Keywords: adrenergic, caveolin, cirrhotic cardiomyopathy
Abstract
Cirrhotic cardiomyopathy is a clinical syndrome in patients with liver cirrhosis characterized by blunted cardiac contractile responses to stress and/or heart rate-corrected QT (QTc) interval prolongation. Caveolin-3 (Cav-3) plays a critical role in cardiac protection and is an emerging therapeutic target for heart disease. We investigated the protective role of cardiac-specific overexpression (OE) of Cav-3 in cirrhotic cardiomyopathy. Biliary fibrosis was induced in male Cav-3 OE mice and transgene negative (TGneg) littermates by feeding a diet containing 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC; 0.1%) for 3 wk. Liver pathology and blood chemistries were assessed, and stress echocardiography, telemetry, and isolated heart perfusion studies to assess adrenergic responsiveness were performed. Cav-3 OE mice showed a similar degree of hyperdynamic contractility, pulmonary hypertension, and QTc interval prolongation as TGneg mice after 3 wk of DDC diet. Blunted systolic responses were shown in both DDC-fed Cav-3 OE and TGneg hearts after in vivo isoproterenol challenge. However, QTc interval prolongation after in vivo isoproterenol challenge was significantly less in DDC-fed Cav-3 OE hearts compared with DDC-fed TGneg hearts. In ex vivo perfused hearts, where circulatory factors are absent, isoproterenol challenge showed hearts from DDC-fed Cav-3 OE mice had better cardiac contractility and relaxation compared with DDC-fed TGneg hearts. Although Cav-3 OE in the heart did not prevent cardiac alterations in DDC-induced biliary fibrosis, cardiac expression of Cav-3 reduced QTc interval prolongation after adrenergic stimulation in cirrhosis.
NEW & NOTEWORTHY Prevalence of cirrhotic cardiomyopathy is up to 50% in cirrhotic patients, and liver transplantation is the only treatment. However, cirrhotic cardiomyopathy is associated with perioperative morbidity and mortality after liver transplantation; therefore, management of cirrhotic cardiomyopathy is crucial for successful liver transplantation. This study shows cardiac myocyte specific overexpression of caveolin-3 (Cav-3) provides better cardiac contractile responses and less corrected QT prolongation during adrenergic stress in a cirrhotic cardiomyopathy model, suggesting beneficial effects of Cav-3 expression in cirrhotic cardiomyopathy.
INTRODUCTION
Patients with liver cirrhosis often present with a hyperdynamic systemic circulation characterized by increased heart rate and cardiac output and reduced systemic vascular resistance and arterial blood pressure (18, 43). Despite an increased basal cardiac output, cirrhotic patients show impaired contractile responsiveness to physiologic or pharmacologic stimuli; a phenomenon called cirrhotic cardiomyopathy (18, 31, 35, 41, 43). Patients with cirrhotic cardiomyopathy are usually asymptomatic at rest; however, overt heart failure can be revealed in stress conditions such as liver transplantation. Cirrhotic cardiomyopathy is also characterized by diastolic dysfunction and electrophysiological abnormalities, including heart rate-corrected QT (QTc) interval prolongation (18, 43). The prevalence of cirrhotic cardiomyopathy is reported in up to 50% of cirrhotic patients, and liver transplantation is the only proven treatment (18, 43). Since cirrhotic cardiomyopathy is associated with perioperative morbidity and mortality after liver transplantation (12, 24, 44), strategies to improve cardiac function are needed.
Caveolae, flask-shaped 50- to 100-nm invaginations of the plasma membrane, are subsets of membrane lipid rafts containing cholesterol and sphingolipids (23). Caveolins are structural proteins essential for caveolae formation and regulation of intracellular signaling and function (23, 26, 29). Although the heart expresses all three isoforms of caveolins (Cav-1, -2, and -3), Cav-3 is necessary for caveolae formation in cardiac myocytes and plays a critical role in cardioprotection (26, 29, 33). Moreover, Cav-3 is an emerging therapeutic target for various heart diseases (26, 29). Previously, we have reported that cardiac-specific overexpression of Cav-3 (Cav-3 OE) prevents ischemia/reperfusion injury (32) and pressure overload and angiotensin II-induced cardiac hypertrophy (10, 16). Furthermore, Cav-3 OE mice have a higher heart rate variability, with lower resting heart rates, and alteration in baseline cardiac conduction and expression levels of specific cardiac ion channels (25).
Feeding of 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) is a well-established mouse model of sclerosing cholangitis and biliary fibrosis (6) that induces the main pathognomonic features of cirrhotic cardiomyopathy after 3 wk including cardiac hypercontractility at baseline, blunted contractile responses to pharmacological stress, and QTc interval prolongation (4, 5). The study aimed to investigate potential protective mechanisms involving Cav-3 OE in the heart on the contractile responses to stress and QTc interval prolongation in DDC-induced cirrhotic cardiomyopathy.
METHODS
Animals and diet.
Animals (C57Bl/6 mice, Jackson laboratory, Bar Harbor, ME) were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC), and protocols approved by the Veterans Affairs San Diego Healthcare System Institutional Animal Care and Use Committee. Biliary fibrosis was induced by feeding 14- to 20-wk-old male mice (25–30 g body wt) with 0.1% DDC (Sigma-Aldrich, St. Louis, MO)-supplemented chow, by mixing 1 g of DDC powder with 1 kg of isocaloric chow (Harlan-Teklad, Madison, WI). A Teklad 4% mouse/rat diet 7001 was used as a normal chow. Cirrhotic cardiomyopathy was assessed with serial echocardiography in mice on the DDC diet for 24 wk. Based on our echocardiography results and previous studies with DDC diet (4, 5), 3 wk of DDC feeding were chosen for the following experiments. Transgenic (TG) mice with cardiac myocyte-specific Cav-3 OE were produced in our laboratory using the α-myosin heavy chain promoter in a C57Bl/6 background (32). Age-matched male transgene-negative (TGneg) siblings were used as controls for Cav-3 OE mice. Animals were kept on a 12-h light-dark cycle in a temperature-controlled room with ad libitum access to food and water.
Liver enzymes, bilirubin, and ammonia levels.
Blood collection was performed by cardiac puncture after 3 wk of diet. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bilirubin levels and plasma ammonia level were analyzed using AU680 chemistry analyzer (Beckman Coulter, Carlsbad, CA) by IDEXX Laboratories (West Sacramento, CA).
Echocardiography.
Transthoracic echocardiography was performed using a Vevo 3100 machine with MX550S transducer (VisualSonics. Toronto, Canada) under isoflurane anesthesia (1–1.5% at a flow rate of 1 L/min oxygen) before and after 3 wk of the DDC diet. Adrenergic stress was tested with an intraperitoneal bolus of 0.01 mg/kg of isoproterenol (Isuprel; Valeant Pharmaceuticals North America, Bridgewater, NJ) followed by echocardiography for 30 min after injection. Fractional shortening (FS) and ejection fraction (EF) were evaluated for systolic function, and E/A and E/e’ ratio were evaluated for diastolic function. Pulmonary acceleration time (PAT) and PAT/pulmonary ejection time (PET) ratio were evaluated to detect pulmonary hypertension (7, 30).
Telemetry.
