Abstract
Transfusion of red blood cells (RBCs) is a common life-saving clinical practice in severely anemic or hemorrhagic patients; however, it may result in serious pathological complications such as transfusion-related acute lung injury. The factors mediating the deleterious effects of RBC transfusion remain unclear. In this study, we tested the effects of washed long-term (RBC-O; >28 days) versus short-term (RBC-F; <14 days) stored RBCs and their supernatants on lung endothelial (EC) permeability under control and inflammatory conditions. RBCs enhanced basal EC barrier function as evidenced by an increase in transendothelial electrical resistance and decrease in permeability for macromolecules. RBCs also attenuated EC hyperpermeability and suppressed secretion of EC adhesion molecule ICAM-1 and proinflammatory cytokine IL-8 in response to LPS or TNF-α. In both settings, RBC-F had slightly higher barrier protective effects as compared with RBC-O. In contrast, supernatants from both RBC-F and RBC-O disrupted the EC barrier. The early phase of EC permeability response caused by RBC supernatants was partially suppressed by antioxidant N-acetyl cysteine and inhibitor of Src kinase family PP2, while addition of heme blocker and inhibition of NOD-like receptor family pyrin domain containing protein 3 (NLRP3), stress MAP kinases, receptor for advanced glycation end-products (RAGE), or Toll-like receptor-4 (TLR4) signaling were without effect. Morphological analysis revealed that RBC supernatants increased LPS- and TNF-α-induced breakdown of intercellular junctions and formation of paracellular gaps. RBC supernatants augmented LPS- and TNF-α-induced EC inflammation reflected by increased production of IL-6, IL-8, and soluble ICAM-1. These findings demonstrate the deleterious effects of RBC supernatants on EC function, which may have a major impact in pathological consequences associated with RBC transfusion.
Keywords: endothelial cells, inflammation, red blood cells, transfusion, transfusion-related acute lung injury, vascular permeability
INTRODUCTION
Over 13 million RBC units are transfused in the United States each year, and red blood cell (RBC) transfusion can be life saving (70). However, RBC transfusion is also associated with serious complications such as transfusion-related acute lung injury (TRALI), which is a life-threatening condition. The incidence of TRALI varies among studies from 0.8 to 15% in patients who receive RBC transfusion (12, 17, 37, 68). Notably, the risk is much higher in critically ill patients who are in a septic condition or on mechanical ventilation (5, 17, 20, 37, 39, 45), which emphasizes the importance of patient factors and the contribution of traditional “two-hit model” for development of TRALI. Two hypotheses are currently proposed to explain the etiology of TRALI: a “two-hit model” and a ‘threshold’ hypothesis (68). The threshold hypothesis postulates that a TRALI develops when stimuli exceed a certain threshold of neutrophil and endothelium activation (10). In turn, the two-hit model postulates that the first hit is introduced by preexisting patient condition (trauma, major surgery, sepsis, etc.), which stimulates adherence of primed neutrophils to the pulmonary endothelium, and the second hit is caused by certain mediators in blood products and activates the endothelium and neutrophils (15, 40, 68). The transfusion-induced second hit is presented by two types: antibody-mediated and nonantibody-mediated type. Antibody-mediated TRALI is thought to be caused by passive transfusion of antibodies (usually human lymphocyte antigen or human neutrophil antigen) from donors (15, 68). Nonantibody-mediated TRALI is thought to be caused by inflammatory mediators in transfused blood products (24, 68). Although previous studies have identified factors associated with two hits such as mechanical ventilation, cardiac surgery, sepsis, and massive transfusion (12, 37, 68), the precise mechanisms how these factors induce neutrophil activation and increase pulmonary endothelial permeability remain largely unknown.
A number of studies have raised concerns over transfusion of old-stored RBCs since they undergo morphologic and functional changes during storage (1, 26, 29, 36, 61, 69). Some characteristic features of RBC storage lesions include vesiculation, depletion of 2,3-DPG and adenosine triphosphate (ATP), decreased levels of nitric oxide (NO), accumulation of microparticles and bioactive lipids, and increased level of free hemoglobin (21, 24, 35). RBC transfusion is also associated with immunosuppression (8, 55), postoperative infections (1, 29, 39, 54, 61), acute kidney injury (69, 72), and acute lung injury (5, 20, 35), and these complications may be more frequent with old RBCs. Interestingly, three large randomized controlled trials have now shown no mortality difference between patients who were transfused old versus fresh RBCs (13, 23, 58). These studies included both cardiac surgery patients and critically ill adults. One of the major criticisms of these studies is that “old” RBCs were in the range of 21 to 28 days old rather than closer to the 42-day expiration mark. In many busy medical centers, it is common to transfuse RBCs closer to the 42-day expiration mark because these RBCs are near expiration and the overall RBC supply is limited. Hence, controversy remains over the impact of RBC storage duration on clinical outcomes beyond mortality and also whether very old RBCs are harmful (47).
Many in vitro and in vivo studies suggested possible mechanisms of RBC transfusion-associated pathologies (40, 42, 68). It was shown that accumulation of bioactive lipids detected in RBC with longer storage duration (49, 52) can cause neutrophil activation through a G protein-coupled receptor on the neutrophil (18) and through the leukotriene B4 receptor BLT2 and activation of protein kinase C on pulmonary endothelium (50). Other studies demonstrated the increased production of microparticles from RBC and platelets during storage, which triggered coagulation through tissue factor signaling and induced the secretion of proinflammatory cytokines (11, 19). In addition to the generation of these soluble mediators, other changes in RBCs such as loss of Duffy antigen expression (34) or impaired release of adenosine-5′-triphosphate (73) also contribute to the development of TRALI. However, critical mediators in RBC transfusates that trigger injury as well as the mechanisms of their action remain to be identified. The conflicting findings from clinical trials further complicate understanding of true risks attributable to RBC storage age (62, 65, 66).
Because endothelial barrier integrity is essential for normal functioning of vascular system, dynamic regulation of endothelial permeability is performed by a number of contractile and noncontractile mechanisms. Activation of RhoA GTPase and its effector, Rho-kinase, leads to inhibitory phosphorylation of myosin phosphatase MYPT1 and increased levels of phosphorylated myosin light chains, a trigger mechanism of endothelial actomyosin contraction leading to cell retraction, disruption of cell-cell junctions and breach of endothelial barrier (31, 63). Activation of stress MAP kinases may also lead to increased endothelial cell (EC) permeability (4, 6). In turn, noncontractile mechanisms of EC hyperpermeability are associated with site-specific phosphorylation of adherens junction proteins VE-cadherin and β-catenin leading to disassembly of adherens junction protein complexes, VE-cadherin disappearance from cell-cell junctions, and internalization (48, 59). The noncontractile mechanism of EC permeability is often triggered by inflammatory agonists and mediated by reactive oxygen species and nonreceptor tyrosine kinases (7, 31). EC monolayer integrity is also affected by endothelial apoptosis (2). A few studies have shown that allogenic RBCs have direct negative impact on pulmonary endothelial function. For instance, the interaction of RBCs with lung endothelial cells (ECs) has been shown to cause endothelial dysfunction via increased generation of reactive oxygen species (32). Moreover, RBC transfusion activates lung endothelium in vivo leading to an elevated level of proinflammatory danger-associated molecular pattern (DAMP) high-mobility group box 1 (HMGB1) in the lung (33). Furthermore, RBCs induce necroptosis in EC with the release of HMGB1 resulting in increased lung inflammation (43). In the present study, we investigated the effects of fresh RBCs and old-stored RBCs and their supernatants on endothelial permeability and inflammation, the two major hallmarks of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). In addition, to recapitulate a clinical scenario of critically ill patients receiving RBC transfusion, we employed a two-hit model where EC dysfunction caused by challenge with inflammatory agonists was followed by incubation with RBCs.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary artery endothelial cells (HPAECs) were purchased from Lonza (Allendale, NJ) and used for experiments at passages 5–9. Cells were maintained in EGM-2 (CC-3156 and CC-4176; Lonza) containing 10% FBS in humidified air 5% CO2 at 37°C and switched to 2% FBS containing EGM-2 for agonist stimulation. The following inhibitors were used in cell culture experiments: reactive oxygen species (ROS) inhibitor N-acetyl cysteine and hemopexin used as heme inhibitor (Sigma, St. Louis, MO); NOD-like receptor family pyrin domain containing protein 3 (NLRP3) inflammasome inhibitor Isoliquiritigenin and TLR4 inhibitor CLI-095 (InvivoGen, San Diego, CA); Erk1,2 inhibitor UO126 (Promega, Madison, WI); receptor for advanced glycation end-products (RAGE) antagonist FPS-ZM1; Src family inhibitor PP2; JAK inhibitor I (CAS 457081-03-7); and p38MAPK inhibitor SB203580 (EMD Millipore, Billerica, MA). Human recombinant TNF-α, IL-6, IL-6-soluble receptor, and ELISA kits for human soluble ICAM-1 (sICAM-1)/IL-8 were obtained from R&D Systems (Minneapolis, MN); lipopolysaccharide (LPS; Escherichia coli O55:B5); and antibodies for human ICAM-1/VCAM-1 were from Santa Cruz Biotechnology (Santa Cruz, CA); VE-cadherin antibody was from Cayman Chemical (Ann Arbor, MI); and antibodies for IκBα and β-tubulin were from Cell Signaling (Danvers, MA). Specificity of all antibodies had been validated by manufacturer and confirmed in our previous studies. Unless specified, all other biochemical reagents were obtained from Sigma (St. Louis, MO).
RBC and supernatant preparation.
