Abstract
Adoptive transfer of immune cells is being actively pursued for cancer treatment. Natural killer (NK) cells, a class of cytotoxic immune cells, generally lack inherent selectivities toward cancer. To bestow tumor-targeting abilities and enhance anticancer efficacy, a new strategy is established to glycoengineer NK cells. Carbohydrate-based ligands for CD22, a marker for B cell lymphoma, are introduced onto NK cells through either metabolic engineering or glyco-polymer insertion. Such NK cells exhibited greatly enhanced cytotoxicities toward CD22+ lymphoma cells in a CD22-dependent manner. Importantly, both CD22+ lymphoma cell lines and primary lymphoma cells from human cancer patients can be effectively killed by the engineered NK cells. Furthermore, glycoengineered NK cells provided significant protection to tumor-bearing mice. Thus, NK cell glycoengineering is an exciting new approach for cancer treatment complementing the current immune cell genetic engineering strategy.
Short abstract
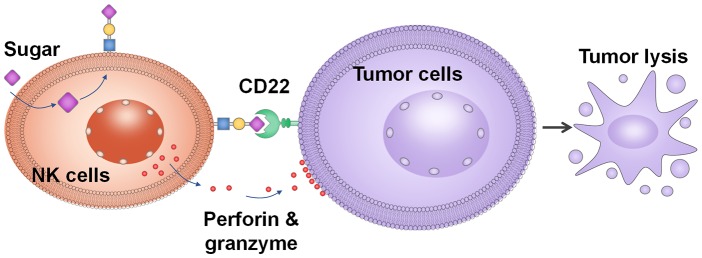
Sweetening natural killer cells enhanced their tumor-killing abilities in vitro and reduced tumor growth in vivo.
Introduction
The chimeric antigen receptor T cells (CAR-T) are breakthrough anticancer therapies with two types of CAR-T cells approved by the FDA for cancer treatment.1,2 However, despite great promise, CAR-T therapies have several limitations.3 Because of the potential immune responses by the host against the foreign major histocompatibility complex (MHC) molecules expressed on the surface of nonautologous T cells,4 the patient’s own T cells need to be extracted, genetically engineered with a chimeric antigen receptor for tumor recognition, expanded into a larger number, and reinfused back to the patient. Such a process is time and resource intensive, which is reflected by the high costs of CAR-T therapy ($475,000).5 In addition, for patients who have been heavily pretreated with chemotherapy and/or radiation, it can be difficult to acquire sufficient numbers of autologous T cells for CAR-T generation. CAR-T may not be produced in time for patients with rapidly developing diseases.
Natural killer (NK) cells are another type of cytotoxic immune cells that are capable of killing tumor cells, providing an attractive alternative to T cell-based therapy.3 NK cells do not express MHC class I molecules on the cell surface. As a result, they can be potentially used as an off-the-shelf cellular therapy with clinical evidence showing that adoptive transfer of allogeneic NK cells is safe to patients.6−8 NK cells can be prepared in a large scale and readily available to patients. On the other hand, NK cells do not have inherent targeting abilities toward cancer cells. To overcome this drawback, NK cells have been genetically engineered with chimeric antigen receptors (CAR-NK).3,9 However, NK cells are known to be notoriously adverse to endogenous gene uptake, resulting in low transgene expression.10 Therefore, new methods need to be developed to enhance the abilities of NK cells to recognize tumor cells.
We have begun to investigate strategies to engineer NK cells and bestow de novo abilities for NK cells to recognize cancer, such as B cell lymphoma. Each year, approximately 70 000 people are diagnosed with B-cell lymphoma in the United States alone. While the anti-CD20 antibody rituximab can be effective,11,12 it does not provide a cure, especially for the indolent lymphoma with annual deaths reaching 20 000.12−15 As native NK cells lack intrinsic affinities toward B cell lymphoma, we envision that if NK cells can be engineered to better recognize lymphoma cells, better therapeutic efficacy may be achieved.
Herein, we report for the first time that glycoengineering of NK cells with 9-O modified sialic acid-based CD22 ligands can significantly improve their abilities to bind and kill CD22+ lymphoma cells. CD22, also known as siglec-2, is a B-cell-restricted antigen, which can serve as a selective target for B cell lymphoma.16−19 The natural ligand on the cell for CD22 is the trisaccharide Neu5Acα2-6Galβ1-4GlcNAc that terminates glycans on the cell surface.20−22 Ground-breaking studies17,21−23 by the Paulson and Nitschke groups showed that the installation of a modified benzoate amide at the C-9 position of sialic acid in CD22 ligands can significantly enhance the binding affinity toward CD22. Furthermore, these compounds are highly selective toward CD22 with little cross-reactivities to other siglecs, such as siglec 7, which is an inhibitory receptor on NK cells.21 Glycan engineering of NK cells with CD22 ligands is an exciting new strategy for anticancer immunotherapy.