Intraperitoneal implantation of a telemetry transmitter (ETA-F10; Data Sciences International, St. Paul, MN) was performed as described previously (25). Mice were recovered for 7–10 days before telemetry. Telemetry recordings were obtained for 48 h before and after 1 and 3 wk of diet using Dataquest ART 4.36 software (Data Sciences International, St. Paul, MN). Adrenergic stress was assessed by intraperitoneal isoproterenol (0.01 mg/kg) injected at 3 wk of diet. Data were analyzed over 4-h periods of day and night: 10 AM-2 PM (day cycle) and 10 PM-2 AM (night cycle) with the Ponemah 6.12 software (Data Sciences International). QTc intervals were obtained using the formula QT/(RR/100)1/2 (45).
Isolated heart perfusion with isoproterenol challenge.
Mice were anesthetized with 60 mg/kg pentobarbital sodium after 3 wk of the DDC diet, and hearts were removed and perfused with Langendorff retrograde perfusion techniques as described previously (27). The hearts were stabilized for 15 min, and then, a bolus of isoproterenol (Isuprel; Valeant Pharmaceuticals, Bridgewater, NJ) was injected through aorta cannula every 5 min at increasing concentrations (100 pM, 1 nM, 10 nM, 30 nM, 100 nM, 300 nM, and 1 µM). Left ventricular (LV) developed pressure, LV dP/dt maximum and minimum, and heart rate (HR) were recorded and analyzed using a data acquisition system (PowerLab) and LabChart 7 software (AD, Colorado Springs, CO).
Cardiac and liver histology.
After 3 wk of diet, samples of liver and heart were fixed in 10% aqueous buffered zinc formalin (Z-Fix; Anatech, Battle Creek, MI) and embedded in paraffin and sectioned at 5 µm. Hematoxylin/eosin (H&E) staining and Masson’s trichrome staining were performed. Sections were photographed using a Keyence Microscope BZ-X710 (Keyence, Laguna Hills, CA). Fibrotic area was quantified on six random nonoverlapping images at low magnification (×10) using ImageJ software.
Statistical analysis.
Investigators were blinded to the genetic background of mice. All values are expressed as means ± SE. Two-way or two-way repeated measure ANOVA followed by post hoc Tukey’s correction for multiple comparisons was used. P < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism Software v7.0 (La Jolla, CA).
RESULTS
DDC diet-induced cirrhotic cardiomyopathy in mice.
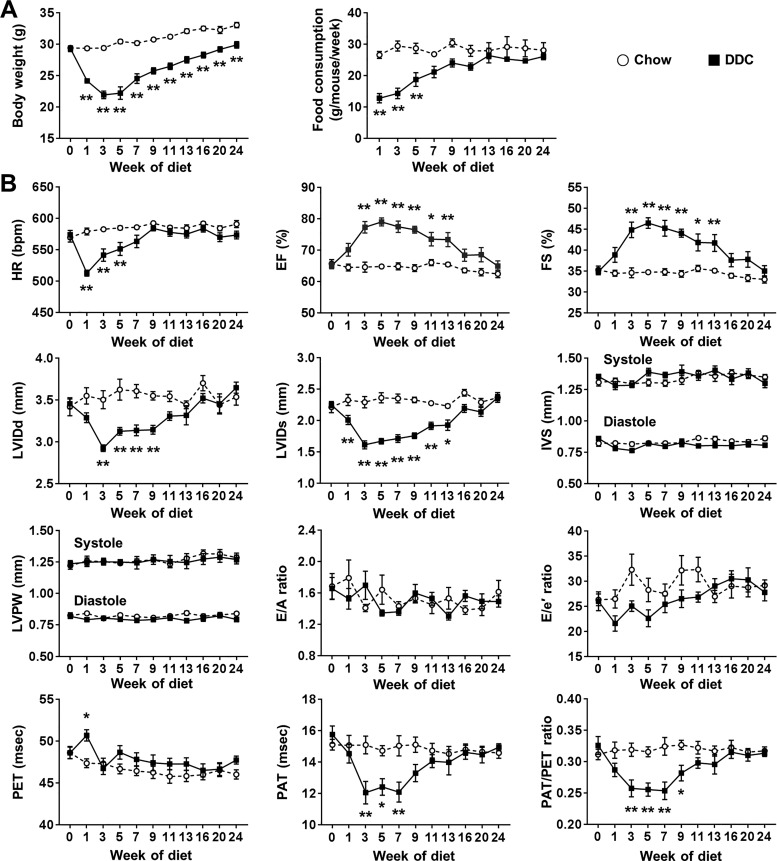
To investigate the time course of the cardiovascular changes in liver cirrhosis, we performed serial echocardiography for 24 wk of DDC diet in wild-type mice (Fig. 1). EF and FS were highest, and PAT/PET ratio was lowest between 3 and 7 wk of DDC diet. Previous studies showed dysregulation of cardiac energetics and metabolism (glycogen accumulation and altered fatty acid metabolism) and electrophysiological abnormalities (QTc interval prolongation) after 3 wk of DDC diet and reversibility of these findings with resolution of liver injury (4, 5). These data suggest the DDC mouse model of liver cirrhosis mimics clinical cirrhotic cardiomyopathy that can be reversed after liver transplantation (31). Therefore, we chose 3 wk of DDC diet for the experiments with Cav-3 OE mice.
Fig. 1.
Body weight and cardiovascular changes during 24 wk of diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet in wild-type mice. A: the changes of body weight and food consumption (n = 8–10 per group). B: the changes of systolic and diastolic function and pulmonary artery flow assessed by echocardiography (n = 8–10 per group). Ejection fraction (EF) and fractional shortening (FS) were highest, and pulmonary acceleration time-to-pulmonary ejection time (PAT/PET) ratio was lowest between 3 and 7 wk of DDC diet. HR, heart rate; LVIDd, left ventricular internal diameter at end diastole; LVIDs, left ventricular internal diameter at end systole; IVS, interventricular septum; LVPW, left ventricular posterior wall; E/A ratio, ratio of the early (E) to late (A) ventricular filling velocities on transmitral Doppler; E/e′ ratio, ratio of early filling velocity on transmitral Doppler (E) to early relaxation velocity on tissue Doppler (e′).Values are means ± SE. *P < 0.05, **P < 0.01, compared with chow-fed mice.
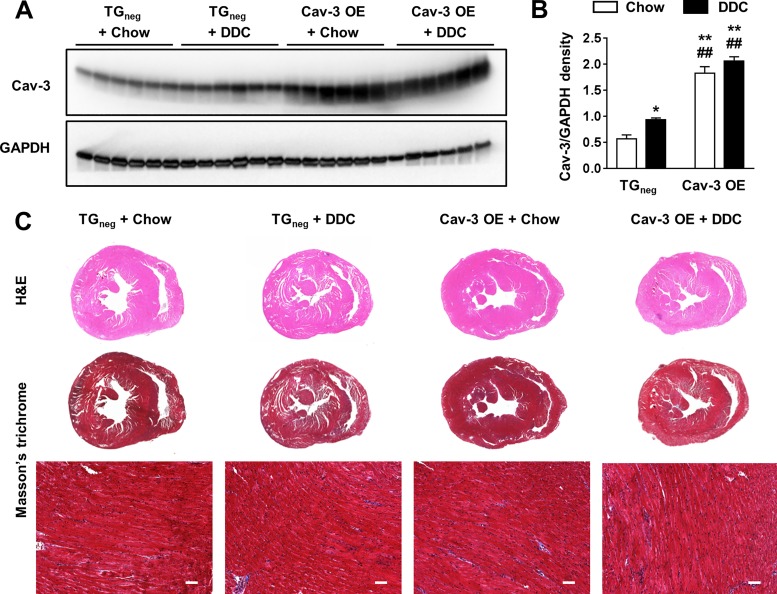
No change in DDC-induced liver cirrhosis in Cav-3 OE mice.