Total of four aphaeresis packed RBC units (blood type O+) from healthy donors in citrate phosphate dextrose (CPD) as an anticoagulant were purchased from BioChemed (Winchester, VA). The RBC units were not leukoreduced but were otherwise collected and stored in accordance with Food and Drug Administration standards for RBC collection and storage. Donor-specific demographic data were not available. The RBC were stored at +4°C. Fresh RBC were defined as having storage duration of less than 14 days after blood collection and old-stored RBCs were defined as having storage duration of more than 28 days. After centrifugation of RBC aliquot at 600 g for 15 min, supernatant was divided and stored for subsequent experiments. After buffy coat was removed by aspiration, the remaining RBCs were washed with phosphate-buffered saline (PBS) and were centrifuged again at 600 g for 15 min. This procedure was repeated three times, and then, RBC were reconstituted with PBS to make a hematocrit of 50%. The washing procedures were performed before each experiment.
Endothelial permeability assays.
Measurement of transendothelial electrical resistance (TER) across human pulmonary artery endothelial cell monolayers was performed the using electric cell-substrate impedance sensing system ECIS-1600 (Applied Biophysics, Troy, NY) as previously described (64). RBCs were washed and reconstituted in PBS to reach a hematocrit of 50% and added to HPAEC monolayers in three different dilutions (1:250, 1:100, and 1:20).
Permeability visualization.
Endothelial permeability to macromolecules was also determined by express permeability testing assay (Xpert) developed by our group (16). Briefly, cells were grown on 18 mm coverslips coated with biotinylated gelatin and placed in 12 wells plate. After agonist stimulations, permeability was evaluated by adding FITC-avidin (25 mg/mL; Invitrogen, Carlsbad, CA) to the culture medium for 2 min followed by two washing steps with PBS and cell fixation with 3.7% formaldehyde in PBS for 10 min at room temperature. Images were acquired using EVOS FL Auto 2 Cell Imaging System (Thermo Fisher Scientific, Hanover Park, IL).
Measurement of cytokines and chemokines.
For cytokine measurement, conditioned medium of HPAEC was collected and centrifuged at 1,500 rpm for 10 min at 4°C to remove cellular debris. Soluble ICAM-1 (sICAM-1) and IL-8 levels were measured using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Absorbance was read at 450 nm using a Victor X5 Multilabel Plate Reader (Perkin Elmer, Waltham, MA).
Western blot analysis.
Protein extracts were subjected to 8% SDS-PAGE and transferred to a polyvinylidene difluoride membrane using a semidry transfer apparatus (Bio-Rad, Hercules, CA) at 17 V for 1 h. The membranes then were incubated with specific antibodies of interest. Equal protein loading was verified by reprobing membranes with β-tubulin antibodies. Immunoreactive proteins were visualized by SuperSignal West Dura chemiluminescence reagent according to the manufacturer’s protocol (Pierce, Rockford, IL).
Immunofluorescence.
Cells were fixed in 3.7% (vol/vol) formaldehyde for 10 min at room temperature followed by washing with PBS and blocked in PBS and Tween-20 containing 1% bovine serum albumin. To visualize the cell junctions, cells were incubated with anti-rabbit VE-cadherin antibody (1:1,000) for 1 h at room temperature. Then, cells were incubated with Alexa Fluor 488-conjugated anti-rabbit IgG antibody (1:500; Invitrogen) and Alexa Fluor 594-conjugated Phalloidin (1:500, Invitrogen). Cell images were captured using EVOS FL Auto 2 cell imaging system and fluorescence intensity and quantitative analysis were performed using an ImageJ program.
Statistical analysis.
Assay results are expressed as means ± SD of three to five independent experiments. Continuous variables were compared using Student’s t test or Mann-Whitney U test depending on normality. For multiple-group comparisons, one-way ANOVA or the Kruskal-Wallis test was used. In all tests, P < 0.05 was considered statistically significant.
RESULTS
Washed RBCs enhance endothelial barrier function.
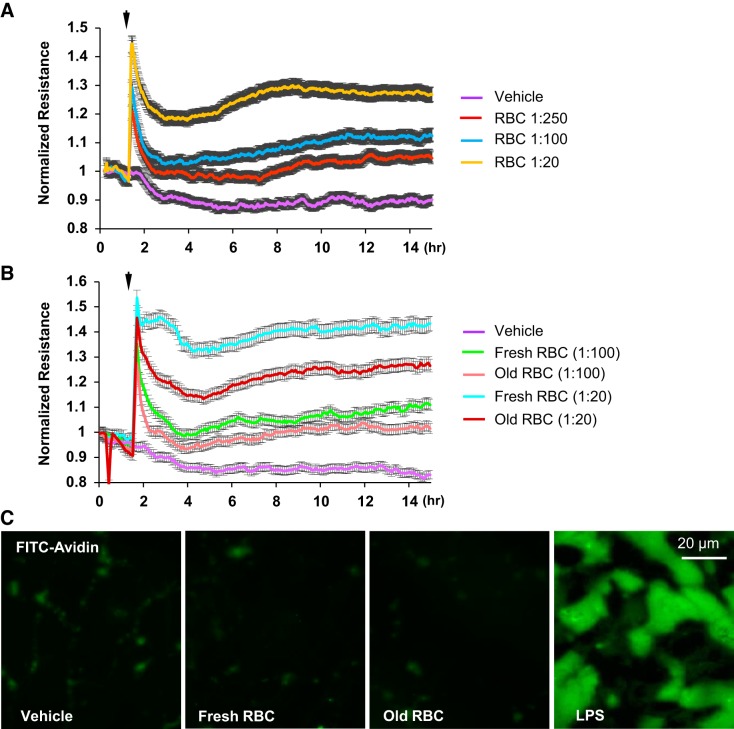
The effects of RBCs on HPAEC barrier function were evaluated by monitoring changes in TER across confluent HAPEC monolayers. In all experiments, only washed RBCs were used. Washed RBCs enhanced HPAEC barrier function in a dose-dependent manner, as reflected by an increase in TER (Fig. 1A). The barrier enhancing effects of RBCs was greater with fresh (less than 14 days of storage) RBC units than with old-stored (more than 28 days of storage) units. Of note, fresh RBC at lower dilutions exhibited similar or more pronounced barrier-enhancing effects than old-stored RBCs at highest concentration (1:20 dilution) (Fig. 1B). Endothelial permeability for macromolecules was further examined by Xpert visualization assay. Addition of both washed fresh and old-stored RBCs did not disrupt EC barrier, while inflammatory agonist LPS used as positive control caused pronounced HPAEC barrier dysfunction (Fig. 1C).
Fig. 1.
Effects of washed red blood cells (RBCs) on human pulmonary artery endothelial cell (HPAEC) barrier function. A and B: time-dependent analysis of transendothelial electrical resistance changes was performed in endothelial cells (ECs) incubated with washed RBC at indicated final dilutions (A) or in ECs incubated with fresh (less than 14 days of storage) and old (more than 28 days of storage) red blood cells (B); n = 5. C: barrier maintenance of pulmonary ECs incubated with fresh or old stored RBCs was confirmed by Xpert visualization assay using FITC-avidin as tracer. Control treatment with LPS (200 ng/mL) caused profound EC hyperpermeability reflected by robust penetration of FITC-avidin across the cell monolayer; n = 5; bar = 20 µm.
Washed RBCs do not induce inflammatory response in HPAECs.
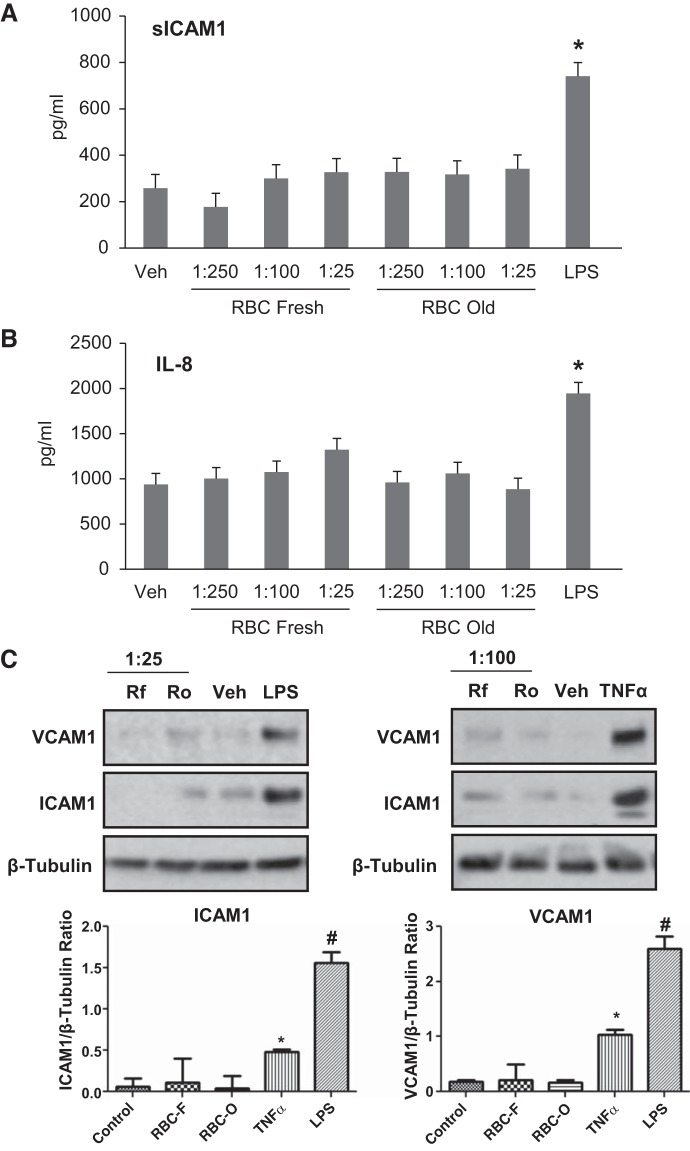
The induction of inflammation in some patients has been attributed to harmful effects of RBC transfusion especially with long-term stored RBCs. To evaluate the role of RBCs in modulating inflammatory responses of HPAECs, fresh and old-stored RBC were first separated from supernatants, washed in PBS and incubated with pulmonary ECs. The levels of soluble ICAM-1 and a neutrophil chemotactic factor, IL-8, reflecting inflammatory activation of ECs were measured by ELISA in the cultured medium. Both short-term (RBC-F) and long-term (RBC-O) RBCs had no effect on sICAM-1 and IL-8 production by HPAECs suggesting that RBC alone did not induce EC inflammatory response (Fig. 2, A and B). Consistently, RBCs did not induce ICAM-1 or VCAM-1 protein expression (Fig. 2C). In contrast, the well-known inflammatory agonists LPS and TNF-α, which were used as control, caused robust IL-8 and sICAM-1 secretion and upregulated ICAM-1 and VCAM-1 expression by ECs.