Results and Discussion
Constructing NK Cells with CD22 Ligands through Glycoengineering
As a proof-of-concept, we selected NK-92 cells, which are a well-established NK cell line24−26 readily expandable to reach clinically useful doses. Furthermore, NK-92 cells have been tested in phase I clinical trials for cancer treatment, exhibiting good safety profiles.27,28
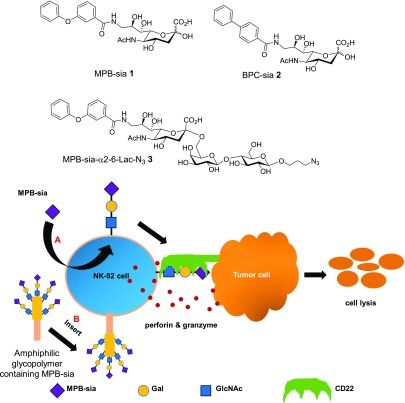
We explored two glycoengineering approaches to introduce CD22 ligands onto NK-92 cells. In the first method, we tested the possibilities of cells to take up exogenous sialic acids and metabolically incorporate the sialic acid into endogenous glycoproteins on the surface of cells. While glycan metabolic engineering has been applied to cells such as cancer,29,30 it is unclear whether NK cells can uptake modified sialic acid (sia) derivatives such as MPB-sia 1 and BPC-sia 2 as precursors and transform them into CD22 ligands through the cellular biosynthesis machinery (Figure 1, method A). In a complementary approach, we synthesized an amphiphilic polymer bearing multiple CD22 ligand trisaccharide 3 (Supplementary Figure 1). This glyco-polymer may directly insert into NK-92 membrane, bestowing CD22 targeting abilities to NK-92 cells (Figure 1, method B).
Figure 1.
Modification of NK-92 with CD22 ligands through glycoengineering. Two methods have been developed. Method A is metabolic glycoengineering using a sialic acid derivative, e.g., MPB-sia 1, which could be metabolized onto the surface of NK-92 cell through the sialic acid biosynthetic pathway. Method B uses a glyco-polymer containing MPB-sia, which could insert into the NK-92 cell membrane presumably because of its amphiphilicity. Both approaches could enhance the ability of targeting and binding of NK-92 cells toward CD22 positive cells resulting in more effective lysis of target cancer cells.
To test metabolic glycoengineering, NK-92 cells were incubated with MPB-sia 1 or BPC-sia 2 supplemented medium as well as that with equal amount of unmodified free sialic acid as a control. Upon removing all free sialic acid or derivatives by thorough washing, the cells were treated with an α2-3,6,8 neuraminidase that can cleave α2-3, α2-6, and α2-8 sialyl linkages. The amounts of free sialic acid and derivatives released were functionalized with 1,2-diamino-4,5-methylenedioxybenzene (DMB)31,32 and quantified by mass spectrometry through comparison with standard compounds. As shown in Table S1, while no MPB-sia 1 was detected in parent cells, incubation of NK-92 cells with MPB-sia 1 led to the detection of significant amounts of MPB-sia (5.2 × 106 molecules/cell) from cells. DMB functionalized BPC-sia was also detected from BPC-sia 2 treated cells. However, the amount of BPC-sia was too small to be accurately quantified, suggesting MPB-sia 1 was more efficiently incorporated into cells.
