Abstract
Background
The clinical characteristics of human metapneumovirus (hMPV)-associated lower respiratory tract infection (LRTI) after allogeneic hematopoietic stem cell transplantation (HSCT) is not well described. We describe the clinical course in eight HSCT recipients suffering from hMPV infection.
Methods
We prospectively included all patients with hMPV-associated LRTI after allogeneic HSCT during a period of 1 year. hMPV was diagnosed by multiplex polymerase chain reaction (PCR) from bronchoalveolar lavage (BAL).
Results
Eight patients with hMPV-associated LRTI were identified from 93 BAL samples. Three of the eight patients had co-infections with other pathogens. The median age of the patients was 45 years [interquartile range (IQR) 36.8–53.5], the median time posttransplant was 473 days (IQR 251–1,165), 5/8 patients had chronic graft-versus-host disease (cGvHD), and 6/8 patients received immunosuppression. Chest computed tomography (CT) scanning showed a ground-glass pattern in 7/8 patients. Seven of eight patients required hospitalization due to severe symptoms and hypoxemia. All were treated with intravenous immunoglobulin (IVIG), which was combined with oral ribavirin in six patients. The mortality rate was 12.5 % (1/8).
Conclusions
hMPV-associated LRTI in allogeneic HSCT recipients are not uncommon and present with unspecific respiratory symptoms, ground-glass pattern in CT scanning, and co-infection.
Keywords: Human metapneumovirus, Posttransplant infection, Treatment, Allogeneic hematopoietic stem cell transplantation, Respiratory virus
Introduction
Respiratory tract infections (RTIs) are common reasons for the consultation of allogeneic hematopoietic stem cell transplantation (HSCT) recipients in outpatient clinics [1]. In recent years, “new” respiratory viruses, such as human metapneumovirus (hMPV) and coronaviruses, were discovered and associated with severe morbidity and mortality in the immunocompromised host [1–3].
hMPV is a single-strand negative RNA virus and belongs to the family of paramyxoviridae, together with respiratory syncytial virus (RSV) and parainfluenza viruses. hMPV can be isolated from respiratory samples from otherwise healthy children with upper/lower (U/L)-RTIs [4]. Seroepidemiological studies indicate a 90 % exposure until adulthood. In HSCT recipients, hMPV-associated LRTIs have been associated with considerable morbidity and mortality [5, 6]. Only a few clinical studies have addressed the clinical presentation, risk factors, and the clinical course.
Methods
Patients
Between June 2009 and June 2010, all patients following allogeneic HSCT with symptoms of LRTI were further examined by computed tomography (CT) scanning and, in case of pulmonary infiltrates, underwent a bronchoalveolar lavage (BAL) with microbiological work-up. LRTI was defined by the presence of coughing, dyspnea, and/or pulmonary infiltrates in CT chest scans [7].
Following an index case of hMPV infection, polymerase chain reaction (PCR) for hMPV was routinely included in the diagnostic work-up.
After diagnosis, clinical and laboratory parameters (including blood count, C-reactive protein, and immunoglobulins) were analyzed and reevaluated prospectively up to 4 weeks. Furthermore, additional clinical and laboratory data were collected from the charts retrospectively. All patients gave written informed consent [approved by the local Institutional Review Board (IRB) EKBB-363/09].
The degree of immunodeficiency was classified according to clinical and laboratory criteria as described previously [8]. Patients with allogeneic HSCT >6 months prior to hMPV diagnosis, acute graft-versus-host disease (aGvHD) grade <2, leukocyte count >2.0 × 109/L, lymphocyte count >0.1 × 109/L, recipients of maintenance immunosuppressive drugs, or T cell or B cell depletion >3 months prior to hMPV diagnosis were classified as moderately immunodeficient. In case of HSCT <6 months prior to hMPV diagnosis, T cell or B cell depletion <3 months prior to diagnosis, aGvHD-grade >2, leukopenia <2.0 × 109/L, lymphopenia <0.1 × 109/L, or hypogammaglobulinemia <4.0 g/L, immunodeficiency was classified severe.