Fig. 2.
Effects of washed red blood cells (RBCs) on human pulmonary artery endothelial cell (HPAEC) inflammatory activation. HPAECs were challenged with vehicle, indicated dilutions of fresh (RBC-F) and old (RBC-O) RBCs, 200 ng/mL LPS or 0.2 ng/mL TNF-α, followed by incubation for 16–20 h. A and B: the conditioned medium was analyzed for soluble ICAM-1 (sICAM-1) (A) and IL-8 (B) using ELISA assay. C: Western blot analysis of ICAM-1 and VCAM-1 expression by HPAECs treated with RBC-F (Rf) and RBC-O (Ro) at indicated dilutions or LPS. Membrane reprobing for β-tubulin was used as normalization control. Bar graphs depict the quantitative densitometry analysis of Western blot data; n = 3. *P < 0.05 vs. nonstimulated control. #P < 0.005.
Effects of pretreatment with washed RBCs on EC permeability caused by LPS and TNF-α.
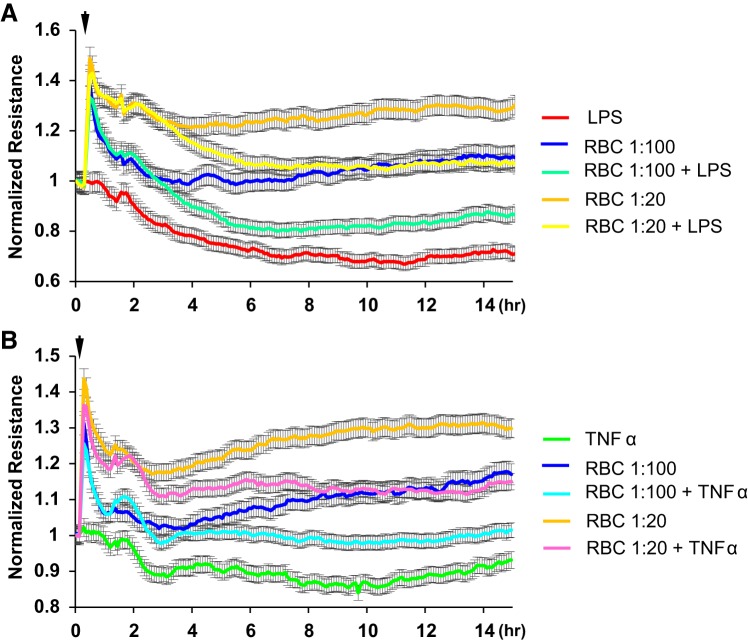
In agreement with results in Fig. 1, the addition of washed RBCs caused rapid (30 min) and dose-dependent TER increase. Subsequent addition of LPS decreased TER in RBC-preincubated endothelial monolayers; however, the TER remained at higher levels as compared with ECs challenged with LPS alone (Fig. 3A). Similar dose-dependent protective effects of RBC pretreatment on EC barrier were observed in TNF-α-stimulated ECs (Fig. 3B).
Fig. 3.
Effect of washed red blood cell (RBC) pretreatment on LPS- or TNF-α-induced human pulmonary artery endothelial cell (HPAEC) barrier disruption. Washed fresh RBCs were added to HPAEC monolayers at indicated dilutions 30 min before challenge with LPS (A) or TNF-α (B). Barrier function was examined by measuring transendothelial electrical resistance changes; n = 4. Arrow indicates the time of LPS or TNF-α stimulation.
Washed RBC posttreatment provides EC barrier protection against inflammatory agonists.
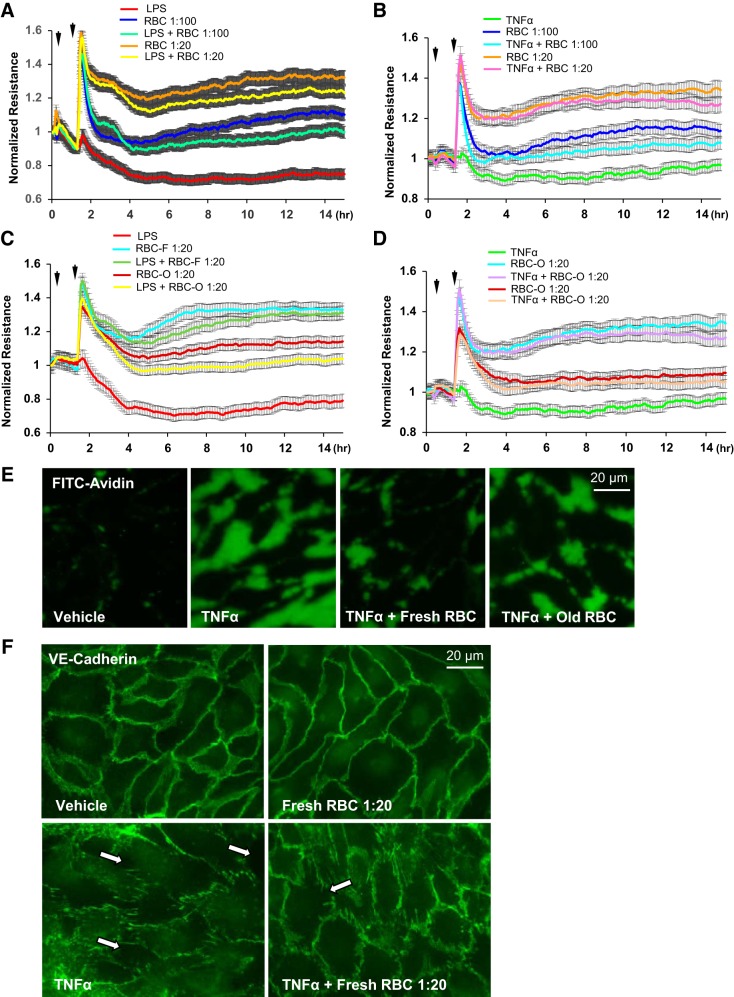
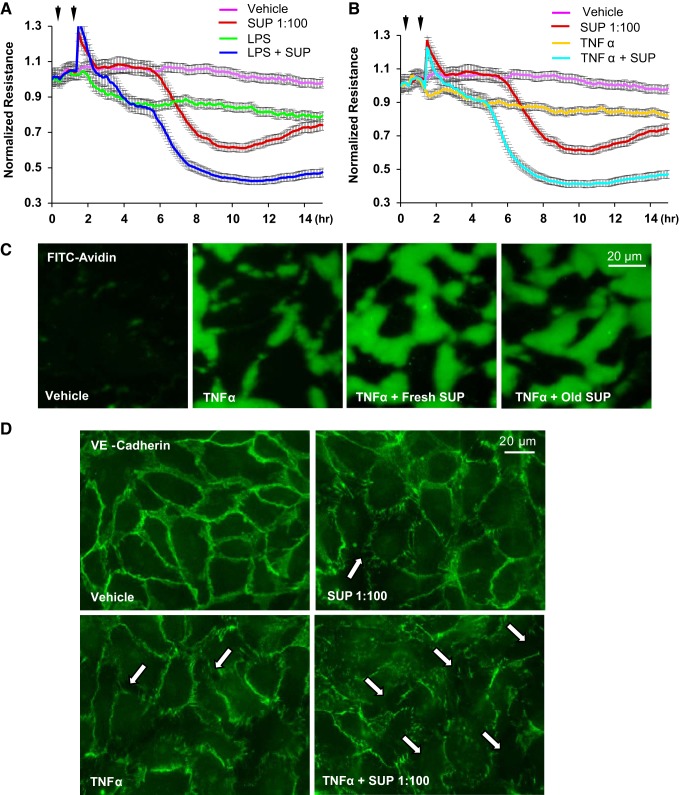
To recapitulate clinically relevant settings, we sought to determine whether washed RBC addition after inflammatory insult inhibits endothelial barrier disruption. Addition of washed RBCs 1 h after EC challenge with LPS or TNF-α was protective against EC barrier dysfunction caused by both LPS and TNF-α (Fig. 4, A and B). Interestingly, the barrier protective effects of RBC-F were greater than effects by same dose of RBC-O, as evidenced by higher TER values (Fig. 4, C and D). RBC-induced protection of endothelial barrier in TNF-α-challenged EC monolayers was further confirmed by Xpert visualization assay (Fig. 4E) Both washed RBC-F and RBC-O attenuated LPS- and TNF-α-induced permeability of HPAEC monolayers (Fig. 4E). EC monolayers immunostaining with antibody to adherence junction protein VE-cadherin showed dramatic disassembly of cell-cell junctions caused by TNF-α, which was markedly attenuated by addition of washed RBCs (Fig. 4F).
Fig. 4.
Effect of washed red blood cell (RBC) posttreatment on LPS- or TNF-α-induced human pulmonary artery endothelial cell (HPAEC) barrier disruption. HPAEC monolayers were incubated with LPS or TNF-α for 1 h followed by addition of RBCs at 1:20 or 1:100 dilution. A and B: effect of posttreatment with fresh RBCs on LPS-induced transendothelial electrical resistance (TER) decline (A) and TNF-α-induced TER decline (B); n = 5. C and D: comparative analysis of endothelial cell (EC) posttreatment with fresh (RBC-F) and old (RBC-O) RBCs on LPS-induced TER decline (C) and TNF-α-induced TER decline (D); n = 5. E: visualization of permeability for FITC-avidin in control HPAEC monolayers and monolayers challenged with TNF-α with or without posttreatment with fresh or old RBCs; n = 3. F: washed RBCs protected HPAECs against TNF-α-induced breakdown of cell junctions monitored by immunofluorescence staining of transmembrane adherence junction protein, VE-cadherin; n = 5; bar = 20 µm.