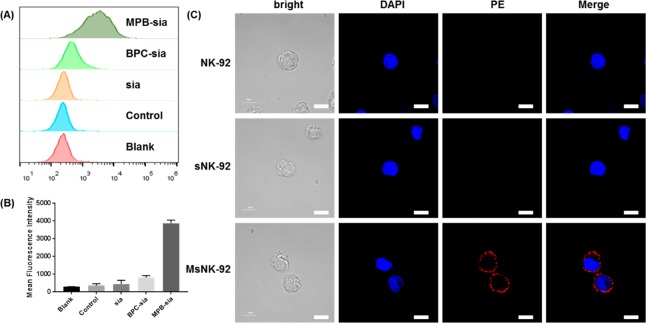
In order to test the function of engineered CD22 ligands on the cell surface, glycoengineered NK-92 cells were treated with CD22 protein followed by a fluorescently labeled anti-CD22 monoclonal antibody (mAb, clone HIB22). The extent of CD22 binding was quantified by flow cytometry analysis. Native NK-92 cells had little binding with CD22 over the background, similar to free sialic acid treated NK-92 cells. While BPC-sia 2 enhanced CD22 binding to NK-92 cells, MPB-sia 1 incubation led to the greatest improvement in cellular binding by CD22 (Figure 2A,B). These results can be explained by the higher affinity of MPB functionalized CD22 ligand with CD2221 and/or the more ready incorporation of MPB-sia onto the cells (Table S1). The engineered cells were then imaged by confocal microscopy. Native NK-92 cells or NK-92 cells treated with free sialic acid (sNK-92) did not present much PE fluorescence upon incubation with CD22 and PE-labeled anti-CD22 mAb (Figure 2C). In contrast, apparent PE fluorescence was observed on the surface of MPB-sia 1 treated cells (MsNK-92), with fluorescence intensities distributed over the whole cell surface (Figure 2C). These results suggest that NK-92 cells could be metabolically glycoengineered with sialic acid derivatives such as MPB-sia 1 to install CD22 ligands on the cell surface.
Figure 2.
MPB-sia can be metabolically engineered onto the surface of NK-92 cell to enhance the binding ability to CD22 protein. (A) Metabolic incorporation of various sialic acid derivatives onto NK-92 cells as measured by flow cytometry. Control represents nonengineered NK-92 cells treated with CD22-Fc and PE-mouse anti human CD22 mAb (Clone HIB22). (B) Quantification of the mean fluorescence intensities of cells upon incubation with various sialic acid derivatives. Mean with SD are presented for n = 3. (C) Confocal microscopy images of NK-92 cells engineered with sialic acid (sNK-92) or MPB-sia 1 (MsNK-92), followed by human CD22-Fc incubation and PE-mouse anti human CD22 mAb staining. Cells were fixed and nuclei were stained with DAPI. Scale bar, 10 μm.
CD22 ligands were metabolically engineered onto NK-92 cells by MPB-sia 1 in a dose- and time-dependent manner (Supplementary Figure 2). Increasing the concentration of MPB-sia 1 enhanced the levels of CD22 ligand expression on the cell surface reaching a maximum at 4 mM of MPB-sia 1 at 24 h. Increasing the incubation time to 48 and 72 h led to higher levels of CD22 binding to NK-92 cells. Cell viability studies showed slight decreases of cell viability when concentrations of MPB-sia 1 were over 4 mM (Supplementary Figure 3). Thus, 2 mM MPB-sia 1 was selected for further study. Next, the persistence of CD22 ligands on engineered NK-92 cells was analyzed. Upon removal of MPB-sia 1 from cell culture medium, 50% of CD22 binding remained on NK-92 cells after 48 h (Supplementary Figure 4). As CD22 prefers α2-6-sia linkages, the levels of α2-6-sia glycans on engineered NK-92 cells were determined by staining with FITC-labeled α2-6-sia binding plant lectin Sambucus nigra lectin (SNA) (Supplementary Figure 5). There were no significant changes of SNA staining before or after glycoengineering, suggesting little influence on the overall amounts of α2-6-sia linkages by MPB-sia 1 incubation.
As an alternative to metabolic glycoengineering, we investigated the possibility of directly inserting CD22 ligands onto the surface of NK-92 cells (Figure 1, method B). In order to accomplish this, a cholesterol-terminated poly(acrylic acid) polymer was synthesized by atom-transfer radical-polymerization (ATRP) with an average molecular weight of 30 kDa (Supplementary Figure 6). The carboxylic acid side chain of the polymer was modified with MPB-sia-α2-6-Lac-N33 through the copper catalyzed azide–alkyne cycloaddition reaction with an average of 100 trisaccharides per polymer chain producing glyco-polymer Chol-P-CD22L1004 (Supplementary Figure 1). Upon incubation of NK-92 cells with the Chol-P-CD22L1004, the cholesterol end of the polymer could insert into the cellular membrane through hydrophobic–hydrophobic interactions, anchoring the polymer onto the cell surface. After 1 h of incubation, the cells were washed followed by treatment with human CD22 and the PE-labeled anti-CD22 mAb. Strong CD22 binding was detected with 2.5 μM polymer (equivalent to 250 μM MPB-sia 1) (Supplementary Figure 7). With the glycopolymer, while the surface CD22 ligand level decreased rapidly (∼85% loss) during the first 24 h after removal of polymer-containing medium, CD22 binding remained detectable for 72 h by FACS analysis (Supplementary Figure 8). Compared to metabolically engineered NK cells (Supplementary Figure 4), the polymer approach was associated with much faster drops in surface CD22 ligand levels. This may be because through metabolic glycoengineering, MPB-sia 1 taken up inside the cells could be continuously modified into CD22 ligands over time.
