The U.S. public identified lowering prescription drug prices as the top health priority for Congress in 2019. That nearly all of the 2020 presidential candidates have introduced proposals for lowering drug prices points to the salience of this issue. The focus of debate over drug prices has largely been on prices that are too high. But what if some drug prices are too low? That is the concern raised in a recent report from the Food and Drug Administration (FDA) on drug shortages.1 In the market for some drugs, the FDA suggests that manufacturers lack incentives to enter the market or invest in manufacturing quality, ultimately resulting in shortages. The FDA’s somewhat paradoxical conclusion is that the US should pay more for some drugs to prevent shortages.
Drug shortages disproportionately affect generic, injectable medications, which have been marketed for decades and have lower prices even when compared to other generics.1 These shortages affect essential drugs (injectable antibiotics, such as vancomycin and cefazolin, chemotherapeutic agents, such as vincristine and doxorubicin, and anesthetics, such as lidocaine and bupivacaine),2 and therefore have major public health consequences, including delays in or omission of doses; use of less effective treatments; increased morbidity; and even death.3,4 Assessing the root causes of and potential solutions to drug shortages is timely because the number of shortages has increased recently, from 60 per week in 2016 to over 100 in 2018..2 Furthermore, with nearly 100 bills introduced in Congress that affect drug pricing, the federal government may intervene in drug markets. Policymakers should consider unintended consequences for drug supply, particularly for medicines prone to shortages.
‘Root causes’ of drug shortages
The FDA identifies three ‘root causes’ of drug shortages. The first is weak financial incentives for manufacturers to produce drugs with limited profitability. More than half of the drugs in shortage experienced declining sales and prices for years before the shortage. Second, markets for generic drugs (which account for two-thirds of shortages) do not recognize and reward sponsors for high-quality manufacturing. Third, the logistical and regulatory complexities of drug manufacturing complicate markets’ ability to restore supply after a disruption. These ‘root causes’ are actually proximal causes. To truly understand the root causes requires knowing why high-quality manufacturing is not rewarded, why markets are slow to respond to shortages, and why prices of generics have decreased.
Generic drug manufacturing and purchasing system
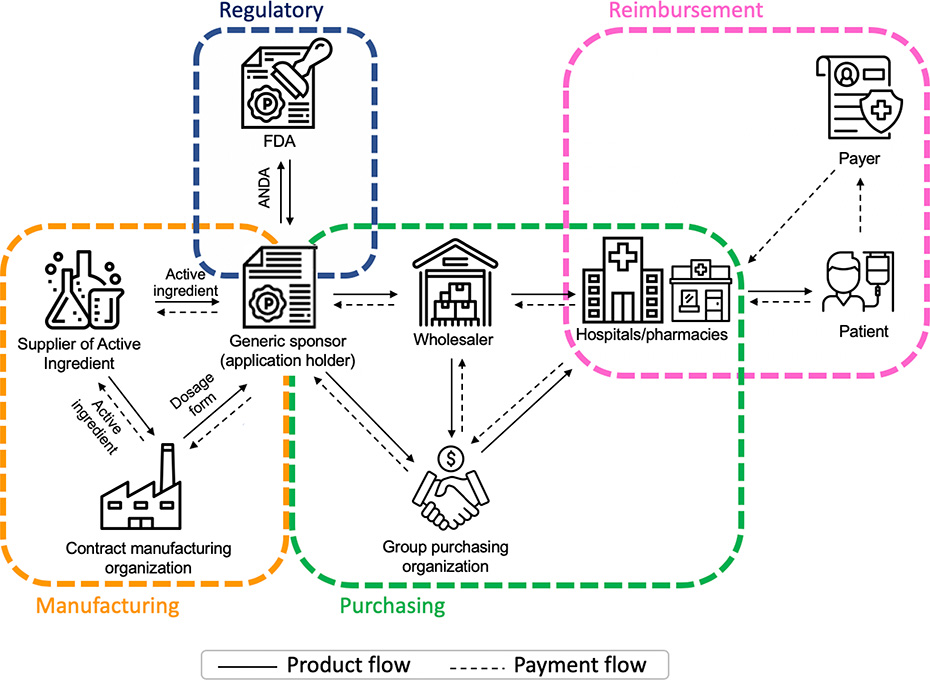
Understanding the complexity of the generic drug manufacturing and purchasing system is key to understanding how shortages occur (Figure). On the manufacturing side, it is increasingly the case that responsibility for obtaining approval, producing the active ingredient, and manufacturing the final dosage form rests not with a single company, but with three companies. Pharmaceutical companies that want to market a generic version for an existing drug (generic sponsors) submit an abbreviated new drug application to the FDA. After FDA approval, generic sponsors may either produce the active ingredient and the final dosage form or outsource production. It is increasingly the case that generic sponsors purchase the active ingredient from an independent supplier, and also have separate agreements with contract manufacturing organizations who produce the final dosage form.