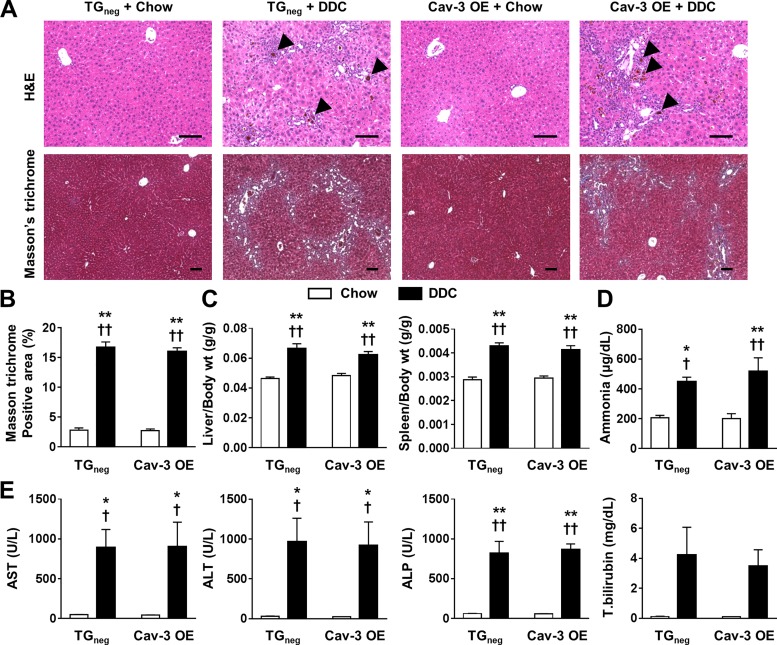
There was no mortality in all groups. After 3 wk of diet, DDC-fed Cav-3 OE mice showed a similar weight loss as TGneg mice (data not shown). DDC-fed Cav-3 OE and TGneg mice showed similar levels of porphyrin accumulation and mononuclear cell infiltration around the portal vein in H&E staining as well as portal-portal bridge fibrosis in Masson’s trichrome staining (Fig. 2A). Liver fibrosis was approximately five times greater in DDC-fed mice compared with chow-fed mice regardless of genotype (Fig. 2B). Liver and spleen weights normalized to body weight were higher in DDC-fed mice regardless of genotype (Fig. 2C). Ammonia and liver enzymes were increased in both DDC-fed Cav-3 OE and TGneg mice (Fig. 2, D and E). These data suggest that cardiac Cav-3 OE does not impact overall liver injury in response to DDC.
Fig. 2.
Liver and spleen changes after 3 wk of diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet in caveolin-3 (Cav-3) overexpression (OE) and transgene negative (TGneg) mice. A: liver histology stained with hematoxylin and eosin (H&E) and Masson’s trichrome. Porphyrin accumulation (arrowhead), mononuclear cell infiltration, and portal-portal bridge fibrosis are shown in H&E and Masson’s trichrome, respectively, in DDC-fed groups. B–D: liver injury determined by (B) fibrotic area by Masson-trichrome staining (n = 6 per group; B), liver and spleen to body weight ratio (n = 10 per group; C), plasma ammonia level (n = 6 per group; D), and serum liver enzymes and total bilirubin level (n = 6 per group; E) were similar between DDC-fed Cav-3 OE and TGneg mice. AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase. Values are means ± SE. Scale bar = 100 µm. *P < 0.05, **P < 0.01 compared with chow-fed TGneg mice. †P < 0.05, ††P < 0.01 compared with chow-fed Cav-3 OE mice.
Echocardiography in DDC cirrhotic cardiomyopathy.
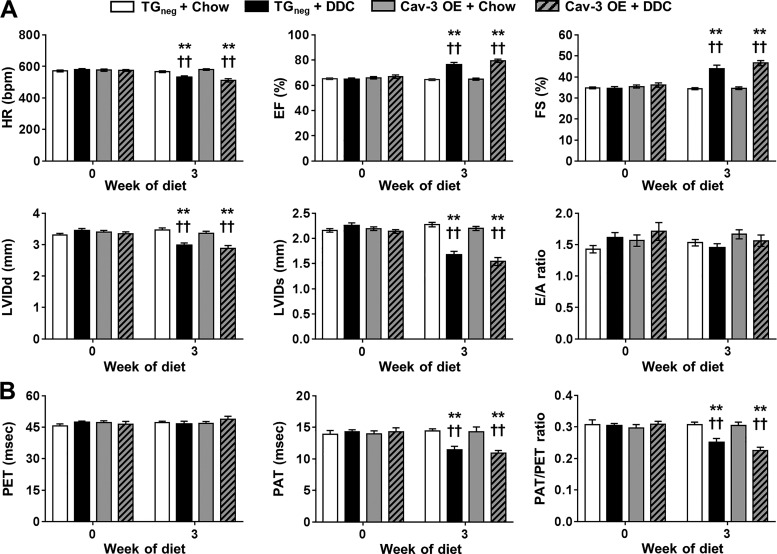
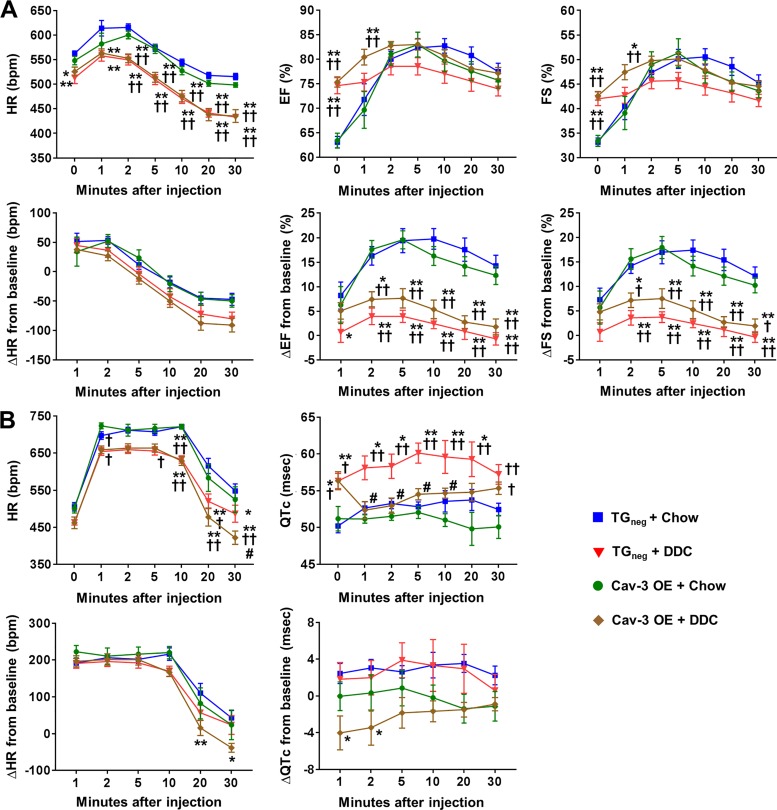
Both DDC-fed Cav-3 OE and TGneg mice demonstrated bradycardia and hyperdynamic contractility of LV (increase of EF and FS) after 3 wk of diet (Fig. 3A) in agreement with previous studies (4, 5). PAT and PAT/PET ratios are sensitive measures of pulmonary hypertension in mice (30). Both DDC-fed Cav-3 OE and TGneg mice showed pulmonary hypertension with decreases in PAT and PAT/PET ratio (Fig. 3B). This result is compatible with previous findings of decreased and PAT/PET ratios in carbon tetrachloride-induced cirrhotic mice (2).