Enhanced Killing of CD22 Positive Cells by Glycoengineered NK-92 Cells
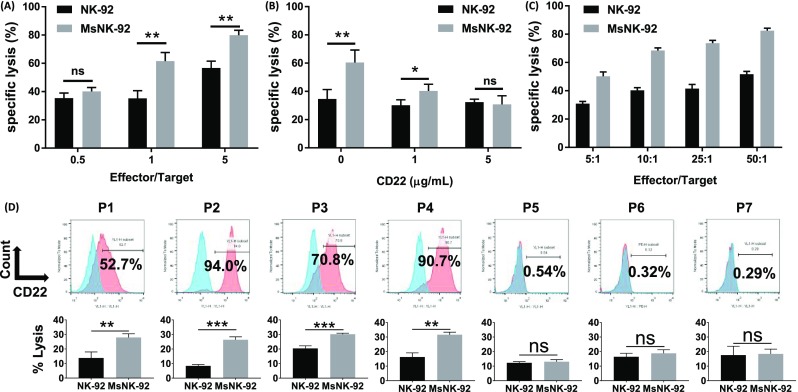
With the increased affinity of glycoengineered NK-92 cells for CD22, their cytotoxicities toward CD22 expressing human lymphoma Raji cells (Supplementary Figure 9) were evaluated. Metabolic glycoengineered NK-92 cells significantly enhanced lysis of target cell Raji compared to unmodified NK-92 at effector/target cell ratio 1 or greater (Figure 3A). To test the CD22 dependence, CD22 protein was added to the mixture of Raji cell and glycoengineered NK-92 cells. With increasing amounts of free CD22 in solution, the abilities of the NK-92 cells to kill Raji cells decreased, which reached the levels of unengineered NK-92 cells with 5 μg/mL of CD22 (Figure 3B). The reduced cytotoxicities in the presence of free CD22 are presumably due to competitive binding of free CD22 protein to engineered NK-92 cells, suggesting CD22 plays an important role in cytotoxicities of engineered NK-92 cells toward CD22+ cancer cells.
Figure 3.
Glycoengineered NK-92 cells could enhance killing of CD22 positive cells. (A) Lysis of Raji-luc cells by NK-92 and MsNK-92. Different effector-to-target cell ratios; P = 0.0049 (E/T = 1) and P = 0.0026 (E/T = 5). (B) Increasing concentration of CD22 reduced the killing activities of engineered MsNK-92 cells, while impacting little the activities of NK-92 cells without glycoengineering; E/T = 1:1, P = 0.0154 (0 μg/mL free CD22 protein), P = 0.0442 (1 μg/mL). (C) Relative killing activities of glycoengineered NK-92 cells against CD22 positive CHO cells as detected by flow cytometry. (D) Significantly enhanced cytotoxicities were bestowed by glycoengineered NK-92 cells toward CD22 high patient-derived leukemic cells (P1–P4) versus those expressing CD22 in low levels (P5–P7). Top row: surface expression of CD22 on patient-derived leukemic samples as determined by flow cytometry. Bottom row: lysis of patient-derived leukemic samples by NK-92 and MsNK-92. P = 0.0077 (P1), P = 0.0002 (P2), P = 0.0009 (P3), P = 0.0014 (P4), P = 0.4439 (P5), P = 0.2907 (P6), and P = 0.8473 (P7). Mean with SD are presented for n = 3. Statistical significances were assessed using Student’s t test. In all figures, ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further confirm the role of CD22 in cytotoxicities of glycoengineered NK-92 cell, Chinese Hamster Ovarian (CHO) cells were genetically engineered to express human CD22 on the cell surface (CHO-hCD22), which were subjected to cytotoxicity assay by glycoengineered NK-92 cells33,34 with wild-type CHO cells (CHO-WT) as the control. Both metabolic glycoengineered and glycopolymer-modified NK-92 cells showed enhanced cytotoxicity toward CHO-hCD22 cells compared to CHO-WT cells (Figure 3C and Supplementary Figure 10), confirming the importance of CD22 in cytotoxicities of engineered NK-92. As the killing activities of both types of NK cells were similar and metabolic engineering requires only the monosaccharide MPB-sia 1 without the need to synthesize trisaccharide 3 and the polymer 4, further investigation was focused on the metabolic glycoengineering approach.