Diagnostics of viral infection
In all patients, BAL samples were analyzed with an established multiplex PCR assay (RespiFinder®) [9]. RespiFinder® is able to detect 14 RNA viruses, one DNA virus, and four bacteria: adenovirus, Bordetella pertussis, Chlamydophila pneumoniae, coronavirus 229E/NL63/OC43, hMPV, influenza A/B, influenza A/H5N1, Legionella pneumophila, Mycoplasma pneumoniae, parainfluenza types 1 to 4, respiratory syncytial virus A/B, and rhinovirus [10]. For infection control reasons, all patients were screened weekly by nasopharyngeal swabs during outpatient follow-up until they became negative with the same assay.
Cytopathological work-up
Standardized cytopathological work-up of BAL samples included absolute cell counts, immunophenotyping of lymphocytes, and immunostaining for RSV, cytomegalovirus (CMV), and influenza virus. Standardized bacteriological cultures to identify bacteria, fungi, and mycobacteria were performed.
Radiological work-up
CT images were analyzed by two independent radiologists being aware of clinical findings, underlying disease, and posttransplant immunodeficient status. Images were compared retrospectively with earlier and later series within a 4-week period of time.
Data analysis
The SPSS 13 software package (http://www.SPSS.com) was used to calculate the medians and interquartile ranges (IQRs). We used non-parametric tests due to the non-normal data distributions.
Results
Patient characteristics
Between June 2009 and June 2010, eight cases of hMPV-associated LRTI were identified among 93 patients undergoing bronchoscopy and BAL for LRTI. The overall incidence of hMPV-associated LRTI was, hence, 8.6 %. The median time posttransplant was 473 days (IQR 251–1,165). Five patients had chronic graft-versus-host disease (cGvHD) and four of these patients had previously biopsy-proven bronchiolitis obliterans and were immunosuppressed with prednisone, tacrolimus, and mycophenolic acid (Table 1). Six cases occurred during March and April 2010. The infections were not epidemiologically related and we could not identify contact exposures between patients. The clinical characteristics at diagnosis are presented in Table 1. The performance score (Karnofsky scale) was decreased by 20 % compared to previous visits (median before = 90 %, median at diagnosis = 70 %). Dry cough and fever were commonly present (in 8/8 and 7/8 patients, respectively) at diagnosis, whereas only half of the patients presented with a runny nose or dyspnea New York Heart Association (NYHA) score >II.
Table 1.
Patient baseline characteristics
| Patient | Age (years) | Gender | Underlying diseasea | Remissionb | Time postTx (days)c | Fever | Cough | Running nose | Dyspnea (NYHA)d | Co-infectione | cGvHDf | BOg | ISh | SIDi | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | B-ALL | Unknown | 9 | 38.5 | + | − | I | No | 0 | No | CsA, Pred | 3 |
IVIGj Ribavirink |
Died |
| 2 | 37 | F | B-ALL | CR | 60 | 38.5 | + | + | I | Rhinovirus | Moderate | No | CsA, Pred | 3 |
IVIGj Ribavirink |
Survived |
| 3 | 36 | F | AML | CR | 1,460 | 37 | + | − | III | M. catarrhalis | 0 | No | None | 0 |
