Abstract
Patients undergoing coronary angiography after myocardial infarction (MI) often develop cardiac and renal dysfunction. We hypothesized that the apolipoprotein A-I mimetic peptide 4F (4F) would prevent those complications. Male Wistar rats were fed a high-cholesterol diet for 8 days. The rats were then anesthetized with isoflurane and randomly divided into five groups: a control group (sham-operated rats), and four groups of rats induced to MI by left coronary artery ligation, the rats in three of those groups being injected 6 h later, with the nonionic contrast agent iopamidol, 4F, and iopamidol plus 4F, respectively. At postprocedure hour 24, we performed the following experiments/tests (n = 8 rats/group): metabolic cage studies; creatinine clearance studies; analysis of creatinine, urea, sodium, potassium, triglycerides, total cholesterol, very low-, low- and high-density lipoproteins (VLDL, LDL, and HDL); immunohistochemistry; histomorphometry; Western blot analysis; and transmission electron microscopy. In another set of experiments (n = 8 rats/group), also performed at postprocedure hour 24, we measured mean arterial pressure, heart rate, heart rate variability, echocardiographic parameters, left ventricular systolic pressure, and left ventricular end-diastolic pressure. 4F protected against MI-induced increases in total cholesterol, triglycerides, and LDL; increased HDL levels; reversed autonomic and cardiac dysfunction; decreased the myocardial ischemic area; minimized renal and cardiac apoptosis; protected mitochondria; and strengthened endothelia possibly by minimizing Toll-like receptor 4 upregulation (thus restoring endothelial nitric oxide synthase protein expression) and by upregulating vascular endothelial growth factor protein expression. 4F-treated animals showed signs of cardiac neovascularization. The nitric oxide-dependent cardioprotection and renoprotection provided by 4F could have implications for post-MI treatment.
Keywords: apolipoprotein A, hypercholesterolemia, contrast media/adverse effects, myocardial infarction, peptides
INTRODUCTION
Myocardial infarction (MI) is a leading cause of death worldwide. Cholesterol fractions such as low- and high-density lipoproteins (LDL and HDL) are among the most commonly measured biomarkers in clinical medicine. Observational studies have shown that LDL and HDL levels have opposing (respectively, positive and negative) associations with the risk of MI (15, 42). A low HDL level is a risk factor for major cardiovascular events (13, 22, 57). In vitro studies and preclinical models have shown that HDL and its main protein constituent apolipoprotein A-I (apoA-I), possess potent vasculoprotective properties, having antioxidant, anti-inflammatory, and antiapoptotic effects on the endothelium (10, 36). There is emerging evidence that HDL augments physiological, hypoxia-mediated angiogenic processes. Infusions of reconstituted HDL in mice have been shown to improve blood flow recovery following ischemia (49). In an elegant study, Prosser et al. (43) found that infusion of apoA-I increased hypoxia-mediated angiogenesis in a murine model of hindlimb ischemia, consistent with the multifunctional regulation of neovascularization observed in vivo, promoting key angiogenic processes during hypoxia. The authors found that, under hypoxic conditions, reconstituted HDL augmented hypoxia-inducible factor 1, α-subunit, as well as increasing expression of vascular endothelial growth facor (VEGF) and VEGF receptor 2. They concluded that apoA-I promotes physiological, hypoxia-mediated angiogenesis. It has also been demonstrated that HDL regulates endothelial progenitor cells by increasing endothelial nitric oxide synthase (eNOS) and preventing apoptosis (37). The synthetic apoA-I mimetic peptide 4F (hereafter called 4F) mimics many of the properties of apoA-I, acting as a mediator of cholesterol efflux and thus countering the proinflammatory effects of LDL (5, 23, 33, 56).
Radiocontrast (RC)-induced acute kidney injury (AKI) continues to be a common cause of hospital-acquired renal injury. Various studies have demonstrated that diabetic nephropathy, volume depletion, hypercholesterolemia, and preexisting renal insufficiency are the major risk factors to the development of radiocontrast-induced AKI (41, 55). It has been demonstrated that nitric oxide (NO) plays an important protective role in the renal response to the administration of radiocontrast material (1, 3). Because NO can mediate the balance between oxygenation and vasodilation in the renal medulla, it could play an important role in the prevention of renal medullary hypoxic injury (7, 8).
In the present study, we attempted to determine whether 4F protects against the cardiac and renal dysfunction seen in a rat model of MI. We used a model of MI followed by radiocontrast administration in an attempt to mimic what occurs in patients suffering an MI, who typically undergo coronary angiography thereafter. We hypothesized that the mechanism of cardiac and renal dysfunction in the MI model is mediated, at least in part, by inflammation, apoptosis, upregulation of Toll-like receptor 4 (TLR4), and downregulation of eNOS. apoA-I regulates angiogenesis, augmenting hypoxia-mediated neovascularization. It is also known that modulators such as apoA-I, eNOS, and VEGF are conditionally regulated, which might partially explain the mechanisms of the vascular biological effects of HDL.
METHODS
Ethical aspects.
The experimental protocol was approved by the Research Ethics Committee of the University of São Paulo School of Medicine (Reference no. 261/13). The animals were handled with care, to minimize discomfort, distress, and pain, in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, 2011 revised).
The authors declare that all supporting data are available within the article data. Requests for detailed analytic methods will be addressed by the corresponding author.
Animals and experimental protocol.
Male Wistar rats, weighing 180–230 g, were obtained from the animal facility of the University of São Paulo School of Medicine, São Paulo, Brazil. Over an 8-day period, the rats were fed a high-cholesterol diet and given ad libitum access to tap water. The rats were randomly divided into five groups: Sham, comprising sham-operated rats; MI, comprising rats anesthetized with isoflurane and subsequently being induced to MI by ligation of the left coronary artery with a 7-0 polypropylene suture, as previously described (51); MI+RC, comprising rats induced to MI (as above) and receiving, 6 h later, a femoral vein injection of the nonionic RC agent iopamidol (2.9 g/kg body wt); MI+4F, comprising rats induced to MI (as above) and receiving, 6 h later, an intraperitoneal injection of 4F (sequence: Ac-DWFKAFYDKVAEKFKEAFNH2, 10 mg/kg body wt; Atlantic Peptides, Lewisburg, PA); and MI+RC+4F, comprising rats induced to MI (as above) and receiving, 6 h later, a femoral vein injection of iopamidol (2.9 g/kg body wt) and an intraperitoneal injection of 4F (10 mg/kg body wt). The dosage of 4F was chosen on the basis of those employed in previous studies demonstrating its anti-inflammatory properties in animal models and in humans (6, 14, 21, 58). All experiments were conducted 24 h after the sham procedure or MI induction. Animals were euthanized with intraperitoneal injections of ketamine and xylazine at three times the normal dosage.
MI induction.
Before surgery, the animals were anesthetized with isoflurane (1.5%). Each animal was then placed in the supine position, and lateral thoracotomy was performed in the left fourth intercostal space, separating the latissimus dorsi and pectoral muscles. A small self-retaining retractor was employed to hold the intercostal space open to visualize the beating heart. The pericardium was then opened with a sterile, flexible cotton-tipped rod. The left anterior descending coronary artery was identified and then occluded 2 mm from its origin between the edge of the left atrium and the pulmonary artery sulcus, with a 7-0 polypropylene suture (Prolene; Ethicon, Somerville, NJ). The infarcted area was immediately identified by the difference in color and the loss of contractile function. The thorax was then closed in two layers with simple interrupted 4-0 monofilament nylon sutures. In the postoperative period, the rats received an intramuscular injection of morphine and benzylpenicillin (30,000 IU). After having fully recovered from the anesthesia and surgery, each rat was returned to its home cage and maintained under standard laboratory conditions.
Study outline.
Using eight animals per group, we performed the following experiments: metabolic cage studies, creatinine clearance studies, analysis of blood and urine, immunohistochemistry, histomorphometry, Western blot analysis, and transmission electron microscopy (to evaluate the heart and kidney mitochondria). After receiving radiocontrast, 4F, or both, the animals were moved to individual cages and maintained on a 12:12-h light-dark cycle with ad libitum access to water only (no food provided). At 24 h after the sham procedure or MI induction, urine samples were collected, water intake was quantified, and urine volume was measured. Animals were then anesthetized with intraperitoneal injections of ketamine (0.05 mg/100 g body wt) and xylazine (0.01 mg/100 g body wt), after which blood samples were collected and the organs perfused with PBS. The kidneys and heart were removed immediately thereafter. Some kidneys and hearts were frozen in liquid nitrogen and stored at −70°C for subsequent immunoblotting. Renal and cardiac tissues were also processed for immunohistochemistry (to quantify cardiac and renal cell apoptosis as well as cardiac angiogenesis), histological analyses, and transmission electron microscopy (to evaluate mitochondria). Biochemical analyses were performed in plasma. The size of the infarct area was determined with the method described by Takagawa et al. (51). In another set of experiments (n = 8 rats/group), also conducted at postprocedure hour 24, we measured mean arterial pressure (MAP), heart rate (HR), heart rate variability, echocardiographic parameters, left ventricular systolic pressure (LVSP), and left ventricular end-diastolic pressure (LVEDP).
Biochemical analysis.
We determined plasma levels of creatinine, urea, sodium, potassium, lactate, triglycerides, total cholesterol, VLDL, LDL, and HDL. To that end, we used an automated analyzer (COBAS; Roche Diagnostics, Indianapolis, IN).
Histopathological sampling and analysis of hearts.