To establish the potential translatability of the glycoengineering strategy, primary lymphoma cells were obtained from lymphoma patients and incubated with glycoengineered NK-92 cells (Figure 3D). For patient-derived lymphoma cells expressing high levels of CD22 (P1–P4 in Figure 3D), significantly enhanced cytotoxicities by glycoengineered NK-92 were observed compared to unmodified NK-92. For patient cells with low levels of CD22 expression (P5–P7), there were no significant changes in cell death when incubated with glycoengineered NK-92 or unmodified NK-92. These results suggested glycoengineering of NK-92 cells with MPB-sia 1 can be a promising strategy to treat patients with CD22 positive B cell lymphoma.
Enhanced Killing Mechanism of Glycoengineered NK-92 Cells against Raji Cells
To gain a deeper understanding of the mode of action against Raji, glycoengineered NK-92 cells were incubated with Calcein-AM-labeled Raji cells and imaged by fluorescence microscopy (Supplementary Figure 11). If NK-92 cells can bind with Raji, cell clusters would be formed around unlabeled NK-92 cells. Significantly higher percentages of glycoengineered NK-92 cells were found clustered with Raji cells compared with native NK-92 cells and Raji cells, which could be attributed to the CD22 ligands on the glycoengineered NK-92 increasing the binding affinity with Raji. Time-lapse imaging further revealed that the NK-92 cells could find Raji cells and kill them after binding (see the time-lapse imaging video in the Supporting Information).
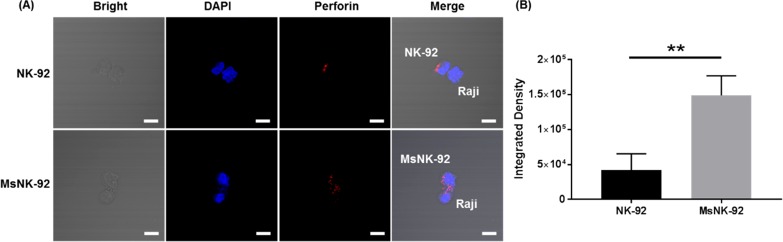
Upon binding with target cells, NK cells can release cytotoxic granules containing perforin or pro-inflammatory cytokines such as IFN-γ to kill the target cells.35,36 Confocal imaging of the NK-92 and Raji cells complexes showed an increased number of cytotoxic granules at the immunological synapse in metabolic glycoengineered NK-92 cells when bound with Raji cells (Figure 4), which indicated glycoengineered NK-92 cells were activated after initial binding. To further confirm the activation of glycoengineered NK-92, we detected the IFN-γ release. The glycoengineered NK-92 generated higher levels of pro-inflammatory cytokine IFN-γ compared with the unengineered parental NK-92 (Supplementary Figure 12).
Figure 4.
Complex formation between NK-92 cells and Raji cells investigated by confocal microscopy. Raji cells and NK-92 cells were coincubated for 1 h, fixed, permeabilized, and stained for perforin (red) to identify cytotoxic granules. Cell nuclei were labeled with DAPI (blue). Scale bar: 10 μm. (A) Representative images of cell–cell complex formation. (B) Perforin (red) fluorescence quantification. P = 0.0071. Mean with SD are presented for n = 3. Statistical significance was assessed using Student’s t test. In all figures, ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Evaluation of Antitumor Effect of Glycoengineered NK-92 Cells In Vivo
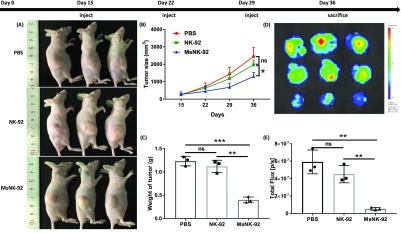
With the promising in vitro results, we analyzed the antitumor effect of glycoengineered NK-92 cells in vivo. Luciferase engineered Raji (Raji-luc) lymphoma cells were injected to nude mice subcutaneously. On days 15, 22, and 29 post tumor inoculation, glycoengineered NK-92 cells were administered intratumorally (Figure 5 and Supplementary Figure 13). As a control, groups of tumor-bearing mice received intratumoral injection of PBS or nonengineered NK-92 cells. The nonengineered NK-92 cells did not show significant protection compared to PBS. In contrast, animals injected with metabolic glycoengineered MsNK-92 cells significantly slowed down tumor growth compared to other groups. Thus, with the help of CD22 binding ligand on NK cell surface, an enhanced antitumor protection was achieved in vivo.
Figure 5.