IVIGj Ribavirink |
Survived |
| 4 | 27 | M | CML | CR | 550 | 38.2 | + | − | III | No | Moderate | Yes | Tac, Pred, MMF | 2 |
IVIGj Ribavirink |
Survived |
| 5 | 57 | F | MM | CR | 315 | 39 | + | + | III | Coronavirus OC53 | Moderate | Yes | Tac, Pred, MMF | 1 |
IVIGj Ribavirink |
Survived |
| 6 | 38 | F | AML | PD | 395 | 39 | + | + | II | No | 0 | No | None | 0 |
IVIGj Ribavirink |
Survived |
| 7 | 54 | M | MM | CR | 2,310 | 38.5 | + | − | III | No | Moderate | Yes | Tac, Pred, MMF | 1 | IVIGj | Survived |
| 8 | 53 | M | NHL | CR | 870 | 38.7 | + | + | II | No | Moderate | Yes | CsA, Pred, MMF | 1 | IVIGj | Survived |
| Median | 45 | 473 | 38.5 | 3 Tac, 3 CsA 4 MMF, 6 Pred |
F female, M male, B-ALL acute lymphatic leukemia, AML acute myeloic leukemia, CML chronic myeloic leukemia, MM multiple myeloma, NHL non-Hodgkin lymphoma, CR complete remission, PD progressive disease
aHematologic disease prior to hematopoietic stem cell transplantation (HSCT)
bState of remission after HSCT
cTime of positive human metapneumovirus (hMPV) polymerase chain reaction (PCR)
dClinical stage according the New York Heart Association (NYHA) score I–IV
eOther pathogens found in bronchoalveolar lavage (BAL)
fChronic graft-versus-host disease grading according to [25]
gBronchiolitis obliterans was biopsy-proven cases only prior to the diagnosis of hMPV infection
hImmunosuppression
iAmount of criteria for severe immunosuppression as suggested by [8]
jStandard dose 0.5 g/kg bodyweight
kStandard dose 600 mg TID
Laboratory findings
At diagnosis, the median total lymphocyte count was significantly lower compared to 4 weeks earlier (778 vs. 1,378/μL, p = 0.04) and the C-reactive protein (CRP) level was significantly higher (41.3 vs. 3.7 mg/L, p = 0.005). Hemoglobin, neutrophil, platelet, and immunoglobulin levels remained unchanged. Four weeks after diagnosis, only IgG levels (8.8 vs. 16.9 g/L, p = 0.032) and reticulocyte count increased (67 vs. 120/μL p = 0.0457) after therapeutic interventions (see below), whereas the hemoglobin, neutrophil, lymphocyte, platelet, IgA, and IgM levels did not change significantly.
Radiologic findings
Prior to BAL, a chest CT scan was performed in each patient. The typical CT findings of two patients are shown in Fig. 1. Although CT findings are unspecific for viral infections, ground-glass pattern opacities were the most frequent findings, detected in 7/8 patients, but nodules with halo (5/8) and alveolar/interstitial infiltrates (4/8) were also common findings. In 6/8 patients, expiratory imaging was performed. In these patients, no air trapping as a sign for active bronchiolitis obliterans was present.
Fig. 1.
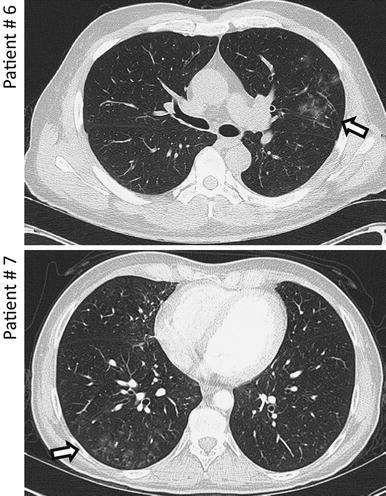
Chest computed tomography (CT) findings at diagnosis. Patients are marked according to the code used in the tables. The arrows indicate ground-glass pattern
Spirometry findings
At the time of diagnosis, spirometry findings indicated a drop in the FEV1 of 20 % of the median as compared to earlier spirometry. CO diffusion capacity was slightly reduced. Parameters were restored to baseline levels 2 months after infection.