Hearts were harvested, washed in potassium chloride (1 M), and stored at −20°C for 1 h in an acrylic heart matrix (Harvard Apparatus, Holliston, MA). The hearts were cut with a razor blade into 1-mm or 2-mm sections, six to nine sections being obtained from the base to the apex. Each section was embedded in paraffin with a standard histological procedure. The paraffin blocks were sectioned at 5-µm intervals with a rotary microtome and stained with Masson-Goldner trichrome staining. The slices were successively incubated in the following solutions: Mayer’s hematoxylin (Merck, Zug, Switzerland); Ponceau-acid fuchsin (Sigma-Aldrich, Buchs, Switzerland); phosphomolybdic acid-orange G (Merck and Sigma-Aldrich, respectively); and Lichtgrün (Sigma-Aldrich). The samples were dehydrated in a graded (ascending) ethanol series and coverslipped with mounting medium (Eukitt; Electron Microscopy Sciences, Hatfield, PA). Images were acquired with a digital scanner (Scanscope CS; Aperio Technologies, Vista, CA), and the dedicated software (ImageScope; Aperio Technologies) was used to measure the scar thickness in the middle of the infarct and to determine the infarct area. The size of the infarct area was calculated as the proportion of the left ventricular area infarcted in relation to the left ventricular area as a whole. The measurements were performed on one section per heart and were then averaged.
Cardiac cell apoptosis.
Sections were processed for terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining with an in situ cell death detection kit (DNase; Roche, Penzberg, Germany) according to the manufacturer’s instructions. Sections were counterstained with 4′,6-diamidino-2-phenylindole, coverslipped, and visualized under fluorescence microscopy. The TUNEL-positive cells were quantified in 30 randomLy chosen fields per slide by an investigator who was blinded to the groups. To obtain the mean numbers of TUNEL-positive myocytes, all fields (0.087 mm2 each) were evaluated and the mean counts per heart calculated.
Renal cell apoptosis.
Renal cell apoptosis was assessed by TUNEL staining with an in situ cell death detection kit (DNase, Roche). The TUNEL-positive cells were quantified in 30 randomLy chosen fields per slide by an investigator who was blinded to the groups. To obtain the mean numbers of TUNEL-positive cells in the tubules, all fields (0.087 mm2 each) were evaluated and the mean counts per kidney calculated.
Transmission electron microscopy to evaluate kidney and heart mitochondria.
To determine the ultrastructural alterations and number of mitochondria, we performed transmission electron microscopy. Kidneys and hearts were minced and fixed in 3% glutaraldehyde. Purified mitochondrial preparations were obtained, pelleted, and fixed in 2.5% glutaraldehyde in PBS (pH 7.4) at 4°C. The specimens were postfixed with 2% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4), dehydrated, embedded in epoxy resin, cut into thin (90-nm) sections, and viewed under transmission electron microscopy. The quantitative analysis was performed in 15 images per sample (magnification, ×15,000 and ×100,000) by an investigator who was blinded to the origin of the samples. The mitochondria were quantified by use of the ImageJ program (National Institutes of Health, Bethesda, MD), and the counts are expressed as mitochondria per the square of 100 micromoles.
Histology.
For CD68 and TLR4 immunostaining in renal and cardiac tissues, respectively, samples were processed in 4-mm paraffin sections. After deparaffinization, endogenous peroxidase activity was blocked with 0.3% H2O2 in water for 10 min at room temperature. Sections were then incubated overnight at 4°C with CD68 antibody (clone ED1, 1:100; AbD Serotec, Oxford, UK) and anti-TLR4 (sc-1615, 1:50; Santa Cruz Biotechnology, Dallas, TX). That was followed by incubation with the secondary antibody (goat anti-mouse IgG; Zymed Laboratories, South San Francisco, CA) at 37°C for 30 min. The reaction product was detected with the avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA). The color reaction was developed with 3,3′-diaminobenzidine (Sigma-Aldrich). To develop the color reaction, the sections were counterstained with methyl green and then with freshly prepared 3,3′-diaminobenzidine solution. Sections were counterstained with hematoxylin and mounted for light microscopy. For all sections, negative controls consisted of replacing the primary antibody with equivalent concentrations of an irrelevant normal rabbit IgG. The sections were examined under light microscopy at a magnification of ×400. To obtain the mean numbers of infiltrating CD68-positive cells in the renal cortical tubulointerstitium, all fields (0.087 mm2 each) were evaluated and the mean counts per kidney were calculated. To obtain the mean numbers of TLR4-stained cells, the optical density was evaluated with computer-assisted image analysis (Image-Pro Plus 6.0; Media Cybernetics, Silver Spring, MD). Six fields were randomLy chosen for each slide, and the mean optical density was calculated. The assays were performed in a blinded manner.
Isolectin staining.
The hearts were arrested in diastole. They were then fixed, dehydrated, and embedded in paraffin. To measure the levels of cardiac angiogenesis in the different groups, paraffin sections of the whole heart were used for Griffonia simplicifolia isolectin B4 (isolectin GS-IB4) staining, according to the manufacturer’s instructions (Vector Laboratories). Photomicrographs of isolectin GS-IB4 staining were obtained. The labeling was visualized with fluorescein, and an antifade reagent (ProLong Gold; Invitrogen, Carlsbad, CA) was used as the mounting medium for paraffin sections of renal and cardiac tissues in multiple immunofluorescence labeling. Eight fields in each section were randomLy chosen for counting vessel profiles at the border and remote area separately. The capillary density was expressed as capillaries per high-power field.
Electrophoresis and immunoblotting.
Kidney and heart samples were run on polyacrylamide minigels. After transfer by electroelution to nitrocellulose membranes (GE Healthcare, Little Chalfont, UK), blots were blocked with 5% nonfat dry milk in Tris-buffered saline. Blots were then incubated overnight with antibodies against eNOS (1:500; Santa Cruz Biotechnology), VEGF (1:200; Santa Cruz Biotechnology), and apoA-I (1:2,500; LifeSpan BioSciences, Seattle, WA). The labeling was visualized with a horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG, 1:2,000, or anti-mouse, 1:2,000; Sigma Chemical) and enhanced chemiluminescence detection (GE Healthcare Life Sciences, Pittsburgh, PA). We scanned the enhanced chemiluminescence films with an imaging system (Alliance 4.2; UVItec, Cambridge, UK). We used densitometry to perform a quantitative analysis of the antibodies, normalizing the bands to α-actin or glyceraldehyde-3-phosphate dehydrogenase expression. Using eight animals per group, we evaluated autonomic function, performed echocardiography, and assessed left ventricular function.
Autonomic evaluations.
One day before MI induction or the sham procedure, rats were anesthetized with isoflurane, after which two catheters, each filled with 0.06 mL of saline, were implanted, one into the femoral artery and the other into the femoral vein. The free ends of the catheters were tunneled under the skin of the back to the level of the shoulder blades. The animals were placed in cages (n = 1 animal/cage) and allowed to recover from surgery. We chose to use small cages to immobilize the animals and thus facilitate the measurement of blood pressure. At 24 h after the sham procedure or MI induction, the arterial cannula was connected to a strain-gauge transducer (Blood Pressure XDCR; Kent Scientific, Litchfield, CT). Arterial pressure signals were recorded over a 30-min period in conscious animals on a microcomputer equipped with an analog-to-digital converter board (WinDaq, 2-kHz; DATAQ, Springfield, OH).
The recorded data were analyzed on a beat-to-beat basis to quantify changes in MAP and HR. Spontaneous blood pressure and HR variability at rest were then estimated by measuring the MAP and HR once every second. Sequential bolus injections (0.1 mL) of phenylephrine (0.25 µg/dose) and sodium nitroprusside (0.5 µg/dose) were given to induce increases or decreases, respectively, in the MAP pressure responses. We calculated baroreflex sensitivity on the basis of the bradycardic and tachycardic responses (expressed in beats·min−1·mmHg−1). In each experiment, the baroreceptor reflex gain was calculated as the ratio of the maximal change in HR (in beats/min) to the maximal change in MAP (in mmHg). At the end of the experiment, the baseline MAP and HR values were determined for the subsequent induction of baroreceptor reflexes.
Echocardiographic measurements.
After the autonomic evaluations had been made in conscious animals, the same animals were anesthetized with ketamine (80 mg/kg body wt) and xylazine (12 mg/kg body wt), after which they were submitted to additional echocardiographic measurements and evaluation of left ventricular function. Echocardiography was performed by an observer who was blinded to the groups, in accordance with the guidelines of the American Society of Echocardiography. Images were obtained with a 10- to 14-MHz linear transducer in an ultrasound scanning system (SEQUOIA 512; Acuson, Mountain View, CA) for measurements of the following: 1) morphometric parameters: left ventricular mass (corrected for body weight) and left ventricular end-diastolic diameter); 2) systolic function parameters: left ventricular ejection fraction (LVEF) and circumferential fiber shortening velocity; 3) diastolic function parameters: left ventricular isovolumetric relaxation time and peak E-wave deceleration time, both of which are HR dependent, divided by the square root of the RR interval; and 4) one global function parameter: the myocardial performance index.
Evaluation of left ventricular function.
A PE-50 catheter was inserted into the right carotid artery and advanced into the left ventricle. Ventricular pressure signals were measured with a strain-gauge transducer (Kent Scientific) and digitally recorded (for 5 min) with the WinDaq data acquisition system (DATAQ). The recorded data were analyzed on a beat-to-beat basis to quantify changes in left ventricular pressure. The following indexes were obtained: HR, left ventricular systolic pressure, and LVEDP.
Statistical analysis.
Differences among the means of multiple parameters were analyzed by one-way ANOVA followed by the Student-Newman-Keuls test. Quantitative data are expressed as means ± SE or as means ± SD (for the autonomic evaluations). Values of P < 0.05 were considered statistically significant. The statistical software employed was GraphPad Prism, version 5 (GraphPad, La Jolla, CA).
RESULTS
Treatment with 4F protects against MI-induced autonomic dysfunction.