In vivo antitumor activity of glycoengineered NK-92 cells against Raji-luc xenograft model. 107 Raji-luc cells were injected subcutaneously into the flanks of Balb/c nude mice. Fifteen days later, the mice were treated with an intratumoral injection of 107 glycoengineered NK-92 cells (MsNK-92 in 50 μL PBS), unengineered NK-92 (NK-92), or PBS buffer (50 μL) once a week. Bioluminescence images (BLI) were acquired with an IVIS Lumina II imaging system. (A) Images of mice with tumor at day 36. (B) Tumor growth curve. (C) Tumor weight measurements. (D) BLI images of the tumor after surgical removal from mice. (E) Quantitative BLI signals of the tumor after surgical removal from mice. Mean with SD are presented. Statistical significance was assessed using Student’s t test. In all figures, ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001. P = 0.0294 (panel B), P = 0.0003 (MsNK-92 vs PBS), P = 0.0010 (MsNK-92 vs NK-92) (panel C), P = 0.0025 (MsNK-92 vs PBS), P = 0.0024 (MsNK-92 vs NK-92) (panel E).
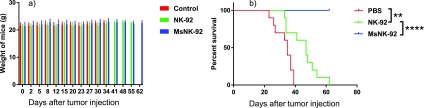
To better mimic the clinical condition, we further evaluated the efficacy of our strategy in a B cell lymphoma model. Raji-luc cells were injected intravenously on day 0, which were followed by intravenous administration of MsNK-92 cells on days 2, 5, 8, 12, and 15. Control mice were administered with either PBS or the same number of the parent NK-92 cells. The mice receiving MsNK-92 cells did not lose weight (Figure 6a), suggesting little toxicities due to systemic administration of cells. The survival of mice was continuously monitored (Figure 6b). While NK-92 cells provided significant protection to mice compared to the PBS group, all mice eventually died by day 62. Excitingly, all mice in the group receiving MsNK-92 cells survived, highlighting the power of the glycoengineering approach.
Figure 6.
In vivo antitumor activity of glycoengineered NK-92 cells against Raji-luc B cell lymphoma. 106 Raji-luc cells were injected intravenously into NOD SCID mice. On days 2, 5, 8, 12, and 15, mice received intravenous injections of 107 glycoengineered NK-92 cells (MsNK-92 in 100 μL PBS), unengineered parent NK-92 (NK-92), or PBS buffer control (100 μL) (n = 10 for each group). (a) The body weights of all mice were continuously monitored. No significant changes in body weight were observed, suggesting little toxicities due to administration of cells. (b) Kaplan–Meier survival curves of mice receiving PBS, NK-92, and MsNK-92 cells. All mice receiving PBS or NK-92 cells died by day 62, while 100% of the mice treated with MsNK-92 survived. Statistical significance was assessed using Student’s t test. **, P < 0.01; ***, P < 0.001.
Conclusion
While NK cells can potentially be cytotoxic against cancer cells, their lack of inherent affinity toward cancer cells is a significant drawback for NK-based therapy. To overcome this, we chemically engineered NK cells to gain novel targeting abilities. Among various strategies and reagents examined, the metabolic glycoengineering with MPB-sia monosaccharide successfully introduced CD22 ligand on NK-92 cells through the sialic acid biosynthetic pathway for B cell lymphoma targeting. The introduced MPB group greatly enhanced the binding ability and killing activity of NK-92 cells against CD22 positive cells in vitro and in mouse tumor models. Moreover, the glycoengineered NK-92 cells exhibited CD22-dependent cytotoxicity against primary lymphoma cells isolated from patients, which highlights its translational potential. The NK cell metabolic glycoengineering approach is simple and effective and can complement well the genetic engineering strategy of chimeric antigen receptors. Although the CD22 ligands on the NK cell surface would eventually become undetectable, the patients can be infused with multiple rounds of engineered NK cells to treat cancer. Studies are underway to further develop the glycoengineering method to enhance the efficacy of NK cell-based immunotherapy.
Acknowledgments
We are grateful to the National Cancer Institute, National Institutes of Health (Grant R01 CA225105), Michigan State University Foundation, the Michigan Economic Development Corporation through the MTRAC program, and Xiamen Nuokangde Biological Technology Co., Ltd (China) for financial support of our work. We thank Dr. J. Paulson (Scripps Research Institute) for kindly providing us the CHO and CHO-hCD22 cells.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.9b00956.
Author Contributions
∇ X.W. and S.L. contributed equally to this work. Z.L. conceived the concept of the metabolic glycoengineering method and supervised the project. X.H. conceived the concept of membrane insertion glycoengineering method and cosupervised the project. X.W., S.L., Z.F., J.W., H.C., Z.L., and X.H. contributed to experimental design. X.W., S.L., Y.T., J.Z., X.Y., N.L., X.W., and T.L. performed the experiments. X.W., S.L., Z.L., and X.H. wrote the manuscript.