Figure.
Generic Drug Manufacturing and Purchasing System
Notes: Abbreviations: FDA=Food and Drug Administration; ANDA=Abbreviated New Drug Application. The figure depicts the generic drug manufacturing and purchasing system, including regulatory approval provided by the FDA; the joint manufacturing enterprise of generic sponsors who hold ANDAs, suppliers of active ingredients and contract manufacturing organizations; drug purchasing by health systems and pharmacies who often go through intermediaries such as group purchasing organizations or wholesalers; and ultimately reimbursement that flows from patients and payers (e.g., insurance companies, Medicare) to the health systems and pharmacies that administer or dispense drugs to patients. Solid arrows indicate the direction of the flow of generic drug products or services, and dotted arrows the flow of payment.
Similarly, the generic drug purchasing system is fragmented. Health systems and pharmacies, who administer or dispense drugs to patients, often purchase drugs through intermediaries, such as wholesalers and group purchasing organizations (GPOs). In addition, payers (e.g., insurers, Medicare) reimburse health systems and pharmacies for drugs purchased using fixed payments (prospective payments to hospitals or maximum allowable cost to pharmacies) creating strong incentives to purchase generics at the lowest price regardless of any differences in the quality of the manufacturing process.
Manufacturing concentration and internationalization
At nearly every point in this system, the market has become more concentrated, meaning a small number of companies account for a large share of the market, and concentration is at the root of shortages. Among manufacturers, concentration is evident among all three actors. First, although policymakers have eliminated many barriers to generic entry, there remains high concentration in the supply of generic products.2 Drug shortages are more likely to occur in markets with only 1 to 3 generic sponsors.1,2,5 Second, because of consolidation of suppliers, competing generic sponsors often rely on a single active ingredient supplier. Third, it is increasingly common for a single contract manufacturer to produce the final dosage forms for all generic sponsors marketing a given product.2 Moreover, 90% of active ingredients and 60% of dosage forms dispensed in the US are manufactured overseas, complicating FDA’s monitoring efforts.1 Market concentration is the underlying reason why markets are so slow in responding to shortages. When production is halted for quality control problems (e.g., the sterile injectables produced by the manufacturing facility are non-sterile or contain metal particulates), there is no alternative facility available.
Purchasing concentration
On the purchasing side, GPOs are highly concentrated; the top four now account for 90% of the market.1 The market power of GPOs has lowered prices for health systems but, according to FDA, has also contributed to a ‘race to the bottom’,1 which has decreased generic sponsors’ profitability, especially in the case of injectables, which are costly-to-manufacture. Importantly, because generic drugs are bioequivalent and exchangeable there is no mechanism in the purchasing system to reward high-quality production even though FDA asserts differences in the quality of manufacturing practices exist and are inextricably linked to shortages. Concentration among intermediaries in the drug purchasing system is a likely culprit in driving the prices of some generics so low that generic sponsors do not see them as profitable.