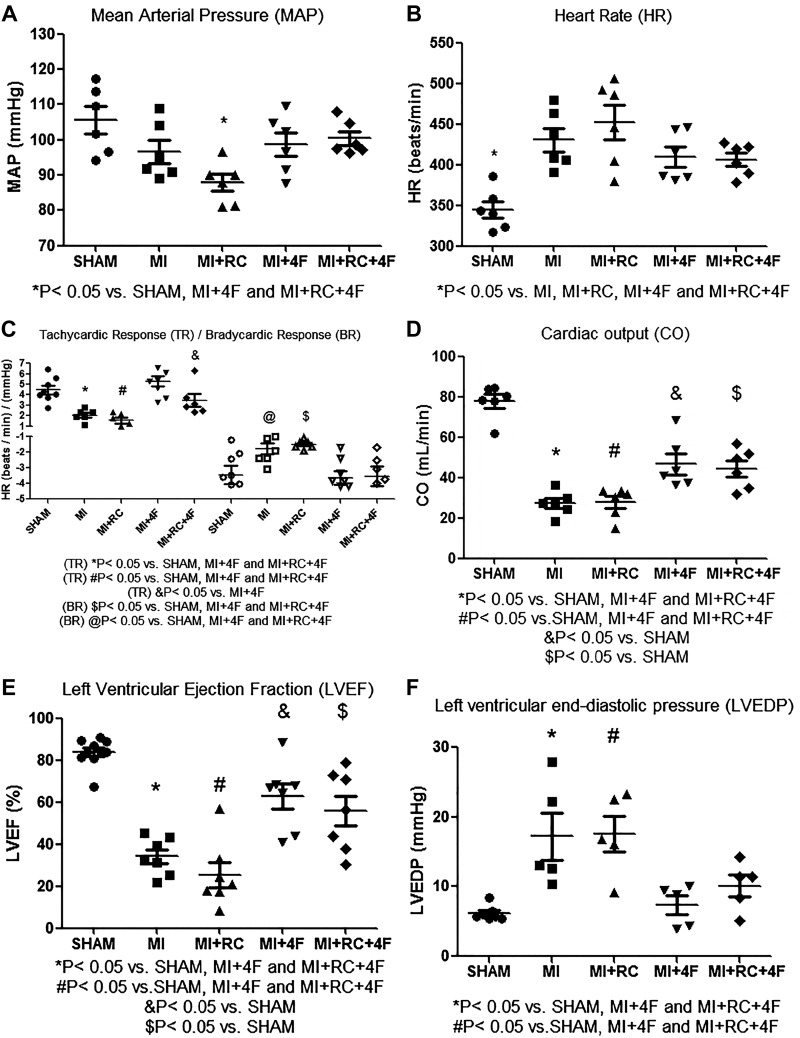
The mean value for MAP (in mmHg) was significantly lower in the MI+RC group rats than in the Sham, MI+4F, and MI+RC+4F groups of rats [87.8 ± 2.4 vs. 105.5 ± 3.8, 98.7 ± 3.4, and 100.4 ± 1.9, respectively (P < 0.05) for all], although it was comparable to that observed for the MI group rats (96.7 ± 3.3), as can be seen in Fig. 1A. At 24 h after the sham procedure or MI induction, the HR was higher in MI and MI+RC group rats than in Sham group of rats, and the mean HR (in beats/min) was partially normalized in the 4F-treated rats: MI+4F (409.3 ± 12) and MI+RC+4F (406.2 ± 8) vs. Sham (344.7 ± 10), MI (430.6 ± 14), and MI+RC (452.3 ± 21), and the differences were significant (P < 0.05 for all), as shown in Fig. 1B. Baroreflex sensitivity, as determined by evaluating the tachycardic and bradycardic responses, was lower in MI rats, indicating that MI induced autonomic dysfunction (Fig. 1C). The baroreflex sensitivity was completely preserved in MI+4F and MI+RC+4F rats, as evidenced by the mean bradycardic response (in beats·min−1·mmHg−1): MI+4F (−4.1 ± 0.5) and MI+RC+4F (−4.1 ± 0.9) vs. Sham (−3.38 ± 0.8), MI (−1.0 ± 0.3), and MI+RC (−1.1 ± 0.2)]; the mean tachycardic response (in beats·min−1·mmHg−1): MI+4F (5.34 ± 0.24) and MI+RC+4F (3.85 ± 0.79) vs. Sham (3.8 ± 0.36), MI (1.95 ± 0.16), and MI+RC (1.85 ± 0.55); and the differences were significant for both parameters (P < 0.05 for all).
Fig. 1.
Apolipoprotein A-I mimetic peptide 4F (4F) protects against myocardial infarction (MI)-induced autonomic and myocardium dysfunction. All studies were performed at 24 h after the sham procedure or MI induction: mean arterial pressure (note that this decreased only in the MI+radiocontrast (RC) group; A); heart rate (B); baroreflex sensitivity, as estimated by determining bradycardic response and tachycardic response at low doses of phenylephrine and sodium nitroprusside; note that treatment with 4F completely restored baroreflex sensitivity (C); cardiac output, measured by echocardiography (D); left ventricular ejection fraction, measured by echocardiography (E); and invasive evaluation of left ventricular function, in which a catheter was inserted into the right carotid artery and advanced into the left ventricle to measure left ventricular end-diastolic pressure (F); (n = 8/group, ANOVA).
Treatment with 4F protects against MI-induced myocardial dysfunction.
At 24 h after ligation of the left coronary artery or sham surgery, the mean cardiac output (in mL/min) was significantly lower in the MI and MI+RC groups than in the Sham group (27.2 ± 2.4 and 27.7 ± 3.0 vs. 77.8 ± 3.3, P < 0.05 for both), although that observed for the MI+4F and MI+RC+4F groups (46.6 ± 5.0 and 44.5 ± 3.9, respectively) was comparable to that observed for the Sham group and significantly different from that observed for the MI and MI+RC groups (P < 0.05 for both), as shown in Fig. 1D. The mean LVEF (in %) was significantly higher in MI+4F and MI+RC+4F rats than in MI and MI+RC rats (62.8 ± 6.1 and 55.8 ± 7.2, respectively, vs. 34.1 ± 3.4 and 25.4 ± 5.9, respectively), whereas it was significantly higher (83.7 ± 2.1) in the sham-operated rats (P < 0.05 for all), as can be seen in Fig. 1E. We found it interesting that 4F administration completely prevented the increase in LVEDP (in mmHg) seen in the untreated rats by 24 h after the sham procedure or MI induction: MI+4F (7.36 ± 1.3) and MI+RC+4F (10.1 ± 1.6) vs. MI (17.2 ± 3.4) and MI+RC (17.6 ± 2.5) (P < 0.05 for all), the mean values obtained for the rats treated with 4F being comparable to that observed for the sham-operated rats (6.16 ± 0.4). The mean LVEDP did not differ among the MI+4F, MI+RC+4F, and Sham groups of rats (Fig. 1F).
Treatment with 4F protects against MI and radiocontrast-induced AKI.
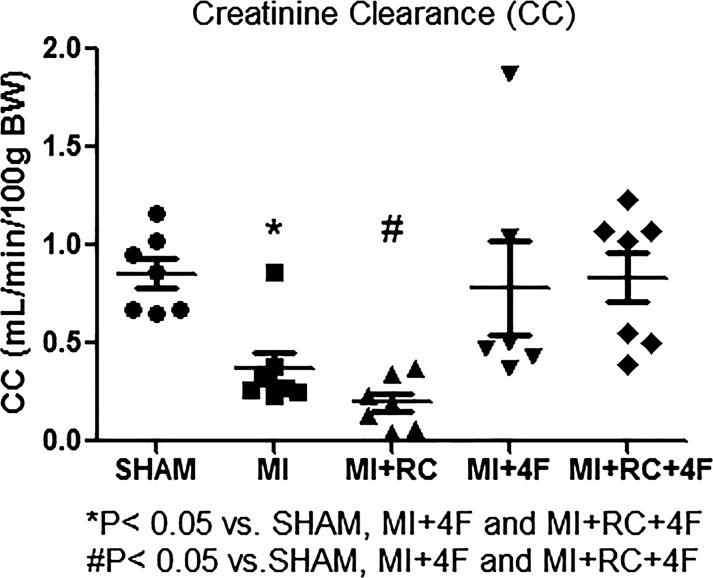
As can be seen in Fig. 2 and Table 1, the mean creatinine clearance and serum creatinine (i.e., renal function), analyzed at 24 h after the sham procedure or MI induction, were significantly lower in MI and MI+RC rats than in all other rats; both of those parameters were also lower in MI+RC rats than in MI rats, although the difference was not statistically significant. Treatment with 4F restored renal function after MI-induced AKI (Table 1).
Fig. 2.
Synthetic apolipoprotein A-I mimetic peptide 4F (4F) protects against myocardial infarction (MI)- and radiocontrast (RC)-induced acute kidney injury. Renal function was assessed by determining creatinine clearance with the formula: creatinine clearance = (Ucreatinine × Uvolume/1,440 min)/Pcreatinine, where Ucreatinine is urinary concentration of creatinine, Uvolume/1,440 min is urine output in microliters per day (1,440 min), and Pcreatinine is plasma concentration of creatinine. Clearance studies were performed in all groups: Sham (sham-operated rats), MI (rats induced to MI by ligation of the left coronary artery), MI+RC (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol), MI+4F (rats induced to MI and receiving, 6 h later, intraperitoneal injection of 4F), and MI+RC+4F (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol and intraperitoneal injection of 4F). All studies were performed 24 h after the sham procedure or MI induction (n = 8/group, ANOVA).
Table 1.