Results of bronchial lavage and co-infections
At the initial diagnosis of hMPV infection, co-infections were observed in three patients: one with rhinovirus in the nasopharyngeal swab, one with Moraxella catarrhalis in BAL, and one with coronavirus OC43 in BAL, respectively. Notably, during outpatient follow-up, nasopharyngeal swab screening could only repeatedly detect hMPV in patients with initial co-infection indicated in previous BAL samplings. No fungal or mycobacterial co-infections were detected. No CMV reactivation was present. The findings of BAL fluids are summarized in Table 2. At the time of diagnosis of hMPV infection, cytology in BAL showed predominant macrophages (67 %) and lymphocytes (10 %). It is important to note that patient 1 was in marrow aplasia at the time of BAL, which explains why only a few lymphocytes (6 × 106/L) were observed (Table 2). For patient 6, BAL was performed for virological diagnosis but, unfortunately, no quantitative differential cell count was done.
Table 2.
Bronchoalveolar lavage findings
| Patient | Age (years)/gender | Macrophages % (cells × 106/L) | Lymphocytes % (cells × 106/L) | Neutrophils % (cells × 106/L) | Eosinophils % (cells × 106/L) | Mast cells/HPF |
|---|---|---|---|---|---|---|
| 1 | 51/F | 84 (97.4) | 5 (6) | 10 (12) | 1.1 (1) | 0 |
| 2 | 37/F | – | – | – | – | – |
| 3 | 36/F | 84 (99.54) | 12 (14) | 2 (2) | 2 (2) | 0 |
| 4 | 27/M | 80 (203) | 15 (38) | 5 (13) | 0 (0) | 0 |
| 5 | 57/F | 38 | 7 | 55 | 0 | 0 |
| 6 | 38/F | – | – | – | – | – |
| 7 | 54/M | 54 (188) | 8 (28) | 38 (132) | 0 (0) | 0 |
| 8 | 53/M | 38 (83.6) | 32 (70) | 30 (66) | 0 (10) | 10 |
| Median | 67 % | 10 % | 20 % | 0 % | 0/HPF |
Patient 2 was diagnosed only by nasopharyngeal swab. No BAL was performed. For patient 6, BAL was available, but no quantitative differential count of leukocytes was performed
F female, M male, HPF high power field at standard microscopy
Treatment and outcome of hMPV-associated LRTI
Treatment
All patients were treated with weekly intravenous immunoglobulin (IVIG) 0.5 g/kg bodyweight. The median treatment duration was 3 weeks. Furthermore—based on expert opinion and Bonney et al. [11]—six patients received oral ribavirin, starting with a loading dose of 600 mg on the first day, 200 mg TID for the next 2 days, with a further increase of the dosage every 2 days until a maximum dose of 600 mg TID was reached. The median duration of ribavirin treatment was 17 days, with four patients receiving the maximum dose for a median time of 1 week. Treatment with ribavirin was based on the decision by the treating physician, depending on the clinical manifestations.
Treatment-related complications
Overall, treatment was well tolerated, with no significant adverse events, except for the requirement of red blood cell (RBC) transfusion support, which was needed in 4/6 ribavirin-treated patients, due to the well-known adverse effect of ribavirin on RBCs and erythropoiesis [12]. Hemoglobin levels started to decrease in ribavirin-treated patients after 7 days of treatment. Due to low hemoglobin levels and associated symptoms of anemia, 4/6 patients treated with ribavirin required a total of 24 erythrocyte transfusions.
Outcome
Seven of eight patients (87.5 %) required hospitalization due to poor clinical condition, indicated by a reduced Karnofsky scale score and a dyspnea NYHA score of III. No further specified score was used to decide on the requirements of hospitalization.
One patient (number 1) required intensive care and assisted ventilation. Despite immediate treatment with IVIG and oral ribavirin, rapid respiratory failure and exhaustion developed and the patient died 39 days after the diagnosis of hMPV pneumonia from multi-organ failure. This patient has been infected within 30 days of transplantation. Surviving patients became asymptomatic (i.e., no coughing or sneezing, dyspnea NYHA score <II) after a median of 21 days. Seven of eight patients were discharged with clinically improved conditions after a median of 13 days. Treatment was stopped when clinical and laboratory improvement was observed or hMPV was no longer detectable in nasopharyngeal swab screening. hMPV in nasopharyngeal swabs could no longer be detected after a median of 2 weeks. Overall, clinical recovery was excellent 2 months after infection, indicated by a 90 % Karnofsky score. Respiratory recovery as assessed by the lung function test was reached in the same time period.