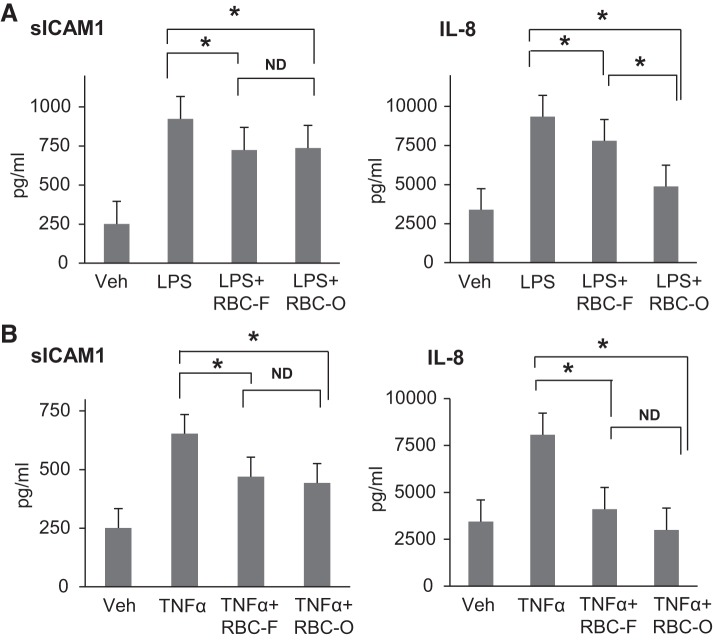
Washed RBCs attenuate LPS- and TNF-α-induced sICAM-1 and IL-8 production by HPAECs.
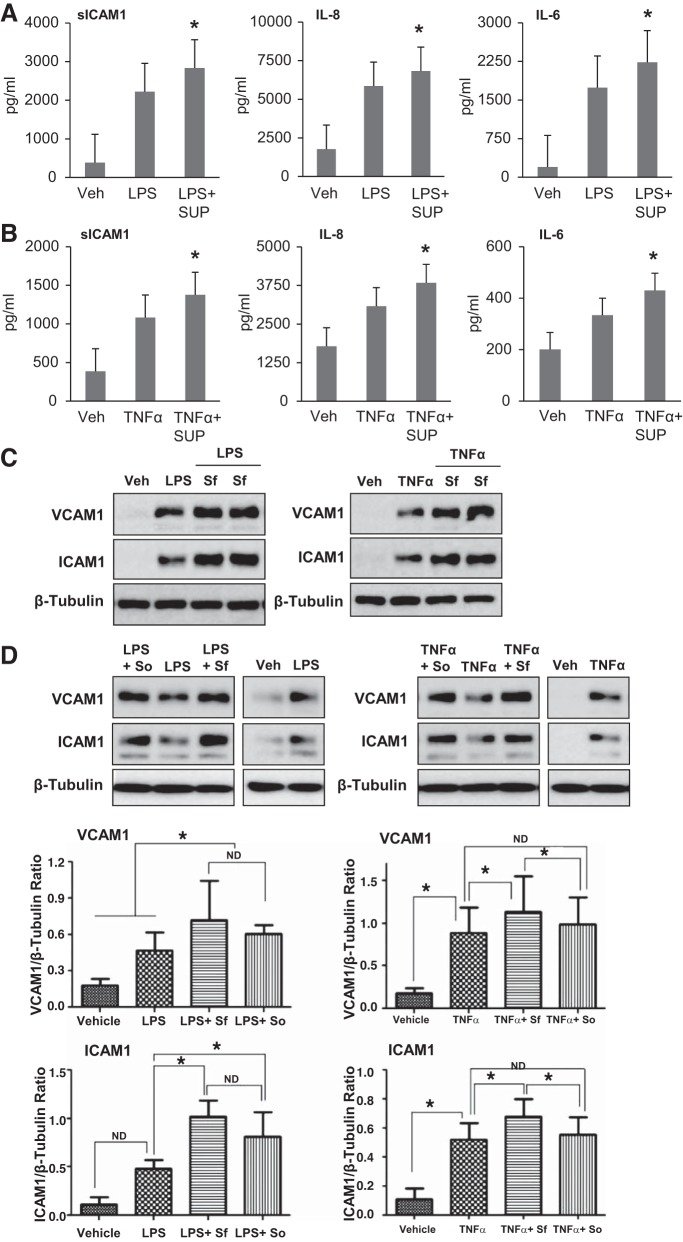
To investigate whether RBCs provide protection against agonist-induced inflammation in HPAECs, we analyzed the levels of EC inflammatory markers, sICAM and IL-8, in conditioned medium after EC challenge with TNF-α or LPS followed by addition of RBC-F or RBC-O. Both fresh and old-stored RBC attenuated LPS- and TNF-α-induced sICAM and IL-8 production (Fig. 5, A and B). There was no significant difference between RBC-F and RBC-O in the degree of sICAM-1 reduction. However, RBC-O caused stronger suppression of IL-8 production after LPS challenge as compared with RBC-F. These interesting observations suggest that washed RBC-O may have relatively higher anti-inflammatory effects than RBC-F at least in our experimental settings. There was also a trend to stronger reduction of IL-8 production by RBC-O, although it did not reach statistically significant difference.
Fig. 5.
Effects of washed red blood cells (RBCs) on LPS- or TNF-α-induced inflammatory responses by human pulmonary artery endothelial cells (HPAECs). HPAEC monolayers were incubated with LPS (A) or TNF-α (B) for 1 h followed by addition of fresh (RBC-F) and old (RBC-O) RBCs (1:100 dilution). The conditioned medium was analyzed for soluble ICAM-1 (sICAM-1) and IL-8 using ELISA assay; n = 5. *P < 0.05 vs. LPS or TNF-α; ND, no statistical difference.
RBC supernatants induce endothelial barrier disruption.
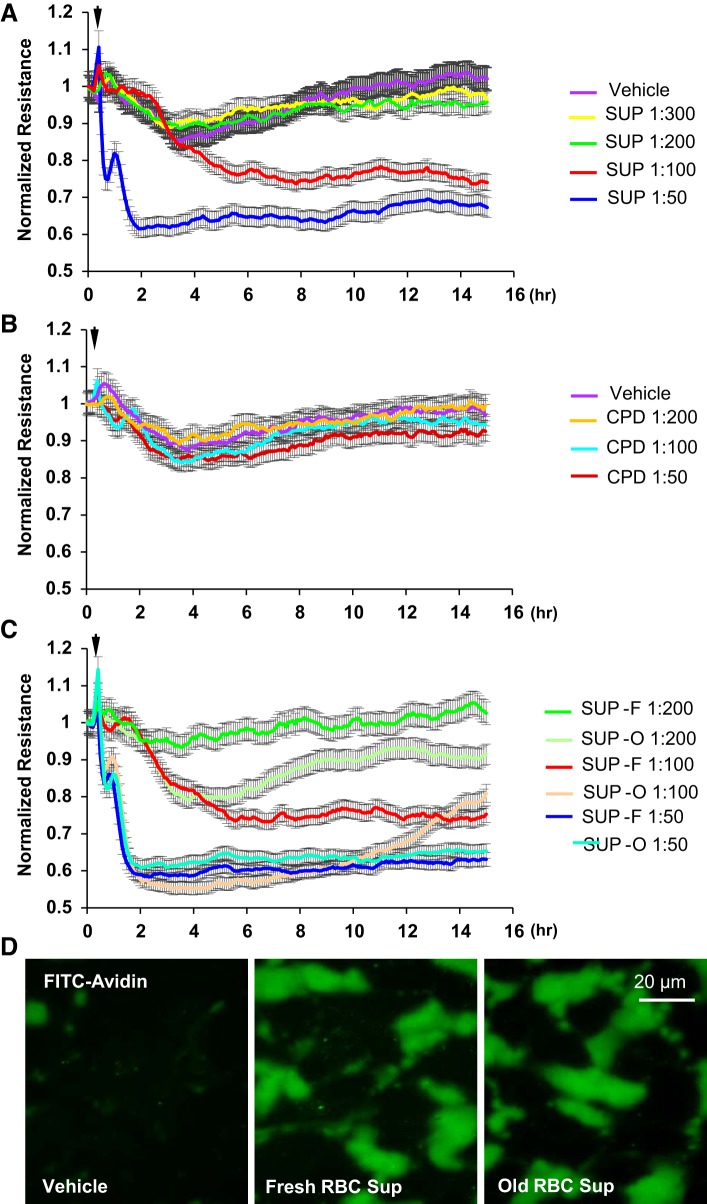
Previous reports have suggested that supernatants from RBCs rather than RBCs themselves are critical mediators of pathologies associated with RBC transfusion (3, 38). Since our results clearly suggest the protective roles of RBCs on EC barrier function, we next analyzed biological activities of RBC supernatants toward pulmonary endothelium. Measurements of TER showed that RBC supernatants decreased TER across HPAEC monolayers in a dose-dependent manner, indicating their pronounced barrier disruptive properties (Fig. 6A). As RBCs contained citrate phosphate dextrose (CPD) solution as an anticoagulant, additional TER experiments were performed with CPD fluid alone as a control. CPD did not change TER thus further demonstrating that increase in permeability is due to RBC supernatants themselves (Fig. 6B). There was no appreciable difference between the magnitude of EC barrier disruptive effects caused by supernatants from fresh RBCs (SUP-F) and old RBC (SUP-O) preparations (Fig. 6C). Of note, although effects of storage on RBC supernatant barrier-disruptive properties were minimal within each RBC unit, the magnitude of EC barrier-disruptive response to RBC supernatants from different donors exhibited much higher variation (data not shown). The deleterious effects of both SUP-F and SUP-O on endothelial barrier were further confirmed by Xpert assay (Fig. 6D).