Biochemical parameters at 24 h after the sham procedure or induction of MI
| Group |
|||||
|---|---|---|---|---|---|
| Parameter | Sham | MI | MI+RC | MI+4F | MI+RC+4F |
| Creatinine, mg/dL | 0.27 ± 0.02 | 0.57 ± 0.04a,b,c | 0.65 ± 0.04a,b,c | 0.38 ± 0.02d | 0.39 ± 0.02e |
| Lactate, mmol/L | 1.54 ± 0.12 | 2.4 ± 0.11f,g,h | 2.66 ± 0.2e,i,j | 1.74 ± 0.2 | 1.87 ± 0.2 |
| Triglycerides, mg/dL | 39.8 ± 8.5 | 114.1 ± 14.6d,k,l | 120.0 ± 13.6d,k,h | 43.9 ± 11.6 | 62.58 ± 13.6 |
| Cholesterol, mg/dL | 95.7 ± 7.5 | 145.0 ± 10.5f,g,h | 154.8 ± 9.42e,i,h | 93.8 ± 11.4 | 100.9 ± 14.0 |
| VLDL, mg/dL | 7.1 ± 0.8 | 23.7 ± 0.95a,b,c | 25.3 ± 1.2a,b,c | 8.62 ± 2.1 | 11.1 ± 2.8 |
| LDL, mg/dL | 35.5 ± 4.2 | 118.5 ± 4.8a,b,c | 120.9 ± 5.4a,b,c | 42.7 ± 7.3f | 62.2 ± 5.2f |
| HDL, mg/dL | 44.0 ± 4.3 | 57.5 ± 10.7 | 42.3 ± 9.9i,h | 77.8 ± 3.9e | 72.6 ± 4.1e |
Data represent means ± SE. MI, myocardial infarction; RC, radiocontrast; 4F, apolipoprotein A-I mimetic peptide 4F; HDL, LDL, and VLDL, high-, low, and very low-density lipoprotein.
P < 0.0001 vs. Sham,
P < 0.0001 vs. MI+4F,
P < 0.0001 vs. MI+RC+4F,
P < 0.001 vs. Sham,
P < 0.01 vs. Sham,
P < 0.05 vs. Sham,
P < 0.05 vs. MI+4F,
P < 0.05 vs. MI+RC+4F,
P < 0.01 vs. MI+4F,
P < 0.01 vs. MI+RC+4F,
P < 0.001 vs. MI+4F,
P < 0.001 vs. MI+RC+4F.
Treatment with 4F protects against MI-induced increases in total cholesterol, triglycerides, and LDL.
As can be seen in Table 1, MI induced increases in the levels of total cholesterol, triglycerides, and LDL. Treatment with 4F protected against those increases, whereas it increased levels of HDL. In addition, 4F treatment appeared to protect the microcirculation, plasma lactate being significantly lower in MI+4F and MI+RC+4F rats than in MI and MI+RC rats (Table 1).
Treatment with 4F decreases the myocardial ischemic area.
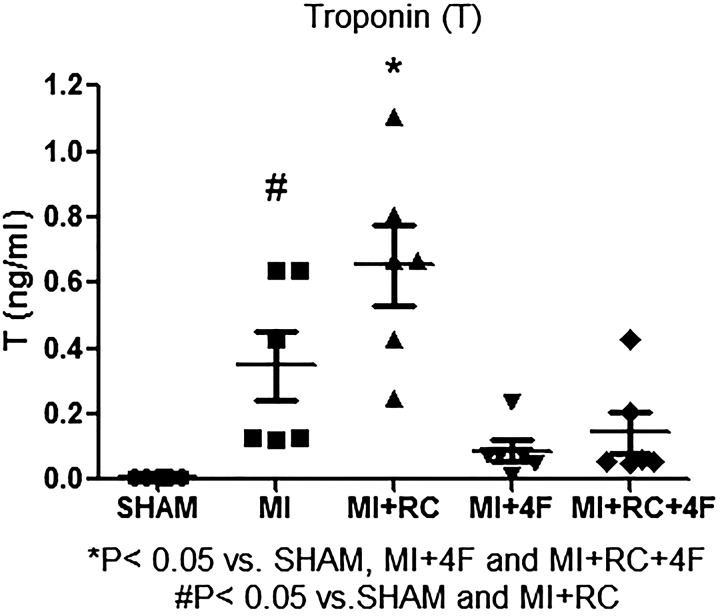
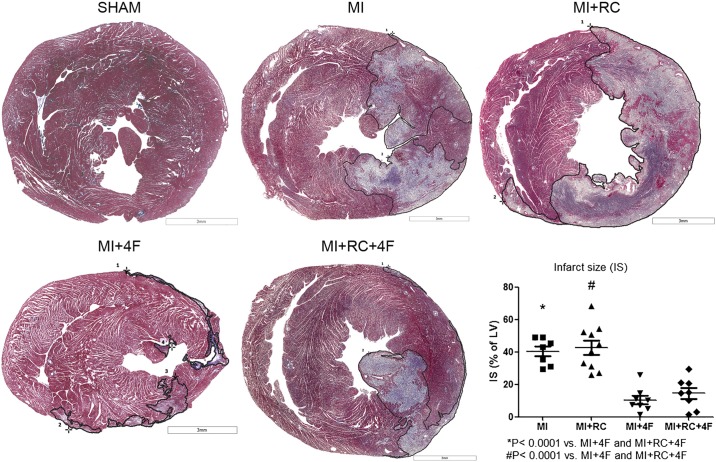
We analyzed the effect of myocardial ischemia by measuring the levels of troponin I and the myocardial ischemic area. Serum levels of troponin I were higher in MI and MI+RC rats than in MI+4F and MI+RC+4F rats (Fig. 3). One unexpected finding was that troponin I levels were higher in the MI+RC group than in the MI group, suggesting that radiocontrast administration increased the ischemic area. The infarct size was measured with Masson–Goldner trichrome staining and expressed as a percentage of the total left ventricular area. As can be seen in Fig. 4, there was no difference between the MI and MI+RC groups in terms of the infarct size. In addition, the histological measurements showed a broad spectrum of myocardial injury, the infarcts in the MI and MI+RC groups ranging from large to small. The 4F-treated animals presented smaller infarcts (Fig. 4).
Fig. 3.
Synthetic apolipoprotein A-I mimetic peptide 4F (4F) decreases serum levels of troponin I after myocardial infarction (MI) and after radiocontrast (RC) administration. Levels of troponin I were measured in all groups: Sham (sham-operated rats); MI (rats induced to MI by ligation of the left coronary artery); MI+RC (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol); MI+4F (rats induced to MI and receiving, 6 h later, intraperitoneal injection of 4F); and MI+RC+4F (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol and intraperitoneal injection of 4F). All studies were performed 24 h after sham procedure or MI induction (n = 8/group, ANOVA).
Fig. 4.
Apolipoprotein A-I mimetic peptide 4F (4F) decreases the ischemic area of the myocardium. All studies were performed 24 h after sham procedure or myocardial infarction (MI) induction. Infarct size was determined by Masson–Goldner trichrome staining and expressed as percent total left ventricular area. Infarct areas were smaller in 4F-treated animals than in untreated animals (n = 8/group, ANOVA).
Treatment with 4F decreases apoptosis in cardiac and renal tissues.
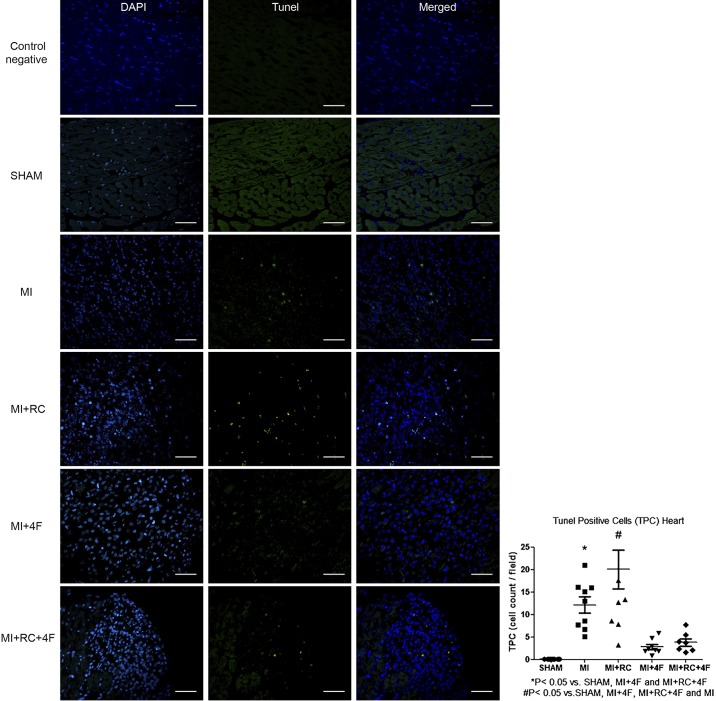
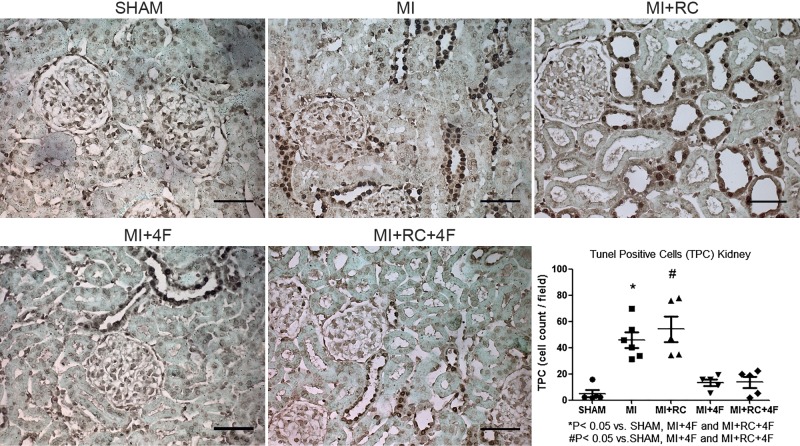
Positive TUNEL immunofluorescence staining in hearts from MI and MI+RC animals showed extensive nuclear changes consistent with apoptotic cell death, whereas the TUNEL-positive cell count was markedly lower in the MI+4F and MI+RC+4F groups, suggesting a profound antiapoptotic effect of 4F treatment (Fig. 5). The number of TUNEL-positive cells was also lower in the MI group than in the MI+RC group. We find it interesting that MI induced apoptosis in renal tissue and that the apoptosis was not aggravated by radiocontrast administration (Fig. 6). Renal tissues from MI and MI+RC rats showed extensive nuclear changes consistent with apoptotic cell death. Treatment with 4F decreased apoptosis by 60%. (Fig. 6).
Fig. 5.