Fig. 3.
Echocardiographic changes after 3 wk of diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet in caveolin-3 (Cav-3) overexpression (OE) and transgene negative (TGneg) mice. A and B: the changes of (A) systolic and diastolic function and (B) pulmonary artery flow were similar between DDC-fed Cav-3 OE and TGneg mice (n = 10 per group). HR, heart rate; EF, ejection fraction; FS, fractional shortening; LVIDd, left ventricular internal diameter at end diastole; LVIDs, left ventricular internal diameter at end systole; E/A ratio, ratio of the early (E) to late (A) ventricular filling velocities on transmitral Doppler; PET, pulmonary ejection time; PAT, pulmonary acceleration time. Values are means ± SE **P < 0.01, compared with chow-fed TGneg mice. ††P < 0.01, compared with chow-fed Cav-3 OE mice.
Telemetry in DDC cirrhotic cardiomyopathy.
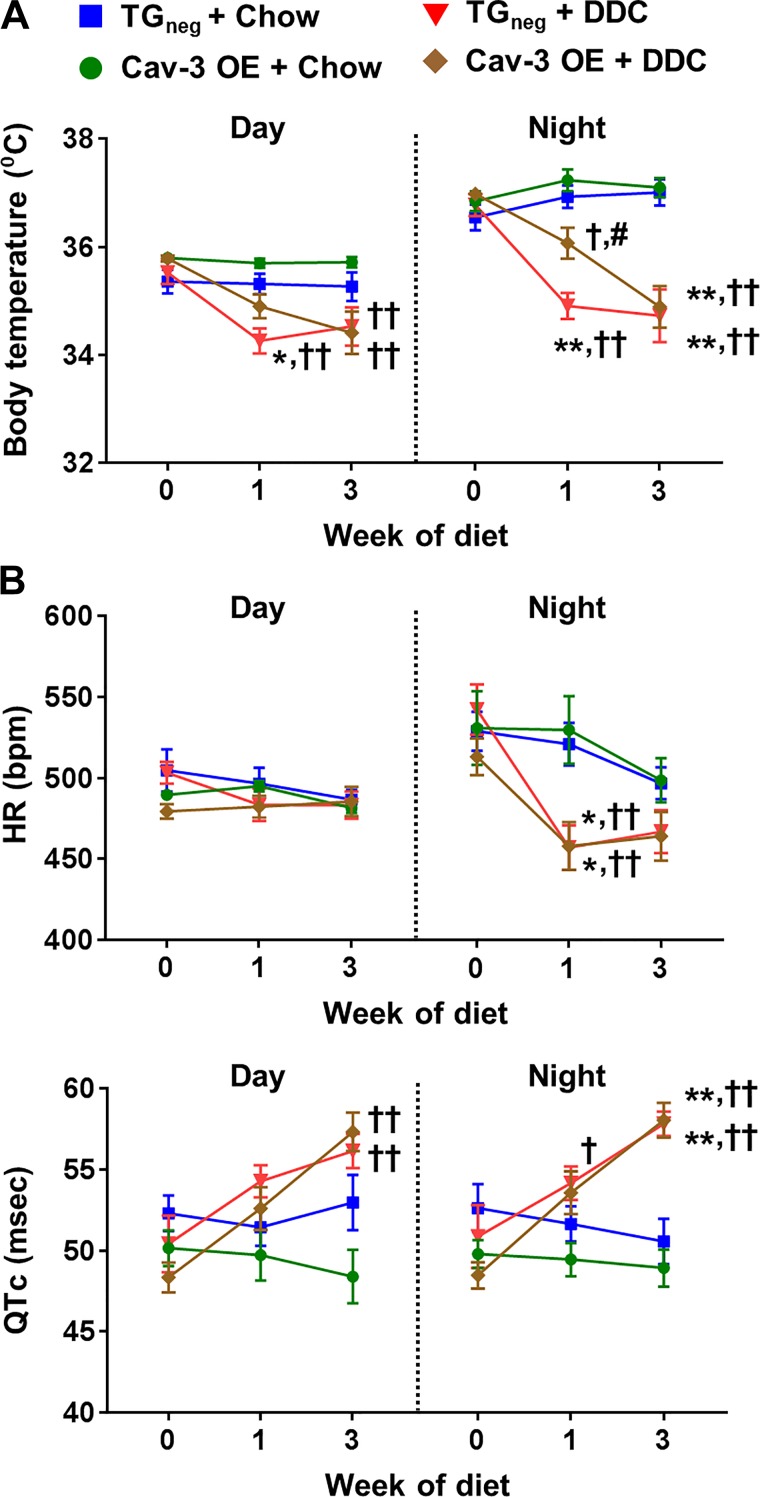
Diurnal changes in body temperature, HR, and QTc interval are shown in Fig. 4. DDC-fed mice showed more hypothermia than chow-fed mice, especially at night (Fig. 4A). Low body temperature was more prominent in DDC-fed TGneg mice than DDC-fed Cav-3 OE mice at 1 wk of DDC diet. After 3 wk of diet, both DDC-fed Cav-3 OE and TGneg mice showed similar degree of hypothermia. There were significant changes in heart rate during the night in both DDC-fed Cav-3 OE and TGneg mice after 1 wk of diet (Fig. 4B). Both DDC-fed Cav-3 OE and TGneg mice showed QTc interval prolongation after 3 wk of diet (Fig. 4B).
Fig. 4.
Changes of body temperature and electrocardiography during day and night after 3 wk of diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet in caveolin-3 (Cav-3) overexpression (OE) and transgene negative (TGneg) mice. A and B: the changes of body temperature (A) and heart rate (HR; B) and corrected QT (QTc) interval were similar between DDC-fed Cav-3 OE and TGneg mice (n = 7–10 per group). Values are means ± SE. *P < 0.05, **P < 0.01, compared with chow-fed TGneg mice. †P < 0.05, ††P < 0.01, compared with chow-fed Cav-3 OE mice. #P < 0.05 compared with DDC-fed TGneg mice.
Stress-induced echocardiography and telemetry in DDC cirrhotic cardiomyopathy.
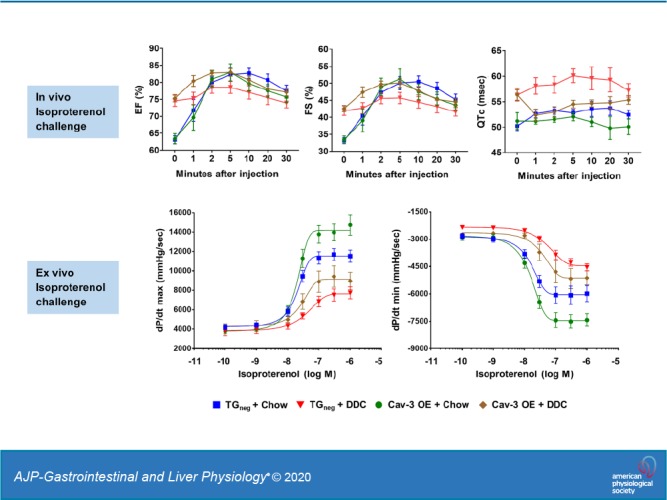
Since cirrhotic cardiomyopathy is characterized by impaired contractile responsiveness to physiologic or pharmacological stimuli (18, 31, 35, 41, 43), mice were challenged with isoproterenol under isoflurane anesthesia after 3 wk of DDC diet (Fig. 5A). The trends of HR changes were similar between the groups after isoproterenol injection with a lower heart rate in DDC-fed groups (Fig. 5A). The increase of EF and FS were similar between chow-fed Cav-3 OE and chow-fed TGneg mice; however, chow-fed Cav-3 OE mice tended to show faster recovery to the baseline than TGneg mice (Fig. 5A). This induction is largely impaired by DDC feeding, suggesting development of cirrhotic cardiomyopathy in both DDC-fed Cav-3 OE and TGneg mice. However, though not significant, DDC-fed Cav-3 OE mice showed a trend of higher ΔEF and ΔFS, suggesting partial protection from cirrhotic cardiomyopathy. At 5 min of isoproterenol injection, which showed peak effects, the mean delta changes of EF from baseline were 19.4% (TGneg + Chow), 3.9% (TGneg + DDC), 19.6% (Cav-3 OE + Chow), and 7.6% (Cav-3 OE + DDC).