Fig. 6.
Effects of red blood cell (RBC) supernatants on human pulmonary artery endothelial cell (HPAEC) barrier function. HPAEC barrier function was examined by measuring transendothelial electrical resistance (TER) changes. A: RBC supernatants tested at different dilutions decreased TER in a dose-dependent manner. B: anticoagulant conservant [citrate phosphate dextrose solution (CPD)] used for RBC storage did not affect basal TER of EC monolayers. C: supernatants from both fresh stored (SUP-F; less then 14 days) and old stored (SUP-O; more than 28 days) RBCs exhibited similar barrier disruptive effects on HPAEC monolayers reflected by sharp TER decline; n = 4. D: barrier-disruptive effects of supernatants from fresh and old stored RBCs were analyzed in Xpert visualization assays using FITC-avidin as a tracer. There was no difference between fresh and old supernatants; n = 5; bar = 20 µm.
Signaling pathways mediating barrier-disruptive effects of RBC supernatants.
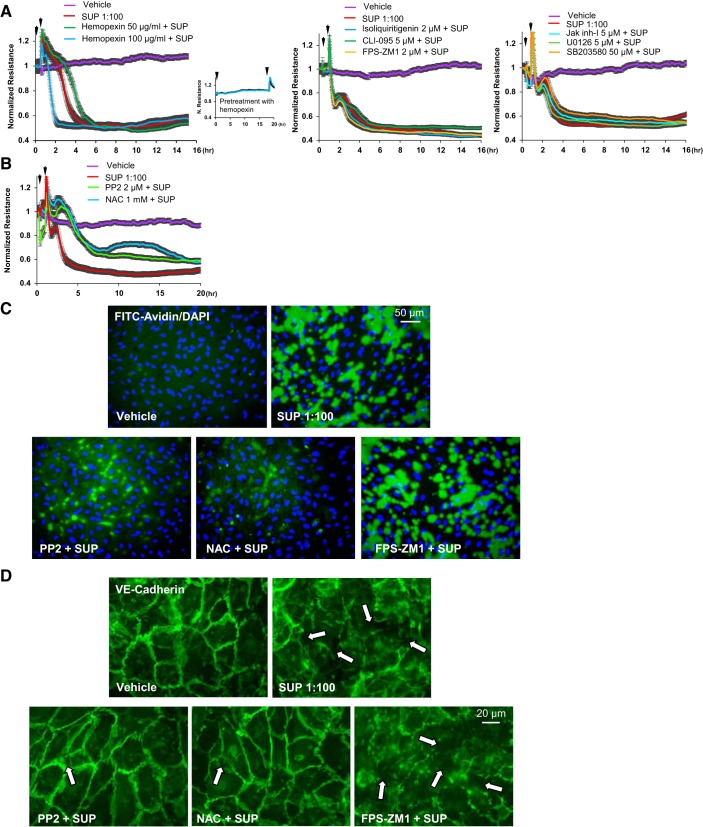
Given the complexity of mechanisms involved in endothelial barrier regulation, we performed a broad inhibitory analysis to identify specific signaling pathways triggering pulmonary endothelial cell permeability induced by RBC supernatant. Since no RhoA activation was detected in supernatant-conditioned ECs (data not shown), in the following studies we focused on other candidate pathways causing endothelial barrier dysfunction. Given the reported barrier disruptive effects of free heme (44), which may be released to supernatants by RBCs undergoing hemolysis during storage, we first tested effects of hemopexin, a known scavenger of heme. While hemopexin alone had no effect on basal EC permeability monitored by TER measurements (Fig. 7A, left, inset), pretreatment with hemopexin did not block profound TER decline caused by RBC supernatant (Fig. 7A, left). EC pretreatment with inhibitor of NLRP3 inflammasome, RAGE, or TLR4 receptor inhibitors also did not affect RBC supernatant-induced TER decline (Fig. 7A, middle). Similarly, pretreatment with inhibitors of JAK, p38, and Erk1,2 MAP kinases was without effect on RBC supernatant-induced EC barrier dysfunction (Fig. 7A, right). In contrast, the acute phase of supernatant-induced EC permeability was attenuated by pharmacologic inhibitor of Src tyrosine kinase and the ROS inhibitor N-acetyl cysteine (NAC) (Fig. 7B). Biophysical assays of EC permeability were further validated by fluorescence-based assay to monitor EC monolayer permeability for macromolecules. The results confirmed TER studies and showed that EC monolayer permeability for the tracer molecule FITC-labeled avidin triggered by RBC supernatant was markedly attenuated by NAC and Src kinase inhibitor PP2, while RAGE inhibitor was without effect (Fig. 7C). Functional permeability assays were further supported by immunofluorescence analysis of cell-cell junction integrity, which showed that robust disassembly of VE-cadherin positive adherens junctions caused by RBC supernatants was preserved by EC pretreatment with NAC and PP2, while the RAGE inhibitor FPS-ZM1 was without effect (Fig. 7D).
Fig. 7.
Analysis of signaling pathways mediating barrier-disruptive effects of red blood cell (RBC) supernatants. A: human pulmonary artery endothelial cells (HPAECs) were pretreated 30 min (first arrow) before RBC supernatant (SUP) addition (second arrow, 1:100 dilution) with vehicle or the following inhibitors: heme inhibitor hemopexin (50 µg/mL and 100 µg/mL) Inset shows transendothelial electrical resistance records in endothelial cell (EC) monolayers treated with hemopexin alone) (left); NOD-like receptor family pyrin domain containing protein 3 (NLRP3) inflammasome inhibitor isoliquiritigenin (2 µM), receptor for advanced glycation end-products (RAGE) antagonist FPS-ZM1 (2 µM), and TLR4 inhibitor CLI-095 (5 µM) (middle); and Jak inhibitor I (5 µM), p38 MAPK inhibitor SB203580 (50 µM), and Erk1,2 inhibitor UO126 (5 µM) (right); n = 5. B: effects of the Src inhibitor PP2 (2 µM) and reactive oxygen species (ROS) inhibitor N-acetyl cysteine (NAC; 1 mM) on TER decline caused by fresh RBC supernatants (1:100 dilution); n = 4. C: effects of ROS (NAC, 1 mM), Src (PP2, 2 µM), and RAGE (FPS-ZM1, 2 µM) inhibitors on EC permeability for macromolecules were evaluated using Xpert visualization assay with FITC-avidin as a tracer (green). Nuclear counterstaining with DAPI (blue) demonstrate equal cell densities in control and experimental groups; n = 5; bar = 50 µm. D: NAC and PP2, but not FPS-ZM1, protected EC monolayers against breakdown of cell junctions caused by RBC supernatants, which was monitored by immunofluorescence staining for VE-cadherin; n = 5; bar = 20 µm.
RBC supernatants exhibit modest proinflammatory effects on HPAECs.
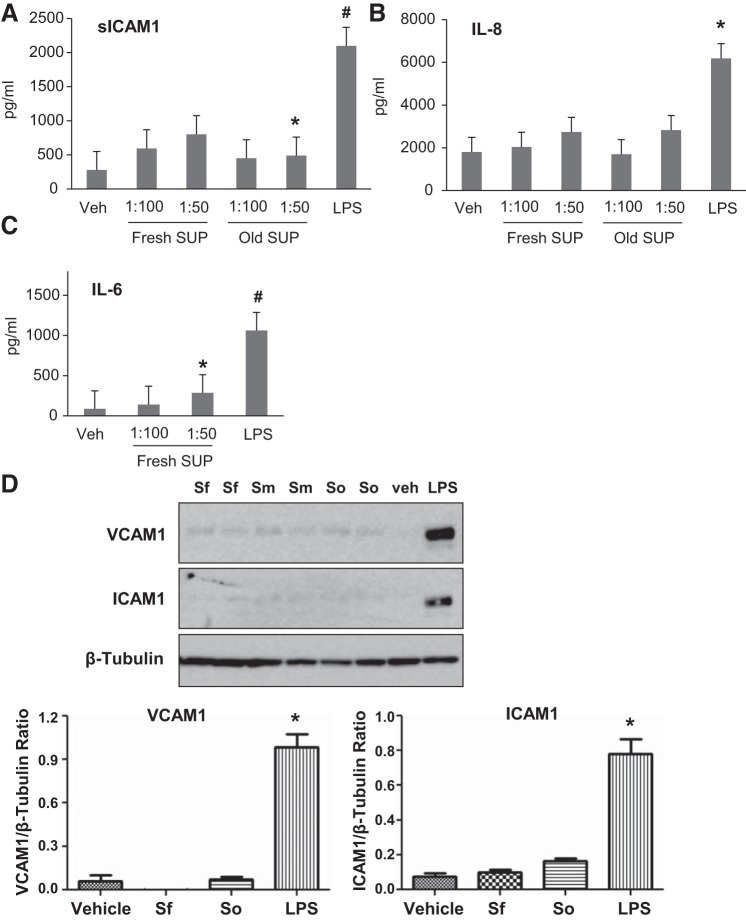
The effects of RBC supernatants on endothelial dysfunction were further assessed by measuring the levels of proinflammatory cytokines and protein expression of EC inflammatory adhesion molecules. ELISA results showed that RBC supernatants caused marginal increase of sICAM and IL-6 only at higher doses (1:50) and did not have effect on IL-8 production (Fig. 8, A–C). Of note, these and lower supernatant doses were sufficient to cause severe barrier disruption (Fig. 6). Western blot analysis also demonstrated that RBC supernatants alone did not trigger expression of ICAM-1 or VCAM-1 in contrast to potentiation effects by LPS, which was used as a positive control (Fig. 8D).