Apolipoprotein A-I mimetic peptide 4F (4F) decreases apoptosis in the heart after myocardial infarction (MI). Representative micrographs from terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assays, showing nuclei (DAPI, blue), TUNEL signals (green), and merged images of myocardium tissue in all groups. All studies were performed 24 h after sham procedure or MI induction. Scale bar, 50 µm; magnification, ×400. Quantitative analysis of apoptosis is shown (n = 7–8/group, ANOVA).
Fig. 6.
Apolipoprotein A-I mimetic peptide 4F (4F) decreases apoptosis in kidneys. Representative micrographs of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) staining of renal sections in all groups. Magnification, ×400 (n = 7–8/group, ANOVA).
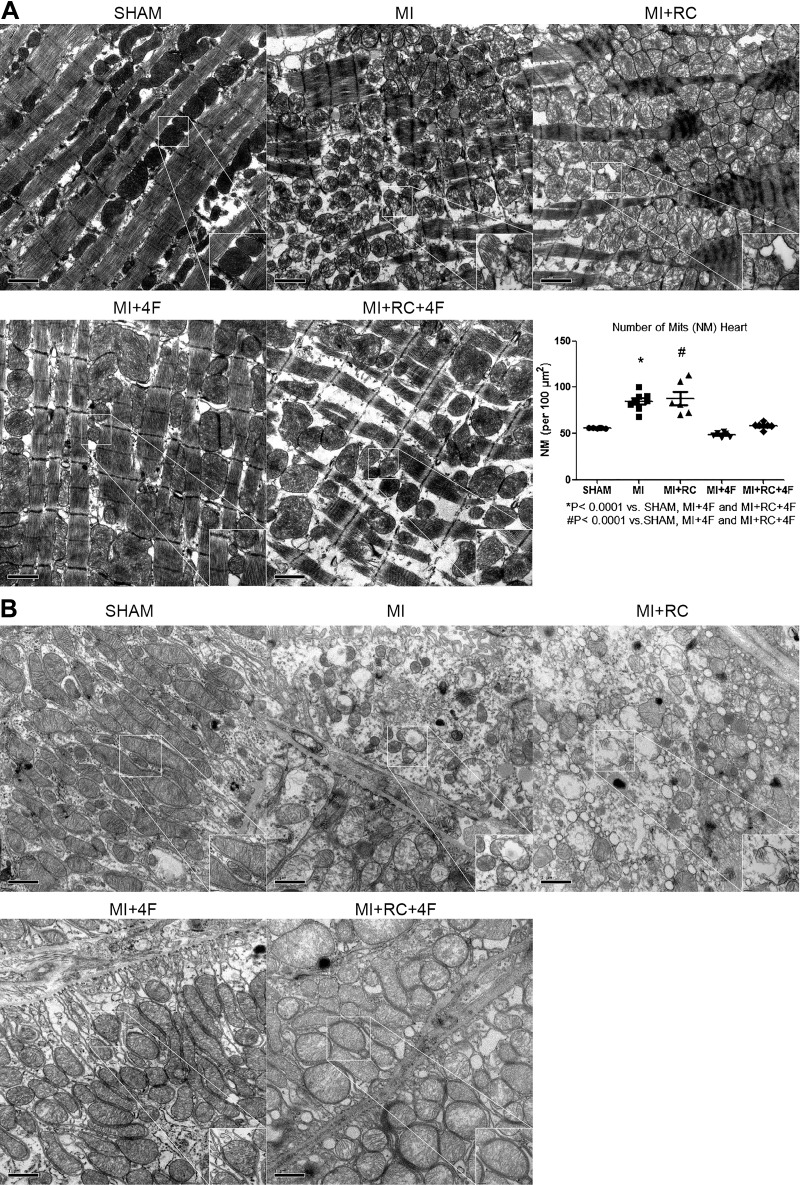
Treatment with 4F protects myocardial and kidney mitochondria.
Transmission electron microscopy revealed increased numbers of mitochondria and disruption of mitochondrial morphology in the rat myocardium after MI (Fig. 7). In the MI+4F and MI+RC+4F groups, mitochondria of uniform size and regular morphology were vertically aligned between the myocardial fibers. In the MI and MI+RC groups, the myocardial fiber network was disrupted, and the number of mitochondria was higher. After MI, the mitochondria became small and round, with an irregular arrangement (Fig. 7A). These phenomena suggest that the function of the myocardial mitochondria is disrupted by MI. Transmission electron microscopy revealed extensive damage to mitochondria in proximal tubular cells after MI (Fig. 7B). In MI and MI+RC rats, the mitochondria were round and swollen, with no cristae, some showing disrupted membranes and the release of matrix material into the cytoplasm. In contrast, the representative samples from Sham, MI+4F, and MI+RC+4F groups showed many elongated mitochondria (Fig. 7B), with structural preservation of cristae on the basal aspect of the tubular cell. A few swollen mitochondria were detected in some cells.
Fig. 7.
Synthetic apolipoprotein A-I mimetic peptide 4F (4F) protects myocardial and kidney mitochondria after myocardial infarction (MI) and after radiocontrast (RC) administration. Representative transmission electron micrograph of hearts and kidneys at magnifications of ×15,000 and ×100,000 in the following groups: MI (rats induced to MI by ligation of left coronary artery); MI+RC (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol); MI+4F (rats induced to MI and receiving, 6 h later, intraperitoneal injection of 4F); and MI+RC+4F (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol and intraperitoneal injection of 4F). Quantitative analysis of mitochondria is shown. A: in the infarcted area of the heart, mitochondria at the border zone appear to be rounder and smaller in shape; note that the number of mitochondria is higher in 4F-treated animals than in treated animals. B: transmission electron microscopy revealed extensive post-MI damage to mitochondria in proximal tubular cells. Representative samples from treated animals show many elongated mitochondria with structural preservation of cristae structure on the basal side of the tubular cell (n = 6/group, ANOVA).
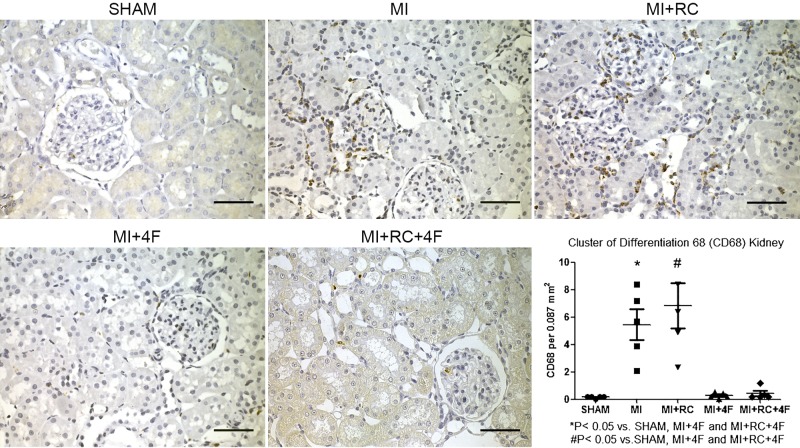
Treatment with 4F decreases macrophage infiltration in renal tissues.
Quantification of CD68 immunostaining (the mean number of CD68-positive cells per 0.087 mm2 field) revealed marked accumulation of CD68 macrophages in the kidneys of the MI and MI+RC group rats (Fig. 8), no such accumulation being observed in Sham, MI+4F, or MI+RC+4F groups of rats: MI (5.45 ± 1.13) and MI+RC (6.84 ± 1.7) vs. Sham (0.17 ± 0.03), MI+4F (0.29 ± 0.09), and MI+RC+4F (0.43 ± 0.19) (P < 0.05 for all). The CD68 immunolabeling was significantly less pronounced in the MI+4F and MI+RC+4F rats than in the MI and MI+RC rats (P < 0.05 for all).
Fig. 8.
Synthetic apolipoprotein A-I mimetic peptide 4F (4F) decreases macrophage infiltration in renal tissues after myocardial infarction (MI) and after radiocontrast (RC) administration. Immunostaining for CD68 in all groups: Sham (sham-operated rats); MI (rats induced to MI by ligation of left coronary artery); MI+RC (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol); MI+4F (rats induced to MI and receiving, 6 h later, intraperitoneal injection of 4F); and MI+RC+4F (rats induced to MI and receiving, 6 h later, femoral vein injection of iopamidol and intraperitoneal injection of 4F). Note the marked accumulation of CD68 macrophages in kidneys of MI and MI+RC groups compared with Sham, MI+4F, and MI+RC+4F groups. Quantitative analysis of CD68-positive cells is shown (n = 8/group, ANOVA).
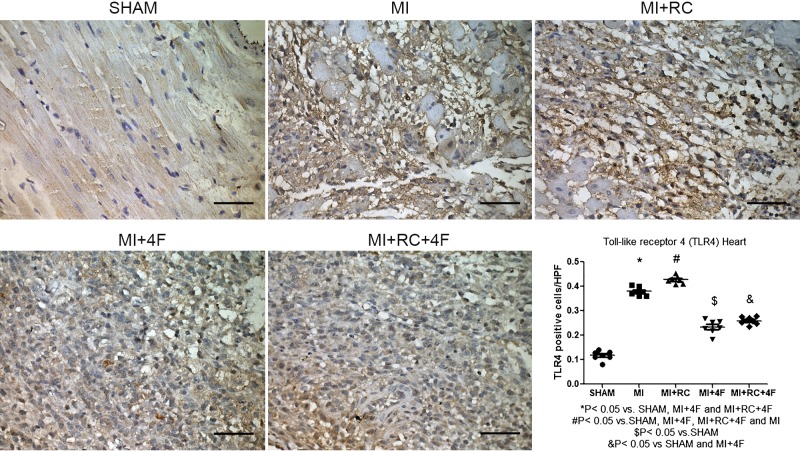
Treatment with 4F protects against MI-induced injury by decreasing TLR4 activation.