Fig. 5.
Isoproterenol challenge in vivo after 3 wk of diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet in caveolin-3 (Cav-3) overexpression (OE) and transgene negative (TGneg) mice. A: the changes of heart rate (HR) and systolic function under isoflurane anesthesia after intraperitoneal injection of 0.01 mg/kg isoproterenol (n = 9–10 per group). The increase of ejection fraction (EF) fractional shortening (FS) after isoproterenol injection were significantly less in both DDC-fed Cav-3 OE and DDC-fed TGneg mice compared with chow-fed mice. B: the changes of HR and corrected QT (QTc) in awake state after intraperitoneal injection of 0.01 mg/kg isoproterenol (n = 7–9 per group). QTc interval of DDC-fed TGneg mice was significantly higher than that of DDC-fed Cav-3 OE mice at 1, 2, 5 and 10 min after isoproterenol injection. Values are means ± SE. *P < 0.05, **P < 0.01, compared with chow-fed TGneg mice. †P < 0.05, ††P < 0.01, compared with chow-fed Cav-3 OE mice. #P < 0.05, compared with DDC-fed TGneg mice.
Isoproterenol was injected intraperitoneally in awake mice after 3 wk of DDC diet to investigate QTc interval changes after adrenergic stimulation. The trends of increase in HR were similar between the four groups after isoproterenol; however, DDC-fed Cav-3 OE mice showed faster recovery to the baseline with significantly lower HR at 30 min compared with other groups (Fig. 5B). Baseline QTc intervals were similar between DDC-fed TGneg (56.3 ms) and DDC-fed Cav-3 OE mice (56.4 ms), which were higher than chow-fed TGneg mice (50.2 ms). However, after isoproterenol injection, DDC-fed TGneg mice showed an increase of QTc interval, whereas DDC-fed Cav-3 OE mice showed decreased QTc intervals. Therefore, the QTc interval of DDC-fed TGneg mice was significantly higher than DDC-fed Cav-3 OE mice at 1, 2, 5, and 10 min after isoproterenol (P = 0.011, P = 0.021, P = 0.014, and P = 0.040, respectively), suggesting a mechanistic role for Cav-3 in reducing QTc interval prolongation after adrenergic stimulation in cirrhosis.
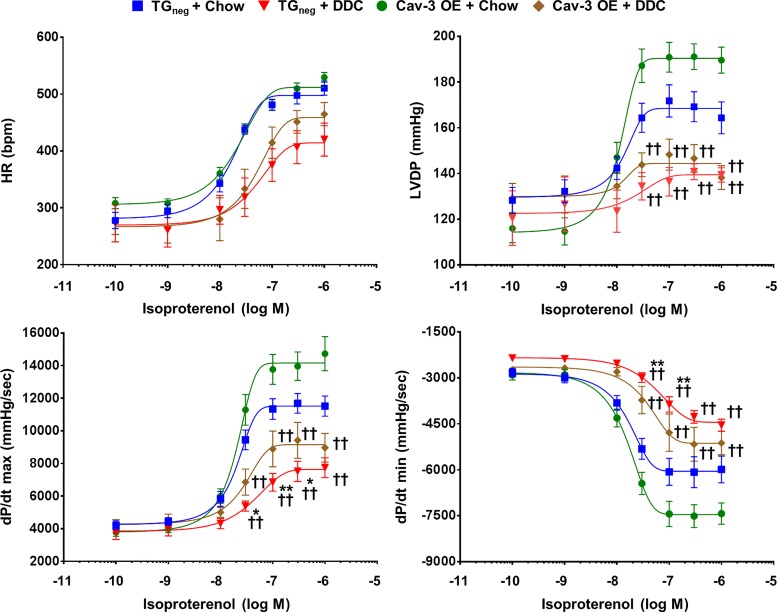
Ex vivo assessment of baseline and stress-induced cardiac function in DDC cirrhotic cardiomyopathy.
Langendorff perfusion with isoproterenol (100 pM to 1 µM) was performed after 3 wk of DDC diet (Fig. 6) to investigate intrinsic contractile responses of hearts to catecholamine stimulation. The Cav-3 OE hearts tended to show better contractility (dP/dtmax) and relaxation (dP/dtmin) compared with TGneg hearts in both chow and DDC diet after isoproterenol administration. When compared with chow-fed TGneg hearts, DDC-fed TGneg hearts showed a significantly lower contractile and relaxation response to 30–300 nM of isoproterenol. However, no significant difference existed in contractility and relaxation between DDC-fed Cav-3 OE hearts and chow-fed TGneg hearts, suggesting a beneficial role for Cav-3 in stress-induced cardiac dysfunction in cirrhosis.
Fig. 6.
Isoproterenol challenge in ex vivo heart after 3 wk of diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet in caveolin-3 (Cav-3) overexpression (OE) and transgene negative (TGneg) mice. Langendorff perfusion with incremental concentrations (100 pM, 1 nM, 10 nM, 30 nM, 100 nM, 300 nM, and 1 µM) of isoproterenol were performed (n = 7–10 per group). At 30–300 nM of isoproterenol, DDC-fed TGneg hearts showed significant less contractility (dPmax/dt) and relaxation (dPmin/dt) compared with chow-fed TGneg hearts, but no significant difference existed between DDC-fed Cav-3 OE hearts and chow-fed TGneg hearts. HR, heart rate; LVDP, left ventricular developed pressure (difference between LV systolic and diastolic pressure). *P < 0.05, **P < 0.01 compared with chow-fed TGneg mice. ††P < 0.01 compared with chow-fed Cav-3 OE mice.
Cardiac histology in DDC cirrhotic cardiomyopathy.
The chow-fed Cav-3 OE hearts showed approximately three times higher Cav-3 protein expression than chow-fed TGneg hearts (P < 0.001). Cav-3 expression was increased 1.6-fold in DDC-fed TGneg hearts compared with chow-fed TGneg hearts (P = 0.041), which is similar to our previous reports of increased Cav-3 expression in daunorubicin-treated rabbit hearts (11). There were no differences in fibrosis of the heart between the four groups (Fig. 7C).
Fig. 7.