Fig. 8.
Effects of red blood cell (RBC) supernatants (SUP) on inflammatory response by human pulmonary artery endothelial cells (HPAECs). HPAECs were challenged with RBC supernatants from fresh and old stored RBCs at 1:50 and 1:100 dilutions, or cells were stimulated with LPS as a positive control. A–C: after 16–20 h, the cultured medium was examined by ELISA for inflammatory markers: soluble ICAM-1 (sICAM-1) (A), IL-8 (B), and IL-6 (C); n = 5. *P < 0.05 and #P < 0.01 vs. nonstimulated control. D: Western blot analysis of VCAM-1 and ICAM-1 expression in total lysates of ECs stimulated with RBC supernatants or LPS. Sf, fresh supernatant, less than 14 days; So, old supernatant, more than 28 days; Sm, supernatant between 14 and 28 days of storage. RBC supernatants alone did not induce inflammatory response. Membrane reprobing for β-tubulin was used as normalization control. Bar graphs depict the quantitative densitometry analysis of Western blot data; n = 4, *P < 0.05 vs. nonstimulated control.
RBC supernatants augment LPS- or TNF-α-induced barrier disruption and inflammation.
It has been noted that critically ill patients are more vulnerable to adverse effects associated with RBC transfusion (36). To test this notion, we evaluated the barrier-disruptive effects of RBC supernatants on ECs after LPS or TNF-α challenge. TER measurements by ECIS showed that RBC supernatants further augmented the TER decrease induced by LPS or TNF-α (Fig. 9, A and B). Xpert assay results also showed that both fresh and old RBC supernatants enhanced endothelial permeability as observed by an increase in TNF-α-induced FITC-avidin penetration across HPAEC monolayers (Fig. 9C). Immunofluorescence staining demonstrated that RBC supernatants augmented TNF-α-induced breakdown of cell junctions revealed by VE-cadherin immunostaining (Fig. 9D). We also evaluated the role of RBC supernatants in exacerbation of LPS- or TNF-α-induced EC inflammation. The results show that RBC supernatants augmented both LPS- and TNF-α-induced production of sICAM-1, IL-8, and IL-6 (Fig. 10, A and B). In agreement with ELISA results, Western blot analysis demonstrated that RBC supernatants augmented LPS- or TNF-α-induced expression of ICAM-1 and VCAM-1 (Fig. 10C). There was no statistical difference between SUP-F and SUP-O in augmenting the LPS- and TNF-α-induced EC inflammatory responses (Fig. 10D).
Fig. 9.
Effects of red blood cell (RBC) supernatants on LPS- and TNF-α-induced endothelial cell (EC) barrier disruption. A and B: human pulmonary artery endothelial cell (HPAEC) monolayers were challenged with LPS (200 ng/mL) (A) or TNF-α (2 ng/mL) (B) with or without addition of supernatants (SUP) from fresh RBC 1 h later. HPAEC barrier function was monitored by transendothelial electrical resistance (TER) changes. RBC supernatants augmented LPS- and TNF-α-induced transendothelial electrical resistance decline; n = 5. C: effects of TNF-α and supernatants from fresh and old RBC on EC permeability for macromolecules were evaluated by Xpert permeability assay. Both fresh and old RBC supernatants further increased EC permeability caused by LPS and TNF-α; n = 5. D: breakdown of cell junctions by TNF-α and RBC supernatants was monitored by immunofluorescence staining of VE-cadherin; n = 4; bar = 20 µm.
Fig. 10.
Effects of red blood cell (RBC) supernatants (SUP) on LPS or TNF-α-induced inflammatory response of human pulmonary artery endothelial cells (HPAECs). A and B: HPAECs were treated with LPS (A) or TNF-α (B) for 1 h followed by addition of fresh RBC supernatants (1:100 dilution) for 16 to 20 h and ELISA analysis of the markers of inflammation: soluble ICAM-1 (sICAM-1), IL-8, and IL-6; n = 3. *P < 0.05 vs. LPS or TNF-α alone. C: Western blot analysis of ICAM-1 and VCAM-1 expression was performed in HPAEC challenged with LPS or TNF-α with or without posttreatment with fresh RBC supernatants. D: effects of fresh and old RBC supernatants on VCAM-1 and ICAM-1 expression caused by LPS or TNF-α. Membrane reprobing for β-tubulin was used as normalization control. Sf, fresh supernatant, less than 14 days; So, old supernatant, more than 28 days Bar graphs depict the quantitative densitometry analysis of Western blot data; n = 3. *P < 0.05.
DISCUSSION
A growing body of evidence indicates that RBC transfusion is not always safe but may have adverse pathological consequences especially in critically ill patients. In addition, a number of observational and meta-analysis studies have suggested that prolonged RBC storage duration may be a risk factor for TRALI and RBC transfusion-related mortality (1, 17, 26, 28, 35, 36). A growing number of studies indicate that pulmonary endothelium is a critical target of RBC transfusion-related injury. Thus the current lack of consensus hypothesis on the direct causal relationship between RBC storage and transfusion-related diseases prompted us to test the effects of RBC on endothelial function.
In the present study, we investigated the effects of RBC on two major functions of EC: maintenance of endothelial barrier integrity and modulation of inflammation, both of which play a crucial role in the pathogenesis of ALI and ARDS. Our results show that irrespective of storage duration, RBC following washing enhanced basal endothelial barrier properties and attenuated barrier disruption caused by LPS and TNF-α. Washed RBCs suppressed the LPS- or TNF-α-induced production of proinflammatory cytokines and EC adhesion molecules ICAM-1 and VCAM-1. The barrier protective properties of RBCs were due to prevention of TNF-α-induced interendothelial junction breakdown and paracellular gap formation. In contrast, RBC supernatants caused increased EC permeability and augmented LPS- or TNF-α-induced inflammatory response by lung ECs. These findings indicate that the critical mediators of RBC transfusion-linked pathologies may not be RBCs per se but instead due to bioactive molecules present in the supernatants. Our results also show that RBCs stored for 4 wk retained barrier protective and anti-inflammatory activities, while supernatants from both short-term and long-term stored RBCs had a similar degree of EC barrier-disruptive and LPS/TNF-α-augmenting proinflammatory effects. These findings indicate that storage duration does not substantially worsen the quality of RBCs and “storage lesion” described by many studies may be due to other factors, such as changing composition of supernatant. Our findings are also clinically relevant since we tested RBC stored up to 4 wk, which is consistent with the current global practice of maximal 3–4 wk of RBC storage for transfusion purposes.
The mechanism of RBC-induced enhancement of endothelial cell barrier function remains to be elucidated. A direct interaction between RBCs and ECs leading to cytoskeleton remodeling may be involved in this process. The maintenance of junctional assembly and sealing of TNF-α-induced paracellular gaps by RBCs suggests in favor of this mechanism. However, we cannot rule out the possible involvement of other pathways such as the contribution of bioactive molecules present in RBCs. Among such molecules, RBCs are a major source of sphingosine 1-phosphate, which is known to play a role in maintaining endothelial barrier integrity (71). Future studies are warranted to determine critical mediators involved in facilitation of RBC-induced endothelial protection.
In agreement with earlier studies, we also found that RBC supernatants possess barrier-disruptive properties (38, 46, 50, 52, 56, 67). Surprisingly, supernatants from fresh RBCs or old RBCs had same degree of deleterious effects on ECs with that of old RBCs, suggesting that these harmful substances are present in RBC from the beginning, opposing the existing theory that they accumulate during storage. Several experiments performed in human lung microvascular ECs showed similar results to HPAEC studies. These experiments showed disruptive effects of RBC supernatants on lung microvascular EC (data not shown). These results suggest general disruptive effects of RBC supernatants on micro- and macrovascular EC monolayers.
The biochemical changes that occur during RBC storage have been considered as possible mediators of TRALI, which include decreased 2,3-DPG (24, 55), cytoskeletal changes in RBCs (9, 24, 55), increased levels of nitric oxide (NO) scavengers such as free hemoglobin (24, 25, 30), microparticle formation (11, 19, 21), increased nontransferrin bound iron (14, 25, 26), and increased bioactive lipids (41, 49–52) and mitochondrial DNA DAMPs (53). Lipidomic analysis have shown that ceramides, glycerophospholipids, and sterols are potential molecules contributing to RBC storage lesion (60). However, accumulated lipids did not induce TRALI in LPS-primed subjects (41). Free iron and heme may also contribute to the harmful effects of RBC transfusion as nitric oxide (NO) scavengers (14, 25, 26). Recent studies have also suggested the role for HMGB1 in RBC-induced EC dysfunction (43). However, most of the aforementioned molecules with adverse effects on vascular endothelium appear after prolonged RBC storage, and this hypothesis appears inconsistent with our data demonstrating a barrier-disruptive effect from fresh supernatant suggesting the initial presence of EC-disruptive mediators in RBC supernatants. Future studies are warranted to identify barrier disruptive and proinflammatory molecules present in the RBC supernatant fraction.
This study identified two cellular pathways: oxidative stress and Src kinase signaling that trigger early phase of EC barrier failure caused by RBC supernatants. Indeed, morphological analysis of EC monolayers demonstrated rapid breakdown of cell-cell contacts (Fig. 7), the events known to be largely regulated by Src (31, 63). Interestingly, other barrier-disruptive mechanisms mediated by RhoA or stress MAP kinases or involvement of NLRP3 inflammasome, RAGE, or TLR4 receptor signaling apparently did not play a role in barrier-disruptive effects by RBC supernatants. Since activation of Src activity in many cases is stimulated by elevation of reactive oxygen species (22), pharmacological inhibition of ROS-Src axis may improve EC dysfunction and clinical outcomes of complications associated with RBC transfusion.