Discussion
During a period of 1 year, we could identify eight cases of hMPV-associated LRTI in patients following allogeneic HSCT, as found in the BAL of patients undergoing bronchoscopy because of respiratory symptoms and infiltrates in CT scan. The literature on hMPV infections following allogeneic or autologous HSCT is still scarce [13–19] and often incomplete. Conditioning regimens, remission status, immunosuppression, and laboratory data, clinical signs and symptoms, and duration of illness, as well as diagnostic microbiological methods, are often not reported.
Our prospective data show that hMPV is not uncommon. Even later than 1 year after transplantation, hospitalization was required in the majority of patients due to a reduction in performance scores and drop in lung function. Initial clinical signs of hMPV infection are unspecific [1, 20] and similar to other viral infections. Chest CT scans predominantly showed a ground-glass pattern, which is also an unspecific and common finding in patients with other viral infections [18]. We, therefore, recommend bronchoscopy for patients suffering from LRTI and showing a ground-glass pattern in the CT scan. This does not only allow for the searching of viral infection using a multiplex PCR, but also for concomitant bacterial infection.
Short interval from transplant to infection, intensive immunosuppression, as well as preexisting pulmonary alterations like bronchiolitis obliterans may affect the clinical course of hMPV, as well as other viral infections [8, 21]. Hence, the importance of single or combinations of risk factors needs to be examined in larger studies.
The outcome of our patients was favorable, except for one patient who suffered from hMPV infection shortly after transplantation (overall survival 87.5 %). A significantly lower mortality rate was observed in our patients compared to the literature, where mortality rates of up to 50 % are reported. However, most of these patients with a poor outcome suffered from hMPV infection which occurred within the first month posttransplant (Table 3). The lack of reports of infections in the later posttransplant period clearly indicates a potential underreporting or underdiagnosis, as only a few centers perform BAL in patients with lower respiratory symptoms. The remarkable better survival in our patients might be due to early diagnosis and the improved “net state of immunity”. Most of our patients had persistent immunosuppressive treatment because of cGvHD or—like in patient 6—immunosuppressive treatment has been stopped only a few weeks before infection and had to be restarted later due to the clinical relapse of cGvHD. We, therefore, speculate that cGvHD and immunosuppressive treatment is a risk factor for clinically relevant hMPV infection.
Table 3.
Review of the literature
| Patient no. | Age | Gender | Basic disease | Type of graft | Time postTx | Co-infection | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | Male | MM | Auto | 1,885 | None | None | Died | Huck et al. [19] |
| 2 | 46 | Male | MM | Allo | 8 | None | Corticosteroids | Died, idiopathic pneumonia | Englund et al. [17] |
| 3 | 54 | Female | MDS | Allo | 22 | None | Aerosolized ribavirin | Died, idiopathic pneumonia and sepsis | |
| 4 | 24 | Female | ALL | Auto | 18 | HSV | None | Survived, but died on relapse of disease | |
| 5 | 30 | Male | AML | Allo | 0 | Parainfluenza virus | Corticosteroids | Died, aGvHD, parainfluenza pneumonia, shock | |
| 6 | 46 | Male | AML | Allo | 13 | None | Corticosteroids | Died, idiopathic pneumonia | |
| 7 | 33 | Female | ALL | Allo | 7 | – | None | Died | Cane et al. [16] |
| 8 | na | Female | CML | Allo | 19 | Pneumonia, RSV | na | Survived | Oliveira et al. [15] |
| 9 | na | Male | NHL | Auto | 2 | Pneumonia | na | Survived | |
| 10 | na | Male | ALL | Allo | 10 | Sepsis, influenza B | na | Died | |