Caveolin-3 (Cav-3) expression and heart changes after 3 wk of diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet in Cav-3 overexpression (OE) and transgene negative (TGneg) mice. A: representative Western blot of Cav-3. B: relative density of Cav-3 normalized to GAPDH showed significant increase of Cav-3 expression in Cav-3 OE groups and 1.6-fold increase in DDC-fed TGneg hearts compared with chow-fed TGneg hearts (n = 6 per group). C: heart histology stained with hematoxylin/eosin (H&E) and Masson’s trichrome were similar between 4 groups. *P < 0.05, **P < 0.01, compared with chow-fed TGneg mice. ##P < 0.01, compared with DDC-fed TGneg mice.
DISCUSSION
Cirrhotic cardiomyopathy is a clinical syndrome in patients with liver cirrhosis characterized by an abnormal and blunted contractile response to stress and/or altered diastolic relaxation with electrophysiological abnormalities in the absence of known cardiac disease (18, 43). Cirrhotic cardiomyopathy is associated with perioperative morbidity and mortality after liver transplantation (12, 13, 24, 44); however, there is no accepted pharmacological treatment for cirrhotic cardiomyopathy (18, 43). This study was the first to investigate cardiac-specific Cav-3 OE as a possible therapeutic target in cirrhotic cardiomyopathy. Although Cav-3 OE in the heart did not alter all cardiac parameters assessed in our DDC model of cirrhotic cardiomyopathy (i.e., hyperdynamic contractility, pulmonary hypertension, and baseline QTc interval prolongation), DDC-fed Cav-3 OE mouse hearts showed better cardiac contractile responses after ex vivo adrenergic stimulation and significantly less QTc interval prolongation after in vivo adrenergic stimulation compared with DDC-fed TGneg hearts.
DDC blocks the rate-limiting step of heme biosynthesis and leads to accumulation of porphyrin (6). We showed that DDC feeding leads to pericholangitis, periductal fibrosis, and portal-portal bridging fibrosis, causing significant duct disease and a biliary type of liver fibrosis as has been shown by others (6). Three weeks of DDC produced the main pathognomonic features of cirrhotic cardiomyopathy, including cardiac hypercontractility at baseline, blunted contractile responses to pharmacological stress, and QTc interval prolongation in line with previous results (4, 5). We are the first to show the development of pulmonary hypertension in the DDC diet model of biliary fibrosis, although it has been partly demonstrated in the carbon tetrachloride-intoxicated liver fibrosis model that has completely distinct pathogenesis (2, 3, 17). Notably, we monitored changes of echocardiography for an extended period, 24 wk, during DDC feeding and revealed the most significant phenotypic changes of cirrhotic cardiomyopathy (i.e., hypercontractility and pulmonary hypertension) between 3 and 7 wk of DDC diet (Fig. 1), which was chosen for testing potential therapeutics of Cav-3.
Liver cirrhosis induces cardiovascular abnormalities. Liver cirrhosis with portal hypertension leads to splanchnic arterial vasodilation, which induces reduced central blood volume with hypotension resulting in baroreceptor-induced activation of the renin-angiotensin-aldosterone system and sympathetic nervous system (18, 43). Therefore, cirrhotic patients show a hyperdynamic circulation with increased EF and cardiac output at rest. However, they show blunted increases in EF and cardiac output under stress conditions (exercise or dobutamine infusion) (18, 31, 35, 41, 43). In our study, DDC-fed Cav-3 OE hearts showed hyperdynamic contractility with blunted increases in EF and FS after in vivo isoproterenol challenge, which is similar to DDC-fed TGneg hearts. However, during ex vivo isoproterenol challenge, DDC-fed Cav-3 OE hearts showed better contractility and relaxation compared with DDC-fed TGneg hearts. Our results may suggest that the intrinsic contractile responses to adrenergic stimulation are improved in DDC-fed Cav-3 OE but the presence of circulatory molecules such as catecholamines under in vivo condition might mask the beneficial effects of Cav-3 in cirrhotic hearts.
Several pathogenic mechanisms are involved in altered contractile function in cirrhotic cardiomyopathy (18, 43). In experimental cirrhosis, abnormalities of the β-adrenergic receptor signaling pathway have been identified. Indeed, a decrease in β-adrenergic receptor density (14), decreased expression of stimulatory G proteins (15), increased expression of inhibitory G proteins (1), and attenuation of adenylate cyclase activity with resultant decreased cAMP generation (1, 14, 15) have been identified. In adult cardiomyocytes, Cav-3 colocalized with β-adrenergic receptors, stimulatory G proteins, and adenylate cyclase, implying a role for cardiac Cav-3 as an organizer of signaling components that regulate cAMP production (9, 22). We showed enhanced isoproterenol responsiveness in isolated and perfused DDC-fed Cav-3 OE hearts compared with DDC-fed TGneg hearts. Potential mechanisms involving “protective” alterations by Cav-3 OE of adrenergic signaling components in cirrhotic cardiomyopathy are beyond the scope of the current study but warrant further investigation.
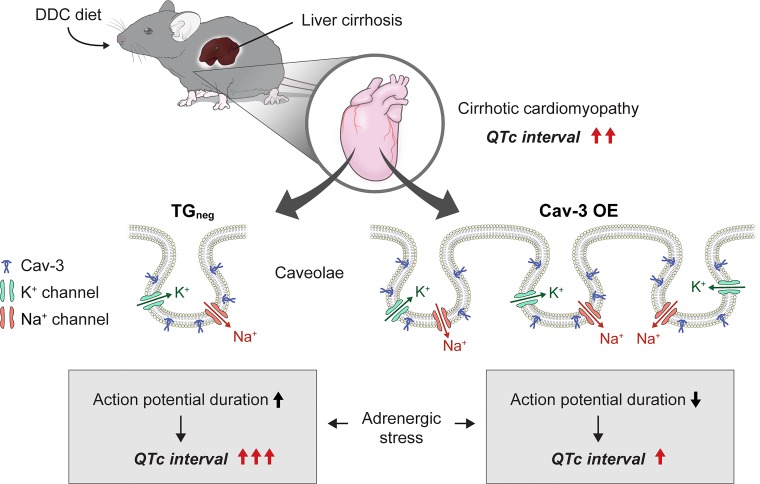
QTc interval prolongation is related to cardiovascular mortality, all-cause mortality, and sudden cardiac death in the general population (20, 21). Compared with the general population, patients with liver cirrhosis were 14 times more likely to suffer a cardiac event after transplantation, and prolonged QTc interval was related to posttransplantation cardiac events (12). QTc interval prolongation also was shown in a DDC-fed mouse model of biliary fibrosis (4, 5). In line with previous results, TGneg and Cav-3 OE mice showed significant QTc interval prolongation after 3 wk of DDC diet compared with chow-fed mice. Although DDC-fed Cav-3 OE mice had similar QTc interval prolongation compared with DDC-fed TGneg mice, they showed reduced QTc interval prolongation after the stress of adrenergic stimulation. The QTc interval was reported to increase progressively during the stress of liver transplantation (28). The mechanisms for QTc interval prolongation are a decrease in repolarizing cardiac membrane currents due to reduction in outward potassium (K+) currents or an increase in depolarizing cardiac currents due to persistent inward sodium (Na+) currents (19). Decreases in delayed rectifier K+ currents and a resultant longer action potential duration have been reported in cirrhotic rat models of bile duct ligation (40). Different caveolin isoforms have been linked with regulation of various ion channels (39, 42); Cav-3 is specifically linked with cAMP-independent localization and regulation of Na+ channels (42). The relation between Cav-3 and QTc interval prolongation was identified in several clinical and experimental studies (8, 36–38). Mutations in the CAV3 gene, which encodes Cav-3, was found in patients with long QT syndrome (38), and the mutant CAV3 increased late Na+ current and a decrease in the inward rectifier K+ current (36–38). The human ether-a-go-go-related (hERG) gene encodes the rapidly activating delayed rectifier K+ channel, and hERG expression in the plasma membrane is regulated by Cav-3 (8). It was recently shown that the Cav-3 mutations linked to the long QT type 9 inherited arrhythmia syndrome affected Cav 1.2-encoded L-type Ca2+ channels highlighting Cav-3′s involvement in cardiac excitability (34). We have shown previously that Cav-3 OE mice have increased expression of Kv1.4, Kv 4.3, and Nav1.5 channels, reduced QTc intervals, and shortened cardiac action potentials (25). Because of the inhibitory role of Cav-3 in QTc interval prolongation, DDC-fed Cav-3 OE mice might show no increase in QTc interval after adrenergic stimulation in our results (Fig. 8). However, further studies are needed to investigate the reasons that Cav-3 OE did not prevent QTc interval prolongation induced by biliary fibrosis but blocked further increases in QTc interval prolongation after adrenergic stimulation.