RBC supernatants also seem to play a role in exacerbating inflammatory responses in EC although they did not induce inflammation on their own. These observations from two-hit models resemble a clinical scenario where adverse effects of RBC transfusion are more pronounced in critically ill patients. Based on our results that RBC supernatants enhance TNF-α or LPS-induced release of proinflammatory cytokines and expression of inflammatory cell adhesion molecules in EC, we can speculate that the deleterious effects of RBC transfusion in such immunocompromised patients could be due to augmented inflammation caused by RBC supernatant. If RBC supernatants are major components of “RBC storage lesions,” washing of RBC units before transfusion of high-risk patients may be beneficial. Indeed, two studies have reported a positive effect of RBC washing (14, 57). Furthermore, in a small randomized trial, washed RBCs showed beneficial effects in coronary artery bypass graft surgery (27). Taken together, these findings suggest that washing of RBCs before transfusion to eliminate deleterious effects of supernatants may prove beneficial at least in a high-risk population such as patients with septic syndrome, patients undergoing cardiac surgery, or individuals with previous history of transfusion reactions.
In summary, the present study demonstrates barrier-enhancing effects of washed RBCs on human pulmonary endothelium and protection against EC barrier dysfunction caused by inflammatory agonists. Our findings reveal contrasting effects of RBCs and their supernatants on pulmonary EC function, which helps to explain the pathological reactions associated with RBC transfusion. Future studies are warranted to elucidate the crucial mediators involved in the effects of RBC on vascular endothelium to better understand the pathogenesis of TRALI and other transfusion-related injuries.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-076259, HL-087823, HL-107920, and HL-130431 and National Institute of General Medical Sciences Grants GM-122940 and GM-114171.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.M., K.A.T., A.A.B., and K.G.B. conceived and designed research; J.K., T.T.T.N., Y.L., C.-O.Z., B.C., and Y.K. performed experiments; J.K., T.T.T.N., Y.L., C.-O.Z., B.C., Y.K., A.A.B., and K.G.B. analyzed data; J.K., T.T.T.N., M.A.M., K.A.T., A.A.B., and K.G.B. interpreted results of experiments; J.K., T.T.T.N., C.-O.Z., and B.C. prepared figures; J.K. drafted manuscript; J.K., T.T.T.N., M.A.M., K.A.T., A.A.B., and K.G.B. edited and revised manuscript; J.K., T.T.T.N., M.A.M., K.A.T., A.A.B., and K.G.B. approved final version of manuscript.
REFERENCES
- 1.Andreasen JJ, Dethlefsen C, Modrau IS, Baech J, Schonheyder HC, Moeller JK, Johnsen SP; North-West Denmark Transfusion Study Group . Storage time of allogeneic red blood cells is associated with risk of severe postoperative infection after coronary artery bypass grafting. Eur J Cardiothorac Surg 39: 329–334, 2011. doi: 10.1016/j.ejcts.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong SM, Wang C, Tigdi J, Si X, Dumpit C, Charles S, Gamage A, Moraes TJ, Lee WL. Influenza infects lung microvascular endothelium leading to microvascular leak: role of apoptosis and claudin-5. PLoS One 7: e47323, 2012. doi: 10.1371/journal.pone.0047323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner JM, Nydam TL, Clarke JH, Banerjee A, Silliman CC, McCarter MD. Red blood cell supernatant potentiates LPS-induced proinflammatory cytokine response from peripheral blood mononuclear cells. J Interferon Cytokine Res 29: 333–338, 2009. doi: 10.1089/jir.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker PM, Verin AD, Booth MA, Liu F, Birukova A, Garcia JG. Differential regulation of diverse physiological responses to VEGF in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1500–L1511, 2001. doi: 10.1152/ajplung.2001.281.6.L1500. [DOI] [PubMed] [Google Scholar]
- 5.Benson AB, Austin GL, Berg M, McFann KK, Thomas S, Ramirez G, Rosen H, Silliman CC, Moss M. Transfusion-related acute lung injury in ICU patients admitted with gastrointestinal bleeding. Intensive Care Med 36: 1710–1717, 2010. doi: 10.1007/s00134-010-1954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 289: L75–L84, 2005. doi: 10.1152/ajplung.00447.2004. [DOI] [PubMed] [Google Scholar]
- 7.Birukova AA, Starosta V, Tian X, Higginbotham K, Koroniak L, Berliner JA, Birukov KG. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl Res 161: 495–504, 2013. doi: 10.1016/j.trsl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology 10, Suppl 1: 208–214, 2005. doi: 10.1080/10245330512331390447. [DOI] [PubMed] [Google Scholar]
- 9.Bosman GJ, Werre JM, Willekens FL, Novotný VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med 18: 335–347, 2008. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 10.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI). Br J Haematol 136: 788–799, 2007. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang AL, Kim Y, Seitz AP, Schuster RM, Lentsch AB, Pritts TA. Erythrocyte-derived microparticles activate pulmonary endothelial cells in a murine model of transfusion. Shock 47: 632–637, 2017. doi: 10.1097/SHK.0000000000000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford L, Jia Q, Subramanian A, Yadav H, Wilson GA, Murphy SP, Pathak J, Schroeder DR, Kor DJ. Characterizing the epidemiology of postoperative transfusion-related acute lung injury. Anesthesiology 122: 12–20, 2015. doi: 10.1097/ALN.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper DJ, McQuilten ZK, Nichol A, Ady B, Aubron C, Bailey M, Bellomo R, Gantner D, Irving DO, Kaukonen KM, McArthur C, Murray L, Pettilä V, French C; TRANSFUSE Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Age of red cells for transfusion and outcomes in critically ill adults. N Engl J Med 377: 1858–1867, 2017. doi: 10.1056/NEJMoa1707572. [DOI] [PubMed] [Google Scholar]
- 14.Cortés-Puch I, Wang D, Sun J, Solomon SB, Remy KE, Fernandez M, Feng J, Kanias T, Bellavia L, Sinchar D, Perlegas A, Solomon MA, Kelley WE, Popovsky MA, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood 123: 1403–1411, 2014. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis BR, McFarland JG. Mechanisms of transfusion-related acute lung injury (TRALI): anti-leukocyte antibodies. Crit Care Med 34, Suppl: S118–S123, 2006. doi: 10.1097/01.CCM.0000214293.72918.D8. [DOI] [PubMed] [Google Scholar]
- 16.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93: 254–263, 2013. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupuis C, Sonneville R, Adrie C, Gros A, Darmon M, Bouadma L, Timsit JF. Impact of transfusion on patients with sepsis admitted in intensive care unit: a systematic review and meta-analysis. Ann Intensive Care 7: 5, 2017. doi: 10.1186/s13613-016-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellison MA, Ambruso DR, Silliman CC. Therapeutic options for transfusion related acute lung injury; the potential of the G2A receptor. Curr Pharm Des 18: 3255–3259, 2012. doi: 10.2174/1381612811209023255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer D, Büssow J, Meybohm P, Weber CF, Zacharowski K, Urbschat A, Müller MM, Jennewein C. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion 57: 2701–2711, 2017. doi: 10.1111/trf.14268. [DOI] [PubMed] [Google Scholar]
- 20.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O’Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med 176: 886–891, 2007. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Lv L, Liu S, Ma G, Su Y. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang 105: 11–17, 2013. doi: 10.1111/vox.12014. [DOI] [PubMed] [Google Scholar]
- 22.Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D, Malik AB. Evidence of a common mechanism of disassembly of adherens junctions through Gα13 targeting of VE-cadherin. J Exp Med 211: 579–591, 2014. doi: 10.1084/jem.20131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, Devereaux PJ, Hirsh J, Warkentin TE, Webert KE, Roxby D, Sobieraj-Teague M, Kurz A, Sessler DI, Figueroa P, Ellis M, Eikelboom JW. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med 375: 1937–1945, 2016. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 24.Hess JR. Red cell changes during storage. Transfus Apheresis Sci 43: 51–59, 2010. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 118: 6675–6682, 2011. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 115: 4284–4292, 2010. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jy W, Gomez-Marin O, Salerno TA, Panos A, Williams D, Shariatmadar S, Johansen ME, Bidot C, Horstman LL, Ahn YS. Transfusion with washed vs. unwashed packed red cells in coronary artery bypass graft (CABG) surgery: major ourcome differences. Blood 124: 2887, 2014. doi: 10.1182/blood.V124.21.2887.2887. [DOI] [Google Scholar]