Positive immunostaining for TLR4, which manifested as a pervasive brown-yellow color in the myocardial cells, was found in the myocardial tissue from rats in all five groups. The mean protein expression of TLR4 (Fig. 9), determined by counting the number of TLR4-positive cells per high-power field, was significantly higher in the MI and MI+RC groups than in the Sham, MI+4F, and MI+RC+4F groups (0.38 ± 0.01 and 0.43 ± 0.01 vs. 0.12 ± 0.01, 0.23 ± 0.01, and 0.26 ± 0.01, respectively, P < 0.05 for all).
Fig. 9.
Toll-like receptor 4 (TLR4) protein expression in the heart is increased after myocardial infarction (MI) and after radiocontrast (RC) administration and is decreased by treatment with apolipoprotein A-I mimetic peptide 4F (4F). Immunostaining for TLR4 protein in all groups. Note that protein expression of TLR4 in myocardium, as determined by counting the number of TLR4-positive cells per high-power field, was higher in MI and MI+RC groups than in MI+4F and MI+RC+4F groups, also being higher in the MI+RC group than in the MI group. Scale bar, 50 µm; magnification, ×400. Quantitative analysis of TLR4 is shown as number of positive cells per high-power field (n = 7–8/group, ANOVA).
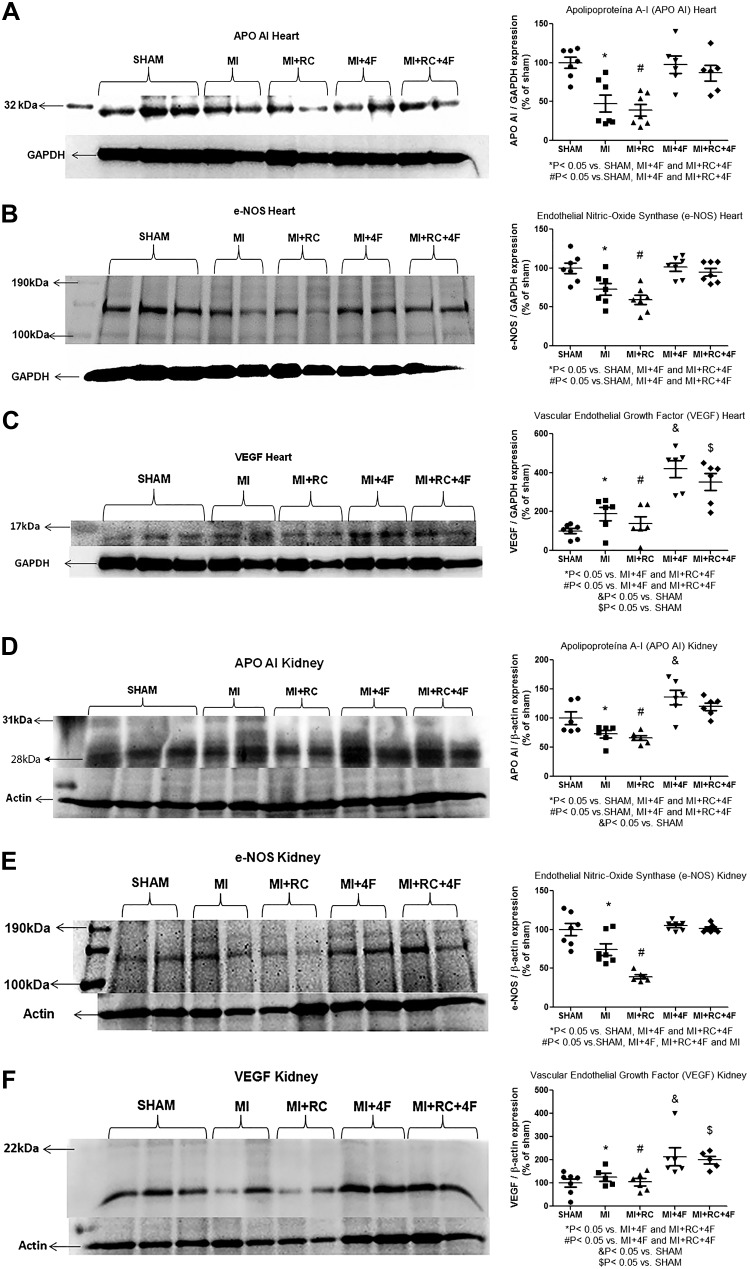
Treatment with 4F induces vascular protection, increasing apoA-I protein expression, in heart and kidneys.
In cardiac tissues, the mean protein expression of apoA-I (in %) was markedly lower in the MI and MI+RC groups than in the Sham, MI+4F, and MI+RC+4F groups (47.6 ± 11.1 and 39.3 ± 7.5 vs. 100.0 ± 7.0, 97.5 ± 11.2, and 86.7 ± 9.9, respectively; P < 0.05 for all), as shown in Fig. 10A. It is noteworthy that apoA-I protein expression recovered completely after treatment with 4F. The mean protein expression of eNOS (in %) was also lower in the MI and MI+RC groups than in the Sham, MI+4F, and MI+RC+4F groups (74.4 ± 7.5 and 39.4 ± 2.5 vs. 100.0 ± 8.0, 104.8 ± 2.3, and 101.5 ± 1.9, respectively; P < 0.05), as can be seen in Fig. 10B. At 24 h after the procedures, VEGF protein expression was also higher in MI and MI+RC rats than in sham-operated rats, although the difference was not statistically significant (Fig. 10C). However, the mean protein expression of VEGF (in %) was markedly higher in the MI+4F and MI+RC+4F groups than in the other groups: MI+4F (420.0 ± 43.6) and MI+RC+4F (352.5 ± 43.7) vs. MI (189.0 ± 35.0), MI+RC (138.9 ± 34.8), and Sham (100.0 ± 13.2) (P < 0.05 for all). In the kidneys, the pattern of apoA-I protein expression was similar to that observed in the heart (Fig. 10). Although the mean apoA-I protein expression in the kidneys (in %) was lower in the MI and MI+RC rats than in the sham-operated rats, the treatment with 4F reversed that effect: MI+4F (136.0 ± 12.7) and MI+RC+4F (120.0 ± 6.6) vs. Sham (100.0 ± 10.9), MI (72.8 ± 6.4), and MI+RC (66.0 ± 4.1) (P = 0.05 for all), as shown in Fig. 10D. In the MI group, the mean protein expression of eNOS in renal tissue (in %) was significantly lower than that observed for the Sham group and was approximately double that observed for the MI+RC group: MI (74.4 ± 7.5) vs. Sham (100.0 ± 7.9) and MI+RC (39.4 ± 2.5) (P < 0.05 for both), as shown in Fig. 10E. Treatment with 4F preserved that expression: MI+4F (104.8 ± 2.3) and MI+RC+4F (101.5 ± 1.9). We find it interesting that, although the mean renal protein expression of VEGF (in %) in the MI and MI+RC groups did not differ from that observed for the Sham group (125.8 ± 17.5 and 104.7 ± 15.7, respectively, vs. 100.0 ± 17.4, P < 0.05), it was significantly lower than that observed for the MI+4F and MI+RC+4F groups (213.5 ± 38.3 and 199.6 ± 16.7, respectively, P < 0.05 for both vs. MI and MI+RC), as depicted in Fig. 10F.
Fig. 10.
Apolipoprotein A-I (apoA-I) mimetic peptide 4F (4F) provides vascular protection in heart and kidneys, increasing apoA-I expression. Semiquantitative immunoblotting of heart and kidney fractions, together with densitometric analysis of samples. We performed the following immunoblots: reacted with anti-apoA-I antibody, revealing a 28-kDa band, in cardiac tissue (A); reacted with anti-endothelial nitric oxide synthase (anti-eNOS) antibody, revealing a 140-kDa band, in cardiac tissue (B); reacted with anti-vascular endothelial growth factor (VEGF) antibody, revealing a 22-kDa band, in cardiac tissue (C); reacted with anti-apoA-I antibody, revealing a 28-kDa band, in renal tissue (D); reacted with anti-eNOS antibody, revealing a 140-kDa band, in renal tissue (E); and reacted with anti-VEGF antibody, revealing a 22-kDa band, in renal tissue (F) (n = 6/group, ANOVA).
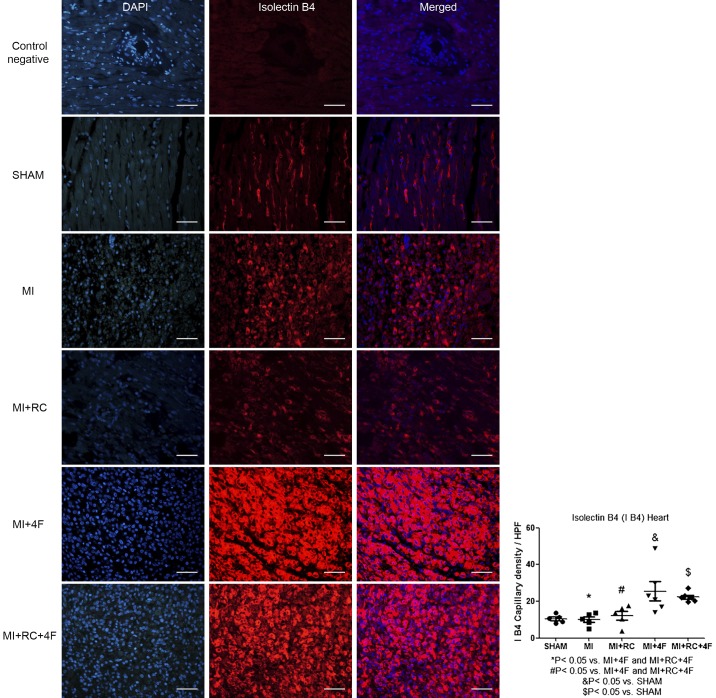
Treatment with 4F increases capillary density in the heart.
In cardiac tissue, capillary density, as determined by immunostaining for isolectin GS-IB4, remained unchanged after MI in the untreated rats (Fig. 11). However, by 24 h after the procedures, the mean capillary density (expressed as capillaries/high-power field) was increased in the treated groups, in which it was significantly greater than that observed for the untreated groups and Sham group: MI+4F (25.5 ± 5.1) and MI+RC+4F (22.3 ± 1.1) vs. MI (10.1 ± 1.5), MI+RC (12.2 ± 2.4), and Sham (10.5 ± 1.0) (P < 0.05 for all).