Fig. 8.
Cartoon depicting possible mechanism of caveolin-3 (Cav-3) overexpression (OE) regulation of corrected QT (QTc) interval prolongation in cirrhotic cardiomyopathy. In diethoxycarbonyl-1,4-dihydrocollidine (DDC)-induced cirrhotic cardiomyopathy animal model, Cav-3 OE is cardioprotective under adrenergic stimulation by reducing QTc interval. Cav-3 plays an essential role in regulating potassium (K+) and sodium (Na+) channels in cardiomyocytes. Cav-3 OE reverses QTc interval prolongation potentially by shortening action potential duration during adrenergic stress.
A limitation of the current study is that we did not investigate mechanisms for the Cav-3 OE-associated improvements in the LV contractile response and reduced QTc interval prolongation after adrenergic stimulation. In our results, Cav-3 expression was increased 1.6-fold in DDC-fed TGneg hearts compared with chow-fed TGneg hearts. This result is consistent with previous reports that structural caveolae and Cav-3 protein were both increased in a rabbit model of daunorubicin cardiotoxicity (11). Therefore, the increase of Cav-3 in the hearts under liver cirrhosis might be a compensatory increase, and further studies for the protective mechanism of Cav-3 OE are needed.
In conclusion, we have described a useful model of DDC induced biliary fibrosis that demonstrates the liver pathology and pathognomonic signs of cirrhotic cardiomyopathy, including a hyperdynamic circulation, blunted stimulated cardiac functional reserve, and prolonged QTc intervals. We have found that cardiac myocyte-specific overexpression of Cav-3 alters the LV contractile responses and QTc interval prolongation seen during adrenergic stress in the DDC cirrhotic cardiomyopathy model. Our results point to beneficial effects of Cav-3 expression in the heart in cirrhotic cardiomyopathy that may lead to future therapies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL091071 (to H. H. Patel) and Veterans Affairs Grant BX001963 (to H. H. Patel).
DISCLOSURES
H. H. Patel has equity as a founder in CavoGene LifeSciences Holdings, LLC. No conflicts of interest, financial or otherwise, are declared by the remaining authors.
AUTHOR CONTRIBUTIONS
S.Y.K., K.H.K., D.M.R., and H.H.P. conceived and designed research; S.Y.K., J.M.S., J.L., and M.D. performed experiments; S.Y.K., K.H.K., J.M.S., and H.H.P. analyzed data; S.Y.K., K.H.K., D.M.R., A.Z.-H., and H.H.P. interpreted results of experiments; S.Y.K. and K.H.K. prepared figures; S.Y.K., B.P.H., D.M.R., and H.H.P. drafted manuscript; S.Y.K., K.H.K., J.M.S., J.L., M.D., B.P.H., D.M.R., A.Z.-H., and H.H.P. edited and revised manuscript; S.Y.K., K.H.K., J.M.S., J.L., M.D., B.P.H., D.M.R., A.Z.-H., and H.H.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Medical Illustration and Design, a part of the medical research support services of Yonsei University College of Medicine, for all artistic support related to this work.
REFERENCES
- 1.Ceolotto G, Papparella I, Sticca A, Bova S, Cavalli M, Cargnelli G, Semplicini A, Gatta A, Angeli P. An abnormal gene expression of the beta-adrenergic system contributes to the pathogenesis of cardiomyopathy in cirrhotic rats. Hepatology 48: 1913–1923, 2008. doi: 10.1002/hep.22533. [DOI] [PubMed] [Google Scholar]
- 2.Das M, Boerma M, Goree JR, Lavoie EG, Fausther M, Gubrij IB, Pangle AK, Johnson LG, Dranoff JA. Pathological changes in pulmonary circulation in carbon tetrachloride (CCl4)-induced cirrhotic mice. PLoS One 9: e96043, 2014. doi: 10.1371/journal.pone.0096043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delire B, Stärkel P, Leclercq I. Animal models for fibrotic liver diseases: what we have, what we need, and what is under development. J Clin Transl Hepatol 3: 53–66, 2015. doi: 10.14218/JCTH.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai MS, Eblimit Z, Thevananther S, Kosters A, Moore DD, Penny DJ, Karpen SJ. Cardiomyopathy reverses with recovery of liver injury, cholestasis and cholanemia in mouse model of biliary fibrosis. Liver Int 35: 1464–1477, 2015. doi: 10.1111/liv.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai MS, Shabier Z, Taylor M, Lam F, Thevananther S, Kosters A, Karpen SJ. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology 51: 2097–2107, 2010. doi: 10.1002/hep.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, Jaeschke H, Zatloukal K, Denk H, Trauner M. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol 171: 525–536, 2007. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in mice. Curr Protoc Mouse Biol 1: 71–83, 2011. doi: 10.1002/9780470942390.mo100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J, Wang T, Li X, Shallow H, Yang T, Li W, Xu J, Fridman MD, Yang X, Zhang S. Cell surface expression of human ether-a-go-go-related gene (hERG) channels is regulated by caveolin-3 protein via the ubiquitin ligase Nedd4-2. J Biol Chem 287: 33132–33141, 2012. doi: 10.1074/jbc.M112.389643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem 280: 31036–31044, 2005. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- 10.Horikawa YT, Panneerselvam M, Kawaraguchi Y, Tsutsumi YM, Ali SS, Balijepalli RC, Murray F, Head BP, Niesman IR, Rieg T, Vallon V, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J Am Coll Cardiol 57: 2273–2283, 2011. doi: 10.1016/j.jacc.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichikawa Y, Zemljic-Harpf AE, Zhang Z, McKirnan MD, Manso AM, Ross RS, Hammond HK, Patel HH, Roth DM. Modulation of caveolins, integrins and plasma membrane repair proteins in anthracycline-induced heart failure in rabbits. PLoS One 12: e0177660, 2017. doi: 10.1371/journal.pone.0177660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josefsson A, Fu M, Björnsson E, Kalaitzakis E. Prevalence of pre-transplant electrocardiographic abnormalities and post-transplant cardiac events in patients with liver cirrhosis. BMC Gastroenterol 14: 65, 2014. doi: 10.1186/1471-230X-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SM, George B, Alcivar-Franco D, Campbell CL, Charnigo R, Delisle B, Hundley J, Darrat Y, Morales G, Elayi SC, Bailey AL. QT prolongation is associated with increased mortality in end stage liver disease. World J Cardiol 9: 347–354, 2017. doi: 10.4330/wjc.v9.i4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Z, Meddings JB, Lee SS. Membrane physical properties determine cardiac beta-adrenergic receptor function in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol 267: G87–G93, 1994. doi: 10.1152/ajpgi.1994.267.1.G87. [DOI] [PubMed] [Google Scholar]
- 15.Ma Z, Miyamoto A, Lee SS. Role of altered beta-adrenoceptor signal transduction in the pathogenesis of cirrhotic cardiomyopathy in rats. Gastroenterology 110: 1191–1198, 1996. doi: 10.1053/gast.1996.v110.pm8613009. [DOI] [PubMed] [Google Scholar]