Potential solutions to drug shortages
Addressing shortages begins with FDA’s regulatory authority. Congress enhanced this authority through the Food and Drug Administration Safety and Innovation Act of 2012, which required all manufacturers of medically important drugs to provide timely notification of supply disruptions or discontinuations. The recently proposed Mitigating Emergency Drug Shortages (MEDS) Act would further enhance this authority by requiring manufacturers, including active ingredient suppliers, to fully disclose the causes, expected duration, and likely impact of shortages; and report contingency plans for essential medications. Notably, the MEDS Act would require the Secretary of Health and Human Services to make recommendations on market-based incentives to promote domestic manufacturing of active ingredients and final dosage forms, and to encourage manufacturing of drugs in shortage. The MEDS Act stops short, however, of requiring any action in response to such recommendations or providing FDA with authority to impose monetary penalties on manufacturers who fail to meet reporting or planning requirements.
However, FDA’s authority to address shortages is limited, as it has no oversight over drug purchasing. A viable solution to drug shortages likely would require FDA to work with payers who bear the costs of drug shortages and can alter the financial incentives of manufacturers and health systems. A coordinated effort could take the form of FDA developing a system for rating generic manufacturers on the maturity of their quality management systems, and payers (e.g., Medicare) creating financial incentives that reward the steady supply of high-quality products.
Yet, involving payers more directly in addressing drug shortages comes with significant challenges. First, there is a need for greater transparency in the private sector contracts between GPOs and generic manufacturers, which FDA views as a black box. Second, it is not obvious that payers are willing to increase payments for drugs even for those in shortage. Third, payers must have a mechanism to directly reward manufacturers for engaging in high-quality production practices. Some options within the current fixed payment reimbursement system, both of which would require legislation, include paybacks for top-rated manufacturers, and federal tax credits for manufacturers investing in domestic injectable manufacturing facilities. Alternatively, payment for some shortage drugs could be pegged to purchasing costs rather than a payment limit.6 Financial incentives for high-quality manufacturing practices should be contingent on the steady supply of product. Ultimately, a blend of legislative, regulatory and financial remedies is needed to ensure an adequate supply of essential medicines in the US.
Supplementary Material
Acknowledgments
Disclosures and funding sources: Hernandez is funded by the National Heart, Lung and Blood Institute (grant number K01HL142847). The authors have no conflicts of interest to disclose.
Contributor Information
Inmaculada Hernandez, Department of Pharmacy and Therapeutics, School of Pharmacy, University of Pittsburgh, Pittsburgh, PA; Center for Pharmaceutical Policy and Prescribing (CP3), University of Pittsburgh, Pittsburgh, PA.
Tina Batra Hershey, Department of Health Policy and Management, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA; Center for Pharmaceutical Policy and Prescribing (CP3), University of Pittsburgh, Pittsburgh, PA.
Julie M. Donohue, Department of Health Policy and Management, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA; Center for Pharmaceutical Policy and Prescribing (CP3), University of Pittsburgh, Pittsburgh, PA.
References
- 1.US Food and Drug Administration Report. Drug Shortages: Root Causes and Potential Solutions. 2019; https://www.fda.gov/drugs/drug-shortages/report-drug-shortages-root-causes-and-potential-solutions. Accessed November 6, 2019.
- 2.Rosenberg M Identifying the Root Causes of Drug Shortages and Finding Enduring Solutions. 2018; https://healthpolicy.duke.edu/sites/default/files/atoms/files/duke-fda_drug_shortages_presentation_slides__0.pdf. Accessed November 6, 2019. [Google Scholar]
- 3.Fox ER, Sweet BV, Jensen V. Drug Shortages: A Complex Health Care Crisis. Mayo Clin Proc.89(3):361–373. [DOI] [PubMed] [Google Scholar]
- 4.Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 2017;317(14):1433–1442. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez I, Sampathkumar S, Good CB, Kesselheim AS, Shrank WH. Changes in Drug Pricing After Drug Shortages in the United States. Ann Intern Med. 2018;170(1):74–76. [DOI] [PubMed] [Google Scholar]
- 6.Drug Shortages: Why they happen and what they mean. Statement before the Senate Finance Committee by Scott Gottlieb. 2011; https://www.finance.senate.gov/imo/media/doc/Gottlieb%20Testimony1.pdf Accessed November 19, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.