- 28.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 358: 1229–1239, 2008. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 29.Leal-Noval SR, Jara-López I, García-Garmendia JL, Marín-Niebla A, Herruzo-Avilés A, Camacho-Laraña P, Loscertales J. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology 98: 815–822, 2003. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Zhao W, Christ GJ, Gladwin MT, Kim-Shapiro DB. Nitric oxide scavenging by red cell microparticles. Free Radic Biol Med 65: 1164–1173, 2013. doi: 10.1016/j.freeradbiomed.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 280: C719–C741, 2001. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 32.Mangalmurti NS, Chatterjee S, Cheng G, Andersen E, Mohammed A, Siegel DL, Schmidt AM, Albelda SM, Lee JS. Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products. Transfusion 50: 2353–2361, 2010. doi: 10.1111/j.1537-2995.2010.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangalmurti NS, Friedman JL, Wang LC, Stolz D, Muthukumaran G, Siegel DL, Schmidt AM, Lee JS, Albelda SM. The receptor for advanced glycation end products mediates lung endothelial activation by RBCs. Am J Physiol Lung Cell Mol Physiol 304: L250–L263, 2013. doi: 10.1152/ajplung.00278.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood 113: 1158–1166, 2009. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martí-Carvajal AJ, Simancas-Racines D, Peña-González BS. Prolonged storage of packed red blood cells for blood transfusion. Cochrane Database Syst Rev 7: CD009330, 2015. doi: 10.1002/14651858.CD009330.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min JJ, Bae JY, Kim TK, Hong DM, Hwang HY, Kim KB, Han KS, Jeon Y. Association between red blood cell storage duration and clinical outcome in patients undergoing off-pump coronary artery bypass surgery: a retrospective study. BMC Anesthesiol 14: 95, 2014. doi: 10.1186/1471-2253-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulder HD, Augustijn QJ, van Woensel JB, Bos AP, Juffermans NP, Wösten-van Asperen RM. Incidence, risk factors, and outcome of transfusion-related acute lung injury in critically ill children: a retrospective study. J Crit Care 30: 55–59, 2015. doi: 10.1016/j.jcrc.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Muszynski JA, Bale J, Nateri J, Nicol K, Wang Y, Wright V, Marsh CB, Gavrilin MA, Sarkar A, Wewers MD, Hall MW. Supernatants from stored red blood cell (RBC) units, but not RBC-derived microvesicles, suppress monocyte function in vitro. Transfusion 55: 1937–1945, 2015. doi: 10.1111/trf.13084. [DOI] [PubMed] [Google Scholar]
- 39.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg 137: 711–716, 2002. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 40.Peters AL, van Hezel ME, Juffermans NP, Vlaar AP. Pathogenesis of non-antibody mediated transfusion-related acute lung injury from bench to bedside. Blood Rev 29: 51–61, 2015. doi: 10.1016/j.blre.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Peters AL, Vervaart MA, van Bruggen R, de Korte D, Nieuwland R, Kulik W, Vlaar AP. Non-polar lipids accumulate during storage of transfusion products and do not contribute to the onset of transfusion-related acute lung injury. Vox Sang 112: 25–32, 2017. doi: 10.1111/vox.12453. [DOI] [PubMed] [Google Scholar]
- 42.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion 25: 573–577, 1985. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 43.Qing DY, Conegliano D, Shashaty MG, Seo J, Reilly JP, Worthen GS, Huh D, Meyer NJ, Mangalmurti NS. Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. Am J Respir Crit Care Med 190: 1243–1254, 2014. doi: 10.1164/rccm.201406-1095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafikova O, Williams ER, McBride ML, Zemskova M, Srivastava A, Nair V, Desai AA, Langlais PR, Zemskov E, Simon M, Mandarino LJ, Rafikov R. Hemolysis-induced lung vascular leakage contributes to the development of pulmonary hypertension. Am J Respir Cell Mol Biol 59: 334–345, 2018. doi: 10.1165/rcmb.2017-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rana R, Fernández-Pérez ER, Khan SA, Rana S, Winters JL, Lesnick TG, Moore SB, Gajic O. Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion 46: 1478–1483, 2006. doi: 10.1111/j.1537-2995.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 46.Serrano K, Pambrun C, Levin E, Devine DV. Supernatant reduction of stored gamma-irradiated red blood cells minimizes potentially harmful substances present in transfusion aliquots for neonates. Transfusion 57: 3009–3018, 2017. doi: 10.1111/trf.14270. [DOI] [PubMed] [Google Scholar]
- 47.Shah A, Brunskill SJ, Desborough MJ, Doree C, Trivella M, Stanworth SJ. Transfusion of red blood cells stored for shorter versus longer duration for all conditions. Cochrane Database Syst Rev 12: CD010801, 2018. doi: 10.1002/14651858.CD010801.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud PO, Romero IA, Weksler B, Gillies MC. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-β1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol 90: 323–332, 2011. doi: 10.1016/j.ejcb.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Silliman CC, Burke T, Kelher MR. The accumulation of lipids and proteins during red blood cell storage: the roles of leucoreduction and experimental filtration. Blood Transfus 15: 131–136, 2017. doi: 10.2450/2017.0314-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silliman CC, Kelher MR, Khan SY, West FB, McLaughlin NJD, Elzi DJ, England K, Bjornsen J, Kuldanek SA, Banerjee A. Supernatants and lipids from stored red blood cells activate pulmonary microvascular endothelium through the BLT2 receptor and protein kinase C activation. Transfusion 57: 2690–2700, 2017. doi: 10.1111/trf.14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion 51: 2549–2554, 2011. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest 101: 1458–1467, 1998. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmons JD, Lee YL, Pastukh VM, Capley G, Muscat CA, Muscat DC, Marshall ML, Brevard SB, Gillespie MN. Potential contribution of mitochondrial DNA damage associated molecular patterns in transfusion products to the development of acute respiratory distress syndrome after multiple transfusions. J Trauma Acute Care Surg 82: 1023–1029, 2017. doi: 10.1097/TA.0000000000001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spadaro S, Taccone FS, Fogagnolo A, Fontana V, Ragazzi R, Verri M, Valpiani G, Greco P, Bianconi M, Govoni M, Reverberi R, Volta CA. The effects of storage of red blood cells on the development of postoperative infections after noncardiac surgery. Transfusion 57: 2727–2737, 2017. doi: 10.1111/trf.14249. [DOI] [PubMed] [Google Scholar]
- 55.Sparrow RL. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus 8, Suppl 3: s26–s30, 2010. doi: 10.2450/2010.005S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparrow RL, Patton KA. Supernatant from stored red blood cell primes inflammatory cells: influence of prestorage white cell reduction. Transfusion 44: 722–730, 2004. doi: 10.1111/j.1537-2995.2004.03113.x. [DOI] [PubMed] [Google Scholar]
- 57.Stapley R, Rodriguez C, Oh JY, Honavar J, Brandon A, Wagener BM, Marques MB, Weinberg JA, Kerby JD, Pittet JF, Patel RP. Red blood cell washing, nitrite therapy, and antiheme therapies prevent stored red blood cell toxicity after trauma-hemorrhage. Free Radic Biol Med 85: 207–218, 2015. doi: 10.1016/j.freeradbiomed.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, Carson JL, Levy JH, Whitman G, D’Andrea P, Pulkrabek S, Ortel TL, Bornikova L, Raife T, Puca KE, Kaufman RM, Nuttall GA, Young PP, Youssef S, Engelman R, Greilich PE, Miles R, Josephson CD, Bracey A, Cooke R, McCullough J, Hunsaker R, Uhl L, McFarland JG, Park Y, Cushing MM, Klodell CT, Karanam R, Roberts PR, Dyke C, Hod EA, Stowell CP. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 372: 1419–1429, 2015. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Z, Li X, Massena S, Kutschera S, Padhan N, Gualandi L, Sundvold-Gjerstad V, Gustafsson K, Choy WW, Zang G, Quach M, Jansson L, Phillipson M, Abid MR, Spurkland A, Claesson-Welsh L. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J Exp Med 209: 1363–1377, 2012. doi: 10.1084/jem.20111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timperio AM, Mirasole C, D’Alessandro A, Zolla L. Red blood cell lipidomics analysis through HPLC-ESI-qTOF: application to red blood cell storage. J Integr OMICS 3: 11–24, 2013. doi: 10.5584/jiomics.v3i1.123. [DOI] [Google Scholar]
- 61.Tornero E, Pereira A, Bravo J, Angulo S, Basora M, Marcos M, Soriano A. Transfusion of packed red blood cells stored >14 days was associated with a higher risk of infection after hip revision arthroplasty. Hip Int 26: 132–137, 2016. doi: 10.5301/hipint.5000324. [DOI] [PubMed] [Google Scholar]
- 62.Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, Lowell CA, Norris PJ, Murphy EL, Weiskopf RB, Wilson G, Koenigsberg M, Lee D, Schuller R, Wu P, Grimes B, Gandhi MJ, Winters JL, Mair D, Hirschler N, Sanchez Rosen R, Matthay MA; TRALI Study Group . Transfusion-related acute lung injury: incidence and risk factors. Blood 119: 1757–1767, 2012. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 64.Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol 281: L565–L574, 2001. doi: 10.1152/ajplung.2001.281.3.L565. [DOI] [PubMed] [Google Scholar]
- 65.Vlaar AP, Binnekade JM, Prins D, van Stein D, Hofstra JJ, Schultz MJ, Juffermans NP. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case-control study. Crit Care Med 38: 771–778, 2010. doi: 10.1097/CCM.0b013e3181cc4d4b. [DOI] [PubMed] [Google Scholar]
- 66.Vlaar AP, Hofstra JJ, Determann RM, Veelo DP, Paulus F, Kulik W, Korevaar J, de Mol BA, Koopman MM, Porcelijn L, Binnekade JM, Vroom MB, Schultz MJ, Juffermans NP. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood 117: 4218–4225, 2011. doi: 10.1182/blood-2010-10-313973. [DOI] [PubMed] [Google Scholar]
- 67.Vlaar AP, Hofstra JJ, Levi M, Kulik W, Nieuwland R, Tool AT, Schultz MJ, de Korte D, Juffermans NP. Supernatant of aged erythrocytes causes lung inflammation and coagulopathy in a “two-hit” in vivo syngeneic transfusion model. Anesthesiology 113: 92–103, 2010. doi: 10.1097/ALN.0b013e3181de6f25. [DOI] [PubMed] [Google Scholar]
- 68.Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: a clinical review. Lancet 382: 984–994, 2013. doi: 10.1016/S0140-6736(12)62197-7. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Li Q, Ma T, Liu X, Wang B, Wu Z, Dang S, Lv Y, Wu R. Transfusion of older red blood cells increases the risk of acute kidney injury after orthotopic liver transplantation: a propensity score analysis. Anesth Analg 127: 202–209, 2018. doi: 10.1213/ANE.0000000000002437. [DOI] [PubMed] [Google Scholar]
- 70.Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 56: 2173–2183, 2016. doi: 10.1111/trf.13676. [DOI] [PubMed] [Google Scholar]
- 71.Xiong Y, Hla T. S1P control of endothelial integrity. Curr Top Microbiol Immunol 378: 85–105, 2014. doi: 10.1007/978-3-319-05879-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg 178: 570–572, 1999. doi: 10.1016/S0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 73.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med 39: 2478–2486, 2011. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]