- 16.Markandeya YS, Phelan LJ, Woon MT, Keefe AM, Reynolds CR, August BK, Hacker TA, Roth DM, Patel HH, Balijepalli RC. Caveolin-3 overexpression attenuates cardiac hypertrophy via inhibition of T-type Ca2+ current modulated by protein kinase Cα in cardiomyocytes. J Biol Chem 290: 22085–22100, 2015. doi: 10.1074/jbc.M115.674945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez AK, Maroni L, Marzioni M, Ahmed ST, Milad M, Ray D, Alpini G, Glaser SS. Mouse models of liver fibrosis mimic human liver fibrosis of different etiologies. Curr Pathobiol Rep 2: 143–153, 2014. doi: 10.1007/s40139-014-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Møller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol 53: 179–190, 2010. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest 115: 2018–2024, 2005. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, Olesen MS, Struijk JJ, Haunsø S, Svendsen JH, Køber L, Gerds TA, Holst AG. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J 35: 1335–1344, 2014. doi: 10.1093/eurheartj/ehu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noseworthy PA, Peloso GM, Hwang SJ, Larson MG, Levy D, O’Donnell CJ, Newton-Cheh C. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol 17: 340–348, 2012. doi: 10.1111/j.1542-474X.2012.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostrom RS, Violin JD, Coleman S, Insel PA. Selective enhancement of beta-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol Pharmacol 57: 1075–1079, 2000. [PubMed] [Google Scholar]
- 23.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, Fix OK, Kay N, Abecassis MI, Gheorghiade M, Flaherty JD. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol 58: 223–231, 2011. doi: 10.1016/j.jacc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Schilling JM, Horikawa YT, Zemljic-Harpf AE, Vincent KP, Tyan L, Yu JK, McCulloch AD, Balijepalli RC, Patel HH, Roth DM. Electrophysiology and metabolism of caveolin-3-overexpressing mice. Basic Res Cardiol 111: 28, 2016. doi: 10.1007/s00395-016-0542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schilling JM, Roth DM, Patel HH. Caveolins in cardioprotection - translatability and mechanisms. Br J Pharmacol 172: 2114–2125, 2015. doi: 10.1111/bph.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.See Hoe LE, Schilling JM, Tarbit E, Kiessling CJ, Busija AR, Niesman IR, Du Toit E, Ashton KJ, Roth DM, Headrick JP, Patel HH, Peart JN. Sarcolemmal cholesterol and caveolin-3 dependence of cardiac function, ischemic tolerance, and opioidergic cardioprotection. Am J Physiol Heart Circ Physiol 307: H895–H903, 2014. doi: 10.1152/ajpheart.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin WJ, Kim YK, Song JG, Kim SH, Choi SS, Song JH, Hwang GS. Alterations in QT interval in patients undergoing living donor liver transplantation. Transplant Proc 43: 170–173, 2011. doi: 10.1016/j.transproceed.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Stary CM, Tsutsumi YM, Patel PM, Head BP, Patel HH, Roth DM. Caveolins: targeting pro-survival signaling in the heart and brain. Front Physiol 3: 393, 2012. doi: 10.3389/fphys.2012.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibault HB, Kurtz B, Raher MJ, Shaik RS, Waxman A, Derumeaux G, Halpern EF, Bloch KD, Scherrer-Crosbie M. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ Cardiovasc Imaging 3: 157–163, 2010. doi: 10.1161/CIRCIMAGING.109.887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J, Genescà J. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol 42: 68–74, 2005. doi: 10.1016/j.jhep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation 118: 1979–1988, 2008. doi: 10.1161/CIRCULATIONAHA.108.788331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsutsumi YM, Kawaraguchi Y, Horikawa YT, Niesman IR, Kidd MW, Chin-Lee B, Head BP, Patel PM, Roth DM, Patel HH. Role of caveolin-3 and glucose transporter-4 in isoflurane-induced delayed cardiac protection. Anesthesiology 112: 1136–1145, 2010. doi: 10.1097/ALN.0b013e3181d3d624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyan L, Foell JD, Vincent KP, Woon MT, Mesquitta WT, Lang D, Best JM, Ackerman MJ, McCulloch AD, Glukhov AV, Balijepalli RC, Kamp TJ. Long QT syndrome caveolin-3 mutations differentially modulate Kv 4 and Cav 1.2 channels to contribute to action potential prolongation. J Physiol 597: 1531–1551, 2019. doi: 10.1113/JP276014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umphrey LG, Hurst RT, Eleid MF, Lee KS, Reuss CS, Hentz JG, Vargas HE, Appleton CP. Preoperative dobutamine stress echocardiographic findings and subsequent short-term adverse cardiac events after orthotopic liver transplantation. Liver Transpl 14: 886–892, 2008. doi: 10.1002/lt.21495. [DOI] [PubMed] [Google Scholar]
- 36.Vaidyanathan R, Van Ert H, Haq KT, Morotti S, Esch S, McCune EC, Grandi E, Eckhardt LL. Inward rectifier potassium channels (Kir2.x) and caveolin-3 domain-specific interaction: implications for Purkinje cell-dependent ventricular arrhythmias. Circ Arrhythm Electrophysiol 11: e005800, 2018. doi: 10.1161/CIRCEP.117.005800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaidyanathan R, Vega AL, Song C, Zhou Q, Tan BH, Berger S, Makielski JC, Eckhardt LL. The interaction of caveolin 3 protein with the potassium inward rectifier channel Kir2.1: physiology and pathology related to long qt syndrome 9 (LQT9). J Biol Chem 288: 17472–17480, 2013. doi: 10.1074/jbc.M112.435370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114: 2104–2112, 2006. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 39.Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. J Biol Chem 280: 11656–11664, 2005. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 40.Ward CA, Ma Z, Lee SS, Giles WR. Potassium currents in atrial and ventricular myocytes from a rat model of cirrhosis. Am J Physiol Gastrointest Liver Physiol 273: G537–G544, 1997. doi: 10.1152/ajpgi.1997.273.2.G537. [DOI] [PubMed] [Google Scholar]
- 41.Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut 49: 268–275, 2001. doi: 10.1136/gut.49.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res 90: 443–449, 2002. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- 43.Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, Afeltra A, Sanyal AJ. Cirrhotic cardiomyopathy. J Am Coll Cardiol 56: 539–549, 2010. doi: 10.1016/j.jacc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 44.Zardi EM, Zardi DM, Chin D, Sonnino C, Dobrina A, Abbate A. Cirrhotic cardiomyopathy in the pre- and post-liver transplantation phase. J Cardiol 67: 125–130, 2016. doi: 10.1016/j.jjcc.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Wu J, King JH, Huang CL, Fraser JA. Measurement and interpretation of electrocardiographic QT intervals in murine hearts. Am J Physiol Heart Circ Physiol 306: H1553–H1557, 2014. doi: 10.1152/ajpheart.00459.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]