| 11 | na | Female | MM | Auto | 277 | RSV | na | Survived | |
| 12 | na | Male | PNH | Allo | 0 | None | na | Survived | |
| 13 | na | Male | CML | Allo | 17 | Pneumonia | na | Survived | |
| 14 | na | Male | CML | Allo | 0 | None | na | Survived | |
| 15 | na | Female | CML | Allo | 0 | None | na | Survived | |
| 16 | na | Female | CML | Allo | 46 | None | na | Survived | |
| 17 | na | Male | CML | Allo | 28 | None | na | Survived | |
| 18 | na | Female | SAA | Allo | 97 | None | na | Survived | |
| 19 | 40 | Female | Hodgkin | Allo | 101 | Influenza A, coronavirus | Oseltamivir | Died on day 112 | Campbell et al. [13] |
| 20 | 58 | Female | na | na | na | None | na | unknown | Franquet et al. [18] |
| 21 | 27 | Male | na | na | na | None | na | unknown | |
| 22 | 58 | Male | na | na | na | None | na | unknown | |
| 23 | 23 | Male | na | na | na | None | na | unknown | |
| 24 | 44 | Male | na | na | na | None | na | unknown | |
| Median | 44 (30–54) |
14 male 10 female |
15 allo 4 auto 5 not available |
17 (4.5–37) | 8 died, 11 survived, 5 unknown |
na information not available, MM multiple myeloma, MDS myelodysplastic syndrome, ALL acute lymphatic leukemia, AML acute myeloic leukemia, CML chronic myeloic leukemia, NHL non-Hodgkin lymphoma, PNH paroxysmal night hemoglobinuria, SAA severe anaplastic anemia, HSV herpes simplex virus, RSV respiratory syncytial virus
We cannot clarify if the treatment with IVIG and ribavirin was responsible for the therapeutic effect and the good outcome due to the lack of a control group. However, case reports and small case series have described favorable outcomes upon treatment with ribavirin and IVIG [11, 22]. IVIG treatment was given in all our cases, but the amount of hMPV-neutralizing antibodies in IVIG formulations remains unclear. Randomized studies on the hMPV treatment are still missing and will be difficult to perform due to the relatively small case number in single centers. Recommended treatment with ribavirin is based on expert opinion [3, 23], which is supported by data from in vitro cell cultures and mouse models [24]. In our center, we use oral ribavirin in severely immunocompromised patients with hMPV infection. This was well tolerated, except for hemolysis with the need for repetitive RBC transfusions. This needs to be considered as a severe side effect. Hence, a careful analysis of the potential risks and benefits is recommended before making the decision to start ribavirin treatment in selected patients with severe infection. Several other treatment strategies were described in the literature. Patients treated with corticosteroids, aerosolized ribavirin, or oseltamivir did not survive [13, 15–19].
Limitations of our study are the relatively small number of patients with hMPV LRTI, the single-center approach, and the lack of a proper control group for our treatment approach. However, due to the availability of clinical and laboratory data, including lung function tests and CT scans, we are able to properly describe the clinical course of hMPV LRTI in recipients of allogeneic HSCT.
In summary, hMPV infection is not uncommon in allogeneic HSCT recipients, even later than 1 year after transplantation. In our case series, most patients suffered from persistent immunosuppression and/or cGvHD. If patients suffer from respiratory symptoms and a ground-glass pattern in the CT scan, we recommend BAL with specific viral testing. If patients are treated with IVIG and ribavirin, the clinical outcome of hMPV infection is good. Due to ribavirin-associated hemolysis, the drug needs to be given with caution. The effectiveness and safety of a combined treatment with IVIG and ribavirin needs to be assessed in prospective therapeutic trials.
Acknowledgments
Conflict of interest
All authors do not have a conflict of interest.
