [PubMed] [Google Scholar]
- 37.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 3: 399–425, 2008. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu CY, Wong HN, Schauerte JA, Kaufman RJ. The protein kinase/endoribonuclease IRE1alpha that signals the unfolded protein response has a luminal N-terminal ligand-independent dimerization domain. J Biol Chem 277: 18346–18356, 2002. doi: 10.1074/jbc.M112454200. [DOI] [PubMed] [Google Scholar]
- 39.Lubamba BA, Jones LC, O’Neal WK, Boucher RC, Ribeiro CM. X-Box-binding protein 1 and innate immune responses of human cystic fibrosis alveolar macrophages. Am J Respir Crit Care Med 192: 1449–1461, 2015. doi: 10.1164/rccm.201504-0657OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makhija L, Krishnan V, Rehman R, Chakraborty S, Maity S, Mabalirajan U, Chakraborty K, Ghosh B, Agrawal A. Chemical chaperones mitigate experimental asthma by attenuating endoplasmic reticulum stress. Am J Respir Cell Mol Biol 50: 923–931, 2014. doi: 10.1165/rcmb.2013-0320OC. [DOI] [PubMed] [Google Scholar]
- 41.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O’Neal WK, Ribeiro CM. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol 6: 639–654, 2013. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martino ME, Olsen JC, Fulcher NB, Wolfgang MC, O’Neal WK, Ribeiro CM. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem 284: 14904–14913, 2009. doi: 10.1074/jbc.M809180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11: 411–418, 2010. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ming J, Ruan S, Wang M, Ye D, Fan N, Meng Q, Tian B, Huang T. A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1. Oncotarget 6: 40692–40703, 2015. doi: 10.18632/oncotarget.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muñoz JP, Ivanova S, Sánchez-Wandelmer J, Martínez-Cristóbal P, Noguera E, Sancho A, Díaz-Ramos A, Hernández-Alvarez MI, Sebastián D, Mauvezin C, Palacín M, Zorzano A. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J 32: 2348–2361, 2013. doi: 10.1038/emboj.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muñoz JP, Zorzano A. Endoplasmic reticulum stress enters a Nogo zone. Sci Transl Med 3: 88ps26, 2011. doi: 10.1126/scitranslmed.3002708. [DOI] [PubMed] [Google Scholar]
- 47.Ngoh GA, Papanicolaou KN, Walsh K. Loss of mitofusin 2 promotes endoplasmic reticulum stress. J Biol Chem 287: 20321–20332, 2012. doi: 10.1074/jbc.M112.359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nozaki J, Kubota H, Yoshida H, Naitoh M, Goji J, Yoshinaga T, Mori K, Koizumi A, Nagata K. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic beta cells. Genes Cells 9: 261–270, 2004. doi: 10.1111/j.1356-9597.2004.00721.x. [DOI] [PubMed] [Google Scholar]
- 49.Park S, Lim Y, Lee D, Elvira R, Lee JM, Lee MR, Han J. Modulation of protein synthesis by eIF2α phosphorylation protects cell from heat stress-mediated apoptosis. Cells 7: 254, 2018. doi: 10.3390/cells7120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patergnani S, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Giorgi C, Marchi S, Missiroli S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P. Calcium signaling around Mitochondria Associated Membranes (MAMs). Cell Commun Signal 9: 19, 2011. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pincus D, Chevalier MW, Aragón T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol 8: e1000415, 2010. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol 305: L912–L933, 2013. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prakash YS, Iyanoye A, Ay B, Sieck GC, Pabelick CM. Store-operated Ca2+ influx in airway smooth muscle: Interactions between volatile anesthetic and cyclic nucleotide effects. Anesthesiology 105: 976–983, 2006. doi: 10.1097/00000542-200611000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol Cell Physiol 272: C966–C975, 1997. doi: 10.1152/ajpcell.1997.272.3.C966. [DOI] [PubMed] [Google Scholar]
- 55.Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim Biophys Acta 1833: 213–224, 2013. doi: 10.1016/j.bbamcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 56.Remondelli P, Renna M. The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front Mol Neurosci 10: 187, 2017. doi: 10.3389/fnmol.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribeiro CM, Lubamba BA. Role of IRE1α/XBP-1 in cystic fibrosis airway inflammation. Int J Mol Sci 18: 118, 2017. doi: 10.3390/ijms18010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O’neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem 280: 17798–17806, 2005. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 59.Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787: 1342–1351, 2009. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86: 369–408, 2006. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 61.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22: 3898–3909, 2003. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol 2010: 1, 2010. doi: 10.1155/2010/830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodríguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155: 172–187, 2013. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vannuvel K, Renard P, Raes M, Arnould T. Functional and morphological impact of ER stress on mitochondria. J Cell Physiol 228: 1802–1818, 2013. doi: 10.1002/jcp.24360. [DOI] [PubMed] [Google Scholar]
- 66.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428–1459, 2002. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright D, Sharma P, Ryu MH, Rissé PA, Ngo M, Maarsingh H, Koziol-White C, Jha A, Halayko AJ, West AR. Models to study airway smooth muscle contraction in vivo, ex vivo and in vitro: implications in understanding asthma. Pulm Pharmacol Ther 26: 24–36, 2013. doi: 10.1016/j.pupt.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Wright DB, Trian T, Siddiqui S, Pascoe CD, Johnson JR, Dekkers BG, Dakshinamurti S, Bagchi R, Burgess JK, Kanabar V, Ojo OO. Phenotype modulation of airway smooth muscle in asthma. Pulm Pharmacol Ther 26: 42–49, 2013. doi: 10.1016/j.pupt.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem 280: 33917–33925, 2005. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 70.Yuan X, Wang J, Li Y, He X, Niu B, Wu D, Lan N, Wang X, Zhang Y, Dai X, Wang X, Liu Z, Li G. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25 -induced endoplasmic reticulum stress in asthma. Sci Rep 8: 7950, 2018. doi: 10.1038/s41598-018-26221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng H, Wu D, Wu X, Zhang X, Zhou Q, Luo Y, Yang X, Chock CJ, Liu M, Yang XO. Leptin promotes allergic airway inflammation through targeting the unfolded protein response pathway. Sci Rep 8: 8905, 2018. doi: 10.1038/s41598-018-27278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci USA 103: 14343–14348, 2006. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]