Fig. 11.
Apolipoprotein A-I mimetic peptide 4F (4F) increases capillary density-to-myocyte area ratio. MI, myocardial infarction; RC, radiocontrast. Representative, double-immunofluorescence images of myocardium stained for endothelial cells [red, Griffonia simplicifolia isolectin B4 (isolectin GS-IB4)] or nuclei (blue, DAPI), together with merged images showing colocalization. Scale bar, 50 µm; magnification, ×400 (n = 7–8/group, ANOVA).
DISCUSSION
Here, we have demonstrated that treatment with 4F reverses the autonomic and cardiac dysfunction occurring after MI. In addition, we found that 4F treatment decreased the myocardial ischemic area, minimized apoptosis, and protected the mitochondria. It is possible that 4F exerts those effects by minimizing TLR4 upregulation (thus protecting the cardiac endothelium), by restoring eNOS protein expression to normal levels and by upregulating VEGF protein expression. Furthermore, cardiac tissues from treated animals were found to contain greater numbers of isolectin GS-IB4-binding vessels, which are indicative of neovascularization. Moreover, we found that administration of radiocontrast increased levels of troponin I, as well as increasing apoptosis and the number of TUNEL-positive cells, in cardiac tissue. However, radiocontrast administration did not increase the myocardial infarct area.
In the present study, the levels of total cholesterol, LDL, and triglycerides at 24 h after the procedures were higher in the MI groups than in the Sham group. Pitt et al. (40) found that patients with acute coronary syndromes showed an increase in LDL levels at 4 days after the onset of the syndrome. Inflammation, endothelial dysfunction, increased thrombogenicity, and plaque vulnerability are crucial mechanisms underlying the complex interplay between cholesterol metabolism and MI (11, 48). Acute coronary syndrome significantly affects the concentration and composition of the lipids and lipoproteins in plasma. The acute-phase response alters not only the concentration of the lipoproteins but also the composition of the circulating LDL and HDL. During the acute-phase response, LDL synthesis is increased (26). In addition, the acute-phase response has quantitative and qualitative effects on HDL and its contents. Inflammation decreases the level of HDL by increasing the activity of endothelial lipase and soluble phospholipase A2 (4). Inflammation associated with hypertriglyceridemia is caused by an increase in lipoprotein production and a decrease in lipoprotein clearance. The increase in triglyceride-rich lipoproteins is secondary to the reesterification of plasma fatty acids. Clearance decreases mainly secondarily to the inhibition of lipoprotein lipase activity (34). Myocardial damage-induced stress increases the adrenergic-mediated lipolysis of the adipocytes (31). The mobilization of free fatty acids and hepatic secretion of VLDL result in elevated triglyceride levels. Golino et al. (20) investigated the effects of acute hypercholesterolemia in a rabbit model of coronary occlusion-reperfusion and detected an association between acute hypercholesterolemia and the no-reflow phenomenon, which is attributed to microvascular injury. One systematic review and meta-analysis showed that LDL-lowering therapy of greater intensity was associated with a greater reduction in risk of all-cause and cardiovascular mortality in patients with elevated baseline levels of LDL (>100 mg/dL) (35). It is known that baseline LDL concentrations are independently associated with MI-induced microvascular injury (45). Elevated LDL levels have been shown to cause inflammation and endothelial dysfunction, major mechanisms in the pathophysiology of ischemia-reperfusion injury, suggesting another link between LDL and microvascular injury (27). In animals and humans with hypercholesterolemia, endothelium-dependent relaxation is reduced and vasoconstriction is increased, a mechanism that might be related to impaired production and endothelial release of NO (24, 32, 50). In the present study, treatment with 4F decreased LDL levels and increased HDL levels, as well as increasing eNOS protein expression, in hearts and kidneys.
We observed an increase in HR at 24 h after the sham procedure or MI induction. We hypothesized that HR would increase as a compensatory mechanism to maintain MAP within the normal range. We found that post-MI treatment with 4F normalized the HR. The baroreflex system is devoted to maintaining cardiovascular homeostasis and preserving blood flow to vital organs (12). In MI, there appears to be a direct relationship between baroreflex sensitivity and survival, reduced survival having been reported in the setting of reduced baroreflex function (17, 39). It is known that eNOS activity is impaired in endothelial cells exposed to native LDL (18). In addition, oxidized LDL particles can independently alter eNOS abundance (28). In the present study, cardiac output, LVEF, and LVEDP were protected by treatment with 4F. That protection might be explained by the 4F-induced decrease in the size of the infarct area.
It has been shown that TLRs play a critical role in the activation of innate and adaptive immunity (2), as well as triggering inflammation, which impairs wound healing in various chronic diseases. Loss of TLR4 provides protection against post-MI ischemia-reperfusion injury and remodeling (16, 52). In addition, mice lacking TLR4 sustain smaller infarcts and exhibit less inflammation after myocardial ischemia-reperfusion injury than do wild-type mice (38). Therefore, modulation of TLR4 signaling is a potential therapeutic target in myocardial ischemic injury. Treatment with 4F might protect cardiac function by decreasing TLR4 expression.
We found that treatment with 4F increased eNOS expression, that increase being accompanied by overexpression of VEGF. We also demonstrated a 4F-induced increase in the number of isolectin GS-IB4-positive cells, which is indicative of neovascularization, in cardiac tissue. Inhibition of angiogenesis is known to lead to myocardial remodeling and dysfunction (54). After MI, neovascularization in the zone adjacent to the ischemic region helps preserve cardiac function and thus attenuates adverse left ventricular remodeling. Neovascularization is orchestrated by interactions among proangiogenic factors, antiangiogenic factors, the extracellular matrix, and endothelial cells in the border zone (19). Increased expression of VEGF and isolectin GS-IB4 along the border of the infarct zone might be responsible for the decreases in cardiomyocyte apoptosis and the number of mitochondria. Within the healing heart, enhanced angiogenesis enables greater delivery of nutrients, bioactive molecules, and oxygen, thereby minimizing scarring.
We found that MI induced a decrease in glomerular filtration (as determined by measuring creatinine clearance), as well as an increase in the numbers of TUNEL- and CD68-positive cells in renal tissue. In addition, MI resulted in decreased expression of apoA-I and eNOS in renal tissue. It is possible that the decrease in cardiac output and LVEF, together with the increase in LVEDP, could have been responsible for the decrease in glomerular filtration, because those changes resulted in renal vasoconstriction similar to that seen in the cardiorenal syndrome. However, there was no decrease in the MAP of the rats induced to MI. We hypothesized that that phenomenon could be related to cross talk between the organs. Ischemic renal diseases have been associated with remote organ inflammation and impaired function (25, 29, 44). Ruparelia et al. (47) demonstrated that MI results in remote organ inflammation, as evidenced by increased mRNA expression of inflammatory cytokines and marked upregulation of vascular cell adhesion molecule 1 in renal glomeruli, together with leukocyte recruitment to and infiltration of the kidneys. Van Dokkum et al. (53) found that rats undergoing unilateral nephrectomy followed by MI showed accelerated renal damage, as evidenced by increased levels of proteinuria and plasma creatinine, as well as focal glomerulosclerosis.
It is well known that the incidence of contrast-induced nephropathy depends on a number of risk factors, especially chronic kidney disease and diabetes mellitus (9, 30, 46), both of which are related to reduced NO production. A single dose of a contrast agent produces little renal injury unless its administration is preceded by a combination of insults that can dysregulate medullary blood flow. Inhibition of NO synthesis can exacerbate renal failure, inducing more extensive necrosis of the medullary thick limbs. In addition, NO plays an important role in increasing medullary oxygenation and regulating blood flow in that region (7, 8). Hypercholesterolemia has also been shown to aggravate contrast-induced nephrotoxicity (3). In the present study, the decrease in eNOS protein expression was more severe in the MI+RC group than in the MI group. The degree of renal dysfunction (as quantified by measuring creatinine clearance and serum creatinine) in the MI and MI+RC groups did not differ from that observed in any of the other groups. Although the quantification of inulin clearance would have been a better method to measure renal function, the animals were severely ill and therefore could not be submitted to the ~2-h surgery required to determine inulin clearance. Treatment with 4F completely restored renal function in the MI+4F and MI+RC+4F groups, possibly because the 4F treatment resulted in normalization of eNOS protein expression in renal tissues.
Perspectives and Significance
Treatment with 4F appears to inhibit inflammation and apoptosis, thereby strengthening endothelia. The NO-dependent cardioprotection and renoprotection provided by 4F could have implications for treatment in the post-MI period.
GRANTS
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant 2010/19012-0) and by the Laboratórios de Investigação Médica (Medical Investigation Laboratories) of the Faculdade de Medicina da Universidade de São Paulo (University of São Paulo School of Medicine). L. Andrade is the recipient of a grant from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development; Grant 301193/2016-9).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.d.S.M. and L.A. conceived and designed research; R.d.S.M., M.C.I., J.M.C.C., T.R.S., P.S.G., and M.R.G. performed experiments; R.d.S.M., M.C.I., J.M.C.C., T.R.S., P.S.G., M.R.G., I.d.L.N., and L.A. analyzed data; R.d.S.M., T.R.S., P.S.G., I.d.L.N., and L.A. interpreted results of experiments; R.d.S.M. prepared figures; R.d.S.M. and L.A. drafted manuscript; R.d.S.M. and L.A. edited and revised manuscript; R.d.S.M., M.C.I., J.M.C.C., T.R.S., P.S.G., M.R.G., I.d.L.N., and L.A. approved final version of manuscript.