References
- 1.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EJ. Viral diagnostics and antiviral therapy in hematopoietic stem cell transplantation. Curr Pharm Des. 2008;14:1997–2010. doi: 10.2174/138161208785061364. [DOI] [PubMed] [Google Scholar]
- 3.Ison MG. Respiratory viral infections in transplant recipients. Antivir Ther. 2007;12:627–638. [PubMed] [Google Scholar]
- 4.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatzidimitriou D, Gavriilaki E, Sakellari I, Diza E. Hematopoietic cell transplantation and emerging viral infections. J Med Virol. 2010;82:528–538. doi: 10.1002/jmv.21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20:245–260. doi: 10.1002/rmv.651. [DOI] [PubMed] [Google Scholar]
- 7.Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 8.Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis. 2008;46:402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 9.Dumoulin A, Widmer AF, Hirsch HH. Comprehensive diagnostics for respiratory virus infections after transplantation or after potential exposure to swine flu A/H1N1: what else is out there? Transpl Infect Dis. 2009;11:287–289. doi: 10.1111/j.1399-3062.2009.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reijans M, Dingemans G, Klaassen CH, et al. RespiFinder: a new multiparameter test to differentially identify fifteen respiratory viruses. J Clin Microbiol. 2008;46:1232–1240. doi: 10.1128/JCM.02294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonney D, Razali H, Turner A, Will A. Successful treatment of human metapneumovirus pneumonia using combination therapy with intravenous ribavirin and immune globulin. Br J Haematol. 2009;145:667–669. doi: 10.1111/j.1365-2141.2009.07654.x. [DOI] [PubMed] [Google Scholar]
- 12.Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- 13.Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis. 2010;201:404–413. doi: 10.1086/651662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debur MC, Vidal LR, Stroparo E, et al. Human metapneumovirus infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2010;12:173–179. doi: 10.1111/j.1399-3062.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira R, Machado A, Tateno A, Boas LV, Pannuti C, Machado C. Frequency of human metapneumovirus infection in hematopoietic SCT recipients during 3 consecutive years. Bone Marrow Transplant. 2008;42:265–269. doi: 10.1038/bmt.2008.153. [DOI] [PubMed] [Google Scholar]
- 16.Cane PA, van den Hoogen BG, Chakrabarti S, Fegan CD, Osterhaus AD. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–310. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- 17.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 18.Franquet T, Rodríguez S, Martino R, Salinas T, Giménez A, Hidalgo A. Human metapneumovirus infection in hematopoietic stem cell transplant recipients: high-resolution computed tomography findings. J Comput Assist Tomogr. 2005;29:223–227. doi: 10.1097/01.rct.0000157087.14838.4c. [DOI] [PubMed] [Google Scholar]
- 19.Huck B, Egger M, Bertz H, et al. Human metapneumovirus infection in a hematopoietic stem cell transplant recipient with relapsed multiple myeloma and rapidly progressing lung cancer. J Clin Microbiol. 2006;44:2300–2303. doi: 10.1128/JCM.00152-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ison MG, Hayden FG. Viral infections in immunocompromised patients: what’s new with respiratory viruses? Curr Opin Infect Dis. 2002;15:355–367. doi: 10.1097/00001432-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Lacey SF, Diamond DJ, Zaia JA. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol Blood Marrow Transplant. 2004;10:433–447. doi: 10.1016/j.bbmt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Safdar A. Immune modulatory activity of ribavirin for serious human metapneumovirus disease: early i.v. therapy may improve outcomes in immunosuppressed SCT recipients. Bone Marrow Transplant. 2008;41:707–708. doi: 10.1038/bmt.2008.80. [DOI] [PubMed] [Google Scholar]
- 23.Fischer SA. Emerging viruses in transplantation: there is more to infection after transplant than CMV and EBV. Transplantation. 2008;86:1327–1339. doi: 10.1097/TP.0b013e31818b6548. [DOI] [PubMed] [Google Scholar]
- 24.Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother. 2006;50:774–777. doi: 10.1128/AAC.50.2.774-777.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]