The authors declare the following competing financial interest(s): Xiamen Nuokangde Biological Technology Co., Ltd is the initiator of this work; Dr. Zhu Li is chairman of the company. The company is collaborating with Xiamen University, Sun Yat-Sen University, and Michigan State University to complete the proof-of-concept of the project and integrate all data into the manuscript for publication. The patent related to this project has been filed (Patent application number: 201810139361.2).
Supplementary Material
References
- Fesnak A. D.; June C. H.; Levine B. L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H.; O’Connor R. S.; Kawalekar O. U.; Ghassemi S.; Milone M. C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- Mehta R. S.; Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front. Immunol. 2018, 9, 283. 10.3389/fimmu.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser R.; Blazar B. R. Acute graft-versus-host disease — biologic process, prevention, and therapy. N. Engl. J. Med. 2017, 377, 2167–2179. 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. E.; Cheung M. C. CAR T-cells: costs, comparisons, and commentary. J. Med. Econ. 2019, 22, 613–615. 10.1080/13696998.2019.1582059. [DOI] [PubMed] [Google Scholar]
- Olson J. A.; Leveson-Gower D. B.; Gill S.; Baker J.; Beilhack A.; Negrin R. S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010, 115, 4293–4301. 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. S.; Soignier Y.; Panoskaltsis-Mortari A.; McNearney S. A.; Yun G. H.; Fautsch S. K.; McKenna D.; Le C.; Defor T. E.; Burns L. J.; Orchard P. J.; Blazar B. R.; Wagner J. E.; Slungaard A.; Weisdorf D. J.; Okazaki I. J.; McGlave P. B. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Curti A.; Ruggeri L.; D’Addio A.; Bontadini A.; Dan E.; Motta M. R.; Trabanelli S.; Giudice V.; Urbani E.; Martinelli G.; Paolini S.; Fruet F.; Isidori A.; Parisi S.; Bandini G.; Baccarani M.; Velardi A.; Lemoli R. M. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011, 118, 3273–3279. 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- Tang X.; Yang L.; Li Z.; Nalin A. P.; Dai H.; Xu T.; Yin J.; You F.; Zhu M.; Shen W.; Chen G.; Zhu X.; Wu D.; Yu J. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [PMC free article] [PubMed] [Google Scholar]
- Matosevic S. Viral and nonviral engineering of natural killer cells as emerging adoptive cancer immunotherapies. J. Immunol. Res. 2018, 2018, 4054815. 10.1155/2018/4054815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotan E.; Aggarwal C.; Smith M. R. Impact of Rituximab (Rituxan) on the treatment of B-cell non-Hodgkin’s lymphoma. Pharm. Ther. 2010, 35, 148–157. [PMC free article] [PubMed] [Google Scholar]
- Bello C.; Sotomayor E. M. Monoclonal antibodies for B-cell lymphomas: rituximab and beyond. Am. Soc. Hematol. Educ. Program. 2007, 2007, 233–242. 10.1182/asheducation-2007.1.233. [DOI] [PubMed] [Google Scholar]
- Castillo J.; Winer E.; Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp. Hematol. 2008, 36, 755–768. 10.1016/j.exphem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin’s lymphoma. Annu. Rev. Med. 2008, 59, 237–250. 10.1146/annurev.med.59.060906.220345. [DOI] [PubMed] [Google Scholar]
- Evans L. S.; Hancock B. W. Non-Hodgkin lymphoma. Lancet 2003, 362, 139–146. 10.1016/S0140-6736(03)13868-8. [DOI] [PubMed] [Google Scholar]
- Ereño-Orbea J.; Sicard T.; Cui H.; Mazhab-Jafari M. T.; Benlekbir S.; Guarné A.; Rubinstein J. L.; Julien J.-P. Molecular basis of human CD22 function and therapeutic targeting. Nat. Commun. 2017, 8, 764. 10.1038/s41467-017-00836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C.; Completo G. C.; Sigal D. S.; Crocker P. R.; Saven A.; Paulson J. C. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 2010, 115, 4778–4786. 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haso W.; Lee D. W.; Shah N. N.; Stetler-Stevenson M.; Yuan C. M.; Pastan I. H.; Dimitrov D. S.; Morgan R. A.; FitzGerald D. J.; Barrett D. M.; Wayne A. S.; Mackall C. L.; Orentas R. J. Anti-CD22–chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013, 121, 1165–1174. 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry T. J.; Shah N. N.; Orentas R. J.; Stetler-Stevenson M.; Yuan C. M.; Ramakrishna S.; Wolters P.; Martin S.; Delbrook C.; Yates B.; Shalabi H.; Fountaine T. J.; Shern J. F.; Majzner R. G.; Stroncek D. F.; Sabatino M.; Feng Y.; Dimitrov D. S.; Zhang L.; Nguyen S.; Qin H.; Dropulic B.; Lee D. W.; Mackall C. L. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018, 24, 20–28. 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. D.; Sgroi D.; Sjoberg E. R.; Stamenkovic I.; Varki A. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J. Biol. Chem. 1993, 268, 7019–7027. [PubMed] [Google Scholar]