REFERENCES
- 1.Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest 94: 1069–1075, 1994. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499–511, 2004. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Andrade L, Campos SB, Seguro AC. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of L-arginine. Kidney Int 53: 1736–1742, 1998. doi: 10.1046/j.1523-1755.1998.00906.x. [DOI] [PubMed] [Google Scholar]
- 4.Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function recent advances. J Am Coll Cardiol 46: 1792–1798, 2005. doi: 10.1016/j.jacc.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 5.Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, Johansson J, Azhar S. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res 51: 1496–1503, 2010. doi: 10.1194/jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 49: 1344–1352, 2008. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J Clin Invest 88: 390–395, 1991. doi: 10.1172/JCI115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332: 647–655, 1995. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 9.Cacoub P, Deray G, Baumelou A, Jacobs C. Nephrotoxicity of low osmolar radiocontrast agents in patients with chronic renal failure. Nephron 48: 324–325, 1988. doi: 10.1159/000184952. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol 23: 1724–1731, 2003. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 11.Carnevale R, Bartimoccia S, Nocella C, Di Santo S, Loffredo L, Illuminati G, Lombardi E, Boz V, Del Ben M, De Marco L, Pignatelli P, Violi F. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 237: 108–116, 2014. doi: 10.1016/j.atherosclerosis.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension 26: 341–347, 1995. doi: 10.1161/01.HYP.26.2.341. [DOI] [PubMed] [Google Scholar]
- 13.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM; SCORE Project Group . Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 24: 987–1003, 2003. doi: 10.1016/S0195-668X(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 14.Datta G, Gupta H, Zhang Z, Mayakonda P, Anantharamaiah GM, White CR. HDL mimetic peptide administration improves left ventricular filling and cardiac output in lipopolysaccharide-treated rats. J Clin Exp Cardiolog 2: 1000172, 2011. doi: 10.4172/2155-9880.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J; The Emerging Risk Factors Collaboration . Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302: 1993–2000, 2009. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallach R, Shainberg A, Avlas O, Fainblut M, Chepurko Y, Porat E, Hochhauser E. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J Mol Cell Cardiol 48: 1236–1244, 2010. doi: 10.1016/j.yjmcc.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Feriani DJ, Coelho-Júnior HJ, de Oliveira JCMF, Delbin MA, Mostarda CT, Dourado PMM, Caperuto ÉC, Irigoyen MCC, Rodrigues B. Pyridostigmine improves the effects of resistance exercise training after myocardial infarction in rats. Front Physiol 9: 53, 2018. doi: 10.3389/fphys.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest 103: 897–905, 1999. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 20.Golino P, Maroko PR, Carew TE. The effect of acute hypercholesterolemia on myocardial infarct size and the no-reflow phenomenon during coronary occlusion-reperfusion. Circulation 75: 292–298, 1987. doi: 10.1161/01.CIR.75.1.292. [DOI] [PubMed] [Google Scholar]
- 21.Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ Res 97: 236–243, 2005. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 335: 136–148, 2007. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam RM, Pourmousa M, Sviridov D, Gordon SM, Neufeld EB, Freeman LA, Perrin BS Jr, Pastor RW, Remaley AT. Structural properties of apolipoprotein A-I mimetic peptides that promote ABCA1-dependent cholesterol efflux. Sci Rep 8: 2956, 2018. doi: 10.1038/s41598-018-20965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan R, Aynedjian HS, Schlondorff D, Bank N. Renal vasoconstriction caused by short-term cholesterol feeding is corrected by thromboxane antagonist or probucol. J Clin Invest 86: 1707–1714, 1990. doi: 10.1172/JCI114895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003. doi: 10.1097/01.ASN.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 26.Kwon SW, Yoon SJ, Kang TS, Kwon HM, Kim JH, Rhee J, Lee SJ, Park JK, Lim JY, Yoon YW, Hong BK. Significance of small dense low-density lipoprotein as a risk factor for coronary artery disease and acute coronary syndrome. Yonsei Med J 47: 405–414, 2006. doi: 10.3349/ymj.2006.47.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Mehta JL. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res 68: 353–354, 2005. doi: 10.1016/j.cardiores.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Liao JK, Shin WS, Lee WY, Clark SL. Oxidized low-density lipoprotein decreases the expression of endothelial nitric oxide synthase. J Biol Chem 270: 319–324, 1995. doi: 10.1074/jbc.270.1.319. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19: 1360–1370, 2008. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med 89: 615–620, 1990. doi: 10.1016/0002-9343(90)90180-L. [DOI] [PubMed] [Google Scholar]
- 31.McCann BS, Magee MS, Broyles FC, Vaughan M, Albers JJ, Knopp RH. Acute psychological stress and epinephrine infusion in normolipidemic and hyperlipidemic men: effects on plasma lipid and apoprotein concentrations. Psychosom Med 57: 165–176, 1995. doi: 10.1097/00006842-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Meredith IT, Yeung AC, Weidinger FF, Anderson TJ, Uehata A, Ryan TJ, Selwyn AP, Ganz P. Role of impaired endothelium-dependent vasodilation in ischemic manifestations of coronary artery disease. Circulation 87, Suppl V: V56–V66, 1993. [Google Scholar]
- 33.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Yu N, Ansell BJ, Datta G, Garber DW, Fogelman AM. Apolipoprotein A-I mimetic peptides. Arterioscler Thromb Vasc Biol 25: 1325–1331, 2005. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- 34.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. HDL as a biomarker, potential therapeutic target, and therapy. Diabetes 58: 2711–2717, 2009. doi: 10.2337/db09-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 319: 1566–1579, 2018. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, Barter PJ. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 111: 1543–1550, 2005. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 37.Noor R, Shuaib U, Wang CX, Todd K, Ghani U, Schwindt B, Shuaib A. High-density lipoprotein cholesterol regulates endothelial progenitor cells by increasing eNOS and preventing apoptosis. Atherosclerosis 192: 92–99, 2007. doi: 10.1016/j.atherosclerosis.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 39.Pinkham MI, Whalley GA, Guild SJ, Malpas SC, Barrett CJ. Arterial baroreceptor reflex control of renal sympathetic nerve activity following chronic myocardial infarction in male, female, and ovariectomized female rats. Am J Physiol Regul Integr Comp Physiol 309: R169–R178, 2015. doi: 10.1152/ajpregu.00026.2015. [DOI] [PubMed] [Google Scholar]
- 40.Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. J Am Coll Cardiol 51: 1440–1445, 2008. doi: 10.1016/j.jacc.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 41.Porter GA. Radiocontrast-induced nephropathy. Nephrol Dial Transplant 9, Suppl 4: 146–156, 1994. [PubMed] [Google Scholar]
- 42.Prospective Studies Collaboration, Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55000 vascular deaths. Lancet 370: 1829–1839, 2007. [Erratum in Lancet 372: 292, 2008]. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 43.Prosser HC, Tan JT, Dunn LL, Patel S, Vanags LZ, Bao S, Ng MK, Bursill CA. Multifunctional regulation of angiogenesis by high-density lipoproteins. Cardiovasc Res 101: 145–154, 2014. doi: 10.1093/cvr/cvt234. [DOI] [PubMed] [Google Scholar]
- 44.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int 63: 600–606, 2003. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 45.Reindl M, Reinstadler SJ, Feistritzer HJ, Theurl M, Basic D, Eigler C, Holzknecht M, Mair J, Mayr A, Klug G, Metzler B. Relation of low-density lipoprotein cholesterol with microvascular injury and clinical outcome in revascularized ST-elevation myocardial infarction. J Am Heart Assoc 6: e006957, 2017. doi: 10.1161/JAHA.117.006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudnick MR, Berns JS, Cohen RM, Goldfarb S. Contrast media-associated nephrotoxicity. Semin Nephrol 17: 15–26, 1997. [PubMed] [Google Scholar]
- 47.Ruparelia N, Digby JE, Jefferson A, Medway DJ, Neubauer S, Lygate CA, Choudhury RP. Myocardial infarction causes inflammation and leukocyte recruitment at remote sites in the myocardium and in the renal glomerulus. Inflamm Res 62: 515–525, 2013. doi: 10.1007/s00011-013-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol 30: 2311–2316, 2010. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 49.Sumi M, Sata M, Miura S, Rye KA, Toya N, Kanaoka Y, Yanaga K, Ohki T, Saku K, Nagai R. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol 27: 813–818, 2007. doi: 10.1161/01.ATV.0000259299.38843.64. [DOI] [PubMed] [Google Scholar]
- 50.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Hirooka Y, Ando S, Takeshita A. Vasodilatory effect of arginine vasopressin is mediated by nitric oxide in human forearm vessels. J Clin Invest 92: 1483–1490, 1993. doi: 10.1172/JCI116726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol (1985) 102: 2104–2111, 2007. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res 102: 257–264, 2008. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 53.van Dokkum RP, Eijkelkamp WB, Kluppel AC, Henning RH, van Goor H, Citgez M, Windt WA, van Veldhuisen DJ, de Graeff PA, de Zeeuw D. Myocardial infarction enhances progressive renal damage in an experimental model for cardio-renal interaction. J Am Soc Nephrol 15: 3103–3110, 2004. doi: 10.1097/01.ASN.0000145895.62896.98. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Wu J, You J, Shi H, Xue X, Huang J, Xu L, Jiang G, Yuan L, Gong X, Luo H, Ge J, Cui Z, Zou Y. HSF1 deficiency accelerates the transition from pressure overload-induced cardiac hypertrophy to heart failure through endothelial miR-195a-3p-mediated impairment of cardiac angiogenesis. J Mol Cell Cardiol 118: 193–207, 2018. doi: 10.1016/j.yjmcc.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int 45: 259–265, 1994. doi: 10.1038/ki.1994.32. [DOI] [PubMed] [Google Scholar]
- 56.White CR, Datta G, Wilson L, Palgunachari MN, Anantharamaiah GM. The apoA-I mimetic peptide 4F protects apolipoprotein A-I from oxidative damage. Chem Phys Lipids 219: 28–35, 2019. doi: 10.1016/j.chemphyslip.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodward M, Brindle P, Tunstall-Pedoe H; SIGN group on risk estimation . Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 93: 172–176, 2007. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF, Chatham J, Anantharamaiah GM, White CR. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol 297: H866–H873, 2009. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]