- Rillahan C. D.; Macauley M. S.; Schwartz E.; He Y.; McBride R.; Arlian B. M.; Rangarajan J.; Fokin V. V.; Paulson J. C. Disubstituted sialic acid ligands targeting siglecs CD33 and CD22 associated with myeloid leukaemias and B cell lymphomas. Chem. Sci. 2014, 5, 2398–2406. 10.1039/c4sc00451e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.; Paulson J. C. CD22 ligands on a natural N-glycan scaffold efficiently deliver toxins to B-lymphoma cells. J. Am. Chem. Soc. 2017, 139, 12450–12458. 10.1021/jacs.7b03208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm S.; Gerlach J.; Brossmer R.; Danzer C.-P.; Nitschke L. The ligand-binding domain of CD22 Is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J. Exp. Med. 2002, 195, 1207–1213. 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Oberoi P.; Oelsner S.; Waldmann A.; Lindner A.; Tonn T.; Wels W. S. Chimeric antigen receptor-engineered NK-92 cells: An off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front. Immunol. 2017, 8, 533. 10.3389/fimmu.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingemann H.; Boissel L.; Toneguzzo F. Natural killer cells for immunotherapy - Advantages of the NK-92 cell line over blood NK cells. Front. Immunol. 2016, 7, 91. 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suck G.; Odendahl M.; Nowakowska P.; Seidl C.; Wels W. S.; Klingemann H. G.; Tonn T. NK-92: an ’off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 485–492. 10.1007/s00262-015-1761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S.; Meagher R.; Swearingen M.; Myint H.; Rich E.; Martinson J.; Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 2008, 10, 625–632. 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- Tonn T.; Schwabe D.; Klingemann H. G.; Becker S.; Esser R.; Koehl U.; Suttorp M.; Seifried E.; Ottmann O. G.; Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Du J.; Meledeo M. A.; Wang Z.; Khanna H. S.; Paruchuri V. D.; Yarema K. J. Metabolic glycoengineering: sialic acid and beyond. Glycobiology 2009, 19, 1382–1401. 10.1093/glycob/cwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.; Hong S.; Rong J.; You Q.; Dai P.; Huang R.; Tan Y.; Hong W.; Xie C.; Zhao J.; Chen X. Bifunctional unnatural sialic acids for dual metabolic labeling of cell-surface sialylated glycans. J. Am. Chem. Soc. 2013, 135, 9244–9247. 10.1021/ja402326z. [DOI] [PubMed] [Google Scholar]
- Lewis A. L.; Nizet V.; Varki A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 11123–11128. 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavdarli S.; Yamakawa N.; Clarisse C.; Aoki K.; Brysbaert G.; Le Doussal J.-M.; Delannoy P.; Guérardel Y.; Groux-Degroote S. Profiling of O-acetylated gangliosides expressed in neuroectoderm derived cells. Int. J. Mol. Sci. 2020, 21, 370. 10.3390/ijms21010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durward M.; Harms J.; Splitter G. Antigen specific killing assay using CFSE labeled target cells. J. Visualized Exp. 2010, 10.3791/2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. K.; Boukhaled G. M.; Condotta S. A.; Mazouz S.; Guthmiller J. J.; Vijay R.; Butler N. S.; Bruneau J.; Shoukry N. H.; Krawczyk C. M.; Richer M. J. Interleukin-10 directly inhibits CD8(+) T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity 2018, 48, 299–312. 10.1016/j.immuni.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genssler S.; Burger M. C.; Zhang C.; Oelsner S.; Mildenberger I.; Wagner M.; Steinbach J. P.; Wels W. S. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology 2016, 5, e1119354. 10.1080/2162402X.2015.1119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelsner S.; Friede M. E.; Zhang C.; Wagner J.; Badura S.; Bader P.; Ullrich E.; Ottmann O. G.; Klingemann H.; Tonn T.; Wels W. S. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy 2017, 19, 235–249. 10.1016/j.jcyt.2016.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.